ABSTRACT
The malaria parasite Plasmodium falciparum contains a nonphotosynthetic plastid organelle that possesses plant-like metabolic pathways. Plants use the plastidial isoprenoid biosynthesis pathway to produce volatile odorants, known as terpenes. In this work, we describe the volatile chemical profile of cultured malaria parasites. Among the identified compounds are several plant-like terpenes and terpene derivatives, including known mosquito attractants. We establish the molecular identity of the odorant receptors of the malaria mosquito vector Anopheles gambiae, which responds to these compounds. The malaria parasite produces volatile signals that are recognized by mosquitoes and may thereby mediate host attraction and facilitate transmission.
IMPORTANCE
Malaria is a key global health concern. Mosquitoes that transmit malaria are more attracted to malaria parasite-infected mammalian hosts. These studies aimed to understand the chemical signals produced by malaria parasites; such an understanding may lead to new transmission-blocking strategies or noninvasive malaria diagnostics.
INTRODUCTION
Malaria remains an enormous burden to human health worldwide. There are over 250 million cases of malaria each year, and nearly 1 million deaths (1). A single protozoan species, Plasmodium falciparum, is responsible for the most severe and deadly cases of this disease. Widespread and emerging drug resistance has contributed to a resurgence of malaria and to increased international attention to malaria control (2, 3). Because P. falciparum is transmitted through the bite of mosquitoes of the genus Anopheles, mosquito vector management has remained a key component of most malaria reduction efforts (4).
Female mosquitoes choose their mammalian hosts based in part on complex chemical cues. Some of these signals, such as carbon dioxide, have been well characterized on a molecular level. For example, carbon dioxide is not only a potent mosquito stimulant but also augments mosquito feeding behaviors and modulates attraction to other human body odors (5). However, Anopheles gambiae strains that lack functional CO2 receptors are still capable of locating human hosts (6), indicating that additional chemical signals also drive host preference. Several recent studies have demonstrated that Plasmodium-infected hosts, including humans and rodents, are more attractive to Anopheles spp. (7–9). Analysis of volatile organic compounds emitted by Plasmodium-infected mice revealed an overall increase in volatiles, including host-derived compounds that enhance mosquito attraction (9).
While female mosquitoes depend on protein-rich blood meals for egg maturation, both male and female mosquitoes are also attracted to and feed from plants. Plant nectar is an important, carbohydrate-rich nutrient source that provides essential energy for flight and, for some mosquito species, overwintering (10, 11). This phytoattraction has been successfully harnessed by malaria control efforts through “attractive nectar baiting” strategies, in which mosquitoes are lured to sugar-water blends spiked with plant volatiles and insecticides (12, 13). Suspected preferred host plants for Anopheles gambiae include Asteracaeae spp. and Ricinus communis (14). Analysis of purified odorants from these plants has revealed enrichment of volatile compounds known as terpenes, including 10-carbon monoterpenes such as pinene and limonene. At low concentrations, these purified terpenes directly mediate attraction of Anopheles spp. (14).
Terpenes are low-vapor-pressure hydrocarbons that belong to a class of compounds known as isoprenoids. Over 200,000 isoprenoids have been described, and this large group of biomolecules exhibits dramatic structural and functional diversity (15). All isoprenoids are produced downstream of two common 5-carbon precursors, isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). Animals and fungi generate isoprenoids through a biosynthetic route that proceeds through mevalonate. In contrast, eubacteria and plastid-containing eukaryotes use an alternate metabolic route, the nonmevalonate or methylerythritol phosphate (MEP) pathway. Plants utilize both the mevalonate and MEP pathways; however, it is the chloroplast-localized MEP pathway that is used for biosynthesis of the terpene volatiles that constitute their characteristic flavors and fragrances (16). For many species of insects, not just mosquitoes, chemodetection of plant-derived terpenes directly modulates herbivory and pollination behaviors (reviewed in reference 17).
The malaria parasite Plasmodium falciparum contains an unusual plastid organelle called the apicoplast, which is of a similar endosymbiotic evolutionary origin as plant chloroplasts (18). While the apicoplast retains several plant-like metabolic pathways, evidence suggests that the MEP pathway may be the only essential function of this organelle during intraerythrocytic development (19–21). In this work, we examined the possibility that, like plants, Plasmodium falciparum parasites might utilize the MEP pathway to produce terpenes. We determined the volatile chemical composition of headspace gas from cultured P. falciparum and thus identified parasite-produced terpene molecules that represent known mosquito phytoattractants. In addition, we established the molecular identity of the Anopheles gambiae odorant receptors that respond to these plant-like terpenes. Together, our studies provide evidence that malaria parasites produce specific volatile compounds, and anopheline mosquitoes that transmit malaria contain the cellular machinery necessary for detecting and responding to these compounds. Thus, plant-like terpenes produced by P. falciparum may represent semiochemicals for mediating anopheline mammalian host preference.
RESULTS
Plant-like volatile compounds in Plasmodium falciparum headspace gas.
We hypothesized that malaria parasites might produce volatile organic compounds, including terpenes. We therefore evaluated the chemical composition of the headspace gas above asynchronous P. falciparum parasites cultured in human red blood cells (RBCs). Because previous studies of volatile emissions from Plasmodium berghei-infected mice (average blood volume, 2 to 4 ml) (9) or low-volume P. falciparum cultures (22) did not detect malaria parasite-specific volatiles, we utilized large-volume cultures (200 ml) to increase the likelihood of detecting small quantities of Plasmodium-produced compounds. In addition, because terpenes are present at low levels in human serum (23), we utilized medium supplemented with a lyophilized serum substitute (Albumax; Invitrogen) which does not contain detectable terpenes. For headspace sampling, we employed solid-phase microextraction (SPME) fibers, which selectively bind and concentrate nonpolar organic compounds, as is typically performed to evaluate plant-derived volatiles (reviewed in reference 24).
