Abstract
Tsetse flies (Glossina spp.), vectors of African trypanosomes, are distinguished by their specialized reproductive biology, defined by adenotrophic viviparity (maternal nourishment of progeny by glandular secretions followed by live birth). This trait has evolved infrequently among insects and requires unique reproductive mechanisms. A key event in Glossina reproduction involves the transition between periods of lactation and nonlactation (dry periods). Increased lipolysis, nutrient transfer to the milk gland, and milk-specific protein production characterize lactation, which terminates at the birth of the progeny and is followed by a period of involution. The dry stage coincides with embryogenesis of the progeny, during which lipid reserves accumulate in preparation for the next round of lactation. The obligate bacterial symbiont Wigglesworthia glossinidia is critical to tsetse reproduction and likely provides B vitamins required for metabolic processes underlying lactation and/or progeny development. Here we describe findings that utilized transcriptomics, physiological assays, and RNA interference–based functional analysis to understand different components of adenotrophic viviparity in tsetse flies.
Keywords: Glossina, tsetse fly, lactation, adenotrophic viviparity, Wigglesworthia
INTRODUCTION
Tsetse (Diptera: Glossinidae)
Tsetse flies (Glossina spp.) are members of Hippoboscoidea, a dipteran superfamily containing Glossinidae (tsetse flies), Hippoboscidae (louse flies, sheep keds), and Streblidae and Nycteribiidae (together known as bat flies). Within the Glossinidae, there are 33 extant taxa composed of 22 species, including five subspecies complexes forming 3 subgenera: Fusca (Austenina Townsend), Palpalis (Nemorhina Robineau-Desvoidy), and Morsitans (Glossina Wiedemann) (described in 74). Morsitans group flies are largely savanna and woodland inhabitants, and Palpalis group flies frequent riverine and lacustrine habitats (116). Fusca group flies largely inhabit the moist forests of West Africa, although G. brevipalpis occurs discontinuously in East Africa, Democratic Republic of the Congo, and Mozambique. The host specificity of the species groups varies; Palpalis group flies are strongly anthropophilic, whereas Morsitans and Fusca are more zoophilic.
Tsetse as Disease Vectors
Male and female adult tsetse flies are obligate blood-feeding vectors of pathogenic trypanosomes. Chief among these are Trypanosoma (Trypanozoon) brucei gambiense and T. b. rhodesiense, which cause fatal diseases in humans if untreated. Human African trypanosomiasis (HAT) caused by T. b. gambiense represents over 90% of cases and occurs in northwest Uganda, extending into the Central African Republic and southern Chad, along the Congo River north of Brazzaville, and by the Atlantic coast between Gabon and Equatorial Guinea (141). HAT caused by T. b. rhodesiense is present east of the Rift Valley, is zoonotic, and causes a more acute disease that is rapidly fatal if untreated. In addition to the impact of HAT, nagana [or, animal African trypanosomiasis (AAT)] caused by T. b. brucei and related trypanosomatids, T. congolense and T. vivax, prevents or limits access to 10 million square kilometers of Africa for cattle farming (124) and has wide implications for land use including constraints on mixed agriculture and lack of animal labor for ploughing (69). Economic losses in cattle production are estimated at US$1–1.2 billion owing to 3 million cattle deaths per year, with total agricultural losses from AAT estimated at US$4.75 billion per year (23). In 2000 the African Union recognized trypanosomiasis as “one of Africa’s greatest constraints to socio-economic development” and began a continent-wide elimination program, Pan African Tsetse Trypanosomiasis Eradication Campaign (PATTEC) (70).
Disease Control Methods
Drugs available for treatment of African trypanosomes are expensive (122). Disease management in late disease stages has a high rate of mortality (66, 99) and a high incidence of failure (22). The ineffectiveness of mammalian vaccines is due to the capacity for antigenic variation by the trypanosomes (59, 65). Active surveillance and patient treatment are essential for disease control but are too expensive to implement at times of low endemicity. Modeling of the different control strategies (whether targeting the animal reservoir, humans, or the vector) shows that vector control is the most efficient method to suppress outbreaks (32, 141). Vector control tools include aerial pesticide sprays (71), topical insecticide applications for animals, traps/targets to reduce local tsetse populations (68), and sterile insect technique for isolated populations (134).
Unique Aspects of Tsetse Biology
A remarkable adaptation within the Hippoboscoidea, including Glossina, is adenotrophic viviparity. The nature of Glossina reproduction means that each female produces only 8–10 progeny during the course of her lifetime. Because of this low reproductive capacity, population control methods are highly successful. Glossinidae, and indeed all Hippoboscoidea, are exclusively hematophagous. This highly restricted nutritional ecology has required the establishment of associations with symbiotic bacteria to provide nutritional supplementation (see sidebar, Wigglesworthia Symbiosis and Its Role in Host Fecundity and Health of Progeny). Recently, the whole genome sequence (WGS) of G. morsitans morsitans has been completed (63), along with the WGS of its obligate endosymbiont, Wigglesworthia glossinidia (3, 112). The availability of genomic knowledge coupled with transcriptomic data and downstream functional analyses has expanded our understanding of tsetse’s reproductive, nutritive, and symbiotic biologies. In this review, we describe the current state of knowledge on tsetse’s reproductive anatomy, processes associated with the Glossina gonotrophic cycle (ovulation, oogenesis, larvigenesis, and parturition), milk production, and the role of tsetse’s obligate symbionts in fecundity.
BASIC REPRODUCTIVE PHYSIOLOGY AND ANATOMY
Insect Reproduction
Animal reproduction can be divided into two major strategies: oviparity and viviparity (17, 90). Oviparous females deposit eggs that undergo embryogenesis outside the female. Viviparous females retain their eggs within the reproductive tract until after progeny emerge from the egg and give birth to live offspring. Viviparity is documented for 11 orders of insects (52, 90) and has evolved independently at least 60 times in several fly lineages (90). Among viviparous species, many are facultative (situational oviparity or viviparity) whereas others display obligate viviparity. Ovoviviparity is a third term used to describe reproductive mechanisms whereby embryos develop in the eggs while still in the mother and progeny emerge rapidly after eggs are deposited. Of interest, most, if not all, ovoviviparous insects can give live birth under specific situations, suggesting ovoviviparous insects should be more appropriately classified as facultatively viviparous. Obligate viviparity is further divided into lecithotrophic (nutrients provided only by the egg yolk) and matrotrophic (nutrients provided by the egg yolk and other routes) forms. There are three divisions of matrotrophic viviparity. In hemocoelous viviparity, embryos and larvae develop within the hemocoel and absorb nutrients from the hemolymph (52, 108). In pseudoplacental viviparity, embryos and larvae develop in terminal ovarian follicles and/or a uterus-like structure, such as the brood sac in cockroaches (52, 108, 123). Nutrients are provided by actively secreting, thickened cells that are usually follicular (cells that surrounded developing oocytes in earlier stages) and/or epithelial (52, 108, 123). In adenotrophic viviparity, embryos and larvae develop in the common oviduct (or uterus) and are nourished through glandular secretions from specialized organs (52); this form is employed by Glossina.
Adenotrophic Viviparity in Flies: An Unusual Characteristic
In Diptera, adenotrophic viviparity is limited to the superfamily Hippoboscoidea, which includes Glossina, the family Sarcophagidae (flesh flies), and the subfamily Mesembrinellinae (Calliphoridae) (49, 90). Within Sarcophagidae, only a single species (Sarcophaga nigriventris) may provide nourishment to progeny, whereas all members of Hippoboscoidea and Mesembrinellinae produce nutrients for their intrauterine larvae (90). Flies belonging to Mesembrinellinae provide milk-like products to their progeny via secretions from ectodermal glands in their enlarged spermathecae (49). Other previously identified lecithotrophic flies deposit larval progeny much larger than the eggs or have expanded spermathecae/accessory glands, suggesting that in utero nourishment among dipterans might occur more frequently than has been officially documented (90).
Members of Hippoboscoidea and S. nigriventris provide nutrient secretions via the accessory glands (milk gland, or uterine gland). In most flies, accessory glands are small and consist of only a few cells (4). In Hippoboscoidea, accessory glands are expanded into a large, branched organ that contains hundreds to thousands of cells, occupies much of the abdominal space, and is connected to the uterus to provision nutrients directly to feeding larvae (52, 84, 90).
