Abstract
The Useful Field of View Test (UFOV1) has been used to examine age-related changes in visual processing and cognition and as an indicator of everyday performance outcomes, particularly driving, for over 20 years. How UFOV performance changes with age and what may impact such changes have not previously been investigated longitudinally. Predictors of change in UFOV performance over a five-year period among control-group participants (n = 690) from the Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) study were examined. Random effects models were estimated with four-subtest total UFOV as the outcome and baseline age, education, gender, race, visual acuity, depressive symptoms, mental status, and self-rated health, as well as attrition, as predictors. UFOV performance generally followed a curvilinear pattern, improving and then declining over time. Only increased age was consistently related to greater declines in UFOV performance over time. UFOV and WAIS-R Digit Symbol Substitution (DSS), a standard measure of cognitive speed, had similar trajectories of change. The implications of these results are discussed.
Keywords: UFOV, useful field of view, longitudinal, random effects, speed of processing
The “functional” or “occupational” visual field was introduced by Sanders (1970) and later defined by Verriest and colleagues (1985) as the visual area over which people perceive information within a brief glance. The Useful Field of View Test (UFOV) was originally developed to examine age-related changes in the functional visual field that were undetectable by standard ophthalmic measures (Bergen & Julesz, 1983; Plude & Hoyer, 1981; Rabbitt, 1965a, 1965b; Scialfa, Kline, & Lyman, 1987; R. Sekuler & Ball, 1986). Further research indicated that although UFOV performance is impacted by visual capacities, the eccentricity of peripheral targets was not an important factor in age-related performance difficulties (Seiple, Szlyk, Yang, & Holopigian, 1996; A. B. Sekuler, Bennett, & Mamelak, 2000). Other researchers concluded that the UFOV would best be conceptualized as efficiency in processing (Ball, Owsley, & Beard, 1990; Owsley, Ball, & Keeton, 1995; A. B. Sekuler, Bennett, & Mamelak, 2000). The current PC-version of the UFOV measures speed of information processing across four tasks (Edwards et al., 2006; Edwards et al., 2005). Performance is more strongly related to measures of cognition than vision, and may tap speed of processing in particular (Ball, Owsley, Sloane, Roenker, & Bruni, 1993; Edwards et al., 2005; Goode et al., 1998).
Utility of the UFOV
The UFOV is of interest because it not only captures age-related cognitive decline, but has ecological validity in predicting everyday outcomes. For example, UFOV performance has been related to performance of instrumental activities of daily living (Owsley, Sloane, McGwin Jr., & Ball, 2002; K. M. Wood et al., 2005) and indices of mobility such as life space, functional reach, and ambulatory abilities (Broman et al., 2004; Owsley & McGwin Jr, 2004; Riolo, 2003; Sims, McGwin, Pulley, & Roseman, 2001; Stalvey, Owsley, Sloane, & Ball, 1999). The test consistently predicts a variety of driving outcomes among older adults such as on-road driving (Myers, Ball, Kalina, Roth, & Goode, 2000; Roenker, Cissell, Ball, Wadley, & Edwards, 2003; J. M. Wood & Troutbeck, 1995), driving-simulator performance (Hoffman, McDowd, Atchley, & Dubinsky, 2005; Rizzo, Reinach, McGehee, & Dawson, 1997) and at-fault crash involvement (Ball & Owsley, 1993; Ball et al., 2006; Owsley et al., 1998; Owsley, Ball, Sloane, Roenker, & Bruni, 1991; Owsley, McGwin, & Ball, 1998). Today the UFOV is commonly used in medical, occupational, and rehabilitation settings, not only with older adults, but also with clinical populations at risk for cognitive impairment, including patients with Alzheimer's disease (Duchek, Hunt, Ball, Buckles, & Morris, 1998; Rizzo, Reinach, McGehee, & Dawson, 1997), stroke (Fisk, Owsley, & Mennemeier, 2002; Marshall et al., 2007), traumatic brain injury (Fisk, Novack, Mennemeier, & Roenker, 2002; Novack et al., 2006), multiple sclerosis (Shawaryn, Schultheis, Garay, & Deluca, 2002), and Parkinson's disease (Uc et al., 2006).
The UFOV is also of interest because evidence suggests that age-related declines in performance can be reversed through cognitive speed of processing training (Ball, Beard, Roenker, Miller, & Griggs, 1988; Ball et al., 2002; R. Sekuler & Ball, 1986; Willis et al., 2006). Such training not only improves UFOV performance, but also transfers to enhanced IADL performance (Edwards et al., 2002; Edwards, Wadley, Vance, Roenker, & Ball, 2005), maintained health-related quality of life (Wolinsky, Unverzagt, Smith, Jones, Stoddard et al., 2006; Wolinsky, Unverzagt, Smith, Jones, Wright et al., 2006), and improved on-road driving performance (Roenker, Cissell, Ball, Wadley, & Edwards, 2003).
Although cross-sectional research clearly indicates age-related differences in UFOV performance (Edwards et al., 2006; A. B. Sekuler, Bennett, & Mamelak, 2000), how UFOV performance changes with age has not been longitudinally examined. Thus, the present study will determine average trajectories of change, inter-individual differences in change, and predictors of change on the UFOV over a five-year period. There are several reasons why it is important to evaluate such data. First, different sources of variance may underlie the initial level of performance and rate of change (MacDonald, Hultsch, Strauss, & Dixon, 2003). Demographic and health-related factors other than age may prove to be influential. Second, determining longitudinal trajectories of UFOV performance among older adults will be helpful for guiding cognitive training interventions, as well as for comparing normal age-related decline to that observed in clinical populations. Third, there has been debate among cognitive aging researchers regarding whether or not the UFOV is primarily a speed of processing measure (Ball, Edwards, & Ross, 2007). Others have emphasized the role of visual attention in tasks somewhat similar to the UFOV (Scalf et al., 2007). This question will be addressed by comparing UFOV performance over time to a standard measure of processing speed, the WAIS-R Digit Symbol Substitution Test (DSS, Wechsler, 1981).
Processing speed is a leading indicator of cognitive aging, especially for memory and spatial ability (e.g., Finkel, Reynolds, McArdle, & Pedersen, 2007; Lemke & Zimprich, 2005). Age strongly predicts performance on cognitive speed measures like the DSS (Hoyer, Stawski, Wasylyshyn, & Verhaeghen, 2004). Moreover, rates of age changes have not been found to vary as a function of demographic or physical health variables (Anstey, Hofer, & Luszcz, 2003; Hoyer, Stawski, Wasylyshyn, & Verhaeghen, 2004; MacDonald, Hultsch, Strauss, & Dixon, 2003; Salthouse, 1992). Variables like education, gender, self-rated health, medical conditions, and depressive symptoms predict level of cognitive speed performance, but not rate of change (Anstey, Hofer, & Luszcz, 2003; MacDonald, Hultsch, Strauss, & Dixon, 2003).
Hypotheses
Based on the research described above, the following hypotheses were generated regarding longitudinal changes in UFOV performance among older adults. First, age was expected to predict both level of performance and rate of change, with older individuals performing less well initially, and declining faster, than relatively younger individuals. Second, we expected demographic and health variables to predict level of UFOV performance, but not rate of change. Third, we expected trajectories of change for UFOV and DSS to show similar patterns. Finally, we expected that changes in DSS would be associated with changes in UFOV, indicating that the two measures tap a similar construct. To test our hypotheses, we used data from the Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) study. The ACTIVE study evaluated the impact of three cognitive training interventions for community-dwelling older adults over a five-year period (see Jobe et al., 2001, for details). Random effects models were specified with total UFOV and DSS scores as criterion variables. Demographic and health variables known to correlate with UFOV and/or DSS performance (Edwards et al., 2006; MacDonald, Hultsch, Strauss, & Dixon, 2003) were chosen as predictors.
