Abstract
Objectives
To validate the hypothesis that men displaying serum PSA slopes ≤2.0 pg/mL/month postprostatectomy, measured with a new immuno-PCR diagnostic test (NADiA® ProsVue™) were at a reduced risk of clinical recurrence as determined by positive biopsy, imaging or death due to prostate cancer.
Methods
From 4 clinical sites, we selected a cohort of 304 men followed up to 17.6 years postprostatectomy for clinical recurrence. We assessed the prognostic value of a PSA slope cutpoint of 2.0 pg/mL/month against established risk factors to identify men at very low risk of clinical recurrence using uni- and multivariate Cox proportional hazards regression and Kaplan-Meier analysis.
Results
The univariate HR (95% CI) of a PSA slope >2.0 pg/mL/month was 18.3 (10.6–31.8), compared to a slope ≤2.0 pg/mL/month (P <0.0001). Median disease-free survival was 4.8 years versus >10 years in the 2 groups (P <0.0001). Multivariate HR for PSA slope with the covariates of preprostatectomy PSA, pathologic stage and Gleason score was 9.8 (5.4–17.8), an 89.8% risk reduction, for men with PSA slopes ≤2.0 pg/mL/month (P <0.0001). Gleason Score (<7 vs. ≥7) was the only other significant predictor (HR 5.4, 2.1–13.8, P = 0.0004).
Conclusions
Clinical recurrence following radical prostatectomy is often difficult to predict since established factors do not reliably stratify risk. We demonstrate that a NADiA ProsVue slope ≤2.0 pg/mL/month postprostatectomy is prognostic for reduced risk of prostate cancer recurrence and adds predictive power to established risk factors.
Keywords: NADiA Pros Vue, PSA, prostate cancer, prostatectomy
INTRODUCTION
Prostate cancer is the most prevalent male malignancy in the United States. With PSA screening, the age at diagnosis has decreased with a concomitant increase in the number of men diagnosed with early-stage or clinically localized disease.1,2 Radical prostatectomy (RP) is an increasingly common treatment for localized prostate cancer perhaps due to wider adoption of robotic-assisted laparoscopic technology. While surgery can result in long-term cancer control,3–6 more men are living longer with prostate cancer. This has resulted in a proportionately larger number of men at risk for cancer recurrence heralded by a rising PSA that exceeds a defined cutpoint (0.2–0.4 ng/mL, or 200–400 pg/mL). Biochemical recurrence (BCR) is conservatively estimated to affect around 50,000 men each year.7 As a result, both physicians and the men treated by RP are interested in estimating the risk of clinical recurrence.
Post-RP risk stratification approaches are based on a number of factors, but rely heavily on pathologic findings such as Gleason score, percentage of grade 4–5 disease, surgical margin involvement, extracapsular extension, seminal vesicle invasion and lymph node involvement. Multivariate models (nomograms) that incorporate these factors predict the likelihood of BCR and are not suitably generalizable for identifying individual men at high or low risk of clinical recurrence, either locally or systemically.8 The serum PSA nadir value following RP can be an indicative factor since undetectable levels <0.01 ng/mL (<10 pg/mL) are associated with better prognosis when BCR is used as the endpoint.9–12 However these assays cannot quantitate post-RP PSA concentrations <10 pg/mL, thus they are unable to distinguish between men that are at increased risk of recurrence and those that may be at reduced risk of recurrence.12–14
The inability to differentiate clinical recurrence risk is unfortunate for several reasons. From a healthcare management perspective, the intensity of serum PSA and urologic follow-up could be reduced in men at low risk for meaningful clinical recurrence. In addition, empiric use of adjuvant therapies could be reduced or eliminated, which could also reduce healthcare costs. From a patient perspective, quality of life would improve with the avoidance of unneeded radiotherapy or hormonal therapy, safer treatment of hypogonadism, and the unquantifiable yet important alleviation of psychological stress.
