Abstract
Context:
Consumption of high-fructose diets promotes hepatic fatty acid synthesis (de novo lipogenesis [DNL]) and an atherogenic lipid profile. It is unclear whether these effects occur independent of positive energy balance and weight gain.
Objectives:
We compared the effects of a high-fructose, (25% of energy content) weight-maintaining diet to those of an isocaloric diet with the same macronutrient distribution but in which complex carbohydrate (CCHO) was substituted for fructose.
Design, Setting, and Participants:
Eight healthy men were studied as inpatients for consecutive 9-day periods. Stable isotope tracers were used to measure fractional hepatic DNL and endogenous glucose production (EGP) and its suppression during a euglycemic-hyperinsulinemic clamp. Liver fat was measured by magnetic resonance spectroscopy.
Results:
Weight remained stable. Regardless of the order in which the diets were fed, the high-fructose diet was associated with both higher DNL (average, 18.6 ± 1.4% vs 11.0 ± 1.4% for CCHO; P = .001) and higher liver fat (median, +137% of CCHO; P = .016) in all participants. Fasting EGP and insulin-mediated glucose disposal did not differ significantly, but EGP during hyperinsulinemia was greater (0.60 ± 0.07 vs 0.46 ± 0.06 mg/kg/min; P = .013) with the high-fructose diet, suggesting blunted suppression of EGP.
Conclusion:
Short-term high-fructose intake was associated with increased DNL and liver fat in healthy men fed weight-maintaining diets.
The prevalence of obesity, diabetes, and nonalcoholic fatty liver disease is increasing worldwide (1–3). Although the etiology of this alarming trend is multifactorial, several studies have pointed to a role of added sugar (4–6) and, in particular, to high-fructose corn syrup (4). Diets containing high amounts of fructose are associated with increased triglyceride (7–12) apolipoprotein B100, and small, dense, low-density lipoprotein (LDL) levels (10), resulting in an atherogenic lipid profile that is associated with an increased risk of cardiovascular disease (13) and diabetes (14, 15). Long-term fructose overfeeding also increases visceral and liver fat (10, 16, 17), which, of themselves, are associated with increased risk of diabetes (18) and cardiovascular disease (19). One metabolic process that may link these deleterious effects is the conversion of fructose to fat (hepatic de novo lipogenesis [DNL]). Compared with glucose, which is primarily metabolized in extrahepatic tissues, fructose is primarily metabolized in the liver, where it bypasses key initial regulatory steps in the glycolytic pathway, thus providing an unregulated source of acetyl coenzyme A for DNL (20).
The potentially deleterious effects of high-carbohydrate diets (21) and in particular diets high in fructose have received considerable attention recently (4, 5, 22–24) and have contributed to recommendations to limit sugar consumption (25). A recent meta-analysis suggested that the deleterious effects of fructose occur only in the presence of overfeeding (26) and most human studies published to date are confounded by the fact that fructose was provided in the context of excess energy intake and weight gain. It is unclear, therefore, whether these effects also occur during energy balance or weight stability. To determine the effects of a high-fructose diet on DNL and liver fat in the absence of overfeeding, we performed inpatient studies in healthy volunteers, strictly controlling for energy intake. We compared the effects of short-term feeding of a diet high in fructose with those of an isocaloric diet in which complex carbohydrate (CCHO) was substituted for fructose, on DNL and liver fat (primary outcomes), and triglycerides, body composition, and hepatic and whole-body insulin sensitivity (secondary outcomes).
Materials and Methods
Participants
Eight healthy men, ages 18–65 years, with body mass index less than 30 kg/m2, were recruited from the community. At screening, participants were required to have a fasting insulin level less than 14 μU/mL, total cholesterol less than 200 mg/dL, and triglycerides less than 150 mg/dL. Key exclusion criteria included evidence of liver disease (alanine transaminase or aspartate transaminase above the upper limit of normal; hepatitis C antibody or hepatitis B surface antigen positive, or liver fat > 5% by proton magnetic resonance spectroscopy [MRS], as described below), fasting glucose greater than 100 mg/dL, current use of any antidiabetic or hypolipidemic agents, or HIV infection. All procedures followed were in accordance with the ethical standards of the University of California, San Francisco and Touro University Institutional Review Boards, and in accordance with the Helsinki Declaration of 1975 as revised in 1983. No patient names, initials, or hospital identification numbers were reported, and written informed consent was obtained from each participant before screening.
