Abstract
BACKGROUND:
Human telomere disease consists of a wide spectrum of disorders, including pulmonary, hepatic, and bone marrow abnormalities. The extent of bone marrow and liver abnormalities in patients with interstitial lung disease (ILD) and short telomeres is unknown.
METHODS:
The lung transplant clinic established a prospective protocol to identify short telomeres in patients with ILD not related to connective tissue disease or sarcoidosis. Patients with short telomeres underwent bone marrow biopsies, liver biopsies, or both as part of the evaluation for transplant candidacy.
RESULTS:
One hundred twenty-seven patients met ILD categorization for inclusion. Thirty were suspected to have short telomeres, and 15 had the diagnosis confirmed. Eight of 13 (53%) patients had bone marrow abnormalities. Four patients had hypocellular marrow associated with macrocytosis and relatively normal blood counts, which resulted in changes to planned immunosuppression at the time of transplant. Four patients with more severe hematologic abnormalities were not listed because of myelodysplastic syndrome (two); monoclonal gammopathy of unclear significance (one); and hypocellular marrow, decreased megakaryocyte lineage associated with thrombocytopenia (one). Seven patients underwent liver biopsies, and six had abnormal liver pathology. These abnormalities did not affect listing for lung transplant, and liver biopsies are no longer routinely obtained.
CONCLUSIONS:
Subclinical bone marrow and liver abnormalities can be seen in patients with ILD and short telomeres, in some cases in the absence of clinically significant abnormalities in peripheral blood counts and liver function tests. A larger study examining the implication of these findings on the outcome of patients with ILD and short telomeres is needed.
Human telomere disease consists of a wide spectrum of disorders, including pulmonary, hepatic, and bone marrow abnormalities (eg, aplastic anemia, acute leukemia).1 Mutations in genes controlling telomere length have incomplete penetrance and can induce single or multiorgan disease, associated with different phenotypes and varying degrees of severity.1,2 Short telomeres and telomerase mutations are important risk factors for familial and sporadic forms of idiopathic pulmonary fibrosis (IPF).3,4 Approximately 15% of patients with familial interstitial pneumonia (FIP) have mutations in telomerase reverse transcriptase (TERT) or telomerase RNA complex (TERC).5 Moreover, about 25% of patients with sporadic IPF have short telomeres in peripheral blood leukocytes, despite no detectable telomerase mutations,6 suggesting that other genetic or nongenetic causes could lead to shortened telomeres. Usual interstitial pneumonia, the histologic hallmark of IPF, is found in 85% of patients with interstitial lung disease (ILD) and short telomeres.7 However, other ILDs7 as well as the combined pulmonary fibrosis emphysema8,9 syndrome have also been reported in association with telomerase mutations and short telomeres.
Prior studies of the manifestations of short telomeres have examined kindreds of affected subjects and found that telomere length and genetic mutations of genes controlling telomere length were associated with aplastic anemia and pulmonary and liver disease.3,10,11 However, the extent of subclinical bone marrow and/or liver disease in patients with ILD and short telomeres has not been previously investigated.
In 2011, a subject suspected of having short telomeres underwent a lung transplant at our institution, which was complicated by severe bone marrow and liver failure. This led our program to establish a comprehensive plan to evaluate subjects with ILD for potential telomeropathy, as defined by short telomeres and any organ dysfunction known to be associated with functional mutations in genes encoding telomerase.12 Here, we report the results of our evaluation and its effectiveness at assessing for telomeropathy and subclinical organ dysfunction in a cohort of patients with ILD undergoing evaluation for lung transplantation.
Material and Methods
Subjects
In September 2011, the lung transplant program at Brigham and Women’s Hospital established clinical guidelines designed to increase the index of suspicion for short telomeres and associated disease(s) in patients referred for consideration of candidacy. Here, we report the results of this intervention. All patients with ILD and two or more visits to the program were included in the study cohort. Patients with sarcoidosis or connective tissue disease (CTD)-associated ILD were excluded. Patients with ILD were suspected to have short telomeres if they had any of the following:
• WBC count, hematocrit level, or platelet count below the lower limit of normal13
• Mean corpuscular volume (MCV) above the upper limit of normal14,15
• Abnormal liver function tests11
• Abnormal coagulation profile
• History or evidence of hepatosplenomegaly on abdominal ultrasonography
• Family history of interstitial pneumonia, self-reported early graying, aplastic anemia, or liver disease
Individuals with suspected short telomeres then underwent telomere length testing. They were diagnosed with short telomeres if telomere length was shorter than the 10th percentile of the reference population. Patients with short telomeres were referred for bone marrow and liver biopsies to further evaluate their candidacy for lung transplantation.
