Abstract
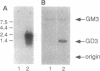
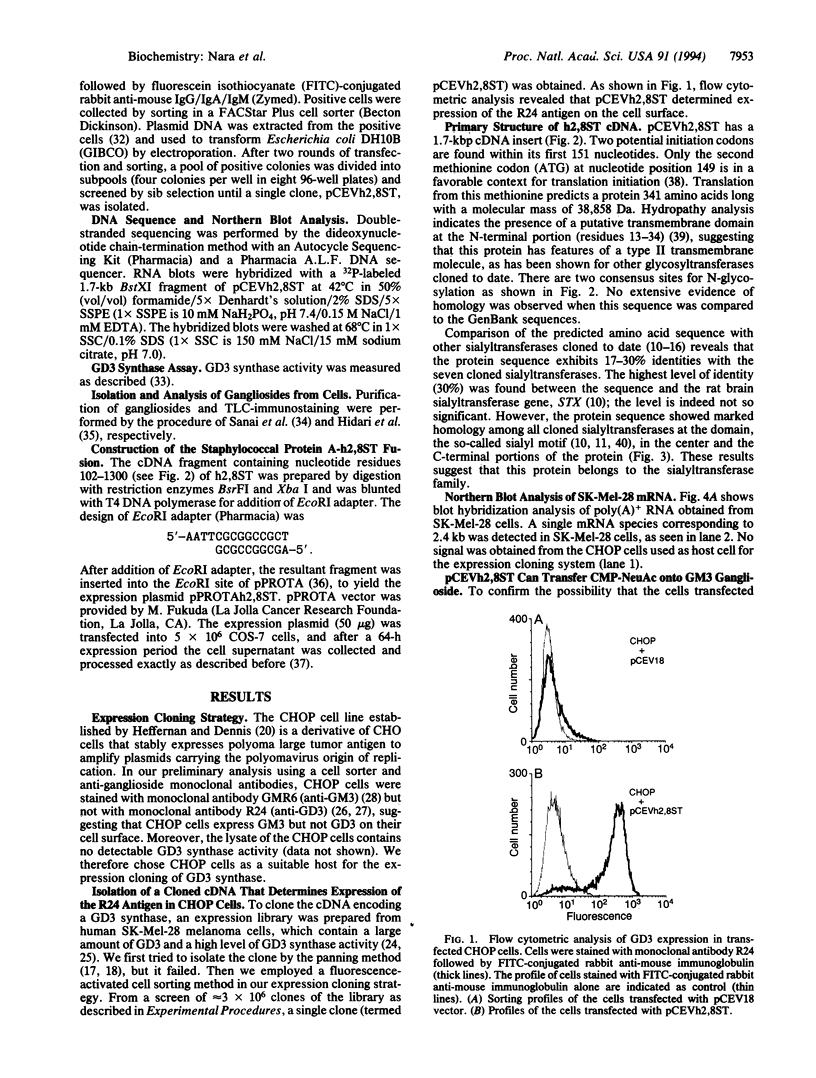
Using an expression cloning approach, we have isolated a cDNA encoding GD3 synthase (CMP-NeuAc:NeuAc alpha 2-3Gal beta 1-4Glc beta 1-1'Cer alpha 2,8-sialyltransferase, EC 2.4.99.8), which is a key regulatory enzyme determining the prominence of the ganglioside biosynthesis pathway. The cloned cDNA encodes a 341-amino acid protein containing a single transmembrane domain at its N-terminal region, suggesting that the protein has a type II transmembrane topology. The sequence of alpha 2,8-sialyltransferase showed a high level of similarity with other sialyltransferases at two conserved regions typical in the sialyltransferase family. Transfected cells containing the cloned cDNA expressed GD3 ganglioside on the cell surface, which was detectable with specific anti-GD3 antibody by immunofluorescence and immunostaining after separation of isolated glycolipids on thin-layer chromatography. The cDNA hybridized to a single mRNA species of 2.4 kb in melanoma cells. This sialyltransferase is distinctive in catalyzing the formation of the alpha 2-8 linkage of sialic acids.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruffo A., Seed B. Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8573–8577. doi: 10.1073/pnas.84.23.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierhuizen M. F., Fukuda M. Expression cloning of a cDNA encoding UDP-GlcNAc:Gal beta 1-3-GalNAc-R (GlcNAc to GalNAc) beta 1-6GlcNAc transferase by gene transfer into CHO cells expressing polyoma large tumor antigen. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9326–9330. doi: 10.1073/pnas.89.19.9326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierhuizen M. F., Mattei M. G., Fukuda M. Expression of the developmental I antigen by a cloned human cDNA encoding a member of a beta-1,6-N-acetylglucosaminyltransferase gene family. Genes Dev. 1993 Mar;7(3):468–478. doi: 10.1101/gad.7.3.468. [DOI] [PubMed] [Google Scholar]
- Bouvier J. D., Seyfried T. N. Ganglioside composition of normal and mutant mouse embryos. J Neurochem. 1989 Feb;52(2):460–466. doi: 10.1111/j.1471-4159.1989.tb09143.x. [DOI] [PubMed] [Google Scholar]
- Carubia J. M., Yu R. K., Macala L. J., Kirkwood J. M., Varga J. M. Gangliosides of normal and neoplastic human melanocytes. Biochem Biophys Res Commun. 1984 Apr 30;120(2):500–504. doi: 10.1016/0006-291x(84)91282-8. [DOI] [PubMed] [Google Scholar]
- Cheresh D. A., Harper J. R., Schulz G., Reisfeld R. A. Localization of the gangliosides GD2 and GD3 in adhesion plaques and on the surface of human melanoma cells. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5767–5771. doi: 10.1073/pnas.81.18.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh D. A., Pierschbacher M. D., Herzig M. A., Mujoo K. Disialogangliosides GD2 and GD3 are involved in the attachment of human melanoma and neuroblastoma cells to extracellular matrix proteins. J Cell Biol. 1986 Mar;102(3):688–696. doi: 10.1083/jcb.102.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Hayakawa H., Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987 Feb 11;15(3):1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dippold W. G., Knuth A., Meyer zum Büschenfelde K. H. Inhibition of human melanoma cell growth in vitro by monoclonal anti-GD3-ganglioside antibody. Cancer Res. 1984 Feb;44(2):806–810. [PubMed] [Google Scholar]
