Abstract
S-allyl cysteine (SAC) is the most abundant compound in aged garlic extracts (AGEs). AGE has been reported to ameliorate the oxidative damage implicated in a variety of diseases. However, the effects of SAC have not been established in liver cirrhosis. The aim of this study was to examine the effect of therapeutic administration of SAC in liver cirrhosis by chronic carbon tetrachloride (CCl4) administration in rats. SAC or other cysteine compounds were administered from 4 weeks when liver fibrosis was confirmed to be in process. CCl4 administration elevated plasma alanine aminotransferase, plasma lipid peroxidation, liver hydroxyproline, and liver transforming growth factor (TGF)-β at 12 weeks. SAC prevented these changes induced by CCl4. Furthermore, SAC improved survival in a dose-dependent manner following consecutive CCl4 administration. The inhibitory mechanisms may be associated with a decrease in the profibrogenic cytokine, TGF-β as well as the antioxidative properties of SAC.
Keywords: aged garlic extracts, carbon tetrachloride, cirrhosis, S-allyl cysteine, TGF-β
Introduction
Liver fibrosis and cirrhosis, its end-stage sequelae, represent a major worldwide health problem. However, there is no appropriate drug for treating liver cirrhosis in safe for a long time. Garlic has been used as a traditional medicine for hundreds of years, and scientific studies have shown that it prevents thrombosis as well as inhibits inflammation and cellular oxidative stress.(1–3) However, the effective molecules in garlic and their pharmacological actions have not been elucidated clearly. Substantial evidence shows that aged garlic extract (AGE) ameliorates the oxidative damage implicated in aging and a variety of diseases such as cancer, cardiovascular alterations, stroke, Alzheimer’s disease, and other age-related degenerative conditions. In addition, AGE has been widely studied for its high antioxidant content and protective health potential.(4) S-allyl cysteine (SAC), a constituent of (AGEs), is a stable and odorless compound. The pharmacokinetic behavior of SAC has been reported.(5) SAC has been shown to be rapidly and easily absorbed in the gastrointestinal tract and distributed mainly plasma, liver, and kidney (T1/2 ≅ 2 h), and the bioavailability was 98% in rats.(5) It has been reported that SAC was mainly excreted into urine in the N-acethyl form.(5) A large number of studies have demonstrated the antioxidant activity of AGE and SAC both in vivo, in various experimental animal models associated with oxidative stress, and in vitro using several methods to scavenge reactive oxygen species or induce oxidative damage.(4) Although it has been reported that SAC protects against doxorubicin toxicity in the liver and heart,(6) gentamicin-induced renal damage,(7) and ischemic brain damage,(8) the effects of SAC on liver fibrosis are still unknown.
We previously reported that SAC attenuates the acute liver injury, and pulmonary fibrosis(9) caused by carbon tetrachloride (CCl4) in rats. The present study aimed to examine the effects of orally administrated SAC on CCl4-induced hepatic fibrosis.
Methods
Reagents
CCl4 and collagenase were purchased from Wako Pure Chem. Ind., Ltd. (Osaka, Japan). N-acetyl cysteine (NAC), l-cysteine (CYS), Dulbecco’s modified Eagle medium (DMEM), and fetal bovine serum (FBS) were obtained from Sigma (St. Louis, MO). Other regents used were of an analytical grade.
Animals
Male Wistar rats (4 weeks old) were purchased from SLC (Shizuoka, Japan) and maintained for 1 week before experimental use. All rats were cared for under the specifications outlined in the Guiding Principles for the Care and Use of Laboratory Animals and approved by the local ethics committee on experimental animal research. CCl4 (2 ml/kg, 25% in corn oil) was intraperitoneally (i.p.) injected into rats twice a week for 12 weeks and more to determine the survival rate (starting numbers of each group: n = 20). Rats were randomly divided into seven groups at 4 weeks after CCl4 injection (Age 8 weeks). Group 1 was the sham group treated intraperitoneally with corn oil only. Group 2 was the CCl4 group treated with CCl4 and water. Groups 3, 4, and 5 were the SAC group treated with CCl4 and SAC (50, 100, or 200 mg/kg/day). Group 6 was the NAC group treated with CCl4 and NAC (600 mg/kg/day). Group 7 was the CYS group treated with CCl4 and CYS (600 mg/kg/day). Drugs such as SAC, NAC, and CYS were administered orally to rats as part of a mixed diet at the indicated dose per day. One week after the last treatment of CCl4 (5 g/kg, i.p.), the animals were sacrificed under urethane anesthesia to collect their blood. After perfusion with ice-cold saline, liver tissue samples were collected. All animals survived throughout the study (Age 16 weeks).
