Solar energy conversion efficiency in important food and biofuel crops provides a baseline for future improvements in conversion efficiency.
Abstract
The conversion efficiency (εc) of absorbed radiation into biomass (MJ of dry matter per MJ of absorbed photosynthetically active radiation) is a component of yield potential that has been estimated at less than half the theoretical maximum. Various strategies have been proposed to improve εc, but a statistical analysis to establish baseline εc levels across different crop functional types is lacking. Data from 164 published εc studies conducted in relatively unstressed growth conditions were used to determine the means, greatest contributors to variation, and genetic trends in εc across important food and biofuel crop species. εc was greatest in biofuel crops (0.049–0.066), followed by C4 food crops (0.046–0.049), C3 nonlegumes (0.036–0.041), and finally C3 legumes (0.028–0.035). Despite confining our analysis to relatively unstressed growth conditions, total incident solar radiation and average growing season temperature most often accounted for the largest portion of εc variability. Genetic improvements in εc, when present, were less than 0.7% per year, revealing the unrealized potential of improving εc as a promising contributing strategy to meet projected future agricultural demand.
Substantial increases in yield are needed to feed and fuel the world’s growing human population. With an estimated population of nine billion people by the middle of this century (Lutz and Samir, 2010) and rising affluence resulting in greater consumption of grain-fed animal products (Cirera and Masset, 2010), different studies predict that, by midcentury, global crop production will need to increase 60% to 120% over 2005 levels without the expansion of agricultural land area (Tilman et al., 2011; Alexandratos and Bruinsma, 2012).
Doubling yields in major food and fuel crops requires considerable effort, especially as yields are beginning to plateau in many major food crops. Yield increases necessary for doubling productivity by midcentury are estimated at 1.16% to 1.31% each year in all cereals (Hall and Richards, 2013), 1.7% per year in wheat (Triticum aestivum; Rosegrant and Agcaoili, 2010), and 2.4% (noncompounding average per year) across all major grain crops (Ray et al., 2013). However, global mean increases from the past 20 to 30 years suggest that yield gains in rice (Oryza sativa) and wheat are approximately 1% (Lopes et al., 2012; Manès et al, 2012; Ray et al., 2013) and declining in some areas of the world (Cassman et al., 2010; Fischer and Edmeades, 2010; Long and Ort, 2010; Ray et al., 2013). Global yearly increases are estimated at 1.3% in soybean (Glycine max) and 1.6% in maize (Zea mays), with similar concerns that yield trends may also be decreasing in some major growing regions (Lobell and Gourdji, 2012; Ray et al., 2013).
Efforts to increase yields in the next few decades must also account for environmental and sustainability goals (Sayer et al., 2013) as well as heightened environmental stresses predicted to occur due to climate change, which are already responsible for some of the stagnation in yield increases. Anthropogenic sources of greenhouse gases have caused an approximately 1°C increase in land surface temperatures since 1900, and global mean surface temperatures are likely to increase by up to 2.4°C to 4.8°C by the end of the century (IPCC, 2013). Drought is also expected to become more frequent and intense in many regions of the world (Dai, 2011; IPCC, 2013). Of the variability present in major food crop yield gains, 30% can be explained by climate change alone (Lobell and Field, 2007), with drastic decreases in barley (Hordeum vulgare), maize, rice, sorghum (Sorghum bicolor), soybean, and wheat yields as average growing season temperatures surpass the temperature optimum for each crop (Lobell and Gourdji, 2012). Current levels of atmospheric CO2 concentration [CO2] are the highest they have been in at least 800,000 years (IPCC, 2013). Elevated [CO2] increases water use efficiency (Ainsworth and Long, 2005, Bernacchi et al., 2007, Leakey et al., 2009), but probably not to an extent that would mitigate the resulting reductions in yield caused by higher temperature and higher vapor pressure deficit (Ort and Long, 2014). Additionally, any fertilization effects on C3 yields due to elevated [CO2] would be at least in part negated by drought and temperature stress, leaving yield increases far from optimal (Long et al., 2006a; Lobell and Gourdji, 2012).
USING PAST LITERATURE TO BETTER UNDERSTAND THE ROLE THAT IMPROVEMENTS IN CONVERSION EFFICIENCY HAVE PLAYED IN INCREASED YIELDS
To double yields in less than 50 years with the additional challenges of climate change, research needs to target yield-related processes that have potential for considerable improvement. The theoretical maximum conversion efficiency (εc) of photosynthetically active radiation (PAR) into plant biomass has been calculated in C3 (0.094) and C4 (0.123) plants in optimal conditions. The calculations are based on the minimal amounts of energy that could be lost due to various steps in transforming intercepted PAR into plant biomass. The steps where energy is lost include light reflection and transmission, photochemical inefficiency resulting from excess energy in absorbed blue light, thermodynamic limitations, carbohydrate synthesis, photorespiration (C3 only), and respiration (Zhu et al., 2008, 2010). Estimated at less than half of the theoretical maximum in C3 and C4 plants in optimal conditions, εc appears to be an ideal candidate for increasing yield potential (Beadle and Long, 1985; Zhu et al., 2010). Moreover, considerable variation is present in εc, as it is sensitive to greenhouse gases and weather-related variables predicted to intensify due to climate change (Sinclair and Muchow, 1999; Slattery et al., 2013). Because εc is seemingly well below its theoretical maximum and is a highly variable parameter across growing environments and crop species, potential methods to improve εc have been identified and reviewed. The most general strategy for improving εc at the leaf level involves improving the efficiency of carboxylation by Rubisco while limiting oxygenation in C3 plants (Zhu et al., 2010; Parry et al., 2011; Raines, 2011; Ainsworth et al., 2012; Evans, 2013). At the canopy level, targets include using altered canopy architecture and antenna size to improve light distribution in dense-canopy crops (Zhu et al., 2010; Ort et al., 2011; Parry et al., 2011; Ainsworth et al., 2012; Reynolds et al., 2012), maximizing nitrogen partitioning, and enhancing spike photosynthesis (Reynolds et al., 2012).
While there is seemingly substantial potential for increasing εc in major food and biofuel crops, judging the effectiveness of each strategy is difficult without baseline estimates of εc and rates of gain to date in individual crops. Sensitivity to environmental conditions implies that using single studies may not be the best method for gauging the status of εc within individual crop species. Therefore, a meta study of the large body of literature that exists on εc should provide insight into the current status of εc in individual crops, the extent that εc varies among food and biofuel crop species, which crops demonstrate greater potential for εc improvements, and inherent characteristics that may be benefitting crops with greater realized εc. Since the literature spans several decades, the extent to which genetic improvements versus climate change have contributed to changes in εc can also be assessed in individual crop species.
