Four leading cell biologists reflect on the controversial nature of the vector for transport between the ER and Golgi apparatus in higher plants.
Abstract
The endoplasmic reticulum (ER) is the gateway to the secretory pathway in all eukaryotic cells. Its products subsequently pass through the Golgi apparatus on the way to the cell surface (true secretion) or to the lytic compartment of the cell (vacuolar protein transport). In animal cells, the Golgi apparatus is present as a stationary larger order complex near the nucleus, and transport between the cortical ER and the Golgi complex occurs via an intermediate compartment which is transported on microtubules. By contrast, higher plant cells have discrete mobile Golgi stacks that move along the cortical ER, and the intermediate compartment is absent. Although many of the major molecular players involved in ER-Golgi trafficking in mammalian and yeast (Saccharomyces cerevisiae) cells have homologs in higher plants, the narrow interface (less than 500 nm) between the Golgi and the ER, together with the motility factor, makes the identification of the transport vectors responsible for bidirectional traffic between these two organelles much more difficult. Over the years, a controversy has arisen over the two major possibilities by which transfer can occur: through vesicles or direct tubular connections. In this article, four leading plant cell biologists attempted to resolve this issue. Unfortunately, their opinions are so divergent and often opposing that it was not possible to reach a consensus. Thus, we decided to let each tell his or her version individually. The review begins with an article by Federica Brandizzi that provides the necessary molecular background on coat protein complexes in relation to the so-called secretory units model for ER-Golgi transport in highly vacuolated plant cells. The second article, written by Chris Hawes, presents the evidence in favor of tubules. It is followed by an article from David Robinson defending the classical notion that transport occurs via vesicles. The last article, by Akihiko Nakano, introduces the reader to possible alternatives to vesicles or tubules, which are now emerging as a result of exciting new developments in high-resolution light microscopy in yeast.
Cell biology textbooks usually give a description of the Golgi apparatus as it is seen and functions in a mammalian cell. This is understandable considering the huge body of literature on this organelle in animal cells, especially in relation to disease. It also reflects and is designed to accommodate the large readership from the medical sciences. With few exceptions (Ito et al., 2014), there are few reviews on the Golgi apparatus that give a balanced account of its structure and function across the whole eukaryotic kingdom.
The plant Golgi apparatus is nevertheless a fascinating organelle that has unique features, especially when compared with its mammalian counterpart. It is polydisperse rather than being present as a continuous perinuclear ribbon, it does not disassemble during mitosis, and it is motile. These characteristics are of great importance, especially because Golgi stacks in plants, along with their protein processing functions, have been termed polysaccharide factories and their activity is essential for formation of a cell wall during cytokinesis and growth. Nevertheless, it is Golgi stack motility that makes bidirectional protein transport between the endoplasmic reticulum (ER) and the Golgi apparatus in plants conceptually more difficult to grasp and therefore even more intriguing than others in other eukaryotic kingdoms.
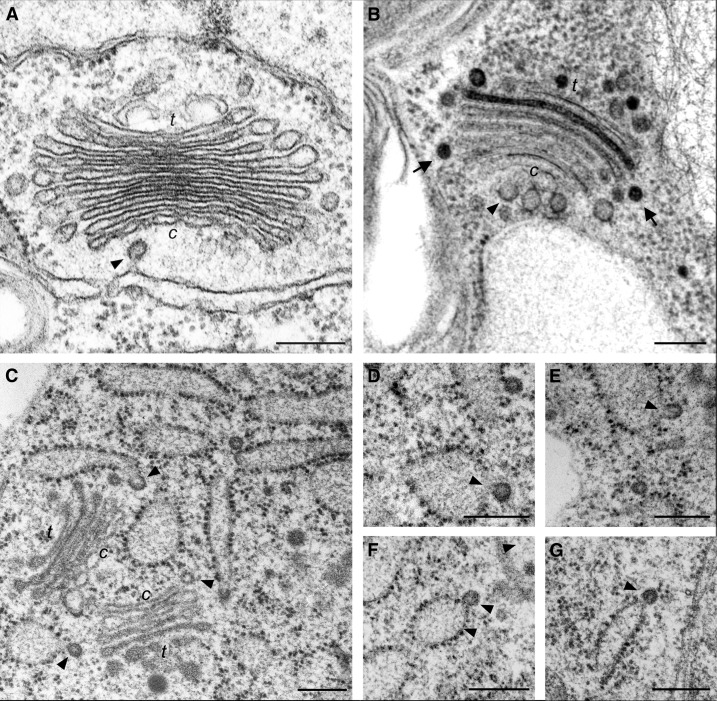
There is general consensus that in all eukaryotes, anterograde protein transport out of the ER is largely dependent upon Coat Protein II (COII) proteins and that retrograde transport both within the Golgi stack and between the Golgi and the ER requires COPI proteins (Szul and Sztul, 2011; Barlowe and Miller, 2013). Because recruitment of proteins in yeasts and mammals culminates in the formation of a transport vesicle, it is logical to assume that this may also be the case for plants. However, whereas there appears to be no doubt about the formation and release of COPI vesicles at the periphery of plant Golgi cisternae (Pimpl et al., 2000; Donohoe et al., 2007), the existence of COPII-coated vesicles and their operation as anterograde transport vectors in the narrow (less than 500 nm) interface between the ER and Golgi apparatus of land plants is a matter of considerable controversy (Hawes et al., 2008; Hawes, 2012). By contrast, there are numerous examples among lower eukaryotes where electron micrographs reveal a Golgi stack immediately adjacent (less than 500 nm) to transitional ER, per definition, a domain of the ER (or nuclear envelope) showing vesicle budding profiles and lacking ribosomes. The best examples for this are probably the yeast Pichia pastoris (Mogelsvang et al., 2003) and the algae Chlamydomonas noctigama (Fig. 1, A and B; Hummel et al., 2007), Tribonema vulgare, and Melosira varians (see Getty Images nos. 169272449 and 128618249; www.gettyimages.com). ER vesiculation profiles have often been recorded for mammalian cells going right back to the early papers of George Palade (for references, see Tartakoff, 2002). Interestingly, in all of these cases, as with the algae just mentioned, classical chemical fixation was sufficient to obtain the images. Therefore, one would expect that higher plants would be no different in this regard. Unexpectedly, this is not the case. So far, only in rapidly frozen samples has it been possible to visualize ER vesiculation profiles. Even then, such images are rare (Fig. 1, C and D; Robinson et al., 2007; Kang and Staehelin, 2008; Langhans et al., 2012).
Figure 1.
Electron microscopy of COPII budding. A and B, Transitional ER plus adjacent Golgi stacks in the green alga C. noctigama as seen in chemically fixed (A) and high-pressure frozen samples (B). The cis-trans (c and t) polarity of the Golgi stacks is clearly visible and so too are budding and released COPII vesicles (arrowheads). Putative COPI vesicles are marked with arrows. C, High-pressure frozen endosperm cell of Arabidopsis. Budding COPII vesicles are marked with arrowheads, and free putative COPII vesicles are marked with arrows. D to G, Collage of COPII budding profiles. Note that many of the buds are at the termini of ER cisternae. Note that the ER in high-pressure frozen samples is, in general, much more dilated than in chemically fixed samples; in C. noctigama, it is extremely dilated (the ER in B can be recognized by the ribosomes at the left of the vacuole-like structure). Bars = 200 nm.
Golgi stacks are invariably associated with tubular ER and only rarely with the edges of cisternae (Sparkes et al., 2009b). Moreover, in highly vacuolated plant cells such as in the leaf epidermis, Golgi stacks move (several micrometers per second) in a stop-and-go fashion along the surface of the ER (Boevink et al., 1998; Nebenführ et al., 1999). This contrasts with the situation in mammalian cells and in the aforementioned algae, where the ER and the Golgi are more or less stationary. So is perhaps Golgi motility the clue to the controversy surrounding COPII vesicle identification in higher plants?
The only alternative to vesicle-mediated transport is through some form of interconnecting tubules, either permanent or more probably temporal in nature. If so, the early secretory pathway of plants would appear to be fundamentally different from that of other eukaryotes. The purpose of this article is to examine whether this conclusion is warranted and valid.
Four scientists who have made major contributions in this area have come together to give their views on the matter. However, their divergent opinions have precluded a joint review. It was therefore decided that their opinions should appear separately. Our paper starts with a contribution from Federica Brandizzi who sets the scene at the molecular level, followed by two articles: one summarizing the data pro tubules (from Chris Hawes) and the other arguing in favor of vesicles (from David Robinson). The final article is from Aki Nakano, whose recent successful application of super high-resolution microscopy on yeast (Saccharomyces cerevisiae) allows for new insights into ER-Golgi trafficking in higher plants. We believe that the plant sciences community cannot fail to benefit from witnessing how four experienced cell biologists perceive the current state of play in this controversy. Although being unable to come to a final agreement on this issue, we dwell in the “Conclusion” on further possible courses of action.
