Incomplete splicing of a mitochondrial gene affects plant growth and development.
Abstract
Mitochondria play an important role in maintaining metabolic and energy homeostasis in the cell. In plants, impairment in mitochondrial functions usually has detrimental effects on growth and development. To study genes that are important for plant growth, we have isolated a collection of slow growth (slo) mutants in Arabidopsis (Arabidopsis thaliana). One of the slo mutants, slo3, has a significant reduction in mitochondrial complex I activity. The slo3 mutant has a four-nucleotide deletion in At3g61360 that encodes a pentatricopeptide repeat (PPR) protein. The SLO3 protein contains nine classic PPR domains belonging to the P subfamily. The small deletion in the slo3 mutant changes the reading frame and creates a premature stop codon in the first PPR domain. We demonstrated that the SLO3-GFP is localized to the mitochondrion. Further analysis of mitochondrial RNA metabolism revealed that the slo3 mutant was defective in splicing of NADH dehydrogenase subunit7 (nad7) intron 2. This specific splicing defect led to a dramatic reduction in complex I activity in the mutant as revealed by blue native gel analysis. Complementation of slo3 by 35S:SLO3 or 35S:SLO3-GFP restored the splicing of nad7 intron 2, the complex I activity, and the growth defects of the mutant. Together, these results indicate that the SLO3 PPR protein is a splicing factor of nad7 intron 2 in Arabidopsis mitochondria.
Mitochondria, the power plants of the cell, are thought to derive from endosymbiotic α-proteobacteria (Gray, 1999). Through evolution, most of the mitochondrial genes have been transferred to the nucleus. Although today’s mitochondria still have their own genomes, the sizes are significantly smaller than those of α-proteobacteria. Nonetheless, the gene expression and protein synthesis machineries in mitochondria still retain similarity to their prokaryotic ancestors.
It has been estimated that 2,000 to 3,000 proteins are located in plant mitochondria (Millar et al., 2005). Among these, only approximately 60 proteins, including ribosomal proteins, subunits of electron transport complexes, and proteins involved in cytochrome c biogenesis, are encoded by the mitochondrial genome (Unseld et al., 1997). It is evident that the biogenesis and function of mitochondria are highly dependent on nuclear genes.
The mitochondrial genome does not contain any RNA polymerase genes. So the transcription of mitochondrial genes relies exclusively on nucleus-encoded RNA polymerases (Hess and Börner, 1999). In addition to transcription, the complex posttranscriptional processes of primary transcripts, including RNA cleavage, editing, and splicing, also require a lot of proteins encoded by the nuclear genes (de Longevialle et al., 2010). In plants, these posttranscriptional processes play an important role in the regulation of mitochondrial gene expression (Binder and Brennicke, 2003). For instance, many plant mitochondrial genes are interrupted by introns. Thus, RNA splicing of a precursor transcript is required for the removal of introns to join two neighboring exons together. In addition to conventional cis-introns, plant mitochondrial genomes contain several trans-split genes. Exons and flanking introns of these genes are scattered around the genome, independently transcribed, and the introns are spliced in trans to produce mature messenger RNA (mRNA). In Arabidopsis (Arabidopsis thaliana) mitochondria, 23 introns, including four in each of NADH dehydrogenase subunit1 (nad1), nad2, nad5, and nad7, three in nad4, and one in each of cytochrome c oxidase subunit2 (cox2), cytochrome c maturation subunit F C-terminus (ccmFc), ribosomal protein large subunit2 (rpl2), and ribosomal protein small subunit3 (rps3), have been described (Bonen, 2008). Among these, nad1, nad2, and nad5 transcripts contain introns that require trans splicing (Malek and Knoop, 1998).
In general, introns are classified into two families, group I and group II, according to conserved RNA structures and splicing mechanisms. Group I introns consist of 10 conserved paired regions, P1 to P10, in the core secondary structure. Splicing of group I introns is processed by two-step phosphoryl transfer reactions using the exogenous guanosine as a cofactor (Kruger et al., 1982; Cech, 1990). Thus far, only one group I intron was found in the cox1 gene of several plants (Cho et al., 1998). Group II introns are characterized by six stem-loop structures (e.g. domains I–VI), and the splicing mechanism resembles that of nuclear premRNA splicing with two transesterification steps and the intron being released as a lariat (Lambowitz and Zimmerly, 2004). Originally, group I and group II introns were capable of self-splicing, which was enhanced by proteins encoded by their introns. These intron-encoded proteins contain reverse transcriptase/endonuclease domains related to intron mobility and a maturase domain involved in splicing. In land plants, almost all introns have lost their intron-encoded proteins. Only two maturase genes, maturase K (matK) and matR, are retained in the genomes of chloroplasts and mitochondria, respectively. Most introns in plant chloroplasts and mitochondria are group II introns, and none of these introns have been demonstrated to self-splice in vitro (de Longevialle et al., 2010). Thus, the splicing of plant organellar group II introns requires additional trans factors encoded by nuclear genes.
Several nucleus-encoded proteins or splicing factors have been implicated in plant mitochondrial RNA splicing. Among them, pentatricopeptide repeat (PPR) proteins, a large family of sequence-specific RNA-binding proteins, have been shown to be required for splicing of mitochondrial introns in Arabidopsis (Barkan and Small, 2014; Brown et al., 2014). The Organelle Transcript Processing43 (OTP43) PPR protein is required for trans splicing of nad1 intron 1 (de Longevialle et al., 2007). The ABSCISIC ACID OVERLY SENSITIVE5 (ABO5) PPR protein is required for cis-splicing of nad2 intron 3 (Liu et al., 2010), and the Buthionine Sulfomixine-Insensitive Roots6 (BIR6) PPR protein is involved in splicing of nad7 intron 1 (Koprivova et al., 2010). The PPR proteins TANG2 and OTP439 are involved in the splicing of nad5 introns 2 and 3, respectively (Colas des Francs-Small et al., 2014). In addition to PPR proteins, the Regulator of Chromosome Condensation1/UV-B Resistance8/Guanine Nucleotide Exchange Factor-Like3 (RUG3) protein is required for the splicing of nad2 introns 2 and 3 (Kühn et al., 2011). What is This Factor 9, a Plant Organellar RNA Recognition domain-containing protein, is required for rpl2 and ccmFc intron splicing (Francs-Small et al., 2012). Arabidopsis mitochondrial transcriptional termination factor15 (mTERF15) is involved in the splicing of nad2 intron 3 (Hsu et al., 2014). Several nucleus-encoded maturases (e.g. nMAT1, nMAT2, and nMAT4) have been implicated in efficient splicing of several transcripts in Arabidopsis mitochondria (Nakagawa and Sakurai, 2006; Keren et al., 2009, 2012; Cohen et al., 2014). The DEAD-box protein Putative Mitochondrial Helicase2 and mitochondrial Chloroplast RNA Splicing Associated Factor-like Splicing Factor1 (mCSF1) are involved in the splicing of multiple introns in Arabidopsis mitochondria (Köhler et al., 2010; Zmudjak et al., 2013).
PPR proteins usually contain 2 to 30 tandem repeats of a degenerate 35-amino acid motif, which folds into a pair of antiparallel helices (Yin et al., 2013; Barkan and Small, 2014). There are 450 PPR proteins in Arabidopsis, which are divided into P and PLS subfamilies according to their structures. Proteins in the P subfamily contain the classic PPR (P) motif, which usually do not have any other conserved domains. Members of the PLS subfamily contain P and longer (L) or shorter (S) variant PPR motifs in the tandem arrays of PPR. The PLS subfamily is further divided into PLS, E, and DYW subclasses based on the presence of E or DYW motifs in the C-terminal sequences (Lurin et al., 2004). Plant PPR proteins are predominantly localized to chloroplasts and mitochondria (Colcombet et al., 2013). Members of the P subfamily have been implicated in RNA cleavage, splicing, stabilization, and translation, whereas the plant-specific PLS subfamily PPR proteins are mainly involved in RNA editing in chloroplasts and mitochondria (Takenaka et al., 2013; Barkan and Small, 2014). Nonetheless, it has been shown that some members of the PLS subfamily are also involved in RNA splicing (Chateigner-Boutin et al., 2011; Ichinose et al., 2012).
We have isolated a collection of Arabidopsis slow growth (slo) mutants. One of the slo mutants, slo1, is defective in a PPR protein of the E subclass that is required for mitochondrial nad4-449 and nad9-328 RNA editing (Sung et al., 2010). Here, we report the characterization of another Arabidopsis slo mutant, slo3, which resembles the slo1 mutant in growth and development. In contrast to SLO1, the SLO3 gene encodes a PPR protein of the P subfamily, which is required for the splicing of nad7 intron 2 in Arabidopsis mitochondria. The defect in nad7 intron 2 splicing leads to a significant reduction in complex I activity, which may be accountable for the retarded growth phenotypes observed in the slo3 mutant.
