Abstract
The crystallin proteins were initially identified as structural proteins of the ocular lens and have been recently demonstrated to be expressed in normal retina. They are dramatically upregulated by a large range of retinal diseases including diabetic retinopathy, age-related macular degeneration, uveitis, trauma and ischemia. The crystallin family of proteins is composed of alpha-, beta- and gamma-crystallin. Alpha-crystallins, which are small heat shock proteins, have received substantial attention recently. This review summarizes the current knowledge of alpha-crystallins in retinal diseases, their roles in retinal neuron cell survival and retinal inflammation, and the regulation of their expression and activity. Their potential role in the development of new treatments for neurodegenerative diseases is also discussed.
Keywords: crystallins, retinal diseases, neurodegeneration, apoptosis
1. Introduction
Crystallins constitute a diverse group of proteins first identified in the ocular lens where they are highly concentrated in differentiated lens fiber cells and augment the refractive power of the transparent lens tissue (Wistow and Piatigorsky, 1988; Bloemendal and de Jong, 1991). In vertebrates, three major classes of crystallins, alpha-, beta-, and gamma-, have been described as accumulating in the lens in a spatially and temporally regulated manner (Lubsen, Aarts et al., 1988; Wistow, 1990; Piatigorsky, 1992). Their expression increases dramatically during differentiation of lens epithelial cells into fibers (Wistow and Piatigorsky, 1988). The two alpha-crystallins (alphaa- and alphaB) belong to the small heat shock protein family of molecular chaperones and appear very early during mouse embryonic development (Sax and Piatigorsky, 1994; Robinson and Overbeek, 1996). In the lens, they are usually found as large aggregates, consisting of two types of subunits, alphaA- and alphaB-crystallins (For reviews see (Wistow and Piatigorsky, 1988; Groenen, Merck et al., 1994)). Both subunits are encoded by separate and single-copy genes in humans. Functionally, the small heat shock proteins and alpha-crystallins share the property of being molecular chaperones (Horwitz, 1992; Jakob, Gaestel et al., 1993), and both convey thermotolerance (Landry, Chretien et al., 1989; Kim, Choi et al., 2007). Members of the β/γ-superfamily, which include beta-crystallins (βA1/A3, βA2, βA4, βB1, βB2 and βB3) and gamma-crystallins (γA–F, and γS, formerly βS), are related to microbial proteins induced by physiological stress (Jaenicke and Slingsby, 2001). The rodent gamma-crystallin gene cluster is comprised of six genes, each encoding a functional protein. In contrast, human genes (ψγγE and ψγγF) are pseudogenes (Meakin, Du et al., 1987), and only the gammaC- and gammaD-crystallins are significantly expressed in lens. Six members of the beta-crystallin gene family are dispersed on three mammalian chromosomes. The human genome has an additional betaB2-crystallin-derived pseudogene linked to the functional betaB2-crystallin locus and conversion to the pseudogene results in human congenital cataract (Vanita et al., 2001). The β/γ proteins share a highly stable structure comprising two domains connected by a connecting peptide. Each domain comprises motifs, each forming a Greek key fold forming a β-sandwich structure. The gamma-crystallins are found as monomers while the beta-crystallins, similarly to alpha-crystallins, associate into higher order complexes.
A series of studies over the past 15 years also demonstrated the expression of crystallins in numerous cell types and tissues other than the lens and their roles in cell survival regulation, including in the central nervous system. Several studies, including a comparative study by Zabel et al. (Zabel, Sagi et al., 2006) identified the crystallins among the proteins altered in various central nervous system neurodegenerative disorders, including those affecting the retina. This review will focus on the novel implication of crystallins, mainly of the alpha subfamily, in the pathogenesis of retinal diseases or retinal complications of systemic diseases (Table 1).
Table 1.
Summary of recent discoveries on the role of crystallins in retinal diseases.
