Abstract
Background
In 2011 the World Health Organization (WHO) recommended parenteral artesunate in preference to quinine as first‐line treatment for people with severe malaria. Prior to this recommendation, many countries, particularly in Africa, had begun to use artemether, an alternative artemisinin derivative. This review evaluates intramuscular artemether compared with both quinine and artesunate.
Objectives
To assess the efficacy and safety of intramuscular artemether versus any other parenteral medication in treating severe malaria in adults and children.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register, CENTRAL (The Cochrane Library), MEDLINE, EMBASE and LILACS, ISI Web of Science, conference proceedings and reference lists of articles. We also searched the WHO clinical trial registry platform, ClinicalTrials.gov and the metaRegister of Controlled Trials (mRCT) for ongoing trials up to 9 April 2014.
Selection criteria
Randomized controlled trials (RCTs) comparing intramuscular artemether with intravenous or intramuscular antimalarial for treating severe malaria.
Data collection and analysis
The primary outcome was all‐cause death.Two authors independently assessed trial eligibility, risk of bias and extracted data. We summarized dichotomous outcomes using risk ratios (RR) and continuous outcomes using mean differences (MD), and presented both measures with 95% confidence intervals (CI). Where appropriate, we combined data in meta‐analyses and assessed the quality of the evidence using the GRADE approach.
Main results
We included 18 RCTs, enrolling 2662 adults and children with severe malaria, carried out in Africa (11) and in Asia (7).
Artemether versus quinine
For children in Africa, there is probably little or no difference in the risk of death between intramuscular artemether and quinine (RR 0.96, 95% CI 0.76 to 1.20; 12 trials, 1447 participants, moderate quality evidence). Coma recovery may be about five hours shorter with artemether (MD ‐5.45, 95% CI ‐7.90 to ‐3.00; six trials, 358 participants, low quality evidence), and artemether may result in fewer neurological sequelae, but larger trials would be needed to confirm this (RR 0.84, 95% CI 0.66 to 1.07; seven trials, 968 participants, low quality evidence). Artemether probably shortens the parasite clearance time by about nine hours (MD ‐9.03, 95% CI ‐11.43 to ‐6.63; seven trials, 420 participants, moderate quality evidence), and may shorten the fever clearance time by about three hours (MD ‐3.73, 95% CI ‐6.55 to ‐0.92; eight trials, 457 participants, low quality evidence).
For adults in Asia, treatment with intramuscular artemether probably results in fewer deaths than treatment with quinine (RR 0.59, 95% CI 0.42 to 0.83; four trials, 716 participants, moderate quality evidence).
Artemether versus artesunate
Artemether and artesunate have not been directly compared in randomized trials in African children.
For adults in Asia, mortality is probably higher with intramuscular artemether (RR 1.80, 95% CI 1.09 to 2.97, two trials,494 participants, moderate quality evidence).
Authors' conclusions
Although there is a lack of direct evidence comparing artemether with artesunate, artemether is probably less effective than artesunate at preventing deaths from severe malaria. In circumstances where artesunate is not available, artemether is an alternative to quinine.
Keywords: Adolescent; Adult; Child; Child, Preschool; Humans; Infant; Africa; Antimalarials; Antimalarials/administration & dosage; Antimalarials/adverse effects; Artemether; Artemisinins; Artemisinins/administration & dosage; Artemisinins/adverse effects; Artesunate; Asia; Injections, Intramuscular; Malaria, Cerebral; Malaria, Cerebral/drug therapy; Malaria, Cerebral/mortality; Malaria, Falciparum; Malaria, Falciparum/drug therapy; Malaria, Falciparum/mortality; Oceania; Quinine; Quinine/administration & dosage; Quinine/adverse effects; Randomized Controlled Trials as Topic
Artemether injection for treating people with severe malaria
In this review, researchers from The Cochrane Collaboration examined the effects of treating people that have severe malaria with artemether injected intramuscularly, and compared it to treatment with other antimalarial drugs given intramuscularly or intravenously. After searching for relevant trials up to 9 April 2014, we included 18 randomized controlled trials that recruited 2662 adults and children and were conducted mainly in Africa and Asia.
What is severe malaria and how might artemether injection reduce deaths
Severe malaria is caused by infection with the Plasmodium parasite, which is transmitted to people through the bite of an infected female Anopheles mosquito. It is a serious medical condition and can cause vomiting, anaemia, convulsions and death. People need to be treated as quickly as possible.
Injection of artesunate is recommended by the World Health Organization (WHO) for treating adults and children that have severe malaria as trials have shown that it results in fewer deaths compared to quinine treatment. Artemether is an alternative artemisinin derivative but is only available as a pre‐mixed oil‐based solution for intramuscular injection. Artemether is now widely available and is used in many African countries, although it is not specifically recommended by the WHO.
What the research says
Artemether versus quinine:
For children in Africa, intramuscular artemether is probably as good as quinine at preventing deaths from severe malaria (moderate quality evidence). Artemether may shorten recovery time from coma by about five hours (low quality evidence), and may reduce the number of children with signs of brain damage at the time of hospital discharge (low quality evidence).
In older children (> 15 years) and adults in Asia, treatment with artemether probably results in fewer deaths than quinine (moderate quality evidence).
Artemether versus artesunate:
In adults from Asia, artesunate probably prevents more deaths than artemether (moderate quality evidence), but no trials have been conducted in young children from Africa.
Authors conclusions
Although there is a lack of direct evidence comparing artemether with artesunate, artemether is probably less effective than artesunate at preventing deaths from severe malaria. In circumstances where artesunate is not available, artemether is an alternative to quinine.
Summary of findings
Summary of findings for the main comparison.
Summary of findings table 1
| Artemether compared with quinine for treating children with severe malaria | |||||
| Patient or population: Children with severe malaria Settings: Malaria‐endemic countries Intervention: Intramuscular artemether Comparison: Intravenous or intramuscular quinine | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Quinine | Artemether | ||||
| Death | 170 per 1000 | 164 per 1000 (129 to 204) | RR 0.96 (0.76 to 1.2) | 1447 (12 trials) | ⊕⊕⊕⊝ moderate1,2,3,4 |
| Coma resolution time | The mean coma resolution time ranged across control groups from 17.4 to 42.4 hours | The mean coma resolution time in the intervention groups was 5.45 hours shorter (7.90 to 3.00 shorter) | ‐ | 358 (6 trials) | ⊕⊕⊝⊝ low3,5,6,7 |
| Neurological sequelae at discharge | 220 per 1000 | 185 per 1000 (145 to 235) | RR 0.84 (0.66 to 1.07) | 968 (7 trials) | ⊕⊕⊝⊝ low 1,2,3,8 |
| Parasite clearance time | The mean parasite clearance time ranged across control groups from 22.4 to 61.25 hours | The mean parasite clearance time in the intervention groups was 9.03 hours shorter (11.43 to 6.63 shorter) | ‐ | 420 (7 trials) | ⊕⊕⊕⊝ moderate1,3,7,9 |
| Fever clearance time | The mean fever clearance time ranged across control groups from 18 to 61.25 hours | The mean fever clearance time in the intervention groups was 3.73 shorter (6.55 to 0.92 shorter) | ‐ | 457 (8 trials) | ⊕⊕⊝⊝ low3,10,11,12 |
| *The assumed risk is the median control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 No serious risk of bias: Trials were variable in their risk of bias, but exclusion of the trials at high or unclear risk of selection bias did not change this result. 2 No serious inconsistency: None of the individual trials found statistically significant effects, and there was no statistical heterogeneity between trials. 3 No serious indirectness: Trials were from West Africa, East Africa and one from India. All were in children with severe malaria (aged under 15 years), and most compared the standard dose of intramuscular artemether with the WHO recommended dose of intravenous quinine. 4 Downgraded by 1 for serious imprecision: These trials, and the overall meta‐analysis are underpowered to detect a difference or to prove equivalence. 5 Downgraded by 2 for serious risk of bias: Four of the six trials were at unclear risk of selection bias. When these four trials are excluded the result becomes non‐significant. 6 No serious inconsistency: Statistically significant differences were only seen in two of the six trials. However, statistical heterogeneity between trials was low and the overall meta‐analysis is statistically significant. 7 No serious imprecision: The result is statistically significant and the overall meta‐analysis is adequately powered to detect this effect. 8 Downgraded by 2 for very serious imprecision: These trials, and the overall meta‐analysis are underpowered to detect a difference or to prove equivalence. The 95% CI is very wide and includes clinically important differences and no effect. 9 Downgraded by 1 for serious inconsistency: The mean difference in parasite clearance time ranged from a two hour increase with artemether to a 15 hour decrease. 10 Downgraded by 1 for serious risk of bias: Four of the seven trials were at unclear risk of selection bias. When these four trials are excluded the result becomes non‐significant. 11 Downgraded by 1 for serious inconsistency: The mean difference in fever clearance time ranged from a 25 hour increase with artemether to an 18 hour decrease. 12 No serious imprecision: The overall meta‐analysis is powered to detect this effect. The result is statistically significant but may not be clinically important.
Summary of findings 2.
Summary of findings table 2
| Artemether compared with quinine for treating adults with severe malaria | |||||
| Patient or population: Adults with severe malaria Settings: Malaria endemic countries Intervention: Intramuscular artemether Comparison: Intravenous or intramuscular quinine | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Quinine | Artemether | ||||
| Death | 208 per 1000 | 123 per 1000 (87 to 173) | RR 0.59 (0.42 to 0.83) | 716 (4 trials) | ⊕⊕⊕⊝ moderate1,2,3,4 |
| Coma resolution time | ‐ | ‐ | Not pooled. Little difference. | 657 (2 trials) | ⊕⊕⊝⊝ low1,5,6, 7 |
| Neurological sequelae at discharge | 4 per 1000 |
12 per 1000 (1 to 111) |
RR 2.92 (0.31 to 27.86) | 560 (1 trial) | ⊕⊕⊝⊝ low7,8 |
| Parasite clearance time | ‐ | ‐ | Not pooled. Little difference apparent. | 716 (4 trials) | ⊕⊕⊕⊝ moderate1,3,6,9 |
| Fever clearance time | ‐ | ‐ | Not pooled. Little difference apparent. | 716 (4 trials) | ⊕⊕⊝⊝ low1,3,6,10 |
| *The assumed risk is the median control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 No serious risk of bias: Trials are generally well conducted and at low risk of bias. 2 No serious inconsistency: Statistically significant differences were only seen in one of the four trials. However, statistical heterogeneity between trials was low and the overall meta‐analysis is statistically significant. 3 No serious indirectness: All four trials compared intramuscular artemether with intravenous quinine in adults; two trials from Thailand, one each from Vietnam and Papua New Guinea 4 Downgraded by 1 for serious imprecision: These trials, and the overall meta‐analysis are very underpowered to detect a difference in mortality or to prove equivalence. 5Hien 1996 VNM and Karbwang 1995 THA reported median coma time for artemether vs. quinine (Hien 1996 VNM: 66 vs. 48, P = 0.003; Karbwang 1995 THA: 48 vs. 48). Downgraded by 1 for inconsistency: One trial found a shorter median coma resolution time with quinine, and one trial found no difference. 6 Downgraded by 1 for imprecision: The data could not be pooled.
7 No serious risk of bias: This single trial was at low risk of bias.
8 Downgraded by 1 for serious imprecision: Neurological sequelae in adults were uncommon. This trial is underpowered to detect or exclude clinically important differences. 9 Two trials found no significant difference between parasite clearance time for artemether vs. quinine (Karbwang 1992 THA: mean 63.6 vs. 61.6, P = 0.85 and Seaton 1998 PNG: median 48 vs. 52, P = 0.381). Two other trials reported significantly shorter median parasite clearance times for artemether vs. quinine (Hien 1996 VNM: 72 vs. 90 P < 0.001 and Karbwang 1995 THA: 54 vs.78, P = 0.007). No serious inconsistency: The two largest trials both found shorter median clearance times with artemether. 10 Three trials (Hien 1996 VNM, Seaton 1998 PNG and Karbwang 1995 THA) reported median fever clearance time for artemether vs. quinine (127 vs. 90, P < 0.001; 32 vs. 48, P = 0.034 and 79 vs. 84, no significant difference). Karbwang 1992 THAreported mean fever clearance time and found a statistically significant reduction of about 30 hours with artemether. Downgraded by 1 for inconsistency: One trial found a shorter median fever clearance time with quinine, and two trials found a shorter time with artemether.
Summary of findings 3.
Summary of findings table 3
| Artemether compared with artesunate for treating adults with severe malaria | |||||
| Patient or population: Adults with severe malaria Settings: Malaria endemic countries Intervention: Intramuscular artemether Comparison: Intravenous or intramuscular artesunate | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Artesunate | Artemether | ||||
| Death | 87 per 1000 | 156 per 1000 (95 to 258) | RR 1.80 (1.09 to 2.97) | 494 (2 trials) | ⊕⊕⊕⊝ moderate1,2,3,4 |
| Coma resolution time | ‐ | ‐ | Not pooled. No significant difference | 494 (2 trials) | ⊕⊕⊕⊝ moderate1,3,5,6 |
| Neurological sequelae at discharge | ‐ | ‐ | ‐ | 0 (0 trials) | ‐ |
| Parasite clearance time | ‐ | ‐ | Not pooled. No significant difference | 494 (2 trials) | ⊕⊕⊕⊝ moderate1,3,6,7 |
| Fever clearance time | ‐ | ‐ | Not pooled. No significant difference | 494 (2 trials) | ⊕⊕⊝⊝ low1,3,6,8 |
| *The assumed risk is the median control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 No serious risk of bias: Trials were generally well conducted and at low risk of bias. 2 No serious inconsistency: There is no statistical heterogeneity 3 No serious indirectness: The two trials were conducted in Vietnam and Thailand and both compared intramuscular artemether with intravenous artesunate in adults. 4 Downgraded by 1 for serious imprecision: These trials, and the overall meta‐analysis are very underpowered to detect a difference in mortality or to prove equivalence. 5Phu 2010 VNM and Vinh 1997 VNM reported median coma resolution time for artemether vs. artesunate (Phu 2010 VNM: 72 vs. 60, P = 0.11; Vinh 1997 VNM: 47 (artemether) vs. 30 (artesunate IM) vs. 24 (artesunate IV). No serious inconsistency: Both trials suggest an advantage with artesunate although not statistically significant. 6 Downgraded by 1 for serious imprecision: We could not pool these data as median data were presented for both trials. 7Phu 2010 VNM and Vinh 1997 VNM reported median parasite clearance time (Phu 2010 VNM: 72 vs. 72, P = 0.97; Vinh 1997 VNM: 30 (artemether) vs. 24 (artesunate IM) vs. 24 (artesunate IV). No serious inconsistency: Both trials found no difference between treatments. 8Phu 2010 VNM and Vinh 1997 VNM reported median fever clearance time (Phu 2010 VNM: 108 vs. 108, P = 0.27; Vinh 1997 VNM: 48 (artemether) vs. 36 (artesunate IM) vs. 30 (artesunate IV). No serious inconsistency: Both trials found no statistically significant difference between artemether and artesunate.
Background
Description of the condition
Malaria is a febrile illness caused by Plasmodium parasites, which are transmitted to humans through the bite of infected female anopheline mosquitoes. Five species of Plasmodium cause this disease in humans, of which P. falciparum is the most common worldwide, and is responsible for almost all of the severe disease and deaths (WHO 2000; WHO 2008).
