Abstract
Background
The Kidney Donor Profile Index (KDPI) is a more precise donor organ quality metric replacing age-based characterization of donor risk. Little prior attention has been paid on the outcomes of lower quality kidneys transplanted into elderly recipients. Although we have previously shown that immunological risks associated with older organs are attenuated by advanced recipient age it remains unknown whether risks associated with lower quality KDPI organs are similarly reduced in older recipients.
Methods
Donor organ quality as measured by the KDPI was divided into quintiles (very high, high, medium, low, and very low quality) and Cox proportional hazards was used to assess graft and recipient survival in first-time adult deceased donor transplant recipients by recipient age.
Results
In uncensored graft survival analysis, recipients >69 years had comparable outcomes if they received low quality compared to medium quality kidneys. Death-censored analysis demonstrated no increased relative risk when low quality kidneys were transplanted into recipients 70–79 years (HR1.11, p=0.19) or >79 years (HR1.08, p=0.59). In overall survival analysis, elderly recipients gained no relative benefit from medium over low quality kidneys (70–79 years: HR1.03, p=0.51; >79 years: HR1.08, p=0.32).
Conclusions
Our analysis demonstrates that transplanting medium quality kidneys into elderly recipients does not provide significant advantage over low quality kidneys.
Keywords: Organ quality, recipient age, KDPI
Introduction
Renal transplant represents the treatment of choice for patients with end-stage renal disease (ESRD) due to reduced morbidity and mortality compared to maintenance dialysis. (1, 2) In recent years higher adjusted rates of ESRD among older patients (3) have been coupled with an increased age of candidates on the waiting list. (4) Although some patients prefer to remain on dialysis and wait for a better quality organ, the utilization of lower quality donor organs has led to shorter waiting times for some recipients. (5) Due to the limited supply of deceased donor organs nationally, it is imperative to succinctly identify the relative hazards associated with lower quality organs to minimize the discard rate overall.
On March 26, 2012, the Kidney Donor Profile Index (KDPI) was made available with every organ offer in the UNOS allocation system. (6) This measure provides a continuous scale estimating the likelihood of graft failure based on ten donor factors known at the time of organ offer. (7) Moreover, the KDPI is more precise than the prior extended-criteria donor (ECD) / standard-criteria donor (SCD) distinction.(8) The KDPI will be integrated to the national allocation scheme in 2014 and will preferentially assign the best quality organs to recipients with the longest projected post-transplant survival.(9) Despite much prior attention given to the best quality organs, less is known regarding outcomes associated with lower quality organs and some transplant professionals have indicated the need for additional education on how to incorporate the KDPI into clinical decision-making.(6)
Advanced donor age has been linked to decreased graft survival in renal transplant recipients overall. (10) However, our previous work has shown that the risk of graft loss associated with older donor organs is attenuated in older recipients (11), perhaps owing to the relative immunosenescence of these patients. (12) However, whether the relative hazards associated with poorer quality organs as measured by the KDPI are similarly reduced by advanced recipient age are heretofore unknown.
As such, we sought to quantify the likelihoods of graft loss and recipient mortality associated with different levels of organ quality among cohorts of increasing recipient age. We hypothesized that advanced recipient age would not increase the hazard of graft loss or recipient survival. Moreover, we set out to test the correlation of organ quality and transplant outcome in the elderly recipient population.
Results
Recipient Characteristics by Age Cohort
Table 1 lists demographic characteristics by recipient age. Female, Black, and Hispanic patients were less likely to be transplanted after age 69. Hypertension-related renal disease increased with recipient age, and diabetes-related disease peaked in the seventh decade but declined thereafter. A smaller proportion of recipients >70 years had been on dialysis >4 years compared to younger age groups.
Table 1.
Demographic characteristics of transplants and transplant recipients by age cohort.
