Abstract
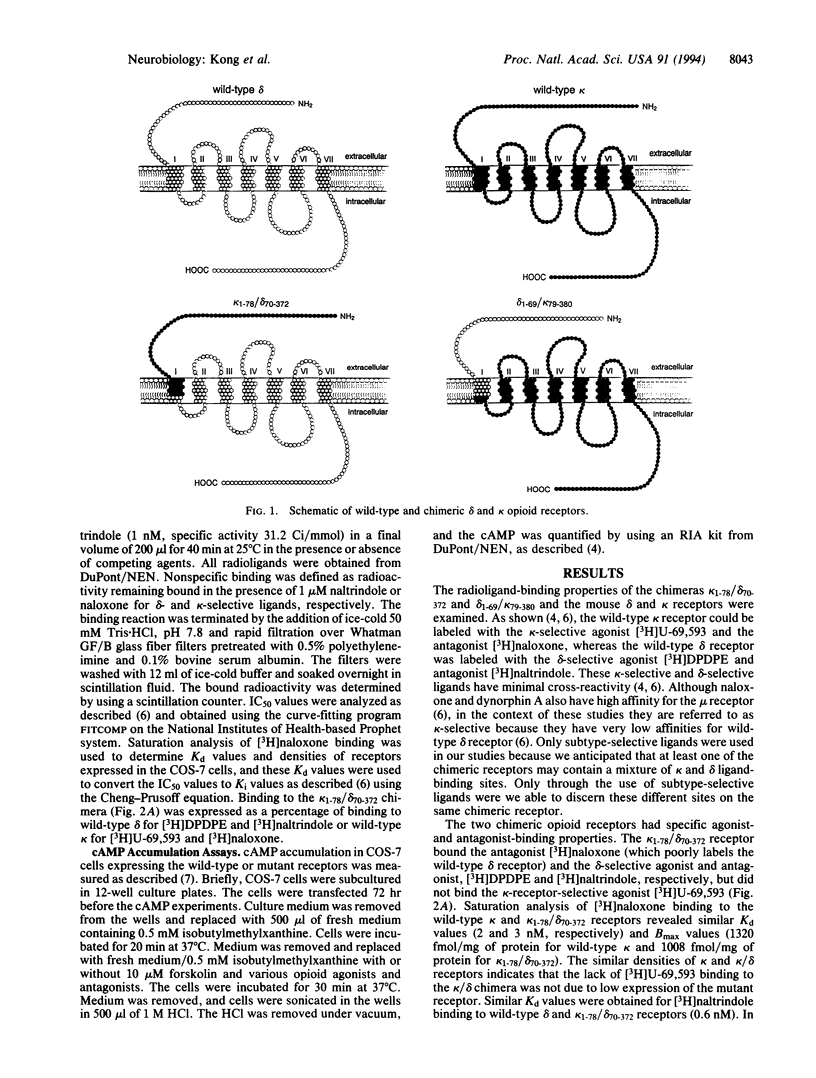
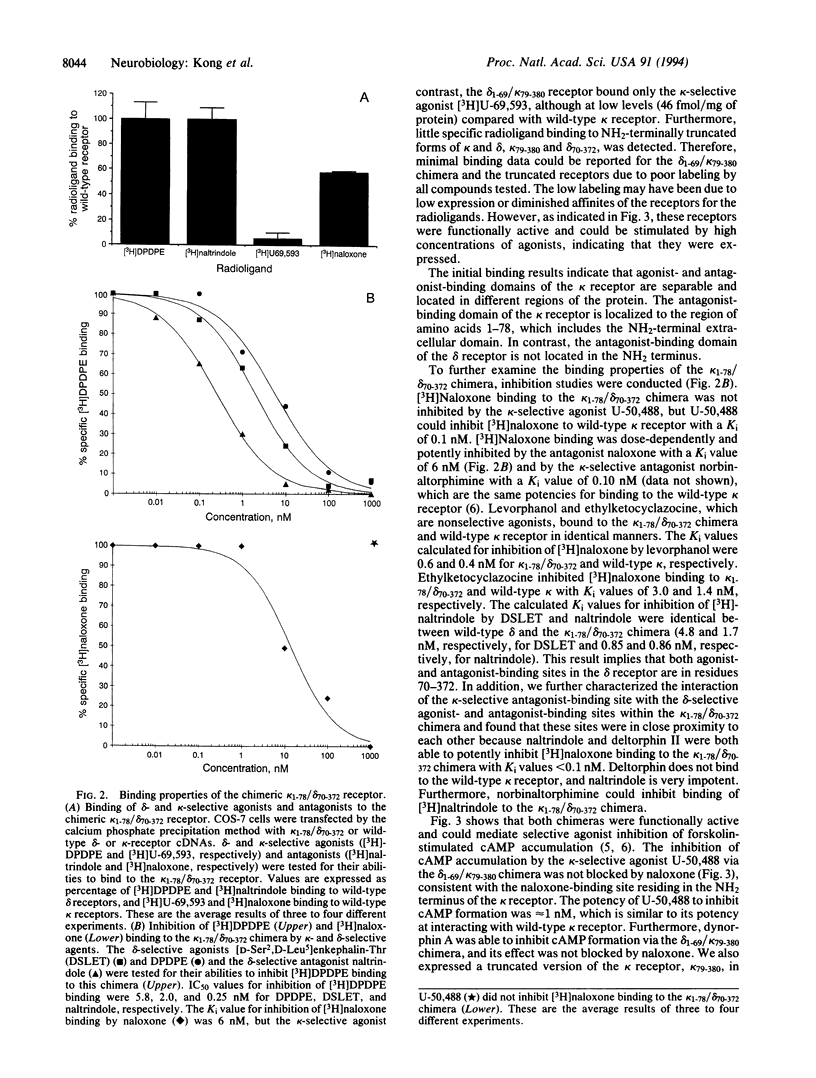
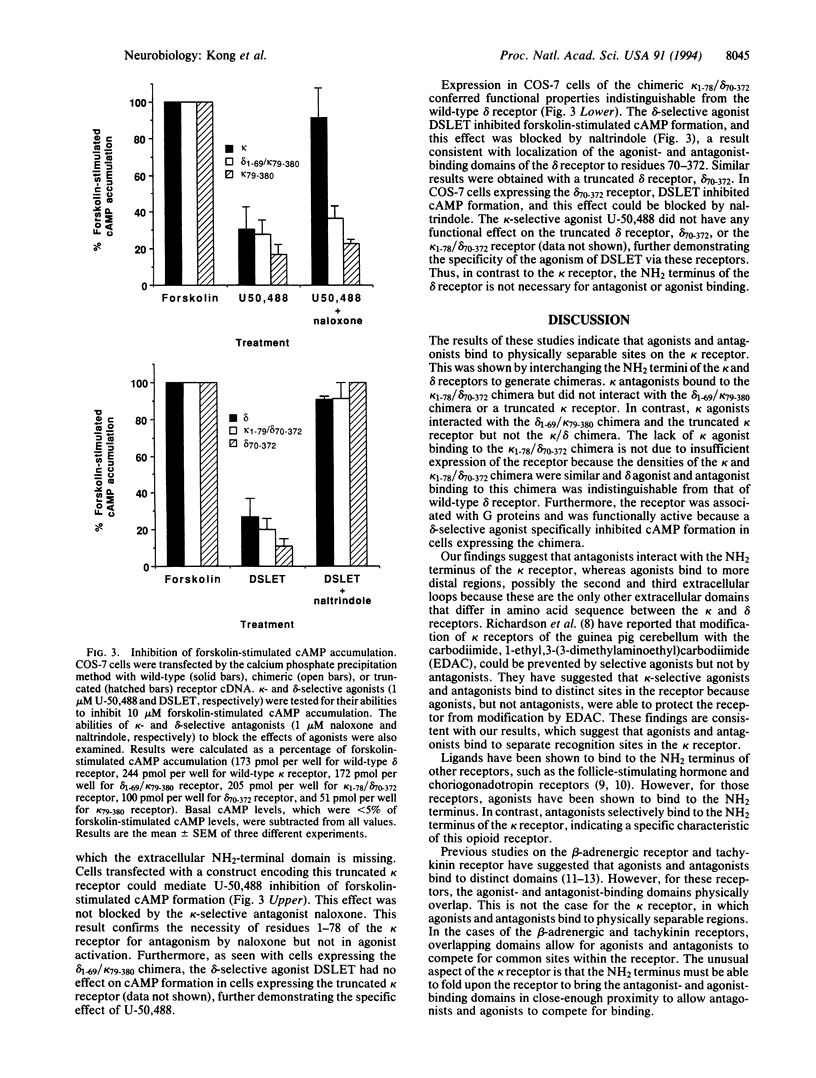
Opium and its derivatives are potent analgesics that can also induce severe side effects, including respiratory depression and addiction. Opioids exert their diverse physiological effects through specific membrane-bound receptors. Three major types of opioid receptors have been described, termed delta, kappa, and mu. The recent molecular cloning of these receptor types opens up the possibility to identify the ligand-binding domains of these receptors. To identify the ligand-binding domains of the kappa and delta receptors, we have expressed in COS-7 cells the cloned mouse delta and kappa receptors and chimeric delta/kappa and kappa/delta receptors in which the NH2 termini have been exchanged. The opioid antagonist naloxone binds potently to wild-type kappa receptor but not to wild-type delta receptor. The kappa/delta chimera bound [3H]naloxone with high affinity. In contrast, the kappa-specific agonist [3H]U-69,593 did not bind to the kappa/delta chimera. These findings indicate that selective agonists and antagonists interact with different recognition sites in the kappa receptor and localize the antagonist-binding domain to the NH2 terminus. Consistent with the results of radioligand-binding studies, the kappa/delta chimera did not mediate kappa-agonist inhibition of cAMP formation. In contrast, the delta/kappa chimera did mediate kappa-agonist inhibition of cAMP formation, but this effect was not blocked by naloxone. Furthermore, a truncated kappa receptor lacking its NH2 terminus was able to mediate agonist inhibition of cAMP accumulation in a naloxone-insensitive manner. This result further indicates that the NH2 terminus of the kappa receptor contains the selective antagonist-binding domain. The ability to dissociate agonist- and antagonist-binding sites will facilitate the development of more specific kappa agonists, which could have analgesic properties devoid of side effects.