Abstract
The error types during brachytherapy (BT) treatments and their occurrence rates are not well known. The limited knowledge is partly attributed to the lack of independent verification systems of the treatment progression in the clinical workflow routine. Within the field of in vivo dosimetry (IVD), it is established that real-time IVD can provide efficient error detection and treatment verification. However, it is also recognized that widespread implementations are hampered by the lack of available high-accuracy IVD systems that are straightforward for the clinical staff to use. This article highlights the capabilities of the state-of-the-art IVD technology in the context of error detection and quality assurance (QA) and discusses related prospects of the latest developments within the field. The article emphasizes the main challenges responsible for the limited practice of IVD and provides descriptions on how they can be overcome. Finally, the article suggests a framework for collaborations between BT clinics that implemented IVD on a routine basis and postulates that such collaborations could improve BT QA measures and the knowledge about BT error types and their occurrence rates.
Modern brachytherapy (BT) is increasingly based on remote afterloading [for high-dose-rate (HDR) BT], the use of three-dimensional (3D) imaging and treatment planning systems (TPSs). These developments have reduced manual procedures, which are well known to be the most frequent source of errors in radiotherapy (RT).1–4 However, BT still typically involves more manual procedures during catheter/applicator insertion, treatment planning and treatment delivery than does external beam RT (EBRT). Also, treatment delivery verification is less advanced in BT than in EBRT. Therefore, BT may be more prone to errors than is EBRT.
The implementation of 3D imaging in BT has moved the field into an era of improved implant geometry and more frequent use of individualized adaptive approaches via dose optimization. With increased conformality of dose to target, the reliability and precision of dose delivery has become even more important. Furthermore, the availability of 3D dose distributions has substantially improved the understanding of the relation between dose and clinical effects, which allows for improved possibilities to navigate treatments according to certain dose constraints in the balancing and prioritization between the target and organ-at-risk (OAR) doses. As a result, the accuracy of delivered dose is becoming increasingly important for optimal employments of target dose escalation and OAR dose minimization protocols. In particular, for patients with target and/or OAR doses close to constraint values, the clinical consequences of dose uncertainties are more pronounced, hence it is crucial to be able to control the accuracy of dose delivery.5 Future treatment delivery verification should therefore address detection of both errors and variations with the aim to improve the overall accuracy of treatment delivery. This needs to be put in context of high dose gradients present in BT treatments that make accurate dose measurements and treatment deliveries challenging, since even small geometric uncertainties or errors may result in large dose discrepancies from the original treatment plan. Such discrepancies may lead to insufficient dose delivered to the target and/or increased OAR doses.
Treatment errors in BT1−3 are categorized into human errors (e.g. incorrectly specified source strength, erroneously connected source transfer guide tubes and gross applicator reconstruction errors) or malfunctions of the equipment (e.g. defective afterloader stepping motor and flaws in the control software). The types of errors during BT treatments and their occurrence rates are not well known. Existing channels of information regarding errors during RT include dedicated databases6–9 and published reports.1–3,10 However, since RT clinics are not necessarily subject to policies that require public reporting in case of detected treatment errors, it is likely that a substantial portion of occurred incidences are left unknown to the RT community. Furthermore, it is common that BT treatments are not monitored with control methods that are independent from the treatment delivery systems, hence it is possible that errors remain undetected during the entire multifraction treatment course.
The recent Vision 20/20 article regarding in vivo dosimetry (IVD),11 argues that IVD provides an effective method of independent treatment verification that leads to an improved patient safety during BT. The article discusses limitations of existing safety and quality assurance (QA) measures and outlines ways in which real-time IVD can aid error detection and QA. The article also establishes that state-of-the-art IVD technology offers various possibilities for high-precision real-time dose rate monitoring during BT treatments and furthermore describes existing error detection methods and their potential role in the clinical workflow.
Despite several advantages, widespread use of the state-of-the-art IVD during BT treatments is limited. IVD is used mostly in research and development of new technologies. Occasional IVD feasibility studies have also been conducted in some clinics.12–24 However, routine implementations of IVD for error detection monitoring and QA of the BT treatments is yet to come. One reason for this may be that the cost vs benefit relation of IVD is not established and that robust IVD systems that do not leave a significant footprint on the treatment workflow are not commercially available. As a consequence, the occurrence rates and dosimetric impact of different treatment errors are largely unknown and so is the extent of effectiveness of existing safety and QA measures.
The aim of this article is to highlight the capabilities of the state-of-the-art IVD technology and describe how they can be implemented in the clinic. The main limitations of current IVD systems and potential solutions are discussed. This article also suggests the role that IVD systems may play in safety and QA procedures, in order to obtain improved knowledge about different sources of uncertainty during BT treatments, treatment error types and their occurrence rates. The focus of this article is on real-time dosimetry, given the capacity for online monitoring of BT treatments and the superior error detection efficiency compared with passive dosimetry.14,17,22,25
UNCERTAINTIES, ERRORS AND THE NEED FOR IN VIVO DOSIMETRY
Uncertainties during brachytherapy
Uncertainty analyses occupy a substantial portion of the literature and cover various aspects of BT treatment workflow. Uncertainties related to treatment planning arise partly from source calibration uncertainties26 and imperfections of the dose calculation protocols. Present TPSs incorporate the American Association of Physicists in Medicine Task Group (TG)-43 dose calculation protocol,27 which assumes that a patient is made of water and disregards tissue heterogeneities and intersource dose attenuation. In addition, present TPSs are subject to uncertainties related to source parameters and the implementation of dose calculation protocols.26,27 Treatment planning uncertainties are further enhanced by interobserver variability in the volumetric delineation of clinical targets and OARs28–30 and by systematic effects of source positioning and applicator reconstructions.31–37 Shortcomings of the TG-43 dose calculation protocol27 are targeted by developments of model-based dose calculation methods, which is in contrast to the TG-43 account for tissue heterogeneities and individualized patient anatomy.38,39
Although dosimetric uncertainties in BT have been well described, there has been a limited overview of how the impact of clinical uncertainties related to, for example, contouring, treatment planning and organ movements affect the treatment outcome. However, recently, a number of investigations have been published, which improve the possibilities to describe BT uncertainty budgets that encompass the entire treatment workflow from source calibration to treatment delivery.5,40 These investigations point towards the importance of geometric variations induced by organ and applicator movements,41 which complicate and potentially compromise accumulated dose calculations in the organs. Inter- and intrafraction deformations of prostate, bladder and rectum have been studied,42–46 and the dosimetric impact owing to more general anatomical variations has been evaluated in a multicentre comparison of cervix BT.41 Organ–applicator movements occurring between patient imaging and treatment delivery stages have been identified as a major source of uncertainty33,47–53 and their impact further analysed.54–57 In cervical cancer BT, inter- and intrafraction uncertainties owing to organ movements and deformations account for 20–25% of the D2cm3 parameter (minimum dose to the most irradiated 2 cm3) per fraction and is the most essential component in the uncertainty budget for OARs.5
Errors during brachytherapy
The knowledge about BT treatment errors comes from published reports1–3,10 and databases, such as ROSIS,7 SAFRON8,9 and ACCIRAD,6 and is limited to the information that clinics are willing and able to share. As demonstrated through phantom and computer-simulated error scenarios,14,17,21,25 several of the reported BT error types could have been detected with real-time IVD, for example, for the reported case of an HDR unit malfunction, where the BT source had broken loose from the guide wire and remained inside the patient.10 If real-time IVD was more frequently implemented in the clinical workflow routine, in addition to consensus recommendations for image-guided BT,36,37,58,59 our knowledge about BT errors and their occurrence rates could improve, provided the reporting is open and honest.
REAL-TIME IN VIVO DOSIMETRY TODAY AND CURRENT CHALLENGES
Dosimetry technology
Summaries of dosimetry technology that have been used for BT during in vivo and phantom measurements are provided by Tanderup et al,11 Cygler et al60 and Baltas et al.61 This section introduces dosimetry technology that is suitable for real-time IVD in BT and is incorporated in recent developments (see Prospects for in vivo dosimetry section).
Semiconductor dosimetry
Semiconductor dosimetry in BT, as in EBRT, is based on diodes and metal-oxide-semiconductor-field-effect-transistor (MOSFET) detectors. Diodes have been incorporated for IVD during BT,12,13,62–64 in the bladder (3-mm outer probe diameter) and/or rectum (7-mm outer probe diameter) manufactured by PTW Fruiberg, Freiberg, Germany. Compared with diode-based dosemeters, the MOSFET detector is a relatively new dosemeter technology in RT. MOSFET detectors are based on miniature n- or p-type silicon devices with small dosimetric sensitive volumes, of submicron thickness. The small sensitive volume allows for dosimetry in steep dose gradients as well as cases of electronic disequilibrium, which are common conditions in BT. It also allows for the construction of small dosemeter probes with approximately 1.3-mm outer diameters that can fit inside small catheters in or near the tumour region, and therefore facilitates IVD inside urethral catheters15,46,65–67 and inside nasopharyngeal applicators.68
Common disadvantages of semiconductor dosemeters are temperature and energy dependences in radiation fields. Diodes are also subject to a significant directional dependence and MOSFETs to a rather limited lifetime.
The temperature dependence of diodes has been reported to range between 0% K−1 and 0.6% K−1.12,69,70 Temperature instability in MOSFETs can be compensated electronically by using a readout current corresponding to the thermostable point71 or a dual-MOSFET-dual-bias device, as realized in the Best Medical Canada (Ottawa, Ontario) commercial MOSFET.72 However, some MOSFET arrays may still exhibit temperature dependence as was reported by Bloemen-van Gurp et al65 who observed an increase of 0.6% K−1 for temperatures between 20 and 37 °C.
Energy dependence in semiconductor devices can be minimized by calibration of the detectors in a reference radiation field of a similar energy as used in patient measurements. Comparisons between MOSFET responses and reference dose rates obtained with Monte Carlo simulations were made for 192Ir source irradiation in water at various angles and radial distances from the point of interest.73 If an energy correction coefficient was applied, the results agreed within 5%, which corresponded to experimental uncertainties. Reniers et al22 measured the variation of the MOSFET response with distance and used a linear fit through the measurements as an energy correction factor.
Fibre-coupled dosimetry
Fibre-coupled dosemeter probes are composed of a detector that is coupled to a fibre optic cable. The detector emits light in proportion to the absorbed dose in the detector volume. Fibre-coupled dosimetry for BT sources has been performed with detectors composed of organic scintillation materials,17,19,74 aluminium oxide crystals (Al2O3:C)14,75 and Ce3+ doped SiO2.76 The small (1 mm) outer diameter of the dosemeter probes and their flexibility allows for IVD, e.g. in urethra19 and BT needles.14
The main disadvantages of fibre-coupled dosemeters are temperature dependence and the stem signal. The stem signal corresponds to irradiation-induced emission of fluorescence and Cerenkov light in the fibre optic cable and is a significant source of background when the source irradiates near the fibre optic cable and relatively far from the detector. The stem signal can be suppressed efficiently using a chromatic removal technique, originally developed for fibre-coupled scintillator dosimetry for EBRT by Fontbonne et al,77 which has been adapted for BT with scintillator78 and Al2O3:C dosimetry.79
Recently, Beddar80 pointed to new evidence for temperature dependence of plastic scintillator sensitivity. The results were confirmed in two other studies,81,82 which demonstrated that the response decreases linearly with increasing temperatures. The studies revealed that the responses of blue BCF-12 and green BCF-60 scintillators from Saint Gobain, Paris France, decreased with temperature by 0.05% K−1 or 0.09% K−1 and 0.55% K−1 or 0.50% K−1, respectively. The studies also showed that the stem signal was not significantly temperature dependent. Edmund and Andersen83 showed that the Al2O3:C radioluminescence response decreases by 0.2% K−1, and by contrast, Carrara et al76 showed that the Ce3+ doped SiO2 response increases by 0.2% K−1.