Fibers were exposed to a controlled atmosphere conditioned by P. falciparum for 48 h and then were desorbed and analyzed via electron impact (EI) gas chromatography-mass spectrometry (GC-MS). As is typical of complex volatile samples, component peaks overlapped and were not well resolved by visual inspection (see Fig. S1 in the supplemental material for representative traces). For this reason, resulting chromatograms were deconvoluted to isolate overlapping peaks and to extract and annotate component mass spectra. When distinguishing parasite-specific compounds, we aimed to identify compounds qualitatively present in parasite-infected samples compared to controls. Therefore, we conservatively selected compounds present in a majority of independent biological replicates of parasite-infected RBC samples and excluded entities also present in either uninfected RBC samples or blank controls that contained neither RBCs nor medium. Four compounds specific to parasite-infected samples were thus identified, including two terpenes (Fig. 1). These identified compounds have previously been identified as typical components of plant essential oils and/or fungal volatile profiles (25–28).
FIG 1 .
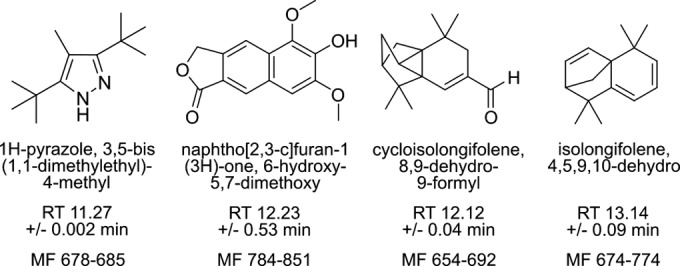
Plasmodium-specific volatile organic compounds. Compounds annotated in three or more P. falciparum-infected SPME sampling replicates (total n = 5) and not in uninfected red blood cell samples (n = 3) or blank controls (n = 6). For each compound, the average retention time (RT) and the range of match factors are indicated. Match factors (MF, 0 to 999) describe how well a sample spectrum agrees with the database spectrum. Values of >650 indicate close identity.
Terpenes are present in malaria-infected erythrocytes.
We identified several entities that were annotated as terpenes and were present exclusively in the headspace gas of malaria parasites and not that of control uninfected erythrocytes or blank samples. Since terpenes with closely related chemical structures give rise to similar mass spectra, variability in compound annotation is typical and expected. The dominant malaria parasite-specific terpenes were annotated as a 15-carbon sesquiterpene (4,5,9,10-dehydro-isolongifolene) and its close derivative (8,9-dehydro-9-formyl cycloisolongifolene) (Fig. 2; see also Fig. S2 in the supplemental material). No commercial standards or known synthesis routes have been described for either compound; however, the structural annotations are supported by consistent database match factors, from 654 to 774.
FIG 2 .
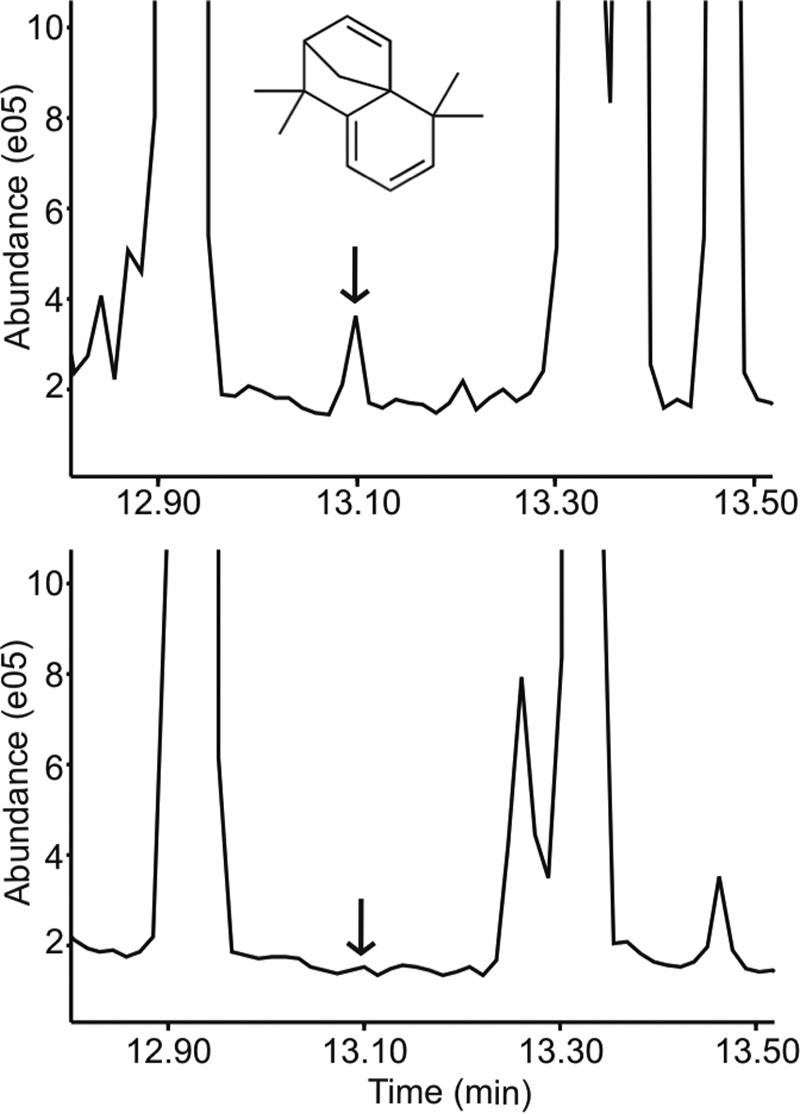
4,5,9,10-Dehydro-isolongifolene is present in the headspace gas of Plasmodium-infected RBCs. (Top) Total ion chromatogram (TIC) of SPME fibers conditioned with headspace gas from P. falciparum-infected human RBCs. Arrow, retention time of 13.101 min (typical of 4,5,9,10-dehydro-isolongifolene). (Bottom) TIC of SPME fibers conditioned with headspace gas from uninfected human RBCs.
In addition, each malaria parasite-infected sample contained at least one 10-carbon monoterpene. Monoterpene annotations varied between samples but included the structurally related compounds limonene and pinanediol (an α-pinene derivative) (see Fig. S3 in the supplemental material). To confirm the identity of these monoterpenes, we extracted nonpolar organic compounds from cultured P. falciparum and performed GC-MS analysis. P. falciparum-infected cultures, but not uninfected RBC or blank controls, contained a single peak suggestive of a monoterpene with a retention time of 2.39 min, identical to that of an α-pinene (monoterpene) standard (Fig. 3). Comparison of the mass spectra of the observed parasite-specific peak with that of a purified standard established that the parasite-specific compound is α-pinene (Fig. 3E), a terpene compound previously shown to be produced by Anopheles-preferred plant species and attractive to A. gambiae (14).
