Abstract
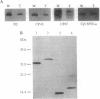
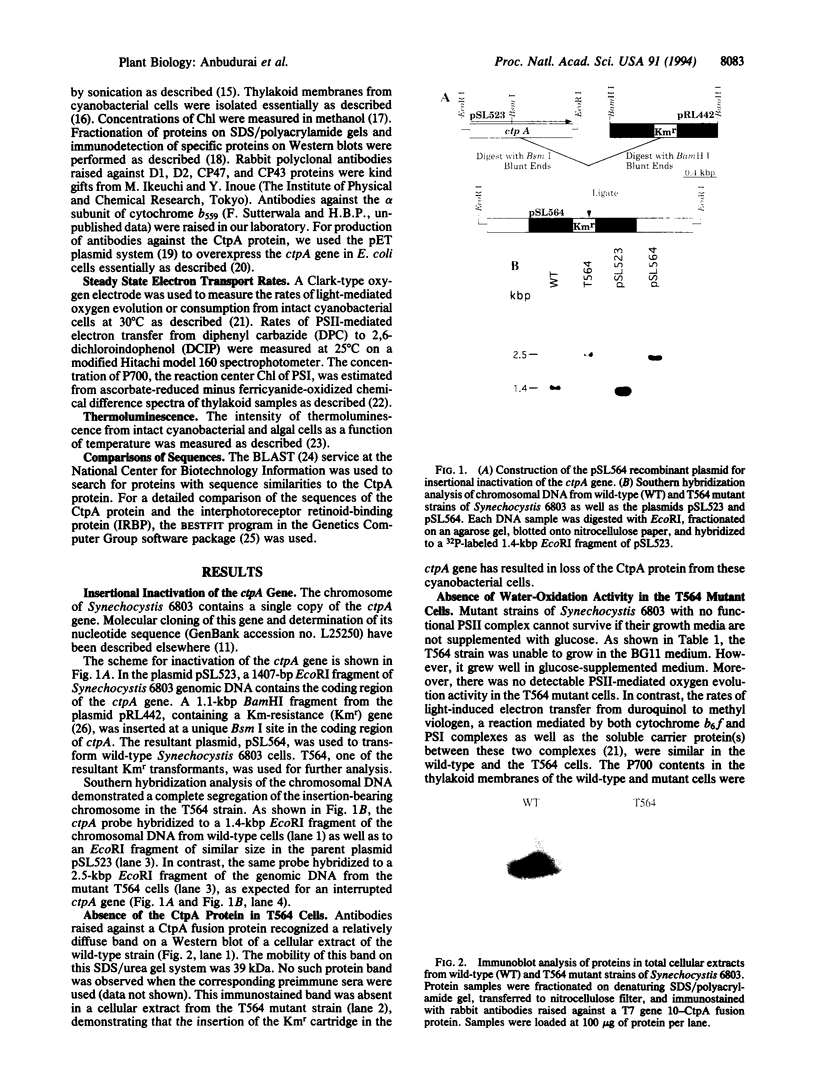
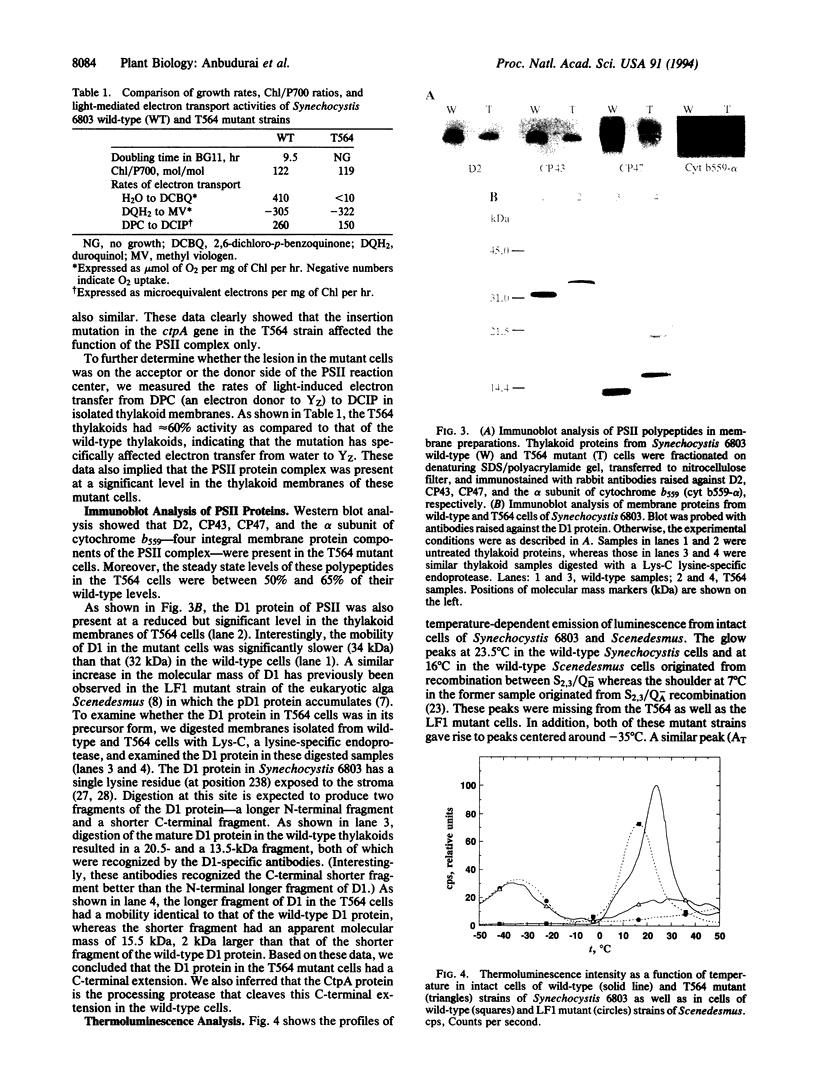
The D1 protein of the photosystem II (PSII) complex in the thylakoid membrane of oxygenic photosynthetic organisms is synthesized as a precursor polypeptide (pD1) with a C-terminal extension. Posttranslational processing of the pD1 protein is essential to establish water oxidation activity of the PSII complex. We have recently identified a gene, ctpA, a mutation in which resulted in a loss of PSII activity in the cyanobacterium Synechocystis sp. PCC 6803. To study the function of the CtpA protein, we inactivated the ctpA gene by inserting a kanamycin-resistance gene into its coding sequence. The resultant mutant strain, T564, had no PSII-mediated water oxidation activity, but it had normal cytochrome b6f and photosystem I activities. Measurements of thermoluminescence profiles and rates of reduction of 2,6-dichlorophenolindophenol indicated that PSII complexes in the mutant cells had functional reaction centers that were unable to accept electrons from water. Immunoblot analysis showed that D1, D2, CP47, CP43, and the alpha subunit of cytochrome b559, five integral membrane proteins of PSII, were present in T564 cells. Interestingly, the D1 protein in the mutant cells was 2 kDa larger than that in wild-type cells, due to the presence of a C-terminal extension. We conclude that the CtpA protein is a processing enzyme that cleaves off the C-terminal extension of the D1 protein. Interestingly, the CtpA protein shows significant sequence similarity to the interphotoreceptor retinoid-binding proteins in the bovine, human, and insect eye systems.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Anbudurai P. R., Pakrasi H. B. Mutational analysis of the PsbL protein of photosystem II in the cyanobacterium Synechocystis sp. PCC 6803. Z Naturforsch C. 1993 Mar-Apr;48(3-4):267–274. doi: 10.1515/znc-1993-3-424. [DOI] [PubMed] [Google Scholar]
- Borst D. E., Redmond T. M., Elser J. E., Gonda M. A., Wiggert B., Chader G. J., Nickerson J. M. Interphotoreceptor retinoid-binding protein. Gene characterization, protein repeat structure, and its evolution. J Biol Chem. 1989 Jan 15;264(2):1115–1123. [PubMed] [Google Scholar]
