Abstract
The maltose regulon (mal regulon) has previously been shown to consist of the mal gene cluster (malMP, malXCD and malAR operons) in Streptococcus pneumoniae. In this study, we have further elucidated the complete mal regulon in S. pneumoniae D39 using microarray analyses and β-galactosidase assays. In addition to the mal gene cluster, the complete mal regulon of S. pneumoniae D39 consists of a pullulanase (PulA), a glucosidase (DexB), a glucokinase (RokB), a PTS component (PtsG) and an amylase (AmyA2). Our microarray studies and β-galactosidase assays further showed that the LacI-family transcriptional regulator MalR represses the expression of the mal regulon in the absence of maltose. Furthermore, the role of the pleiotropic transcriptional regulator CcpA in the regulation of the mal regulon in the presence of maltose was explored. Our microarray analysis with a ΔccpA strain showed that CcpA only represses the expression of the malXCD operon and the pulA gene in the presence of maltose. Hence, we extend the mal regulon now consisting of pulA, dexB, rokB, ptsG and amyA2 in addition to malMP, malXCD and malAR operons.
Introduction
Streptococcus pneumoniae is a Gram-positive, alpha-hemolytic, facultative anaerobic member of the genus Streptococcus and a significant human pathogen [1]. It is present in the nasopharynx asymptomatically and may spread to various parts of the human body to cause numerous diseases including pneumonia, meningitis, septicemia and otitis media [2,3]. For successful survival and pathogenesis, it needs to acclimatize itself to changing nutritional circumstances inside the human body and make use of the available resources. Among these resources, carbohydrates are of utmost utility for pneumococcus, as it uses them as a carbon source for growth and survival [4]. Regulatory mechanisms of different sugars and carbon sources have been studied in S. pneumoniae [5–11].
The existence of many sugar-specific PTSs (phosphotransferase systems) confers bacteria the ability of metabolizing different carbon sources [12]. Bacteria have the ability to ferment several β-glucosides such as cellobiose, aesculin, arbutin and salicin, mostly present in plants [13]. A plant storage glycan, starch, is made of glucose monomers joined via α-1, 4 glycosidic linkages with additional branches introduced by α-1,6 bound glucose moieties [14]. Breakdown products of starch are maltose and maltodextrins. Maltose is a disaccharide formed from two units of glucose joined with an α(1→4) bond [15], whereas maltodextrins consist of glucose units connected in chains of variable length [16]. Previously, maltose-dependent gene regulation has been a topic of research in S. pneumoniae. These studies established the malXCD, malMP and malAR operons (mal gene cluster) as the maltose regulon (mal regulon), where MalXCD and MalMP are involved in maltosaccharide uptake and utilization [17,18]. malXCD and malMP are regulated by a transcriptional repressor, MalR, which binds explicitly to two operator sequences located in the promoter regions of the malXCD and malMP operons [17].
The studies on maltose regulation in S. pneumoniae have so far shown only the mal gene cluster (malXCD, malMP and malAR operons) as a part of the mal regulon, whereas in this study we have explored the maltose-mediated gene regulation through microarray studies and β-galactosidase assays, and identified the complete mal regulon regulated by the transcriptional repressor MalR in S. pneumoniae. The complete mal regulon consists of nine genes, which encode for ABC transporters (MalXCD), a maltose utilization enzyme (MalA), an amylomaltase (MalM), a phosphorylase (MalP), a glucose-specific PTS system (PtsG), a glucosidase (DexB), an amylase (AmyA2), a glucokinase (RokB) and a pullulanase (PulA). Furthermore, the role of the transcriptional regulator CcpA in the regulation of the mal regulon has also been investigated by the use of DNA microarray analyses.
Material and Methods
Bacterial strains, growth conditions and DNA modification
Bacterial strains and plasmids used in this study are listed in Table 1. M17 broth [19] supplemented with 0.5% (w/v) glucose was used for growing S. pneumoniae D39 [20] in tubes or on blood agar plates supplemented with 1% (v/v) defibrinated sheep blood in micro-aerophilic conditions at 37°C. For β-galactosidase assays, derivatives of S. pneumoniae D39 were grown in M17 medium supplemented with different sugars (Glucose and maltose) with various concentrations (w/v) as mentioned in the Results, and cells were harvested at mid-exponential growth phase. For selection on antibiotics, media were supplemented with the following concentrations of antibiotics; spectinomycin: 150 μg/ml and tetracycline: 1 μg/ml for S. pneumoniae; and ampicillin: 100 μg/ml for E. coli. All bacterial strains used in this study were stored in 10% (v/v) glycerol at -80°C. All DNA manipulations in this study were done as described before [21]. For PCR amplification, chromosomal DNA of S. pneumoniae D39 wild-type [20] was used. Primers used in this study are based on the sequence of the D39 genome [20] and listed in S1 Table.
Table 1. List of strains and plasmids used in this study.
| Strain/plasmid | Description | Source |
|---|---|---|
| S. pneumoniae | ||
| D39 | Serotype 2 strain. cps 2 | Laboratory of P. Hermans. |
| ΔccpA | D39 ΔccpA; SpecR | [38] |
| MA200 | D39 ΔmalR; SpecR | This study |
| MA201 | D39 ΔbgaA::PmalM-lacZ; TetR | This study |
| MA202 | D39 ΔbgaA::PmalX-lacZ; TetR | This study |
| MA203 | D39 ΔbgaA::PdexB-lacZ; TetR | This study |
| MA204 | D39 ΔbgaA::ProkB-lacZ; TetR | This study |
| MA205 | D39 ΔbgaA::PptsG-lacZ; TetR | This study |
| MA206 | D39 ΔbgaA::PamyA2-lacZ; TetR | This study |
| MA207 | D39 ΔbgaA::PpulA-lacZ; TetR | This study |
| MA208 | D39 ΔbgaA::PmalX-M-lacZ; TetR | This study |
| MA209 | MA200 ΔbgaA::PmalM-lacZ; TetR | This study |
| MA210 | MA200 ΔbgaA::PmalX-lacZ; TetR | This study |
| MA211 | MA200 ΔbgaA::PdexB-lacZ; TetR | This study |
| MA212 | MA200 ΔbgaA::ProkB-lacZ; TetR | This study |
| MA213 | MA200 ΔbgaA::PptsG-lacZ; TetR | This study |
| MA214 | MA200 ΔbgaA::PamyA2-lacZ; TetR | This study |
| MA215 | MA200 ΔbgaA::PpulA-lacZ; TetR | This study |
| MA216 | MA200 ΔbgaA::PZn-malR; TetR | This study |
| E. coli | ||
| EC1000 | KmR; MC1000 derivative carrying a single copy of the pWV1 repA gene in glgB | [64] |
| Plasmids | ||
| pPP2 | AmpR TetR; promoter-less lacZ. For replacement of bgaA with promoter lacZ fusion. Derivative of pTP1 | [22] |
| pKB01_sfgfp(Bs) | bla tet bgaA PZn-sfgfp(Bs) | [24] |
| pMA201 | pPP2 PmalM-lacZ | This study |
| pMA202 | pPP2 PmalX-lacZ | This study |
| pMA203 | pPP2 PdexB-lacZ | This study |
| pMA204 | pPP2 ProkB-lacZ | This study |
| pMA205 | pPP2 PptsG-lacZ | This study |
| pMA206 | pPP2 PamyA2-lacZ | This study |
| pMA207 | pPP2 PpulA-lacZ | This study |
| pMA208 | bla tet bgaA PZn-malR | This study |
Construction of a malR mutant
ΔmalR was constructed by allelic replacement with a spectinomycin-resistance marker. Briefly, primers malR-1/malR-2 and malR-3/malR-4 were used to generate PCR fragments of the left and right flanking regions of malR, respectively. PCR products of the left and right flanking regions of malR contain AscI and NotI restriction sites, respectively, as does the spectinomycin-resistance gene. The spectinomycin-resistance gene was amplified with primer pair Spec-F/Spec-R from the plasmid pORI38. Then, by restriction and ligation, the left and right flanking regions of malR were fused to the spectinomycin-resistance gene. The resulting ligation products were transformed to S. pneumoniae D39 wild-type and selection of the mutant strains was done on the appropriate concentration of spectinomycin. Spectinomycin-resistant clones were further examined for the presence of the malR deletion by colony PCR and DNA sequencing.