Whereas scintillation and Ce3+ doped SiO2 detectors do not exhibit energy dependence for high-energy BT sources, Al2O3:C dosemeter probes have shown some energy-response artefacts.75 Monte Carlo simulations performed by Andersen et al75 demonstrated that a dosemeter probe placed inside a stainless steel BT needle would over-respond with respect to water with magnitudes depending on the source-to-detector distance at an approximately constant rate.
Important challenges for in vivo dosimetry
Widespread implementation of high-precision IVD systems in the clinical routine is limited by the potential introduction of added risks and discomfort to the patient as well as the extra workload and potential interference with the existing clinical workflow. The extra workload is linked to dosemeter calibration, reconstruction of dosemeter position in patient images, and placement and securing of dosemeter probe etc. The accepted extra workload depends on the user. One clinic may consider a daily 20-min dosemeter calibration routine acceptable, whereas another would require a maximum 5-min investment for all IVD-related stages. Also, BT clinics would most likely require that the implementation of the IVD system did not reduce or disturb the focus on patient care.
A common problem in IVD is the poor knowledge of the exact dosemeter position. For instance, discrepancies >30% between measured and expected dose rates have been observed during IVD14,19,21,62,63 and have been attributed to poor correspondence between the dosemeter position during the patient image acquisition and that during the treatment delivery owing to patient movements during the transfer between imaging and treatment locations.19,21,63 Since BT dose distributions are characterized by steep gradients, the uncertainties in the detector position may generate substantial dose uncertainties. Positional uncertainties <1.5 mm for the dosemeter and individual source positions are required in order to be able to detect source displacement errors of 5 mm.25 However, with some substandard image quality and potential geometric variations in the anatomy, it is not trivial to achieve positional uncertainties better than 1.5 mm during IVD measurements. As a result, IVD implementations must incorporate unrestrictive error criteria in order to avoid false alarms, which in turn allows only for the detection of gross errors of >50% and can lead to situations where smaller errors are undetected and may cause harm to the patient.
Compromised dosemeter positions, where the position during the treatment delivery did not correspond to that acquired from patient images, have been indicated during IVD in the urethra,12,19 the rectum,12,13,62,63,84 and source applicators.14,21 Compromised dosemeter positions may be caused by erroneous reconstructions owing to poor image quality and/or by dosemeter shifts from their initial placement with respect to the adjacent tissue, for example, owing to patient movements during transport between locations. Such dosemeter shifts are difficult to model and can result in too optimistic uncertainty budgets. In addition, compromised dosemeter positions make it difficult, if not impossible, to relate measured dose rates with planned dose rate calculations. As a result, false error alarms may be generated if comparisons between measured and planned dose rates rely on a static a priori reconstruction of the dosemeter position. IVD would therefore provide only limited warranty for a treatment termination unless risks of such false errors are eliminated.
The extra workload and potential interference with the clinical workflow are linked to manual procedures of the IVD implementation and the lack of automated dosimetry procedures. For instance, most in vivo measurements require a manual insertion of the dosemeter probe into source applicators or catheters placed in OARs. Although the dosemeter placement procedures may be straightforward, they may require preparations by the doctor in the operating theatre,14,21 and thus reduce the dissemination of IVD in BT.
In order to learn more about errors that occur during BT treatments and their rates, IVD systems must be implemented more commonly in the clinical routine, and they must be able to provide highly accurate comparisons between measured and expected dose rates to improve their sensitivity to smaller errors. The main challenge is therefore to develop IVD systems that are both accurate and leave a negligible footprint in the clinical workflow. Such IVD systems should also provide well-known energy responses if the dosemeter is placed near a boundary of heterogeneity in the medium or if the dose field is perturbed significantly by medium heterogeneities.
ERROR DETECTION CRITERIA
Errors during BT result in discrepancies between the planned and delivered treatments. The errors may occur when source positions and/or dwell times deviate from those in the treatment plan and result in erroneous dose rate distributions in the target and OARs. Although patient images can be used to detect independent movements between target/OARs and source applicators,51 further source position error types, in addition to dwell time errors, may be detected with real-time dosimetry, as will be described in the Future directions section. This section will therefore discuss error detection mainly by means of time resolved dosimetry.
Real-time dosemeters can translate measured dose rates into source-to-detector distances (see Source localization and real-time applicator reconstruction section), hence real-time IVD may monitor treatment parameters that can be derived from time-resolved dose rates and source positioning, e.g. source dwell times, source coordinates and dose rates at the dosemeter position.
Furthermore, positioning technology (see Detector positioning technology section) provides time-resolved spatial coordinates of the dosemeter probe, which allows for real-time monitoring of geometric changes in the anatomy during the treatment delivery.
The BT source coordinates and dwell times defined in the treatment plan and the coordinates of the dosemeter probe can be used to calculate expected dose rates and derived treatment parameter values. Measured parameter values may be treated as surrogates for the treatment delivery, hence the integrity of the planned treatment may be evaluated based on comparisons between the expected and measured parameter values. Therefore, discrepancies between measured and expected treatment parameters may serve as a surrogate for discrepancies between the planned and delivered treatments.
An error criterion for BT defines the level of discrepancy between measured and expected treatment parameters that is required in order to declare a treatment error. This section discusses different error criteria that could be adapted for IVD during BT.
Fixed discrepancy criterion
Errors may be declared if monitored treatment parameters, for example, measured dose rates or source positions, differ from expected values by a pre-defined fixed discrepancy level. Such fixed discrepancy criteria have been implemented during patient measurements and phantom experiments.12,17 Waldhäusl et al12 performed IVD in the rectum and bladder during intracavitary BT and compared measured and expected doses calculated based on image reconstructed distances between the source applicators and dosemeter probe positions and the dwell times defined in the treatment plan. The BT treatments were investigated in detail if measured and expected doses differed by >10%. Therriault-Proulx et al17 simulated real-time IVD in the rectal wall and urethra during phantom experiments of HDR BT treatment plans where positioning errors were imposed. The numbers of true and false errors detected were investigated for individual source dwell positions for fixed discrepancy criteria between 3% and 20%.
Statistical discrepancy criterion
For statistically based discrepancy criteria, the discrepancy between measured and expected treatment parameter values is expressed in terms of all known sources of uncertainty, for example, the number of measured and calculated standard uncertainties added in quadrature. The dominating source of uncertainty in BT is the positional uncertainty,14,25 which depends on the source-to-detector distance, and should therefore not be excluded from the uncertainty budget.
Andersen et al14 performed real-time IVD in the tumour region during interstitial pulsed dose rate (PDR) BT for cervical cancer and compared measured dose rates with calculations based on the treatment plan. The comparison was based on the statistical discrepancy criterion, which declared a treatment error if the dose rate difference was >2.58 standard deviations, which corresponded to a p-value of 0.01. Similar concepts have been implemented during phantom measurements.14,18,22,25,85 Nakano et al86 adapted statistically based discrepancy criteria during phantom experiments using HDR BT sources, where the discrepancy was based on measured and expected source coordinates rather than on dose rates.
Treatment progression snapshots
The integrity of the planned treatment could be assessed after an initial portion of the treatment progression by comparing the level of agreement between monitored and planned treatment parameter values.
This error detection technique was proposed by Sheikh-Bagheri and Munro87 who monitored the BT source position during HDR treatments using X-ray fluoroscopy with a C-arm. A treatment error would be declared if the source position in the fluoroscopy image snapshot would not agree with the treatment plan. The same error detection principle for source localization has also been suggested for a pinhole camera,88 an array of fibre-coupled scintillators20 and imaging with 192Ir photons.89
Optimal error detection criterion
In BT, the relative magnitudes of measurement and positional uncertainties depend on the source-to-detector distance.14 For instance, dose rate uncertainties for individual source dwell positions may range between 3% and 26% in a single treatment plan owing to the impact of the positional uncertainties for the source and dosemeter probe.25 Fixed discrepancy criteria would declare an error whenever the discrepancy between measured and expected treatment parameter values exceed the pre-defined limit, e.g. 10%. As stated,25 such an error criterion is more susceptible to false-positive error declarations when the source-to-detector distance is small and where the uncertainties are potentially >10% and to false-negative error declarations when the distance is large and the uncertainties are potentially <10%. As a result, statistical discrepancy criteria provide more confidence in the error declaration for IVD during BT than do fixed discrepancy criteria.
Error criteria based on treatment progression snapshots would be able to detect potential treatment errors that have occurred up to the instance of monitoring and would not be sensitive to errors that could occur at a later stage during treatment. Real-time IVD can monitor the agreement between measured and planned parameter values, hence statistical discrepancy criteria would be more suitable for BT than would treatment progression snapshots.
Treatment errors could be declared if too large doses were measured at International Commision on Radiation Units and Measurements (ICRU) reporting rectal and bladder points.90,91 For instance, a statistical discrepancy criterion could be defined in order to monitor the dose at reporting points. However, dose estimates at ICRU reporting points have been shown to be unreliable given practical difficulties to position the dosemeter probe accurately in the steep dose gradient regions.12,92,93 A further argument against error criteria based on dose rates at ICRU reporting points is that modern BT is adapting volumetric dosimetry during treatment planning.94,95
PROSPECTS FOR IN VIVO DOSIMETRY
Recent developments have improved the precision and capabilities of dosimetry technology (see Novel dosimetry technology section) and also provided partial solutions to the main challenges in IVD (see Some solutions to main challenges section). They also provide important contributions to the efforts in bringing accurate and user friendly IVD systems to the clinic, with the aim towards widespread IVD implementations and a better knowledge about error types and their rates in BT.
Novel dosimetry technology
Multipoint dosemeters
State-of-the-art single-point real-time dosemeters are abundant and play an important role for IVD during BT given their favourable characteristics, including small diameters and small detector volumes.11 However, dosemeter probes with several point detectors arranged in a line or surface arrays are becoming more common. One advantage with detector arrays is that one dosemeter probe alone allows for simultaneous dose rate measurements at several spatial points. Additionally, dose rate measurements at several points allow for real-time source localization (see Source localization and real-time applicator reconstruction section).
Recently developed linear arrays of MOSFET and plastic scintillator detectors with small outer diameters (approximately 1 mm) are an improvement on the former diode arrays, for example, by PTW Freiburg, which has a 7-mm diameter that limits the possible dosimetery sites to large cavities, e.g. rectum.
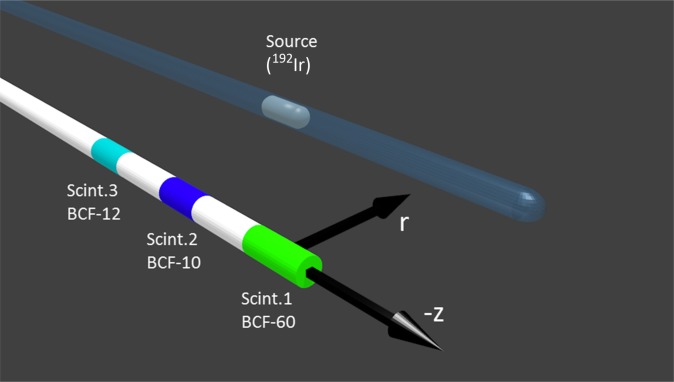
The extension of plastic scintillation dosimetry from one to multiple scintillating elements per optical probe was made possible with a mathematical formalism introduced by Archambault et al,96 which allows for optical separation of the different light emitting elements that compose the total optical signal. A multipoint plastic scintillation detector (mPSD) using three scintillator elements (Figure 1) measures dose rates in water with high accuracies, as demonstrated by Therriault-Proulx et al97 for a 6-MV external photon beam and for a 192Ir HDR BT source.18 A weighting function that accounts for the difference in signal-to-noise ratio between multiple measurement points was tested for BT source irradiations at source-to-detector radial distances (r) from 1 to 5 cm and for a range over 10 cm along the longitudinal axis (z). The measurements demonstrated a reliable way to improve the error detection capacity of the mPSD.
Figure 1.