Demographic and laboratory characteristics of those who were suspected of having short telomeres and those who were not are listed in e-Table 1 (453.4KB, pdf) . An analysis of the results of the implementation of this protocol was conducted with approval from the Institutional Review Board (Protocol# 2011-P-002391/1).
Diagnosis of ILD and Clinical Information
A review of medical records including CT scanning and existing surgical lung biopsy results was used to determine the diagnosis of ILD. Hematology, chemistry, and pathology results were obtained through a review of the computerized medical records.
Telomere Length by Flow Fluorescence In Situ Hybridization
Telomere length analysis in peripheral blood lymphocytes was performed with a Clinical Laboratory Improvement Amendments-approved test performed at Repeat Diagnostics Laboratory, Vancouver, Canada.16,17 The control population for this test consisted of 835 subjects identified only by age and sex; no other demographic or medical information was available.17 The age distribution of the control population was as follows: 0 to 40 years, 398; 41 to 50 years, 80; 51 to 70 years, 155; and > 70 years, 202 control subjects. Telomere length was defined as short if it was under the 10th percentile of age-matched control subjects, which represented the statistical outlier of telomere length compared with the control population (details of the study population are in e-Table 1 (453.4KB, pdf) of Reference 14).
Sequencing and Mutation Analysis
Seven subjects consented to genetic testing. Sequencing and mutational analysis for TERT and TERC were performed in a Clinical Laboratory Improvement Amendments-approved laboratory.
Statistical Analysis
Results are expressed as mean ± SD for n number of samples. Analysis of difference between groups was conducted using an analysis of variance, t test, or Fisher exact test as appropriate. A two-sided P value < .05 was used for statistical significance. All analysis was done using GraphPad Prism software (GraphPad 5.0).
Results
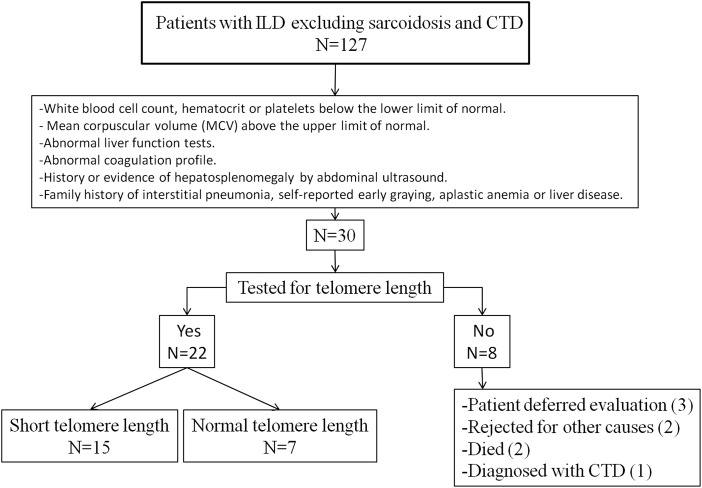
From September 2011 until December 2013, 127 patients met our preestablished inclusion criteria of ILD without evidence of sarcoidosis or CTD and at least two visits to our lung transplant clinic (Fig 1, Table 1). Most patients (60.6%) were diagnosed with IPF. Based on our predefined screening criteria, 30 patients (23.6%) were suspected of having short telomeres, and 22 of 127 (17.3%) patients underwent telomere length testing. Eight patients did not undergo telomere testing for the following reasons: Three patients were too well for transplant and elected to defer testing, two patients died, two patients were rejected for transplant for other causes, and one patient was diagnosed with CTD during the evaluation process. Macrocytosis (86.3%) was the most common finding that led to telomere testing, followed by a family history of early graying and FIP (e-Table 2 (453.4KB, pdf) ).
Figure 1 –
Study flow diagram. CTD = connective tissue disease; ILD = interstitial lung disease.
TABLE 1 ] .
Summary of Study Patients
| Interstitial Pneumonia | IPF | NSIP | HP | Unclassified IP | CPFE | Total |
| No. (%)a | 77 (60.6) | 12 (9.4) | 5 (3.9) | 19 (14.9) | 14 (11) | 127 |
| Tested for telomere length,b No. (%) | 11 (14.2) | 1 (8.3) | 2 (40) | 5 (26.3) | 3 (21.4) | 22 (17.3) |
| Confirmed short telomeres,c No. (%) | 9 (81.8) | 0 (0) | 2 (100) | 2 (40) | 2 (66.6) | 15 (68.2) |
CPFE = combined pulmonary fibrosis emphysema; HP = hypersensitivity pneumonitis; IP = interstitial pneumonia; IPF = idiopathic pulmonary fibrosis; NSIP = nonspecific interstitial pneumonia.