Construction of promoter lacZ fusions and β-galactosidase assays
Chromosomal transcriptional lacZ-fusions to the malM (spd-1933), malX (spd-1934), dexB (spd-0311), rokB (spd-0580), ptsG (spd-0661), amyA2 (spd-1215) and pulA (spd-0250) promoters were constructed in the integration plasmid pPP2 [22] with primer pairs mentioned in S1 Table, leading to plasmids pMA201, pMA202, pMA203, pMA204, pMA205, pMA206, pMA207 and pMA208, respectively. These constructs were further introduced into D39 wild-type resulting in strains MA201, MA202, MA203, MA204, MA205, MA206, MA207 and MA208, respectively. pMA201, pMA202, pMA203, pMA204, pMA205, pMA206 and pMA207 were also transformed into the malR deletion strain resulting in strains MA209, MA210, MA211, MA212, MA213, MA214 and MA215, respectively. All plasmid constructs were checked by PCR and DNA sequencing. β-galactosidase activity was measured as described before [23] using cells grown in M17 medium with appropriate sugars and harvested in the mid-exponential growth phase (S1 Fig).
Complementation of malR
malR was PCR amplified using primer pair MalR-comp-1/ MalR-comp-2 and cloned into EcoRI and BamHI sites of pKB01_sfgfp(Bs) [24], giving pMA208. pMA208 was transformed into ΔmalR strain resulting in strain MA216.
RNA extraction, reverse transcription (RT)-PCR and purification for quantitative RT-PCR
Total RNA was isolated from S. pneumoniae D39 wild-type, ΔmalR and malR-comp strains grown in GM17 (0.5% Glucose + M17) as described [25]. The RNA sample was treated with 2U of RNase free Dnase I (Invitrogen, Paisley, United Kingdom) to remove any DNA contamination. First, strand cDNA synthesis was performed on RNA [25,26]. cDNA (2 μl) was amplified in a 20 μl reaction volume that contained 3 pmol of each primer (S1 Table) and the reactions were performed in triplicate [25]. The transcription level of specific genes was normalized to gyrA transcription, amplified in parallel with gyrA-F and gyrA-R primers. The results were interpreted using the comparative CT method [27].
Microarray analysis
For DNA microarray analysis of the transcriptional response to maltose, the transcriptome of S. pneumoniae D39 wild-type, grown in replicates in GM17 (0.5% Glucose + M17) medium was compared to that grown in MM17 (0.5% Maltose + M17) medium. To analyze the effect of malR deletion on the transcriptome of S. pneumoniae, D39 wild-type and its isogenic malR mutant were grown in replicates in GM17 medium and harvested at mid-exponential growth phase. All other procedures regarding the DNA microarray experiment were performed essentially as described before [28,29]. Similarly, to observe the impact of ccpA on the global gene expression of S. pneumoniae and specifically on the mal regulon, S. pneumoniae D39 wild-type and its isogenic ccpA mutant were grown in replicates in MM17 (0.5% Maltose + M17) medium and harvested at the mid-exponential phase of the growth. All other procedures regarding the DNA microarray experiment were performed essentially as described before [28,29].
DNA microarray data were analyzed as done before [25,30]. For the identification of differentially expressed genes a Bayesian p-value of <0.001 and a fold change cut-off of 2 was applied. Microarray data have been submitted to GEO (Gene Expression Omnibus) database under the accession number GSE65550.
Results
Maltose-dependent gene regulation in S. pneumoniae
To elucidate the effect of maltose on the transcriptome of S. pneumoniae, a microarray aided comparison of D39 wild-type grown in MM17 (0.5% Maltose + M17) to that grown in GM17 (0.5% Glucose + M17) was performed. D39 wild-type and D39 ΔmalR strains grow similarly in GM17 (0.5% Glucose + M17) and MM17 (0.5% Maltose + M17) (S1 Fig). Table 2 summarizes the transcriptome changes observed in S. pneumoniae in the presence of maltose. The presence of maltose in the medium resulted in the upregulation of many genes and operons including the mal gene cluster (malXCD, malAR and malMP) after applying the criteria of ≥ twofold difference and p-value <0.001. Upregulation of the mal gene cluster in the presence of maltose not only corroborates the previous results [17] but also indicates that the conditions used in this study to explore the maltose-dependent genes in S. pneumoniae are appropriate. Expression of the cel gene cluster (spd_0277–0283) [31] was increased in the presence of maltose. The cel gene cluster codes for proteins that are putatively involved in cellobiose utilization [31]. It has previously been shown in S. pneumoniae D39 that the expression of the cel gene cluster is mediated by cellobiose and the transcriptional regulator CelR activates the expression of the cel gene cluster in the presence of cellobiose [31,32]. Expression of some other carbohydrate utilization genes was also affected in the presence of maltose as shown in Table 2. These genes are dexB, rokB, ptsG and amyA2. dexB encodes a 1,6-alpha-glucosidase that hydrolyzes α-1,6-glucosidic linkage at the non-reducing end of dextran or isomaltooligosaccharides to produce glucose [33]. rokB codes for a putative glucokinase that has been named the RokB protein [34]. ptsG encodes a glucose-specific EII permease (EIIGlc), but the deletion of ptsG did not affect glucose uptake in Corynebacterium glutamicum suggesting the presence of some other glucose system [35]. amyA2 encodes an alpha amylase that has been suggested to be a virulence factor in Group A Streptococcus (GAS) [36]. The involvement of these genes in the carbohydrate metabolism and the altered expression of these genes in the presence of maltose stimulated us to further investigate the role of these genes.
Table 2. Summary of transcriptome comparison of S. pneumoniae D39 wild-type grown in MM17 (0.5% Maltose + M17) and GM17 (0.5% Glucose + M17).
| a D39 tag | b Function | c Ratio |
|---|---|---|
| spd_0277 | 6-phospho-beta-glucosidase, CelA | 9.3 |
| spd_0279 | PTS system, IIB component, CelB | 6.5 |
| spd_0280 | Transcriptional regulator, CelR | 5.8 |
| spd_0281 | PTS system, IIA component, CelC | 6.2 |
| spd_0282 | Hypothetical protein | 4.9 |
| spd_0283 | PTS system, IIC component, CelD | 3.2 |
| spd_0311 | Glucan 1,6-alpha-glucosidase, DexB | 3.4 |
| spd_0580 | Glucokinase, RokB | 2.4 |
| spd_0661 | PTS system, IIABC components, PtsG | 4.6 |
| spd_0662 | Hypothetical protein | 3.0 |
| spd_1215 | Alpha-amylase, AmyA2 | 6.6 |
| spd_1932 | Glycogen phosphorylase family protein, MalP | 6.2 |
| spd_1933 | Amylomaltase, MalM | 6.2 |
| spd_1934 | Maltose/maltodextrin ABC transporter, MalX | 4.0 |
| spd_1935 | Maltodextrin ABC transporter, permease protein, MalC | 3.6 |
| spd_1936 | Maltodextrin ABC transporter, permease protein, MalD | 2.9 |
aGene numbers refer to D39 locus tags.
bD39 annotation/TIGR4 annotation [20].
cRatio represents the fold increase in the expression of genes in MM17 as compared to GM17.
