Abstract
Organismal lifespan is a complex trait that is governed by both its genetic makeup as well as the environmental conditions. The improved socioeconomic condition of humans has led to many lifestyle changes that in turn have altered the demography that includes postponement of procreation. Late age progeny is shown to suffer from many congenital diseases. Hence, there is a need to identify and evaluate natural molecules that could enhance reproductive health span. We have used the well-established model organism, Drosophila melanogaster, and ascertained the consequence of diet supplementation with curcumin. Flies reared on curcumin-supplemented diet had significantly higher lifespan. The progeny of flies reared on curcumin had a higher viability. The activity of a key mitochondrial enzyme—aconitase was significantly higher in flies reared on curcumin-supplemented diet. The results suggest that curcumin can not only correct a key step in the citric acid cycle and help in the release of additional energy but also permanently correct developmental and morphogenetic processes.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-014-9702-8) contains supplementary material, which is available to authorized users.
Keywords: Curcumin, Aconitase, Glycogen, Triglycerides, pAkt/Akt, CG9510, AKHR, Drosophila
Introduction
Prolonged reproductive lifespan is an important fitness trait in all species engaged in repeated reproduction. However, there is a progressive decline in the functional capacity of physiological processes in all the living systems (Yarian et al. 2006)—a process called aging. Changing human lifestyle since the late twentieth century has led to postponement of procreation. Across biological systems, reproduction at later ages is shown to be on the declining trend. Further, children born to parents of older ages are shown to suffer from many congenital diseases. It is uncertain that the progress made in saving lives is due to improved health in advanced age and, hence, an important topic for research (Vaupel 2010). Furthermore, there is a need to address not only the problems associated with reproduction at later ages and suggest potential remedies but also suggest possible ways of producing viable, healthy, and fertile progeny at older ages as offspring viability is shown to decline with increasing parental age in Drosophila (Hercus and Hoffmann 2000; Kern et al. 2001). Fortunately, a sizable fraction of many signaling pathways regulating aging in short-lived organisms is shown to extend lifespan in mammals as well (Kenyon 2010). However, how different pathways and processes act together to promote or retard the aging process is not entirely clear (Sahin and DePinho 2010). Drosophila melanogaster has been in the forefront of research on aging interventions. Our focus here is to utilize the information from studies that have attempted to understand the dynamics of life-history traits through dietary manipulations (Tatar et al. 2014) in this model organism and elaborate further as direct genetical interventions in humans are not possible due to technical and ethical reasons (Lee et al. 2010). A number of studies in the recent past have shown the beneficial effects of nutraceuticals—secondary compounds produced by plants in promoting healthy aging in invertebrate models through reduced oxidative damage (Dong et al. 2012). Aloe vera (Chandrashekara and Shakarad 2011), blueberry (Peng et al. 2012), and green tea (Li et al. 2007, 2008) extract supplementation of fly diet increased the lifespan through increased activity of antioxidant enzymes—superoxide dismutase and catalase. Spermidine supplementation of ordinary fly food increased the mean lifespan of flies up to 30 % (Eisenberg et al. 2009). Curcumin supplementation of sucrose-yeast diets extended the fly lifespan through upregulation of superoxide dismutase (Shen et al. 2012) and protection against oxidative stress (Lee et al. 2010; Soh et al. 2013).
It is evident that the primary mode of action of nutraceuticals seems to be through scavenging of reactive oxygen species from the mitochondria and other cellular sources. In a study that attempted to assess the damage to (mitochondrial) proteins during the course of aging in flies, it was found that only aconitase activity was reduced due to its increased carbonylation and oxidative damage. Further, supplementation of fly diet with fluoroacetate—a competitive inhibitor of aconitase activity—reduced the lifespan of flies in a dose-dependent manner (Das et al. 2001), thus establishing the causal link between aconitase activity and longevity. Aconitase is an important protein in the mitochondrial matrix. Furthermore, mitochondrial protein density was shown to increase by 25 % in flies under diet restriction (DR), demonstrating the importance of the mitochondrial electron transport chain function in the lifespan extension upon DR in Drosophila (Zid et al. 2009). However, dietary restrictions are shown to genuinely extend lifespan in flies (Grandison et al. 2009) at a cost to reproduction (Partridge et al. 2005), supporting the adaptive response hypothesis involving a shift of resources during short periods of famine away from reproduction toward increased somatic maintenance (Shanley and Kirkwood 2000). If indeed the resource shifting adaptive hypothesis is true, then it is possible that the lifespan extension by nutraceuticals could also be due to diversion of energy reserves from reproduction to somatic maintenance. Unfortunately, none of the studies that have used nutraceutical supplementation have ascertained their effect on fecundity, and it is entirely possible that the fecundity of the flies was significantly reduced. Alternatively, it is possible that the mitochondrial activity is increased, thus making additional energy available for somatic maintenance and/or reproduction. Through this research, we set out to test these two not necessarily mutually exclusive hypotheses by supplementing larval diet with curcumin. Curcumin supplementation in larval diet significantly increased pre-adult viability, adult longevity, fertility, and vertical climbing ability. The increased adult life-history trait values were associated with increased levels of glycogen and pAkt and aconitase activity but decreased CG9510, AKHR, and tryglyceride levels.
Materials and methods
Fly husbandry
Wild-type Canton S strain of D. melanogaster flies used in this study were obtained from the National Drosophila Stock Center at University of Mysore, Mysore, Karnataka, India. Flies were maintained on standard banana-jaggery medium (SM) (Chandrashekara and Shakarad 2011) under standard laboratory conditions of 24 ± 1 °C temperature, 75 ± 5 % relative humidity, and 12:12 L:D cycle (SLC). Flies were maintained in a 2 week discrete generation cycle for ten generations before being used in this study. The adult density was regulated at about 100 flies per half-pint bottle with 25 mL of SM. There were a total of ten bottles. Flies from ten bottles were combined into a single breeding cage, hereafter referred to as parental cage (PC).
Preparation of curcumin-supplemented diet
A total of 2.5 L of SM was prepared following the procedure of Chandrashekara and Shakarad (2011) and split into five batches of 500 mL each. For the control group, SM was poured into the bottles. For the curcumin-supplemented media, either 5, 10, 25, or 50 μM of curcumin obtained from Sigma-Aldrich, USA, was added and mixed thoroughly just before pouring into the bottles. All bottles were plugged with nonadsorbent cotton and media was allowed to set under room temperature. All other laboratory reagents used in this study were purchased from Merck, unless otherwise mentioned.
Generation of assay flies and egg to adult viability assay
The eggs obtained from PC on a laying plate were dispensed into different treatment media bottles at a density of ∼100 eggs/bottle with 45 mL media. Ten bottles each for the five treatment groups of SM, SM + 5, 10, 25, or 50 μM curcumin were set up. All bottles were incubated at SLC till the emergence of flies. The emerging flies were sorted based on gender under light diethyl ether anesthesia and held as virgins in holding bottles containing SM till being used in further assays. The flies emerging from different treatments are designated as FA, FB, FC, FD, and FE, to indicate progeny raised on SM and SM with 5, 10, 25, or 50 μM of curcumin, respectively. All assays were carried out on SM using mated flies. The total number of flies that emerged from each bottle was used to calculate the viability of flies emerging from each treatment group.
Longevity assay of actively reproducing progeny (F) flies
The longevity of reproducing female and male FA, FB, FC, FD, and FE flies was assessed in mixed sex cultures (Handa et al. 2014). The flies were sorted into vials containing ∼4 mL SM under light diethyl ether anesthetization. A density of four females and four males was maintained per vial. Ten vials were set up per treatment. Three replicate sets were assayed over a period of 1 year. In total, 30 vials of four mixed sex pairs were assayed per treatment. Census was carried out on a daily basis. The surviving flies were transferred to fresh SM vials every alternate day. The dead flies were aspirated out, gender identified, and recorded. The census was continued till the death of the last fly. In total, 600 males and 600 females were used for the longevity assay.