Severe malaria is diagnosed on the basis of a positive blood slide or antigen test for malaria, plus the presence of clinical or laboratory features of vital organ dysfunction. These include impaired consciousness, coma, convulsions, respiratory distress, shock (systolic blood pressure < 70 mmHg in adults, < 50 mmHg in children), jaundice, haemoglobinuria, hypoglycaemia, severe metabolic acidosis or anaemia (WHO 2010). Cerebral malaria is one form of severe malaria, where the patient has some impairment of consciousness and cognition. This can vary from slight disorientation through to deep coma where the patient is unconscious and unrousable. Even with correct treatment cerebral malaria can cause a mortality rate of up to 20%, and a small proportion of people that survive infection can have persistent neurological sequelae (Jaffar 1997).
People living in malaria‐endemic regions can develop a naturally acquired immunity to malaria through repeated exposure to the parasite over five to 10 years (Doolan 2009). This partial immunity is protective against the most severe forms of the disease, and as a consequence, in high transmission settings mortality from severe malaria is highest in young children and decreases with increasing age (WHO 2010).
The World Health Organization (WHO) currently recommends parenteral artesunate as the first‐line treatment for severe malaria, followed by a complete course of an effective artemisinin‐based combination therapy (ACT) as soon as the patient can take oral medications (WHO 2010). The WHO based their recommendation on evidence from two large multi‐centre clinical trials that demonstrated the superiority of intravenous artesunate over the standard treatment quinine (Dondorp 2005; Dondorp 2010). A Cochrane Review of available data concluded that treating people that have severe malaria with artesunate instead of quinine would reduce the risk of death by 39% in adults and 24% in children (Sinclair 2011).
Description of the intervention
Artesunate is only one of a number of antimalarials derived from artemisinin, which is extracted from the herb Artemesia annua and is the active ingredient in a Chinese herbal remedy for fever. Once ingested or injected, artemisinin derivatives undergo conversion to dihydroartemisinin, the active metabolite, which has a broad spectrum of activity against the blood stage asexual Plasmodium parasites (Navaratnam 2000; ter Kuile 1993). Artemisinin derivatives clear parasites from the peripheral blood quicker than other antimalarials, but only artesunate has been shown to impact mortality.
Unlike artesunate, artemether is poorly soluble in water and the parenteral formulation is only available as a pre‐mixed oil‐based solution for intramuscular injection (80 mg/mL for use in adults and 40 mg/mL for children). The standard dose is 3.2 mg/kg on admission followed by 1.6 mg/kg once daily until oral therapy is tolerated (WHO 2010). Peak plasma concentrations typically occur around six hours after intramuscular injection, but in severely ill children with poor peripheral perfusion, absorption can be highly erratic (Karbwang 1997; Mithwani 2004; Murphy 1997).
Conversely, artesunate is supplied as a dry powder for mixing with sodium bicarbonate prior to either intravenous or intramuscular injection (WHO 2010). Compared with artemether, the absorption of artesunate is more reliable with peak plasma concentrations following intramuscular injection occurring at around one hour (Hien 2004; Illet 2002; Nealon 2002). These more favourable pharmacokinetic properties of artesunate moved research attention away from artemether and artesunate now has a stronger evidence‐base and is the preferred therapy (Sinclair 2011; WHO 2010).
Why it is important to do this review
A number of African countries incorporated intramuscular artemether into their national guidelines prior to the WHO recommendation for artesunate. Systematic reviews concluded that intramuscular artemisinin derivatives (including both artesunate and artemether) were not inferior to quinine in preventing deaths from malaria, but were safer and easier to administer (AQMSG 2001; Kyu 2009; McIntosh 2000).
Following the WHO recommendation for artesunate as the preferred treatment for severe malaria, there is a need to re‐evaluate the role of intramuscular artemether in the management of severe malaria in adults and children.
Objectives
To assess the efficacy and safety of intramuscular artemether versus any other parenteral medication in the treatment of severe malaria in adults and children.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs).
Types of participants
Adults and children (under 15 years of age) with severe malaria.
Types of interventions
Intervention
Intramuscular artemether
Control
Any other parenteral medication for the treatment of severe malaria
Types of outcome measures
Primary outcomes
Death from any cause
Secondary outcomes
Coma resolution time
Neurological sequelae (such as blindness, deafness, hemiplegia and others)
Time to hospital discharge
Parasite clearance time
Fever clearance time
Need for blood transfusion
Severe anaemia
Adverse events (including hypoglycaemia, tinnitus, nausea, vomiting, haematological and cardiac‐related adverse events)
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press and in progress).
Electronic searches
Databases
We searched the following databases on 09 April 2014 using the search terms detailed in Table 6: Cochrane Infectious Diseases Group Specialized Register; Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 4), published in The Cochrane Library; MEDLINE (1966 to April 2014); EMBASE (1974 to April 2014), LILACS (1982 to April 2014) and ISI Web of Science (1900 to April 2014). We also searched the WHO clinical trial registry platform, ClinicalTrials.gov and the metaRegister of Controlled Trials (mRCT) up to 09 April 2014 for ongoing trials using 'artemether', 'severe malaria', 'complicated malaria', 'artesunate', 'arteether', and 'child*' as search terms.
Table 1.
Search strategy
| Search set | CIDG SR1 | CENTRAL | MEDLINE2 | Embase2 | LILACS2 | ISI Web of Science |
| 1 | malaria | Malaria ti, ab, MeSH | Malaria ti, ab, MeSH | Malaria ti, ab, Emtree | malaria | malaria |
| 2 | artemether | Artemether ti, ab | Artemether ti, ab | Artemether ti, ab, Emtree | artemether | artemether |
| 3 | Artemisinin* | Artemisinin* ti, ab | Artemisinin* ti, ab | Artemisinin* ti, ab | Artemisinin* | Artemisinin* |
| 4 | intramuscular | Intramuscular ti, ab | Intramuscular ti, ab | Intramuscular ti, ab | intramuscular | intramuscular |
| 5 | parenteral | Injections, Intramuscular [MeSH] | Injections, Intramuscular [MeSH] | Intramuscular drug administration [Emtree] | parenteral | parenteral |
| 6 | 2 or 3 | Parenteral ti, ab | Parenteral ti, ab | Parenteral drug administration [Emtree] | 2 or 3 | 2 or 3 |
| 7 | 4 or 5 | 2 or 3 | 2 or 3 | 2 or 3 | 4 or 5 | 4 or 5 |
| 8 | 1 and 5 and 7 | 4 or 5 or 6 | 4 or 5 or 6 | 4 or 5 or 6 | 1 and 5 and 7 | 1 and 5 and 7 |
| 9 | ‐ | 1 and 7 and 8 | 1 and 7 and 8 | 1 and 7 and 8 | ‐ | Randomised clinical trial* |
| 10 | ‐ | ‐ | ‐ | ‐ | ‐ | 8 and 9 |
1Cochrane Infectious Diseases Group Specialized Register. 2Search terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Lefebvre 2011).
Searching other resources
Conference proceedings
We searched relevant proceedings of the following meetings for trial information; Multilateral Initiative on Malaria (MIM) Pan‐African Malaria Conference, European Congress of Tropical Medicine and American Society of Tropical Medicine and Hygiene (09 April 2014).
Researchers
We contacted researchers working in the field and the WHO for unpublished and ongoing trials.
Reference lists
We checked the reference lists of existing reviews and of all trials identified by the above methods.
Data collection and analysis
Selection of studies
Two authors (EE and EEE) independently screened the literature search results and obtained the full reports of such potentially relevant trials. EE and EEE independently applied the inclusion criteria to the full reports using an eligibility form and scrutinized publications to ensure each trial was included only once. We resolved any disagreements through discussion with a third author and, when necessary, by consulting a member of the Cochrane Infectious Diseases Group (CIDG) editorial team. Also, we listed the excluded studies and the reasons for their exclusion.
Data extraction and management
EE and EEE independently extracted data using a specifically developed piloted data extraction form. We resolved any disagreements through discussion with all of the review authors and, when necessary, by consulting a member of the CIDG editorial team. We contacted the corresponding publication author in the case of unclear information or missing data. For each outcome we aimed to extract the number of participants randomized and the number analysed in each treatment group. For dichotomous outcomes, we recorded the number of participants experiencing the event and the number assessed in each treatment group. For continuous outcomes, we extracted arithmetic means and standard deviations for each treatment group, together with the numbers assessed in each group.
Where baseline proportions of participants in the intervention and control arms in whom antipyretics were administered varied, we only included trials that reported fever clearance time and provided additional information about antipyretics use at baseline for participants in both intervention and control arm to avoid confounding in the summary estimate for fever clearance. Where there was significant difference between antipyretic use at baseline in intervention and control arms, we only reported fever clearance time in a table. We defined cure rates in this review as time from first dose to first negative parasite reading for two consecutive readings.
Assessment of risk of bias in included studies
EE and EEE independently assessed the risk of bias of each trial using a risk of bias form. We attempted to contact the trial authors if this information was not specified or if it was unclear. We resolved any disagreements by discussion between review authors. Six components were assessed: generation of the randomization sequence, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other biases (such as the trial stopped early). We categorized our judgements as either 'low', 'high', or 'unclear' risk of bias, and described our reasons for doing so.
Measures of treatment effect
We calculated results using risk ratios for dichotomous data, and mean difference values for continuous data, and presented these effect estimates with 95% confidence intervals (CI). We treated time‐to‐event outcomes as continuous data and accordingly mean difference calculated from mean time in intervention versus control groups.
Unit of analysis issues
For multiple arm trials, we combined all relevant experimental intervention groups of the trial into a single group, and also combined all relevant control intervention groups into a single control group. For dichotomous outcomes, both the sample sizes and the numbers of people with events were added across groups. For continuous outcomes, we combined means and standard deviations using methods described in the Cochrane Handbook for Systematic Reviews of Interventions.
Dealing with missing data
We analysed data according to the intention‐to treat principle (all randomized participants should be analysed in the groups to which they were originally assigned). If there were discrepancies between the number randomized and the number analysed, we calculated the percentage loss to follow‐up for each treatment group and reported this information.
However, if for some trials it was unclear whether there was loss to follow‐up, we entered the number analysed into Review Manager (RevMan) whenever these figures were available. By attempting to carry out a complete case analysis, we avoided making assumptions about the outcomes of participants lost to follow‐up. Where possible, we contacted authors for missing data.
Assessment of heterogeneity
We looked for statistical heterogeneity by inspecting the forest plots for overlapping CIs, applying the Chi2 test (P < 0.10 considered statistically significant) and the I2 statistic (I2 value < 50% used to denote moderate levels of heterogeneity).
Assessment of reporting biases
We planned to construct funnel plots to look for evidence of publication bias provided we included a sufficient number of trials to make this informative.
Data synthesis
We analysed the data using Review Manager (RevMan). In the first instance, we applied a fixed‐effect meta‐analysis. However, if we detected moderate heterogeneity but still considered it appropriate to combine the trials, we then used a random‐effects approach. Where heterogeneity was very high such that meta‐analysis was not appropriate, we displayed the results in forest plots or tables but did not combine the results. Where data were only presented as medians and ranges, we presented the results in tables.
We presented the main results of the review alongside a GRADE appraisal of the quality of evidence in the 'Summary of Findings' tables.
Subgroup analysis and investigation of heterogeneity
We grouped the analysis and results by children and adults. We reported results by whether the studies were carried out in Africa or in Asia. We examined whether loading dose or quinine influenced outcomes.
Sensitivity analysis
We conducted a sensitivity analysis to investigate the robustness of the results to the risk of bias components by including only trials that concealed the allocation and had low incomplete outcome data (< 10%).
Assessment of the quality of evidence
We assessed the quality of the evidence following the GRADE approach and defined 'quality' as an assessment of our confidence in the estimates of effect (Guyatt 2008).
Results
Description of studies
See the Characteristics of included studies section for details of the included trials.
Results of the search
We conducted the literature search up to 09 April 2014 and identified 77 references (see Figure 1).
Figure 1.
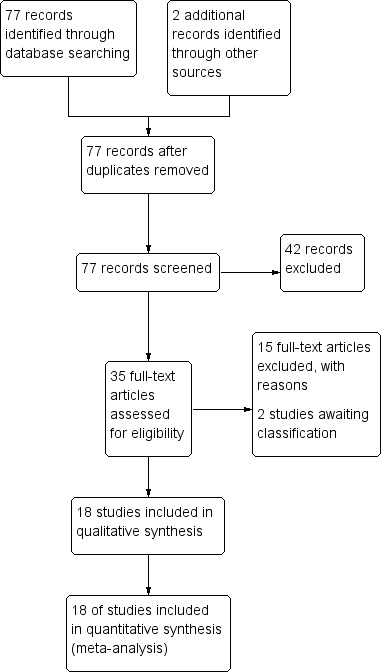
Study flow diagram.
Included studies
We included 18 RCTs, enrolling 2662 participants, in this review. Twelve trials enrolled children only (1447 participants aged between six months and 15 years), and six trials enrolled older children and adults (1215 participants aged between 13 and 79 years).
Location
The trials in children were primarily conducted in Africa: Nigeria (five trials), Sudan (two trials), the Gambia (one trial), Kenya (one trial), Malawi (one trial), and Mali (one trial); with only one trial from Asia (India).
Five adult trials were conducted in Asia: Vietnam (three trials), and Thailand (two trials); and one in Oceania; Papua New Guinea (one trial). We have attached a three letter country code to each trial ID to aid forest plot interpretation.
Interventions
All 12 trials in children compared artemether with quinine. Artemether was given by intramuscular injection, with a loading dose of 3.2 mg/kg body weight followed by maintenance doses of 1.6 mg/kg for three to six days (see Table 7 for details). Only three trials followed this with oral therapy once tolerated (Murphy 1996 KEN; Taylor 1998 MWI; van Hensbroek 1996 GMB). For quinine, nine trials administered the WHO recommended loading dose of 20 mg/kg of intravenous or intramuscular quinine followed by a maintenance dose of 10 mg/kg. However, van Hensbroek 1996 GMB administered the maintenance dose at 12‐hourly intervals instead of eight‐hour intervals (see Table 7).
Table 2.