Study design
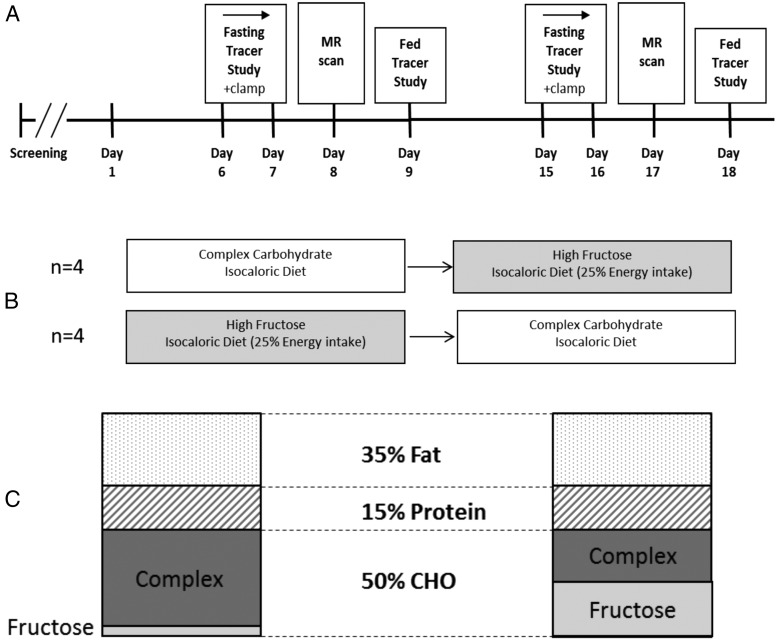
Participants were hospitalized in the University of California, San Francisco Clinical and Translational Science Institute Clinical Research Center (CRC) at San Francisco General Hospital (SFGH) for 18 consecutive days (Figure 1A). They were fed weight-maintaining diets with fixed amounts of protein (15% of total energy intake), fat (35%), and carbohydrate (50%). Diets high in fructose (20–25% of energy intake as fructose in the form of a specially formulated fructose-containing beverage) or containing an isocaloric amount of CCHO substituted for fructose were fed during consecutive 9-day periods (Figure 1, B and C). Complex carbohydrates provided in both the CCHO and high-fructose diets were from solid foods including cereal, bread, pasta, rice, potato, and baked items developed for this protocol to standardize the menus, but in variable amounts to meet the goal of providing 50% of total calories from carbohydrate. The CCHO diet provided 5% total calories as fructose in the form of fruit and sucrose baked into muffins and cookies. The CCHO diet provided 28 g per day of fiber and the high-fructose diet, 17 g per day of fiber. All foods were provided to study participants, and nonstudy foods were not allowed. The order of feeding was not randomized; the first four participants consumed the CCHO diet first, followed by the high-fructose diet. The other four participants were fed the diets in the reverse order.
Figure 1.
Details of study design. A, Overview of the study protocol depicting the timing of the stable isotope infusions, euglycemic-hyperinsulinemic clamp, and magnetic resonance studies. B, Four participants started with the complex carbohydrate diet followed by the high-fructose diet; the other four were fed the diets in the opposite sequence. C, Both diets had identical energy and macronutrient content; the complex carbohydrate diet had a very low fructose content (5% of total energy intake) whereas fructose accounted for half of the total carbohydrate intake on the high-fructose diet (25% of total energy intake).
Participants were weighed each morning, and energy intake was adjusted as needed to maintain a constant weight. Whole-body fat and lean body mass were measured on day 2 (baseline) and at the end of each dietary period by dual-energy x-ray absorptiometry (Hologic Discovery Wi, New Bedford, MA). All meals and snacks were prepared in the metabolic kitchen of the CRC. Participants were allowed to leave the CRC only when accompanied by a member of the research staff.
Stable isotope tracer studies of DNL and hepatic and peripheral insulin sensitivity
On the sixth day of each dietary period, participants fasted after 1700 h. At 1900 h, iv catheters were placed in each arm for infusion of the isotope solutions and periodic blood sampling. At 2000 h, a primed constant infusion of Na [1-13C] acetate (0.35 g/h) was started for measurement of fasting DNL. At 0430 h the following day, primed, continuous infusions of [U-13C] glucose (1.2 mg/kg/h), and [2-13C] glycerol (15 mg/kg lean body mass/h) were started for measurement of endogenous glucose production (EGP) and gluconeogenesis, respectively. Blood samples were obtained every 10 minutes between 0800 and 0830 h for steady-state fasting measurements. The isotope infusions continued, and a 3-hour euglycemic-hyperinsulinemic clamp (27) was started at 1000 h. After collection of fasting blood samples, insulin was infused at a rate of 40 mIU/m2·min. Blood samples were collected at 5-minute intervals from a retrograde iv line placed in a hand that was warmed in a heated box at 50–55°C. Whole-blood glucose concentrations were measured in real time (YSI Stat 2300 glucose analyzer, Yellow Springs, OH). A variable infusion of 20% dextrose (labeled with 0.6% [U-13C] glucose) was adjusted to maintain blood glucose concentrations at the baseline level. Additional blood samples were collected at 30-minute intervals for post-hoc batch analysis of insulin. During the final 30 minutes of the clamp, blood samples were collected every 10 minutes for determination of EGP and gluconeogenesis under conditions of steady-state hyperinsulinemia.
Substrate oxidation by indirect calorimetry
Oxygen consumption and carbon dioxide production were measured by indirect calorimetry (DeltaTrac II metabolic cart, SensorMedics, Yorba Linda, CA) for calculation of resting energy expenditure, and substrate oxidation rates (28).
Stable isotope tracer study of DNL during feeding
On the ninth day of each dietary period, after fasting overnight, participants began consuming hourly liquid meals at 0600 h. The liquid meals were formulated to simulate the participant's assigned diet (CCHO or fructose) and continued until midnight. At 1000 h, another primed constant infusion of labeled acetate (as described above) was started. Indirect calorimetry was repeated periodically before and during the feeding study. Overall substrate utilization during feeding was calculated by averaging eight 15–25-minute measurements of indirect calorimetry (0700–0800, 0900–1000, 1100–1200, 1300–1400, 1500–1600, 1700–1800, 1900–2000, and 2100–2200 h.).
Liver fat by magnetic resonance spectroscopy
Magnetic resonance (MR) exams were performed on a 3 Tesla scanner (GE Healthcare, Waukesha, WI). The proton MRS was acquired from a 20-cc voxel placed in the liver, using a 64-acquisition time series for water-suppressed spectra and an 8-acquisition time series for water-unsuppressed spectra with a repetition time of 2500 milliseconds and an echo time of 27–38 milliseconds. Further details are published (29). All spectra were acquired in the late afternoon to early evening, generally from 1700–1800 h. A standardized snack was provided at 1530 h. The MR spectra were postprocessed as described elsewhere (29) to reduce the effects of respiratory motion and to generate peak areas for lipids and water. These measures were corrected for differential MR transverse relaxation time decay by using global values for transverse relaxation time of 66 milliseconds for lipids and 30 milliseconds for water, based on measurements in healthy controls seen at this MR facility. The MRS-measured liver fat was calculated as total lipids/(total lipids + unsuppressed water). Paired data were not available for one subject due to a technical issue with the scanner hardware on the day of the test.