Fertility assay
Five hundred virgin female and 500 virgin male flies from the respective holding bottles of the five treatment (FA, FB, FC, FD, and FE) groups were recombined into five independent progeny breeding cages (FCs) and allowed to mate and age. The flies in these five FCs were maintained on SM and were given fresh food every alternate day. The identity and purity of the FC was strictly observed. Fertility of the aging progeny (F) flies was assayed at 3, 10, 20, 30, 40, and 50 day adult ages. On the designated age, eggs were collected on a laying plate from FCs and dispensed into ten bottles each, at a density of 100 eggs/bottle with 45 mL SM. All the 50 bottles were incubated at SLC. The number of emerging flies from each of the ten bottles for progeny groups FA, FB, FC, FD, and FE was recorded. The effective fertility was calculated as the average of the sum total of adults that emerged. Since each of the ten replicate bottles per treatment had 100 exact eggs dispensed, the average is represented as percent fertility.
Vertical climbing assay
The ability to move against gravity and climb is suggested to indicate the level of physical fitness of test animals (Lee et al. 2010). Vertical climbing ability of male flies that emerged from different treatment bottles was assessed. Twenty male flies per treatment group were collected and transferred to the empty, 0–15 cm graduated vial. The vial was gently tapped and placed in vertical position. The number of flies that crossed the 15 cm mark in 30 s was counted. Three trials were conducted on each set of 20 flies. The vertical climbing was assessed on 3, 10, 20, 30, 40, and 50 day adult ages. The data is expressed as the percentage of flies that crossed the 15 cm mark.
Larval feeding rate assay
The eggs obtained from PC were transferred at a density of 50 eggs/6 mL SM and allowed to develop till early third instar. The early third instar larvae were removed from the SM vials and used in the feeding rate assay. The larvae were individually transferred to an assay petri plate of 5 cm diameter containing 10 mL of either liquid SM (SM without agar) or liquid SM supplemented with 10 μM of curcumin and allowed 5 s for acclimation. The feeding rate was measured as the mean number of sclerite retractions in two consecutive 10 s intervals. The average of the two rates was taken as the feeding rate of that larva. Twenty larvae were assayed for each of the two treatment groups. The feeding rate assays were replicated four times. A total of 160 larvae were assayed for feeding rate.
Egg to adult development time, dry weight, and lipid content assay
The egg to adult development time was assessed in the SM and SM supplemented with 10 μM of curcumin. Eggs obtained from PC on a laying plate were collected with the help of a moistened brush and dispensed into 10 cm (L) × 2.5 cm (D) glass vials containing 6 mL either SM or SM supplemented with 10 μM of curcumin at a density of 50 eggs per vial. Twenty such vials were set up per treatment group and incubated at SLC. Once the pupae darkened, the vials were checked every 4 h and any eclosed adults were removed and sexed and the time of their eclosion was recorded. The four hourly checks were continued until three consecutive days passed with no eclosion recorded from any vial. The egg to adult development time was calculated by taking midpoint of the egg laying window as the 0th hour and the midpoint of two successive four hourly checks as the time of emergence.
The flies from development time assay, on emergence, were etherized and sorted based on gender into groups of ten individuals and transferred to prelabeled clean empty dry vials, dried at 70 °C for 36 h, and weighed to the nearest microgram using a microbalance again to obtain dry weight. The dry flies were transferred to corresponding prelabeled 1.5 mL Eppendroff microcenrtifuge tubes containing 1.2 mL diethyl ether and put on a gel rocker set to 2,000 rpm to extract ether soluble lipids. The lipids were extracted over 36–40 h with two ether changes at 12 h intervals. After the last ether change, the flies were washed in excess of ether, dried for 2 h at room temperature, and weighed to obtain lipid-free weight of flies. The dry weight and lipid-free dry weight were used to obtain the lipid content in the flies. Five replicate vials per sex per treatment were set up. The development time, dry weight, and lipid content assay were replicated four times.
Estimation of energy content and enzyme activity
Protein preparation
Twenty female flies were collected from each treatment. Protein extraction from the flies was conducted by following a standard protocol (Clark and Keith 1988) with modifications. In brief, flies were homogenized in 100 μL of protein extraction buffer (0.01 M Na2PO4, 1 mM EDTA, pH 7.4). The homogenates were centrifuged at 3,000 rpm for 6 min at 4 °C. Twenty-five microliters of the supernatant was added to 1.5 mL of Bradford reagent (Sigma). Absorbance was read at 600 nm. A calibration curve was obtained using bovine serum albumin (BSA) as a standard.
Estimation of glycogen
Flies were killed by rapid immersion in >95 °C water without prior modification to their behavior so as to denature enzymes and prevent postmortem degradation of glycogen. Animals were then stored overnight at −70 °C. The tissue from 20 female flies was pooled for the assay of glycogen. Glycogen levels were determined as described by using a standard protocol (Palanker et al. 2009) with minor modifications. In brief, flies were homogenized in ice-cold homogenization buffer (20 mM HEPES, 0.2 % sodium azide, 0.2 mM PMSF, and 1 mM EDTA, pH 7.0) and centrifuged at 17,000 rpm for 3 min at 4 °C, and 25 μL of the supernatant was incubated with 100 μL glucose reagent (Sigma) to measure the glucose level, or 100 μL glucose reagent and 0.5 U amyloglucosidase (Sigma) to measure the glycogen level. The samples were incubated at 37 °C for 30 min and the absorbance was measured at 540 nm. Glucose and glucose plus glycogen amounts were determined using a standard curve and were normalized to the amount of protein. The amount of glycogen was determined by subtracting the amount of glucose from the glucose plus glycogen level. Each sample was assayed thrice and the mean used in the statistical analysis.
Triglyceride measurements
Triglyceride levels were determined by using a standard protocol (Palanker et al. 2009) with minor modifications. Twenty female flies were homogenized in 100 μL phosphate buffered saline (PBS) and 0.5 % Tween 20 and immediately incubated at 70 °C for 5 min. The heat-treated homogenate was centrifuged, and 25 μL supernatant was incubated with Triglyceride Reagent (Sigma) for 30 min at 37 °C. Samples were then incubated with Free Glycerol Reagent (Sigma) for 5 min at 37 °C and assayed at 525 nm using a Beckman DU 640 spectrophotometer. Triglyceride levels were normalized to protein amounts in each homogenate and experiments were repeated three times.
Measurement of aconitase activity
Since the fat bodies are known to impede in the enzyme assays, we have used mated male flies for assaying aconitase activity. We followed the protocol of Das et al. (2001) to measure the aconitase activity. The activity of aconitase (EC 4.2.1.3) is measured on the basis of the conversion of citrate into α-oxoglutarate in association with the reduction of NADP, as described by Kennedy et al. (1983). The mitochondrial pellets (obtained by following the methods of Das et al. 2001) were sonicated four times in a Branson 2200 sonicator for 30 s each in a buffer consisting of 154 mM Tris-HCl, pH 7.4, and 5 mM citrate. The reaction mixture consisted of 25 μg protein in 27 mM Tris-HCl, pH 7.4, 5 mM sodium citrate, 0.2 mM NADP, 0.6 mM MnCl2, and 1 U of isocitrate dehydrogenase. One unit of enzyme activity is defined as the amount of enzyme that catalyzes the formation of 1 μmol of isocitrate from citrate per min at pH 7.4 at 30 °C. The formation of NADPH was followed over time at 30 °C at a wavelength of 340 nm using a Beckman DU 640 spectrophotometer (Das et al. 2001).