| Characteristic | 18–29 years (N = 10,675) | 30–39 years (N=20,504) | 40–49 years (N=31,661) | 50–59 years (N=38,692) | 60–69 years (N=26,641) | 70–79 years (N=5,372) | >79 years (N=1,766) | |
|---|---|---|---|---|---|---|---|---|
| Sex | Female | 0.43 | 0.42 | 0.39 | 0.39 | 0.39 | 0.35 | 0.29 |
| Male | 0.57 | 0.58 | 0.61 | 0.61 | 0.61 | 0.65 | 0.71 | |
| Ethnicity | White | 0.44 | 0.45 | 0.48 | 0.51 | 0.57 | 0.65 | 0.76 |
| Black | 0.32 | 0.34 | 0.32 | 0.30 | 0.25 | 0.19 | 0.13 | |
| Hispanic | 0.17 | 0.14 | 0.12 | 0.12 | 0.11 | 0.10 | 0.06 | |
| Asian | 0.04 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.04 | |
| Other | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.01 | 0.01 | |
| Cause of Renal Disease | Diabetes | 0.04 | 0.12 | 0.18 | 0.28 | 0.34 | 0.27 | 0.19 |
| Hypertension | 0.13 | 0.21 | 0.22 | 0.21 | 0.22 | 0.29 | 0.38 | |
| PCKD | 0.01 | 0.03 | 0.10 | 0.12 | 0.09 | 0.08 | 0.07 | |
| Other | 0.81 | 0.62 | 0.49 | 0.38 | 0.35 | 0.36 | 0.37 | |
| Missing | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 | |
| Angina | Yes | 0.00 | 0.00 | 0.01 | 0.02 | 0.03 | 0.04 | 0.04 |
| No | 0.14 | 0.14 | 0.15 | 0.17 | 0.19 | 0.22 | 0.25 | |
| Missing | 0.85 | 0.85 | 0.84 | 0.81 | 0.78 | 0.74 | 0.71 | |
| CVD | Yes | 0.01 | 0.01 | 0.02 | 0.02 | 0.02 | 0.03 | 0.03 |
| No | 0.76 | 0.78 | 0.76 | 0.76 | 0.76 | 0.72 | 0.70 | |
| Missing | 0.23 | 0.21 | 0.23 | 0.23 | 0.23 | 0.26 | 0.27 | |
| Hypertension | Yes | 0.56 | 0.64 | 0.64 | 0.64 | 0.64 | 0.62 | 0.59 |
| No | 0.21 | 0.15 | 0.14 | 0.13 | 0.12 | 0.12 | 0.11 | |
| Missing | 0.23 | 0.21 | 0.22 | 0.23 | 0.25 | 0.26 | 0.30 | |
| HCV | Positive | 0.03 | 0.05 | 0.09 | 0.09 | 0.05 | 0.03 | 0.02 |
| Negative | 0.87 | 0.85 | 0.81 | 0.81 | 0.85 | 0.87 | 0.86 | |
| Missing | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.12 | |
| PVD | Yes | 0.01 | 0.02 | 0.03 | 0.04 | 0.06 | 0.06 | 0.04 |
| No | 0.87 | 0.86 | 0.86 | 0.86 | 0.86 | 0.88 | 0.91 | |
| Missing | 0.12 | 0.12 | 0.11 | 0.09 | 0.08 | 0.06 | 0.05 | |
| Diabetes | Yes | 0.05 | 0.15 | 0.23 | 0.36 | 0.44 | 0.37 | 0.27 |
| No | 0.88 | 0.77 | 0.70 | 0.59 | 0.52 | 0.61 | 0.71 | |
| Missing | 0.08 | 0.08 | 0.07 | 0.05 | 0.04 | 0.02 | 0.02 | |
| Dialysis Vintage | None or <1 yr | 0.24 | 0.23 | 0.25 | 0.26 | 0.26 | 0.26 | 0.26 |
| 1–2 years | 0.18 | 0.15 | 0.16 | 0.15 | 0.16 | 0.17 | 0.18 | |
| 2–4 years | 0.29 | 0.27 | 0.27 | 0.28 | 0.30 | 0.30 | 0.33 | |
| >4 years | 0.29 | 0.35 | 0.32 | 0.30 | 0.28 | 0.26 | 0.23 | |
| Functional Status | No Assist | 0.79 | 0.78 | 0.76 | 0.74 | 0.74 | 0.77 | 0.77 |
| Some Assist | 0.11 | 0.12 | 0.13 | 0.15 | 0.15 | 0.15 | 0.15 | |
| Total Assist | 0.01 | 0.02 | 0.02 | 0.03 | 0.03 | 0.02 | 0.02 | |
| Missing | 0.09 | 0.09 | 0.09 | 0.09 | 0.08 | 0.06 | 0.05 | |
| BMI | Normal | 0.50 | 0.42 | 0.35 | 0.31 | 0.29 | 0.33 | 0.37 |
| Underweight | 0.08 | 0.04 | 0.02 | 0.02 | 0.02 | 0.01 | 0.01 | |
| Overweight | 0.23 | 0.28 | 0.32 | 0.35 | 0.38 | 0.41 | 0.43 | |
| Obese | 0.11 | 0.15 | 0.18 | 0.21 | 0.22 | 0.19 | 0.14 | |
| Severe or Morbid Obese | 0.06 | 0.09 | 0.10 | 0.10 | 0.08 | 0.05 | 0.04 | |
| Missing | 0.02 | 0.02 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | |
| PRA | 0 | 0.42 | 0.39 | 0.42 | 0.44 | 0.45 | 0.47 | 0.50 |
| 1–20 | 0.23 | 0.24 | 0.24 | 0.24 | 0.23 | 0.22 | 0.20 | |
| 21–40 | 0.05 | 0.06 | 0.05 | 0.05 | 0.05 | 0.04 | 0.04 | |
| 41–60 | 0.04 | 0.04 | 0.04 | 0.03 | 0.03 | 0.03 | 0.02 | |
| 61–80 | 0.04 | 0.04 | 0.04 | 0.03 | 0.02 | 0.02 | 0.02 | |
| 81–100 | 0.11 | 0.12 | 0.09 | 0.07 | 0.06 | 0.04 | 0.03 | |
| Missing | 0.11 | 0.11 | 0.12 | 0.14 | 0.16 | 0.18 | 0.19 | |
Values expressed in proportions (columns add to 1.0 within each variable category). All variables significantly different (p<0.05) across age cohorts by Pearson Chi-Square analysis. PCKD: Polycystic Kidney Disease.