FIG 3 .
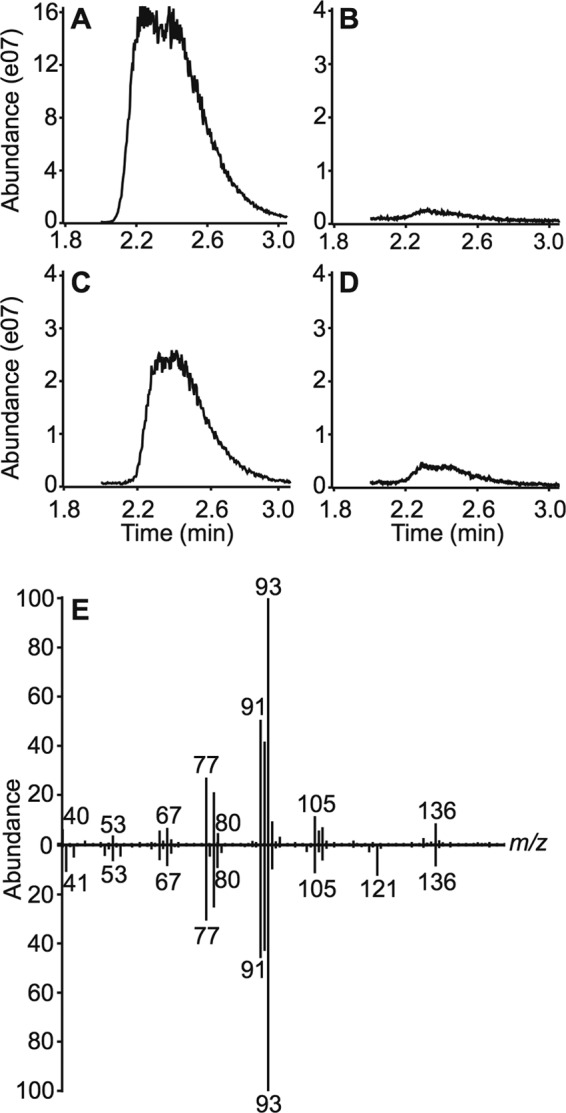
Pinene is produced in malaria parasites by de novo isoprenoid biosynthesis. (A to D) Extracted ion chromatograms (EIC) of the total ion chromatograms (TIC) at m/z 93, the base peak in the ion spectrum of pinene. (A) α-(+)-Pinene standard (positive control); (B) uninfected RBCs (negative control); (C) Plasmodium-infected RBCs; (D) Plasmodium-infected RBCs treated with 5 µM fosmidomycin (inhibitor of parasite isoprenoid biosynthesis). (E) EI mass spectra of the observed pinene peak from P. falciparum RBCs in panel C (top) compared to the purified α-(+)-pinene standard in panel A (bottom).
Terpenes are produced by de novo isoprenoid biosynthesis in malaria parasites.
To evaluate whether terpenes in malaria parasite-infected samples were produced de novo by the parasite, we utilized fosmidomycin, a phosphonic acid antibiotic that inhibits the first dedicated enzyme of the MEP pathway, deoxyxylulose phosphate reductoisomerase (19). Previous metabolic profiling of fosmidomycin-treated parasites has established that fosmidomycin reduces concentrations of isoprenoid precursors in P. falciparum (29). Upon fosmidomycin treatment of cultured P. falciparum, pinene peak abundance decreased dramatically (Fig. 3D). Proteomic studies of mature RBCs have indicated that these cells do not possess the enzymatic machinery to produce the isoprenoid precursors required for terpene synthesis (30, 31). In addition, RBCs do not appear to contain substantial stores of IPP, since malaria parasites that cannot produce IPP themselves are unable to survive (21, 29). Together, this evidence strongly supports that the monoterpenes emitted by Plasmodium-infected RBCs arise from the MEP pathway of the malaria parasite.
Anopheles odorant receptors respond to malaria parasite-produced terpenes.
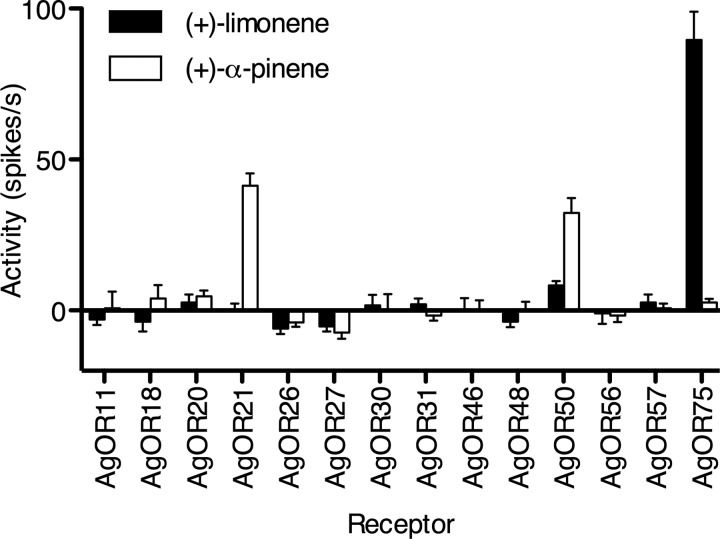
P. falciparum is transmitted person to person through the bite of anopheline mosquitoes. To locate plant and mammalian nutrient sources, A. gambiae detects volatile compounds via signals through ligand-gated voltage channels known as odorant receptors (AgORs) (32). Electrophysiological and behavioral studies have indicated that A. gambiae detects and is attracted to plant volatiles. While high concentrations of terpenes often repel mosquitoes, pinene and limonene at low concentrations directly attract A. gambiae and are the dominant volatile organic compounds found in the extracts of mosquito-preferred plant species (14).
To determine the biochemical mechanism by which A. gambiae detects plant- and malaria parasite-produced terpenes, we assayed a panel of mosquito odorant receptors (AgORs) for pinene and/or limonene ligand-activated electrical activity. Using the Drosophila melanogaster “empty neuron” in vivo expression system (33, 34), we found that AgOR75 was dramatically stimulated by (+)-limonene, while AgORs 21 and 50 were substantially stimulated by pinene (Fig. 4; see also Fig. S4 in the supplemental material). These odorant receptors are differentially expressed in Anopheles chemosensory tissues. Specifically, AgORs 21 and 50 are highly expressed in both male and female antennae (35). These studies confirmed that the primary African malaria vector mosquito can distinguish monoterpenes produced by P. falciparum. In addition, our studies establish the molecular identity of the monoterpene-specific odorant receptors of A. gambiae.