Ovarian, Milk Gland, and Uterine Structure
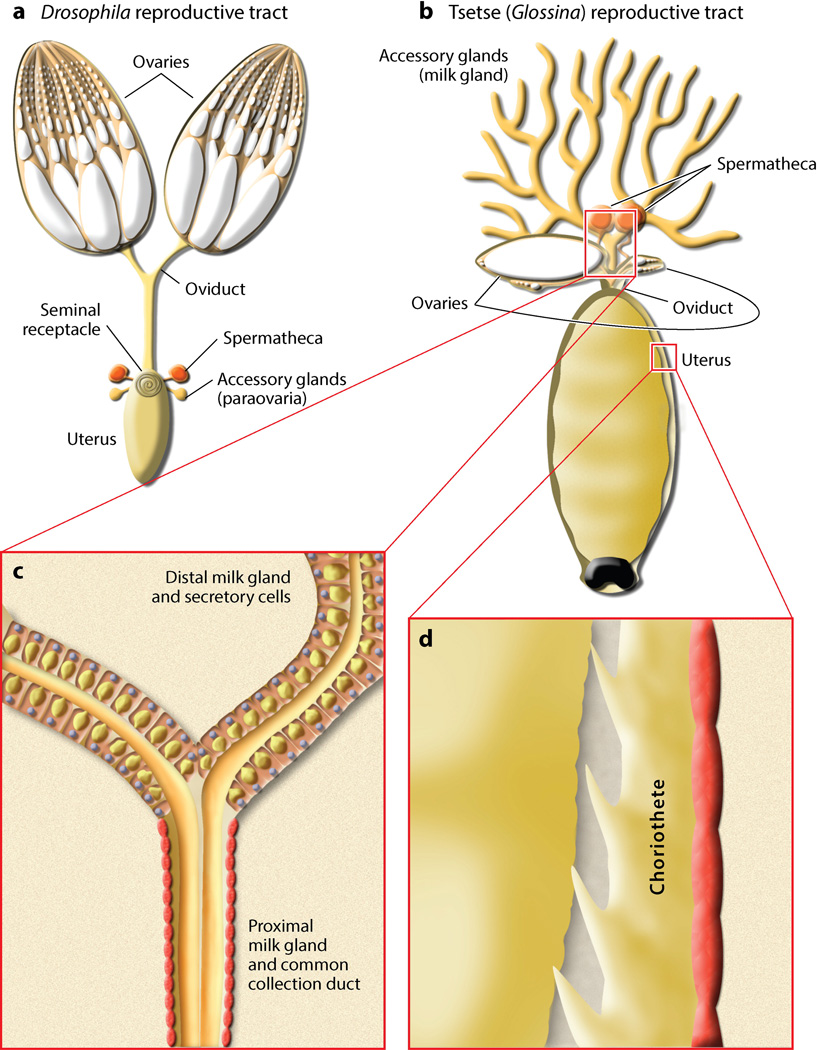
Three critical adaptations that allow tsetse, along with other members of Hippoboscoidea, to harbor and feed intrauterine larvae are the reduction of ovaries and the expansion of the milk gland and the reproductive tract (Figure 1a,b). Tsetse ovaries have a reduced ovariole number, two ovarioles per ovary, relative to ovaries from other dipterans, which can contain dozens of ovarioles (Figure 1a,b). The milk gland that connects to the uterus expands throughout the abdomen as bifurcating tubules and is a permutation of the female accessory glands or paraovaria found in other flies/insects (Figure 1a,b). This structure consists of the common collecting duct and the proximal and distal tubules (104, 130, 131). The distal milk gland is composed of large columnar and pyramidal cells, which distend as secretory reservoirs accumulate milk secretions. The proximal section contains underdeveloped organelles associated with secretory components (104) (Figure 1c). The differential structure of the distal and proximal sections suggests that secretory products are produced in the distal tubules and that the proximal section likely acts as a conduit for milk transfer. The muscular common collecting duct has been suggested as a regulator of milk flow into the uterus and to the larvae (131). The uterus is greatly expanded compared with the reproductive tract of other flies (106). The uterine wall is covered by tracheated muscle lined by a layer of squamous epithelium with cuticular intima (104, 131). A ridged, tongue-like structure with cuticular horns known as the choriothete is distinct from the uterus and causes a thickening of the anteroventral side of the uterine wall due to underlying epithelium with cuboidal cells, considerable connective and muscle tissue, and tight folding of projections toward the uterine lumen (104, 115, 128). The cuboidal cells of the choriothete appear to be secretory (104, 115, 128). Two functions for the choriothete have been suggested. First, the choriothete may facilitate removal of the egg chorion and larval cuticle both mechanically and through the secretion of sticky mucoproteins (104, 115, 128). Second, the choriothete may serve as an anchor for the larva throughout lactation to ensure correct posture for feeding (115, 128) (Figure 1d).
Figure 1.
Comparative morphology of dipteran reproductive organs. Illustrations of reproductive tract morphology from (a) Drosophila and (b) Glossina. (c) Magnified view of the Glossina milk gland tubules highlights the change in tubule and secretory cell physiology in the transition between the distal and proximal milk gland. (d) Magnified representation of the intrauterine wall and choriothete structure.
Life Cycle of Tsetse Flies
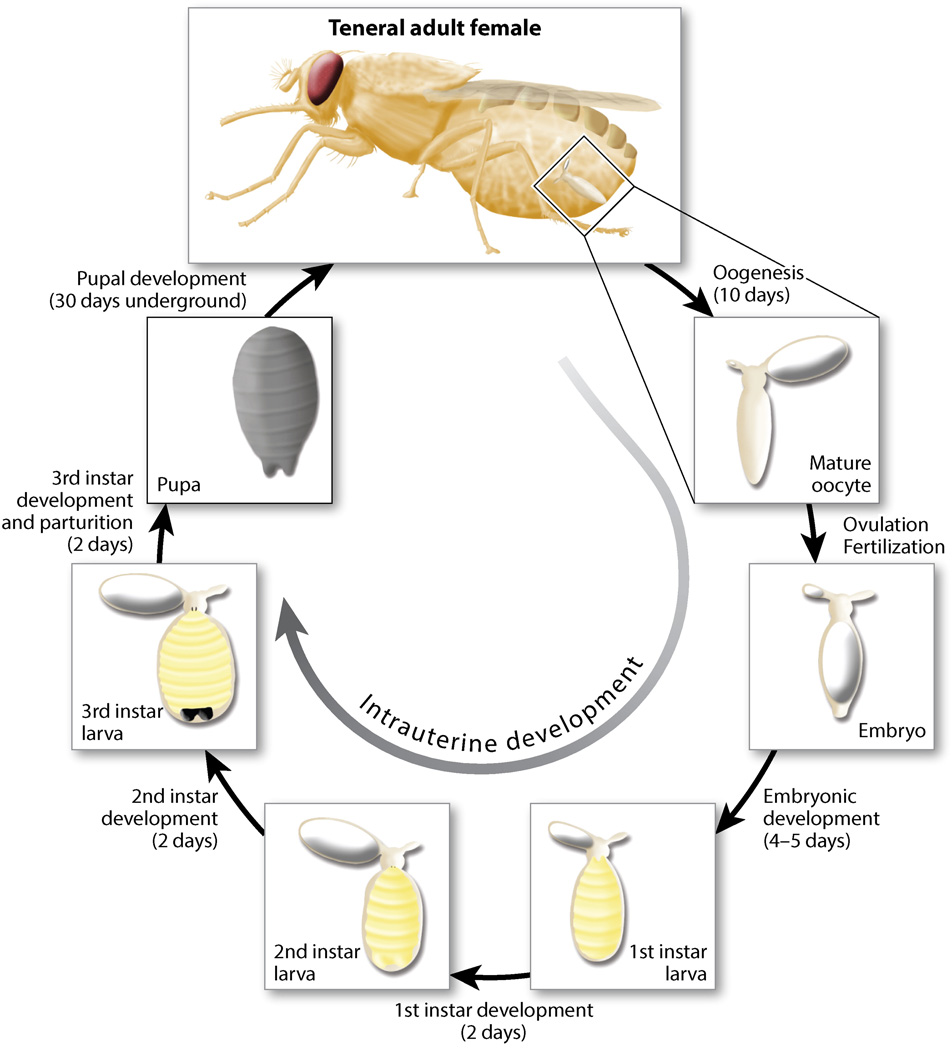
Females deposit a single larva per gonotrophic cycle (131). The first gonotrophic cycle begins when teneral tsetse females emerge from the puparium. At this time, the first oocyte begins development in the right ovary. Mating typically occurs 3–5 days after adult emergence and sperm are stored in the spermathecae until fertilization during ovulation. The first ovulation occurs ~10 days after adult emergence, followed by intrauterine embryogenesis and larvigenesis (Figure 2). During intrauterine embryonic and first instar larval development, a second oocyte begins development within the left ovary. Embryogenesis takes 3–4 days and is followed by 5–6 days of larval development (120, 131). The second oocyte matures before larval development is completed. Females give birth to their first progeny ~20 days posteclosion. Larvae are deposited on an appropriate substrate and burrow into the ground to pupariate within 1–2 h (131), and adults eclose after 30 days. The fully developed second oocyte in the left ovary is ovulated 20–35 min after larviposition. This development cycle allows deposition of the second larva 9–10 days after the first birth. The duration of progeny development depends on diet and environmental factors, including blood meal availability and host type (120, 131) as well as exposure to abiotic/biotic stressors that interfere with oogenesis or milk production (61).
Figure 2.
Schematic of the first gonotrophic cycle of a Glossina morsitans female under optimal environmental and nutritional conditions. The different stages of oogenesis, embryogenesis, and larvigenesis within the Glossina reproductive tract (ovaries and uterus) are shown.
OOGENESIS AND OVULATION IN TSETSE
Oogenesis
Individual oocyte development in tsetse is similar to that observed in other flies within the brachyceran suborder. The viviparous nature of tsetse’s reproductive biology adds a layer of complexity to the system and places significant constraints on the resources utilized for oogenesis in terms of physical space, nutrition, and timing owing to intrauterine larval development (26). Oogenic development in tsetse is conserved relative to that in other related dipteran species and is detailed for a number of fly species (2, 47, 62, 72, 73, 94). Only a single oocyte develops at a time while the remaining three ovarian follicles are held in a state of arrest that is broken upon ovulation of the mature oocyte. Oocyte development alternates between the right and left ovaries during each gonotrophic cycle (118, 119). The mechanism by which tsetse develop only a single oocyte when all ovarioles are exposed to the same hormonal, nutritional, and chemical milieus remains unknown.
A key stage of oocyte development is vitellogenesis, which is the synthesis, secretion, and uptake of yolk proteins. Brachyceran fly species produce lipase-derived yolk proteins (57, 117). Most species have multiple yolk protein genes, two to five depending on the species (19, 57, 87). However, tsetse is unique in that it carries only a single yolk protein gene orthologous to Drosophila yp2 (6). In addition to the reduction in yolk protein genes, the tissue specificity of tsetse yolk protein genes differs from that of most brachyceran flies such as Drosophila, in which yolk proteins are produced by both the fat body and ovarian follicle cells (20, 46, 64, 67, 142). In tsetse, yolk protein is synthesized only by the follicle cells of the developing oocyte (6, 58, 62), as in Stomoxys calcitrans (29, 60). The Drosophila yolk protein gene (yp2) is expressed in a follicle-cell-specific manner (48), suggesting that follicle-specific expression for yp2 orthologs likely occurs in most brachyceran flies.
Regulation of oogenesis in Diptera is a complex process and integrates signaling from multiple sources. The regulatory mechanisms associated with oogenesis in the Nematocera (primarily mosquitoes) are well characterized owing both to their significance as vectors and to the phenomenon of anautogeny (the requirement of a blood meal to initiate oogenesis) (reviewed in 7, 107). This process is regulated via signals from ecdysteroids (E), juvenile hormones (JH), insulin/insulin-like growth factors, and target of rapamycin (TOR), as well as peptide hormones such as ovarian ecdysiotropic hormone (OEH) and oostatic hormone (18, 21, 43, 50, 53–55, 84).
Comparative analysis of the literature on regulation of oogenesis in brachyceran species is less conclusive. All the aforementioned signaling pathways associated with oogenesis in Nematocera are also necessary for brachyceran oogenesis (1, 28, 33, 109, 110, 126, 127). However, these pathways function differently within the context of Brachycera. In most brachycerans (under optimal conditions), oocyte development is a constitutive process rather than an “on” or “off” state as observed in anautogenous mosquitoes. This is not to say that the process is unconditional, as nutritional status influences brachyceran oogenesis (125). Little is known regarding hormonal regulation of oogenesis in tsetse. As observed in other related flies, JH appears to play a significant role in oogenesis in tsetse. Flies subject to the removal of the biosynthetic source of JH (the corpora allata) soon after eclosion are unable to provide viable oocytes. This effect is reversible by the ectopic application of a JH analog (42). Further analysis is needed to understand the mechanisms by which oogenesis in tsetse is regulated.