Samples
Blood samples were centrifuged at 12,000 g for 5 min, and the supernatant was stored at −80°C until use. Part of the left lobe was excised, fixed in 10% buffered formaldehyde, and embedded in paraffin blocks. The remaining liver was immediately frozen in liquid nitrogen and then stored at −80°C until use. Serial 4-µm liver sections were subjected to Azan-Mallory staining.
Analysis of liver enzyme activities and lipid peroxides in plasma, and hepatic malondialdehyde
Plasma enzyme activities of alanine aminotransferase (ALT) were determined using an automatic analyzer. Plasma lipid peroxides (LPO) were measured using an enzyme-linked immunosorbent assay (ELISA) kit (LPO-586TM, OXIS International, Portland, OR). An equal volume of plasma was mixed with 20 mM phosphate buffer (pH 7.4) containing 0.5 M butylated hydroxytoluene. Each admixture was centrifuged at 3,000 g for 10 min at 4°C to remove debris. An aliquot of each sample was removed to determine the protein concentration. The remainder of the sample was used to measure malondialdehyde (MDA) and 4-hydroxy-2-nonenal (HNE) levels with the BIOXYTECH® LPO-586TM assay (OXIS International) according to the manufacturer’s instructions. This assay is based on the reaction of a chromogenic reagent, N-methyl-2-phenylindole, with MDA and 4-hydroxyalkenales at 45°C, resulting in a stable chromophore with a maximal absorbance at 586 nm. Hepatic total MDA levels were analyzed by the Colorimetric TBARS Microplate Assay Kit (Oxford Biomedical Research, Inc. Oxford, MI). Briefly, the liver tissue (about 200 mg) was deproteinized with 5% sulfosalicylic acid and centrifuged at 10,000 g for 15 min at 4°C. The supernatant was used for the assay according to the manufacturer’s instructions.
Hydroxyproline contents of liver tissues
A modified method of Kivirikko et al.(10) was used to determine the hydroxyproline content of the left lobe in each rat. The left lobe was lyophilized using a freeze-drying system. Freeze-dried left lobes were hydrolyzed with 5 ml 6N HCl at 100°C for 20 h. Resultant hydrolysates were neutralized with NaOH and adjusted to pH 7–8. Aliquots (500 µl) were oxidized by incubation with 0.2 M chloramine-T solution for 120 min at 0°C, followed by addition of 3.6 M thiosulfate in boiling water for 30 min and then extraction with 3 ml toluene. Aliquots (250 µl) were reacted with Ehrlich’s reagent for 30 min at room temperature. Reacted products were measured at 550 nm on a spectrophotometer.
Western blot analysis of liver tissues
Frozen liver tissues were homogenized in sample buffer (50 mM Tris-HCl, pH 6.8, 2% sodium dodecyl sulfate (SDS), 10 mM dithiothreitol, 10% glycerol, and 1 mM phenylmethylsulfonyl fluoride). Lysates were centrifuged at 12,000 g at 4°C for 15 min. Proteins (30 µg) of soluble and precipitated fractions were electrophoretically separated by 7.5–15% SDS-polyacrylamide gel electrophoresis and then electroblotted onto polyvinylidene difluoride membranes. The membranes were blocked with 5% skim milk and then incubated with an anti-desmin polyclonal antibody (Abcam, Cambridge, UK), anti-α-SMAβ1 monoclonal antibody (Oxford Biomedical Research, Oxford, MI), anti-tissue Inhibitor of metalloproteinase 1 (TIMP-1) monoclonal antibody (R&D Systems, Minneapolis, MN), or anti-collagen-1 antibody (Abcam). Membranes were then incubated with secondary anti-rabbit, -mouse, or -goat IgG (Dako Cytomation; Kyoto, Japan), followed by incubation for 2 min with ECL solution and then exposure to X-ray film. Western blots were quantified using Scion Image ver. 1.63.
Measurement of matrix metalloproteinase activity
The activity of matrix metalloproteinases (MMPs) was detected by gelatin zymography using Novex Zymogram Gels, Tris-Glycine SDS Sample Buffer, Zymogram Renaturing Buffer, and Zymogram Developing Buffer (Invitrogen, Carlsbad, CA). In brief, after lung tissue proteins (50 µg) of soluble and precipitated fractions were electrophoretically separated on gels, the gels were incubated in renaturing buffer at room temperature for 1 h and then in developing buffer at 37°C for 16 h. To stop the reaction, a specific protease inhibitor was added to the developing buffer. Quantitative analysis of the gelatinolytic enzyme was performed using Scion Image ver. 1.63.