Maltose-dependent expression of dexB, rokB, ptsG and amyA2 in addition to malMP and malXCD
To further confirm our maltose-dependent microarray results, we decided to study the expression of the selected genes (dexB, rokB, ptsG and amyA2) that may have a role in the utilization of maltose in addition to the mal gene cluster (malMP and malXCD). There is another gene, pulA (encoding a pullulanase), which is proposed to be a part of the mal regulon in S. pneumoniae [37]. Although we could not observe any change in the expression of pulA in our maltose microarray, we still decided to pursue our investigation with pulA to further confirm its role.
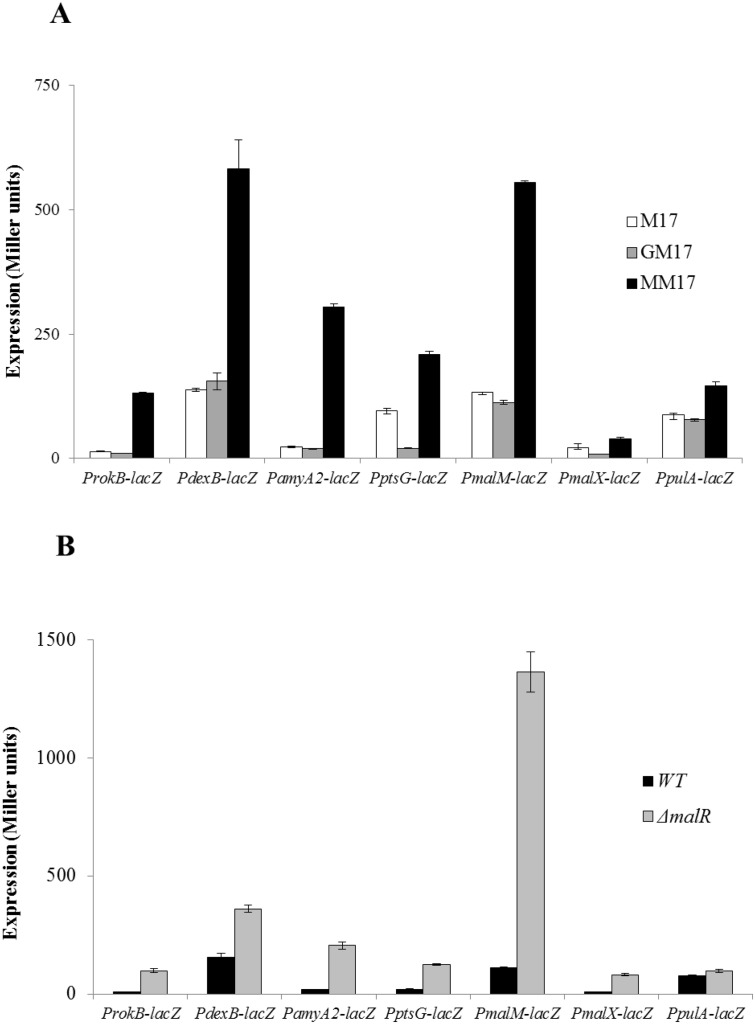
We made promoter lacZ-fusions of these genes (malM, malX, pulA, dexB, rokB, ptsG and amyA2) and transformed these lacZ-fusions into S. pneumoniae D39 wild-type. β-galactosidase assays were performed with the strains containing these lacZ-fusions. β-galactosidase assay data showed markedly increased activities of PmalX-lacZ, PmalM-lacZ, PdexB-lacZ, ProkB-lacZ, PptsG-lacZ and PamyA2-lacZ in the presence of maltose compared to glucose (Fig 1A). However, no significant change in the activity of PpulA-lacZ in the presence of maltose was observed (Fig 1A). These results confirm our maltose microarray results suggesting that the expression of malMP, malXCD, dexB, rokB, ptsG and amyA2 is dependent on maltose.
Fig 1. Expression levels (in Miller units) of ProkB-lacZ, PdexB-lacZ, PamyA2-lacZ, PptsG-lacZ, PmalM-lacZ, PmalX-lacZ and PpulA-lacZ.
A) in D39 wild-type grown in M17 (without any added sugar), GM17 (0.5% Glucose + M17) and MM17 (0.5% maltose + M17) B) in D39 wild-type and ΔmalR grown in GM17 (0.5% Glucose + M17). Standard deviations of three independent replicates are indicated in bars.
Maltose derepresses, while glucose and other tested sugars, represses the expression of malXCD operon
To further demonstrate the role of other sugars in the regulation of the mal gene cluster, the S. pneumoniae D39 wild-type strain containing PmalM-lacZ was grown in the presence of different sugars in M17 medium and subsequently, β-galactosidase assays were performed. The results indicate that the expression of PmalM-lacZ was highest in the presence of maltose and much lower in the presence of all other tested sugars including glucose (Table 3). This data confirms that maltose activates the expression of the PmalM-lacZ and other tested carbon sources do not play a role in the activation of PmalM-lacZ.
Table 3. Expression levels (in Miller units) of PmalM-lacZ transcriptional fusion in the D39 wild-type grown in M17 medium with different added sugars (0.5% w/v).
| β-galactosidase Activity (Miller Units) | |
|---|---|
| Sugars | PmalM-lacZ |
| M17 | 132 (4) |
| Arabinose | 129 (3) |
| Cellobiose | 133 (5) |
| Fructose | 144 (9) |
| Galactose | 151 (9) |
| Glucose | 98 (11) |
| Lactose | 146 (2) |
| Maltose | 525 (41) |
| Mannose | 153 (5) |
| Mannitol | 148 (9) |
| Raffinose | 121 (3) |
| Sorbitol | 146 (5) |
| Sucrose | 124 (12) |
| Trehalose | 190 (6) |
Standard deviation of three independent replicates is given in parentheses.
MalR is a transcriptional repressor of the mal regulon
MalR, a LacI family transcriptional regulator, has been shown to regulate the expression of malXCD and malMP operons [18]. Here, we show that expression of dexB, rokB, ptsG and amyA2 is also increased in the presence of maltose. To study whether MalR is involved in the regulation of these genes, we constructed an isogenic mutant of malR by replacing the malR gene with a spectinomycin-resistance marker. PmalX-lacZ, PmalM-lacZ, PdexB-lacZ, ProkB-lacZ, PptsG-lacZ, PpulA-lacZ and PamyA2-lacZ transcriptional lacZ-fusions were transformed into ΔmalR. β-galactosidase assays were performed with the strains containing these lacZ-fusions grown in GM17 (0.5% Glucose + M17) medium (Fig 1B). Our β-galactosidase assay data showed that repression on these promoters was relieved in ΔmalR in the presence of glucose, except for PpulA-lacZ, whose activity did not change significantly. This data not only confirms the results of Nieto et al. [17] but also indicates that dexB, rokB, ptsG and amyA2 are regulated by MalR and that these genes are part of the mal regulon.