Ovulation
Ovulation in tsetse is coordinated by the mating and pregnancy status of the fly. Females carrying an intrauterine embryo or larva will not ovulate and will hold the next developed oocyte until parturition or abortion. The presence of a developing or developed oocyte within the ovaries inhibits oogenesis in the other three follicles. This stasis is broken upon parturition or abortion of the primary offspring and ovulation of the penultimate oocyte, which occurs within 24 h of parturition. This cycle implies that the presence of an intrauterine offspring directly or indirectly inhibits ovulation and oogenesis beyond the penultimate oocyte. Detailed physiological analyses have determined the mechanisms regulating ovulation in tsetse. The two key inputs regulating ovulation are mating status and oocyte development. Mating status is determined by mechanical stimuli resulting from copulation, which must occur for at least 1.5–2 h (27, 120).
The mating status of a female does not trigger ovulation, as most females mate approximately 2–3 days posteclosion and do not ovulate until their first oocyte is ready at 8–10 days posteclosion (131). Injection of hemolymph or ovary/oviduct extracts from mated ovulating females into mature virgin females stimulates ovulation, which indicates the presence of a hemolymph or reproductive tissue-borne regulatory factor (28, 113).The presence of a mature oocyte in mated females appears to stimulate the release of an ovulation-stimulating factor into the hemolymph. This substance may be synthesized within the median neurosecretory cells (MNCs) and transported to a neurohemal organ, and it appears to require cyclic AMP as a second messenger (36). A study in G. austeni showed that ablation of the brain MNCs before or after mating in teneral females disrupts ovulation (45). The development of new proteomic and metabolomic analyses will advance these classic physiological studies and will facilitate the identification of factors regulating ovulation in tsetse.
TSETSE LACTATION AND UNDERLYING MECHANISMS
Structural Changes in the Milk Gland Associated with Lactation
Milk production is a complex process requiring massive physiological changes to support the growth of a larva that increases over 100-fold in dry mass over a 6-day period (37, 80). These changes occur in two major organs: the milk gland and the fat body. The fat body undergoes a two- to threefold increase in lipid content before the first lactation cycle and again following each parturition in subsequent dry phases. These lipid reserves are broken down and mobilized to the milk gland during lactation (4, 5, 11, 81). Structural changes in the milk gland are inverse to those of the fat body (37). Distal milk gland tubules are 30–40 µm wide prior to lactation and increase to 80–100 µm at the midpoint of lactation (37). Following parturition, the tubules revert to their prelactation width (37). The increased size of the milk gland corresponds to increased levels of rough endoplasmic reticulum and Golgi apparatuses (37, 56), which function in the production of milk proteins (111, 129, 131), and enlarged secretory reservoirs filled with milk products (8, 37).
Milk products are added to the secretory reservoirs by merocrine secretion (87, 131). A porous plug termed the rete retains the reservoir contents (56, 86). The volume of the secretory reservoirs increases nearly 100-fold between parturition and day 6 of the subsequent pregnancy cycle (8, 37, 86). A net loss of materials from the reservoir is observed during the remainder of the second pregnancy cycle (days 6–8) (56, 86). Histochemical analyses indicate that the primary contents of the secretory reservoir are proteins and phospholipids (86). These contents are comparable to the gut contents of third instar larvae with the exception that there are high levels of triacylglycerides (TAGs) in larvae, suggesting a conversion of phospholipids to TAGs, which are easily stored in the larval gut (31).
Metabolic Aspects Underlying Tsetse Lactation
During larvigenesis, a combination of amino acids, free fatty acids (FFAs), and diacylglycerols (DAGs) is transported to the milk gland via the hemolymph to support milk synthesis (5, 81, 97, 131). These nutrients are generated either directly from blood meals ingested during larvigenesis or indirectly from the breakdown of lipid reserves (75, 96, 106, 134). Lipids are the primary nutritional component of milk during the early phases of pregnancy, and proteins are critical in late lactation as lipid reserves decline (82, 96, 132).DAG, which is transported by lipophorin from the fat body to the milk gland, is the main lipid moiety provided for milk fat synthesis (15, 76, 101, 105). FFAs in the hemolymph are directly absorbed by the milk gland through passive diffusion and/or an uncharacterized protein-mediated mechanism (75, 121, 133). Lipids are likely directly incorporated into the milk to act as the milk fat source. Free amino acids are transferred into the milk gland through uncharacterized amino acid transporters (76, 79, 95, 96, 105). A key aspect of tsetse’s metabolic physiology is the reliance on proline, rather than sugars, as a hemolymph-borne energy source (24, 25, 88). Proline is obtained primarily from digested blood and by conversion of alanine to proline via the breakdown of fat body lipids (24, 25, 88, 89). Thus, amino acids fulfill dual roles by providing energy to support milk production and functioning as building blocks for milk protein synthesis.
Proteinaceous Components of Tsetse Milk
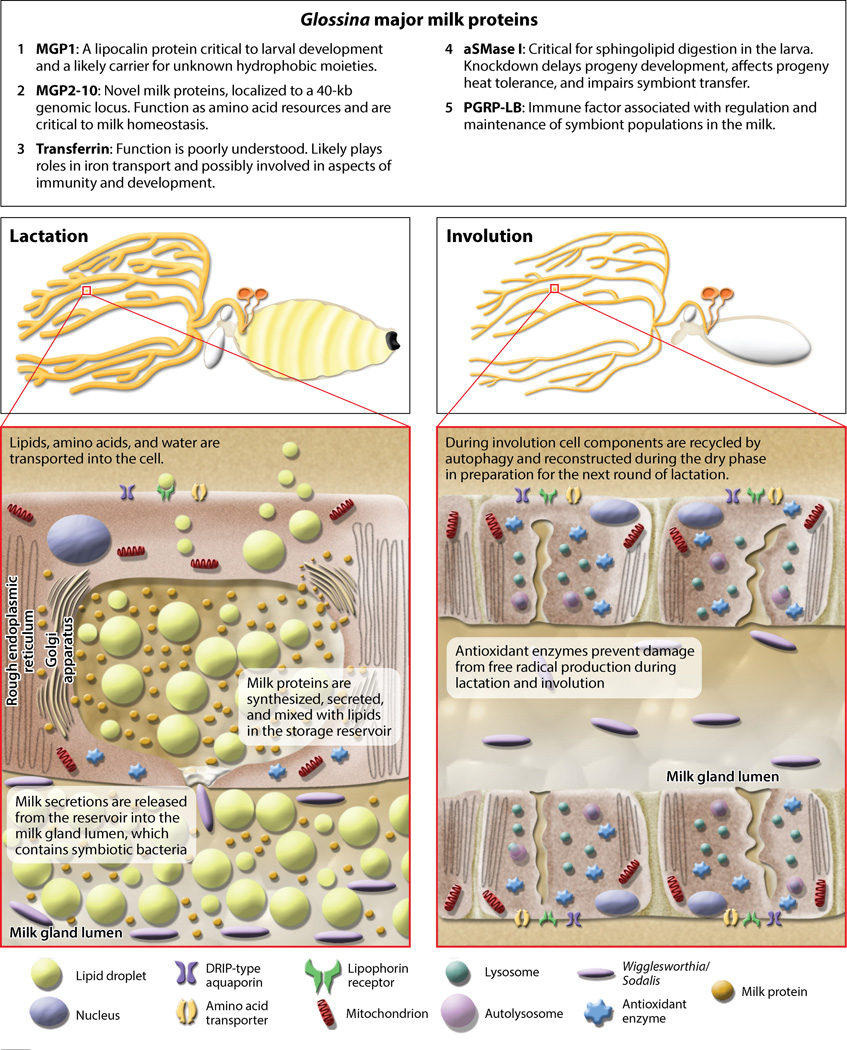
We have provided in Figure 3 a synopsis of the roles of the major proteins found in tsetse milk. Twelve major milk proteins in G. morsitans have been identified. These proteins represent 47% of all female gene transcripts during lactation and less than 4% during the dry period (12). Osir et al. (102) discovered the first milk protein, Milk Gland Protein 1 (MGP1), and Attardo et al. (6, 8) determined its full sequence. Following the identification of MGP1, a family of unrelated tsetse-specific milk proteins, MGP2–10, was identified (12, 144). Proteomic analysis of larval gut contents validated their role as milk components (12). In addition, analyses of the milk transcriptome/proteome identified an acid sphingomyelinase (aSMase1) and a transferrin protein as major constituents of tsetse milk. The aSMase1 protein is activated by the low pH of the larval gut and aids in lipid digestion (13). Little is known about the physiological role of transferrin in the milk (51, 126). In addition to the major milk proteins, proteomic analysis identified multiple minor constituents (12). One of these is peptidoglycan recognition protein-LB, PGRP-LB (135), which suppresses immune function to facilitate successful transfer of bacterial symbionts between mother and offspring.
Figure 3.
Overview of Glossina lactation. Top: Description of major milk proteins. Bottom left: Diagram of a reproductive tract during lactation, featuring milk gland secretory cells undergoing milk generation and secretion (inset). Bottom right: Diagram of a reproductive tract during the dry period after birth, featuring milk gland secretory cells undergoing involution (inset).
Transcriptional Regulation of Milk Proteins
Milk protein gene expression is tightly regulated and shows a rapid increase in transcript abundance during lactation, followed by an abrupt decline within 24 h of parturition (4, 12). The completion of the WGS from G. morsitans has aided analysis of gene regulatory sequences. However, the inability to generate transgenic tsetse flies because of their reproductive physiology required the analysis of tsetse regulatory elements in the heterologous Drosophila system (4). Transformation of Drosophila with the tsetse mgp1 regulatory sequence fused to a reporter gene showed conservation in tissue-specific expression between species. Drosophila bearing this construct show reporter gene expression restricted to the female accessory glands. Comparative bioinformatic analysis of the minimal mgp1 promoter sequence and putative regulatory sequences from the other MGP genes identified a single conserved transcription factor binding site for the homeodomain transcription factors. Differential RNA-seq analysis and tissue-specific qPCR analyses revealed a candidate homeodomain factor, ladybird late (lbl), whose knockdown yielded a reduction in fecundity owing to reduced milk protein levels (4). LBL therefore likely represents at least one of the factors regulating the expression of tsetse milk protein genes.