Stable isotope analyses and calculations
Chylomicrons were separated by ultracentrifugation (30 minutes at 33 000 rpm) and the separated very low-density lipoprotein (17 hours at 40 000 rpm) was used to isolate triglycerides by thin-layer chromatography. For determination of DNL and gluconeogenesis, palmitate and glucose isotopomer distribution (30) and tracer-to-tracee ratios were determined by gas chromatography–mass spectrometry. EGP (rate of appearance of glucose) was calculated using standard dilution techniques (31). Fasting DNL was calculated as the average of plateau values measured at 0600, 0800, and 1000 h. The integrated DNL during feeding was calculated as the area under the curve (AUC) of measurements over 14 hours. Glycogenolysis was calculated by subtracting gluconeogenesis from EGP.
Measures of lipid and glucose metabolism
Serum insulin concentrations were measured by RIA (Linco Research, Inc., St. Charles, MO). Whole-body insulin-mediated glucose uptake during the final hour of the clamp was calculated using both infused and endogenously produced glucose and adjusted for steady-state insulin levels. Fasting samples collected on the seventh day of each feeding period were collected for measurement of lipids in the clinical laboratory at SFGH. Postprandial triglyceride levels were also measured at 1400 and 1500 h during the tracer/feeding study and averaged.
Statistical methods
Data are presented as the mean ± SEM unless otherwise noted. For normally distributed data, results during fructose and CCHO feeding were compared by paired t test. Nonnormally distributed data were analyzed by Wilcoxon signed rank sum test. Statistical analyses were performed using SigmaStat version 3.5 (Systat Software, Inc., Point Richmond, CA). A two-sided P < 0.05 was considered statistically significant.
Results
Clinical characteristics of participants
At screening, average age was 42 ± 3 years; body mass index, 24.4 ± 1.6 kg/m2; fasting insulin, 5.0 ± 1.7 μU/mL; glucose, 78 ± 2 mg/dL; and triglycerides, 68 ± 4 mg/dL. Four participants were African American, two caucasian, one Latino, and one of mixed race. No participants smoked cigarettes, and none took medications or dietary supplements during the inpatient study. All eight participants completed the 18-day inpatient study and tolerated the diets and all procedures. The diets were generally well tolerated, with no participants exhibiting symptoms of fructose malabsorption.
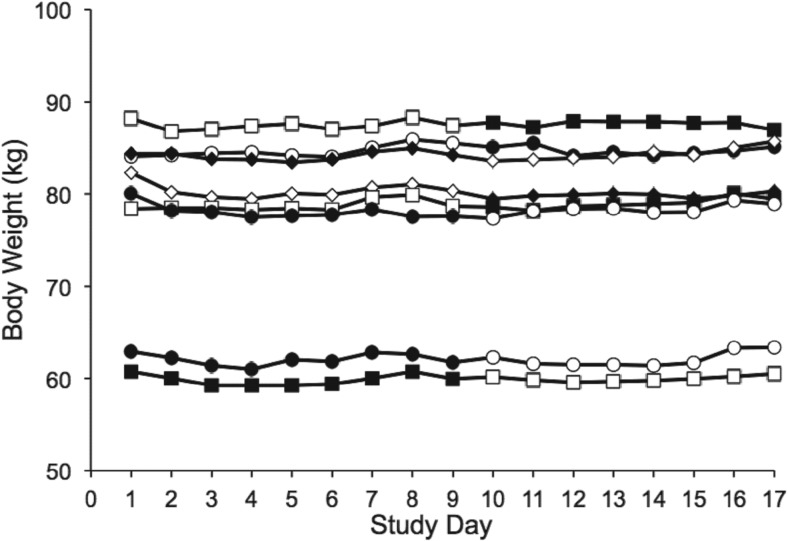
Weight and body composition
Weight stability was maintained in all participants (Figure 2), as was overall body fat content (baseline, 20.0 ± 0.02%; CCHO, 20.3 ± 0.02%; P = .39 vs baseline; fructose, 20.4 ± 0.02%; P = .84 vs baseline).
Figure 2.
Daily weight, obtained after overnight fasting, in the individual participants during the 18-day inpatient study. Open symbols depict weights obtained during complex carbohydrate feeding, filled symbols during high-fructose feeding. Weight remained stable in each participant studied, regardless of the order in which the diets were fed.
Lipid and glucose metabolism values for each dietary period
Fasting lipid, glucose, and insulin levels did not differ significantly between dietary periods (Table 1). Likewise, fasting EGP, gluconeogenesis, and glycogenolysis, measured by stable isotope tracers, and net glucose oxidation by indirect calorimetry did not differ significantly (Table 1).
Table 1.