Recently, we developed an immuno-polymerase chain reaction (IPCR) assay for total serum PSA and validated its analytical performance in human samples.15 Our nucleic acid detection immunoassay (NADiA®) incorporates a double-stranded (ds) DNA label for high-sensitivity analyte detection and resists non-specific binding to improve precision. The limit of quantification (LOQ) is 0.00065 ng/mL (0.65 pg/mL), more than an order of magnitude lower than the most sensitive commercial PSA assays. Further, the assay employs a calculation of PSA slope (PSA change over time) as a unique prognostic factor. In pilot studies using NADiA PSA, we found that 90% of men without BCR have serum PSA concentrations below 5 pg/mL in the first several months following RP.16 In addition, the superior sensitivity and precision of this assay provided a unique opportunity to evaluate the kinetics of PSA at these very low post-RP concentrations. We found that PSA change over time (slope) exhibited first-order kinetics in non-recurring men and that the slope (in pg/mL/month) was significantly different from men that clinically recurred and that slope was a stronger prognostic factor than PSA nadir.17 These studies determined the potential clinical utility of a NADiA PSA slope cutpoint of 2.0 pg/mL/month and formed the hypothesis that men displaying a slope ≤2.0 pg/mL/month post-RP could be at a reduced risk of clinical recurrence.
The study protocol was reviewed with the US Food and Drug Administration through the pre-IDE process. Its objective was to validate the hypothesis that men displaying PSA slopes ≤2.0 pg/mL/month post-RP were at a reduced risk of clinical recurrence in an appropriately powered case-cohort designed trial.18 The results provided the basis for United States 510(k) clearance of NADiA ProsVue™ as a post-RP prognostic marker.
PATIENTS AND METHODS
Study design
The required sample size assuming a type I error rate of 5%, 80% power, and a univariate hazard ratio (HR) for PSA slope of 1.4 (estimated from pilot studies) based on a two-sided test was 262. Assuming 20% prevalence for recurrence, a minimum of 52 men with recurrence was required. A cohort random sampling of eligible men was performed within four strata arising from categorization as above or below median patient age (61.4 years) and median sample storage time (13.5 years). The final selected study population consisted of 64 men with clinical recurrence and 240 controls (prevalence 21.1%). Post-RP PSA slope (ProsVue result) was calculated in units of pg/mL vs. time in months for each man using least squares linear regression. ProsVue results were then expressed as a binary categorical variable with the cutpoint set at 2.0 pg/mL/month. ProsVue results ≤2.0 were pre-specified for identifying men at reduced risk of recurrence, therefore results >2.0 identified men “not at reduced risk of recurrence.”
Study population
The study was prospectively designed and incorporated archived serum samples from men treated by RP at four investigational sites (Duke University, Eastern Virginia Medical School, Memorial Sloan-Kettering Cancer Center, and the University of Washington) during 1990–2001. Institutional review boards at all sites approved the study protocol. Study samples were de-identified except for dates of birth, death, and clinical procedures, which were used to calculate age at RP, time to recurrence or length of follow-up. Inclusion criteria consisted of men with biopsy-confirmed prostate cancer treated by RP with a PSA value in the first post-RP sample <0.1 ng/mL (<100 pg/mL) using standard-of-care immunoassays for PSA. Three serum samples obtained between 1.5 and 20 months post-RP were required with at least a two-month interval between each sample draw date. Documentation of pre-RP PSA, pathologic stage and Gleason score was required to serve as covariates in multivariate analyses. Findings of positive surgical margins, extracapsular extension, seminal vesicle invasion, bladder neck invasion and positive lymph nodes were also recorded if documentation was available. Men categorized by the sites as non-recurrent required a minimum post-RP follow-up of 8 years. Exclusion criteria were radiation treatment administered in the first 12 months post-RP, androgen deprivation therapy administered in the first 20 months post-RP, and serum samples stored longer than 20 years.
Criteria for response
Men categorized by the sites as recurrent required documented evidence of local recurrence by biopsy, evidence of metastases by imaging (MRI, CT, bone scan, or 111In capromab pendetide immunoscintigraphy in conjunction with CT), or death due to prostate cancer. Men categorized as non-recurrent were followed after RP a median of 11.0 (IQR, 9.6–12.9) years vs. a median of 4.7 (IQR, 2.7–8.4) years for men with clinically recurrent disease. Men with BCR without documented evidence of disease (stage D0) were categorized as non-recurrent.
