ABSTRACT
Nipah virus and Hendra virus are emerging, highly pathogenic, zoonotic paramyxoviruses that belong to the genus Henipavirus. They infect humans as well as numerous mammalian species. Both viruses use ephrin-B2 and -B3 as cell entry receptors, and following initial entry into an organism, they are capable of rapid spread throughout the host. We have previously reported that Nipah virus can use another attachment receptor, different from its entry receptors, to bind to nonpermissive circulating leukocytes, thereby promoting viral dissemination within the host. Here, this attachment molecule was identified as heparan sulfate for both Nipah virus and Hendra virus. Cells devoid of heparan sulfate were not able to mediate henipavirus trans-infection and showed reduced permissivity to infection. Virus pseudotyped with Nipah virus glycoproteins bound heparan sulfate and heparin but no other glycosaminoglycans in a surface plasmon resonance assay. Furthermore, heparin was able to inhibit the interaction of the viruses with the heparan sulfate and to block cell-mediated trans-infection of henipaviruses. Moreover, heparin was shown to bind to ephrin-B3 and to restrain infection of permissive cells in vitro. Consequently, treatment with heparin devoid of anticoagulant activity improved the survival of Nipah virus-infected hamsters. Altogether, these results reveal heparan sulfate as a new attachment receptor for henipaviruses and as a potential therapeutic target for the development of novel approaches against these highly lethal infections.
IMPORTANCE
The Henipavirus genus includes two closely related, highly pathogenic paramyxoviruses, Nipah virus and Hendra virus, which cause elevated morbidity and mortality in animals and humans. Pathogenesis of both Nipah virus and Hendra virus infection is poorly understood, and efficient antiviral treatment is still missing. Here, we identified heparan sulfate as a novel attachment receptor used by both viruses to bind host cells. We demonstrate that heparin was able to inhibit the interaction of the viruses with heparan sulfate and to block cell-mediated trans-infection of henipaviruses. Moreover, heparin also bound to the viral entry receptor and thereby restricted infection of permissive cells in vitro. Consequently, heparin treatment improved survival of Nipah virus-infected hamsters. These results uncover an important role of heparan sulfate in henipavirus infection and open novel perspectives for the development of heparan sulfate-targeting therapeutic approaches for these emerging infections.
INTRODUCTION
Nipah virus (NiV) and the closely related Hendra virus (HeV) are emerging zoonotic pathogens that have been classified in the new Henipavirus genus, within the Paramyxoviridae family. Both viruses cause considerable morbidity and mortality in numerous mammalian species, including humans. HeV first appeared in 1994 in Australia (1), while NiV emerged in Southeast Asia in 1998 (2), where it continues to cause regular outbreaks with very high mortality rates, between 50 and 100% (3). The natural hosts for both viruses are fruit bats (Pteropidae family), with a wide distribution in Australia, Southeast Asia, India, and Africa. Potential new virus spillovers thus present a constant risk for future outbreaks (3). The endotheliotropism of these henipaviruses is responsible for systemic infections with generalized vasculitis and may be associated with severe acute respiratory syndrome and encephalitis (3). Both viruses are classified as biosafety level 4 (BSL4) pathogens and present important biosecurity threats (4). There is currently neither a vaccine nor approved treatment against human henipavirus infection.
Henipaviruses have two membrane glycoproteins: the attachment protein (G), which binds the ephrin-B2 (EFN-B2) and/or EFN-B3 entry receptor, which are common to both NiV and HeV (5–7), and the fusion protein (F), which is responsible for virus entry into the cell cytoplasm via fusion of viral and cellular membranes. NiV has been found to use another unknown attachment receptor to bind to nonpermissive circulating leukocytes, thereby promoting viral dissemination within the host and trans-infection of permissive target cells (8). This attachment molecule was sensitive to proteolytic degradation, and virus internalization was not required for cell-mediated trans-infection. As proteoglycans are used by several viruses for binding to target cells (9, 10), we investigated their potential role as a henipavirus attachment receptor.
Heparan sulfate (HS) is a complex and sulfated polysaccharide of the glycosaminoglycan (GAG) family that is linked to ubiquitous core proteins of the cell surface and extracellular matrix of all eukaryotes. HS is composed of a repetition of a d-glucuronic acid/N-acetyl-d-glucosamine disaccharide motif that can be further modified by addition of sulfate groups. HS has the ability to bind to a vast repertoire of proteins and is involved in many physiological as well as pathological processes (11). In addition, the long carbohydrate chains of HS provide easily accessible binding sites for a wide range of pathogens, including viruses, bacteria, and parasites (11, 12). Commercial heparin (HP), which is widely used for its anticoagulant properties, is a GAG that is chemically related to HS (13, 14) and displays protein-binding properties similar to HS.
In this study, we show that both NiV and HeV can use HS as an attachment receptor to mediate trans-infection. In addition, HS facilitates henipavirus infection in cis, and heparin can compete with HS for binding to the virus, thereby limiting both trans-infection and infection itself. Finally, heparin significantly reduced NiV infection in hamsters, suggesting its potential use to treat henipavirus infections.
RESULTS
Henipavirus uses HS for trans-infection.
We first asked whether leukocytes could capture HeV and transmit it to susceptible cells in trans without becoming infected themselves (Fig. 1A). As we previously found for NiV (8), peripheral blood lymphocytes (PBLs) also transmit cell-attached HeV to susceptible cells, indicating that trans-infection is a mechanism shared by both members of the Henipavirus genus.
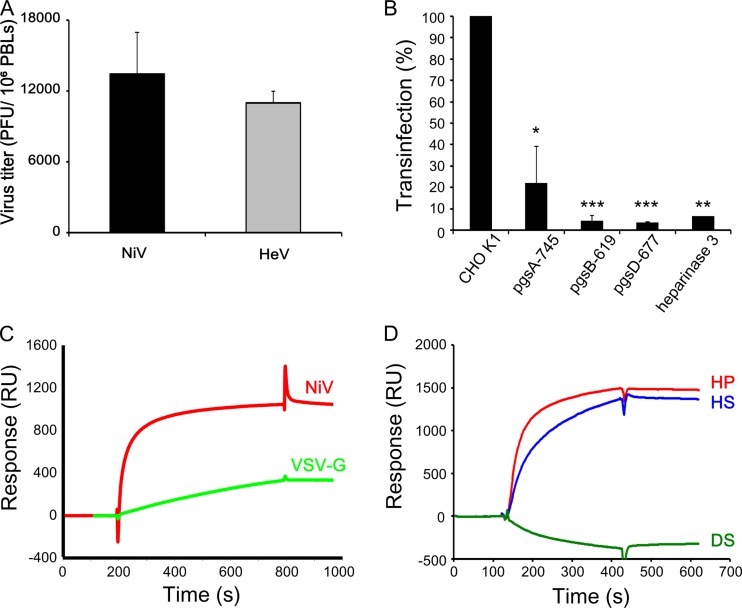
FIG 1 .