These analyses used primary literature to compare the mean εc among and within several food and biofuel crop species (Table I). Additionally, the relationships between εc and environmental and genetic variables were examined over several decades within major food crops. Briefly, studies containing εc (also referred to as radiation use efficiency) measurements under relatively unstressed growing conditions were collected. εc values were extracted from the resulting 164 studies (Supplemental Table S1). εc is generally calculated as the slope of crop accumulated biomass (in terms of mass or energy) versus intercepted or absorbed solar energy or PAR by the canopy. For the purpose of these analyses, all values were standardized to units of MJ of dry matter per MJ of absorbed PAR before statistically testing differences at α = 0.1. An additional aim was to estimate rates of gain in εc due to breeding and to determine whether εc variation in major food crops over time was more significantly associated with breeding or climate variables. When available, crop information and growing conditions from each study were used as independent variables in multiple regression analyses. These variables included year of release (YOR), mean annual [CO2] for the years that the experiments were conducted, mean growing season temperature (T), available incident solar radiation during the growing season (St), the amount of precipitation (rain and irrigation) available during the growing season, and plant density (maize only). Varieties included in the analyses were indicated for each crop and subgroup (Supplemental Table S2). In food crops with significant positive correlations between εc and YOR, the regression coefficient was used to determine the time to double or reach the maximum εc, assuming no major changes in trends due to genetic or environmental factors. (For a fully detailed explanation of the methods used in this study, see Supplemental Materials and Methods S1.)
Table I. Important food and C4 biofuel crop species used in εc analyses.
Species further divided into genetic components are indicated. Biomass energy content for vegetative (V) and combined vegetative and reproductive (V+R) stages used for converting εc values to energy units are indicated for each crop.
| Species | Common Name | Type | Food or Energy Crop | Groups by Cultivar, Species, or Hybrid | Energy Content | Energy Content Data Source | |
|---|---|---|---|---|---|---|---|
| V | V+R | ||||||
| MJ kg−1 | |||||||
| Z. mays | Maize | C4 | Botha | 17.0 | 17.0 | Penning de Vries et al. (1989) | |
| S. bicolor | Sorghum | C4 | Botha | Energy/biomass/forage grain | 17.6 | 17.3 | Amthor et al. (1994) |
| S. tuberosum | Potato | C3 | Food | 17.0 | 15.8 | Penning de Vries et al. (1989) | |
| O. sativa | Rice | C3 | Food | New hybrids | 15.1 | 15.9 | Penning de Vries et al. (1989) |
| indica | |||||||
| japonica | |||||||
| Basmati | |||||||
| T. aestivum | Wheat | C3 | Food | Spring | 17.0 | 16.6 | Penning de Vries et al. (1989) |
| Winter | |||||||
| H. vulgare | Barley | C3 | Food | 16.1 | 15.6 | McKendry (2002; V); Sinha et al. (1982; V+R) | |
| A. hypogaea | Peanut | C3 | Food | 17.9 | 23.3 | Penning de Vries et al. (1989) | |
| G. max | Soybean | C3 | Food | 18.1 | 19.8 | Amthor et al. (1994) | |
| Cicer arietinum | Chickpea | C3 | Food | 17.9 | 18.6 | Penning de Vries et al. (1989) | |
| Cajanus cajan | Pigeonpea | C3 | Food | 17.9 | 18.4 | Penning de Vries et al. (1989) | |
| P. virgatum | Switchgrass | C4 | Energy | 17.4 | 17.4 | McKendry (2002) | |
| Saccharum spp. | Sugarcane | C4 | Energy | 17.4 | 17.4 | Botha (2009) | |
| Miscanthus spp. | Miscanthus | C4 | Energy | M. giganteus | 18.5 | 18.5 | McKendry (2002) |
| M. sinensis | |||||||
Maize studies completely overlapped from food to energy analyses. Sorghum food and energy cultivars were separated and analyzed in the respective analyses.
STATUS OF εC IN MAJOR FOOD CROP SPECIES
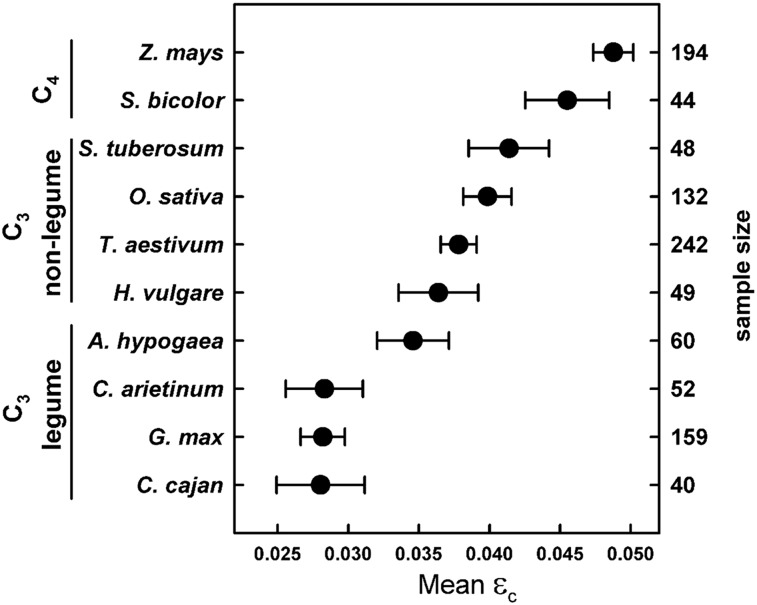
Previous estimates putting εc means in food crops at approximately one-third to one-half of the maximum (Beadle and Long, 1985; Zhu et al., 2010) were consistent with the results from this study, with the exception of many legumes demonstrating values below one-third the C3 maximum. Maize, a highly developed and intensively grown crop, had the greatest mean εc among food crops included in the analysis (0.0488; Fig. 1; Table II) but was still less than one-half the predicted maximum of 0.123 (Zhu et al., 2010). Grain sorghum was slightly lower than maize (0.0455; P = 0.10; Fig. 1) and was only 37% of the maximum. C3 nonlegume crop means were significantly lower than C4 means and ranged from 40% to 45% of the predicted maximum, with the greatest C3 mean in potato (Solanum tuberosum; 0.0414; Fig. 1; Table II). Except for peanut (Arachis hypogaea), which had a mean εc of 0.0346 (Fig. 1; Table II) and was approximately 38% of the maximum, all legume means were approximately 0.028 and 31% of the possible maximum for C3 crops (Fig. 1; Table II).