FEDERICA BRANDIZZI: THE SECRETORY UNITS MODEL FOR ER PROTEIN TRANSPORT IN HIGHLY VACUOLATED CELLS
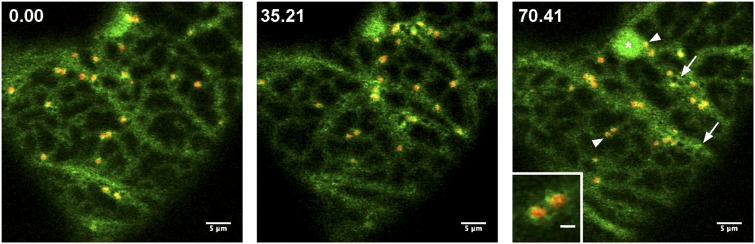
In live-cell imaging analyses, the Arabidopsis (Arabidopsis thaliana) COPII coat components (Sec13, Sec23, Sec24, and Sec31), when expressed in highly vacuolated leaf epidermal cells in tobacco (Nicotiana tabacum) and Arabidopsis, have been found in punctate structures that are associated with the ER and move with the Golgi stacks (Stefano et al., 2006; Hanton et al., 2007, 2009; Sieben et al., 2008; Wei and Wang, 2008; Faso et al., 2009; Takagi et al., 2013; Tanaka et al., 2013), which, in fully expanded plant cells, are highly dispersed and motile (Boevink et al., 1998; Stefano et al., 2014). The punctae labeled by the COPII coat proteins are commonly indicated as ER exit sites (ERESs). With the exception of Sec13, which has also been found at the nuclear envelope (Yang et al., 2005), Sec23, Sec24, and Sec31 are predominantly localized at such areas. Three-dimensional projection reconstruction of confocal images followed by rendering analyses have shown that Sec16 is localized in cup-like structures where the ER assumes a high-degree curvature (Takagi et al., 2013; Fig. 2), supporting the intriguing possibility for specific requirements of ER membrane curvature for the ERES establishment and maintenance. In a Sec24A partial loss-of-function mutant, a functional fluorescent protein fusion to Sec24A has been identified also in bright structures of unknown identity (Faso et al., 2009). It has been hypothesized that such structures may represent ERESs in formation or protein aggregates (Faso et al., 2009). The COPII-recruiting guanosine triphosphate hydrolyzing-protein (GTPase) Sar1 has been found at the ERESs but also over the ER network to a variable degree that may depend on the specific Sar1 isoform (Hanton et al., 2008). The distribution of Sec16, Sar1, and the COPII coat proteins drastically differs from that of the Sar1-guanine nucleotide exchange factor Sec12, which has been found distributed largely at the ER (Bar-Peled and Raikhel, 1997; daSilva et al., 2004; Yang et al., 2005). Similar to other eukaryotic cells, transport of proteins in plant cells may occur by bulk flow, as demonstrated for soluble proteins (Crofts et al., 1999; Phillipson et al., 2001), as well as in dependence of signals that can be present in the transmembrane domain (Brandizzi et al., 2002a; Schoberer et al., 2014), or through the presence of specific motifs that are recognized by COPII proteins.
Figure 2.
Golgi cisternae (rat sialyltransferase transmembrane domain and cytosolic tail fused to the yellow fluorescent protein, red) and the ERES marker (SEC16-GFP, green) visualized in tobacco leaf epidermal cells. Images from time-lapse sequence acquired at the cortical region of tobacco leaf epidermal cell with a Zeiss LSM510 confocal microscope. The Sec16 marker distributes at the peri-Golgi area (arrowheads) as well as to structures of unknown identity that are not associated with the Golgi marker (arrows; Takagi et al., 2013). The structures labeled by Sec16 can assume a ring-like shape (Takagi et al., 2013). Time of frames in the sequence is indicated at the left-hand corner of images (seconds). *, A chloroplast that is visible through chlorophyll autofluorescence. Bars = 5 and 1 μm (inset).
As it would be expected for specialized ER export domains in which cargo is packaged for export and release to the Golgi, ERESs are dynamic entities. Photobleaching experiments on fluorescent protein fusions to Sec13 and Sec24 have shown a high degree of turnover of these proteins on and off ERESs. Functional analyses on the effect of Sec16A loss-of-function mutation on COPII assembly in live cells have also demonstrated that Sec16A is involved in the dynamic association of coat components onto the ER, because in its absence, Sec24 and Sec13 were found to cycle on and off the ERESs to a much faster rate than in wild-type cells (Takagi et al., 2013). These findings support the possibility that Sec16 has a regulatory role on the COPII coat assembly, likely by influencing the GTPase activity of Sar1, which recruits the outer COPII coat components (Takagi et al., 2013). Increase in ERES size and number was verified when ER export competent membrane cargo was expressed transiently in tobacco leaf epidermal cells compared with ER export incompetent membrane cargo and bulk flow cargo (Hanton et al., 2008), supporting that the establishment and maintenance of ERESs are responsive to the cell’s necessity to export membrane and cargo from the ER. It will be interesting to test in the future whether modulation of ERES number and size depends on Sec16 and its functional interactions with COPII proteins.
A striking feature of the ERESs is their movement. The subcellular distribution of ERESs with respect to Golgi stacks has been debated for quite some time (Brandizzi and Barlowe, 2013). Although a transient association of the COPII machinery with the Golgi apparatus is plausible if partially coated COPII carriers are linked with the Golgi membrane before the COPII coat is completely shed (Langhans et al., 2012), a recent study on the subcellular localization and function of a plant Sec16 homolog has revealed new insights that further support an association of ERESs and motile Golgi (daSilva et al., 2004; Takagi et al., 2013). In particular, through fluorescence recovery after photobleaching analyses using a functional fluorescent protein to Sec16A, Sec16A was found to undergo dynamic binding and release from the membranes to slower rates compared with those of the outer COPII coat components such as Sec13, which was found to interact with Sec16 together with Sec31, and Sec24 (Takagi et al., 2013). If the punctate distribution of fluorescent protein fusion to COPII components observed in live-cell imaging studies were the result of association of these proteins with Golgi membranes, then Sec16 should show dynamics on and off membranes similar to the outer COPII components. However, the evidence that Sec16 cycles on and off the membranes at a slower rate compared with Sec24 and Sec13 and that the pool of membrane-associated protein significantly differs between Sec16 and Sec13 or Sec24 (Takagi et al., 2013) supports that a subpopulation of Sec16 is excluded from the coat of formed COPII carriers and labels ERESs that face the Golgi apparatus. The discrete steady-state localization of Sec16A at ERESs that are associated with the Golgi stacks supports that the plant ER/Golgi interface is uniquely organized such that ERESs move together with associated Golgi stacks. In this model, known as the secretory-units model (daSilva et al., 2004), ERESs would facilitate ER export to the Golgi at a Golgi-facing surface that is relatively static. Such organization does not exclude the possibility that non-Golgi-associated ERESs may also exist. In this light, it is possible that the ERES/Golgi unit may encounter ERESs that are not associated with a Golgi stack and eventually associate with it. It is similarly possible that non-Golgi-associated ERESs may assemble to form new Golgi stacks. The association of ERESs with the Golgi could also favor efficient retrograde transport mediated by cargo carriers such as COPI vesicles that have been clearly visualized in plant cells (Pimpl et al., 2000; Donohoe et al., 2007). ADP-ribosylation factor1, the GTPase involved in COPI coat assembly and dissociation, as well as coatomer, has been localized at the plant Golgi (Stefano et al., 2006; Matheson et al., 2007). It is possible to hypothesize that COPI vesicles might fuse proximally to COPII-enriched ERESs. Retrograde transport of membrane from the Golgi at the ER-Golgi interface close to the ERES region could facilitate fast retrieval and concentration of soluble N-ethylmaleimide-sensitive factor activating protein receptor (SNARE) proteins and other components of the machinery necessary for anterograde transport from the ER towards the Golgi.
The close spatial relationship between Golgi and ER and the evidence for a continuous exchange of Golgi enzymes with the ER (Brandizzi et al., 2002b) raise the question on whether the close association of ERESs with Golgi could have a role in holding the ER and the Golgi in close association. Such organization would facilitate ER protein export to a motile organelle. That the plant ER and the Golgi are attached has been demonstrated through the application of optical laser tweezers through which induced movement of tweezer-pulled Golgi stacks caused movement of the ER (Sparkes et al., 2009a). It is possible that ERESs and Golgi may be tethered by a proteinaceous mesh, which could not only facilitate the ER-Golgi directionality of COPII carriers, but also hold the two organelles together. It may also be that ERESs have a more direct role in the ER-Golgi interaction. It is also possible that the transient state between formation and dissipation of COPII carriers could enable the formation of a dynamic bridge that is sufficient to hold the Golgi in place on ERESs. Similar to the Golgi biogenesis model proposed earlier (Donohoe et al., 2013), the dynamic attachment of the ER and the Golgi would be facilitated during cisternae biogenesis whereby the most ER-proximal cisterna of the Golgi would be produced de novo by partial or complete fusion of COPII carriers and then mature into a distal cisterna via retrograde recycling of membranes and proteins.
CHRIS HAWES: LET IT BE TUBES
What Can EM Tell Us?
Back in the 1970s and 1980s, prior to the discovery of COPI and COII vesicles, various electron microscope (EM) studies of the plant endomembrane system suggested that there may be direct membrane connections between the ER and Golgi bodies, which were often termed dicytosomes in those days (Mollenhauer at al., 1976; Harris, 1979). To enable selective enhancement of the membranes of these organelles and to permit studies in three dimensions using thick sections and high-voltage electron microscopy, osmium impregnation techniques combined with either zinc iodide or potassium ferricyanide, to enhance the deposition of osmium on membranes and within the lumen of EM tubules and ER/Golgi cisternae, were often applied (Harris, 1979; Hepler, 1981). This resulted in a number of reports suggesting that the ER was directly connected to Golgi bodies via membrane-bounded tubules and that Golgi bodies themselves presented many tubules at the margins of their cisternae.
In our laboratory, we undertook a limited survey of ER-Golgi interactions in a number of tissues using the zinc iodide osmium impregnation technique (Juniper et al., 1982) and concluded that in maize (Zea mays) root caps, bean (Phaseolus vulgaris) root tips, and leaves (probably erroneously), the ER and Golgi were not connected. However, in the heavily protein-secreting glands of the Venus flytrap (Dionea muscipula), after stimulation of secretion, we observed numerous tubular extensions from cisternal rims, including cis-cisternae, that appeared to interconnect with the ER. At the same time, a study of the Golgi apparatus in developing wheat (Triticum aestivum) endosperm (Parker and Hawes, 1982), using high-voltage electron microscopy of thick sections of osmium-impregnated tissue, reported fine peripheral cisternal tubules connecting the cis-Golgi as well as other cisternae to the ER. This was also reported in developing bean and mung bean (Vigna radiata) seeds (Harris, 1979; Harris and Oparka, 1983). In wheat, these fine tubules were often much thinner than an ER tubule and, unless heavily osmium impregnated, under normal contrasting conditions in the EM, would unlikely scatter sufficient electrons to be readily visible. More recently, we reported ER-Golgi connections in tobacco leaves using osmium impregnation (Brandizzi et al., 2002b). Of course, the presence of tubules in electron micrographs does not prove they are involved in transport, retrograde, or anterograde but does show perhaps a more intimate relationship between the two organelles than is often assumed. Likewise, it has been shown that the ER surface itself is highly motile (Runions et al., 2006). Therefore, conceptually, there should be no problem in envisaging membrane flow from the ER through to the Golgi.