FIG 4 .
Anopheles gambiae odorant receptors respond to malaria terpenes. Anopheles odorant receptors were expressed in the Drosophila empty neuron in vivo expression system and exposed to (+)-limonene and α-(+)-pinene. (+)-Limonene specifically activated receptor 75. α-(+)-Pinene activated receptors 21 and 50 (n = 6; error bars indicate standard errors of the means).
DISCUSSION
Our studies indicated that Plasmodium falciparum malaria parasites produce a repertoire of plant-like volatile compounds. These compounds may represent interspecies chemical signals, or semiophores, that modulate the attraction of vector mosquitoes to hosts. Among the parasite-specific compounds we identified, terpenes are bioavailable molecules that readily pass through membranes and partition into alveolar gas in the lung. Terpenes, likely from dietary sources, have previously been identified in exhaled breath samples of humans (36). Upon malaria parasite infection, parasite-produced terpenes are likely to be detected outside infected individuals, since the total number of parasites in a typical infected human well exceeds the number sampled in culture in such studies (37, 38).
Previous studies have suggested that P. falciparum infection of Anopheles spp. mosquitoes may reduce fitness and alter feeding behaviors (39–41). Over time, selective pressures might enrich for mosquitoes with a decreased tendency to feed from malaria parasite-infected individuals. Therefore, any chemical signals that increase attraction of mosquitoes to infected individuals must be difficult to select against and resistant to evolutionary pressures. This hypothesis is consistent with the finding that malaria infection increases production of typical mammalian host odorants (9). Our studies suggest an additional strategy by P. falciparum for overcoming selection against biting infected hosts, in which the malaria parasite compensates by imitating the volatile components of plants preferred by Anopheles spp. The parasite thus hijacks a highly selected signaling response that is necessary for mosquito nectar feeding behavior and survival. Since Plasmodium infection increases nectar attraction in Anopheles (42), the parasite appears to facilitate transmission both by generating a mosquito chemoattractant and by sensitizing the mosquito to detect this signal. Interruption of parasite-mediated volatile signaling to mosquitoes will be a potent means of blocking this critical step in the malaria life cycle.
P. falciparum has well-characterized biosynthetic machinery to produce isoprenoid building blocks and prenyl diphosphates (43–45). In other systems, such as plants, terpenes are produced by terpene synthases, which generate terpenes by catalyzing intramolecular cyclization of prenyl diphosphate substrates (46). This promiscuous reaction typically produces a variety of chemically related terpene variants from a single enzyme, a cardinal feature of this enzyme class (47). Consistent with this product diversity, the large protein family of terpene synthases (Pfam 01397) exhibits remarkable sequence diversity. Our studies strongly suggest that terpenes are produced de novo in P. falciparum, since chemical inhibition of parasite-specific isoprenoid biosynthesis reduces terpene production. No unambiguous terpene synthase ortholog is present in P. falciparum, based on domain or phylogenetic analyses, but is likely to be represented among the nearly one-half of the parasite genes that remain unannotated. The diversity of terpenes present in P. falciparum-conditioned gas suggests that there is at least one monoterpene and one sesquiterpene synthase.
Here, we have reported a repertoire of volatile organic compounds that are specific to P. falciparum-infected cultures. These compounds are not likely to represent all possible malaria parasite-specific volatiles, because our conservative data filtering necessarily excluded compounds that are parasite specific but exhibit significant biological variability. The volatile fingerprint of P. falciparum represents not only a target for the development of inhibitors that will interrupt malaria transmission, but also an untapped strategy for malaria diagnostics. The parasite-specific compounds we have identified may represent volatile biomarkers of malaria infection. Ongoing studies will establish the presence and identity of these compounds in human P. falciparum infection.
MATERIALS AND METHODS:
Plasmodium falciparum culture and strains.
All P. falciparum strains were cultured in vitro in human erythrocytes (48) at 2% hematocrit. The culture conditions were as described previously (29), with the following modifications: we used a 5% O2–5% CO2–90% N2 atmosphere in RPMI 1640 medium supplemented with 27 mM sodium bicarbonate, 11 mM glucose, 5 mM HEPES, 1 mM sodium pyruvate, 0.37 mM hypoxanthine, 0.01 mM thymidine, 0.25 mg/ml gentamicin (Goldbio), and 0.5% Albumax (Invitrogen). Wild-type strain 3D7 (MRA-102) was obtained from the Malaria Research and Reference Reagent Resource Center (MR4). 3D7-IG was kindly provided by Daniel Goldberg, Washington University School of Medicine.
Headspace sampling.
Plasmodium falciparum strain 3D7-MR4 was cultured in a cell bioreactor bag (GE Life Sciences) for 48 h with a volume of 200 ml at 2% hematocrit and 2% parasitemia (infected erythrocytes/total erythrocytes). The culture was injected into the bag via syringe through a liquid injection port in a sterile environment. Uninfected samples contained erythrocytes and medium, and blank controls represented sampling from empty bags without medium or erythrocytes. The two injection ports with attached airtight filters were then used to fill the bag with a 5% O2–5% CO2–90% N2 atmosphere. The biobag was secured to a tilting plate and connected to 0.63-in. sterile plastic tubing (Cole Parmer) through two injection ports. The ends of the tubing were connected to Luer pieces, which were secured to the biobag ports by using Parafilm. Of note, the biobag ports do not contain Luer locks, but all other pieces of tubing in the system are connected with interlocking Luer pieces. One piece of tubing was connected directly to a Bio-Rad Econo pump, and the other was fed through an airtight hole in a 250-ml medium bottle (Kimax). The bottle also contained openings for fiber insertion and outgoing plastic tubing. This tubing was connected to the other end of the peristaltic pump, completing the closed loop. A carboxen-polydimethylsiloxane SPME microfiber (Sigma-Aldrich), inside a manual holder, was placed through an adaptor into the medium bottle. Parafilm was used to secure the fiber and fiber holder in place and provide an airtight seal. Each experiment was performed in a temperature-maintained 37°C room for optimal malaria parasite growth. Sampling was initiated by opening the clamps on the two biobag injection ports, initiating peristalsis, and extending the fiber from inside the holder to its exposed position in the bottle. The fiber was exposed to the sampling conditions for 48 h. After sampling, the fiber was resheathed and analyzed by GC-MS as detailed below.