Co-morbid hypertension, diabetes, angina, and peripheral vascular disease increased with recipient age. The proportion of HCV positive patients was highest among recipients in the fifth and sixth decades. Recipients >70 years were also more likely to be overweight but less likely to be severely/morbidly obese. An increased proportion of recipients >70 years also had PRA = 0 whereas a lower proportion of these recipients had a PRA in the higher ranges (i.e., 61–80 and 81–100).
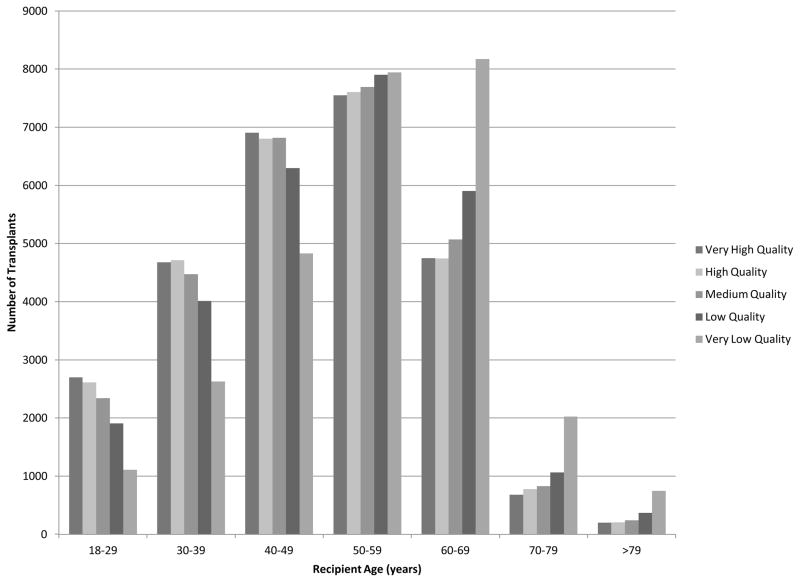
Although the majority of very low quality organs were transplanted into recipients younger than 70 years, recipients 70 years and over received these organs with higher frequency (Figure 1). The probability of receiving a very low quality organ increased dramatically with age from 10% among ages 18–29 years, to 38% among ages 70–79 years, and 42% in recipients 79 years and over (p < 0.0001). Conversely, the likelihood of receiving a very high quality organ decreased with recipient age, where recipients 70–79 years and >79 years received 13% and 11% of these organs, respectively (Table 2). Similar trends were observed in the transplantation of high quality organs among older recipients.
Figure 1. Number of Transplants by Organ Quality and Recipient Age.
The majority of organs, include those of low and very low quality, are transplanted into younger recipients. However, the probability of receiving lower quality organs increases with age.
Table 2.
Distribution of Deceased Donor Renal Transplants by Organ Quality by Recipient Age (1995–2010)
| Distribution of Transplants by Organ Quality by Recipient Age Cohort (1995–2010)
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organ Quality Category | 18–29 years | 30–39 years | 40–49 years | 50–59 years | 60–69 years | 70–79 years | >79 years | |||||||
| N | P | N | P | N | P | N | P | N | P | N | P | N | P | |
| Very High Quality (81–100th %-tile) | 2699 | 0.25 | 4678 | 0.23 | 6907 | 0.22 | 7550 | 0.20 | 4748 | 0.17 | 680 | 0.13 | 199 | 0.11 |
|
| ||||||||||||||
| High Quality (61–80th %-tile) | 2612 | 0.24 | 4713 | 0.23 | 6806 | 0.21 | 7606 | 0.20 | 4743 | 0.17 | 776 | 0.14 | 207 | 0.12 |
|
| ||||||||||||||
| Medium Quality (41–60th %-tile) | 2344 | 0.22 | 4472 | 0.22 | 6821 | 0.22 | 7691 | 0.20 | 5070 | 0.18 | 828 | 0.15 | 239 | 0.14 |
|
| ||||||||||||||
| Low Quality (21–40th %-tile) | 1909 | 0.18 | 4012 | 0.20 | 6296 | 0.20 | 7902 | 0.20 | 5906 | 0.21 | 1062 | 0.20 | 371 | 0.21 |
|
| ||||||||||||||
| Very Low Quality (0–20th %-tile) | 1111 | 0.10 | 2629 | 0.13 | 4831 | 0.15 | 7943 | 0.21 | 8174 | 0.29 | 2026 | 0.38 | 750 | 0.42 |
Unadjusted Graft and Patient Survival by Organ Quality and Recipient Age Cohort
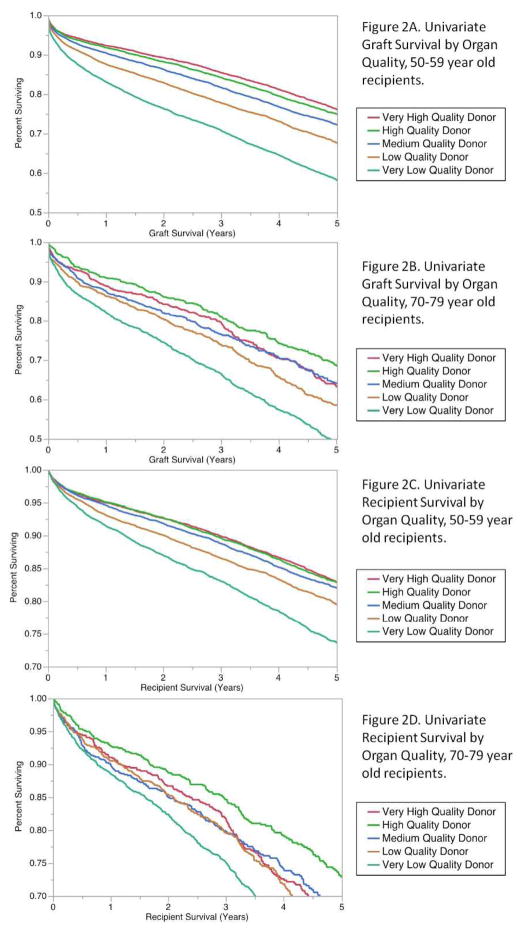