| Disease | Crystallins | Changes | Associated proteins, effects or localization |
|---|---|---|---|
| Diabetes (Kumar et al., 2005; Fort et al., 2009) | All crystallins (αA and αB, β and γ) | Upregulation | Bcl-Xs, Bax, caspases |
| Retinopathy of prematurity (OIR) (Kase et al., 2010) | αB-crystallin | Upregulation | Hif1a, VEGF |
| Optic nerve injury (Munemasa et al., 2009) | αA and αB-crystallins | Downregulagion (protective when overexpressed) | Expressed in GC |
| Autoimmune uveitis (Rao et al., 2008: Saraswathy and Rao, 2009) | αA and β-crystallins; NOT αB | Upregulation | MnSOD (no interaction proven): interaction with nitrated Cyto c |
| Endophthalmitis (bacterial infection) (Whiston et al., 2008) | αB-crystallin | Increased cleaved form | Increased caspase-3 |
| Ischemia (Kalesnykas et al. 2008) | αB-crystallin | Upregulation | In GC (along with other hsps) |
| Age-related Macular degeneration (Umeda et al., 2005) | βb2-crystallin | Accumulation in Drusen | No interaction but lots of proteins in Drusen |
| Chemically induced hypoxia (Yaung et al., 2008) | αA and αB- crystallin | Dose responsive (low dose = Upregulation: = high dose Downregulation) | Mitochondrial localization |
2. Crystallins in retinal diseases
Recent studies have shown increased levels of alpha-crystallins in different models of acutely induced retinal degeneration, including those due to light toxicity and retinal trauma (Sakaguchi, Miyagi et al., 2003; Vazquez-Chona, Song et al., 2004; Steele, Inman et al., 2006). In both models, alphaA- and alphaB-crystallin mRNAs and proteins increased within the first few days following the insult, suggesting that alpha-crystallins play an important role in the early phases of those retinal degenerations. One of the observations demonstrating the relevance to human pathologies has been the discovery that alpha-crystallins are concentrated in drusen in monkey and human retinas, a specific deposit associated with age-related macular degeneration (Johnson, Brown et al., 2005; Umeda, Suzuki et al., 2005). Understanding the functions and regulation of the crystallins in retina, especially in the context of disease conditions, has greatly improved by the use of transgenic mice combined with animal models of several neurodegenerative diseases. Original characterization of alpha-crystallin knockout animals showed the only anomalies presented in alphaA-crystallin knockouts were smaller lenses, coupled with late and progressive opacification (Brady, Garland et al., 1997). No obvious perinatal defects were detected in mice lacking alphaB-crystallin, and their lenses remained transparent. However, as they aged, alphaB-crystallin homozygous knockout mice show postural defects and other health problems from progressive myopathy (Brady, Garland et al., 2001). Studies using knockout animals have shown that alphaA- and/or alphaB-crystallins are crucial in other retinal pathologies including hypoxia, Staphylococcus aureus induced endophthalmitis and uveitis (Rao, Saraswathy et al., 2008; Whiston, Sugi et al., 2008; Yaung, Kannan et al., 2008). In all of these studies, no obvious defects were noticed in non-pathological conditions, but the absence of alphaA- and/or alphaB-crystallins dramatically enhanced pathological features. In both the endophthalmitis and uveitis models, AlphaA- and alphaB-crystallin knockout animals showed increased retinal cell death as measured by TUNEL positive cell counts. In the endophthalmitis model, increase retinal folding and bleeding were also observed in absence of alphaB-crystallin and correlated with decreased retinal function analyzed by electro-retinogram. In the uveitis model, histological analysis demonstrated that absence of either one of the alpha-crystallin led to a large increase in retinal degeneration marked by decrease cell number in both inner and outer retinal layers. These results suggest that crystallins have conserved adaptive roles in retinal stress conditions and are essential for retinal cell survival in such conditions.
The role of crystallins in retinal complications of systemic diseases has been studied particularly in diabetes, where crystallin upregulation appears to be a hallmark of diabetic retinopathy. Multiple studies have now demonstrated that both alphaA- and alphaB-crystallins are upregulated in the retina from several animal models of diabetes including streptozotocin and alloxan induced diabetes, spontaneously diabetic OLETF, and rats with diabetes due to a high fat diet (Kumar, Haseeb et al., 2005; Kim, Choi et al., 2007; Wang, Wu et al., 2007; Fort, Freeman et al., 2009). The upregulation of alpha-crystallin in several different models of diabetes strongly suggests that alpha-crystallins somehow play a role in the pathophysiology of the disease.
Kase et al. (2010) recently demonstrated that in addition to its neuroprotective role described above, alphaB-crystallin plays an important role in other aspects of retinal pathophysiology, including retinal vascular integrity through modulation of vascular endothelial growth factor (VEGF). The authors showed that alphaB-crystallin was essential for the regulation of VEGF function, especially for the regulation of vascular permeability, neovascularization and angio-genesis as demonstrated using the oxygen induced retinopathy (OIR) and choroidal neovascularization (CNV) models.
3. Crystallins and inflammation
As mentioned previously, crystallins are involved in numerous pathologies, particularly those showing major inflammatory response, such as multiple sclerosis. The eye is an immune-privileged site where inflammation is inhibited to prevent damage to the delicate ocular tissues and preserve vision. In conditions such as endophthalmitis, uveitis or retinal trauma, the immune privilege is overcome and inflammation is induced within the eye, leading to retinal degeneration. Studies of these pathologies in wild-type and alpha-crystallin knockout animals strongly suggest that alpha-crystallin overexpression could be a protective mechanism to prevent retinal cell death during inflammation (Rao, Saraswathy et al., 2008; Whiston, Sugi et al., 2008). AlphaA- and alphaB-crystallins were also crucial in preventing ultraviolet light (UVA)-induced photoreceptor apoptosis in the retina (Liu, Schlosser et al., 2004). It has also been proposed that alpha-crystallin upregulation in these diseases could be part of the mechanism leading to neurodegeneration because antibodies against alphaA- and/or alphaB-crystallins are found in serum from patients with uveitis and multiple sclerosis, as well as in mice with experimental autoimmune encephalomyelitis (Ousman, Tomooka et al., 2007; Chen, Holland et al., 2008). However, autoimmune attack on alphaB-crystallin is not the direct cause of tissue damage. Rather, it worsens the severity of damage by simultaneously eliminating anti-inflammatory actions of alpha-crystallins, and reducing the ability of alpha-crystallin to inhibit programmed cell death in glial cells (Ousman, Tomooka et al., 2007).