RESULTS
Phenotypic Analysis of Arabidopsis slo3 Mutant
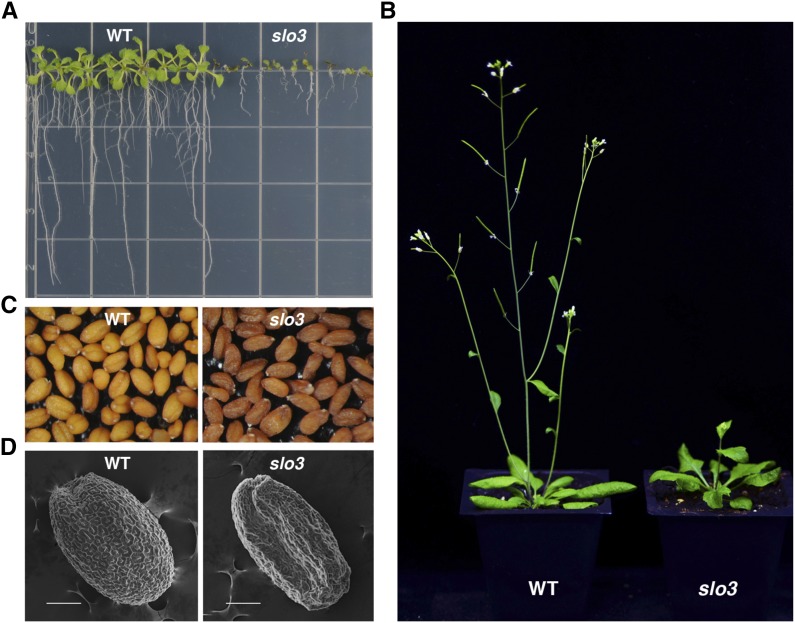
To study genes that are important for plant growth, we isolated several slo mutants from a collection of Arabidopsis transfer DNA (T-DNA) insertion lines (Sung et al., 2010). One of the slo mutants, slo3, has late germination, retarded growth, and delayed development phenotypes. When grown on tissue culture medium, the root length of slo3 was significantly shorter than that of the wild type (Fig. 1A). The development of true leaves was also significantly delayed in slo3 compared with that of the wild type (Fig. 1A). When grown in soil for 6 weeks, the wild-type Arabidopsis plant was mature and well developed, but the slo3 mutant was just about to bolt, and most of the rosette leaves were curly (Fig. 1B). A mature slo3 mutant plant could grow to about two-thirds of the height of the wild type, and did not display obvious defects in flowers or siliques. However, the seeds of the slo3 mutant were darker and shrunken compared with those of the wild type (Fig. 1, C and D).
Figure 1.
Phenotypic analysis of Arabidopsis slo3 mutants. A, Ten-day-old Arabidopsis wild-type (WT) and slo3 seedlings grown on tissue culture medium. B, Six-week-old Arabidopsis wild-type and slo3 plants grown in soil. C, Morphology of wild-type and slo3 seeds under light microscope. The mutant seeds are darker and shrunken compared with the wild type. D, Scanning electron micrographs of wild-type and slo3 seeds. Scale bars = 100 μm.
The Primary Root of slo3 Has Fewer Proliferating Cells
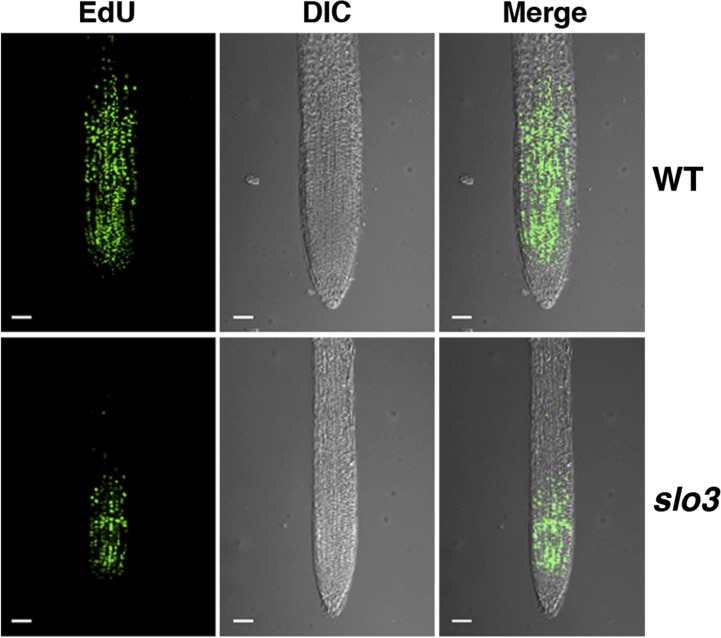
We used the ethynyl deoxyuridine (EdU)-based proliferation assay to compare cell division in roots of 7-d-old wild-type and slo3 seedlings. The modified nucleoside EdU is incorporated during DNA synthesis, which provides a rapid and robust assay for detection of cell proliferation in plant tissues (Kotogány et al., 2010). During the period (30 min) of EdU labeling, the primary root of the wild type has a lot more newly synthesized DNA than that of slo3 (Fig. 2). DNA synthesis mainly occurred in the cell division zone close to the root tip. The EdU assay revealed that the primary root of slo3 had fewer proliferating cells, which would result in a slow growth phenotype of the mutant.
Figure 2.
EdU staining of primary roots from 7-d-old wild-type (WT) and slo3 seedlings. The green fluorescent signals represent newly synthesized DNA during the period (30 min) of EdU staining. The results indicate that the slo3 mutant root has significantly fewer proliferating cells than that of the wild type. Scale bars = 50 μm. DIC, Differential interference contrast.
Identification of the SLO3 Gene
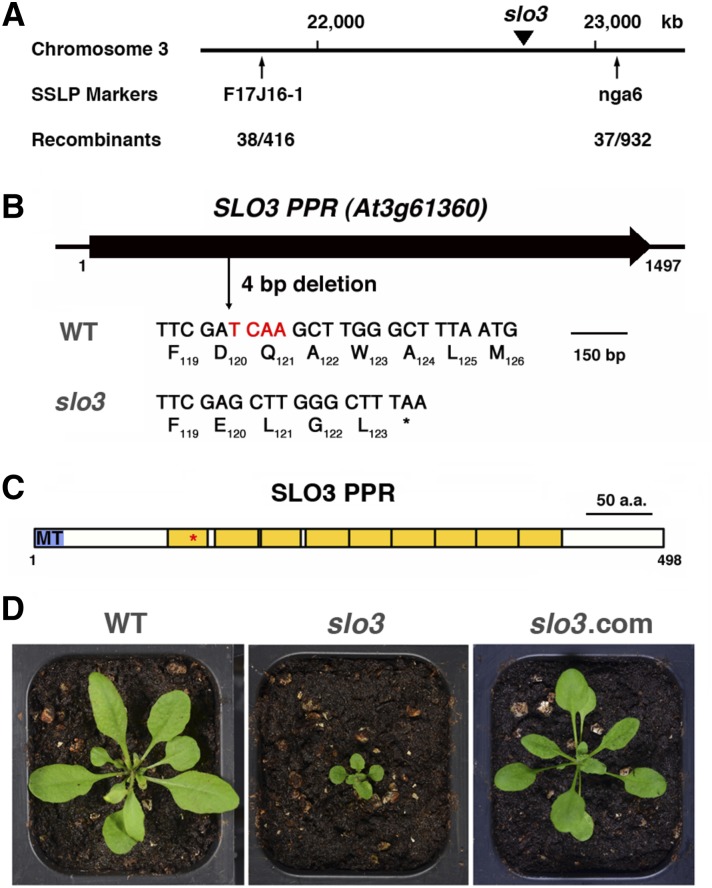
Genetic analysis of the slo3 mutant indicated that slo3 was a recessive mutant, but its phenotype did not cosegregate with the T-DNA insertion. We thus made a cross between the slo3 mutant (Columbia-0 [Col-0] background) and Landsberg erecta (Ler), and used map-based cloning to identify the molecular lesion of slo3. Simple sequence length polymorphism (SSLP) analysis of F2 mutant plants allowed us to assign the slo3 mutation to the vicinity of SSLP marker nga6 on the bottom arm of chromosome 3 (Fig. 3A). To facilitate the identification of the SLO3 gene, we sequenced the whole genome of slo3 and compared it with that of the reference genome. On the bottom arm of chromosome 3, we found a four-nucleotide deletion in the coding region of At3g61360 in the slo3 mutant. The deletion would change the reading frame of At3g61360 after the 119th amino acid residue and produced a stop codon at the position of the 124th residue in the slo3 mutant (Fig. 3B). Reverse transcription (RT)-PCR analysis revealed that steady-state levels of slo3 mutant transcripts were comparable with those of the wild type (Supplemental Fig. S1). Thus, the four-nucleotide deletion does not significantly affect the stability of the slo3 mutant transcripts. The SLO3 (At3g61360) gene encodes a PPR protein of 498 amino acid residues (Fig. 3C). The SLO3 PPR protein, a member of the P subfamily, contains nine PPR domains and a putative presequence for mitochondrion targeting. The premature stop codon derived from the slo3 mutant transcripts is located in the first PPR domain (Fig. 3C). A protein BLAST search revealed that SLO3 homologs were found in angiosperms, but not in gymnosperms or nonvascular plants (data not shown). Alignment of SLO3 homologs from Arabidopsis, Populus trichocarpa, Solanum lycopersicum, Glycine max, rice (Oryza sativa), Sorghum bicolor, and maize (Zea mays) revealed that these proteins were highly conserved between dicotyledon and monocotyledon plants (Supplemental Fig. S2).
