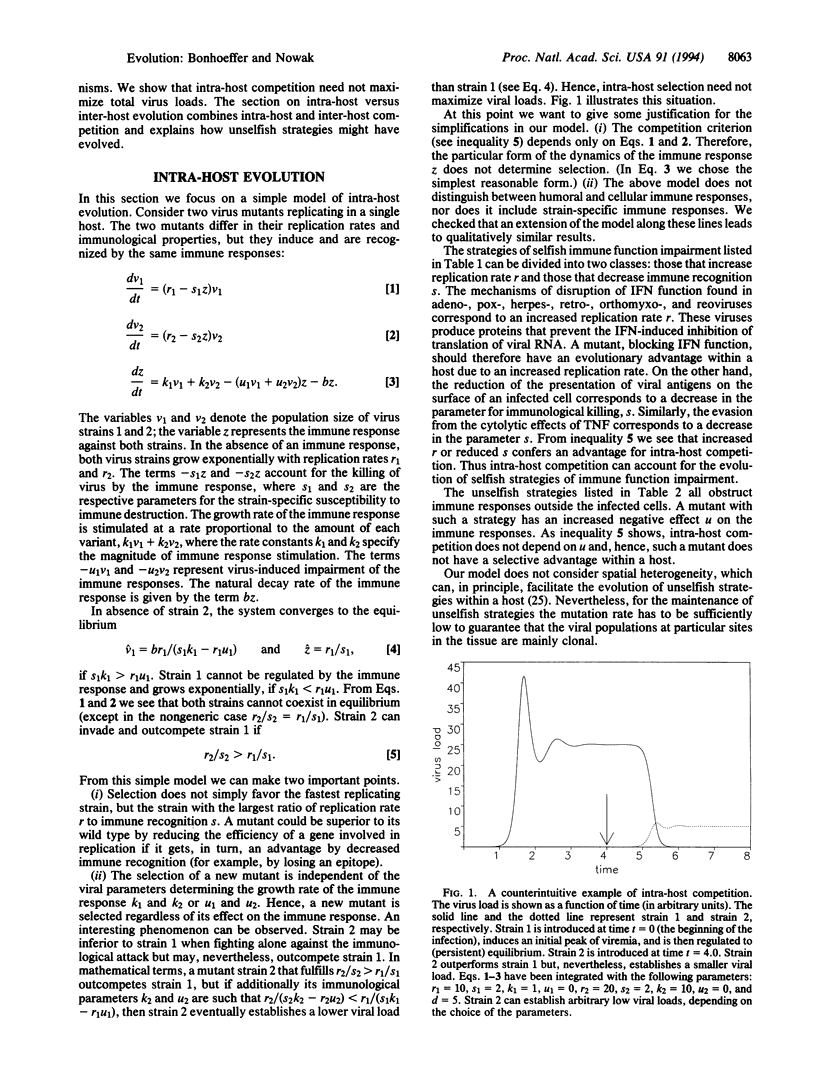
Abstract
We investigate the evolution of viral strategies to counteract immunological attack. These strategies can be divided into two classes: those that impair the immune response inside or at the surface of a virus-infected cell and those that impair the immune response outside an infected cell. The former strategies confer a "selfish" individual selective advantage for intra-host competition among viruses. The latter strategies confer an "unselfish" selective advantage to the virus population as a group. A mutant, defective in the gene coding for the extracellular immune function-impairment strategy, may be protected from immune attack because the wild-type virus in the same host successfully impairs the host's immune function. Such "unselfish" defense strategies are neutral with respect to intra-host competition. We present simple models of viral intra-host and combined inter- and intra-host evolution. We show that selfish strategies can evolve by intra-host evolution. Unselfish strategies may evolve if inter-host selection pressures outweigh intra-host selection, suggesting that such strategies can only evolve in viruses with low mutation rates.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht J. C., Fleckenstein B. New member of the multigene family of complement control proteins in herpesvirus saimiri. J Virol. 1992 Jun;66(6):3937–3940. doi: 10.1128/jvi.66.6.3937-3940.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcamí A., Smith G. L. A soluble receptor for interleukin-1 beta encoded by vaccinia virus: a novel mechanism of virus modulation of the host response to infection. Cell. 1992 Oct 2;71(1):153–167. doi: 10.1016/0092-8674(92)90274-g. [DOI] [PubMed] [Google Scholar]
- Anderson R. M., May R. M. Coevolution of hosts and parasites. Parasitology. 1982 Oct;85(Pt 2):411–426. doi: 10.1017/s0031182000055360. [DOI] [PubMed] [Google Scholar]
- Beattie E., Tartaglia J., Paoletti E. Vaccinia virus-encoded eIF-2 alpha homolog abrogates the antiviral effect of interferon. Virology. 1991 Jul;183(1):419–422. doi: 10.1016/0042-6822(91)90158-8. [DOI] [PubMed] [Google Scholar]
- Bhat R. A., Thimmappaya B. Two small RNAs encoded by Epstein-Barr virus can functionally substitute for the virus-associated RNAs in the lytic growth of adenovirus 5. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4789–4793. doi: 10.1073/pnas.80.15.4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne H., Smith G., Beck S., Minson T. A complex between the MHC class I homologue encoded by human cytomegalovirus and beta 2 microglobulin. Nature. 1990 Oct 25;347(6295):770–772. doi: 10.1038/347770a0. [DOI] [PubMed] [Google Scholar]
- Chao L., Levin B. R. Structured habitats and the evolution of anticompetitor toxins in bacteria. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6324–6328. doi: 10.1073/pnas.78.10.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding L. R. Virus proteins that counteract host immune defenses. Cell. 1992 Oct 2;71(1):5–7. doi: 10.1016/0092-8674(92)90259-f. [DOI] [PubMed] [Google Scholar]