4. Crystallins and apoptosis
The first demonstrated non-structural function of crystallins was the protective chaperone function. Recent studies confirm that the increased alpha-crystallin expression in retinal diseases is neuroprotective rather than part of the disease mechanism, and that this neuroprotective effect of alphaA- and alphaB-crystallins occurs through several anti-apoptotic pathways. First, alphaA- and alphaB-crystallin expression increases following retinal insults that coincide with increased Bax, caspase-3 and other proteins involved in apoptosis and neurodegeneration (Vazquez-Chona, Song et al., 2004). Second, alphaB-crystallin is abundantly expressed in breast and kidney cancers (Holcakova, Hernychova et al., 2008; Sitterding, Wiseman et al., 2008). AlphaB-crystallin also inhibits apoptosis induced by various stimuli, including DNA-damaging agents such as TNF-alpha and Fas (Mehlen, Kretz-Remy et al., 1996a; Mehlen, Schulze-Osthoff et al., 1996b) and growth factor deprivation, and by disrupting the proteolytic activation of caspase-3 (Kamradt, Chen et al., 2001; Kamradt, Chen et al., 2002). Kamradt et al. (Kamradt, Lu et al., 2005) also showed that alphaB-crystallin inhibits TRAIL (Tumor necrosis factor-related apoptosis-inducing ligand) induced apoptosis through suppression of caspase-3 activation. In this study, the authors showed that restoration of alphaB-crystallin expression in alphaB-crystallin deficient cells was sufficient to protect them from TRAIL-induced apoptosis, and that alphaB-crystallin knockdown by siRNA was sufficient to sensitize cancer cells to TRAIL-induced apoptosis. Another group showed that inhibition of stress-induced apoptosis by alphaB-crystallin could be due to its interaction with pro-apoptotic members of the Bcl-2 family, Bax and Bcl-Xs, and their resulting sequestration in the cytoplasm (Mao, Liu et al., 2004). AlphaA-crystallin overexpression in the retina during experimental uveitis was shown to have a neuroprotective effect on photoreceptor cells mediated by the interaction of alphaA-crystallin with cytochrome c and procaspase-3, thus preventing the activation of the latter and subsequent cell death induction (Rao, Saraswathy et al., 2008). AlphaB-crystallin confers stress-induced-apoptosis resistance to several retinal cell types including retinal pigment epithelium, pericytes, endothelial cells and astrocytes (Alge, Priglinger et al., 2002; Nagaraj, Oya-Ito et al., 2005; Ousman, Tomooka et al., 2007). All of these data strongly suggest that overexpression of crystallins, especially the alpha-crystallins, could be a protective mechanism for neurons to block apoptosis.
Interestingly, very few studies have directly examined the differential mechanisms of action of both alpha-crystallins. Liu et al (Liu, Schlosser et al., 2004) showed that alphaB-crystallin abrogates UVA-induced apoptosis in lens epithelial cells through repression of UVA-induced activation of the RAF/MEK/ERK pathway, whereas alphaA-crystallin activates the Akt survival pathway to counteract the UVA-induced apoptosis. They also showed that calcium-activated RAF/MEK/ERK signaling pathway mediates p53-dependent apoptosis and is abrogated by alphaB-crystallin through inhibition of Ras activation (Li, Liu et al., 2005). While these results demonstrate that the two alpha-crystallins have, at least in part, differential mechanisms of action against stress-induced apoptosis in the lens, it needs to be elucidated if such specificities also applies to non-lenticular tissues.
5. Crystallins regulation
In the lens, alpha-crystallin expression is mainly regulated at the transcription level, particularly via transcription factors of the Maf family, especially L-maf and c-maf (Cvekl and Duncan, 2007; Kataoka, 2007). Recent observations suggest that crystallin expression and activity are also regulated during diseases by modulation of their translation rate (Fort et al. unpublished data) as well as through post-translational modifications, such as cleavage leading to inactivation (Whiston, Sugi et al., 2008). Further, the assembly of alphaB-crystallin into large homo- and hetero-oligomeric chaperone complexes is negatively regulated by stress-induced phosphorylation on serine residues 19, 45, and 59, and the dissociated complexes have diminished chaperone activity (Ito, Okamoto et al., 1997; Ito, Kamei et al., 2001). A recent study suggested that alphaB-crystallin expression could be induced in astrocytes in culture by TGFβ1 and TGFβ2, linking this pathway to the hypoxia-induced overexpression of crystallin in retinal astrocytes (Yu, Fuchshofer et al., 2007). Furthermore, alpha-crystallin expression in the retina was down-regulated following steroid induced ocular hypertension related to ganglion cell death (Miyara, Shinzato et al., 2008).
6. Post-translational modifications of crystallins
Protein functions are tightly regulated by diverse mechanisms including through post-translational modifications. Post-translational modifications such as deamidation, racemization, truncation and phosphorylation have been long reported in the lens, specifically during aging (Lampi, Ma et al., 1998; Shih, Lampi et al., 1998). Post-translational modifications such as phosphorylation alters the surface charge of the protein, leading to conformational changes, which in turn may alter protein–protein interactions and impair chaperone function (Aquilina, Benesch et al., 2004). In the retina of diabetic rats gamma-crystallins undergo post-translational modifications, such as tyrosine nitration, while alphaB-crystallin is differentially phosphorylated (Kim, Choi et al., 2007; Zhan, Du et al., 2008). Kim et al also demonstrated that in spontaneously diabetic OLETF rats, increased expression of alpha-crystallins correlates with increased phosphorylation on serine residues 45 and 59, which negatively regulates alpha-crystallin chaperone activity (Ito, Okamoto et al., 1997; Ito, Kamei et al., 2001). Preferential glycation of alpha-crystallin was also observed in diabetic rat and human lens (Perry, Swamy et al., 1987; Swamy and Abraham, 1987; Swamy, Tsai et al., 1993) and correlated with increased size of aggregates formed by alpha-crystallins (Swamy, Tsai et al., 1993), leading to decreased chaperone activity of the protein (Cherian and Abraham, 1995; Shroff, Cherian-Shaw et al., 2000). All these studies demonstrate that the detailed identification of these modifications in retina is crucial to understand the function and regulation of alpha-crystallins in diseases such as diabetes and may lead to new therapeutic approaches.