Percent of total.
Percent of No.
Percent of tested.
There were no statistically significant differences in age or demographic characteristics between patients who did not meet criteria for telomere testing and those who were suspected to have short telomeres. Patients who were suspected of having short telomeres had significantly higher MCV and lower platelet counts (e-Table 1 (453.4KB, pdf) ).
Targeted Testing for Short Telomeres in a Cohort of Patients With ILD
Using the previously established criteria predictive of telomeropathy14 described previously we prospectively assessed 22 patients with ILD being evaluated for lung transplantation and found that 15 (68.2%) had telomeres shorter than the 10th percentile predicted for age (Table 1).
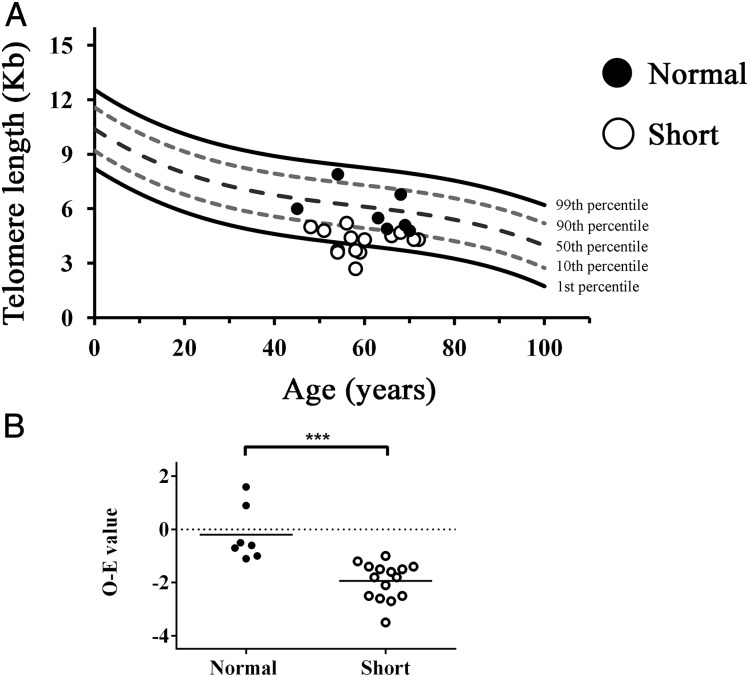
As expected, telomere length was significantly shorter in the cohort diagnosed with short telomeres compared with those who had normal telomere length (Fig 2). The average difference in lymphocyte telomere length between patient and expected age-adjusted 50th percentile value was −1.94 ± 0.69 kb for patients with short telomeres as compared with −0.2 ± 1.03 kb in those with normal telomere length (P < .001).
Figure 2 –
Lymphocyte telomere length in patients with interstitial lung disease and suspected short telomeres. Telomere length as measured by flow fluorescence in situ hybridization is shown for patients with short telomeres (○) and those with normal telomere length (●). A, Lymphocyte telomere length of tested subjects shown relative to indicated percentiles of a reference cohort as reported in the clinical testing by Repeat Diagnostics (Vancouver, Canada). B, Mean O − E (50th percentile age-adjusted telomere length) for subjects with interstitial lung disease with (○) and without (●) short telomeres. E = expected; O = observed.
The average age of patients tested for short telomeres was 60.2 ± 7.7 years (range, 45-72 years); 15 of 22 (68.2%) patients were men (Table 2). There was no statistically significant difference in RBC indexes and platelet counts between 15 patients with short telomeres and the seven patients with normal telomere length; however, this could be because of the small number of subjects in each group. In subjects with short telomeres, median hemoglobin and MCV levels were 13.5 g/dL and 97.9 μm3, indicating that for most patients, changes in peripheral blood counts were relatively mild.
TABLE 2 ] .
