Abstract
Objective
The stability and composition of intestinal flora plays a vital role in human wellbeing throughout life from as early as birth. Over the past 50 years, several studies have been conducted to evaluate the effect of probiotic administration in pediatric gastroenterology. This document aims to provide a recommendation score on probiotic utilization in pediatric gastroenterology, together with a review of current knowledge concerning its benefits, tolerability, and safety.
Study Design
Published literature was selected without study design restriction: clinical guidelines, meta-analyses, randomized controlled trials (RCTs), cohort studies, outcomes research and case–controlled studies were selected using the following MESH-validated terms: probiotics, diarrhea, acute diarrhea, antibiotic-associated diarrhea, traveler’s diarrhea, bacterial diarrhea, nosocomial diarrhea, prophylactic diarrhea, Helicobacter pylori infection, colic, infantile colic, necrotizing enterocolitis (NEC), inflammatory bowel disease, constipation, and allergy. Once the validity and the quality of results were evaluated, a recommendation score and level of evidence were assigned for pediatric gastrointestinal-related conditions, according to the updated Evidence-Based Medicine guidelines: 1a for systematic review (SR) of RCTs, 1b for individual RCT, 1c for SR and individual RCT, 2a for SR of cohort studies, 2b for individual cohort studies, 2c for outcomes research, and 3a for SR of case-control studies.
Results and Conclusions
The Latin American Expert group consensus recommends the use of the following probiotics for pediatric gastrointestinal conditions: prevention of acute infectious diarrhea (AID): 1b for Bifidobacterium lactis, Lactobacillus rhamnosus GG (LGG), and L. reuteri; prevention of nosocomial diarrhea: 1 b for B. lactis Bb12, B. bifidum, LGG and Streptococcus thermophiles; treatment of AID: 1a for LGG and S. boulardii, 1b for L. reuteri; prevention of antibiotic-associated diarrhea: 1b for LGG and S. boulardii; prevention of traveler’s diarrhea: 1b for S. boulardii; prevention of infantile colic: 1a for L. reuteri DSM 17938; treatment of infantile colic: 1b for L. reuteri DSM 17938; prevention of NEC: 1a for B. breve, mixtures of Bifidobacterium and Streptococcus, LGG, L. acidophilus and L. reuteri DSM 17938; induction and maintenance of remission in ulcerative colitis: 1b for VSL#3; improving symptoms of irritable bowel syndrome: 2c for LGG and VSL#3.
Key Points
| Certain probiotics have demonstrated efficacy and are widely used for preventing and treating medical conditions involving the gastrointestinal tract in children. |
| Lactobacillus rhamnosus GG (LGG), L. reuteri and Saccharomyces boulardii are the best studied probiotics and have been shown to be most effective as treatment if introduced early in the course of the disease. |
| Due to strain specificity, only clinically tested probiotics can be recommended to treat specific indications in children. |
Introduction
The stability and composition of intestinal flora plays a vital role in good health and wellbeing of a human being throughout life from as early as birth.
In order to improve the microbial intestinal environment, several studies have been carried out to evaluate the effect of probiotic administration for the prevention and treatment of various medical conditions. This paper aims to provide a detailed review of scientific evidence based on the use of probiotics in pediatrics, along with current knowledge concerning its benefits, tolerability, and safety. This position paper was conceived with the objective to develop a consensus document that may unify and guide pediatric healthcare providers in the management of probiotics in Latin America, while incorporating the critical variable of the benefit of probiotic use.
Objectives
The purpose of this review was to update scientific evidence and grade of recommendation to develop future guidance in the medical use of probiotics in pediatric patients. Three main objectives were established by the working group:
To develop evidence-based guidelines for probiotic use in pediatric patients through a critical and comprehensive literature review.
To provide a useful tool for probiotic use aimed at general practitioners, pediatricians, and pediatric gastroenterologists.
To contribute to the rational clinical use of probiotics in pediatric diseases, supported by scientific evidence.
Methodology
The present consensus guidelines paper is a result of the discussions of the Latin American (LATAM) expert consensus group representing ten Latin-American countries: Argentina, Bolivia, Brazil, Chile, Columbia, El Salvador, Guatemala, Mexico, Peru, and Venezuela.
The topics selected for this paper were centered on probiotic use in children in the following indications: acute infectious diarrhea (AID), antibiotic-associated diarrhea (AAD), traveler’s diarrhea, Helicobacter pylori infection, infantile colic, necrotizing enterocolitis (NEC), inflammatory bowel disease (IBD), and functional gastrointestinal disorders; e.g., irritable bowel syndrome (IBS), constipation, and allergy. Relevant clinical questions were used as a basis for discussion and topics were divided between authors according to their field of expertise in the aforementioned various childhood diseases.
The protocol for evidence research was established using the following validated ‘Medical Subject Headings’ (MeSH®) terms: probiotics, diarrhea, acute diarrhea, AAD, traveler’s diarrhea, bacterial diarrheal, nosocomial diarrhea, prophylactic diarrhea, Helicobacter pylori, infant colic, infantile colic, NEC, IBD, constipation, and allergy.
A literature search was conducted using PubMed, MEDLINE, Embase, and the Cochrane database of systematic reviews (SRs) in Spanish and English, covering the period February 1965–October 2014. The patient age range was 0–18 years.
Published literature was selected without study design restriction to include SRs and meta-analyses, clinical guidelines, randomized controlled trials (RCTs), cohort studies and case–control studies. Narrative reviews were not used; neither were case series and case reports, nor observational studies. Only articles with satisfactory methodology—including clinical guidelines—and which clearly responded to these questions were selected and included in our review.
Other papers fulfilling the criteria were also included in the review; these included papers found through searching the bibliographies of reviews, and papers that were already known to the authors but not found in the literature searches, or subsequently suggested by the peer reviewers.
Since the majority of evaluated articles were RCTs, we referred to the User Guidelines for Medical Literature and the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system for grading evidence [1–5] to validate papers selected for critical review by answering the questions shown in Table 1. Assessment of each article was completed by at least two independent evaluators and discrepancies were discussed within the entire group and resolved using scientific consensus. Once the validity and the quality of results were evaluated, a recommendation grade and level of evidence were assigned according to the updated guidelines established by the Oxford Centre for Evidence-Based Medicine (CEBM) (Table 2) [6].
Table 1.
Questions for evaluating randomized controlled trials (RCTs)
| I. Evaluation of RCT validity |
| Was treatment randomly administered to patients? |
| Was a comprehensive and evolutionary control conducted? |
| Was analysis done on all patients participating in the RCT? |
| Was a blinding procedure maintained for the administered treatment? |
| Were the study groups similar? |
| II. Evaluation of RCT results |
| What was the scope of the treatment’s effect? |
| How accurately was the treatment’s effect measured? |
| III. Applicability of results |
| Can these results be applied when treating my patients? |
| Were all outcome variables found to be clinically important? |
| Were benefits higher than undesirable effects? |
Table 2.
Oxford Centre for Evidence-Based Medicine—levels of evidence. Adapted from CEBM [6]
| Level of evidence | Therapy/prevention, etiology/harm |
|---|---|
| 1a | SR (with homogeneitya) of RCTs |
| 1b | Individual RCT (with narrow CI) |
| 1c | All or noneb |
| 2a | SR (with homogeneitya) of cohort studies |
| 2b | Individual cohort study (including low-quality RCT, e.g. <80 % follow-up) |
| 2c | ‘Outcomes’ research; ecological studies |
| 3a | SR (with homogeneitya) of case-control studies |
| 3b | Individual case-control study |
CI confidence interval, RCT randomized controlled trial, SR systematic review
aHomogeneity denotes a systematic review that is free of worrisome variations (heterogeneity) in the directions and degrees of results between individual studies. Not all systematic reviews with statistically significant heterogeneity need be worrisome, and not all worrisome heterogeneity need be statistically significant
bMet when all patients died before the treatment became available, but some now survive on it; or when some patients died before the treatment became available, but none now die on it
Definition and History of Probiotics
Probiotics are live microorganisms that, when administered in adequate amounts, confer a health benefit on the host [7] while enhancing the properties of intestinal flora [8]. Lilly and Stillwell [9] coined the term probiotics, and, in 1974, Parker gave it its current significance [10].
It should be emphasized that the FAO/WHO definition of probiotics provided by the Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO) does not mention the human origin of the bacterial strain among the criteria for the selection and definition of probiotics, but instead classify according to effect caused [7].
Nowadays, probiotics use is increasingly widespread; however, indication of their use has been evidenced since ancient times. Over 2000 years ago in Rome in 76 AD, Pliny the Elder used fermented milk to treat diarrhea. The term ‘probiotic’ (bios: life; pro: in favor of) was used for the first time in the 1960s, even though the beneficial effect of certain bacteria had been studied for over a century [5]. In 1906, Tissier [11] observed that significant colonization of bifidobacteria in stool provided protection against the development of diarrhea in children and, in 1908, Eli Metchnikoff, Professor at the Pasteur Institute in Paris and Nobel laureate of Medicine and Physiology, illustrated the health benefits of fermented yogurt (Lactobacillus bulgaricus) [12–14].
In recent years, both consumers and the medical community have developed an increased interest in the potential benefits of probiotics, which is amplified by a growing body of research and literature.
Probiotics may be registered as food supplements or drugs, depending on efficacy and safety evidence provided by manufacturers to Health Evaluation Authorities [15]. Probiotics are available as capsules, tablets, packets, or powders and are contained in various fermented foods; in addition, probiotic products may contain a single microorganism or a mixture of several species [16–19]. Among the scientific community, probiotics are designated by nomenclature agreement, based on their genus, species, and an alphanumeric designation, for example, Saccharomyces boulardii CNCM I-745, L. casei DN-114 001 or L. rhamnosus GG (LGG).
A variety of bacteria have been studied to explore their probiotic effect, including various Lactobacillus and Bifidobacterium strains, which are normal inhabitants of a healthy intestinal flora as well as the yeast S. boulardii and some bacillus species. Criteria for probiotic use are listed in Table 3 [20]. When evaluating this research, it is important to note that the effects of any bacteria are strain-specific, meaning the data from research relates only to the specific strain being evaluated [10, 15, 18, 19].
Table 3.
Criteria for use as a probiotic. Adapted from Borchers et al. [20]
| The organism must be fully identified: genus, species and strain |
| No pathogenic effects and toxicity, and must not be associated with disease or be carrying antibiotic resistance genes |
| It must be viable and stable (at least briefly) in the gastrointestinal tract, and resistant to bile acids and digestive enzymes |
| It must adhere to mucosal surface and colonize the intestine (at least briefly) |
| It must be stable during processing and storage |
| It must have a sufficient number of viable cells |
| It must undergo in vivo and in vitro trials to prove any attributed probiotic effect and documented clinical benefit |
Mechanisms of Action
The mechanisms of action of probiotics are summarized in Table 4. The proposed mechanisms for the protective effects of probiotics include direct hostility towards pathogens through competitive adherence to the mucosa and epithelium; strengthening of the gut epithelial barrier and modulation of the immune system to convey an advantage to the host by restoring normal intestinal flora [21]; inhibiting Clostridium difficile through an antisecretory effect [22]; production of intestinal mucin; synthesis of bacteriocins and other antimicrobial molecules; restoration of close enterocyte bonds (by re-establishing intestinal permeability); secretion of immunologic defensins; interaction with dendritic cells, Toll-like receptors (TLRs) and intracellular inflammatory pathways; activation of macrophages and natural killer (NK) cells [23]; stimulation of lymphoid tissue associated with the gut (GALT) [24]; and modulation of innate and adaptive immunity involving immunoglobulins and cytokines [25].