7. Crystallins and therapies
Intravitreal injections of alpha-crystallin has a positive outcome on retinal cell survival post-trauma such as in optic nerve crush. In these studies, alpha-crystallin administration prior to, or at the time of the injury, prevented retinal ganglion cell axon degeneration and decreased microglial activation, as shown by prevention of the upregulation of TNF-alpha and iNOS production (Wu et al. 2009; Ying et al. 2008). Interestingly, another set of publications demonstrated that fragments of alpha- or beta-crystallin could regulate crystallin functions, including their chaperone function. These studies showed that peptides of alphaA-, alphaB- and betaA1/A3-crystallin could regulate the oligomerization of crystallins and their refractive properties as well as the chaperone activity of alpha-crystallin (Santhoshkumar et al. 2009; Tanaka, JBC, 2008). Together, these studies suggest that the protective effect of crystallins on retinal cells could be obtained by local injection of specific small peptides corresponding to sequences of alpha-crystallins, an interesting approach which would have multiple advantages, including size, specificity of action and stability.
8. Beta- and gamma-crystallins and retinal diseases
In addition to the major emphasis on crystallins of the alpha-subfamily, numerous studies have demonstrated that beta- and gamma-crystallins were also playing critical roles in retinal diseases. Beta- and gamma-crystallin expression increases in animal models of retinal diseases such as glaucoma, retinitis pigmentosa, light-induced damage and diabetic retinopathy (Organisciak, Darrow et al., 2006; Piri, Song et al., 2007; Fort, Freeman et al., 2009). Studies of both experimental and genetically elevated intraocular pressure demonstrated decreased levels of expression of beta-crystallins including βB2- and βA3/A1-crystallins (Ahmed, Brown et al., 2004; Naskar and Thanos, 2006; Steele, Inman et al., 2006; Piri, Song et al., 2007), and decreased gamma-crystallin mRNA levels (Steele, Inman et al., 2006). Organisciak et al. (Organisciak, Darrow et al., 2006) also showed that beta-and gamma-crystallins are upregulated prior to photoreceptor loss in genetic animal models of retinitis pigmentosa, while studies by Fort et al. and Saraswathy et al. (Fort, Freeman et al., 2009; Saraswathy and Rao, 2009) showed increased expression of beta- and/or gamma-crystallins, respectively, in diabetic retinopathy and experimental autoimmune uveitis models. In the light-induced toxicity model, beta- and gamma-crystallins are induced after 3 h, but after 8 h beta-crystallin levels returned to normal and gamma-crystallins were down-regulated (Sakaguchi, Miyagi et al., 2003; Organisciak, Darrow et al., 2006). Another study demonstrated that 24 h of recovery led to increased levels of αA- and βB3-crystallins (Tanito, Haniu et al., 2006). While the exact role of beta- and gamma-crystallin proteins in these diseases remains to be fully elucidated, these studies demonstrated that beta- and gamma-crystallins can promote ganglion cell axon regrowth. βB2-crystallin was first shown to be highly expressed in regenerating ganglion cells in which they have an autocrine effect promoting retinal ganglion cell axon regrowth (Liedtke, Schwamborn et al., 2007). This was among the first evidence suggesting that beta-crystallin proteins could also be secreted and be neuroprotective through other mechanisms. A subsequent study then demonstrated that this effect was related to increased inflammation and activation of the CNTF-STAT3 pathway (Fischer, Hauk et al., 2008). The authors showed that gamma- but not alpha-crystallins had a similar action, demonstrating again the differential function of crystallins in retina and retinal diseases. However, several of these studies found that beta- and gamma-crystallins, like alpha-crystallins, were targeted for post-translational modifications (Organisciak, Darrow et al., 2006; Tanito, Haniu et al., 2006; Liedtke, Schwamborn et al., 2007; Fort, Freeman et al., 2009).
Collectively, these studies show that beta- and gamma-crystallin expression can be regulated at both transcriptional and translational levels and they vary with disease duration. These studies also strongly suggest that crystallin content and modifications occur as a function of the type of retinal degeneration and that each individual crystallin can be affected in a different manner or extent. Interestingly, it has also been reported that beta-crystallins can play a critical role in normal retinal development as demonstrated by the retinal abnormalities observed when βA3/A1-crystallin gene is mutated (Gehlbach, Hose et al., 2006). The authors also showed that βA3/A1-crystallin was involved in the initial patterning and subsequent remodeling of the retinal vasculature during development (Sinha, Klise et al., 2008).
9. Conclusion
Alpha-crystallin expression and functions are altered in nearly every neurodegenerative disorder, demonstrating the emerging concept that these proteins are key factors in the pathogenesis of central nervous system diseases. Alpha-crystallins control critical aspects of cell biology (cell survival regulation through inhibition of apoptosis) and integrated biology (angiogenesis regulation by controlling the expression and localization of vascular endothelial growth factor). Alpha-crystallin functions are regulated by adaptive and maladaptive post-translational modifications (Fig. 1). Understanding this evolutionarily conserved general response to metabolic stresses could lead to new therapeutic opportunities for a wide range of neurovascular disorders.
