Abstract
Background:
Anemia is a prevalent situation in patients with chronic kidney disease (CKD) and can be well managed with erythropoiesis-stimulating agents (ESAs). Continuous erythropoietin receptor activator (CERA) has a long half-life that allows to be administered once monthly. The lowest recommended dose for patients with non dialysis CKD is 120 μg per month. The objectives were to assess the efficacy of subcutaneous monthly dosing of CERA in CKD stages 4 and 5 not on dialysis, and to determine the equivalent dose to epoetin β and darbepoetin α.
Methods:
This is a cohort study. A 30-patient group that ESAs was changed to CERA (μg/month) was used as treatment group. We used the following clinically-based equivalent dosing: epoetin β (IU/week) and darbepoetin α (μg/week): 3000/15= 50; 4000/20=75; 6000/30=100; 8000/40=150. Another group of 30 patients with similar characteristics was used as control group and received the same epoetin β and darbepoetin α doses.
Results:
The mean CERA initial dose and at 6 months was 81.9 ± 35.2 and 82.0 ± 37.82 μg/month (p=0.37). The mean erythropoietin resistance index (ERI) and hemoglobin at baseline and at 6 months in the CERA group and in the control group were not statistically significant.
Conclusion:
Monthly dosing treatment with CERA is safe and effective. A dose of 75-100 μg/month is enough to maintain stable levels of hemoglobin. Hippokratia 2014; 18 (4): 315-318.
Keywords: Anemia, CERA, continuous erythropoietin receptor activator, darbepoetin α, epoetin β
Introduction
Anemia is a frequent complication in chronic kidney disease (CKD) that progresses as the glomerular filtration rate (GFR) decreases, requiring treatment in many cases from CKD stages 3 and 41,2.
There are several treatment options with erythropoiesis-stimulating agents (ESAs) that have helped to improve the quality of life of these patients. Continuous erythropoietin receptor activator (CERA) is a new ESA that using a polyethylene glycol acid group that confers it a lower affinity for the endogenous erythropoietin receptor, grants the drug a longer half-life3-5. This is particularly beneficial in the outpatient CKD population, patients on peritoneal dialysis or those with kidney transplants, who usually receive ESAs subcutaneously.
The summary of product characteristics of CERA recommends using the same monthly dose for patients with CKD previously receiving doses <8000 IU/week of epoetin β or <40 μg/week of darbepoetin α, which is an excessively wide range for this population.
The objectives of the present study were to assess the efficacy of subcutaneous monthly dosing of CERA in patients with stages 4 and 5 CKD not on dialysis, and to determine the equivalent dose of CERA to epoetin β and darbepoetin α.
Material and Methods
Selection of patients
In this retrospective study we selected 30 consecutive patients and switched their regular ESA for CERA (methoxy polyethylene glycol-epoetin beta, MIRCERA®, Hoffman-La Roche Ltd, Basel, Switzerland) according to the equivalent doses provided in Table 1, based on previous unpublished data. We subsequently selected 30 additional patients who comprised the control group. All patients included in the study signed an informed consent form. Follow-up period was 6 months.
Table 1. Dose conversion to continuous erythropoietin receptor activator (CERA).
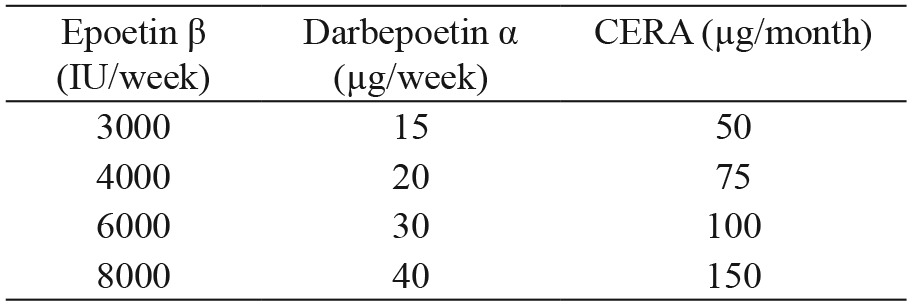
CERA: continuous erythropoietin receptor activator, IU: international units, μg: micrograms.