GC-MS analysis of SPME fiber extracts.
Samples were analyzed on an Agilent 7890A gas chromatograph interfaced with an Agilent 5975C mass spectrometer. The GC column used for the study was an Agilent HP-5MS column (30 m, 0.25-mm inner diameter [i.d.], 0.25-µm film thickness). Samples were injected in a splitless mode with injector and transfer line temperatures set at 300°C. A linear temperature gradient was started with an initial temperature of 60°C, held for 2 min, increased to 300°C at 10°C/min, and held for 1 min. The ion source temperature, electron energy, and emission current were set at 230°C, 70 eV, and 300 µA, respectively, to obtain EI mass spectra.
Manual analysis of GC-MS data.
The raw data were analyzed by using the automated mass spectral deconvolution and identification system (AMDIS), which provides an output of the GC trace with a deconvoluted mass spectrum extracted from each trace. Each mass spectrum represents a potential compound at a specific point in the trace. Every sample (headspace gas and parasite extract) yielded an average of 700 mass spectra per analysis. The structures of the compounds in each GC peak were identified by database search (NIST Mass Spectrum Library) using AMDIS software. Background peaks that represented known biologically irrelevant contaminants, such as polysiloxane arising from SPME fibers, were excluded from further analysis, as were compounds that did not possess consistent, parasite-unique ion spectra at given retention times.
Saponin lysis of P. falciparum cultures.
Parasites were freed from RBCs through lysis with saponin at a final concentration of 0.1% (vol/vol), followed by centrifugation at 2,500 rpm and resuspension in 4 ml of phosphate-buffered saline (PBS). Pellets were washed in an additional 1 ml of PBS, followed by centrifugation at 14,000 for 1 min. Dry pellets were stored at −80°C until analysis.
Organic extraction.
Extraction of isolated parasite cells was performed as described for the original Folch procedure (49), with the following modifications. Saponin-lysed parasite pellets were suspended in 1 ml of 2:1 (vol/vol) chloroform-methanol. The suspensions were sonicated for 30 s and then iced for 30 s for 3 cycles. Samples were vortexed at 600 rpm for 1 h after sonication. Samples were then centrifuged at 14,000 rpm, and the supernatant was recovered. Three hundred microliters of 0.9% NaCl was added to induce phase separation of the sample. The organic phase was recovered for analysis. The organic phase was not evaporated after extraction, in order to avoid loss of volatile compounds.
GC-MS analysis of extracted samples.
GC-MS analyses were conducted on a Thermo ISQ 1300 GC-MS system with the Xcalibur operation system (San Jose, CA, USA). Separation was achieved with a Thermo 30-m TG SQC column (0.25-mm i.d., 0.25-μm film thickness) at a flow rate of 1 ml/min with He as the carrier gas. The GC temperature was started at 50°C for 2 min, raised to 150°C at 10°C/min, and then to the final temperature of 300°C at a rate of 20°C/min. The samples were injected in a splitless mode, and the EI mass spectra were acquired in the mass range of 40 to 450 Da at a rate of 0.2 s/scan. The injector, transfer line, and ion source temperatures were set at 240°C, 250°C, and 210°C, respectively.
Single-unit electrophysiological recordings.
All experiments were performed on adult female flies, 5 days after eclosion. Flies were reared at 25°C in an incubator with a 12-h light-dark cycle. “Empty neuron” recordings were from flies of genotype w Δhalo/Δhalo Or22a-GAL4/UAS-AgOrX. The ab3A mutant flies and Or22a-GAL4 and UAS-AgOr transgenic lines were described previously (32). Fourteen AgOrs (AgOr11, -18, -20, -21, -26, -27, -30, -31, -46, -48, -50, -56, -57, and -75), previously found to respond to terpenes (32), were selected to test their responsiveness to additional terpene compounds [α-(+)-pinene, Sigma-Aldrich catalog no 26870; β-(+)-pinene, no. 80607; α-(−)-pinene, no. 305715; β-(−)-pinene, no. 402753; R-(+)-limonene, no. 183164; S-(−)-limonene, no. 218367]. Odorants were diluted in paraffin oil (10−2, vol/vol), and odor stimuli (50 µl applied to a filter disc) were delivered from a Pasteur pipette via a 500-ms pulse of air (200 ml/min) into the main air stream (2,000 ml/min), as described previously (32). Extracellular single-unit recordings were performed essentially as described elsewhere (32). Briefly, electrical activity of the olfactory receptor neurons (ORNs) was recorded extracellularly by placing a sharp electrode filled with Ringer solution into a sensillum, and the reference electrode filled with the same Ringer solution was placed in the eye. AC signals (300 to 2,000 Hz) were recorded on an Iso-DAM amplifier (World Precision Instruments) and digitized at 5 kHz with an Axoscope 10.2 apparatus (Molecular Devices). ORN spike responses were quantified offline and averaged from 6 different neurons. Baseline spike frequency (calculated from spike activity 1 s prior to odor stimulus) was subtracted from the result.