Illustration of a multipoint plastic scintillation detector system composed of three different types of scintillators (Scint) that were used for phantom experiments with a 192Ir high-dose-rate brachytherapy source.18 Reproduced from Therriault-Proulx et al18 with permission from the American Association of Physicists in Medicine.
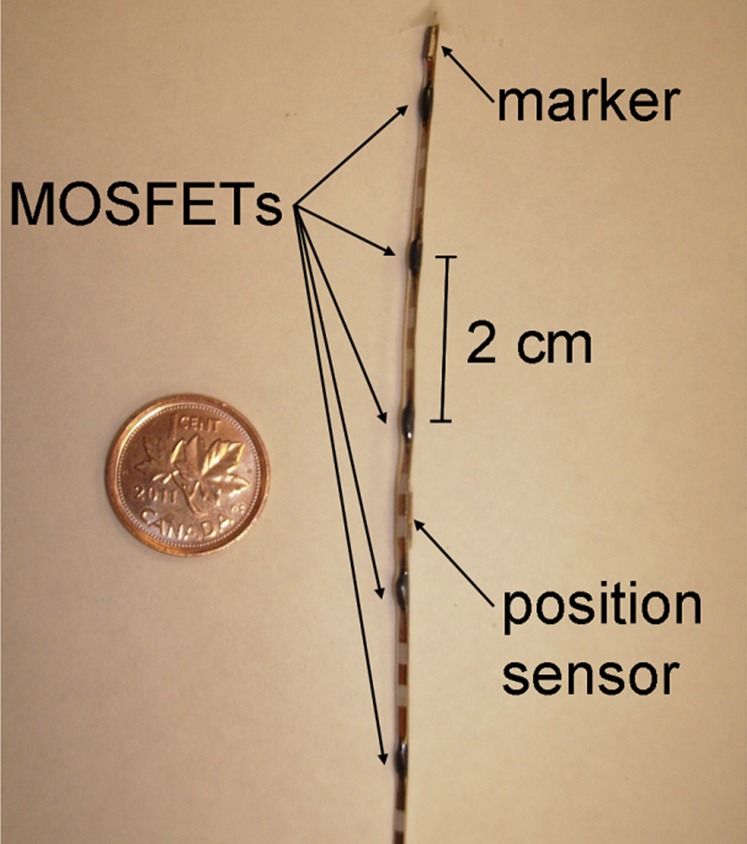
A line array, including five microMOSFET detectors (Figure 2) has recently been realized for the radiation positioning system (RADPOS), in order to allow for simultaneous real-time dosimetry at five spatial points.46,98 The RADPOS probe is 1.3 mm in diameter and can fit inside a Foley catheter or in an interstitial catheter. The probe incorporates a positioning sensor (see Detector positioning technology section) and a radio-opaque marker, both of which are visible on radiographs and aid probe localization procedures based on patient images. The probe has been used in vivo during permanent prostate implant BT by Cherpak et al.46 Next improvements to the RADPOS should address further miniaturization of the RADPOS probe.
Figure 2.
Radiation positioning system detector composed of five micrometal-oxide-semiconductor-field-effect-transistor (MOSFET) radiation detectors, one electromagnetic positioning sensor and a radio-opaque marker that has been used in vivo during permanent prostate implant brachytherapy.46 Reproduced from Cherpak et al46 with permission from Elsevier.
Cartwright et al20 developed a plastic endorectal applicator made of 16 individual plastic scintillator BrachyFOD™ detectors forming a two-dimensional array along the surface of the applicator. The applicator also incorporated radio-opaque markers to facilitate dosemeter reconstruction based on radiographs.
This kind of rigid applicator provides a displacement between the rectum and target regions and allows for simultaneous real-time dosimetry at 16 measurement points located at a submillimetre distance from the rectal wall. In-phantom measurements demonstrated accurate dose rate mapping along the rectal wall for a moving 192Ir HDR BT source.
Detector positioning technology
Real-time dosimetry combined with time-resolved monitoring of the dosemeter probe position is possible with the RADPOS dosemeter (Figure 2).46,98 The computer-controlled system combines multipoint real-time dosimetry with an electromagnetic positioning device and includes a software program that allows sampling of position and dose rates either manually or automatically in user-defined time intervals. The position coordinates of the detector are determined by monitoring the response of the motion sensor to a pulsed 3D magnetic field generated by a 3D-Guidance™ DC magnetic field transmitter (Ascension Technology Corporation; Burlington, VT). The transmitted magnetic field is measured by the detector located in the RADPOS probe with a frequency of up to 20 Hz. A specially developed algorithm converts the measurements into x, y and z coordinates of the detector as well as the azimuth, elevation and roll angles of rotation. Numerical data as well as graphical display are available with the software and can be viewed on the host computer.
Source localization and real-time applicator reconstruction
Time-resolved source localization methods have been developed for the localization of moving sources and BT seeds by means of real-time dosimetry technology,13,18,20,86,99 spectroscopy,24 electronic portal imaging devices,100 2D diode arrays101 and pinhole cameras.88,102–104 These methods are mainly used for BT QA protocols, although some of them have also been implemented for real-time IVD probes during phantom experiments.
Source localization algorithms adapted for BT have been based on triangulation,101 multiparametric fit techniques,105 least square fitting,13,24,99 weighted dosemeter outputs,18 iterative techniques,20 fitted functions of the detector reading with respect to the source-to-detector distance,86 fitting of Lorentz class functions to measured dose profiles100 and specific image reconstruction techniques for pinhole cameras.88 Nakano et al,99 demonstrated that the redundancy of dosimetry points increases the source localization accuracy.
3D localization of a moving 192Ir HDR BT source was performed in a water phantom by Therriault-Proulx et al18 with single-fibre three-point mPSD (see Novel dosimetry technology section) and an algorithm based on the weighted dosemeter light outputs of the individual scintillator elements. The parallel dosemeter probe and source catheters were positioned from 1 to 5 cm in the radial direction, and the source dwelled at positions along the longitudinal detector axis. The source localization accuracy was better than 1 and 2 mm for 69% and 87% of the dwell positions, respectively. Furthermore, the mPSD measured the uncertainty of the source position in the longitudinal direction to be 0.32 ± 0.06 mm in the fixed water phantom geometry, leading to the conclusion that 0.5-mm lower limit of source localization accuracy should be expected when using this technique in vivo.
Source localization techniques have been employed mainly for BT QA protocols;24,88,101–104 however, they have an interesting potential for IVD implementations in BT with the state-of-the-art dosimetry technology and should therefore be further investigated. For instance, Nakano et al86,99 have suggested methods for backscatter and tissue heterogeneity corrections using multiple dosemeter points, which could be relevant if the dosemeter probe had been calibrated under irradiation conditions significantly different from the in vivo scenario.
Palvolgyi106,107 used multiparametric fit techniques105 to develop methods to reconstruct intracavitary and interstitial applicators. The techniques were based on pre-determined applicator template files and specific reference points of the applicators (e.g. tandem tip and needle base) identified with C-arm radiographs. The same techniques could be employed for source applicator reconstructions if source localization coordinates in individual applicators acquired with real-time IVD were used as applicator reference points. Therefore, IVD could potentially provide an independent verification of target–applicator reconstruction uncertainties in the treatment planning.31
Some solutions to main challenges
Developments of tools and methods that increase the possibilities to perform routine and precise IVD have been sparsely represented in the literature and do not cover all aspects of the clinical workflow. Some solutions to important challenges (see Important challenges for in vivo dosimetry section) are described in this section and suggestions for further developments are provided.
Dosemeter placement tools
Independent dosemeter shifts with respect to the organ within which it was initially positioned may be avoided with proper implementation tools that stabilize the dosemeter position and assure that it remains unchanged with respect to the closest tissue in spite of patient transfers between locations,108,109 vaginal packing and/or source applicator clamping devices,47–49,52 potential patient posture changes108 and OAR deformations.42,43,49 If the tools assure a stable dosemeter position, further patient images and dosemeter reconstructions are not required, hence a less resource-demanding implementation of IVD can be provided.
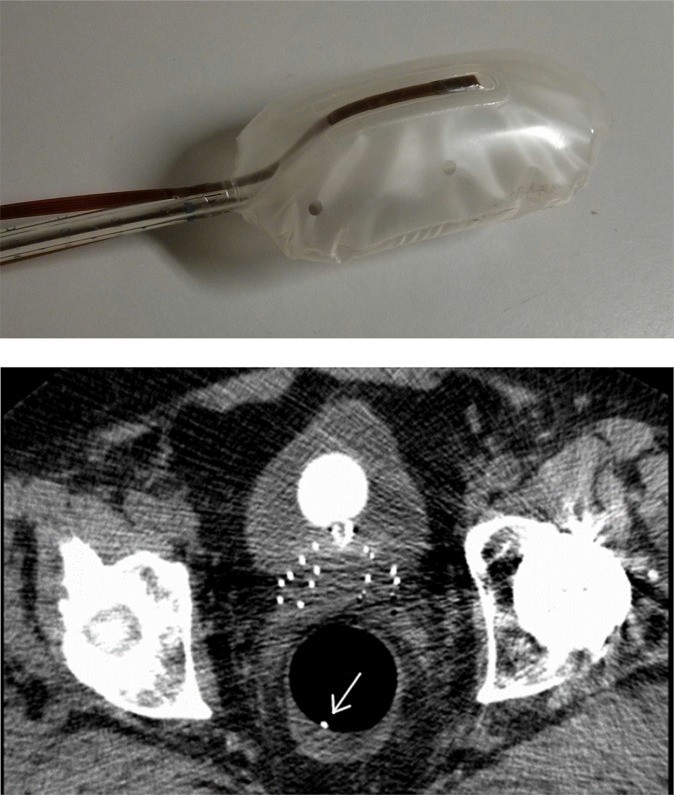
One solution is to embed single-point dosemeters at the surface of endorectal balloons (Figure 3) [Rosenfeld A, University of Wollongong, 2014, personal communication].110,111 The balloons both immobilize the prostate during the treatment and allow for real-time IVD at well-defined points near the rectal wall. The dose rate to the rectal wall can be monitored in real time and compared with the expected dose rates from the TPS based on CT image data sets.
Figure 3.
Top: RadiaDyne rectal balloon with dual-MOSkin™ detectors embedded on the top and bottom of the balloon. Bottom: slice of the CT scan showing needles in prostate and rectum with a rectal balloon with a posterior dual-MOSkin visible on a CT scan (white dot indicated with the arrow). The anterior dual-MOSkin is not visible in that 0.8-mm CT slice.
Hardcastle et al110 performed real-time rectal wall dosimetry during hypothetical prostate intensity-modulated radiotherapy (IMRT) treatments in a phantom using dual face-to-face MOSkins™ (Centre of Medical Radiation Physics, Wollongong, NSW) embedded within the lining of RadiaDyne (RadiaDyne, Houston, TX) endorectal balloon. The measurements revealed that anterior rectal wall doses were 2.6% and 3.2% lower than calculations for 3D conformed radiotherapy and IMRT plans, respectively, which was attributed to limitations of the TPS. Encouraged by the successful application for EBRT, the same dual MOSkin device [Rosenfeld A, University of Wollongong, 2014, personal communication] (Figure 3) was used for prostate immobilization and real-time IVD of the rectal wall for HDR BT patients. The measured anterior rectal wall doses were 13–43% lower than those of the planned ones, whereas the measured posterior rectal wall doses were 1–30% above the planned values. The planned doses were calculated based on reconstructions of the dual-MOSFET positions from 0.8-mm slice CT images. The very large discrepancies originated from patient movements that generated mismatches between the dosemeter positions reconstructed from the CT images and those several hours later during the treatment delivery, as well as from the varying rectal filling status, which could shift the balloon. The problem with a moving dosemeter position may be targeted with alternative endorectal balloon designs or implementation procedures and/or with dosemeter positioning methods presented in the Knowledge of dosimeter position section.