Fig. 1.
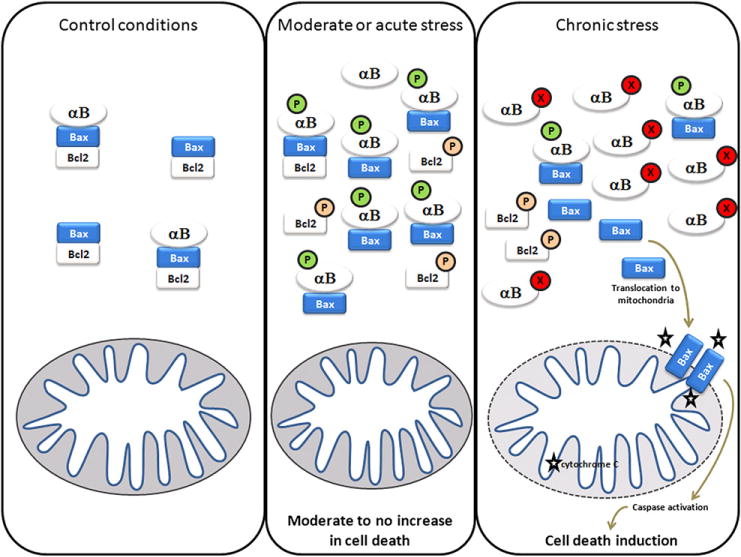
Model of alpha-crystallin functions during chronic (protective) and acute (impaired) stress conditions. In control conditions, anti-apoptotic proteins such as bcl-2 interact and prevent the activation of pro-apoptotic proteins such as Bax. When cells are exposed to a moderate or acute stress, different signaling pathways can be activated leading to the inactivation of the anti-apoptotic member of that complex. Parallel pathways lead to increase AlphaB-crystallin (and alphaA-crystallin) expression as well as anti-apoptotic activity (including phosphorylation) through interaction and inhibition of pro-apoptotic proteins such as Bax and Bcl-Xs. In chronic stress conditions such as diabetes, retinopathy of prematurity or uveitis, alpha-crystallins undergo post-translational modifications such as nitration, glycation, deamidation which inhibit their anti-apoptotic function and lead to cell death induction. (P: phosphorylation; X: unidentified post-translational modification).
References
- Ahmed F, Brown KM, et al. Microarray analysis of changes in mRNA levels in the rat retina after experimental elevation of intraocular pressure. Invest Ophthalmol Vis Sci. 2004;45(4):1247–1258. doi: 10.1167/iovs.03-1123. [DOI] [PubMed] [Google Scholar]
- Alge CS, Priglinger SG, et al. Retinal pigment epithelium is protected against apoptosis by alphaB-crystallin. Invest Ophthalmol Vis Sci. 2002;43(11):3575–3582. [PubMed] [Google Scholar]
- Aquilina JA, Benesch JL, et al. Phosphorylation of alphaB-crystallin alters chaperone function through loss of dimeric substructure. J Biol Chem. 2004;279(27):28675–28680. doi: 10.1074/jbc.M403348200. [DOI] [PubMed] [Google Scholar]
- Bloemendal H, de Jong WW. Lens proteins and their genes. Prog Nucleic Acid Res Mol Biol. 1991;41:259–281. doi: 10.1016/s0079-6603(08)60012-4. [DOI] [PubMed] [Google Scholar]
- Brady JP, Garland D, et al. Targeted disruption of the mouse alpha A-crystallin gene induces cataract and cytoplasmic inclusion bodies containing the small heat shock protein alpha B-crystallin. Proc Natl Acad Sci U S A. 1997;94(3):884–889. doi: 10.1073/pnas.94.3.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady JP, Garland DL, et al. AlphaB-crystallin in lens development and muscle integrity: a gene knockout approach. Invest Ophthalmol Vis Sci. 2001;42(12):2924–2934. [PubMed] [Google Scholar]
- Chen L, Holland GN, et al. Associations of seroreactivity against crystallin proteins with disease activity and cataract in patients with uveitis. Invest Ophthalmol Vis Sci. 2008;49(10):4476–4481. doi: 10.1167/iovs.08-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherian M, Abraham EC. Decreased molecular chaperone property of alpha-crystallins due to posttranslational modifications. Biochem Biophys Res Commun. 1995;208(2):675–679. doi: 10.1006/bbrc.1995.1391. [DOI] [PubMed] [Google Scholar]
- Cvekl A, Duncan MK. Genetic and epigenetic mechanisms of gene regulation during lens development. Prog Retin Eye Res. 2007;26(6):555–597. doi: 10.1016/j.preteyeres.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer D, Hauk TG, et al. Crystallins of the beta/gamma-superfamily mimic the effects of lens injury and promote axon regeneration. Mol Cell Neurosci. 2008;37(3):471–479. doi: 10.1016/j.mcn.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Fort PE, Freeman WM, et al. The retinal proteome in experimental diabetic retinopathy: up-regulation of crystallins and reversal by systemic and periocular insulin. Mol Cell Proteomics. 2009;8(4):767–779. doi: 10.1074/mcp.M800326-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlbach P, Hose S, et al. Developmental abnormalities in the Nuc1 rat retina: a spontaneous mutation that affects neuronal and vascular remodeling and retinal function. Neuroscience. 2006;137(2):447–461. doi: 10.1016/j.neuroscience.2005.08.084. [DOI] [PubMed] [Google Scholar]
- Groenen PJ, Merck KB, et al. Structure and modifications of the junior chaperone alpha-crystallin. From lens transparency to molecular pathology. Eur J Biochem. 1994;225(1):1–19. doi: 10.1111/j.1432-1033.1994.00001.x. [DOI] [PubMed] [Google Scholar]
- Holcakova J, Hernychova L, et al. Identification of alphaB-crystallin, a biomarker of renal cell carcinoma by SELDI-TOF MS. Int J Biol Markers. 2008;23(1):48–53. doi: 10.1177/172460080802300108. [DOI] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci U S A. 1992;89(21):10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Kamei K, et al. Phosphorylation-induced change of the oligomerization state of alpha B-crystallin. J Biol Chem. 2001;276(7):5346–5352. doi: 10.1074/jbc.M009004200. [DOI] [PubMed] [Google Scholar]