Method
Participants
ACTIVE participants were required to be at least 65 years old and meet health and functional criteria. Exclusionary criteria were: a) significant functional impairment; b) diagnosis of dementia or Mini-Mental State Examination score less than 23; c) any medical condition with a high probability of death or functional decline; d) far visual acuity worse than 20/50; e) difficulties with language and communication; or f) inability to participate in testing or training sessions. Data from control group participants only (N = 698) were examined. Four participants were excluded because they did not provide information for two of the predictors (self-rated health and depressive symptoms) at baseline or any of the follow-up sessions. An additional four participants were excluded because they did not provide baseline UFOV data and failed to complete the UFOV at more than one follow-up session. The final sample (N = 690) included 181 males and 509 females who were 65 to 94 years of age (M = 73.95, SD = 5.95). Most participants were either Caucasian (71.9%) or African American (26.8%). Years of education ranged from sixth grade to doctoral level, with an average level of 13.38 years, corresponding to “some college or vocational training.”
Measures
Center for Epidemiological Studies – Depression Scale (CES-D)
The short form of the CES-D is a 20-item questionnaire that assesses depressive symptoms (Radloff, 1977). Respondents rate the frequency of their feelings over the week preceding the assessment, from 0 (rarely or none of the time) to 3 (most of the time). Higher scores indicate greater depressive symptoms.
DSS
WAIS-R DSS assesses perceptual and motor processing speed (Wechsler, 1981). Participants receive a grid of 93 empty squares with a number (1 though 9) above each square, as well as a key of symbols that correspond to each number. In 90 seconds, participants must substitute as many symbols for numbers as possible. For the current analyses, scores were the number of substitutions completed correctly.
Far visual acuity
Far visual acuity was measured by standard procedure, using any corrective lenses normally worn by participants. An ETDRS chart was displayed by a Good-Lite model 600A light box (Good-Lite, 2007). Using the ACTIVE scoring method, 10 points are given for each line read correctly. Scores can range from 0 to 90 and can be converted into Snellen or LogMAR equivalents.
Mini-Mental State Examination (MMSE)
The MMSE contains 30 items that assess orientation, memory, attention and language (Folstein, Folstein, & McHugh, 1975). Scores can range from 0 to 30, with higher scores indicating better performance. Baseline MMSE scores ranged from 24 to 30 in the current study, demonstrating the range restriction imposed by the ACTIVE criteria.
Self-Rated Health
Participants rated their health on a scale of 1 = excellent to 5 = poor.
UFOV
The present study used the four-subtest, personal computer, touch screen version of the UFOV with test-retest reliability of 0.74 (Edwards et al., 2005). In each subtest, targets are displayed at durations ranging from 16.67 – 500 ms. Scores are the display durations at which participants accurately perform each subtest 75% of the time. The first subtest requires participants to identify a target (a silhouette of a truck or car) presented in a fixation box in the center of the screen. The second subtest measures speed of processing for a divided attention task, involving identification of the center target and simultaneous localization of a peripheral target (a silhouette of a car). The third subtest is identical to the second subtest, but also includes visual distractors (triangles of the same size and luminance as the peripheral target). The fourth subtest is the most demanding. Two targets are presented in the central fixation box (two cars, two trucks, or one car and one truck) and the participant must indicate whether these targets are the same or different. Simultaneous localization of a peripheral target embedded in distractors is also required. Participants indicate their responses to each subtest (identifying and localizing targets) by touching the screen. Total scores for the four-subtest UFOV can range from 66.68 to 2000 ms.
Attrition
Attrition was dummy coded so that participants without fifth annual follow-up data were coded as dropouts, and participants who provided fifth annual data were coded as non-dropouts.
Procedure
Participants completed several measurements, including demographic information, MMSE, and far visual acuity, during an initial in-person screening visit. Those who qualified for the ACTIVE study returned for a baseline visit during which cognitive assessments, including the UFOV, were administered. Follow-up assessments were conducted approximately two months (post-test), one year (first annual), two years (second annual), three years (third annual), and five years (fifth annual) after baseline. The MMSE, CES-D, DSS, UFOV, visual acuity test, and self-rated health measure were administered at each annual follow-up.
Statistical Analyses
Pearson correlations were calculated between baseline four-subtest total UFOV, DSS number correct, CES-D scores, MMSE scores, far visual acuity, self-rated health, age, and years of education. Then, we examined attrition effects using a MANOVA with attrition status as the independent variable, to see whether participants who dropped out before the last assessment had different characteristics than participants who remained. Dependent variables were the following, measured at baseline: CES-D, MMSE, four-subtest total UFOV, far visual acuity, DSS number correct, self-rated health, age, and years of education.
Next, total UFOV and DSS scores from each assessment period were re-scaled to z-scores based upon performance at baseline. It should be emphasized that the scales for DSS and UFOV had opposite signs; that is, higher DSS scores, but lower UFOV scores, indicated better performance. A series of random effects models were used to examine associations between age, attrition status, race, education, visual acuity, self-rated health, MMSE, CES-D, and five-year changes in UFOV and DSS performance. In each model, time was centered at baseline. Model fit was evaluated using -2 Log Likelihood (-2LL), and changes in -2LL from one model to another were evaluated using χ2, where df = the difference in model parameters. Pseudo-R2 values for each model were calculated based on the proportion reduction in random variance components above and beyond the model immediately preceding it. For example, residual pseudo-R2 values were calculated for the unconditional growth models via the following equation: (residual estimate for unconditional means model – residual estimate for unconditional growth model)/residual estimate for unconditional means model.
We first examined an unconditional means model and three baseline unconditional growth models for UFOV: a fixed and random linear time model; a fixed quadratic time (time2) and random linear time model; and a random quadratic time model. Quadratic time was considered because plots of the means for UFOV suggested a curvilinear pattern over the five time points. The unconditional growth model that yielded the smallest -2LL statistic was used in subsequent conditional models.
Next, we added age, which was centered at the sample mean, and attrition status (coded as 0 = non-dropout and 1 = dropout) to the model as fixed-effect predictors. Linear time effects were assessed via predictor*time interactions (e.g., age*time). Third, we incorporated quadratic time effects for age and attrition (e.g., age*time2) into the model. A fourth model then built upon the third model, controlling for age and attrition while examining other baseline demographic and health predictors. These predictors were: race (coded 0 = white and 1 = other), gender (0 = male and 1 = female), years of education, self-rated health, MMSE, CES-D, and visual acuity. Continuous variables were centered at their sample means. For seven individuals, CES-D and/or self-rated health scores were substituted from later waves due to missing baseline data. The fourth model did not include interaction terms for the additional demographic and health predictors; linear time effects for these predictors were tested in a fifth model, and quadratic time effects were tested in a sixth model.
Then, we ran each of the above models with DSS as the outcome instead of UFOV; the DSS models were kept identical to the UFOV models for easier comparisons. We estimated random effects correlations for DSS and UFOV from the best-fitting models, using a two-stage procedure available in SAS Proc Mixed for outputting Bayesian intercepts and slopes. Finally, because some studies have examined UFOV subtest 2 as a separate outcome, or have calculated composite scores using only the latter three subtests (Edwards et al., 2006; Hoffman, McDowd, Atchley, & Dubinsky, 2005), we estimated each random effects model with both three-subtest total UFOV and just subtest 2 as outcomes.
Results
Correlations and Attrition Analyses
Correlations between continuous measures at baseline are shown in Table 1. Participants completed an average of 5 follow-up sessions, with a mean follow-up length of 3.53 years (SD = 1.75). In all, 452 participants (101 men and 351 women) provided data at the fifth annual follow-up visit, and the other 238 individuals were coded as dropouts. Dropouts completed an average of 3.36 follow-up sessions, with a mean follow-up length of 1.46 years (SD = 1.19). Table 2 shows the number of individuals who dropped out at each assessment due to death or participant, family, or study staff decisions. Participants most commonly reported not continuing participation due to lack of interest (26%), being too busy (22%), being ill (16%), or being caregiver for a family member (7%). Study staff decactivated participants most commonly due to being unable to reach the participant (79%), the participant repeatedly missing study visits (9%), or passive refusal by the participant (6%). Family decisions indicate that a relative would not allow the participant to speak to study staff for interviewing or scheduling.