Characteristics of trials comparing artemether and quinine in children
| Trial ID | Year of study | Age limits | Quinine dosing schedule | Artemether dosing schedule | ||||
| Loading dose | Maintainance | Follow‐on therapy | Loading dose | Maintainance | Follow‐on therapy | |||
| Adam 2002 SDN | 2002 | 'Children' | 20 mg/kg IV | 10 mg/kg IV every eight hours for 72 hours | Oral quinine for 7 days | 3.2 mg/kg IM | 1.6 mg/kg IM once daily for 4 days | None |
| Aguwa 2010 NGA | 2007 | 6 months to 12 yrs | 20 mg/kg IV or IM | 10 mg/kg IV/IM every eight hours | None | 3.2 mg/kg IM | 1.6 mg/kg IM once daily for 2 days | None |
| Huda 2003 IND | 2001 | < 14 yrs | 20 mg/kg IV | 10 mg/kg IV every eight hours | Quinine to complete 7 days | 1.6 mg/kg IM twice daily | 1.6 mg/kg IM once daily for 5 days | None |
| Minta 2005 MLI | 2004 | 3 months to 15 yrs | 20 mg/kg IV | 10 mg/kg IV every eight hours | Quinine 10 mg/kg every eight hours | 3.2mg/kg IM twice daily | 1.6 mg/kg IM once daily for 4 days | None |
| Murphy 1996 KEN | 1996 | 5 months to 12 yrs | 20 mg/kg IV | 10 mg/kg IV every eight hours | SP once | 3.2 mg/kg IM | 1.6 mg/kg IM once daily for 4 days | SP once |
| Ojuawo 1998 NGA | 1998 | Mean age about 4 yrs | 10 mg/kg IV | 10 mg/kg IV every eight hours | Quinine to complete 7 days | 3.2 mg/kg IM | 1.6 mg/kg IM 12 hrs later, then once daily for 2 days | None |
| Olumese 1999 NGA | 1999 | 11 months to 5 yrs | 20mg/kg IV | 10mg/kg IV every eight hours | Quinine to complete 7 days | 3.2 mg/kg IM | 1.6 mg/kg IM once daily for 4 days | None |
| Osonuga 2009 NGA | 2009 | 1 to 12yrs | 10 mg/kg IV | 10 mg/kg IV every eight hours | Quinine to complete 7 days | 1.6 mg/kg IM twice daily | 1.6 mg/kg IM once daily for 4 days | None |
| Satti 2002 SDN | 1996 | 3 months to 15yrs | 10 mg/kg IV | 10 mg/kg IV every eight hours | Quinine to complete 7 days | 1.6 mg/kg IM twice daily | 1.6 mg/kg IM once daily for 4 days | None |
| Taylor 1998 MWI | 1994 | Mean age of 3 yrs | 20 mg/kg IV | 10 mg/kg IV every eight hours for at least 2 doses | SP once | 3.2 mg/kg IM | 1.6 mg/kg IM once daily for 2 days at least | SP once |
| van Hensbroek 1996 GMB | 1994 | 1 to 9yrs | 20 mg/kg IV | 10 mg/kg IV every twelve hours | Quinine to complete 5 days | 3.2 mg/kg IM | 1.6 mg/kg IM once daily for 3 days | SP once1 |
| Walker 1993 NGA | 1993 | 1 to 5yrs | 20 mg/kg IV | 10 mg/kg IV every eight hours | Quinine to complete 7 days | 3.2 mg/kg IM | 1.6 mg/kg IM once daily for 4 days | None |
IM = intramuscular; IV = intravenous; SP = sulphadoxine‐pyrimethamine.
1Only in the second and third years of the study.
In adults, four trials compared artemether with quinine (Hien 1996 VNM; Karbwang 1992 THA; Karbwang 1995 THA; Seaton 1998 PNG) and two trials compared artemether with artesunate (Phu 2010 VNM; Vinh 1997 VNM). Artemether was given intramuscularly, over three to seven days with slight variations in dosing (see Table 8 and Table 9) and all four trials administered quinine at the WHO‐recommended loading dose.
Table 3.
Characteristics of trials comparing artemether and quinine in adults
| Trial ID | Year of study | Age limits | Quinine dosing schedule | Artemether dosing schedule | ||||
| Loading dose | Maintainance | Follow‐on therapy | Loading dose | Maintainance | Follow‐on therapy | |||
| Hien 1996 VNM | 1996 | 15 to 79 yrs | 20 mg/kg IM | 10 mg/kg IM every eight hours | Quinine or mefloquine to complete 7 days | 4 mg/kg IM | 2 mg/kg IM once daily for 4 days | Quinine or mefloquine to complete 7 days |
| Karbwang 1992 THA | 1991 | 15 to 45 yrs | 20 mg/kg IV | 10 mg/kg every eight hours for 7 days | Quinine to complete 7 days | 160 mg IM | 80 mg IM once daily for 6 days | None |
| Karbwang 1995 THA | 1994 | 15 to 55 yrs | 20 mg/kg IV | 10 mg/kg every eight hours for 7 days | Quinine to complete 7 days | 160 mg IM | 80 mg IM once daily for 6 days | None |
| Seaton 1998 PNG | 1995 | > 12 yrs | 20 mg/kg IV | 10 mg/kg IV every eight hours | Quinine to complete 7 days | 3.2 mg/kg IM | 1.6 mg/kg IM once daily for 4 days | None |
IM = intramuscular; IV = intravenous.
Table 4.
Characteristics of studies comparing artemether and artesunate in adults
| Trial ID | Year of study | Age limits | Artemether dosing schedule | Artesunate dosing schedule | ||||
| Loading dose | Maintainance | Follow‐on therapy | Loading dose | Maintainance | Follow‐on therapy | |||
| Phu 2010 VNM | 2003 | 15 to 77 yrs | 3.2 mg/kg IM | 1.6 mg/kg IM daily | None | 2.4 mg/kg IM | 1.2 mg/kg IM once daily | 2 mg/kg of artesunate to complete 7 days |
| Vinh 1997 VNM | 1994 | 15 to 66 yrs | 200 mg IM | 100 mg IM once daily for 3 days | Mefloquine once | 120 mg IM or IV | 60 mg IM or IV once daily for 3 days | Mefloquine once |
IM = intramuscular; IV = intravenous.
Supportive care
All 12 trials in children reported measurement of blood glucose on admission, but only nine trials reported any subsequent active monitoring for hypoglycaemia.
Only one trial in adults reported measuring blood glucose on admission and monitored hypoglycaemia up to 24 hours after admission (Hien 1996 VNM).
Outcome measures
All 18 trials reported death, a measure of coma resolution, fever clearance and parasite clearance as outcomes. Eleven trials reported neurological sequelae at discharge. Only two trials (Aguwa 2010 NGA; Phu 2010 VNM) reported duration of hospital stay and two trials (Hien 1996 VNM; Olumese 1999 NGA) reported on the number of children requiring blood transfusions. Eleven trials (Adam 2002 SDN; Hien 1996 VNM; Huda 2003 IND; Karbwang 1992 THA; Karbwang 1995 THA; Minta 2005 MLI; Murphy 1996 KEN; Phu 2010 VNM; Seaton 1998 PNG; van Hensbroek 1996 GMB; Walker 1993 NGA) reported on adverse events including episodes of hypoglycaemia. We have listed the outcome definitions used in the included trials in Table 10.
Table 5.
Definitions of outcome measures used in the review
| Trial ID | Coma resolution time | Fever clearance time | Parasite clearance time | Hypoglycaemia |
| Adam 2002 SDN | Mean value (h) reported and defined as a Blantyre coma score of 5 recorded for at least 24 hours | Mean value (h) reported and defined as the time after which the temperature remained normal (axillary temperature < 37.5°C) | Mean value (h) reported and defined as the time passed from admission and start of treatment until two consecutive negative smears. Blood films repeated every 8 hours. | Number of episodes (n/N) reported but not defined |
| Aguwa 2010 NGA | Proportions with coma resolution on D3 reported but not defined | Proportions with fever clearance on D3 and D14 reported and defined as body temperature ≤ 37.5°C after commencement of treatment | Proportions with parasite clearance on D3 and D14. Parasite clearance was taken as adequate clinical and parasitological response (ACPR) at days 3 and 14. Parasite count taken on D0, D3 and D14. | Not reported |
| Hien 1996 VNM | Median value (h) reported and defined as the time to reach a score of 15 on the Glasgow Coma Scale | Median value (h) reported but not defined. | Median value (h) reported and defined as the time to Assessed every 4 hours for the first 24 hours and every 6 hours until three consecutive negative blood smears | Number of episodes (n/N) reported but not defined |
| Huda 2003 IND | Glasgow coma scale was used in grading the level of consciousness of the patients every eight hours |
Mean value (h) reported and defined as time to clearance of fever | Mean value (h) reported but not defined | Not reported |
| Karbwang 1992 THA | Unclear if values reported are means or medians (h) | Mean value (h) reported and defined as time for the temperature to fall below 37.5°C and remain that value for 72 hours | Mean value (h) reported and defined as the time for the parasite count to fall below the level of microscopic detection (thick film) | Not reported |
| Karbwang 1995 THA | Median value (h) reported and defined as the time taken for the patients to recover completely from unconciousness | Mean value (h) reported and defined as time for the temperature to fall below 37.5°C and remain that value for 72 hours | Median value (h) reported and defined as the time taken for parasite count to fall below the level of microscopic detection (thick film) | Not reported |
| Minta 2005 MLI | Mean value (h) reported and defined as the time to normalization of consciousness | Mean value (h) reported but not defined | Mean value (h) reported and defined as time till negative parasitaemia result | Not reported |
| Murphy 1996 KEN | Median value (h) reported but not described | Median value (h) reported but not described | Median value (h) reported but not described. Every four hours until clearance | Not reported |
| Ojuawo 1998 NGA | Mean value (h) reported and defined as the interval between onset of therapy and the attainment of full consciousness | Mean value (h) reported and defined as the interval between the onset of therapy and the time the body temperature is ≤ 37°C and remained so | Defined as two successive thick blood films done at 12 hours interval are negative for asexual forms of plasmodium species | Not reported |
| Olumese 1999 NGA | Mean value (h) reported and defined as time to regain full consciousness | Mean value (h) reported and defined as the time for temperature to fall below 37.5°C and remain so for at least 48 hours | Mean value (h) reported and defined as the time from start of drug administration to the first of two consecutive negative thick smears remaining negative until day 7 |
Not reported |
| Osonuga 2009 NGA | Mean value (h) reported and defined as time to attainment of a Blantyre score of 5 for at least 24 hours from initiation of treatment | Mean value (h) reported but not defined | Mean value (h) reported but not defined. Thick and thin film done on D0 and repeated on Days 3, 7 and 14 | Not reported |
| Phu 2010 VNM | Median value (h) reported and defined as time to Glasgow coma score of 15. | Median value (h) reported and defined as the time for temperature to fall below 37.5°C and remain so | Median value (h) reported and defined as the time to clear all parasites | Number of episodes (n/N) reported but not defined |
| Satti 2002 SDN | Mean value (h) reported and defined as time to regaining consciousness | Mean value (h) reported and defined as the time for temperature to fall below 37.5°C | Mean value (h) reported and defined as time to clear parasites measured every six hours till clearance | Not reported |
| Seaton 1998 PNG | Median value (h) reported but not defined | Median value (h) reported and defined as a temperature <37.5 °C on two successive readings | Median value (h) reported and defined as as the time at which the blood films were negative for P. falciparum for at least eight hours | Number of episodes (n/N) reported but not defined |
| Taylor 1998 MWI | Median value (h) reported and defined as time required for a child to achieve a Blantyre Coma Score of 5 |
Median value (h) reported and defined as the time at which the rectal or axillary temperature dropped below 37.5°C and remained < 37.5°C for 24 consecutive hours | Median value (h) reported and defined as the time at which the first of two negative (0 parasites/200 WBC) thick blood films was prepared. Every four hours till clearance | Not reported |
| van Hensbroek 1996 GMB | Median value (h) reported and defined as time to regain full consciousness | Median value (h) reported and defined as time needed for the rectal temperature to fall below 38.0°C for at least 24 hours | Median value (h) reported and defined as time needed for all parasites to clear relative to parasite density at admission and assessed every 12 hours till clearance | Number of episodes (n/N) reported and defined as a blood glucose level below 40 mg/dL (2.2 mmol/L) |
| Vinh 1997 VNM | Median value (h) reported and defined as time to regain full consciousness | Median value (h) reported and defined as time for axillary temperature to fall to, and remain for ≥ 24 hours at 37.5°C or lower | Median value (h) reported and defined as time to clear parasites | Not reported |
| Walker 1993 NGA | Mean value (h) reported but not defined | Mean value (h) reported | Mean value (h) reported and defined as the time for parasitaemia to be cleared and to remain so up to Day 7. Assessed every six hours during period of coma and then every 12 hours. | Not reported |
WBC = white blood cell.
Other outcomes reported by trials which we did not include in this review were time to death (Murphy 1996 KEN; Taylor 1998 MWI; van Hensbroek 1996 GMB), survival rate (Karbwang 1992 THA; Karbwang 1995 THA), cause of death (Karbwang 1992 THA), fatality rate (Vinh 1997 VNM), time to ambulation (Olumese 1999 NGA; Phu 2010 VNM), time to sit unaided and time to drink (Phu 2010 VNM; Walker 1993 NGA), time to eating (Phu 2010 VNM), gametocyte carriage (Adam 2002 SDN), recrudescence (Adam 2002 SDN; Minta 2005 MLI; Taylor 1998 MWI; Walker 1993 NGA), 28th day cure rate (Satti 2002 SDN; van Hensbroek 1996 GMB; Walker 1993 NGA). Other outcomes included duration of parenteral treatment and time for plasma lactate levels to fall below 2.5 mmol/L (Hien 1996 VNM).
Excluded studies
We excluded 14 trials and listed the reasons for their exclusion in the 'Characteristics of excluded studies' section.
Risk of bias in included studies
See Figure 2 for a summary of the risk of bias assessments. We presented further details in the 'Characteristics of included studies' tables.
Figure 2.
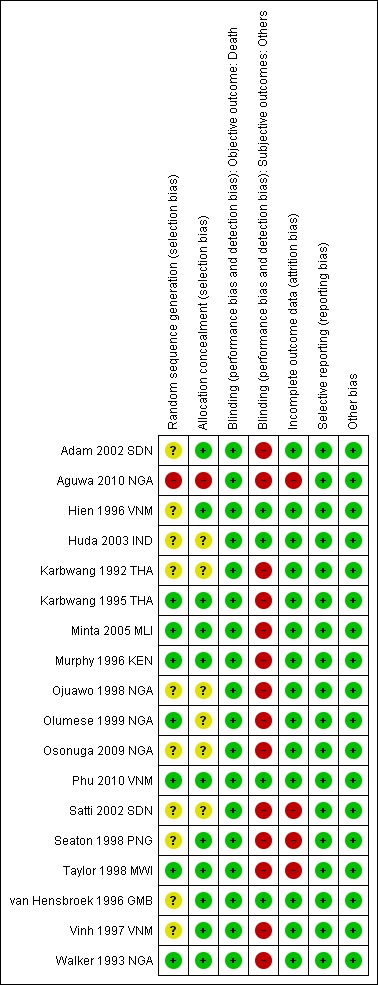
Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.
Allocation
Seven trials were at low risk of bias regarding the generation of allocation sequence while one trial was at high risk of bias (Aguwa 2010 NGA). Ten trials were at unclear risk of bias because review authors did not provide enough information to permit us to make a judgement.
Eleven trials were at low risk of bias regarding allocation concealment and the remaining trials provided insufficient information to make a judgement.
Blinding
In all trials, except Hien 1996 VNM and Phu 2010 VNM, investigators and participants were aware of treatment allocation. Participants were also not blind to the intervention as two different routes (intramuscular (artemether) and intravenous (quinine) were used to administer the interventions. Blinding was unlikely to affect the assessment of outcome death in all trials. In one trial, microscopists were blinded to the intervention and clinical status of the patients (Huda 2003 IND). The other subjective outcomes were thus at high risk of bias in all open included trials or at unclear risk of bias where trial provided did not provide information.
Incomplete outcome data
Fourteen trials reported no losses to follow‐up. The remaining four trials (Aguwa 2010 NGA; Satti 2002 SDN; Seaton 1998 PNG; Taylor 1998 MWI) reported over 10 per cent attrition in either one or both trial arms. Two trials used the per protocol number of participants as a denominator in the analysis (Taylor 1998 MWI; Seaton 1998 PNG). The other two trials used the number of participants randomized as the denominator in the analysis.
Selective reporting
We did not detect any evidence of selective outcome reporting.
Other potential sources of bias
We did not identify any other sources of bias.
Effects of interventions
See: Table 1; Table 2; Table 3
Artemether versus quinine
Children
Twelve trials were conducted in children, Eleven in Africa and one in Asia. All used loading doses of quinine.