Figure 3.
Molecular characterization of Arabidopsis slo3. A, The slo3 mutation was mapped to the bottom arm of chromosome 3 between SSLP markers F17J16-1 and nga6. B, The slo3 mutant has a four-nucleotide deletion in the coding region of At3g61360. The deletion changes the amino acid codons 120 to 123 and generates a stop codon at the 124th position. The At3g61360 gene encodes a PPR protein of the P subfamily. C, Schematic diagram of Arabidopsis SLO3 PPR protein. MT, Mitochondrial targeting sequence; yellow boxes, PPR domains. The asterisk indicates the position of a premature stop codon derived from the slo3 mutant transcript. D, Complementation of slo3 by 35S:SLO3 complementary DNA (cDNA). Plants shown are 24-d-old wild type (WT), slo3, and slo3 complemented by 35S:SLO3 (slo3.com) grown in soil.
Complementation of slo3 by 35S:SLO3 and 35S:SLO3-GFP cDNAs
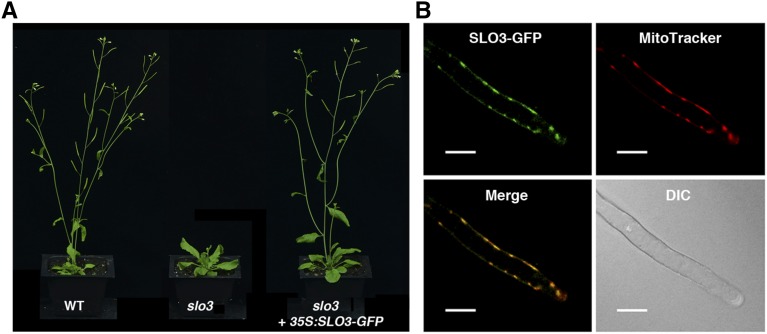
To further prove that the slo3 allele is responsible for the mutant phenotypes, we constructed plant expression vectors harboring 35S:SLO3 or 35S:SLO3-GFP cDNAs. The constructs were transformed into slo3 plants to test if they could rescue the mutant phenotypes. When we screened T1 seeds from both transgenic events on selective medium, successful transformants had a growth phenotype similar to that of the wild type, indicating that 35S:SLO3 and 35S:SLO3-GFP were able to rescue the slo3 mutant. Both 35S:SLO3 and 35S:SLO3-GFP complementation lines were carried to T3 homozygosity and used for further analysis. A representative complementation line, slo3 complemented by 35S:SLO3, is shown in Figure 3D. Similarly, the 35:SLO3-GFP transgene was able to restore the growth phenotype of slo3 to that of the wild type (Fig. 4A). Together, these results confirmed that the slo3 mutant is caused by a loss-of-function mutation in the At3g61360 gene.
Figure 4.
Arabidopsis SLO3-GFP is localized to the mitochondrion. A, Six-week-old Arabidopsis wild type (WT), slo3, and slo3 complemented by 35S:SLO3-GFP grown in soil. B, Subcellular localization of SLO3-GFP in the root hair of a 35S:SLO3-GFP transgenic plant. Mitochondria were visualized by MitoTracker orange staining. The green fluorescent signals of SLO3-GFP colocalized with the orange fluorescent signals of MitoTracker. DIC, Differential interference contrast. Scale bars = 20 μm.
The SLO3 PPR Protein Is Localized to the Mitochondrion
The SLO3 PPR contains an amino-terminal sequence to target the protein to mitochondria according to SUBAcon (http://suba3.plantenergy.uwa.edu.au/suba-app/flatfile.html?id=AT3G61360.1), which integrates multiple software predictions (Hooper et al., 2014). The phenotypes of slo3 also implicated that the SLO3 PPR could be localized to the mitochondrion. We showed that the 35:SLO3-GFP construct was able to complement the slo3 mutant (Fig. 4A), indicating that the SLO3-GFP fusion protein is functional in planta. Confocal microscopy was used to observe the GFP fluorescent signals in the roots of these complemented plants. MitoTracker orange was used to stain the roots as a control for mitochondrial localization. In the root hair of a 35:SLO3-GFP-complemented line, the green fluorescent signals colocalized with the MitoTracker orange fluorescent signals (Fig. 4B). Similar results were observed in root epidermal cells (Supplemental Fig. S3). Although the TargetP program (http://www.cbs.dtu.dk/services/TargetP/) predicts that the SLO3 PPR protein is localized to the chloroplast or mitochondrion, we could not observe the green fluorescent signals of the SLO3-GFP in the chloroplast (Supplemental Fig. S3). These results suggest that the SLO3-GFP fusion protein is localized to the mitochondrion, and SUBAcon is more accurate in the prediction of SLO3 subcellular localization.
The slo3 Mutant Is Defective in Splicing of nad7 Intron 2
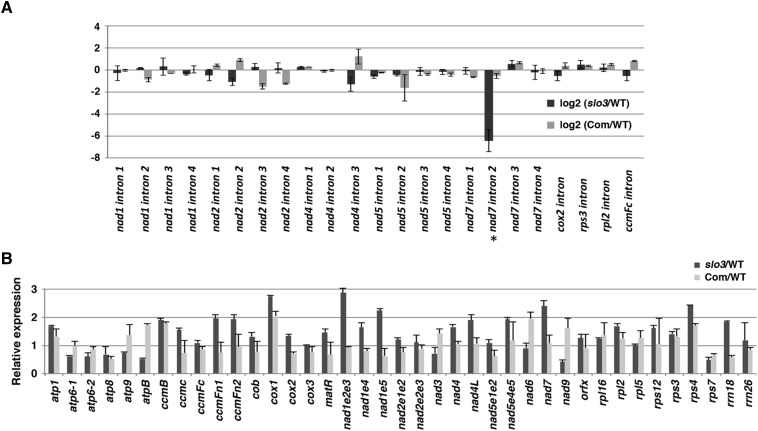
It is well known that many plant PPR proteins are involved in chloroplast or mitochondrial RNA metabolism (Barkan and Small, 2014). The phenotypes of the slo3 mutant and the subcellular localization of the SLO3-GFP imply that the SLO3 PPR protein may play a role in mitochondrial RNA metabolism. To test this hypothesis, we designed experiments to examine mitochondrial RNA editing, splicing, and the abundance of mitochondrial transcripts in the slo3 mutant. We used RT-PCR and bulk sequencing of the amplified cDNAs to compare the editing efficiencies of mitochondrial transcripts in slo3 and the wild type. Of the more than 500 mitochondrial RNA editing sites examined (Sung et al., 2010), we did not observe any significant difference between slo3 and the wild type (data not shown). For RNA splicing analysis, we used quantitative RT-PCR assays to examine the ratio of spliced to unspliced RNA for each mitochondrial intron in wild-type, slo3, and 35S:SLO3-GFP-complemented plants. Of the 23 introns examined, the splicing efficiency of nad7 intron 2 was dramatically decreased in the slo3 mutant, which was restored in the complemented plants (Fig. 5A). The quantitative RT-PCR results also revealed that the reduced slo3/wild-type ratio for nad7 intron 2 splicing was due to both an increase in unspliced and a decrease in spliced nad7 transcripts in the slo3 mutant (Supplemental Fig. S4). Compared with those of the wild type, the splicing efficiencies of the other introns were not significantly affected in the slo3 mutant (Fig. 5A).
Figure 5.
Splicing efficiency and abundance of mitochondrial transcripts in the slo3 mutant. A, Quantitative RT-PCR analysis of intron-containing mitochondrial transcripts. The histogram shows the log2 ratio of spliced to unspliced RNA in slo3 and complemented plants (Com) as compared with the corresponding wild type (WT). The splicing efficiency of nad7 intron 2 (indicated by asterisk) was dramatically reduced in the slo3 mutant. This specific splicing defect was restored in the complemented plants. B, Quantitative RT-PCR analysis of mitochondrial transcripts. The histogram shows the relative fold change of mitochondrial transcripts in slo3 and complemented plants as compared with the corresponding wild type. All of the quantifications were normalized to the nuclear gene Actin2 (ACT2). atp1, ATP synthase subunit1; cob, cytochrome c biogenesis; orfx, open reading frame x.