α-SMA and desmin immunochemistry
Liver tissues were fixed in formalin and embedded in paraffin. The tissues were cut serially into 5-µm sections, deparaffinized, and heated in a microwave in citrate buffer (0.1 M, pH 6.0) for 15 min at 600 W, and then cooled at room temperature for 60 min. The sections were treated with 0.3% H2O2 for 5 min and preincubated with 10% normal goat serum (Vector Laboratories, Burlingame, CA) in phosphate-buffered saline with Tween® 20 (PBST). Double staining for α-SMA (monoclonal mouse anti-human SMA antibody, 1:100; DAKO Cytomation, Glostrup, Denmark) and desmin (rabbit polyclonal anti-desmin antibody, 1:500; Abcam, Tokyo, Japan) was performed on each of these sections at 4°C overnight. Secondary antibodies were fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG (1:100; Sigma) or phycoerythrin (PE)-conjugated affini-pure F(ab’)2 fragment donkey anti-rabbit IgG (H + L) (1:100; Jackson Immuno Research, PA). The sections were viewed under a confocal laser scanning microscope (LSM510; Carl Zeiss, Tokyo, Japan) using filters for PE (red) and FITC (green).
Statistical analysis
Data are expressed as the means ± SEM. Survival data were analyzed by a Kaplan-Meier method. Statistical analysis was performed using analysis of variance (ANOVA), followed by appropriate post-hoc tests including the Scheffe’s correction. P values of <0.05 were considered statistically significant.
Results
Survival rate
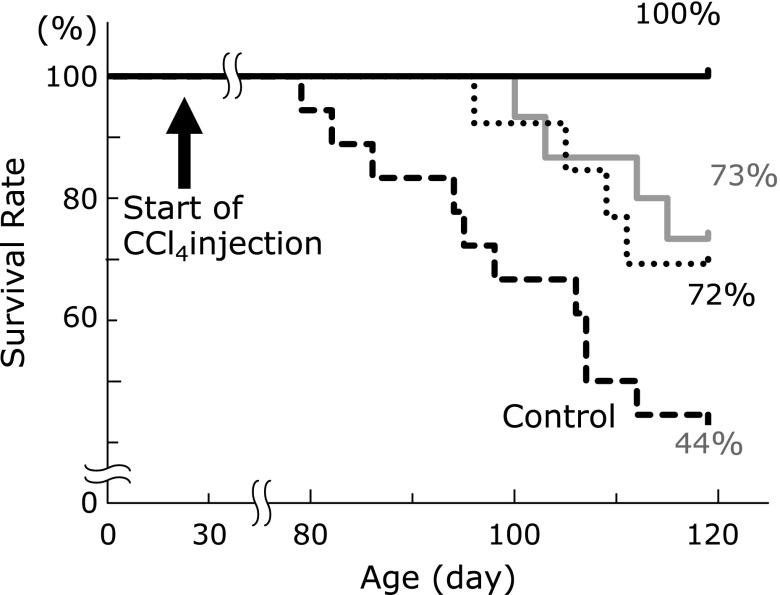
Azan-Mallory positive staining of the liver was found from 3 weeks after CCl4 administration (data not shown). SAC or NAC was treated from 4 weeks (age 56 days) after CCl4 (after the established fibrosis). The survival rate of rats was compared between those with or without SAC treatment at 13 weeks (age 120 days) after the start of CCl4 injections (Fig. 1). SAC significantly and dose-dependently improved the survival rate of rats.
Fig. 1.
Effects of SAC treatment on the survival rate. The survival rate of rats was compared with or without drug treatment at 120 days after the start of CCl4 injections. Statistical analysis was performed using the chi-square test. Black large dotted line, Control; gray solid line, NAC (600 mg/kg/day); black dotted line, SAC (200 mg/kg/day); black solid line, SAC (600 mg/kg/day). SAC (200 mg/kg/day) and NAC (600 mg/kg/day); p<0.05. SAC (600 mg/kg/day); p<0.01.
