Abstract
In studies of immune aging, naïve T cells frequently take center stage. Describing the complexity of the human naïve T cell repertoire remains a daunting task; however, emerging data suggest that homeostatic mechanisms are robust enough to maintain a large and diverse CD4 T cell repertoire with age. Compartment shrinkage and clonal expansions are challenges for naïve CD8 T cells. In addition to population aspects, identification of potentially targetable cellular defects is receiving renewed interest. The last decade has seen remarkable progress in identifying genetic and biochemical pathways that are pertinent for aging in general and that are instructive to understand naïve T cell dysfunction. One hallmark sets naïve T cell aging apart from most other tissues except stem cells: they initiate but do not complete differentiation programs towards memory cells. Maintaining quiescence and avoiding differentiation may be the ultimate challenge to maintain the functions unique for naïve T cells.
Introduction
Aging, defined as progressive functional decline over time, affects all organ systems and is the major cause of, or at least contributes to, most diseases in the adult. The immune system is a prime example; immune competence declines with age, causing increased morbidity and mortality from infections, as well as being a factor in the increased incidence of malignancies (1–3). Less intuitively, the aging immune system is also more inclined to elicit nonspecific inflammation, which accelerates degenerative diseases, most prominently seen in cardiovascular and neurodegenerative disorders (4–6). Moreover, immune aging can impair tolerance mechanisms and is a risk factor for autoimmunity (7, 8). Generally known as “immunosenescence”, this term is too narrow to reflect the multitude of mechanisms involved and may even be misleading, implying cellular senescence as the main pathological event.
Hallmarks of Aging
To describe our current understanding of the aging process in its complexity, López-Otin and colleagues define cellular and molecular hallmarks that describe common pathways which, in turn, signify aging over a range of tissues and species: stem cell exhaustion limiting regenerative capacity; various forms of genomic instability including telomere attrition, DNA damage, mitochondrial dysfunction and epigenetic changes; loss of proteostasis; nutritional sensing; cellular senescence; and altered intercellular communication (Table 1) (9). In this review, we will discuss how these general aging mechanisms help explain age-associated changes in the immune system and, conversely, how studies on T cell aging can expand this conceptual framework. We will focus exclusively on human naïve T cells and refer to recent broader reviews for comprehensive reading on immune aging (10–14).
Table 1.
Comparison of pathways pertinent in general aging to findings in T cell aging and differentiation
| General aging hallmarks (modified from Lopez-Otin et al.) |
Naïve T cell aging hallmarks (human) |
Hallmarks associated with naïve T cell activation/ differentiation |
|---|---|---|
| Decline in regenerative capacity/stem cell exhaustion) | Thymic involution, no evidence for homeostatic proliferation exhaustion | N/A |
| Telomeric attrition | Telomeric attrition | Telomere attrition |
| Genetic instability/DNA damage | Defective DNA repair responses associated with premature naïve T cell aging in rheumatoid arthritis | Increased DNA damage compared to naïve T cells |
| Epigenetic alterations | No data | Poised effector genes |
| Cellular senescence | Inconclusive evidence | Induction of p16 with naïve T cell activation |
| Metabolic processes/nutrient sensing/insulin-IGF pathway | No data | Aerobic glycolysis/oxidative phosphorylation pathways strictly linked with activation/differentiation |
| Mitochondrial dysfunction | No data | Increased mitochondrial capacity |
| Loss of proteostasis | No data in humans, autophagy defects in mouse | Autophagy involved in T cell differentiation |
| Loss of stem-cellness due to partial lineage commitment | Partial differentiation into memory cells (miR expression patterns, expression of CD25 and CD95) | Full differentiation into memory T cells |
Age and regenerative capacity – Maintaining the size of the naïve T cell pool
As pointed out by Lopez-Otin et al. (9), a decline in regenerative capacity is a well-appreciated hallmark of aging, and attrition of stem cells with age is a universal finding in virtually all tissues (Table 1). To prevent stem cell exhaustion, mechanisms are in place to preserve cell quiescence (15). Failure of these mechanisms leads to premature exhaustion and accelerates the aging process. The adaptive immune system is special, in that generation of novel naïve T cells is entirely dependent on thymic function. Since thymic output peaks at puberty and progressively declines thereafter, thymic involution may be independent of and precede stem cell aging. The naïve T cell emerges as a quasi-stem cell regenerating the T cell system, and principles of stem cell aging apply to naïve T cell aging.
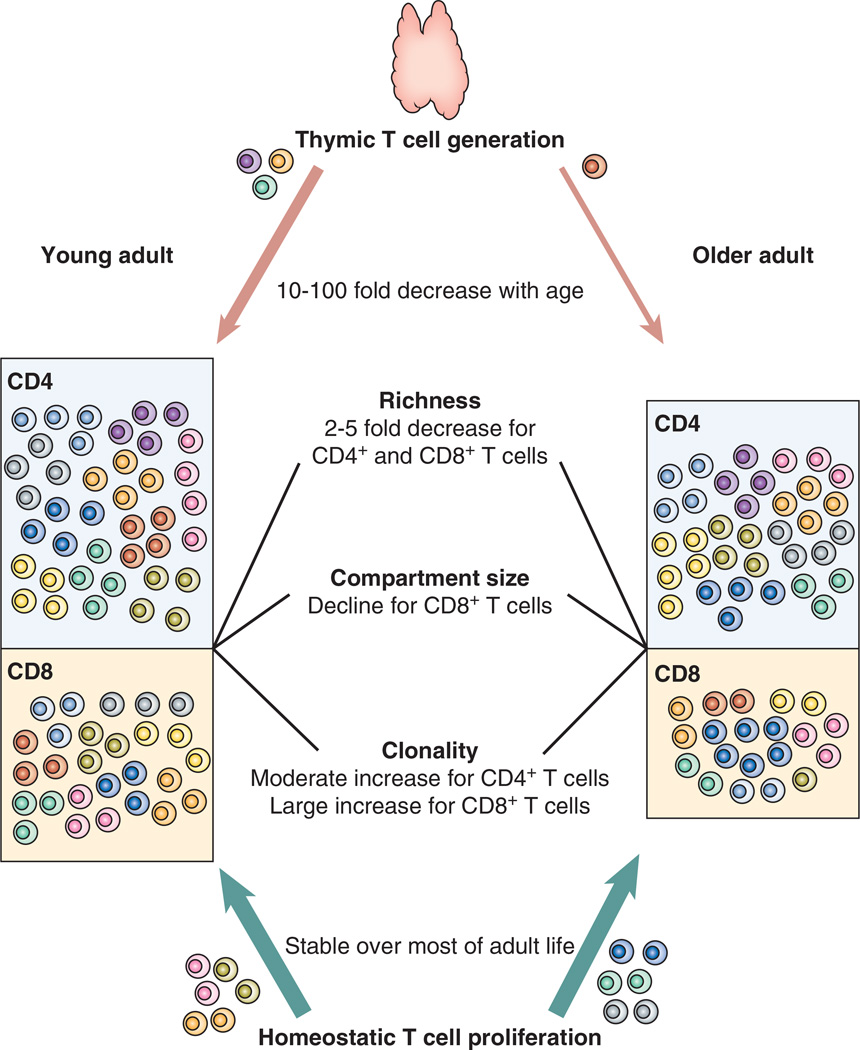
The dramatic loss of the thymus prompted a natural supposition that thymic involution is responsible for the age-associated failure of the adaptive immune system (16, 17). Indeed, the naïve T cell compartment in the mouse is dependent on thymic emigrants throughout life. Insufficient production of new cells by the thymus during aging is associated with compartment shrinkage and eventually leads to holes in the murine T cell repertoire (18, 19). Several lines of recent evidence have challenged the importance of thymic involution in human immune aging (20). While vital for building a T cell repertoire during the growing phases of the host, thymic output appears unnecessary for repertoire maintenance during adulthood and T cell regeneration is nearly entirely derived from homeostatic proliferation of the existing T cell pool, which is sufficient to maintain a large compartment of naïve CD4 T cells (Figure 1) (21).