Water Transfer into the Milk Gland
In addition to proteins and lipids, water represents the third and most abundant component of the milk (31). Ten genes coding for aquaporins (AQPs), transmembrane proteins that act as water channels, were identified in the tsetse genome—two more than are known from any other insect (14). Two AQP genes, Drosophila integral protein A (DripA) and DripB, are highly expressed in the milk gland (14). Suppression of expression of either DripA or DripB had only a minor impact on progeny production. However, combined suppression of their expression drastically reduced progeny output (14). Progeny from AQP-deficient mothers showed a significant reduction in water content (14). These results indicate that AQPs play an important role in milk hydration.
Hormonal Regulation of Milk Production, Larvigenesis, and Birth
The tsetse lactation/larvigenesis cycle is hormonally regulated. Cross talk among JH, E, IIS, the poorly defined parturition hormone (PH), and various neurosecretory factors is necessary to orchestrate lactation and parturition (11, 34, 41, 42, 77, 78, 83). Topical application of the developmental hormones JH or E induces larval abortion in G. morsitans (34). These larvae abort as second or third instars, lack complete cuticle melanization, and are unable to pupariate. The mechanism behind this phenomenon remains poorly characterized. However, larvae can survive for several days in utero in a starved female, arguing against starvation as a mechanism for the rapid abortion induced by E or JH analogs (34).
Like Drosophila, tsetse express two JH receptor paralogs, the bHLH PAS family transcription factors Methoprene tolerant (Met) and germ-cell expressed (gce) (10). The presence of duplicate JH receptors is thus conserved within Muscomorpha (137), and these proteins regulate divergent target genes as in Drosophila (40, 93). Investigators have examined JH action during tsetse reproduction by manipulating hormone titer (11, 34, 41, 42, 78, 83). Allatectomy (CAX), or removal of the corpus allatum, has been a popular approach for removing circulating JH in adults. As shown by Ejezie & Davey (42), allatectomized G. austeni did not produce offspring unless given exogenous JH. The timing of CAX was crucial—early allatectomized flies failed even to produce vitellogenic oocytes and late CAX occasionally allowed flies to produce some offspring. In contrast, Langley& Pimley (83) observed no effect of CAX on G. morsitans reproduction, perhaps reflecting inherent differences among tsetse species or surgical technique.
Lipid homeostasis between stored TAGs and milk-borne lipids is critical for tsetse lactation (5). The release dynamics of adipokinetic hormone (AKH), a lipid-synthesis-stimulating factor from the corpus cardiaca, closely follows the pregnancy cycle, showing a dramatic increase to promote lipid breakdown during mid-pregnancy (11, 105, 106). Both the AKH system and Brummer lipase, a downstream target of the IIS pathway, are required for tsetse lactation; knockdown of either inhibits the breakdown and utilization of stored lipids (5). Additionally, JH can affect milk production. The average volume of the corpora allata in G. austeni fluctuates in accordance with the milk gland, reaching maximum size 2 days before both maximal milk gland size and synthetic activity during each cycle (41). CAX induces fat body hypertrophy and suppresses milk production; JH replacement therapy restores the capacity for lactation (42). A recent molecular analysis demonstrated that lipogenic genes, lipid levels, and JH-/IIS-associated gene transcripts show maximal levels at the onset of lactation, followed by minimal levels at larviposition (11).These results suggest that JH plays an important role in maintaining lipid stores and lipogenesis by the fat body that precedes lactation. Thus, lack of JH likely interferes with the transition between dry and lactating periods, particularly by affecting lipid homeostasis, impairing milk gland function (11, 42).
Parturition
The identity of PH, a factor that induces larval expulsion by pregnant females, remains enigmatic. Some evidence suggests that both the mother and the larva play active roles in orchestrating the timing of parturition (35, 38).With regard to humoral factors, a uterine-derived substance with PH activity elicits abortion when it is injected during early pregnancy and parturition when injected during late pregnancy, but only in neck-ligated flies, suggesting the requirement of additional input from the central nervous system (39). PH activity was also observed in uterine extracts from six Glossina species and even in extracts from the common oviduct of the flesh fly Sarcophaga bullata (20). The final E peak during pregnancy has been postulated to initiate the release of PH (114), and E application alone increases both the frequency and the amplitude of uterine muscular contractions in vitro (3). Thus, PH activity perhaps results from the integration of several distinct molecules or pathways rather than a single, elusive bioactive compound.
Antioxidant Production During Lactation Protects Milk Gland Function and Maintains Fecundity
Tsetse females are fertile throughout their entire life span and undergo multiple pregnancies without showing significant reproductive senescence (77, 91). This is surprising because the tsetse milk gland generates a substantial amount of milk in a short period (12), which likely yields oxidative stress and damage. Tsetse females appear to manage reproduction-associated oxidative stress by upregulating antioxidant enzymes (Figure 3). Suppression of this response increased accumulation of oxidative damage and reduced reproductive output because of a diminished ability to produce milk proteins during subsequent reproductive cycles (91). When this response is suppressed in early reproductive cycles, cumulative damage and reproductive senescence occur (91). Thus, the antioxidant response is a critical mechanism that facilitates maximum reproductive output and prevents premature reproductive senescence due to oxidative stress during tsetse lactation (91).
COMPARATIVE ASPECTS OF MATROTROPHIC VIVIPARITY AND LACTATION
Matrotrophic Viviparity and Lactation-Like Systems Among Insects
Research on the adenotrophic viviparous biology outside of Glossina for other closely related Hippoboscoidea is sparse (85). Structurally, the milk glands from sheep keds and tsetse flies are quite similar; the only difference is that the secretory reservoir of sheep keds is bilobed (85). Histochemical analyses of the secretory reservoir suggest that sheep ked milk is similar to tsetse milk (85). Variation in milk gland cell size and the abundance of endoplasmic reticulum from sheep ked glands is similar to that seen in tsetse during lactation (85). In contrast to tsetse, sheep keds have higher milk lipid content and a reduced dependence on stored nutrients derived from early blood meals, as female sheep keds are wingless and live in constant, direct contact with the host, allowing more frequent feeding by the mother.
Diptera outside the Hippoboscoidea, specifically S. nigriventris and members of Mesembrinellinae, likely produce milk products via accessory glands and the spermathecae, respectively (49, 91). Other nondipteran insects provide nourishment to developing embryos and larvae through hemocoelous or pseudoplacental viviparous mechanisms. Select dermapterans (52); the viviparous cockroach, Diploptera punctata (124, 143); aphids (16); and certain psocopterans (52, 108) undergo pseudoplacental viviparity (52). Hemocoelous viviparity in strepsipterans and dipterans has been documented (52, 108). Recent studies on the dermapteran Arixenia esau revealed that it uses a two-phase reproductive strategy termed pseudoplacento-uterotrophic viviparity, in which nutrients are provided to progeny in both the terminal ovarian follicle and the uterus (132). Two separate cell types associated with these tissues are responsible for nutrient production in Arixenia lactation (132). In D. punctata, developing progeny remain in the mother’s brood sac and are provided milk-like substances via secretory cells in the brood sac walls (124, 143). For aphids, nutrients are provided through the amnio-serosal membrane that immediately surrounds the developing embryo (16). Outside these examples, little is known about nutrient transfer in other viviparous insect systems.
Similarities in Lactation Among Vertebrates and Invertebrates
Tsetse lactation shares many analogous features with mammalian lactation. First, tsetse flies undergo punctuated periods of milk synthesis and related metabolic activities, followed by rapid involution preceding an extended dry period (37, 131). Second, lipocalins are a conserved class of proteins found in tsetse and mammalian milk and likely transport specific hydrophobic moieties to the developing offspring (6). Third, sphingomyelinase activity in both tsetse and mammalian milk has been documented (13, 100). The tsetse MGP2–10 proteins appear analogous to mammalian caseins; they act as a major source of amino acids and phosphate and are critical to milk homeostasis (12). Unlike the caseins, the MGP2–10 family likely does not transport calcium, as only trace amounts of calcium are detected in tsetse milk (31). This finding is unsurprising because insects synthesize a chitin-based exoskeleton rather than a calcium-based endoskeleton (12). Another distinguishing feature of tsetse milk is that it lacks sugar, which is usually abundant in the milk of most mammals (31). This difference likely reflects tsetse’s dependence on amino acids rather than glucose as a nutrient source within the blood. Finally, both mammalian milk and tsetse milk provide bacterial symbionts to juvenile progeny (see sidebar, Wigglesworthia Symbiosis and Its Role in Host Fecundity and Health of Progeny, above). These parallels are likely due to the fact that although tsetse and mammals are invertebrates and vertebrates, respectively, both are eukaryotic organisms that must provide nursing offspring similar milk-borne nutrients when no other food is available or utilized.
CONCLUSION
Tsetse flies are one of the most unique insect vectors owing to their adenotrophic viviparous reproduction. This peculiar reproductive strategy yields very few progeny and is an excellent target for population control. Recent studies, particularly the completed tsetse genome project and its associated functional genomics projects (4, 12, 14, 63, 91), along with previous biochemical and physiological studies, have helped elucidate the underpinnings of tsetse reproduction. The Wigglesworthia symbionts are also critical to reproduction and progeny development. The mechanisms by which this bacterial association is critical to tsetse physiology have begun to be established (3, 92, 103, 112, 136, 138, 139). The processes of tsetse reproduction and reliance on its symbionts provide targets for methods to suppress tsetse fecundity, including: (a) interfering with digestion and nutrient mobilization to disrupt milk production, (b) suppressing milk protein expression, (c) inhibiting the tsetse stress response during reproduction to disrupt subsequent gonotrophic cycles, and (d) disrupting the synthesis of vitamins and cofactors by Wigglesworthia. These targets could be exploited to generate tsetse-specific population control tools, thus reducing the prevalence of African trypanosomiasis.
Many aspects of tsetse reproductive physiology are analogous to those of mammalian reproduction. This begs the question of whether tsetse can be used as a model system to study lactation and maternal-derived nutrition. Availability of the sequenced tsetse genome (63) in combination with transcriptomic and physiological studies establishes the foundation for tsetse as a model organism. Initial studies of the role of the microbiome in immune development indicate that tsetse will find utility as a model to address complex questions associated with maternally derived nutrient provisioning (138, 139). Further studies regarding multigenerational effects in relation to changes in milk content and transfer of environmental toxicants in milk will benefit from the adoption of tsetse as a model species for lactation biology.
WIGGLESWORTHIA SYMBIOSIS AND ITS ROLE IN HOST FECUNDITY AND HEALTH OF PROGENY.