An alternative to dosemeters embedded in endorectal balloons has been presented by Cartwright et al20 (see Multipoint dosimeters section), which incorporates 16 PSDs near the surface of a rectal probe. The performance of the rectal probe has been published for controlled phantom experiments, and its stability with respect to adjacent anatomy would be revealed in clinical implementations.
Inflatable balloons attached to intracavitary applicators have been used during BT in order to increase the distance between the source positions and the bladder and/or rectum.112–114 Similar to the abovementioned application for rectal wall dosimetry, real-time vaginal wall dosimetry at a well-defined point could be facilitated if IVD probes were embedded in the surface of the intracavitary inflatable balloons.
IVD in urethra has been performed with dosemeter probes positioned inside standard urethral catheters.15,19,46 In such IVD procedures, the dosemeter probe is in contact with liquids from the patient, hence substantial sterilization is required to prevent infection. As discussed,11 specially designed urethral catheters with a closed-end lumen dedicated for housing a dosemeter probe would reduce time and resources necessary for proper sterilization of the dosemeter and eliminate the risk of infection. Inspired by IVD probes embedded in endorectal balloons [Rosenfeld A, University of Wollongong, 2014, personal communication],110,111 a dosemeter probe could be moulded within a specially designed urethral catheter in order to allow for IVD in the urethra or at the bladder neck. It should be emphasized that a small outer diameter of the dosemeter probe and a small detector volume are required in order to allow for IVD at the surface of the catheter into which the probe is incorporated. For this reason, several kinds of MOSFETs46,67 and fibre-coupled dosemeters18,19,75,76 are ideal, in contrast to most commercial diodes that have bulky packaging and thus do not facilitate a dosimetry point near the surface of the OAR. The MOSkin detector recently introduced by the Centre for Medical Radiation Physics, University of Wollongong, Wollongong, NSW, Australia, is particularly suitable in this case, given its 0.07-mm water equivalent depth of sensitive volume.115
Since the endorectal balloons incorporated by Hardcastle et al110 and Rosenfeld [Rosenfeld A, University of Wollongong, 2014, personal communication] were air filled, the MOSkin detectors were placed near the boundary of heterogeneity in the medium. The phantom results by Hardcastle et al110 revealed that the measured doses were lower than the TPS calculations, as expected, given that the TPS did not take the heterogeneity into account. The results therefore indicated that it is feasible to perform dosimetry in conditions with strong electronic disequilibrium. However, the dosimetric accuracy of IVD systems at non-equilibrium conditions should be further studied for BT. Alternatively for rectal wall dosimetry, the inflatable balloons discussed in this section could be filled with water, rather than air, in order to avoid non-equilibrium conditions, as was done for the endorectal balloon by Wootton et al.111
It is important to note that the positions of IVD probes inserted into BT source applicators, or embedded in the surface of inflatable balloons attached to intracavitary applicators, are correlated with the applicator positions, hence most dosemeters (with the exception of RADPOS) would not be able to reveal inter- or intrafraction organ–applicator movements, for example, caused by perineal oedema,51 vaginal packing and/or source applicator clamping effects47–49,52 or varying filling status of the rectum and/or bladder.42,43,49 On the other hand, dosemeters inserted into catheters positioned in OARs are minimally correlated with source applicator movements, hence they could allow for the detection of organ–applicator movements. Examples are rectal dosemeter probes or dosemeters embedded in the surface of endorectal balloons or urethral catheters. Furthermore, since IVD in OARs directly monitors the dose to such organs, it provides greater confidence in meeting their dose constraints.
Knowledge of dosemeter position
Any a priori reconstruction method is subject to the risk of compromising effects that invalidate the original dosemeter position. For instance, gas filling in the rectum may shift the position of the endorectal balloon with an embedded dosemeter, [Rosenfeld A, University of Wollongong, 2014, personal communication] and sudden patient movements during treatment delivery may shift the position of the endorectal dosemeter probe.20 Updated information about the dosemeter position with respect to the original reconstruction would therefore be helpful in order to eliminate errors in expected dose calculations owing to the shifted dosemeter positions.
One way to update the dosemeter position is to exploit real-time treatment planning equipment during treatment delivery. For phantom experiments of HDR BT, Tenconi et al23 attached MOSkin detectors to a transrectal ultrasound (TRUS) probe, which was used for treatment planning and dosemeter position reconstruction and remained in place throughout the treatment delivery. With this implementation, sudden positional shifts of the TRUS probe with respect to the patient's anatomy, for example, owing to unexpected changes in posture, could be monitored online during the treatment. If shifts were observed, the MOSkin detector coordinates could be redefined and new reference dose rates calculated.
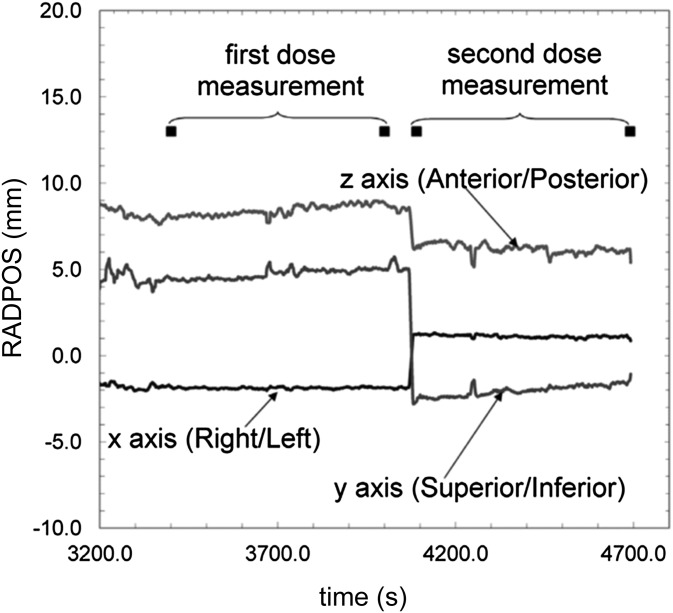
Recently, Cherpak et al46 performed multipoint IVD combined with time-resolved dosemeter positioning inside the urethra during permanent prostate implant BT for 16 patients, using the RADPOS probe (see Detector positioning technology section). The RADPOS was able to indentify prostate movements caused by needle insertion and prostate dosimetry effects caused by the TRUS probe removal. Positional changes of the RADPOS sensor ranging from 1.4 to 9.7 mm were measured owing to removal of the TRUS probe. The maximum integral dose in the prostatic urethra ranged from 89 to 195 Gy, and the measured dose before and after TRUS removal differed from −66% to 36%. The authors concluded that changes in the position of the urethra, including those owing to the removal of the TRUS probe, can be significant, as shown in Figure 4, and should be quantified to evaluate the influence on dose distributions. The in vivo study demonstrated that detector positioning technology allows for real-time dosemeter localization during BT treatments, hence eliminating the reliance on a priori dosemeter reconstructions and drastically reduceing the risk of compromised a priori expected dose rate calculations, for example, owing to unexpected dosemeter shifts.
Figure 4.
The radiation positioning system (RADPOS) position measurements for one patient in the study by Cherpak et al46 taken after completed implantation. Black squares represent the time of the initial and final metal-oxide-semiconductor-field-effect-transistor readings taken for the first measurement period [with transrectal ultrasound (TRUS) probe in place] and the second measurement period (after the TRUS probe was removed). The change in position from the removal of the probe can be seen in all coordinates at approximately t = 4100 s. Reproduced from Cherpak et al46 with permission from Elsevier.
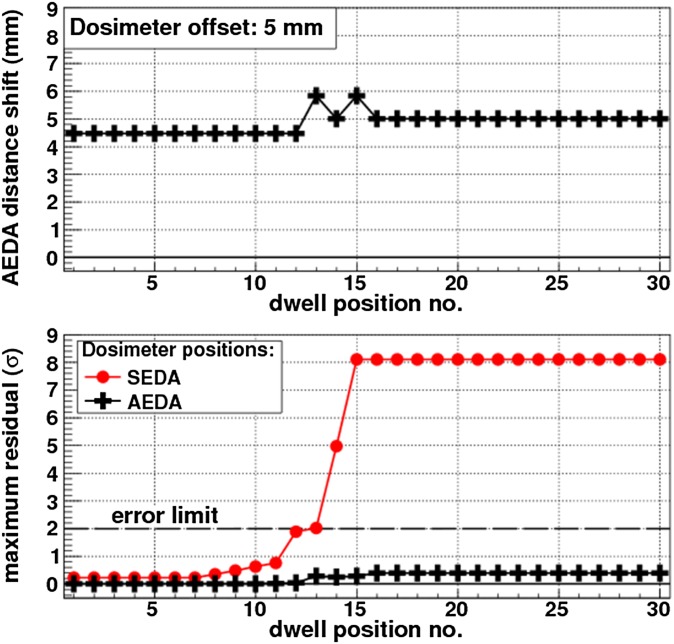
Kertzscher et al21 performed real-time IVD with fibre-coupled Al2O3:C single point dosimetry79 during PDR BT of locally advanced cervical cancer that included tandem-ring and needle applicators. Comparisons between measured and planned dose rates revealed large discrepancies, which indicated that the a priori dosemeter position reconstruction had been compromised. The dosemeter shifts generated false alarms when an error detection algorithm was used that relied on a priori reconstructions,14,25 referred to as a static error detection algorithm (SEDA). The problem was solved with an adaptive error detection algorithm (AEDA). Rather than relying on a priori reconstructions, the AEDA determined the dosemeter position in a data-driven approach, in which the level of correspondence between dose rate distributions of the measurement and calculations of simulated alternative dosemeter positions were quantified. The alternative position that yielded the highest correspondence provided the most likely dosemeter position. The AEDA yielded the most accurate dosemeter coordinates, which were then used for the reference calculations in order to evaluate the treatment. The in vivo implementation demonstrated that the AEDA correctly declared false errors generated when the dosemeter probe had been misreconstructed, whereas the SEDA erroneously declared true errors. Computer-simulated true error scenarios corresponding to guide tube swap errors and individual needle shifts demonstrated that the AEDA had high error detection efficiency and in contrast to the SEDA, eliminated all simulated false errors represented by mispositioned dosemeters (Figure 5). Furthermore, the AEDA provided geometrical feedback, which indicated potential small systematic reconstruction variations in the treatment plan. In summary, the AEDA offers improved guidance in decision-making in the event of potential errors detected with IVD, since it is independent from potential systematic errors of detector positioning technology and it eliminates false errors related to inaccurate a priori dosemeter reconstructions, for example, owing to poor image quality or dosemeter shifts caused by patient movements during transfer between various locations in the hospital.
Figure 5.
Example of adaptive error detection algorithm (AEDA) and static error detection algorithm (SEDA) responses to a simulated false error treatment case, where the dosemeter was shifted by 5 mm. The top plot shows the accurate geometric feedback of the most viable accurate dosemeter position provided by the AEDA, the AEDA distance shift. The bottom plot shows that the maximum residual from the AEDA response does not break the statistical error criterion, whereas that from the SEDA does. The maximum residual is expressed in units of one standard deviation of the total uncertainty budget. Reproduced from Kertzscher et al21 with permission from American Association of Physicists in Medicine.
Automation
The automation of manual IVD procedures could substantially reduce the extra workload and potential interference with the clinical workflow. For instance, resources required by the clinical staff could be minimized if a dosemeter probe was already embedded in the BT source applicator or even eliminated if the afterloader incorporated a thin dosemeter probe in a dedicated stepping motor such that the probe could be inserted into source catheters in an automated procedure during the treatment.
Further IVD developments and in vivo feasibility studies could be encouraged if a direct communication with the afterloader control system was provided. Such a communication would send an indication signal for the start time of each dwell position in order to assure that the measured and planned dose rates were in phase, and thus would not require visual inspections of the control plots. That way, the indication signal would allow for straightforward developments of fully automated IVD software that were robust against treatment interruptions. Furthermore, if the digital imaging and communications in medicine (DICOM) files from the TPS were exported independently to the afterloader and the IVD data acquisition system, a communication line would also allow for an assessment of the treatment plans prior to treatment delivery. If a discrepancy was noticed, it meant that a wrong or defective treatment plan had been exported to the afterloader or the IVD system.