Figure 1. Naïve T cell homeostasis and age.
Thymic T cell regeneration is quantitatively irrelevant throughout adult life and homeostatic proliferation is responsible for maintaining the size of the naïve T cell compartment. While only thymic T cell generation can add novel naïve T cells and enrich diversity, homeostatic T cell proliferation can sustain the richness of the TCR repertoire (i.e. the total number of T cells with different TCR sequences), while peripheral selection during homeostatic proliferation may result in increasing unevenness, i.e., increasing inequalities in clonal sizes and clonal expansions of selected few clones. Age-associated changes in these metrics between CD4 and CD8 T cells of young (<35 years) and older (65–80 year-old) healthy adults are illustrated.
Surgery removing or reducing the thymus in early childhood changes the composition of the T cell compartment, mimicking immune aging in young adults, but only in individuals chronically infected with CMV (22). Similarly, the relative expansion of memory and effector T cell populations and the relative decline in naïve CD4 T cells, attributed to age in earlier studies, is entirely due to chronic CMV stimulation and not found in older individuals who do not have antibodies to CMV (23). More importantly, data on T cell turnover and decline in T cell receptor excisions (TRECs) best fits to the model that T cell generation in the adult relies mostly, if not entirely, on homeostatic proliferation of existing naïve T cells. Despite a decrease in daily thymic output by a factor of 10 to 100 during adult life, peripheral naïve T cell turnover does not measurably increase suggesting that that peripheral proliferation does not need to compensate for thymic failure and that even in early adulthood, thymic output contributes little to the maintenance of the size of the naïve T cell compartment (24–26). Only in the 8th and 9th decade of life does the frequency of cycling cells increase, probably as a consequence of increased cell death rather than lack of thymic function (27, 28).
Peripheral expansion of naïve T cells is driven by tonic TCR signals and homeostatic cytokines, in particular by IL-7 (29, 30). IL-7 concentrations do not decline with age and therefore do not become limiting, consistent with the observation that the CD4 T cell compartment does not reduce in size. IL-2 stimulation may also play a role since naïve CD4 T cells increasingly express the high-affinity IL-2 receptor CD25 with age, and become IL-2 responsive (31). IL-7 drives T cell proliferation without inducing a switch to a memory phenotype in vitro (32). CD31, expressed on a subset of naïve CD4 T cells, identifies recent thymic emigrants or at least cells that have not peripherally proliferated. TREC concentrations decrease slightly in CD31+ CD4 T cells with age consistent with only limited replication in this population (33). As one would expect, the CD31+ population progressively shrinks with age, consistent with the idea that increasingly more T cells participate in proliferation and lose CD31 (33, 34). In parallel, the CD31− naïve T cell population increases in size and its TREC content more drastically declines (33). The loss of CD31 expression in naïve T cells is generally not associated with the acquisition of phenotypic markers of memory T cells.
In contrast to CD4 T cells, the naïve CD8 T cell compartment shrinks with age (Figure 1) (23, 35). The reason for this subset difference is unclear. Theoretically, homeostatic proliferation conditions are not worse for CD8 T cells. On the contrary, CD8 T cells should receive more signals from abundant MHC class I than CD4 cells receive from restrictedly expressed MHC class II molecules. Indeed, compared with CD4 T cells, naïve CD8 T cells develop a higher degree of clonal size inequality with frequent clonal expansions, indicating that naïve CD8 T cells do proliferate at a higher rate than CD4 T cells (Figure 1) (36). Shrinking of the naïve CD8 T cell compartment may therefore not be due to defective regeneration. Alternatively, naïve CD8 T cells may acquire phenotypic memory markers due to increased turnover. In murine models, such virtual memory T cells preferentially develop in the CD8 compartment (37, 38). However, TCR sequencing studies do not support the notion that development of CD8 virtual memory T cells is a frequent event in older humans. Clonal expansions originating in the naïve CD8 T cell compartment largely maintain a naïve phenotype and only a small fraction of these clones express memory cell markers. Moreover, TCR diversity in CD8 memory T cells is about tenfold lower than that of CD4 memory T cells, which would not be expected if a larger fraction of CD8 than CD4 naïve T cells is converted into T cells with a memory phenotype (36).
Alternatively, increased proliferation may be associated with increased cell death, causing depletion of naïve CD8 T cells. In modeling naïve T cell division and survival, Reynolds and colleagues propose that increased IL-7-driven proliferation, possibly due to a lower threshold for IL-7 receptor signaling, leads to abrupt declines in compartment size several years later (39). Taking this thought one step further, tonic TCR and homeostatic cytokine stimulation need to be optimally tuned to find the balance between providing sufficient signals for survival and replacement without triggering increased turnover (38). Excess cytokines, such as those produced in inflammatory conditions, increased expression of cytokine receptors such as CD25, or lowering of TCR and cytokine receptor thresholds may be detrimental for homeostasis and accelerate immune aging, similar to stem cells where cellular quiescence is important for delaying stem cell exhaustion and aging (9, 40). Accordingly, accelerated aging has been described in several autoimmune diseases (7, 8).