Estimation of pAkt/Akt
An equal number of control and curcumin-treated flies were stored in prechilled PBS pH 7.4 containing protease inhibitors (Sigma) at −20 °C till further use. Extracts were prepared by homogenization (Kimble/Kontes Motor Cordless 749540-0000) followed by centrifugation at 4,000 rpm for 5 min at 4 °C. Supernatants were transferred to fresh tubes and quantified using Bradford reagent (Sigma). An equal amount of extracts from the control and curcumin treated flies was run on SDS-PAGE at 150 V for 45 min. Gel was transferred to nitrocellulose membrane (Amersham) using Mini Trans-Blot cell apparatus (Bio-Rad) for 2 h at 50 V. For Western blotting, the membrane was incubated with PBS containing 3 % BSA followed by overnight incubation at 4 °C with primary antibodies anti-rabbit Akt (1:1,000) from Thermo Scientific and anti-rabbit pAkt (1:1,000) donated by Dr. Rajesh Rajaiah of Maryland University. The following day, the membrane was washed thrice with PBS containing 0.01 % of Tween-20 followed by incubation with secondary anti-rabbit HRP 1:1,000 (Sigma) for 1 h at RT. Thereafter, the membrane was again washed with PBS containing 0.01 % of Tween-20 prior to development. Blot was developed using Luminol reagent (Millipore) as per the manufacturer’s instructions. The relative band intensity ratio (wherever mentioned) was calculated using NIH ImageJ software.
Total RNA isolation and semiconservative PCR of AKHR, CG9510, and RPL32
Total RNA isolation was done from an equal number of control and curcumin-treated flies. cDNA was freshly prepared from quantified RNA as per manual’s instructions (Thermo Scientific). PCR was performed using AKHR fwd: 5′-TCCATCACCGTGTACAGCAT-3′ and AKHR rev: 5′-GAGCGATATGCAGACCATCA-3′ and CG9510 fwd: 5′-GGTTGTACGACACAGTGAAG-3′ and CG9510 rev: 5′-CTGGCGTCCAAATCATCC-3′ (Iijima et al. 2009; Heinrichsen et al. 2013). Normalization was done using RPL32 gene with fwd: 5′-CCGCTTCAAGGGACAGTATC-3′ and rev: 5′-GACAATCTCCTTGCGCTTCT-3′ primers.
Statistical analyses
In all cases other than survival function analysis, the means were used as the units of analysis. One-way analysis of variance was used to assess the significance of difference between means. The difference among treatment means was compared using Tukey-Kramer minimum significant difference (MSDα0.05) test (Sokal and Rohlf 1995). The difference between adult survival curves was analyzed using Kaplan-Meier log-rank test (Fisher and van Belle 1993). Graphical presentations of all results except survival probability values are indicated as mean + SEM.
Results
Egg to adult viability
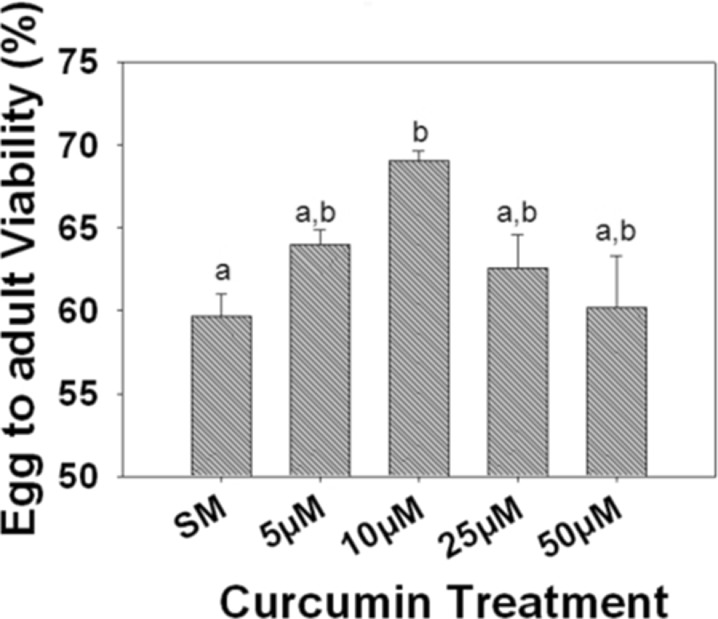
There was a significant main effect of treatment on the egg to adult viability (F4, 10 = 4.2797, p = 0.0383). The viability was lowest in the SM when compared to the SM supplemented with different concentrations of curcumin. Further, supplementing SM with 10 μM of curcumin resulted in a significantly higher viability when compared to the viability in SM but not significantly different from the viability in SM supplemented with other concentrations of curcumin (Fig. 1).
Fig. 1.
Mean (±SE) egg to adult viability. There was a significant effect of curcumin treatment on egg to adult viability (F 4, 8 = 4.2797, p = 0.038). Viability was significantly higher in media supplemented with 10 μM curcumin. Bars with the same lowercase letters are not significantly different, while those having different lowercase letters are significantly different (Tukey-Kramer MSDα0.05 = 8.8821)
Longevity of actively reproducing progeny (F) flies
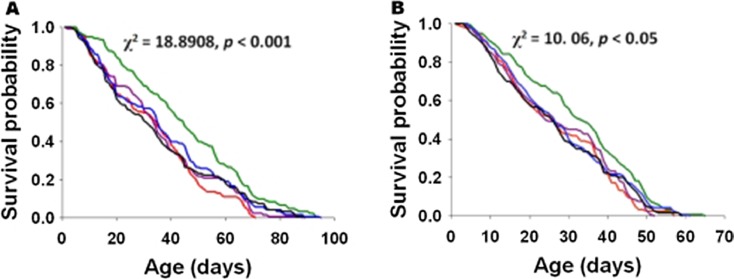
There was significant sex (F1, 2 = 165.2173, p = 0.006), treatment (F4, 8 = 17.2628, p = 0.0005), and sex × treatment interaction (F4, 8 = 29.582, p = 0.0000) effect on the average longevity (Table 1). Further, there was a significant sex and treatment effect on the median (F1, 2 = 56.738, p = 0.0171 and F4, 8 = 3.8317, p = 0.0502, respectively) and maximum (F1, 2 = 28.6376, p = 0.0332 and F4, 8 = 3.9573, p = 0.0465, respectively) longevity. Average, median, and maximum are descriptive statistics that provide insight into the overall trend but do not provide insight into the biological process (Chandrashekara and Shakarad 2011). We, therefore, compared the survival probabilities of flies reared on different concentrations of curcumin. The survival rates of both female and male flies were significantly influenced by curcumin supplementation (Fig. 2).
Table 1.
Mean, median, and maximum longevity (days) of adult Canton S (Drosophila melanogaster) adults supplemented with/without curcumin. The data was analyzed using Tukey-Kramer MSDα0.05
| Treatment | Number | Female | Male | ||||
|---|---|---|---|---|---|---|---|
| Mean | Median | Maximum | Mean | Median | Maximum | ||
| SM | 120 | 33.77 | 35 | 76 | 27.14 | 26 | 57 |
| SM + 5 M | 120 | 35.67 | 34 | 88 | 27.68 | 24.5 | 53 |
| SM + 10 M | 120 | 45.37 | 45 | 95 | 32.34 | 33.5 | 65 |
| SM + 25 M | 120 | 36.99 | 35.5 | 95 | 28.21 | 27 | 61 |
| SM + 50 M | 120 | 34.86 | 31.5 | 89 | 26.81 | 26 | 59 |
| F | 23.68 | 4.489 | 3.576 | 9.82 | 2.51 | 2.67 | |
| p | 0.0001 | 0.034 | 0.059 (NS) | 0.003 | NS | NS | |
| Tukey-Kramer MSDα0.05 | 22.58a | 25.11 | 11.02 | ||||
SM standard media
aTukey-Kramer MSDα0.05 values for comparison of difference among treatments are provided
Fig. 2.