Inclusion criteria were prevalent patients who attended, with advanced chronic kidney disease (ACKD), a monographic clinic (specialized in patients with stage 4 and 5 CKD not on dialysis with a GFR lower than 30 ml/min/1.73 m2 body surface) and receiving from 3000 to 8000 IU/week of epoetin β, or equivalent doses of darbepoetin α (15-40 μg/week), with stable hemoglobin (variation in hemoglobin level less than ± 0.5 g/dL in the three months prior to initiation of the study) and stable iron deposits, demonstrated by a transferrin saturation index greater than 20%. The exclusion criteria were: suspected or confirmed active bleeding, active malignancy, hospital admission or severe inflammatory disease in the three months prior to initiation of the study.
Variables analyzed
We identified age, sex, etiology of kidney disease, presence of arterial hypertension defined as blood pressure greater than 130/85 mmHg or treatment with antihypertensive drugs regardless of the blood pressure value, diabetes mellitus, weight, height, body mass index and weekly doses of epoetin β or darbepoetin α.
Laboratory tests were performed every three months, starting from the three months prior to initiation of the study, these tests included hemoglobin (g/L), hematocrit (%), ferritin (μg/L), Transferrin saturation index (TSI, %), kidney function expressed by means of serum creatinine levels (mg/dL) and estimated glomerular filtration rate (eGFR) calculated by means of the CKD-EPI formula, serum albumin (g/dL) and proteinuria (g/24h).
Using the same time intervals, the erythropoietin resistance index (ERI) (IU/kg/week/g/dL) or equivalent dose of darbepoetin α using a dose conversion ratio of 1:200 was calculated. For the estimation of the ERI in patients treated with CERA, the mean equivalent doses of CERA and the rest of the ESAs were estimated according to table 1 (1 μg/week: 225 IU/week) with respect to epoetin β.
In every visit, patients were questioned about possible adverse effects. Intravenous (IV) iron was administered as ferric hydroxide sucrose (5 mL vial containing 20 mg/mL of elemental iron) at varying intervals to maintain a TSI > 20% and ferritin > 100 μg/L.
Statistical analysis
Normally distributed quantitative variables are expressed as mean and standard deviation (SD) and qualitative variables as percentages. Student's t-test for independent samples was used to compare quantitative variables and the chi-square test was used for qualitative variables. P-values less than 0.05 were considered significant. Data processing and analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 15.0 (SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristics
The baseline characteristics of the overall population and of each individual subgroup (CERA and control) are shown in Table 2. Percentage of patients receiving epoetin β was 46.6% (28 patients received a mean dose of 3678 ± 1067 IU/week). Percentage of patients receiving darbepoetin α was 53.3% (32 patients), mean dose of 31.0 ± 12.9 μg/week. Both ESAs were administered subcutaneously.
Table 2. Demographic and clinical baseline data of the study group consisting of patients receiving CERA and control group receiving epoetin β and darbepoetin α.
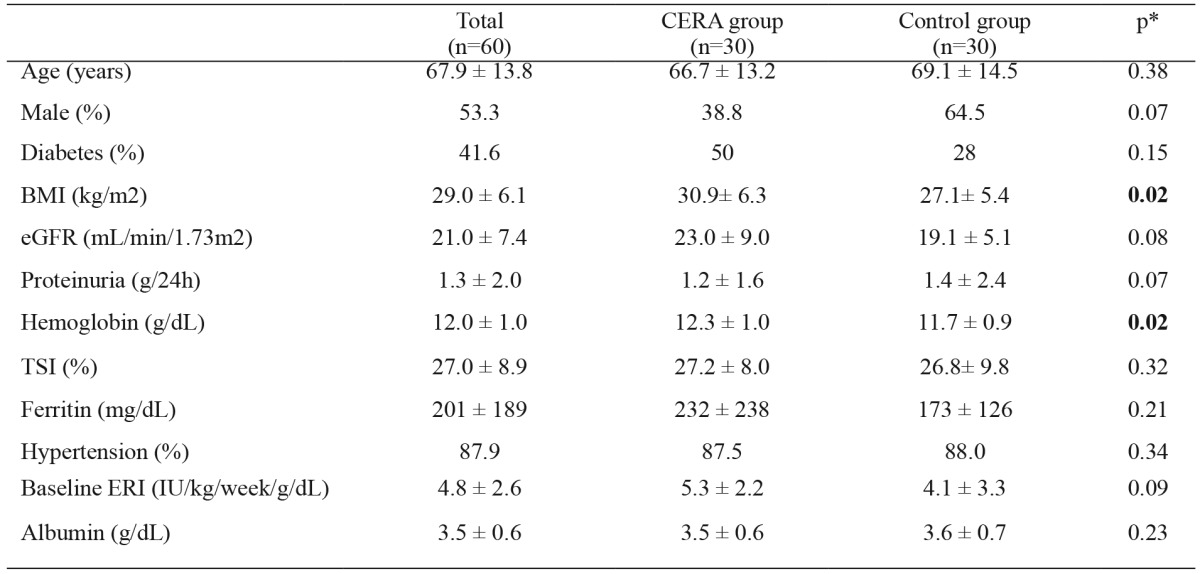
p*: p value between data from CERA and control groups, NS: non significant, CERA: continuous erythropoietin receptor activator, BMI: body mass index, eGFR: glomerular filtration rate, TSI: transferrin saturation index, ERI: erythropoietin resistance index.