In all recipients up to 69 years, we found that decreasing organ quality predicted worse graft survival and patient survival (only recipients 50–59 years shown). However, for ages 70–79 years and >79 years we found increased variability in recipient outcome by organ quality. For example, very high quality organs were not predictive of the best graft survival at most time points following transplant (Figure 2). Albeit less pronounced, similar patterns were observed when analyzing death-censored graft survival comparing older and younger recipients. Similarly, when examining overall recipient survival, organ quality appeared more predictive of recipient mortality among younger recipients, but very high quality organs were not associated with the best patient survival in recipients aged 70–79 years.
Figure 2. Uncensored Graft and Patient Survival by Organ Quality Category (recipients 50–59 years and 70–79 years only).
Graft survival decreases with decreasing organ quality in recipients 50–59 years old (Figure 2A), however, the highest quality kidneys do not predict the best graft survival in 70–79 year old recipients. (Figure 2B). Moreover, recipient survival also decreases with decreasing organ quality in recipients 50–59 years (Figure 2C), however, the highest quality donor kidneys do not predict the best survival in 70–79 year old recipients (Figure 2D).
Additionally, because the 70 and over population appeared to have several distinguishing characteristics compared to younger groups (lower sensitization profile, more males, and fewer patients on dialysis >4 years) we performed sub-analyses for these characteristics and concluded that these factors likely did not account for age-related differences in recipients 70 and older (not shown).
Adjusted Uncensored Graft Survival
Multivariable analysis explored hazard ratios for graft loss by organ quality and recipient age using medium quality (3rd quintile KDPI) kidneys as the reference group (Table 3). Among all recipients <70 years, we found statistically significant differences in graft survival by organ quality category. However, for recipients 70–79 years we found no significantly increased risk associated with transplantation of low quality organs (HR 1.05, 95% CI 0.97–1.14, p = 0.22) compared to medium quality organs. Although recipients >79 years showed a trend towards worsening graft survival with declining organ quality, all but very low quality kidneys achieved statistical significance (very high quality: HR 0.90, 95% CI 0.75–1.07, p = 0.24; high quality: HR 0.89, 95% CI 0.74–1.06, p = 0.20; low quality: HR 1.08, 95% CI 0.94–1.24, p = 0.28).
Table 3.
Multivariate Hazard Ratios for Graft Failure, Death-Censored Graft Failure, and Recipient Mortality by Recipient Age Cohort. Reference = medium quality kidneys (KDPI 51–60th percentile; HR =1.00 – not shown).