Death: there was no overall difference in all‐cause mortality between intramuscular artemether and intravenous quinine (RR 0.96, 95% CI 0.76 to 1.20; 12 trials, 1447 participants, Analysis 1.1). However, these 12 trials were too small to detect or exclude clinically important differences, and the overall meta‐analysis remains significantly underpowered to prove equivalence (see Table 11 and Table 12). The current total sample size has adequate power to exclude effects as large as seven extra deaths per 100 patients.
Table 6.
Optimal information size calculations; dichotomous outcomes
| Outcome | Type of test | Proportion in control group3 | Proportion in Intervention group | Estimated RR | Total sample size1,2 |
| Death | Superiority | 0.17 | 0.136 | 0.80 | 3514 |
| Equivalence | 0.17 | 0.14 to 0.204 | ‐ | 6592 | |
| Neurological sequelae | Superiority | 0.25 | 0.20 | 0.80 | 2184 |
| Equivalence | 0.25 | 0.22 to 0.284 | ‐ | 8760 |
1 These calculation were performed using a power calculator available at: http://www.sealedenvelope.com/power/ 2 All calculation were performed for a power of 80% and an α error of 0.05. 3 The proportion in the control group is taken from the median control group risk across trials. 4 A maximum 3% risk difference was chosen to represent equivalence.
Table 7.
Optimal information size calculations; continuous outcomes
| Outcome | Type of test | Mean in control group3 | Mean in Intervention group4 | SD of outcome | Total sample size1,2 |
| Coma resolution time | Superiority | 25 | 19 | 20 | 350 |
| Equivalence | 25 | 19 to 31 | 20 | 382 | |
| Parasite clearance time | Superiority | 42 | 36 | 20 | 350 |
| Equivalence | 42 | 36 to 48 | 20 | 382 | |
| Fever clearance time | Superiority | 48 | 42 | 20 | 350 |
| Equivalence | 48 | 36 to 54 | 20 | 382 |
1 These calculations were performed using a power calculator available at: http://www.sealedenvelope.com/power/ 2 All calculation were performed for a power of 80% and an α error of 0.05. 3 The mean in the control group is taken from the median control group across studies. 4 A six‐hour time difference was chosen to represent a clinically important benefit.
Analysis 1.1.
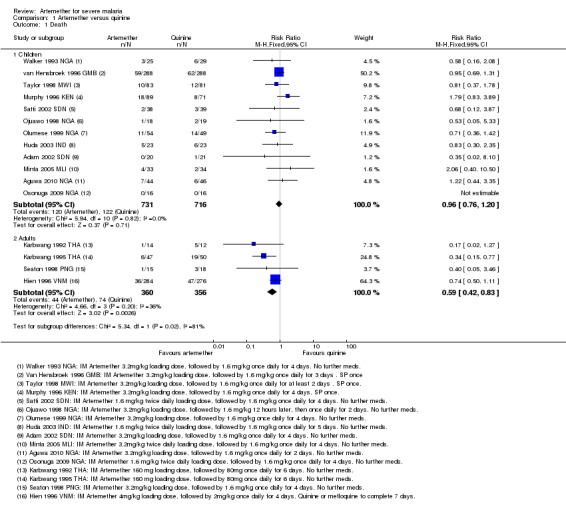
Comparison 1 Artemether versus quinine, Outcome 1 Death.
Coma resolution time: the mean coma resolution time was about five hours shorter with artemether (26 hours) compared with quinine (30.55 hours) (MD ‐5.45 hours, 95% CI ‐7.90 to ‐3.00; six trials, 358 participants, Analysis 1.3). This effect was largest in trials without adequate allocation concealment and therefore at unclear or high risk of bias. A sensitivity analysis excluding these trials found no significant difference between groups. In addition, three trials reported median time to coma resolution (see Table 13). Two trials found no significant difference (Murphy 1996 KEN; Taylor 1998 MWI), and one trial found the median time to be longer with artemether (26 hours versus 20 hours, P = 0.046, van Hensbroek 1996 GMB). Two other trials (Minta 2005 MLI; Osonuga 2009 NGA) reported mean coma resolution time as mean (SD) but the data was not normally distributed and so we have reported this an additional table (Table 13).
Table 8.
Additional data: Artemether versus quinine in children
| Pre‐specified outcome | Trial reported outcome | Trial | No. of participants | Artemether | Quinine | Comparative results reported in article |
| Coma resolution time (h) | Median (IQR) |
Murphy 1996 KEN | 160 | 12 (2.8 to 96) | 13 (2.8 to 96) | Not significantly different |
| Median (IQR) |
van Hensbroek 1996 GMB | 576 | 26 (15 to 48) | 20 (12 to 43) | P = 0.046 | |
| Median (IQR) |
Taylor 1998 MWI | 164 | 18 (8 to 30) | 20 (10 to 54) | Not significantly different | |
| Coma recovery (%) on Day 3 | Aguwa 2010 NGA | 90 | 15.9% | 21.4% | RR = 0.763 (95% CI 0.065 to 9.015) | |
| Mean (SD) | Osonuga 2009 NGA | 32 | 4.5 (13.05) | 9 (24.59) | P = 0.523 | |
| Mean (SD) | Minta 2005 MLI | 67 | 30.57 (29.02) | 25.15 (31.62) | P = 0.53 | |
| Time to hospital discharge | % spending less than one week in hospital |
Aguwa 2010 NGA | 90 | 61.76% | 71.74% | P = 0.829 |
| Fever clearance (h) | Median (IQR) |
Murphy 1996 KEN | 160 | 32 (4 to 86) | 32 (4 to 96) | Not significantly different |
| van Hensbroek 1996 GMB | 576 | 30 (16 to 48) | 33 (12‐60) | P = 0.8 | ||
| Taylor 1998 MWI | 164 | 31 (24 to 52) | 45 (33 to 60) | "Significant" | ||
| Fever clearance (%) on Day 3 | Aguwa 2010 NGA | 90 | 90.0% | 87.7% | P = 0.753 | |
| Parasite clearance (h) | Median (IQR) |
Murphy 1996 KEN | 160 | 39.5 (24 to 45) | 48 (37 to 56) |
P < 0.001 |
| van Hensbroek 1996 GMB | 576 | 48 (36 to 60) | 60 (48 to 72) | P < 0.001 | ||
| Taylor 1998 MWI | 164 | 32 (25 to 36) | 40 (32 to 48) | 'significant' | ||
| Parasite clearance (%) on Day 3 | Aguwa 2010 NGA | 90 | 99.0% n = 44 |
96.8% n = 46 |
P = 0.422 | |
| Needing blood transfusion | ‐ | van Hensbroek 1996 GMB | ‐ | ‐ | ‐ | "The two groups were similar in terms of the need for blood transfusions,and the incidence of secondary bacterial infections (data not shown)." |
IQR = interquartile range.
Analysis 1.3.
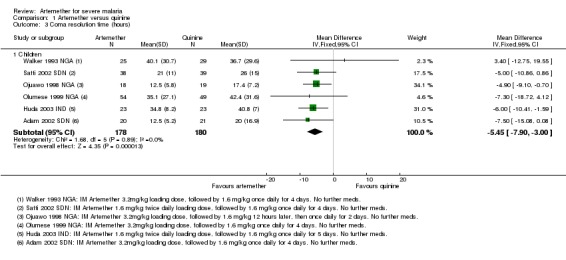
Comparison 1 Artemether versus quinine, Outcome 3 Coma resolution time (hours).
Neurological sequelae: there was no overall difference in the risk of neurological sequelae at hospital discharge between artemether (185 per 1000) and quinine (220 per 1000) (RR 0.84, 95% CI 0.66 to 1.07; seven trials, 968 participants, Analysis 1.4). Again these trials were too small to enable us to confidently detect or exclude what may be clinically important differences between treatments (see Table 11). The overall meta‐analysis is adequately powered to exclude effects larger than eight additional sequelae per 100 patients.
Analysis 1.4.
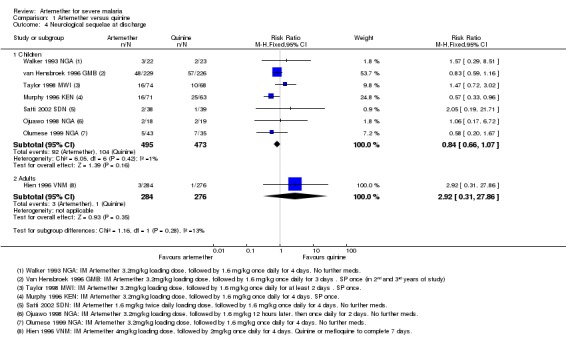
Comparison 1 Artemether versus quinine, Outcome 4 Neurological sequelae at discharge.
Three trials continued to monitor patients with neurological sequelae after hospital discharge. Satti 2002 SDN found no difference at day seven, Taylor 1998 MWI found most sequelae had resolved and van Hensbroek 1996 GMB found no difference at day 28 (Analysis 1.5).
Analysis 1.5.
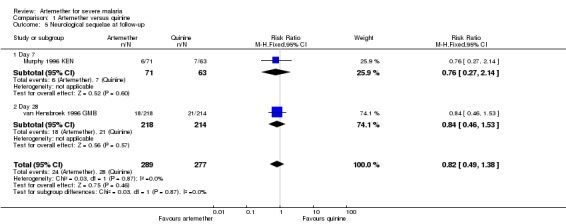
Comparison 1 Artemether versus quinine, Outcome 5 Neurological sequelae at follow‐up.
Parasite clearance time (PCT): the mean parasite clearance time in children was approximately nine hours shorter with artemether (MD ‐9.03 hours, 95% CI ‐11.43 to ‐6.63; seven trials, 420 participants, Analysis 1.6. The statistical significance of this result remained after we excluded trials at unclear or high risk of selection bias. The mean parasite clearance times for artemether and quinine were 36.25 and 43.18 hours respectively.
Analysis 1.6.
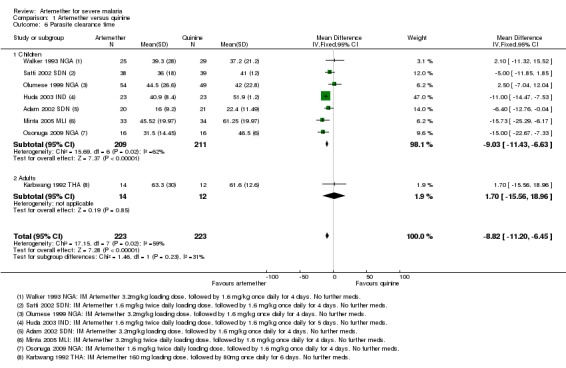
Comparison 1 Artemether versus quinine, Outcome 6 Parasite clearance time.
Three additional trials reported median parasite clearance time and all showed a statistically significant benefit with artemether (see Table 13). One trial (Ojuawo 1998 NGA) expressed parasite clearance as the proportion of patients with parasite clearance at 72 hours and at seven days, with no statistically significant differences between groups (see Analysis 1.7). Trials differed with respect to the frequency with which they repeated malaria blood smears (see Table 10).
Analysis 1.7.
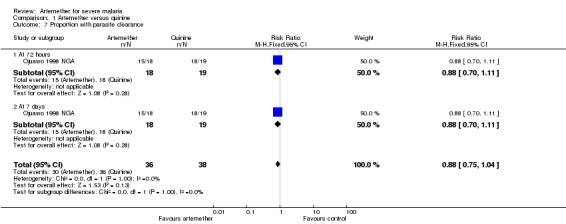
Comparison 1 Artemether versus quinine, Outcome 7 Proportion with parasite clearance.
Fever clearance time (FCT): eight trials reported mean fever clearance time with a statistically significant reduction of about three hours with artemether overall (MD ‐3.73 hours, 95% CI ‐6.55 to ‐0.92; eight trials, 457 participants, Analysis 1.8). The mean fever clearance times for artemether and quinine were 43.69 and 46.26 hours respectively. However, only two of the individual trials showed a statistically significant difference between the groups. Three trials in children reported median fever clearance time and two trials found no statistically significant difference between the two groups (see Table 13).
Analysis 1.8.
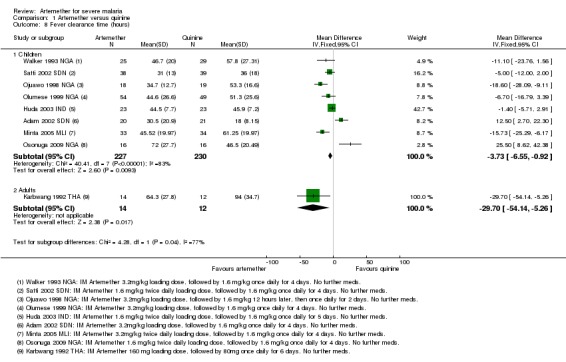
Comparison 1 Artemether versus quinine, Outcome 8 Fever clearance time (hours).
The definitions of fever varied across the included trials. Six trials used a cut off of body temperature less than 37.5°C from initiation of treatment to define fever clearance (see Table 10).
Need for blood transfusion: one trial reported on the number of patients requiring blood transfusions for severe malarial anaemia in both artemether and quinine arms (Olumese 1999 NGA) (see Analysis 1.9). No statistically significant difference was observed between both arms (RR 1.27, 95% CI 0.62 to 2.59; 103 participants, one trial).
Analysis 1.9.
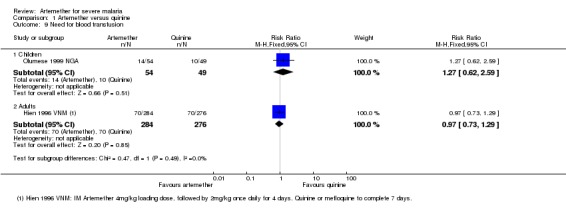
Comparison 1 Artemether versus quinine, Outcome 9 Need for blood transfusion.
Adverse effects: six trials reported on the frequency of adverse events (Adam 2002 SDN; Huda 2003 IND; Minta 2005 MLI; Murphy 1996 KEN; van Hensbroek 1996 GMB; Walker 1993 NGA). Two trials reported no adverse events had occurred during the trial duration (see Table 14). One trial reported the absence of adverse events in the artemether arm (Minta 2005 MLI). No trial reported discontinuation of medication.
Table 9.