trans-Infection with henipaviruses requires the expression of HS. (A) Lymphocyte-mediated trans-infection by NiV and HeV. PBLs were incubated with either NiV or HeV, washed, cultured for 24 h, and then transferred to Vero cell monolayers, which were used for the determination of the viral titers after 4 days of coculture, using infectious center assays. (B) CHO-K1 cells, treated or not with heparinase 3, and three HS-deficient CHO lines, pgsA-745, pgsB-619, and pgsD-677, were incubated with NiV and analyzed for their capacities to transmit infection to susceptible Vero cells in trans. Results are expressed as a percentage of inhibition compared to results with untreated cells ± SD. *, P < 0.05; ***, P < 0.001 (Mann-Whitney U test). (C) SPR analysis of the binding of MLV pseudotyped either with VSV-G (green) or with NiV glycoproteins G and F (red) to HP-activated sensor chip surfaces. (D) SPR analysis of the binding of NiV G and F pseudoparticles to surfaces activated by either HS (blue), DS (green), or HP (red). The binding response, in RU, was recorded as a function of time; results from 1 of 3 experiments are presented.
The trans-infection by leukocytes can also be mimicked in CHO cells (8), which are resistant to henipavirus infection due to the lack of entry receptors EFN-B2 and -B3 (7). The treatment of CHO-K1 cells with heparinase 3 inhibited their trans-infection properties by >90%. Accordingly, CHO-derived cell lines that lacked expression of HS because of their deficiency in GAG biosynthesis enzymes (15) were also highly deficient in their ability to mediate trans-infection of NiV (Fig. 1B). To further characterize the NiV-GAG interaction, we compared the ability of virus particles pseudotyped with NiV G and F glycoproteins or vesicular stomatitis virus G-protein (VSV-G) (8) to bind surfaces coated with the HS analog heparin by using surface plasmon resonance (SPR). The strong hyperbolic association shape observed during the injection phase of the sensorgram for NiV, but not for VSV pseudoparticules, clearly indicated a much stronger binding affinity of NiV-pseudotyped viral particles (Fig. 1C), underlining the importance of NiV glycoproteins in the analyzed interaction. To assess the specificity of the NiV-GAG interaction toward HS, by using SPR we compared the binding of NiV-pseudotyped virus to surfaces displaying HS, HP, or dermatan sulfate (DS), another sulfated GAG (Fig. 1D). Although NiV-pseudotyped virus efficiently bound to HP and HS, we did not detect any interaction with the DS surface, suggesting an implication of specific carbohydrate features. Altogether, these results suggested that NiV could specifically use HS as an attachment receptor to promote efficient trans-infection.
Heparin is a competitive inhibitor of henipavirus trans-infection.
As NiV-pseudotyped virus binds heparin in addition to HS (Fig. 1D), we next analyzed the effect of heparin on the ability of PBLs and CHO-K1 to mediate NiV trans-infection. Heparin reduced the trans-infection property of PBLs and CHO-K1 cells by 80% and 90%, respectively (Fig. 2A). Strikingly, heparin was also active when applied after contact with the virus, and both pretreatment and posttreatment with heparin were effective in inhibiting human PBL-mediated trans-infection of either NiV or HeV (Fig. 2B). This inhibition reflects the known capacity of heparin to bind multiple cell surface proteins due to its high negative charge (14). Furthermore, in a pretreatment regimen, heparin also prevented the binding of NiV to CHO-K1 cells by 99%, as measured by cell-associated viral RNA, while the residual binding to the HS-defective pgsA-745 CHO cell line remained unaffected (Fig. 2C). These results indicated the ability of heparin to elute viral particles from cell surface HS by competition.
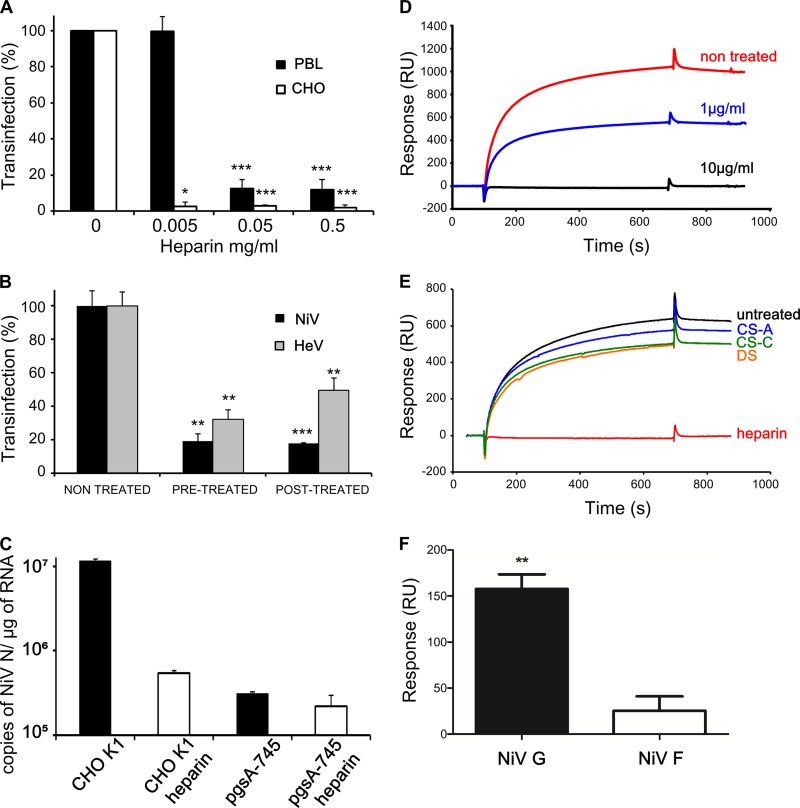
FIG 2 .
Heparin inhibits the interaction between henipavirus and HS. (A) PBLs or CHO-K1 cells were incubated for 30 min with the indicated doses of heparin before being put into contact with NiV. (B) PBLs were treated for 30 min with 0.5 mg/ml of heparin before contact with either NiV or HeV (pretreatment) or after 1 h of incubation with the virus (posttreatment). trans-Infection of Vero cells was than determined as described for Fig. 1. Results are expressed as a percentage of inhibition compared to results in untreated cells, ± the SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Mann-Whitney U test). (C) Analysis of NiV binding to CHO-K1 and HS-deficient CHO-pgsA-745 cells, pretreated or not with heparin (0.5 mg/ml). Cells were put into contact with NiV for 24 h, and the number of viral RNA (N gene) copies was determined by RT-qPCR. (D) SPR analysis of NiV pseudoparticle binding to an HP-activated surface in the absence or presence of soluble heparin at 1 µg/ml or 10 µg/ml. (E) SPR analysis of the binding of NiV pseudoparticles to HP-activated sensor chips, after preincubation with either PBS or 10 µg/ml of soluble heparin, CS-A, CS-C, or DS. (F) SPR analysis of the binding of NiV pseudoparticles, expressing either NiV-G or NiV-F protein, to HP-activated sensor chips. The binding response, in RU, was recorded as a function of time after removal of the background provided by nonpseudotyped particles. Results are presented as the averages of 3 separate experiments (± standard errors of the means) and reflect statistically significant binding of NiV-G to HP. **, P < 0.01 (one-sample t test).