The most common etiology of chronic kidney disease was diabetic nephropathy (32.8%), followed by unknown etiology (19%) and chronic glomerulonephritis (15%), showing no significant differences between both study groups.
Clinical course of each group
The mean baseline dose of CERA was 81.9 ± 35.2 μg/month and 82.0 ± 37.8 μg/month six months later (p = 0.37). In the control group mean baseline dose of epoetin β was 3264 ± 1685 IU/week and 3866 ± 1683 IU/week at 6 months (p = 0.12). Mean dose of darbepoetin α was 18.5 ± 8.7 μg/week at baseline and 19.4 ± 8.9 μg/week at 6 months (p =0.11).
Table 3 shows the variations of the GFR estimated by the CKD-EPI formula and the change in hemoglobin levels in both groups during the follow-up period. Hemoglobin levels remained stable and there were no significant changes in the eGFR in either of the two study groups.
Table 3. Change in hemoglobin, kidney function and erythropoietin resistance index in both study groups during the follow-up period.
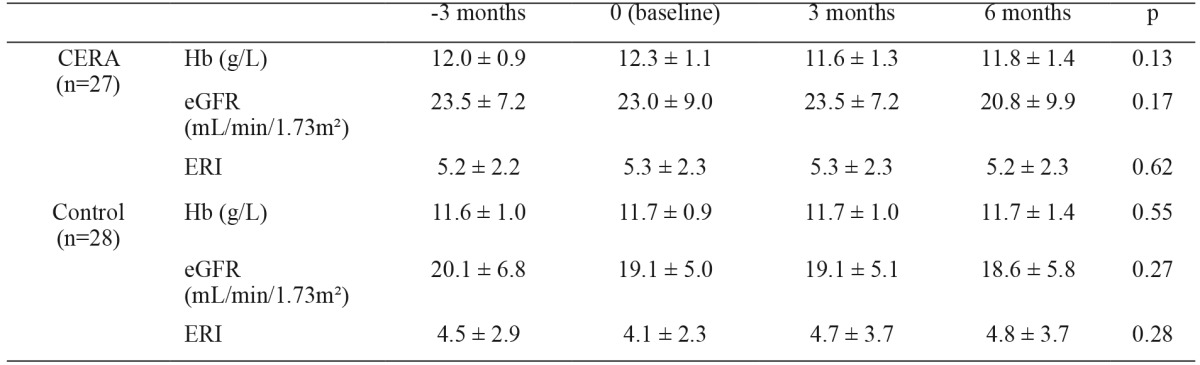
CERA: continuous erythropoietin receptor activator, Hb: Hemoglobin, eGFR: glomerular filtration rate estimated by CKD-EPI equation, ERI erythropoietin resistance index.
During the 6 months of follow up, three patients from the CERA group and two patients from the control group started dialysis treatment and dropped-out from the study. No adverse effects related to ESAs were observed in either of the two study groups.
Discussion
To our knowledge, this is the first study that establishes dose equivalences between monthly administration of CERA and more frequent dosing of epoetin β or darbepoetin α in patients with ACKD.
The summary of product characteristics of CERA establishes a fixed dose of 120 μg/month for patients receiving either <8000 IU/week of epoetin β, or <40 μg/week of darbepoetin α. In view of these results, we propose the use of a mean dose of 80-85 μg/month in patients with advanced CKD (ACKD), which is approximately 30% lower than that established in the drug's summary of product characteristics. This would allow the 75 and 100 mcg dose formulations to be adjusted according to the patient weight and hemoglobin level.
Most studies looking into the clinical use of CERA have been designed to successfully demonstrate the efficacy and safety of the drug in patients with ACKD, those on peritoneal dialysis or hemodialysis and kidney transplant recipients6-11. However, there are very few studies on dose equivalence between CERA and other ESAs. The only published study to date on dose equivalence in ACKD shows results similar to ours12.