- Bowyer J. R., Packer J. C., McCormack B. A., Whitelegge J. P., Robinson C., Taylor M. A. Carboxyl-terminal processing of the D1 protein and photoactivation of water-splitting in photosystem II. Partial purification and characterization of the processing enzyme from Scenedesmus obliquus and Pisum sativum. J Biol Chem. 1992 Mar 15;267(8):5424–5433. [PubMed] [Google Scholar]
- Carpenter S. D., Ohad I., Vermaas W. F. Analysis of chimeric spinach/cyanobacterial CP43 mutants of Synechocystis sp. PCC 6803: the chlorophyll-protein CP43 affects the water-splitting system of Photosystem II. Biochim Biophys Acta. 1993 Sep 13;1144(2):204–212. doi: 10.1016/0005-2728(93)90174-e. [DOI] [PubMed] [Google Scholar]
- Dalbey R. E., Von Heijne G. Signal peptidases in prokaryotes and eukaryotes--a new protease family. Trends Biochem Sci. 1992 Nov;17(11):474–478. doi: 10.1016/0968-0004(92)90492-r. [DOI] [PubMed] [Google Scholar]
- Debus R. J. The manganese and calcium ions of photosynthetic oxygen evolution. Biochim Biophys Acta. 1992 Oct 16;1102(3):269–352. doi: 10.1016/0005-2728(92)90133-m. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diner B. A., Ries D. F., Cohen B. N., Metz J. G. COOH-terminal processing of polypeptide D1 of the photosystem II reaction center of Scenedesmus obliquus is necessary for the assembly of the oxygen-evolving complex. J Biol Chem. 1988 Jun 25;263(18):8972–8980. [PubMed] [Google Scholar]
- Elhai J., Wolk C. P. A versatile class of positive-selection vectors based on the nonviability of palindrome-containing plasmids that allows cloning into long polylinkers. Gene. 1988 Aug 15;68(1):119–138. doi: 10.1016/0378-1119(88)90605-1. [DOI] [PubMed] [Google Scholar]
- Gong H. S., Ohad I. The PQ/PQH2 ratio and occupancy of photosystem II-QB site by plastoquinone control the degradation of D1 protein during photoinhibition in vivo. J Biol Chem. 1991 Nov 5;266(31):21293–21299. [PubMed] [Google Scholar]
- Gonzalez-Fernandez F., Kittredge K. L., Rayborn M. E., Hollyfield J. G., Landers R. A., Saha M., Grainger R. M. Interphotoreceptor retinoid-binding protein (IRBP), a major 124 kDa glycoprotein in the interphotoreceptor matrix of Xenopus laevis. Characterization, molecular cloning and biosynthesis. J Cell Sci. 1993 May;105(Pt 1):7–21. doi: 10.1242/jcs.105.1.7. [DOI] [PubMed] [Google Scholar]