Accordingly, soluble heparin was able to compete and inhibit NiV-pseudotyped binding to immobilized HP or HS by SPR (Fig. 2D and data not shown) with 50% and ~100% reductions observed for 1 µg/ml and 10 µg/ml heparin, respectively. Notably, other GAGs found on cell surface proteoglycans, such as chondroitin sulfate A (CS-A), CS-C, and DS, were devoid of inhibition properties (Fig. 2E), revealing the specificity of the inhibition of NiV binding to HS by heparin. Finally, to determine which of two NiV membrane glycoproteins, F or G, was responsible for binding to heparin, we analyzed HP binding of pseudotyped NiV expressing either NiV-F or -G by SPR (Fig. 2F). Significantly more binding was obtained with NiV-G than with NiV-F (Fig. 2F), and results were further confirmed using HeLa cells transfected with either NiV-G or -F (data not shown). We concluded from these results that NiV trans-infection requires binding to cell surface HS via NiV-G, a process that can be inhibited by competition with heparin.
HS facilitates henipavirus infection in cis.
As GAGs may play multiple roles in viral infection (11, 16), we next investigated whether the HS could be directly involved in henipavirus infection in cis, in addition to its role in trans-infection. Permissive Vero cells were treated with heparinase 3, to remove cell surface HS, prior to the infection with NiV. This resulted in a modest but significant reduction in the number of infected cells at 3 days postinfection (Fig. 3A). Furthermore, the inhibition of HS sulfation in Vero cells after treatment with sodium chlorate significantly reduced infection with both NiV (Fig. 3B) and HeV (Fig. 3C), the latter being much more sensitive to sodium chlorate pretreatment than the former. The obtained results therefore suggest that HS sulfation status is determinant for virus attachment to cells and subsequent infectivity.
FIG 3 .
HS plays a role in henipavirus infection. (A) Vero cells were either treated with heparinase 3 or left untreated prior to NiV infection. Titration was performed 3 days later in a plaque assay. (B and C) Vero cells were treated with increasing concentrations of sodium chlorate for 48 h and then infected with either NiV (B) or HeV (C); titration was performed 3 days later in a plaque assay. Results are expressed as a percentage of results for nontreated controls from triplicate cultures, ± the SD. *, P < 0.05; **, P < 0.01; ***, P < 0.01 (Mann-Whitney U test).
Heparin inhibits infection in vitro.
We then tested whether soluble GAGs could interfere with henipavirus infection in cis. Heparin pretreatment of Vero cells reproducibly reduced the number of cells infected by either NiV or HeV (Fig. 4A). Furthermore, pretreatment of either the cells or the virus was efficient, even with very low concentrations (5 µg/ml) (Fig. 4B). To distinguish the contribution of heparin-mediated inhibition of NiV-G binding to cell surface HS from binding to an EFN-B2/3 entry receptor, HS-negative CHO-pgsA-745 cells stably expressing either EFN-B2 or -B3 (7) were pretreated with 0.5 µg/ml of heparin and infected with NiV (Fig. 4C). Heparin treatment modestly, but significantly, reduced the percentage of infected cells from both cell lines, indicating that this molecule may also directly inhibit or delay the binding of NiV to its entry receptors EFN-B2 and -B3. This effect may be the consequence of direct heparin binding to EFN-B2 and/or -B3, as recently suggested (17). Indeed, SPR analysis suggested that both CHO-pgsA-745–EFN-B2 and -B3 cells bound to heparin, in contrast to nontransfected CHO-pgsA-745 cells (Fig. 4D), and proportionally to the level of expressed EFN-B2 and -B3 transcripts (Fig. 4E). Accordingly, soluble recombinant EFN-B3 bound to heparin in the SPR assay as well (Fig. 4F), fitting a 1:1 Langmuir binding model and yielding a Kd of 16 ± 6 nM (mean ± standard deviation [SD]) for the interaction. Additional SPR analysis showed interactions of EFN-B3 with both HS and heparin, but not with CS (data not shown). Thus, by its ability to compete with HS for binding to NiV-G and to interfere with EFN-B2/B3 receptors, heparin can restrict both trans- and cis-infection by henipavirus.
FIG 4 .
Heparin inhibits infection and limits viral binding to EFN-B2 and -B3. (A) Vero cells were treated with heparin for 30 min before infection with NiV or HeV. Results are expressed as a percentage relative to results in nontreated controls. (B) Vero cells were treated with increasing concentrations of heparin for 30 min before infection with NiV; alternatively, virus was incubated with heparin before contact with cells. Viral titration was performed via a plaque assay, and results are expressed as a percentage of the results in nontreated controls. (C) NiV infection of CHO-pgsA-745–EFN-B2 or –EFN-B3 cells, pretreated with heparin (0.5 mg/ml; 30 min, 37°C) and analyzed in a plaque assay. Results are expressed as the mean percentage relative to results in nontreated controls ± the SD from 8 different experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.01 (Mann-Whitney U test). (D) SPR analysis of binding of CHO-pgsA-745, CHO-pgsA-745–EFN-B2, and CHO-pgsA-745–EFN-B3 to heparin. Results are presented as means ± SD of 4 independent experiments. (E) Quantification results for EFN-B2 and EFN-B3 mRNA expression levels in stably transfected CHO-pgsA-745 cells via RT-qPCR. (F) SPR analysis of the binding of soluble EFN-B3 (0 to 250 nM, as indicated), injected over HP-activated sensor chips; results from triplicate experiments were analyzed. The binding response, in RU, was recorded as a function of time.
Heparin treatment restricts Nipah virus infection in animals.
Well known for its anticoagulant properties, heparin binds and activates anti-thrombin III (AT-III) through a specific pentasaccharide sequence (18, 19). To test the antiviral effect of heparin during NiV infection in vivo and to avoid potential hemorrhagic complications, we produced heparin lacking anticoagulant activity by using periodate oxidation (PO-heparin), which alters the integrity of the AT-III-binding pentasaccharide motif (13). Since PO-heparin inhibited lymphocyte-mediated NiV trans-infection in vitro similarly to heparin (Fig. 5A), we tested its antiviral properties in the golden hamster model of NiV infection, which closely reproduces the NiV pathogenesis seen in humans (20). While all nontreated animals succumbed to infection in less than 6 days, survival in the PO-heparin-treated group increased moderately (P = 0.017) (Fig. 5B), thus suggesting a biological relevance for NiV-HS interaction and revealing potential antiviral properties of heparin-like molecules in vivo.
FIG 5 .