Obviously, these data are based on measurements of peripheral blood lymphocytes, which represent less than 3% of the total T cells in the body. A recent study by Thome et al. quantified the T cell subset composition at nine anatomic tissue sites in 56 individual organ donors over a wide age range (41). Naïve T cells were only found in blood and lymphoid tissue. The distribution variance of naïve CD4 and CD8 T cells between circulation and lymphoid tissue was low. The impact of age on naïve CD8 T cell decline was similar in blood and tissue, while the blood frequencies of naïve CD4 T cells may underestimate an age-associated decline in the lymphoid tissue. However, these tissue data show relative frequencies and may therefore reflect increased frequencies of T effector memory cells, in particular since CMV-positive donors were not excluded. In conclusion, homeostatic proliferation appears to be sufficient for maintaining a sizable naïve CD4 T cell compartment while the naïve CD8 compartment declines in size, possibly as a consequence of increased cell loss rather than defective generation (Figure 1). A lack of regenerative capacity and a decline in immune-competent cells does not appear to cause the immune defects in adaptive immune responses, in particular the CD4 T cell-dependent B cell responses after vaccination.
Age and regenerative capacity – Maintaining richness of the naïve T cell pool while avoiding clonality
T cell replenishment is unique in that it not only has to maintain compartment size, but also TCR diversity. Unlike in B cells, generation of new T cell specificities entirely relies on thymic production and cannot be accomplished by expansion and modification of naïve T cells. For T cell homeostatic mechanisms to maintain a diverse repertoire, at least three conditions must be met:
the initial clonal size in the naïve T cell compartment must be of sufficient size to confer robustness to loss of T cells;
the initial TCR repertoire must be diverse and redundant, such that loss of some clones does not generate holes in the repertoire;
peripheral T cell homeostasis must be non-selective.
The first two factors are highly inter-dependent and are influenced by the size of the species; conditions for mice and humans are therefore very different. Initial clonal size depends on intra-thymic proliferation after TCR rearrangement, as well as the peripheral proliferation within the compartment that is at least partially empty, i.e., in the fetus as well as during the subsequent growth period up to early adulthood. Data on increased naïve T cell proliferation in newborns support the notion that recent thymic emigrants proliferate in the periphery to establish a minimal clonal size. More than 10% of cord blood T cells of infants born prematurely in the third trimester, when the human thymus starts to be active, are in the cell cycle (42). By fetal maturity this frequency has declined, but is still much higher than in young adults. Data on naïve T cell turnover in children are not available, but clonal expansion of thymic emigrants may reach clonal sizes of 100 cells, a number that is consistent with TREC measurements in CD31+ T cells which presumably have proliferated little or not at all after the initial seeding from the thymus (33). To determine how age affects TCR diversity in the adult, we performed an in silico simulation based on current estimates of kinetic parameters and clonal sizes (43). Even under the extreme scenario that thymic production stops at age 20 years and compartment size shrinks, we found that diversity is only mildly reduced over lifetime, as long as homeostatic proliferation is not highly selective.
Recent progress in next-generation sequencing has allowed testing of these predictions and estimating the overall richness of the naïve TCR repertoire. Initial estimates hovered around a few million different TCRβ chains, an estimate of the same magnitude as in the mouse, but much lower than those derived from concentrations of TRECs (44–46). The major challenge of these sequencing studies continues to be extrapolating from the small analyzed sample to the entire compartment of close to 1012 T cells, while not excluding infrequent sequences as possible PCR or sequencing errors. Using a nonparametric analysis and multiple sampling, we arrived at a much higher richness estimate of close to 100 million different naïve TCRβ chain sequences in young adults suggesting that the human TCR repertoire is extremely diverse and it would require a massive contraction with age to be functionally relevant (36). However, we only found a modest two- to five-fold contraction in repertoire richness for both the naïve CD4 and CD8 compartments, when we analyzed replicate samples of highly purified naïve T cells from lymphopheresis samples of 70- to 85-year-old individuals. While these individuals are certainly above average in their health status, these data document that peripheral homeostatic mechanisms are able to sustain a diverse repertoire. Based on these studies we predict that thymic involution does not have a detrimental influence on TCR diversity with age (Figure 1). Differences in study design may explain why our conclusion contradicts several earlier and also more recent studies that found a larger degree in diversity decline with age. Most of the studies that describe a major contraction in the repertoire based their analyses on total T cell population instead of purified naïve and memory T cell subsets. The observed repertoire contraction could therefore only reflect a lower proportion of naïve cells in the sample (46). Moreover, richness in older individuals is often underestimated because clonal sizes become increasingly non-Gaussian-distributed with clonally expanded T cells occurring even within the naïve repertoire. The increased clonality may result in a low estimate of richness if the repertoire is not analyzed in sufficient depth. Estimates from studies in particular from the era prior to next-generation sequencing therefore reflect the increased clonality while not allowing conclusions on richness (28).
Increased clonality is frequent with higher age, in particular, in the naïve CD8 compartment (Figure 1); some naïve T cell clones can reach a size usually only seen for memory T clones (36). Clonal selection may occur due to higher avidity to self-antigens, which in fact has been shown in animal studies (47), or increased responsiveness to cytokines, which we have shown in simulation experiments, but which has not been studied in vivo. The increasing unevenness in clonal sizes in older individuals is likely to bias the repertoire of T cells responding to a new antigenic challenge; more frequent T cell clones will out compete less frequent specificities. In principle, such a mechanism would be similar to what has been described as “original antigenic sin” where T cell responses are biased towards previous encounters of related antigens, although in this case the bias would derive from uneven homeostatic proliferation, presumably due to self-recognition (48). One possible complication from excessive clonal expansion is a collapse of the repertoire diversity as predicted by in silico simulation (43). Moreover, owing to the increased fitness of peripherally selected clonotypes, ongoing thymic activity or thymic rejuvenation would not be efficacious to prevent or restore such a scenario since new thymic emigrants will not be able to compete (49). We predict that healthy immune aging will require prevention of increasing clonality. Since clonality is variable, valuable information should be learned from understanding why clonality occurs more in some older individuals than others.
Age and telomere attrition in naïve T cells
Attrition of telomeres is an accepted hallmark of aging and is generally attributed to replicative history (Table 1). Like stem cells, naïve T cells are able to express telomerase, but their telomeres shorten with age (50). The current interpretation is that stimuli triggering homeostatic proliferation are not sufficient to induce telomerase expression, and that telomere shortening is a consequence of homeostatic turnover. It is intuitive to postulate that telomere attrition at least in part accounts for defective T cell responses in older individuals (51). The ability to exhibit a proliferative burst with more than ten cell divisions within a short timeframe is an absolute requirement for an effective naïve T cell response, and telomeric erosion will curtail this burst. Keeping turnover low as long as it is sufficient to replace cell loss is therefore desirable and a prerequisite of healthy aging, with the additional benefit of avoiding clonality as discussed above. However, a replicative history does not appear to be the only or major cause for telomere attrition. In early studies by Weng and Hodes, age-associated telomere attrition in naïve T cells paralleled that of memory T cells, although the kinetics of these two populations are vastly different (52). Moreover, telomere length did not differ between CD31+ and proliferated CD31neg naïve T cells. In addition to being sensitive to replication, telomeres are also subject to DNA damage, even more so than genomic DNA. Since DNA repair mechanisms at telomeres are limited by shelterin (a telomere-specific protein complex) (53), DNA damage tends to persist and could be responsible for age-associated telomere attrition irrespective of proliferation.