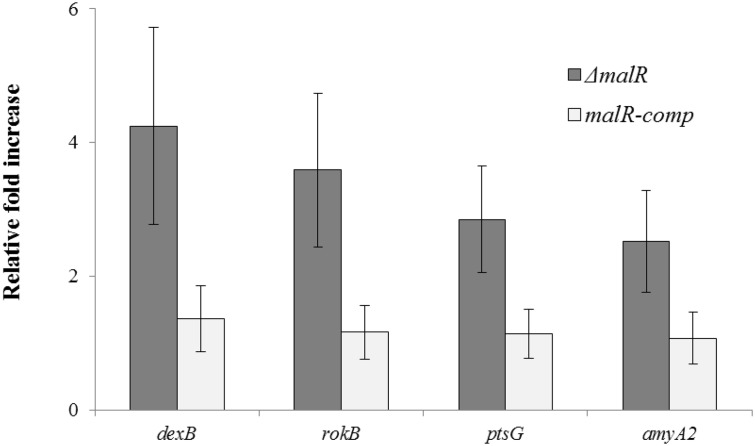
To further confirm the role of MalR as a transcriptional repressor of dexB, rokB, ptsG and amyA2, we complemented malR gene in ΔmalR strain and preformed quantitative RT-PCR on these genes. The results of quantitative RT-PCR show that the expression of dexB, rokB, ptsG and amyA2 increased significantly in ΔmalR strain (Fig 2), whereas the wild-type expression was restored when malR gene was complemented in ΔmalR strain. These results further confirm that MalR acts as a transcriptional repressor of the mal regulon consisting of pulA, dexB, rokB, ptsG and amyA2 in addition to malMP, malXCD and malAR operons.
Fig 2. The relative expression of dexB, rokB, ptsG and amyA2 in ΔmalR and malR-comp (malR complemented in ΔmalR strain) strains compared to D39 wild-type grown in GM17 (0.5% Glucose + M17).
The expression of the genes dexB, rokB, ptsG and amyA2 was normalized with housekeeping gene gyrA. Results represent mean and standard deviation of three independent experiments. The fold increase is relative to the expression in D39 wild-type.
DNA microarray analysis with a ΔmalR mutant
To confirm our β-galactosidase assays results and to elucidate the impact of malR deletion on the global gene expression of S. pneumoniae, DNA microarray analysis was performed with D39 wild-type against its isogenic malR mutant grown in GM17 (0.5% Glucose + M17) medium. GM17 medium was used to grow the strains as our β-galactosidase assays showed that the expression of the maltose-responsive genes was lower in the presence of glucose. Table 4 summarizes the results of transcriptome changes induced in S. pneumoniae due to the deletion of malR. The malR deletion did not have an extensive effect on the transcriptome of S. pneumoniae. After choosing the criterion of ≥ twofold difference as the threshold change and a p-value <0.001, malXCD, malMP, amyA2, ptsG, dexB and rokB (MalR regulon) were upregulated significantly in the ΔmalR strain and no other bigger responses were observed in the transcriptome. This data is in accordance with the β-galactosidase data mentioned above. This data further suggests that MalR is a negative transcriptional regulator of the mal regulon (malXCD, malMP, amyA2, ptsG, dexB and rokB). No change in the expression of pulA was observed which might indicate the role of another transcriptional regulator in the regulation of pulA.
Table 4. Summary of transcriptome comparison of S. pneumoniae D39 wild-type and ΔmalR grown in GM17 (0.5% Glucose + M17).
| a D39 Tag | b Function | c Ratio |
|---|---|---|
| spd_0311 | Glucan 1,6-alpha-glucosidase, DexB | 2.2 |
| spd_0580 | Glucokinase, RokB | 3.0 |
| spd_0661 | PTS system, IIABC components, PtsG | 1.8 |
| spd_0662 | Hypothetical protein | 3.7 |
| spd_1215 | Cytoplasmic alpha-amylase, AmyA2 | 6.8 |
| spd_1933 | Amylomaltase, MalM | 6.3 |
| spd_1934 | Maltose/maltodextrin ABC transporter, MalX | 2.9 |
| spd_1938 | Maltose operon transcriptional repressor, MalR | -2.9 |
aGene numbers refer to D39 locus tags.
bD39 annotation/TIGR4 annotation [20].
cRatio represents the fold increase in the expression of genes in ΔmalR as compared to the wild-type.
Role of CcpA in maltose-dependent gene regulation
CcpA is a global transcriptional regulator that causes repression of genes involved in the utilization of non-preferred sugars in the presence of a preferred sugar [38–40]. To study the role of CcpA in the regulation of the mal regulon, we analyzed the promoter regions of the malXCD, malMP, pulA, amyA2, ptsG, dexB and rokB genes for the presence of a CcpA binding site (cre box). Interestingly, a CcpA binding site was found in the malX and pulA promoter regions, suggesting a putative role of CcpA in the regulation of malXCD and pulA. To determine the functionality of the CcpA binding site in the promoter regions of malX and pulA, and to find the global effect of ccpA on the gene expression of S. pneumoniae in the presence of maltose, we performed microarray comparison of D39 ΔccpA with D39 wild-type grown in MM17 (0.5% Maltose + M17) medium. After choosing the criterion of ≥ twofold difference as the threshold change in expression and a p-value <0.001, the results of our microarray analysis demonstrated that deletion of ccpA led to the upregulation of the malXCD operon and the pulA gene in the presence of maltose (Table 5). Upregulation of the malXCD operon explains why we could not observe strikingly increased activity of PmalX-lacZ in ΔmalR compared to the wild-type (Fig 1B). pulA was 26fold upregulated in ΔccpA in the presence of maltose which suggests that pulA is repressed by CcpA in the presence of maltose and also explains why we could not see derepression of pulA in ΔmalR in the presence of glucose or increased expression of pulA in the presence of maltose. There were also a number of other genes that were differentially expressed in ΔccpA in the presence of maltose. These genes have been grouped into COG functional categories according to the putative function of respective proteins (Table 6). Most of these genes are carbohydrate transport and metabolism genes, which suggests that the repression on genes caused by CcpA is relieved in the absence of CcpA, as most of the genes belonging to category G are upregulated (32 out of 45). There are also genes that are involved in energy production and conversion. Amino acid transport and metabolism genes also form a major group among the genes differentially expressed in our microarray analysis.
Table 5. List of the mal regulon genes regulated in transcriptome comparison of S. pneumoniae D39 wild-type and ΔccpA grown in MM17 (0.5% Maltose + M17).
| a D39 Tag | b Function | c Ratio |
|---|---|---|
| spd_0250 | Pullulanase, extracellular, PulA | 26.8 |
| spd_0661 | PTS system, IIABC components, PtsG | 3.1 |
| spd_1935 | Maltose/maltodextrin ABC transporter, MalC | 3.7 |
| spd_1936 | Maltose/maltodextrin ABC transporter, MalD | 3.5 |
| spd_1937 | Maltodextrose utilization protein, MalA | 1.9 |
| spd_1938 | Maltose operon transcriptional repressor, MalR | 2.5 |
aGene numbers refer to D39 locus tags.
bD39 annotation [20].
cRatio represents the fold increase in the expression of genes in ΔccpA as compared to the wild-type.