Clinical samples
Serum samples from 433 men treated by RP were available for this study. Samples were stored at ≤–70 °C in each site’s biorepository. The sample dra w dates and the PSA immunoassay method used were documented in medical records. Samples from 24 men could not be used because the men either did not meet protocol eligibility or were excluded because secondary treatment was administered during the sample collection period. A total of 1,227 archived samples from the 409 men eligible for cohort selection were shipped on dry ice.
NADiA PSA assay
NADiA is a re-engineered IPCR assay that utilizes a non-native dsDNA label for analyte detection (IRIS International, Inc.). Details of the NADiA PSA assay procedure have been previously described.15 Briefly, the monoclonal antibody (MAb)-DNA conjugate is reacted with sample in a microtiter plate format to form a first immune complex with PSA. The complex is then captured onto paramagnetic microparticles coated with the second capture MAb. Excess reporter MAb-DNA conjugate is removed from the microparticles by several cycles of magnetic capture and re-suspension. The specifically bound DNA label is amplified using PCR and the quantification cycles (Cq) of standards and samples are measured by qPCR. Cq values are calculated from the calibration curve and converted to PSA concentrations (pg/mL). ProsVue results are calculated from three NADiA PSA values using ProsVue Software. The linear range of the NADiA PSA assay extends beyond its reportable range (0.65–100 pg/mL), and intra-assay precision is <9.0%.15
Statistical analyses
The primary outcome measure was clinical recurrence-free survival defined as being alive with no detectable disease on biopsies or imaging studies. Univariate and multivariate Cox proportional hazards regression models were performed to determine the predictive capability of the binary expression of ProsVue results and covariates for clinical recurrence-free survival. Supportive survival analyses employed the Kaplan-Meier method with results of both Wilcoxon and log-rank tests reported. Sensitivity, specificity and positive and negative predictive values (PPV, NPV) were calculated via a standard 2x2 contingency table of clinical recurrence category vs. ProsVue risk category and exact binomial 95% confidence intervals (CI) were determined for each parameter. Comparisons of clinicodemographic factors between recurrent and non-recurrent groups were performed using the Wilcoxon rank sum test for continuous variables, and the Pearson chi-square or Fisher's Exact tests, as appropriate, for categorical variables. SAS V9.1 (SAS Institute, Cary, NC), JMP v5.01 (SAS Institute), and NCSS 2007 (NCSS Inc., Kaysville, UT) were used for the statistical analyses. All tests were two-sided and P <0.05 was considered significant.
RESULTS
Study population
Patient characteristics are listed in Table 1. For discrete covariates, category frequencies are provided, and for continuously valued covariates, mean, median and interquartile ranges (IQR) are given. Overall, 56.1% of men had organ-confined disease, and 3% had evidence of nodal spread. Forty-six percent had Gleason scores of 6 or lower, whereas 76.6% had PSA values ≤10 ng/mL at the time of RP. Comparing the recurrent and non-recurrent groups, pre-RP PSA, tumor volume, pT3/pT4 disease, Gleason score ≥7, positive surgical margins, extracapsular extension, seminal vesicle invasion and bladder neck invasion tended to be higher in value or percentage incidence in the recurrent vs. non-recurrent groups, indicating a higher proportion of aggressive cancers in the clinically recurrent group.
Table 1.