We also used quantitative RT-PCR assays to compare the abundance of mitochondrial transcripts in wild-type, slo3, and 35S:SLO3-GFP-complemented plants. Separate primer sets designed to detect different transcription units of the trans splicing genes (e.g. nad1, nad2, and nad5) were included in the analysis. These assays covered 33 protein-coding and 2 ribosomal RNA (rRNA) genes of the Arabidopsis mitochondrial genome. We did not detect significant transcript reduction in any of the mitochondrial genes examined that could potentially lead to reduced activities of respiratory complexes or other mitochondrial functions (Fig. 5B). By contrast, significant increases in the accumulation of several mitochondrial transcripts were observed in the slo3 mutant as compared with wild-type or complemented plants (Fig. 5B). It is likely that these are secondary effects caused by the loss of nad7 intron 2 splicing in slo3.
RNA Editing of the Unspliced nad7 Was Not Affected in slo3
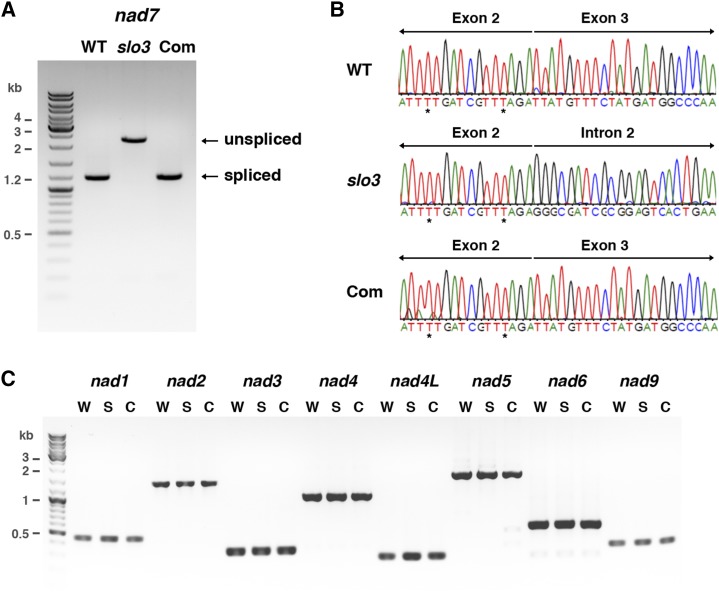
The Arabidopsis nad7 gene contains four introns, and the length of intron 2 is 1,063 bp. RT-PCR analysis with primers that would amplify the 1,185-bp full-length nad7 cDNA in the wild type revealed that the majority of nad7 transcripts in the slo3 mutant were about 2.2 kb (Fig. 6A). Sequencing analysis confirmed that the 2.2-kb products were the unspliced form of the nad7 transcript retaining the entire intron 2 (Fig. 6B). By contrast, fully processed nad7 transcripts were the predominant forms present in wild-type and complemented plants (Fig. 6, A and B). These results further confirmed that the splicing of nad7 intron 2 was severely impaired in the slo3 mutant. Sequencing analysis of these RT-PCR products also allowed us to compare the editing status of fully processed and intron 2-containing nad7 transcripts in the wild type and slo3, respectively. The editing of all sites located in the exons of nad7 was not affected in the slo3 mutant (Fig. 6B; data not shown). It has been shown that RNA editing in trans-introns is involved in the splicing reaction in wheat (Triticum aestivum) mitochondria (Farré et al., 2012). Interestingly, Arabidopsis nad7 intron 2 contains an editing site near the junction of intron 2-exon 3. Although the nad7 intron 2 is not a trans-intron, it is still of interest to know if the status of this particular editing site is affected in the slo3 mutant. We used RT-PCR to amplify the intron 2-containing nad7 transcripts in the wild type with primers located in exon 2 and exon 3 (Supplemental Fig. S5). Bulk sequencing analysis of these unspliced transcripts revealed that the C-to-U editing of the nad7 intron 2 editing site was not affected in the slo3 mutant (Supplemental Fig. S5). Thus, the lack of nad7 intron 2 splicing was not due to the absence of an RNA editing factor rather than a splicing factor in the slo3 mutant.
Figure 6.
RT-PCR and sequence analysis of nad7 transcripts in the slo3 mutant. A, Detection of fully processed nad7 transcripts by RT-PCR. The primers used for this experiment were located in exons 1 and 5, which would generate the 1,185-bp RT-PCR products for fully processed nad7 transcripts. Instead of 1,185 bp, the majority of the RT-PCR products in slo3 were the 2,248-bp unspliced form. Fully processed nad7 transcripts were restored in complemented plants (Com). B, Sequence histograms of RT-PCR products showing the junctions of spliced (exon 2-exon 3) and unspliced (exon 2-intron 2) nad7 transcripts from wild-type (WT), slo3, and complemented plants. Sequence analysis of the RT-PCR products confirmed that the 2,248-bp unspliced form contained exons 1 to 5 and the entire intron 2 of nad7 in the slo3 mutant. Asterisks indicate RNA editing sites. C, RT-PCR analysis of nad1, nad2, nad3, nad4, nad4L, nad5, nad6, and nad9 transcripts in wild type (W), slo3 (S), and complemented (C) plants. The primers used for this experiment were designed to amplify full-length or fully processed nad transcripts. These results confirmed that the splicing of nad7 intron 2 was specifically affected in slo3, whereas other mitochondrial nad transcripts were processed and accumulated normally in the mutant.
In addition to nad7, we used similar RT-PCR assays with primers designed to detect fully processed or full-length transcripts of other mitochondrial nad genes. The results indicated that the expression of nad1, nad2, nad3, nad4, nad4L, nad5, nad6, and nad9 was not affected in the slo3 mutant (Fig. 6C). Taken together, these results suggest that the slo3 mutant has a specific defect in the splicing of nad7 intron 2.
Prediction of SLO3 PPR Recognition Sites in nad7 Intron 2
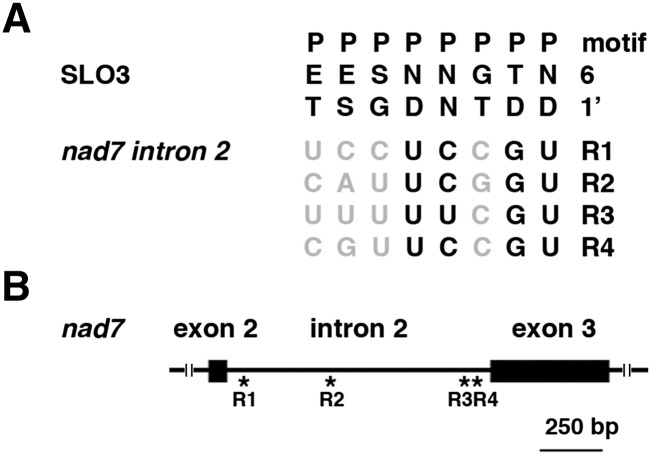
It has been proposed that PPR proteins may recognize RNA sequences via a one PPR motif-one nucleotide module, and the amino acids at particular positions (6 and 1′) in each repeat determine the nucleotide-binding specificity (Barkan et al., 2012). We used bioinformatics predictions (https://www.cs.colostate.edu/∼approve/) based on a combinatorial amino acid code for RNA recognition by PPR proteins (Barkan et al., 2012) to predict the SLO3 PPR binding sites in nad7 intron 2. We have identified four candidate RNA sequences that SLO3 PPR may bind in nad7 intron 2 (Fig. 7A). Interestingly, three of the four candidate sites are located in the vicinity of intron-exon junctions (Fig. 7B). Further experiments are required to test if the SLO3 PPR protein can really bind to these candidate sequences.
Figure 7.
Predictions of SLO3 PPR binding sites in nad7 intron 2. A, The 6/1′ amino acid combinations for each PPR (P) motif were aligned to four RNA sequences (R1 to R4) in nad7 intron 2 that SLO3 might bind. Nucleotides that match the expectation with high correlations are shown in black (Barkan et al., 2012). The nad7 intron 2 has 1,063 nucleotides, and R1 to R4 encompass nucleotides 28 to 35, 391 to 398, 1,039 to 1,046, and 1,044 to 1,051 of the intron, respectively. B, Schematic diagram of nad7 intron 2. Asterisks indicate the relative positions of R1 to R4 in the intron.