Table 6. Number of genes significantly affected in D39 ΔccpA as compared to the D39 wild-type grown in MM17 (0.5% Maltose + M17).
| Functional categories | Total | Up | Down |
|---|---|---|---|
| C: Energy production and conversion | 13 | 8 | 5 |
| D: Cell cycle control, cell division, chromosome partitioning | 0 | 0 | 0 |
| E: Amino acid transport and metabolism | 11 | 5 | 6 |
| F: Nucleotide transport and metabolism | 15 | 2 | 13 |
| G: Carbohydrate transport and metabolism | 45 | 32 | 13 |
| H: Coenzyme transport and metabolism | 4 | 3 | 1 |
| I: Lipid transport and metabolism | 10 | 2 | 8 |
| J: Translation, ribosomal structure and biogenesis | 24 | 2 | 22 |
| K: Transcription | 13 | 10 | 3 |
| L: Replication, recombination and repair | 5 | 3 | 2 |
| M: Cell wall/membrane/envelope biogenesis | 9 | 8 | 1 |
| O: Posttranslational modification, protein turnover, chaperones | 9 | 6 | 3 |
| P: Inorganic ion transport and metabolism | 5 | 3 | 2 |
| Q: Secondary metabolites biosynthesis, transport and catabolism | 2 | 1 | 1 |
| R: General function prediction only | 18 | 9 | 9 |
| S: Function unknown | 21 | 15 | 6 |
| T: Signal transduction mechanisms | 10 | 7 | 3 |
| U: Intracellular trafficking, secretion, and vesicular transport | 1 | 0 | 1 |
| V: Defense mechanisms | 4 | 3 | 1 |
| Others | 34 | 19 | 15 |
| Total number of genes | 253 | 138 | 115 |
Genes affected with more than 2 fold in D39 ΔccpA as compared to the D39 wild-type are shown as COG functional categories.
Prediction and confirmation of a MalR operator site in maltose-responsive genes
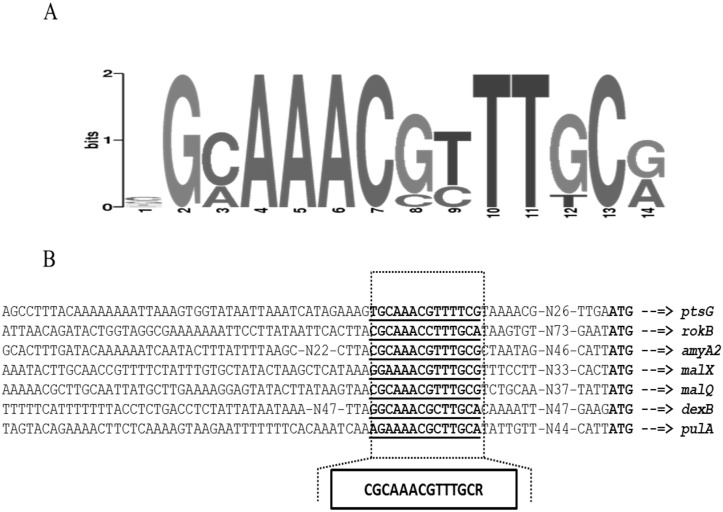
Previously, the MalR operator site has been identified by using footprint analysis in the promoter regions of malX (5’-CGCAAACGTTTTCC-3’) and malM (5’-CGCAAACGTTTGCGT- 3’) [17]. Using these sites, we generated a weight matrix of the MalR operator site (5’-CGCAAACGTTTKSG-3’) through Genome-2D software (Fig 3A) [41]. This weight matrix was further used to perform genome-wide search in S. pneumoniae D39 to find more MalR operator sites by using Genome-2D software. Interestingly, our bioinformatics analysis revealed the presence of the MalR operator site in the promoter regions of pulA, dexB, rokB, ptsG and amyA2 (Fig 3A). These observations are consistent with our transcriptome analysis with ΔmalR and further confirm the role of MalR in the regulation of these genes. We also generated a weight-matrix using these DNA sequences, which serve as the MalR operator site in these promoters (Fig 3B) and searched the MalR operator sites in other streptococci. Our in silico analysis with this MalR operator site indicates the conservation of the MalR operator site in other sequenced strains of S. pneumoniae available in the KEGG database. Streptococcus agalactiae, Streptococcus dysgalactiae, Streptococcus equi, Streptococcus gallolyticus, Streptococcus mitis, Streptococcus pyogenes, Streptococcus sanguinis, Streptococcus suis and Streptococcus uberis also encode a putative mal regulon but gene composition of the mal regulon may vary from S. pneumoniae.
Fig 3.
(A) Weight matrix of the identified MalR operator site in the ProkB, PdexB, PamyA2, PptsG, PmalM, PmalX and PpulA in S. pneumoniae D39 (B) Position of a MalR operator site in ProkB, PdexB, PamyA2, PptsG, PmalM, PmalX and PpulA in S. pneumoniae D39. Transcription start sites are bold while MalR operator sites are bold-underlined.
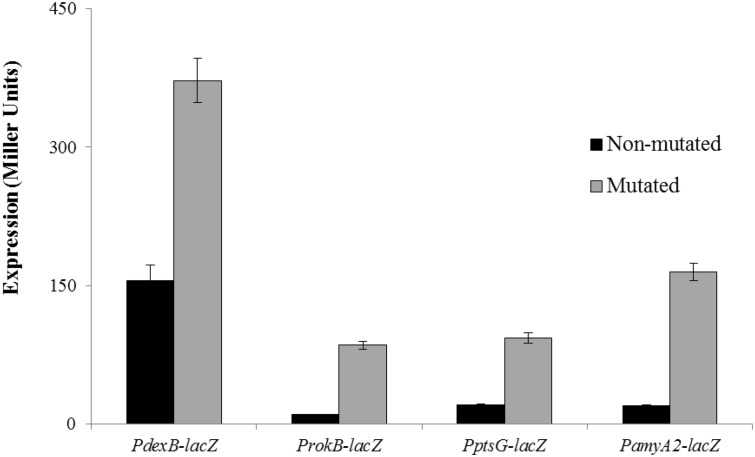
To further confirm the proposed MalR operator sites in the promoter regions of dexB, rokB, ptsG and amyA2, we mutated few bases in the proposed MalR operator sites present in the promoter regions of dexB (5’-GGCAAACGCTTGCA-3’ to 5’-GGCGAATACTTGCA-3’), rokB (5’-CGCAAACCTTTGCA-3’ to 5’-CGCGCATCTTTGCA-3’), ptsG (5’-TGCAAACGTTTTCG-3’ to TGCACACATCTTCG) and amyA2 (5’-CGCAAACGTTTGCG-3’ to 5’-CGCCGAATTTTGCG-3’). These mutated promoter regions of dexB, rokB, ptsG and amyA2 were fused with lacZ and β-galactosidase assays were performed. The expression of the mutated promoters was significantly higher as compared to that of the non-mutated in the presence of glucose. The expression of the mutated promoters in the wild-type was comparable to that of non-mutated promoters in ΔmalR in the presence of glucose (Fig 4). These results suggest that the MalR operator sites present in the promoter regions of dexB, rokB, ptsG and amyA2 are functional and act as MalR operator sites.
Fig 4. Expression levels (in Miller units) of mutated and non-mutated ProkB-lacZ, PdexB-lacZ, PamyA2-lacZ and PptsG-lacZ in D39 wild-type grown in GM17 (0.5% Glucose + M17).
Standard deviations of three independent replicates are indicated in bars.