Clinicopathologic characteristics of the studied population
| Characteristic | N | Pct. | Mean | Median (IQR) |
|---|---|---|---|---|
| Age at RP (years) | 304 | – | 61.3 | 61.4 (59.6–66.1) |
| Percent tumor volume | 65 | – | 18.3 | 15.0 (6.5–25.0) |
| Pre-RP treatment | 59 | 19.4 | ||
| Pre-RP PSA (ng/mL) | ||||
| ≤4.0 | 70 | 23.0 | ||
| 4.0–10.0 | 163 | 53.6 | ||
| 10.1–20.0 | 57 | 18.8 | ||
| >20.0 | 14 | 4.6 | ||
| Pathologic GS | ||||
| <6 | 44 | 14.5 | ||
| 6 | 96 | 31.6 | ||
| 7 | 126 | 41.4 | ||
| >7 | 38 | 12.5 | ||
| Pathologic stage | ||||
| T0 | 2 | 0.7 | ||
| T2 | 168 | 55.4 | ||
| T3 | 120 | 39.4 | ||
| T4 | 14 | 4.6 | ||
| Other pathologic findings1 | ||||
| Surgical margin positive | 95 | 31.3 | ||
| Extracapsular extension | 125 | 41.4 | ||
| Seminal vesicle involvement | 37 | 12.4 | ||
| Bladder neck invasion | 18 | 11.0 | ||
| Lymph node involvement | 8 | 3.0 | ||
| Race1 | ||||
| African-American | 25 | 8.3 | ||
| Asian | 6 | 2.0 | ||
| Caucasian | 270 | 89.7 | ||
| Ethnicity1 | ||||
| Hispanic | 8 | 4.4 | ||
| Non-Hispanic | 173 | 95.6 | ||
Data for these characteristics were not documented in all 304 men in the study
NADiA PSA results
Median sampling times across the study cohort were 4.9, 8.5 and 12.8 months post-RP. Median NADiA PSA concentrations in the first post-RP serum samples were 3.1 (IQR 1.8–6.6) and 14.1 (IQR 4.1–48.4) pg/mL in the non-recurrent and recurrent groups, respectively (P <0.0001). Median PSA values in the second and third samples showed minimal change over time in the non-recurrent group but showed a significant rise across the three sampling points in the recurrent group. Median ProsVue results calculated from the three samples were 0.03 (IQR, −0.04–0.24) pg/mL/month in the non-recurrent group compared to 5.6 (1.6–22.0) in the clinically recurrent group (P <0.0001).
Efficacy analysis
Clinical recurrence was documented in 64/304 (21.1%) men; 15 initially recurred with biopsy-proven localized cancer and 49 initially recurred with distant metastases. Skeletal metastases was the initial presentation in 24/64 (37.5%) cases. Of the men that recurred, 46/64 (71.9%) were correctly classified as “not at reduced risk” of recurrence based on a ProsVue result >2.0 pg/mL/month. Of the men that did not recur, 227/240 (94.5%) were correctly classified as “at reduced risk” of recurrence based on a ProsVue result ≤2.0. Although the purpose of the study was to validate the hypothesis that ProsVue results ≤2.0 pg/mL/month identified men at a reduced risk of clinical recurrence, the sensitivity, specificity, PPV and NPV for clinical recurrence were also determined. Sensitivity (95% CI) was 71.9% (59.2–82.4%) at 94.6% (90.9–97.1%) specificity. PPV and NPV were 78.0% (65.3–87.7%) and 92.7% (88.6–96.5%), respectively at the 21.1% prevalence of recurrence in this study.
NADiA ProsVue results and pathological variables
The risk of clinical recurrence in relation to ProsVue results and pathological variables is listed in Table 2. A ProsVue result ≤2.0 was significantly associated with a reduced risk of clinically recurrent prostate cancer by univariate Cox proportional hazards regression analysis (HR 18.3, 95% CI, 10.6–31.8, P <0.0001). Most covariates and other pathological variables were also significantly associated with clinical recurrence using univariate analysis. In the multivariate model that included pre-RP PSA, pathological stage, and Gleason score as covariates, the ProsVue HR was minimally attenuated to 9.8 (95% CI, 5.4–17.8, P <0.0001) and was an independent predictor of recurrence risk. The inverse of the HR yields a ratio of 0.102 for an 89.8% reduction in risk of recurrence for men with a ProsVue result ≤2.0. Of the covariates, only Gleason score was significantly associated with recurrence risk (HR 5.4, 95% CI, 2.1–13.8, P = 0.0004) for an 81.4% reduction in risk for men with Gleason score <7.
Table 2.