- Gunnery S., Rice A. P., Robertson H. D., Mathews M. B. Tat-responsive region RNA of human immunodeficiency virus 1 can prevent activation of the double-stranded-RNA-activated protein kinase. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8687–8691. doi: 10.1073/pnas.87.22.8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S. L., Frank I., Yee A., Cohen G. H., Eisenberg R. J., Friedman H. M. Glycoprotein C of herpes simplex virus type 1 prevents complement-mediated cell lysis and virus neutralization. J Infect Dis. 1990 Aug;162(2):331–337. doi: 10.1093/infdis/162.2.331. [DOI] [PubMed] [Google Scholar]
- Holmes E. C., Zhang L. Q., Simmonds P., Ludlam C. A., Brown A. J. Convergent and divergent sequence evolution in the surface envelope glycoprotein of human immunodeficiency virus type 1 within a single infected patient. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4835–4839. doi: 10.1073/pnas.89.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu D. H., de Waal Malefyt R., Fiorentino D. F., Dang M. N., Vieira P., de Vries J., Spits H., Mosmann T. R., Moore K. W. Expression of interleukin-10 activity by Epstein-Barr virus protein BCRF1. Science. 1990 Nov 9;250(4982):830–832. doi: 10.1126/science.2173142. [DOI] [PubMed] [Google Scholar]
- Imani F., Jacobs B. L. Inhibitory activity for the interferon-induced protein kinase is associated with the reovirus serotype 1 sigma 3 protein. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7887–7891. doi: 10.1073/pnas.85.21.7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings S. R., Rice P. L., Kloszewski E. D., Anderson R. W., Thompson D. L., Tevethia S. S. Effect of herpes simplex virus types 1 and 2 on surface expression of class I major histocompatibility complex antigens on infected cells. J Virol. 1985 Dec;56(3):757–766. doi: 10.1128/jvi.56.3.757-766.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katze M. G., Tomita J., Black T., Krug R. M., Safer B., Hovanessian A. Influenza virus regulates protein synthesis during infection by repressing autophosphorylation and activity of the cellular 68,000-Mr protein kinase. J Virol. 1988 Oct;62(10):3710–3717. doi: 10.1128/jvi.62.10.3710-3717.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotwal G. J., Isaacs S. N., McKenzie R., Frank M. M., Moss B. Inhibition of the complement cascade by the major secretory protein of vaccinia virus. Science. 1990 Nov 9;250(4982):827–830. doi: 10.1126/science.2237434. [DOI] [PubMed] [Google Scholar]
- Levin B. R., Bull J. J. Short-sighted evolution and the virulence of pathogenic microorganisms. Trends Microbiol. 1994 Mar;2(3):76–81. doi: 10.1016/0966-842x(94)90538-x. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Shenk T. Adenovirus virus-associated RNA and translation control. J Virol. 1991 Nov;65(11):5657–5662. doi: 10.1128/jvi.65.11.5657-5662.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May R. M., Anderson R. M. Population biology of infectious diseases: Part II. Nature. 1979 Aug 9;280(5722):455–461. doi: 10.1038/280455a0. [DOI] [PubMed] [Google Scholar]