SUPPLEMENTAL MATERIAL
Total ion chromatograph (TIC) of Plasmodium parasite and red blood cell volatile organic compounds. (Top) Typical chromatograph of SPME fibers exposed to Plasmodium falciparum-infected red blood cells. Marked peaks represent dominant compounds in each trace, many of which represent known contaminant compounds (asterisks, silyated background compounds). Red, parasite-specific peaks from a representative parasite sample compared to the uninfected control. (Bottom) Typical chromatograph of SPME fibers exposed to uninfected red blood cells. (A) Hydrogen azide; (B) n-butane; (C) n-hexane; (D) toluene; (E) 2,4-dimethyl heptane; (F) 2,3-dimethyl heptane; (G) 4-methyl octane; (H) methoxy-phenyl-oxime; (I) benzaldehyde; (J) octanal; (K) 2-ethyl 1-hexanol; (L) 2,3,6,7-tetramethyl octane; (M) dodecane; (N) 1,4-dimethyl-trans-cyclooctane. Download
Terpenes exclusive to headspace gas of infected red blood cells. (Top) Overlay of typical extracted ion chromatograms for the base peak ion of 4,5,9,10-dehydro-isolongifolene, m/z 143. The arrow indicates the retention time of 4,5,9,10-dehydro-isolongifolene, 13.1 min. (Bottom) Overlay of typical extracted ion chromatograms for the parent peak ion of 8,9-dehydro-9-formyl cycloisolongifolene, m/z 230. The arrow indicates the retention time of 8,9-dehydro-9-formyl cycloisolongifolene, 12.1 min. Download
Heat map of terpene annotation predictions. Each column represents compounds identified from SPME sampling replicates from five independent biological samples of P. falciparum-infected RBCs. Not represented are compounds also present in RBCs or identified solely from nonpolar organic extractions. The gray scale represents the confidence in the match ID given by NIST, based on the similarity between the ion spectra in the sample and the reference spectra from the NIST library. White represents 0% probability, indicating the spectrum was not found in the TIC, and black represents 80% probability (the highest match of all annotated compounds), indicating the terpene was found in the TIC with an ion spectrum that closely matched the reference spectrum in the NIST library. Heat map includes all entities annotated as terpenes; monoterpenes are indicated in boldface. Download
Anopheles odorant receptor stimulation by parasite monoterpenes. Responses of Anopheles receptors in the presence of additional terpene isomers. (−)-Limonene had no major differences in its activation profile from (+)-limonene, and α-(−)-pinene, β-(+)-pinene, and β-(−)-pinene had no major differences in their activation profiles from that for α-(+)-pinene. Download
ACKNOWLEDGMENTS
This work was supported by the Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital (MD-LI-2011-171), NIH NIAID R01AI103280, a March of Dimes Basil O’Connor Starter Scholar Research Award to A. R. Odom, and the Doris Duke Charitable Foundation. J. R. Crowley was supported by grants P41RR000954, P60 DK020579, and P30DK056341. C.-Y. Su and J. R. Carlson were supported by grants from the NIH. M. Kelly was supported through an HHMI Undergraduate Science Education Grant and the Amgen Scholars Program. C. Schaber was supported by a Monsanto Excellence Fund Graduate Fellowship.
We thank Allison Rhodes for technical assistance in annotating entities from GC-MS traces. We are grateful to Jeffrey Henderson and Daniel Cuthbertson for critical reading of the manuscript.
For the experiments carried out in Plasmodium falciparum, A. Odom and M. Kelly conceived and designed the experiments; A. Odom, M. Kelly, Jan Crowley, F.-F. Hsu, and C. Schaber performed the experiments and analyzed the data. For the Anopheles gambiae odorant receptor experiments, J. R. Carlson and C.-Y. Su conceived and designed the experiments, C.-Y. Su performed the experiments, and C.-Y. Su and J. R. Carlson analyzed the data. M. Kelly, C.-Y. Su, J. R. Carlson, C. Schaber, and A. Odom wrote the manuscript.
We declare we have no conflicts of interest.
Footnotes
Citation Kelly M, Su C-Y, Schaber C, Crowley JR, Hsu F-F, Carlson JR, Odom AR. 2015. Malaria parasites produce volatile mosquito attractants. mBio 6(2):e00235-15. doi:10.1128/mBio.00235-15.
REFERENCES
- 1.World Health Organization 2012. WHO malaria report 2012. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Dondorp AM, Fairhurst RM, Slutsker L, MacArthur JR, Guerin PJ, Wellems TE, Ringwald P, Newman RD, Plowe CV. 2011. The threat of artemisinin-resistant malaria. N Engl J Med 365:1073–1075. doi: 10.1056/NEJMp1108322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fairhurst RM, Nayyar GM, Breman JG, Hallett R, Vennerstrom JL, Duong S, Ringwald P, Wellems TE, Plowe CV, Dondorp AM. 2012. Artemisinin-resistant malaria: research challenges, opportunities, and public health implications. Am J Trop Med Hyg 87:231–241. doi: 10.4269/ajtmh.2012.12-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization 2013. Malaria entomology and vector control. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 5.Dekker T, Geier M, Cardé RT. 2005. Carbon dioxide instantly sensitizes female yellow fever mosquitoes to human skin odours. J Exp Biol 208:2963–2972. doi: 10.1242/jeb.01736. [DOI] [PubMed] [Google Scholar]
- 6.McMeniman CJ, Corfas RA, Matthews BJ, Ritchie SA, Vosshall LB. 2014. Multimodal integration of carbon dioxide and other sensory cues drives mosquito attraction to humans. Cell 156:1060–1071. doi: 10.1016/j.cell.2013.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lacroix R, Mukabana WR, Gouagna LC, Koella JC. 2005. Malaria infection increases attractiveness of humans to mosquitoes. PLoS Biol 3:e298. doi: 10.1371/journal.pbio.0030298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornet S, Nicot A, Rivero A, Gandon S. 2013. Malaria infection increases bird attractiveness to uninfected mosquitoes. Ecol Lett 16:323–329. doi: 10.1111/ele.12041. [DOI] [PubMed] [Google Scholar]
- 9.De Moraes CM, Stanczyk NM, Betz HS, Pulido H, Sim DG, Read AF, Mescher MC. 2014. Malaria-induced changes in host odors enhance mosquito attraction. Proc Natl Acad Sci U S A 111:11079–11084. doi: 10.1073/pnas.1405617111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster WA. 1995. Mosquito sugar feeding and reproductive energetics. Annu Rev Entomol 40:443–474. doi: 10.1146/annurev.en.40.010195.002303. [DOI] [PubMed] [Google Scholar]