Plasma ALT and LPO, and hepatic total MDA
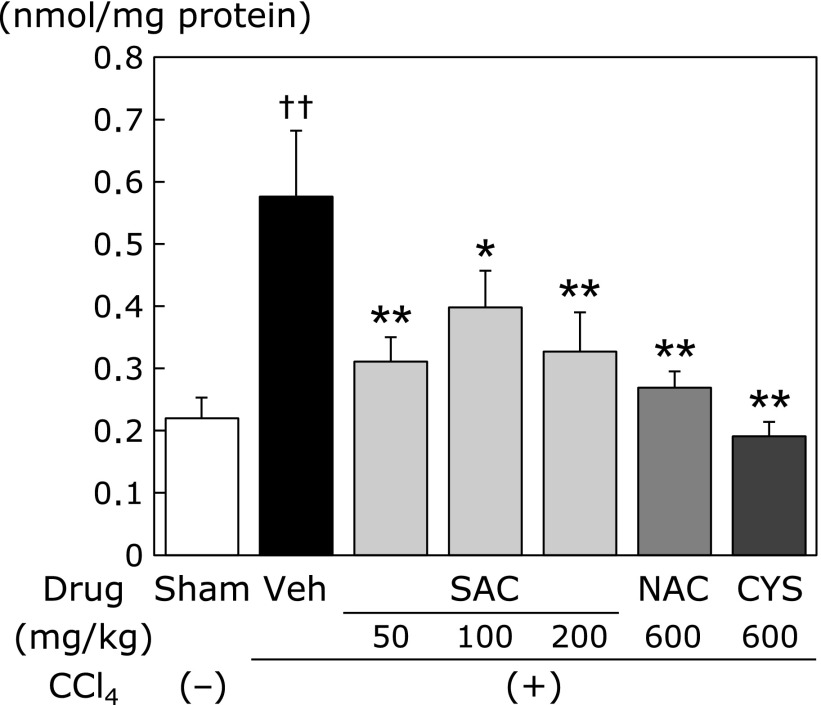
Although the plasma levels of ALT (Fig. 2A) and LPO (Fig. 2B) were increased in the CCl4 group compared with those in the sham group, SAC significantly reduced these increases in a dose-dependent manner. NAC and CYS did not reduce the CCl4-induced increase of plasma LPO. However, SAC reduced the plasma LPO concentration significantly. Hepatic total MDA levels were significantly increased in the CCl4 group. Drug administration groups (SAC, NAC, and CYS groups) significantly decreased the increases to similar extent (Fig. 3).
Fig. 2.
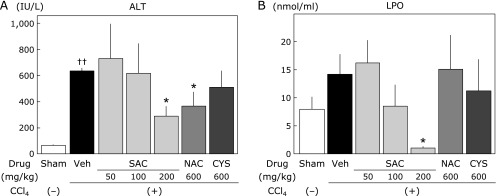
Effects of cysteine compounds on plasma ALT and LPO levels following CCl4 administration. CCl4 (2 ml/kg, 25% in corn oil) was intraperitoneally injected into rats twice a week for 12 weeks. Rats were randomly divided into 7 groups at 4 weeks after CCl4 injection. SAC, NAC, and CYS were administered to rats as a mixed diet of the expressed dose per day. At 1 week after the final CCl4 treatment, the animals were sacrificed, and assays were performed as described in the Materials and Methods. (A) Plasma ALT levels. (B) Plasma LPO (MDA + HNE) levels. Data are presented as the means ± SEM of at least 5 animals. ††p<0.01 vs the sham group. *p<0.05 vs the vehicle group.
Fig. 3.
Effects of cysteine compounds on hepatic MDA levels following CCl4 administration. CCl4 (2 ml/kg, 25% in corn oil) was intraperitoneally injected into rats twice a week for 12 weeks. Rats were randomly divided into 7 groups at 4 weeks after CCl4 injection. SAC, NAC, and CYS were administered to rats as a mixed diet of the expressed dose per day. At 1 week after the final CCl4 treatment, the animals were sacrificed, and assays were performed as described in the Materials and Methods. Data are presented as the means ± SEM of at least 5 animals. ††p<0.01 vs the sham group. *p<0.05, **p<0.01 vs the vehicle group.