| Recipient Age (years) | Organ Quality (KDPI Quintile) | |||
|---|---|---|---|---|
| Very High (0–20th percentile) | High (21–40th percentile) | Low (61–80th percentile) | Very Low (81–100th percentile) | |
| Uncensored Graft Failure (HR (95% CI), p value) | ||||
| 30–39 | 0.78 (0.74–0.81) p<0.001 |
0.84 (0.80–0.88) p<0.001 |
1.13 (1.08–1.18) p<0.001 |
1.41 (1.34–1.47) p<0.001 |
| 40–49 | 0.78 (0.75–0.81) p<0.001 |
0.82 (0.79–0.85) p<0.001 |
1.10 (1.06–1.14) p<0.001 |
1.51 (1.45–1.56) p<0.001 |
| 50–59 | 0.80 (0.77–0.83) p<0.001 |
0.80 (0.77–0.84) p<0.001 |
1.13 (1.09–1.16) p<0.001 |
1.41 (1.37–1.45) p<0.001 |
| 60–69 | 0.80 (0.77–0.83) p<0.001 |
0.80 (0.77–0.84) p<0.001 |
1.13 (1.09–1.16) p<0.001 |
1.41 (1.37–1.45) p<0.001 |
| 70–79 | 0.88 (0.80–0.97) p=0.009 |
0.77 (0.70–0.85) p<0.001 |
1.05 (0.97–1.14) p=0.22 |
1.42 (1.33–1.51) p<0.001 |
| >79 | 0.90 (0.75–1.07) p=0.24 |
0.89 (0.74–1.06) p=0.20 |
1.08 (0.94–1.24) p=0.28 |
1.26 (1.13–1.41) p<0.001 |
| Death-Censored Graft Failure (HR (95% CI), p value) | ||||
| 30–39 | 0.75 (0.71–0.79) p<0.001 |
0.81 (0.78–0.86) p<0.001 |
1.17 (1.11–1.22) p<0.001 |
1.47 (1.47–1.55) p<0.001 |
| 40–49 | 0.72 (0.69–0.76) p<0.001 |
0.78 (0.74–0.82) p<0.001 |
1.16 (1.12–1.21) p<0.001 |
1.62 (1.56–1.69) p<0.001 |
| 50–59 | 0.66 (0.63–0.70) p<0.001 |
0.75 (0.71–0.79) p<0.001 |
1.21 (1.16–1.27) p<0.001 |
1.75 (1.68–1.82) p<0.001 |
| 60–69 | 0.64 (0.60–0.69) p<0.001 |
0.70 (0.65–0.75) p<0.001 |
1.22 (1.15–1.29) p<0.001 |
1.89 (1.80–1.98) p<0.001 |
| 70–79 | 0.68 (0.55–0.83) p<0.001 |
0.75 (0.62–0.91) p=0.003 |
1.11 (0.95–1.28) p=0.19 |
1.90 (1.69–2.12) p<0.001 |
| >79 | 0.46 (0.28–0.70) p<0.001 |
1.03 (0.73–1.41) p=0.88 |
1.08 (0.82–1.41) p=0.59 |
1.63 (1.33–2.01) p<0.001 |
| Recipient Mortality (HR (95% CI), p value) | ||||
| 30–39 | 0.84 (0.78–0.91) p<0.001 |
0.88 (0.82–0.95) p=0.002 |
1.04 (0.96–1.13) p=0.31 |
1.26 (1.15–1.37) p<0.001 |
| 40–49 | 0.87 (0.82–0.91) p<0.001 |
0.89 (0.85–0.94) p<0.001 |
1.01 (0.96–1.07) p=0.61 |
1.33 (1.25–1.40) p<0.001 |
| 50–59 | 0.85 (0.82–0.89) p<0.001 |
0.87 (0.83–0.90) p<0.001 |
1.08 (1.04–1.12) p=0.002 |
1.34 (1.29–1.39) p<0.001 |
| 60–69 | 0.86 (0.82–0.90) p<0.001 |
0.85 (0.82–0.89) p<0.001 |
1.10 (1.06–1.14) p<0.001 |
1.25 (1.20–1.29) p<0.001 |
| 70–79 | 0.93 (0.84–1.03) p=0.16 |
0.77 (0.69–0.85) p<0.001 |
1.03 (0.94–1.13) p=0.51 |
1.29 (1.20–1.39) p<0.001 |
| >79 | 0.99 (0.82–1.18) p=0.87 |
0.89 (0.73–1.07) p=0.24 |
1.08 (0.93–1.25) p=0.31 |
1.19 (1.05–1.34) p=0.006 |
When comparing the hazards for graft loss associated within each organ quality category by age group, we found that recipient age alone did not appear to increase the relative hazard of graft loss. In fact, for high quality organs, the relative hazard of graft loss appeared to decrease after age 69 for low quality kidneys. Additionally, among all recipients of very low quality kidneys, the relative risk of graft loss appeared to be the lowest among recipients >79 years old (HR 1.26, 95% CI 1.13–1.41, p < 0.001).