Respiratory Complex I Activity Is Significantly Reduced in slo3
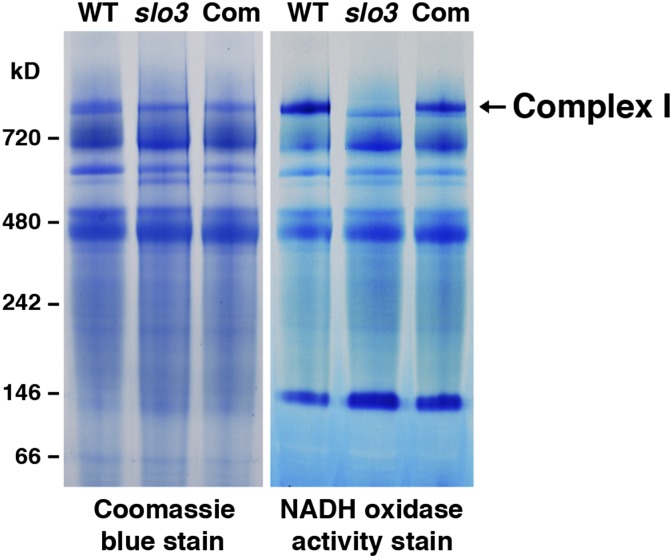
Nad7 is a subunit of the mitochondrial electron transport chain complex I (NADH dehydrogenase). The splicing defect in nad7 may directly affect the activity of complex I in the slo3 mutant. We used blue native (BN) PAGE (BN-PAGE) and in-gel activity staining to examine the activity of complex I in wild-type, slo3, and 35S:SLO3-GFP-complemented plants. Compared with that of the wild type, the activity of complex I was significantly reduced in the slo3 mutant (Fig. 8). In complemented plants, the complex I activity was restored to levels similar to those of the wild type (Fig. 8). These results further confirmed that the primary defect in nad7 intron 2 splicing would result in dysfunctional mitochondria in the slo3 mutant.
Figure 8.
The activity of complex I is significantly reduced in the slo3 mutant. Crude membrane extracts isolated from wild-type (WT), slo3, and slo3-complemented (Com) plants were separated by BN-PAGE followed by Coomassie Blue staining (left) or an in-gel activity stain for NADH dehydrogenase (right).
DISCUSSION
Group II introns in land plant mitochondrial genomes seem to have lost their self-splicing ability. Thus, the removal of these introns requires the participation of maturases, specific splicing factors, and more general splicing factors. The angiosperm mitochondrial genome contains only one maturase gene, matR, located within nad1 intron 4 (Wahleithner et al., 1990), and its role in splicing has yet to be established. By contrast, several nMATs have been shown to be involved in the splicing of mitochondrial introns (Nakagawa and Sakurai, 2006; Keren et al., 2009, 2012; Cohen et al., 2014). In contrast to the hallmark intron-encoded maturase that acts as an intron-specific splicing factor, these nMATs seem to target multiple unrelated introns in plant mitochondria. In addition to nMATs, several nucleus-encoded factors, including PPR proteins, have been shown to be involved in the splicing of mitochondrial introns in plants (Brown et al., 2014).
PPR proteins are known to play important roles in various steps of organellar RNA metabolism in plants (Barkan and Small, 2014). We have identified a PPR protein of the P subfamily, SLO3 (At3g61360), which is required for the splicing of nad7 intron 2 in Arabidopsis mitochondria. The SLO3 protein does not contain any recognizable RNA-binding motifs other than the PPR domains. The slo3 mutant has a 4-bp deletion in the coding region of SLO3, which creates a premature stop codon in the first PPR domain. In general, mRNAs containing premature termination codons will be degraded via the nonsense-mediated decay pathway that is highly conserved in eukaryotic organisms (Chang et al., 2007; Stalder and Mühlemann, 2008). RT-PCR analysis revealed that the abundance of slo3 mutant transcripts was similar to that of the wild type (Supplemental Fig. S1). It is known that plant nonsense-mediated decay pathways can be triggered by 3′ untranslated region (UTR)-located introns or long 3′UTRs (Kerényi et al., 2008). Arabidopsis SLO3 is an intronless gene, and the slo3 mutant transcripts do not contain any introns. Although slo3 mRNAs are expected to have long 3′UTRs after the premature termination codon, these mutant transcripts may contain stabilizing cis elements or distinct secondary/tertiary structures to escape from nonsense-mediated decay. Even if the slo3 mutant transcripts were translatable, the synthesized peptide would only contain part of the first PPR domain. The truncated SLO3 protein, if it does exist in the slo3 mutant, would not retain any intact PPR domain. It is very likely that slo3 is a null mutant.
We examined several aspects of mitochondrial RNA metabolism in slo3 and identified that the defect in nad7 intron 2 splicing was the primary cause of the mutant phenotypes. Nad7 is a subunit of respiratory complex I that is highly conserved among eukaryotes and prokaryotes. As expected, the impairment in nad7 intron 2 splicing leads to a significant reduction in complex I activity in the slo3 mutant (Fig. 8). In addition to the defect in nad7 intron 2 splicing, increases in the levels of several mitochondrial transcripts were observed in the slo3 mutant (Fig. 5B). However, these changes in mitochondrial transcript levels are not specific to slo3. Similar results have been reported in Arabidopsis abo5, rug3, and mterf15 mutants that are impaired in the splicing of nad2 transcripts (Liu et al., 2010; Kühn et al., 2011; Hsu et al., 2014); the nmat4 mutant that is defective in nad1 premRNA processing and maturation (Cohen et al., 2014); the slo1 mutant that has completely lost RNA editing at nad4-449 and nad9-328 (Sung et al., 2010); and the NADH dehydrogenase (ubiquinone) fragment S subunit 4 (ndufs4) mutant that is impaired in a nucleus-encoded complex I subunit (Meyer et al., 2009). Therefore, the increased mitochondrial transcript levels in slo3 may be due to a secondary effect in response to reduced complex I activity, rather than a direct effect caused by the loss of function of SLO3. Since we could not detect any major defects in RNA splicing, editing, and accumulation of other mitochondrial nad transcripts, the defect in nad7 intron 2 splicing might be accountable for the reduction of complex I activity and growth retardation in the slo3 mutant.
It is likely that multiple splicing factors may be involved in the removal of a specific intron in plant mitochondria (Brown et al., 2014). Previously, Arabidopsis nMAT2 was shown to be involved in the splicing of multiple mitochondrial introns, including nad7 intron 2 (Keren et al., 2009). The splicing of nad7 intron 2 is partially lost in the nMat2 mutant (Keren et al., 2009). In addition, a more general splicing factor, mCSF1, has been shown to be involved in the processing of many mitochondrial introns, including nad7 intron 2 (Zmudjak et al., 2013). Here, we demonstrate that the SLO3 PPR protein is specifically involved in the splicing of nad7 intron 2. It is conceivable that the removal of nad7 intron 2 may require a splice complex involving SLO3, nMat2, and mCSF1. It will be interesting to examine how these proteins, and possibly other unidentified splicing factors, work in concert to specifically remove nad7 intron 2 in Arabidopsis mitochondria.
The Arabidopsis mitochondrial nad7 gene contains five exons separated by four introns. In addition to splicing factors required for the splicing of nad7 intron 2, the BIR6 PPR protein (At3g48250) of the P subfamily has been implicated to be involved in the splicing of nad7 intron 1 (Koprivova et al., 2010). Although both SLO3 and BIR6 PPR proteins are members of the P subfamily, sequence alignment of these two proteins revealed that they are not closely related to each other (data not shown). It has been shown that PPR proteins may bind RNA via a one PPR domain-one nucleotide module (Yin et al., 2013; Barkan and Small, 2014). The specific effects of BIR6 and SLO3 on the splicing of nad7 intron 1 and intron 2, respectively, may derive primarily from the sequence-specific binding to single-stranded RNA of PPR tracts in these two proteins. Nevertheless, the splicing defects of nad7 introns 1 (in bir6) and 2 (in slo3) are sufficient to explain the dramatic reduction of fully processed nad7 transcripts, leading to a reduced complex I activity and growth retardation in the mutants.
Little is known about the structure and function of the mitochondrion-encoded Nad7 in plants. In Arabidopsis, respiratory complex I contains more than 40 subunits encoded by both nuclear and mitochondrial genes (Klodmann et al., 2010; Meyer, 2012). It is known that the multiprotein subunits in complex I form an L-shaped structure, which is composed of the hydrophilic matrix arm (or peripheral arm) and the hydrophobic membrane arm (Klodmann et al., 2010; Meyer et al., 2011). The matrix arm contains the NADH-oxidizing activity, and the membrane arm contains the ubiquinone-binding site. It is still not clear how these subunits are assembled into a functional complex. It has been proposed that Arabidopsis complex I subunit proteins will form subcomplexes, which are analogous to the N module (dehydrogenase domain), Q module (ubiquinone reduction module or hydrogenase doamin), and P module (the membrane arm) of complex I from heterotrophic eukaryotes (Klodmann et al., 2010). Nad7 is a member of the Q module (or the 140-kD subcomplex) of complex I that connects the dehydrogenase domain to the membrane arm, whereas most of the other Nad subunits encoded by mitochondrial genes are located in the membrane arm (Klodmann et al., 2010; Meyer, 2012).
In addition to Arabidopsis mutants that are defective in nad7, the well-characterized cytoplasmic male sterile2 mutant of Nicotiana sylvestris has lost the nad7 gene (Gutierres et al., 1997; Dutilleul et al., 2003, 2005). Recently, a PPR protein of the E subfamily, small kernel 1 (SMK1), has been shown to be responsible for the editing of nad7 transcripts in maize and rice (Li et al., 2014). The smk1 mutant has dramatically reduced complex I activity and abnormal embryo and endosperm development (Li et al., 2014). The molecular defects in these mutants implicate that Nad7 is important for complex I activity in plants. As it is literally impossible to create mutants for mitochondrial genes, these surrogate mutants may be used to study the functions of Nad7 in the assembly and activity of complex I in plants (Colas des Francs-Small and Small, 2014).