Table 4.
Summary of mechanisms of action of probiotics
| Immunomodulation | Increase in the number of immunoglobulin-secreting cells in the intestinal mucosa |
| Facilitates transport of antigens to the submucosal lymphocytes ensuring a more immediate immune reaction [25] | |
| Antibacterial action | Production of antibacterial substances |
| Action against common pathogens (E. coli, Clostridium difficile and Salmonella spp.) [21] | |
| Competitive exclusion | Competes with adhesion of pathogens to the intestinal mucosa |
| Colonization of the intestine with beneficial bacteria [23] |
Total fecal lgA and polio antivirus are increased significantly with live bifidobacteria. Kaila et al. [26] illustrated that LGG significantly increased the humoral immune response to rotavirus enteritis, and more so with live probiotics than inactive ones; in addition, adhesion of Bifidobacteria Bb12 improves in the presence of LGG in healthy children and during episodes of diarrhea, suggesting that the action of the two probiotics might be synergistic.
S. boulardii CNCM I-745 has several mechanisms of action which may be classified into three main areas: luminal action, trophic action, and mucosal—anti-inflammatory signaling effects. Within the intestinal lumen, S. boulardii may interfere with pathogenic toxins (especially C. difficile toxin A and B), preserve cellular physiology, interfere with pathogen attachment, interact with normal microbiota or assist in reestablishing short-chain fatty acid levels. S. boulardii also may act as an immune regulator (either acting as an immune stimulant or by reducing pro-inflammatory response), both within the lumen and systemically [27].
Results
A flow chart presenting the literature search results and selection process is shown in Fig. 1. The initial electronic search identified 219 articles, plus another 18 were identified via other sources. After de-duplication, 209 records remained, and of these, 17 were excluded due to the content being non-relevant (n = 3) or due to being published in a language other than English, French or Spanish (n = 14). Of the remaining 192 papers, full text examination led to another 20 being excluded (Fig. 1). Another six papers were identified by peer reviewers and added at the post-submission stage. The final number of papers included in the review was 178, and of these, 74 provided guidance on the use of probiotics in children. The papers were divided into the specified indications and discussed in the following sections.
Fig. 1.
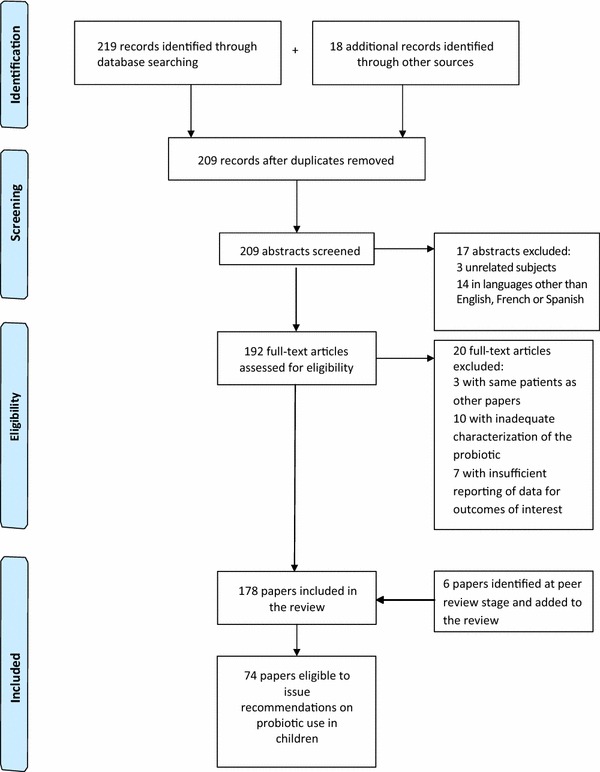
Flow chart showing papers identified and evaluated for this review
Clinical Applications of Probiotics in Gastroenterology
Acute Infectious Diarrhea (AID)
Prevention of AID
Breast milk provides the best protection against infectious gastrointestinal disease in infants. Attempts have been made to adapt the ingredients of infant formulas made from cow’s milk by adding probiotics and/or prebiotics to emulate the immunological development of breastfed children. In a study conducted in India, Saran et al. fed infants with fermented milk for 6 months, resulting in significant weight gain and 50 % reduction of infectious diarrhea [28]. Sazawal et al. [29] used milk fortified with B. lactis HN019 and galacto-oligosaccharides, resulting in higher serum iron levels even when groups received iso-caloric diets with the same iron content, a ten percent decrease in all diarrhea types was also observed. This effect on morbidity prevention was attributed to a better absorption through beneficial changes in the intestinal flora with the side effect of preventing morbidities. Although the majority of trials showed a positive trend in the prevention of AID, data on evidence to support the routine use of probiotics to prevent infectious diarrhea was not found to be consistent [30]. Three major RCTs provide evidence of a statistically significant but clinically questionable effect of certain probiotic strains [LGG, L. reuteri-American Type Culture Collection (ATCC) 55730, B. lactis Bb12] in the prevention of community-acquired diarrhea [31]. No study has suggested an adverse secondary effect of a probiotic-enriched formula in healthy children.

Prevention of Nosocomial Diarrhea
In 1994, Saavedra et al. [32] reported that Streptococcus (Str.) thermophilus and B. bifidum (renamed B. lactis) reduced the incidence of nosocomial diarrhea in a small group of children admitted for an extended period in a healthcare institute for chronic patients. Szajewska [33] showed that LGG also reduces nosocomial infections, particularly in rotavirus gastroenteritis; however, in a double-blind RCT with 220 children, Mastretta et al. [34] did not find any statistically significant protective effect of LGG in nosocomial rotavirus infection. In 2004, a multicenter, double-blind RCT conducted by Chouraqui et al. evaluated the efficacy of a milk formula supplemented with viable B. lactis strain Bb 12 (BbF) among 90 healthy children living in residential nurseries or foster care centers; the study did not show a reduction in prevalence of diarrhea with living B. lactis Bb12-fortified formula compared with placebo [28.3 vs 38.7 %; relative risk (RR) 0.7 (95 % confidence interval (CI) 0.4–1.3)] [35]. A double-blind RCT with 971 infants administered B. breve C50 and Str. thermophilus 065 fermented formula for 4–6 months. The infants fed with the fermented formula had normal growth and less severe episodes of diarrhea, with fewer reports of dehydration, fewer doctor visits, and less prescription of oral rehydration solution (ORS) [36]. The authors concluded that seven children needed a probiotic treatment in order to prevent healthy children from developing nosocomial rotavirus gastroenteritis. However, the protective effect in preventing nosocomial diarrhea becomes less significant if the incidence of episodes per patient and per month accounts for more than the total percentage of patients with diarrhea [37].

Treatment of AID
Diagnostics and therapeutics of AID must be based on the pathophysiological consequences of the disease: water and electrolyte loss and gastrointestinal ecosystem alteration [37].
Probiotic administration can protect intestinal microbiota against AID; in 2005, Shamir et al. showed a reduction in the duration of acute gastroenteritis from 1.96 ± 1.24 to 1.43 ± 0.71 days (p = 0.017), with the addition of 109 colony-forming units (CFU) of Str. thermophilus, B. lactis, L. acidophilus, 10 mg of zinc and 0.3 g of fructo-oligosaccharide per day [38]. In addition, several studies have demonstrated the efficacy of LGG in reducing the duration of acute viral diarrhea and AID, as well as a reduction in length of hospitalization, in both eutrophic and severely malnourished children [39, 40]. Guarino et al. [41] also demonstrated a significant reduction in rotavirus shedding. In a prospective European RCT using LGG (1010 CFU/250 mL) to supplement ORS in 287 children with acute diarrhea, the results showed a significant decrease in the duration of diarrhea close to 10 % (mean duration of 123 h in the placebo group compared with 110 h in the intervention group) with an improved response in the rotavirus group [42]. Shornikova et al. [43] evaluated the efficacy of L. reuteri ATCC 55730 in 66 children hospitalized with rotavirus diarrhea randomized into three groups: a placebo group and two groups with different doses of L. reuteri (107 and 1010 CFU/g once a day for 5 days). The probiotic reduced duration of diarrhea with a dose-dependent effect (2.5 days in the placebo group vs 1.9 and 1.5 in the L. reuteri groups). In a recent meta-analysis, Szajewska et al. [44] evaluated two RCTs (n = 196) using L. reuteri DSM 17938 and three RCTs (n = 156) on the evaluation of L. reuteri ATCC 55730 administration among hospitalized children aged 3–60 months; compared with placebo or no treatment, DSM 17938 significantly reduced the duration of diarrhea (mean difference −32 h, 95 % confidence interval (CI) −41 to −24) and increased the chance of cure on Day 3 (RR 3.5, 95 % CI 1.2–10.8, random effects model). Similar results were obtained with the original strain, L. reuteri ATCC 55730 [44].
Other probiotics such as L. acidophilus LB in a product containing heat-killed Lactobacillus have also demonstrated effectiveness in reducing the duration of the AID when tested on 73 children with acute diarrhea (50 % rotavirus positive) [45]. Comparable results were obtained in a double-blind study in 87 Polish children with AID treated with a mixture of three strains of L. rhamnosus (573L/1, 573L/2, 573L/3) all at a dose of 1.2 × 1010 CFU twice daily for 5 days; L. rhamnosus reduced the duration of rotavirus diarrhea (76 ± 35 h vs 115 ± 67 h (p = 0.03), but not of other types of diarrhea [46]. Intestinal colonization by administration with these strains was 80 and 41 % at 5 and 14 days, respectively. The intervention also shortened the time required for intravenous rehydration [15 ± 14 vs 38 ± 33 h (p = 0.006)], although factors such as the variability of care may have influenced the result [46]. At least three clinical trials in developing countries deny the beneficial effect of LGG and L. acidophilus in acute diarrhea or severe diarrhea, probably related to the difference in etiological profile [47–49]. No decrease in the duration of diarrhea with a mixture of L. acidophilus, B. bifidum (B. lactis) and L. bulgaricus was observed [50]. The strain L. paracasei ST11 did not reduce the volume of stool in rotavirus infection but improved the results of cumulative stool output (225 ± 218 vs 381 ± 240 mL/kg), stool frequency (27.9 ± 17 vs 42.5 ± 26), and ORS intake (180 ± 207 vs 331 ± 236 mL/kg) in children with less severe non-rotavirus diarrhea compared with those receiving placebo treatment in Bangladeshi children [51]. Two meta-analyses concluded that most of the studies were conducted in the developed world, and that LGG, L. acidophilus and L. bulgaricus had no efficacy [52, 53]. In an SR of double blind RCTs, Szajewska and Mrukowicz also found that the duration of viral diarrhea was significantly reduced (by about 17 h or 0.7 days) compared with controls [54]. The effectiveness of LGG appears to be connected to the logarithm of the dose (>1011 as the most effective dose) [53]. A Cochrane review of 63 RCTs and 8014 participants (56 trials recruited infants and young children) showed the beneficial effect of probiotics in combination therapy with ORS in reducing the duration of diarrhea, although the size of the effect varied considerably between studies [55]. The average of the effect was significant for mean duration (MD) of diarrhea (MD 24.76 h; 95 % CI 15.9–33.6; n = 4,555, 35 trials); diarrhea lasting ≥4 days (RR 0.41; 95 % CI 0.32–0.53; n = 2,853, 29 trials); and stool frequency on Day 2 (MD 0.80; 95 % CI 0.45–1.14; n = 2,751, 20 trials). The two most commonly studied probiotics were LGG (13 RCTs) and S. boulardii (10 RCTs) [55]. The first double-blind, prospective, randomized study of S. boulardii (a non-pathogenic yeast) was completed over 15 years ago: diarrhea persisted for more than 7 days in 12 % of the placebo group and in 3 % of the probiotic group [56]. Since then, several other double-blind, prospective randomized trials conducted on S. boulardii in children with acute gastroenteritis have consistently shown a significant improvement compared with placebo. A consecutive series of 130 Mexican children with AID from 3 months to 3-years-old were treated with ORS plus placebo or with ORS plus S. boulardii 600 mg/day for 5 days. A significant decrease in the number of stools occurred from the second day following probiotic use. After 48 h, the percentage of children considered cured was almost 50 % (vs 8 % in the placebo group) and, at Day 4, the percentage cured was up to 95 % (vs 50 % in the placebo group) [57]. Kurugol and Koturoglu [58] treated 200 children with acute diarrhea with 250 mg of S. boulardii or placebo for 5 days: the duration of diarrhea and length of hospitalization was reduced by about 24 h. Villarruel and colleagues [59] showed similar results in 88 children treated in ambulatory care in Argentina. This study concluded that diarrhea persisted for more than 7 days in 27 % of the control group, compared with 7 % of the group treated with S. boulardii for 6 days, with a greater effect if treatment was initiated during the first 2 days of diarrhea. In addition, studies of diarrheal parasitic infections showed that S. boulardii improved feeding tolerance in children with chronic Giardia lamblia [60]. This probiotic is also shown to be effective in amoebiasis and AIDS-associated diarrhea [61, 62]. An open RCT in Pakistani children with acute infectious gastroenteritis demonstrated that administration of 500 mg of S. boulardii for 5 days significantly reduced stool frequency and duration of diarrhea (3.5 vs 4.8 days, p = 0.001) and resulted, 2 months later, in a 50 % decrease in re-infection rate with a 30 % bodyweight increase [63].