Univariable and multivariate Cox regression analysis of the risk of clinical recurrence according to the post-RP ProsVue slope and pathologic variables
| Clinicopathological variables | N | Median years of follow-up | No. Events | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||||
| NADiA ProsVue result | |||||||
| ≤2.0 pg/mL/month | 245 | 10.6 | 18 | 1.0 | 1.0 | ||
| >2.0 pg/mL/month | 59 | 7.8 | 46 | 18.3 (10.6–31.8) | <0.0001 | 9.8 (5.4–17.8) | <0.0001 |
| Pre-RP PSA level (ng/mL) | |||||||
| Continuous variable | 304 | 8.6 | 64 | 1.1 (1.03–1.08) | <0.0001 | 1.0 (0.98–1.03) | 0.647 |
| Pathological Gleason score | |||||||
| 4, 5, 6 | 140 | 10.9 | 5 | 1.0 | 1.0 | ||
| 7, 8, 9 | 164 | 10.0 | 59 | 12.1 (4.8–30.1) | <0.0001 | 5.4 (2.1–13.8) | 0.0004 |
| Pathological stage | |||||||
| pT0–pT2 | 170 | 10.8 | 13 | 1.0 | 1.0 | ||
| pT3–pT4 | 134 | 9.7 | 51 | 5.8 (3.2–10.7) | <0.0001 | 1.7 (0.9–3.4) | 0.105 |
|
| |||||||
|
Other pathological findings1
| |||||||
| Percent tumor volume | |||||||
| Continuous variable | 65 | 10.1 | 16 | 1.0 (1.0–1.1) | 0.059 | ND2 | |
| Surgical margins | |||||||
| Negative | 209 | 10.5 | 27 | 1.0 | |||
| Positive | 95 | 9.9 | 37 | 3.3 (2.0–5.4) | <0.0001 | ND | |
| Extracapsular extension | |||||||
| Absent | 177 | 10.8 | 15 | 1.0 | |||
| Present | 125 | 9.7 | 48 | 5.3 (3.0–9.5) | <0.0001 | ND | |
| Seminal vesicle invasion | |||||||
| Absent | 261 | 10.5 | 40 | 1.0 | |||
| Present | 37 | 8.8 | 24 | 5.3 (3.2–8.8) | <0.0001 | ND | |
| Bladder neck invasion | |||||||
| Absent | 146 | 10.5 | 24 | 1.0 | |||
| Present | 18 | 9.8 | 7 | 2.8 (1.2–6.5) | 0.016 | ND | |
Data for other pathologic findings were not documented in all 304 patients;
ND = not included in multivariate model
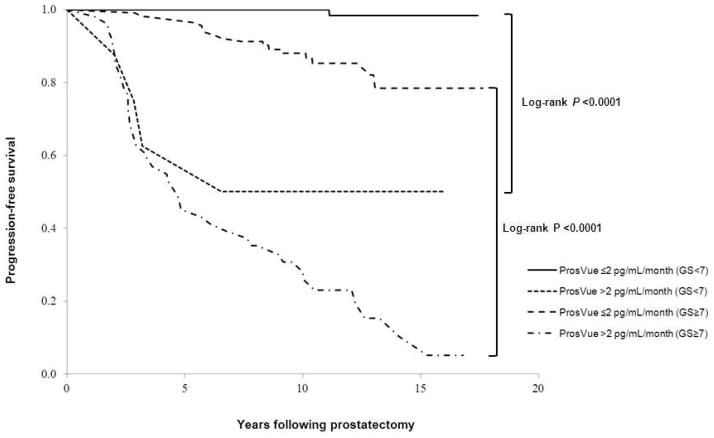
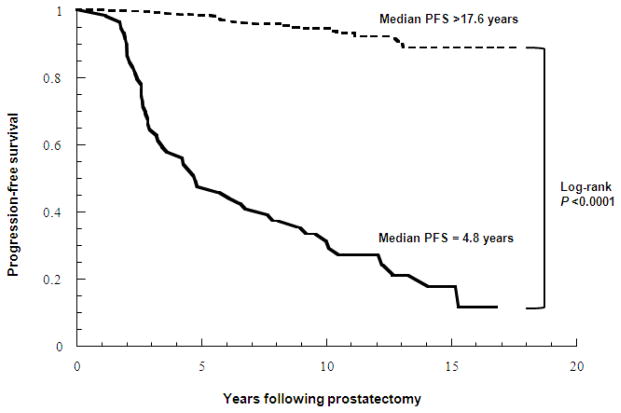
Figure 1 is a Kaplan-Meier plot of clinical recurrence-free survival based on the ProsVue cutpoint of 2.0 pg/mL/month. The plots diverge within the first two years of follow-up (Wilcoxon P <0.0001) and continue to separate due to fewer clinically recurrent events in men with ProsVue results ≤2.0. Median clinical recurrence-free survival was 4.8 years vs. >17.6 years when ProsVue results were >2.0 and ≤2.0, respectively (log-rank P <0.0001). Figure 2 is a Kaplan-Meier plot of clinical recurrence-free survival based on the ProsVue cutpoint in groups of men with pathological Gleason scores <7 (N=140) and ≥7 (N=164). The plots for the groups with Gleason <7 and ≥7 and ProsVue results >2.0 also diverge within the first two years post-RP, whereas the separation of the plots for the groups with Gleason <7 and ≥7 and ProsVue results ≤2.0 diverge more slowly due to fewer clinically recurrent events in men with ProsVue results ≤2.0 (log-rank P <0.0001 for Gleason scores <7 and ≥7).