Most complex I mutants show some form of growth retardation (de Longevialle et al., 2007; Keren et al., 2009, 2012; Liu et al., 2010; Kühn et al., 2011; Haïli et al., 2013; Wydro et al., 2013; Zmudjak et al., 2013; Cohen et al., 2014; Colas des Francs-Small et al., 2014; Hsu et al., 2014). We used EdU staining to demonstrate that a smaller cell proliferation zone in the slo3 mutant root may be accountable for the short root phenotype of the mutant. The slo3 mutant plants contain dysfunctional mitochondria with reduced complex I activity. This raises an interesting question as to how perturbation of mitochondrial function may affect the size and activity of the root apical meristem. Plant growth is primarily determined by meristems with the plant hormone auxin orchestrating many of the underlying processes (Durbak et al., 2012). It has been shown that perturbation of mitochondrial function may negatively affect auxin signaling (Kerchev et al., 2014). In addition to maintaining metabolic and energy homeostasis of the plant cell, the functional state of mitochondria may directly interact with auxin signaling pathways to regulate plant growth and development.
The other plant hormones including gibberellic acid, brassinosteroids, and abscisic acid are also involved in the regulation of cell proliferation in the root apical meristem (Benková and Hejátko, 2009). Interestingly, the Arabidopsis abo5 mutant that has enhanced sensitivity to abscisic acid in both postgermination seedling growth and root growth is defective in a PPR protein involved in the splicing of mitochondrial nad2 intron 3 (Liu et al., 2010). The delayed germination of an Arabidopsis complex I mutant, ndufs4, can be rescued by application of GA (Meyer et al., 2009). It will be interesting to further investigate how the perturbation of mitochondrial functions may affect the actions of these plant hormones to regulate plant growth.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) ecotype Col-0, slo3 mutant, and 35S:SLO3 or 35S:SLO3-GFP transgenic plants were grown on one-half-strength Murashige and Skoog (MS) plates (MS salts [Sigma-Aldrich], pH adjusted to 5.7 with 1 N KOH, 0.8% [w/v] agar) containing 2% (w/v) Suc or in the soil in the growth chamber on a 16-h-light/8-h-dark cycle at 23°C.
Mutant Screen and Genetic Mapping of slo3
We isolated a collection of slo mutants from T-DNA insertion lines of the Col-0 genotype (Sung et al., 2010). Genetic analysis revealed that the slo3 mutant was not linked to T-DNA insertion. We generated an F2 population derived from a cross between the slo3 mutant and Ler. F2 seedlings were grown on one-half-strength MS + 2% (w/v) Suc medium and scored for slow root growth. Genomic DNA was isolated from individual F2 plants showing the mutant phenotype. SSLP markers described in the Monsanto Col-0 and Ler polymorphism database (http://www.arabidopsis.org/Cereon) were used to map the slo3 mutation to the bottom arm of chromosome 3.
Next-Generation Sequencing of slo3
Genomic DNA extracted from slo3 mutant seedlings was sent to Yourgene Bioscience (www.yourgene.com.tw/) for whole-genome sequencing. DNA libraries were prepared from 5 μg of genomic DNA using a TruSeq DNA Sample Preparation Kit (Illumina) according to the manufacturer’s instruction. Ten nanomolar DNA libraries mixed with denaturation solution and hybridization buffer was loaded onto an Illumina HiSEquation 2000 flowcell, and run for 100 cycles using the paired-end module. Data were processed using the Illumina CASAVA pipeline (version 1.8) to convert the results to FASTQ files. Illumina HiSEquation 2000 reads from slo3 were mapped against the The Arabidopsis Information Resource 10 reference genome using the Maq short-read mapping software with default settings for paired-end analysis (Li and Durbin, 2009). The sequences corresponding to the bottom arm of chromosome 3 were checked visually using the Integrative Genomics Viewer (https://www.broadinstitute.org/igv/; Thorvaldsdóttir et al., 2013).
EdU Labeling of Arabidopsis Roots
Roots of 7-d-old Arabidopsis wild-type and slo3 intact seedlings were submerged into 10 μm EdU (Invitrogen, Click-iT EdU Imaging Kit, C10337) in one-half-strength MS plus 2% (w/v) Suc liquid medium, and placed in the 23°C growth chamber for 30 min. Tissue fixation, permeabilization, and EdU detection were performed according to the manufacturer’s experimental protocols. The EdU-labeled roots were observed under confocal laser scanning microscope 510 META Zeiss.
RNA Extraction, RT-PCR, and RNA Editing Analysis
Total RNA was isolated from 2-week-old Arabidopsis seedlings as described previously (Tseng et al., 2013). The RNA was treated with DNaseI prior to use in the assays. For RT-PCR analysis of SLO3 and Elongation Factor 1α, the first strand of cDNA was synthesized using SuperScriptIII (Invitrogen) and oligo-deoxythymine according to the manufacturer’s instructions. The following primers were used for PCR: SLO3, 5′-AATGGTCATCCATTCCCAAA-3′ and 5′-CGTGCGAGAATGTGAAGTGT-3′; and Elongation Factor 1α, 5′-GTTTCACATCAACATTGTGGTCATTGG-3′ and 5′-GAGTACTTGGGGGTAGTGGCATCC-3′. For analysis of mitochondrial RNA editing, we used RT-PCR and bulk sequencing of the amplified cDNAs as described previously (Sung et al., 2010). To examine the editing site located in nad7 intron 2, we used RT-PCR with primers 5′-GAGGGACTGAGAAATTAATAGAGTACA-3′ and 5′-TGGTACCTCGCAATTCAAAA-3′ located in nad7 exon 2 and exon 3, respectively, to amplify wild-type and slo3 cDNAs. In the wild type, the amplified RT-PCR products including cDNAs with and without intron 2 were separated by agarose gel electrophoresis. The band corresponding to intron 2-containing cDNAs was sliced out from gels. These intron 2-containing cDNAs were purified with an Illustra GFX PCR DNA and gel band purification kit (GE Healthcare Life Sciences) and subjected to sequencing analysis.
Analyses of Mitochondrial RNA Splicing and Transcript Abundance
To quantify the splicing of mitochondrial mRNAs, quantitative RT-PCR was performed using specific primers designed for intron-exon regions (unspliced forms) and exon-exon regions (spliced forms) of each gene (de Longevialle et al., 2007; Koprivova et al., 2010). The sequences of the primers used for quantitative RT-PCR analysis of mitochondrial intron splicing are listed in Supplemental Table S1. Quantitative RT-PCR analysis of mitochondrial mRNAs and rRNAs was performed as described previously (Koprivova et al., 2010; Kühn et al., 2011). The sequences of the primers used for quantitative RT-PCR analysis of mitochondrial genes are listed in Supplemental Table S2. All of the quantifications were normalized to the nuclear gene ACT2. The quantitative RT-PCRs were performed in triplicate for each sample in three independent experiments. For RT-PCR analysis of mitochondrial nad transcripts, the first strand of cDNA was synthesized using SuperScriptIII (Invitrogen) and random hexamer primers as described previously (Colas des Francs-Small et al., 2014). The sequences of the primers used are listed in Supplemental Table S3.
Scanning Electron Microscopy
For scanning electron microscopy, Arabidopsis seeds of the wild type and slo3 were dried with Hitachi HCP-2 critical point dryer. After coating with Hitachi E-1010 ion sputter, the samples were observed under an FEI Quanta 200 scanning electron microscope at 20 kV.
Complementation of Arabidopsis slo3 Mutant by 35S:SLO3 and 35S:SLO3-GFP
RT-PCR with a forward primer 5′-CACCATGCTTCAGAAGATCTCCTC-3′ and two different reverse primers, 5′-CTACATTAGCTGTATTTCAG-3′ and 5′-CATTAGCTGTATTTCAGGAG-3′, was used to generate full-length SLO3 cDNAs with and without a stop codon, respectively. The PCR products were cloned into a Gateway pENTR/D-TOPO vector (Invitrogen) and verified by sequencing. To generate a 35S:SLO3 construct, the full-length SLO3 cDNA with a stop codon was introduced into a Gateway destination vector pGWB502Ω. The full-length SLO3 cDNA without a stop codon was used to generate a SLO3-GFP fusion construct in a Gateway destination vector pGWB505. The resulting constructs containing the hygromycin selectable marker were transformed into Agrobacterium tumefaciens GV3101 and were introduced into Arabidopsis slo3 mutant by floral dip. In both events, T1 plants that were resistant to hygromycin had an appearance similar to that of the wild type, indicating that the transformed 35S:SLO3 and 35S:SLO3-GFP cDNAs were able to complement the slo3 mutant. Several independent 35S:SLO3 and 35S:SLO3-GFP complementation lines were grown to T3 homozygosity for further analysis.