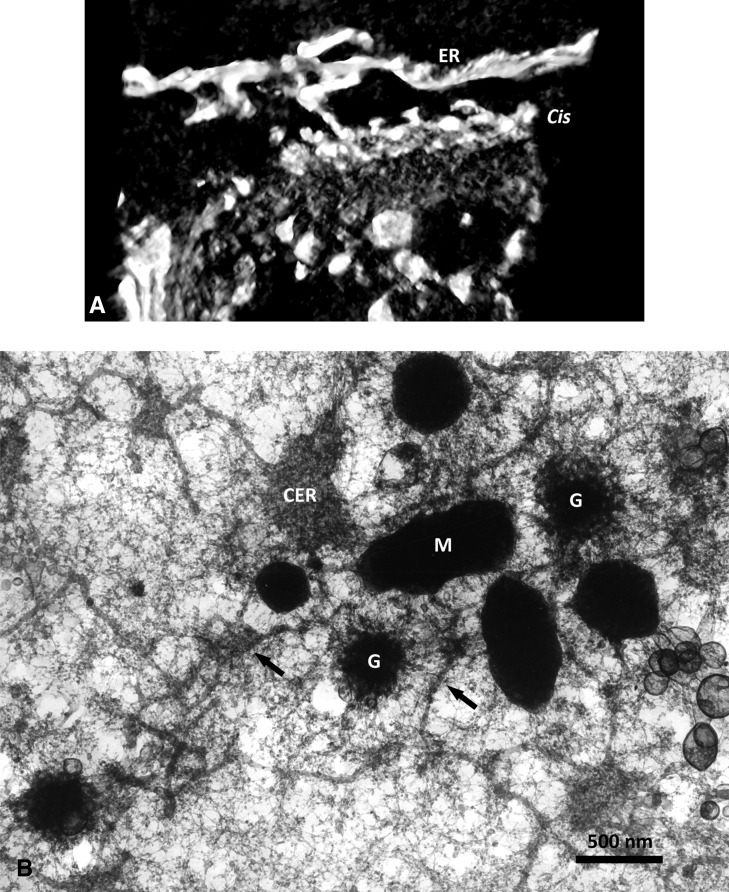
In contrast to these results, there are relatively few reports of vesicles budding from the ER or existing at the interface between the ER and Golgi, although Schnepf and Christ (1980) suggested that a vesicle flow exits in the epithelial cells of the nectaries of Asclepias curassavica. Only in a few publications using ultra-rapid high-pressure freeze fixation followed by freeze substitution and electron tomography have COPII vesicles been shown budding from the ER (Kang and Staehelin, 2008; Hawes, 2012; Donohoe et al., 2013), mainly in root meristem cells. An example in developing Arabidopsis endosperm tissue can be seen in Figure 1, C to G, of this article. Unfortunately, three-dimensional analysis and reconstruction by electron tomography of data such as these still relies on manual tracing of images, as autosegmentation algorithms cannot as yet differentiate the subtle differences in contrast in such electron micrographs to permit totally unbiased autosegmented reconstructions. However, it is possible to observe tubular connections between ER and cis-Golgi in tomograms of osmium-impregnated root material (C. Hawes, unpublished data; Fig. 3A). At this stage, it should be noted that lack of COPII vesicles is not restricted to plants but has been reported in several microorganisms. Likewise, depletion of COPII components does not always inhibit cargo transport from the ER in yeasts and animals (for references, see Mironov, 2014).
Figure 3.
A, Maximum-intensity projection in negative contrast of a stack of thin sections from a tomogram of a pea (Pisum sativum) root tip Golgi body and associated ER impregnated by the osmium zinc iodide technique. The reconstruction is presented at an angle to show a clear tubular connection between the ER and cis-Golgi. B, Inside face view of a dry-cleaved carrot (Daucus carota) suspension culture cell. The cell had been fixed on a coated EM grid, dehydrated, and critical point dried prior to dry cleaving on double-sided tape. The view onto the plasma membrane shows dark mitochondria (M), complete Golgi stacks in face view (G), cisternal ER (CER), and tubular ER (arrows). Note the huge difference between the diameter of a Golgi body and ER tubules.
An argument has been made on the lack of COPII EM images based around the calculation that, in reality, there are very few COPII vesicles at any one time in the ER-Golgi interface and that, in any one thin section, at most, only one vesicle would be seen (Langhans et al., 2012). This argument of course only holds true if such vesicles do exist. If they don’t, then they obviously would not be seen. Likewise, if tubules are transient in nature, they would rarely be seen in conventional electron micrographs and, when caught in cross section, would appear to be vesicular! An obvious experiment where COPII vesicles should be seen is in the reformation of Golgi after brefeldin A (BFA)-induced reabsorption into the ER. One such study on tobacco Bright Yellow2 (BY2) cells reported buds on the ER surfaces, which were infrequent, and tubular vesicular clusters, representing the earliest observable stage of stack regeneration cells (Langhans et al., 2007). These clusters immunolabeled for COPI coat components but not for COPII proteins. Considering the number of Golgi stacks in such cells, which is in the hundreds, if COPII vesicles exist, then it is surprising that none were seen in these experiments.
Of course, the get-out-of-jail card and perhaps a lazy answer to all of these discrepancies in the ultrastructural literature is simply to state that data from chemically fixed material is artifactual in nature due to slow fixation rates and only data from ultra-rapidly frozen freeze-substituted material is acceptable. This of course ignores the fact that, in chemically fixed material, it is easy to visualize both COPI and clathrin coats. So, why not COPII coats, especially as they are relatively easy to visualize in chemically fixed cells of algae such as C. noctigama (Hummel et al., 2007)? Two other golden rules of thin-section transmission electron microscopy also have to be remembered: (1) A thin section presents a two-dimensional image, and thus a tubule in cross section can easily be misinterpreted as a vesicle; and (2) Any biological material has to scatter sufficient electrons to form an image. Thus, a membrane in transverse section, spanning 70 nm of resin, scatters sufficient electrons to form a classic unit-membrane image, whereas the same stained membrane in face view may not present sufficient heavy-metal stain molecules and thus be electron lucent and not form an image; thus, fine tubules and membranes in face view can be missed. Selective-membrane staining techniques overcome this latter limitation. Of course, other EM techniques exist such as freeze-fracture or freeze-fracture deep etch, which should reveal structured exit sites on ER and COPII coats, but as far as I am aware, apart from the occasional image showing clathrin-coated vesicles and COPI vesicles (Coleman et al., 1987; Andreeva et al., 1998, no such images of COPII structures have been published in plants.
Has Live-Cell Imaging Helped?
Our initial observations on Golgi and ER in living leaf epidermal cells let us observe, for the first time, the dynamic nature of the organelles and the fact that Golgi bodies in leaves appeared to move over the surface of the ER (Boevink et al., 1998). This led us to propose the hoover model of Golgi bodies traveling over the ER surface sucking up vesicles produced by the ER, thus making the serious, but all too common, mistake of assuming that the plant ER-Golgi interface would function exactly the same as the mammalian ER in the production of COPII vesicles. However, over the past decade or so, we have refined our ideas and developed the secretory unit concept of ERESs and Golgi bodies traveling as single units around the cell with the motile surface of the ER (daSilva et al., 2004; Langhans et al., 2012). Such advances were made possible by the revolution in live-cell imaging offered by fluorescent protein technology and direct organelle labeling combined with techniques such as photobleaching and photoactivation of fluorescent probes. This enabled a range of experiments to be undertaken on the ER-Golgi interface, and contrary to what is often stated, it was shown there is no real evidence that transport between the ER and individual Golgi bodies only takes places when stacks are stationary (the stop-and-go model; Brandizzi et al., 2002b). We demonstrated that in a fluorescence recovery after photobleaching experiment, recovery of fluorescence of a Golgi membrane protein could be demonstrated in moving Golgi, indicating a continual transfer of protein from ER to Golgi (daSilva et al., 2004). Subsequently, laser manipulation of Golgi has demonstrated that when captured and translated through the cytoplasm by an infrared laser beam, Golgi bodies almost always drag a tubule of ER behind them (Sparkes et al., 2009c). One of the conclusions from this work was that the ER and Golgi are closely associated and tethered together, not via the cytoskeleton, but most likely by a number of structural proteins such as the Golgins/Golgi matrix proteins (Hawes et al., 2008). In studies of Golgi destruction and reformation in tobacco leaf cells, we showed that with either a genetic block of secretion or inhibition by BFA, reabsorption of the Golgi back into the ER was an ordered process, starting with the release of translocated Golgins into the cytoplasm followed by sequential reabsorption of trans-, medial, and cis-membranes into the ER. However, a cis-located matrix component, CASP, remained associated with ERESs, and we suggest that this protein may be part of the tethering complex holding the Golgi to the ER (Osterrieder et al., 2010; Schoberer et al., 2010). How this ordered membrane transport back to the ER is mediated is not known; perhaps some of the cisternal associated Golgi-ER tubules are involved in this pathway.
Interestingly, Golgi bodies in vacuolated tissue such as leaf epidermal cells are only ever associated with curved membranes of the ER such as tubules or more rarely the edges of cisternae (Sparkes et al., 2009b) and are never seen on the flat faces of ER cisternae. This situation has also been reported for yeast, where ERESs were associated with high-curvature domains containing the membrane-curving ER protein reticulon1 (Okamoto et al., 2012), and we have preliminary evidence that in Arabidopsis, reticulons may interact with SEC12, the Sar1-guanine nucleotide exchange factor that recruits the GTPase to the ER membrane as part of the COPII coat-building process (Kriechbaumer and Hawes, unpublished data). Thus, we can conclude that plant ERESprobably requires a curved ER surface on which to form.
Due to the nature of fluorescence and the diffraction limit of the microscopies used, an artificial impression is given in typical confocal micrographs of the diameter of ER tubules, compared with the diameter of a Golgi body (around 1 µm), giving a ratio of approximately 1:2. However, when observed by EM techniques such as dry cleave to show the cortical ER, whose tubes are in reality are around 30 to 90 nm, and associated Golgi, it is obvious that this ratio is more like 1:8, even taking into account the fact that cis-Golgi cisternae tend to be smaller in diameter than the rest of the stack (Fig. 3B). Thus, ERESs have to form on a relatively restricted area of highly curved membrane and not a flat surface, and perhaps the requirement for membrane bending proteins to produce a bud is lessened. Also, an ERES would need to be linear in structure along roughly 200 nm of ER tubule. Could such a structure produce sufficient 70-nm vesicles to transfer the required protein and membrane to a Golgi body? Such calculations have not yet been made.