Sensitivity and specificity
The confidence levels of the treatment status messages provided by the error detection system can be expressed in terms of the sensitivity and specificity. These statistical measures provide the proportions of correctly identified positives (sensitivity) and correctly identified negatives (specificity). Ideally, an error detection system would be able to identify all errors (100% sensitivity) and not generate any false errors (100% specificity). However, as found by Therriault-Proulx et al,17 the numbers of false errors increased when the fixed discrepancy criterion decreased from 20% to 3% (see Fixed discrepancy criterion section), that is, when the sensitivity increased. The results demonstrate the relation of sensitivity and specificity with Type I and Type II errors, which quantify the false alarm rate and the inability to detect errors, respectively. Similar results have been obtained by Reniers et al.22 A thorough assessment of the sensitivity and specificity measures for the error detection system implemented for IVD would raise the confidence in the decision-making in the clinic. Such assessments would also allow for an improved understanding of the error detection system and the error criteria implemented (see Error detection criteria section) and facilitate comparisons between IVD systems.
Future directions
Widespread IVD implementation is hampered partially owing to the resources required in order to operate current dosimetry systems and the related training of clinical personnel. Furthermore, since the nature of BT treatment errors and their occurrence rates are not well known, BT practitioners are not necessarily convinced that treatment incidents may occur in their clinics. Wider acceptance for IVD among BT practitioners could be obtained if straightforward state-of-the-art IVD technology (see Novel dosimetry technology section) was more available and if solutions to current challenges for IVD (see Some solutions to main challenges section) were fully incorporated.
Table 1 provides the important aspects for IVD and several of the advantages that the state-of-the-art systems can provide for BT. Although each aspect could be further developed, the major challenge for IVD is to combine them into a packaged solution. The goal with a packaged solution would be to provide QA for the BT treatment and the capability to detect as many treatment error types as possible. A comprehensive list of the role of IVD for QA and error detection is provided in Table 1 by Tanderup et al.11 As a complement, Table 2 lists the role for QA and error detection of IVD and imaging, summarizes known levels of uncertainty and provides likelihood estimates of the error types and their related effects. Since there is no systematic overview of the incidence and effects of different BT errors, the scoring of the BT errors have been made according to the authors' best estimate. Current QA strategies for treatment error prevention and uncertainty limitation include manual checklists, and in some advanced settings, real-time or near-real-time imaging, which can result in online treatment modifications, such as catheter repositioning or treatment plan modifications. Real-time imaging has the advantage of addressing intra- and interfraction uncertainties, which is the major uncertainty component in BT, while it in general is challenging to use IVD to comprehensively measure the dose to an entire organ or tumour. However, as indicated in Table 2, real-time imaging is not capable of detecting a range of error scenarios. Since several error scenarios can be detected with IVD, real-time imaging and IVD represent complementary strategies for quality control in BT, and both have strong potentials for future developments. The combined use of real-time imaging and IVD in large patient studies could therefore potentially help in minimizing BT treatment uncertainty levels and allow for systematic overviews of the probabilities and effects of error scenarios (see further discussion below).
Table 1.
Important aspects for in vivo dosimetry (IVD) and their main advantages
| Essential aspects for IVD | Aim | Existing developments | Further developments |
|---|---|---|---|
| Target/OAR dosimetry | Dose monitoring; error detection; QA of treatment plan; dose escalation control | Dose monitoring; gross error detection | Improvement of dosimetric accuracy of IVD in target/OAR |
| Sterilization | Eliminate risk for infection | In-house solutions for sterilization of catheters that contain dosemeter probes | Integrated IVD catheters in source applicators and IVD catheters dedicated for specific anatomical sites |
| Spatial accuracy | Fixate dosemeter with respect to target, OAR or source applicator; stabilize anatomical region; maximize dosimetric accuracy | Dosemeter probes embedded into endorectal balloons | Develop specialized equipment for specific anatomical sites |
| Identification of source position | Error detection; geometric feedback for brachytherapy source; facilitate independent catheter reconstruction | Multipoint dosemeters | Investigate full in vivo potential; optimize and adapt various existing algorithms (see Detector positioning technology section) |
| Identification of dosemeter position | Validate calculated reference dose rates; identify target, OAR or source applicator movements; geometric feedback for dosemeter; QA of treatment planning system reconstruction | Detector positioning technology; adaptive error detection algorithm | Further in vivo performance studies |
| Statistical error criterion | Improve sensitivity and specificity for error detection algorithms | Comparisons between measured and planned dose rates or source positions, considering all known sources of uncertainty | Thorough sensitivity and specificity assessments; further studies of uncertainties for IVD |
| Automation | Minimize resource requirements and interference with clinical workflow | None available | Afterloaded dosemeter; communication line between afterloader and IVD system |
OAR, organ at risk; QA, quality assurance.
Existing developments provided in this article are mentioned, and suggestions for further developments provided.
Table 2.
Error detection capacities (detectability) of real-time in vivo dosimetry (IVD) and real-time imaging, uncertainty levels of each error scenario (quality item), and the probabilities and effects of the error scenarios
| Quality item | Detectability |
Uncertainty level5,40 | Error probability and effect (authors' opinion) |
||
|---|---|---|---|---|---|
| Real-time IVD | Real-time imaging | Probability of error | Effecta | ||
| Source calibration | ✔b | 2% | Low | Low–high | |
| Afterloader source positioning and dwell time (non-patient specific)c | ✔d | 4% | – | – | |
| Afterloader malfunction | ✔d | – | Low | Low–high | |
| Patient identification | ✔e | – | Low | High | |
| Correct treatment plan | ✔f | – | Low | High | |
| Intra- and interfraction organ/applicator movementc | ✔d,g | ✔ | 10–25% for organs at risk; >10–25% for target if high-dose-rate needle movements uncorrected | – | – |
| Applicator reconstruction and fusion errors | ✔d,h | ✔ | 4% | Intermediate | Low–intermediate |
| Applicator length/source indexer length | ✔d,i | – | Intermediate | Low–high | |
| Source step size (patient specific) | ✔d,i | – | Low | High | |
| Interchanged guide tubes | ✔d | – | Intermediate | Low–high | |
| Recording of dosec | ✔j | 3–5% | – | – | |
The table is a complement to Table 1 by Tanderup et al.11 The uncertainty levels are taken from Tanderup et al5 and Kirisits et al.40 The probabilities and effects were estimated by the authors.
Refers to the potential effect on dose administration.
If the dosemeter calibration is independent from the actual source.
Quality item that corresponds to an uncertainty in the treatment, which is always present and therefore does not have an occurrence likelihood, rather than an error.
Quality items for which detectability strongly would benefit from two or more point detectors, as stated or indicated in Andersen et al,14 Therriault-Proulx et al,17,18 Cartwright et al,20 Kertzscher et al,21 Reniers et al,22 Kertzscher et al,25 Cherpak et al46 and Nakano et al.99
Wrong patient identification could be detected, for example, by comparing the patient's anatomy acquired during the treatment with the anatomy from the treatment plan.
If digital imaging and communications in medicine (DICOM) was exported independently to the afterloader and the IVD data acquisition system (see second paragraph in the Automation section.).
If IVD dosemeter probe is not attached to source applicator (see last paragraph in the Dosemeter placement tools section).
May be detected with source localization and applicator reconstruction (see Source localization and real-time applicator reconstruction section), dedicated algorithms for real-time IVD (see fourth paragraph in the Knowledge of dosemeter position section) and positioning technology (see third paragraph in the Knowledge of dosemeter position section).
May be detected by means of comparisons between measured and expected dose rates or source positions.
Relevant if treatment planning system dose calculations are inaccurate, for example, if significant tissue heterogeneity and/or dose field perturbation effects are insufficiently accounted for by TPS (see seventh paragraph in Dosimeter placement tools section).
Several important issues could be targeted if more BT clinics implemented IVD in their treatment workflow on a routine basis:
the capacity of the state-of-the-art dosimetry systems in the in vivo environment in terms of error detection and online in vivo QA
potential limitations of IVD systems and necessary areas of improvements in terms of technological developments, accuracy, practicality, performance of algorithms, implementation tools and methodologies and user friendly software
improved knowledge about BT treatment errors and their occurrence rates
the highlighting of potential changes or improvements necessary to the clinical infrastructure and treatment procedures, for example, via incidence learning database structures,116 which would reduce uncertainties and systematic variability in BT
increased reporting of treatment errors and uncertainties for the BT community.
A comprehensive cost–benefit study is required to provide a clear assessment of the necessity of IVD for BT. Conducting such a study requires collaborations between multiple clinics, which implemented routine IVD and targeted the abovementioned issues. Such a multicentre study would likely have a large impact on the industry, since it relies on adaptation of the state-of-the-art technology (see Novel dosimetry technology section) into commercial products (see Some solutions to main challenges section).
One potential and useful tool for a detailed analysis of the data collected in a cost–benefit study is the adaptation of failure modes and effects analysis (FMEA). Swamidas et al117 and Wilkinson and Kolar118 have suggested the introduction of FMEA into BT. FMEA is a quality management and risk assessment tool that identifies the steps in a system process, for example, treatment planning procedure, that have the highest likelihood for failure or injury.119 Wilkinson and Kolar118 performed FMEA for treatment planning of HDR BT and quantified the likelihood for failure or injury within the following categories: imaging/registration, catheter reconstruction, dwell position activity, dose points/normalization, optimization/dose and evaluation. The study resulted in some discrepancies between the FMEA results and nationally reported failures, which indicated that failure reporting was insufficient.
Since current real-time IVD is capable of online verification of the agreement between planned and delivered treatments and the monitoring of BT treatment parameters, such as OAR doses, IVD could provide with input parameters into an FMEA study. Provided an open and honest reporting of treatment outcomes, IVD can play an important role in a systematic approach to quantify the risks related to several aspects of BT treatments. Detailed risk evaluations with FMEA could ultimately guide the focus for improvement of various aspects of BT treatments, for example, by comparing clinical workflow routines and identifying aspects that pose higher risks than others. Furthermore, a quantification of risks and failure modes based on abundant IVD data could potentially improve the understanding of effects seen in retrospective BT studies that are currently not well understood.
CONCLUSIONS
The past decade has witnessed significant progress with regard to IVD detectors and demonstration that real-time dosimetry is possible. However, these new developments have not yet translated into improved clinical routine, and there is currently considerable potential to make progress with regard to increased utilization of IVD for error detection. The prerequisite for broad dissemination of routine IVD is that the error detection sensitivity and specificity is significantly improved while the workload is kept at a level that makes IVD cost effective. In this context, the major issues to address are: control of detector positioning, development of error detection algorithms and improved and automated workflow. The perspective of addressing these challenges is to make independent treatment monitoring possible in a large number of patients.
FUNDING
SB was partially supported by the National Cancer Institute through an R01 grant (CA120198-01A2). AR was supported by The Australian National Health and Medical Research Cancel, grants NN1000557 and 573428. The work of KT and GK has been funded by The Danish Cancer Society and CIRRO—The Lundbeck Foundation Center for Interventional Research in Radiation Oncology and The Danish Council for Strategic Research.
Acknowledgments
ACKNOWLEDGMENTS
The authors would like to thank Claus E. Andersen, Centre for Nuclear Technologies, Technical University of Denmark, for his support and Ola Holmberg, International Atomic Energy Agency, for helpful discussions regarding the current status of error reporting in radiotherapy. AR would like to thank Dean Cutajar, Marco Petasecca, Michael Lerch and Michael Weaver, Centre for Medical Radiation Physics, University of Wollongong, Wollongong, NSW, Australia; Joseph Bucci, St George Cancer Care Centre, Sydney, Australia; Mauro Carrera et al, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy; and Jan Jakubek and Stanislav Pospisil et al, Institute of Experimental and Applied Physics, Czech Technical University, Prague Czech Republic; and Vladimir Perevertaylo, SPA BIT, for their contributions.
