Abstract
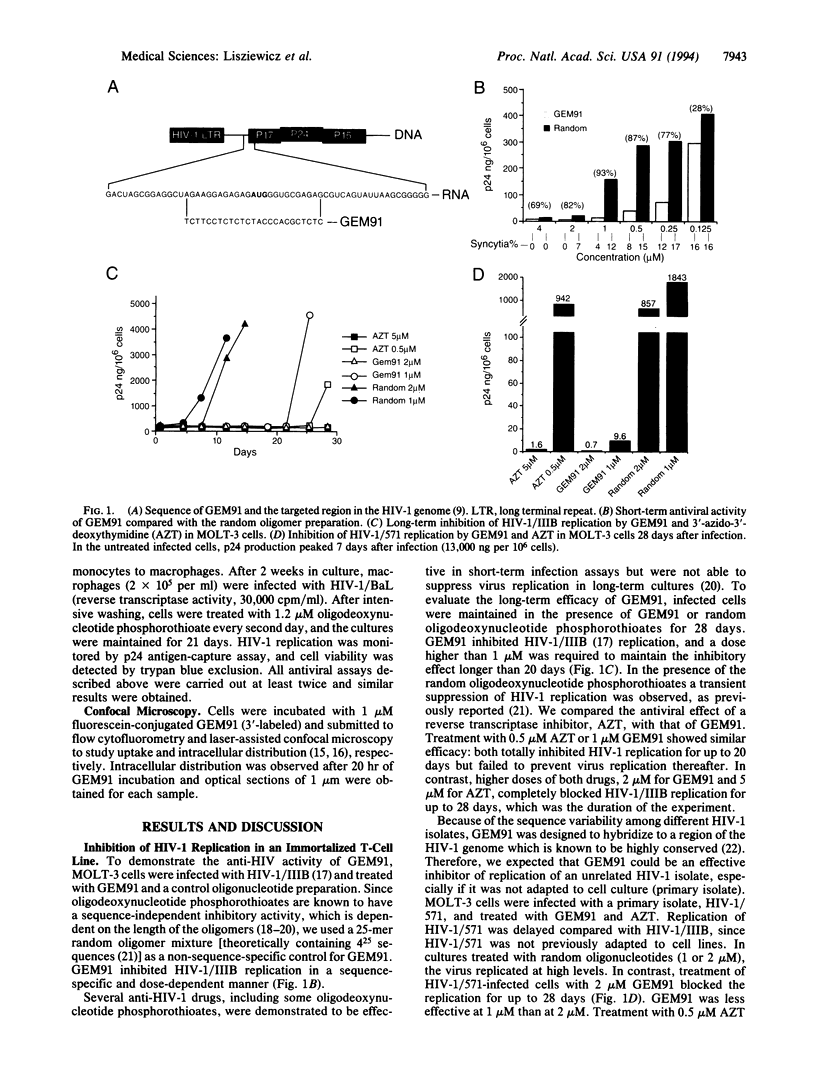
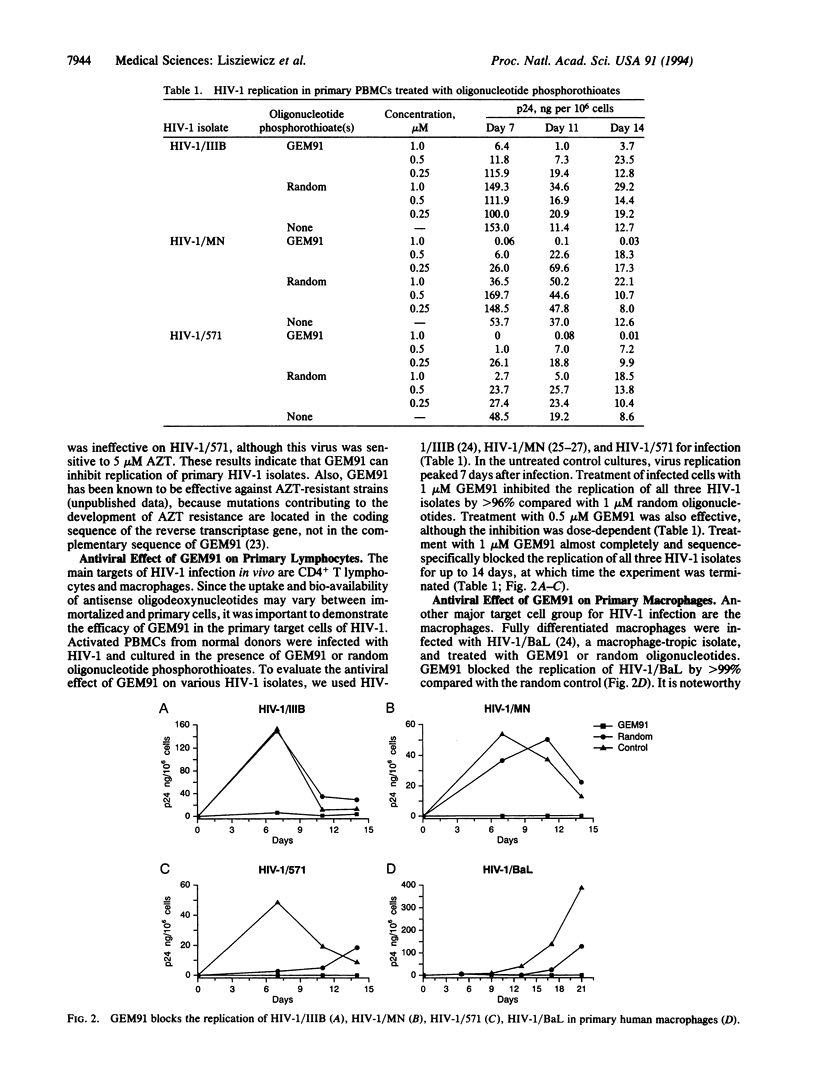
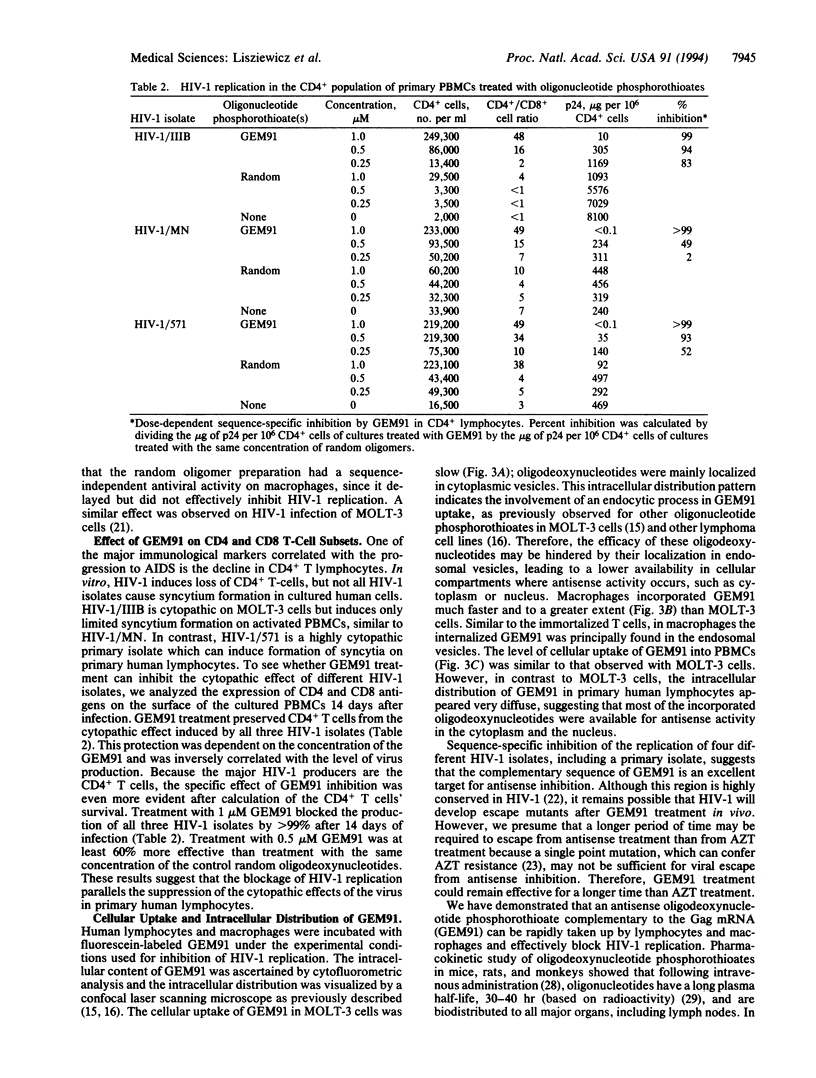
Gene-expression modulator 91 (GEM91) is a 25-nt antisense oligodeoxynucleotide phosphorothioate complementary to the Gag mRNA of human immunodeficiency virus type 1 (HIV-1). Cellular uptake and intracellular distribution of GEM91 within cells suggest that this oligomer is readily available for antisense activity. GEM91 inhibited HIV-1 replication in a dose-dependent and sequence-specific manner. In a comparative study, 2 microM GEM91 was as effective as 5 microM 3'-azido-3'-deoxythymidine in blocking virus replication during the 28-day treatment of an HIV-1-infected T-cell line. GEM91 also completely inhibited (> 99%) the growth of three different HIV-1 isolates in primary lymphocytes and prevented the cytopathic effect of the virus in primary CD4+ T cells. Similarly, treatment with GEM91 for 3 weeks of HIV-1/BaL-infected primary macrophages blocked virus replication. Based on GEM91 anti-HIV-activity, safety, and pharmacokinetic profile in animals, a clinical trial was started using this compound as an antisense oligonucleotide drug for the treatment of the acquired immunodeficiency syndrome.
Full text
PDF
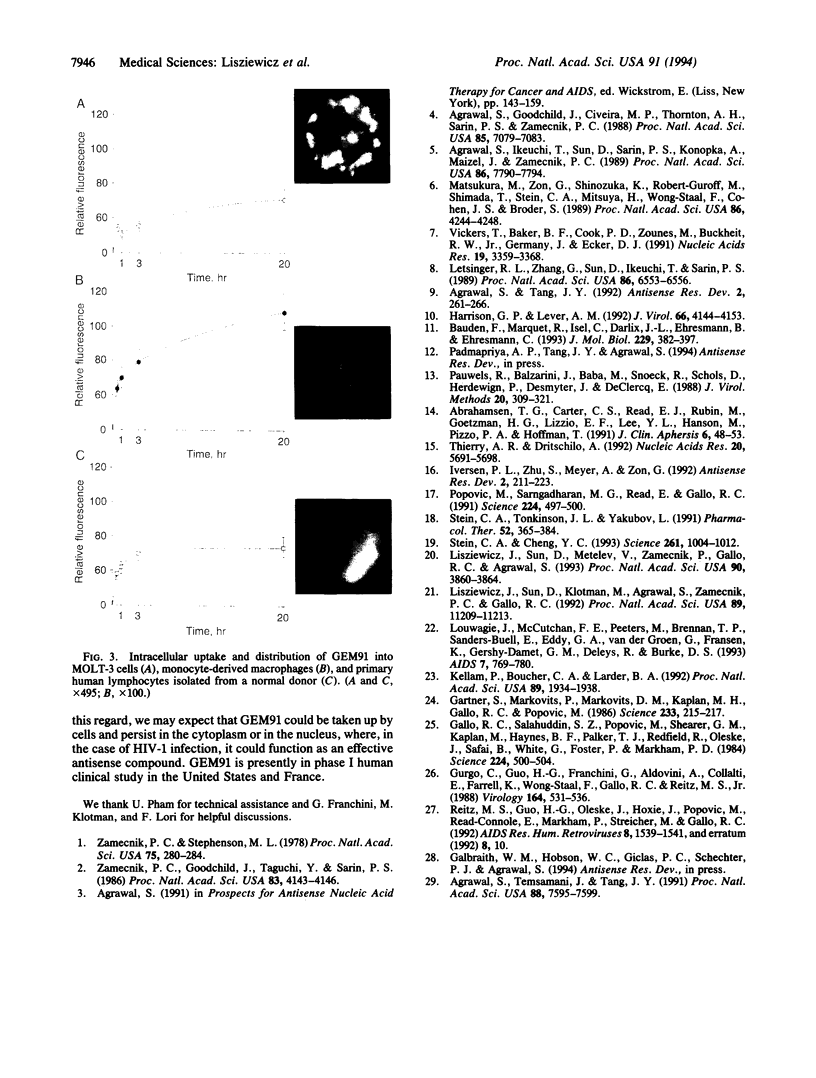
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamsen T. G., Carter C. S., Read E. J., Rubin M., Goetzman H. G., Lizzio E. F., Lee Y. L., Hanson M., Pizzo P. A., Hoffman T. Stimulatory effect of counterflow centrifugal elutriation in large-scale separation of peripheral blood monocytes can be reversed by storing the cells at 37 degrees C. J Clin Apher. 1991;6(1):48–53. doi: 10.1002/jca.2920060110. [DOI] [PubMed] [Google Scholar]