Adverse event monitoring and reporting
| Study ID | Sample size | Clinical symptoms monitoring | Biochemistry | Haematological | Electrocardiogram | Additional comments on adverse events |
| Adam 2002 SDN | 41 | Not reported | Not reported | Not reported | Not reported | "Neurological deficits were not observed in any patient during the follow‐up period" |
| Aguwa 2010 NGA | 90 | Not reported | Not reported | Not reported | Not reported | None |
| Hien 1996 VNM | 560 | Clinical assessment every 4 hours for the first 24 hours and 6 hourly afterwards | Blood glucose, lactate and cytokine levels measured 4, 8, 12 and 24 hours after admission | Full blood count on admission | Pre‐treatment and 12 hours after initiation of treatment on Day 0, 4 hours after last dose and at discharge | None |
| Huda 2003 IND | 46 | Lumbar puncture Chest x‐ray on day 0 |
Blood Glucose, Renal Function Test, Liver Function Test and Serum Electrolyte on Days 0 and 3 |
Full Blood Count on Days 0 and 3 | Day 0 | "No serious side effects of either of the drugs were observed in our study...... Closer and more frequent monitoring and larger sample size would have probably revealed more subtle adverse drug effects." |
| Karbwang 1992 THA | 26 | Clinical evaluation daily for at least 7 days Lumbar puncture Chest x‐ray on day 0 |
Biochemistry on Days 0, 2, 4 and 7 | Full Blood Count on Days 0, 2, 4 and 7 | On admission for all patients; then once daily and every six hours for quinine and artemether patients respectively | "The side effects in the quinine group were dizziness and vertigo. No side effects were detected with artemether". |
| Karbwang 1995 THA | 102 | Clinical evaluation on admission and twice daily for at least 7 days Lumbar puncture Chest x‐ray on day 0 |
Biochemistry on Days 0, 2, 4 and 7 | FBC on Days 0, 2, 4 and 7 | On admission for all patients; then once daily and every six hours for quinine and artemether patients respectively | QTc prolongation and tinnitus were the major adverse events in Quinine arm. Mild transient pain at injection site for approximately 15 mins after artemether treatment. |
| Minta 2005 MLI | 67 | Clinical examination daily on Days 1 to7, and 14 | Blood glucose on Days 1, 2, 3, 5, 7 and 14 Urea and Serum Electrolyte, transaminases, phosphatases on Days 1, 3, 5, 7 and 14 |
FBC on Days 1, 3, 5, 7 and 14 | Once daily on Days 1, 3, 5, 7 and 14 | None |
| Murphy 1996 KEN | 160 | Clinical assessment on admission, then at six, then 12 hour intervals till discharge | Blood glucose, urea, electrolytes, blood gases and when clinically indicated | FBC on Day 0 and when clinically indicated Blood cultures on Day 0 |
On admission and at 6, 24, 30, 48 and 54 hours | None |
| Ojuawo 1998 NGA | 37 | Clinical assessment on Day 0 | Urea and electrolyte Blood sugar and liver function test on Day 0 |
FBC on Day 0 | None | None |
| Olumese 1999 NGA | 103 | Clinical assessments on Days 0, 3, 7, 14, 28 | Blood glucose, Urea and creatinine, electrolytes on Days 0, 3, 7, 14, 28 | WBC count on Days 0, 3, 7, 14, 28 | None | "No adverse reactions to the two drugs were recorded during the study". |
| Osonuga 2009 NGA | 32 | Clinical examination on Days 0 to 7 and 14 | None | None | None | None |
| Phu 2010 VNM | 370 | Clinical examination on admission Chest x‐ray on admission Lumbar puncture |
Blood urea nitrogen, serum creatinine, aspartate aminotransferase, alanine transaminase, plasma lactate | Full blood count on admission | None | None |
| Satti 2002 SDN | 77 | Clinical evaluation on admission and every six hours on Days 0 to 4, and then once daily on Days 14, 21 and 28 | Blood glucose, serum creatinine, serum aspartate, aminotransferase on Day 0 | WBC, Haemoglobin on Days 0 and 3 | None | None |
| Seaton 1998 PNG | 33 | Chest X‐ray on admission | Renal and Liver function tests on admission, Days 3 and 7 | Full Blood Count on Days 0, 3 and 7 | None | None |
| Taylor 1998 MWI | 183 | CSF collected on admission | Blood glucose, Blood pH, on D0 (every four hours for the first 24 hours |
Haematocrit every eight hours Full Blood Count, urea and electrolytes on Days 0, 3, 7 and 28 |
On admission, 6, 48,54 and 96 hours | "Of the initial 127 patients on whom serial electrocardiographic tracings were made, more patients in the quinine group showed prolongation of the corrected QT intervals after treatment, but the differences were not statistically or clinically significant." "There were no significant differences between the two treatment groups in terms of adverse effects associated with antimalarial treatment (i.e. new signs and symptoms which develop within seven days of the start of treatment)." |
| van Hensbroek 1996 GMB | 576 | Clinical examination on Day 0 Lumbar puncture on admission |
Blood glucose on admission, after 4hours and 12 hours | PCV, Haemoglobin, Blood culture on Day 0 | None | None |
| Vinh 1997 VNM | 124 | Clinical examination on admission | Blood glucose, serum creatinine, serum bilirubin on admission | Full blood count on admission | None | None |
| Walker 1993 NGA | 54 | Clinical examination twice daily Spinal taps |
Urea and Electrolyte, on days 3, 7, 14, 28 | PCV on days 3, 7, 14, 28 | On admission, at 4 and 6 hours | None |
Only two trials reported episodes of hypoglycaemia (Analysis 1.10). Other adverse effects reported were QT prolongation, local skin reaction at the injection site, abscess, urticarial rash, pruritus, supraventricular tachycardia and urinary tract infection.. However, these trials were insufficiently powered to detect differences in adverse events. The trials had similar definitions of adverse events (included only adverse effects that could not be attributable to malaria).
Analysis 1.10.
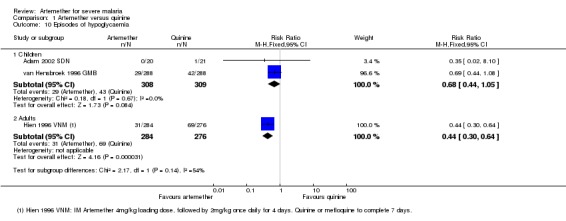
Comparison 1 Artemether versus quinine, Outcome 10 Episodes of hypoglycaemia.
Time to hospital discharge: none of the included trials reported time to discharge. One trial reported the proportion of patients that spent less than one week in hospital and found no significant difference between groups (see Table 13).
Adults
Four trials were conducted in adults, three in Asia and one in Oceania. All used loading doses of quinine.
Death: artemether resulted in fewer deaths compared with quinine (RR 0.59, 95% CI 0.42 to 0.83; four trials, 716 participants, Analysis 1.1) from trials conducted mostly in Asia.
Coma resolution time: three trials reported a measure of coma resolution time. Hien 1996 VNM reported median coma resolution time, which was shorter in the quinine arm. Karbwang 1995 THA found both arms to be comparable in terms of coma resolution time. The third trial, Karbwang 1992 THA reported mean coma resolution time but the data were incompletely reported (see Table 15).
Table 10.
Additional data: Artemether versus quinine in adults
| Pre‐specified outcome | Trial reported outcome | Trial | No. of participants | Artemether | Quinine | Comparative results reported in article |
| Coma resolution time (h) | Median (IQR) | Hien 1996 VNM | 560 | 66 (30 to 132) | 48 (20 to 84) | P = 0.003 |
| Median (Range) | Karbwang 1995 THA | 97 | 48 (6 to 144) | 48 (6 to 144) | Not significantly different | |
| Fever clearance (h) | Median (IQR) | Hien 1996 VNM | 560 | 127 (60 to 216) | 90 (54 to 144) | < 0.001 |
| Median (Range) | Seaton 1998 PNG | 33 | 32 (20 to 112) | 48 (28 to 88) | P = 0.034 | |
| Median (Range) | Karbwang 1995 THA | 97 | 79 (16 to 147) | 84 (36 to 144) | Not significantly different | |
| Parasite clearance (h) | Median (IQR) | Hien 1996 VNM | 560 | 72 (54 to 102) | 90 (66 to 108) | < 0.001 |
| Median (range) | Seaton 1998 PNG | 33 | 48(4 to 72) | 52 (12 to112) | P = 0.381 | |
| Median (Range) | Karbwang 1995 THA | 97 | 54 (30 to 164) | 78 (18 to 168) | P = 0.007 |
IQR = interquartile range.
Neurological sequelae: only one trial Hien 1996 VNM reported neurological sequelae at discharge. Four neurological sequelae were reported with no difference between groups (one trial; 560 participants, Analysis 1.4).
Parasite clearance time (PCT): one trial reported mean parasite clearance time but showed no statistically significant difference between artemether and quinine (MD 1.70 hours, 95% CI ‐15.56 to 18.96; 26 participants, one trial, Analysis 1.6). Three other trials reported median parasite clearance time. Two trials reported a significantly shorter time to clearance of parasites with artemether (Hien 1996 VNM; Karbwang 1995 THA; see Table 13). Seaton 1998 PNG found no significant difference between artemether and quinine with respect to parasite clearance time.
Trials differed with respect to the frequency with which they repeated malaria blood smears (see Table 10).
Fever clearance time (FCT): four trials reported a measure of fever clearance time. Karbwang 1992 THA reported mean fever clearance time and found a statistically significant reduction of about 30 hours with artemether (MD ‐29.7 hours, 95% CI ‐54.14 to ‐5.26; 26 participants, one trial). The other three trials in adults reported median fever clearance time and two (Hien 1996 VNM; Seaton 1998 PNG) reported a statistically significant reduction in fever clearance time in favour of quinine and artemether respectively. Karbwang 1995 THA found both groups were comparable (see Table 15).
The definitions of fever varied across the included trials. Three trials used a cut off of body temperature less than 37.5°C from initiation of treatment to define fever clearance (see Table 10).
Need for blood transfusion: one trial reported on the number of patients requiring blood transfusions for severe malarial anaemia in both artemether and quinine arms (Hien 1996 VNM) (see Analysis 1.9). No statistically significant difference was observed between both arms (RR 0.97, 95% CI 0.73 to 1.29; 560 participants, one trial).
Adverse events: one trial reported episodes of hypoglycaemia (Analysis 1.10). Other adverse effects reported were abscess, induration at injection site, leg discomfort, chest infection and gastrointestinal bleeding. However, these trials were insufficiently powered to detect differences in adverse events. The trials had similar definitions of adverse events (included only adverse effects that could not be attributable to malaria).
Artemether versus artesunate
Adults
Two trials were conducted in adults; both from Asia.
Death: only two trials directly compared intramuscular artemether and intravenous artesunate. Overall, the risk of all‐cause mortality was significantly higher following treatment with artemether (RR 1.80, 95% CI 1.09 to 2.97; 494 participants, two trials, Analysis 2.1). However, both trials were too small to detect or exclude clinically important differences, and the overall meta‐analysis remains significantly underpowered to prove superiority (see Table 11 and Table 12).
Analysis 2.1.

Comparison 2 Artemether versus artesunate, Outcome 1 Death.
Coma resolution time: two trials reported median coma resolution times. Both trials reported a shorter coma resolution time with artesunate. However, these differences were not statistically significant (Table 16).
Table 11.
Additional data: Artemether versus artesunate in adults
| Pre‐specified outcome | Trial reported outcome | Trial | No. of participants | Artemether | Artesunate IM | Artesunate IV | Comparative results reported in article |
| Coma resolution time (h) | Median (range) | Phu 2010 VNM | 370 | 72(2 to 2232) n = 184 | 60(4 to 2136) n = 186 | ‐ | P = 0.11 |
| Median (95% CI) | Vinh 1997 VNM | 124 | 47 (31 to 63) | 30 (18 to 42) | 24 (4 to 44) | ‐ | |
| Fever clearance (h) | Median (range) | Phu 2010 VNM | 370 | 108 (0 to 888) n = 184 | 108 (0 to 888) n = 186 | ‐ | P = 0.27 |
| Median (95% CI) | Vinh 1997 VNM | 124 | 48 (38 to 58) | 36 (30 to 42) | 30 (18 to 42) | ‐ | |
| Parasite clearance (h) | Median (range) | Phu 2010 VNM | 370 | 72 (2 to 204) |
72 (7 to 330) |
‐ | P = 0.97 |
| Median (95% CI) | Vinh 1997 VNM | 124 | 30 (26 to 34) | 24 (15 to 33) | 24 (15 to 33) | Not statistically significant |
IM = intramuscular; IV = intravenous.
Parasite clearance time: two trials found no overall difference in median parasite clearance time (Phu 2010 VNM; Vinh 1997 VNM).
Fever clearance time:Phu 2010 VNM found no statistically significant difference in median fever clearance time between intramuscular artemether and intravenous artesunate. The additional small trial (Vinh 1997 VNM) found a benefit in favour of artesunate although this was not statistically significant.
Need for blood transfusion:Phu 2010 VNM found no difference between treatments with respect to the need for blood transfusion in adult severe malaria patients (RR 1.01, 95% CI 0.78 to 1.32; 370 participants, one trial, Analysis 2.2).
Analysis 2.2.
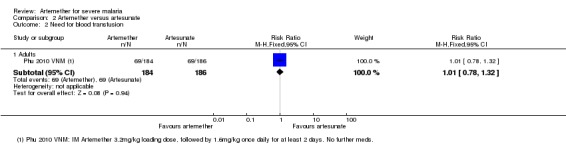
Comparison 2 Artemether versus artesunate, Outcome 2 Need for blood transfusion.
Adverse effects: only Phu 2010 VNM reported adverse events. The risk of hypoglycaemia was significantly higher in adults treated with intramuscular artemether compared with artesunate (RR 1.70. 95% CI 0.4 to 7.24; 370 participants, one trial, Analysis 2.4).
Analysis 2.4.
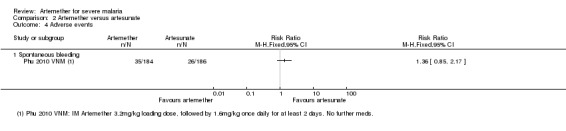
Comparison 2 Artemether versus artesunate, Outcome 4 Adverse events.
Discussion
Summary of main results
We included 18 RCTs, enrolling 2662 children and adults with severe malaria. Eleven trials were conducted in Africa and seven trials were undertaken in Asia.
Artemether versus quinine
For children (trials mostly conducted in Africa), there is probably little or no difference in the risk of death between intramuscular artemether and quinine (moderate quality evidence). Artemether may shorten the coma recovery time by about five hours (low quality evidence), and may reduce the number of children with subsequent neurological sequelae (low quality evidence). Artemether probably shortens the parasite clearance time by about nine hours (moderate quality evidence), and may shorten the fever clearance time by about three hours (low quality evidence).
For older children (> 15 years) and adults in Asia, artemether probably reduces deaths compared with quinine (moderate quality evidence), but larger trials are required to have full confidence in this finding.
Artemether versus artesunate
Artemether and artesunate have only been compared in two trials in adults from Asia, and mortality is probably higher with intramuscular artemether (moderate quality evidence) but larger trials are required to have confidence in this finding.
Overall completeness and applicability of evidence
Although 16 trials directly compared artemether versus quinine, none were adequately powered to detect clinically important differences. The total number of participants included in these trials (2163 participants) remains far short of the 7429 participants included in trials of artesunate versus quinine. The majority of data comparing artemether and quinine were from trials conducted in sub‐Saharan Africa where artemether is most widely used.
The two trials directly comparing artemether and artesunate were conducted in adults in Asia, and the results are therefore poorly applicable to children in Africa. However, in the absence of direct comparisons in children, the low quality evidence of equivalence between artemether and quinine suggests that artesunate will be as superior to artemether as it has been shown to be superior to quinine.
Artemether is prone to erratic and partial absorption and takes longer to achieve peak plasma concentrations as demonstrated in animal and human studies. These pharmacokinetic attributes make artemether that is injected intramuscularly less readily available in the human body and may explain the difference in outcomes between artesunate and intramuscular artemether we have observed in this review.
Quality of the evidence
We assessed the quality of the evidence using the GRADE approach and have presented it in three 'Summary of Findings' tables (Table 1; Table 2; Table 3).
The evidence of equivalence between intramuscular artemether and intravenous quinine in children is of moderate quality due to the small sample sizes of the included trials and the overall lack of power to fully exclude clinically important differences.
Similarly, we downgraded the evidence for reductions in mortality in adults treated with artemether compared with quinine, and artesunate compared with artemether, to moderate due to imprecision. Larger trials are needed to have full confidence in these effects.
Authors' conclusions
Although there is a lack of direct evidence comparing artemether with artesunate, artemether is probably less effective than artesunate at preventing deaths from severe malaria. In circumstances where artesunate is not available, artemether is an alternative to quinine.