Of course, it is generally accepted that COPII components are required in plants for some, if not all, transport between the ER and Golgi. As described above, it is easily demonstrated that inhibition of the formation of a COPII coat by expressing a nonhydrolysable form of the coat-initiating GTPase SAR1p results in the disruption of Golgi stack homeostasis and resorption of Golgi membrane back into the ER (Osterrieder et al., 2010). However, coating of a membrane patch to promote curvature and concentrate cargo at the tip of a transiently produced tubule is a distinct possibility. It has been shown in vitro that COPII components can tubulate liposome membrane (Bacia et al., 2011), and it has even been suggested that COPII coat components have sufficient flexibility to form 300-nm tubular structures that can accommodate large filamentous cargo such as procollagen fibrils (Miller and Schekman, 2013). Such tubes could easily span the narrow greater-than-300-nm interface between ER and cis-Golgi in plants.
DAVID G. ROBINSON: THE ODDS ARE STACKED IN FAVOR OF VESICLES
The Mobile Secretory Unit: What Are the Consequences for Bidirectional ER-Golgi Traffic?
In the frequently used leaf epidermal system, it is well established that COPII-fluorescence labeling always colocalizes with fluorescent Golgi markers, whether the Golgi stacks are mobile or not (daSilva et al., 2004; Hanton et al., 2009). For most researchers, punctate fluorescent COPII signals on the surface of the ER are synonymous with ERESs, but this may not necessarily be so because the visualization of COPII binding does not actually reveal the actual exit event. Langhans et al. (2012) have also questioned the fidelity of COPII-fluorescence labeling in recording ERESs on the surface of the ER and have suggested instead that the signals may instead represent prefusion COPII vesicles lying in the interface between ERESs and the cis-Golgi. Despite these caveats, it seems that anterograde traffic from the ER is restricted to a domain of the ER immediately adjacent to a Golgi stack and is embodied in the concept of the secretory unit (daSilva et al., 2004; Hanton et al., 2009). It now appears that retrograde traffic between the Golgi and the ER is also spatially restricted, because it has been demonstrated that the SNAREs on target membrane (t-SNAREs) required for COPI vesicle fusion with the ER localize to ER domains immediately underneath Golgi stacks (Lerich et al. 2012). This unique feature of the secretory pathway in plants probably serves the purpose of control and regulation, because the problem of stochastic release of COPII vesicles into the cytosol and their subsequent capture by Golgi stacks is avoided.
The concept of the secretory unit received great support from laser-trapping technology, which demonstrated that ER tubules moved with individual Golgi stacks when the latter were displaced (Sparkes et al., 2009a). This key observation could be interpreted as proof of direct membrane continuities between the ER and the cis-Golgi, but in the opinion of the majority, it is a consequence of the existence of tethering/matrix proteins that not only anchor the Golgi stack to the ER surface (Latijnhouwers et al., 2005; Osterrieder, 2012), but also maintain the integrity of the Golgi stack (Ito et al., 2014). An important feature of these experiments is that the Golgi stacks were immobilized by actin inhibitors, obviously a prerequisite for capturing otherwise mobile Golgi stacks. In this situation, interlocking matrix protein interactions between the ER and the Golgi are probably comparable to the real-life situation of a stop period in Golgi travel. However, it remains unclear as to whether the actual exit of anterograde cargo from the ER (i.e. vesicle budding [or COPII tube formation]) is restricted to the stationary phase or occurs continuously during Golgi movement. We also do not know whether antero- and retrograde transport are separate or coordinated, synchronized events. Lerich et al. (2012) have suggested that prefusion clusters of COPII and COPI vesicles might accompany mobile Golgi stacks, but the colocalization of Golgi stacks with the SNARE of the Qc-type syntaxin of plants72 (SYP72) of the t-SNARE fusion complex only in the immobilized condition suggests that entry of COPI retrograde cargo into the ER is restricted to stationary Golgi stacks.
Why Are COPII Vesicles Difficult to Visualize in Higher Plants?
COPI vesicles have been detected at the periphery of Golgi cisternae in higher plants (Pimpl et al., 2000; Donohoe et al., 2007). Although their positive identification as retrograde carriers to the ER in plants awaits confirmation, it is very likely that they do fulfill this function. This being so, there seems to be no a priori reason for questioning the participation of COPII vesicles in anterograde ER-Golgi traffic, yet their visualization in higher plant cells has proved difficult. In mammalian cells, there is a transport intermediate between the ER and the perinuclear immobile Golgi complex known as the ER-Golgi intermediate compartment (ERGIC; Appenzeller-Herzog and Hauri, 2006). It is this structure rather than the Golgi apparatus that is engaged in bidirectional COPII/COPI-mediated trafficking. During its formation (apparently through homotypic COPII vesicle fusion) and before it begins moving in the direction of the Golgi complex, it lies close to the surface of the ER (200–500 nm distant) in the immediate vicinity of ER export sites. Nevertheless, free COPII-coated carriers/vesicles have been reported on several occasions in the ER/ERGIC interface (Zeuschner et al., 2006; Hughes et al., 2009; Witte et al., 2011). In support of these observations, it has been recently demonstrated that loss of function of a cytosolic protein complex (transmembrane tyrosine receptor kinase-fused gene), which forms aggregates that temporarily trap COPII carriers in the ER/ERGIC interface, causes free COPII carriers to accumulate throughout the cytoplasm (Johnson et al., 2015).
A similarly narrow interface exists between ER and the cis-Golgi (the probable ERGIC equivalent in plants), so why the problem in seeing COPII vesicles in thin sections of higher plant cells? Langhans et al. (2012) have attributed this to the relatively small numbers of transport vesicles (at the very most 20) in the interface at any one time, which extrapolates to only a single vesicle in a thin section. There may be other contributing factors, for example, the speed of vesicle transport and, obviously if bidirectional transport is restricted to the stationary phase of Golgi movement, the timing of transport. If so, the chances of visualizing transport vesicles in the ER-Golgi interface will be seriously affected by the mobile status of the Golgi stack at the moment of fixation.
Have Gap-Spanning Tubules Been Observed in the Interface between the ER and cis-Golgi?
This can be answered with a categorical no, despite opposing claims made in some recent reviews (Sparkes et al., 2009a; Stefano et al., 2014). A careful scrutiny of the electron micrographs in the papers cited in these reviews (for details, see “Chris Hawes: Let It Be Tubes”) in support of direct membrane continuity at the ER/Golgi interface reveals that the connections are not at the interface but are, in fact, lateral connections between undefined but probably median Golgi cisternae and the ER. Moreover, the frequency of such continuities is enhanced under nonphysiological conditions, e.g. cold (Mollenhauer at al., 1976) or BFA (Ritzenthaler et al., 2002) treatments. The physiological significance of such lateral connections remains obscure, especially because it is not in harmony with the glycoprotein processing reactions that are supposed to occur in a sequential manner through the Golgi stack (from cis to trans). Also, lateral connections of this type are difficult to reconcile with Golgi stacks gliding over the surface of the ER. Finally, if tubular connections between the ER/nuclear envelope and the cis-face of a Golgi stack do exist, one would expect that the chances of visualizing them would be greater in those cases where the Golgi is immobile. However, such structures have never been seen in algal cells with naturally stationary Golgi stacks nor in higher plant cells where the Golgi has been immobilized through actin inhibitors.
What Are the Advantages of Vesicles?
Vesicles allow organelles to communicate among themselves and with the cell exterior (via the plasma membrane). With such transport vectors, cargo molecules can be transferred between organelles without disturbing their integrity. By excluding certain molecules and including others, vesicles also allow for a high degree of selectivity in intracellular transport. The efficient sorting of proteins into vesicles is to a great extent related to coat proteins at their surface that interact with several different types of integral transmembrane proteins: cargo receptors, helper proteins, such as the p24 proteins, and SNAREs. All of the coat proteins (COPI, COPII, and clathrin) can polymerize to form spherical structures (cages) and thus have the inherent ability to form vesicles, although it is true that COPII does it differently to COPI and clathrin (Hughson, 2010). However, the special properties of the COPII coat allow the incorporation of cargos of different sizes, something that cannot be achieved with clathrin or COPI vesicles. Therefore, COPII vesicles of different sizes can accommodate export of different cargo from the ER (Miller and Schekman, 2013) without the need for direct tubular connections. Finally, by concentrating SNAREs into a small amount of membrane, the efficiency of vesicle fusion with a specific target compartment is increased. The inhibition of vesicle formation can lead to uncontrolled fusion of organelles mediated by SNAREs on vesicle membrane and t-SNAREs as seen for the Golgi-ER hybrid structures formed in the presence of BFA (Elazar et al., 1994; Ritzenthaler et al., 2002).
By contrast, tubular contacts (irrespective of their longevity), or direct contacts between organelles culminating in fusion and then fission (as, for example, in hug-and-kiss models; Kurokawa et al., 2014), have the inherent caveat that an unspecific mixing of organelle contents may occur. Another problem is that if there is direct contact between the lumina of two adjacent compartments, how can pH differences be maintained? This is particularly important in the case of the endoplasmic reticulum protein retention (ERD2) receptor recognizing the tetrapeptide motif lys-asp-glu-leu (KDEL) receptor, which, in mammalian cells, is thought to bind to its KDEL-ligands at an acid pH (pH 6.7) in the cis-Golgi and to release them at the higher pH (pH 7.2) of the ER lumen (Wilson et al., 1993; Majoul et al., 1998; Paroutis et al., 2004). As is the case with the mannosyl-6 P receptor in mammalian endosomes, apparently only a relatively small shift in pH is sufficient to cause ligand release. A similar pH gradient of about 0.5 pH units between the ER and the Golgi also exists in plants (Martiniére et al., 2013), and it has recently been shown that the binding of COPII proteins to a plant ERD2 homolog is favored at neutral pH conditions, whereas the recruitment of COPI proteins to ERD2 is more optimal at an acidic pH (Montezinos et al., 2014).