- Agrawal S., Goodchild J., Civeira M. P., Thornton A. H., Sarin P. S., Zamecnik P. C. Oligodeoxynucleoside phosphoramidates and phosphorothioates as inhibitors of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7079–7083. doi: 10.1073/pnas.85.19.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal S., Ikeuchi T., Sun D., Sarin P. S., Konopka A., Maizel J., Zamecnik P. C. Inhibition of human immunodeficiency virus in early infected and chronically infected cells by antisense oligodeoxynucleotides and their phosphorothioate analogues. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7790–7794. doi: 10.1073/pnas.86.20.7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal S., Tang J. Y. GEM 91--an antisense oligonucleotide phosphorothioate as a therapeutic agent for AIDS. Antisense Res Dev. 1992 Winter;2(4):261–266. doi: 10.1089/ard.1992.2.261. [DOI] [PubMed] [Google Scholar]
- Agrawal S., Temsamani J., Tang J. Y. Pharmacokinetics, biodistribution, and stability of oligodeoxynucleotide phosphorothioates in mice. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7595–7599. doi: 10.1073/pnas.88.17.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudin F., Marquet R., Isel C., Darlix J. L., Ehresmann B., Ehresmann C. Functional sites in the 5' region of human immunodeficiency virus type 1 RNA form defined structural domains. J Mol Biol. 1993 Jan 20;229(2):382–397. doi: 10.1006/jmbi.1993.1041. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Kaplan M. H., Gallo R. C., Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986 Jul 11;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Gurgo C., Guo H. G., Franchini G., Aldovini A., Collalti E., Farrell K., Wong-Staal F., Gallo R. C., Reitz M. S., Jr Envelope sequences of two new United States HIV-1 isolates. Virology. 1988 Jun;164(2):531–536. doi: 10.1016/0042-6822(88)90568-5. [DOI] [PubMed] [Google Scholar]