Subcellular Localization of SLO3-GFP Fusion Protein
The 35S:SLO3-GFP construct was transformed into Arabidopsis wild-type plants by floral dip. Several independent 35S:SLO3-GFP transgenic lines were carried to T3 homozygosity. Roots from these stable transgenic Arabidopsis plants overexpressing the SLO3-GFP fusion protein were stained with MitoTracker orange (Molecular Probes) and observed under confocal laser scanning microscope 510 META Zeiss.
BN-PAGE and Analysis of Complex I Activity
Eight-day-old Arabidopsis seedlings were used for preparation of crude Arabidopsis membrane extracts and analysis of mitochondria complex I activity according to Pineau et al. (2008). Twenty micrograms of total proteins from crude membrane extracts was loaded in each lane and separated by BN-PAGE (Invitrogen, BN1002BOX). The gels were stained by Coomassie Blue R-250 (Sigma) or subjected to an in-gel complex I activity assay. To detect the activity of NADH dehydrogenase, the gel was immersed in a staining buffer (0.2 mm NADH, 1 mm nitroblue tetrazolium, 50 mm MOPS, adjusted to pH 7.6 with KOH) for 20 min at room temperature. The staining solution was replaced by a fixation solution (30% [v/v] methanol, 10% [v/v] acetic acid]) to stop the reaction.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers NM_116000, ERP64851, XP_004237781, XP_003554843, XP_002282912, XP_002438925, AFW69255, and NP_001058444.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. RT-PCR analysis of Arabidopsis SLO3 transcripts in wild-type, slo3, and complemented plants.
Supplemental Figure S2. Alignment of SLO3 proteins from different plants.
Supplemental Figure S3. Subcellular localization of SLO3-GFP in 35S:SLO3-GFP-complemented plants.
Supplemental Figure S4. Quantitative RT-PCR of spliced and unspliced nad7 transcripts in slo3.
Supplemental Figure S5. Analysis of RNA editing in nad7 intron 2.
Supplemental Table S1. Primers used for quantitative RT-PCR analysis of mitochondrial intron splicing.
Supplemental Table S2. Primers used for quantitative RT-PCR analysis of mitochondrial genes.
Supplemental Table S3. Primers used for RT-PCR analysis of nad transcripts in Arabidopsis mitochondria.
Supplementary Material
Acknowledgments
We thank Tsuyoshi Nakagawa for the pGWB vectors and Mei-Jane Fang for assistance in confocal microscopy.
Glossary
- PPR
pentatricopeptide repeat
- T-DNA
transfer DNA
- EdU
ethynyl deoxyuridine
- Col-0
Columbia-0
- Ler
Landsberg erecta
- SSLP
simple sequence length polymorphism
- cDNA
complementary DNA
- RT
reverse transcription
- BN
blue native
- UTR
untranslated region
- MS
Murashige and Skoog
Footnotes
This work was supported by Academia Sinica (grant no. 98-CDA-L04).
Articles can be viewed without a subscription.
References
- Barkan A, Rojas M, Fujii S, Yap A, Chong YS, Bond CS, Small I (2012) A combinatorial amino acid code for RNA recognition by pentatricopeptide repeat proteins. PLoS Genet 8: e1002910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan A, Small I (2014) Pentatricopeptide repeat proteins in plants. Annu Rev Plant Biol 65: 415–442 [DOI] [PubMed] [Google Scholar]
- Benková E, Hejátko J (2009) Hormone interactions at the root apical meristem. Plant Mol Biol 69: 383–396 [DOI] [PubMed] [Google Scholar]
- Binder S, Brennicke A (2003) Gene expression in plant mitochondria: transcriptional and post-transcriptional control. Philos Trans R Soc Lond B Biol Sci 358: 181–188, discussion 188–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonen L. (2008) Cis- and trans-splicing of group II introns in plant mitochondria. Mitochondrion 8: 26–34 [DOI] [PubMed] [Google Scholar]
- Brown GG, Colas des Francs-Small C, Ostersetzer-Biran O (2014) Group II intron splicing factors in plant mitochondria. Front Plant Sci 5: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech TR. (1990) Self-splicing of group I introns. Annu Rev Biochem 59: 543–568 [DOI] [PubMed] [Google Scholar]
- Chang YF, Imam JS, Wilkinson MF (2007) The nonsense-mediated decay RNA surveillance pathway. Annu Rev Biochem 76: 51–74 [DOI] [PubMed] [Google Scholar]
- Chateigner-Boutin AL, des Francs-Small CC, Delannoy E, Kahlau S, Tanz SK, de Longevialle AF, Fujii S, Small I (2011) OTP70 is a pentatricopeptide repeat protein of the E subgroup involved in splicing of the plastid transcript rpoC1. Plant J 65: 532–542 [DOI] [PubMed] [Google Scholar]
- Cho Y, Qiu YL, Kuhlman P, Palmer JD (1998) Explosive invasion of plant mitochondria by a group I intron. Proc Natl Acad Sci USA 95: 14244–14249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Zmudjak M, Colas des Francs-Small C, Malik S, Shaya F, Keren I, Belausov E, Many Y, Brown GG, Small I. , et al. (2014) nMAT4, a maturase factor required for nad1 pre-mRNA processing and maturation, is essential for holocomplex I biogenesis in Arabidopsis mitochondria. Plant J 78: 253–268 [DOI] [PubMed] [Google Scholar]
- Colas des Francs-Small C, Falcon de Longevialle A, Li Y, Lowe E, Tanz SK, Smith C, Bevan MW, Small I (2014) The pentatricopeptide repeat proteins TANG2 and ORGANELLE TRANSCRIPT PROCESSING439 are involved in the splicing of the multipartite nad5 transcript encoding a subunit of mitochondrial complex I. Plant Physiol 165: 1409–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colas des Francs-Small C, Small I (2014) Surrogate mutants for studying mitochondrially encoded functions. Biochimie 100: 234–242 [DOI] [PubMed] [Google Scholar]
- Colcombet J, Lopez-Obando M, Heurtevin L, Bernard C, Martin K, Berthomé R, Lurin C (2013) Systematic study of subcellular localization of Arabidopsis PPR proteins confirms a massive targeting to organelles. RNA Biol 10: 1557–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Longevialle AF, Meyer EH, Andrés C, Taylor NL, Lurin C, Millar AH, Small ID (2007) The pentatricopeptide repeat gene OTP43 is required for trans-splicing of the mitochondrial nad1 Intron 1 in Arabidopsis thaliana. Plant Cell 19: 3256–3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Longevialle AF, Small ID, Lurin C (2010) Nuclearly encoded splicing factors implicated in RNA splicing in higher plant organelles. Mol Plant 3: 691–705 [DOI] [PubMed] [Google Scholar]
- Durbak A, Yao H, McSteen P (2012) Hormone signaling in plant development. Curr Opin Plant Biol 15: 92–96 [DOI] [PubMed] [Google Scholar]
- Dutilleul C, Driscoll S, Cornic G, De Paepe R, Foyer CH, Noctor G (2003) Functional mitochondrial complex I is required by tobacco leaves for optimal photosynthetic performance in photorespiratory conditions and during transients. Plant Physiol 131: 264–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutilleul C, Lelarge C, Prioul JL, De Paepe R, Foyer CH, Noctor G (2005) Mitochondria-driven changes in leaf NAD status exert a crucial influence on the control of nitrate assimilation and the integration of carbon and nitrogen metabolism. Plant Physiol 139: 64–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farré JC, Aknin C, Araya A, Castandet B (2012) RNA editing in mitochondrial trans-introns is required for splicing. PLoS ONE 7: e52644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francs-Small CC, Kroeger T, Zmudjak M, Ostersetzer-Biran O, Rahimi N, Small I, Barkan A (2012) A PORR domain protein required for rpl2 and ccmF(C) intron splicing and for the biogenesis of c-type cytochromes in Arabidopsis mitochondria. Plant J 69: 996–1005 [DOI] [PubMed] [Google Scholar]
- Gray MW. (1999) Evolution of organellar genomes. Curr Opin Genet Dev 9: 678–687 [DOI] [PubMed] [Google Scholar]
- Gutierres S, Sabar M, Lelandais C, Chétrit P, Diolez P, Degand H, Boutry M, Vedel F, de Kouchkovsky Y, De Paepe R (1997) Lack of mitochondrial and nuclear-encoded subunits of complex I and alteration of the respiratory chain in Nicotiana sylvestris mitochondrial deletion mutants. Proc Natl Acad Sci USA 94: 3436–3441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haïli N, Arnal N, Quadrado M, Amiar S, Tcherkez G, Dahan J, Briozzo P, Colas des Francs-Small C, Vrielynck N, Mireau H (2013) The pentatricopeptide repeat MTSF1 protein stabilizes the nad4 mRNA in Arabidopsis mitochondria. Nucleic Acids Res 41: 6650–6663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess WR, Börner T (1999) Organellar RNA polymerases of higher plants. Int Rev Cytol 190: 1–59 [DOI] [PubMed] [Google Scholar]