Kaplan-Meier survival probability curves for FA (red), FB (purple), FC (olive green), FD (blue), and FE (black). Comparison of age-dependent survival curves of female (A) and male (B) flies indicated highly significant differences
Fertility
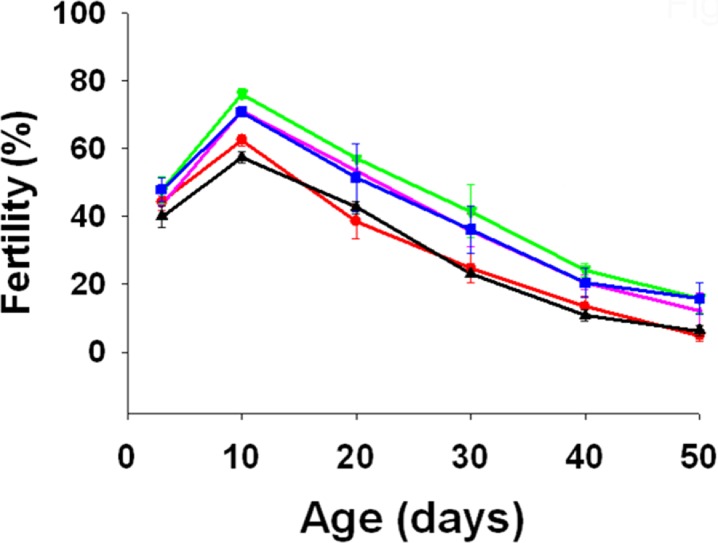
The age of the flies significantly influenced their fertility (F5, 10 = 123.0682, p = 0.0000), in that, fertility of flies declined with increasing age. However, supplementing media with curcumin significantly increased the fertility of the flies (F4, 8 = 12.7575, p = 0.0015). Overall, 10 day old flies had the highest fertility compared to other ages and FC had higher fecundity over other flies (Fig. 3).
Fig. 3.
Mean (±SE) fertility of FA (red), FB (purple), FC (olive green), FD (blue), and FE (black) at different adult ages. Curcumin supplementation significantly increased fertility (F 4, 8 = 12.7575, p = 0.0015)
Vertical climbing ability
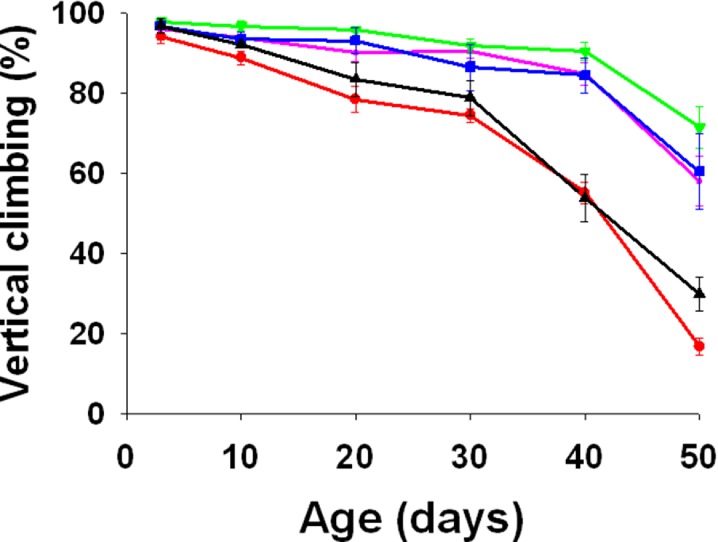
There was a significant decline in the physical activity with increasing age of the flies (F5, 10 = 91.8434, p = 0.0000). However, there was a significant increase in the physical activity of the flies due to diet supplementation with curcumin (F4, 8 = 21.271, p = 0.0003). Further, there was a significant age × treatment interaction effect (F20, 40 = 9.1018, p = 0.0000). The flies reared on low to moderate supplementation of curcumin had significantly higher activity during later ages compared to flies reared on standard media and media supplemented with 50 μM curcumin (Tukey-Kramer MSD = 16.7208), while there was no significant difference in young and middle ages (Fig. 4).
Fig. 4.
Mean (±SE) of the percentage of FA (red), FB (purple), FC (olive green), FD (blue), and FE (black) flies climbing beyond 15 cm height. Flies reared on media supplemented with low to moderate curcumin were significantly more agile during old ages than flies reared on standard medium or media with high concentration of curcumin (F 20, 40 = 9.1018, p = 0.0000)
Larval feeding rate
There was no significant effect of curcumin supplementation on larval feeding rate (F1, 3 = 0.3738, p = 0.584; Supplementary Fig. 1).
Egg to adult development time, dry weight, and lipid content
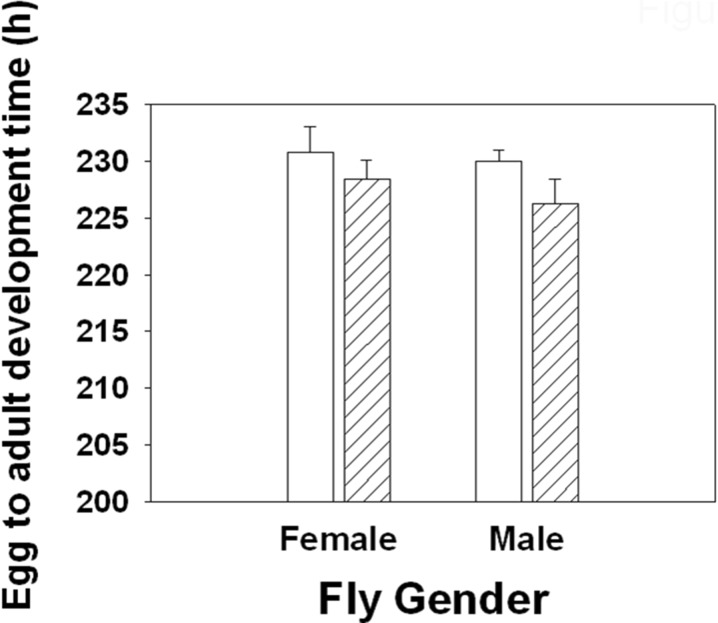
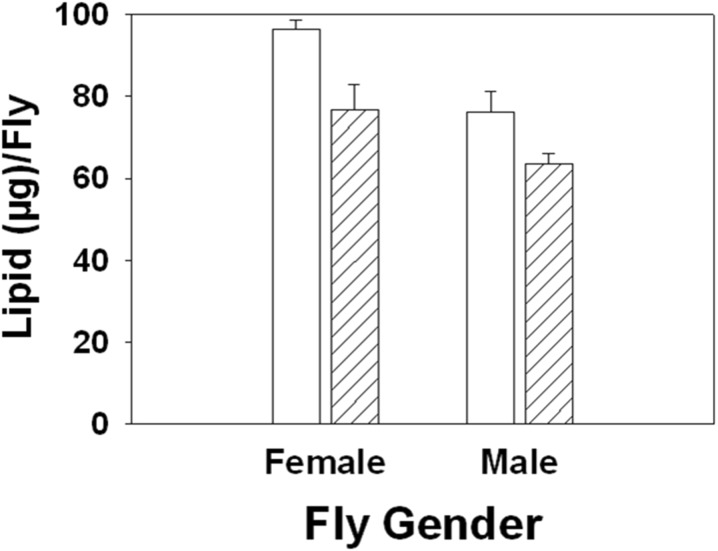
Curcumin supplementation significantly reduced the development time (F1, 3 = 9.736, p = 0.05). On average, FC completed development 3 h earlier than FA (Fig. 5). However, curcumin supplementation had no significant effect on the dry weight of the flies (F1, 3 = 0.018, p = 0.903; Supplementary Fig. 2) but had a significant effect on the lipid content at emergence (F1, 3 = 16.971, p = 0.026). Overall, FC had a lower lipid level than FA (Fig. 6).
Fig. 5.
Mean (±SE) of egg to adult development time of female and male flies raised on SM and SM + 10 μM curcumin. Curcumin supplementation significantly decreased development time (F 1, 3 = 9.736, p = 0.05)
Fig. 6.