Table 1. Intercorrelations between Relevant Continuous Measures at Baseline.
| Measure | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| 1. Years of age | — | 0.08* | -0.35** | -0.17** | 0.11** | -0.36** | 0.42** | -0.08* |
| 2. CES-Da | — | -0.23** | -0.23** | 0.34** | -0.06 | 0.16** | -0.25** | |
| 3. DSS number correct | — | 0.37** | -0.30** | 0.20** | -0.46** | 0.27** | ||
| 4. MMSE total | — | -0.22** | 0.17** | -0.30** | 0.28** | |||
| 5. Self-rated healtha | — | -0.13** | 0.23** | -0.23** | ||||
| 6. Visual acuity | — | -0.25** | 0.05 | |||||
| 7. 4-subtest total UFOVa | — | -0.24** | ||||||
| 8. Years of education | — |
p < 0.05.
p < 0.01.
Note. CES-D = Center for Epidemiological Studies – Depression Scale; DSS = WAIS-R Digit Symbol Substitution; MMSE = Mini-Mental State Examination; UFOV = Useful Field of View Test.
Higher scores indicate more depression, worse health, or poorer performance.
Table 2. Numbers of Dropouts Among Study Participants at Each Assessment.
| Last Useful Field of View Test Assessment | Reason | ||||
|---|---|---|---|---|---|
|
| |||||
| Death | Participant Decision | Site Decision | Family Decision | Total | |
| Post-Test | 1 | 32 | 7 | 0 | 40 |
| First Annual | 15 | 35 | 11 | 2 | 63 |
| Second Annual | 16 | 19 | 9 | 1 | 45 |
| Third Annual | 6 | 21 | 5 | 0 | 32 |
| Fifth Annual | 22 | 14 | 21 | 1 | 58 |
A MANOVA revealed that dropouts were significantly older, less educated, and more depressed at baseline than non-dropouts; dropouts also had significantly lower MMSE scores, UFOV scores, and self-rated health (Table 3). A second MANOVA with gender as a between-subjects factor revealed no significant interaction between gender and attrition.
Table 3. Baseline Characteristics of Dropouts and Non-Dropouts.
| Non-dropouts | Dropouts | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | N | M | SD | N | M | SD |
| Years of age* | 452 | 73.58 | 5.86 | 238 | 74.65 | 6.09 |
| CES-Da * | 452 | 4.70 | 4.54 | 238 | 5.70 | 5.37 |
| DSS number correct | 450 | 39.94 | 11.69 | 237 | 39.24 | 11.01 |
| MMSE total* | 452 | 27.52 | 1.92 | 238 | 26.89 | 2.06 |
| Self-rated healtha* | 452 | 2.54 | 0.87 | 238 | 2.84 | 0.84 |
| Visual acuity* | 452 | 73.42 | 11.74 | 238 | 71.41 | 12.66 |
| 4-subtest total UFOVa* | 448 | 919.67 | 274.38 | 238 | 1008.34 | 307.61 |
| Years of education* | 452 | 13.58 | 2.58 | 238 | 13.00 | 2.89 |
Note. Individuals who did not complete baseline UFOV or DSS did complete these tests at later assessments. CES-D = Center for Epidemiological Studies – Depression Scale; DSS = WAIS-R Digit Symbol Substitution; MMSE = Mini-Mental State Examination; UFOV = Useful Field of View Test.
Higher scores indicate more depression, worse health, or poorer performance.
Mean difference p < 0.05.
Unconditional Growth Models for UFOV and DSS
Unconditional growth models with linear time, centered at baseline, showed significantly improved fit over unconditional means models for UFOV, χ2(3) = 31.24, p < 0.01, and DSS, χ2(3) = 108.1, p < 0.01. Although the main effect of linear time was not significant for UFOV (p = 0.52), linear time was significant for DSS (p < 0.001). Quadratic time was then added to the model as a fixed effect. For UFOV, the linear and quadratic time model showed improved fit over the linear only model, χ2(1) = 109.38, p < 0.001, and quadratic and linear time were both significant at p < 0.001. The same held true for DSS: the linear and quadratic time model had improved fit relative to the linear model, χ2(1) = 83.62, p < 0.01, and random and quadratic time were significant (p < 0.01). A random quadratic unconditional model was considered as an alternative, but this model showed worse fit than the fixed quadratic/random linear models for both UFOV (-2LL difference = -6.83) and DSS (-2LL difference = -21.54). Based on these results, fixed quadratic and random linear time models formed the basis for subsequent conditional models. For models with UFOV as the outcome, we were unable to estimate the variance component associated with the quadratic effect. Therefore, the models have variance components for intercept and linear time, but not quadratic time.
Conditional Growth Models for UFOV
The random effects models for UFOV are summarized in Table 4. Model 1 shows the linear and quadratic unconditional growth model that was discussed above.
Table 4. Standardized Total UFOV Performance over Time as Predicted by Baseline Demographic and Health Variables.
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Variable | Estimate | S.E. | Estimate | S.E. | Estimate | S.E. | Estimate | S.E. |
| Fixed Effects | ||||||||
| Intercept | -0.120** | 0.036 | -0.195** | 0.039 | -0.197** | 0.039 | 0.027 | 0.083 |
| Time | -0.144** | 0.017 | -0.166** | 0.018 | -0.164** | 0.019 | -0.163** | 0.019 |
| Time2 | 0.032** | 0.004 | 0.036** | 0.004 | 0.036** | 0.004 | 0.035** | 0.004 |
| Attrition | 0.220** | 0.066 | 0.236** | 0.067 | 0.096 | 0.063 | ||
| Attrition*time | 0.074** | 0.023 | -0.028 | 0.066 | -0.025 | 0.066 | ||
| Age | 0.075** | 0.005 | 0.077** | 0.005 | 0.066** | 0.005 | ||
| Age*time | 0.004** | 0.001 | -0.001 | 0.003 | -0.001 | 0.003 | ||
| Attrition*time2 | 0.038 | 0.023 | 0.037 | 0.023 | ||||
| Age*time2 | 0.001* | 0.001 | 0.001* | 0.001 | ||||
| CES-D | 0.010 | 0.006 | ||||||
| Education | -0.034** | 0.011 | ||||||
| MMSE | -0.087** | 0.015 | ||||||
| Gender | -0.034 | 0.067 | ||||||
| Race | -0.212** | 0.065 | ||||||
| Self-rated health | 0.086* | 0.035 | ||||||
| Visual acuity | -0.007* | 0.003 | ||||||
| Random Effects | ||||||||
| Residual95% confidence | 0.207** | 0.006 | 0.207** | 0.007 | 0.207** | 0.007 | 0.207** | 0.007 |
| Lower | 0.194 | 0.195 | 0.194 | 0.194 | ||||
| Upper | 0.220 | 0.221 | 0.220 | 0.220 | ||||
| Var (intercept) 95% confidence | 0.782** | 0.048 | 0.558** | 0.036 | 0.557** | 0.035 | 0.459** | 0.030 |
| Lower | 0.695 | 0.493 | 0.492 | 0.404 | ||||
| Upper | 0.882 | 0.632 | 0.631 | 0.523 | ||||
| Var (time) 95% confidence | 0.005** | 0.001 | 0.005** | 0.001 | 0.005** | 0.001 | 0.004** | 0.001 |
| Lower | 0.004 | 0.003 | 0.003 | 0.003 | ||||
| Upper | 0.009 | 0.008 | 0.008 | 0.008 | ||||
| Corr (int, time) | 0.016* | 0.106 | 0.003 | 0.005 | 0.004 | 0.005 | 0.003 | 0.005 |
| 95% confidence | ||||||||
| Lower | 0.003 | -0.007 | -0.007 | -0.007 | ||||
| Upper | 0.029 | 0.014 | 0.015 | 0.013 | ||||
| a Pseudo-R2 | ||||||||
| Residual | 0.099 | 0.001 | 0.003 | < 0.001 | ||||
| Intercept | 0.060 | 0.287 | 0.002 | 0.098 | ||||
| Time | 0.173 | 0.001 | 0.007 | |||||
| Corr (int, time) | 0.789 | 0.174 | -0.416 | |||||
| Model Fit | ||||||||
| Log likelihood | 6063.655 | 5818.353 | 5811.016 | 5694.044 | ||||
| AIC | 6077.655 | 5840.353 | 5837.016 | 5734.044 | ||||
| BIC | 6119.972 | 5906.851 | 5915.604 | 5854.949 | ||||
p < 0.05, two-tailed.