Tsetse’s vertebrate blood diet is rich in protein and lipids but low in specific micronutrients such as vitamins. To supplement their diet, all tsetse species and individuals harbor the enteric symbiont Wigglesworthia, a member of Gammaproteobacteria, intracellularly in bacteriocytes that form the bacteriome organ in the anterior midgut (3, 136). The symbiont is also present extracellularly in the lumen of the milk gland (9) and is transferred in milk to colonize the milk gland and gut bacteriome organs of the progeny. The Wigglesworthia-tsetse association is ancient and, as a consequence of its strict vertical transmission, displays concordant evolution with host species phylogeny (30). The WGS of Wigglesworthia from two tsetse species, G. brevipalpis and G. morsitans, have been determined, and both were found to be reduced to near 700 kb, approximately 10 times smaller than in most other Proteobacteria (3, 112). Despite this size reduction, the Wigglesworthia genome has retained the ability to synthesize B vitamins, including B1 (thiamine), B2 (riboflavin), B3 (nicotinamide), B5 (pantothenic acid), B6 (pyridoxine), and B9 (folic acid) (3, 112). Females that receive tetracycline-supplemented blood meals to clear Wigglesworthia cannot support larval development and abort their progeny (98, 99, 104). Loss of fecundity can be partially recovered by dietary supplementations with micronutrients from yeast extracts or B vitamins (92, 98, 103). Beyond the nutritional role Wigglesworthia plays in host fecundity, Wigglesworthia presence during larval development is essential for immune system maturation (138–140). Adult progeny that lacked Wigglesworthia during larval development were immunocompromised, characterized by deficient hematopoiesis (139) and gut immune integrity (138, 140), and were highly susceptible to trypanosome infections.
SUMMARY POINTS.
Viviparity occurs in many insect systems, but adenotrophic viviparity is relatively rare, only definitively documented among members of the dipteran superfamily Hippoboscoidea and the subfamily Mesembrinellinae. The organ that produces the milk-like secretions differs between the two independent occurrences of adenotrophic viviparity evolution.
Adenotrophic viviparity in Hippoboscoidea features three major morphological changes: an expanded uterus to support the development of the intrauterine larva, an expanded accessory gland that secretes milk, and reduced ovariole development.
Tsetse larvae require substantial maternal transfer for development, resulting in a 50% reduction in maternal lipid reserves. Disruption of nutrient transfer results in delayed parturition or abortion.
Milk secretions contain 12 major proteins, which are expressed in accordance with the lactation cycle. During lactation, the major milk proteins represent 47% of the mother’s transcriptional output.
Tsetse’s obligate symbiont, Wigglesworthia glossinidia, is transmitted to the intrauterine larva via milk. In the absence of Wigglesworthia, emerging adult progeny are immunocompromised and infertile. Sterility resulting from the absence of Wigglesworthia can be rescued through dietary supplementation with yeast extract and B complex vitamins.
Tsetse lactation is functionally analogous to mammalian lactation, as are the major proteins involved. This is unsurprising because the basic nutritional requirements for most eukaryotes are equivalent.
FUTURE ISSUES.
Regulation of oogenesis: What are the roles of hormonal and nutritional inputs (e.g., JH, E, insulin-like peptides, oostatic hormone, amino acid signaling) in the regulation of oogenesis? How does the tsetse fly restrict oocyte development to a single oocyte per gonotrophic cycle?
Regulation of ovulation and parturition: Which hemolymph-borne factors appear to regulate ovulation and parturition in tsetse? From what tissue do these factors originate? How is their activity regulated during oogenesis and larvigenesis?
Regulation of lactation-specific products: How is homeodomain factor activity regulated? What factors beyond a single homeodomain factor regulate milk production?
Prevention of larval movement until birth: What mechanisms are responsible for suppressing the movement of a larva (other than feeding) within the uterus, as an active, motile larva could damage the uterus? This reduced movement may be due to delayed neural development (44).
Role of obligate symbiosis in fecundity and host immunity: Which B vitamins and other symbiont-produced factors are required for milk production and maturation of larval immunity? For which host pathways and/or metabolites are these vitamins essential for maintaining functionality?
Convergent evolution: What are the common mechanisms of milk generation among insects, and what situations facilitated the transition from oviparity to matrotrophic viviparity? How is this transition similar to that which occurred in vertebrates?
Harnessing adenotrophic viviparity for vector control: Can vulnerabilities in the reproductive cycle be exploited to manage tsetse populations? Can compounds that interfere with milk protein regulation or Wigglesworthia fitness be used as tsetse-specific insecticides?
ACKNOWLEDGMENTS
The breadth of topics discussed in this review was wide, and we apologize to investigators whose work was not cited because of page constraints. Results presented in this review were supported by the National Institutes of Health awards AI081774 and F32AI093023 and by the Ambrose Monell Foundation. J.B.B. and G.M.A. contributed equally to this manuscript.
Glossary
- Adenotrophic viviparity
a form of matrotrophic viviparity in which progeny are provided nutrients within a uterus through glandular secretions until live birth
- Matrotrophic viviparity
nutrients during gestation are provided by mechanisms beyond that of the egg yolk
- Milk gland
common term used to describe the tsetse fly accessory gland; also known as the uterine gland
- E
ecdysteroids
- JH
juvenile hormone
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Joshua B. Benoit, Email: joshua.benoit@uc.edu.
Geoffrey M. Attardo, Email: geoffrey.attardo@yale.edu.
Aaron A. Baumann, Email: baumanna@janelia.hhmi.org.
Veronika Michalkova, Email: veronika.michalkova@savba.sk.
Serap Aksoy, Email: serap.aksoy@yale.edu.
LITERATURE CITED
- 1.Adams TS. The role of juvenile hormone in housefly ovarian follicle morphogenesis. J. Insect Physiol. 1974;20:263–276. doi: 10.1016/0022-1910(74)90058-4. [DOI] [PubMed] [Google Scholar]
- 2.Adams TS, Reinecke JP. The reproductive physiology of the screwworm, Cochliomyia hominivorax (Diptera: Calliphoridae). I. Oogenesis. J. Med. Entomol. 1979;15:472–483. doi: 10.1093/jmedent/15.5-6.472. [DOI] [PubMed] [Google Scholar]
- 3. Akman L, Yamashita A, Watanabe H, Oshima K, Shiba T, et al. Genome sequence of the endocellular obligate symbiont of tsetse flies, Wigglesworthia glossinidia. Nat. Genet. 2002;32:402–407. doi: 10.1038/ng986. Provides the first genome sequence for Wigglesworthia.
- 4.Attardo GM, Benoit JB, Michalkova V, Patrick KR, Krause TB, Aksoy S. The homeodomain protein ladybird late regulates synthesis of milk proteins during pregnancy in the tsetse fly (Glossina morsitans) PLOS Negl. Trop. Dis. 2014;10:e2645. doi: 10.1371/journal.pntd.0002645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attardo GM, Benoit JB, Michalkova V, Yang G, Roller L, et al. Analysis of lipolysis underlying lactation in the tsetse fly, Glossina morsitans. Insect Biochem. Mol. Biol. 2012;42:360–370. doi: 10.1016/j.ibmb.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Attardo GM, Guz N, Strickler-Dinglasan P, Aksoy S. Molecular aspects of viviparous reproductive biology of the tsetse fly (Glossina morsitans morsitans): regulation of yolk and milk gland protein synthesis. J. Insect Physiol. 2006;52:1128–1136. doi: 10.1016/j.jinsphys.2006.07.007. Conducts the first in-depth molecular analyses of tsetse milk proteins.
- 7.Attardo GM, Hansen IA, Raikhel AS. Nutritional regulation of vitellogenesis in mosquitoes: implications for anautogeny. Insect Biochem. Mol. Biol. 2005;35:661–675. doi: 10.1016/j.ibmb.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Attardo GM, Lohs C, Heddi A, Alam UH, Yildirim S, Aksoy S. Analysis of milk gland structure and function in Glossina morsitans: milk protein production, symbiont populations and fecundity. J. Insect Physiol. 2008;54:1236–1242. doi: 10.1016/j.jinsphys.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balmand S, Lohs C, Aksoy S, Heddi A. Tissue distribution and transmission routes for the tsetse fly endosymbionts. J. Invertebr. Pathol. 2013;112:S116–S122. doi: 10.1016/j.jip.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baumann A, Barry J, Wang S, Fujiwara Y, Wilson TG. Paralogous genes involved in juvenile hormone action in Drosophila melanogaster. Genetics. 2010;185:1327–1336. doi: 10.1534/genetics.110.116962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baumann A, Benoit JB, Michalkova V, Mireji PO, Attardo GM, et al. Juvenile hormone and insulin signaling pathways regulate lipid levels during lactation and dry periods of tsetse fly pregnancy. Mol. Cell. Endocrinol. 2012;372:30–41. doi: 10.1016/j.mce.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Benoit JB, Attardo GM, Michalkova V, Bohova J, Zhang Q, et al. A novel highly divergent protein family from a viviparous insect identified by RNA-seq analysis: a potential target for tsetse fly-specific abortifacients. PLOS Genet. 2014;10:e1003874. doi: 10.1371/journal.pgen.1003874. Performs a comprehensive transcriptome analysis to compare dry and lactating periods during tsetse pregnancy.