Summary of Demographic and Laboratory Data of Patients Who Underwent Telomere Length Analysis
| Telomere Length | Tested for Telomere Length (N = 22) | Short Telomeres (n = 15) | Normal Telomere Length (n = 7) |
| Age, mean ± SD | 60.2 ± 7.7 | 59.3 ± 7.0 | 62.0 ± 9.2 |
| Sex, male, No. (%) | 15 (68.2) | 10 (66.6) | 5 (71.4) |
| Hematologic analysis | |||
| Hemoglobin, g/dL | 14.0 ± 1.7 | 13.5 ± 1.7 | 15.1 ± 1.4 |
| RBC count, 106/μL | 4.33 ± 0.54 | 4.14 ± 0.46 | 4.74 ± 0.37 |
| MCV, μm3 | 96.9 ± 6.6 | 97.9 ± 7.2 | 95.0 ± 4.9 |
| Platelets, 103/μL | 215 ± 63 | 211 ± 73 | 223 ± 33 |
| Liver abnormalitiesa | 6 | 4 | 2 |
| Family history | |||
| Fibrosis | 10 | 7 | 3 |
| Early graying | 10 | 8 | 2 |
| Cirrhosis/aplastic anemia | 1 | 1 | 0 |
MCV = mean corpuscular volume.
Liver abnormalities: abnormal liver function tests or liver ultrasonography.
In patients with ILD referred for lung transplantation and who were tested for telomere length, macrocytosis had a sensitivity of 68.4% and a positive predictive value of 86.6% in identifying subjects with short telomeres. The prevalence of macrocytosis in patients with short telomeres was 86.6% (13 of 15 patients). The two patients who had short telomeres without macrocytosis had a history of FIP and early graying, and one subject also had abnormal liver function tests (Table 2, e-Table 3 (453.4KB, pdf) ).
Subclinical Bone Marrow Abnormalities in Patients With ILD and Short Telomeres
Short telomeres have been associated with aplastic anemia, cytopenias, and macrocytosis.10 To investigate the degree of associated marrow dysfunction, patients with short telomeres underwent a bone marrow biopsy. Among the 15 patients with short telomeres, 13 (87%) underwent a bone marrow biopsy, and two patients were still completing their lung transplant evaluations at the time of manuscript preparation. Bone marrow abnormalities were found in eight of 13 (61.5%) patients (Table 3). Of the eight patients with bone marrow abnormalities, four demonstrated hypocellular marrow alone, two demonstrated findings consistent with early myelodysplastic syndrome, one patient had hypocellular marrow with decreased megakaryocyte lineage, and one patient was noted to have increased plasma cells and was ultimately diagnosed with monoclonal gammopathy of undetermined significance (MGUS). In this group of eight patients with abnormal bone marrow findings, the median hemoglobin level was 12.9 g/dL (range, 11.3-17 g/dL). Two of 15 subjects with short telomeres had a low platelet count (13.3%), and all subjects had normal WBC counts. Only one of the 13 patients who underwent bone marrow biopsy was taking immunosuppressive therapy (mycophenolate mofetil); however, this patient was noted to have peripheral blood count abnormalities that preceded the initiation of immunosuppressive therapy. No complications developed as a result of the bone marrow biopsies. Of note, patients who had normal bone marrow biopsies but slightly abnormal peripheral blood counts were ultimately diagnosed with anemia of chronic disease.
TABLE 3 ] .
Outcome of Evaluation of Short Telomeres on Lung Transplant Candidacy
| Sex, Age, y | Diagnosis | Peripheral Blood | Bone Marrow | Liver Function | Liver Biopsya | Transplant Candidacy |
| M, 72 | IPF | Macrocytosis | Hypocellular | Normal | … | Listed no induction |
| M, 58 | IPF | Normal | Normal | Normal | Marked regenerative changes | Listed |
| M, 54 | CPFE | Macrocytosis | Hypocellular | Normal | … | Listed no induction |
| F, 68 | IPF | Anemia, macrocytosis | Normal | Normal | Patchy mild portal chronic inflammation, spotty hepatocyte necrosis, and marked regenerative changes | Listed |
| M, 71 | IPF | Anemia, macrocytosis | Plasmacytoma/MGUS | Normal | Mild regenerative changes | Not listed MGUS |
| M, 58 | IPF | Anemia, macrocytosis | Hypocellular marrow. Erythroid dyspoiesis with megaloblastoid changesb | Normal | … | Not listed early MDS |
| M, 58 | IPF | Anemia, macrocytosis, thrombocytopenia | Hypocellular with decreased megakaryocytes | Normal | … | Not listed. Decreased megakaryocytes on bone marrow biopsy |
| F, 59 | IPF | Normal | Normal | Elevated enzymes, ALK P | Normal | Listed |
| F, 66 | HP | Anemia, macrocytosis | Normal | Normal | Regenerative changes | Listed |
| F, 51 | HP | Macrocytosis | Hypocellular | Low total protein | Regenerative changes | Listed no induction |
| M, 48 | U | Macrocytosis | Normal | Normal | … | Listed |
| M, 60 | CPFE | Macrocytosis | Hypocellular | Normal enzymes. Fatty liverc | Mild steatohepatitis. Moderate macrovesicular steatosis. Focal pericellular fibrosis. No cirrhosis. | Listed no induction |
| M, 57 | U | Macrocytosis, thrombocytopenia | Hypocellular marrow/karyotypic changesd | Normal | … | Not listed (early MDS and severe right ventricular dysfunction) |
ALK P = alkaline phosphatase; F = female; M = male; MDS = myelodysplastic syndrome; MGUS = monoclonal gammopathy of undetermined significance; U = unclassified interstitial pneumonia. See Table 1 legend for expansion of other abbreviations.