Larger, adequately powered clinical trials are necessary for conclusive evidence on the relative effects of artemether. However, given the low bioavailability of artemether when given intramuscularly, it is unlikely that these trials will be done or indeed whether they are necessary.
Acknowledgements
The academic editor for this review was Dr Michael Eisenhut; Dr David Sinclair provided support in the preparing the 'Summary of findings' tables.
The protocol for this review was developed during the Reviews for Africa Fellowship Programme organized by the Nigerian Branch of the South Africa Cochrane Centre in July 2012. The UK Department for International Development (DFID) supports this programme through the Effective Health Care Research Consortium (EHCRC) at the Liverpool School of Tropical Medicine (LSTM). This document is an output from a project funded by UKaid for the benefit of developing countries. The views expressed are not necessarily those of UKAid or the Department for International Development (DFID).
The editorial base for the Cochrane Infectious Diseases Group is funded by UKaid from the UK Government for the benefit of developing countries.
Data and analyses
Comparison 1.
Artemether versus quinine
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Children | 12 | 1447 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.76, 1.20] |
| 1.2 Adults | 4 | 716 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.42, 0.83] |
| 2 Death: Time since admission to hospital | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Death within 24 hours | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.02, 8.10] |
| 3 Coma resolution time (hours) | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Children | 6 | 358 | Mean Difference (IV, Fixed, 95% CI) | ‐5.45 [‐7.90, ‐1.00] |
| 4 Neurological sequelae at discharge | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Children | 7 | 968 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.66, 1.07] |
| 4.2 Adults | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [0.31, 27.86] |
| 5 Neurological sequelae at follow‐up | 2 | 566 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.49, 1.38] |
| 5.1 Day 7 | 1 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.27, 2.14] |
| 5.2 Day 28 | 1 | 432 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.46, 1.53] |
| 6 Parasite clearance time | 8 | 446 | Mean Difference (IV, Fixed, 95% CI) | ‐8.82 [‐11.20, ‐6.45] |
| 6.1 Children | 7 | 420 | Mean Difference (IV, Fixed, 95% CI) | ‐9.03 [‐11.43, ‐6.63] |
| 6.2 Adults | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [‐15.56, 18.96] |
| 7 Proportion with parasite clearance | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.75, 1.04] |
| 7.1 At 72 hours | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.70, 1.11] |
| 7.2 At 7 days | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.70, 1.11] |
| 8 Fever clearance time (hours) | 9 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Children | 8 | 457 | Mean Difference (IV, Fixed, 95% CI) | ‐3.73 [‐6.55, ‐0.92] |
| 8.2 Adults | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐29.70 [‐54.14, ‐5.26] |
| 9 Need for blood transfusion | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Children | 1 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.62, 2.59] |
| 9.2 Adults | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.73, 1.29] |
| 10 Episodes of hypoglycaemia | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Children | 2 | 617 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.44, 1.05] |
| 10.2 Adults | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.30, 0.64] |
| 11 Adverse events | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 QT prolongation | 2 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.10 [1.33, 7.19] |
| 11.2 Local skin reactions | 1 | 576 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.03, 0.50] |
| 11.3 Abscess | 2 | 1136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.04, 0.90] |
| 11.4 Urticarial rash | 1 | 576 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.15] |
| 11.5 Supraventricular tachycardia | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.01, 4.59] |
| 11.6 Pruritus | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.13] |
| 11.7 Urinary tract infection | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.46 [0.15, 81.36] |
| 11.8 Induration at injection site | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.44 [0.94, 253.49] |
| 11.9 Leg discomfort | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.22, 2.16] |
| 11.10 Chest infection | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.81, 1.53] |
| 11.11 GI bleeding | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.52, 1.20] |
Analysis 1.2.
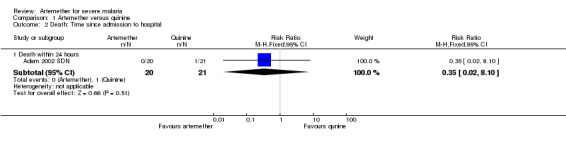
Comparison 1 Artemether versus quinine, Outcome 2 Death: Time since admission to hospital.
Analysis 1.11.
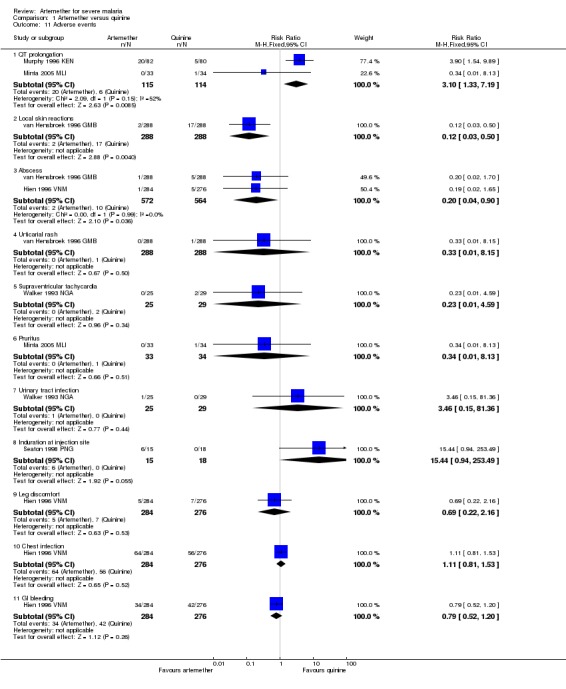
Comparison 1 Artemether versus quinine, Outcome 11 Adverse events.
Comparison 2.
Artemether versus artesunate
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Adults | 2 | 494 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.09, 2.97] |
| 2 Need for blood transfusion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Adults | 1 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.78, 1.32] |
| 3 Episodes of hypoglycaemia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Spontaneous bleeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Analysis 2.3.
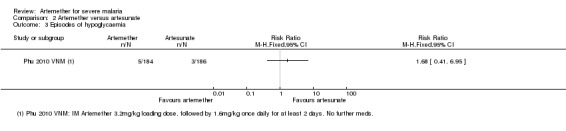
Comparison 2 Artemether versus artesunate, Outcome 3 Episodes of hypoglycaemia.
Differences between protocol and review
In the protocol, we specified that we would include only trials in children (aged < 15 years). However, we amended the inclusion criteria to include trials in adults and children.
We said we would explore data by drug regimen, type of severe malaria (cerebral versus non‐cerebral malaria), time since admission to hospital, length of follow‐up and geographical region, but data were insufficient.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Trial design: Open label RCT Trial dates: November 2001 to January 2002 | |
| Participants | Number of participants: 41 children enrolled Inclusion criteria: Children with severe malaria (age range not stated); cerebral malaria, repeated convulsions severe anaemia with haemoglobin <5g/dL, hyper parasitaemia (parasite count > 100,000 rings/µL or combinations of these criteria. Exclusion criteria: None stated |
|
| Interventions | 1. Intramuscular artemether (Kunming Pharmaceuticals; China)
2. Intravenous quinine
|
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
|
|
| Notes | Location: Outpatient clinic in New Halfa, Eastern Sudan Transmission: "meso‐endemic" Funding: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Each child was randomized". No further details provided. |
| Allocation concealment (selection bias) | Low risk | "Envelopes containing the assigned treatment were opened sequentially at the time when each patient was recruited to the study". |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | Described as open‐label. However, lack of blinding is unlikely to bias an objective outcome like death. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | High risk | An open‐label trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up were reported. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: RCT Trial dates: July to October 2007 |
|
| Participants | Number of participants: 90 children enrolled Inclusion criteria: Children between six months and 12 years of age presenting with fever (> 37.5°C ) and P. falciparum infection with one or more general danger signs of severe or complicated malaria based on the WHO criteria for severe malaria Exclusion criteria: Serious concomitant illness, for example, sickle cell anaemia, HIV, tuberculosis and other chronic diseases, severe malnutrition, known hypersensitivity to one of the trial drugs. |
|
| Interventions | 1. Intramuscular artemether (Paluther; May and Baker)
2.Intravenous or intramuscular quinine (Quinimax; Sanofi)
|
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
|
|
| Notes | Location: Federal Medical Centre, Birnin Kudu, Jigawa State of Nigeria Transmission: Stable perennial transmission Funding: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Patients were assigned to receive quinine if the last digit of their hospital identification number was odd and to receive artemether if the last digit of their hospital identification number was even or zero". |
| Allocation concealment (selection bias) | High risk | Trial authors did not describe any methods of allocation concealment, and this would not be possible using this randomization method. |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | No blinding was described. However, lack of blinding is unlikely to bias an objective outcome like death. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | High risk | No blinding is described, and blinding would not be feasible. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses to follow‐up at day 14 were > 10% in both trial arms. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: Double blind RCT Trial dates: Not stated |
|
| Participants | Number of participants: 560 adults aged 15 to 79 years enrolled Inclusion criteria: Patients were included in the trial if they (or an accompanying relative) gave informed consent, had asexual forms of P. falciparum on a peripheral‐blood smear, were older than 14 years, were not in the first trimester of pregnancy, were not intravenous drug users, had received less than 3 g of quinine or two doses of artemisinin or a derivative in the previous 48 hours, and had one or more of the following: a score on the Glasgow Coma Scale of less than 11 (indicating cerebral malaria); anaemia (hematocrit, 20 percent), with a parasite count exceeding 100,000 parasites/mm3 on a peripheral‐blood smear; jaundice (serum bilirubin, 2.5 mg/dL (50 mmol per litre)), with a parasite count of more than 100,000 parasites/mm3 on a peripheral‐blood smear; renal impairment (urine output, 400 mL per 24 hours; and serum creatinine, 3 mg/dL (250 mmol/L)); hypoglycaemia (blood glucose, 40 mg/dL (2.2 mmol/L)); hyperparasitaemia (10% parasitaemia); and systolic blood pressure below 80 mmHg with cool extremities (indicating shock). Exclusion criteria: None stated |
|
| Interventions | 1.Intramuscular artemether (Kunming Pharmaceutical)
2.Intramuscular quinine
|
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
|
|
| Notes | Location: Special Research Ward, Centre for Tropical Diseases, Ho Chi Minh City, Vietnam Transmission: Not stated Funding: Wellcome Trust |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial authors provided no information on methods of sequence generation. |
| Allocation concealment (selection bias) | Low risk | "The drugs for each patient were placed in a coded sealed envelope and the envelopes were randomized in blocks of 20. Once a patient was enrolled in the study the envelope was opened". |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | "To maintain blinding, a separate team of nurses, who were not otherwise involved with the care of the study patients, drew up and gave the injections. The drugs were kept in an opaque packet in a locked cabinet during the study". Both interventions were administered by intramuscular injection so blinding was feasible." |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | Low risk | "To maintain blinding, a separate team of nurses, who were not otherwise involved with the care of the study patients, drew up and gave the injections. The drugs were kept in an opaque packet in a locked cabinet during the study". Both interventions were administered by intramuscular injection so blinding was feasible." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up were recorded. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: Open RCT Trial dates: April 2000 to July 2001 |
|
| Participants | Number: 46 children aged 6 months to 12 years enrolled Inclusion criteria: Asexual forms of P. falciparum from peripheral blood smear. One or more clinical manifestations of severe malaria present which included – cerebral malaria, severe anaemia (haemoglobin < 5 g/dL or hematocrit < 15%) metabolic abnormalities (hypoglycaemia: plasma glucose < 40 mg/dL or < 2.2 mmol/L), algid malaria (associated with peripheral circulatory failure or shock), black‐water fever, renal failure, spontaneous bleeding (thrombocytopenia, DIC), pulmonary edema and jaundice. Exclusion criteria: History of having received artemether /quinine within 24 hours preceding admission. Severe protein energy malnutrition or clinical/laboratory evidence of other significant illness not attributable to severe malaria. |
|
| Interventions | 1. Intramuscular artemether
2. Intravenous quinine
Supportive therapy was given to all patients. |
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review: None |
|
| Notes | Location: Inpatient unit of Department of Pediatrics, and Parasitology laboratory, Department of Microbiology, Uttar Pradesh, India Transmission: Unknown Funding: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial authors provided no information on methods of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Trial authors provided no information on allocation concealment. |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | An open‐label trial is unlikely to bias an objective outcome like death. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | Low risk | "The slides for malarial parasites were transported to the parasitology laboratory where the person examining the slides was unaware of the clinical status of the patient and also the treatment assignment group". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up were recorded. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: RCT Trial dates: May to December 1991 |
|
| Participants | Number of participants: 26 adults aged 15 to 45 years enrolled Inclusion criteria: Patients with severe falciparum malaria (WHO definition) with no history of antimalarials within 24 hours prior to admission, aged between 15 to 45 years and weighed 45 to 60kg Exclusion criteria: None stated |
|
| Interventions | 1. Intramuscular artemether (Arthermin®)
2. Intravenous quinine
|
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
|
|
| Notes | Location: Prapokklao Hospital, Chantaburi, Thailand Transmission: Not stated Funding: Support from United Medical Ltd., Bangkok (provided artemether) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The patients were randomized to receive either quinine or artemether". |
| Allocation concealment (selection bias) | Unclear risk | Trial authors provided no information on allocation concealment. |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | Trial authors provided no information on blinding. However, lack of blinding is unlikely to bias an objective outcome like death. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | High risk | Trial authors provided no information on blinding, however it may not be feasible due to different routes of administration for both interventions. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up reported. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: RCT Trial dates:1992 to 1994 |
|
| Participants | Number of participants: 102 adults aged between 15 and 55 years enrolled Inclusion criteria: Male and female (non‐pregnant) patients with severe falciparum malaria (WHO definition) with no history of antimalarial treatment within 24 hours before admission aged 15 to 65 years and weighing 45 to 75kg Exclusion criteria: Patients with concurrent diseases were excluded |
|
| Interventions | 1. Intramuscular artemether
2. Intravenous quinine
|
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
|
|
| Notes | Location: Prapokklao Hospital, Chantaburi, Thailand Transmission: Not stated Funding: UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomization at WHO Office. |
| Allocation concealment (selection bias) | Low risk | "Each treatment was enclosed in a sealed envelope, which was opened only after the physician in charge had decided to recruit the patient into the study". |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | Trial authors provided no information on blinding. However, lack of blinding is unlikely to bias an objective outcome like death. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | High risk | Trial authors provided no information on blinding, however it may not be feasible due to different routes of administration for both interventions. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up about 5%. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: Open RCT Trial dates: June 1993 to February 1994 and June 1994 to December 1994 |
|
| Participants | Number of participants: 67 children aged three months to 15 years enrolled Inclusion criteria: Fever (core temperature ≥ 38 °C), positive blood smear for P. falciparum with ≥ 0.1% of parasitized erythrocytes, one major criterion or two minor criteria for severe malaria cases (WHO criteria) and parental consent Exclusion criteria: Patients with infection who had been treated within 24 hours with quinine or intramuscular injection was not eligible. |
|
| Interventions | 1. Intramuscular artemether (Paluther)
2. Intravenous quinine
|
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
|
|
| Notes | Location: Gabriel Touré's Hospital, Mali Transmission: Unknown Funding: Rhône‐Poulenc Rorer Doma (France) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomization by clinical monitor. |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes used to conceal allocation. |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | An open‐label trial is unlikely to bias an objective outcome like death. No blinding is described, and blinding would not be feasible. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | High risk | An open‐label trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up reported. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: Open RCT Trial dates: Not stated |
|
| Participants | Number: 160 children aged five months to 12 years enrolled Inclusion criteria: Children were admitted to the trial if they had P. falciparum asexual parasitaemia, were comatose and parental consent was obtained. Exclusion criteria: Children were excluded if there was evidence of a pre‐existing neurological deficit, head injury, or history of recent treatment with antimalarial drugs other than chloroquine. |
|
| Interventions | 1. Intramuscular artemether (Paluther, Rhône‐Poulenc)
2. Intravenous quinine (Laboratoires Renaudin, Paris)
|
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
|
|
| Notes | Location: Kenya Medical Research Institute (KEMRI) Coastal Research Unit, Kilifi district hospital, Kenya. Transmission: Unknown Funding:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally‐coded unique trial numbers. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes prepared by the clinical monitor. |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | An open‐label trial is unlikely to bias an objective outcome like death. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | High risk | An open‐label trial. Blinding unlikely as artemether and quinine were given by 2 different routes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 40 patients (14 from artemether arm and 26 from quinine arm) excluded. Mostly for not meeting inclusion criteria. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: RCT Trial dates: Not stated |