Mean (±SE) of lipid content (at emergence) in female and male flies raised on SM and SM + 10 μM curcumin. Curcumin supplementation significantly decreased lipid content (F 1, 3 = 16.971, p = 0.026)
Energy molecules and enzyme activity
Glycogen content
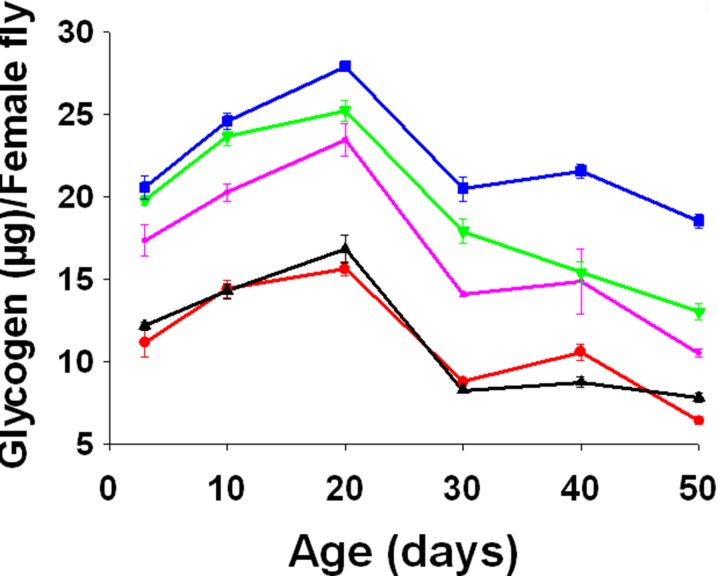
There was a significant age (F5, 10 = 262.1424, p = 0.0000), treatment (F4, 8 = 258.2476, p = 0.0000), and age × treatment interaction (F20, 40 = 3.5477, p = 0.0003) effect on the glycogen content. The FB, FC, and FD flies had significantly higher glycogen compared to FA and FE flies (Tukey-Kramer MSD = 3.4088) (Fig. 7).
Fig. 7.
Mean (±SE) of glycogen content per female fly at different adult ages in FA (red), FB (purple), FC (olive green), FD (blue), and FE (black). Curcumin supplementation significantly increased glycogen content (F 4, 8 = 258.2476, p = 0.0000)
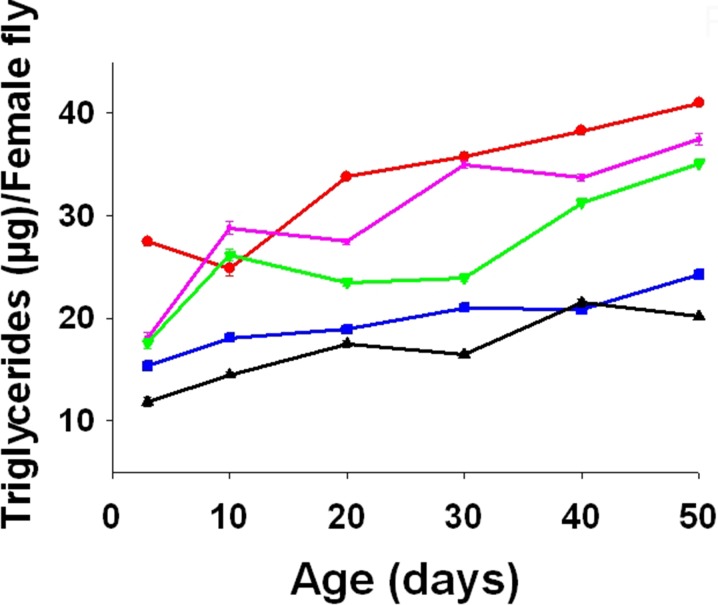
Triglyceride content
There was a significant age (F5, 10 = 41.2765, p = 0.0000), treatment (F4, 8 = 56.5896, p = 0.0000), and age × treatment interaction (F20, 40 = 5.7977, p = 0.0000) effect on triglyceride content (Tukey-Kramer MSD = 1.6509). The triglyceride content was inversely related to the curcumin concentration in the media (Fig. 8).
Fig. 8.
Mean (±SE) of triglycerides content per female fly at different adult ages in FA (red), FB (purple), FC (olive green), FD (blue), and FE (black). Curcumin supplementation significantly decreased triglycerides content (F 4, 8 = 56.5896, p = 0.0000)
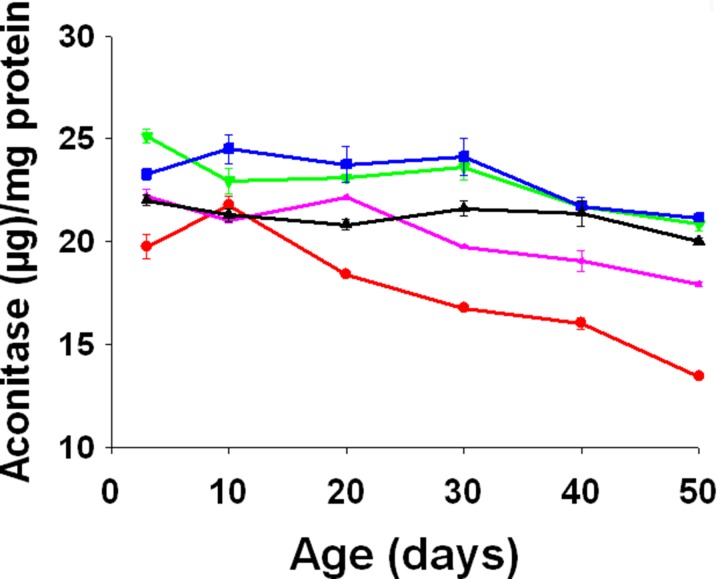
Aconitase activity
There was a significant age (F5, 10 = 97.2914, p = 0.0000), treatment (F4, 8 = 160.5529, p = 0.0000), and age × treatment interaction (F20, 40 = 7.2945, p = 0.0000) effect on the aconitase activity. The FC flies had significantly higher aconitase activity compared to FA and FE flies during early life (Tukey-Kramer MSD = 2.4513) (Fig. 9).
Fig. 9.
Mean (±SE) of aconitase content per milligram of protein at different adult ages in FA (red), FB (purple), FC (olive green), FD (blue), and FE (black). Curcumin supplementation significantly increased aconitase content (F 4, 8 = 160.5529, p = 0.0000)
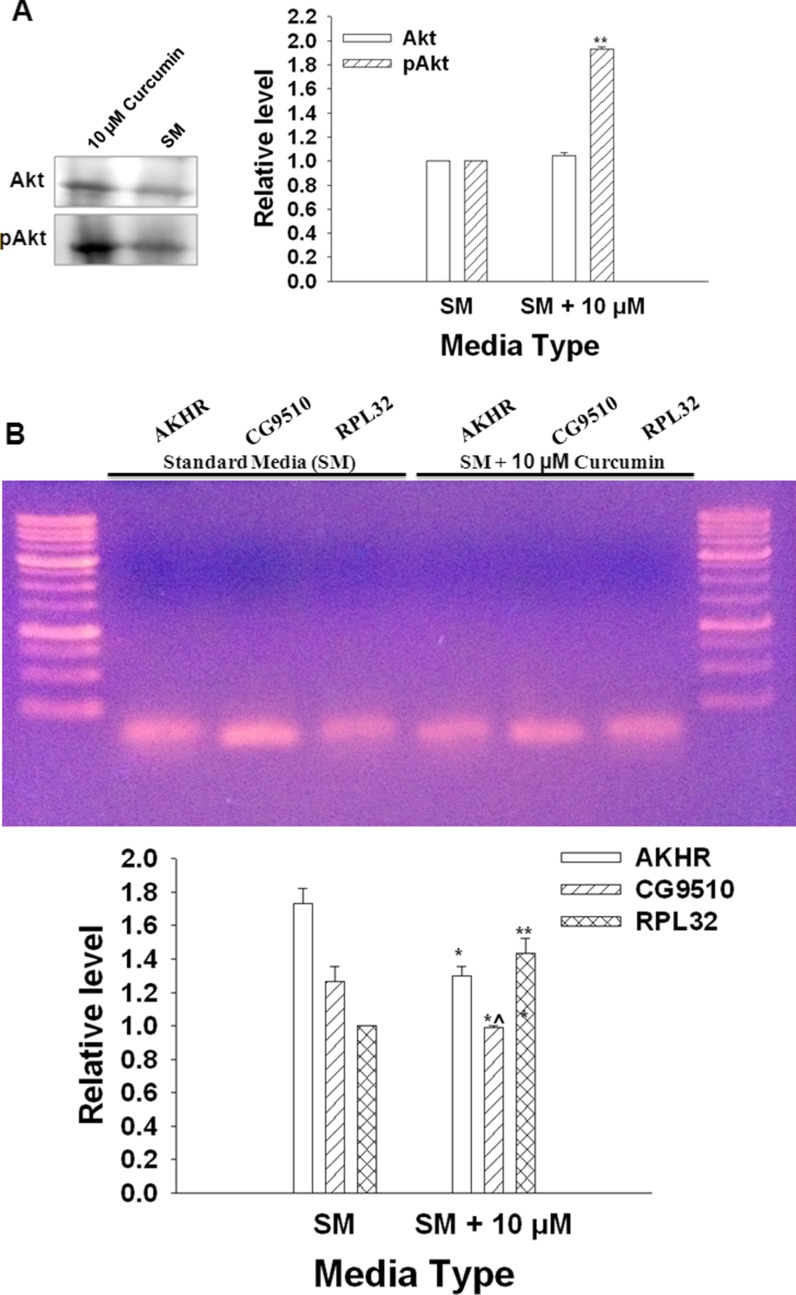
pAkt/Akt ratio
The pAkt/Akt ratio was significantly higher in adult flies that emerged from media supplemented with 10 μM curcumin (FC) than those that emerged from SM (FA) (Fig. 10A).