Age and DNA damage in naïve T cells
Accumulation of genetic damage in general is a characteristic hallmark of aging in various tissues and settings (Table 1) (9, 54, 55). Its relevance is highlighted by the finding that many premature aging syndromes are caused by underlying defects in DNA repair pathways (56). DNA damage responses in naïve CD4 T cells have not been widely studied in context of aging; with the exception of the accelerated immune aging in patients with rheumatoid arthritis that has been linked to defects in telomerase induction after activation and defects in the MRE-ATM DNA repair pathway, both of which rendered naïve CD4 T cells susceptible to apoptosis (57–60). There is evidence that the defects identified in patients with rheumatoid arthritis are also relevant for physiological aging. DNA damage, as quantified by comet assay, gradually increases with advancing age in memory T cells from healthy controls. In comparison, DNA damage remains low in naïve CD4 T cells up to 65 years, and increased DNA damage comparable to memory T cells is only found in some individuals in their seventh decade of life. The consequences of damage to mitochondrial DNA, also a hallmark of aging, have been explored even less for T cell aging (61, 62). mtDNA is particularly sensitive to cumulative DNA damage for a number of reasons. It is exposed to reactive oxygen species, whose major producer is the mitochondrion; it lacks protective histones; and its repair mechanisms are less efficacious.
Metabolic pathways in T cells – Tailored to T cell needs
Taking a wider view, telomeric erosion, mitochondrial dysfunction, in part caused by mtDNA damage, and DNA damage responses are closely intertwined with cell metabolism (63, 64). Deregulated nutrient sensing is associated with organismal aging in many model systems (65). The insulin/IGF-1 signaling pathway has been noted as a major aging-controlling pathway that is active in various species throughout evolution (9). In contrast, regulation of metabolic pathways in T cells is less dependent on availability of insulin or IGF-1, but integral to T cell function, complicating the application of metabolic concepts in general aging to immune aging (Table 1). The decisions of whether aerobic glycolysis or oxidative phosphorylation predominates and which fuel is being utilized are strictly linked to T cell activation and differentiation (65–67). Similar to tumor cells, aerobic glycolysis provides the building blocks enabling proliferation and T cell expansion, an essential element in particular for the naïve T cell response. Metabolic pathways are closely intertwined with signaling events after TCR stimulation, e.g., reactive oxygen species produced as a result of mitochondrial activity play a key role in TCR-induced signaling cascades. Moreover, many signaling molecules involved in T cell activation are also involved in the regulation of metabolic activity. Equally important, autophagy is a key mechanism controlling T cell differentiation and survival (68, 69). Since the metabolic pathways and their relationship to signaling pathways in basic T cell biology are increasingly understood, it can be expected that these insights will be fertile ground for immune aging studies. However, the scope of such studies will likely be different from the nutrition sensing studies in general aging research that try to curtail metabolic activity to prolong longevity, as exemplified with calorie restriction. While data on naïve T cells are lacking, initial studies have proven the value of understanding metabolic pathways to explain defects in T memory cell function (70). In elderly CD4 memory T cells, the increased expression of dual specific phosphatase 4 that terminates nuclear ERK signaling and impairs proliferation and effector functions, is the result of increased activation of the metabolic master regulator AMPK (71). In the case of CD8 effector T cells that lack the expression of CD28, but regain CD45RA and that have features of cellular senescence, inhibition of p38 signaling induced autophagy and restored energy-dependent functions (72). Finally, at least shown for murine T cells, T cell aging is associated with a defect in chaperone-mediated autophagy (69).
Cellular senescence – Does it play a role in naïve T cells?
Cellular senescence is a classical aging hallmark (Table 1). In addition to stable cell cycle arrest, senescent cells show characteristic phenotypic and functional changes, including the production of inflammatory cytokines (73). While senescence was originally attributed to telomeric erosion, non-telomeric DNA damage can also trigger it by derepressing the INK4/ARF locus. The INK4A/ARF locus comprises overlapping reading frames of two genes, p16(INK4a) and p19(ARF) that play important roles as cell cycle inhibitors in regulating cell growth and senescence and are mutated in various forms of cancer (74, 75). In contrast to other forms of cellular senescence, p16-induced senescence is not associated with an inflammatory phenotype (76). Derepression of INK4/ARF and transcription of p16 is seen with age in a variety of tissues (77). T cells are no exception to this rule and even show higher expression of p16 than most other cells (78). However, p16 expression is also induced after T cell activation and appears to be a physiological mechanism to curtail clonal expansion particularly in an immune response of naïve T cells (79). Whether naïve T cells develop features of senescence in the classical sense is undetermined. As discussed above, there is no evidence that T cell turnover decreases with age; if anything, it increases in the very old. Important for the topic of this review, the evidence that increased constitutive expression of p16 in T cells with age includes naïve T cells is only indirect and inconclusive (78). Older individuals may accumulate effector T cells, in particular in the setting of chronic latent infection such as CMV. These cells are, by nature of their differentiation state, less prone to proliferation but are otherwise fully functional (80, 81). T cell exhaustion, as found with chronic, actively replicating viral infections, is characterized by the expression of cell surface molecules such as PD1 and TIM3 on memory and effector cell populations and very different from cellular senescence (82).
Functional alterations in naïve T cells - A consequence of being no longer completely naïve?