Antiviral effect of PO-heparin. (A) In vitro comparison of the inhibitory effects of heparin and PO-heparin (0.5 mg/ml) on the trans-infection ability of leukocytes treated before contact with NiV. The NiV titer was measured in an infectious center assay, and results are expressed as the percentage of inhibition compared to results in untreated cells. (B) Groups of 5 hamsters were either left untreated or treated daily by subcutaneous injections of PO-heparin (10 mg/kg) for 12 days. Animals were infected intraperitoneally with 500 LD50s of NiV on the first day of treatment and followed for 3 weeks. The results are expressed as the percentage of surviving animals in each group. Survival was significantly increased in the group of treated animals. *, P = 0.017 (Mantel-Cox test).
DISCUSSION
The rapid dissemination of Nipah virus and Hendra virus in an infected host may play a critical role in the high pathogenicity of henipaviruses. Our previous work demonstrated that human peripheral blood lymphocytes are not permissive to NiV infection but that they could capture the virus and transmit it to susceptible target cells in trans (8). In contrast to human lymphocytes, specific subsets of porcine lymphocytes could be infected with NiV and thus participate in the transmission of the virus in the swine host, also in cis (21). Low levels of viral replication were detected in human dendritic cells, suggesting that this cell population could contribute to transmission of NiV both in cis and in trans (8). Recently, a CD169-dependent trans-infection pathway in dendritic cells was demonstrated for henipaviruses (22); however, the molecular mechanism of NiV trans-infection mediated by lymphocytes, which do not express CD169, has remained obscure.
This report reveals HS to be a cell surface attachment receptor for both NiV and HeV that is implicated in the trans-infection properties of human leukocytes as well as in henipavirus infection in cis. HS has been shown to bind many different viruses by interacting with either one or more viral glycoproteins. Interactions between HS and the HIV-1 envelope glycoprotein gp120 (23, 24) and with vaccinia virus A27L protein (25) have been demonstrated. Furthermore, HS can bind measles virus hemagglutinin (H) (26) and the fusion (F) proteins of respiratory syncytial virus (RSV) (27) and human metapneumovirus (28). In the cases of bovine RSV and canine distemper virus, both H and F seem to interact with HS (29, 30). Our results suggest that NiV-G interacts with HS. Although VSV-G protein has been shown to interact with HS (31), our SPR analysis indicated that binding of NiV-G to HS had a higher affinity. Further studies using recombinant soluble viral G protein should give better insights into the biochemical characteristics of this interaction and identify binding sites on both ligands. HS has been previously suggested to capture, protect, and transmit HIV-1 (9) and human T-cell lymphotropic virus type 1 (HTLV-1) (10) in trans. Similarly to what was shown for HIV-1 (23), the other sulfated GAGs, such as CS or DS, were inactive, implying that structural features beyond charge density on the polysaccharide backbone could be important for the activity. The implication is that different HS-binding sites may be involved, as shown recently for papillomavirus, for which sequential engagement of three different HS-binding sites leads to virus attachment, interaction with the receptor, and entry into the cell (32). Likewise, HS stabilizes varicella-zoster virus, making it more accessible for binding to its entry receptor (33). Thus, similar to its role in other viral infections, HS could improve the contact of henipavirus with host cells so as to help the virus reach the cellular receptors EFN-B2 and -B3 (summarized schematically in Fig. 6).
FIG 6 .
Schematic presentation of possible implications of heparan sulfate and heparin in henipavirus infection. (A) NiV and HeV interact with their entry receptors, EFN-B2 and -B3, and with HS. While the first interaction is important for virus infection in cis, the second leads to infection in trans and may facilitate virus dissemination in the host. In addition, HS may help the virus to reach its entry receptors and accumulate on the cell surface and/or stabilize the interaction with EFN (B2 and B3). (B) Heparin binds henipavirus G-protein as well as ephrin receptors and may thus displace the virus from the cell surface and prevent it from reaching its entry receptors. Consequently, heparin inhibits infection in trans and restrains direct infection in cis, respectively.
We observed that heparin inhibits henipavirus trans-infection in vitro. Owing to its capacity to bind multiple cell surface proteins via negatively charged sulfated groups (14), heparin could prevent henipavirus attachment to cell surface HS. As it is more sulfated than HS, it could also bind viruses with higher affinity than HS and thus act as an efficient competitive inhibitor for virus binding. Furthermore, heparin significantly reduces direct virus infection in cis, in addition to affecting infection in trans. This effect may also result from the interaction between heparin and henipavirus receptors, as evidenced by SPR for EFN-B3 and in agreement with a recent report (17). These results allowed us to propose a model for the role of HS in henipavirus binding and entry and possible interference of heparin with viral infection (Fig. 6). In addition to the inhibition of henipavirus infection by binding to either viral G glycoprotein or viral entry receptors, heparin may significantly limit virus spread within the organism by blocking binding to the attachment receptor HS and consecutive trans-infection of permissive cells.
The mechanism by which a heparin molecule devoid of anticoagulation properties (13) restrains virus infectivity in vivo most likely depends on the combination of its different biological activities. In addition to affecting henipavirus infection in cis and in trans, heparin could constrain inflammation and consequent tissue damage by binding various inflammatory molecules. Indeed, both heparin and HS bind to important immunoregulatory molecules, such as the chemokine CXCL10 (34), which is strongly upregulated during NiV infection (35). Moreover, as heparin is a heavily sulfated electronegative molecule, it might enhance the antiadhesive properties of the endothelium against leukocytes (36). This effect may rely on the ability of heparin to bind directly to several adhesion molecules that are expressed during inflammation, including L-selectin (37), CD11b/MAC1 (38), and P-selectin (39). Finally, heparin-like derivatives can stabilize the endothelium (40) and help in blocking the passage of both humoral and cellular immune factors, as well as viruses, through the blood-brain barrier (14, 41). This process may also contribute to the antiviral effect observed during in vivo experiments, together providing a “proof of concept” for further development of this antiviral approach.
The heparin-mediated inhibition of henipavirus infection both in vitro and in vivo highlights the antiviral potential of this GAG, which is well tolerated and has already been used in the clinical environment as an anticoagulant for more than 50 years. Indeed, heparin treatment reduces NiV infection in a hamster animal model, thus opening interesting therapeutic perspectives to complement treatment of this highly lethal infection. Additionally, the acute nature of henipavirus infection makes it more prone to the regulatory action of heparin, compared to some chronic infections, including HIV or HTLV, where heparin showed in vitro antiviral activity (9, 10). The HS mimetic PI-88 has already been shown to have significant beneficial effect in vivo in the outcome of dengue virus and encephalitic flavivirus infections (42). The use of derivatives that mimic the heparin/HS structure (43), synthetic antilipopolysaccharide peptides that bind HS moieties on cell surfaces (44), or polyanionic compounds with longer half-lives in vivo (40), devoid of anticoagulant activity and with potentially higher affinity to henipavirus G-protein, may further improve therapeutic effects.