REFERENCES
- 1.Lopez PO, Andreo P, Cosset J-M, Dutreix A, Landberg T. Prevention of accidental exposures to patients undergoing radiation therapy. ICRP Publications 86, Annals of the ICRP. New York, NY: Permagon; 2000. [Google Scholar]
- 2.Ashton LP, Cosset J-M, Levin V, Martinez A, Nag S. Prevention of high-dose-rate brachytherapy accidents. ICRP Publications 97, Annals of the ICRP. New York, NY: Permagon; 2004. [Google Scholar]
- 3.IAEA Safety Report Series 17. Lessons learned from accidental exposures in radiotherapy. Vienna, Austria: IAEA. IAEA Safety Reports Series; 2000. [Google Scholar]
- 4.Radiotherapy risk profile. Geneva, Switzerland: WHO Press; 2008. [Google Scholar]
- 5.Tanderup K, Nesvacil N, Pötter R, Kirisits C. Uncertainties in image guided adaptive cervix cancer brachytherapy: impact on planning and prescription. Radiother Oncol 2013; 107: 1–5. [DOI] [PubMed] [Google Scholar]
- 6.Chambrette V, Hardy S, Nenot J-C. Irradiation accidents. Establishment of a data base “ACCIRAD” at the IPSN [in French]. Radioprotection 2001; 36: 477–510. [Google Scholar]
- 7.Cunningham J, Coffey M, Knöös T, Holmberg O. Radiation Oncology Safety Information System (ROSIS)–profiles of participants and the first 1074 incident reports. Radiother Oncol 2010; 97: 601–7. doi: 10.1016/j.radonc.2010.10.023 [DOI] [PubMed] [Google Scholar]
- 8.Holmberg O, Malone J, Rehani M, McLean D, Czarwinski R. Current issues and actions in radiation protection of patients. Eur J Radiol 2010; 76: 15–19. doi: 10.1016/j.ejrad.2010.06.033 [DOI] [PubMed] [Google Scholar]
- 9.IAEA. Radiation protection of patients (RPOP). Safety in Radiation Oncology - SAFRON [cited 2 March 2014]. Available from: https://rpop.iaea.org/RPOP/RPoP/Modules/login/safron-register.htm
- 10.IAEA. Radiation protection of patients (RPOP). Accident Prevention [Internet] [cited 2 March 2014]. Available from: https://rpop.iaea.org/RPOP/RPoP/Content/InformationFor/HealthProfessionals/2_Radiotherapy/AccidentPrevention.htm
- 11.Tanderup K, Beddar S, Andersen CE, Kertzscher G, Cygler JE. In vivo dosimetry in brachytherapy. Med Phys 2013; 40: 1–15. doi: 10.1118/1.4810943 [DOI] [PubMed] [Google Scholar]
- 12.Waldhäusl C, Wambersie A, Pötter R, Georg D. In-vivo dosimetry for gynaecological brachytherapy: physical and clinical considerations. Radiother Oncol 2005; 77: 310–17. [DOI] [PubMed] [Google Scholar]
- 13.Tanderup K, Christensen JJ, Granfeldt J, Lindegaard JC. Geometric stability of intracavitary pulsed dose rate brachytherapy monitored by in vivo rectal dosimetry. Radiother Oncol 2006; 79: 87–93. doi: 10.1016/j.radonc.2006.02.016 [DOI] [PubMed] [Google Scholar]
- 14.Andersen CE, Nielsen SK, Lindegaard JC, Tanderup K. Time-resolved in vivo luminescence dosimetry for online error detection in pulsed dose-rate brachytherapy. Med Phys 2009; 36: 5033–43. [DOI] [PubMed] [Google Scholar]
- 15.Cygler JE, Saoudi A, Perry G, Morash C, E C. Feasibility study of using MOSFET detectors for in vivo dosimetry during permanent low-dose-rate prostate implants. Radiother Oncol 2006; 80: 296–301. doi: 10.1016/j.radonc.2006.07.008 [DOI] [PubMed] [Google Scholar]
- 16.Zhang R, Gladstone DJ, Jarvis LA, Strawbridge RR, Jack Hoopes P, Friedman OD, et al. Real-time in vivo Cherenkoscopy imaging during external beam radiation therapy. J Biomed Opt 2013; 18: 1–3. doi: 10.1117/1.JBO.18.11.110504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Therriault-Proulx F, Briere TM, Mourtada F, Aubin S, Beddar S, Beaulieu L. A phantom study of an in vivo dosimetry system using plastic scintillation detectors for real-time verification of 192Ir HDR brachytherapy. Med Phys 2011; 38: 2542–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Therriault-Proulx F, Beddar S, Beaulieu L. On the use of a single-fiber multipoint plastic scintillation detector for 192Ir high-dose-rate brachytherapy. Med Phys 2013; 40: 1–10. doi: 10.1118/1.4803510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suchowerska N, Jackson M, Lambert J, Yin YB, Hruby G, McKenzie DR. Clinical trials of a urethral dose measurement system in brachytherapy using scintillation detectors. Int J Radiat Oncol Biol Phys 2011; 79: 609–15. doi: 10.1016/j.ijrobp.2010.03.030 [DOI] [PubMed] [Google Scholar]
- 20.Cartwright LE, Suchowerska N, Yin Y, Lambert J, Haque M, McKenzie DR. Dose mapping of the rectal wall during brachytherapy with an array of scintillation dosimeters. Med Phys 2010; 37: 2247–55. [DOI] [PubMed] [Google Scholar]
- 21.Kertzscher G, Andersen CE, Tanderup K. Adaptive error detection for HDR/PDR brachytherapy: guidance for decision making during real-time in-vivo point dosimetry. Med Phys 2014; 41: 1–11. [DOI] [PubMed] [Google Scholar]
- 22.Reniers B, Landry G, Eichner R, Hallil A, Verhaegen F. In vivo dosimetry for gynaecological brachytherapy using a novel position sensitive radiation detector: feasibility study. Med Phys 2012; 39: 1925–35. [DOI] [PubMed] [Google Scholar]
- 23.Tenconi C, Carrara M, Borroni M, Cerrotta A, Cutajar D, Petasecca M, et al. TRUS-probe integrated MOSkin detectors for rectal wall in vivo dosimetry in HDR brachytherapy: in phantom feasibility study. Radiat Meas 2014; in press. [Google Scholar]
- 24.Rosenfeld AB, Cutajar DL, Lerch MLF, Takacs GJ, Brady J, Braddock T, et al. In vivo dosimetry and seed localization in prostate brachytherapy with permanent implants. IEEE Trans Nucl Sci 2004; 51: 3013–18. [Google Scholar]
- 25.Kertzscher G, Andersen CE, Siebert F-A, Nielsen SK, Lindegaard JC, Tanderup K. Identifying afterloading PDR and HDR brachytherapy errors using real-time fiber-coupled Al(2)O(3):C dosimetry and a novel statistical error decision criterion. Radiother Oncol 2011; 100: 456–62. doi: 10.1016/j.radonc.2011.09.009 [DOI] [PubMed] [Google Scholar]
- 26.DeWerd LA, Ibbott GS, Meigooni AS, Mitch MG, Rivard MJ, Stump KE, et al. A dosimetric uncertainty analysis for photon-emitting brachytherapy sources: report of AAPM Task Group No. 138 and GEC-ESTRO. Med Phys 2011; 38: 782–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rivard MJ, Coursey BM, DeWerd LA, Hanson WF, Saiful Huq M, Ibbott GS, et al. Update of AAPM Task Group No. 43 Report: a revised AAPM protocol for brachytherapy dose calculations. Med Phys 2004; 31: 633–74. [DOI] [PubMed] [Google Scholar]
- 28.Kirisits C, Siebert F-A, Baltas D, De Brabandere M, Hellebust TP, Berger D, et al. Accuracy of volume and DVH parameters determined with different brachytherapy treatment planning systems. Radiother Oncol 2007; 84: 290–7. doi: 10.1016/j.radonc.2007.06.010 [DOI] [PubMed] [Google Scholar]
- 29.Hellebust TP, Tanderup K, Lervåg C, Fidarova E, Berger D, Malinen E, et al. Dosimetric impact of interobserver variability in MRI-based delineation for cervical cancer brachytherapy. Radiother Oncol 2013; 107: 13–19. doi: 10.1016/j.radonc.2012.12.017 [DOI] [PubMed] [Google Scholar]
- 30.Petrič P, Hudej R, Rogelj P, Blas M, Tanderup K, Fidarova E, et al. Uncertainties of target volume delineation in MRI guided adaptive brachytherapy of cervix cancer: a multi-institutional study. Radiother Oncol 2013; 107: 6–12. [DOI] [PubMed] [Google Scholar]
- 31.Hellebust TP, Tanderup K, Bergstrand ES, Knutsen BH, Røislien J, Olsen DR. Reconstruction of a ring applicator using CT imaging: impact of the reconstruction method and applicator orientation. Phys Med Biol 2007; 52: 4893–904. doi: 10.1088/0031-9155/52/16/012 [DOI] [PubMed] [Google Scholar]
- 32.Tanderup K, Hellebust TP, Lang S, Granfeldt J, Pötter R, Lindegaard JC, et al. Consequences of random and systematic reconstruction uncertainties in 3D image based brachytherapy in cervical cancer. Radiother Oncol 2008; 89: 156–63. doi: 10.1016/j.radonc.2008.06.010 [DOI] [PubMed] [Google Scholar]
- 33.De Leeuw AA, Moerland MA, Nomden C, Tersteeg RH, Roesink JM, Jürgenliemk-Schulz IM. Applicator reconstruction and applicator shifts in 3D MR-based PDR brachytherapy of cervical cancer. Radiother Oncol 2009; 93: 341–6. doi: 10.1016/j.radonc.2009.05.003 [DOI] [PubMed] [Google Scholar]
- 34.Haack S, Nielsen SK, Lindegaard JC, Gelineck J, Tanderup K. Applicator reconstruction in MRI 3D image-based dose planning of brachytherapy for cervical cancer. Radiother Oncol 2009; 91: 187–93. doi: 10.1016/j.radonc.2008.09.002 [DOI] [PubMed] [Google Scholar]
- 35.Wills R, Lowe G, Inchley D, Anderson C, Beenstock V, Hoskin P. Applicator reconstruction for HDR cervix treatment planning using images from 0.35 T open MR scanner. Radiother Oncol 2010; 94: 346–52. [DOI] [PubMed] [Google Scholar]
- 36.Haie-Meder C, Pötter R, Van Limbergen E, Briot E, De Brabandere M, Dimopoulos J, et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I): concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol 2005; 74: 235–45. [DOI] [PubMed] [Google Scholar]
- 37.Hellebust TP, Kirisits C, Berger D, Pérez-Calatayud J, De Brabandere M, De Leeuw A, et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group: considerations and pitfalls in commissioning and applicator reconstruction in 3D image-based treatment planning of cervix cancer brachytherapy. Radiother Oncol 2010; 96: 153–60. [DOI] [PubMed] [Google Scholar]
- 38.Beaulieu L, Carlsson Tedgren A, Carrier J-F, Davis SD, Mourtada F, Rivard MJ, et al. Report of the Task Group 186 on model-based dose calculation methods in brachytherapy beyond the TG-43 formalism: current status and recommendations for clinical implementation. Med Phys 2012; 39: 6208–36. doi: 10.1118/1.4747264 [DOI] [PubMed] [Google Scholar]
- 39.Rivard MJ, Venselaar JL, Beaulieu L. The evolution of brachytherapy treatment planning. Med Phys 2009; 36: 2136–53. [DOI] [PubMed] [Google Scholar]