Seven subjects underwent liver biopsies, since it was believed not to influence management.
Could represent early myelodysplastic syndrome.
By abdominal ultrasonography.
Early myelodysplastic syndrome. Trisomy 1q and monosomy 7q.
Subclinical Liver Abnormalities in Patients With ILD and Short Telomeres
In previous reports, liver fibrosis has been associated with IPF, especially in patients with dyskeratosis congenita.18 To investigate the extent of subclinical liver disease in our cohort of patients with ILD and short telomeres, seven patients underwent liver biopsies, five of whom had no prior evidence of liver disease such as abnormal liver function tests or fatty liver. Of the remaining two patients, one had fatty liver evidence on abdominal ultrasonography and the other had abnormal liver function tests (Table 3). Liver biopsy specimen was abnormal in six of seven (86%) patients. Of the six patients with an abnormal liver biopsy specimen, four patients demonstrated evidence of regenerative changes alone, and two patients demonstrated evidence of hepatitis and steatosis without cirrhosis. One of these patients had evidence of fatty liver on abdominal ultrasonography. One patient had bleeding after liver biopsy that resolved without intervention, and no other complications of biopsies were observed.
Impact of Evaluation on Lung Transplant Candidacy
Of the 13 patients with short telomeres who underwent bone marrow biopsies, nine were accepted and listed for lung transplantation, with planned modification of standard immunosuppression (no induction) for those with hypocellular bone marrow. Of the four patients not deemed suitable candidates for lung transplantation, two had changes consistent with myelodysplastic syndrome on bone marrow biopsy, one patient had MGUS, and one patient had a hypocellular marrow, rare megakaryocytes on bone marrow biopsy and thrombocytopenia. Liver biopsies did not have any impact on the evaluation process (Table 3).
Identification of Telomerase Mutations
Of our cohort of patients with short telomeres, seven patients agreed to genetic testing. Of the seven patients who underwent genetic testing, two patients were noted to have coding mutations in TERT believed likely to be pathogenic (one patient was noted to have a mutation [c.2768C > T] resulting in a substitution of leucine for proline at position 923, and the other patient was noted to have a mutation [c.2225G > A] resulting in a histidine replacing an arginine at position 742), three patients were noted to have a previously described nonsynonymous coding variant in TERT (Ala 1062 Thr), and two patients had no detectable mutations in either TERT or TERC (Table 4). Of the two patients with likely pathogenic mutations in TERT, one patient (c.2768C > T) was listed for lung transplant, and the other (c.2225G > A) was rejected because of bone marrow findings consistent with early myelodysplastic syndrome.
TABLE 4 ] .
Evaluation for Telomerase Mutation in Patients With Short Telomeres
| Sex, Age, y | Diagnosis | Bone Marrow | Mutation TERT/TERC |
| F, 51 | Hypersensitivity pneumonitis | Hypocellular | R742H |
| M, 58 | Idiopathic pulmonary fibrosis | Hypocellular | P923L |
| M, 58 | Idiopathic pulmonary fibrosis | Normal | A1062T |
| M, 54 | Combined pulmonary fibrosis emphysema | Hypocellular | A1062T |
| M, 60 | Combined pulmonary fibrosis emphysema | Hypocellular | A1062T |
| M, 48 | Unclassified interstitial pneumonia | Normal | No mutation identified |
| M, 57 | Unclassified interstitial pneumonia | Hypocellular marrow/karyotypic changesa | No mutation identified |
A1062T = alanine to threonine substitution at residue 1062 in TERT; P923L = proline to leucine substitution at residue 923 in TERT; R742H = arginine to histidine substitution at residue 742 in TERT. See Table 3 legend for expansion of other abbreviations.
Trisomy 1q and monosomy 7q.