Effects of CCHO Versus High-fructose Diet on Lipid and Glucose Metabolism
| CCHO Diet | High-fructose Diet | P Value | |
|---|---|---|---|
| Fasting lipids, mg/dL | |||
| Total cholesterol | 166 ± 10 | 167 ± 11 | .83 |
| LDL cholesterol | 107 ± 8 | 109 ± 10 | .69 |
| HDL cholesterol | 39 ± 2 | 38 ± 2 | .49 |
| Triglycerides | 102 ± 7 | 100 ± 7 | .67 |
| Fasting glucose, mg/dL | 75.2 ± 0.8 | 77.3 ± 0.9 | .21 |
| Glucose during clamp; last 30 min, mg/dL | 75.3 ± 1.4 | 75.0 ± 1.6 | .48 |
| Fasting insulin, μU/mL | 8.8 ± 0.9 | 9.0 ± 1.1 | .85 |
| Insulin during clamp; last 30 min, μU/mL | 77.5 ± 4.7 | 83.2 ± 4.8 | .21 |
| Total glucose uptake during clamp, mg/kg per min per μU/dL | 9.49 ± 1.50 | 10.33 ± 1.07 | .25 |
| Glucose oxidation by indirect calorimetry, mg/kg/min | |||
| Fasting | 1.50 ± 0.26 | 1.50 ± 0.18 | .98 |
| Hyperinsulinemic clamp | 2.79 ± 0.28 | 2.69 ± 0.39 | .79 |
| Nonoxidative glucose disposal | 51% | 66% | |
| Endogenous glucose production, Ra glucose; mg/kg/min | |||
| Fasting | 1.70 ± 0.08 | 1.95 ± 0.17 | .11 |
| Hyperinsulinemic clamp | 0.46 ± 0.06 | 0.60 ± 0.07 | .01 |
| Suppression by insulin | 71% | 66% | |
| Gluconeogenesis, mg/kg per min | |||
| Fasting | 0.56 ± 0.04 | 0.56 ± 0.04 | .96 |
| Hyperinsulinemic clamp | 0.02 ± 0.004 | 0.02 ± 0.004 | .83 |
| Suppression by insulin | 96% | 96% | |
| Glycogenolysis, mg/kg per min | |||
| Fasting | 1.14 ± 0.06 | 1.39 ± 0.14 | .06 |
| Hyperinsulinemic clamp | 0.44 ± 0.06 | 0.58 ± 0.06 | .01 |
| Suppression by insulin | 60% | 54% | |
Abbreviations: HDL, high-density lipoprotein; Ra, rate of appearance.
Data are mean ± sem. P-values by paired t test.
Insulin-mediated glucose uptake measured during a euglycemic hyperinsulinemic clamp did not differ significantly in the two dietary periods (Table 1). However, EGP during the clamp was significantly greater with the fructose diet (P = .012 vs CCHO), suggesting a blunting in the ability of insulin to suppress EGP. This difference was driven by a significantly higher rate of glycogenolysis (P = .013), whereas gluconeogenesis was equally suppressed (96%) with the two diets.
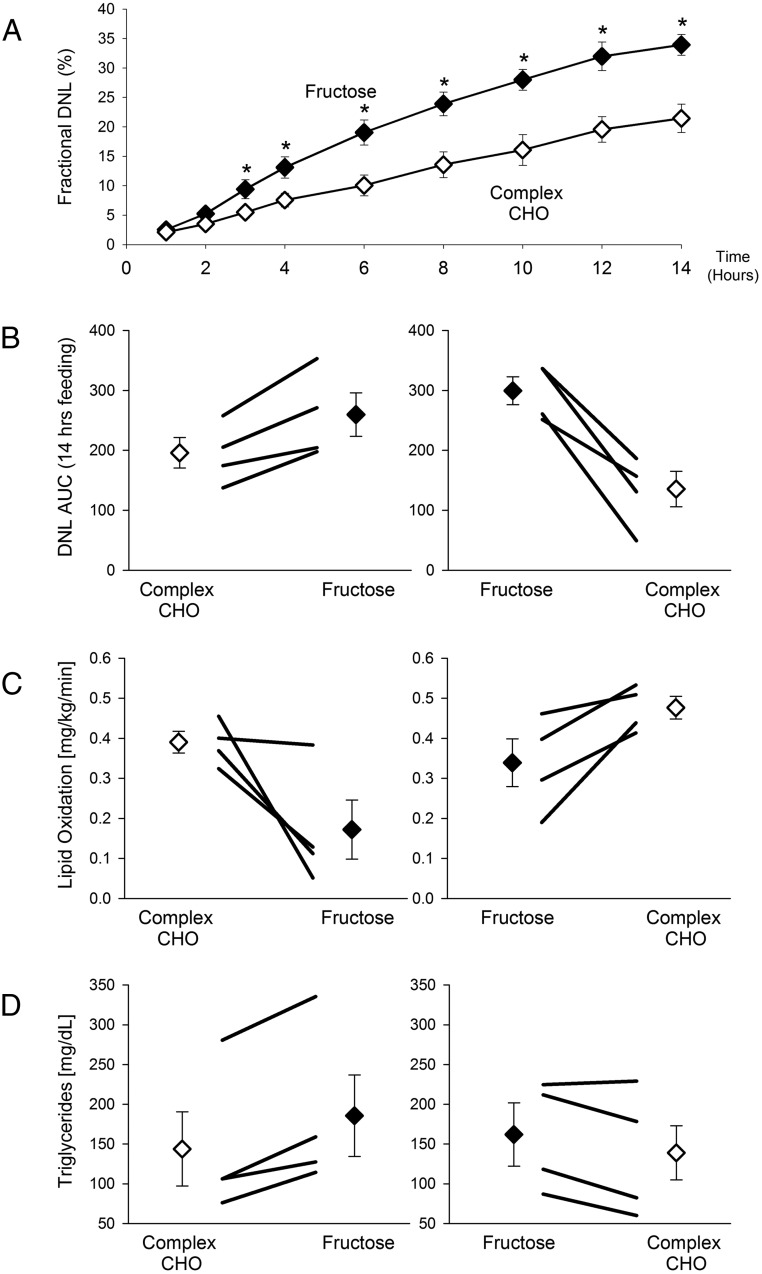
Fasting rates of fractional hepatic DNL were slightly but not significantly higher with the fructose diet (9.3 ± 1.8% fructose vs 6.4 ± 1.0% CCHO; P = .21). In contrast, DNL during feeding was significantly greater with the high-fructose diet, beginning 3 hours after initiation of feeding and continuing throughout a 14-hour tracer/feeding study (Figure 3A). The average AUC for integrated DNL over this period was significantly greater with the high-fructose diet, compared with the CCHO diet (277 ± 21 vs 162 ± 22; P = .002). In each participant, DNL-AUC was greater with the-high- fructose diet, regardless of the order in which the diets were administered (Figure 3B)
Figure 3.