Bidirectional protein trafficking between the ER and the Golgi apparatus also entails the movement of membrane. In the anterograde direction, this serves the purpose of replenishing membrane lost at the trans-face of the Golgi stack through release of the trans-Golgi network (Viotti et al., 2010) and continually drives the process termed cisternal maturation. In the retrograde direction, it is a consequence of receptor recycling. While vesicles clearly fulfill this requirement, it is less easy to see how tubes can achieve it. If ERES and cis-Golgi cisternae briefly touch each other, fuse, and rapidly separate, there can be no net movement of membrane at all. This is a stringent interpretation of the hug-and-kiss model of Kurokawa et al. (2014). However, if there is an active uptake of membrane (a patch of previously COPII-coated ERESs) together with soluble cargo as the cis-Golgi docks onto ERES, the model should perhaps be renamed hug and bite.
As new data becomes available, researchers are often compelled to revise their standpoints on particular issues. My initial interpretation of ER-Golgi traffic, based on immunofluorescent studies in tobacco BY2 cells (Yang et al., 2005), led me to believe that ERESs were greatly in excess of Golgi stacks. Switching to leaf epidermal cells and perhaps more stringent localization criteria via transient expression of X-fluorescent protein tagged, convinced me of the validity of the secretory unit concept. Nevertheless, I was never in doubt about vesicles, and I maintain that the unique features of the secretory unit, i.e. mobile Golgi stacks with a narrow interface to the ER, are not necessarily an impediment to COP vesicles as mediators for bidirectional protein traffic between the ER and the Golgi apparatus in higher plants. By contrast, direct membrane continuities between the ER and the Golgi have physiological drawbacks, and there is no convincing evidence for their existence, even in organisms where Golgi stacks remain permanently stationary.
AKIHIKO NAKANO: A COMMON MECHANISM FOR ER-TO-GOLGI TRAFFIC: A VIEW FROM SUPERRESOLUTION LIVE-IMAGING MICROSCOPY
How proteins traffic between the ER and the Golgi apparatus is an interesting and controversial issue. Problems have arisen in part through limitations of time and space resolution in observing the two organelles. My opinions section will focus on how we have tackled this problem by superresolution live imaging.
The budding yeast S. cerevisiae has been used as an ideal model system to study molecular mechanisms of membrane trafficking, because it is amenable to both genetics and biochemistry. In addition, its simplicity in organellar organization offers advantages in live imaging. We have applied high-speed confocal microscopy (Nakano, 2002) to S. cerevisiae and have made many discoveries that would have never been possible without live imaging at high spatiotemporal resolutions. Here, I will describe first what we learned from S. cerevisiae and then move on to compare similarly obtained data from plant and animal cells.
Golgi cisternae do not stack in S. cerevisiae (Glick and Nakano, 2009; Suda and Nakano, 2012), but not all budding yeasts have this feature. P. pastoris has, for example, stacked Golgi. This peculiar property of S. cerevisiae provides a wonderful opportunity to observe individual Golgi cisternae in living cells. Our and Ben Glick’s groups demonstrated that the yeast Golgi cisternae change properties from cis to medial and then to trans over time (Losev et al., 2006; Matsuura-Tokita et al., 2006). This gave strong support for the cisternal maturation model of intra-Golgi protein transport (Glick and Nakano, 2009; Nakano and Luini, 2010).
During the course of our studies on S. cerevisiae, we found that the microscopic method we were using (combination of a high-speed spinning-disc confocal scanner and a high-sensitivity camera system) had a great potential in improving resolution not only in time, but also in space (Matsuura-Tokita et al., 2006). I will skip the details here, but briefly, accurate image acquisition and minute data processing by deconvolution allow for amazing superresolution beyond the diffraction limit (Kurokawa et al., 2013). We have achieved 50- to 60-nm resolution in three-dimensional space with the time resolution of a few seconds per volume (the spec is further rising now). This method has been given the acronym SCLIM, standing for superresolution confocal live imaging microscopy (Kurokawa et al., 2013).
With such a high spatiotemporal resolution, we next tried to understand how cis-Golgi cisternae form. According to a classic cisternal maturation model, newly formed COPII vesicles containing cargo fuse with each other, and cis-Golgi proteins would join via COPI vesicles. First, we observed that the fluorescence of COPII coats, indicators of ERESs, shows a very dynamic behavior. The ERESs enlarge and shrink and are often very mobile. They appear to be stabilized when approaching high-curvature ER domains, such as the saddle-shaped surface of the ER sheet edge and along the ER tubules (Fig. 4). On the Golgi side, cis-cisternae show a significantly high probability of staying in the vicinity of the ERESs, whereas trans-cisternae do not (Okamoto et al., 2012).
Figure 4.
Organization of the ERESs in the budding yeast S. cerevisiae. Left, Dual-color three-dimensional image of Sec13-GFP (ERES marker, green) and monomeric red fluorescent protein-Sec12 (bulk ER marker, red) obtained by SCLIM. Right, Two-dimensional slice image taken from the three-dimensional data. ERESs localize at the high-curvature domains of the ER, such as along tubules and at the edge of the sheet. Bar = 1 μm.
A more detailed analysis of their behavior unveiled that the cis-Golgi frequently approaches the ERESs, keeps contact for a few seconds, and then leaves there. In addition, the fluorescence intensity of COPII often goes down upon this contact, suggesting an uncoating event during this process. The trans-Golgi cisternae also approach the ERESs but do not share the same tendency for a collapse of the COPII coat (Kurokawa et al., 2014). We reasoned that what we later termed the hug-and-kiss behavior of the cis-Golgi toward the ERESs indicates the capture of newly forming COPII vesicles followed by their uncoating and subsequent fusion with the cis-Golgi. To confirm this, we set up a system to pulse chase the cargo by live imaging. Fluorescent cargo that is synthesized and accumulated in the ER at a high temperature (39°C) proceeds to the secretory process upon shift down to a low temperature (25°C). Cargo first relocates to the ERESs and then gradually moves to the Golgi apparatus. When cis-Golgi approaches the ER, the cargo signal overlaps with the cis-Golgi signal, and then the cis-Golgi leaves the ER together with the cargo (Kurokawa et al., 2014). Thus, we conclude that cargo is delivered from the ERESs to the cis-Golgi through such a hug-and-kiss event. This seems to be a safer and more efficient way to send cargo to the destination than by releasing free COPII vesicles into the cytosol. It also explains why it has been so difficult to observe COPII vesicles by electron microscopy. Now, this hug-and-kiss delivery of cargo from the ER to the Golgi raises many new questions. (1) Do the COPII signals seen in our experiments represent clusters of COPII vesicles or patches of COPII coat? We do not know at the moment. Their sizes and shapes vary dynamically, suggesting that they do not correspond to individual single COPII buds. Considering the flexible nature of the COPII coat (Miller and Schekman, 2013), they could be either clusters of COPII buds or large patches of COPII coat, as has been proposed for clathrin coats. (2) Is the COPII vesicle formation completed before or during the hug and kiss? In other words, when does the fission of COPII vesicles occur? This is a good question. As the sorting of cargo from ER resident proteins must occur during the budding event, discontinuity would be desired. But as has been discussed for the presence of tubular connections in the Golgi stacks (Glick and Nakano, 2009; Nakano and Luini, 2010), a physical discontinuity of membrane may not be necessarily required for sorting of proteins. (3) If the hug-and-kiss mechanism ensures efficient and safe transfer of cargo, why are COPII vesicles necessary? We are not proposing that COPII vesicles are not released at all. At least, in certain in vitro or cell-free reconstructed systems, COPII vesicles do form in a Sar1 GTPase-dependent manner (Oka and Nakano, 1994; Barlowe et al., 1994; Matsuoka et al., 1998; Sato and Nakano, 2004; Tabata et al., 2009). In sec17 (soluble NSF attachment protein) and sec18 (N-ethylmaleimide sensitive factor) mutants, which are defective in vesicle fusion, numerous vesicles including COPII vesicles accumulate in the cytosol (Novick et al., 1980). However, releasing free vesicles in a large amount would be wasteful and even dangerous. Under normal conditions, we believe that complete release of COPII vesicles is maintained to a minimum. (4) If the cis-Golgi acts as a preexisting compartment to capture cargo from the ER, is there a stable pool? How does this model reconcile with the cisternal maturation from cis to trans? In our previous work (Matsuura-Tokita et al., 2006), we stated that the cis-Golgi appears to form de novo. However, with improved resolution, it looks more likely that small structures containing a cis-Golgi marker move around and grow over time before entering the maturation phase. We usually use Suppressor of Erd2 Deletion (Sed5; the counterpart of SYP31/SYP32 of plants and syntaxin5 of mammals) as a cis-Golgi marker, but considering the findings we have made in tobacco BY2 cells (Ito et al., 2012), the yeast Sed5 compartment may represent both a sorting platform like the ERGIC as well as the first enzymatic station of the glycosylation factory (see also below). (5) Is a hug-and-kiss mechanism specific to yeast? This is also a frequently asked question. We believe that the answer is no. In the remaining part of my essay, I would like to proceed to a comparison between yeast and other eukaryotes.
Plant cells have beautiful stacks of the Golgi apparatus, which are largely associated with the ER. To try to address how Golgi stacks are assembled and maintained in tobacco BY2 cells, we set up a system for live imaging in which Golgi stacks are disassembled by BFA treatment and reformed after BFA removal (Ito et al., 2012). While most of Golgi markers were absorbed into the ER upon BFA treatment as previously reported (Takeuchi et al., 2000, 2002; Ritzenthaler et al., 2002), we realized that some of the cis-Golgi markers (SYP31 and Retrieval Protein Endoplasmic Reticulum 1B) remained in the cytosol as small punctate structures. Another cis-Golgi marker, ERD2, diffuses into the ER together with medial and trans-markers, suggesting that there are two different classes of cis-Golgi proteins. Upon BFA wash, the small punctate structures of SYP31 nucleate to build new cisternae, to which other Golgi proteins then join in a cis-to-trans sequence. The small punctate structures of SYP31 are in the close vicinity of the ERESs but do not completely overlap with them (Ito et al., 2012).