- Dippold W. G., Lloyd K. O., Li L. T., Ikeda H., Oettgen H. F., Old L. J. Cell surface antigens of human malignant melanoma: definition of six antigenic systems with mouse monoclonal antibodies. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6114–6118. doi: 10.1073/pnas.77.10.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drickamer K. A conserved disulphide bond in sialyltransferases. Glycobiology. 1993 Feb;3(1):2–3. doi: 10.1093/glycob/3.1.2. [DOI] [PubMed] [Google Scholar]
- Furukawa K., Arita Y., Satomi N., Eisinger M., Lloyd K. O. Tumor necrosis factor enhances GD3 ganglioside expression in cultured human melanocytes. Arch Biochem Biophys. 1990 Aug 15;281(1):70–75. doi: 10.1016/0003-9861(90)90414-t. [DOI] [PubMed] [Google Scholar]
- Furukawa K., Yamaguchi H., Oettgen H. F., Old L. J., Lloyd K. O. Two human monoclonal antibodies reacting with the major gangliosides of human melanomas and comparison with corresponding mouse monoclonal antibodies. Cancer Res. 1989 Jan 1;49(1):191–196. [PubMed] [Google Scholar]
- Gillespie W., Kelm S., Paulson J. C. Cloning and expression of the Gal beta 1, 3GalNAc alpha 2,3-sialyltransferase. J Biol Chem. 1992 Oct 15;267(29):21004–21010. [PubMed] [Google Scholar]
- Grundmann U., Nerlich C., Rein T., Zettlmeissl G. Complete cDNA sequence encoding human beta-galactoside alpha-2,6-sialyltransferase. Nucleic Acids Res. 1990 Feb 11;18(3):667–667. doi: 10.1093/nar/18.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakomori S. Bifunctional role of glycosphingolipids. Modulators for transmembrane signaling and mediators for cellular interactions. J Biol Chem. 1990 Nov 5;265(31):18713–18716. [PubMed] [Google Scholar]
- Heffernan M., Dennis J. W. Polyoma and hamster papovavirus large T antigen-mediated replication of expression shuttle vectors in Chinese hamster ovary cells. Nucleic Acids Res. 1991 Jan 11;19(1):85–92. doi: 10.1093/nar/19.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidari K. I., Kawashima I., Tai T., Inagaki F., Nagai Y., Sanai Y. In vitro synthesis of disialoganglioside (GD1 alpha) from asialo-GM1 using sialyltransferases in rat liver Golgi vesicles. Eur J Biochem. 1994 Apr 1;221(1):603–609. doi: 10.1111/j.1432-1033.1994.tb18772.x. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hoyer L. L., Roggentin P., Schauer R., Vimr E. R. Purification and properties of cloned Salmonella typhimurium LT2 sialidase with virus-typical kinetic preference for sialyl alpha 2----3 linkages. J Biochem. 1991 Sep;110(3):462–467. doi: 10.1093/oxfordjournals.jbchem.a123603. [DOI] [PubMed] [Google Scholar]
- Inoue N., Kinoshita T., Orii T., Takeda J. Cloning of a human gene, PIG-F, a component of glycosylphosphatidylinositol anchor biosynthesis, by a novel expression cloning strategy. J Biol Chem. 1993 Apr 5;268(10):6882–6885. [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Schreurs J., Gorman D. M., Maruyama K., Ishii A., Yahara I., Arai K., Miyajima A. Cloning of an interleukin-3 receptor gene: a member of a distinct receptor gene family. Science. 1990 Jan 19;247(4940):324–327. doi: 10.1126/science.2404337. [DOI] [PubMed] [Google Scholar]
- Kasahara K., Guo L., Nagai Y., Sanai Y. Enzymatic assay of glycosphingolipid sialyltransferase using reverse-phase thin-layer chromatography. Anal Biochem. 1994 Apr;218(1):224–226. doi: 10.1006/abio.1994.1164. [DOI] [PubMed] [Google Scholar]
- Kotani M., Ozawa H., Kawashima I., Ando S., Tai T. Generation of one set of monoclonal antibodies specific for a-pathway ganglio-series gangliosides. Biochim Biophys Acta. 1992 Jul 21;1117(1):97–103. doi: 10.1016/0304-4165(92)90168-t. [DOI] [PubMed] [Google Scholar]