|
| Participants | Number of participants: 37 children enrolled (age range not stated) Inclusion criteria: Children with unrousable coma, asexual forms of P. falciparum parasitaemia and no other identifiable cause of coma. Exclusion criteria: None stated |
|
| Interventions | 1. Intramuscular artemether
2. Intravenous quinine
|
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review: None |
|
| Notes | Location: University of Ilorin Teaching Hospital, Nigeria Transmission: Unknown Funding: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned to either of the two treatment modalities". |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement. |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | Unlikely to be biased whether blinding was done or not. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | High risk | No information about blinding provided by trial authors. Blinding unlikely as artemether and quinine were given by 2 different routes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up recorded. |
| Selective reporting (reporting bias) | Low risk | Most relevant outcomes reported. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: Open label RCT Trial dates: Not stated |
|
| Participants | Number of participants: 103 children aged 11 months to five years enrolled Inclusion criteria: Children aged six months to 5 years satisfying the WHO criteria for cerebral malaria, viz. unrousable coma lasting more than 30 minutes (with or without convulsions) with the presence of peripheral P. falciparum parasitaemia were included in the trial. Exclusion criteria: None stated |
|
| Interventions | 1. Intramuscular artemether
2. Intravenous quinine (Lemquine®)
Loading dose quinine was omitted in patients with a positive history of quinine or mefloquine ingestion in the preceding 24 hours before hospital presentation. |
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
|
|
| Notes | Location: Emergency Paediatric ward, University College Hospital, Ibadan, Nigeria Transmission: Unknown Funding: World Bank/UNDP/WHO special fund for Research and Training in Tropical Diseases (TDR) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation. |
| Allocation concealment (selection bias) | Unclear risk | Methods not described by trial authors. |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | Unlikely to be biased whether blinding was done or not. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | High risk | No information of blinding reported by authors. Blinding unlikely as artemether and quinine were given by 2 different routes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up recorded. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: RCT Trial dates: Not stated |
|
| Participants | Number: 32 children aged one to 12 years enrolled Inclusion criteria: Children aged one to 12 years, with fever (temperature > 37.5°C), presence of convulsion, vomiting, hypoglycaemia, anaemia and headache. Informed consent obtained from the parents and guardians. Assurance that patients will be resident within catchments of trial for follow‐up. Absence of concomitant illness such as bronchopneumonia, typhoid, meningitis, urinary tract infection. Exclusion criteria: History of blood transfusion in the last two months, presence of concomitant illness, history of previous allergy to quinine and artemether. |
|
| Interventions | 1. Intramuscular artemether (Rhône‐Poulence, Rorer France)
2. Intravenous quinine (Evans)
Treatment with quinine was for a total of seven days. |
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review: None |
|
| Notes | Location: Overcomers Specialist Clinic Ileshan and General Hospital Ikenne, Nigeria Transmission: Unknown Funding: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The children were randomly allocated into 2 treatment groups; treatment Q and A for quinine and artemether respectively". |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment provided by trial authors. |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | Unlikely to be biased whether blinding was done or not. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | High risk | No information of blinding reported by authors. Blinding unlikely as artemether and quinine were given by two different routes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition about 6% and not likely to affect outcomes. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. Authors have published three outcomes in three different publications from the same trial. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: Double blind RCT Trial dates: May 1996 to June 2003 |
|
| Participants | Number of participants: 370 adults aged between 15 and 77 years enrolled Inclusion criteria: Peripheral blood smears had asexual forms of P. falciparum and had at least one of the following severe complications: cerebral malaria (Glasgow Coma Score was less than 11), renal acute failure (oliguria and serum creatinine > 250 μmol/L), jaundice (total serum bilirubin > 50 μmol/L) with a parasite count of more than 100,000/μL or with serum creatinine > 250 μmol/L, hypoglycaemia (blood glucose < 2.2 mmol/L), anaemia (haematocrit < 20%) with a parasite count of more than 100,000/μL, hyperparasitaemia (parasite count > 500,000/μL), hyperlactataemia (plasma lactate > 4 mmol/L), metabolic acidosis (standard base excess > ‐ 5 mmol/L, base deficit < 10 mmol/L) and shock (systolic blood pressure < 80 mmHg with cool extremities). Exclusion criteria: Patients were not included if they were < 14 years, were pregnant in the first trimester, were known intravenous drug abusers, had received more than 3 g of quinine or two doses of any artemisinin derivatives in the previous 48 hours before admission, had a past history of allergy to any artemisinin derivatives, or if known to be HIV positive. |
|
| Interventions | 1.Intramuscular artemether (Kunming Pharmaceutical Company, Kunming, China)
2.Intramuscular artesunate (Guilin No 2 Pharmaceutical Factory, Guangxi,China)
|
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
|
|
| Notes | Location: Hospital for Tropical Diseases, Ho Chi Minh City, Vietnam Transmission: Not stated Funding: Wellcome Trust, UK |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization was generated from random number tables". |
| Allocation concealment (selection bias) | Low risk | "Labels with the name of drug for each patient were put in coded sealed opaque envelopes, and the envelopes were randomized in blocks of 20. Once a patient was enrolled in the study the envelope was opened". |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | "An independent team of nurses, not otherwise involved in the study or responsible for the care of these patients, open the envelope, randomized the patient and prepared the injection. Neither the treating physicians, study doctors and nurses, or patients knew which anti‐malarial drugs was administered". |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | Low risk | "An independent team of nurses, not otherwise involved in the study or responsible for the care of these patients, open the envelope, randomized the patient and prepared the injection. Neither the treating physicians, study doctors and nurses, or patients knew which anti‐malarial drugs was administered". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up recorded. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: RCT Trial dates: May 1995 to June 1996 |
|
| Participants | Number: 77 children enrolled aged three months to 15 years Inclusion criteria: Children who satisfied the WHO criteria for diagnosis of cerebral malaria (such as unrousable coma for at least half an hour following convulsions, positive blood film for malaria, exclusion of other causes of encephalopathy and informed consent by parent or guardian). Exclusion criteria: Concomitant acute illness such as pneumonia, meningitis or acute renal failure and any contraindication to IM injection. |
|
| Interventions | 1.Intramuscular artemether
2. Intravenous quinine
Treatment with quinine was for a total of seven days |
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
|
|
| Notes | Location: Khartoum Children's Emergency Hospital and Ahmed Gasim Specialist Hospital for Children, Sudan Transmission: Unknown Funding:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The cases were randomly allocated into two groups". |
| Allocation concealment (selection bias) | Unclear risk | No information provided by trial authors about allocation concealment. |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | Unlikely to be biased whether blinding was done or not. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | High risk | No information of blinding reported by authors. Blinding unlikely as artemether and quinine were given by 2 different routes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | > 10% of participants in each arm dropped out before Day 28. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: Open‐label, RCT Trial dates: June 1992 to May 1995 |
|
| Participants | Number: 33 adults aged above 12 years Inclusion criteria: Blood smear showed asexual forms of P. falciparum; in addition to fulfilling one or more of the WHO criteria for severe or complicated malaria Exclusion criteria: Patients under the age of 12 years, pregnant women, those who had received parenteral antimalarial treatment prior to admission and those with a co‐existent bacterial, viral, fungal or mixed malarial infection were excluded. |
|
| Interventions | 1. Intramuscular artemether (Rhone‐Poulenc Rorer)
2. Intravenous Quinine (Medipharma)
|
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:None |
|
| Notes | Location:Port Moresby General Hospital, Papua New Guinea Transmission: Seasonal/Sporadic Funding:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomly assigned to treatment with quinine or artemether. |
| Allocation concealment (selection bias) | Low risk | Envelopes containing the assigned treatment were opened sequentially when a patient was recruited. |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | Unlikely to be biased whether blinding was done or not. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | High risk | No information of blinding reported by authors. Blinding unlikely as artemether and quinine were given by two different routes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Differential proportion of withdrawals from both arms (25% versus 10%). |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: Open label RCT Trial dates: January 1992 to June 1994 |
|
| Participants | Number: 183 children enrolled (age range not stated) Inclusion criteria: Children with asexual forms of P. falciparum detected in a peripheral blood smear, and a Blantyre Coma Score ≤ 2, and if no other cause of fever or altered consciousness could be discovered. Exclusion criteria: None stated |
|
| Interventions | 1. Intramuscular artemether
2. Intravenous quinine
|
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
|
|
| Notes | Location: Paediatric ward at the Queen Elizabeth Central Hospital, Malawi Transmission: Unknown Funding: UNDP/World Bank/WHO special programme for research and training in tropical diseases (TDR) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization. |
| Allocation concealment (selection bias) | Low risk | "Randomized treatment assignments were prepared in blocks of ten by the sponsoring agency.Following initial stabilization, diagnosis and examination, a sealed envelope containing the treatment group was opened, and the patient was allocated to treatment". |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | An open‐label trial is unlikely to bias an objective outcome like death. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | High risk | An open‐label trial. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Differential proportion of withdrawals from both arms (13% versus 8%). |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: Open label trial Trial dates: 1992 to 1994 |
|
| Participants | Number: 576 children aged one to nine years enrolled Inclusion criteria: Unconscious children one to nine years of age with a Blantyre coma score of 2 or less, asexual forms of P. falciparum were identified on a thick blood film, and a parent or guardian gave informed consent. Exclusion criteria: Patients with diseases other than malaria at the time of admission and those who recovered consciousness immediately after correction of hypoglycaemia or within one hour if they were convulsing on admission. Patients treated with quinine before admission. |
|
| Interventions | 1. Intramuscular artemether (Paluther, Rhone‐Poulenc)
2. Intravenous quinine (Rotexmedica, Germany)
An oral dose of approximately 1.25 mg/kg pyrimethamine and 25 mg/kg sulfadoxine was given to both arms to reduce recrudescence (in the 2nd and 3rd years of the trial). |
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
|
|
| Notes | Location: Royal Victoria Hospital and Sibanor Health Centre,Banjul Gambia Transmission:Unknown Funding:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial authors do not provide details of sequence generation |
| Allocation concealment (selection bias) | Low risk | "The treatment code for each child was stored in a sealed envelope that was opened after the admission procedure was completed and parental consent had been obtained". |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | An open‐label trial is unlikely to bias an objective outcome like death. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | Low risk | "Each blood film was examined by two independent observers who were unaware of the treatment code". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up were recorded. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: Open RCT Trial dates: March 1992 to September 1994 |
|
| Participants | Number: 124 adults aged between 16 and 66 years Inclusion criteria: 15 years of age or older, with clinical symptoms and signs of malaria and the presence of asexual forms of P. falciparum in their peripheral blood. In addition to having at least one of the following signs: (i) unrousable coma (Glasgow coma score < ll); (ii) hypoglycaemia (blood glucose < 2.2 mmol/L (40 mg %); (iii) acute renal failure (plasma creatinine > 265.2 µmol/L (3 mg %) with or without oliguria); (iv) jaundice (total bilirubin > 51.3 mol/L (3 mg %)) with parasitaemia > 100,000/µL or with plasma creatinine >1.5 mg %; (v) anaemia (haematocrit < 20%) with parasitaemia > 100000/µL; (vi) shock (systolic arterial pressure < 80 mmHg with a thready pulse and cold clammy extremities); and (vii) hyper parasitaemia >500000/µL. Exclusion criteria: Patients were excluded from the trial if prior treatment with more than 3 g of quinine or two doses of artemisinin or a derivative had been recorded by the peripheral health care worker. Pregnant patients in the first trimester, and patients with concomitant diseases (such as active tuberculosis, bacterial meningitis), or mixed infections with P. vivax were also excluded from the trial. |
|
| Interventions | 1.Intramuscular artemether (Kunming Pharmaceutical, Yunnan, China)
2.Intramuscular artesunate (Guilin No. 2 Pharmaceutical Factory, Guangxi, China)
3. Intravenous artesunate (Guilin No. 2 Pharmaceutical Factory, Guangxi, China)
All patients received 750 mg mefloquine (Lariam®, Roche) as a single dose after regaining consciousness or at day 4. |
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
|
|
| Notes | Location: Tan Phu regional Hospital, Vietnam Transmission: Endemic Funding: Roche Asian Research Foundation, Hong Kong |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial authors do not provide details about random sequence generation. |
| Allocation concealment (selection bias) | Low risk | "When a patient fulfilled the enrolment criteria, a sealed envelope containing the code for the treatment regimen was opened to allocate him/her to one of the following 4 treatment groups". |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | Described as open‐label. However, lack of blinding is unlikely to bias an objective outcome like death. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | High risk | An open‐label trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up were reported. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: Open RCT Trial dates: Not stated |
|
| Participants | Number: 54 children aged one to five years enrolled Inclusion criteria: Patients were admitted if they satisfied the strict WHO definition of cerebral malaria. Exclusion criteria: None stated |
|
| Interventions | 1. Intramuscular artemether
2. Intravenous quinine
|
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
|
|
| Notes | Location: University College Hospital, Ibadan, Nigeria Transmission:Unknown Funding: World Bank/UNDP/WHO special fund for Research and Training in Tropical Diseases (TDR) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers used. |
| Allocation concealment (selection bias) | Low risk | "Each child was then assigned a random number from a list prepared by an independent collaborator and thus allocated at random to receive either intramuscular artemether or intravenous quinine". |
| Blinding (performance bias and detection bias) Objective outcome: Death | Low risk | An open‐label trial is unlikely to bias an objective outcome like death. |
| Blinding (performance bias and detection bias) Subjective outcomes: Others | High risk | "This was a randomized, open, controlled study in which no attempt was made to ‘blind’ the investigators, as the test drug and the control drug were given by 2 different routes". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only one patient excluded from fever clearance time outcome assessment because of urinary tract infection. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aceng 2005 | Intervention was rectal artemisinin. |
| Bhattacharya 1997 | Not a RCT. |
| Bunnag 1992 | Drug‐resistant malaria not severe malaria. |
| Dunyo 2011 | Uncomplicated malaria. |
| Falade 2007 | Comparison not parenteral treatment. |
| Fargier 1999 | Not a RCT. |
| Karbwang 1994 | Comparison not parenteral treatment. |
| Karunajeewa 2006 | Not a RCT. |
| Myint 1987 | Not a RCT. |
| Osonuga 2006 | No desired review outcomes. |
| Reham 2012 | Patients not randomly selected (non‐probability consecutive sampling used). |
| Rehman 2013 | Design is a case‐control prospective study. |
| Shwe 1988 | Not a RCT. |
| Shwe 1992 | Participants in the artemether arm also received single dose of mefloquine. |
| White 1992 | Participants not randomized. |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Multicentre trial |
| Participants | Information not available |
| Interventions | Information not available |
| Outcomes | Information not available |
| Notes | Information not available |
| Methods | RCT |
| Participants | 105 adults enrolled |
| Interventions | Information not available |
| Outcomes | Information not available |
| Notes | Information not available |
Contributions of authors
Ekpereonne Esu (EE) and Emmanuel E. Effa (EEE) identified and extracted data from eligible trials for this review. EE entered data into Review Manager (RevMan). EEE and EE performed risk of bias assessment and analysed data. EE prepared the 'Summary of findings' tables and the first draft of the review. All authors read, gave input to all sections and approved the final version.