Adjusted Death-censored Graft Survival
Death-censored (DWFG) analysis demonstrated that improved organ quality predicted significantly better graft survival for recipients <70 years old. In recipients 70–79 years old, we found an increased but non-significant relative hazard of DWFG graft loss associated with low quality kidneys (HR 1.11, 95% CI 0.95–1.28, p = 0.19). For recipients >79 years old, although there was a clear death-censored graft survival benefit afforded by the transplantation of very high quality kidneys (HR 0.46, 95% CI 0.28–0.70, p < 0.001), no significant benefit was associated with high quality kidneys (HR 1.03, 95% 0.73–1.41, p = 0.88). For high and low quality kidneys, 70–79 year old recipients had a lower relative hazard of DWFG graft loss compared to the youngest recipients. Additionally, for very low and low quality organs we found that the oldest recipients (>79 years) had the smallest relative hazard of death-censored graft failure for any age group (Table 3). When comparing the relative hazards for graft loss by organ quality and recipient age group, we observed similar trends in death-censored as in uncensored analysis. Moreover, among all recipients of very low quality kidneys we found that patients >79 years had relative hazards lower than 50–79 year olds and comparable to recipients 40–49 years old.
Adjusted Overall Recipient Survival
Among recipients in the 50–69 years old, the receipt of a low quality kidney appeared to significantly decrease patient survival (Table 3). However, for recipients 70–79 years, low quality kidneys were not significantly associated with increased risk of death (HR 1.03, 95% CI 0.94–1.13, p = 0.51). A similar finding was observed for recipients >79 years who received low quality kidneys (HR 1.08, 95% CI 0.93–1.25, p = 0.31). Moreover, in this cohort we observed a non-significant overall survival benefit associated with high (HR 0.89, 95% CI 0.73–1.07, p = 0.24) and very high quality kidneys (HR 0.99, 95% CI 0.82–1.18, p = 0.87). Interestingly, although low quality kidneys significantly increased the relative hazards of uncensored and death-censored graft loss overall in recipients <50 years, there was no significantly increased hazard of mortality for recipients 30–39 years (HR 1.04, 95% CI 0.96–1.13, p = 0.31) or 40–49 years (HR 1.01, 95% CI 0.96–1.07, p = 0.61).
Compared to all recipients of high and low quality kidneys, recipients aged 70–79 years had a relative risk of mortality equal to or below the risk of death as recipients <50 years. Additionally, 70–79 year old recipients of very low quality kidneys had a lower relative hazard of death compared to the recipients in the fifth and sixth decades of life, and recipients >79 years had the lowest relative hazard of death of any age group (HR 1.19, 95% CI 1.05–1.34, p = 0.006).
Discussion
Our findings demonstrate a more modest relative hazard of uncensored and death-censored graft loss associated with low quality kidneys compared to medium quality kidneys in recipients > 70 years. We have also shown that advanced recipient age alone does not increase the relative risk of graft loss associated with low quality KDPI (61–80th percentile) kidneys. With respect to overall survival, we found that medium quality kidneys provided a more modest relative survival benefit compared to low quality kidneys in recipients aged 70 years and over. Moreover, these findings do not seem attributable to differences in recipient characteristics such as lower degree of sensitization, more males, and fewer patients who have spent >4 years on dialysis. Because our results are expressed in terms of hazard ratios, it is important to note that they refer to age-specific relative (in contrast to absolute) risks.
Immunoscenescence may contribute to the decreased relative hazards associated with low quality kidneys. Previously, we were able to show that the augmented immune response to older organs is blunted if older organs are placed into older recipients. (11), (12) However, given the non-significant benefit of very high quality kidneys in uncensored graft survival in recipients >79 years, it is possible that the decreased overall life expectancy in this population masked the potential impact of organ quality in this group. In contrast, it was also interesting to note that recipients >79 years did have a significant DWFG survival benefit associated with very high quality kidneys. Because this group has an increased proportion of non-sensitized patients (not shown) it is plausible that this provides some explanation for these differences. However, the inability of these patients to enjoy the complete lifetime of higher quality KDPI kidneys may support the allocation of higher KDPI kidneys into older recipients.
Heaphy et al. also investigated the interaction between donor and recipient characteristics in kidney transplantation. (13) Similar to our work, they questioned whether current concepts of “donor risk” were uniform for all recipients, and found using a different large dataset that, “the impact of donor risk varies dramatically with respect to recipient...age.” However, unlike their work (which compared associated risks of organ quality between age groups above and below 60 years) our study has examined the effect of organ quality within age groups. Furthermore, because our study sub-stratified recipients 60 years and over by decade, we were able to discern interaction between donor and recipient factors that were not detected when grouping recipients into above vs. below 60 years. In our analysis, we found that recipients 60–69 years old did derive a statistically significant graft and recipient survival benefit from medium compared to low quality kidneys, whereas patients older than 69 years did not.
We believe there are several important implications of our findings. Transplant programs have heightened regulatory oversight compared to other medical and surgical disciplines, with the risks of “underperformance” potentially leading to some centers to choose more risk-averse behaviors including declining offers from organs of low or very low quality. (14) Our results suggest that the utilization of these organs in older recipients may occur without significant detriment to outcomes. It is also known that kidneys with a higher KDPI have an increased likelihood of being discarded, with recent estimates being between 15–30% for KDPI scores of 61st-80th percentile (i.e. our “low quality” group). (15) However, our results suggest that some of these organs with an increased risk for discard may be used safely in older recipients leading to an improved utilization and potentially shorter waiting times.