p < 0.01, two-tailed.
Note. UFOV = Useful Field of View Test; MMSE = Mini-Mental State Examination; CES-D = Center for Epidemiological Studies – Depression Scale.
Pseudo-R2 values for each model are based on the proportion reduction in random variance components above and beyond the model immediately preceding it.
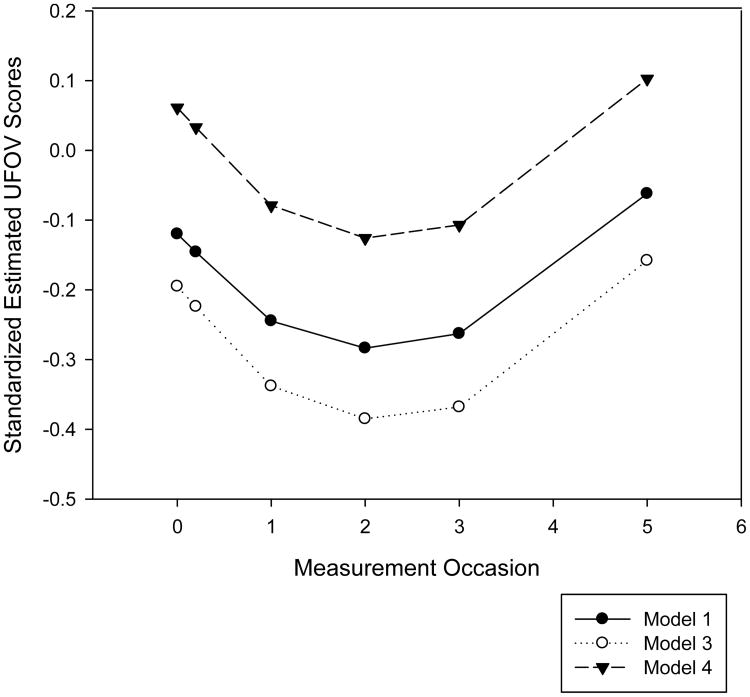
Model 2, which included centered age and attrition as fixed predictors, showed significantly improved fit over Model 1, χ2(4) = 245.30, p < 0.01. Age, attrition, an age*time interaction, and an attrition*time interaction were statistically significant. For each year older than the mean age of 73.58, estimated baseline UFOV scores were higher (i.e., slower) by 0.08 standard deviations. Independent of age, study dropouts performed an estimated 0.22 standard deviations worse than non-dropouts. Figure 1 shows estimated UFOV trajectories with and without controlling for age and attrition, suggesting that performance is underestimated when these variables are not taken into account. This figure also illustrates that estimated UFOV performance generally improved from baseline to second annual, then declined.
Figure 1.
Estimated standardized Useful Field of View Test (UFOV) performance over time without covariates (Model 1), controlling for age and attrition (Model 3), and controlling for age, attrition, and demographic and health variables (Model 4).
Model 3 incorporated quadratic time effects for age and attrition. This model had significantly better fit than Model 2, χ2(2) = 7.34, p < 0.05, although the magnitude of the fit improvement was relatively small. The main effects for attrition and age, as well as the quadratic age*time2 interaction, were statistically significant, indicating that the linear age*time effect changed over time. However, age did not appear to impact the linear rate of change instantaneous at baseline, as the linear age*time interaction was not significant in Model 3. Model 4, which included baseline demographic and health predictors, showed significantly improved fit over Model 3, χ2(6) = 116.72, p < 0.01. Independent of age and attrition, faster baseline UFOV performance was associated with being White, more years of education, and better visual acuity, MMSE performance, and self-rated health. The main effect for age and the age*time2 interaction remained statistically significant, but the main effect for attrition did not. Gender and CES-D were not significantly associated with UFOV performance. No additional linear or quadratic time interactions were statistically significant in Models 5 and 6 (not shown), and these models did not show improved fit over Model 4. Age was the only predictor that showed consistent associations with rate of change in UFOV.
Conditional Growth Models for DSS
The results of the random effects models for DSS are summarized in Table 5. Linear and quadratic time effects were statistically significant in Model 1, similar to Model 1 for UFOV. Model 2 showed significantly improved fit over Model 1, χ2(4) = 151.87, p < 0.01. Main effects and linear interactions for age and attrition were significant; older individuals and dropouts made significantly fewer correct substitutions at baseline and also experienced faster performance declines. None of the quadratic predictor*time interactions were significant in Model 3. Moreover, Model 3 did not show improved fit over Model 2, χ2(2) = -0.83, p < 0.01, in contrast to Model 3 for UFOV.
Table 5. Standardized DSS Number Correct over Time as Predicted by Baseline Demographic and Health Variables.
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Variable | Estimate | S.E. | Estimate | S.E. | Estimate | S.E. | Estimate | S.E. |
| Fixed Effects | ||||||||
| Intercept | 0.080* | 0.037 | 0.205** | 0.042 | 0.206** | 0.042 | -0.162** | 0.062 |
| Time | 0.076** | 0.014 | 0.087** | 0.015 | 0.085** | 0.015 | 0.084** | 0.015 |
| Time2 | -0.027** | 0.003 | -0.029** | 0.003 | 0.028** | 0.003 | -0.028** | 0.003 |
| Attrition | -0.365** | 0.071 | -0.373** | 0.072 | -0.186** | 0.064 | ||
| Attrition*time | -0.045* | 0.019 | 0.001 | 0.055 | 0.001 | 0.055 | ||
| Age | -0.054** | 0.006 | -0.054* | 0.006 | -0.045** | 0.005 | ||
| Age*time | -0.004** | 0.001 | -0.005 | 0.002 | -0.005 | 0.002 | ||
| Attrition*time2 | -0.016 | 0.019 | -0.016 | 0.019 | ||||
| Age*time2 | -0.001 | 0.001 | -0.001 | 0.001 | ||||
| CES-D | -0.019** | 0.006 | ||||||
| Education | 0.051** | 0.012 | ||||||
| MMSE | 0.089** | 0.016 | ||||||
| Gender | 0.380** | 0.068 | ||||||
| Race | 0.468** | 0.066 | ||||||
| Self-rated health | -0.161** | 0.036 | ||||||
| Visual acuity | 0.004 | 0.003 | ||||||
| Random Effects | ||||||||
| Residual95% confidence | 0.136** | 0.004 | 0.136** | 0.004 | 0.136** | 0.004 | 0.136** | 0.004 |
| Lower | 0.128 | 0.128 | 0.128 | 0.128 | ||||
| Upper | 0.145 | 0.145 | 0.145 | 0.145 | ||||
| Var (intercept) 95% confidence | 0.853** | 0.049 | 0.706** | 0.041 | 0.706** | 0.041 | 0.499** | 0.030 |
| Lower | 0.762 | 0.630 | 0.630 | 0.465 | ||||
| Upper | 0.956 | 0.792 | 0.792 | 0.590 | ||||
| Var (time) 95% confidence | 0.004** | 0.001 | 0.004** | 0.001 | 0.004** | 0.001 | 0.004** | 0.001 |
| Lower | 0.003 | 0.002 | 0.002 | 0.002 | ||||
| Upper | 0.006 | 0.006 | 0.006 | 0.006 | ||||
| Corr (int, time) 95% confidence | 0.015** | 0.093 | 0.007 | 0.005 | 0.007 | 0.005 | 0.008* | 0.004 |
| Lower | 0.005 | -0.002 | -0.002 | 0.001 | ||||
| Upper | 0.026 | 0.016 | 0.016 | 0.017 | ||||
| a Pseudo-R2 | ||||||||
| Residual | 0.137 | 0.001 | < 0.001 | -0.001 | ||||
| Intercept | 0.037 | 0.172 | < 0.001 | 0.258 | ||||
| Time | 0.152 | 0.001 | < 0.001 | |||||
| Corr (int, time) | 0.564 | 0.003 | 0.397 | |||||
| Model Fit | ||||||||
| Log likelihood | 5102.383 | 4950.521 | 4949.691 | 4765.666 | ||||
| AIC | 5116.383 | 4972.521 | 4975.691 | 4803.666 | ||||
| BIC | 5158.725 | 5039.058 | 5054.325 | 4918.593 | ||||
p < 0.05, two-tailed.