More recently, two international guidelines on diarrheal management in children addressed the use of probiotics in acute diarrhea with ORS; experts concluded that LGG and S. boulardii reduced the duration of diarrhea by 1 day, giving evidence level 1+ for these two probiotics only [64, 65]. Finally, the Probiotics and Prebiotics Global Guidelines published by the World Gastroenterology Organization (WGO) in 2011 confirmed the use of the above-mentioned probiotics in the management of children with acute diarrhea with ORS, level of evidence 1a for the same probiotics only [66].
Regarding the treatment of AID in children, the LATAM consensus estimates that a decrease in the duration of diarrhea, as well as hospitalization length, is an important benefit from the standpoint of social and economic development in acute infectious gastroenteritis in children. While there have been numerous published clinical studies evaluating different probiotics in the treatment of acute gastroenteritis, tests differ in relation to the strains tested, dosages, methodological quality, definitions of diarrhea and the results obtained. Most studies show statistically significant effects in terms of clinical benefit, with greater effect on viral diarrhea [31]. In general, published meta-analyses conclude that the duration of diarrhea episodes is shortened by approximately 24 h (17–30 h) with the selected strains of lactobacilli and LGG, L. acidophilus, L. bulgaricus, L. reuteri, and S. boulardii [31, 33, 52, 54, 55, 67]. In addition, studies have proven the increased effectiveness of probiotics when they were administered in the early stages of the disease; in at least three studies in children with acute diarrhea of different etiologies, probiotic use reduced the average duration of episodes by 57 % (35–71 %) [44, 64, 68]. Only LGG and S. boulardii have Evidence Level 1+ for the treatment of acute diarrhea with ORS in children [64–66]. In developing countries, for varieties of lactobacilli, we found few study-based assays in the community while we observed sufficient data on S. boulardii.

Antibiotic-Associated Diarrhea (AAD)
Antibiotic treatment alters the gastrointestinal microflora, which produces various clinical symptoms, particularly diarrhea. AAD incidence in children is approximately 10 % in first-line treatment, regardless of the reason for antibiotic administration [69]; children under 2 years of age are more likely to experience an episode of AAD, especially those treated with antibiotics or antibiotic combinations such as amoxillin–clavulanic acid (23 %) [70]. Nevertheless, the large majority of AAD cases are mild to moderate and rarely require hospitalization. According to the meta-analysis performed by Szajeweska in 2006, probiotics reduce the risk of AAD in children. Analysis of subgroups of children to whom probiotics were administered preventively showed that reduction in AAD risk was mainly associated with the use of LGG (95 % CI 0.15–0.6), S. boulardii (95 % CI 0.07–0.6), or B. lactis and Str. thermophilus (95 % CI 0.3–0.95) [70]. These data indicated that one of seven patients who develop diarrhea during antibiotic treatment would benefit from AAD prevention if they were to simultaneously receive any of these probiotics.
The use of S. boulardii has proven to be the only effective method of preventing diarrhea caused by C. difficile [71]. Randomized controlled trials provide moderate evidence of a beneficial effect produced by LGG, B. lactis, Str. thermophilus and S. boulardii. Nonetheless, there is no evidence to support the use of probiotics in the prevention of recurring symptoms caused by C. difficile [31].
Potential effects of probiotics in preventing AAD in children require more studies for routine indication. Such studies should focus on probiotic strains that have proved to be beneficial such as LGG, or S. boulardii, as well as on dosages that have showed to be more effective. There are not enough publications on treating children with severe AAD with probiotics.

Traveler’s Diarrhea
The past two decades have seen an unprecedented, sustained growth in international travel. Although much of the growth represents increased travel to Europe and Asia, travel to South America has also increased. Traveler’s diarrhea is a common condition with major economic and health impacts. In traveling children, the etiologies of diarrhea are likely to be similar to those described for diarrhea in adults [71, 72]. Children who travel are at risk of developing the same well known illnesses that affect adult travelers [73]. In the assessment of the ill pediatric traveler, physicians must consider geographic, seasonal, and environmental factors and assess compliance to pre-travel advice. Treatment recommendations for pediatric traveler’s diarrhea have come from expert opinion, anecdotal evidence, limited case reports, the World Health Organization, The Medical Letter [71, 72] and extrapolation from treatment recommendations for traveler’s diarrhea in adults [73].
Various randomized clinical studies have evaluated the efficacy of probiotics in the prevention of this medical condition. One trial using L. acidophilus and two other trials using LGG showed negative results [74–76]. An RCT using S. boulardii illustrated a significantly beneficial effect in terms of reduced incidence of diarrhea, which was shown to be dose dependent, strongly dependent on adherence, and which tended to differ according to geographical location [77].
According to Mackell [73], the best treatment choice for the pediatric traveler must address a combination of efficacy, adherence, and cost.

Helicobacter pylori Infection
Current interest in probiotics as therapeutic agents against H. pylori is stimulated not only by the clinical data showing efficacy of some probiotics in different gastrointestinal diseases but also by the increasing resistance of pathogenic bacteria to antibiotics, thus the interest in developing alternative therapies. Several clinical trials have evaluated the role of probiotics in adults and children colonized with H. pylori. Studies on L. johnsonii, S. boulardii ± inulin or L. acidophilus LB and L. gasseri OLL2716 (LG21) indicate that they decrease colonization density, while maintaining lower levels of the pathogen in the gastric mucosa but that they do not eradicate H. pylori [78–80]. However, in some studies with probiotics combined with treatment regimens using proton pump inhibitors (PPIs) and antibiotics, eradication rates moderately increased. For LGG the eradication rate was 69 % (0.98 RR, 95 % CI 0.7–1.4) [81], for L casei DN-114 001 it was 84.6 % (95 % CI 71.2–95.5) vs 57.5 % in the control group (p = 0.0045) [82], for B. animalis and L. casei it was 45.5 vs 37.5 % with control (p = 0.345) [83], for L. reuteri ATCC 55730 85 vs 80 % with control (p > 0.05) [84], for a supplement containing L. casei DN-114001, L. bulgaricus, and S. thermophilus, eradiation rates were 88.5 vs 51.5 % with no supplementation (p < 0.01) [85], and a study using S. boulardii showed an eradication rate of 93.3 vs 80.9 % with control (p = 0.750) [86].
Finally, the same studies evaluated the occurrence of adverse events during treatment and observed a non-significant decrease in symptoms. Only the open trial by Hurduc and collaborators showed that the incidence of side effects was significantly reduced in the S. boulardii group: 30.9 % in the control vs 8.3 % in the probiotic group (p = 0.047) [86].
In conclusion, there is currently a lack of sufficient evidence to recommend the use of probiotics in this area.

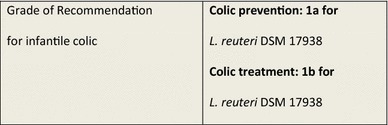
Infant Colic
Infantile colic consists of repeated distress periods of irritability and crying for indiscernible reasons for more than 3 h a day, 3 days a week and for 3 weeks or more [87]. It affects between 5 and 19 % of newborns and infants in the first months of life and creates a frustrating situation for parents and caregivers. The following differential diagnoses should be considered: cow’s milk protein allergy, gastro-esophageal reflux disease, intestinal hypermotility, and hormonal problems [88].
Despite the fact that some experiments have been performed with simeticone [89] and home remedies [90–93] have attempted to resolve this common problem, at the moment, no treatment has proven to be fully effective. Medical literature provides one SR [94] and eight randomized, double-blind, controlled studies using probiotics, seven of them with L. reuteri DSM 17938 in nursing infants [95–101] and a sixth one with formulas supplemented with two different probiotic strains, B lactis and Str. thermophiles [102].
The SR and published RCTs show that administration of L. reuteri DSM 17938 led to improved infantile colic symptoms in comparison with simeticone in exclusively breastfed children [94–101]. The included trials were supported by the manufacturer of the probiotic strain under study, which raises the possibility of bias; however, the likelihood is small since the trials were fully investigator-initiated and data controlled with transparent disclosure of potential conflicts of interest by the respective authors.
The RCT published in 2010 by Savino and colleagues [97] showed that L. reuteri DSM 17938 improved symptoms of infantile colic—as shown by a greater proportion of early breastfed infants with ≥50 % reduction in crying time from baseline vs placebo at Days 7 (p = 0.006), 14 (p = 0.007) and 21 (p = 0.036)—and was well tolerated. More recently, Sung and colleagues described a large, well designed RCT of L. reuteri for the management of infant colic in a broad community of breastfed and formula-fed infants in Australia [101]. In total, 167 breastfed infants were randomized to receive L. reuteri or placebo. At multiple follow-up intervals, the authors found no improvement in the duration of crying time in infants who received probiotic compared with placebo, in fact, infants receiving the studied probiotic cried significantly more [101].
In the earlier study [97], breastfeeding mothers were required to avoid cow’s milk, while the study by Sung et al. [101] did not have this requirement, so environmental factors may have played a role in the differences in outcomes between these two studies. Furthermore, the later study excluded infants with colic due to cow’s milk protein allergy and included infants treated with PPIs [101]. Indeed, 21 subjects in the probiotics group and 24 in the placebo group received PPIs and so may have been suffering from gastroesophageal reflux and not infantile colic. Therefore, the lack of significant difference between the two groups in the study by Sung et al. could be due to undeclared allocation bias. Recruiting infants treated with concomitant drugs (such as PPIs) and dietary approaches (probiotic and hypoallergenic formulas) introduced confounding factors that needed to be evaluated individually in order to perform an appropriate multivariate regression analysis and to distinguish the effects of the tested probiotic from those of the drugs.