Renal transplantation in older recipients not only improves morbidity and mortality (16) but also has been shown to impact quality of life. (17) One single-institution study found that non-diabetics had a significantly higher health-related quality of life following renal transplantation. (18) Given that co-morbid diabetes is lower among older recipients, it is possible that this finding may be generalizable to a large number of this population. Our findings may also aid in educating older recipients regarding the relative hazards associated with the transplantation of select donor organs of lesser quality.
The introduction of organ quality metrics into national organ allocation policy has the potential to dictate the availability of organs to certain age groups. Although the KDPI’s prediction value has been described as having moderate predictive accuracy, it is worth cautioning that the quantitative risks of graft loss vary among recipient subgroups. It is therefore valuable to consider these differences when advising patients and accepting organ offers in light of the available evidence. Although our analysis does not account for every possible risk factor associated with kidney transplant, we do hope to better characterize the risks of death and graft loss empirically associated with different levels of organ quality as measured by the KDPI. We emphasize here that for older recipients all decreased quality organs (as defined by the KDPI) do not translate to similar alterations in risk of graft loss or death as they do for younger recipients.
Because a smaller number of transplants occur in patients over 70 years, it is possible that our findings imply a type II statistical error. However, our study had in each cohort of age and organ quality a sufficient number of events to detect a statistically significant difference. Additionally, the measure we used for organ quality does not include every quality or risk factor related to graft failure, but only those currently included in the KDPI. Another potential explanation for our findings could be center-level volume and experience differences in the transplantation of recipients > 70 years. However, because our data set did not provide information at the institutional level, we were unable to analyze this as a potential cause. Moreover, we did not seek to quantify the survival benefits afforded by the transplantation of different levels of organ quality in each subgroup. We believe this is an important clinical question worthy of further study.
In conclusion, we report that low quality deceased donor kidneys as measured by the KDPI have no additional relative hazard of graft loss or mortality in recipients 70 years and over. This information should be used to inform clinicians and older candidates on the benefits of transplantation of low quality deceased donor kidneys and to assist in guiding national organ allocation policy.
Materials and Methods
We utilized United Network for Organ Sharing (UNOS) data as provided in the Standard Research and Analysis (STAR) file to analyze outcomes in 137,311 adult patients who received deceased donor renal transplants between January 1, 1995 and December 31, 2010 with follow-up through October 12, 2012. Exclusion criteria included recipients younger than 18 years of age, those with any prior organ transplant, recipients of en-bloc renal transplants, multi-organ transplants, and those for whom a donor Kidney Donor Risk Index (KDRI) could not be calculated.
Independent variables included donor organ quality represented by KDPI quintiles (i.e., “very high quality”: KDRI 0.34–0.94, KDPI 1st-20th percentile; “high quality”: KDRI 0.95–1.11, KDPI 21st-40th percentile; “medium quality”: KDRI 1.12–1.30, KDPI 41st-60th percentile; “low quality”: KDRI 1.31–1.53, KDPI 61st-80th percentile; and, “very low quality”: KDRI 1.54–3.19, KDPI 81st-100th percentile). Donor factors entering into the calculation of the KDRI are listed in SDC, Table 1. Cold ischemia time was also considered as potentially predictive of outcomes but was not included in the analysis because there were no significant differences across organ quality groups. Primary outcomes were graft failure (counted as return to dialysis and recipient death), death-censored graft failure or DWFG (where only return to dialysis was counted as graft failure), and overall recipient survival. We divided recipients into age-specific strata by decade for independent analyses.
Separate multivariable Cox proportional hazards models were used to assess relative risks of donor organ quality by age group on dependent variables and to control for covariates. We considered for inclusion fourteen recipient- and transplant-specific covariates including: sex, ethnicity, cause of renal disease, dialysis vintage, hypertension, diabetes, angina, cerebrovascular disease (CVD), peripheral vascular disease (PVD), anti-HCV positivity (HCV), functional status, body mass index (BMI) category, peak panel reactive antibody (PRA), and organ quality category. Stepwise backward selection was used to create a unique model for each age stratum and outcome with criteria for inclusion being p<0.05.
All statistical analyses were performed with JMP, Version 10. SAS Institute Inc., Cary, NC, 1989–2007.
Acknowledgments
This work was supported in part by Health Resources and Services Administration contract 234-2005-37011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Abbreviations
- CVD
Cerebrovasular disease
- DWFG
Death-with-functioning-graft
- ESRD
End-stage renal disease
- ECD
Extended-criteria donor
- HR
Hazard ratio
- CI
confidence interval
- HCV
Hepatitis C
- KDPI
Kidney Donor Profile Index
- KDRI
Kidney Donor Risk Index
- PRA
Panel reactive antibody
- PVD
Peripheral vascular disease
- SCD
Standard-criteria donor
- STAR
Standard Transplant Analysis and Research
- UNOS
United Network for Organ Sharing
Footnotes
Disclosure:
The authors of this manuscript have no conflicts of interest to disclose to Transplantation.