p < 0.01, two-tailed.
Note. DSS = WAIS-R Digit Symbol Substitution; MMSE = Mini-Mental State Examination; CES-D = Center for Epidemiological Studies – Depression Scale.
Pseudo-R2 values for each model are based on the proportion reduction in random variance components above and beyond the model immediately preceding it.
Model 4 showed significantly improved fit over Model 3, χ2(6) = 184.03, p < 0.01. Age, attrition, MMSE, self-rated health, CES-D, race, gender, and education significantly predicted level of DSS performance, while visual acuity did not. Being female and White was associated with better performance, as were lower CES-D scores and greater self-rated health, years of education, and MMSE scores. None of the linear or quadratic interactions for age and attrition were significant, although the age*time interaction was marginal (p = 0.06).
Model 5 (not shown), which included linear interaction terms for the demographic and health variables that were added in Model 4, had significantly better fit than Model 4, χ2(7) = 39.24, p < 0.01. However, Model 6 (not shown) did not show improved fit. The only additional linear or quadratic interaction to reach significance in Model 5 was MMSE*time, t = -2.28, p = 0.02, indicating that individuals with lower baseline MMSE scores declined faster on DSS. Because only one interaction was significant, and because Model 5 did not represent an improvement for UFOV, the details of Model 5 for DSS are not reported. Change trajectories for both UFOV and DSS were calculated using Model 4 coefficients to maximize comparability.
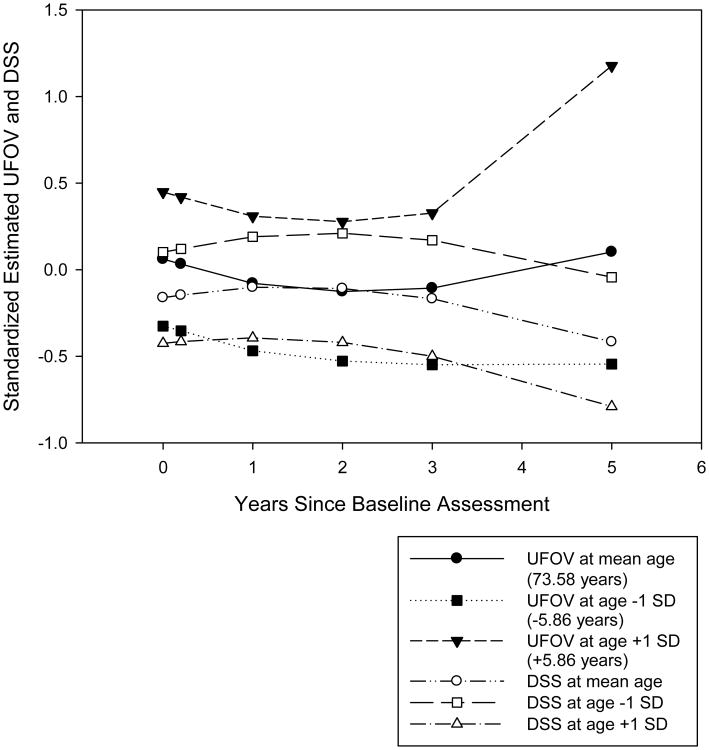
Figure 2 compares these change trajectories for UFOV and DSS performance by age. Despite some differences between the models in terms of predictors and significance levels, the trajectories are similar in shape and position. As with UFOV, significant residual and intercept variance remained unexplained for each DSS model (p's < 0.01). Individual differences in intercept and linear change were generated from Model 4 for UFOV and DSS. Individual differences at intercept were significantly correlated (r = -0.37, p < 0.01), as were the individual differences in linear change (r = -0.28, p < 0.01). In both cases, poorer performance at intercept or linear declines in functioning for the UFOV and DSS were correlated.
Figure 2.
Estimated standardized Useful Field of View Test (UFOV) and WAIS-R Digit Symbol Substitution (DSS) performance over time by age, using Model 4 equations.
Three-Subtest Total UFOV and Subtest 2
All random effects models for UFOV were re-run using the three-subtest total and only subtest 2 as outcomes. For the three-subtest total and subtest 2, the best-fitting unconditional growth model was again the linear and quadratic time model that was chosen for the 4-subtest total. Linear time was not significant in the linear time-only model for the three-subtest composite (p = 0.55), but was significant for subtest 2 (p < 0.02). Quadratic time was significant for both outcomes (p's < 0.001). For all conditional models, the results were very similar to the findings for the four-subtest composite. Main effects for age, race, education, visual acuity, MMSE, and self-rated health, as well as the age*time2 interaction, were significantly associated with the three-subtest composite and subtest 2; gender, CES-D, and other linear and quadratic interactions were not significant.
Discussion
We examined trajectories of change in four subtest-total UFOV scores over a five-year period. Average UFOV performance changed in a curvilinear fashion, improving and then declining (Figure 2). Our first hypothesis that increased age would be associated with lower baseline UFOV performance and with greater declines over time, independent of the covariates, was supported. Demographic and health variables like MMSE scores, race, education, vision, and self-rated health were associated with level of UFOV performance, but were not related to rate of change, supporting our second hypothesis. These findings are consistent with studies of other speed of processing measures.
We also found some support for our third hypothesis that speed of processing would be a primary component of the UFOV. Trajectories of change for UFOV and DSS showed similar overall patterns (Figure 2). Moreover, random effects for DSS were significantly correlated with random effects for UFOV, which suggests that both measures reflect the same cognitive domain. However, there were significant individual differences in the rate of change in UFOV and DSS performance, as evidenced by the significant random slope (i.e., time) variance in the models. Schaie (1989) found similar variability in Finding A's and Identical Pictures tests, which also measure speed of processing.
Many of the variables that predicted UFOV performance also predicted DSS performance. However, visual acuity was significantly associated with UFOV, but not DSS. This finding is intuitive, as UFOV involves more visual processing than DSS. CES-D, gender, and MMSE*time were associated with DSS, but not UFOV; some of these differences may have occurred because DSS involves motor coordination, while UFOV is strictly perceptual. The current findings were in line with Edwards et al.(2006), who found that age, vision, MMSE, and self-rated health, but not gender, were associated with UFOV performance. Age has consistently emerged as a strong predictor of between and within-person declines on cognitive speed measures (MacDonald, Hultsch, Strauss, & Dixon, 2003). Moreover, other studies have found that cross-sectional covariates like education, gender, self-rated health, depressive symptoms, and memory influence level of cognitive performance, but not rate of change (Anstey, Hofer, & Luszcz, 2003; MacDonald, Hultsch, Strauss, & Dixon, 2003).