Evidence supporting the prophylactic use of L. reuteri DSM 17938 during the first 3 months of life was shown by Indrio et al. in an RCT investigating the prophylactic use of L. reuteri DSM 17938 to prevent onset of functional gastrointestinal disorders, showing a reduction in the onset of daily crying time, regurgitation, and constipation [100].
One well recognized limitation of all the studies of infant colic is the need for a more objective way of measuring duration of crying rather than relying on the parents’ compliance to establish this outcome. The Saavedra et al. study [102] showed that supplementing formulas with B. lactis and Str. thermophilus was well tolerated and decreased colic episodes in children, possibly by modifying patterns of fermentation, which leads to less gas or water formation, which in turn may affect gastrointestinal tolerance, although this remains speculative.
In addition to clinical effects, use of L. reuteri DSM 17938 was associated with decreased private and public costs for the management of this condition [100].
Necrotizing Enterocolitis (NEC)
NEC is the most common gastrointestinal emergency in newborns and a major cause of morbidity and mortality in preterm infants [103], especially in those with very low birth weight (VLBW) <1500 g [104, 105]. Incidence of NEC is variable depending on countries and neonatal care units.
Recently there has been increasing interest in testing the potential benefits of probiotics in preterm infants in terms of preventing NEC [106]. The mechanisms by which probiotics may protect infants at high risk of developing NEC and/or sepsis include providing a barrier preventing the migration of bacteria and their products through the mucosa [107, 108], the competitive exclusion of potential pathogens [109], the modification of the host response to microbial products [110], the increased response of the mucosal IgA, the improvement of enteral nutrition, which inhibits pathogen growth and stimulation of immune responses while regulating TLRs, the nuclear κB factor and inflammatory cytokine production [111, 112].
Four meta-analyses on administration of probiotics to newborns with VLBW have been published [104, 113–115]. Deshpande et al. observed that with gestational age under 33 weeks there was a lower risk of mortality of any origin and of NEC by 53 and 64 %, respectively, in newborns who received probiotics, when compared with a control group [104]. The risk of sepsis did not differ significantly between groups. Additionally, the Cochrane review [113] reported that enteral supplementation of certain probiotics reduced the risk of NEC and mortality in preterm infants weighing less than 1500 g with no evidence of significant reduction of nosocomial sepsis. However, both of these meta-analyses have shown that not all probiotics tested were equally effective. The combinations used in the meta-analyses by Bin-Nun et al. [114] (B. infantis plus Str. thermophilus plus B. bifidus) and Lin et al. [115] (L. acidophilus plus B. infantis) were the most effective [116].
Alfaleh et al. reached the same conclusions but the results cannot be extrapolated to newborns weighing <1000 g because of lack of data in this high-risk group [116, 117]. The more recent meta-analysis published by Deshpande et al. in 2010 includes four new RCTs (n = 783), in relation to the one published in 2007 by the same author, one of them being a multicenter study [118]. The analysis revealed a highly statistically significant decrease in the risk of severe NEC and death (p < 0.00001) [104]. The strains evaluated were B. breve, S. boulardii, mixtures of Bifidobacterium and Streptococcus, LGG, and L. acidophilus. In addition, among all studies, the use of probiotics was described as safe and well tolerated [104, 113–115].
Another study showed that the prophylactic use of L. reuteri resulted in a statistically significant reduction in rates of NEC in children: NEC decreased 15.1 to 2.5 % (p = 0.0475), the need for surgery or death due to NEC was reduced from 8.2 to 2.5 % (p = 0.1774), and no adverse events related to the use of L. reuteri were reported [119].
In conclusion, the use of probiotics significantly reduces the risk of severe forms of NEC and death. Further studies are needed to answer important questions such as dose, strain, more efficient forms of administration and long-term safety for use in patients at risk of NEC.

Inflammatory Bowel Disease (IBD)
The concept of dysbiosis, an imbalance between ‘protective’ and ‘harmful’ intestinal bacteria, is gaining credibility among multiple etiologic factors of IBD. Nowadays, the rationale for administering strains of live ‘beneficial’ bacteria for IBD is based largely on the premise of dysbiosis. The most important attribute that makes probiotics appealing for use in IBD is their ability to regulate the host mucosal immune response, since it is well known that immune and epithelial cells of the small intestine can discriminate between various microorganisms through the activation of TLRs [120].
Many in vitro and animal studies favor this hypothesis. The literature published on adults emphasizes the role of probiotics in maintaining remission of IBD, especially in pouchitis [121]. A 2-year follow-up study on LGG administration was performed in children with Crohn’s disease in remission and resulted in a decreased relapse rate of 31 % in the probiotic group vs 17 % in the placebo group with non-significant statistical difference. There was also no difference in the duration of time until relapse [122]. However, despite a lack of evidence regarding any benefits, almost 80 % of children affected by IBD regularly consume probiotics [123]. In a randomized pilot study on the induction and maintenance of remission in children with ulcerative colitis, the proprietary preparation VSL#3 (a high-concentration mixture of probiotic bacterial strains) has been found to be as effective as adjuvant therapy, both in inducing and maintaining remission [124].
Although the incidence of pouchitis among Latin American children is increasing, our group did not find sufficient evidence in the literature among the pediatric population to give a recommendation on this pediatric indication.

Functional Gastrointestinal Disorders: Irritable Bowel Syndrome (IBS)
Functional gastrointestinal disorders are among society’s most common conditions, affecting millions of people of all ages, including children. At any one time, approximately two out of five persons will be affected by a functional gastrointestinal disorder. Furthermore, as gastrointestinal disorders often overlap, many patients could be affected by more than one functional gastrointestinal disorder simultaneously [125]. In the criteria for the definition of functional gastrointestinal disorders—the Rome III [126] consensus process 2006—the disorders are grouped by type of chronic or recurrent symptoms of functional origin. IBS is a functional gastrointestinal disorder characterized by abdominal discomfort or pain associated with defecation or change in bowel habits in absence of organic disease. IBS pathogenesis is multi-factorial and involves altered reactivity with increased intestinal motility and secretion with intraluminal stimuli such as food, inflammation, bacteria, or extra-intestinal elements such as emotional factors. IBS can also be linked to associated mechanisms such as lactose intolerance, fructose, malabsorption of biliary salts, bacterial overgrowth, and increased short-chain fatty acids [127]. There is no curative treatment for IBS, but symptom relief and periods of remission are achievable. Probiotics reduce the manifestations of functional disorders through modification of enzymatic and metabolic function. Studies have shown the efficacy of probiotics in IBS in adults but data in children are limited. Two studies in children with LGG showed controversial results. A 6-week RCT of LGG compared with placebo undertaken by Bausserman and Michail [128] showed negative results in 50 children and young adults (aged 6–20 years). Conversely, in another RCT with 37 participants, those in the LGG group were more likely to have treatment success than those in the placebo group and had reduced frequency of pain (p = 0.02), but not severity of pain [129]. Finally, Guandalini et al. [130] studied 59 children and concluded that VSL#3 is safe and more effective than placebo in ameliorating symptoms and improving quality of life in children affected by IBS.

Constipation
Probiotics alter the composition of the feces of healthy individuals. Only one randomized trial has been completed for children with constipation; the addition of LGG to lactulose as standard treatment offered no additional benefit [131]. More recently, a systemic evaluation and updated evidence on the efficacy and safety of probiotic supplementation for the treatment of constipation was performed in 2010 by Chmielewska and Szajewska [132]. The authors concluded that the scarce amount of data published to date do not yet provide sufficient scientific evidence to support a general recommendation about the use of probiotics for treatment of functional constipation. In the absence of new available data on the use of probiotics, the authors concluded that the use of probiotics for this condition should be considered investigational.

Allergy
Much of the literature on probiotics and allergy originates from Finland. Several publications report significant reductions in the SCORing Atopic Dermatitis (SCORAD) score with administration of B. lactis BB-12 and LGG ATCC 53103 [133, 134]. Perinatal administration of LGG ATCC 53103 leads to decreased atopic eczema, even up to the age of 7 years [135, 136]. Simultaneous treatment with pre- and probiotics (a mixture of four strains and galacto-oligosaccharides) administered to pregnant women for 2–4 weeks before delivery and to children over 6 months showed no effect on the cumulative incidence of allergic diseases at the age of 2 years compared with placebo, but tended to reduce disease associated with allergic reactions, and a significant reduction in atopic eczema was observed [137]. Taylor and colleagues [138], however, questioned the role of probiotics in allergy prevention, and reported that early supplementation with L. acidophilus did not reduce the risk of atopic dermatitis (AD) in infants at high risk and was even associated with increased allergic sensitivity in children. The pathogenesis of AD seems to involve deterioration of the intestinal barrier, considering that supplementation with probiotics can stabilize said function and reduce gastrointestinal symptoms in children with AD [139]. However, the composition of the intestinal microbiota in children with atopic eczema and dermatitis syndrome with and without food allergy was similar. As a result, there is no conclusive evidence regarding the role of the intestinal microbiota in relation to the development of infant food allergy in children with atopic eczema [140]. Moreover, the data from Brouwer et al. [141] shows that neither L. rhamnosus nor LGG had influence on the SCORAD score, sensitization, inflammatory parameters or cytokine production. The data on probiotics and allergy need further clarification, as the positive data are reported by different groups from Finland, but have not been confirmed by others—probably because of geographic or genetic differences playing a detrimental role among this population. In at least one study, L. rhamnosus and B. lactis improved only children sensitized to food, suggesting a genetic influence on the efficacy of probiotics in these children [142]. Treatment with LGG has been shown to reduce the intensity of atopic eczema and symptoms of hyper-IgE dermatitis syndrome but not in IgE-sensitized children [143]. Rosenfeldt et al. [144] showed that a combination of L rhamnosus 19070-2 and L. reuteri DSM 122460 was beneficial in the treatment of AD. The effect was more pronounced in patients with positive skin test response and increased levels of IgE [141]. By 1997, it had been suggested that probiotic bacteria may encourage endogenous barrier mechanisms in patients with AD and food allergy, and by reducing intestinal inflammation, may act as a useful tool in the treatment of food allergy [133]. However, authors of the latest Cochrane review concluded that there was inconclusive evidence for giving prebiotics to prevent allergic disorders in infants [145].
Probiotics in Marketed Products and Infant Formulas
There are an increasing number of probiotics emerging in a variety of distribution channels, especially in internet-based sales. At the same time, one of the main issues concerns the quality control and safety of these products commercialized in the market. In other words, there are more and more supplements with scarce scientific evidence of their medicinal potency, no or little control of their composition, no control of their shelf-life, and no knowledge of side effects or supplement–drug interactions [146].
The food industry uses microbial supplements, mainly in milk or yogurt drinks. Some of these microbial supplements are also sold in capsules, increasingly blurring the line between food and drug. Some probiotics claiming health benefits in dairy foods have been well documented, after identifying and isolating their microorganisms. One study identified mislabeling in 47 % of dietary supplements and 40 % milk products tested [147]. The poor labeling of dietary supplements is a global problem [148].
In the case of all marketed probiotics and formulas, investigations into the mechanism of action of specific strains should be mandatory along with conducting clinical studies on these products since the in vitro effects of a strain may be contrary to those observed in vivo [149]. The effects demonstrated with one strain cannot be extrapolated to another, even if they belong to the same species. Only a specific strain and its commercially ‘controlled’ product for which convincing data are available should be recommended for medical use.
Probiotic bacterial strains include lactobacilli (LGG, L. reuteri, L. johnsonii) [150] and bifidobacteria (B. lactis Bb 12), as well as strains of Escherichia coli (E. coli Nissle 1917) [151] and certain enterococci strains (although this agent has been linked to the transfer of resistance encoded and transmitted by plasmids) [152].