Figure 1.
Kaplan-Meier plot of clinical progression-free survival probabilities for patients categorized as at reduced risk for clinical recurrence (dashed line) and not at reduced risk for recurrence (solid line) by ProsVue results.
Figure 2.
Kaplan-Meier plot of clinical progression-free survival probabilities for patients categorized as at reduced risk for clinical recurrence and not at reduced risk for recurrence based on ProsVue results in relation to groups of men with pathologic Gleason score <7 and ≥7 prostate cancers.
In the Gleason score ≥7 subset, sensitivity was 71.2% (57.9–82.2%) at 91.4% (84.4–96.0%) specificity and PPV and NPV were 82.4% (69.1–91.6%) and 85.0% (77.0–91.0%) respectively. Multivariate Cox proportional hazards regression analysis including pre-RP PSA and pathological stage (T3-T4 vs. T0-T2) as covariates showed minimal attenuation of the HR for ProsVue to 8.1 (95% CI, 4.5–14.7, P <0.0001) and it remained the strongest independent predictor of recurrence risk.
COMMENT
Prostate cancer is a complex disease, and it has proved difficult to predict clinical outcomes accurately in individual men following RP.8 Previous studies have linked undetectable PSA levels <0.01 ng/mL (<10 pg/mL) post-RP to relapse-free survival12 and nadir values to a lower likelihood for BCR.14 However, the risk of BCR is not informative as a predictor of clinical endpoints because of the unpredictable kinetics of post-RP PSA values after a BCR event occurs.19–23 For this reason, the NADiA ProsVue pre-IDE protocol stipulated that efficacy must be defined by clinically documented prostate cancer recurrence rather than BCR. Patients with ProsVue results ≤2.0 pg/mL/month enjoyed a ~90% reduction in risk of clinical recurrence in multivariate analyses. The multivariate model included pre-RP PSA, pathological stage and Gleason score and demonstrated that a ProsVue result ≤2.0 was the most powerful independent prognostic factor for identifying men at reduced risk for clinical recurrence (HR 9.8, P <0.0001). While Gleason score was also found to be a prognostic factor (HR 5.4), ProsVue demonstrated significant utility for identifying men at low risk of clinical recurrence in the subset of men having Gleason score ≥7 prostate cancer.
Limitations of this study include its reliance on archived serum samples. Because of this, the timing of serum sampling was not standardized and ProsVue calculations were determined from varying post-RP timepoints. However, this reflects real-world usage of PSA for post-treatment monitoring since routine testing does not always occur at fixed intervals. Further analysis of our data showed that the prognostic performance of ProsVue was not significantly different when all three NADiA PSA samples were collected within or outside of a one year time period (P = 0.17, data not shown). To address potential selection bias, we compared the demographic and clinicopathological characteristics of the men selected for this study against those of >10,000 RP cases in three published series.6,24,25 Results revealed similar distributions of demographic and clinicopathological characteristics to the men in this study. Importantly, the pre-IDE protocol was not designed to assess the utility of ProsVue results for identifying men at high risk for clinical recurrence and it was not intended to select men for additional post-RP treatment.