- Mold C., Bradt B. M., Nemerow G. R., Cooper N. R. Epstein-Barr virus regulates activation and processing of the third component of complement. J Exp Med. 1988 Sep 1;168(3):949–969. doi: 10.1084/jem.168.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak M. A., Anderson R. M., McLean A. R., Wolfs T. F., Goudsmit J., May R. M. Antigenic diversity thresholds and the development of AIDS. Science. 1991 Nov 15;254(5034):963–969. doi: 10.1126/science.1683006. [DOI] [PubMed] [Google Scholar]
- Nowak M. A., May R. M., Anderson R. M. The evolutionary dynamics of HIV-1 quasispecies and the development of immunodeficiency disease. AIDS. 1990 Nov;4(11):1095–1103. doi: 10.1097/00002030-199011000-00007. [DOI] [PubMed] [Google Scholar]
- Nowak M. A., May R. M. Superinfection and the evolution of parasite virulence. Proc Biol Sci. 1994 Jan 22;255(1342):81–89. doi: 10.1098/rspb.1994.0012. [DOI] [PubMed] [Google Scholar]
- Ray C. A., Black R. A., Kronheim S. R., Greenstreet T. A., Sleath P. R., Salvesen G. S., Pickup D. J. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1 beta converting enzyme. Cell. 1992 May 15;69(4):597–604. doi: 10.1016/0092-8674(92)90223-y. [DOI] [PubMed] [Google Scholar]
- Siliciano R. F., Lawton T., Knall C., Karr R. W., Berman P., Gregory T., Reinherz E. L. Analysis of host-virus interactions in AIDS with anti-gp120 T cell clones: effect of HIV sequence variation and a mechanism for CD4+ cell depletion. Cell. 1988 Aug 12;54(4):561–575. doi: 10.1016/0092-8674(88)90078-5. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Davis T., Wignall J. M., Din W. S., Farrah T., Upton C., McFadden G., Goodwin R. G. T2 open reading frame from the Shope fibroma virus encodes a soluble form of the TNF receptor. Biochem Biophys Res Commun. 1991 Apr 15;176(1):335–342. doi: 10.1016/0006-291x(91)90929-2. [DOI] [PubMed] [Google Scholar]
- Smith G. L. Virus strategies for evasion of the host response to infection. Trends Microbiol. 1994 Mar;2(3):81–88. doi: 10.1016/0966-842x(94)90539-8. [DOI] [PubMed] [Google Scholar]
- Spriggs M. K., Hruby D. E., Maliszewski C. R., Pickup D. J., Sims J. E., Buller R. M., VanSlyke J. Vaccinia and cowpox viruses encode a novel secreted interleukin-1-binding protein. Cell. 1992 Oct 2;71(1):145–152. doi: 10.1016/0092-8674(92)90273-f. [DOI] [PubMed] [Google Scholar]
- Townsend A., Bastin J., Gould K., Brownlee G., Andrew M., Coupar B., Boyle D., Chan S., Smith G. Defective presentation to class I-restricted cytotoxic T lymphocytes in vaccinia-infected cells is overcome by enhanced degradation of antigen. J Exp Med. 1988 Oct 1;168(4):1211–1224. doi: 10.1084/jem.168.4.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton C., Mossman K., McFadden G. Encoding of a homolog of the IFN-gamma receptor by myxoma virus. Science. 1992 Nov 20;258(5086):1369–1372. doi: 10.1126/science.1455233. [DOI] [PubMed] [Google Scholar]
- Wold W. S., Gooding L. R. Region E3 of adenovirus: a cassette of genes involved in host immunosurveillance and virus-cell interactions. Virology. 1991 Sep;184(1):1–8. doi: 10.1016/0042-6822(91)90815-s. [DOI] [PubMed] [Google Scholar]