L. acidophilus LB has shown antibacterial activity against E. coli. However, if E. coli is present in the gastrointestinal tract of the host before L. acidophilus LB is administered, like in the case of acute gastroenteritis, their antibacterial activity can be strongly reduced [153]. Adhesion of Bifidobacteria Bb 12 improves in the presence of LGG, both in healthy children and during episodes of diarrhea, suggesting synergy between the two strains [154].
To date, few publications on the clinical effects of probiotic supplementation in infant formulas, follow-up formulas, and special medical foods have addressed this subject. It should be noted that some studies describe an adequate safety profile and studies also report that the use of probiotic supplementation does not affect infant growth [155–157]. Yet, although evidence on the benefits in NEC, treatment and prevention of AID, AAD, and atopy and allergy have been published, it must be stressed that these benefits are rare and strain-specific, and thus conclusions are limited until more scientific data become available—a situation that prevents us from drawing definitive conclusions on the subject.

Safety and Side Effects
Probiotics are considered ‘generally recognized as safe’ (GRAS) and well tolerated in humans. The most common adverse effects include bloating and flatulence; however, these are typically mild and subside with continued use [158]. One theoretical concern associated with probiotics includes the potential for these viable organisms to move from the gastrointestinal tract and cause systemic infections. Although rare, probiotic-related bacteremia and fungemia have been reported, particularly in neonates [159–163]. It is estimated that the risk of developing bacteremia from ingested lactobacilli probiotics is <1 per 1 million users [164], and the risk of developing fungemia from S. boulardii is estimated at 1 per 5.6 million users [165].
Large-scale epidemiological studies on probiotic use in countries where their use is widespread show low rates of systemic infection between 0.05 and 0.40 % [166]. Of all documented invasive infections, most of them occur mainly in immune-compromised adults [164].
Lactobacilli have caused cases of sepsis, meningitis, and infections in other organs in adults [167, 168]; however, invasive infections in infants and children are extremely rare [164]. Nevertheless, bacteremia has been attributed to Lactobacillus supplementation in children [169] and in neonates [160, 161, 163]. In a review, Enache-Angoulvant and Hennequin found evidence of sepsis in children with short bowel syndrome treated with Lactobacillus and S. boulardii-related fungemia was reported in about 50 patients, with central venous catheterization as the main risk factor [170]. Fungemia was also observed in patients with a central venous catheter hospitalized in beds adjacent to patients treated with the yeast [171]. There were no reports of probiotic translocation from the gastrointestinal tract into the systemic circulation. Fungemia has also been reported with S. cerevisiae in two newborns, one of whom was being treated with the agent and the other contracted the fungemia from the first [162]. These cases highlight that probiotic supplementation should be used with caution in children with central catheters and those with known or potentially compromised intestinal mucosal integrity or those with underdeveloped immune systems.
To minimize the risk of side effects such as bacteremia and fungemia, studies may be performed using non-viable or inactivated preparations. These modified probiotic preparations may be the best choice in high-risk situations. The effects of these agents may go beyond the gastrointestinal tract to distant areas, such as the urogenital and respiratory mucosa, and it may not be necessary to administer intact probiotic organisms to achieve benefits. At basic research level, probiotic products such as secreted proteins or DNA can block inflammation and prevent death of intestinal epithelial cells [172].
Limitations
Among the seventy-four studies that met our inclusion criteria and were included in the recommendations, the quality varied and almost all of the included trials had methodological limitations. The most common problems were lack of description of randomization procedures and group allocation and blinding. In general terms, individual publication bias was not evaluated by our reviewers.
Conclusions
Probiotics have demonstrated efficacy in preventing and treating various medical conditions, particularly those involving the gastrointestinal tract in children. In addition, probiotics are a helpful tool in specific infectious, inflammatory and functional disorders, but it is important to note that evidence indicates strain specificity in each case. Certain probiotics have been widely used for a variety of disorders and data supports their increased use. Available literature shows a statistically significant benefit in decreasing intensity, duration and number of consultations for acute gastroenteritis caused by various infectious agents, mostly viral and parasitic-related illnesses, when specific probiotics are combined with ORS.
LGG, L. reuteri and S. boulardii are the best studied probiotics. Some lactobacilli strains and S. boulardii have proven to be most effective if treatment is introduced early. Another particular application is NEC, where probiotics B. breve, and specific mixtures of Bifidobacterium, Streptococcus, and Lactobacillus strains significantly reduce the risk of severe forms and associated mortality.
Due to strain specificity, only clinically tested probiotics can be recommended to treat pediatric patients.
Acknowledgments
Dr. Maruy acknowledges consulting honoraria from Biocodex. None of the other authors have competing interests.
Editorial assistance—editing and styling the manuscript in preparation for submission and assistance with post-submission revisions—was provided by Mary Hines of Springer Healthcare and funded by Biocodex.
Conflicts of interest
The Advisory Committee of the Latin-American (LATAM) expert consensus group coordinated the development of this Consensus Statement with an unrestricted grant from Biocodex. The opinions expressed in this Consensus Statement represent only those of the Advisory Committee.
Footnotes
On behalf of the Latin-American (LATAM) expert consensus group.
References
- 1.Guyatt GH, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaeschke R, et al. Users’ guides to the medical literature. III. How to use an article about a diagnostic test. A. Are the results of the study valid? Evidence-Based Medicine Working Group. JAMA. 1994;271:389–391. doi: 10.1001/jama.271.5.389. [DOI] [PubMed] [Google Scholar]
- 3.Laupacis A, et al. Users’ guides to the medical literature. V. How to use an article about prognosis. Evidence-Based Medicine Working Group. JAMA. 1994;272:234–237. doi: 10.1001/jama.272.3.234. [DOI] [PubMed] [Google Scholar]
- 4.Levine M, et al. Users’ guides to the medical literature. IV. How to use an article about harm. Evidence-Based Medicine Working Group. JAMA. 1994;271:1615–1619. doi: 10.1001/jama.271.20.1615. [DOI] [PubMed] [Google Scholar]
- 5.Oxman AD, et al. Users’ guides to the medical literature. VI. How to use an overview. Evidence-Based Medicine Working Group. JAMA. 1994;272:1367–1371. doi: 10.1001/jama.272.17.1367. [DOI] [PubMed] [Google Scholar]
- 6.CEBM. Oxford Centre for Evidence-Based Medicine—levels of evidence. Centre for Evidence-Based Medicine. 2009. http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed 15 July 2014.
- 7.FAO/WHO. Working group on drafting guidelines for the evaluation of probiotics in food. Guidelines for the evaluation of probiotics in food. 2002. ftp://ftp.fao.org/es/esn/food/wgreport2.pdf. Accessed 2 Sept 2010.
- 8.Huff BA. Caveat emptor. “Probiotics” might not be what they seem. Can Fam Physician. 2004;50:583–587. [PMC free article] [PubMed] [Google Scholar]
- 9.Lilly DM, Stillwell RH. Probiotics: growth-promoting factors produced by microorganisms. Science. 1965;147:747–748. doi: 10.1126/science.147.3659.747. [DOI] [PubMed] [Google Scholar]
- 10.Parker RB. Probiotics, the other half of the antibiotic story. Animal Nutr Health. 1974;29:4–8. [Google Scholar]
- 11.Tissier H. Tritement des infections intestinales par la methode de translormation de la flore bacterienne de l’intestin. C R Soc Biol. 1906;60:359–361. [Google Scholar]
- 12.FAO/WHO. Joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food. 2001. ftp://ftp.fao.org/docrep/fao/009/a0512e/a0512e00.pdf. Accessed 2 Sept 2010.
- 13.Czerucka D, et al. Review article: yeast as probiotics—Saccharomyces boulardii. Aliment Pharmacol Ther. 2007;26:767–778. doi: 10.1111/j.1365-2036.2007.03442.x. [DOI] [PubMed] [Google Scholar]
- 14.Lajolo F. Alimentos funcionales: Aspectos Científicos y Regulatorios. Dieta y Salud. 2003;10:2–7. [Google Scholar]
- 15.ANMAT. Alimentos Funcionales Probióticos. 2010. http://www.anmat.gov.ar/consumidores/alimentos/Alimentos_Funcionales_Probioticos.pdf. Accessed 30 Oct 2014.
- 16.Elmer GW, et al. Biotherapeutic agents. A neglected modality for the treatment and prevention of selected intestinal and vaginal infections. JAMA. 1996;275:870–876. doi: 10.1001/jama.275.11.870. [DOI] [PubMed] [Google Scholar]
- 17.Gorbach SL. Prebióticos y lácteos fermentados: sus beneficios para la salud. Infect Dis Clin Pract Esp. 1995;3:2–7. [Google Scholar]
- 18.Henriquez Moya M, Moreno C. Probióticos: Legislación en Marcha. Dirección Nacional de Alimentos. Secretaria de Agricultura, Ganadería, Pesca y Alimentos. 2009. http://www.alimentosargentinos.gov.ar/0-3/revistas/r_39/articulos/Probioticos.htm. Accessed 2 Sept 2010.
- 19.WGO. Probióticos y Prebióticos. World Gastroenterology Organisation. 2008. http://www.worldgastroenterology.org/assets/downloads/es/pdf/guidelines/19_probioticos_prebioticos_es.pdf. Accessed 2 Sept 2010.