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen Y., Mestek A., Liu J., Hurley J. A., Yu L. Molecular cloning and functional expression of a mu-opioid receptor from rat brain. Mol Pharmacol. 1993 Jul;44(1):8–12. [PubMed] [Google Scholar]
- Evans C. J., Keith D. E., Jr, Morrison H., Magendzo K., Edwards R. H. Cloning of a delta opioid receptor by functional expression. Science. 1992 Dec 18;258(5090):1952–1955. doi: 10.1126/science.1335167. [DOI] [PubMed] [Google Scholar]
- Fong T. M., Cascieri M. A., Yu H., Bansal A., Swain C., Strader C. D. Amino-aromatic interaction between histidine 197 of the neurokinin-1 receptor and CP 96345. Nature. 1993 Mar 25;362(6418):350–353. doi: 10.1038/362350a0. [DOI] [PubMed] [Google Scholar]
- Gether U., Johansen T. E., Snider R. M., Lowe J. A., 3rd, Nakanishi S., Schwartz T. W. Different binding epitopes on the NK1 receptor for substance P and non-peptide antagonist. Nature. 1993 Mar 25;362(6418):345–348. doi: 10.1038/362345a0. [DOI] [PubMed] [Google Scholar]
- Kieffer B. L., Befort K., Gaveriaux-Ruff C., Hirth C. G. The delta-opioid receptor: isolation of a cDNA by expression cloning and pharmacological characterization. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12048–12052. doi: 10.1073/pnas.89.24.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong H., Raynor K., Yasuda K., Moe S. T., Portoghese P. S., Bell G. I., Reisine T. A single residue, aspartic acid 95, in the delta opioid receptor specifies selective high affinity agonist binding. J Biol Chem. 1993 Nov 5;268(31):23055–23058. [PubMed] [Google Scholar]