Altogether, this study demonstrates a previously unrecognized HS-henipavirus interaction involved in both NiV and HeV infection and dissemination within the host. It may allow henipaviruses to bind to different circulating cells, to use them for transport for efficient spreading within the host organism, and for target cells, to allow accumulation on their surface, enhancing the efficacy of infection in cis. These results reveal heparan sulfate as a potential therapeutic target for the development of novel approaches against these highly lethal infections. In this context, heparin or its derivatives may be used in a metaphylaxis approach of virus-exposed and potentially infected animals, before the appearance of clinical symptoms, during regular Hendra equine epizootics in Australia (3).
MATERIALS AND METHODS
Ethics statement.
Venous blood from anonymous healthy human volunteers was obtained from the Blood Transfusion Centre (Etablissement Francais du Sang, Lyon, France) in accordance with its guidelines, with informed written consent from each volunteer.
All animals were handled in strict accordance with good animal practices as defined by the French National Charter on the ethics of animals experiments, and all efforts were made to minimize suffering. Animal work was approved by the regional ethical committee CECCAPP (P4_2010_008), and experiments were performed in the INSERM Jean Mérieux BSL4 laboratory in Lyon, France (French Animal regulation commitee number A69 387 05 02).
Cell culture.
The CHO cell line K1 and CHO pgsA-745 cells stably transfected with human ephrin-B2 and -B3, (pgsA-EFNB2 and pgsA-EFNB3; all generously provided by B. Lee [UCLA, United States] [7]) were maintained in F-12 medium (Invitrogen) supplemented with 10% fetal calf serum (FCS), 100 U/ml penicillin, 0.1 mg streptomycin, 10 mM HEPES, and 2 mM l-glutamine at 37°C in 5% CO2. Vero E6, HS-deficient CHO-K1 cells CHO pgsA-745, CHO pgsB-619, and CHO pgsD-677 cells (15), HeLa cells, and stable transfected HeLa cells with phCMV-Nipah-G-Neo and phCMV-Nipah-F-Neo, expressing NiV-G and -F proteins, respectively (HeLa-G and HeLa-F), were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen) supplemented as described above. Human peripheral blood was obtained from 20 different healthy donors from the Blood Transfusion Centre (Lyon, France). Peripheral blood mononuclear cells were isolated by density Ficoll/Hypaque gradient centrifugation and then centrifuged through a 50% Percoll gradient (Pharmacia Fine Chemicals) for 20 min at 400 × g. PBLs were recovered from the high-density fraction. For SPR analysis, cells were detached by Versene treatment and washed twice with HBS-P buffer (10 mM HEPES, 150 mM NaCl, 0.005% surfactant P20, pH 7.4).
Virus infection and titration.
Nipah virus (isolate UMMC1; GenBank accession number AY029767) (42), recombinant NiV expressing enhanced green fluorescent protein (45), and Hendra virus (Australia/horse/1994) obtained from Porton Down Laboratory, United Kingdom, were prepared on Vero-E6 cells as described previously (46), and infection virus was used in the INSERM Jean Mérieux BSL4 laboratory in Lyon, France. All cells were infected at a multiplicity of infection (MOI) of 1, for 1 h at 37°C, washed twice, and observed by inverted and/or fluorescence microscopy daily or harvested for RNA isolation or for use in trans-infection assays. At the indicated times postinfection, 150 µl of cell culture supernatant was collected and frozen prior to viral titration. Viral titration was performed by plaque assay as detailed elsewhere (47). The viral infection level in cocultures of leukocytes and CHO cells with Vero cells was determined using a previously described infectious center assay in 6-well plates (48), after 20 min of formaldehyde fixation and crystal violet staining. Alternatively, in heparin-mediated inhibition assays, titrations were directly performed by plaque assay, using Vero cells or CHO-pgsA745–EFN-B2 or -B3 cell monolayers. Pseudotyped viral particles, containing either NiV-G and/or -F or control VSV-G were produced using Friend’s murine leukemia virus (MLV) particles and 293T cells, as described previously (8).
Treatment of cells with sodium chlorate.
Vero cell monolayers were grown to confluence and maintained in DMEM supplemented with 10% FCS and sodium chlorate (NaClO3; 25, 50 and 75 mM) for 48 h to inhibit HS sulfation in cells, as rapid turnover of proteoglycans in the cells (47) required prolonged incubation with NaClO3. Cells were then detached by using trypsin–0.05% EDTA, distributed into new 6-well tissue culture plates, and grown to confluence in the presence of the same range of concentrations of NaClO3 and infected with either NiV or HeV (50 or 100 PFU/well). Following 72 h of incubation in CMC/DMEM supplemented with 3% FCS and NaClO3, virus was titrated by using crystal violet staining.
trans-infection assay.
Cells were put in contact with virus (MOI, 1) for 1 h at 37°C, 5% CO2. After two washes with phosphate-buffered saline (PBS), cells were cultured at 37°C for 24 h and then collected and washed, and 10-fold serial dilutions were added to cell monolayers of Vero cells for determination of cell-associated infectious NiV in a infectious center assay, as described previously (8). In some experiments, cells were incubated for 1 h at 37°C with 10 U/ml of heparinase 3 (Sigma) and washed 3 times before contact with NiV. Treatment with heparin (porcine intestinal mucosa; Sigma) of either cells or virus and pseudotyped viral particles was performed for 30 min at 37°C. In heparin pretreatment experiments, after incubation with heparin, cells were thoroughly washed before adding the virus. In heparin posttreatment experiments, NiV-resistant cells were initially put in contact with either NiV or HeV for 1 h and incubated with heparin afterward, for 30 min at 37°C, washed, and analyzed in a trans-infection assay as described above.
SPR-based binding assays.
SPR experiments were performed on a BIAcore X apparatus (for analysis of cells and pseudotyped viral particles) or a Biacore 3000 apparatus (for pseudotyped viral particles and recombinant ephrin-B3), using CM4 (for pseudotyped virus and recombinant EFN-B3) and CM5 (for cells) sensor chips and HBS-P buffer. HS (Celsus, Cincinnati, OH, United States), HP (Sigma), and DS (Sigma) were biotinylated and immobilized on Biacore sensor chips, as described before (49). Briefly, two flow cells were activated with a mix of 0.2 M N-ethyl-N′-(diethylaminopropyl)-carbodiimide (EDC) and 0.05 M N-hydroxysuccinimide (NHS). Then, streptavidin (50 µg/ml in 10 mM acetate buffer [pH 4.5]) was injected over the activated flow cells, to obtain an immobilization level of ~2,500 response units (RU). One of these flow cells served as a negative control, while biotinylated HP, HS, and DS were injected on the other flow cells, to obtain suitable immobilization levels (50 to 70 RU for recombinant human ephrin-B3 Fc and pseudotyped viral particles analysis; 200 RU for cells). Interaction assays were performed at a flow rate of 5 µl/min and involved 5- to 10-min injections of sample over the HP and negative-control surfaces, followed by a 5-min washing step with HBS-P buffer to allow dissociation of the complexes formed. At the end of each cycle, GAG surfaces were regenerated by sequential injections of 0.05% SDS (1 min) and 2 M NaCl (2.5 min). Typical sample concentrations used were 10 µg/ml for recombinant human ephrin-B3 Fc (R&D Systems), 1 × 107 to 2.4 × 107 particles/ml for pseudotyped viral particles and 0.5 × 106 to 0.7 × 106 cells/ml for CHO-pgsA-745 and HeLa cell transfectants. The sensorgrams shown correspond to on-line subtraction of the negative-control signal from the GAG surface signal. Competition assays were performed by preincubating NiV pseudoparticles with GAGs (1 to 10 µg/ml, final concentration) for 15 min prior to injection.