Figure 1.
Calculated εc means in 10 major food crops. Crops are organized by C4, C3 nonlegume, and C3 legume categories. Sample size is shown on the right axis. Error bars represent 90% confidence intervals.
Table II. Numerical data from mean εc analyses in major food crops.
Species, common name, photosynthetic type, and any groups or specifications within species are indicated. εc means and se are reported along with sample size (n) for each species and group within species.
| Species | Common Name | Type | Groups by Cultivar, Species, or Hybrid | Mean εc | se | n |
|---|---|---|---|---|---|---|
| Z. mays | Maize | C4 | 0.0488 | 0.001 | 194 | |
| S. bicolor | Sorghum | C4 | Grain type only | 0.0455 | 0.002 | 44 |
| S. tuberosum | Potato | C3 | 0.0414 | 0.002 | 48 | |
| O. sativa | Rice | C3 | 0.0399 | 0.001 | 132 | |
| New hybrids | 0.0472 | 0.002 | 29 | |||
| indica | 0.0442 | 0.002 | 25 | |||
| japonica | 0.0388 | 0.002 | 57 | |||
| Basmati | 0.0273 | 0.003 | 21 | |||
| T. aestivum | Wheat | C3 | 0.0378 | 0.001 | 242 | |
| Spring | 0.0352 | 0.001 | 105 | |||
| Winter | 0.0399 | 0.001 | 137 | |||
| H. vulgare | Barley | C3 | 0.0364 | 0.002 | 49 | |
| A. hypogaea | Peanut | C3 | 0.0346 | 0.002 | 60 | |
| G. max | Soybean | C3 | 0.0282 | 0.001 | 159 | |
| C. arietinum | Chickpea | C3 | 0.0283 | 0.002 | 52 | |
| C. cajan | Pigeonpea | C3 | 0.0280 | 0.002 | 40 |
One caveat is that this study omitted values from the literature that included belowground biomass, with the exception of peanut and potato, because (1) studies basing εc measurements on total aboveground and belowground biomass were minimal and (2) belowground harvesting methods differed greatly and, therefore, may have skewed the results. εc is estimated to increase by 10% to 20% when accounting for belowground biomass in annual plants (Sinclair and Muchow, 1999), which would result in an approximate increase of 0.01 in εc in food crops from this analysis, but this still would not account for the large disparity between measured and theoretical values. The greatest C3 εc was in potato, which included belowground biomass but was still only 44% of the theoretical maximum. However, the omission of belowground biomass in the calculation of εc could have contributed to the disproportionately lower εc in legume crops if belowground biomass energy is greater in legumes compared with other crops. Indeed, belowground biomass (roots and nodules) of soybean contains more energy (18.3 MJ kg−1) than sorghum belowground tissue (16.7 MJ kg−1; Amthor et al., 1994). Based on energy contents reported on a per area basis by Amthor et al. (1994), accounting for soybean belowground biomass would increase whole-plant energy content by almost 6%. However, this would only increase soybean εc to approximately 0.03, which is still well below the εc of C3 nonlegume crops (Fig. 1; Table II).
Additionally, legume εc may have been affected by nitrogen fixation, the costs of which have been determined and vary by study. One study reports a 5% greater photon energy requirement for nitrogen fixation compared with the combined cost of NH4+ and NO3− assimilation that occurs in most nonlegume crops (Andrews et al., 2009). In terms of carbon usage, the proportion of assimilated carbon that is diverted to the nodules for nitrogen fixation is reported as 7% to 19% (Gordon et al., 1987; Vessey et al., 1988; Hansen et al., 1992, 1993; Fujikake et al., 2003; Ito et al., 2006). Correcting for these costs on εc would result in a range of 0.03 to 0.034 for the legumes in this study (excluding peanut). These values are still lower than nonlegume εc values (Fig. 1; Table II), but other factors related to nitrogen fixation may also limit legumes, such as the delay in forming mature nodules early in the growing season (Andrews et al., 2009). Therefore, increasing the efficiency of nitrogen fixation may represent an additional means to improve εc in legumes that has previously received little attention with regard to increasing photosynthetic efficiency.
Peanut was the anomaly within the legume group, with εc more similar to nonlegume C3 crops than to legumes. Although a portion of belowground biomass was included in the analyses of all peanut studies included, this only comprised the fruiting bodies growing underground and did not include the rest of the root biomass. A more likely explanation for the disparity between peanut and other legumes in this study was the difference in reported energy contents. Peanut whole-plant energy content was 1.2 times greater than that in the rest of the legumes (Table I), which corresponded to an approximately 1.2 times greater efficiency (Fig. 1; Table II).
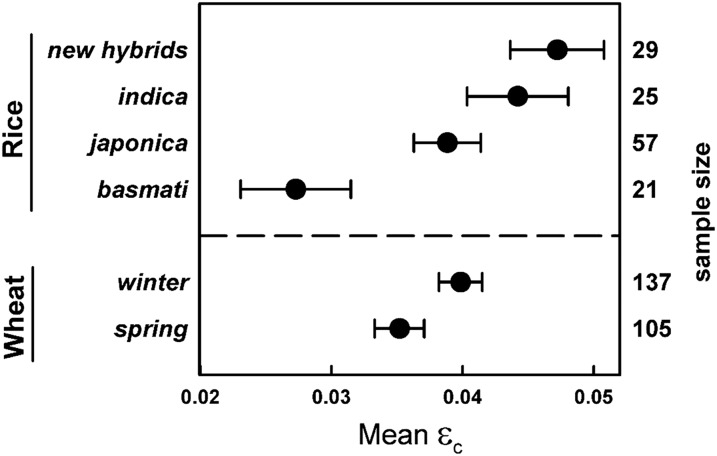
εC VARIES AMONG GROUPS WITHIN RICE AND WHEAT, BUT THIS MAY BE THE RESULT OF VARYING GROWTH CONDITIONS
Significant differences in εc were evident within the subgroups of rice and wheat (Fig. 2) but may have been confounded by differences in growth environments (Supplemental Table S3). At approximately one-half of the C3 theoretical maximum, the εc of new hybrid (0.0472) and indica (0.0442) rice varieties was significantly greater than that of japonica (0.0388) and basmati rice (0.0273; Fig. 2). New hybrid rice εc was not significantly different from maize εc (P = 0.51), and neither new hybrid nor indica εc was significantly different from grain sorghum εc (P = 0.49 and P = 0.72, respectively). However, a negative relationship between available St and subgroup εc suggested that the significantly greater εc in new hybrid and indica varieties may be associated with growth conditions rather than genetic enhancements (Supplemental Table S3). Significant differences were also evident between the εc means of spring wheat (0.0352) and winter wheat (0.0399; P < 0.01; Fig. 2; Table II). However, mean St and T were once again lower in winter wheat compared with spring wheat (Supplemental Table S3), the effects of which are discussed below.