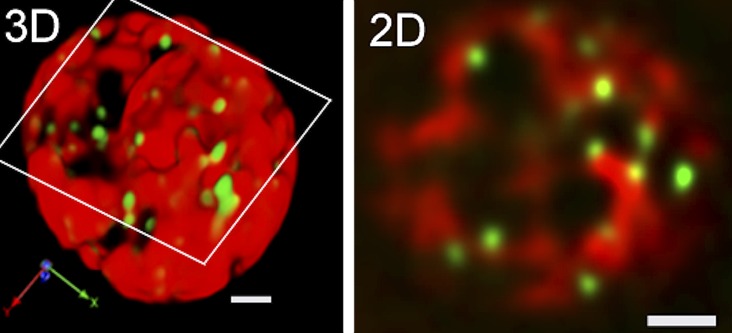
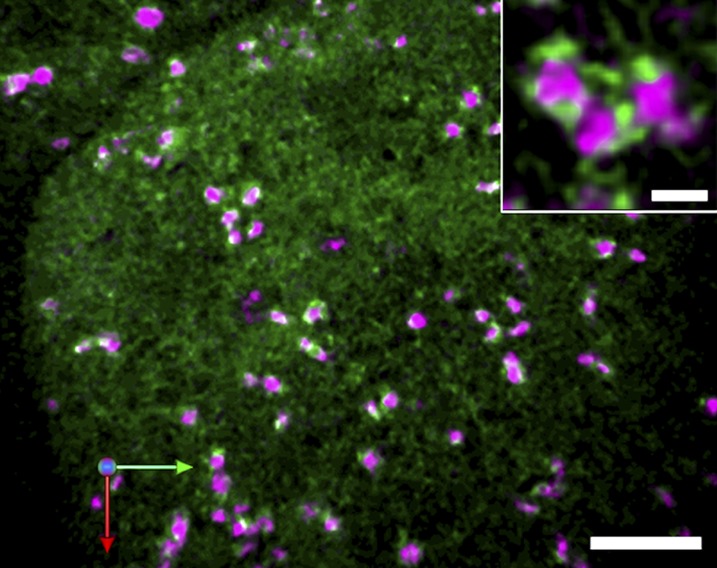
All of these observations suggest that the SYP31 compartment represents a scaffold to build the Golgi stack around, like the ERGIC of mammalian cells. The other type of cis-Golgi, in which ERD2 resides, may be a little distal to the SYP31 compartment. Simultaneous imaging of SYP31 and ERD2 shows their slightly different localizations in the stack (Y. Ito, unpublished data). Distinction of the cis-most cisterna of the Golgi from the later compartments in terms of function has also been reported by electron tomography. The cis-most cisterna is proposed to function as a protein-sorting platform, whereas the later compartments are involved in the biosynthetic activities of the Golgi (Donohoe et al., 2013). Regarding the relationship with the ERESs, it should also be mentioned that, in our study of BY2 cells, the number of ERESs is larger than that of cis-Golgi cisternae (Fig. 5). The ERESs without associated Golgi stacks are extremely mobile and may become stable when they encounter the Golgi (Ito et al., 2012).
Figure 5.
The cis-Golgi cisternae (monomeric red fluorescent protein-SYP31, magenta) and ERESs (SEC13-yellow fluorescent protein, green) visualized in tobacco BY2 cells. Confocal images were captured by SCLIM and reconstructed into three dimensions with deconvolution. A typical trajectory image from a three-dimensional time-lapse movie is shown. Almost all of the Golgi stacks were associated with bright spots of ERESs. From the magnified image (inset), we could observe the ERESs surrounding the cis-Golgi making ring-shaped fluorescent patterns. Bars = 5 and 1 μm (inset).
Consideration of the role the ERGIC plays for a subset of cis-Golgi compartments leads to the notion that it may be a very general feature of ER-to-Golgi trafficking (Ito et al., 2014; Kurokawa et al., 2014). It is widely accepted that the ERGIC is a typical structure of vertebrate cells, which have radial organization of microtubules, and receives cargo from the ERESs in the cell periphery before transporting it to the Golgi ribbon in the centrosomal region. Other organisms that do not have such astral patterns of microtubules, e.g. invertebrates, plants, and fungi, are believed to lack the ERGIC. However, as discussed above, plant cells appear to have a structure functionally related to the ERGIC. Drosophila melanogaster cells, which have dispersed Golgi, have also been reported to have an ERGIC-like structure (Witte et al., 2011). Furthermore, even in the yeast S. cerevisiae, we now propose that the mobile Sed5 structures that show a hug-and-kiss action toward ERESs may be regarded as ERGIC-like (Kurokawa et al., 2014). The ERGIC of mammalian cells is closely associated with the ERESs (Budnik and Stephens, 2009) and so is the D. melanogaster ERGIC (Witte et al., 2011). A SCLIM image of ERESs and cis-Golgi in a BY2 cell is presented in Figure 5. Altogether, the currently available data envisage a common mechanism of cargo delivery in the spatially close relationship of ERESs and the ERGIC (or its counterpart). Such an association of ERESs and the ERGIC is relatively stable in the cases of mammals, D. melanogaster, plants, and the yeast P. pastoris but is transient in S. cerevisiae. Interaction of these two compartments must require special molecular machinery, and we have proposed a role for the tethering factor Vesicle Transport (YusoU is transport in Japanese) factor; yeast counterpart of p115) in the association of ERESs and the cis-Golgi. Interestingly, when the Uso1 function is compromised by a temperature-sensitive mutation, the action of cis-Golgi is frozen during the hug-and-kiss event (Kurokawa et al., 2014).
There are still many problems remaining. Obviously, the observation of COPII vesicles in the spatiotemporal resolution good enough to distinguish individual dynamics has urgent priority for us. There is biochemical evidence that cargoes are selected during COPII vesicle budding (Sato and Nakano, 2005) and ER-Golgi shuttling proteins are retrieved back from the Golgi to the ER via COPI vesicles (Cosson et al., 1998; Sato et al., 2001). These events also await a revisit by live imaging. We are now in the process of developing the second-generation SCLIM, which will provide us with far-better images of what is going on in the narrow interface between the ER and the Golgi apparatus.
CONCLUSION
After having gone through the information and arguments presented above, it will not come as a surprise to the reader that the authors found themselves unable to reach a consensus about the modality of membrane traffic between the ER and the Golgi apparatus in higher plants. While there is no question that COPII proteins are essential for this process, doubt continues as to whether vesicles are the vectors of bidirectional traffic between the two organelles. It is clear that fluorescence microscopy, immunolabeling, and live cell imaging in combination have revolutionized our conceptual understanding of the structure and functioning of the eukaryotic Golgi apparatus and its relationship with the ER. However, the present superresolution fluorescence microscopic techniques such as structured illumination microscopy, stimulated emission depletion microscopy, and photoactivated localization microscopy are not sufficient to unequivocally identify discrete individual COPII vesicles. On the other hand, new techniques such as SCLIM are emerging that have the potential for even higher superresolution not only in space, but also in time and may well bring about a breakthrough in understanding what really is going on in living cells. Of course, electron microscopy is still the most powerful technique to solve the finer structural details of the interface between the ER and the Golgi whether in chemically fixed or frozen samples. Thus, perhaps a combination of ultra-rapid freezing and freeze substitution combined with selective membrane staining and one of the new high-resolution three-dimensional scanning electron microscopy technologies such as focussed ion beam-scanning electron microscopy or serial block face imaging might solve some of the mysteries of the plant ER-Golgi interface and help settle the controversy explored in this article. Unfortunately, irrespective of the method of preparation and the type of imaging, the downside with electron microscopy is that only single Golgi stacks can be visualized, with the caveat that the one being visualized might temporarily not be engaged in trafficking with the ER.
There is also an issue with the experimental systems being used. Beautiful live-cell imaging data has been obtained with the leaf epidermis system, but obviously it will be interesting to explore other cell types. While it seems that the situation in tobacco BY2 cells is not too different from that of leaf epidermal cells, there are other cell types such as in the meristem or in the endosperm during cellularization whose secretion status is unclear and where it is difficult, especially by conventional live-cell imaging, to recognize a close spatial relationship between the ER and Golgi stacks, let alone being able to say anything about Golgi motility due to the sheer density of the cytoplasm. On the other hand, such cell types, because of their relatively high cytoplasm/cell volume ratio, freeze well and are therefore most suitable for electron microscopy studies. The reverse is true for leaf epidermal cells, which are excellent objects for live-cell imaging, but present an almost insurmountable obstacle for ultra-rapid freezing. It remains to say that, even with the ideal plant cell, to capture the complexity of bidirectional ER-Golgi traffic in plant cells, correlative light and electron microscopy is necessary. Establishing this technique for studies on plants is going to be a major challenge for the future.
Glossary
- ER
endoplasmic reticulum
- ERES
endoplasmic reticulum exit site
- ERGIC
endoplasmic reticulum-Golgi intermediate compartment
- BFA
brefeldin A
Footnotes
This work was supported by the Chemical Sciences, Geosciences, and Biosciences Division, Office of Basic Energy Sciences, Office of Science, U.S. Department of Energy (award no. DE–FG02–91ER20021 to F.B.) and the National Science Foundation (grant no. MCB1243792 to F.B.).