Fig. 10.
A The Western blot indicating comparative levels of Akt and pAkt proteins in adult flies (at emergence) raised on SM and SM + 10 μM curcumin. The pAkt levels (1.93 ± 0.021) are significantly higher (n = 3, **p < 0.001) in the flies raised on media supplemented with 10 μM curcumin compared to Akt level (1.046 ± 0.020). B Expression levels of AKHR, CG9510, and RPL32 (semiconservative PCR method). AKHR and CG9510 levels were normalized to RPL32 levels. There was a significant decline in the expression of AKHR (0.99 ± 0.012) and CG9510 (1.3 ± 0.02), even though the level of RPL32 (1.433 ± 0.088) was significantly higher due to curcumin treatment (n = 3, **p < 0.001, *^p < 0.02, *p < 0.05, Student’s t test). The corresponding levels in the flies raised on SM were AKHR (1.267 ± 0.088), CG9510 (1.733 ± 0.088), and RPL32 (1.0 ± 0.00)
AKHR and CG9510 expression
There was a significant decrease in the expression levels of AKHR and CG9510 levels in FC flies as compared to FA flies (Fig. 10B).
Discussion
Lifespan—the time lag between birth and death of an organism—is an important evolutionary adaptation (Carey 2003). Several studies in the past have successfully enhanced the lifespan of model organisms by dietary supplementation with reactive oxygen species (ROS)-scavenging substances (Howitz et al. 2003; Wood et al. 2004). However, in most diet manipulation studies (Chippindale et al. 1993; Chapman and Partridge 1996; Tu and Tatar 2003) barring that of Chandrashekara and Shakarad (2011), the correlated response to increased longevity has been the reduced fecundity. Any manipulation that reduces fecundity is going to affect the fitness of the organism(s). Further, the current lifestyle changes demand that we identify and evaluate molecules that could potentially increase reproductive health span. In the present study, low to moderate curcumin supplementation of D. melanogaster larval diet significantly increased lifespan. The increased lifespan was not due to increased body weight or energy levels (Supplementary Fig. 2 and Fig. 6) or decreased fertility; in contrast, FC flies had higher fertility (Fig. 3). Soh et al. (2013) also reported increased fertility in flies fed with curcumin during larval stages. Lifespan extension by curcumin is associated with lower time-dependent death rate (Fig. 2). Lifespan extension due to lower rate of time-dependent death rate as a result of resveratrol (Chandrashekara and Shakarad 2011; Wang et al. 2013) and A. vera extract (Chandrashekara and Shakarad 2011) and curcumin (Lee et al. 2010; Shen et al. 2012; Soh et al. 2013) supplementation was reported for independent D. melanogaster populations whose genetic backgrounds were either different or the same from those used in the present study. In the present study, the gender of the flies influenced the magnitude of response to curcumin supplementation. Sex and genetic background-dependent lifespan extension by curcumin has also been observed by Lee et al. (2010). Curcumin-supplemented diet leads to an increase in superoxide dismutase activity (Shen et al. 2012), suggesting the better scavenging of ROS. However, the progressive decline in the probability of survival with increasing age is mainly attributed to damage of macromolecules (Martin and Grotewiel 2006; Valko et al. 2007) leading to malfunction of the mitochondria (Partridge and Gems 2002), thus hampering energy production and availability. Aconitase—a key mitochondrial protein—activity was reduced both in aging Drosophila (Das et al. 2001) and mice (Yarian et al. 2006). Flies given curcumin-supplemented diet had significantly higher aconitase than flies given nonsupplemented diet (Fig. 7). An increase in the amount of aconitase could possibly have increased the glycolysis and citric acid cycle, thereby ensuring the normal flow of electrons and thus making additional energy available for somatic maintenance and/or reproduction. Curcumin was shown to readily transfer electron or donate H-atom two phenolic sites to scavenge free radicals in animal cell cultures (Barzegar and Moosavi-Movahedi 2011). Further, Soh et al. (2013) showed that flies raised on curcumin-supplemented diet resisted lethal doses of paraquat in a stage-specific manner.
Insects have to expend energy constantly, and triglycerides and glycogen are the energy reserves in animal cells (Arrese and Soulages 2010). Flies reared on curcumin-supplemented diet had significantly higher glycogen (Fig. 7)—a polymeric form of glucose—that can be readily degraded to be used as glycolytic fuel (Steele 1982) as indicated by higher pAkt/Akt ratio (Fig. 10A). However, other studies showed that loss of function in Akt prolonged lifespan in mice (Nojima et al. 2013) and humans (Mercken et al. 2013). Although Akt is known to play important roles in the signaling pathways of several cellular functions including nutrient metabolism, cell growth, apoptosis, and survival (Song et al. 2005), none of the seven Akt1 single-nucleotide polymorphisms (SNPs) studied were significantly associated with longevity in humans (Nygaard et al. 2013). The difference in our results and those of Nojima et al. (2013) and Mercken et al. (2013) could be due to different experimental organisms with greatly differing genetic architecture and/or due to specific effects of curcumin. Contrary to earlier findings of reduced CG9510 (Heinrichsen et al. 2013) and AKHR (Iijima et al. 2009) activity leading to increased triglyceride levels, flies from FC had significantly lower triglycerides (Fig. 8) despite having reduced CG9510 and AKHR activity (Fig. 10B), suggesting the increased hydrolysis of triglycerides through activation of lipases by lipid storage droplet protein-1 (Patel et al. 2005; Arrese et al. 2006). Our results clearly indicate that curcumin specifically seems to enhance the energy utilization mechanisms rather than energy accumulation processes. The beneficial effects of curcumin supplementation are further borne out by the increased number of viable progeny (Fig. 3) perhaps due to improved vitellogenesis and/or improved microtubule network during meiosis in aging oocytes, as increasing maternal age leads to decline in vitellogenesis (Carlson and Harshman 1999) and weakening of microtubule network in aging oocytes (Schatten et al. 1999). Further, flies reared on curcumin-supplemented diet had significantly greater vertical climbing ability (Fig. 4) especially at older ages suggesting better general health condition of the flies, perhaps due to improved neuromuscular organization (Soh et al. 2013) and neuroprotective activity as in the case of resveratrol (Wang et al. 2002, 2004; Araki et al. 2004; Han et al. 2004; Parker et al. 2005; Mokni et al. 2007; Chandrashekara and Shakarad 2011). Alzheimer’s disease model genotypes of Drosophila adult flies showed up to 75 % improved lifespan and locomotor activity and reduced neurotoxicity when fed with curcumin-supplemented diet throughout adult life (Caesar et al. 2012). The improved health condition of the flies given curcumin-supplemented diet is also reflected by increased egg to adult viability. An independent study using flies with different genetic backgrounds and different diet compositions also reported that curcumin supplementation had no detrimental effect on egg-hatching rate (Soh et al. 2013).