Figure 2.
Calculated εc means for categories within rice (top) and wheat (bottom). Sample size is shown on the right axis. Error bars represent 90% confidence intervals.
εCs IN BIOENERGY CROPS
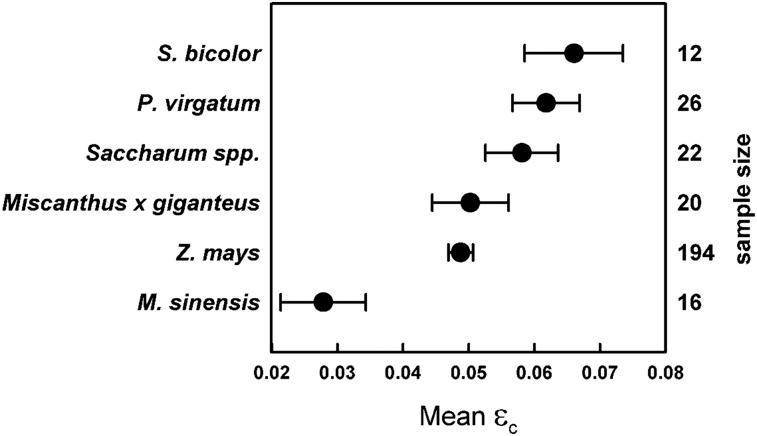
Not surprisingly, C4 crop εc was almost always greater than C3 εc due to inherent properties of C4 photosynthesis. C4 plants concentrate CO2 at the site of carboxylation, thereby inhibiting energy losses to photorespiration and increasing the maximum potential εc as compared with C3 plants (Zhu et al., 2008, 2010). However, εc was often greater in C4 energy crops compared with C4 food crops. At 0.066, nongrain sorghum varieties had the greatest εc mean of the energy crops included and reached 54% of the theoretical mean for C4 crops (Fig. 3; Table III). Switchgrass (Panicum virgatum) εc was not significantly lower than sorghum εc (P = 0.44) and was also greater than 50% of theoretical (0.0618; Fig. 3; Table III). Sugarcane (Saccharum officinarum) was similar to sorghum (P = 0.16) and switchgrass (P = 0.42), with a mean of 0.0581 (Fig. 3; Table III), and was not significantly different from the Miscanthus × giganteus mean of 0.0503 (P = 0.11; Fig. 3; Table III). Maize εc was the second lowest of the bioenergy crops (0.0488; Fig. 3; Table III), despite having the highest εc of the food crops (Fig. 1; Table II). Miscanthus sinensis had the lowest mean of the bioenergy crops (0.027) and was only 23% of the maximum theoretical for C4 crops (Fig. 3; Table III).
Figure 3.
Calculated εc means in major C4 biofuel crops. Sample size is shown on the right axis. Error bars represent 90% confidence intervals.
Table III. Numerical data from mean εc analyses in C4 bioenergy crops.
Species, common name, photosynthetic type, and any groups or specifications within species are indicated. εc means and se are reported along with sample size (n) for each species and group within species.
| Species | Common Name | Type | Groups by Cultivar, Species, or Hybrid | Mean εc | se | n |
|---|---|---|---|---|---|---|
| Z. mays | Maize | C4 | 0.0488 | 0.001 | 194 | |
| S. bicolor | Sorghum | C4 | Energy, biomass, forage | 0.0660 | 0.005 | 12 |
| P. virgatum | Switchgrass | C4 | 0.0618 | 0.003 | 26 | |
| Saccharum spp. | Sugarcane | C4 | 0.0581 | 0.003 | 22 | |
| Miscanthus spp. | Miscanthus | C4 | M. giganteus | 0.0503 | 0.004 | 20 |
| M. sinensis | 0.0279 | 0.004 | 16 |
The apparent disparity between C4 food and bioenergy crop εc may be the result of plant growth habit. Unlike the annual C4 food crops, most of the C4 energy crops were perennial grasses, which are expected to demonstrate greater aboveground biomass than annuals early in the season. Once established, perennials such as M. giganteus and switchgrass draw upon belowground reserves from the previous season to facilitate growth after emergence that is independent of absorbed radiation (Dohleman et al., 2012). This would inflate εc in perennial C4 biofuel crops during early crop growth. A comparison by Ceotto et al. (2013) of the C3 perennial giant reed (Arundo donax) and the C4 annual energy crop sorghum supported this point. εc in giant reed, which demonstrates photosynthetic rates typical of C3 plants (Balota et al., 2008), was greater than the εc of the C4 annual sweet sorghum (Ceotto et al., 2013). However, the intercept of the relationship between aboveground biomass and intercepted radiation in giant reed was greater than zero, suggesting that rhizome energy contributions to aboveground biomass were increasing shoot growth rates independent of radiation absorption (Ceotto et al., 2013).