It is common for patients with stage 4 and 5 of ACKD to show fluctuations of their hemoglobin levels during short periods of time; these are mainly caused by a progression in their kidney disease, but can also be a consequence of other added factors such as inflammation, infections, malnutrition, arteriosclerosis or hospital admissions13-15. Such cases may require higher doses of ESAs. Thus, we consider that the most appropriate parameter to assess the anemia and its response to treatment is the erythropoietin resistance index (ERI), as shown in several studies16-18. In our study, the ERI calculated in both groups remained stable throughout the follow-up period, therefore confirming the validity of the equivalent doses proposed in this study, as both the ERI and the hemoglobin level are two effective parameters of assessment in this respect. The ERI in patients treated with CERA was calculated using a correction factor derived from our preliminary experience (unpublished data), which is 1:225 with respect to epoetin β. Although this dose equivalence should be corroborated in other studies, we may assume its validity as the possible bias is constant and the clinical significance lies within its variations over time. In patients treated with CERA, there were no changes in the ERI during the study period.
The two study groups were not totally homogeneous as the levels of darbepoetin α were higher than the equivalent levels of epoetin β in the CERA group. However, there were no significant differences between the CERA group and the control group, therefore making it possible to compare both study groups. We also found that the overall doses of darbepoetin α were higher than the doses of epoetin β, which we put down to a treatment bias resulting from the tendency to prescribe darbepoetin α to patients with greater ESAs requirements, allowing higher doses to be administered at longer intervals. In any case, we consider that this does not invalidate the study since in the group treated with CERA there were patients treated with both types of ESAs.
CERA showed to be a safe drug as no drug-related side effects were recorded during our follow-up period. A very low proportion of mild adverse effects are documented in previous publications, such as headache or vomiting19. Possible medium or long-term adverse effects remain to be assessed.
Our study determines a more exact and stratified ESAs dose conversion for patients with stage 4 and 5 of chronic kidney disease than that which appears in the drug's summary of product characteristics, and we consider it may be useful in clinical practice when dealing with this group of patients.
Nevertheless, we are aware that our study has some limitations: first, the follow-up period was short and probably with a longer follow-up results could have been more accurate. Second, the small sample size. Third, the computation of equivalent dose of ESAs used in their study. Fourth, there is a lack of homogeneity among their study groups. Fifth is the period of inclusion criteria was aleatory chosen and longer or shorter periods could have offered different results. Sixth, three months interval can be a long time period especially to determine variations in hematocrit and hemoglobin. For all that reasons, further studies are needed to confirm these results.
In view of these results, we conclude that monthly dosing treatment with C.E.R.A. is both safe and effective in maintaining hemoglobin levels in patients with stage 4 and 5 CKD.
For most patients with ACKD, the proposed dose of 75 to 100 μg/month is sufficient to maintain stable hemoglobin levels, which may represent a significant saving with respect to the dose indicated in the summary of product characteristics.
Conflict of interest
The authors declare no conflicts of interest related to the contents of this article.
References
- 1.McClellan W, Aronoff SL, Bolton WK, Hood S, Lorber DL, Tang KL, et al. The prevalence of anemia in patients with chronic kidney disease. Curr Med Res Opin. 2004;20:1501–1510. doi: 10.1185/030079904X2763. [DOI] [PubMed] [Google Scholar]
- 2.Kazmi WH, Kausz AT, Khan S, Abichandani R, Ruthazer R, Obrador GT, et al. Anemia: an early complication of chronic renal insufficiency. Am J Kidney Dis. 2001;38:803–812. doi: 10.1053/ajkd.2001.27699. [DOI] [PubMed] [Google Scholar]