- 13.Benoit JB, Attardo GM, Michalkova V, Takac P, Bohova J, Aksoy S. Sphingomyelinase activity in mother’s milk is essential for juvenile development: a case from lactating tsetse flies. Biol. Reprod. 2012;87:1–10. doi: 10.1095/biolreprod.112.100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benoit JB, Hansen IA, Attardo GM, Michalkova V, Mireji PO, et al. Aquaporins are critical for provision of water for lactation and progeny hydration to maintain tsetse fly reproductive success. PLOS Negl. Trop. Dis. 2014;8:e2517. doi: 10.1371/journal.pntd.0002517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benoit JB, Yang G, Krause TB, Patrick KR, Aksoy S, Attardo GM. Lipophorin acts as a shuttle of lipids to the milk gland during tsetse fly pregnancy. J. Insect Physiol. 2011;57:1553–1561. doi: 10.1016/j.jinsphys.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bermingham J, Wilkinson TL. Embryo nutrition in parthenogenetic viviparous aphids. Physiol. Entomol. 2009;34:103–109. [Google Scholar]
- 17.Blackburn DG. Viviparity and oviparity: evolution and reproductive strategies. In: Knobil TE, editor. Encyclopedia of Reproduction. Vol. 4. London: Academic; 1999. pp. 994–1003. [Google Scholar]
- 18.Borovsky D, Song Q, Ma MC, Carlson DA. Biosynthesis, secretion, and immunocytochemistry of trypsin modulating oostatic factor of Aedes aegypti. Arch. Insect Biochem. Physiol. 1994;27:27–38. [Google Scholar]
- 19.Bownes M. Three genes for three yolk proteins in Drosophila melanogaster. FEBS Lett. 1979;100:95–98. doi: 10.1016/0014-5793(79)81138-2. [DOI] [PubMed] [Google Scholar]
- 20.Briers T, Huybrechts R. Control of vitellogenin synthesis by ecdysteroids in Sarcophaga bullata. Insect Biochem. 1984;14:121–126. [Google Scholar]
- 21.Brown MR, Clark KD, Gulia M, Zhao Z, Garczynski SF, et al. An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA. 2008;105:5716–5721. doi: 10.1073/pnas.0800478105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brun R, Schumacher R, Schmid C, Kunz C, Burri C. The phenomenon of treatment failures in human African trypanosomiasis. Trop. Med. Int. Health. 2001;6:906–914. doi: 10.1046/j.1365-3156.2001.00775.x. [DOI] [PubMed] [Google Scholar]
- 23.Budd LT. DFID-Funded Tsetse and Trypanosome Research and Development Since 1980, Vol. 2: Economic Analysis. London: Dep. Int. Dev.; 1999. [Google Scholar]
- 24.Bursell E. Synthesis of proline by the fat body of the tsetse fly (Glossina morsitans). Metabolic pathways. Insect Biochem. 1977;7:427–434. [Google Scholar]
- 25.Bursell E. The role of proline in energy metabolism. In: Downer RGH, editor. Energy Metabolism in Insects. New York: Springer; 1981. pp. 135–154. [Google Scholar]
- 26.Buxton P. The Natural History of Tsetse Flies. London: H.K. Lewis & Co.; 1955. [Google Scholar]
- 27.Chaudhury MF, Dhadialla TS. Evidence of hormonal control of ovulation in tsetse flies. Nature. 1976;260:243–244. doi: 10.1038/260243a0. [DOI] [PubMed] [Google Scholar]
- 28.Chaudhury MFB, Dhadialla TS, Kunyiha RW. Evidence of neuroendocrine relationships between mating and ovulation in the tsetse fly, Glossina morsitans morsitans. Insect Sci. Appl. 1981;1:161–166. [Google Scholar]
- 29.Chen AC, Kim HR, Mayer RT, Norman JO. Vitellogenesis in the stable fly, Stomoxys calcitrans. Comp. Biochem. Physiol. B. 1987;88:897–903. [Google Scholar]
- 30.Chen X, Li S, Aksoy S. Concordant evolution of a symbiont with its host insect species: molecular phylogeny of genus Glossina and its bacteriome-associated endosymbiont, Wigglesworthia glossinidia. J. Mol. Evol. 1999;48:49–58. doi: 10.1007/pl00006444. [DOI] [PubMed] [Google Scholar]
- 31.Cmelik SHW, Bursell E, Slack E. Composition of the gut contents of third-instar tsetse larvae (Glossina morsitans Westwood) Comp. Biochem. Physiol. 1969;29:447–453. [Google Scholar]
- 32.Davis S, Aksoy S, Galvani A. A global sensitivity analysis for African sleeping sickness. Parasitology. 2011;138:516–526. doi: 10.1017/S0031182010001496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Loof A, Bylemans D, Schoofs L, Janssen I, Spittaels K, et al. Folliculostatins, gonadotropins and a model for control of growth in the grey fleshfly, Neobellieria (Sarcophaga) bullata. Insect Biochem. Mol. Biol. 1995;25:661–667. doi: 10.1016/0965-1748(95)00005-g. [DOI] [PubMed] [Google Scholar]
- 34.Denlinger DL. Insect hormones as tsetse abortifacients. Nature. 1975;253:347–348. doi: 10.1038/253347a0. [DOI] [PubMed] [Google Scholar]
- 35.Denlinger DL. Who controls the rhythm of tsetse parturition: mother or larva? Bull. Entomol. Res. 1983;8:25–28. [Google Scholar]
- 36.Denlinger DL, Chaudhury MF, Dhadialla TS. Cyclic AMP is a likely mediator of ovulation in the tsetse fly. Experientia. 1978;34:1296–1297. doi: 10.1007/BF01981428. [DOI] [PubMed] [Google Scholar]
- 37. Denlinger DL, Ma W-C. Dynamics of the pregnancy cycle in the tsetse Glossina morsitans. J. Insect Physiol. 1974;20:1015–1019. 1021–1026. doi: 10.1016/0022-1910(74)90143-7. Describes the changes in the larval size and milk gland size during the course of pregnancy.
- 38.Denlinger DL, Saini RK, Chaudhury MFB. Parturition in the tsetse fly Glossina morsitans: pattern of activity, sound production and evidence for control by the mother’s brain. J. Insect Physiol. 1983;29:715–721. [Google Scholar]
- 39.Denlinger DL, Ždárek J. A hormone from the uterus of the tsetse fly, Glossina morsitans stimulates parturition and abortion. J. Insect Physiol. 1996;43:135–142. doi: 10.1016/s0022-1910(96)00089-3. [DOI] [PubMed] [Google Scholar]
- 40.Dubrovsky EB, Dubrovskaya VA, Bernardo T, Otte V, DiFilippo R, Bryan H. The Drosophila FTZ-F1 nuclear receptor mediates juvenile hormone activation of E75A gene expression through an intracellular pathway. J. Biol. Chem. 2011;286:33689–33700. doi: 10.1074/jbc.M111.273458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ejezie GC, Davey KG. Changes in the neurosecretory cells, corpus cardiacum and corpus allatum during pregnancy in Glossina austeni Newst. (Diptera, Glossinidae) Bull. Entomol. Res. 1974;64:247–256. [Google Scholar]
- 42.Ejezie GC, Davey KG. Some effects of allatectomy in female tsetse, Glossina austeni. J. Insect Physiol. 1976;22:1743–1749. doi: 10.1016/0022-1910(76)90068-8. [DOI] [PubMed] [Google Scholar]
- 43.Fallon AM, Hagedorn HH, Wyatt GR, Laufer H. Activation of vitellogenin synthesis in the mosquito Aedes aegypti by ecdysone. J. Insect Physiol. 1974;20:1815–1823. doi: 10.1016/0022-1910(74)90211-x. [DOI] [PubMed] [Google Scholar]
- 44.Farris SM, Rio RV. Brain development in an insect with extensive maternal care, the tsetse fly Glossina morsitans (Diptera: Glossinidae); Front. Behav. Neurosci. Conf. Abstr.: Tenth Int. Congr. Neuroethol; 2012. [Google Scholar]
- 45.Foster WA. Surgical inhibition of ovulation and gestation in the tsetse fly Glossina austeni Newst. (Dipt Glossinidae) Bull. Entomol. Res. 1974;63:483–493. [Google Scholar]
- 46.Fourney RM, Pratt GF, Harnish DG, Wyatt GR, White BN. Structure and synthesis of vitellogenin and vitellin from Calliphora erythrocephala. Insect Biochem. 1982;12:311–321. [Google Scholar]
- 47.French A, Hoopingarner R. Gametogenesis in the house fly, Musca domestica. Ann. Entomol. Soc. Am. 1965;58:650–657. doi: 10.1093/aesa/58.5.650. [DOI] [PubMed] [Google Scholar]
- 48.Garabedian MJ, Hung MC, Wensink PC. Independent control elements that determine yolk protein gene expression in alternative Drosophila tissues. Proc. Natl. Acad. Sci. USA. 1985;82:1396–1400. doi: 10.1073/pnas.82.5.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guimaraes JH. A systematic revision of the Mesembrinellidae, stat. nov. (Diptera, Cyclorrhapha) Arq. Zool. 1977;29:1–109. [Google Scholar]
- 50.Gulia-Nuss M, Robertson AE, Brown MR, Strand MR. Insulin-like peptides and the target of rapamycin pathway coordinately regulate blood digestion and egg maturation in the mosquito Aedes aegypti. PLOS ONE. 2011;6:e20401. doi: 10.1371/journal.pone.0020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guz N, Attardo GM, Wu Y, Aksoy S. Molecular aspects of transferrin expression in the tsetse fly (Glossina morsitans morsitans) J. Insect Physiol. 2007;53:715–723. doi: 10.1016/j.jinsphys.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hagan HR. Embryology of Viviparous Insects. New York: Ronald Press; 1951. [Google Scholar]
- 53.Hagedorn HH, O’Connor JD, Fuchs MS, Sage B, Schlaeger DA, Bohm MK. The ovary as a source of alpha-ecdysone in an adult mosquito. Proc. Natl. Acad. Sci. USA. 1975;72:3255–3259. doi: 10.1073/pnas.72.8.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hagedorn HH, Shapiro JP, Hanaoka K. Ovarian ecdysone secretion is controlled by a brain hormone in an adult mosquito. Nature. 1979;282:92–94. doi: 10.1038/282092a0. [DOI] [PubMed] [Google Scholar]
- 55.Hansen IA, Attardo GM, Park JH, Peng Q, Raikhel AS. Target of rapamycin-mediated amino acid signaling in mosquito anautogeny. Proc. Natl. Acad. Sci. USA. 2004;101:10626–10631. doi: 10.1073/pnas.0403460101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hecker H, Moloo SK. Quantitative morphological changes of the uterine gland cells in relation to physiological events during a pregnancy cycle in Glossina morsitans morsitans. J. Insect Physiol. 1983;29:651–658. [Google Scholar]
- 57.Hens K, Lemey P, Macours N, Francis C, Huybrechts R. Cyclorraphan yolk proteins and lepidopteran minor yolk proteins originate from two unrelated lipase families. Insect Mol. Biol. 2004;13:615–623. doi: 10.1111/j.0962-1075.2004.00520.x. [DOI] [PubMed] [Google Scholar]
- 58.Hens K, Macours N, Claeys I, Francis C, Huybrechts R. Cloning and expression of the yolk protein of the tsetse fly Glossina morsitans morsitans. Insect Biochem. Mol. Biol. 2004;34:1281–1287. doi: 10.1016/j.ibmb.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 59.Horn D, McCulloch R. Molecular mechanisms underlying the control of antigenic variation in African trypanosomes. Curr. Opin. Microbiol. 2010;13:700–705. doi: 10.1016/j.mib.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Houseman JG, Morrison PE. Absence of female-specific protein in the hemolymph of stable fly Stomoxys calcitrans (L.) (Diptera: Muscidae) Arch. Insect Biochem. Physiol. 1986;3:205–213. [Google Scholar]
- 61.Hu CY, Rio RVM, Medlock J, Haines LR, Nayduch D, et al. Infections with immunogenic trypanosomes reduce tsetse reproductive fitness: potential impact of different parasite strains on vector population structure. PLOS Negl. Trop. Dis. 2008;2:e192. doi: 10.1371/journal.pntd.0000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huebner E, Tobe SS, Davey KG. Structural and functional dynamics of oogenesis in Glossina austeni: general features, previtellogenesis and nurse cells. Tissue Cell. 1975;7:297–317. doi: 10.1016/0040-8166(75)90007-5. [DOI] [PubMed] [Google Scholar]
- 63. International Glossina Genome Initiative. Genome sequence of the tsetse fly (Glossina morsitans): vector of African trypanosomiasis. Science. 2014;344:380–386. doi: 10.1126/science.1249656. Provides the genome sequence for Glossina morsitans.