TRENDS IN MAJOR FOOD CROP εC OVER THE PAST FEW DECADES
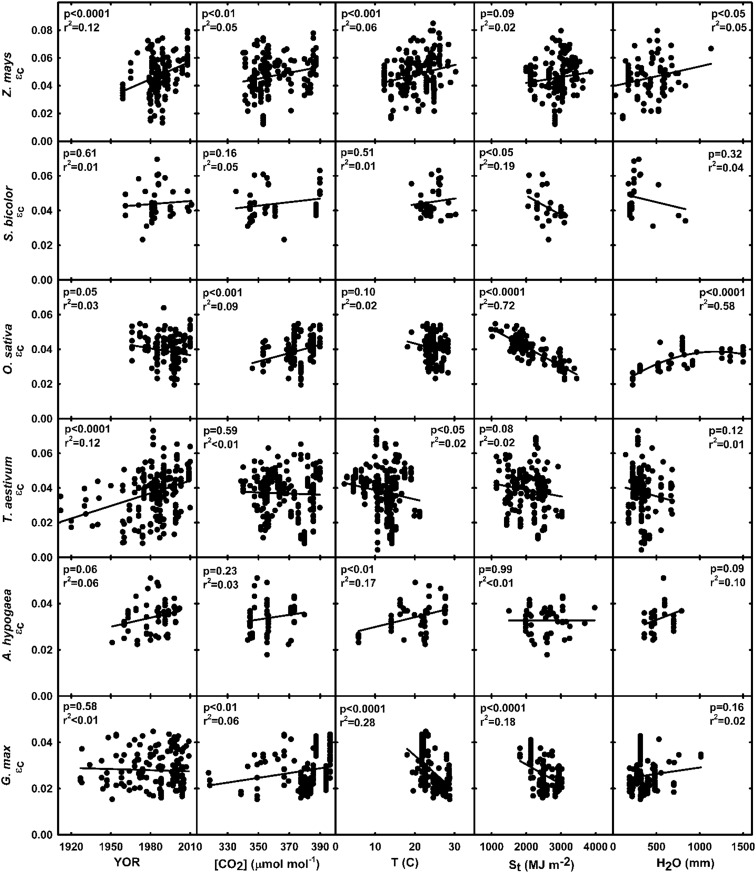
Prior to conducting multiple regression analyses, the individual relationships between εc and independent variables were determined in six major food crops (Fig. 4). In multiple regression analyses, the best model was selected using the lowest corrected Akaike information criterion (AICc) score, and inclusion of an independent variable in the model demonstrated that the variable accounted for a large portion of the variability in εc for that crop. The variables included in the final model closely matched corresponding partial correlation coefficients, demonstrating that colinearity between independent variables was not a significant factor in the multiple regression results (Supplemental Table S4).The most commonly included variables were T and St, which were included in five linear multiple regression models each (Table IV). YOR was only included in four models, and [CO2] only in one model (Table IV). The AICc order of single variables included in the final model indicated relative correlation strengths for each variable. T was the first variable included in three of the models (peanut, soybean, and wheat), St in two of the models (rice and sorghum), and YOR in one model (maize; Table IV). Although density was also included in the maize analyses, there was no significant correlation between maize εc and density, even though selection for density tolerance is shown to correlate with increasing yield (Duvick, 2005). Additionally, substituting density for YOR did not result in a significant correlation between density and εc. Due to reduced sample size, precipitation available during the growing season was only included in the analyses for soybean but was not in the final selected model.
Figure 4.
Relationships between εc and individual independent variables in six major food crops. Independent variables included YOR, mean annual [CO2] during the measurement period, T, available St, and water available during the growing season as precipitation and irrigation (H2O). Lines represent least-squares regression, with corresponding significance levels and correlation coefficients in each graph. εc versus density in maize is not shown.
Table IV. Multiple regression analyses of εc in six major food crops.
The final model and ranking of variables included in the final model were determined using the lowest AICc. Variable coefficients and significance were determined using the final model.
| Crop | Variable Rank | Modela | AICc | Coefficient (10−3) | P |
|---|---|---|---|---|---|
| Peanut (n = 51) | YOR, St, T | −540.1 | <0.001 | ||
| 1 | T | −536.0 | 0.426 | <0.001 | |
| 2 | YOR | −531.8 | 0.123 | <0.05 | |
| 3 | St | −526.6 | −0.00238 | 0.12 | |
| Soybean (n = 117) | YOR, [CO2], St, T | −1,270.2 | <0.0001 | ||
| 1 | T | −1,231.8 | −1.04 | <0.0001 | |
| 2 | St | −1,226.3 | −0.00824 | <0.0001 | |
| 3 | [CO2] | −1,204.3 | 0.0524 | <0.05 | |
| 4 | YOR | −1,201.3 | 0.0293 | 0.21 | |
| Rice (n = 102) | St, T | −1,133.8 | <0.0001 | ||
| 1 | St | −1,125.0 | −0.00960 | <0.0001 | |
| 2 | T | −1,014.3 | −0.729 | <0.01 | |
| Wheat (n = 159) | YOR, St, T | −1,451.2 | <0.0001 | ||
| 1 | T | −1,447.9 | −0.746 | <0.001 | |
| 2 | YOR | −1,438.4 | 0.105 | <0.05 | |
| 3 | St | −1,437.8 | −0.00284 | 0.13 | |
| Sorghum (n = 23) | 1 | St | −228.3 | −0.00739 | 0.11 |
| Maize (n = 149) | YOR, T | −1,347.8 | <0.0001 | ||
| 1 | YOR | −1,346.0 | 0.346 | <0.0001 | |
| 2 | T | −1,344.0 | 0.521 | <0.01 |
Independent variables included YOR, mean annual [CO2] during the measurement period, T, and available St. Water available as precipitation and irrigation was included when sample size changed by less than 10% after including it in the analyses. Density was included in maize analyses but was not in the selected model.
When St was included in a model, the simple regression coefficient was always negative (Table IV). Negative correlations of εc with St within several food crops reinforced the notion that available light in excess of photosynthetic capacity decreases εc (Sinclair and Muchow, 1999; Slattery et al., 2013). Lowering St to a point near light saturation at the top of the canopy increases εc but ultimately depresses yield potential, due to less overall energy available to the crop. Therefore, altering pigment concentrations or canopy architecture in canopies with high leaf area indices can optimize light availability among leaf layers and decrease wasted incident radiation (Long et al., 2006b; Ort et al., 2011; Drewry et al., 2014). This should have been evident in new rice varieties bred for reduced tillering, as fewer tillers was hypothesized to alter canopy structure, allowing greater penetration of light in the canopy and, therefore, greater εc (Peng et al., 2008). In fact, εc was greatest in the new hybrid varieties (Table II; Fig. 2). However, these new hybrid varieties were also grown in relatively dim conditions as compared with the other subgroups (Supplemental Table S3), preventing any conclusions regarding the effectiveness to this approach in conditions where incident light oversaturates photosynthetic capacity. Nonetheless, frequent negative relationships between εc and St in food crops present a solid argument for improving light distribution and use in dense food crop canopies.