- Harrison G. P., Lever A. M. The human immunodeficiency virus type 1 packaging signal and major splice donor region have a conserved stable secondary structure. J Virol. 1992 Jul;66(7):4144–4153. doi: 10.1128/jvi.66.7.4144-4153.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen P. L., Zhu S., Meyer A., Zon G. Cellular uptake and subcellular distribution of phosphorothioate oligonucleotides into cultured cells. Antisense Res Dev. 1992 Fall;2(3):211–222. doi: 10.1089/ard.1992.2.211. [DOI] [PubMed] [Google Scholar]
- Kellam P., Boucher C. A., Larder B. A. Fifth mutation in human immunodeficiency virus type 1 reverse transcriptase contributes to the development of high-level resistance to zidovudine. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1934–1938. doi: 10.1073/pnas.89.5.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letsinger R. L., Zhang G. R., Sun D. K., Ikeuchi T., Sarin P. S. Cholesteryl-conjugated oligonucleotides: synthesis, properties, and activity as inhibitors of replication of human immunodeficiency virus in cell culture. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6553–6556. doi: 10.1073/pnas.86.17.6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisziewicz J., Sun D., Klotman M., Agrawal S., Zamecnik P., Gallo R. Specific inhibition of human immunodeficiency virus type 1 replication by antisense oligonucleotides: an in vitro model for treatment. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11209–11213. doi: 10.1073/pnas.89.23.11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisziewicz J., Sun D., Metelev V., Zamecnik P., Gallo R. C., Agrawal S. Long-term treatment of human immunodeficiency virus-infected cells with antisense oligonucleotide phosphorothioates. Proc Natl Acad Sci U S A. 1993 May 1;90(9):3860–3864. doi: 10.1073/pnas.90.9.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louwagie J., McCutchan F. E., Peeters M., Brennan T. P., Sanders-Buell E., Eddy G. A., van der Groen G., Fransen K., Gershy-Damet G. M., Deleys R. Phylogenetic analysis of gag genes from 70 international HIV-1 isolates provides evidence for multiple genotypes. AIDS. 1993 Jun;7(6):769–780. doi: 10.1097/00002030-199306000-00003. [DOI] [PubMed] [Google Scholar]
- Matsukura M., Zon G., Shinozuka K., Robert-Guroff M., Shimada T., Stein C. A., Mitsuya H., Wong-Staal F., Cohen J. S., Broder S. Regulation of viral expression of human immunodeficiency virus in vitro by an antisense phosphorothioate oligodeoxynucleotide against rev (art/trs) in chronically infected cells. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4244–4248. doi: 10.1073/pnas.86.11.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels R., Balzarini J., Baba M., Snoeck R., Schols D., Herdewijn P., Desmyter J., De Clercq E. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J Virol Methods. 1988 Aug;20(4):309–321. doi: 10.1016/0166-0934(88)90134-6. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Reitz M. S., Jr, Guo H. G., Oleske J., Hoxie J., Popovic M., Read-Connole E., Markham P., Streicher H., Gallo R. C. On the historical origins of HIV-1 (MN) and (RF). AIDS Res Hum Retroviruses. 1992 Sep;8(9):1539–1541. doi: 10.1089/aid.1992.8.1539. [DOI] [PubMed] [Google Scholar]