- 11.Gu W, Müller G, Schlein Y, Novak RJ, Beier JC. 2011. Natural plant sugar sources of anopheles mosquitoes strongly impact malaria transmission potential. PLoS One 6:e15996. doi: 10.1371/journal.pone.0015996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beier JC, Müller GC, Gu W, Arheart KL, Schlein Y. 2012. Attractive toxic sugar bait (ATSB) methods decimate populations of Anopheles malaria vectors in arid environments regardless of the local availability of favoured sugar-source blossoms. Malar J 11:31. doi: 10.1186/1475-2875-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nyasembe VO, Tchouassi DP, Kirwa HK, Foster WA, Teal PE, Borgemeister C, Torto B. 2014. Development and assessment of plant-based synthetic odor baits for surveillance and control of malaria vectors. PLoS One 9:e89818. doi: 10.1371/journal.pone.0089818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nyasembe VO, Teal PE, Mukabana WR, Tumlinson JH, Torto B. 2012. Behavioural response of the malaria vector Anopheles gambiae to host plant volatiles and synthetic blends. Parasit Vectors 5:234. doi: 10.1186/1756-3305-5-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gershenzon J, Dudareva N. 2007. The function of terpene natural products in the natural world. Nat Chem Biol 3:408–414. doi: 10.1038/nchembio.2007.5. [DOI] [PubMed] [Google Scholar]
- 16.Gutensohn M, Nagegowda DA, Dudareva N. 2013. Involvement of compartmentalization in monoterpene and sesquiterpene biosynthesis in plants, p 155–169. In Bach TJ, Rohmer M (ed), Isoprenoid synthesis in plants and microorganisms. Springer-Verlag, New York, NY. [Google Scholar]
- 17.Hick AJ, Luszniak MC, Pickett JA. 1999. Volatile isoprenoids that control insect behaviour and development. Nat Prod Rep 16:39–54. doi: 10.1039/a705984a. [DOI] [Google Scholar]
- 18.Van Dooren GG, Striepen B. 2013. The algal past and parasite present of the apicoplast. Annu Rev Microbiol 67:271–289. doi: 10.1146/annurev-micro-092412-155741. [DOI] [PubMed] [Google Scholar]
- 19.Jomaa H, Wiesner J, Sanderbrand S, Altincicek B, Weidemeyer C, Hintz M, Türbachova I, Eberl M, Zeidler J, Lichtenthaler HK, Soldati D, Beck E. 1999. Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science 285:1573–1576. doi: 10.1126/science.285.5433.1573. [DOI] [PubMed] [Google Scholar]
- 20.Odom AR, Van Voorhis WC. 2010. Functional genetic analysis of the Plasmodium falciparum deoxyxylulose 5-phosphate reductoisomerase gene. Mol Biochem Parasitol 170:108–111. doi: 10.1016/j.molbiopara.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeh E, DeRisi JL. 2011. Chemical rescue of malaria parasites lacking an apicoplast defines organelle function in blood-stage Plasmodium falciparum. PLoS Biol 9:e1001138. doi: 10.1371/journal.pbio.1001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong RP, Flematti GR, Davis TM. 2012. Investigation of volatile organic biomarkers derived from Plasmodium falciparum in vitro. Malar J 11:314. doi: 10.1186/1475-2875-11-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashley DL, Bonin MA, Cardinali FL, McCraw JM, Wooten JV. 1994. Blood concentrations of volatile organic compounds in a nonoccupationally exposed US population and in groups with suspected exposure. Clin Chem 40:1401–1404. [PubMed] [Google Scholar]
- 24.Yang C, Wang J, Li D. 2013. Microextraction techniques for the determination of volatile and semivolatile organic compounds from plants: a review. Anal Chim Acta 799:8–22. doi: 10.1016/j.aca.2013.07.069. [DOI] [PubMed] [Google Scholar]
- 25.Xie Z, Zhao Z, Wu G, Ye X, Shi L, Guo J. 2012. Component analysis of the root exudates at different growth stages in chili pepper. Acta Agric Boreali Occidentalia Sinica 8:031 (In Chinese.) [Google Scholar]
- 26.Chen C, Hostettler FD. 1969. Phenolic constituents of elm wood: 2-naphthoic acid derivatives from Ulmus thomasii. Tetrahedron 25:3223–3229. doi: 10.1016/S0040-4020(01)82854-4. [DOI] [Google Scholar]
- 27.Ze-Kun L. 2012. GC-MS analysis of volatile oils from Bupleurum chinense DC. f. vanheurckii (Muell.-Arg.) Shan et Y. Li. J Med Plants Res 6:926–928. doi: 10.5897/JMPR11.1174. [DOI] [Google Scholar]
- 28.Dũng NX, Chính TD, Rãng DD, Leclercq PA. 1994. Chemical composition of the leaf oil of Alpinia breviligulata Gagnep. from Vietnam. J Essent Oil Res 6:181–182. doi: 10.1080/10412905.1994.9698350. [DOI] [Google Scholar]
- 29.Zhang B, Watts KM, Hodge D, Kemp LM, Hunstad DA, Hicks LM, Odom AR. 2011. A second target of the antimalarial and antibacterial agent fosmidomycin revealed by cellular metabolic profiling. Biochemistry 50:3570–3577. doi: 10.1021/bi200113y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alvarez-Llamas G, de la Cuesta F, Barderas MG, Zubiri I, Posada-Ayala M, Vivanco F. 2013. Characterization of membrane and cytosolic proteins of erythrocytes, p 71–80. In Vivanco F (ed), Vascular proteomics. Humana Press, New York, NY. [DOI] [PubMed] [Google Scholar]
- 31.Goodman SR, Daescu O, Kakhniashvili DG, Zivanic M. 2013. The proteomics and interactomics of human erythrocytes. Exp Biol Med (Maywood) 238:509–518. doi: 10.1177/1535370213488474. [DOI] [PubMed] [Google Scholar]
- 32.Carey AF, Wang G, Su CY, Zwiebel LJ, Carlson JR. 2010. Odorant reception in the malaria mosquito Anopheles gambiae. Nature 464:66–71. doi: 10.1038/nature08834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hallem EA, Ho MG, Carlson JR. 2004. The molecular basis of odor coding in the Drosophila antenna. Cell 117:965–979. doi: 10.1016/j.cell.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 34.Dobritsa AA, van der Goes van Naters W, Warr CG, Steinbrecht RA, Carlson JR. 2003. Integrating the molecular and cellular basis of odor coding in the drosophila antenna. Neuron 37:827–841. doi: 10.1016/S0896-6273(03)00094-1. [DOI] [PubMed] [Google Scholar]