Multiple regression analyses indicated that, in addition to St, T accounted for the greatest proportion of the variation in εc in most food crops that were otherwise classified as experiencing optimal growth conditions. T was negatively correlated with rice, wheat, and soybean εc (Table IV). Positive correlations were evident between εc and T in maize and peanut (Table IV), but as temperatures continue to rise with predicted changes in climate, most likely all food crops will begin to suffer decreases in εc. Even in peanut, a crop where increases in T were positively correlated with εc in this analysis, recent studies have found that expected increases in T will result in decreases in photosynthesis that will not be alleviated by elevated [CO2] (Prasad et al., 2003). A similar result was found in soybean grown in the field under elevated [CO2] and elevated T, where elevated [CO2] had little effect on photosynthesis when T reached above optimal (Ruiz-Vera et al., 2013). While increasing temperatures can negatively affect crop growth and development in many ways, specific inhibitions to photosynthesis at the leaf level include decreased Rubisco specificity to CO2, limited ribulose 1,5-bisphosphate regeneration, and destabilization of Rubisco activase, which can have severe implications on C3 and even C4 photosynthesis at very high but increasingly frequent temperatures (for review, see Ainsworth and Ort, 2010). Therefore, mitigating these harmful effects on leaf photosynthesis through transgenic approaches should be a priority along with improving leaf photosynthetic efficiency.
Improving εc stress tolerance is becoming increasingly important, as current work to increase yields is making crops more sensitive to detrimental climate change effects. Breeding for greater yields in optimal conditions has resulted in greater sensitivity to the environment (i.e. greater yield instability in less favorable conditions) in maize (Lobell et al., 2014) and soybean (Koester et al., 2014; Rincker et al., 2014). This may explain the lack of significant correlations between εc and density in maize. Although εc was expected to increase with density, a greater sensitivity to temperature and, therefore, vapor pressure deficit could negate those benefits (Lobell et al., 2014). It is also more difficult for newer, high-yielding cultivars of wheat to realize maximum yields in the field as T stress becomes more common (Gourdji et al., 2013). Since εc is sensitive to the environment (Table IV; Sinclair and Muchow, 1999; Slattery et al., 2013), improving stress tolerance deserves even greater attention going forward in order to mitigate the negative effects of climate change on yield.
PROJECTED TIME TO DOUBLE CURRENT εC VALUES AND REACH THEORETICAL MAXIMUM εC IN MAJOR FOOD CROPS
Positive correlation of εc with YOR was limited across food crops and only demonstrated rates of increase of less than 0.7% per year. Relationships between εc and YOR determined in the absence of environmental variability in wheat (Shearman et al., 2005; Sadras et al., 2012) and soybean (Koester et al., 2014) were reported as less than 0.65% gain per year in εc and were consistent with the rates from this study. At best, these rates are half of the rates of yield increase necessary to double crop production by midcentury (Rosegrant and Agcaoili, 2010; Hall and Richards, 2013; Ray et al., 2013). Consequently, projections of when εc would double or reach the theoretical maximum suggest that the rate of genetic advancements to the present are not enough to double εc by the middle of the century in the crops studied. In maize, the food crop with the greatest mean (Fig. 1) and greatest rate of increase with YOR (Table V), εc would not double until the year 2134, and the maximum would be reached approximately 70 years later (Table V). In peanut, the estimated time for εc to double was approximately 250 years, while reaching the maximum would not occur for at least 400 years (Table V). The year of doubling εc in wheat was 2357, whereas reaching the maximum would occur in 2391 (Table V). Due to having the low absolute value of εc and the lowest rate of gain in soybean, doubling εc would take approximately one millennium (Table V). These projections, based on trends in εc spanning several decades, demonstrated that breeding and biotechnology to date have not necessarily selected for increasing εc as a high priority. Thus, there is potentially a large amount of room for improvement in this key factor and, therefore, yield potential.
Table V. Summary of εc trends and projections in major food crops.
Positive trends in εc from multiple regression analyses were used to project the year in which εc will double and reach the theoretical maximum, assuming no changes in the coefficients due to climate change, breeding intensity, etc.
| Crop | YOR Slope | Year of Doubling | Year of Maximum |
|---|---|---|---|
| year−1 × 10−3 | |||
| Maize | 0.346 | 2134 | 2176 |
| Wheat | 0.105 | 2357 | 2391 |
| Peanut | 0.123 | 2273 | 2317 |
| Soybean | 0.029 | 2966 | 2986 |
CONCLUSION
As greater increases in yields are needed to feed and fuel the world’s population, targets such as εc are key to reaching these goals. This assessment aimed to determine the mean εc in several important food and biofuel crops, test the key contributors to variation in εc, and determine genetic trends in εc. As expected, mean εc values in food crops were greatest in C4, followed by nonlegume C3, and were lowest in legume C3 plants. All food crop means were lower than one-half the theoretical maximum. Bioenergy crop εc means were much greater than those in food crops, and some, including the energy crops sorghum and switchgrass, exceeded 50% of the maximum for C4 grasses. However, εc values for perennial grasses may have been inflated if measured during the growing season interval when stored rhizome reserves are mobilized and contribute to aboveground biomass accumulation. Reported variation in εc was found to be generally negatively correlated with St and T. Positive correlations with YOR were only present in a few food crops, and rates of increase were relatively low, suggesting that εc will not double in most crops before the middle of the century at the current rate of increase.
While these findings show that there has been little progress to date in improving εc, the fact that εc has room for improvement and is receiving increasing amounts of attention is promising. Targets for improving εc in various manners have already been identified and reviewed (Amthor, 2010, Zhu et al., 2010; Parry et al., 2011; Raines, 2011; Ainsworth et al., 2012; Reynolds et al., 2012; Evans, 2013) and have the potential to greatly alter the current trends in εc improvement. This study emphasizes the importance of using strategies that improve nitrogen fixation efficiency in legumes, canopy light distribution, and tolerance to higher temperatures to increase genetic gains and limit detrimental environmental effects on εc. As these strategies are implemented to improve εc and, therefore, yield potential, these εc means and trends will serve as a baseline to track the relative success of each approach.
Supplemental Data
The following supplemental materials are available.
Supplemental Table S1. Data sources used in analyses.
Supplemental Table S2. Varieties included in crop and subgroup analyses.
Supplemental Table S3. Means and ranges of independent variables used in analyses.
Supplemental Table S4. Partial correlation coefficients from regression analyses.
Supplemental Materials and Methods S1. Detailed description of data collection, manipulation, and analysis.
Supplementary Material
Acknowledgments
We thank Dr. Adam Davis for providing advice on the statistical analyses used in this study and Dr. Elizabeth Ainsworth for advice on the meta-analysis procedures.
Glossary
- [CO2]
CO2 concentration
- εc
conversion efficiency
- PAR
photosynthetically active radiation
- YOR
year of release
- T
mean growing season temperature
- St
incident solar radiation during the growing season
- AICc
corrected Akaike information criterion
Footnotes
This work was supported by the U.S. Department of Agriculture Agricultural Research Service.