Histological findings and hydroxyproline concentration in the liver
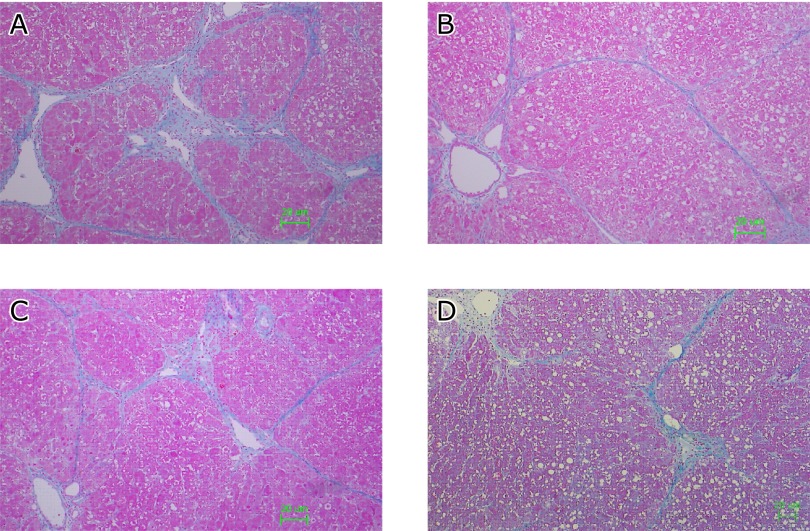
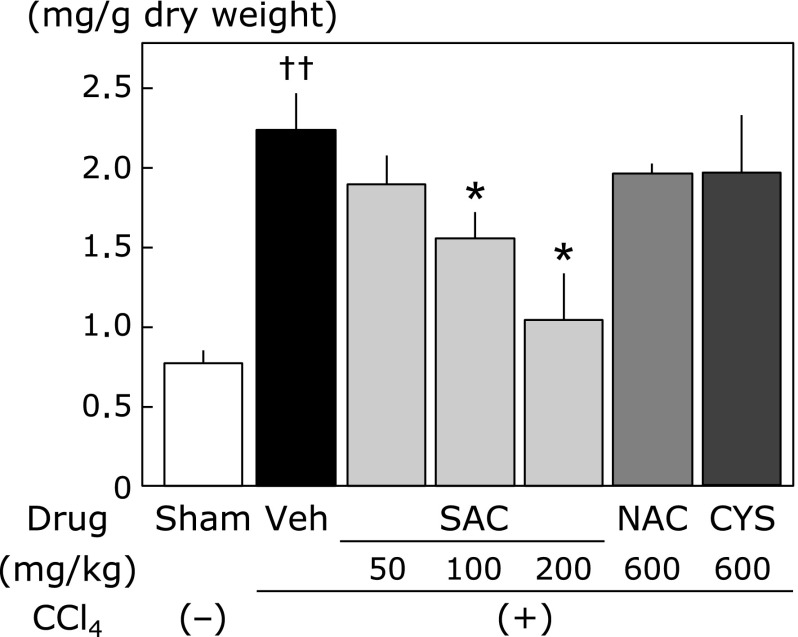
Fig. 4A shows representative Azan-Mallory staining of the liver with repeated CCl4 administration that induced necrosis of hepatic cells, fatty acid changes, infiltration of inflammatory cells, and extensive bridging fibrosis. Liver tissue was remarkably protected from CCl4-induced fibrosis by SAC (200 mg/kg) (Fig. 4B). Similarly, CCl4-induced fibrosis was significantly inhibited by NAC (Fig. 4C) and CYS (600 mg/kg) (Fig. 4D). Hydroxyproline contents are shown in Fig. 5. NAC and CYS did not reduce the CCl4-induced increase of hydroxyproline content. However, hydroxyproline contents induced by CCl4 were significantly reduced in liver tissues by SAC in a dose-dependent manner.
Fig. 4.
Effect of cysteine compounds on liver histology of hepatic fibrosis. Representative images of Azan-Mallory staining of the liver at 12 weeks after CCl4 administration. The animals were treated as described in the legend for Fig. 2. The animals were sacrificed and the right lobe was fixed and embedded in paraffin. (A) Vehicle. (B) SAC (200 mg/kg). (C) NAC (600 mg/kg). (D) CYS (600 mg/kg).
Fig. 5.
Hydroxyproline levels in the liver. The amount of collagen in the liver is expressed as the content of hydroxyproline. Data are presented as the means ± SEM of at least 5 animals. ††p<0.01 vs the sham group. *p<0.05 vs the vehicle group.
Effect of cysteine compounds on TGF-β levels in the liver
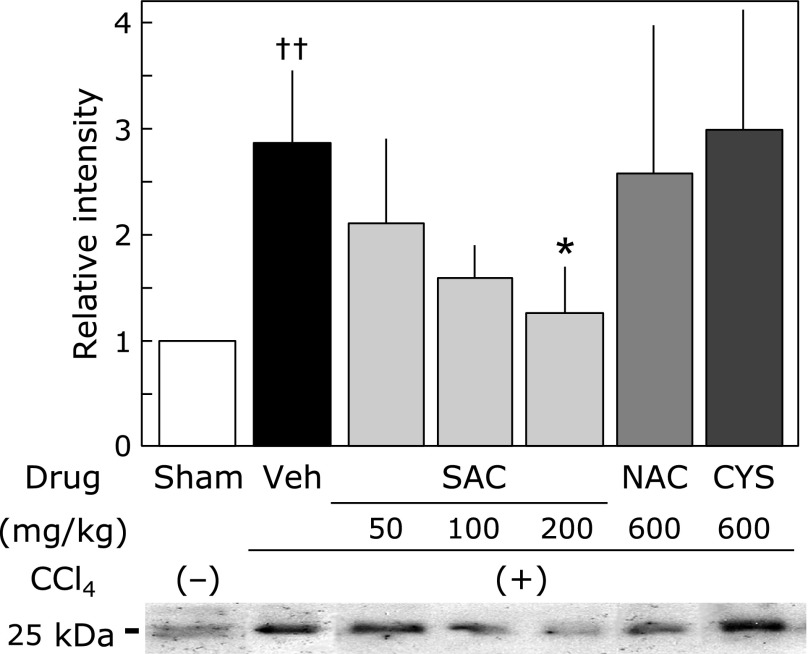
Fig. 6 shows TGF-β levels in the liver as determined by western blot analysis. The TGF-β levels of the control group was significantly increased, by approximately compared with those of the sham group. SAC (200 mg/kg) significantly reduced the CCl4-induced increase of TGF-β in the liver.