- Hara H., Yamamoto Y., Higashitani A., Suzuki H., Nishimura Y. Cloning, mapping, and characterization of the Escherichia coli prc gene, which is involved in C-terminal processing of penicillin-binding protein 3. J Bacteriol. 1991 Aug;173(15):4799–4813. doi: 10.1128/jb.173.15.4799-4813.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M. R., Grubb J. H., Sly W. S. C-terminal processing of human beta-glucuronidase. The propeptide is required for full expression of catalytic activity, intracellular retention, and proper phosphorylation. J Biol Chem. 1993 Oct 25;268(30):22627–22633. [PubMed] [Google Scholar]
- Lind L. K., Shukla V. K., Nyhus K. J., Pakrasi H. B. Genetic and immunological analyses of the cyanobacterium Synechocystis sp. PCC 6803 show that the protein encoded by the psbJ gene regulates the number of photosystem II centers in thylakoid membranes. J Biol Chem. 1993 Jan 25;268(3):1575–1579. [PubMed] [Google Scholar]
- Mannan R. M., Whitmarsh J., Nyman P., Pakrasi H. B. Directed mutagenesis of an iron-sulfur protein of the photosystem I complex in the filamentous cyanobacterium Anabaena variabilis ATCC 29413. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10168–10172. doi: 10.1073/pnas.88.22.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder J. B., Goloubinoff P., Edelman M. Molecular architecture of the rapidly metabolized 32-kilodalton protein of photosystem II. Indications for COOH-terminal processing of a chloroplast membrane polypeptide. J Biol Chem. 1984 Mar 25;259(6):3900–3908. [PubMed] [Google Scholar]
- Metz J., Nixon P., Diner B. Nucleotide sequence of the psbA3 gene from the cyanobacterium Synechocystis PCC 6803. Nucleic Acids Res. 1990 Nov 25;18(22):6715–6715. doi: 10.1093/nar/18.22.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon P. J., Trost J. T., Diner B. A. Role of the carboxy terminus of polypeptide D1 in the assembly of a functional water-oxidizing manganese cluster in photosystem II of the cyanobacterium Synechocystis sp. PCC 6803: assembly requires a free carboxyl group at C-terminal position 344. Biochemistry. 1992 Nov 10;31(44):10859–10871. doi: 10.1021/bi00159a029. [DOI] [PubMed] [Google Scholar]