RNA isolation and RT-qPCR.
RNA was isolated from cells and plasma by using an RNeasy minikit (Qiagen) in RLT buffer, according to the manufacturer’s instructions. Reverse transcription (RT) was performed on 0.5 µg of total RNA by using oligo(dT) and random hexamer oligonucleotide primers (iScript cDNA synthesis kit; Bio-Rad) and run in a Biometra T-Gradient PCR device, and cDNAs were diluted 1/10. Quantitative PCR (qPCR) was performed with cDNA samples by using Platinum SYBR green qPCR SuperMix-UDG with a ROX kit (Invitrogen). qPCR was run on the StepOne Plus PCR system (Applied Biosystems) as follows: 95°C for 5 min and 40 cycles of 95°C for 15 s and 60°C for 1 min, followed by a melting curve of up to 95°C at 0.8°C intervals. All samples were run in duplicate, and the results were analyzed using StepOne software v2.1. The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was used as a housekeeping gene to normalize the samples. GAPDH and standard references for the corresponding genes were included in each run to check for RNA integrity, RNA load, and inter-PCR variation. After normalization, the results were expressed as the number of mRNA copies of the gene of interest per microgram of analyzed RNA. All calculations were done using the ΔΔCT model (50), and experiments were performed according to the MIQE guideline (51). Primer used included the following: NiV N forward, GGCAGGATTCTTCGCAACCATC, and reverse, GGCTCTTGGGCCAATTTCTCTG; murine GAPDH forward, GCATGGCCTTCCGTGTCC, and reverse, TGTCATCATACTTGGCAGGTTTCT; EFN-B2 forward, CAAGTTCTGCTGGATCAA, and reverse, GATGTTGTTCCCCGAATG, EFN-B3 forward, ATGGAAAGAGACCGAGGG, and reverse, GAGGTTGCATTGCTGGTG.
Production of heparin devoid of anticoagulant activity (PO-heparin).
To eliminate its anticoagulant properties, heparin was treated with periodate, as previously described (52). Briefly, 100 mg of heparin from porcine intestine (185.8 USP units/mg; Sigma) was dissolved in 900 µl of 0.1 M NaIO4 in 0.05 M sodium acetate buffer (pH 5) and stirred at 4°C for 3 days. The unreacted NaIO4 was then neutralized by addition of glycerol (25 µl), dialyzed against H2O, and lyophilized. The sample was resuspended in 800 µl of 0.2 M NaBH4 in 0.25 M ammonium bicarbonate and incubated for a further 3 h at 4°C. After acidification with glacial acetic acid (to eliminate any remaining NaBH4), the reaction mixture was neutralized by addition of NaOH (1 M), dialyzed against water, and lyophilized.
Infection of hamsters.
Eight-week-old golden hamsters (Mesocricetus auratus; Janvier, France) were anesthetized and infected intraperitoneally with 0.4 ml containing NiV (500 50% lethal doses [LD50s] preincubated with heparin [0.5 mg/ml; 30 min at 37°C]). Groups of 5 animals were treated daily subcutaneously with the PO-heparin (10 mg/kg of body weight) for 12 days, starting from the day of infection. Animals were followed daily for 3 weeks.
Statistical analysis.
Data are expressed as means ± SD or as the percentage of survival. Statistical analyses were performed using Mann-Whitney U test, one-sample t test, and Mantel-Cox test within GraphPad’s Prism4 software.
ACKNOWLEDGMENTS
This work was supported by INSERM, ANR-09-MIEN-018-01, and ANR-ASTRID-2011. C.M. was supported by Cluster of Infectiology Rhône-Alpes, and K.D. was supported by a DGA/INSERM research fellowship.
We thank I. Bally and N. Thielens, from the IBS platform of the Partnership for Structural Biology and the Institut de Biologie Structurale in Grenoble for assistance and access to the Biacore facility and members of the group “Immunobiology of Viral Infections” for their help during the realization of the study. In addition, we thank the LabEx ECOFECT (ANR-11-LABX-0048) of Université de Lyon, France, as well as I. Grosjean from the CelluloNet facility (SFR Bioscience Gerland-Lyon-Sud, UMS344/US8) for their help. We are also grateful to B. Lee (UCLA, USA) for providing us EFN-B2- and -B3-expressing CHO cells and to P. Lawrence (CIRI Lyon) for English editing. We also thank D. Cornec, F. Jacquot, A. Duthey, A. Valve, L. Barrot, and other biosafety team members from INSERM BSL4 “Jean Mérieux” for their assistance.
Footnotes
Citation Mathieu C, Dhondt KP, Châlons M, Mély S, Raoul H, Negre D, Cosset F, Gerlier D, Vivès RR, Horvat B. 2015. Heparan sulfate-dependent enhancement of henipavirus infection. mBio 6(2):e02427-14. doi:10.1128/mBio.02427-14.