- 20.Borchers AT, et al. Probiotics and immunity. J Gastroenterol. 2009;44:26–46. doi: 10.1007/s00535-008-2296-0. [DOI] [PubMed] [Google Scholar]
- 21.Indriyani S. Effects of live versus heat-killed probiotics on acute diarrhea in young children. Indriyani Paediatr Indones. 2012;52:249–254. [Google Scholar]
- 22.Chen X, et al. Saccharomyces boulardii inhibits ERK1/2 mitogen-activated protein kinase activation both in vitro and in vivo and protects against Clostridium difficile toxin A-induced enteritis. J Biol Chem. 2006;281:24449–24454. doi: 10.1074/jbc.M605200200. [DOI] [PubMed] [Google Scholar]
- 23.Lutgendorff F, et al. The role of microbiota and probiotics in stress-induced gastro-intestinal damage. Curr Mol Med. 2008;8:282–298. doi: 10.2174/156652408784533779. [DOI] [PubMed] [Google Scholar]
- 24.Donkor ON, et al. Cytokine profile and induction of T helper type 17 and regulatory T cells by human peripheral mononuclear cells after microbial exposure. Clin Exp Immunol. 2012;167:282–295. doi: 10.1111/j.1365-2249.2011.04496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guarino A, et al. Probiotics as prevention and treatment for diarrhea. Curr Opin Gastroenterol. 2009;25:18–23. doi: 10.1097/MOG.0b013e32831b4455. [DOI] [PubMed] [Google Scholar]
- 26.Kaila M, et al. A prospective study of humoral immune responses to cow milk antigens in the first year of life. Pediatr Allergy Immunol. 1994;5:164–169. doi: 10.1111/j.1399-3038.1994.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 27.McFarland LV. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J Gastroenterol. 2010;16:2202–2222. doi: 10.3748/wjg.v16.i18.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saran S, et al. Use of fermented foods to combat stunting and failure to thrive. Nutrition. 2002;18:393–396. doi: 10.1016/s0899-9007(01)00790-0. [DOI] [PubMed] [Google Scholar]
- 29.Sazawal S, et al. Efficacy of milk fortified with a probiotic Bifidobacterium lactis (DR-10TM) and prebiotic galacto-oligosaccharides in prevention of morbidity and on nutritional status. Asia Pac J Clin Nutr. 2004;13:S28. [Google Scholar]
- 30.Agostoni C, et al. Probiotic bacteria in dietetic products for infants: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2004;38:365–374. doi: 10.1097/00005176-200404000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Szajewska H, et al. Probiotics in gastrointestinal diseases in children: hard and not-so-hard evidence of efficacy. J Pediatr Gastroenterol Nutr. 2006;42:454–475. doi: 10.1097/01.mpg.0000221913.88511.72. [DOI] [PubMed] [Google Scholar]
- 32.Saavedra JM, et al. Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. Lancet. 1994;344:1046–1049. doi: 10.1016/s0140-6736(94)91708-6. [DOI] [PubMed] [Google Scholar]
- 33.Szajewska H, et al. Efficacy of LactobacillusGG in prevention of nosocomial diarrhea in infants. J Pediatr. 2001;138:361–365. doi: 10.1067/mpd.2001.111321. [DOI] [PubMed] [Google Scholar]
- 34.Mastretta E, et al. Effect of Lactobacillus GG and breast-feeding in the prevention of rotavirus nosocomial infection. J Pediatr Gastroenterol Nutr. 2002;35:527–531. doi: 10.1097/00005176-200210000-00013. [DOI] [PubMed] [Google Scholar]
- 35.Chouraqui JP, et al. Acidified milk formula supplemented with bifidobacterium lactis: impact on infant diarrhea in residential care settings. J Pediatr Gastroenterol Nutr. 2004;38:288–292. doi: 10.1097/00005176-200403000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Thibault H, et al. Effects of long-term consumption of a fermented infant formula (with bifidobacterium breve c50 and Streptococcus thermophilus 065) on acute diarrhea in healthy infants. J Pediatr Gastroenterol Nutr. 2004;39:147–152. doi: 10.1097/00005176-200408000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Pawlowski SW, et al. Diagnosis and treatment of acute or persistent diarrhea. Gastroenterology. 2009;136:1874–1886. doi: 10.1053/j.gastro.2009.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shamir R, et al. Evaluation of a diet containing probiotics and zinc for the treatment of mild diarrheal illness in children younger than one year of age. J Am Coll Nutr. 2005;24:370–375. doi: 10.1080/07315724.2005.10719487. [DOI] [PubMed] [Google Scholar]
- 39.Isolauri E, et al. A human Lactobacillus strain (Lactobacillus casei sp strain GG) promotes recovery from acute diarrhea in children. Pediatrics. 1991;88:90–97. [PubMed] [Google Scholar]
- 40.Shornikova AV, et al. A trial in the Karelian Republic of oral rehydration and LactobacillusGG for treatment of acute diarrhoea. Acta Paediatr. 1997;86:460–465. doi: 10.1111/j.1651-2227.1997.tb08913.x. [DOI] [PubMed] [Google Scholar]
- 41.Guarino A, et al. Oral bacterial therapy reduces the duration of symptoms and of viral excretion in children with mild diarrhea. J Pediatr Gastroenterol Nutr. 1997;25:516–519. doi: 10.1097/00005176-199711000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Guandalini S, et al. LactobacillusGG administered in oral rehydration solution to children with acute diarrhea: a multicenter European trial. J Pediatr Gastroenterol Nutr. 2000;30:54–60. doi: 10.1097/00005176-200001000-00018. [DOI] [PubMed] [Google Scholar]
- 43.Shornikova AV, et al. Bacteriotherapy with Lactobacillus reuteri in rotavirus gastroenteritis. Pediatr Infect Dis J. 1997;16:1103–1107. doi: 10.1097/00006454-199712000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Szajewska H, et al. Meta-analysis: Lactobacillus reuteri strain DSM 17938 (and the original strain ATCC 55730) for treating acute gastroenteritis in children. Benef Microbes. 2014;5:285–293. doi: 10.3920/BM2013.0056. [DOI] [PubMed] [Google Scholar]
- 45.Simakachorn N, et al. Clinical evaluation of the addition of lyophilized, heat-killed Lactobacillus acidophilus LB to oral rehydration therapy in the treatment of acute diarrhea in children. J Pediatr Gastroenterol Nutr. 2000;30:68–72. doi: 10.1097/00005176-200001000-00020. [DOI] [PubMed] [Google Scholar]
- 46.Szymanski H, et al. Treatment of acute infectious diarrhoea in infants and children with a mixture of three Lactobacillus rhamnosus strains—a randomized, double-blind, placebo-controlled trial. Aliment Pharmacol Ther. 2006;23:247–253. doi: 10.1111/j.1365-2036.2006.02740.x. [DOI] [PubMed] [Google Scholar]
- 47.Costa-Ribeiro H, et al. Limitations of probiotic therapy in acute, severe dehydrating diarrhea. J Pediatr Gastroenterol Nutr. 2003;36:112–115. doi: 10.1097/00005176-200301000-00021. [DOI] [PubMed] [Google Scholar]
- 48.Salazar-Lindo E, et al. Lactobacillus casei strain GG in the treatment of infants with acute watery diarrhea: a randomized, double-blind, placebo controlled clinical trial [ISRCTN67363048] BMC Pediatr. 2004;4:18. doi: 10.1186/1471-2431-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kowalska-Duplaga K, et al. Efficacy of Trilacin the treatment of acute diarrhoea in infants and young children—a multicentre, randomized, double-blind placebo-controlled study. Pediatria Współczesna. 2004;3:295–299. [Google Scholar]
- 50.Khanna V, et al. Efficacy of tyndalized Lactobacillus acidophilus in acute diarrhea. Indian J Pediatr. 2005;72:935–938. doi: 10.1007/BF02731667. [DOI] [PubMed] [Google Scholar]
- 51.Sarker SA, et al. Lactobacillus paracasei strain ST11 has no effect on rotavirus but ameliorates the outcome of nonrotavirus diarrhea in children from Bangladesh. Pediatrics. 2005;116:e221–e228. doi: 10.1542/peds.2004-2334. [DOI] [PubMed] [Google Scholar]
- 52.Huang JS, et al. Efficacy of probiotic use in acute diarrhea in children: a meta-analysis. Dig Dis Sci. 2002;47:2625–2634. doi: 10.1023/a:1020501202369. [DOI] [PubMed] [Google Scholar]
- 53.Van Niel CW, et al. Lactobacillus therapy for acute infectious diarrhea in children: a meta-analysis. Pediatrics. 2002;109:678–684. doi: 10.1542/peds.109.4.678. [DOI] [PubMed] [Google Scholar]
- 54.Szajewska H, Mrukowicz JZ. Probiotics in the treatment and prevention of acute infectious diarrhea in infants and children: a systematic review of published randomized, double-blind, placebo-controlled trials. J Pediatr Gastroenterol Nutr. 2001;33(Suppl 2):S17–S25. doi: 10.1097/00005176-200110002-00004. [DOI] [PubMed] [Google Scholar]
- 55.Allen SJ, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010;(11):CD003048. doi:10.1002/14651858.CD003048.pub3. [DOI] [PMC free article] [PubMed]
- 56.Höchter W, et al. Saccharomyces boulardii bei akuter Erwachsenendiarrhoe. Münch Med Wschr. 1990;132:188–192. [Google Scholar]
- 57.Cetina-Sauri G, Sierra Basto G. Therapeutic evaluation of Saccharomyces boulardii in children with acute diarrhea. Ann Pediatr. 1994;41:397–400. [Google Scholar]
- 58.Kurugol Z, Koturoglu G. Effects of Saccharomyces boulardii in children with acute diarrhoea. Acta Paediatr. 2005;94:44–47. doi: 10.1111/j.1651-2227.2005.tb01786.x. [DOI] [PubMed] [Google Scholar]
- 59.Villarruel G, et al. Saccharomyces boulardii in acute childhood diarrhoea: a randomized, placebo-controlled study. Acta Paediatr. 2007;96:538–541. doi: 10.1111/j.1651-2227.2007.00191.x. [DOI] [PubMed] [Google Scholar]
- 60.Castañeda C, et al. Effects of Saccharomyces boulardii in children with chronic diarrhea, especially cases due to giardiasis. Rev Mex Pueric Pediatr. 1995;2:12–16. [Google Scholar]
- 61.Mansour-Ghanaei F, et al. Efficacy of saccharomyces boulardii with antibiotics in acute amoebiasis. World J Gastroenterol. 2003;9:1832–1833. doi: 10.3748/wjg.v9.i8.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saint-Marc T, et al. Efficacy of Saccharomyces boulardii in the treatment of diarrhea in AIDS. Ann Med Interne (Paris) 1991;142:64–65. [PubMed] [Google Scholar]
- 63.Billoo AG, et al. Role of a probiotic (Saccharomyces boulardii) in management and prevention of diarrhoea. World J Gastroenterol. 2006;12:4557–4560. doi: 10.3748/wjg.v12.i28.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gutierrez Castrellon P, et al. An evidence based Iberic-Latin American guideline for acute gastroenteritis management in infants and prescholars. An Pediatr (Barc) 2010;72:220 e1–20. [DOI] [PubMed]
- 65.National Collaborating Centre for Women’s and Children’s Health. Diarrhoea and vomiting caused by gastroenteritis: diagnosis, assessment and management in children younger than 5 years. National Institute for Health and Clinical Excellence (NICE). 2009. Accessed 30 Oct 2014.
- 66.WGO. Probióticos y Prebióticos. World Gastroenterology Organisation. 2011. http://www.worldgastroenterology.org/assets/export/userfiles/2012%20Probiotics_NEW%20FINAL_sp.pdf. Accessed 30 Oct 2014.