In conclusion, our results suggests that curcumin supplementation not only enhances ROS scavenging (Barzegar and Moosavi-Movahedi 2011; Shen et al. 2012), neuroprotection (Caesar et al. 2012), and neuromuscular organization (Soh et al. 2013) but also increases the efficiency of enzymes involved in energy metabolism and, thus, makes additional energy available for somatic maintenance and/or reproduction, truly making it the sanjeevani—infusing life not only into those that are fed with curcumin but into their progeny that are raised on curcumin-unsupplemented diet as well.
Electronic supplementary material
(GIF 83 kb)
(GIF 73 kb)
Acknowledgments
We thank two anonymous referees for their helpful comments on the earlier versions of the manuscript and suggesting additional experiments on pAkt/Akt ratio and fat metabolism related genes. We also thank Dr. Rajesh Rajaiah, Department of Microbiology and Immunology, University of Maryland, USA, for providing us the pAKT antibody; Dr. Rajagopal Raman, Gut Biology Laboratory, Department of Zoology, University of Delhi; Dr. K. Natarajan, Ambedkar Centre for Biomedical Research, University of Delhi for providing the chemicals, buffers, and instruments required in Akt/pAkt, AKHR, and CG9510 Western blot and PCR experiments; and Ms. Nalini Mishra and numerous research trainees for their assistance in the research. MS thanks the University of Delhi and Council of Scientific and Industrial Research, Government of India, for financial assistance. SP thanks the University Grants Commission, Government of India, for DSKPDF fellowship.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
K.T.C., S.P., and M.S. designed the research; K.T.C and S.P. performed the research; and K.T.C., S.P., and M.S. analyzed the data and wrote the paper.
Abbreviations
- DR
Diet restriction
- SM
Standard banana-jaggery medium
- SLC
Standard laboratory conditions
- RT
Room temperature
- PC
Parental fly cage
- FC
Progeny fly cage
- FA
Progeny raised on SM
- FB
Progeny raised on SM supplemented with 5 μM curcumin
- FC
Progeny raised on SM supplemented with 10 μM curcumin
- FD
Progeny raised on SM supplemented with 25 μM curcumin
- FE
Progeny raised on SM supplemented with 50 μM curcumin
References
- Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- Arrese EL, Soulages JL. Insect fat body: energy, metabolism, and regulation. Annu Rev Entomol. 2010;55:207–255. doi: 10.1146/annurev-ento-112408-085356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrese EL, Patel RT, Soulages JL. The main triglyceride-lipase from the insect fat body is an active phospholipase A(1): identification and characterization. J Lipid Res. 2006;47:2656–2667. doi: 10.1194/jlr.M600161-JLR200. [DOI] [PubMed] [Google Scholar]
- Barzegar A, Moosavi-Movahedi AA. Intracellular ROS protection efficiency and free radical-scavenging activity of curcumin. PLoS ONE. 2011;6(10):e26012. doi: 10.1371/journal.pone.0026012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caesar I, Jonson M, Nilsson KPR, Thor S, Hammarström P. Curcumin promotes A-beta fibrillation and reduces neurotoxicity in transgenic Drosophila. PLoS ONE. 2012;7(2):e31424. doi: 10.1371/journal.pone.0031424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JR (2003) Life span: a conceptual overview. In: Carey JR, Tuljapurkar S (eds) Life span: evolutionary, ecological, and demographic perspectives. Population Council and Development Review 29(Suppl):1–18
- Carlson KA, Harshman LG. Extended longevity lines of Drosophila melanogaster: characterization of oocyte stages and ovariole numbers as a function of age and diet. J Gerontol A Biol. 1999;54:B432–B440. doi: 10.1093/gerona/54.10.B432. [DOI] [PubMed] [Google Scholar]
- Chandrashekara KT, Shakarad MN (2011) Aloe vera or resveratrol supplementation in larval diet delays adult aging in the fruit fly, Drosophila melanogaster. J Gerontol A Biol Sci Med Sci 66(9):965–971 [DOI] [PubMed]
- Chapman T, Partridge L. Female fitness in Drosophila melanogaster: an interaction between the effect of nutrition and of encounter rate with males. Proc R Soc Lond B Biol Sci. 1996;263:755–759. doi: 10.1098/rspb.1996.0113. [DOI] [PubMed] [Google Scholar]
- Chippindale AK, Leroi AM, Kim SB, Rose MR. Phenotypic plasticity and selection in Drosophila life history evolution. 1. Nutrition and the cost of reproduction. J Evol Biol. 1993;6:171–193. doi: 10.1046/j.1420-9101.1993.6020171.x. [DOI] [Google Scholar]
- Clark AG, Keith LE. Variation among extracted lines of Drosophila melanogaster in triacylglycerol and carbohydrate storage. Genetics. 1988;119:595–607. doi: 10.1093/genetics/119.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das N, Levine RL, Orr WC, Sohal RS. Selectivity of protein oxidative damage during aging in Drosophila melanogaster. Biochem J. 2001;360:209–216. doi: 10.1042/0264-6021:3600209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Guha S, Sun X, Cao M, Wang X, Zou S. Nutraceutical interventions for promoting healthy aging in invertebrate models. Oxidative Med Cell Longev. 2012 doi: 10.1155/2012/718491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg T, Knauer H, Schauer A, Büttner S, Ruckenstuhl C, Carmona-Gutierrez D, Ring J, Schroeder S, Magnes C, Antonacci L, Fussi H, Deszcz L, Hartl R, Schraml E, Criollo A, Megalou E, Weiskopf D, Laun P, Heeren G, Breitenbach M, Grubeck-Loebenstein B, Herker E, Fahrenkrog B, Fröhlich KU, Sinner F, Tavernarakis N, Minois N, Kroemer G, Madeo F. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11(11):1305–1314. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- Fisher LD, van Belle G. Biostatistics: a methodology for health sciences. New York: Wiley; 1993. pp. 786–843. [Google Scholar]
- Grandison RC, Piper MD, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462:1061–1064. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YS, Zheng WH, Bastianetto S, Chabot JG, Quirion R. Neuroprotective effects of resveratrol against beta-amyloid-induced neurotoxicity in rat hippocampal neurons: involvement of protein kinase C. Br J Pharmacol. 2004;141:997–1005. doi: 10.1038/sj.bjp.0705688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa J, Chandrashekara KT, Kashyap K, Sageena G, Shakarad MN (2014) Gender based disruptive selection maintains body size polymorphism in Drosophila melanogaster. J Biosci 39:609–620. doi:10.1007/s12038-014-9452-x [DOI] [PubMed]
- Heinrichsen ET, Zhang RJE, Ngo J, Diop S, Bodmer R, Joiner WJ, Metallo CM, Haddad GG (2013) Metabolic and transcriptional response to a high-fat diet in Drosophila melanogaster. Mol Metabol 3:42–54. doi. 10.1007/s12038-014-9452-x [DOI] [PMC free article] [PubMed]
- Hercus MJ, Hoffmann AA. Maternal and grandmaternal age influence offspring fitness in Drosophila. Proc R Soc Lond B. 2000;267:2105–2110. doi: 10.1098/rspb.2000.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Iijima K, Zhao L, Shenton C, Iijima-Ando K. Regulation of energy stores and feeding by neuronal and peripheral CREB activity in Drosophila. PLoS ONE. 2009;4(12):e8498. doi: 10.1371/journal.pone.0008498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MC, Emptage MH, Dreyer JL, Beinert H. The role of iron in activation-inactivation of aconitase. J Biol Chem. 1983;258:11098–11105. [PubMed] [Google Scholar]