Postprandial fractional DNL, lipid oxidation rates, and triglyceride levels. Data obtained during the high-fructose diet are shown as closed symbols, and those during CCHO feeding as open symbols. A, Postprandial DNL. On day 9 of each dietary period, after fasting overnight, participants began consuming hourly liquid meals at 0600 h. Blood samples were collected periodically throughout the 18-h feeding study. Asterisks denote that DNL during feeding was significantly greater with the high-fructose diet, beginning at 3 h and continuing throughout the tracer/feeding study (P < .05 at each timepoint). B, Integrated AUC for DNL (AUC-DNL). For each participant the AUC-DNL was higher with the high-fructose diet, independent of the order in which the diets were administered. Average AUC-DNL was significantly greater with the fructose diet (P = .002 vs CCHO). C, Lipid oxidation rates. Indirect calorimetry was performed eight times during the feeding study for calculation of lipid oxidation rate. The average rate of lipid oxidation was significantly lower with the high-fructose diet (P = .005 vs CCHO). D, Average postprandial triglyceride levels were significantly higher during high-fructose feeding (P = .002 vs CCHO). Although the magnitude of differences on the two diets varied from participant to participant, in each case the direction of the differences was consistent.
Average net whole-body postprandial lipid utilization, measured by indirect calorimetry, was significantly lower after the high-fructose diet compared with CCHO diet (0.34 ± 0.06 vs 0.43 ± 0.02 mg/kg/min; P = .005; Figure 3C). This effect was observed in all participants, regardless of the order in which the diets were administered. Likewise, postprandial carbohydrate utilization was consistently and significantly higher with the high-fructose diet (2.71 ± 0.27 vs 2.52 ± 0.16 mg/kg/min for CCHO; P = .001). Average postprandial triglyceride levels were higher during fructose compared with CCHO feeding in seven of eight participants (172 ± 29 vs 140 ± 28 mg/dL; P = .002; Figure 3D).
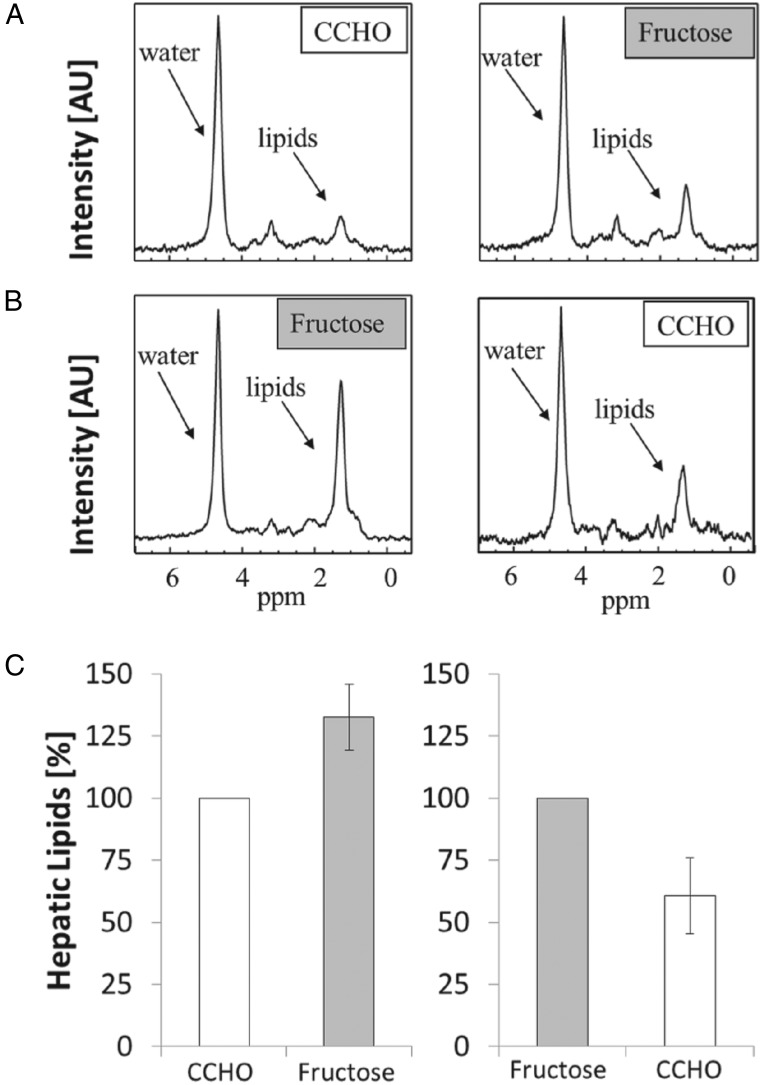
Liver fat
In all seven participants who underwent paired studies of liver fat by magnetic resonance spectroscopy (MRS), liver fat was greater with the high-fructose diet compared with the CCHO diet (fructose median, 137% of CCHO; first quartile, 116%; third quartile, 183%; P = .016; Figure 4). The median percentage of liver fat for the high-fructose diet was 1.02% (range, 0.53–3.07%), whereas the median percentage of liver fat for the CCHO diet was 0.70% (range, 0.39–2.37%). As was the case for DNL, higher liver fat with the fructose diet was observed regardless of the order in which the diets were fed.