- 64.Isaac PG, Bownes M. Ovarian and fat-body vitellogenin synthesis in Drosophila melanogaster. Eur. J. Biochem. 1982;123:527–534. doi: 10.1111/j.1432-1033.1982.tb06563.x. [DOI] [PubMed] [Google Scholar]
- 65.Jackson AP, Berry A, Aslett M, Allison HC, Burton P, et al. Antigenic diversity is generated by distinct evolutionary mechanisms in African trypanosome species. Proc. Natl. Acad. Sci. USA. 2012;109:3416–3421. doi: 10.1073/pnas.1117313109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jannin J, Cattand P. Treatment and control of human African trypanosomiasis. Curr. Opin. Infect. Dis. 2004;17:565–571. doi: 10.1097/00001432-200412000-00009. [DOI] [PubMed] [Google Scholar]
- 67.Jensen PV, Hansen BL, Hansen GN, Thomsen E. Vitellogenin and vitellin from the blowfly Calliphora vicina: occurrence, purification and antigenic characterization. Insect Biochem. 1981;11:129–135. [Google Scholar]
- 68.Joja LL, Okoli UA. Trapping the vector: community action to curb sleeping sickness in southern Sudan. Am. J. Public Health. 2001;91:1583–1585. doi: 10.2105/ajph.91.10.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jordan AM. Trypanosomiasis Control and African Rural Development. London: Longman; 1986. [Google Scholar]
- 70.Kabayo JP. Aiming to eliminate tsetse from Africa. Trends Parasitol. 2002;18:473–475. doi: 10.1016/s1471-4922(02)02371-1. [DOI] [PubMed] [Google Scholar]
- 71.Kgori PM, Modo S, Torr SJ. The use of aerial spraying to eliminate tsetse from the Okavango Delta of Botswana. Acta Trop. 2006;99:184–199. doi: 10.1016/j.actatropica.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 72.King RC. Ovarian Development in Drosophila melanogaster. New York: Academic; 1970. [Google Scholar]
- 73.King RC, Aggarwal SK, Aggarwal U. The development of the female Drosophila reproductive system. J. Morphol. 1968;124:143–166. doi: 10.1002/jmor.1051240203. [DOI] [PubMed] [Google Scholar]
- 74.Krafsur ES. Tsetse flies: genetics, evolution, and role as vectors. Infect. Genet. Evol. 2009;9:124–141. doi: 10.1016/j.meegid.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Langley PA, Bursell E. Role of fat body and uterine gland in milk synthesis by adult female Glossina morsitans. Insect Biochem. 1980;10:11–17. [Google Scholar]
- 76. Langley PA, Bursell E, Kabayo J, Pimley RW, Trewen MA, Marshall J. Hemolymph lipid transport from fat body to uterine gland in pregnant females of Glossina morsitans. Insect Biochem. 1981;11:225–231. Conducts critical research on nutrient metabolism during tsetse fly lactation (see also References 78–83).
- 77.Langley PA, Clutton-Brock TH. Does reproductive investment change with age in tsetse flies, Glossina morsitans morsitans (Diptera: Glossinidae)? Funct. Ecol. 1998;12:866–870. [Google Scholar]
- 78.Langley PA, Felton T, Oouchi H. Juvenile hormone mimics as effective sterilants for the tsetse fly Glossina morsitans morsitans. Med. Vet. Entomol. 1988;2:29–35. doi: 10.1111/j.1365-2915.1988.tb00046.x. [DOI] [PubMed] [Google Scholar]
- 79.Langley PA, Pimley RW. Utilization of U-14C amino acids or U-14C protein by adult Glossina morsitans during in utero development of larva. J. Insect Physiol. 1974;20:2157–2170. doi: 10.1016/0022-1910(74)90041-9. [DOI] [PubMed] [Google Scholar]
- 80.Langley PA, Pimley RW. Quantitative aspects of reproduction and larval nutrition in Glossina morsitans morsitans Westw. (Diptera: Glossinidae) fed in vitro. Bull. Entomol. Res. 1975;65:129–142. [Google Scholar]
- 81.Langley PA, Pimley RW. Influence of diet on synthesis and utilization of lipids for reproduction by the tsetse fly Glossina morsitans. J. Insect Physiol. 1979;25:79–86. [Google Scholar]
- 82.Langley PA, Pimley RW. Storage and mobilisation of nutriment for uterine milk synthesis by Glossina morsitans. J. Insect Physiol. 1979;25:193–197. [Google Scholar]
- 83.Langley PA, Pimley RW. A role of juvenile hormone and the effects of so-called anti-juvenile hormones in Glossina morsitans. J. Insect Physiol. 1986;32:727–731. [Google Scholar]
- 84.Lea AO. Some relationships between environment, corpora allata, and egg maturation in aedine mosquitoes. J. Insect Physiol. 1963;9:793–809. [Google Scholar]
- 85.Lenoble BJ, Denlinger DL. The milk gland of the sheep ked, Melophagus ovinus: a comparison with Glossina. J. Insect Physiol. 1982;28:165–172. [Google Scholar]
- 86.Ma WC, Denlinger DL, Jarlfors U, Smith DS. Structural modulations in the tsetse fly milk gland during a pregnancy cycle. Tissue Cell. 1975;7:319–330. doi: 10.1016/0040-8166(75)90008-7. [DOI] [PubMed] [Google Scholar]
- 87.Martinez A, Bownes M. The sequence and expression pattern of the Calliphora erythrocephala yolk protein A and B genes. J. Mol. Evol. 1994;38:336–351. doi: 10.1007/BF00163151. [DOI] [PubMed] [Google Scholar]
- 88.McCabe CT, Bursell E. Interrelationships between amino acid and lipid metabolism in the tsetse fly, Glossina morsitans. Insect Biochem. 1975;5:781–789. [Google Scholar]
- 89.McCabe CT, Bursell E. The metabolism of digestive products in the tsetse fly, Glossina morsitans. Insect Biochem. 1975;5:769–779. [Google Scholar]
- 90. Meier R, Kotrba M, Ferrar P. Ovoviviparity and viviparity in the Diptera. Biol. Rev. Camb. Philos. Soc. 1999;74:199–258. Reviews fly reproductive mechanisms.
- 91.Michalkova V, Benoit JB, Attardo GM, Medlock J, Aksoy S. Amelioration of reproduction-associated oxidative stress in a viviparous insect is critical to prevent reproductive senescence. PLOS ONE. 2014;9:e87554. doi: 10.1371/journal.pone.0087554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Michalkova V, Benoit JB, Weiss BL, Attardo GM, Aksoy S. Obligate symbiont-generated vitamin B6 is critical to maintain proline homeostasis and fecundity in tsetse flies. Appl. Environ. Microbiol. 2014;80:5844–5853. doi: 10.1128/AEM.01150-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Minakuchi C, Zhou X, Riddiford LM. Krüppel homolog 1 (Kr-h1) mediates juvenile hormone action during metamorphosis of Drosophila melanogaster. Mech. Dev. 2008;125:91–105. doi: 10.1016/j.mod.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moloo SK. Oocyte differentiation and vitellogenesis in Glossina morsitans Westw. Acta Trop. 1971;28:334–340. [PubMed] [Google Scholar]
- 95.Moloo SK. Aspects of the nutrition of adult female Glossina morsitans during pregnancy. J. Insect Physiol. 1976;22:563–567. doi: 10.1016/0022-1910(76)90177-3. [DOI] [PubMed] [Google Scholar]
- 96.Moloo SK. Storage of nutriments by adult female Glossina morsitans and their transfer to the intra-uterine larva. J. Insect Physiol. 1976;22:111–115. doi: 10.1016/0022-1910(76)90120-7. [DOI] [PubMed] [Google Scholar]
- 97.Nogge G. Sterility in tsetse flies (Glossina morsitans Westwood) caused by loss of symbionts. Experientia. 1976;32:995–996. doi: 10.1007/BF01933932. [DOI] [PubMed] [Google Scholar]
- 98.Nogge G. Significance of symbionts for the maintenance of an optimal nutritional state for successful reproduction in hematophagous arthropods. Parasitology. 1981;82:101–104. [Google Scholar]
- 99.Nok AJ. Arsenicals (melarsoprol), pentamidine and suramin in the treatment of human African trypanosomiasis. Parasitol. Res. 2003;90:71–79. doi: 10.1007/s00436-002-0799-9. [DOI] [PubMed] [Google Scholar]
- 100.Nyberg L, Farooqi A, Blackberg L, Duan RD, Nilsson A, Hernell O. Digestion of ceramide by human milk bile salt-stimulated lipase. J. Pediatr. Gastroenterol. Nutr. 1998;27:560–567. doi: 10.1097/00005176-199811000-00013. [DOI] [PubMed] [Google Scholar]
- 101.Ochanda JO, Osir EO, Nguu EK, Olembo NK. Lipophorin from the tsetse fly, Glossina morsitans morsitans. Comp. Biochem. Physiol. B. 1991;99:811–814. doi: 10.1016/0305-0491(91)90146-5. [DOI] [PubMed] [Google Scholar]
- 102. Osir EO, Kotengo M, Chaudhury MFB, Otieno LH. Structural studies on the major milk gland protein of the tsetse fly, Glossina morsitans morsitans. Comp. Biochem. Physiol. B. 1991;99:803–809. doi: 10.1016/0305-0491(91)90145-4. Discovers the partial amino acid sequence for the first identified tsetse milk protein.