Fig. 6.
Effect of cysteine compounds on TGF-β levels in the liver. Tissue lysates was evaluated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis as described in the Materials and Methods. Animals were treated as described in Fig. 2. The relative increases of the intensity compared with the sham group are expressed as percentages. Values are means ± SEM (n = 5–8). *p<0.05 vs the CCl4 group. ††p<0.01 vs the sham group.
Activation of hepatic stellate cells
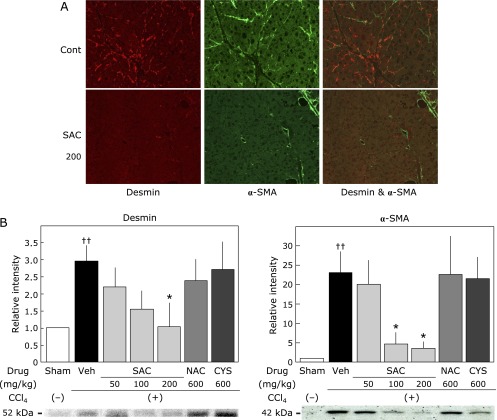
Double immunofluorescence staining (anti-desmin labeled with FITC, and anti-α-SMA labeled with PE) (Fig. 7A) indicated that a fine network pattern of stellate cells (red) existed in the livers treated with CCl4 alone for 12 weeks (control). Double-positive cells, presumably activated stellate cells, were observed in the control group. Conversely, the number of desmin-positive stellate cells and α-SMA-positive activated stellate cells was lower in the SAC-treated group. Fig. 7B shows the desmin levels in liver tissues. Desmin levels in the CCl4 group were significantly increased compared with those in the sham group. The CCl4-induced increase of desmin levels was significantly and dose-dependently reduced by SAC. α-SMA expression in the CCl4 group was approximately 23-fold higher than that in the sham group. This increase of α-SMA expression was significantly reduced in SAC-treated groups (100 mg/kg and 200 mg/kg).
Fig. 7.
Effect of cysteine compounds on hepatic stellate cell activation in the liver. (A) Double immunofluorescence staining (anti-desmin labeled with FITC and anti-α-SMA labeled with PE) indicated a fine network pattern of stellate cells in the livers treated with CCl4 alone for 12 weeks (Control). Double-positive cells, presumably activated stellate cells, were observed in the control group. Conversely, desmin-positive (red) and α-SMA-positive (green) cells were hardly observed in SAC-treated groups (SAC 200). (B) Effect of cysteine compounds on the expression of desmin and α-SMA in the liver. Tissue lysates were evaluated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis as described in the Materials and Methods. Animals were treated as described in the legend to Fig. 2. The relative increases of the intensity compared with the sham group are expressed as percentages. Values are the means ± SEM (n = 5–8). *p<0.05 vs the CCl4 group. ††p<0.01 vs the sham group. See online version figure.
Fibrolytic activity
Fig. 8A shows hepatic pro-MMP9 activities as determined by gelatin zymography. Pro-MMP9 activities in the CCl4 group were approximately 5-fold higher than those in the sham group. This increase of pro-MMP9 activities was significantly reduced in the SAC (200 mg/kg)-treated group and NAC (600 mg/kg)-treated group. Fig. 8B shows TIMP-1 levels in the liver. TIMP-1 expression in the CCl4 group was approximately 2.5-fold higher than that in the sham group, which was reduced in SAC-treated groups (100 mg/kg and 200 mg/kg).
Fig. 8.
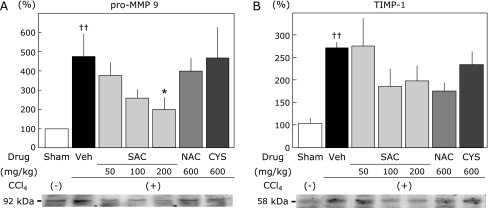
Effect of cysteine compounds on pro-MMP9 and TIMP-1 activities in the liver. (A) The protease activity of pro-MMP9 was detected by gelatin zymography. (B) TIMP-1 activity was detected by western blot analysis. Relative increases compared with the sham group are presented. Values are the means ± SEM (n = 5–8). *p<0.05 vs CCl4 group. ††p<0.01 vs the sham group.
Discussion
In this study, we showed that oral administration of SAC after CCl4 treatment resulted in marked inhibition of the development of liver fibrosis and cirrhosis as well as mortality. There are few reports on the action of amino acids in hepatic pathophysiology. Dietary l-glycine attenuates CCl4-induced liver fibrosis in rats(11) and l-leucine stimulates the secretion of hepatocyte growth factor by hepatic stellate cell activation.(12) Thus, the data presented here on SAC provides new information on the pharmacological action of amino acids in liver fibrosis (Supplemental Fig. 1*).