- McFarland K. C., Sprengel R., Phillips H. S., Köhler M., Rosemblit N., Nikolics K., Segaloff D. L., Seeburg P. H. Lutropin-choriogonadotropin receptor: an unusual member of the G protein-coupled receptor family. Science. 1989 Aug 4;245(4917):494–499. doi: 10.1126/science.2502842. [DOI] [PubMed] [Google Scholar]
- Raynor K., Kong H., Chen Y., Yasuda K., Yu L., Bell G. I., Reisine T. Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors. Mol Pharmacol. 1994 Feb;45(2):330–334. [PubMed] [Google Scholar]
- Richardson A., Simon J., Barnard E. A. Protection by opioid ligands against modification of the opioid receptor by a carbodiimide. Biochem Pharmacol. 1992 Apr 1;43(7):1415–1419. doi: 10.1016/0006-2952(92)90197-q. [DOI] [PubMed] [Google Scholar]
- Sprengel R., Braun T., Nikolics K., Segaloff D. L., Seeburg P. H. The testicular receptor for follicle stimulating hormone: structure and functional expression of cloned cDNA. Mol Endocrinol. 1990 Apr;4(4):525–530. doi: 10.1210/mend-4-4-525. [DOI] [PubMed] [Google Scholar]
- Strader C. D., Sigal I. S., Candelore M. R., Rands E., Hill W. S., Dixon R. A. Conserved aspartic acid residues 79 and 113 of the beta-adrenergic receptor have different roles in receptor function. J Biol Chem. 1988 Jul 25;263(21):10267–10271. [PubMed] [Google Scholar]
- Yasuda K., Raynor K., Kong H., Breder C. D., Takeda J., Reisine T., Bell G. I. Cloning and functional comparison of kappa and delta opioid receptors from mouse brain. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6736–6740. doi: 10.1073/pnas.90.14.6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda K., Rens-Domiano S., Breder C. D., Law S. F., Saper C. B., Reisine T., Bell G. I. Cloning of a novel somatostatin receptor, SSTR3, coupled to adenylylcyclase. J Biol Chem. 1992 Oct 5;267(28):20422–20428. [PubMed] [Google Scholar]