The presence of a significant age*time2 interaction for UFOV indicates that the linear age*time effect changes over time, suggesting that age-related changes in UFOV performance are quadratic in nature rather than linear. Research aimed at establishing norms for longitudinal UFOV performance in older adults should factor in the curvilinear nature of these changes, especially given that similar results were obtained for UFOV subtest 2 and the three-subtest total. However, it should be emphasized that the results for the predictor*time interactions, as well as the correlations between intercepts and slopes, depend largely on the centering of time (in this case, at baseline). Further research is needed to fine-tune the estimates of age-related changes in UFOV and DSS performance.
Our results were limited by selection criteria for participation in the ACTIVE study, which included relatively high physical and cognitive functioning. ACTIVE participants were predominantly female and predominantly white, so the race and gender effects we found may not be reliable. In addition, data were not missing at random. Over a third of the original participants dropped out of the study, and dropouts had poorer baseline functioning than non-dropouts. Non-dropouts tended to remain healthy throughout the study period; for example, 84.5% of non-dropouts rated their health as good or better at baseline, and 81.6% did so again at fifth annual. Therefore, adjusting the models for attrition was important.
Another study limitation was that the maximum follow-up interval of the ACTIVE trial was 5 years (M = 3.53 years, SD = 1.75). This may not be long enough to accurately estimate change trajectories or fully eliminate practice effects. Because UFOV performance has not previously been studied longitudinally, the optimal follow-up length for longitudinal research is unknown. Practice effects may have contributed to the initial improvements seen in UFOV and DSS performance. Rabbitt, Diggle, Holland, and McInnes (2004) examined practice effects on tests of intelligence, vocabulary, and verbal memory over a 17-year period and found that practice effects attenuated performance declines for 3-5 years and remained significant for up to 7 years. Training gains for older adults on the DSS have long been documented (Beres & Baron, 1981; Hinton-Bayre & Geffen, 2005), and UFOV performance also improves with practice (Richards, Bennett, & Sekuler, 2006). We added a practice effect variable—total number of follow-up assessments for each person, as an indicator of exposure to the tests—to the random effects models for UFOV and DSS, but none of the results changed appreciably. The quadratic time variable may have provided a sufficient adjustment for practice effects. Rabbitt and colleagues (2004) found that, after adjustments for practice and drop-out, performance declined with age, other demographic variables were not related to decline, and variability between individuals increased with age. All of these observations were seen in the current study for UFOV, and the former two observations were seen with DSS.
While the current study focused on normative UFOV changes across time, prior analyses have examined the impact of speed of processing training on longitudinal UFOV performance relative to the control group. Wolinsky and colleagues (2006) found that the effect sizes for speed of processing training, measured in standard deviations of improvement between the trained group and the control group, was 1.46 at post-test, 1.21 at first annual, 0.87 at second annual, and 0.76 at fifth annual. Ball, Ross, Roth, and Edwards (under review) found that the number of training sessions completed, as well as the completion of booster sessions, had an additive effect on attenuating declines in UFOV performance relative to the control group. Increased age, however, was associated with smaller training gains. These findings along with our results suggest that the UFOV is sensitive to speed of processing declines that occur with normal aging, and that speed of processing training may attenuate these declines.
Future studies should compare longitudinal UFOV changes to longitudinal changes in other speed of processing measures besides the DSS. Our analyses should be replicated with other samples and populations, including different racial and ethnic groups and people with different health conditions. Considering that the UFOV has been used to evaluate the driving capacity of neurologically impaired populations, including adults with Alzheimer's disease (Duchek, Hunt, Ball, Buckles, & Morris, 1998; Rizzo, Reinach, McGehee, & Dawson, 1997) and stroke (Fisk, Owsley, & Mennemeier, 2002; Marshall et al., 2007), trajectories of decline should be examined in impaired populations as well. It would be of interest to evaluate how sensitive the UFOV is to terminal cognitive decline. Additionally, neuro-imaging studies would be informative for elucidating the neural correlates of UFOV performance to see if there are similarities to correlates of the functional field of view task outlined by Scalf and colleagues (2007).
In conclusion, our results indicate that the UFOV is primarily a measure of speed of processing and that age may be an important predictor of change in UFOV performance even when attrition, baseline physical and mental health, visual acuity, education, race, and gender are taken into account. Current versions of the UFOV can be administered by personal computer, which is practical for clinical and research settings (Edwards et al., 2005). The UFOV can identify people who might benefit from speed of processing training, which enhances everyday functioning and health-related quality of life (Ball, Edwards, & Ross, 2007; Roenker, Cissell, Ball, Wadley, & Edwards, 2003; Wolinsky, Unverzagt, Smith, Jones, Wright et al., 2006).
Acknowledgments
The ACTIVE study was supported by grants from the National Institute on Aging and the National Institute of Nursing Research to Hebrew Rehabilitation Center for the Aged U01 NR04507, Indiana University School of Medicine U01 NR04508, Johns Hopkins University U01 AG14260, New England Research Institutes U01 AG14282, Pennsylvania State University U01 AG14263, University of Alabama-Birmingham U01 AG14289, and University of Florida U01 AG014276. The authors would like to thank the entire ACTIVE team. The Center for Translational Research on Aging and Mobility is supported by an Edward R. Roybal Center grant 5 P30 AG022838. Brent J. Small is supported by National Institute on Aging grant R03 AG024082.
Karlene Ball and Daniel Roenker are stockholders and consultants to Visual Awareness, Inc., the company which owns the patents to the Useful Field of View test and the speed of processing training program software. Jerri Edwards has worked as a consultant to Visual Awareness, Inc.
Footnotes
UFOV is a registered trademark of Visual Awareness, Inc.
Contributor Information
Melissa Lunsman, University of South Florida.
Jerri D. Edwards, University of South Florida
Ross Andel, University of South Florida.