REFERENCES
- 1.Murray K, Selleck P, Hooper P, Hyatt A, Gould A, Gleeson L, Westbury H, Hiley L, Selvey L, Rodwell B, Ketterer P. 1995. A morbillivirus that caused fatal disease in horses and humans. Science 268:94–97. doi: 10.1126/science.7701348. [DOI] [PubMed] [Google Scholar]
- 2.Chua KB, Bellini WJ, Rota PA, Harcourt BH, Tamin A, Lam SK, Ksiazek TG, Rollin PE, Zaki SR, Shieh W, Goldsmith CS, Gubler DJ, Roehrig JT, Eaton B, Gould AR, Olson J, Field H, Daniels P, Ling AE, Peters CJ, Anderson LJ, Mahy BW. 2000. Nipah virus: a recently emergent deadly paramyxovirus. Science 288:1432–1435. doi: 10.1126/science.288.5470.1432. [DOI] [PubMed] [Google Scholar]
- 3.Marsh GA, Wang LF. 2012. Hendra and Nipah viruses: why are they so deadly? Curr Opin Virol 2:242–247. doi: 10.1016/j.coviro.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Lam SK. 2003. Nipah virus—a potential agent of bioterrorism? Antiviral Res 57:113–119. doi: 10.1016/S0166-3542(02)00204-8. [DOI] [PubMed] [Google Scholar]
- 5.Bonaparte MI, Dimitrov AS, Bossart KN, Crameri G, Mungall BA, Bishop KA, Choudhry V, Dimitrov DS, Wang L-F, Eaton BT, Broder CC. 2005. Ephrin-B2 ligand is a functional receptor for Hendra virus and Nipah virus. Proc Natl Acd Sci U S A 102:10652–10657. doi: 10.1073/pnas.0504887102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Negrete OA, Levroney EL, Aguilar HC, Bertolotti-Ciarlet A, Nazarian R, Tajyar S, Lee B. 2005. EphrinB2 is the entry receptor for Nipah virus, an emergent deadly paramyxovirus. Nature 436:401–405. doi: 10.1038/nature03838. [DOI] [PubMed] [Google Scholar]
- 7.Negrete OA, Wolf MC, Aguilar HC, Enterlein S, Wang W, Mühlberger E, Su SV, Bertolotti-Ciarlet A, Flick R, Lee B.. 2006. Two key residues in ephrinB3 are critical for its use as an alternative receptor for Nipah virus. PLoS Pathog 2:e7. doi: 10.1371/journal.ppat.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathieu C, Pohl C, Szecsi J, Trajkovic-Bodennec S, Devergnas S, Raoul H, Cosset FL, Gerlier D, Wild TF, Horvat B. 2011. Nipah virus uses leukocytes for efficient dissemination within a host. J Virol 85:7863–7871. doi: 10.1128/JVI.00549-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bobardt MD, Saphire AC, Hung H-C, Yu X, Van der Schueren B, Zhang Z, David G, Gallay PA. 2003. Syndecan captures, protects, and transmits HIV to T lymphocytes. Immunity 18:27–39. doi: 10.1016/S1074-7613(02)00504-6. [DOI] [PubMed] [Google Scholar]
- 10.Piñon JD, Klasse PJ, Jassal SR, Welson S, Weber J, Brighty DW, Sattentau QJ. 2003. Human T-cell leukemia virus type 1 envelope glycoprotein gp46 interacts with cell surface heparan sulfate proteoglycans. J Virol 77:9922–9930. doi: 10.1128/JVI.77.18.9922-9930.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vivès RR, Lortat-Jacob H, Fender P. 2006. Heparan sulphate proteoglycans and viral vectors: ally or foe? Curr Gene Ther 6:35–44. doi: 10.2174/156652306775515565. [DOI] [PubMed] [Google Scholar]
- 12.Bernfield M, Götte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. 1999. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem 68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 13.Bourin MC, Lindahl U. 1993. Glycosaminoglycans and the regulation of blood coagulation. Biochem J 289:313–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lever R, Page CP. 2002. Novel drug development opportunities for heparin. Nat Rev Drug Discov 1:140–148. doi: 10.1038/nrd724. [DOI] [PubMed] [Google Scholar]
- 15.Rostand KS, Esko JD. 1997. Microbial adherence to and invasion through proteoglycans. Infect Immun 65:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spillmann D. 2001. Heparan sulfate: anchor for viral intruders? Biochimie 83:811–817. doi: 10.1016/S0300-9084(01)01290-1. [DOI] [PubMed] [Google Scholar]
- 17.Holen HL, Zernichow L, Fjelland KE, Evenroed IM, Prydz K, Tveit H, Aasheim H-C. 2011. Ephrin-B3 binds to a sulfated cell-surface receptor. Biochem J 433:215–223. doi: 10.1042/BJ20100865. [DOI] [PubMed] [Google Scholar]
- 18.Lam LH, Silbert JE, Rosenberg RD. 1976. The separation of active and inactive forms of heparin. Biochem Biophys Res Commun 69:570–577. doi: 10.1016/0006-291X(76)90558-1. [DOI] [PubMed] [Google Scholar]
- 19.Kusche M, Bäckström G, Riesenfeld J, Petitou M, Choay J, Lindahl U. 1988. Biosynthesis of heparin. O-sulfation of the antithrombin-binding region. J Biol Chem 263:15474–15484. [PubMed] [Google Scholar]
- 20.Wong KT, Grosjean I, Brisson C, Blanquier B, Fevre-Montange M, Bernard A, Loth P, Georges-Courbot M-C, Chevallier M, Akaoka H, Marianneau P, Lam SK, Wild TF, Deubel V. 2003. A golden hamster model for human acute Nipah virus infection. Am J Pathol 163:2127–2137. doi: 10.1016/S0002-9440(10)63569-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stachowiak B, Weingartl HM. 2012. Nipah virus infects specific subsets of porcine peripheral blood mononuclear cells. PLoS One 7:e30855. doi: 10.1371/journal.pone.0030855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akiyama H, Miller C, Patel HV, Hatch SC, Archer J, Ramirez NG, Gummuluru S. 2014. Virus particle release from glycosphingolipid-enriched microdomains is essential for dendritic cell-mediated capture and transfer of HIV-1 and henipavirus. J Virol 88:8813–8825. doi: 10.1128/JVI.00992-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mondor I, Ugolini S, Sattentau QJ. 1998. Human immunodeficiency virus type 1 attachment to HeLa CD4 cells is CD4 independent and gp120 dependent and requires cell surface heparans. J Virol 72:3623–3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vivès RR, Imberty A, Sattentau QJ, Lortat-Jacob H. 2005. Heparan sulfate targets the HIV-1 envelope glycoprotein gp120 coreceptor binding site. J Biol Chem 280:21353–21357. doi: 10.1074/jbc.M500911200. [DOI] [PubMed] [Google Scholar]
- 25.Chung CS, Hsiao JC, Chang YS, Chang W. 1998. A27L protein mediates vaccinia virus interaction with cell surface heparan sulfate. J Virol 72:1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terao-Muto Y, Yoneda M, Seki T, Watanabe A, Tsukiyama-Kohara K, Fujita K, Kai C. 2008. Heparin-like glycosaminoglycans prevent the infection of measles virus in SLAM-negative cell lines. Antiviral Res 80:370–376. doi: 10.1016/j.antiviral.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Feldman SA, Audet S, Beeler JA. 2000. The fusion glycoprotein of human respiratory syncytial virus facilitates virus attachment and infectivity via an interaction with cellular heparan sulfate. J Virol 74:6442–6447. doi: 10.1128/JVI.74.14.6442-6447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang A, Masante C, Buchholz UJ, Dutch RE. 2012. Human metapneumovirus (HMPV) binding and infection are mediated by interactions between the HMPV fusion protein and heparan sulfate. J Virol 86:3230–3243. doi: 10.1128/JVI.06706-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujita K, Miura R, Yoneda M, Shimizu F, Sato H, Muto Y, Endo Y, Tsukiyama-Kohara K, Kai C. 2007. Host range and receptor utilization of canine distemper virus analyzed by recombinant viruses: involvement of heparin-like molecule in CDV infection. Virology 359:324–335. doi: 10.1016/j.virol.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 30.Karger A, Schmidt U, Buchholz UJ. 2001. Recombinant bovine respiratory syncytial virus with deletions of the G or SH genes: G and F proteins bind heparin. J Gen Virol 82:631–640. [DOI] [PubMed] [Google Scholar]