Thirteen men had ProsVue results >2.0 pg/mL/month but showed no clinical evidence of disease recurrence after a minimum 8-year follow-up (i.e., false positive result). In three (23.1%) men, the PSA rise was not sustained and all values declined to <0.05 ng/mL as determined by standard PSA assays and remained <0.05 ng/mL throughout follow-up. Ten men were staged as D0 based on BCR and negative imaging findings, 8 of whom received salvage RT and/or ADT. None of these men had clinical disease recurrence or died from prostate cancer during follow-up.
It should be noted that the men in this study underwent RP between 1991 and 2001 and Gleason grade patterns were assigned by each institution according to the grading system used at that time. The criteria for Gleason grading was refined in 200526 therefore we attempted to adjust for these changes so that our results would better correlate with men classified by today’s criteria. We combined Gleason score 4, 5 and 6 tumors into one group (≤6). Most cases initially reported as pattern 2 would now be graded pattern 3 since pathologists seldom assign a grade less than 3 to tumors. Scores for higher-grade tumors were unified to account for certain patterns originally graded as pattern 3 that would now be graded as pattern 4. We were unable to adjust for any Gleason 3+3=6 tumors that might have been upgraded to 3+4=7 using the new scoring system.
CONCLUSIONS
This is the first study of a prognostic assay for risk stratification of clinical prostate cancer recurrence with >10 years median follow-up. NADiA ProsVue is also the first assay to receive FDA clearance based on linear slope of tumor marker concentrations over time. A ProsVue result was the most powerful indicator of reduced risk of clinical recurrence and added prognostic value to established risk factors. ProsVue testing could possibly reduce healthcare costs by reducing the intensity of follow-up in men identified at a reduced risk for recurrence. Further studies will assess method performance and define the role of the assay in risk models and nomograms.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Tiwari RC, Murray T, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Moul JW, Wu H, Sun L, et al. Epidemiology of radical prostatectomy for localized prostate cancer in the era of prostate-specific antigen: An overview of the Department of Defense Center for Prostate Disease Research national database. Surgery. 2002;132:213–219. doi: 10.1067/msy.2002.125315. [DOI] [PubMed] [Google Scholar]
- 3.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 4.Liu L, Coker AL, Du XL, et al. Long-term survival after radical prostatectomy compared to other treatments in older men with local/regional prostate cancer. J Surg Oncol. 2008;97:583–591. doi: 10.1002/jso.21028. [DOI] [PubMed] [Google Scholar]
- 5.Gretzer MB, Trock BJ, Han M, et al. A critical analysis of the interpretation of biochemical failure in surgically treated patients using the American Society for Therapeutic Radiation and Oncology criteria. J Urol. 2002;168:1419–1422. doi: 10.1016/S0022-5347(05)64464-3. [DOI] [PubMed] [Google Scholar]
- 6.Roehl KA, Han M, Ramos CG, et al. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004;172:910–914. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 7.Moul JW. The evolving definition of advanced prostate cancer. Rev Urol. 2004;6(suppl 8):S10–S17. [PMC free article] [PubMed] [Google Scholar]
- 8.Shariat SF, Kattan MW, Vickers AJ, et al. Critical review of prostate cancer predictive tools. Future Oncology. 2009;5:1555–1584. doi: 10.2217/fon.09.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witherspoon LR, Lapeyrolerie T. Sensitive prostate specific antigen measurements identify men with long disease-free intervals and differentiate aggressive from indolent cancer recurrences within two years after radical prostatectomy. J Urol. 1997;157:1322–1328. [PubMed] [Google Scholar]