- Kozak M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J Biol Chem. 1991 Oct 25;266(30):19867–19870. [PubMed] [Google Scholar]
- Kukowska-Latallo J. F., Larsen R. D., Nair R. P., Lowe J. B. A cloned human cDNA determines expression of a mouse stage-specific embryonic antigen and the Lewis blood group alpha(1,3/1,4)fucosyltransferase. Genes Dev. 1990 Aug;4(8):1288–1303. doi: 10.1101/gad.4.8.1288. [DOI] [PubMed] [Google Scholar]
- Larsen R. D., Ernst L. K., Nair R. P., Lowe J. B. Molecular cloning, sequence, and expression of a human GDP-L-fucose:beta-D-galactoside 2-alpha-L-fucosyltransferase cDNA that can form the H blood group antigen. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6674–6678. doi: 10.1073/pnas.87.17.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen R. D., Rajan V. P., Ruff M. M., Kukowska-Latallo J., Cummings R. D., Lowe J. B. Isolation of a cDNA encoding a murine UDPgalactose:beta-D-galactosyl- 1,4-N-acetyl-D-glucosaminide alpha-1,3-galactosyltransferase: expression cloning by gene transfer. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8227–8231. doi: 10.1073/pnas.86.21.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. C., Kurosawa N., Hamamoto T., Nakaoka T., Tsuji S. Molecular cloning and expression of Gal beta 1,3GalNAc alpha 2,3-sialyltransferase from mouse brain. Eur J Biochem. 1993 Sep 1;216(2):377–385. doi: 10.1111/j.1432-1033.1993.tb18155.x. [DOI] [PubMed] [Google Scholar]
- Livingston B. D., Paulson J. C. Polymerase chain reaction cloning of a developmentally regulated member of the sialyltransferase gene family. J Biol Chem. 1993 Jun 5;268(16):11504–11507. [PubMed] [Google Scholar]
- Nagai Y., Nakaishi H., Sanai Y. Gene transfer as a novel approach to the gene-controlled mechanism of the cellular expression of glycosphingolipids. Chem Phys Lipids. 1986 Dec 15;42(1-3):91–103. doi: 10.1016/0009-3084(86)90045-9. [DOI] [PubMed] [Google Scholar]
- Nagata Y., Yamashiro S., Yodoi J., Lloyd K. O., Shiku H., Furukawa K. Expression cloning of beta 1,4 N-acetylgalactosaminyltransferase cDNAs that determine the expression of GM2 and GD2 gangliosides. J Biol Chem. 1992 Jun 15;267(17):12082–12089. [PubMed] [Google Scholar]
- Nakaishi H., Sanai Y., Shiroki K., Nagai Y. Analysis of cellular expression of gangliosides by gene transfection. I: GD3 expression in myc-transfected and transformed 3Y1 correlates with anchorage-independent growth activity. Biochem Biophys Res Commun. 1988 Jan 29;150(2):760–765. doi: 10.1016/0006-291x(88)90456-1. [DOI] [PubMed] [Google Scholar]
- Nakakuma H., Sanai Y., Shiroki K., Nagai Y. Gene-regulated expression of glycolipids: appearance of GD3 ganglioside in rat cells on transfection with transforming gene E1 of human adenovirus type 12 DNA and its transcriptional subunits. J Biochem. 1984 Nov;96(5):1471–1480. doi: 10.1093/oxfordjournals.jbchem.a134976. [DOI] [PubMed] [Google Scholar]
- Paulson J. C., Colley K. J. Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J Biol Chem. 1989 Oct 25;264(30):17615–17618. [PubMed] [Google Scholar]
- Pukel C. S., Lloyd K. O., Travassos L. R., Dippold W. G., Oettgen H. F., Old L. J. GD3, a prominent ganglioside of human melanoma. Detection and characterisation by mouse monoclonal antibody. J Exp Med. 1982 Apr 1;155(4):1133–1147. doi: 10.1084/jem.155.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan S., Lloyd K. O. Glycosylation pathways in the biosynthesis of gangliosides in melanoma and neuroblastoma cells: relative glycosyltransferase levels determine ganglioside patterns. Cancer Res. 1992 Oct 15;52(20):5725–5731. [PubMed] [Google Scholar]