- 40.Kirisits C, Rivard MJ, Baltas D, Ballester F, De Brabandere M, van der Laarse R, et al. Review of clinical brachytherapy uncertainties: analysis guidelines of GEC-ESTRO and the AAPM. Radiother Oncol 2014; 110: 199–212. doi: 10.1016/j.radonc.2013.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nesvacil N, Tanderup K, Hellebust TP, De Leeuw A, Lang S, Mohamed S, et al. A multicentre comparison of the dosimetric impact of inter- and intra-fractional anatomical variations in fractionated cervix cancer brachytherapy. Radiother Oncol 2013; 107: 20–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buchali A, Koswig S, Dinges S, Rosenthal P, Salk J, Lackner G, et al. Impact of the filling status of the bladder and rectum on their integral dose distribution and the movement of the uterus in the treatment planning of gynaecological cancer. Radiother Oncol 1999; 52: 29–34. [DOI] [PubMed] [Google Scholar]
- 43.Hellebust TP, Dale E, Skjønsberg A, Rune DR. Inter fraction variations in rectum and bladder volumes and dose distributions during high dose rate brachytherapy treatment of the uterine cervix investigated by repetitive CT-examinations. Radiother Oncol 2001; 60: 273–80. [DOI] [PubMed] [Google Scholar]
- 44.Holloway CL, Racine M-L, Cormack RA, O’Farrell DA, Viswanathan AN. Sigmoid dose using 3D imaging in cervical-cancer brachytherapy. Radiother Oncol 2009; 93: 307–10. doi: 10.1016/j.radonc.2009.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jamema SV, Mahantshetty U, Tanderup K, Malvankar D, Sharma S, Engineer R, et al. Inter-application variation of dose and spatial location of D(2cm(3)) volumes of OARs during MR image based cervix brachytherapy. Radiother Oncol 2013; 107: 58–62. doi: 10.1016/j.radonc.2013.01.011 [DOI] [PubMed] [Google Scholar]
- 46.Cherpak AJ, Cygler JE, E C, Perry G. Real-time measurement of urethral dose and position during permanent seed implantation for prostate brachytherapy. Brachytherapy 2013; 13: 169–77. doi: 10.1016/j.brachy.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 47.Hoskin PJ, Cook M, Bouscale D, Cansdale J. Changes in applicator position with fractionated high dose rate gynaecological brachytherapy. Radiother Oncol 1996; 40: 59–62. [DOI] [PubMed] [Google Scholar]
- 48.Kim RY, Meyer JT, Spencer SA, Meredith RF, Jennelle RLS, Salter MM. Major geometric variations between intracavitary applications in carcinoma of the cervix: high dose rate vs. low dose rate. Int J Radiat Oncol 1996; 35: 1035–8. [DOI] [PubMed] [Google Scholar]
- 49.Bahena JH, Martinez A, Yan D, Mele E, Edmunson G, Brown D, et al. Spatial reproducibility of the ring and tandem high-dose rate cervix applicator. Int J Radiat Oncol Biol Phys 1998; 41: 13–19. [DOI] [PubMed] [Google Scholar]
- 50.Datta NR, Kumar S, Das KJ, Pandey CM, Halder S, Ayyagari S. Variations of intracavitary applicator geometry during multiple HDR brachytherapy insertions in carcinoma cervix and its influence on reporting as per ICRU report 38. Radiother Oncol 2001; 60: 15–24. [DOI] [PubMed] [Google Scholar]
- 51.Hoskin PJ, Bownes PJ, Ostler P, Walker K, Bryant L. High dose rate afterloading brachytherapy for prostate cancer: catheter and gland movement between fractions. Radiother Oncol 2003; 68: 285–8. [DOI] [PubMed] [Google Scholar]
- 52.Wulf J, Popp K, Oppitz U, Baier K, Flentje M. Positional variability of a tandem applicator system in HDR brachytherapy for primary treatment of cervix cancer. Analysis of the anatomic pelvic position and comparison of the applicator positions during five insertions. Strahlenther Onkol 2004; 180: 216–24. [DOI] [PubMed] [Google Scholar]
- 53.Beriwal S, Kim H, Coon D, Mogus R, Heron DE, Li X, et al. Single magnetic resonance imaging vs magnetic resonance imaging/computed tomography planning in cervical cancer brachytherapy. Clin Oncol (R Coll Radiol) 2009; 21: 483–7. doi: 10.1016/j.clon.2009.03.007 [DOI] [PubMed] [Google Scholar]
- 54.Anderson C, Lowe G, Wills R, Inchley D, Beenstock V, Bryant L, et al. Critical structure movement in cervix brachytherapy. Radiother Oncol 2013; 107: 39–45. doi: 10.1016/j.radonc.2013.01.006 [DOI] [PubMed] [Google Scholar]
- 55.Dinkla AM, Pieters BR, Koedooder K, Meijnen P, van Wieringen N, van der Laarse R, et al. Deviations from the planned dose during 48 hours of stepping source prostate brachytherapy caused by anatomical variations. Radiother Oncol 2013; 107: 106–11. doi: 10.1016/j.radonc.2012.12.011 [DOI] [PubMed] [Google Scholar]
- 56.Lang S, Nesvacil N, Kirisits C, Georg P, Dimopoulos JC, Federico M, et al. Uncertainty analysis for 3D image-based cervix cancer brachytherapy by repetitive MR imaging: assessment of DVH-variations between two HDR fractions within one applicator insertion and their clinical relevance. Radiother Oncol 2013; 107: 26–31. [DOI] [PubMed] [Google Scholar]
- 57.Rey F, Chang C, Mesina C, Dixit N, Kevin Teo B-K, Lin LL. Dosimetric impact of interfraction catheter movement and organ motion on MRI/CT guided HDR interstitial brachytherapy for gynecologic cancer. Radiother Oncol 2013; 107: 112–16. doi: 10.1016/j.radonc.2012.12.013 [DOI] [PubMed] [Google Scholar]
- 58.Pötter R, Haie-Meder C, Van Limbergen E, Barillot I, De Brabandere M, Dimopoulos J, et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol 2006; 78: 67–77. [DOI] [PubMed] [Google Scholar]
- 59.Dimopoulos JCA, Petrow P, Tanderup K, Petric P, Berger D, Kirisits C, et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (IV): basic principles and parameters for MR imaging within the frame of image based adaptive cervix cancer brachytherapy. Radiother Oncol 2012; 103: 113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cygler JE, Tanderup K, Beddar S, Perez-Calatayud J. In vivo dosimetry in brachytherapy. In: Comprehensive brachytherapy: physical and clinical aspects. Boca Raton, FL: Taylor & Francis; 2012. pp. 379–96. [Google Scholar]
- 61.Baltas D, Sakelliou L, Zamboglou N. The physics of modern brachytherapy for oncology. Boca Raton, FL: Taylor & Francis Group; 2007. [Google Scholar]
- 62.Seymour EL, Downes SJ, Fogarty GB, Izard MA, Metcalfe P. In vivo real-time dosimetric verification in high dose rate prostate brachytherapy. Med Phys 2011; 38: 4785–94. [DOI] [PubMed] [Google Scholar]
- 63.Allahverdi M, Sarkhosh M, Aghili M, Jaberi R, Adelnia A, Geraily G. Evaluation of treatment planning system of brachytherapy according to dose to the rectum delivered. Radiat Prot Dosimetry 2012; 150: 312–15. doi: 10.1093/rpd/ncr415 [DOI] [PubMed] [Google Scholar]
- 64.Alecu R, Alecu M. In-vivo rectal dose measurements with diodes to avoid misadministrations during intracavitary high dose rate brachytherapy for carcinoma of the cervix. Med Phys 1999; 26: 768–70. [DOI] [PubMed] [Google Scholar]
- 65.Bloemen-van Gurp EJ, Murrer LH, Haanstra BK, van Gils FC, Dekker AL, Mijnheer BJ, et al. In vivo dosimetry using a linear Mosfet-array dosimeter to determine the urethra dose in 125I permanent prostate implants. Int J Radiat Oncol Biol Phys 2009; 73: 314–21. doi: 10.1016/j.ijrobp.2008.08.040 [DOI] [PubMed] [Google Scholar]
- 66.Bloemen-van Gurp EJ, Haanstra BK, Murrer LH, van Gils FC, Dekker AL, Mijnheer BJ, et al. In vivo dosimetry with a linear MOSFET array to evaluate the urethra dose during permanent implant brachytherapy using iodine-125. Int J Radiat Oncol Biol Phys 2009; 75: 1266–72. doi: 10.1016/j.ijrobp.2009.04.042 [DOI] [PubMed] [Google Scholar]
- 67.Gambarini G, Carrara M, Tenconi C, Mantaut N, Borroni M, Cutajar D, et al. Online in vivo dosimetry in high dose rate prostate brchytherapy with MOSkin detectors: in phantom feasibility study. Appl Radiat Isot 2014; 83: 222–6. doi: 10.1016/j.apradiso.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 68.Qi Z-Y, Deng X-W, Cao X, Huang S-M, Lerch M, Rosenfeld A. A real-time in vivo dosimetric verification method for high-dose rate intracavitary brachytherapy of nasopharyngeal carcinoma. Med Phys 2012; 39: 6757–63. doi: 10.1118/1.4758067 [DOI] [PubMed] [Google Scholar]
- 69.Van Dam J, Leunens G, Dutreix A. Correlation between temperature and dose rate dependence of semiconductor response; influence of accumulated dose. Radiother Oncol 1990; 19: 345–51. [DOI] [PubMed] [Google Scholar]
- 70.Grussel E, Rikner G. Evaluation of temperature effects in p-type silicon detectors. Phys Med Biol 1986; 31: 527–34. [Google Scholar]
- 71.Buehler MG, Blaes BR. On-chip p-MOSFET dosimetry. IEEE Trans Nucl Sci 1993; 40: 1442–9. [Google Scholar]
- 72.Soubra M, Cygler JE, Mackay G. Evaluation of a dual bias dual metal oxide-silicon semiconductor field effect transistor detector as radiation dosimeter. Med Phys 1994; 21: 567–72. [DOI] [PubMed] [Google Scholar]
- 73.Zilio VO, Joneja OP, Popowski Y, Rosenfeld A, Chawla R. Absolute depth-dose-rate measurements for an 192Ir HDR brachytherapy source in water using MOSFET detectors. Med Phys 2006; 33: 1532–9. [DOI] [PubMed] [Google Scholar]
- 74.Lambert J, McKenzie DR, Law S, Elsey J, Suchowerska N. A plastic scintillation dosimeter for high dose rate brachytherapy. Phys Med Biol 2006; 51: 5505–16. doi: 10.1088/0031-9155/51/21/008 [DOI] [PubMed] [Google Scholar]
- 75.Andersen CE, Nielsen SK, Greilich S, Helt-Hansen J, Lindegaard JC, Tanderup K. Characterization of a fiber-coupled Al2O3:C luminescence dosimetry system for online in vivo dose verification during 192Ir brachytherapy. Med Phys 2009; 36: 708–18. [DOI] [PubMed] [Google Scholar]
- 76.Carrara M, Cavatorta C, Borroni M, Tenconi C, Cerrotta A, Fallai C, et al. Characterization of a Ce3+ doped SiO2 optical dosimeter for dose measurements in HDR brachytherapy. Radiat Meas 2013; 56: 312–15. [Google Scholar]
- 77.Fontbonne JM, Iltis G, Ban G, Battala A, Vernhes JC, Tillier J, et al. Scintillating fiber dosimeter for radiation therapy accelerator. IEEE Trans Nucl Sci 2002; 49: 2223–7. [Google Scholar]
- 78.Therriault-Proulx F, Beddar S, Briere TM, Archambault L, Beaulieu L. Technical note: removing the stem effect when performing Ir-192 HDR brachytherapy in vivo dosimetry using plastic scintillation detectors: a relevant and necessary step. Med Phys 2011; 38: 2176–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kertzscher G, Andersen CE, Edmund JM, Tanderup K. Stem signal suppression in fiber-coupled Al2O3:C dosimetry for 192Ir brachytherapy. Radiat Meas 2011; 46: 2020–4. [Google Scholar]