Comparison of Telomere Length in Multiple Cellular Subtypes
Previous reports have shown differences in telomere attrition between different leukocyte cellular subtypes, with natural killer (NK) (NK/T cells) showing the largest change in non-age-adjusted measurements and naive lymphocytes showing the most decline in age-adjusted length in patients with telomerase deficiency compared with their unaffected relatives.17 Telomere length was measured using flow fluorescence in situ hybridization in six different leukocyte subtypes (lymphocytes, granulocytes, naive T cells [CD45RA+], memory T cells [CD45RA−], B cells [CD20], and NK cells [CD57]), and a subtype analysis of peripheral blood leukocytes was performed. First, in our cohort of patients with normal or short telomere length, we compared telomere length and age-adjusted telomere length in all six leukocyte subtypes. Results showed a statistically significant difference in age-adjusted and absolute telomere length in all leukocyte subtypes (e-Figs 1A, 1B (453.4KB, pdf) , respectively). Further, in the subgroup of patients with short telomeres, we found that granulocyte age-adjusted telomere length was significantly shorter than telomere length in all other leukocyte subtypes (e-Fig 1C (453.4KB, pdf) ). In this relatively small group, an analysis of telomere length and age-adjusted telomere length in all six leukocyte subtypes did not show any statistically significant difference between patients with bone marrow abnormalities and those with normal bone marrow biopsy results (e-Fig 2 (453.4KB, pdf) , e-Table 4 (453.4KB, pdf) ).
Discussion
We report significant subclinical marrow and liver abnormalities in our cohort of patients with ILD and short telomeres referred for lung transplantation. This group of patients showed important bone marrow abnormalities, in some cases in the absence of clinically significant changes in peripheral blood counts.
Others have shown that short telomere syndrome can present with lower RBC counts, leukopenia, thrombocytopenia, abnormal liver function, increased MCV, and early graying.14 We used these criteria to identify a subset of patients who had a high likelihood of having telomere disease. Our protocol led to the detection of short telomeres in 68.2% of patients in whom short telomere syndrome was suspected.
Short telomeres and telomeropathies are a spectrum of diseases that can include lung, bone marrow, and liver disease with incomplete penetrance. Macrocytosis15 has previously been associated with short telomere syndrome; however, the extent of bone marrow involvement in patients with mild peripheral blood findings has not been previously described. Our data show the presence of significant abnormalities in > 50% of tested patients even though in some cases peripheral blood abnormalities were minimal.
Previous reports have shown that significant liver abnormalities can exist in patients with short telomeres in the absence of dyskeratosis congenita.3,10 In the series from Alder et al,3 3% of patients with IPF had concomitant liver cirrhosis. Our data show that in subjects with ILD associated with short telomeres, liver biopsy results were abnormal in most patients, even in the absence of peripheral blood abnormalities. In six of seven (86%) patients, liver biopsy results showed abnormalities, with regenerative changes being the most common. However, more significant findings of inflammation, steatosis, and fibrosis were also observed. In our series, none of the patients had cirrhosis, and the biopsy findings did not influence the decision-making process regarding lung transplant eligibility. These findings led us to modify our protocol, and we no longer obtain liver biopsy samples.
The constellation of lung disease and bone marrow and liver abnormalities is highly suggestive of germline mutations in telomerase. Typically, in these instances, telomere length is less than the first percentile predicted for age.7 Genetic sequencing of TERT and TERC in seven patients with short telomeres revealed novel and likely pathogenic mutations in two patients. Three patients with short telomeres were noted to have a previously described nonsynonymous coding variant (Ala 1062 Thr) in TERT, for which there are somewhat conflicting data on the pathogenicity. Estimates of the allelic frequency of this mutation range from 0.6% in an ethnically diverse population19 to 2.5% in a white population.2 In vitro analyses have previously demonstrated that heterozygosity in the Ala 1062 Thr variant of TERT does not result in significant decrease in telomerase activity.3 In contrast, another study has demonstrated that the Ala 1062 Thr variant of TERT can result in a decrease in telomerase enzymatic activity via haploinsufficiency.19 These findings raise the possibility of mutations in other genes involved in telomere length or, perhaps, global effects of oxidative stress on telomere length.20,21
Telomere length in different cellular subtypes in peripheral blood has been studied in the context of the presence or absence of TERT and TERC mutations. Our data demonstrate that different cellular subtypes have similar differences in telomere length. This analysis demonstrates two important findings. First, telomere length in granulocytes is believed to be reflective of myeloid precursor telomere length, since granulocytes do not undergo cell division.22 Granulocyte age-adjusted telomere length was significantly shorter than lymphocytes, naive and memory T cells, B cells, and NK cells. Second, and surprisingly, granulocyte age-adjusted telomere length could not differentiate between patients with and without marrow abnormalities.