Sources of support
Internal sources
University of Calabar, Nigeria.
Liverpool School of Tropical Medicine, UK.
External sources
Department for International Development, UK.
Declarations of interest
None known.
Unchanged
References
References to studies included in this review
- Adam I, Idris HM, Mohamed‐Ali AA, Aelbasit, Elbashir MI. Comparison of intramuscular artemether and intravenous quinine in the treatment of Sudanese children with severe falciparum malaria. East African Medical Journal 2002;79(12):621‐5. [DOI] [PubMed] [Google Scholar]
- Aguwa CN, Ukwe CV, Adibe MO. A comparative study of quinine and artemether in the treatment of severe malaria in Nigerian children. Tropical Journal of Pharmaceutical Research 2010;9(1):11‐7. [Google Scholar]
- Tran TH, Day NP, Nguyen HP, Nguyen TH, Tran TH, Pham PL, et al. A controlled trial of artemether or quinine in Vietnamese adults with severe falciparum malaria. New England Journal of Medicine 1996;335(2):76‐83. [DOI] [PubMed] [Google Scholar]
- Huda SN, Shahab T, Ali SM, Afzal K, Khan HM. A comparative clinical trial of artemether and quinine in children with severe malaria. Indian Pediatrics 2003;40(10):939‐45. [PubMed] [Google Scholar]
- Karbwang J, Sukontason K, Rimchala W, Namsiripongpun W, Tin T, Auprayoon P, et al. Preliminary report: a comparative clinical trial of artemether and quinine in severe falciparum malaria. Southeast Asian Journal of Tropical Medicine and Public Health 1992;23(4):768‐72. [PubMed] [Google Scholar]
- Karbwang J, Tin T, Rimchala W, Sukontason K, Namsiripongpun V, Thanavibul A, et al. Comparison of artemether and quinine in the treatment of severe falciparum malaria in south‐east Thailand. Transactions of the Royal Society of Tropical Medicine and Hygiene 1995;89(6):668‐71. [DOI] [PubMed] [Google Scholar]
- Minta D, Sissoko M, Sidibe I, Dolo A, Poudiougou B, Dembele M, et al. Efficacy and safety of artemether in the treatment of severe and complicated malaria in Mali [Efficacite et tolerance de l'artemether dans le traitement du paludisme grave et complique au Mali]. Mali Médical 2005;20(1‐2):28‐32. [PubMed] [Google Scholar]
- Murphy S, English M, Waruiru C, Mwangi I, Amukoye E, Crawley J, et al. An open randomized trial of artemether versus quinine in the treatment of cerebral malaria in African children. Transactions of the Royal Society of Tropical Medicine and Hygiene 1996;90(3):298‐301. [DOI] [PubMed] [Google Scholar]
- Ojuawo A, Adegboye AR, Oyewalo O. Clinical response and parasite clearance in childhood cerebral malaria: A comparison between intramuscular artemether and intravenous quinine. East African Medical Journal 1998;75:450‐2. [Google Scholar]
- Olumese PE, Björkman A, Gbadegesin RA, Adeyemo AA, Walker O. Comparative efficacy of intramuscular artemether and intravenous quinine in Nigerian children with cerebral malaria. Acta Tropica 1999;73(3):231‐6. [DOI] [PubMed] [Google Scholar]
- Osonuga O, Osonuga AA, Osonuga IO, Osonuga A. Temperature changes in the course of treatment of severe malaria patients with artemether and quinine. Asian Journal of Medical Sciences 2011;2(1):41‐5. [Google Scholar]; Osonuga OA, Osonuga AA, Osonuga IO, Osonuga A. Comparison of Coma Resolution Time in the Course of Treating Childrenwith Severe Falciparum Malaria with Quinine and Artemether. World Journal of Medical Sciences 2011;6(2):42‐45. [Google Scholar]; Osonuga OA, Osonuga IO. Parasitaemia changes in the course of treatment of severe malaria patients with artemether and quinine (A preliminary study). Macedonian Journal of Medical Sciences 2009;2(4):319‐23. [Google Scholar]
- Phu NH, Tuan PQ, Day N, Mai NT, Chau TT, Chuong LV, et al. Randomized controlled trial of artesunate or artemether in Vietnamese adults with severe falciparum malaria. Malaria Journal 2010;9:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satti GM, Elhassan SH, Ibrahim SA. The efficacy of artemether versus quinine in the treatment of cerebral malaria. Journal of the Egyptian Society of Parasitology 2002;32(2):611‐23. [PubMed] [Google Scholar]
- Seaton RA, Trevett AJ, Wembri JP, Nwokolo N, Naraqi S, Black J, et al. Randomized comparison of intramuscular artemether and intravenous quinine in adult, Melanesian patients with severe or complicated, Plasmodium falciparum malaria in Papua New Guinea. Annals of Tropical Medicine and Parasitology 1998;92(2):133‐9. [PubMed] [Google Scholar]
- Taylor TE, Wills BA, Courval JM, Molyneux ME. Intramuscular artemether vs intravenous quinine: an open, randomized trial in Malawian children with cerebral malaria. Tropical Medicine and International Health 1998;3(1):3‐8. [DOI] [PubMed] [Google Scholar]
- Hensbroek MB, Onyiorah E, Jaffar S, Schneider G, Palmer A, Frenkel J, et al. A trial of artemether or quinine in children with cerebral malaria. New England Journal of Medicine 1996;335(2):69‐75. [DOI] [PubMed] [Google Scholar]
- Ha V, Nguyen NH, Tran TB, Bui MC, Nguyen HP, Tran TH, et al. Severe and complicated malaria treated with artemisinin, artesunate or artemether in Viet Nam. Transactions of the Royal Society of Tropical Medicine and Hygiene 1997;91(4):465‐7. [DOI] [PubMed] [Google Scholar]
- Walker O, Salako LA, Omukhodion SI, Sowunmi A. An open randomized comparative study of intramuscular artemether and intravenous quinine in cerebral malaria in children. Transactions of the Royal Society of Tropical Medicine and Hygiene 1993;87(5):564‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Aceng JR, Byarugaba JS, Tumwine JK. Rectal artemether versus intravenous quinine for the treatment of cerebral malaria in children in Uganda: randomised clinical trial. British Medical Journal 2005;330(7487):334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya PC, Pai‐dhungat AJ, Patel K. Artemether in moderate to severe malaria: a multicenter trial in India. Southeast Asian Journal of Tropical Medicine and Public Health 1997;28(4):736‐40. [PubMed] [Google Scholar]
- Bunnag D, Karbwang J, Harinasuta T. Artemether in the treatment of multiple drug resistant falciparum malaria. Southeast Asian Journal of Tropical Medicine and Public Health 1992;23(4):762‐7. [PubMed] [Google Scholar]
- Dunyo S, Sirugo G, Sesay S, Bisseye C, Njie F, Adiamoh M, et al. Randomized trial of safety and effectiveness of chlorproguanil‐dapsone and lumefantrine‐artemether for uncomplicated malaria in children in the Gambia. PLoS One 2011;6(6):e17371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falade CO, Fadero FF, Happi CT, Dada‐Adegbola HO, Gbotosho GO, Ayede I, et al. Dihydroartemisinin suppository in moderately severe malaria: comparative efficacy of dihydroartemisinin suppository versus intramuscular artemether followed by oral sulfadoxine‐pyrimethamine in the management of moderately severe malaria in Nigerian children. American Journal of Tropical Medicine and Hygiene 2007;76(1):1‐6. [PubMed] [Google Scholar]
- Fargier JJ, Louis FJ, Duparc S, Hounsinou C, Ringwald P, Danis M. Comparative study of artemether and quinine in severe Plasmodium falciparum malaria in adults and older children in Cameroon. Médicine Tropicale 1999;59(2):151‐6. [PubMed] [Google Scholar]
- Karbwang J, Na‐Bangchang K, Wattanakoon Y, Thanavibul A, Harinasuta T. Artemether 5 versus 7 day regimen for severe falciparum malaria. Southeast Asian Journal ofTropical Medicine and Public Health 1994;25(4):702‐6. [PubMed] [Google Scholar]
- Karunajeewa HA, Reeder J, Lorry K, Dabod E, Hamzah J, Page‐Sharp M, et al. Artesunate suppositories versus intramuscular artemether for treatment of severe malaria in children in Papua New Guinea. Antimicrobial Agents and Chemotherapy 2006;50(3):968‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pe Than Myint, Tin Shwe. A controlled clinical trial of artemether (qinghaosu derivative) versus quinine in complicated and severe falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 1987;81(4):559‐61. [DOI] [PubMed] [Google Scholar]
- Osonuga OA, Osonuga IO, Akinsomisoye OS, Raji Y, Ademowo OG. Packed cell volume changes in the treatment of severe malarial patients with artemether and quinine (a preliminary study). Journal of Medical Sciences 2006;6(5):853‐7. [Google Scholar]
- Rehman HU, Bilal H, Ali Q. Comparative study of intramuscular artemether and intravenous quinine in the treatment of cerebral malaria in children. Pakistan Paediatric Journal 2012;36(2):75‐80. [Google Scholar]
- Rehman MU, Shrestha B, Zehri T, Thapa S. Efficacy of quinine versus artemether in the treatment of severe malaria. Journal of the Nepal Health Research Council 2013;11(23):17‐21. [PubMed] [Google Scholar]
- Tin Shwe, Pe Than Myint, Ye Htut, Win Myint, Lin Soe. The effect of mefloquine artemether compared with quinine on patients with complicated falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 1988;82(5):665‐6. [DOI] [PubMed] [Google Scholar]
- Shwe T, Hla KK. The effect of artemether plus mefloquine on Myanmar patients with complicated falciparum malaria. Southeast Asian Journal of Tropical Medicine and Public Health 1992;23(Suppl 4):117‐21. [PubMed] [Google Scholar]
- White NJ, Waller D, Crawley J, Nosten F, Chapman D, Brewster D, et al. Comparison of artemether and chloroquine for severe malaria in Gambian children. Lancet 1992;339(8789):317‐21. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Danis M, Chandenier J, Doumbo O, Kombila M, Kouame J, Louis F, et al. Results obtained with i.m. artemether versus i.v. quinine in the treatment of severe malaria in a multi‐centre study in Africa. Japan Journal of Tropical Medicine and Hygiene 1996;24(Suppl 1):93‐6. [Google Scholar]
- Faiz MA, Rahman E, Hossain MA, Rahman MR, Yunus EB, Samad R, et al. A randomized controlled trial comparing artemether and quinine in the treatment of cerebral malaria in Bangladesh. Indian Journal of Malariology 2001;38(1‐2):9‐18. [PubMed] [Google Scholar]
Additional references
- Artemether‐Quinine Meta‐analysis Study Group. A meta‐analysis using individual patient data of trials comparing artemether with quinine in the treatment of severe falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 2001;95(6):637‐50. [DOI] [PubMed] [Google Scholar]
- Dondorp A, Nosten F, Stepniewska K, Day N, White N, South East Asian Quinine Artesunate Malaria Trial (SEAQUAMAT) group. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet 2005;366(9487):717‐25. [DOI] [PubMed] [Google Scholar]
- Dondorp AM, Fanello CI, Hendriksen IC, Gomes E, Seni A, Chhaganlal KD, et al. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open‐label, randomised trial. Lancet 2010;376(9753):1647‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolan DL, Dobaño C, Baird JK. Acquired immunity to malaria. Clinical Microbiology Reviews 2009;22(1):13‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. British Medical Journal 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hien TT, Davis TM, Chuong LV, Ilett KF, Sinh DX, Phu NH, et al. Comparative pharmacokinetics of intramuscular artesunate and artemether in patients with severe falciparum malaria. Antimicrobial Agents and Chemotherapy 2004;48(2):871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilett KF, Batty KT, Powell SM, Binh TQ, Thu le TA, Phuong HL, et al. The pharmacokinetic properties of intramuscular artesunate and rectal dihydroartemisinin in uncomplicated falciparum malaria. British Journal of Clinical Pharmacology 2002;53(1):23‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffar S, Hensbroek M, Palmer A, Schneider G, Greenwood B. Predictors of a fatal outcome following childhood cerebral malaria. American Journal of TropicalMedicine and Hygiene 1997;57(1):20–4. [DOI] [PubMed] [Google Scholar]
- Karbwang J, Na‐Bangchang K, Congpuong K, Molunto P, Thanavibul A. Pharmacokinetics and bioavailability of oral and intramuscular artemether. European Journal of Clinical Pharmacology 1997;52(4):307–10. [DOI] [PubMed] [Google Scholar]
- Kyu HH, Fernández E. Artemisinin derivatives versus quinine for cerebral malaria in African children: a systematic review. Bulletin of the World Health Organization 2009;87(12):896‐904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- McIntosh HM, Olliaro P. Artemisinin derivatives for treating severe malaria. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD000527] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithwani S, Aarons L, Kokwaro GO, Majid O, Muchohi S, Edwards G, et al. Population pharmacokinetics of artemether and dihydroartemisinin following single intramuscular dosing of artemether in African children with severe falciparum malaria. British Journal of Clinical Pharmacology 2004;57(2):146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SA, Mberu E, Muhia D, English M, Crawley J, Waruiru C, et al. The disposition of intramuscular artemether in children with cerebral malaria; a preliminary study. Transactions of the Royal Society of Tropical Medicine and Hygiene 1997;91(3):331‐4. [DOI] [PubMed] [Google Scholar]
- Navaratnam V, Mansor SM, Sit NW, Grace J, Li Q, Olliaro P. Pharmacokinetics of artemisinin‐type compounds. Clinical Pharmacokinetics 2000;39(4):255‐70. [DOI] [PubMed] [Google Scholar]
- Nealon C, Dzeing A, Müller‐Römer U, Planche T, Sinou V, Kombila M, et al. Intramuscular bioavailability and clinical efficacy of artesunate in Gabonese children with severe malaria. Antimicrobial Agents and Chemotherapy 2002;46(12):3933–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
- Sinclair D, Donegan S, Lalloo DG. Artesunate versus quinine for treating severe malaria. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD005967.pub3] [DOI] [PubMed] [Google Scholar]
- ter Kuile F, White NJ, Holloway P, Pasvol G, Krishna S. Plasmodium falciparum: in vitro studies of the pharmacodynamic properties of drugs used for the treatment of severe malaria. Experimental Parasitology 1993;76(1):85–95. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Transactions of the Royal Society of Tropical Medicine and Hygiene 2000;94(Suppl 1):S1–90. [PubMed] [Google Scholar]
- World Health Organization. The global burden of disease: 2004 update. Geneva: World Health Organization, 2008. [Google Scholar]
- World Health Organization. Roll Back Malaria Department. Guidelines for the treatment of malaria. Second Edition. Geneva: World Health Organization, 2010. [Google Scholar]