- 3.Canaud B, Mingardi G, Braun J, Aljama P, Kerr PG, Locatelli F, et al. Intravenous C.E.R.A. maintains stable haemoglobin levels in patients on dialysis previously treated with darbepoetin alfa: results from STRIATA, a randomized phase III study. Nephrol Dial Transplant. 2008;23:3654–3661. doi: 10.1093/ndt/gfn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Del Vecchio L, Cavalli A, Locatelli F. Methoxypolyethylene glycol-epoetin beta for the treatment of anemia associated with chronic kidney disease. Drugs Today (Barc) 2008;44:577–584. doi: 10.1358/dot.2008.44.8.1241306. [DOI] [PubMed] [Google Scholar]
- 5.Macdougall IC. Recent advances in erythropoietic agents in renal anemia. Semin Nephrol. 2006;26:313–318. doi: 10.1016/j.semnephrol.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Macdougall IC, Walker R, Provenzano R, de Alvaro F, Locay HR, Nader PC, et al. C.E.R.A. corrects anemia in patients with chronic kidney disease not on dialysis: results of a randomized clinical trial. Clin J Am Soc Nephrol. 2008;3:337–347. doi: 10.2215/CJN.00480107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esposito C, Abelli M, Sileno G, Migotto C, Torreggiani M, Serpieri N, et al. Effects of continuous erythropoietin receptor activator (CERA) in kidney transplant recipients. Transplant Proc. 2012;44:1916–1917. doi: 10.1016/j.transproceed.2012.05.063. [DOI] [PubMed] [Google Scholar]
- 8.Selby NM, Fonseca SA, Fluck RJ, Taal MW. Hemoglobin variability with epoetin beta and continuous erythropoietin receptor activator in patients on peritoneal dialysis. Perit Dial Int. 2012;32:177–182. doi: 10.3747/pdi.2010.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Locatelli F, Del Vecchio L. Optimizing the management of renal anemia: challenges and new opportunities. Kidney Int Suppl. 2008:S33–S37. doi: 10.1038/ki.2008.525. [DOI] [PubMed] [Google Scholar]
- 10.Cano F, Alarcon C, Azocar M, Lizama C, Maria Lillo A, Delucchi A, et al. Continuous EPO receptor activator therapy of anemia in children under peritoneal dialysis. Pediatr Nephrol. 2011;26:1303–1310. doi: 10.1007/s00467-011-1846-5. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt RJ. Methoxy polyethylene glycol-epoetin beta: worth waiting for or a novelty worn off? Expert Opin Pharmacother. 2009;10:1509–1514. doi: 10.1517/14656560902997982. [DOI] [PubMed] [Google Scholar]
- 12.Minutolo R, Zamboli P, Chiodini P, Mascia S, Vitiello S, Stanzione G, et al. Conversion of darbepoetin to low doses of CERA maintains hemoglobin levels in non-dialysis chronic kidney disease patients. Blood Purif. 2010;30:186–194. doi: 10.1159/000321486. [DOI] [PubMed] [Google Scholar]
- 13.Hojs R, Bevc S, Ekart R. Biomarkers in hemodialysis patients. Adv Clin Chem. 2012;57:29–56. doi: 10.1016/b978-0-12-394384-2.00002-4. [DOI] [PubMed] [Google Scholar]
- 14.Nusair MB, Rajpurohit N, Alpert MA. Chronic Inflammation and Coronary Atherosclerosis in Patients with End-Stage Renal Disease. Cardiorenal Med. 2012;2:117–124. doi: 10.1159/000337082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ronco C, Cicoira M, McCullough PA. Cardiorenal syndrome type 1: pathophysiological crosstalk leading to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure. J Am Coll Cardiol. 2012;60:1031–1042. doi: 10.1016/j.jacc.2012.01.077. [DOI] [PubMed] [Google Scholar]
- 16.López-Gómez JM, Portolós JM, Aljama P. Factors that condition the response to erytropoietin in patients on hemodialysis and their relation to mortality. Kidney Int Suppl. 2008;111:S75–S81. doi: 10.1038/ki.2008.523. [DOI] [PubMed] [Google Scholar]
- 17.López-Gómez JM, Pórez-Flores I, Jofró R, Carretero D, Rodríguez-Benitez P, Villaverde M, et al. Presence of a failed kidney transplant in patients who are on hemodialysis is associated with chronic inflammatory state and erytropoietin resistance. J Am Soc Nephrol. 2004;15:2494–2501. doi: 10.1097/01.ASN.0000137879.97445.6E. [DOI] [PubMed] [Google Scholar]
- 18.Kaysen GA, Müller HG, Ding J, Chertow GM. Challenging the validity of the EPO index. Am J Kidney Dis. 2006;47:157–166. doi: 10.1053/j.ajkd.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Macdougal IC, Robson R, Opatrna S, Liogier X, Pannier A, Jordan P, et al. Pharmacokinetics and Pharmacodynamics of intravenous and subcutaneous continuous erythropoietin receptor activator (C.E.R.A.) in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2006;1:1211–1215. doi: 10.2215/CJN.00730306. [DOI] [PubMed] [Google Scholar]