References
- Andreeva AV, Kutuzov MA, Evans DE, Hawes CR (1998) The structure and function of the Golgi apparatus: a hundred years of questions. J Exp Bot 49: 1281–1291 [Google Scholar]
- Appenzeller-Herzog C, Hauri HP (2006) The ER-Golgi intermediate compartment (ERGIC): in search of its identity and function. J Cell Sci 119: 2173–2183 [DOI] [PubMed] [Google Scholar]
- Bacia K, Futai E, Prinz S, Meister A, Daum S, Glatte D, Briggs JA, Schekman R (2011) Multibudded tubules formed by COPII on artificial liposomes. Sci Rep 1: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Peled M, Raikhel NV (1997) Characterization of AtSEC12 and AtSAR1: Proteins likely involved in endoplasmic reticulum and Golgi transport. Plant Physiol 114: 315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherdt M, Schekman R (1994) COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 77: 895–907 [DOI] [PubMed] [Google Scholar]
- Barlowe CK, Miller EA (2013) Secretory protein biogenesis and traffic in the early secretory pathway. Genetics 193: 383–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boevink P, Oparka K, Santa Cruz S, Martin B, Betteridge A, Hawes C (1998) Stacks on tracks: the plant Golgi apparatus traffics on an actin/ER network. Plant J 15: 441–447 [DOI] [PubMed] [Google Scholar]
- Brandizzi F, Barlowe C (2013) Organization of the ER-Golgi interface for membrane traffic control. Nat Rev Mol Cell Biol 14: 382–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandizzi F, Frangne N, Marc-Martin S, Hawes C, Neuhaus J-M, Paris N (2002a) The destination for single-pass membrane proteins is influenced markedly by the length of the hydrophobic domain. Plant Cell 14: 1077–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandizzi F, Snapp EL, Roberts AG, Lippincott-Schwartz J, Hawes C (2002b) Membrane protein transport between the endoplasmic reticulum and the Golgi in tobacco leaves is energy dependent but cytoskeleton independent: evidence from selective photobleaching. Plant Cell 14: 1293–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnik A, Stephens DJ (2009) ER exit sites: localization and control of COPII vesicle formation. FEBS Lett 583: 3796–3803 [DOI] [PubMed] [Google Scholar]
- Coleman J, Evans D, Hawes C, Horsley D, Cole L (1987) Structure and molecular organization of higher plant coated vesicles. J Cell Sci 88: 35–45 [Google Scholar]
- Cosson P, Lefkir Y, Démollière C, Letourneur F (1998) New COP1-binding motifs involved in ER retrieval. EMBO J 17: 6863–6870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofts AJ, Leborgne-Castel N, Hillmer S, Robinson DG, Phillipson B, Carlsson LE, Ashford DA, Denecke J (1999) Saturation of the endoplasmic reticulum retention machinery reveals anterograde bulk flow. Plant Cell 11: 2233–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- daSilva LL, Snapp EL, Denecke J, Lippincott-Schwartz J, Hawes C, Brandizzi F (2004) Endoplasmic reticulum export sites and Golgi bodies behave as single mobile secretory units in plant cells. Plant Cell 16: 1753–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe BS, Kang BH, Gerl MJ, Gergely ZR, McMichael CM, Bednarek SY, Staehelin LA (2013) Cis-Golgi cisternal assembly and biosynthetic activation occur sequentially in plants and algae. Traffic 14: 551–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe BS, Kang BH, Staehelin LA (2007) Identification and characterization of COPIa- and COPIb-type vesicle classes associated with plant and algal Golgi. Proc Natl Acad Sci USA 104: 163–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elazar Z, Orci L, Ostermann J, Amherdt M, Tanigawa G, Rothman JE (1994) ADP-ribosylation factor and coatomer couple fusion to vesicle budding. J Cell Biol 124: 415–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faso C, Chen YN, Tamura K, Held M, Zemelis S, Marti L, Saravanan R, Hummel E, Kung L, Miller E, et al. (2009) A missense mutation in the Arabidopsis COPII coat protein Sec24A induces the formation of clusters of the endoplasmic reticulum and Golgi apparatus. Plant Cell 21: 3655–3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick BS, Nakano A (2009) Membrane traffic within the Golgi apparatus. Annu Rev Cell Dev Biol 25: 113–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanton SL, Chatre L, Matheson LA, Rossi M, Held MA, Brandizzi F (2008) Plant Sar1 isoforms with near-identical protein sequences exhibit different localisations and effects on secretion. Plant Mol Biol 67: 283–294 [DOI] [PubMed] [Google Scholar]
- Hanton SL, Chatre L, Renna L, Matheson LA, Brandizzi F (2007) De novo formation of plant endoplasmic reticulum export sites is membrane cargo induced and signal mediated. Plant Physiol 143: 1640–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanton SL, Matheson LA, Chatre L, Brandizzi F (2009) Dynamic organization of COPII coat proteins at endoplasmic reticulum export sites in plant cells. Plant J 57: 963–974 [DOI] [PubMed] [Google Scholar]
- Harris N. (1979) Endoplasmic reticulum in developing seeds of Vicia faba: a high voltage electron microscope study. Planta 146: 63–69 [DOI] [PubMed] [Google Scholar]
- Harris N, Oparka K (1983) Connections between dictyosomes, ER and GERL in cotyledons of mung bean (Vigna radiata L.). Protoplasma 114: 93–102 [Google Scholar]
- Hawes C. (2012) The ER/Golgi interface: is there anything in-between. Front Plant Sci 3: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes C, Osterrieder A, Hummel E, Sparkes I (2008) The plant ER-Golgi interface. Traffic 9: 1571–1580 [DOI] [PubMed] [Google Scholar]
- Hepler PK. (1981) The structure of the endoplasmic reticulum revealed by osmium tetroxide-potassium ferricyanide staining. Eur J Cell Biol 26: 102–111 [PubMed] [Google Scholar]
- Hughes H, Budnik A, Schmidt K, Palmer KJ, Mantell J, Noakes C, Johnson A, Carter DA, Verkade P, Watson P, et al. (2009) Organisation of human ER-exit sites: requirements for the localisation of Sec16 to transitional ER. J Cell Sci 122: 2924–2934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughson FM. (2010) Copy coats: COPI mimics clathrin and COPII. Cell 142: 19–21 [DOI] [PubMed] [Google Scholar]
- Hummel E, Schmickl R, Hinz G, Hillmer S, Robinson DG (2007) Brefeldin A action and recovery in Chlamydomonas are rapid and involve fusion and fission of Golgi cisternae. Plant Biol (Stuttg) 9: 489–501 [DOI] [PubMed] [Google Scholar]
- Ito Y, Uemura T, Nakano A (2014) Formation and maintenance of the Golgi apparatus in plant cells. Int Rev Cell Mol Biol 310: 221–287 [DOI] [PubMed] [Google Scholar]
- Ito Y, Uemura T, Shoda K, Fujimoto M, Ueda T, Nakano A (2012) cis-Golgi proteins accumulate near the ER exit sites and act as the scaffold for Golgi regeneration after brefeldin A treatment in tobacco BY-2 cells. Mol Biol Cell 23: 3203–3214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, Bhattacharya N, Hanna M, Pennington JG, Schuh AL, Wang L, Otegui MS, Stagg SM, Audhya A (2015) TFG clusters COPII-coated transport carriers and promotes early secretory pathway organization. EMBO J 34: 811–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juniper BE, Hawes CR, Horne JC (1982) The relationships between the dictyosomes and the forms of endoplasmic reticulum in plant cell with different export programmes. Bot Gaz 143: 135–145 [Google Scholar]
- Kang BH, Staehelin LA (2008) ER-to-Golgi transport by COPII vesicles in Arabidopsis involves a ribosome-excluding scaffold that is transferred with the vesicles to the Golgi matrix. Protoplasma 234: 51–64 [DOI] [PubMed] [Google Scholar]
- Kurokawa K, Ishii M, Suda Y, Ichihara A, Nakano A (2013) Live cell visualization of Golgi membrane dynamics by super-resolution confocal live imaging microscopy. Methods Cell Biol 118: 235–242 [DOI] [PubMed] [Google Scholar]
- Kurokawa K, Okamoto M, Nakano A (2014) Contact of cis-Golgi with ER exit sites executes cargo capture and delivery from the ER. Nat Commun 5: 3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhans M, Hawes C, Hillmer S, Hummel E, Robinson DG (2007) Golgi regeneration after brefeldin A treatment in BY-2 cells entails stack enlargement and cisternal growth followed by division. Plant Physiol 145: 527–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhans M, Meckel T, Kress A, Lerich A, Robinson DG (2012) ERES (ER exit sites) and the “secretory unit concept”. J Microsc 247: 48–59 [DOI] [PubMed] [Google Scholar]
- Latijnhouwers M, Hawes C, Carvalho C (2005) Holding it all together? Candidate proteins for the plant Golgi matrix. Curr Opin Plant Biol 8: 632–639 [DOI] [PubMed] [Google Scholar]
- Lerich A, Hillmer S, Langhans M, Scheuring D, van Bentum P, Robinson DG (2012) ER import sites and their relationship to ER exit sites: a new model for bidirectional ER-Golgi transport in higher plants. Front Plant Sci 3: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losev E, Reinke CA, Jellen J, Strongin DE, Bevis BJ, Glick BS (2006) Golgi maturation visualized in living yeast. Nature 441: 1002–1006 [DOI] [PubMed] [Google Scholar]
- Majoul I, Sohn K, Wieland FT, Pepperkok R, Pizza M, Hillemann J, Söling HD (1998) KDEL receptor (Erd2p)-mediated retrograde transport of the cholera toxin A subunit from the Golgi involves COPI, p23, and the COOH terminus of Erd2p. J Cell Biol 143: 601–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinière A, Desbrosses G, Sentenac H, Paris N (2013) Development and properties of genetically encoded pH sensors in plants. Front Plant Sci 4: 523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson LA, Hanton SL, Rossi M, Latijnhouwers M, Stefano G, Renna L, Brandizzi F (2007) Multiple roles of ARF1 in plant cells include spatially-regulated recruitment of coatomer and elements of the Golgi matrix. Plant Physiol 143: 1615–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka K, Orci L, Amherdt M, Bednarek SY, Hamamoto S, Schekman R, Yeung T (1998) COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell 93: 263–275 [DOI] [PubMed] [Google Scholar]