Brent J. Small, University of South Florida
Karlene K. Ball, University of Alabama at Birmingham
Daniel L. Roenker, Western Kentucky University
References
- Anstey KJ, Hofer SM, Luszcz MA. Cross-sectional and longitudinal patterns of dedifferentiation in late-life cognitive and sensory function: The effect of age, ability, attrition, and occasion of measurement. Journal of Experimental Psychology: General. 2003;132(3):470–487. doi: 10.1037/0096-3445.132.3.470. [DOI] [PubMed] [Google Scholar]
- Ball KK, Beard BL, Roenker DL, Miller RL, Griggs DS. Age and visual search: Expanding the useful field of view. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 1988;5(12):2210–2219. doi: 10.1364/josaa.5.002210. [DOI] [PubMed] [Google Scholar]
- Ball KK, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, et al. Effects of cognitive training interventions with older adults: A randomized controlled trial. JAMA. 2002;288:2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball KK, Edwards JD, Ross LA. The impact of speed of processing training on cognitive and everyday functions. Journals of Gerontology, Series B, Psychological Sciences and Social Sciences. 2007;62B:19–31. doi: 10.1093/geronb/62.special_issue_1.19. [DOI] [PubMed] [Google Scholar]
- Ball KK, Owsley C. The Useful Field of View Test: A new technique for evaluating age- related declines in visual function. Journal of the American Optometric Association. 1993;64(1):71–79. [PubMed] [Google Scholar]
- Ball KK, Owsley C, Beard BL. Clinical visual perimetry underestimates peripheral field problems in older adults. Clinical Vision Sciences. 1990;5:113–125. [Google Scholar]
- Ball KK, Owsley C, Sloane ME, Roenker DL, Bruni JR. Visual attention problems as a predictor of vehicle crashes in older drivers. Investigative Ophthalmology and Visual Science. 1993;34(11):3110–3123. [PubMed] [Google Scholar]
- Ball KK, Roenker DL, McGwin G, Wadley VG, Edwards JD, Raleigh R, et al. Can high-risk older drivers be identified through performance-based measures in a Department of Motor Vehicles setting? Journal of the American Geriatrics Society. 2006;54:77–84. doi: 10.1111/j.1532-5415.2005.00568.x. [DOI] [PubMed] [Google Scholar]
- Ball KK, Ross LA, Roth DL, Edwards JD under review. Maintaining processing speed improvements with booster sessions: How much training is enough? Psychology and Aging [Google Scholar]
- Beres CA, Baron A. Improved digit symbol substitution by older women as a result of extended practice. Journal of Gerontology. 1981;36(5):591–597. doi: 10.1093/geronj/36.5.591. [DOI] [PubMed] [Google Scholar]
- Bergen JR, Julesz B. Parallel versus serial processing in rapid pattern discrimination. Nature. 1983;303(5919):696–698. doi: 10.1038/303696a0. [DOI] [PubMed] [Google Scholar]
- Broman AT, West SK, Munoz B, Bandeen-Roche K, Rubin GS, Turano KA. Divided visual attention as a predictor of bumping while walking: The Salisbury Eye Education. Investigative Ophthalmology and Visual Science. 2004;45(9):2955–2960. doi: 10.1167/iovs.04-0219. [DOI] [PubMed] [Google Scholar]
- Clay OJ, Wadley VG, Edwards JD, Roth DL, Roenker DL, Ball KK. Cumulative meta-analysis of the relationship between useful field of view and driving performance in older adults: Current and future implications. Optometry and Vision Science. 2005;82(8):724–731. doi: 10.1097/01.opx.0000175009.08626.65. [DOI] [PubMed] [Google Scholar]
- Duchek JM, Hunt L, Ball KK, Buckles V, Morris JC. Attention and driving performance in Alzheimer's disease. Journal of Gerontology: Psychological and Social Sciences. 1998;53(2):130–141. doi: 10.1093/geronb/53b.2.p130. [DOI] [PubMed] [Google Scholar]
- Edwards JD, Ross LA, Wadley VG, Clay OJ, Crowe M, Roenker DL, et al. The Useful Field of View Test: Normative data for older adults. Archives of Clinical Neuropsychology. 2006;21:275–286. doi: 10.1016/j.acn.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Edwards JD, Vance DE, Wadley VG, Cissell GM, Roenker DL, Ball KK. The reliability and validity of the Useful Field of View Test as administered by personal computer. Journal of Clinical and Experimental Neuropsychology. 2005;27:529–543. doi: 10.1080/13803390490515432. [DOI] [PubMed] [Google Scholar]
- Edwards JD, Wadley VG, Myers R, Roenker DL, Cissell GM, Ball KK. Transfer of a speed of processing intervention to near and far cognitive functions. Gerontology. 2002;48:329–340. doi: 10.1159/000065259. [DOI] [PubMed] [Google Scholar]
- Edwards JD, Wadley VG, Vance DE, Roenker DL, Ball KK. The impact of speed of processing training on cognitive and everyday performance. Aging & Mental Health. 2005;9:262–271. doi: 10.1080/13607860412331336788. [DOI] [PubMed] [Google Scholar]
- Finkel D, Reynolds CA, McArdle JJ, Pedersen NL. Age changes in processing speed as a leading indicator of cognitive aging. Psychology and Aging. 2007;22(3):558–568. doi: 10.1037/0882-7974.22.3.558. [DOI] [PubMed] [Google Scholar]
- Fisk GD, Novack T, Mennemeier M, Roenker DL. Useful field of view after traumatic brain injury. Journal of Head Trauma Rehabilitation. 2002;17(1):16–25. doi: 10.1097/00001199-200202000-00004. [DOI] [PubMed] [Google Scholar]
- Fisk GD, Owsley C, Mennemeier M. Vision, attention, and self-reported driving behaviors in community-dwelling stroke survivors. Archives of Physical Medicine and Rehabilitation. 2002;83(4):469–477. doi: 10.1053/apmr.2002.31179. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Good-Lite. 2007 Retrieved August 16, 2007, from http://www.good-lite.com/
- Goode KT, Ball KK, Sloane ME, Roenker DL, Roth DL, Myers RS, et al. Useful field of view and other neurocognitive indicators of crash risk in older adults. Journal of Clinical Psychology in Medical Settings. 1998;5(4):425–440. [Google Scholar]
- Hinton-Bayre A, Geffen G. Comparability, reliability, and practice effects on alternate forms of the Digit Symbol Substitution and Symbol Digit Modalities Tests. Psychological Assessment. 2005;17(2):237–241. doi: 10.1037/1040-3590.17.2.237. [DOI] [PubMed] [Google Scholar]
- Hoffman L, McDowd JM, Atchley P, Dubinsky R. The role of visual attention in predicting driving impairment in older adults. Psychology and Aging. 2005;20(4):610–622. doi: 10.1037/0882-7974.20.4.610. [DOI] [PubMed] [Google Scholar]
- Hoyer WJ, Stawski RS, Wasylyshyn C, Verhaeghen P. Adult age and Digit Symbol Substitution performance: A meta-analysis. Psychology and Aging. 2004;19(1):211–214. doi: 10.1037/0882-7974.19.1.211. [DOI] [PubMed] [Google Scholar]
- Jobe JB, Smith DM, Ball KK, Tennstetdt SL, Marsiske M, Willis SL, et al. ACTIVE: A cognitive intervention trial to promote independence in older adults. Controlled Clinical Trials. 2001;22(4):453–479. doi: 10.1016/s0197-2456(01)00139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke U, Zimprich D. Longidudinal changes in memory performance and processing speed in old age. Aging, Neuropsychology, and Cognition. 2005;12:57–77. [Google Scholar]
- MacDonald SWS, Hultsch DF, Strauss E, Dixon RA. Age-related slowing of Digit Symbol Substitution revisited: What do longitudinal age changes reflect? Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2003;58B:187–194. doi: 10.1093/geronb/58.3.p187. [DOI] [PubMed] [Google Scholar]
- Marshall SC, Molnar F, Man-Son-Hing M, Blair R, Brosseau L, Finestone HM, et al. Predictors of driving ability following stroke: A systematic review. Topics in Stroke Rehabilitation. 2007;14(1):98–114. doi: 10.1310/tsr1401-98. [DOI] [PubMed] [Google Scholar]
- Myers RS, Ball KK, Kalina TD, Roth DL, Goode KT. The relationship of Useful Field of View and other screening instruments to on-road driving performance. Perceptual and Motor Skills. 2000;91(1):279–290. doi: 10.2466/pms.2000.91.1.279. [DOI] [PubMed] [Google Scholar]