References
- Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 165: 351–371 [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Ort DR (2010) How do we improve crop production in a warming world? Plant Physiol 154: 526–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth EA, Yendrek CR, Skoneczka JA, Long SP (2012) Accelerating yield potential in soybean: potential targets for biotechnological improvement. Plant Cell Environ 35: 38–52 [DOI] [PubMed] [Google Scholar]
- Alexandratos N, Bruinsma J (2012) World Agriculture towards 2030/2050: The 2012 Revision. Food and Agriculture Organization, Rome [Google Scholar]
- Amthor JS. (2010) From sunlight to phytomass: on the potential efficiency of converting solar radiation to phyto-energy. New Phytol 188: 939–959 [DOI] [PubMed] [Google Scholar]
- Amthor JS, Mitchell RJ, Runion GB, Rogers HH, Prior SA, Wood CW (1994) Energy content, construction cost and phytomass accumulation of Glycine max (L.) Merr. and Sorghum bicolor (L.) Moench grown in elevated CO2 in the field. New Phytol 128: 443–450 [DOI] [PubMed] [Google Scholar]
- Andrews M, Lea PJ, Raven JA, Azevedo RA (2009) Nitrogen use efficiency. 3. Nitrogen fixation: genes and costs. Ann Appl Biol 155: 1–13 [Google Scholar]
- Balota M, Payne WA, Rooney W, Rosenow D (2008) Gas exchange and transpiration ratio in Sorghum. Crop Sci 48: 2361–2371 [Google Scholar]
- Beadle CL, Long SP (1985) Photosynthesis: is it limiting to biomass production? Biomass 8: 119–168 [Google Scholar]
- Bernacchi CJ, Kimball BA, Quarles DR, Long SP, Ort DR (2007) Decreases in stomatal conductance of soybean under open-air elevation of [CO2] are closely coupled with decreases in ecosystem evapotranspiration. Plant Physiol 143: 134–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botha FC. (2009) Energy yield and cost in a sugarcane biomass system. Proc Aust Soc Sugar Cane Technol 31: 1–10 [Google Scholar]
- Cassman KG, Grassini P, van Wart J (2010) Crop yield potential, yield trends, and global food security in a changing climate. In Hillel D, Rosenzweig C, eds, Handbook of Climate Change and Agroecosystems: Impacts, Adaptation, and Mitigation. Imperial College Press, London, pp 37–51 [Google Scholar]
- Ceotto E, Di Candilo M, Castelli F, Badeck FW, Rizza F, Soave C, Volta A, Villani G, Marletto V (2013) Comparing solar radiation interception and use efficiency for the energy crops giant reed (Arundo donax L.) and sweet sorghum (Sorghum bicolor L. Moench). F Crop Res 149: 159–166 [Google Scholar]
- Cirera X, Masset E (2010) Income distribution trends and future food demand. Philos Trans R Soc Lond B Biol Sci 365: 2821–2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai A. (2011) Drought under global warming: a review. Wiley Interdiscip Rev Clim Chang 2: 45–65 [Google Scholar]
- Dohleman FG, Heaton EA, Arundale RA, Long SP (2012) Seasonal dynamics of above- and below-ground biomass and nitrogen partitioning in Miscanthus × giganteus and Panicum virgatum across three growing seasons. Glob Change Biol Bioenergy 4: 534–544 [Google Scholar]
- Drewry DT, Kumar P, Long SP (2014) Simultaneous improvement in productivity, water use, and albedo through crop structural modification. Glob Change Biol 20: 1955–1967 [DOI] [PubMed] [Google Scholar]
- Duvick DN. (2005) Genetic progress in yield of United States maize (Zea mays L.). Maydica 50: 193–202 [Google Scholar]
- Evans JR. (2013) Improving photosynthesis. Plant Physiol 162: 1780–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer RA, Edmeades GO (2010) Breeding and cereal yield progress. Crop Sci 50: S-85–S-98 [Google Scholar]
- Fujikake H, Yamazaki A, Ohtake N, Sueyoshi K, Matsuhashi S, Ito T, Mizuniwa C, Kume T, Hashimoto S, Ishioka NS, et al. (2003) Quick and reversible inhibition of soybean root nodule growth by nitrate involves a decrease in sucrose supply to nodules. J Exp Bot 54: 1379–1388 [DOI] [PubMed] [Google Scholar]
- Gordon AJ, Mitchell DF, Ryle GJA, Powell CE (1987) Diurnal production and utilization of photosynthate in nodulated white clover. J Exp Bot 38: 84–98 [Google Scholar]
- Gourdji SM, Mathews KL, Reynolds M, Crossa J, Lobell DB (2013) An assessment of wheat yield sensitivity and breeding gains in hot environments. Proc R Soc B Biol Sci 280: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AJ, Richards RA (2013) Prognosis for genetic improvement of yield potential and water-limited yield of major grain crops. Field Crops Res 143: 18–33 [Google Scholar]
- Hansen AP, Yoneyama T, Kouchi H (1992) Short-term nitrate effects on hydroponically-grown soybean cv. Bragg and its supernodulating mutant. J Exp Bot 43: 9–14 [Google Scholar]
- Hansen AP, Yoneyama T, Kouchi H, Martin P (1993) Respiration and nitrogen fixation of hydroponically cultured Phaseolus vulgaris L. cv. OAC Rico and a supernodulating mutant. Planta 1993: 538–545 [Google Scholar]
- IPCC (2013) Summary for policymakers. In Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM, eds, Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, UK, pp 3–29 [Google Scholar]
- Ito S, Ohtake N, Sueyoshi K, Ohyama T (2006) Allocation of photosynthetic products in soybean during early stages of nodule formation. Soil Sci Plant Nutr 52: 438–443 [Google Scholar]
- Koester RP, Skoneczka JA, Cary TR, Diers BW, Ainsworth EA (2014) Historical gains in soybean (Glycine max Merr.) seed yield are driven by linear increases in light interception, energy conversion, and partitioning efficiencies. J Exp Bot 65: 3311–3321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leakey ADB, Ainsworth EA, Bernacchi CJ, Rogers A, Long SP, Ort DR (2009) Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. J Exp Bot 60: 2859–2876 [DOI] [PubMed] [Google Scholar]
- Lobell DB, Field CB (2007) Global scale climate-crop yield relationships and the impacts of recent warming. Environ Res Lett 2: 014002 [Google Scholar]