- Matsuura-Tokita K, Takeuchi M, Ichihara A, Mikuriya K, Nakano A (2006) Live imaging of yeast Golgi cisternal maturation. Nature 441: 1007–1010 [DOI] [PubMed] [Google Scholar]
- Miller EA, Schekman R (2013) COPII: a flexible vesicle formation system. Curr Opin Cell Biol 25: 420–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov AA. (2014) ER-Golgi transport could occur in the absence of COPII vesicles. Nat Rev Mol Cell Biol 15: 1. [DOI] [PubMed] [Google Scholar]
- Mogelsvang S, Gomez-Ospina N, Soderholm J, Glick BS, Staehelin LA (2003) Tomographic evidence for continuous turnover of Golgi cisternae in Pichia pastoris. Mol Biol Cell 14: 2277–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollenhauer HH, Morré DJ, Vanderwoude WJ (1976) Endoplasmic reticulum-Golgi apparatus associations in maize root tips. Mikroskopie 31: 257–272 [PubMed] [Google Scholar]
- Montezinos JC, Pastor-Cantizano N, Robinson DG, Marcote MJ, Aniento F (2014) Arabidopsis p24δ5 and p24δ9 facilitate COPI-dependent Golgi-to-ER transport of the K/HDEL receptor ERD2. Plant J 80: 1014–1030 [DOI] [PubMed] [Google Scholar]
- Nakano A. (2002) Spinning-disk confocal microscopy: a cutting-edge tool for imaging of membrane traffic. Cell Struct Funct 27: 349–355 [DOI] [PubMed] [Google Scholar]
- Nakano A, Luini A (2010) Passage through the Golgi. Curr Opin Cell Biol 22: 471–478 [DOI] [PubMed] [Google Scholar]
- Nebenführ A, Gallagher LA, Dunahay TG, Frohlick JA, Mazurkiewicz AM, Meehl JB, Staehelin LA (1999) Stop-and-go movements of plant Golgi stacks are mediated by the acto-myosin system. Plant Physiol 121: 1127–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P, Field C, Schekman R (1980) Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell 21: 205–215 [DOI] [PubMed] [Google Scholar]
- Oka T, Nakano A (1994) Inhibition of GTP hydrolysis by Sar1p causes accumulation of vesicles that are a functional intermediate of the ER-to-Golgi transport in yeast. J Cell Biol 124: 425–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Kurokawa K, Matsuura-Tokita K, Saito C, Hirata R, Nakano A (2012) High-curvature domains of the endoplasmic reticulum (ER) are important for the organization of ER exit sites in Saccharomyces cerevisiae. J Cell Sci 125: 3412–3420 [DOI] [PubMed] [Google Scholar]
- Osterrieder A. (2012) Tales of tethers and tentacles: golgins in plants. J Microsc 247: 68–77 [DOI] [PubMed] [Google Scholar]
- Osterrieder A, Hummel E, Carvalho CM, Hawes C (2010) Golgi membrane dynamics after induction of a dominant-negative mutant Sar1 GTPase in tobacco. J Exp Bot 61: 405–422 [DOI] [PubMed] [Google Scholar]
- Parker ML, Hawes CR (1982) The Golgi apparatus in developing endosperm of wheat (Triticum aestivum L.). Planta 154: 277–283 [DOI] [PubMed] [Google Scholar]
- Paroutis P, Touret N, Grinstein S (2004) The pH of the secretory pathway: measurement, determinants, and regulation. Physiology (Bethesda) 19: 207–215 [DOI] [PubMed] [Google Scholar]
- Phillipson BA, Pimpl P, daSilva LL, Crofts AJ, Taylor JP, Movafeghi A, Robinson DG, Denecke J (2001) Secretory bulk flow of soluble proteins is efficient and COPII dependent. Plant Cell 13: 2005–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimpl P, Movafeghi A, Coughlan S, Denecke J, Hillmer S, Robinson DG (2000) In situ localization and in vitro induction of plant COPI-coated vesicles. Plant Cell 12: 2219–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzenthaler C, Nebenführ A, Movafeghi A, Stussi-Garaud C, Behnia L, Pimpl P, Staehelin LA, Robinson DG (2002) Reevaluation of the effects of brefeldin A on plant cells using tobacco Bright Yellow 2 cells expressing Golgi-targeted green fluorescent protein and COPI antisera. Plant Cell 14: 237–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DG, Herranz MC, Bubeck J, Pepperkok R, Ritzenthaler C (2007) Membrane dynamics in the early secretory pathway. Crit Rev Plant Sci 26: 199–225 [Google Scholar]
- Runions J, Brach T, Kühner S, Hawes C (2006) Photoactivation of GFP reveals protein dynamics within the endoplasmic reticulum membrane. J Exp Bot 57: 43–50 [DOI] [PubMed] [Google Scholar]
- Sato K, Nakano A (2004) Reconstitution of coat protein complex II (COPII) vesicle formation from cargo-reconstituted proteoliposomes reveals the potential role of GTP hydrolysis by Sar1p in protein sorting. J Biol Chem 279: 1330–1335 [DOI] [PubMed] [Google Scholar]
- Sato K, Nakano A (2005) Dissection of COPII subunit-cargo assembly and disassembly kinetics during Sar1p-GTP hydrolysis. Nat Struct Mol Biol 12: 167–174 [DOI] [PubMed] [Google Scholar]
- Sato K, Sato M, Nakano A (2001) Rer1p, a retrieval receptor for endoplasmic reticulum membrane proteins, is dynamically localized to the Golgi apparatus by coatomer. J Cell Biol 152: 935–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnepf E, Christ P (1980) Unusual transfer cells in the epithelium of the nectaries of Asclepias curassavica L. Protoplasma 105: 135–148 [Google Scholar]
- Schoberer J, Liebminger E, Vavra U, Veit C, Castilho A, Dicker M, Maresch D, Altmann F, Hawes C, Botchway SW, et al. (2014) The transmembrane domain of N-acetylglucosaminyltransferase I is the key determinant for its Golgi subcompartmentation. Plant J 80: 809–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoberer J, Runions J, Steinkellner H, Strasser R, Hawes C, Osterrieder A (2010) Sequential depletion and acquisition of proteins during Golgi stack disassembly and reformation. Traffic 11: 1429–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieben C, Mikosch M, Brandizzi F, Homann U (2008) Interaction of the K+-channel KAT1 with the coat protein complex II coat component Sec24 depends on a di-acidic endoplasmic reticulum export motif. Plant J 56: 997–1006 [DOI] [PubMed] [Google Scholar]
- Sparkes I, Runions J, Hawes C, Griffing L (2009a) Movement and remodeling of the endoplasmic reticulum in nondividing cells of tobacco leaves. Plant Cell 21: 3937–3949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkes IA, Frigerio L, Tolley N, Hawes C (2009b) The plant endoplasmic reticulum: a cell-wide web. Biochem J 423: 145–155 [DOI] [PubMed] [Google Scholar]
- Sparkes IA, Ketelaar T, de Ruijter NC, Hawes C (2009c) Grab a Golgi: laser trapping of Golgi bodies reveals in vivo interactions with the endoplasmic reticulum. Traffic 10: 567–571 [DOI] [PubMed] [Google Scholar]
- Stefano G, Hawes C, Brandizzi F (2014) ER: the key to the highway. Curr Opin Plant Biol 22: 30–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano G, Renna L, Chatre L, Hanton SL, Moreau P, Hawes C, Brandizzi F (2006) In tobacco leaf epidermal cells, the integrity of protein export from the endoplasmic reticulum and of ER export sites depends on active COPI machinery. Plant J 46: 95–110 [DOI] [PubMed] [Google Scholar]
- Suda Y, Nakano A (2012) The yeast Golgi apparatus. Traffic 13: 505–510 [DOI] [PubMed] [Google Scholar]
- Szul T, Sztul E (2011) COPII and COPI traffic at the ER-Golgi interface. Physiology 26: 348–354 [DOI] [PubMed] [Google Scholar]
- Tabata KV, Sato K, Ide T, Nishizaka T, Nakano A, Noji H (2009) Visualization of cargo concentration by COPII minimal machinery in a planar lipid membrane. EMBO J 28: 3279–3289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi J, Renna L, Takahashi H, Koumoto Y, Tamura K, Stefano G, Fukao Y, Kondo M, Nishimura M, Shimada T, et al. (2013) MAIGO5 functions in protein export from Golgi-associated endoplasmic reticulum exit sites in Arabidopsis. Plant Cell 25: 4658–4675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi M, Ueda T, Sato K, Abe H, Nagata T, Nakano A (2000) A dominant negative mutant of sar1 GTPase inhibits protein transport from the endoplasmic reticulum to the Golgi apparatus in tobacco and Arabidopsis cultured cells. Plant J 23: 517–525 [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Ueda T, Yahara N, Nakano A (2002) Arf1 GTPase plays roles in the protein traffic between the endoplasmic reticulum and the Golgi apparatus in tobacco and Arabidopsis cultured cells. Plant J 31: 499–515 [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Nishimura K, Kawamukai M, Oshima A, Nakagawa T (2013) Redundant function of two Arabidopsis COPII components, AtSec24B and AtSec24C, is essential for male and female gametogenesis. Planta 238: 561–575 [DOI] [PubMed] [Google Scholar]
- Tartakoff AM. (2002) George Emil Palade: charismatic virtuoso of cell biology. Nat Rev Mol Cell Biol 3: 871–876 [DOI] [PubMed] [Google Scholar]
- Viotti C, Bubeck J, Stierhof YD, Krebs M, Langhans M, van den Berg W, van Dongen W, Richter S, Geldner N, Takano J, et al. (2010) Endocytic and secretory traffic in Arabidopsis merge in the trans-Golgi network/early endosome, an independent and highly dynamic organelle. Plant Cell 22: 1344–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T, Wang A (2008) Biogenesis of cytoplasmic membranous vesicles for plant potyvirus replication occurs at endoplasmic reticulum exit sites in a COPI- and COPII-dependent manner. J Virol 82: 12252–12264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DW, Lewis MJ, Pelham HR (1993) pH-dependent binding of KDEL to its receptor in vitro. J Biol Chem 268: 7465–7468 [PubMed] [Google Scholar]
- Witte K, Schuh AL, Hegermann J, Sarkeshik A, Mayers JR, Schwarze K, Yates JR III, Eimer S, Audhya A (2011) TFG-1 function in protein secretion and oncogenesis. Nat Cell Biol 13: 550–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YD, Elamawi R, Bubeck J, Pepperkok R, Ritzenthaler C, Robinson DG (2005) Dynamics of COPII vesicles and the Golgi apparatus in cultured Nicotiana tabacum BY-2 cells provides evidence for transient association of Golgi stacks with endoplasmic reticulum exit sites. Plant Cell 17: 1513–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuschner D, Geerts WJ, van Donselaar E, Humbel BM, Slot JW, Koster AJ, Klumperman J (2006) Immuno-electron tomography of ER exit sites reveals the existence of free COPII-coated transport carriers. Nat Cell Biol 8: 377–383 [DOI] [PubMed] [Google Scholar]