- 67.Sazawal S, et al. Efficacy of probiotics in prevention of acute diarrhoea: a meta-analysis of masked, randomised, placebo-controlled trials. Lancet Infect Dis. 2006;6:374–382. doi: 10.1016/S1473-3099(06)70495-9. [DOI] [PubMed] [Google Scholar]
- 68.Francavilla R, et al. Randomised clinical trial: Lactobacillus reuteri DSM 17938 vs. placebo in children with acute diarrhoea—a double-blind study. Aliment Pharmacol Ther. 2012;36:363–369. doi: 10.1111/j.1365-2036.2012.05180.x. [DOI] [PubMed] [Google Scholar]
- 69.Turck D, et al. Incidence and risk factors of oral antibiotic-associated diarrhea in an outpatient pediatric population. J Pediatr Gastroenterol Nutr. 2003;37:22–26. doi: 10.1097/00005176-200307000-00004. [DOI] [PubMed] [Google Scholar]
- 70.Szajewska H, et al. Probiotics in the prevention of antibiotic-associated diarrhea in children: a meta-analysis of randomized controlled trials. J Pediatr. 2006;149:367–372. doi: 10.1016/j.jpeds.2006.04.053. [DOI] [PubMed] [Google Scholar]
- 71.Advice for travelers. Treat Guidel Med Lett. 2004;2(21):33–40. [PubMed]
- 72.Advice for travelers. Med Lett Drugs Ther. 1996;38(969):17–20. [PubMed]
- 73.Mackell S. Traveler’s diarrhea in the pediatric population: etiology and impact. Clin Infect Dis. 2005;41(Suppl 8):S547–S552. doi: 10.1086/432950. [DOI] [PubMed] [Google Scholar]
- 74.Hilton E, et al. Efficacy of LactobacillusGG as a diarrheal preventive in travelers. J Travel Med. 1997;4:41–43. doi: 10.1111/j.1708-8305.1997.tb00772.x. [DOI] [PubMed] [Google Scholar]
- 75.Katelaris PH, et al. Lactobacilli to prevent traveler’s diarrhea? N Engl J Med. 1995;333:1360–1361. doi: 10.1056/NEJM199511163332016. [DOI] [PubMed] [Google Scholar]
- 76.Oksanen PJ, et al. Prevention of travellers’ diarrhoea by LactobacillusGG. Ann Med. 1990;22:53–56. doi: 10.3109/07853899009147242. [DOI] [PubMed] [Google Scholar]
- 77.Kollaritsch H, et al. Prevention of traveler’s diarrhea with Saccharomyces boulardii. Results of a placebo controlled double-blind study. Fortschr Med. 1993;111:152–156. [PubMed] [Google Scholar]
- 78.Boonyaritichaikij S, et al. Long-term administration of probiotics to asymptomatic pre-school children for either the eradication or the prevention of Helicobacter pylori infection. Helicobacter. 2009;14:202–207. doi: 10.1111/j.1523-5378.2009.00675.x. [DOI] [PubMed] [Google Scholar]
- 79.Cruchet S, et al. Effect of the ingestion of a dietary product containing Lactobacillus johnsonii La1 on Helicobacter pylori colonization in children. Nutrition. 2003;19:716–721. doi: 10.1016/s0899-9007(03)00109-6. [DOI] [PubMed] [Google Scholar]
- 80.Gotteland M, et al. Effect of regular ingestion of Saccharomyces boulardii plus inulin or Lactobacillus acidophilus LB in children colonized by Helicobacter pylori. Acta Paediatr. 2005;94:1747–1751. doi: 10.1111/j.1651-2227.2005.tb01848.x. [DOI] [PubMed] [Google Scholar]
- 81.Szajewska H, et al. Randomized, double-blind, placebo-controlled trial: effect of LactobacillusGG supplementation on Helicobacter pylori eradication rates and side effects during treatment in children. J Pediatr Gastroenterol Nutr. 2009;48:431–436. doi: 10.1097/mpg.0b013e318182e716. [DOI] [PubMed] [Google Scholar]
- 82.Sykora J, et al. Effects of a specially designed fermented milk product containing probiotic Lactobacillus casei DN-114 001 and the eradication of H. pylori in children: a prospective randomized double-blind study. J Clin Gastroenterol. 2005;39:692–698. doi: 10.1097/01.mcg.0000173855.77191.44. [DOI] [PubMed] [Google Scholar]
- 83.Goldman CG, et al. Effect of a probiotic food as an adjuvant to triple therapy for eradication of Helicobacter pylori infection in children. Nutrition. 2006;22:984–988. doi: 10.1016/j.nut.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 84.Lionetti E, et al. Lactobacillus reuteri therapy to reduce side-effects during anti-Helicobacter pylori treatment in children: a randomized placebo controlled trial. Aliment Pharmacol Ther. 2006;24:1461–1468. doi: 10.1111/j.1365-2036.2006.03145.x. [DOI] [PubMed] [Google Scholar]
- 85.Sýkora J, et al. Supplements of one week triple drug therapy with special probiotic Lactobacillus casei imunitass (Strain DN-114 000) and Streptococcus thermophilus and Lactobacillus bulgaricus in the eradication of H. pylori-colonized children: a prospective randomised trial [abstract P0900] J Pediatr Gastroenterol Nutr. 2004;39:S400. [Google Scholar]
- 86.Hurduc V, et al. A randomized, open trial evaluating the effect of Saccharomyces boulardii on the eradication rate of Helicobacter pylori infection in children. Acta Paediatr. 2009;98:127–131. doi: 10.1111/j.1651-2227.2008.00977.x. [DOI] [PubMed] [Google Scholar]
- 87.Hyman PE, et al. Childhood functional gastrointestinal disorders: neonate/toddler. Gastroenterology. 2006;130:1519–1526. doi: 10.1053/j.gastro.2005.11.065. [DOI] [PubMed] [Google Scholar]
- 88.Savino F. Focus on infantile colic. Acta Paediatr. 2007;96:1259–1264. doi: 10.1111/j.1651-2227.2007.00428.x. [DOI] [PubMed] [Google Scholar]
- 89.Metcalf TJ, et al. Simethicone in the treatment of infant colic: a randomized, placebo-controlled, multicenter trial. Pediatrics. 1994;94:29–34. [PubMed] [Google Scholar]
- 90.Akcam M, Yilmaz A. Oral hypertonic glucose solution in the treatment of infantile colic. Pediatr Int. 2006;48:125–127. doi: 10.1111/j.1442-200X.2006.02182.x. [DOI] [PubMed] [Google Scholar]
- 91.Danielsson B, Hwang CP. Treatment of infantile colic with surface active substance (simethicone) Acta Paediatr Scand. 1985;74:446–450. doi: 10.1111/j.1651-2227.1985.tb11001.x. [DOI] [PubMed] [Google Scholar]
- 92.Gupta SK. Update on infantile colic and management options. Curr Opin Investig Drugs. 2007;8:921–926. [PubMed] [Google Scholar]
- 93.Savino F, et al. A randomized double-blind placebo-controlled trial of a standardized extract of Matricariae recutita, Foeniculum vulgare and Melissa officinalis (ColiMil) in the treatment of breastfed colicky infants. Phytother Res. 2005;19:335–340. doi: 10.1002/ptr.1668. [DOI] [PubMed] [Google Scholar]
- 94.Anabrees J, et al. Probiotics for infantile colic: a systematic review. BMC Pediatr. 2013;13:186. doi: 10.1186/1471-2431-13-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Szajewska H, et al. Lactobacillus reuteri DSM 17938 for the management of infantile colic in breastfed infants: a randomized, double-blind, placebo-controlled trial. J Pediatr. 2013;162:257–262. doi: 10.1016/j.jpeds.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 96.Savino F, et al. Lactobacillus reuteri (American Type Culture Collection Strain 55730) versus simethicone in the treatment of infantile colic: a prospective randomized study. Pediatrics. 2007;119:e124–e130. doi: 10.1542/peds.2006-1222. [DOI] [PubMed] [Google Scholar]
- 97.Savino F, et al. Lactobacillus reuteri DSM 17938 in infantile colic: a randomized, double-blind, placebo-controlled trial. Pediatrics. 2010;126:e526–e533. doi: 10.1542/peds.2010-0433. [DOI] [PubMed] [Google Scholar]
- 98.Chau K, et al. Probiotics for infantile colic: a randomized, double-blind, placebo-controlled trial investigating Lactobacillus reuteri DSM 17938. J Pediatr. 2015;166(74–8):e1. doi: 10.1016/j.jpeds.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 99.Savino F, et al. Preventive effects of oral probiotic on infantile colic: a prospective, randomised, blinded, controlled trial using Lactobacillus reuteri DSM 17938. Benef Microbes. 2014:1–7. [Epub ahead of print]. [DOI] [PubMed]
- 100.Indrio F, et al. Prophylactic use of a probiotic in the prevention of colic, regurgitation, and functional constipation: a randomized clinical trial. JAMA Pediatr. 2014;168:228–233. doi: 10.1001/jamapediatrics.2013.4367. [DOI] [PubMed] [Google Scholar]
- 101.Sung V, et al. Treating infant colic with the probiotic Lactobacillus reuteri: double blind, placebo controlled randomised trial. BMJ. 2014;348:g2107. doi: 10.1136/bmj.g2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Saavedra JM, et al. Long-term consumption of infant formulas containing live probiotic bacteria: tolerance and safety. Am J Clin Nutr. 2004;79:261–267. doi: 10.1093/ajcn/79.2.261. [DOI] [PubMed] [Google Scholar]
- 103.Fernandez JI, De las Cuevas T. Enterocolitis necrotizante neonatal. Bol Pediatr. 2006;46:172–178. [Google Scholar]
- 104.Deshpande G, et al. Probiotics for prevention of necrotising enterocolitis in preterm neonates with very low birthweight: a systematic review of randomised controlled trials. Lancet. 2007;369:1614–1620. doi: 10.1016/S0140-6736(07)60748-X. [DOI] [PubMed] [Google Scholar]
- 105.Samanta M, et al. Prophylactic probiotics for prevention of necrotizing enterocolitis in very low birth weight newborns. J Trop Pediatr. 2009;55:128–131. doi: 10.1093/tropej/fmn091. [DOI] [PubMed] [Google Scholar]
- 106.Millar M, et al. Probiotics for preterm infants? Arch Dis Child Fetal Neonatal Ed. 2003;88:F354–F358. doi: 10.1136/fn.88.5.F354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mattar AF, et al. Effect of probiotics on enterocyte bacterial translocation in vitro. Pediatr Surg Int. 2001;17:265–268. doi: 10.1007/s003830100591. [DOI] [PubMed] [Google Scholar]
- 108.Orrhage K, Nord CE. Factors controlling the bacterial colonization of the intestine in breastfed infants. Acta Paediatr. 1999;88:47–57. doi: 10.1111/j.1651-2227.1999.tb01300.x. [DOI] [PubMed] [Google Scholar]
- 109.Reid G, et al. Can bacterial interference prevent infection? Trends Microbiol. 2001;9:424–428. doi: 10.1016/s0966-842x(01)02132-1. [DOI] [PubMed] [Google Scholar]
- 110.Duffy LC. Interactions mediating bacterial translocation in the immature intestine. J Nutr. 2000;130:432S–436S. doi: 10.1093/jn/130.2.432S. [DOI] [PubMed] [Google Scholar]
- 111.Martin CR, Walker WA. Probiotics: role in pathophysiology and prevention in necrotizing enterocolitis. Semin Perinatol. 2008;32:127–137. doi: 10.1053/j.semperi.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 112.Schanler RJ. Probiotics and necrotising enterocolitis in premature infants. Arch Dis Child Fetal Neonatal Ed. 2006;91:F395–F397. doi: 10.1136/adc.2005.092742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Alfaleh K, Bassler D. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2008;(1):CD005496. doi:10.1002/14651858.CD005496.pub2.. [DOI] [PubMed]
- 114.Bin-Nun A, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr. 2005;147:192–196. doi: 10.1016/j.jpeds.2005.03.054. [DOI] [PubMed] [Google Scholar]
- 115.Lin HC, et al. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2005;115:1–4. doi: 10.1542/peds.2004-1463. [DOI] [PubMed] [Google Scholar]
- 116.Wolvers D, et al. Guidance for substantiating the evidence for beneficial effects of probiotics: prevention and management of infections by probiotics. J Nutr. 2010;140:698S–712S. doi: 10.3945/jn.109.113753. [DOI] [PubMed] [Google Scholar]
- 117.Alfaleh K, et al. Probiotics reduce the risk of necrotizing enterocolitis in preterm infants: a meta-analysis. Neonatology. 2010;97:93–99. doi: 10.1159/000235684. [DOI] [PubMed] [Google Scholar]
- 118.Deshpande G, et al. Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics. 2010;125:921–930. doi: 10.1542/peds.2009-1301. [DOI] [PubMed] [Google Scholar]