- Novack T, Banos JH, Alderson AL, Schneider JJ, Weed W, Blankenship J, et al. UFOV performance and driving ability following traumatic brain injury. Brain Injury. 2006;20(5):455–461. doi: 10.1080/02699050600664541. [DOI] [PubMed] [Google Scholar]
- Owsley C, Ball KK, Keeton DM. Relationship between visual sensitivity and target localization in older adults. Vision Research. 1995;35(4):579–587. doi: 10.1016/0042-6989(94)00166-j. [DOI] [PubMed] [Google Scholar]
- Owsley C, Ball KK, McGwin G, Sloane ME, Roenker DL, White MF, et al. Visual processing impairment and risk of motor vehicle crash among older adults. JAMA. 1998;279(14):1083–1088. doi: 10.1001/jama.279.14.1083. [DOI] [PubMed] [Google Scholar]
- Owsley C, Ball KK, Sloane ME, Roenker DL, Bruni JR. Visual/cognitive correlates of vehicle accidents in older drivers. Psychology and Aging. 1991;6:403–415. doi: 10.1037//0882-7974.6.3.403. [DOI] [PubMed] [Google Scholar]
- Owsley C, McGwin G, Ball KK. Vision impairment, eye disease, and injurious motor vehicle crashes in the elderly. Ophthalmic Epidemiology. 1998;5(2):101–113. doi: 10.1076/opep.5.2.101.1574. [DOI] [PubMed] [Google Scholar]
- Owsley C, McGwin G., Jr Association between visual attention and mobility in older adults. Journal of the American Geriatrics Society. 2004;52:1901–1906. doi: 10.1111/j.1532-5415.2004.52516.x. [DOI] [PubMed] [Google Scholar]
- Owsley C, Sloane ME, McGwin G, Jr, Ball KK. Timed instrumental activities of daily living tasks: Relationship to cognitive function and everyday performance assessments in older adults. Gerontology. 2002;48:254–265. doi: 10.1159/000058360. [DOI] [PubMed] [Google Scholar]
- Plude DJ, Hoyer WJ. Adult age differences in visual search as a function of stimulus mapping and processing load. Journal of Gerontology. 1981;36(5):598–604. doi: 10.1093/geronj/36.5.598. [DOI] [PubMed] [Google Scholar]
- Rabbitt P. Age and discrimination between complex stimuli. In: Welford A, Birren JE, editors. Behavior, Aging and the Nervous System. Springfield, IL: C.C. Thomas; 1965a. [Google Scholar]
- Rabbitt P. An age decrement in the ability to ignore irrelevant information. Journal of Gerontology. 1965b;20:233–238. doi: 10.1093/geronj/20.2.233. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Diggle P, Holland F, McInnes L. Practice and drop-out effects during a 17-year longitudinal study of cognitive aging. Journals of Gerontology Series B, Psychological Sciences and Social Sciences. 2004;59B:84–97. doi: 10.1093/geronb/59.2.p84. [DOI] [PubMed] [Google Scholar]
- Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Richards E, Bennett PJ, Sekuler AB. Age related differences in learning with the useful field of view. Vision Research. 2006;46(25) doi: 10.1016/j.visres.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Riolo L. Attention contributes to functional reach test scores in older adults with history of falling. Physical & Occupational Therapy in Geriatrics. 2003;22:15–29. [Google Scholar]
- Rizzo M, Reinach S, McGehee D, Dawson J. Simulated car crashes and crash predictors in drivers with Alzheimer's disease. Archives of Neurology. 1997;54(5):545–551. doi: 10.1001/archneur.1997.00550170027011. [DOI] [PubMed] [Google Scholar]
- Roenker DL, Cissell GM, Ball KK, Wadley VG, Edwards JD. Speed-of-processing and driving simulator training result in improved driving performance. Human Factors. 2003;45(2):218–233. doi: 10.1518/hfes.45.2.218.27241. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. What do adult age differences in the Digit Symbol Substitution Test reflect? Journals of Gerontology Series B, Psychological Sciences and Social Sciences. 1992;47(3):121–128. doi: 10.1093/geronj/47.3.p121. [DOI] [PubMed] [Google Scholar]
- Sanders AF. Some aspects of the selective process in the functional visual field. Ergonomics. 1970;13(1):101–117. doi: 10.1080/00140137008931124. [DOI] [PubMed] [Google Scholar]
- Scalf PE, Colcombe SJ, McCarley JS, Erickson KI, Alvarado M, Kim JS, et al. The neural correlates of an expanded functional field of view. Journals of Gerontology Series B, Psychological Sciences and Social Sciences. 2007;62B(special issue 1):32–44. doi: 10.1093/geronb/62.special_issue_1.32. [DOI] [PubMed] [Google Scholar]
- Schaie KW. Perceptual speed in adulthood: Cross-sectional and longitudinal studies. Psychology and Aging. 1989;4(4):443–453. doi: 10.1037//0882-7974.4.4.443. [DOI] [PubMed] [Google Scholar]
- Scialfa CT, Kline DW, Lyman BJ. Age differences in target identification as a function of retinal location and noise level: Examination of the useful field of view. Psychology and Aging. 1987;2(1):14–19. doi: 10.1037//0882-7974.2.1.14. [DOI] [PubMed] [Google Scholar]
- Seiple W, Szlyk JP, Yang S, Holopigian K. Age-related functional field losses are not eccentricity dependent. Vision Research. 1996;36:1859–1866. doi: 10.1016/0042-6989(95)00288-x. [DOI] [PubMed] [Google Scholar]
- Sekuler AB, Bennett PJ, Mamelak M. Effects of aging on the useful field of view. Experimental Aging Research. 2000;26:103–120. doi: 10.1080/036107300243588. [DOI] [PubMed] [Google Scholar]
- Sekuler R, Ball KK. Visual localization: Age and practice. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 1986;3(6):864–867. doi: 10.1364/josaa.3.000864. [DOI] [PubMed] [Google Scholar]
- Shawaryn MA, Schultheis MT, Garay E, Deluca J. Assessing functional status: Exploring the relationship between the multiple sclerosis functional composite and driving. Archives of Physical Medicine and Rehabilitation. 2002;83(8):1123–1129. doi: 10.1053/apmr.2002.33730. [DOI] [PubMed] [Google Scholar]
- Sims R, McGwin G, Pulley L, Roseman JM. Mobility impairments in crash-involved older drivers. Journal of Aging and Health. 2001;13(3):430–438. doi: 10.1177/089826430101300306. [DOI] [PubMed] [Google Scholar]
- Stalvey BT, Owsley C, Sloane ME, Ball KK. The Life Space Questionnaire: A measure of the extent of mobility of older adults. Journal of Applied Gerontology. 1999;18(4):460–478. [Google Scholar]
- Uc EY, Rizzo M, Anderson SW, Sparks J, Rodnitzky RL, Dawson J. Impaired visual search in drivers with Parkinson's disease. Annals of Neurology. 2006;60(4):407–413. doi: 10.1002/ana.20958. [DOI] [PubMed] [Google Scholar]
- Verriest G, Bailey I, Calabria G, Campos EC, Crick RP, Enoch JM, et al. The occupational visual field. Paper presented at the 6th International Visual Field Symposium 1985 [Google Scholar]
- Wechsler D. WAIS-R Manual. New York: The Psychological Corporation; 1981. [Google Scholar]
- Willis SL, Tennstetdt SL, Marsiske M, Ball KK, Elias J, Koepke KM, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296(23):2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky FD, Unverzagt FW, Smith DM, Jones R, Stoddard A, Tennstetdt SL. The ACTIVE cognitive training trial and health-related quality of life: Protection that lasts for 5 years. Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2006;61:1324–1329. doi: 10.1093/gerona/61.12.1324. [DOI] [PubMed] [Google Scholar]
- Wolinsky FD, Unverzagt FW, Smith DM, Jones R, Wright E, Tennstetdt SL. The effects of the ACTIVE cognitive training trial on clinically relevant declines in health-related quality of life. Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2006;61B(5):S281–S287. doi: 10.1093/geronb/61.5.s281. [DOI] [PubMed] [Google Scholar]
- Wood JM, Troutbeck R. Elderly drivers and simulated visual impairment. Optometry and Vision Science: Official Publication of the American Academy of Optometry. 1995;72(2):115–124. doi: 10.1097/00006324-199502000-00010. [DOI] [PubMed] [Google Scholar]
- Wood KM, Edwards JD, Wadley VG, Clay OJ, Ball KK, Roenker DL. Sensory and cognitive factors influencing functional ability in older adults. Gerontology. 2005;51:131–141. doi: 10.1159/000082199. [DOI] [PubMed] [Google Scholar]