- 80.Beddar S. On possible temperature dependence of plastic scintillator response. Med Phys 2012; 39: 6522. doi: 10.1118/1.4748508 [DOI] [PubMed] [Google Scholar]
- 81.Buranurak S, Andersen CE, Beierholm AR, Lindvold LR. Temperature variations as a source of uncertainty in medical fiber-coupled organic plastic scintillator dosimetry. Radiat Meas 2013; 56: 307–11. [Google Scholar]
- 82.Wootton L, Beddar S. Temperature dependence of BCF plastic scintillation detectors. Phys Med Biol 2013; 58: 2955–67. doi: 10.1088/0031-9155/58/9/2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Edmund JM, Andersen CE. Temperature dependence of the Al2O3:C response in medical luminescence dosimetry. Radiat Meas 2007; 42: 177–89. [Google Scholar]
- 84.Haughey A, Coalter G, Mugabe K. Evaluation of linear array MOSFET detectors for in vivo dosimetry to measure rectal dose in HDR brachytherapy. Australas Phys Eng Sci Med 2011; 34: 361–6. doi: 10.1007/s13246-011-0084-2 [DOI] [PubMed] [Google Scholar]
- 85.Able CM, Bright M, Frizzell B. Quality control of high-dose-rate brachytherapy: treatment delivery analysis using statistical process control. Int J Radiat Oncol Biol Phys 2013; 85: 828–33. doi: 10.1016/j.ijrobp.2012.05.016 [DOI] [PubMed] [Google Scholar]
- 86.Nakano T, Suchowerska N, Bilek MM, McKenzie DR, Ng N, Kron T. High dose-rate brachytherapy source localization: positional resolution using a diamond detector. Phys Med Biol 2003; 48: 2133–46. [DOI] [PubMed] [Google Scholar]
- 87.Sheikh-Bagheri D, Munro P. A Monte Carlo study of verification imaging in high dose rate brachytherapy. Med Phys 1998; 25: 404–14. [DOI] [PubMed] [Google Scholar]
- 88.Duan J, Macey DJ, Pareek PN, Brezovich IA. Real-time monitoring and verification of in vivo high dose rate brachytherapy using a pinhole camera. Med Phys 2001; 28: 167–73. [DOI] [PubMed] [Google Scholar]
- 89.Verhaegen F, Palefsky S, Rempel D, Poon E. Imaging with Iridium photons: an application in brachytherapy. Proc SPIE 2007; 6510: 1–11. [Google Scholar]
- 90.Chassagne D, Dutreix A, Almond P, Burgers J, Busch M, Joslin C. ICRU Report No. 38. Dose and volume specification for reporting intracavitary therapy in gynaecology. Bethesda, MD: International Commissioning on Radiation Units and Measurements; 1985. [Google Scholar]
- 91.Pötter R, Van Limbergen E, Gerstner N, Wambersie A. Survey of the use of the ICRU 38 in recording and reporting cervical cancer brachytherapy. Radiother Oncol 2001; 58: 11–18. [DOI] [PubMed] [Google Scholar]
- 92.Clark BG, Souhami L, Roman TN, Evans MDC, Pla C. Rectal complications in patients with carcinoma of the cervix treated with concomitant cisplatin and external beam irradiation with high dose rate brachytherapy: a dosimetric analysis. Int J Radiat Oncol Biol Phys 1994; 28: 1243–50. [DOI] [PubMed] [Google Scholar]
- 93.Datta NR, Basu R, Das KJ, Rajasekar D, Pandey CM, Ayyagari S. Problems in reporting doses and volumes during multiple high-dose-rate intracavitary brachytherapy for carcinoma cervix as per ICRU Report 38: a comparative study using flexible and rigid applicators. Gynecol Oncol 2003; 91: 285–92. [DOI] [PubMed] [Google Scholar]
- 94.Pelloski CE, Palmer M, Chronowski GM, Jhingran A, Horton J, Eifel PJ. Comparison between CT-based volumetric calculations and ICRU reference-point estimates of radiation doses delivered to bladder and rectum during intracavitary radiotherapy for cervical cancer. Int J Radiat Oncol Biol Phys 2005; 62: 131–7. [DOI] [PubMed] [Google Scholar]
- 95.Tanderup K, Nielsen SK, Nyvang G-B, Pedersen EM, Røhl L, Aagaard T, et al. From point A to the sculpted pear: MR image guidance significantly improves tumour dose and sparing of organs at risk in brachytherapy of cervical cancer. Radiother Oncol 2010; 94: 173–80. [DOI] [PubMed] [Google Scholar]
- 96.Archambault L, Therriault-Proulx F, Beddar S, Beaulieu L. A mathematical formalism for hyperspectral, multipoint plastic scintillation detectors. Phys Med Biol 2012; 57: 7133–45. doi: 10.1088/0031-9155/57/21/7133 [DOI] [PubMed] [Google Scholar]
- 97.Therriault-Proulx F, Archambault L, Beaulieu L, Beddar S. Development of a novel multi-point plastic scintillation detector with a single optical transmission line for radiation dose measurement. Phys Med Biol 2012; 57: 7147–59. doi: 10.1088/0031-9155/57/21/7147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cherpak A, Ding W, Hallil A, Cygler JE. Evaluation of a novel 4D in vivo dosimetry system. Med Phys 2009; 36: 1672–9. [DOI] [PubMed] [Google Scholar]
- 99.Nakano T, Suchowerska N, McKenzie DR, Bilek MM. Real-time verification of HDR brachytherapy source location: implementation of detector redundancy. Phys Med Biol 2005; 50: 319–27. [DOI] [PubMed] [Google Scholar]
- 100.Smith RL, Taylor ML, McDermott LN, Haworth A, Millar JL, Franich RD. Source position verification and dosimetry in HDR brachytherapy using an EPID. Med Phys 2013; 40: 1–12. doi: 10.1118/1.4823758 [DOI] [PubMed] [Google Scholar]
- 101.Espinoza A, Beeksma B, Petasecca M, Fuduli I, Porumb C, Cutajar D, et al. The feasibility study and characterization of a two-dimensional diode array in “magic phantom” for high dose rate brachytherapy quality assurance. Med Phys 2013; 40: 1–10. [DOI] [PubMed] [Google Scholar]
- 102.Safavi-Naeini M, Han Z, Cutajar D, Guatelli S, Petasecca M, Lerch ML, et al. BrachyView, a novel inbody imaging system for HDR prostate brachytherapy: design and Monte Carlo feasibility study. Med Phys 2013; 40: 1–10. doi: 10.1118/1.4808360 [DOI] [PubMed] [Google Scholar]
- 103.Petasecca M, Loo KJ, Safavi-Naeini M, Han Z, Metcalfe PE, Meikle S, et al. BrachyView: proof-of-principle of a novel in-body gamma camera for low dose-rate prostate brachytherapy. Med Phys 2013; 40: 1–9. doi: 10.1118/1.4794487 [DOI] [PubMed] [Google Scholar]
- 104.Batic M, Burger J, Cindro V, Kramberger G, Mandic I, Mikuz M, et al. A system for localization of high dose rate 192 Ir source during brachytherapy treatment with silicon detectors. Proceedings of the IEEE Conference on Nuclear Science Symposium; Orlando, FL. Orlando, FL: IEEE, 2009. pp. 3794–800.
- 105.Nelder JA, Mead R. A simplex method for function minimization. Comput J 1965; 7: 308–13. [Google Scholar]
- 106.Palvolgyi J. Multiparametric fit method in reconstruction of Fletcher-Suit-Delclos applicator. Med Phys 2006; 33: 69. [DOI] [PubMed] [Google Scholar]
- 107.Palvolgyi J. Multi-parametric fit method in reconstruction of brachytherapy needles. Phys Med 2013; 29: 397–402. doi: 10.1016/j.ejmp.2012.04.001 [DOI] [PubMed] [Google Scholar]
- 108.Thomadsen BR, Shahabi S, Stitt JA, Buchler DA, Fowler JF, Paliwal BR, et al. High dose rate intracavitary brachytherapy for carcinoma of the cervix: the Madison system: II. Procedural and physical considerations. Int J Radiat Oncol Biol Phys 1992; 24: 349–57. [DOI] [PubMed] [Google Scholar]
- 109.Seppenwoolde Y, Kolkman-Deurloo I-K, Sipkema D, de Langen M, Praag J, Jansen P, et al. HDR prostate monotherapy: dosimetric effects of implant deformation due to posture change between TRUS- and CT-imaging. Radiother Oncol 2008; 86: 114–19. doi: 10.1016/j.radonc.2007.11.004 [DOI] [PubMed] [Google Scholar]
- 110.Hardcastle N, Cutajar DL, Metcalfe PE, Lerch MLF, Perevertaylo VL, Tomé WA, et al. In vivo real-time rectal wall dosimetry for prostate radiotherapy. Phys Med Biol 2010; 55: 3859–71. doi: 10.1088/0031-9155/55/13/019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wootton L, Kudchadker R, Lee A, Beddar S. Real-time in vivo rectal wall dosimetry using plastic scintillation detectors for patients with prostate cancer. Phys Med Biol 2014; 59: 647–60. doi: 10.1088/0031-9155/59/3/647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Eng TY, Fuller CD, Cavanaugh SX, Blough MM, Sadeghi A, Herman T. Significant rectal and bladder dose reduction via utilization of Foley balloon catheters in high-dose-rate tandem and ovoid intracavitary brachytherapy of the uterine cervix. Int J Radiat Oncol Biol Phys 2004; 59: 174–8. doi: 10.1016/j.ijrobp.2003.09.090 [DOI] [PubMed] [Google Scholar]
- 113.Malaker K, Shukla V, D’Souza H, Weatherburn H. Minimizing urinary bladder radiation dose during brachytherapy for carcinoma of the cervix using balloon inflation technique. Int J Radiat Oncol Biol Phys 2005; 61: 257–66. doi: 10.1016/j.ijrobp.2004.05.004 [DOI] [PubMed] [Google Scholar]
- 114.Rai B, Patel FD, Chakraborty S, Sharma SC, Kapoor R, Aprem AS. Bladder-rectum spacer balloon in high-dose-rate brachytherapy in cervix carcinoma. Int J Radiat Oncol Biol Phys 2013; 85: 217–22. doi: 10.1016/j.ijrobp.2012.09.037 [DOI] [PubMed] [Google Scholar]
- 115.Kwan IS, Rosenfeld AB, Qi ZY, Wilkinson D, Lerch MLF, Cutajar DL, et al. Skin dosimetry with new MOSFET detectors. Radiat Meas 2008; 43: 929–32. [Google Scholar]
- 116.Ford EC, Fong de Los Santos L, Pawlicki T, Sutlief S, Dunscombe P. Consensus recommendations for incident learning database structures in radiation oncology. Med Phys 2012; 39: 7272–90. doi: 10.1118/1.4764914 [DOI] [PubMed] [Google Scholar]
- 117.Swamidas JV, Sharma S, Mahantshetty UM, Khanna N, Somesan V, Deshpande DD, et al. Risk assessment in intracavitary brachytherapy based on failure mode and effective analysis. Brachytherapy 2010; 9: S48. [Google Scholar]
- 118.Wilkinson DA, Kolar MD. Failure modes and effects analysis applied to high-dose-rate brachytherapy treatment planning. Brachytherapy 2013; 12: 382–6. doi: 10.1016/j.brachy.2013.03.002 [DOI] [PubMed] [Google Scholar]
- 119.Rath F. Tools for developing a quality management program: proactive tools (process mapping, value stream mapping, fault tree analysis, and failure mode and effects analysis). Int J Radiat Oncol Biol Phys 2008; 71: S187–90. doi: 10.1016/j.ijrobp.2007.07.2385 [DOI] [PubMed] [Google Scholar]