- 35.Pitts R, Rinker DC, Jones PL, Rokas A, Zwiebel LJ. 2011. Transcriptome profiling of chemosensory appendages in the malaria vector Anopheles gambiae reveals tissue- and sex-specific signatures of odor coding. BMC Genomics 12:271. doi: 10.1186/1471-2164-12-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips M, Herrera J, Krishnan S, Zain M, Greenberg J, Cataneo RN. 1999. Variation in volatile organic compounds in the breath of normal humans. J Chromatogr B Biomed Sci Appl 729:75–88. doi: 10.1016/S0378-4347(99)00127-9. [DOI] [PubMed] [Google Scholar]
- 37.Lu JZ, Lee PJ, Waters NC, Prigge ST. 2005. Fatty acid synthesis as a target for antimalarial drug discovery. Comb Chem High Throughput Screen 8:15–26. doi: 10.2174/1386207053328192. [DOI] [PubMed] [Google Scholar]
- 38.Adu-Gyasi D, Adams M, Amoako S, Mahama E, Nsoh M, Amenga-Etego S, Baiden F, Asante KP, Newton S, Owusu-Agyei S. 2012. Estimating malaria parasite density: assumed white blood cell count of 10,000/mu l of blood is appropriate measure in Central Ghana. Malar J 11:238. doi: 10.1186/1475-2875-11-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson RA, Knols BG, Koella JC. 2000. Plasmodium falciparum sporozoites increase feeding-associated mortality of their mosquito hosts Anopheles gambiae sl. Parasitology 120:329–333. doi: 10.1017/S0031182099005570. [DOI] [PubMed] [Google Scholar]
- 40.Koella JC. 1999. An evolutionary view of the interactions between anopheline mosquitoes and malaria parasites. Microbes Infect 1:303–308. doi: 10.1016/S1286-4579(99)80026-4. [DOI] [PubMed] [Google Scholar]
- 41.Rossignol PA, Ribeiro JM, Spielman A. 1986. Increased biting rate and reduced fertility in sporozoite-infected mosquitoes. Am J Trop Med Hyg 35:277–279. [DOI] [PubMed] [Google Scholar]
- 42.Nyasembe VO, Teal PE, Sawa P, Tumlinson JH, Borgemeister C, Torto B. 2014. Plasmodium falciparum infection increases Anopheles gambiae attraction to nectar sources and sugar uptake. Curr Biol 24:217–221. doi: 10.1016/j.cub.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cassera MB, Gozzo FC, Fabio L, Merino EF, Del Portillo HA, Peres VJ, Almeida IC, Eberlin MN, Wunderlich G, Wiesner J, Jomaa H, Kimura EA, Katzin AM. 2004. The methylerythritol phosphate pathway is functionally active in all intraerythrocytic stages of Plasmodium falciparum. J Biol Chem 279:51749–51759. doi: 10.1074/jbc.M408360200. [DOI] [PubMed] [Google Scholar]
- 44.Jordão FM, Gabriel HB, Alves JM, Angeli CB, Bifano TD, Breda A, de Azevedo MF, Basso LA, Wunderlich G, Kimura EA, Katzin AM. 2013. Cloning and characterization of bifunctional enzyme farnesyl diphosphate/geranylgeranyl diphosphate synthase from Plasmodium falciparum. Malar J 12:184. doi: 10.1186/1475-2875-12-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Artz JD, Wernimont AK, Dunford JE, Schapira M, Dong A, Zhao Y, Lew J, Russell RG, Ebetino FH, Oppermann U, Hui R. 2011. Molecular characterization of a novel geranylgeranyl pyrophosphate synthase from plasmodium parasites. J Biol Chem 286:3315–3322. doi: 10.1074/jbc.M109.027235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bohlmann J, Meyer-Gauen G, Croteau R. 1998. Plant terpenoid synthases: molecular biology and phylogenetic analysis. Proc Natl Acad Sci U S A 95:4126–4133. doi: 10.1073/pnas.95.8.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Degenhardt J, Köllner TG, Gershenzon J. 2009. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 70:1621–1637. doi: 10.1016/j.phytochem.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 48.Trager W, Jensen JB. 1976. Human malaria parasites in continuous culture. Science 193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 49.Folch J, Lees M, Sloane stanley GH. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Total ion chromatograph (TIC) of Plasmodium parasite and red blood cell volatile organic compounds. (Top) Typical chromatograph of SPME fibers exposed to Plasmodium falciparum-infected red blood cells. Marked peaks represent dominant compounds in each trace, many of which represent known contaminant compounds (asterisks, silyated background compounds). Red, parasite-specific peaks from a representative parasite sample compared to the uninfected control. (Bottom) Typical chromatograph of SPME fibers exposed to uninfected red blood cells. (A) Hydrogen azide; (B) n-butane; (C) n-hexane; (D) toluene; (E) 2,4-dimethyl heptane; (F) 2,3-dimethyl heptane; (G) 4-methyl octane; (H) methoxy-phenyl-oxime; (I) benzaldehyde; (J) octanal; (K) 2-ethyl 1-hexanol; (L) 2,3,6,7-tetramethyl octane; (M) dodecane; (N) 1,4-dimethyl-trans-cyclooctane. Download
Terpenes exclusive to headspace gas of infected red blood cells. (Top) Overlay of typical extracted ion chromatograms for the base peak ion of 4,5,9,10-dehydro-isolongifolene, m/z 143. The arrow indicates the retention time of 4,5,9,10-dehydro-isolongifolene, 13.1 min. (Bottom) Overlay of typical extracted ion chromatograms for the parent peak ion of 8,9-dehydro-9-formyl cycloisolongifolene, m/z 230. The arrow indicates the retention time of 8,9-dehydro-9-formyl cycloisolongifolene, 12.1 min. Download
Heat map of terpene annotation predictions. Each column represents compounds identified from SPME sampling replicates from five independent biological samples of P. falciparum-infected RBCs. Not represented are compounds also present in RBCs or identified solely from nonpolar organic extractions. The gray scale represents the confidence in the match ID given by NIST, based on the similarity between the ion spectra in the sample and the reference spectra from the NIST library. White represents 0% probability, indicating the spectrum was not found in the TIC, and black represents 80% probability (the highest match of all annotated compounds), indicating the terpene was found in the TIC with an ion spectrum that closely matched the reference spectrum in the NIST library. Heat map includes all entities annotated as terpenes; monoterpenes are indicated in boldface. Download
Anopheles odorant receptor stimulation by parasite monoterpenes. Responses of Anopheles receptors in the presence of additional terpene isomers. (−)-Limonene had no major differences in its activation profile from (+)-limonene, and α-(−)-pinene, β-(+)-pinene, and β-(−)-pinene had no major differences in their activation profiles from that for α-(+)-pinene. Download