Discussion and Conclusions
Maltose is one of the sugars that pneumococcus can utilize as a sole carbon source [42]. However, the effect of maltose on the transcriptome of S. pneumoniae was never explored. Moreover, the complete regulon of MalR in S. pneumoniae is also not known. In this study, we explored the effect of maltose on the transcriptome of S. pneumoniae and we have shown that the mal regulon in S. pneumoniae not only consists of the mal gene cluster but also ptsG, dexB, amyA2, pulA and rokB. Furthermore, we have studied the role of CcpA in the regulation of the mal regulon. The complete mal regulon encodes the proteins, which are putatively involved in the maltose transport and utilization. Maltose might enter in the cell by a PTS component (PtsG) and/or a maltose transporter (MalXCD) and, is converted into maltose-6-P. Maltose-6-P can either be converted into D-glucose-6P or it may be converted back into maltose by SPD-0662 [43]. This maltose is further converted into α-D-glucose by MalM (an amylomaltase), whereas the starch present inside the cell can be converted into amylose by MalP (Glycogen phosphorylase family protein) or can be converted into a dextrin by the pullulanase (PulA) and α-amylase (AmyA2) [43]. This dextrin can be converted into α-D-glucose, which is further converted into α-D-glucose-6-P by a glucokinase (RokB) [43].
The mal regulon has been well-studied in different Gram-negative bacteria including the model organism E. coli [44–46] and represents a classical model for positive regulation of transcription. The mal regulon in Gram-negative bacteria consists of three operons (malEFG, malK-lamB-malM and malPQ) and a couple of non-essential genes (malS and malZ) [45,47]. The regulatory mode of the mal regulon in E. coli and several other Gram-negative bacteria is similar and depends on two regulatory proteins, i.e. the cAMP receptor protein (CRP) and the specific maltose induced activator MalT. Genes involved in maltodextrin uptake and metabolism have been the nucleus of the studies in Gram-positive bacteria including S. pneumoniae [47–49]. The regulatory mode of the pneumococcal mal regulon was proposed to be different from that of E. coli on the basis of information available from different studies [48,50]. The projected mechanism for induction of the maltose operons of E. coli involves the binding of the activated allosteric MalT protein to target sequences located upstream of the promoter boxes, which is in contrast with the proposed model of transcriptional regulation of the mal regulon in S. pneumoniae [51]. The regulatory mode of the S. pneumoniae mal regulon is similar to that of the some Gram-positive bacteria. For example, in Streptomyces coelicolor, the transcription of the malEFG gene cluster was induced by maltose and the deletion of malR led to the derepression on malEFG caused by glucose [52]. Similarly, the mal regulon in Streptococcus mutans consists of the malQ-glgP operon, malXFGK operon and the malT gene, and repressed by the transcriptional regulator MalR in the absence of maltose [53–55]. However, some Gram-positive bacteria also possess the mal regulon, which is regulated in a similar fashion as in E. coli, i.e. the mal regulon is positively transcriptionally regulated. Prime example is of Lactococcus lactis where MalR acts as a transcriptional activator of the mal regulon [56]. Sulfolobus acidocaldarius also represents an example of the regulation of the mal regulon similar to that of E. coli and K. pneumoniae [57]. Notably, in S. pneumoniae, there are some other genes that are expressed in the presence of maltose and regulated by MalR. These genes are dexB, ptsG, rokB and amyA2.
Altered expression of dexB, ptsG, malMP and malXCD was also observed in a previous study, where a transcriptome comparison of D39 ΔbguR with D39 wild-type in the presence of glucose was performed [32]. BguR is a transcriptional repressor that represses the expression of the bgu operon in the absence of cellobiose [32]. It has been shown that the deletion of bguR not only derepresses the expression of the bgu operon but also the expression of dexB, ptsG, malMP and malXCD in the presence of glucose. Moreover, expression of ptsG, malMP and malXCD was also increased when the transcriptome of S. pneumoniae grown in CM17 (0.5% Cellobiose + M17) was compared to that grown in GM17 (0.5% Glucose + M17) [32]. These findings also suggest the putative role of ptsG, malMP and malXCD in the utilization of cellobiose. Further investigations focusing on the role of cellobiose and transcriptional regulator BguR in the regulation of the mal regulon will be required to elaborate the role of the ptsG, malMP and malXCD in cellobiose metabolism. Moreover, the regulatory mechanism of the maltose/maltodextrin-induced genes in E. coli was complicated after the identification of non-maltosaccharide inducers and the connection to other regulatory circuits [58,59]. Our multi-sugar experiment clarifies any possible doubts that may have been attributed to other inducers of the mal gene cluster in S. pneumoniae.
A number of carbohydrate metabolism/ utilization genes have been shown to play a role in the virulence status of S. pneumoniae. The neuraminidases (NanA and NanB) and hyaluronate lyase (HylA) are among the ones that are well-studied. There is still a large number of carbohydrate metabolism/ utilization genes that might play a role in the virulence status of S. pneumoniae and require more attention. PulA (a cell wall-anchored pullulanase) and MalX (the lipid-bound solute binding protein) are among the six putative pneumococcal virulence factors that are proposed to be involved in α-glucan metabolism [60]. Extracellular glycogen in S. pneumoniae is depolymerzied into maltodextrins by the pullulanase (PulA) and some of these degradation products can be transported into the cell through PtsG or MalXCD [61]. The presence of PulA and MalX as the only extracellular components of S. pneumoniae’s α-glucan metabolizing machinery, make them vital for the utilization of exogenous glycogen [61]. The extracellular localization of PulA and MalX also suggests that these two proteins may work in conjunction to each other as PulA might degrade the glycogen and MalX will help in the transport of the degradation products of glycogen. Therefore, this partner system may play a very significant role in the pathogenesis of S. pneumoniae.
MalR in S. pneumoniae belongs to the LacI family of transcriptional repressors. It has a helix-turn-helix (HTH) domain and a LacI-sugar binding domain. This family of transcriptional regulators consists of a transcriptional factor mostly involved in the carbohydrate catabolic pathways, and generally, sugars or their phosphorylated counterparts are the effector molecules of these transactional regulators [62]. LacI-family transcriptional regulators are mostly transcriptional repressors, while some may act as both transcriptional repressor and activator. The DNA-binding affinity of a LacI-family transcriptional regulator changes on binding with an effector ligand [63]. A high conservation of this regulatory system through evolution can be observed by the similarities found between MalR and the other members of the family, even at the operator sequence among Gram-positive and Gram-negative bacteria [17]. However, there may be some differences among the genes that are regulated by them. The mode of transcription regulation for the mal regulon in S. pneumoniae demonstrates a substantial difference with the positively regulated genes in the Gram-negative enteric bacteria, which suggests that the evolution of structural and regulatory genes for these operons may have followed different pathways [47].
Supporting Information
Oval indicates the time points on which cultures were harvested for transcriptome analysis.
(TIF)
(DOCX)
Data Availability
Microarray data is available on GEO under accession number GSE65550.
Funding Statement
This work was supported by University of Groningen.