- Ito H, Okamoto K, et al. Phosphorylation of alphaB-crystallin in response to various types of stress. J Biol Chem. 1997;272(47):29934–29941. doi: 10.1074/jbc.272.47.29934. [DOI] [PubMed] [Google Scholar]
- Jaenicke R, Slingsby C. Lens crystallins and their microbial homologs: structure, stability, and function. Crit Rev Biochem Mol Biol. 2001;36(5):435–499. doi: 10.1080/20014091074237. [DOI] [PubMed] [Google Scholar]
- Jakob U, Gaestel M, et al. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993;268(3):1517–1520. [PubMed] [Google Scholar]
- Johnson PT, Brown MN, et al. Synaptic pathology, altered gene expression, and degeneration in photoreceptors impacted by drusen. Invest Ophthalmol Vis Sci. 2005;46(12):4788–4795. doi: 10.1167/iovs.05-0767. [DOI] [PubMed] [Google Scholar]
- Kamradt MC, Chen F, et al. The small heat shock protein alpha B-crystallin negatively regulates cytochrome c- and caspase-8-dependent activation of caspase-3 by inhibiting its autoproteolytic maturation. J Biol Chem. 2001;276(19):16059–16063. doi: 10.1074/jbc.C100107200. [DOI] [PubMed] [Google Scholar]
- Kamradt MC, Chen F, et al. The small heat shock protein alpha B-crystallin negatively regulates apoptosis during myogenic differentiation by inhibiting caspase-3 activation. J Biol Chem. 2002;277(41):38731–38736. doi: 10.1074/jbc.M201770200. [DOI] [PubMed] [Google Scholar]
- Kamradt MC, Lu M, et al. The small heat shock protein alpha B-crystallin is a novel inhibitor of TRAIL-induced apoptosis that suppresses the activation of caspase-3. J Biol Chem. 2005;280(12):11059–11066. doi: 10.1074/jbc.M413382200. [DOI] [PubMed] [Google Scholar]
- Kase S, He S, et al. AlphaB crystallin regulation of angiogenesis by modulation of VEGF. Blood. 2010;115(6):3398–3406. doi: 10.1182/blood-2009-01-197095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka K. Multiple mechanisms and functions of maf transcription factors in the regulation of tissue-specific genes. J Biochem. 2007;141(6):775–781. doi: 10.1093/jb/mvm105. [DOI] [PubMed] [Google Scholar]
- Kim YH, Choi MY, et al. Protein kinase C delta regulates anti-apoptotic alphaB-crystallin in the retina of type 2 diabetes. Neurobiol Dis. 2007;28(3):293–303. doi: 10.1016/j.nbd.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Kumar PA, Haseeb A, et al. Elevated expression of alphaA- and alphaB-crystallins in streptozotocin-induced diabetic rat. Arch Biochem Biophys. 2005;444(2):77–83. doi: 10.1016/j.abb.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Lampi KJ, Ma Z, et al. Age-related changes in human lens crystallins identified by two-dimensional electrophoresis and mass spectrometry. Exp Eye Res. 1998;67(1):31–43. doi: 10.1006/exer.1998.0481. [DOI] [PubMed] [Google Scholar]
- Landry J, Chretien P, et al. Heat shock resistance conferred by expression of the human HSP27 gene in rodent cells. J Cell Biol. 1989;109(1):7–15. doi: 10.1083/jcb.109.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DW, Liu JP, et al. Calcium-activated RAF/MEK/ERK signaling pathway mediates p53-dependent apoptosis and is abrogated by alpha B-crystallin through inhibition of RAS activation. Mol Biol Cell. 2005;16(9):4437–4453. doi: 10.1091/mbc.E05-01-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke T, Schwamborn JC, et al. Elongation of axons during regeneration involves retinal crystallin beta b2 (crybb2) Mol Cell Proteomics. 2007;6(5):895–907. doi: 10.1074/mcp.M600245-MCP200. [DOI] [PubMed] [Google Scholar]
- Liu JP, Schlosser R, et al. Human alphaA- and alphaB-crystallins prevent UVA-induced apoptosis through regulation of PKCalpha, RAF/MEK/ERK and AKT signaling pathways. Exp Eye Res. 2004;79(6):393–403. [PubMed] [Google Scholar]
- Lubsen NH, Aarts HJ, et al. The evolution of lenticular proteins: the beta-and gamma-crystallin super gene family. Prog Biophys Mol Biol. 1988;51(1):47–76. doi: 10.1016/0079-6107(88)90010-7. [DOI] [PubMed] [Google Scholar]
- Mao YW, Liu JP, et al. Human alphaA- and alphaB-crystallins bind to Bax and Bcl-X(S) to sequester their translocation during staurosporine-induced apoptosis. Cell Death Differ. 2004;11(5):512–526. doi: 10.1038/sj.cdd.4401384. [DOI] [PubMed] [Google Scholar]