- SVENNERHOLM L. CHROMATOGRAPHIC SEPARATION OF HUMAN BRAIN GANGLIOSIDES. J Neurochem. 1963 Sep;10:613–623. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]
- Sanai Y., Oda K., Nagai Y. Induction of GD3 ganglioside by adenovirus E1A gene 13S- and 12S-mRNA products in rat 3Y1 cells. J Biochem. 1990 May;107(5):740–742. doi: 10.1093/oxfordjournals.jbchem.a123118. [DOI] [PubMed] [Google Scholar]
- Sanchez-Lopez R., Nicholson R., Gesnel M. C., Matrisian L. M., Breathnach R. Structure-function relationships in the collagenase family member transin. J Biol Chem. 1988 Aug 25;263(24):11892–11899. [PubMed] [Google Scholar]
- Sariola H., Aufderheide E., Bernhard H., Henke-Fahle S., Dippold W., Ekblom P. Antibodies to cell surface ganglioside GD3 perturb inductive epithelial-mesenchymal interactions. Cell. 1988 Jul 15;54(2):235–245. doi: 10.1016/0092-8674(88)90556-9. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Watanabe E., Kawashima K., Sekine S., Dohi T., Oshima M., Hanai N., Nishi T., Hasegawa M. Expression cloning of a novel Gal beta (1-3/1-4) GlcNAc alpha 2,3-sialyltransferase using lectin resistance selection. J Biol Chem. 1993 Oct 25;268(30):22782–22787. [PubMed] [Google Scholar]
- Seed B., Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci U S A. 1987 May;84(10):3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai T., Kawashima I., Furukawa K., Lloyd K. O. Monoclonal antibody R24 distinguishes between different N-acetyl- and N-glycolylneuraminic acid derivatives of ganglioside GD3. Arch Biochem Biophys. 1988 Jan;260(1):51–55. doi: 10.1016/0003-9861(88)90423-7. [DOI] [PubMed] [Google Scholar]
- Thampoe I. J., Furukawa K., Vellvé E., Lloyd K. O. Sialyltransferase levels and ganglioside expression in melanoma and other cultured human cancer cells. Cancer Res. 1989 Nov 15;49(22):6258–6264. [PubMed] [Google Scholar]
- Tsuji S., Arita M., Nagai Y. GQ1b, a bioactive ganglioside that exhibits novel nerve growth factor (NGF)-like activities in the two neuroblastoma cell lines. J Biochem. 1983 Jul;94(1):303–306. doi: 10.1093/oxfordjournals.jbchem.a134344. [DOI] [PubMed] [Google Scholar]
- Uchida Y., Tsukada Y., Sugimori T. Enzymatic properties of neuraminidases from Arthrobacter ureafaciens. J Biochem. 1979 Nov;86(5):1573–1585. doi: 10.1093/oxfordjournals.jbchem.a132675. [DOI] [PubMed] [Google Scholar]
- Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993 Apr;3(2):97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J., Lee E. U., McEntee K., Lai P. H., Paulson J. C. Primary structure of beta-galactoside alpha 2,6-sialyltransferase. Conversion of membrane-bound enzyme to soluble forms by cleavage of the NH2-terminal signal anchor. J Biol Chem. 1987 Dec 25;262(36):17735–17743. [PubMed] [Google Scholar]
- Wen D. X., Livingston B. D., Medzihradszky K. F., Kelm S., Burlingame A. L., Paulson J. C. Primary structure of Gal beta 1,3(4)GlcNAc alpha 2,3-sialyltransferase determined by mass spectrometry sequence analysis and molecular cloning. Evidence for a protein motif in the sialyltransferase gene family. J Biol Chem. 1992 Oct 15;267(29):21011–21019. [PubMed] [Google Scholar]
- Wickner W. T., Lodish H. F. Multiple mechanisms of protein insertion into and across membranes. Science. 1985 Oct 25;230(4724):400–407. doi: 10.1126/science.4048938. [DOI] [PubMed] [Google Scholar]
- van Echten G., Sandhoff K. Ganglioside metabolism. Enzymology, Topology, and regulation. J Biol Chem. 1993 Mar 15;268(8):5341–5344. [PubMed] [Google Scholar]