In this study, SAC had a more preventive effect against fibrosis than did NAC and CYS. SAC, but not NAC, inhibited the deposition of collagens and the expression of desmin, α-SMA, and TGF-β. It has been reported that NAC prevents experimental cirrhosis by inhibiting oxidative stress and downregulating the profibrogenic cytokine TGF-β.(13) However, the drug treatments in our study were started from 4 weeks after CCl4 administration when fibrosis was observed histologically. Therefore, the preventive effects of SAC on the promotion of fibrosis may be synergistically caused by its antioxidative and anti-fibrogenic effects. In fact, the increase of plasma LPOs (Fig. 2B) and hepatic TGF-β (Fig. 6) did not decrease following NAC or CYS treatment under the progress of fibrosis. The increases of hepatic MDA levels were decreased to same extent in all drug-administered groups (Fig. 3). In other words, oxidative stress in the liver might improve similarly in all groups. In addition, inhibition of fibrotic formation (TGF-β production) by repeated CCl4 injection may be systemically and totally resulted in decrease of lipid peroxidation by SAC (200 mg/kg). By definition, progressive fibrosis occurs when the rate of matrix synthesis exceeds the rate of matrix degradation.(14) Furthermore, the MMP/TIMP balance is thought to play a pivotal role in the development of liver fibrosis, but their direct interactions in vivo have not yet been clarified. SAC, but not NAC and CYS, inhibited both pro-MMP9 and TIMP1 expression (Fig. 7). The finding that SAC inhibited hydroxyproline contents (Fig. 5) and TGF-β levels (Fig. 6C) suggested the involvement of the fibrolytic system.
Many reports have considered that hepatic stellate cells are central to the fibrotic process. It has been suggested that hepatic stellate cells are also a source of MMPs, indicating their participation in matrix remodeling.(15–17) SAC reduced the number of hepatic stellate cells (Fig. 8) and their α-SMA expression and activation status (Fig. 6A and B), which may have resulted from the decrease of MMPs.
SAC might markedly improve the survival rate by altering the balance of fibrolytic enzymes and fibrogenesis in liver fibrosis formation induced by CCl4. However, it is unclear whether SAC directly inhibits the activation of hepatic stellate cells and/or the relationship between Kupffer cells and hepatic stellate cells. SAC inhibits the release of cytokines/chemokines that induce neutrophil recruitment, activation of key transcription factors including nuclear factor (NF)-κB, and activator protein-1, thereby augmenting inflammatory responses and tissue damage. Furthermore, it has been shown that SAC inhibits NF-κB activation induced by tumor necrosis factor-α and H2O2 in human T lymphocytes.(18) Because our preliminary study revealed that SAC showed no effect on platelet-derived growth factor-dependent activation of hepatic stellate cells in vitro, the anti-fibrotic mechanisms might potentially involve some types of cells indirectly.
In conclusion, SAC significantly resolved CCl4-induced liver fibrosis with a decrease in the profibrogenic cytokine TGF-β as well as the antioxidative properties of SAC. SAC may be a better therapeutic agent for inhibition of liver fibrosis than other cysteine compounds.
Acknowledgments
We thank Ms. F. Kobayashi and Ms. K. Nakagawa at Osaka City University Medical School and Ms. Nishikawa at Okayama University for their excellent technical assistance. This study was supported in part by grant no. AS231Z03328G (A-STEP, Adaptable and Seamless Technology Transfer Program through Target driven R&D) from the Japan Science and Technology Agency.
Abbreviations
- AGEs
aged garlic extracts
- ALT
alanine aminotransferase
- CCl4
carbon tetrachloride
- CYS
l-cysteine
- DMEM
Dulbecco’s modified Eagle medium
- FBS
fetal bovine serum
- HNE
4-hydroxy-2-nonenal
- LPO
lipid peroxides
- MDA
malondialdehyde
- MMP
matrix metalloproteinase
- NAC
N-acetyl cysteine
- SAC
S-allyl cysteine
- TGF
transforming growth factor
- TIMP-1
tissue inhibitor of metalloproteinase
Conflict of Interest
No potential conflicts of interest were disclosed.