- 31.Guibinga GH, Miyanohara A, Esko JD, Friedmann T. 2002. Cell surface heparan sulfate is a receptor for attachment of envelope protein-free retrovirus-like particles and VSV-G pseudotyped MLV-derived retrovirus vectors to target cells. Mol Ther 5:538–546. doi: 10.1006/mthe.2002.0578. [DOI] [PubMed] [Google Scholar]
- 32.Richards KF, Bienkowska-Haba M, Dasgupta J, Chen XS, Sapp M. 2013. Multiple heparan sulfate binding site engagements are required for the infectious entry of human papillomavirus type 16. J Virol 87:11426–11437. doi: 10.1128/JVI.01721-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu Z, Gershon MD, Ambron R, Gabel C, Gershon AA. 1995. Infection of cells by varicella zoster virus: inhibition of viral entry by mannose 6-phosphate and heparin. Proc Natl Acad Sci U S A 92:3546–3550. doi: 10.1073/pnas.92.8.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ranjbaran H, Wang Y, Manes TD, Yakimov AO, Akhtar S, Kluger MS, Pober JS, Tellides G. 2006. Heparin displaces interferon-gamma-inducible chemokines (IP-10, I-TAC, and Mig) sequestered in the vasculature and inhibits the transendothelial migration and arterial recruitment of T cells. Circulation 114:1293–1300. doi: 10.1161/CIRCULATIONAHA.106.631457. [DOI] [PubMed] [Google Scholar]
- 35.Mathieu C, Guillaume V, Sabine A, Ong KC, Wong KT, Legras-Lachuer C, Horvat B. 2012. Lethal Nipah virus infection induces rapid overexpression of CXCL10. PLoS One 7:e32157. doi: 10.1371/journal.pone.0032157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Floris S, van den Born J, van der Pol SM, Dijkstra CD, De Vries HE. 2003. Heparan sulfate proteoglycans modulate monocyte migration across cerebral endothelium. J Neuropathol Exp Neurol 62:780–790. [DOI] [PubMed] [Google Scholar]
- 37.Koenig A, Norgard-Sumnicht K, Linhardt R, Varki A. 1998. Differential interactions of heparin and heparan sulfate glycosaminoglycans with the selectins. Implications for the use of unfractionated and low molecular weight heparins as therapeutic agents. J Clin Invest 101:877–889. doi: 10.1172/JCI1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salas A, Sans M, Soriano A, Reverter JC, Anderson DC, Piqué JM, Panés J. 2000. Heparin attenuates TNF-alpha induced inflammatory response through a CD11b dependent mechanism. Gut 47:88–96. doi: 10.1136/gut.47.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skinner MP, Lucas CM, Burns GF, Chesterman CN, Berndt MC. 1991. GMP-140 binding to neutrophils is inhibited by sulfated glycans. J Biol Chem 266:5371–5374. [PubMed] [Google Scholar]
- 40.Urbinati C, Chiodelli P, Rusnati M. 2008. Polyanionic drugs and viral oncogenesis: a novel approach to control infection, tumor-associated inflammation and angiogenesis. Molecules 13:2758–2785. doi: 10.3390/molecules13112758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bobardt MD, Salmon P, Wang L, Esko JD, Gabuzda D, Fiala M, Trono D, Van der Schueren B, David G, Gallay PA. 2004. Contribution of proteoglycans to human immunodeficiency virus type 1 brain invasion. J Virol 78:6567–6584. doi: 10.1128/JVI.78.12.6567-6584.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee E, Pavy M, Young N, Freeman C, Lobigs M. 2006. Antiviral effect of the heparan sulfate mimetic, PI-88, against dengue and encephalitic flaviviruses. Antiviral Res 69:31–38. doi: 10.1016/j.antiviral.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Cagno V, Donalisio M, Civra A, Volante M, Veccelli E, Oreste P, Rusnati M, Lembo D. 2014. Highly sulfated k5 Escherichia coli polysaccharide derivatives inhibit respiratory syncytial virus infectivity in cell lines and human tracheal-bronchial histocultures. Antimicrob Agents Chemother 58:4782–4794. doi: 10.1128/AAC.02594-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krepstakies M, Lucifora J, Nagel CH, Zeisel MB, Holstermann B, Hohenberg H, Kowalski I, Gutsmann T, Baumert TF, Brandenburg K, Hauber J, Protzer U. 2012. A new class of synthetic peptide inhibitors blocks attachment and entry of human pathogenic viruses. J Infect Dis 205:1654–1664. doi: 10.1093/infdis/jis273. [DOI] [PubMed] [Google Scholar]
- 45.Yoneda M, Guillaume V, Ikeda F, Sakuma Y, Sato H, Wild TF, Kai C. 2006. Establishment of a Nipah virus rescue system. Proc Natl Acad Sci U S A 103:16508–16513. doi: 10.1073/pnas.0606972103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guillaume V, Wong KT, Looi RY, Georges-Courbot M-C, Barrot L, Buckland R, Wild TF, Horvat B. 2009. Acute Hendra virus infection: analysis of the pathogenesis and passive antibody protection in the hamster model. Virology 387:459–465. doi: 10.1016/j.virol.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 47.Guillaume V, Lefeuvre A, Faure C, Marianneau P, Buckland R, Lam SK, Wild TF, Deubel V. 2004. Specific detection of Nipah virus using real-time RT-PCR (TaqMan). J Virol Methods 120:229–237. doi: 10.1016/j.jviromet.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 48.Horvat B, Rivailler P, Varior-Krishnan G, Cardoso A, Gerlier D, Rabourdin-Combe C. 1996. Transgenic mice expressing human measles virus (MV) receptor CD46 provide cells exhibiting different permissivities to MV infections. J Virol 70:6673–6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vivès RR, Sadir R, Imberty A, Rencurosi A, Lortat-Jacob H. 2002. A kinetics and modeling study of RANTES(9-68) binding to heparin reveals a mechanism of cooperative oligomerization. Biochemistry 41:14779–14789. doi: 10.1021/bi026459i. [DOI] [PubMed] [Google Scholar]
- 50.Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. 2009. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 52.Ono K, Ishihara M, Ishikawa K, Ozeki Y, Deguchi H, Sato M, Hashimoto H, Saito Y, Yura H, Kurita A, Maehara T. 2002. Periodate-treated, non-anticoagulant heparin-carrying polystyrene (NAC-HCPS) affects angiogenesis and inhibits subcutaneous induced tumour growth and metastasis to the lung. Br J Cancer 86:1803–1812. doi: 10.1038/sj.bjc.6600307. [DOI] [PMC free article] [PubMed] [Google Scholar]