- Lobell DB, Gourdji SM (2012) The influence of climate change on global crop productivity. Plant Physiol 160: 1686–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobell DB, Roberts MJ, Schlenker W, Braun N, Little BB, Rejesus RM, Hammer GL (2014) Greater sensitivity to drought accompanies maize yield increase in the U.S. Midwest. Science 344: 516–519 [DOI] [PubMed] [Google Scholar]
- Long SP, Ainsworth EA, Leakey ADB, Nӧsberger J, Ort DR (2006a) Food for thought: lower-than-expected crop yield stimulation with rising CO2 concentrations. Science 312: 1918–1921 [DOI] [PubMed] [Google Scholar]
- Long SP, Ort DR (2010) More than taking the heat: crops and global change. Curr Opin Plant Biol 13: 241–248 [DOI] [PubMed] [Google Scholar]
- Long SP, Zhu XG, Naidu SL, Ort DR (2006b) Can improvement in photosynthesis increase crop yields? Plant Cell Environ 29: 315–330 [DOI] [PubMed] [Google Scholar]
- Lopes MS, Reynolds MP, Manes Y, Singh RP, Crossa J, Braun HJ (2012) Genetic yield gains and changes in associated traits of CIMMYT spring bread wheat in a “historic” set representing 30 years of breeding. Crop Sci 52: 1123–1131 [Google Scholar]
- Lutz W, Samir KC (2010) Dimensions of global population projections: what do we know about future population trends and structures? Philos Trans R Soc Lond B Biol Sci 365: 2779–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manès Y, Gomez HF, Puhl L, Reynolds M, Braun HJ, Trethowan R (2012) Genetic yield gains of the CIMMYT international semi-arid wheat yield trials from 1994 to 2010. Crop Sci 52: 1543–1552 [Google Scholar]
- McKendry P. (2002) Energy production from biomass. Part 1. Overview of biomass. Bioresour Technol 83: 37–46 [DOI] [PubMed] [Google Scholar]
- Ort DR, Long SP (2014) Limits on yields in the Corn Belt. Science 344: 484–485 [DOI] [PubMed] [Google Scholar]
- Ort DR, Melis A (2011) Optimizing antenna size to maximize photosynthetic efficiency. Plant Physiol 155: 79–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry MAJ, Reynolds M, Salvucci ME, Raines C, Andralojc PJ, Zhu XG, Price GD, Condon AG, Furbank RT (2011) Raising yield potential of wheat. II. Increasing photosynthetic capacity and efficiency. J Exp Bot 62: 453–467 [DOI] [PubMed] [Google Scholar]
- Peng S, Khush GS, Virk P, Tang Q, Zou Y (2008) Progress in ideotype breeding to increase rice yield potential. Field Crops Res 108: 32–38 [Google Scholar]
- Penning de Vries FWT, Jansen DM, ten Berge HFM, Bakema A (1989) Simulation of Ecophysiological Processes of Growth in Several Annual Crops. Centre for Agricultural Publishing and Documentation, Wageningen, The Netherlands [Google Scholar]
- Prasad PVV, Boothe KJ, Allen LH Jr, Thomas JMG (2003) Super-optimal temperatures are detrimental to peanut (Arachis hypogaea L.) reproductive processes and yield at both ambient and elevated carbon dioxide. Glob Change Biol 9: 1775–1787 [Google Scholar]
- Raines CA. (2011) Increasing photosynthetic carbon assimilation in C3 plants to improve crop yield: current and future strategies. Plant Physiol 155: 36–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray DK, Mueller ND, West PC, Foley JA (2013) Yield trends are insufficient to double global crop production by 2050. PLoS ONE 8: e66428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds M, Foulkes J, Furbank R, Griffiths S, King J, Murchie E, Parry M, Slafer G (2012) Achieving yield gains in wheat. Plant Cell Environ 35: 1799–1823 [DOI] [PubMed] [Google Scholar]
- Rincker K, Nelson R, Specht J, Sleper D, Cary T, Cianzio SR, Casteel S, Conley S, Chen P, Davis V, et al. (2014) Genetic improvement of US soybean in maturity groups II, III, and IV. Crop Sci 54: 1419 [Google Scholar]
- Rosegrant M, Agcaoili M (2010) Global Food Demand, Supply, and Price Prospects to 2010. International Food Policy Research Institute, Washington, DC [Google Scholar]
- Ruiz-Vera UM, Siebers M, Gray SB, Drag DW, Rosenthal DM, Kimball BA, Ort DR, Bernacchi CJ (2013) Global warming can negate the expected CO2 stimulation in photosynthesis and productivity for soybean grown in the midwestern United States. Plant Physiol 162: 410–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadras VO, Lawson C, Montoro A (2012) Photosynthetic traits in Australian wheat varieties released between 1958 and 2007. Field Crops Res 134: 19–29 [Google Scholar]
- Sayer J, Sunderland T, Ghazoul J, Pfund JL, Sheil D, Meijaard E, Venter M, Boedhihartono AK, Day M, Garcia C, et al. (2013) Ten principles for a landscape approach to reconciling agriculture, conservation, and other competing land uses. Proc Natl Acad Sci USA 110: 8349–8356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman VJ, Scott RK, Foulkes MJ (2005) Physiological processes associated with wheat yield progress in the UK. Crop Sci 45: 175–185 [Google Scholar]
- Sinclair TR, Muchow RC (1999) Radiation use efficiency. Adv Agron 65: 215–265 [Google Scholar]
- Sinha SK, Bhargava SC, Goel A (1982) Energy as the basis of harvest index. J Agric Sci 99: 237–238 [Google Scholar]
- Slattery RA, Ainsworth EA, Ort DR (2013) A meta-analysis of responses of canopy photosynthetic conversion efficiency to environmental factors reveals major causes of yield gap. J Exp Bot 64: 3723–3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilman D, Balzer C, Hill J, Befort BL (2011) Global food demand and the sustainable intensification of agriculture. Proc Natl Acad Sci USA 108: 20260–20264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey JK, Walsh KB, Layzell DB (1988) Can a limitation in phloem supply to nodules account for the inhibitory effect of nitrate on nitrogenase activity in soybean? Physiol Plant 74: 137–146 [Google Scholar]
- Zhu XG, Long SP, Ort DR (2008) What is the maximum efficiency with which photosynthesis can convert solar energy into biomass? Curr Opin Biotechnol 19: 153–159 [DOI] [PubMed] [Google Scholar]
- Zhu XG, Long SP, Ort DR (2010) Improving photosynthetic efficiency for greater yield. Annu Rev Plant Biol 61: 235–261 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.