Supplementary Material
References
- 1.Ide N, Lau BH. Garlic compounds protect vascular endothelial cells from oxidized low density lipoprotein-induced injury. J Pharm Pharmacol. 1997;49:908–911. doi: 10.1111/j.2042-7158.1997.tb06134.x. [DOI] [PubMed] [Google Scholar]
- 2.Ide N, Nelson AB, Lau BH. Aged garlic extract and its constituents inhibit Cu(2+)-induced oxidative modification of low density lipoprotein. Planta Med. 1997;63:263–264. doi: 10.1055/s-2006-957668. [DOI] [PubMed] [Google Scholar]
- 3.Amagase H, Petesch BL, Matsuura H, Kasuga S, Itakura Y. Intake of garlic and its bioactive components. J Nutr. 2001;131:955S–962S. doi: 10.1093/jn/131.3.955S. [DOI] [PubMed] [Google Scholar]
- 4.Colín-González AL, Santana RA, Silva-Islas CA, Chánez-Cárdenas ME, Santamaria A, Maldonado PD.The antioxidant mechanisms underlying the aged garlic extract- and S-allylcysteine-induced protection. Oxid Med Cell Longev 2012; 2012: 907162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagae S, Ushijima M, Hatono S, et al. Pharmacokinetics of the garlic compound S-allylcysteine. Planta Med. 1994;60:214–217. doi: 10.1055/s-2006-959461. [DOI] [PubMed] [Google Scholar]
- 6.Mostafa MG, Mima T, Ohnishi ST, Mori K. S-allylcysteine ameliorates doxorubicin toxicity in the heart and liver in mice. Planta Med. 2000;66:148–151. doi: 10.1055/s-2000-11124. [DOI] [PubMed] [Google Scholar]
- 7.Maldonado PD, Barrera D, Rivero I, et al. Antioxidant S-allylcysteine prevents gentamicin-induced oxidative stress and renal damage. Free Radical Bio Med. 2003;35:317–324. doi: 10.1016/s0891-5849(03)00312-5. [DOI] [PubMed] [Google Scholar]
- 8.Numagami Y, Sato S, Ohnishi ST. Attenuation of rat ischemic brain damage by aged garlic extracts: A possible protecting mechanism as antioxidants. Neurochem Int. 1996;29:135–143. doi: 10.1016/0197-0186(95)00117-4. [DOI] [PubMed] [Google Scholar]
- 9.Mizuguchi S, Takemura S, Minamiyama Y, et al. S-allyl cysteine attenuated CCl4-induced oxidative stress and pulmonary fibrosis in rats. Biofactors. 2006;26:81–92. doi: 10.1002/biof.5520260108. [DOI] [PubMed] [Google Scholar]
- 10.Kivirikko KI, Prockop DJ. Hydroxylation of proline in synthetic polypeptides with purified protocollagen hydroxylase. J Biol Chem. 1967;242:4007–4012. [PubMed] [Google Scholar]
- 11.Rivera CA, Bradford BU, Hunt KJ, et al. Attenuation of CCl4-induced hepatic fibrosis by GdCl3 treatment or dietary glycine. Am J Physiol Gastrointest Liver Physiol. 2001;281:G200–G207. doi: 10.1152/ajpgi.2001.281.1.G200. [DOI] [PubMed] [Google Scholar]
- 12.Tomiya T, Inoue Y, Yanase M, et al. Leucine stimulates the secretion of hepatocyte growth factor by hepatic stellate cells. Biochem Biophys Res Commun. 2002;297:1108–1111. doi: 10.1016/s0006-291x(02)02339-2. [DOI] [PubMed] [Google Scholar]
- 13.Galicia-Moreno M, Rodríguez-Rivera A, Reyes-Gordillo K, et al. N-acetylcysteine prevents carbon tetrachloride-induced liver cirrhosis: role of liver transforming growth factor-beta and oxidative stress. Eur J Gastroen Hepat. 2009;21:908–914. doi: 10.1097/MEG.0b013e32831f1f3a. [DOI] [PubMed] [Google Scholar]
- 14.Bedossa P, Paradis V. Liver extracellular matrix in health and disease. J Pathol. 2003;200:504–515. doi: 10.1002/path.1397. [DOI] [PubMed] [Google Scholar]
- 15.Imai K, Sato M, Kojima N, et al. Storage of lipid droplets in and production of extracellular matrix by hepatic stellate cells (Vitamin A-storing cells) in Long-Evans cinnamon-like colored (LEC) rats. Anat Rec. 2000;258:338–348. doi: 10.1002/(SICI)1097-0185(20000401)258:4<338::AID-AR2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 16.Imai K, Sato T, Senoo H. Adhesion between cells and extracellular matrix with special reference to hepatic stellate cell adhesion to three-dimensional collagen fibers. Cell Struct Funct. 2000;25:329–336. doi: 10.1247/csf.25.329. [DOI] [PubMed] [Google Scholar]
- 17.Neubauer K, Saile B, Ramadori G. Liver fibrosis and altered matrix synthesis. Can J Gastroenterol. 2001;15:187–193. doi: 10.1155/2001/870205. [DOI] [PubMed] [Google Scholar]
- 18.Geng Z, Rong Y, Lau BH. S-allyl cysteine inhibits activation of nuclear factor kappa B in human T cells. Free Radic Biol Med. 1997;23:345–350. doi: 10.1016/s0891-5849(97)00006-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.