References
- 1. Ryan KJ, Ray CG. Sherris Medical Microbiology. USA: McGraw Hill; 2004. [Google Scholar]
- 2. Klugman KP, Chien YW, Madhi SA. Pneumococcal pneumonia and influenza: a deadly combination. Vaccine. 2009;27 Suppl 3: C9–C14. 10.1016/j.vaccine.2009.06.007 [DOI] [PubMed] [Google Scholar]
- 3. Pettigrew MM, Gent JF, Revai K, Patel JA, Chonmaitree T. Microbial interactions during upper respiratory tract infections. Emerg Infect Dis Print. 2008;14: 1584–1591. 10.3201/eid1410.080119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buckwalter CM, King SJ. Pneumococcal carbohydrate transport: food for thought. Trends Microbiol. 2012; 10.1016/j.tim.2012.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Afzal M, Shafeeq S, Kuipers OP. LacR is a repressor of lacABCD and LacT is an activator of lacTFEG, constituting the lac gene cluster in Streptococcus pneumoniae. Appl Environ Microbiol. 2014;80: 5349–5358. 10.1128/AEM.01370-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Afzal M, Shafeeq S, Kuipers OP. Ascorbic acid-dependent gene expression in Streptococcus pneumoniae and the activator function of the transcriptional regulator UlaR2. Front Microbiol. 2015;6: 72 10.3389/fmicb.2015.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Afzal M, Shafeeq S, Henriques-Normark B, Kuipers OP. UlaR activates expression of the ula operon in Streptococcus pneumoniae in the presence of ascorbic acid. Microbiol Read Engl. 2015;161: 41–49. 10.1099/mic.0.083899-0 [DOI] [PubMed] [Google Scholar]
- 8. Afzal M, Shafeeq S, Ahmed H, Kuipers OP. Sialic acid-mediated gene expression in Streptococcus pneumoniae and the role of NanR as a transcriptional activator of the nan gene cluster. Appl Environ Microbiol. 2015; 10.1128/AEM.00499-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Iyer R, Camilli A. Sucrose metabolism contributes to in vivo fitness of Streptococcus pneumoniae. Mol Microbiol. 2007;66: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rosenow C, Maniar M, Trias J. Regulation of the alpha-galactosidase activity in Streptococcus pneumoniae: characterization of the raffinose utilization system. Genome Res. 1999;9: 1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marion C, Stewart JM, Tazi MF, Burnaugh AM, Linke CM, Woodiga SA, et al. Streptococcus pneumoniae can utilize multiple sources of hyaluronic acid for growth. Infect Immun. 2012;80: 1390–1398. 10.1128/IAI.05756-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deutscher J, Francke C, Postma PW. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol Mol Biol Rev MMBR. 2006;70: 939–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kilic AO, Tao L, Zhang Y, Lei Y, Khammanivong A, Herzberg MC. Involvement of Streptococcus gordonii beta-glucoside metabolism systems in adhesion, biofilm formation, and in vivo gene expression. J Bacteriol. 2004;186: 4246–4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brown WH. Introduction to organic chemistry. 3rd ed Hoboken, NJ: Wiley; 2005. [Google Scholar]
- 15. Sugisawa H, Edo H. The Thermal Degradation of Sugars I. Thermal Polymerization of Glucose. J Food Sci. 1966;31: 561–565. 10.1111/j.1365-2621.1966.tb01905.x [DOI] [Google Scholar]
- 16. Bender DA. A dictionary of food and nutrition. 2nd ed Oxford [U.K.]; New York: Oxford University Press; 2005. [Google Scholar]
- 17. Nieto C, Espinosa M, Puyet A. The maltose/maltodextrin regulon of Streptococcus pneumoniae. Differential promoter regulation by the transcriptional repressor MalR. J Biol Chem. 1997;272: 30860–30865. [DOI] [PubMed] [Google Scholar]
- 18. Nieto C, Puyet A, Espinosa M. MalR-mediated regulation of the Streptococcus pneumoniae malMP operon at promoter PM. Influence of a proximal divergent promoter region and competition between MalR and RNA polymerase proteins. J Biol Chem. 2001;276: 14946–14954. [DOI] [PubMed] [Google Scholar]
- 19. Terzaghi BE, Sandine W.E. Improved medium for lactic streptococci and their bacteriophages. Appl Environ Microbiol. 1975;29: 807–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lanie JA, Ng WL, Kazmierczak KM, Andrzejewski TM, Davidsen TM, Wayne KJ, et al. Genome sequence of Avery’s virulent serotype 2 strain D39 of Streptococcus pneumoniae and comparison with that of unencapsulated laboratory strain R6. J Bacteriol. 2007;189: 38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kloosterman TG, Bijlsma JJE, Kok J, Kuipers OP. To have neighbour’s fare: extending the molecular toolbox for Streptococcus pneumoniae. Microbiol Read Engl. 2006;152: 351–359. [DOI] [PubMed] [Google Scholar]
- 22. Halfmann A, Hakenbeck R, Bruckner R. A new integrative reporter plasmid for Streptococcus pneumoniae. FEMS Microbiol Lett. 2007;268: 217–224. [DOI] [PubMed] [Google Scholar]
- 23. Israelsen H, Madsen SM, Vrang A, Hansen EB, Johansen E. Cloning and partial characterization of regulated promoters from Lactococcus lactis Tn917-lacZ integrants with the new promoter probe vector, pAK80. Appl Environ Microbiol. 1995;61: 2540–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Overkamp W, Beilharz K, Detert Oude Weme R, Solopova A, Karsens H, Kovács ÁT, et al. Benchmarking various green fluorescent protein variants in Bacillus subtilis, Streptococcus pneumoniae, and Lactococcus lactis for live cell imaging. Appl Environ Microbiol. 2013;79: 6481–6490. 10.1128/AEM.02033-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shafeeq S, Yesilkaya H, Kloosterman TG, Narayanan G, Wandel M, Andrew PW, et al. The cop operon is required for copper homeostasis and contributes to virulence in Streptococcus pneumoniae. Mol Microbiol. 2011;81: 1255–1270. 10.1111/j.1365-2958.2011.07758.x [DOI] [PubMed] [Google Scholar]
- 26. Yesilkaya H, Manco S, Kadioglu A, Terra VS, Andrew PW. The ability to utilize mucin affects the regulation of virulence gene expression in Streptococcus pneumoniae. FEMS Microbiol Lett. 2008;278: 231–235. [DOI] [PubMed] [Google Scholar]
- 27. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. NatProtoc. 2008;3: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 28. Shafeeq S, Afzal M, Henriques-Normark B, Kuipers OP. Transcriptional profiling of UlaR-regulated genes in Streptococcus pneumoniae. Genomics Data. 2015;4: 57–59. 10.1016/j.gdata.2015.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Afzal M, Manzoor I, Kuipers OP. A fast and reliable pipeline for bacterial transcriptome analysisCase study: Serine-dependent gene regulation in Streptococcus pneumoniae. J Vis Exp. 2015; 10.3791/52649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shafeeq S, Kloosterman TG, Kuipers OP. Transcriptional response of Streptococcus pneumoniae to Zn(2+) limitation and the repressor/activator function of AdcR. Metallomics. 2011;3: 609–618. 10.1039/c1mt00030f [DOI] [PubMed] [Google Scholar]
- 31. Shafeeq S, Kloosterman TG, Kuipers OP. CelR-mediated activation of the cellobiose-utilization gene cluster in Streptococcus pneumoniae. Microbiol Read Engl. 2011;157: 2854–2861. 10.1099/mic.0.051359-0 [DOI] [PubMed] [Google Scholar]
- 32. Shafeeq S, Kuipers OP, Kloosterman TG. Cellobiose-mediated gene expression in Streptococcus pneumoniae: a repressor function of the novel GntR-type regulator BguR. PloS One. 2013;8: e57586 10.1371/journal.pone.0057586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saburi W, Mori H, Saito S, Okuyama M, Kimura A. Structural elements in dextran glucosidase responsible for high specificity to long chain substrate. Biochim Biophys Acta. 2006;1764: 688–698. 10.1016/j.bbapap.2006.01.012 [DOI] [PubMed] [Google Scholar]