Figure 4.
Liver fat, measured by MRS, was higher with the high-fructose diet than the CCHO diet, regardless of diet order. A, MR spectra from a representative participant after the CCHO diet, then after the high-fructose diet, demonstrating the increase in the lipid peaks with fructose. B, MR spectra from another participant with the opposite diet order. In both cases, the lipid peaks are higher with the fructose diet than with the CCHO diet. All spectra are scaled to the water levels. C, Median values for liver fat were significantly higher with the high-fructose diet when compared with the CCHO diet (P = .016). Error bars show first and third quartiles. All participants showed higher liver fat after the high-fructose diet vs the CCHO diet.
Discussion
There is increasing evidence that links excessive consumption of fructose in the form of sugar-sweetened beverages to greater total energy intake, weight gain, and a higher risk of obesity, fatty liver disease, and an atherogenic lipid profile (7–11, 13, 17). Recent studies have demonstrated that consumption of hypercaloric diets rich in fructose results in increases in circulating triglycerides and small, dense low-density lipoprotein; accumulation of visceral and liver fat; and insulin resistance (10, 32). The conversion of sugar to fat in the liver (DNL) may be one of the mechanisms leading to these changes after high-fructose intake. We have previously shown that carbohydrate overfeeding and high-carbohydrate diets stimulate hepatic DNL and increase triglyceride levels in healthy volunteers (31, 33). However, in these earlier studies we could not exclude the possibility that the lipogenic effects of fructose were due in part to positive energy balance, which also characterizes most of the published fructose metabolic studies (10, 11, 34, 35) and may in fact be a result of appetite stimulation or absence of appetite suppression induced by fructose consumption (24). Therefore, we asked whether fructose would stimulate DNL and lead to deleterious effects in the absence of overfeeding. To answer this question, we compared the effects of consuming two isocaloric diets with either high- or low-fructose content on hepatic DNL, hepatic and whole-body insulin sensitivity, and liver fat content. Notably, in these studies and for the first time in humans, neutral energy balance was maintained and overall carbohydrate intake was in keeping with current recommendations and identical for each diet. Indeed, both weight and body composition remained stable over the 18-day study period. We observed that, even during apparent neutral energy balance and with a relatively short follow-up period, consumption of a diet high in fructose was consistently associated with higher rates of DNL and circulating triglycerides during feeding and modestly higher liver fat, regardless of the diet sequence.
Some long-term studies of fructose overfeeding have reported increases in fasting glucose and triglyceride levels (36, 37); in contrast, in the current study, fasting glucose, insulin, and lipid levels were similar after each dietary period. In addition, extrahepatic insulin sensitivity as measured by euglycemic-hyperinsulinemic clamp was not significantly different between the two dietary phases. These results differ from previous studies in animals and humans that show an increase in extrahepatic insulin resistance after long-term ad libitum feeding of a fructose-rich diet (32, 35). However, it remains unclear whether the worsening of extrahepatic insulin sensitivity in these long-term studies was due to chronic overfeeding, weight gain associated with fructose intake, or a combination of these factors. Although we saw no evidence of extrahepatic insulin resistance, the blunted suppression of EGP during hyperinsulinemia suggests that only 1 week of high-fructose feeding, even during neutral energy balance, is sufficient to induce hepatic insulin resistance. Hepatic insulin resistance has also been reported after 3 weeks' consumption of fructose-containing beverages (32). In our study we extended this finding by identifying blunted suppression of the glycogenolytic component of EGP, whereas gluconeogenesis was equally suppressed (by 98%) with both diets.
In our study, the high-fructose diet consistently produced higher fractional hepatic DNL. Hepatic DNL not only produces new fatty acids but also prevents fatty acid oxidation in the liver. When the rate of carbohydrate conversion to fatty acids by DNL is high, the concentration of malonyl coenzyme A (an intermediary substrate from DNL) is increased, which prevents entry and oxidation of long-chain fatty acids in the mitochondrion (38). This effect of hepatic DNL is reflected in the results of our study, in which rates of whole-body lipid oxidation were consistently lower during fructose feeding. The reduction of hepatic fat oxidation and corresponding increase in carbohydrate utilization can result in an overall positive hepatic lipid balance, even in the presence of weight maintenance. Indeed, our study shows that the high-fructose diet was associated with significantly higher hepatic DNL, circulating postprandial triglyceride levels, and liver fat.
This study has several limitations. The sample size was modest, our design did not include a washout period between the two dietary phases, the order in which the diets were fed was not randomly assigned, the postprandial DNL may be considered as an acute response to the high- and low-fructose feeding, and we studied only men. Most of the fructose in the high-fructose diet was fed in liquid, rather than solid form, which may have resulted in more rapid absorption of carbohydrate during high-fructose feeding and affected the rate of presentation of carbohydrate to the liver. Although this difference would be considered a limitation in study design, it more closely reflects the way in which simple and complex carbohydrates are typically ingested outside of the research setting. Likewise, the use of frequent liquid meals during the tracer/feeding study, providing the liver with a steady stream of carbohydrate, may have produced a rate of DNL that is different from the rate that would have been seen had participants consumed larger, less frequent meals that included solid foods. Finally, although our participants did not exhibit symptoms of fructose malabsorption, it is possible that subclinical malabsorption occurred. However, malabsorption of fructose, if anything, would have blunted the DNL response.