References
- 1.Knoll GA. Is kidney transplantation for everyone? The example of the older dialysis patient. Clin J Am Soc Nephrol. 2009 Dec;4(12):2040–4. doi: 10.2215/CJN.04210609. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999 Dec 2;341(23):1725–30. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Renal Data System. USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of diabetes and Digestive and Kidney Disease; Bethesda, MD: 2011. [Google Scholar]
- 4.Rao PS, Merion RM, Ashby VB, et al. Renal transplantation in elderly patients older than 70 years of age: results from the Scientific Registry of Transplant Recipients. Transplantation. 2007 Apr 27;83(8):1069–74. doi: 10.1097/01.tp.0000259621.56861.31. [DOI] [PubMed] [Google Scholar]
- 5.Sung RS, Guidinger MK, Leichtman AB, et al. Impact of the expanded criteria donor allocation system on candidates for and recipients of expanded criteria donor kidneys. Transplantation. 2007 Nov 15;84(9):1138–44. doi: 10.1097/01.tp.0000287118.76725.c1. [DOI] [PubMed] [Google Scholar]
- 6.OPTN/UNOS Kidney Transplantation Committee Report to the Board of Directors; November 12–13, 2012; Saint Louis, MO. [Google Scholar]
- 7.Rao PS, Schaubel DE, Guiding MK, et al. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009 Jul 27;88(2):231–6. doi: 10.1097/TP.0b013e3181ac620b. [DOI] [PubMed] [Google Scholar]
- 8.Metzger RA, Delmonico FL, Feng S, et al. Expanded criteria donors for kidney transplantation. Am J Transplant. 2003;3( Suppl 4):114–25. doi: 10.1034/j.1600-6143.3.s4.11.x. [DOI] [PubMed] [Google Scholar]
- 9. [Accessed 9/19/2013];OPTN/UNOS Board approved significant revisions to deceased donor kidney allocation policy. OPTN Press Release. June 25, 2013. Available at: http://optn.transplant.hrsa.gov/news/newsDetail.asp?id=1600.
- 10.Chavalitdhamrong D, Gill J, Takemoto S, et al. Patient and graft outcomes from deceased donors age 70 years and older: an analysis of the Organ Procurement Transplant Network/United Network of Organ Sharing database. Transplantation. 2008 Jun 15;85(11):1573–9. doi: 10.1097/TP.0b013e31817059a1. [DOI] [PubMed] [Google Scholar]
- 11.Tullius SG, Tran H, Guleria I, et al. The combination of donor and recipient age is critical in determining host immunoresponsiveness and renal transplant outcome. Ann Surg. 2010 Oct;252(4):662–74. doi: 10.1097/SLA.0b013e3181f65c7d. [DOI] [PubMed] [Google Scholar]
- 12.Tullius SG, Milford E. Kidney allocation and the aging immune response. N Engl J Med. 2011 Apr 7;364(14):1369–70. doi: 10.1056/NEJMc1103007. [DOI] [PubMed] [Google Scholar]
- 13.Heaphy EL, Goldfarb DA, Poggio ED, et al. The impact of deceased donor kidney risk significantly varies by recipient characteristics. Am J Transplant. 2013 Apr;13(4):1001–11. doi: 10.1111/ajt.12154. [DOI] [PubMed] [Google Scholar]
- 14.VanWagner LB, Skaro AI. Program-specific Reports: Implications and Impacton Program Behavior. Curr Opin Organ Transplant. 2013 Apr;18(2):210–215. doi: 10.1097/MOT.0b013e32835f07f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart D, Samana C, Cherikh, et al. Has Displaying KDPI in DonorNet Led to a Spike in Discard Rates for Lower Quality Kidneys? American Transplant Congress Meeting Abstracts. Abstract number 301. Am J Transplant. 2013 May;13(5) [Google Scholar]
- 16.Macrae J, Friedman AL, Friedman EA, et al. Live and deceased donor kidney transplantation in patients aged 75 years and older in the United States. Int Urol Nephrol. 2005;37(3):641–8. doi: 10.1007/s11255-004-0010-6. [DOI] [PubMed] [Google Scholar]
- 17.Humar A, Denny R, Matas AJ, et al. Graft and quality of life outcomes in older recipients of a kidney transplant. Exp Clin Transplant. 2003 Dec;1(2):69–72. [PubMed] [Google Scholar]
- 18.Dukes JL, Seelam S, Lentine KL, et al. Health-related quality of life in kidney transplant patients with diabetes. Clin Transplant. 2013 Aug 1; doi: 10.1111/ctr.12198. [DOI] [PubMed] [Google Scholar]