- Meakin SO, Du RP, et al. Gamma-crystallins of the human eye lens: expression analysis of five members of the gene family. Mol Cell Biol. 1987;7(8):2671–2679. doi: 10.1128/mcb.7.8.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlen P, Kretz-Remy C, et al. Human hsp27, Drosophila hsp27 and human alphaB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these proteins against TNFalpha-induced cell death. EMBO J. 1996a;15(11):2695–2706. [PMC free article] [PubMed] [Google Scholar]
- Mehlen P, Schulze-Osthoff K, et al. Small stress proteins as novel regulators of apoptosis. Heat shock protein 27 blocks Fas/APO-1- and staurosporine-induced cell death. J Biol Chem. 1996b;271(28):16510–16514. doi: 10.1074/jbc.271.28.16510. [DOI] [PubMed] [Google Scholar]
- Miyara N, Shinzato M, et al. Proteomic analysis of rat retina in a steroid-induced ocular hypertension model: potential vulnerability to oxidative stress. Jpn J Ophthalmol. 2008;52(2):84–90. doi: 10.1007/s10384-007-0507-5. [DOI] [PubMed] [Google Scholar]
- Munemasa Y, Kwong JM, et al. The role of alphaA- and alphaB-crystallins in the survival of retinal ganglion cells after optic nerve axotomy. Invest Ophthalmol Vis Sci. 2009;50(8):3869–3875. doi: 10.1167/iovs.08-3138. [DOI] [PubMed] [Google Scholar]
- Nagaraj RH, Oya-Ito T, et al. Dicarbonyl stress and apoptosis of vascular cells: prevention by alphaB-crystallin. Ann N Y Acad Sci. 2005;1043:158–165. doi: 10.1196/annals.1333.020. [DOI] [PubMed] [Google Scholar]
- Naskar R, Thanos S. Retinal gene profiling in a hereditary rodent model of elevated intraocular pressure. Mol Vis. 2006;12:1199–1210. [PubMed] [Google Scholar]
- Organisciak D, Darrow R, et al. Genetic, age and light mediated effects on crystallin protein expression in the retina. Photochem Photobiol. 2006;82(4):1088–1096. doi: 10.1562/2005-06-30-RA-599. [DOI] [PubMed] [Google Scholar]
- Ousman SS, Tomooka BH, et al. Protective and therapeutic role for alphaB-crystallin in autoimmune demyelination. Nature. 2007;448(7152):474–479. doi: 10.1038/nature05935. [DOI] [PubMed] [Google Scholar]
- Perry RE, Swamy MS, et al. Progressive changes in lens crystallin glycation and high-molecular-weight aggregate formation leading to cataract development in streptozotocin-diabetic rats. Exp Eye Res. 1987;44(2):269–282. doi: 10.1016/s0014-4835(87)80011-8. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Lens crystallins. Innovation associated with changes in gene regulation. J Biol Chem. 1992;267(7):4277–4280. [PubMed] [Google Scholar]
- Piri N, Song M, et al. Modulation of alpha and beta crystallin expression in rat retinas with ocular hypertension-induced ganglion cell degeneration. Brain Res. 2007;1141:1–9. doi: 10.1016/j.brainres.2006.11.095. [DOI] [PubMed] [Google Scholar]
- Rao NA, Saraswathy S, et al. Elevated retina-specific expression of the small heat shock protein, alphaA-crystallin, is associated with photoreceptor protection in experimental uveitis. Invest Ophthalmol Vis Sci. 2008;49(3):1161–1171. doi: 10.1167/iovs.07-1259. [DOI] [PubMed] [Google Scholar]
- Robinson ML, Overbeek PA. Differential expression of alpha A- and alpha B-crystallin during murine ocular development. Invest Ophthalmol Vis Sci. 1996;37(11):2276–2284. [PubMed] [Google Scholar]
- Sakaguchi H, Miyagi M, et al. Intense light exposure changes the crystallin content in retina. Exp Eye Res. 2003;76(1):131–133. doi: 10.1016/s0014-4835(02)00249-x. [DOI] [PubMed] [Google Scholar]
- Santhoshkumar P, Murugesan R, et al. Deletion of (54)FLRAPSWF(61) residues decreases the oligomeric size and enhances the chaperone function of alphaB-crystallin. Biochemistry. 2009;48(23):5066–5073. doi: 10.1021/bi900085v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraswathy S, Rao NA. Mitochondrial proteomics in experimental autoimmune uveitis oxidative stress. Invest Ophthalmol Vis Sci. 2009 doi: 10.1167/iovs.08-2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sax CM, Piatigorsky J. Expression of the alpha-crystallin/small heat-shock protein/molecular chaperone genes in the lens and other tissues. Adv Enzymol Relat Areas Mol Biol. 1994;69:155–201. doi: 10.1002/9780470123157.ch5. [DOI] [PubMed] [Google Scholar]
- Shih M, Lampi KJ, et al. Cleavage of beta crystallins during maturation of bovine lens. Mol Vis. 1998;4:4. [PubMed] [Google Scholar]
- Shroff NP, Cherian-Shaw M, et al. Mutation of R116C results in highly oligomerized alpha A-crystallin with modified structure and defective chaperone-like function. Biochemistry. 2000;39(6):1420–1426. doi: 10.1021/bi991656b. [DOI] [PubMed] [Google Scholar]