- 34. Shafeeq S, Kloosterman TG, Rajendran V, Kuipers OP. Characterization of the ROK-family transcriptional regulator RokA of Streptococcus pneumoniae D39. Microbiol Read Engl. 2012;158: 2917–2926. 10.1099/mic.0.062919-0 [DOI] [PubMed] [Google Scholar]
- 35. Krause FS, Henrich A, Blombach B, Krämer R, Eikmanns BJ, Seibold GM. Increased glucose utilization in Corynebacterium glutamicum by use of maltose, and its application for the improvement of L-valine productivity. Appl Environ Microbiol. 2010;76: 370–374. 10.1128/AEM.01553-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shelburne SA, Keith DB, Davenport MT, Beres SB, Carroll RK, Musser JM. Contribution of AmyA, an extracellular alpha-glucan degrading enzyme, to group A streptococcal host-pathogen interaction. Mol Microbiol. 2009;74: 159–174. 10.1111/j.1365-2958.2009.06858.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Novichkov PS, Laikova ON, Novichkova ES, Gelfand MS, Arkin AP, Dubchak I, et al. RegPrecise: a database of curated genomic inferences of transcriptional regulatory interactions in prokaryotes. Nucleic Acids Res. 2010;38: D111–8. 10.1093/nar/gkp894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carvalho SM, Kloosterman TG, Kuipers OP, Neves AR. CcpA ensures optimal metabolic fitness of Streptococcus pneumoniae. PloS One. 2011;6: e26707 10.1371/journal.pone.0026707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lulko AT, Buist G, Kok J, Kuipers OP. Transcriptome analysis of temporal regulation of carbon metabolism by CcpA in Bacillus subtilis reveals additional target genes. J Mol Microbiol Biotechnol. 2007;12: 82–95. [DOI] [PubMed] [Google Scholar]
- 40. Zomer AL, Buist G, Larsen R, Kok J, Kuipers OP. Time-resolved determination of the CcpA regulon of Lactococcus lactis subsp. cremoris MG1363. J Bacteriol. 2007;189: 1366–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Baerends RJS, Smits WK, de Jong A, Hamoen LW, Kok J, Kuipers OP. Genome2D: a visualization tool for the rapid analysis of bacterial transcriptome data. Genome Biol. 2004;5(5): R37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bidossi A, Mulas L, Decorosi F, Colomba L, Ricci S, Pozzi G, et al. A functional genomics approach to establish the complement of carbohydrate transporters in Streptococcus pneumoniae. PLoSOne. 2012;7: e33320 10.1371/journal.pone.0033320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kanehisa M, Goto S, Sato Y, Kawashima M, Furumichi M, Tanabe M. Data, information, knowledge and principle: back to metabolism in KEGG. Nucleic Acids Res. 2014;42: D199–205. 10.1093/nar/gkt1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. M A Bloch O Raibaud. Comparison of the malA regions of Escherichia coli and Klebsiella pneumoniae. Journal of Bacteriology. 1987;168: 1220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Boos W, Shuman H. Maltose/maltodextrin system of Escherichia coli: transport, metabolism, and regulation. Microbiol Mol Biol Rev MMBR. 1998;62: 204–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schwartz M. The Maltose Regulon: Escherichia coli and Salmonella typhimurium. American Society for Microbiology, Washington, D. C; 1987. [Google Scholar]
- 47. Puyet A, Espinosa M. Structure of the maltodextrin-uptake locus of Streptococcus pneumoniae. Correlation to the Escherichia coli maltose regulon. J Mol Biol. 1993;230: 800–811. 10.1006/jmbi.1993.1202 [DOI] [PubMed] [Google Scholar]
- 48. Lacks S. Genetic regulation of maltosaccharide utilization in Pneumococcus. Genetics. 1968;60: 685–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Weinrauch Y, Lacks SA. Nonsense mutations in the amylomaltase gene and other loci of Streptococcus pneumoniae. Mol Gen Genet MGG. 1981;183: 7–12. [DOI] [PubMed] [Google Scholar]
- 50. Stassi DL, Dunn JJ, Lacks SA. Nucleotide sequence of DNA controlling expression of genes for maltosaccharide utilization in Streptococcus pneumoniae. Gene. 1982;20: 359–366. [DOI] [PubMed] [Google Scholar]
- 51. Vidal-Ingigliardi D, Richet E, Raibaud O. Two MalT binding sites in direct repeat. A structural motif involved in the activation of all the promoters of the maltose regulons in Escherichia coli and Klebsiella pneumoniae. J Mol Biol. 1991;218: 323–334. [DOI] [PubMed] [Google Scholar]
- 52. Van Wezel GP, White J, Young P, Postma PW, Bibb MJ. Substrate induction and glucose repression of maltose utilization by Streptomyces coelicolor A3(2) is controlled by malR, a member of the lacl-galR family of regulatory genes. Mol Microbiol. 1997;23: 537–549. [DOI] [PubMed] [Google Scholar]
- 53. Kilic AO, Honeyman AL, Tao L. Overlapping substrate specificity for sucrose and maltose of two binding protein-dependent sugar uptake systems in Streptococcus mutans. FEMS Microbiol Lett. 2007;266: 218–223. 10.1111/j.1574-6968.2006.00522.x [DOI] [PubMed] [Google Scholar]
- 54. Sato Y, Okamoto-Shibayama K, Azuma T. The malQ gene is essential for starch metabolism in Streptococcus mutans. J Oral Microbiol. 2013;5: 10.3402/jom.v5i0.21285. Print 2013. 10.3402/jom.v5i0.21285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Webb AJ, Homer KA, Hosie AHF. Two closely related ABC transporters in Streptococcus mutans are involved in disaccharide and/or oligosaccharide uptake. J Bacteriol. 2008;190: 168–178. 10.1128/JB.01509-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Andersson U, Rådström P. Physiological function of the maltose operon regulator, MalR, in Lactococcus lactis. BMC Microbiol. 2002;2: 28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wagner M, Wagner A, Ma X, Kort JC, Ghosh A, Rauch B, et al. Investigation of the malE promoter and MalR, a positive regulator of the maltose regulon, for an improved expression system in Sulfolobus acidocaldarius. Appl Environ Microbiol. 2014;80: 1072–1081. 10.1128/AEM.03050-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ehrmann M, Boos W. Identification of endogenous inducers of the mal regulon in Escherichia coli. J Bacteriol. 1987;169: 3539–3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Reidl J, Römisch K, Ehrmann M, Boos W. MalI, a novel protein involved in regulation of the maltose system of Escherichia coli, is highly homologous to the repressor proteins GalR, CytR, and LacI. J Bacteriol. 1989;171: 4888–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hava DL, Camilli A. Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol Microbiol. 2002;45: 1389–1406. [PMC free article] [PubMed] [Google Scholar]
- 61. Abbott DW, Higgins MA, Hyrnuik S, Pluvinage B, Lammerts van Bueren A, Boraston AB. The molecular basis of glycogen breakdown and transport in Streptococcus pneumoniae. Mol Microbiol. 2010;77: 183–199. 10.1111/j.1365-2958.2010.07199.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nguyen CC, Saier MH. Phylogenetic, structural and functional analyses of the LacI-GalR family of bacterial transcription factors. FEBS Lett. 1995;377: 98–102. [DOI] [PubMed] [Google Scholar]
- 63. Swint-Kruse L, Matthews KS. Allostery in the LacI/GalR family: variations on a theme. Curr Opin Microbiol. 2009;12: 129–137. 10.1016/j.mib.2009.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Leenhouts K, Buist G, Bolhuis A, ten Berge A, Kiel J, Mierau I, et al. A general system for generating unlabelled gene replacements in bacterial chromosomes. Mol Gen Genet MGG. 1996;253: 217–224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Oval indicates the time points on which cultures were harvested for transcriptome analysis.
(TIF)
(DOCX)
Data Availability Statement
Microarray data is available on GEO under accession number GSE65550.