- Stein C. A., Cheng Y. C. Antisense oligonucleotides as therapeutic agents--is the bullet really magical? Science. 1993 Aug 20;261(5124):1004–1012. doi: 10.1126/science.8351515. [DOI] [PubMed] [Google Scholar]
- Stein C. A., Tonkinson J. L., Yakubov L. Phosphorothioate oligodeoxynucleotides--anti-sense inhibitors of gene expression? Pharmacol Ther. 1991 Dec;52(3):365–384. doi: 10.1016/0163-7258(91)90032-h. [DOI] [PubMed] [Google Scholar]
- Thierry A. R., Dritschilo A. Intracellular availability of unmodified, phosphorothioated and liposomally encapsulated oligodeoxynucleotides for antisense activity. Nucleic Acids Res. 1992 Nov 11;20(21):5691–5698. doi: 10.1093/nar/20.21.5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers T., Baker B. F., Cook P. D., Zounes M., Buckheit R. W., Jr, Germany J., Ecker D. J. Inhibition of HIV-LTR gene expression by oligonucleotides targeted to the TAR element. Nucleic Acids Res. 1991 Jun 25;19(12):3359–3368. doi: 10.1093/nar/19.12.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamecnik P. C., Goodchild J., Taguchi Y., Sarin P. S. Inhibition of replication and expression of human T-cell lymphotropic virus type III in cultured cells by exogenous synthetic oligonucleotides complementary to viral RNA. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4143–4146. doi: 10.1073/pnas.83.12.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamecnik P. C., Stephenson M. L. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc Natl Acad Sci U S A. 1978 Jan;75(1):280–284. doi: 10.1073/pnas.75.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]