- Sinha D, Klise A, et al. BetaA3/A1-crystallin in astroglial cells regulates retinal vascular remodeling during development. Mol Cell Neurosci. 2008;37(1):85–95. doi: 10.1016/j.mcn.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitterding SM, Wiseman WR, et al. AlphaB-crystallin: a novel marker of invasive basal-like and metaplastic breast carcinomas. Ann Diagn Pathol. 2008;12(1):33–40. doi: 10.1016/j.anndiagpath.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Steele MR, Inman DM, et al. Microarray analysis of retinal gene expression in the DBA/2J model of glaucoma. Invest Ophthalmol Vis Sci. 2006;47(3):977–985. doi: 10.1167/iovs.05-0865. [DOI] [PubMed] [Google Scholar]
- Swamy MS, Abraham EC. Lens protein composition, glycation and high molecular weight aggregation in aging rats. Invest Ophthalmol Vis Sci. 1987;28(10):1693–1701. [PubMed] [Google Scholar]
- Swamy MS, Tsai C, et al. Glycation mediated lens crystallin aggregation and cross-linking by various sugars and sugar phosphates in vitro. Exp Eye Res. 1993;56(2):177–185. doi: 10.1006/exer.1993.1025. [DOI] [PubMed] [Google Scholar]
- Tanito M, Haniu H, et al. Identification of 4-hydroxynonenal-modified retinal proteins induced by photooxidative stress prior to retinal degeneration. Free Radic Biol Med. 2006;41(12):1847–1859. doi: 10.1016/j.freeradbiomed.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Umeda S, Suzuki MT, et al. Molecular composition of drusen and possible involvement of anti-retinal autoimmunity in two different forms of macular degeneration in cynomolgus monkey (Macaca fascicularis) Faseb J. 2005;19(12):1683–1685. doi: 10.1096/fj.04-3525fje. [DOI] [PubMed] [Google Scholar]
- Vanita Sarhadi V, et al. A unique form of autosomal dominant cataract explained by gene conversion between beta-crystallin B2 and its pseudogene. J Med Genet. 2001;38(6):392–396. doi: 10.1136/jmg.38.6.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Chona F, Song BK, et al. Emporal changes in gene expression after injury in the rat retina. Invest Ophthalmol Vis Sci. 2004;45(8):2737–2746. doi: 10.1167/iovs.03-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YD, Wu JD, et al. Comparative proteome analysis of neural retinas from type 2 diabetic rats by two-dimensional electrophoresis. Curr Eye Res. 2007;32(10):891–901. doi: 10.1080/02713680701593702. [DOI] [PubMed] [Google Scholar]
- Whiston EA, Sugi N, et al. AlphaB-crystallin protects retinal tissue during staphylococcus aureus-induced endophthalmitis. Infect Immun. 2008;76(4):1781–1790. doi: 10.1128/IAI.01285-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistow G. Evolution of a protein superfamily: relationships between vertebrate lens crystallins and microorganism dormancy proteins. J Mol Evol. 1990;30(2):140–145. doi: 10.1007/BF02099940. [DOI] [PubMed] [Google Scholar]
- Wistow GJ, Piatigorsky J. Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Annu Rev Biochem. 1988;57:479–504. doi: 10.1146/annurev.bi.57.070188.002403. [DOI] [PubMed] [Google Scholar]
- Wu N, Wang YH, et al. Alpha-Crystallin downregulates the expression of TNF-alpha and iNOS by activated rat retinal microglia in vitro and in vivo. Ophthalmic Res. 2009;42(1):21–28. doi: 10.1159/000219681. [DOI] [PubMed] [Google Scholar]
- Yaung J, Kannan R, et al. Exacerbation of retinal degeneration in the absence of alpha crystallins in an in vivo model of chemically induced hypoxia. Exp Eye Res. 2008;86(2):355–365. doi: 10.1016/j.exer.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying X, Zhang J, et al. Alpha-crystallin protected axons from optic nerve degeneration after crushing in rats. J Mol Neurosci. 2008;35(3):253–258. doi: 10.1007/s12031-007-9010-1. [DOI] [PubMed] [Google Scholar]
- Yu AL, Fuchshofer R, et al. Hypoxia/reoxygenation and TGF-beta increase alphaB-crystallin expression in human optic nerve head astrocytes. Exp Eye Res. 2007;84(4):694–706. doi: 10.1016/j.exer.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Zabel C, Sagi D, et al. Comparative proteomics in neurodegenerative and non-neurodegenerative diseases suggest nodal point proteins in regulatory networking. J Proteome Res. 2006;5(8):1948–1958. doi: 10.1021/pr0601077. [DOI] [PubMed] [Google Scholar]
- Zhan X, Du Y, et al. Targets of tyrosine nitration in diabetic rat retina. Mol Cell Proteomics. 2008;7(5):864–874. doi: 10.1074/mcp.M700417-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