- 119.Hunter C, et al. Effect of routine probiotic, Lactobacillus reuteri DSM 17938, use on rates of necrotizing enterocolitis in neonates with birthweight <1000 grams: a sequential analysis. BMC Pediatr. 2012;12:142. doi: 10.1186/1471-2431-12-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kadowaki N, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620–1633. doi: 10.1053/j.gastro.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 122.Bousvaros A, et al. A randomized, double-blind trial of Lactobacillus GG versus placebo in addition to standard maintenance therapy for children with Crohn’s disease. Inflamm Bowel Dis. 2005;11:833–839. doi: 10.1097/01.mib.0000175905.00212.2c. [DOI] [PubMed] [Google Scholar]
- 123.Day AS, et al. Use of complementary and alternative medicines by children and adolescents with inflammatory bowel disease. J Paediatr Child Health. 2004;40:681–684. doi: 10.1111/j.1440-1754.2004.00510.x. [DOI] [PubMed] [Google Scholar]
- 124.Miele E, et al. Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. Am J Gastroenterol. 2009;104:437–443. doi: 10.1038/ajg.2008.118. [DOI] [PubMed] [Google Scholar]
- 125.Talley NJ. Functional gastrointestinal disorders as a public health problem. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2008;20(Suppl 1):121–129. doi: 10.1111/j.1365-2982.2008.01097.x. [DOI] [PubMed] [Google Scholar]
- 126.Drossman DA, Dumitrascu DL, Rome III. New standard for functional gastrointestinal disorders. J Gastrointestin Liver Dis. 2006;15:237–241. [PubMed] [Google Scholar]
- 127.Karantanos T, et al. Current insights in to the pathophysiology of Irritable Bowel Syndrome. Gut Pathog. 2010;2:3. doi: 10.1186/1757-4749-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bausserman M, Michail S. The use of Lactobacillus GG in irritable bowel syndrome in children: a double-blind randomized control trial. J Pediatr. 2005;147:197–201. doi: 10.1016/j.jpeds.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 129.Gawronska A, et al. A randomized double-blind placebo-controlled trial of Lactobacillus GG for abdominal pain disorders in children. Aliment Pharmacol Ther. 2007;25:177–184. doi: 10.1111/j.1365-2036.2006.03175.x. [DOI] [PubMed] [Google Scholar]
- 130.Guandalini S, et al. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr. 2010;51:24–30. doi: 10.1097/MPG.0b013e3181ca4d95. [DOI] [PubMed] [Google Scholar]
- 131.Banaszkiewicz A, Szajewska H. Ineffectiveness of Lactobacillus GG as an adjunct to lactulose for the treatment of constipation in children: a double-blind, placebo-controlled randomized trial. J Pediatr. 2005;146:364–369. doi: 10.1016/j.jpeds.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 132.Chmielewska A, Szajewska H. Systematic review of randomised controlled trials: probiotics for functional constipation. World J Gastroenterol. 2010;16:69–75. doi: 10.3748/wjg.v16.i1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Majamaa H, Isolauri E. Probiotics: a novel approach in the management of food allergy. J Allergy Clin Immunol. 1997;99:179–185. doi: 10.1016/s0091-6749(97)70093-9. [DOI] [PubMed] [Google Scholar]
- 134.Isolauri E, et al. Probiotics in the management of atopic eczema. Clin Exp Allergy. 2000;30:1604–1610. doi: 10.1046/j.1365-2222.2000.00943.x. [DOI] [PubMed] [Google Scholar]
- 135.Kalliomaki M, et al. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357:1076–1079. doi: 10.1016/S0140-6736(00)04259-8. [DOI] [PubMed] [Google Scholar]
- 136.Kalliomaki M, et al. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet. 2003;361:1869–1871. doi: 10.1016/S0140-6736(03)13490-3. [DOI] [PubMed] [Google Scholar]
- 137.Kukkonen K, et al. Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:192–198. doi: 10.1016/j.jaci.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 138.Taylor AL, et al. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J Allergy Clin Immunol. 2007;119:184–191. doi: 10.1016/j.jaci.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 139.Rosenfeldt V, et al. Effect of probiotics on gastrointestinal symptoms and small intestinal permeability in children with atopic dermatitis. J Pediatr. 2004;145:612–616. doi: 10.1016/j.jpeds.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 140.Kendler M, et al. Comparison of fecal microflora in children with atopic eczema/dermatitis syndrome according to IgE sensitization to food. Pediatr Allergy Immunol. 2006;17:141–147. doi: 10.1111/j.1399-3038.2005.00371.x. [DOI] [PubMed] [Google Scholar]
- 141.Brouwer ML, et al. No effects of probiotics on atopic dermatitis in infancy: a randomized placebo-controlled trial. Clin Exp Allergy. 2006;36:899–906. doi: 10.1111/j.1365-2222.2006.02513.x. [DOI] [PubMed] [Google Scholar]
- 142.Sistek D, et al. Is the effect of probiotics on atopic dermatitis confined to food sensitized children? Clin Exp Allergy. 2006;36:629–633. doi: 10.1111/j.1365-2222.2006.02485.x. [DOI] [PubMed] [Google Scholar]
- 143.Viljanen M, et al. Probiotics in the treatment of atopic eczema/dermatitis syndrome in infants: a double-blind placebo-controlled trial. Allergy. 2005;60:494–500. doi: 10.1111/j.1398-9995.2004.00514.x. [DOI] [PubMed] [Google Scholar]
- 144.Rosenfeldt V, et al. Effect of probiotic Lactobacillus strains in children with atopic dermatitis. J Allergy Clin Immunol. 2003;111:389–395. doi: 10.1067/mai.2003.389. [DOI] [PubMed] [Google Scholar]
- 145.Osborn DA, Sinn JK. Prebiotics in infants for prevention of allergic disease and food hypersensitivity. Cochrane Database Syst Rev. 2007;(4):CD006474. [DOI] [PubMed]
- 146.Petroczi A, et al. Mission impossible? Regulatory and enforcement issues to ensure safety of dietary supplements. Food Chem Toxicol. 2011;49:393–402. doi: 10.1016/j.fct.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 147.Temmerman R, et al. Identification and antibiotic susceptibility of bacterial isolates from probiotic products. Int J Food Microbiol. 2003;81:1–10. doi: 10.1016/s0168-1605(02)00162-9. [DOI] [PubMed] [Google Scholar]
- 148.Elliot E, Teversham K. An evaluation of nine probiotics available in South Africa, August 2003. S Afr Med J. 2004;94:121–124. [PubMed] [Google Scholar]
- 149.Ibnou-Zekri N, et al. Divergent patterns of colonization and immune response elicited from two intestinal Lactobacillus strains that display similar properties in vitro. Infect Immun. 2003;71:428–436. doi: 10.1128/IAI.71.1.428-436.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Braegger C, et al. Supplementation of infant formula with probiotics and/or prebiotics: a systematic review and comment by the ESPGHAN committee on nutrition. J Pediatr Gastroenterol Nutr. 2011;52:238–250. doi: 10.1097/MPG.0b013e3181fb9e80. [DOI] [PubMed] [Google Scholar]
- 151.Henker J, et al. Probiotic Escherichia coli Nissle 1917 versus placebo for treating diarrhea of greater than 4 days duration in infants and toddlers. Pediatr Infect Dis J. 2008;27:494–499. doi: 10.1097/INF.0b013e318169034c. [DOI] [PubMed] [Google Scholar]
- 152.Yameen MA, et al. Nasal and perirectal colonization of vancomycin sensitive and resistant enterococci in patients of paediatrics ICU (PICU) of tertiary health care facilities. BMC Infect Dis. 2013;13:156. doi: 10.1186/1471-2334-13-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bernet MF, et al. Lactobacillus acidophilus LA 1 binds to cultured human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut. 1994;35:483–489. doi: 10.1136/gut.35.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Juntunen M, et al. Adherence of probiotic bacteria to human intestinal mucus in healthy infants and during rotavirus infection. Clin Diagn Lab Immunol. 2001;8:293–296. doi: 10.1128/CDLI.8.2.293-296.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Urban MF, et al. Growth of infants born to HIV-infected women when fed a biologically acidified starter formula with and without probiotics. South Afr J Clin Nutr. 2008;21:28–32. [Google Scholar]
- 156.Weizman Z, Alsheikh A. Safety and tolerance of a probiotic formula in early infancy comparing two probiotic agents: a pilot study. J Am Coll Nutr. 2006;25:415–419. doi: 10.1080/07315724.2006.10719554. [DOI] [PubMed] [Google Scholar]
- 157.Gibson RA, et al. Safety of supplementing infant formula with long-chain polyunsaturated fatty acids and Bifidobacterium lactis in term infants: a randomised controlled trial. Br J Nutr. 2009;101:1706–1713. doi: 10.1017/S0007114508084080. [DOI] [PubMed] [Google Scholar]
- 158.Probiotics. Med Lett Drugs Ther. 2007;49(1267):66–8. [PubMed]
- 159.Snydman DR. The safety of probiotics. Clin Infect Dis. 2008;46(Suppl 2):S104-11 (discussion S44–51). [DOI] [PubMed]
- 160.Broughton RA, et al. Neonatal meningitis due to Lactobacillus. Pediatr Infect Dis. 1983;2:382–384. doi: 10.1097/00006454-198309000-00012. [DOI] [PubMed] [Google Scholar]
- 161.Kunz AN, et al. Two cases of Lactobacillus bacteremia during probiotic treatment of short gut syndrome. J Pediatr Gastroenterol Nutr. 2004;38:457–458. doi: 10.1097/00005176-200404000-00017. [DOI] [PubMed] [Google Scholar]
- 162.Perapoch J, et al. Fungemia with Saccharomyces cerevisiae in two newborns, only one of whom had been treated with ultra-levura. Eur J Clin Microbiol Infect Dis. 2000;19:468–470. doi: 10.1007/s100960000295. [DOI] [PubMed] [Google Scholar]
- 163.Thompson C, et al. Lactobacillus acidophilus sepsis in a neonate. J Perinatol. 2001;21:258–260. doi: 10.1038/sj.jp.7200509. [DOI] [PubMed] [Google Scholar]
- 164.Borriello SP, et al. Safety of probiotics that contain lactobacilli or bifidobacteria. Clin Infect Dis. 2003;36:775–780. doi: 10.1086/368080. [DOI] [PubMed] [Google Scholar]
- 165.Karpa KD. Probiotics for Clostridium difficile diarrhea: putting it into perspective. Ann Pharmacother. 2007;41:1284–1287. doi: 10.1345/aph.1K228. [DOI] [PubMed] [Google Scholar]
- 166.Fedorak RN, Madsen KL. Probiotics and prebiotics in gastrointestinal disorders. Curr Opin Gastroenterol. 2004;20:146–155. doi: 10.1097/00001574-200403000-00017. [DOI] [PubMed] [Google Scholar]
- 167.Mackay AD, et al. Lactobacillus endocarditis caused by a probiotic organism. Clin Microbiol Infect. 1999;5:290–292. doi: 10.1111/j.1469-0691.1999.tb00144.x. [DOI] [PubMed] [Google Scholar]
- 168.Rautio M, et al. Liver abscess due to a Lactobacillus rhamnosus strain indistinguishable from L. rhamnosus strain GG. Clin Infect Dis. 1999;28:1159–1160. doi: 10.1086/514766. [DOI] [PubMed] [Google Scholar]
- 169.Cabana MD, et al. Probiotics in primary care pediatrics. Clin Pediatr (Phila) 2006;45:405–410. doi: 10.1177/0009922806289614. [DOI] [PubMed] [Google Scholar]
- 170.Enache-Angoulvant A, Hennequin C. Invasive Saccharomyces infection: a comprehensive review. Clin Infect Dis. 2005;41:1559–1568. doi: 10.1086/497832. [DOI] [PubMed] [Google Scholar]
- 171.Cassone M, et al. Outbreak of Saccharomyces cerevisiae subtype boulardii fungemia in patients neighboring those treated with a probiotic preparation of the organism. J Clin Microbiol. 2003;41:5340–5343. doi: 10.1128/JCM.41.11.5340-5343.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jijon H, et al. DNA from probiotic bacteria modulates murine and human epithelial and immune function. Gastroenterology. 2004;126:1358–1373. doi: 10.1053/j.gastro.2004.02.003. [DOI] [PubMed] [Google Scholar]