- Nyhus K. J., Ikeuchi M., Inoue Y., Whitmarsh J., Pakrasi H. B. Purification and characterization of the photosystem I complex from the filamentous cyanobacterium Anabaena variabilis ATCC 29413. J Biol Chem. 1992 Jun 25;267(18):12489–12495. [PubMed] [Google Scholar]
- Pakrasi H. B., Williams J. G., Arntzen C. J. Targeted mutagenesis of the psbE and psbF genes blocks photosynthetic electron transport: evidence for a functional role of cytochrome b559 in photosystem II. EMBO J. 1988 Feb;7(2):325–332. doi: 10.1002/j.1460-2075.1988.tb02816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravnikar P. D., Debus R., Sevrinck J., Saetaert P., McIntosh L. Nucleotide sequence of a second psbA gene from the unicellular cyanobacterium Synechocystis 6803. Nucleic Acids Res. 1989 May 25;17(10):3991–3991. doi: 10.1093/nar/17.10.3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla V. K., Stanbekova G. E., Shestakov S. V., Pakrasi H. B. The D1 protein of the photosystem II reaction-centre complex accumulates in the absence of D2: analysis of a mutant of the cyanobacterium Synechocystis sp. PCC 6803 lacking cytochrome b559. Mol Microbiol. 1992 Apr;6(7):947–956. doi: 10.1111/j.1365-2958.1992.tb01544.x. [DOI] [PubMed] [Google Scholar]
- Silber K. R., Keiler K. C., Sauer R. T. Tsp: a tail-specific protease that selectively degrades proteins with nonpolar C termini. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):295–299. doi: 10.1073/pnas.89.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud R. M., Kossiakoff A. A., Chambers J. L. Mechanisms of zymogen activation. Annu Rev Biophys Bioeng. 1977;6:177–193. doi: 10.1146/annurev.bb.06.060177.001141. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Taguchi F., Yamamoto Y., Inagaki N., Satoh K. Recognition signal for the C-terminal processing protease of D1 precursor protein in the photosystem II reaction center. An analysis using synthetic oligopeptides. FEBS Lett. 1993 Jul 12;326(1-3):227–231. doi: 10.1016/0014-5793(93)81796-3. [DOI] [PubMed] [Google Scholar]
- Zhang L., Pakrasi H. B., Whitmarsh J. Photoautotrophic growth of the cyanobacterium Synechocystis sp. PCC 6803 in the absence of cytochrome c553 and plastocyanin. J Biol Chem. 1994 Feb 18;269(7):5036–5042. [PubMed] [Google Scholar]