The aforementioned limitations notwithstanding, it is striking that in each participant postprandial DNL, carbohydrate oxidation, and triglyceride levels, as well as liver fat, were higher after consumption of the high-fructose diet, compared with the CCHO diet. Moreover, these effects occurred regardless of the order in which the diets were consumed and in the absence of changes in weight or total body fat. Although our high-fructose diet provided slightly more than the ninety fifth percentile of reported fructose intake for young U.S. men, this amount was within the range of intake documented for high-fructose consumers (39). The observation that substitution of CCHO for fructose, even for this short period, was associated with reductions in all of these outcomes suggests a potential role for fructose in metabolic abnormalities and the development of fatty liver. Additional studies are required to examine the cumulative response to chronic high-fructose intake or fructose restriction.
In summary, in this controlled inpatient feeding study, consumption of a high-fructose diet for 9 days was associated with statistically significantly higher levels of hepatic postprandial DNL, triglycerides, and carbohydrate oxidation; modestly higher liver fat; and a blunted suppression of EGP by insulin, compared with an isocaloric diet in which CCHO was substituted for fructose. In contrast, we saw no significant effects of a high-fructose diet on fasting DNL, lipids, glucose, or insulin; or on insulin-mediated glucose uptake. Our results demonstrate for the first time that even during neutral energy balance, carbohydrate quality can affect hepatic lipid metabolism.
Acknowledgments
We are enormously grateful for the support provided by the nursing, bionutrition, and laboratory staff of the Clinical and Translational Science Institute Clinical Research Center at San Francisco General Hospital. We also thank Kimber Stanhope, PhD and Bernadette Marriott, PhD for critical discussion and suggestions; Arianna Pham, PA and Davis Tang, BA (College of Osteopathic Medicine, Touro University California); and Michelle Nyström, MBA (Department of Radiology and Biomedical Imaging, University of California, San Francisco) for technical assistance.
Author Contributions: J.M.S., S.M.N., M.J.W., N.B., M.S., and K.M. designed research; J.M.S. and K.M. obtained funding; J.M.S., S.M.N., M.J.W., J.L.P., M.E.W., L.A.H., T.P.B., M.N.R., M.S., and K.M. conducted research; J.M.S., S.M.N., M.J.W., A.D., V.W.T., N.B., M.S., and K.M. analyzed and interpreted data; J.M.S., S.M.N., M.J.W., M.S., and K.M. wrote the paper with critical revision by J.M.S., S.M.N., M.J.W., V.W.T., N.B., M.N.R., M.S., and K.M.; J.M.S. has primary responsibility for the final content. All authors read and approved the final article.
This work was supported by the National Institutes of Health (NIH) (DK078133), American Diabetes Association (1-08-CR-56), and NIH/National Center for Advancing Translational Sciences University of California San Francisco–Clinical and Translational Science Institute (UL 1 TR000004).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- area under the curve
- CCHO
- complex carbohydrate
- CRC
- Clinical Research Center
- DNL
- de novo lipogenesis
- EGP
- endogenous glucose production
- LDL
- low-density lipoprotein
- MR
- magnetic resonance
- MRS
- magnetic resonance spectroscopy
- SFGH
- San Francisco General Hospital.
References
- 1. Swinburn BA, Sacks G, Hall KD, et al. The global obesity pandemic: Shaped by global drivers and local environments. Lancet. 2011;378(9793):804–814. [DOI] [PubMed] [Google Scholar]
- 2. Lam DW, LeRoith D. The worldwide diabetes epidemic. Curr Opin Endocrinol Diabetes Obes. 2012;19(2):93–96. [DOI] [PubMed] [Google Scholar]
- 3. Schattenberg JM, Schuppan D. Nonalcoholic steatohepatitis: The therapeutic challenge of a global epidemic. Curr Opin Lipidol. 2011;22(6):479–488. [DOI] [PubMed] [Google Scholar]
- 4. Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79(4):537–543. [DOI] [PubMed] [Google Scholar]
- 5. Lim JS, Mietus-Snyder M, Valente A, Schwarz JM, Lustig RH. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol. 2010;7(5):251–264. [DOI] [PubMed] [Google Scholar]
- 6. Te Morenga L, Mallard S, Mann J. Dietary sugars and body weight: Systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ. 2012;346:e7492. [DOI] [PubMed] [Google Scholar]
- 7. Bantle JP, Raatz SK, Thomas W, Georgopoulos A. Effects of dietary fructose on plasma lipids in healthy subjects. Am J Clin Nutr. 2000;72(5):1128–1134. [DOI] [PubMed] [Google Scholar]
- 8. Teff KL, Elliott SS, Tschöp M, et al. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab. 2004;89(6):2963–2972. [DOI] [PubMed] [Google Scholar]
- 9. Chong MF, Fielding BA, Frayn KN. Mechanisms for the acute effect of fructose on postprandial lipemia. Am J Clin Nutr. 2007;85(6):1511–1520. [DOI] [PubMed] [Google Scholar]
- 10. Stanhope KL, Schwarz JM, Keim NL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119(5):1322–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lê KA, Ith M, Kreis R, et al. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am J Clin Nutr. 2009;89(6):1760–1765. [DOI] [PubMed] [Google Scholar]