- 103.Pais R, Lohs C, Wu Y, Wang J, Aksoy S. The obligate mutualist Wigglesworthia glossinidia influences reproduction, digestion, and immunity processes of its host, the tsetse fly. Appl. Environ. Microbiol. 2008;74:5965–5974. doi: 10.1128/AEM.00741-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pellegrini A, Bigliardi E, Bechi N, Paulesu L, Lehane MJ, Avanzati AM. Fine structure of the female reproductive system in a viviparous insect, Glossina morsitans morsitans (Diptera, Glossinidae) Tissue Cell. 2011;43:1–7. doi: 10.1016/j.tice.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 105.Pimley RW, Langley PA. Hormonal control of lipid synthesis in the fat body of the adult female tsetse fly, Glossina morsitans. J. Insect Physiol. 1981;27:839–847. [Google Scholar]
- 106.Pimley RW, Langley PA. Hormone stimulated lipolysis and proline synthesis in the fat body of the adult tsetse fly, Glossina morsitans. J. Insect Physiol. 1982;28:781–789. [Google Scholar]
- 107.Raikhel AS, Kokoza VA, Zhu J, Martin D, Wang SF, et al. Molecular biology of mosquito vitellogenesis: from basic studies to genetic engineering of antipathogen immunity. Insect Biochem. Mol. Biol. 2002;32:1275–1286. doi: 10.1016/s0965-1748(02)00090-5. [DOI] [PubMed] [Google Scholar]
- 108.Retnakaran A, Percy J. Fertilization and special modes of reproduction. In: Kerkut GA, Gilbert LI, editors. Comprehensive Insect Physiology, Biochemistry and Pharmacology. Vol. 1. Oxford, UK: Pergamon; 1985. pp. 231–294. [Google Scholar]
- 109.Richard DS, Rybczynski R, Wilson TG, Wang Y, Wayne ML, et al. Insulin signaling is necessary for vitellogenesis in Drosophila melanogaster independent of the roles of juvenile hormone and ecdysteroids: Female sterility of the chico1 insulin signaling mutation is autonomous to the ovary. J. Insect Physiol. 2005;51:455–464. doi: 10.1016/j.jinsphys.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 110.Riddiford LM. How does juvenile hormone control insect metamorphosis and reproduction? Gen. Comp. Endocrinol. 2012;179:477–484. doi: 10.1016/j.ygcen.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 111.Riddiford LM, Dhadialla TS. Protein synthesis by the milk gland and fat body of the tsetse fly, Glossina pallidipes. Insect Biochem. 1990;20:493–500. [Google Scholar]
- 112.Rio RV, Symula RE, Wang J, Lohs C, Wu YN, et al. Insight into the transmission biology and species-specific functional capabilities of tsetse (Diptera: Glossinidae) obligate symbiont Wigglesworthia. mBio. 2012;3:e00240. doi: 10.1128/mBio.00240-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Robert A, Grillot JP, Guilleminot J, Raabe M. Experimental and ultrastructural study of the control of ovulation and parturition in the tsetse fly Glossina fuscipes (Diptera) J. Insect Physiol. 1984;30:671–679. [Google Scholar]
- 114.Robert A, Strambi A, Strambi C. Haemolymph ecdysteroid levels in female tsetse fly Glossina fuscipes (Diptera) during the first reproductive cycle: a comparison between virgin and mated females. J. Insect Physiol. 1985;32:665–671. [Google Scholar]
- 115.Roberts MJ. The role of the choriothete in tsetse flies. Parasitology. 1972;64:23–36. doi: 10.1017/s0031182000044619. [DOI] [PubMed] [Google Scholar]
- 116.Rogers D, Robinson T. Tsetse distribution. In: Maudlin I, Holmes P, Miles M, editors. The Trypanosomiases. Oxford, UK: CABI; 2004. pp. 139–179. [Google Scholar]
- 117.Sappington TW. The major yolk proteins of higher Diptera are homologs of a class of minor yolk proteins in Lepidoptera. J. Mol. Evol. 2002;55:470–475. doi: 10.1007/s00239-002-2342-0. [DOI] [PubMed] [Google Scholar]
- 118.Saunders DS. Ovaries of Glossina morsitans. Nature. 1960;185:121–122. doi: 10.1038/185121b0. [DOI] [PubMed] [Google Scholar]
- 119.Saunders DS. Studies on ovarian development in tsetse flies (Glossina, Diptera) Parasitology. 1961;51:545–564. doi: 10.1017/s0031182000070797. [DOI] [PubMed] [Google Scholar]
- 120.Saunders DS, Dodd CWH. Mating, insemination, and ovulation in the tsetse fly, Glossina morsitans. J. Insect Physiol. 1972;18:187–198. [Google Scholar]
- 121.Schwenk RW, Holloway GP, Luiken JJ, Bonen A, Glatz JF. Fatty acid transport across the cell membrane: regulation by fatty acid transporters. Prostaglandins Leukot. Essent. Fatty Acids. 2010;82:149–154. doi: 10.1016/j.plefa.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 122.Sondergaard L, Mauchline D, Egetoft P, White N, Wulff P, Bownes M. Nutritional response in a Drosophila yolk protein gene promoter. Mol. Gen. Genet. 1995;248:25–32. doi: 10.1007/BF02456610. [DOI] [PubMed] [Google Scholar]
- 123.Stay B, Coop AC. ‘Milk’ secretion for embryogenesis in a viviparous cockroach. Tissue Cell. 1974;6:669–693. doi: 10.1016/0040-8166(74)90009-3. [DOI] [PubMed] [Google Scholar]
- 124.Steelman CD. Effects of external and internal arthropod parasites on domestic livestock production. Annu. Rev. Entomol. 1976;21:155–178. doi: 10.1146/annurev.en.21.010176.001103. [DOI] [PubMed] [Google Scholar]
- 125.Strickler-Dinglasan PM, Guz N, Attardo G, Aksoy S. Molecular characterization of iron binding proteins from Glossina morsitans morsitans (Diptera: Glossinidae) Insect Biochem. Mol. Biol. 2006;36:921–933. doi: 10.1016/j.ibmb.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Terashima J, Bownes M. Translating available food into the number of eggs laid by Drosophila melanogaster. Genetics. 2004;167:1711–1719. doi: 10.1534/genetics.103.024323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Thomson TC, Johnson J. Inducible somatic oocyte destruction in response to rapamycin requires wild-type regulation of follicle cell epithelial polarity. Cell Death Differ. 2010;17:1717–1727. doi: 10.1038/cdd.2010.49. [DOI] [PubMed] [Google Scholar]
- 128.Tobe SS, Davey KG. The choriothete of Glossina austeni Newst. Bull. Entomol. Res. 1971;61:363–368. [Google Scholar]
- 129.Tobe SS, Davey KG. Autoradiographic study of protein synthesis in abdominal tissues of Glossina austeni. Tissue Cell. 1974;6:255–268. doi: 10.1016/0040-8166(74)90052-4. [DOI] [PubMed] [Google Scholar]
- 130.Tobe SS, Davey KG, Huebner E. Nutrient transfer during the reproductive cycle in Glossina austeni Newst.: histology and histochemistry of the milk gland, fat body, and oenocytes. Tissue Cell. 1973;5:633–650. doi: 10.1016/s0040-8166(73)80050-3. [DOI] [PubMed] [Google Scholar]
- 131. Tobe SS, Langley PA. Reproductive physiology of Glossina. Annu. Rev. Entomol. 1978;23:283–307. doi: 10.1146/annurev.en.23.010178.001435. Reviews tsetse reproductive physiology.
- 132.Tworzydlo W, Kisiel E, Bilinski SM. Embryos of the viviparous dermapteran, Arixenia esau develop sequentially in two compartments: terminal ovarian follicles and the uterus. PLOS ONE. 2013;8:e64087. doi: 10.1371/journal.pone.0064087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Van der Horst DJ, Ryan RO. Lipid transport. In: Gilbert LI, editor. Insect Molecular Biology and Biochemistry. Amsterdam: Elsevier; 2012. pp. 317–345. [Google Scholar]
- 134.Vreysen MJ, Saleh KM, Ali MY, Abdulla AM, Zhu ZR, et al. Glossina austeni (Diptera: Glossinidae) eradicated on the island of Unguja, Zanzibar, using the sterile insect technique. J. Econ. Entomol. 2000;93:123–135. doi: 10.1603/0022-0493-93.1.123. [DOI] [PubMed] [Google Scholar]
- 135.Wang J, Aksoy S. PGRP-LB is a maternally transmitted immune milk protein that influences symbiosis and parasitism in tsetse’s offspring. Proc. Natl. Acad. Sci. USA. 2012;109:10552–10557. doi: 10.1073/pnas.1116431109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang J, Weiss BL, Aksoy S. Tsetse fly microbiota: form and function. Front. Cell. Infect. Microbiol. 2013;3:69. doi: 10.3389/fcimb.2013.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang SL, Baumann A, Wilson TG. Drosophila melanogaster Methoprene-tolerant (Met) gene homologs from three mosquito species: members of PAS transcriptional factor family. J. Insect Physiol. 2007;53:246–253. doi: 10.1016/j.jinsphys.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Weiss BL, Maltz M, Aksoy S. Obligate symbionts activate immune system development in the tsetse fly. J. Immunol. 2012;188:3395–3403. doi: 10.4049/jimmunol.1103691. Studies the role of symbionts for immune development (see also References 139 and 140).
- 139.Weiss BL, Wang J, Aksoy S. Tsetse immune system maturation requires the presence of obligate symbionts in larvae. PLOS Biol. 2011;9:e1000619. doi: 10.1371/journal.pbio.1000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Weiss BL, Wang J, Maltz MA, Wu Y, Aksoy S. Trypanosome infection establishment in the tsetse fly gut is influenced by microbiome-regulated host immune barriers. PLOS Pathog. 2013;9:e1003318. doi: 10.1371/journal.ppat.1003318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Welburn SC, Fèvre EM, Coleman PG, Odiit M, Maudlin I. Sleeping sickness: a tale of two diseases. Trends Parasitol. 2001;17:19–24. doi: 10.1016/s1471-4922(00)01839-0. [DOI] [PubMed] [Google Scholar]
- 142.White NM, Bownes M. Cloning and characterization of three Musca domestica yolk protein genes. Insect Mol. Biol. 1997;6:329–341. doi: 10.1046/j.1365-2583.1997.00187.x. [DOI] [PubMed] [Google Scholar]
- 143.Williford A, Stay B, Bhattacharya D. Evolution of a novel function: nutritive milk in the viviparous cockroach, Diploptera punctata. Evol. Dev. 2004;6:67–77. doi: 10.1111/j.1525-142x.2004.04012.x. [DOI] [PubMed] [Google Scholar]
- 144.Yang G, Attardo GM, Lohs C, Aksoy S. Molecular characterization of two novel milk proteins in the tsetse fly (Glossina morsitans morsitans) Insect Mol. Biol. 2010;19:253–262. doi: 10.1111/j.1365-2583.2009.00987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]