- Kenyon CJ. The genetics of aging. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Kern S, Ackermann M, Stearns SC, Kawecki TJ. Decline in offspring viability as a manifestation of aging in Drosophila melanogaster. Evolution. 2001;55(9):1822–1831. doi: 10.1111/j.0014-3820.2001.tb00831.x. [DOI] [PubMed] [Google Scholar]
- Lee KS, Lee BS, Semnani S, Avanesian A, Um CY, Jeon HJ, Seong KM, Yu K, Min KJ, Jafari M. Curcumin extends lifespan, improves health span, and modulates the expression of age-associated aging genes in Drosophila melanogaster. Rejuvenation Res. 2010;13(5):561–570. doi: 10.1089/rej.2010.1031. [DOI] [PubMed] [Google Scholar]
- Li YM, Chan HYE, Huang Y, Chen ZY. Green tea catechins upregulate superoxide dismutase and catalase in fruit flies. Mol Nutri Food Res. 2007;51(5):546–554. doi: 10.1002/mnfr.200600238. [DOI] [PubMed] [Google Scholar]
- Li YM, Chan HYE, Yao XQ, Huang Y, Chen ZY. Green tea catechins and broccoli reduce fat-induced mortality in Drosophila melanogaster. J Nutri Biochem. 2008;19(6):376–383. doi: 10.1016/j.jnutbio.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Martin I, Grotewiel MS. Oxidative damage and age related functional declines. Mech Ageing Dev. 2006;127(5):411–423. doi: 10.1016/j.mad.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Mercken EM, Crosby SD, Lamming DW, JeBailey L, Krzysik-Walker S, Villareal DT, Capri M, Franceschi C, Zhang Y, Becker K, Sabatini DM, de Cabo R, Fontana L. Calorie restriction in humans inhibits the PI3K/AKT pathway and induces a younger transcription profile. Aging Cell. 2013;12:645–651. doi: 10.1111/acel.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokni M, Elkahoui S, Limam F, Amri M, Aouani E. Effect of resveratrol on antioxidant enzyme activities in the brain of healthy rat. Neurochem Res. 2007;32:981–987. doi: 10.1007/s11064-006-9255-z. [DOI] [PubMed] [Google Scholar]
- Nojima A, Yamashita M, Yoshida Y, Shimizu I, Ichimiya H, Kamimura N, Kobayashi Y, Ohta S, Ishii N, Minamino T. Haploinsufficiency of Akt1 prolongs the lifespan of mice. PLoS ONE. 2013;8(7):e69178. doi: 10.1371/journal.pone.0069178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygaard M, Soerensen M, Flachsbart F, Mengel-From J, Tan Q, Schreiber S, Nebel A, Christensen K, Christiansen L. Akt1 fails to replicate as a longevity-associated gene in Danish and German nonagenarians and centenarians. Eu J Human Genet. 2013;21:574–577. doi: 10.1038/ejhg.2012.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanker L, Tennessen JM, Lam G, Thummel CS. Drosophila HNF4 regulates lipid mobilization and beta-oxidation. Cell Metab. 2009;9(3):228–239. doi: 10.1016/j.cmet.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JA, Arango M, Abderrahmane S, Lambert E, Tourette C, Catoire H, Néri C. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nat Genet. 2005;37:349–350. doi: 10.1038/ng1534. [DOI] [PubMed] [Google Scholar]
- Partridge L, Gems D. Mechanisms of ageing: public or private? Nature Rev Genet. 2002;3:165–175. doi: 10.1038/nrg753. [DOI] [PubMed] [Google Scholar]
- Partridge L, Piper MDW, Mair W. Dietary restriction in Drosophila. Mech Ageing Dev. 2005;126:938–950. doi: 10.1016/j.mad.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Patel RT, Soulages JL, Hariharasundaram B, Arrese EL. Activation of the lipid droplet controls the rate of lipolysis of triglycerides in the insect fat body. J Biol Chem. 2005;280:22624–22631. doi: 10.1074/jbc.M413128200. [DOI] [PubMed] [Google Scholar]
- Peng C, Zuo Y, Kwan KM, Liang Y, Ma KY, Chan HYE, Huang Y, Yu H, Chen ZY. Blueberry extract prolongs lifespan of Drosophila melanogaster. Exp Gerontol. 2012;47(2):170–178. doi: 10.1016/j.exger.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Sahin E, DePinho RA. Linking functional decline of telomeres, mitochondria and stem cells during aging. Nature. 2010;464:520–528. doi: 10.1038/nature08982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatten H, Chakrabarti A, Hedrick J. Centrosome and microtubule instability in aging Drosophila cells. J Cell Biochem. 1999;74:229–241. doi: 10.1002/(SICI)1097-4644(19990801)74:2<229::AID-JCB9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Shanley DP, Kirkwood TBL. Calorie restriction and aging: a life-history analysis. Evolution. 2000;54(3):740–750. doi: 10.1111/j.0014-3820.2000.tb00076.x. [DOI] [PubMed] [Google Scholar]
- Shen LR, Xiao F, Yuan P, Chen Y, Gao QK, Parnell LD, Meydani M, Ordovas JM, Li D, Lai CQ. Curcumin-supplemented diets increase superoxide dismutase activity and mean lifespan in Drosophila. Age. 2012 doi: 10.1007/s11357-012-9438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh JW, Marowsky N, Nichols TJ, Rahman AM, Miah T, Sarao P, Khasawneh R, Unnikrishan A, Heydari AR, Silver RB, Arking R. Curcumin is an early-acting stage specific inducer of extended functional longevity in Drosophila. Exp Gerontol. 2013;48:229–239. doi: 10.1016/j.exger.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf JF. Biometry: the principles and practices of statistics in biological sciences. New York: WH Freeman; 1995. pp. 179–450. [Google Scholar]
- Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele JE. Glycogen-phosphorylase in insects. Insect Biochem. 1982;12:131–147. doi: 10.1016/0020-1790(82)90001-4. [DOI] [Google Scholar]
- Tatar M, Post S, Yu K (2014) Nutrient control of Drosophila longevity. TEM. http://dx.doi.org/10.1016/j.tem.2014.02.006 [DOI] [PMC free article] [PubMed]
- Tu MP, Tatar M. Juvenile diet restriction and the aging and reproduction of adult Drosophila melanogaster. Aging Cell. 2003;2:327–333. doi: 10.1046/j.1474-9728.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell B. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Vaupel JW. Biodemography of human aging. Nature. 2010;464:536–542. doi: 10.1038/nature08984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Xu J, Rottinghaus GE, Simonyai A, Lubahn D, Sun GY, Sun AY. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res. 2002;958:439–447. doi: 10.1016/S0006-8993(02)03543-6. [DOI] [PubMed] [Google Scholar]
- Wang Q, Yu S, Simonyai A, Rottinghaus GE, Sun GY, Sun AY. Resveratrol protects against neurotoxicity induced by kainic acid. Neurochem Res. 2004;29:2105–2112. doi: 10.1007/s11064-004-6883-z. [DOI] [PubMed] [Google Scholar]
- Wang C, Wheeler CT, Alberico T, Sun X, Seeberger J, Laslo M, Spangler E, Kern B, de Cabo R, Zou S. The effect of resveratrol on lifespan depends on both gender and dietary nutrient composition in Drosophila melanogaster. Age. 2013;35:69–81. doi: 10.1007/s11357-011-9332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D (2004) Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 430:686–689 [DOI] [PubMed]
- Yarian CS, Toroser D, Sohal RS. Aconitase is the main functional target of aging in the citric acid cycle of kidney mitochondria from mice. Mech Age Dev. 2006;127:79–84. doi: 10.1016/j.mad.2005.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zid BM, Rogers AN, Katewa SD, Vargas MA, Kolipinski MC, Lu TA, Benzer S, Kapahi P. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell. 2009;139:149–160. doi: 10.1016/j.cell.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(GIF 83 kb)
(GIF 73 kb)