- Hooper CM, Tanz SK, Castleden IR, Vacher MA, Small ID, Millar AH (2014) SUBAcon: a consensus algorithm for unifying the subcellular localization data of the Arabidopsis proteome. Bioinformatics 30: 3356–3364 [DOI] [PubMed] [Google Scholar]
- Hsu YW, Wang HJ, Hsieh MH, Hsieh HL, Jauh GY (2014) Arabidopsis mTERF15 is required for mitochondrial nad2 intron 3 splicing and functional complex I activity. PLoS ONE 9: e112360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose M, Tasaki E, Sugita C, Sugita M (2012) A PPR-DYW protein is required for splicing of a group II intron of cox1 pre-mRNA in Physcomitrella patens. Plant J 70: 271–278 [DOI] [PubMed] [Google Scholar]
- Kerchev PI, De Clercq I, Denecker J, Mühlenbock P, Kumpf R, Nguyen L, Audenaert D, Dejonghe W, Van Breusegem F (2014) Mitochondrial perturbation negatively affects auxin signaling. Mol Plant 7: 1138–1150 [DOI] [PubMed] [Google Scholar]
- Keren I, Bezawork-Geleta A, Kolton M, Maayan I, Belausov E, Levy M, Mett A, Gidoni D, Shaya F, Ostersetzer-Biran O (2009) AtnMat2, a nuclear-encoded maturase required for splicing of group-II introns in Arabidopsis mitochondria. RNA 15: 2299–2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren I, Tal L, des Francs-Small CC, Araújo WL, Shevtsov S, Shaya F, Fernie AR, Small I, Ostersetzer-Biran O (2012) nMAT1, a nuclear-encoded maturase involved in the trans-splicing of nad1 intron 1, is essential for mitochondrial complex I assembly and function. Plant J 71: 413–426 [DOI] [PubMed] [Google Scholar]
- Kerényi Z, Mérai Z, Hiripi L, Benkovics A, Gyula P, Lacomme C, Barta E, Nagy F, Silhavy D (2008) Inter-kingdom conservation of mechanism of nonsense-mediated mRNA decay. EMBO J 27: 1585–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klodmann J, Sunderhaus S, Nimtz M, Jänsch L, Braun HP (2010) Internal architecture of mitochondrial complex I from Arabidopsis thaliana. Plant Cell 22: 797–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler D, Schmidt-Gattung S, Binder S (2010) The DEAD-box protein PMH2 is required for efficient group II intron splicing in mitochondria of Arabidopsis thaliana. Plant Mol Biol 72: 459–467 [DOI] [PubMed] [Google Scholar]
- Koprivova A, des Francs-Small CC, Calder G, Mugford ST, Tanz S, Lee BR, Zechmann B, Small I, Kopriva S (2010) Identification of a pentatricopeptide repeat protein implicated in splicing of intron 1 of mitochondrial nad7 transcripts. J Biol Chem 285: 32192–32199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotogány E, Dudits D, Horváth GV, Ayaydin F (2010) A rapid and robust assay for detection of S-phase cell cycle progression in plant cells and tissues by using ethynyl deoxyuridine. Plant Methods 6: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger K, Grabowski PJ, Zaug AJ, Sands J, Gottschling DE, Cech TR (1982) Self-splicing RNA: autoexcision and autocyclization of the ribosomal RNA intervening sequence of Tetrahymena. Cell 31: 147–157 [DOI] [PubMed] [Google Scholar]
- Kühn K, Carrie C, Giraud E, Wang Y, Meyer EH, Narsai R, des Francs-Small CC, Zhang B, Murcha MW, Whelan J (2011) The RCC1 family protein RUG3 is required for splicing of nad2 and complex I biogenesis in mitochondria of Arabidopsis thaliana. Plant J 67: 1067–1080 [DOI] [PubMed] [Google Scholar]
- Lambowitz AM, Zimmerly S (2004) Mobile group II introns. Annu Rev Genet 38: 1–35 [DOI] [PubMed] [Google Scholar]
- Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25: 1754–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XJ, Zhang YF, Hou M, Sun F, Shen Y, Xiu ZH, Wang X, Chen ZL, Sun SS, Small I. , et al. (2014) Small kernel 1 encodes a pentatricopeptide repeat protein required for mitochondrial nad7 transcript editing and seed development in maize (Zea mays) and rice (Oryza sativa). Plant J 79: 797–809 [DOI] [PubMed] [Google Scholar]
- Liu Y, He J, Chen Z, Ren X, Hong X, Gong Z (2010) ABA overly-sensitive 5 (ABO5), encoding a pentatricopeptide repeat protein required for cis-splicing of mitochondrial nad2 intron 3, is involved in the abscisic acid response in Arabidopsis. Plant J 63: 749–765 [DOI] [PubMed] [Google Scholar]
- Lurin C, Andrés C, Aubourg S, Bellaoui M, Bitton F, Bruyère C, Caboche M, Debast C, Gualberto J, Hoffmann B, et al. (2004) Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell 16: 2089–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek O, Knoop V (1998) Trans-splicing group II introns in plant mitochondria: the complete set of cis-arranged homologs in ferns, fern allies, and a hornwort. RNA 4: 1599–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer EH. (2012) Proteomic investigations of complex I composition: how to define a subunit? Front Plant Sci 3: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer EH, Solheim C, Tanz SK, Bonnard G, Millar AH (2011) Insights into the composition and assembly of the membrane arm of plant complex I through analysis of subcomplexes in Arabidopsis mutant lines. J Biol Chem 286: 26081–26092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer EH, Tomaz T, Carroll AJ, Estavillo G, Delannoy E, Tanz SK, Small ID, Pogson BJ, Millar AH (2009) Remodeled respiration in ndufs4 with low phosphorylation efficiency suppresses Arabidopsis germination and growth and alters control of metabolism at night. Plant Physiol 151: 603–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AH, Heazlewood JL, Kristensen BK, Braun HP, Møller IM (2005) The plant mitochondrial proteome. Trends Plant Sci 10: 36–43 [DOI] [PubMed] [Google Scholar]
- Nakagawa N, Sakurai N (2006) A mutation in At-nMat1a, which encodes a nuclear gene having high similarity to group II intron maturase, causes impaired splicing of mitochondrial NAD4 transcript and altered carbon metabolism in Arabidopsis thaliana. Plant Cell Physiol 47: 772–783 [DOI] [PubMed] [Google Scholar]
- Pineau B, Layoune O, Danon A, De Paepe R (2008) L-galactono-1,4-lactone dehydrogenase is required for the accumulation of plant respiratory complex I. J Biol Chem 283: 32500–32505 [DOI] [PubMed] [Google Scholar]
- Stalder L, Mühlemann O (2008) The meaning of nonsense. Trends Cell Biol 18: 315–321 [DOI] [PubMed] [Google Scholar]
- Sung TY, Tseng CC, Hsieh MH (2010) The SLO1 PPR protein is required for RNA editing at multiple sites with similar upstream sequences in Arabidopsis mitochondria. Plant J 63: 499–511 [DOI] [PubMed] [Google Scholar]
- Takenaka M, Zehrmann A, Verbitskiy D, Härtel B, Brennicke A (2013) RNA editing in plants and its evolution. Annu Rev Genet 47: 335–352 [DOI] [PubMed] [Google Scholar]
- Thorvaldsdóttir H, Robinson JT, Mesirov JP (2013) Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform 14: 178–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng CC, Lee CJ, Chung YT, Sung TY, Hsieh MH (2013) Differential regulation of Arabidopsis plastid gene expression and RNA editing in non-photosynthetic tissues. Plant Mol Biol 82: 375–392 [DOI] [PubMed] [Google Scholar]
- Unseld M, Marienfeld JR, Brandt P, Brennicke A (1997) The mitochondrial genome of Arabidopsis thaliana contains 57 genes in 366,924 nucleotides. Nat Genet 15: 57–61 [DOI] [PubMed] [Google Scholar]
- Wahleithner JA, MacFarlane JL, Wolstenholme DR (1990) A sequence encoding a maturase-related protein in a group II intron of a plant mitochondrial nad1 gene. Proc Natl Acad Sci USA 87: 548–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wydro MM, Sharma P, Foster JM, Bych K, Meyer EH, Balk J (2013) The evolutionarily conserved iron-sulfur protein INDH is required for complex I assembly and mitochondrial translation in Arabidopsis [corrected]. Plant Cell 25: 4014–4027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin P, Li Q, Yan C, Liu Y, Liu J, Yu F, Wang Z, Long J, He J, Wang HW, et al. (2013) Structural basis for the modular recognition of single-stranded RNA by PPR proteins. Nature 504: 168–171 [DOI] [PubMed] [Google Scholar]
- Zmudjak M, Colas des Francs-Small C, Keren I, Shaya F, Belausov E, Small I, Ostersetzer-Biran O (2013) mCSF1, a nucleus-encoded CRM protein required for the processing of many mitochondrial introns, is involved in the biogenesis of respiratory complexes I and IV in Arabidopsis. New Phytol 199: 379–394 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.