- 12. Hudgins LC, Parker TS, Levine DM, Hellerstein MK. A dual sugar challenge test for lipogenic sensitivity to dietary fructose. J Clin Endocrinol Metab. 2011;96(3):861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kannel WB, Vasan RS. Triglycerides as vascular risk factors: New epidemiologic insights. Curr Opin Cardiol. 2009;24(4):345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Basu S, Yoffe P, Hills N, Lustig RH. The relationship of sugar to population-level diabetes prevalence: An econometric analysis of repeated cross-sectional data. PloS One. 2013;8(2):e57873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Romaguera D, Norat T, Wark PA, et al. Consumption of sweet beverages and type 2 diabetes incidence in European adults: Results from EPIC-InterAct. Diabetologia. 2013;56(7):1520–1530. [DOI] [PubMed] [Google Scholar]
- 16. Maersk M, Belza A, Stødkilde-Jørgensen H, et al. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: A 6-mo randomized intervention study. Am J Clin Nutr. 2012;95(2):283–289. [DOI] [PubMed] [Google Scholar]
- 17. Zivkovic AM, German JB, Sanyal AJ. Comparative review of diets for the metabolic syndrome: Implications for nonalcoholic fatty liver disease. Am J Clin Nutr. 2007;86(2):285–300. [DOI] [PubMed] [Google Scholar]
- 18. Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology. 2010;51(2):679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Targher G, Marra F, Marchesini G. Increased risk of cardiovascular disease in non-alcoholic fatty liver disease: Causal effect or epiphenomenon? Diabetologia. 2008;51(11):1947–1953. [DOI] [PubMed] [Google Scholar]
- 20. Samuel VT. Fructose induced lipogenesis: From sugar to fat to insulin resistance. Trends Endocrinol Metab. 2011;22(2):60–65 . [DOI] [PubMed] [Google Scholar]
- 21. Bazzano LA, Hu T, Reynolds K, et al. Effects of Low-Carbohydrate and Low-Fat Diets: A Randomized Trial. Ann Intern Med. 2014;161:309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Ruyter JC, Olthof MR, Seidell JC, Katan MB. A trial of sugar-free or sugar-sweetened beverages and body weight in children. New Engl J Med. 2012;367(15):1397–1406. [DOI] [PubMed] [Google Scholar]
- 23. Ebbeling CB, Feldman HA, Chomitz VR, et al. A randomized trial of sugar-sweetened beverages and adolescent body weight. New Engl J Med. 2012;367(15):1407–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Page KA, Chan O, Arora J, et al. Effects of fructose vs glucose on regional cerebral blood flow in brain regions involved with appetite and reward pathways. JAMA. 2013;309(1):63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Johnson RK, Appel LJ, Brands M, et al. Dietary sugars intake and cardiovascular health: A scientific statement from the American Heart Association. Circulation. 2009;120(11):1011–1020. [DOI] [PubMed] [Google Scholar]
- 26. Wang DD, Sievenpiper JL, de Souza RJ, et al. Effect of fructose on postprandial triglycerides: A systematic review and meta-analysis of controlled feeding trials. Atherosclerosis. 2014;232:125–133. [DOI] [PubMed] [Google Scholar]
- 27. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–E223. [DOI] [PubMed] [Google Scholar]
- 28. Ferrannini E. The theoretical bases of indirect calorimetry: A review. Metabolism. 1988;37(3):287–301. [DOI] [PubMed] [Google Scholar]
- 29. Noworolski SM, Tien PC, Merriman R, Vigneron DB, Qayyum A. Respiratory motion-corrected proton magnetic resonance spectroscopy of the liver. Magn Reson Imaging. 2009;27(4):570–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hellerstein MK, Neese RA. Mass isotopomer distribution analysis at eight years: Theoretical, analytic, and experimental considerations. Am J Physiol. 1999;276(6 Pt 1):E1146–E1170. [DOI] [PubMed] [Google Scholar]
- 31. Schwarz JM, Neese RA, Turner S, Dare D, Hellerstein MK. Short-term alterations in carbohydrate energy intake in humans. Striking effects on hepatic glucose production, de novo lipogenesis, lipolysis, and whole-body fuel selection. J Clin Invest. 1995;96(6):2735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aeberli I, Hochuli M, Gerber PA, et al. Moderate amounts of fructose consumption impair insulin sensitivity in healthy young men: A randomized controlled trial. Diabetes Care. 2013;36(1):150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schwarz JM, Linfoot P, Dare D, Aghajanian K. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am J Clin Nutr. 2003;77(1):43–50. [DOI] [PubMed] [Google Scholar]
- 34. Sobrecases H, Lê KA, Bortolotti M, et al. Effects of short-term overfeeding with fructose, fat and fructose plus fat on plasma and hepatic lipids in healthy men. Diabetes Metab. 2010;36(3):244–246. [DOI] [PubMed] [Google Scholar]
- 35. Tappy L, Lê KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90(1):23–46. [DOI] [PubMed] [Google Scholar]
- 36. Ngo Sock ET, Lê KA, Ith M, Kreis R, Boesch C, Tappy L. Effects of a short-term overfeeding with fructose or glucose in healthy young males. Br J Nutr. 2010;103(7):939–943. [DOI] [PubMed] [Google Scholar]
- 37. Silbernagel G, Machann J, Unmuth S, et al. Effects of 4-week very-high-fructose/glucose diets on insulin sensitivity, visceral fat and intrahepatic lipids: an exploratory trial. Br J Nutr. 2011;106(1):79–86. [DOI] [PubMed] [Google Scholar]
- 38. McGarry JD. Banting lecture 2001: Dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51(1):7–18. [DOI] [PubMed] [Google Scholar]
- 39. Marriott BP, Cole N, Lee E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J Nutr. 2009;139(6):1228S–1235S. [DOI] [PubMed] [Google Scholar]