Naïve T cells have a unique feature that is not appreciated as a general aging hallmark for all tissues, but which they share with stem cells (Table 1). To stay truly naïve (or to remain a fully pluripotent stem cell), naïve T cells as well as stem cells need to avoid differentiation. Stem cells develop an altered lineage potential with age: children and young adults have a dominant lymphoid lineage commitment in contrast to older individuals who are biased towards myeloid differentiation. Changes in lineage commitment and partial differentiation in stem cells are related to DNA damage responses and the expression of the transcription factor BATF (83). Similarly, naïve T cells can differentiate without having encountered exogenous antigen (37). Su and colleagues described that healthy individuals carry memory T cells to antigens they have never encountered in life (84). These cells may be an example of cross-reactivity rather than the result of nonspecific differentiation of naïve T cells. However, virtual memory CD8 T cells have been repeatedly identified in mice (85, 86). What drives the clonal expansion of these cells in addition to recognition of self-antigen is not known; nor is it clear if they are also present in CD4 T cell populations or even at all in humans. TCR sequencing studies have shown that clonal expansion occurs in the human naïve T cell compartment, and more so for CD8 than for CD4 T cells (36). The majority of such clonally expanded cells keep a naïve phenotype, with expression of CD45RA, CD28, CD27 and CCR7; however, a small fraction of some clones also expresses typical memory markers. On a broader scale, expression of CD25 on naïve CD4 T cells and CD95 on a subset of naïve CD8 T cells with age indicates partial activation and/or differentiation (26, 31). Transcriptional and, even more so, epigenetic profiling could address whether naïve T cells have begun to differentiate with age and have transcriptional signatures reminiscent of memory genes or an epigenetic landscape in which T effector genes are poised. Surprisingly, conclusive gene expression studies examining the impact of age on naïve T cells in humans are missing; most of the array studies have either analyzed total CD4 and CD8 T cells or focused on memory T cells, where phenotypic changes of CD28 loss and expression of NK cell surface markers with age have sparked particular interest (87). The same is true for epigenetic studies. Alterations in DNA methylation patterns have been described with age, but only for unfractionated CD4 T cells (88). It remains to be determined whether they reflect T cell differentiation or age-associated changes in naïve and/or memory T cells.
Supporting evidence for the partial differentiation hypothesis comes from identification of signaling alterations in older T cells (89). Reduced miR-181a expression is at least in part the cause for subdued TCR signaling in naïve cells with age. miR-181a controls the expression of DUSP6 and possibly other phosphatases. Loss of miR-181a with age therefore increases DUSP6 activity, which in turn curtails ERK phosphorylation after TCR stimulation (90). Interestingly, miR-181a expression is regulated along a developmental pathway, being highest in thymocytes and lowest in memory and effector T cells (91). Since TCR signaling in memory T cells relies less on the LAT-ERK, and more on the DIg-p38 pathway (92), the lower concentration of miR-181a is not so important for memory cell activation, but is detrimental for aged naïve T cells. To determine whether age-related changes in differentiation-dependent miRNA species are a general theme for naïve T cell aging, we profiled two other miRNAs that are known to change in expression with T cell activation and differentiation. Both miR-146a and miR-21 are expressed at higher concentrations in memory than naïve T cells (93). Our preliminary data suggest that expression levels in naïve CD8 T cells are age-dependent, with older naïve T cells more closely resembling memory T cells (unpublished observations). These data suggest that aging affects miRNA species that regulate T cell differentiation pathways, and that old naïve T cells acquire features of a memory cell without maturing completely. Partial differentiation is an aging hallmark that is shared between stem cells and naïve T cells (Table 1). Obviously, such partial differentiation may have detrimental consequences since the naïve T cells may have lost what is essential for their function, such as the ability to rapidly proliferate and clonally expand, while not fully having gained memory functions such as the rapid expression of poised effector genes. Epigenetic studies will be needed to determine whether loss of quiescence and entering the differentiation pathway to memory or effector T cells is an important component of naïve T cell aging.
Conclusion
Since thymic T cell production only minimally contributes to T cell generation throughout adult life, naïve T cells have to be self-sufficient in regenerating themselves to maintain compartment size. Additional complexity comes from the need to also preserve a diverse repertoire. On a positive note, the system is quite effective in maintaining a diverse repertoire with a huge number of different TCR sequences that should be sufficient to recognize all possible antigenic peptides. However, peripheral fitness selection causes expansions of selected clones that can bias T cell responses. On the cellular level, rules governing the aging of naïve T cells are also in part similar to those of stem cells. Telomere attrition occurs in both cell types in spite of their ability to express telomerase, likely due a combination of cumulative DNA damage and replicative history. Non-telomeric DNA instability also contributes to naïve T cell aging; at least in patients with rheumatoid arthritis, defective DNA repair mechanisms in naïve CD4 T cells contribute to the accelerated immune aging seen in this disease. Equally or maybe even more important than cellular senescence or exhaustion, incomplete differentiation towards memory and effector T cells in part explains the defects in functions characteristic for naïve T cells. In fact, senescence and differentiation pathways are not independent. Regulation of clonal expansion in naïve T cells and differentiation into memory T cells involves pathways also concerned with cellular senescence and DNA damage responses. Preliminary evidence suggests that symmetries between T cell aging and differentiation also exist for regulatory programs controlled by miRNA expression. How far this concept can be expanded to include other aging hallmarks, in particular in the recently emerged fields of T cell metabolism and autophagy, remains to be seen. Interventions to keep naïve T cells quiescent would therefore be equally beneficial to prevent functional decline as well repertoire distortion due to clonal expansions.
Acknowledgment
This work was supported by the National Institutes of Health (R01 AR042547, R01 HL117913, R01 AI044142, R01 AI108906 and P01 HL058000 to CMW and R01 AI108891, R01 AG045779, U19 AI090019 and U19 AI057266 to JJG).