- 10.Doherty AP, Bower J, Smith GL, et al. Undetectable ultrasensitive PSA after radical prostatectomy for prostate cancer predicts relapse-free survival. Br J Cancer. 1999;83:1432–1436. doi: 10.1054/bjoc.2000.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong SK, Park HZ, Lee WK, et al. Prognostic significance of undetectable ultrasensitive prostate-specific antigen nadir after radical prostatectomy. Urology. 2010;76:723–727. doi: 10.1016/j.urology.2010.03.081. [DOI] [PubMed] [Google Scholar]
- 12.Shen S, Lepor H, Yaffee R, et al. Ultrasensitive serum prostate specific antigen nadir accurately predicts the risk of early relapse after radical prostatectomy. J Urol. 2005;173:777–80. doi: 10.1097/01.ju.0000153619.33446.60. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu F, Matsuyama Y, Tominaga T, et al. Inadequacy of prostate-specific antigen doubling time estimates calculated using an ultrasensitive assay of prostate-specific antigen for biochemical failure after radical prostatectomy. Urol Int. 2007;79:356–360. doi: 10.1159/000109723. [DOI] [PubMed] [Google Scholar]
- 14.Taylor JA, Koff SG, Dauser DA, et al. The relationship of ultrasensitive measurements of prostate-specific antigen levels to prostate cancer recurrence after radical prostatectomy. BJU Int. 2006;98:540–543. doi: 10.1111/j.1464-410X.2006.06294.x. [DOI] [PubMed] [Google Scholar]
- 15.McDermed JE, Sanders R, Fait S, et al. Nucleic acid detection immunoassay for prostate specific antigen based on immuno-PCR methodology. Clin Chem. 2012;58:732–740. doi: 10.1373/clinchem.2011.170290. [DOI] [PubMed] [Google Scholar]
- 16.McDermed J, Klem R, Saunders R, et al. Total PSA (tPSA) level post-prostatectomy (RP) measured with the NADIA® ultrasensitive PSA assay and the risk for biochemical recurrence (BCR) Clin Chem. 2008;54(Suppl):C-117. [Google Scholar]
- 17.Moul JW, Semmes OJ, Vessella RL, McDermed JE, Lilja H. Prognostic impact of post-prostatectomy PSA slope determined with a novel, nucleic acid detection immunoassay (NADiA ProsVue) for total PSA. J Clin Oncol. 2011;29(suppl 7):abstr 47. [Google Scholar]
- 18.Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73:1–11. [Google Scholar]
- 19.Moreira DM, Presti JC, Aronson WJ, et al. Natural history of persistently elevated prostate specific antigen after radical prostatectomy: Results from the SEARCH database. J Urol. 2009;182:2250–2256. doi: 10.1016/j.juro.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 20.D’Amico AV, Moul JW, Carroll PR, et al. Surrogate marker for prostate cancer-specific mortality following radical prostatectomy or radiation therapy. J Natl Cancer Inst. 2003;95:1376–1383. doi: 10.1093/jnci/djg043. [DOI] [PubMed] [Google Scholar]
- 21.Amling CL, Bergstralh EJ, Blute ML, et al. Defining prostate specific antigen progression after radical prostatectomy: what is the most appropriate cut point? J Urol. 2001;165:1146–1151. [PubMed] [Google Scholar]
- 22.Stephenson AJ, Kattan MW, Eastham JA, et al. Defining biochemical recurrence of prostate cancer after radical prostatectomy: a proposal for a standardized definition. J Clin Oncol. 2006;24:3973–3978. doi: 10.1200/JCO.2005.04.0756. [DOI] [PubMed] [Google Scholar]
- 23.Shinghal R, Yemoto C, McNeal JE, et al. Biochemical recurrence without PSA progression characterizes a subset of patients after radical prostatectomy. Urology. 2003;61:380–385. doi: 10.1016/s0090-4295(02)02254-9. [DOI] [PubMed] [Google Scholar]
- 24.Sengupta S, Blute ML, Bagniewski SM, et al. After radical retropubic prostatectomy ‘insignificant’ prostate cancer has a risk of progression similar to low-risk ‘significant’ prostate cancer. BJU Int. 2008;101:170–174. doi: 10.1111/j.1464-410X.2007.07270.x. [DOI] [PubMed] [Google Scholar]
- 25.Freedland SJ, Kane CJ, Presti JC, Jr, et al. Comparison of preoperative prostate specific antigen density and prostate specific antigen for predicting recurrence after radical prostatectomy: Results from the SEARCH data base. J Urol. 2003;169:969–973. doi: 10.1097/01.ju.0000051400.85694.bb. [DOI] [PubMed] [Google Scholar]
- 26.Epstein JI, Allsbrook WC, Jr, Amin MB, Egevad L. The ISUP Grading Committee: The International Society of Urological Pathology (ISUP) Consensus Conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol. 2005;29:228–242. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]