There are several limitations to the present study. First, we did not test the entire cohort of patients for telomere length; it is conceivable that other patients in the cohort who did not meet our testing criteria have short telomeres. Since these tests were performed in a clinical setting, it was not justifiable to test subjects in whom we did not have a clinical suspicion of short telomeres. Second, the cohort examined included patients with advanced lung disease, who may reflect a skewed population with a higher predilection toward organ dysfunction. Finally, and importantly, there is a lack of longitudinal follow-up. It is of interest to know whether the changes detected in marrow and/or liver biopsies will result in the future development of clinically significant cytopenias, hematologic malignancies, or liver failure in patients with ILD and short telomeres. This question remains unanswered and merits additional longitudinal follow-up studies.
Since these patients had end-stage lung disease and limited life expectancy, these findings were unlikely to progress and develop into clinically significant disorders in the absence of lung transplantation. A recent report by Silhan et al23 documents the increased risks of hematologic abnormalities, particularly thrombocytopenias, in patients with short telomeres and telomerase mutations after lung transplantation. Drugs used to treat patients after transplantation, such as immunosuppressive and antiviral medications, can lead to declines in peripheral blood counts, and it is possible that those with a hypocellular marrow prior to transplant are at higher risk of developing cytopenias when they receive these drugs. Limited information is available regarding hematologic complications after lung transplant, but neutropenia is known to be associated with graft failure and poor outcomes after kidney transplant.24 Furthermore, infection is one of the leading causes of death in the first year after transplant, and coexisting cytopenias could complicate treatment of infections posttransplant.25 In the absence of significant changes in peripheral blood count or features suggestive of myelodysplastic syndrome or predisposition to other hematologic malignancies, patients with short telomeres and bone marrow dysfunction are candidates for lung transplant in our program; however, we have implemented changes to planned immunosuppression by avoiding induction at the time of transplant. Our limited experience highlights the need to determine the outcome of patients with telomeropathies, particularly regarding bone marrow or liver dysfunction, and the development of secondary hematologic malignancies or susceptibility to infections in the setting of solid organ transplantation.
Conclusions
In summary, our study shows that predetermined noninvasive testing can identify a subset of patients with ILD who should be tested for short telomeres. Further, seemingly mild peripheral blood abnormalities were associated with significant bone marrow pathology and regenerative changes on liver biopsy. These results increase our understanding of telomeropathies and have potentially important implications for patients with interstitial lung disease. A larger study is indicated to further examine the prevalence of these findings and their potential implications for patients with short telomeres who undergo lung transplantation.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: S. E.-C. is the guarantor of the paper and takes responsibility for the integrity of the work as a whole, from inception to published article. I. O. R., P. C. C., H. J. G., and S. E.-C. contributed to conception and design; G. G., Y. C., C. M., G. M. H., B. A. R., and S. E.-C. contributed to analysis and interpretation; and G. G., I. O. R., Y. C., C. M., P. C. C., B. A. R., H. J. G., and S. E.-C. contributed to drafting the manuscript for important intellectual content.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design of the study, data collection and analysis, or in the preparation of the manuscript.
Other contributions: We thank Diana Toledo, CGC, genetic counselor at the BWH Pulmonary Genetics Center for her assistance in the genetic characterization and counseling of our patients. We also thank Bernadette R. Gochuico, MD, for helpful discussions and suggestions and Geraldine Aubert, PhD, for help with generating figures.
Additional information: The e-Figures and e-Tables can be found in the Supplemental Materials section of the online article.
ABBREVIATIONS
- CTD
connective tissue disease
- FIP
familial interstitial pneumonia
- ILD
interstitial lung disease
- IPF
idiopathic pulmonary fibrosis
- MCV
mean corpuscular volume
- MGUS
monoclonal gammopathy of unknown significance
- NK
natural killer
- TERT
telomerase reverse transcriptase
- TERC
telomerase RNA complex
Footnotes
FOR EDITORIAL COMMENT SEE PAGE 1450
Drs Goldberg and El-Chemaly contributed equally to this manuscript.