References
- 1.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 2.Levin MJ. Immune senescence and vaccines to prevent herpes zoster in older persons. Curr Opin Immunol. 2012;24:494–500. doi: 10.1016/j.coi.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Fulop T, Larbi A, Kotb R, de Angelis F, Pawelec G. Aging, immunity, and cancer. Discov Med. 2011;11:537–550. [PubMed] [Google Scholar]
- 4.Morrisette-Thomas V, Cohen AA, Fulop T, Riesco E, Legault V, Li Q, Milot E, Dusseault-Belanger F, Ferrucci L. Inflamm-aging does not simply reflect increases in pro-inflammatory markers. Mech Ageing Dev. 2014;139:49–57. doi: 10.1016/j.mad.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howcroft TK, Campisi J, Louis GB, Smith MT, Wise B, Wyss-Coray T, Augustine AD, McElhaney JE, Kohanski R, Sierra F. The role of inflammation in age-related disease. Aging (Albany NY) 2013;5:84–93. doi: 10.18632/aging.100531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S4–S9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 7.Goronzy JJ, Li G, Yang Z, Weyand CM. The janus head of T cell aging - autoimmunity and immunodeficiency. Front Immunol. 2013;4:131. doi: 10.3389/fimmu.2013.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goronzy JJ, Weyand CM. Immune aging and autoimmunity. Cell Mol Life Sci. 2012;69:1615–1623. doi: 10.1007/s00018-012-0970-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol. 2013;13:875–887. doi: 10.1038/nri3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kogut I, Scholz JL, Cancro MP, Cambier JC. B cell maintenance and function in aging. Semin Immunol. 2012;24:342–349. doi: 10.1016/j.smim.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Goronzy JJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nat Immunol. 2013;14:428–436. doi: 10.1038/ni.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montecino-Rodriguez E, Berent-Maoz B, Dorshkind K. Causes, consequences, and reversal of immune system aging. J Clin Invest. 2013;123:958–965. doi: 10.1172/JCI64096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikolich-Zugich J. Aging of the T cell compartment in mice and humans: from no naive expectations to foggy memories. J Immunol. 2014;193:2622–2629. doi: 10.4049/jimmunol.1401174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol. 2013;14:329–340. doi: 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chinn IK, Blackburn CC, Manley NR, Sempowski GD. Changes in primary lymphoid organs with aging. Semin Immunol. 2012;24:309–320. doi: 10.1016/j.smim.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmer DB. The effect of age on thymic function. Front Immunol. 2013;4:316. doi: 10.3389/fimmu.2013.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blackman MA, Woodland DL. The narrowing of the CD8 T cell repertoire in old age. Curr Opin Immunol. 2011;23:537–542. doi: 10.1016/j.coi.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hale JS, Boursalian TE, Turk GL, Fink PJ. Thymic output in aged mice. Proc Natl Acad Sci U S A. 2006;103:8447–8452. doi: 10.1073/pnas.0601040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qi Q, Zhang DW, Weyand CM, Goronzy JJ. Mechanisms shaping the naive T cell repertoire in the elderly - thymic involution or peripheral homeostatic proliferation? Exp Gerontol. 2014;54:71–74. doi: 10.1016/j.exger.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.den Braber I, Mugwagwa T, Vrisekoop N, Westera L, Mogling R, de Boer AB, Willems N, Schrijver EH, Spierenburg G, Gaiser K, Mul E, Otto SA, Ruiter AF, Ackermans MT, Miedema F, Borghans JA, de Boer RJ, Tesselaar K. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity. 2012;36:288–297. doi: 10.1016/j.immuni.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Sauce D, Larsen M, Fastenackels S, Duperrier A, Keller M, Grubeck-Loebenstein B, Ferrand C, Debre P, Sidi D, Appay V. Evidence of premature immune aging in patients thymectomized during early childhood. J Clin Invest. 2009;119:3070–3078. doi: 10.1172/JCI39269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wertheimer AM, Bennett MS, Park B, Uhrlaub JL, Martinez C, Pulko V, Currier NL, Nikolich-Zugich D, Kaye J, Nikolich-Zugich J. Aging and cytomegalovirus infection differentially and jointly affect distinct circulating T cell subsets in humans. J Immunol. 2014;192:2143–2155. doi: 10.4049/jimmunol.1301721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Boer RJ, Perelson AS. Quantifying T lymphocyte turnover. J Theor Biol. 2013;327:45–87. doi: 10.1016/j.jtbi.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallace DL, Zhang Y, Ghattas H, Worth A, Irvine A, Bennett AR, Griffin GE, Beverley PC, Tough DF, Macallan DC. Direct measurement of T cell subset kinetics in vivo in elderly men and women. J Immunol. 2004;173:1787–1794. doi: 10.4049/jimmunol.173.3.1787. [DOI] [PubMed] [Google Scholar]
- 26.Westera L, van Hoeven V, Drylewicz J, Spierenburg G, van Velzen JF, de Boer RJ, Tesselaar K, Borghans JAM. Lymphocyte maintenance during healthy aging requires no substantial alterationas in cellular turnover. Aging Cell. 2015 doi: 10.1111/acel.12311. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sauce D, Larsen M, Fastenackels S, Roux A, Gorochov G, Katlama C, Sidi D, Sibony-Prat J, Appay V. Lymphopenia-driven homeostatic regulation of naive T cells in elderly and thymectomized young adults. J Immunol. 2012;189:5541–5548. doi: 10.4049/jimmunol.1201235. [DOI] [PubMed] [Google Scholar]
- 28.Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, Witkowski J, Fulbright J, Weyand CM, Goronzy JJ. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174:7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- 29.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol. 2005;174:6571–6576. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 31.Pekalski ML, Ferreira RC, Coulson RM, Cutler AJ, Guo H, Smyth DJ, Downes K, Dendrou CA, Castro Dopico X, Esposito L, Coleman G, Stevens HE, Nutland S, Walker NM, Guy C, Dunger DB, Wallace C, Tree TI, Todd JA, Wicker LS. Postthymic expansion in human CD4 naive T cells defined by expression of functional high-affinity IL-2 receptors. J Immunol. 2013;190:2554–2566. doi: 10.4049/jimmunol.1202914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soares MV, Borthwick NJ, Maini MK, Janossy G, Salmon M, Akbar AN. IL-7-dependent extrathymic expansion of CD45RA+ T cells enables preservation of a naive repertoire. J Immunol. 1998;161:5909–5917. [PubMed] [Google Scholar]
- 33.Kilpatrick RD, Rickabaugh T, Hultin LE, Hultin P, Hausner MA, Detels R, Phair J, Jamieson BD. Homeostasis of the naive CD4+ T cell compartment during aging. J Immunol. 2008;180:1499–1507. doi: 10.4049/jimmunol.180.3.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kohler S, Thiel A. Life after the thymus: CD31+ and CD31- human naive CD4+ T-cell subsets. Blood. 2009;113:769–774. doi: 10.1182/blood-2008-02-139154. [DOI] [PubMed] [Google Scholar]
- 35.Czesnikiewicz-Guzik M, Lee WW, Cui D, Hiruma Y, Lamar DL, Yang ZZ, Ouslander JG, Weyand CM, Goronzy JJ. T cell subset-specific susceptibility to aging. Clin Immunol. 2008;127:107–118. doi: 10.1016/j.clim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qi Q, Liu Y, Cheng Y, Glanville J, Zhang D, Lee JY, Olshen RA, Weyand CM, Boyd SD, Goronzy JJ. Diversity and clonal selection in the human T-cell repertoire. Proc Natl Acad Sci U S A. 2014;111:13139–13144. doi: 10.1073/pnas.1409155111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sprent J, Surh CD. Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nat Immunol. 2011;12:478–484. doi: 10.1038/ni.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Renkema KR, Li G, Wu A, Smithey MJ, Nikolich-Zugich J. Two separate defects affecting true naive or virtual memory T cell precursors combine to reduce naive T cell responses with aging. J Immunol. 2014;192:151–159. doi: 10.4049/jimmunol.1301453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reynolds J, Coles M, Lythe G, Molina-Paris C. Mathematical Model of Naive T Cell Division and Survival IL-7 Thresholds. Front Immunol. 2013;4:434. doi: 10.3389/fimmu.2013.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, Scadden DT. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 41.Thome JJ, Yudanin N, Ohmura Y, Kubota M, Grinshpun B, Sathaliyawala T, Kato T, Lerner H, Shen Y, Farber DL. Spatial Map of Human T Cell Compartmentalization and Maintenance over Decades of Life. Cell. 2014;159:814–828. doi: 10.1016/j.cell.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schonland SO, Zimmer JK, Lopez-Benitez CM, Widmann T, Ramin KD, Goronzy JJ, Weyand CM. Homeostatic control of T-cell generation in neonates. Blood. 2003;102:1428–1434. doi: 10.1182/blood-2002-11-3591. [DOI] [PubMed] [Google Scholar]
- 43.Johnson PL, Yates AJ, Goronzy JJ, Antia R. Peripheral selection rather than thymic involution explains sudden contraction in naive CD4 T-cell diversity with age. Proc Natl Acad Sci U S A. 2012;109:21432–21437. doi: 10.1073/pnas.1209283110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warren RL, Freeman JD, Zeng T, Choe G, Munro S, Moore R, Webb JR, Holt RA. Exhaustive T-cell repertoire sequencing of human peripheral blood samples reveals signatures of antigen selection and a directly measured repertoire size of at least 1 million clonotypes. Genome Res. 2011;21:790–797. doi: 10.1101/gr.115428.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robins HS, Campregher PV, Srivastava SK, Wacher A, Turtle CJ, Kahsai O, Riddell SR, Warren EH, Carlson CS. Comprehensive assessment of T-cell receptor beta-chain diversity in alphabeta T cells. Blood. 2009;114:4099–4107. doi: 10.1182/blood-2009-04-217604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Britanova OV, Putintseva EV, Shugay M, Merzlyak EM, Turchaninova MA, Staroverov DB, Bolotin DA, Lukyanov S, Bogdanova EA, Mamedov IZ, Lebedev YB, Chudakov DM. Age-related decrease in TCR repertoire diversity measured with deep and normalized sequence profiling. J Immunol. 2014;192:2689–2698. doi: 10.4049/jimmunol.1302064. [DOI] [PubMed] [Google Scholar]
- 47.Rudd BD, Venturi V, Li G, Samadder P, Ertelt JM, Way SS, Davenport MP, Nikolich-Zugich J. Nonrandom attrition of the naive CD8+ T-cell pool with aging governed by T-cell receptor:pMHC interactions. Proc Natl Acad Sci U S A. 2011;108:13694–13699. doi: 10.1073/pnas.1107594108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McMichael AJ. The original sin of killer T cells. Nature. 1998;394:421–422. doi: 10.1038/28738. [DOI] [PubMed] [Google Scholar]
- 49.Johnson PL, Goronzy JJ, Antia R. A population biological approach to understanding the maintenance and loss of the T-cell repertoire during aging. Immunology. 2014;142:167–175. doi: 10.1111/imm.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin Y, Damjanovic A, Metter EJ, Nguyen H, Truong T, Najarro K, Morris C, Longo DL, Zhan M, Ferrucci L, Hodes RJ, Weng NP. Age-associated telomere attrition of lymphocytes in vivo is co-ordinated with changes in telomerase activity, composition of lymphocyte subsets and health conditions. Clin Sci (Lond) 2015;128:367–377. doi: 10.1042/CS20140481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lord JM, Akbar AN, Kipling D. Telomere-based therapy for immunosenescence. Trends Immunol. 2002;23:175–176. doi: 10.1016/s1471-4906(02)02170-1. [DOI] [PubMed] [Google Scholar]
- 52.Weng NP, Levine BL, June CH, Hodes RJ. Human naive and memory T lymphocytes differ in telomeric length and replicative potential. Proc Natl Acad Sci U S A. 1995;92:11091–11094. doi: 10.1073/pnas.92.24.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sfeir A, de Lange T. Removal of shelterin reveals the telomere end-protection problem. Science. 2012;336:593–597. doi: 10.1126/science.1218498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moskalev AA, Shaposhnikov MV, Plyusnina EN, Zhavoronkov A, Budovsky A, Yanai H, Fraifeld VE. The role of DNA damage and repair in aging through the prism of Koch-like criteria. Ageing Res Rev. 2013;12:661–684. doi: 10.1016/j.arr.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 55.Cavanagh MM, Weyand CM, Goronzy JJ. Chronic inflammation and aging: DNA damage tips the balance. Curr Opin Immunol. 2012;24:488–493. doi: 10.1016/j.coi.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burtner CR, Kennedy BK. Progeria syndromes and ageing: what is the connection? Nat Rev Mol Cell Biol. 2010;11:567–578. doi: 10.1038/nrm2944. [DOI] [PubMed] [Google Scholar]
- 57.Fujii H, Shao L, Colmegna I, Goronzy JJ, Weyand CM. Telomerase insufficiency in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2009;106:4360–4365. doi: 10.1073/pnas.0811332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shao L, Fujii H, Colmegna I, Oishi H, Goronzy JJ, Weyand CM. Deficiency of the DNA repair enzyme ATM in rheumatoid arthritis. J Exp Med. 2009;206:1435–1449. doi: 10.1084/jem.20082251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weyand CM, Fujii H, Shao L, Goronzy JJ. Rejuvenating the immune system in rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:583–588. doi: 10.1038/nrrheum.2009.180. [DOI] [PubMed] [Google Scholar]
- 60.Hohensinner PJ, Goronzy JJ, Weyand CM. Telomere dysfunction, autoimmunity and aging. Aging Dis. 2011;2:524–537. [PMC free article] [PubMed] [Google Scholar]
- 61.Park CB, Larsson NG. Mitochondrial DNA mutations in disease and aging. J Cell Biol. 2011;193:809–818. doi: 10.1083/jcb.201010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ross OA, Hyland P, Curran MD, McIlhatton BP, Wikby A, Johansson B, Tompa A, Pawelec G, Barnett CR, Middleton D, Barnett YA. Mitochondrial DNA damage in lymphocytes: a role in immunosenescence? Exp Gerontol. 2002;37:329–340. doi: 10.1016/s0531-5565(01)00200-5. [DOI] [PubMed] [Google Scholar]
- 63.Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M, Cooper M, Kotton D, Fabian AJ, Walkey C, Maser RS, Tonon G, Foerster F, Xiong R, Wang YA, Shukla SA, Jaskelioff M, Martin ES, Heffernan TP, Protopopov A, Ivanova E, Mahoney JE, Kost-Alimova M, Perry SR, Bronson R, Liao R, Mulligan R, Shirihai OS, Chin L, DePinho RA. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–365. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464:520–528. doi: 10.1038/nature08982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol. 2013;31:259–283. doi: 10.1146/annurev-immunol-032712-095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pollizzi KN, Powell JD. Integrating canonical and metabolic signalling programmes in the regulation of T cell responses. Nat Rev Immunol. 2014;14:435–446. doi: 10.1038/nri3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu XJ, Araki K, Li SZ, Han JH, Ye LL, Tan WG, Konieczny BT, Bruinsma MW, Martinez J, Pearce EL, Green DR, Jones DP, Virgin HW, Ahmed R. Autophagy is essential for effector CD8(+) T cell survival and memory formation. Nature Immunology. 2014;15:1152–1161. doi: 10.1038/ni.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Valdor R, Mocholi E, Botbol Y, Guerrero-Ros I, Chandra D, Koga H, Gravekamp C, Cuervo AM, Macian F. Chaperone-mediated autophagy regulates T cell responses through targeted degradation of negative regulators of T cell activation. Nat Immunol. 2014;15:1046–1054. doi: 10.1038/ni.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taub DD, Hesdorffer CS, Ferrucci L, Madara K, Schwartz JB, Goetzl EJ. Distinct energy requirements for human memory CD4 T-cell homeostatic functions. FASEB J. 2013;27:342–349. doi: 10.1096/fj.12-217620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu M, Li G, Lee WW, Yuan M, Cui D, Weyand CM, Goronzy JJ. Signal inhibition by the dual-specific phosphatase 4 impairs T cell-dependent B-cell responses with age. Proc Natl Acad Sci U S A. 2012;109:E879–E888. doi: 10.1073/pnas.1109797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Henson SM, Lanna A, Riddell NE, Franzese O, Macaulay R, Griffiths SJ, Puleston DJ, Watson AS, Simon AK, Tooze SA, Akbar AN. p38 signaling inhibits mTORC1-independent autophagy in senescent human CD8(+) T cells. J Clin Invest. 2014;124:4004–4016. doi: 10.1172/JCI75051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123:966–972. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sharpless NE, DePinho RA. The INK4A/ARF locus and its two gene products. Curr Opin Genet Dev. 1999;9:22–30. doi: 10.1016/s0959-437x(99)80004-5. [DOI] [PubMed] [Google Scholar]
- 75.Sperka T, Wang J, Rudolph KL. DNA damage checkpoints in stem cells, ageing and cancer. Nat Rev Mol Cell Biol. 2012;13:579–590. doi: 10.1038/nrm3420. [DOI] [PubMed] [Google Scholar]
- 76.Coppe JP, Rodier F, Patil CK, Freund A, Desprez PY, Campisi J. Tumor suppressor and aging biomarker p16(INK4a) induces cellular senescence without the associated inflammatory secretory phenotype. J Biol Chem. 2011;286:36396–36403. doi: 10.1074/jbc.M111.257071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim WY, Sharpless NE. The regulation of INK4/ARF in cancer and aging. Cell. 2006;127:265–275. doi: 10.1016/j.cell.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 78.Liu Y, Sanoff HK, Cho H, Burd CE, Torrice C, Ibrahim JG, Thomas NE, Sharpless NE. Expression of p16(INK4a) in peripheral blood T-cells is a biomarker of human aging. Aging Cell. 2009;8:439–448. doi: 10.1111/j.1474-9726.2009.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Migliaccio M, Alves PM, Romero P, Rufer N. Distinct mechanisms control human naive and antigen-experienced CD8+ T lymphocyte proliferation. J Immunol. 2006;176:2173–2182. doi: 10.4049/jimmunol.176.4.2173. [DOI] [PubMed] [Google Scholar]
- 80.Weng NP, Akbar AN, Goronzy J. CD28(−) T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30:306–312. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wallace DL, Masters JE, De Lara CM, Henson SM, Worth A, Zhang Y, Kumar SR, Beverley PC, Akbar AN, Macallan DC. Human cytomegalovirus-specific CD8(+) T-cell expansions contain long-lived cells that retain functional capacity in both young and elderly subjects. Immunology. 2011;132:27–38. doi: 10.1111/j.1365-2567.2010.03334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Akbar AN, Henson SM. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat Rev Immunol. 2011;11:289–295. doi: 10.1038/nri2959. [DOI] [PubMed] [Google Scholar]
- 83.Wang J, Sun Q, Morita Y, Jiang H, Gross A, Lechel A, Hildner K, Guachalla LM, Gompf A, Hartmann D, Schambach A, Wuestefeld T, Dauch D, Schrezenmeier H, Hofmann WK, Nakauchi H, Ju Z, Kestler HA, Zender L, Rudolph KL. A differentiation checkpoint limits hematopoietic stem cell self-renewal in response to DNA damage. Cell. 2012;148:1001–1014. doi: 10.1016/j.cell.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 84.Su LF, Kidd BA, Han A, Kotzin JJ, Davis MM. Virus-specific CD4(+) memory-phenotype T cells are abundant in unexposed adults. Immunity. 2013;38:373–383. doi: 10.1016/j.immuni.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chiu BC, Martin BE, Stolberg VR, Chensue SW. Cutting edge: Central memory CD8 T cells in aged mice are virtual memory cells. J Immunol. 2013;191:5793–5796. doi: 10.4049/jimmunol.1302509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, Jameson SC, Kedl RM. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009;206:435–448. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen G, Lustig A, Weng NP. T cell aging: a review of the transcriptional changes determined from genome-wide analysis. Front Immunol. 2013;4:121. doi: 10.3389/fimmu.2013.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reynolds LM, Taylor JR, Ding J, Lohman K, Johnson C, Siscovick D, Burke G, Post W, Shea S, Jacobs DR, Jr, Stunnenberg H, Kritchevsky SB, Hoeschele I, McCall CE, Herrington DM, Tracy RP, Liu Y. Age-related variations in the methylome associated with gene expression in human monocytes and T cells. Nat Commun. 2014;5:5366. doi: 10.1038/ncomms6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goronzy JJ, Li G, Yu M, Weyand CM. Signaling pathways in aged T cells - a reflection of T cell differentiation, cell senescence and host environment. Semin Immunol. 2012;24:365–372. doi: 10.1016/j.smim.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li G, Yu M, Lee WW, Tsang M, Krishnan E, Weyand CM, Goronzy JJ. Decline in miR-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nat Med. 2012;18:1518–1524. doi: 10.1038/nm.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li QJ, Chau J, Ebert PJ, Sylvester G, Min H, Liu G, Braich R, Manoharan M, Soutschek J, Skare P, Klein LO, Davis MM, Chen CZ. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007;129:147–161. doi: 10.1016/j.cell.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 92.Adachi K, Davis MM. T-cell receptor ligation induces distinct signaling pathways in naive vs. antigen-experienced T cells. Proc Natl Acad Sci U S A. 2011;108:1549–1554. doi: 10.1073/pnas.1017340108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Monticelli S. MicroRNAs in T helper cell differentiation and plasticity. Semin Immunol. 2013;25:291–298. doi: 10.1016/j.smim.2013.10.015. [DOI] [PubMed] [Google Scholar]