FUNDING/SUPPORT: Dr El-Chemaly is funded by the National Institutes of Health (NIH) [Grant R21 HL119902-01] and the American Thoracic Society/American Lung Association foundation grant. Drs Hunninghake and Rosas are supported by the NIH [Grant R01 HL111024 to Dr Hunninghake and Grants U01HL105371 and P01HL114501 to Dr Rosas].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Calado RT, Young NS. Telomere diseases. N Engl J Med. 2009;361(24):2353-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Chemaly S, Ziegler SG, Calado RT, et al. Natural history of pulmonary fibrosis in two subjects with the same telomerase mutation. Chest. 2011;139(5):1203-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alder JK, Chen JJ, Lancaster L, et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci U S A. 2008;105(35):13051-13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armanios MY, Chen JJ, Cogan JD, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356(13):1317-1326. [DOI] [PubMed] [Google Scholar]
- 5.Tsakiri KD, Cronkhite JT, Kuan PJ, et al. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci U S A. 2007;104(18):7552-7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cronkhite JT, Xing C, Raghu G, et al. Telomere shortening in familial and sporadic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178(7):729-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diaz de Leon A, Cronkhite JT, Katzenstein AL, et al. Telomere lengths, pulmonary fibrosis and telomerase (TERT) mutations. PLoS ONE. 2010;5(5):e10680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alder JK, Guo N, Kembou F, et al. Telomere length is a determinant of emphysema susceptibility. Am J Respir Crit Care Med. 2011;184(8):904-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nunes H, Monnet I, Kannengiesser C, et al. Is telomeropathy the explanation for combined pulmonary fibrosis and emphysema syndrome? Report of a family with TERT mutation. Am J Respir Crit Care Med. 2014;189(6):753-754. [DOI] [PubMed] [Google Scholar]
- 10.Calado RT, Regal JA, Kleiner DE, et al. A spectrum of severe familial liver disorders associate with telomerase mutations. PLoS ONE. 2009;4(11):e7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talbot-Smith A, Syn WK, MacQuillan G, Neil D, Elias E, Ryan P. Familial idiopathic pulmonary fibrosis in association with bone marrow hypoplasia and hepatic nodular regenerative hyperplasia: a new “trimorphic” syndrome. Thorax. 2009;64(5):440-443. [DOI] [PubMed] [Google Scholar]
- 12.Armanios M. Telomeres and age-related disease: how telomere biology informs clinical paradigms. J Clin Invest. 2013;123(3):996-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamaguchi H, Calado RT, Ly H, et al. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med. 2005;352(14):1413-1424. [DOI] [PubMed] [Google Scholar]
- 14.Diaz de Leon A, Cronkhite JT, Yilmaz C, et al. Subclinical lung disease, macrocytosis, and premature graying in kindreds with telomerase (TERT) mutations. Chest. 2011;140(3):753-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kozlitina J, Garcia CK. Red blood cell size is inversely associated with leukocyte telomere length in a large multi-ethnic population. PLoS ONE. 2012;7(12):e51046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baerlocher GM, Vulto I, de Jong G, Lansdorp PM. Flow cytometry and FISH to measure the average length of telomeres (flow FISH). Nat Protoc. 2006;1(5):2365-2376. [DOI] [PubMed] [Google Scholar]
- 17.Aubert G, Baerlocher GM, Vulto I, Poon SS, Lansdorp PM. Collapse of telomere homeostasis in hematopoietic cells caused by heterozygous mutations in telomerase genes. PLoS Genet. 2012;8(5):e1002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dokal I. Dyskeratosis congenita in all its forms. Br J Haematol. 2000;110(4):768-779. [DOI] [PubMed] [Google Scholar]
- 19.Calado RT, Regal JA, Hills M, et al. Constitutional hypomorphic telomerase mutations in patients with acute myeloid leukemia. Proc Natl Acad Sci U S A. 2009;106(4):1187-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Opresko PL, Fan J, Danzy S, Wilson DM, III, Bohr VA. Oxidative damage in telomeric DNA disrupts recognition by TRF1 and TRF2. Nucleic Acids Res. 2005;33(4):1230-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong JY, De Vivo I, Lin X, Fang SC, Christiani DC. The relationship between inflammatory biomarkers and telomere length in an occupational prospective cohort study. PLoS ONE. 2014;9(1):e87348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weng N. Interplay between telomere length and telomerase in human leukocyte differentiation and aging. J Leukoc Biol. 2001;70(6):861-867. [PubMed] [Google Scholar]
- 23.Silhan LL, Shah PD, Chambers DC, et al. Lung transplantation in telomerase mutation carriers with pulmonary fibrosis. Eur Respir J. 2014;44(1):178-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hurst FP, Belur P, Nee R, et al. Poor outcomes associated with neutropenia after kidney transplantation: analysis of United States Renal Data System. Transplantation. 2011;92(1):36-40. [DOI] [PubMed] [Google Scholar]
- 25.Yusen RD, Christie JD, Edwards LB, et al. ; International Society for Heart and Lung Transplantation. The Registry of the International Society for Heart and Lung Transplantation: thirtieth adult lung and heart-lung transplant report–2013; focus theme: age. J Heart Lung Transplant. 2013;32(10):965-978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement