Abstract
Two independent biological replicates of estrogen depletion were employed with differing drug treatment conditions. Data Set I consisted of 9-month-old New Zealand white female rabbits treated as follows: sham-operated (n=11), ovariectomized (OVX; n=12), OVX+200 μg kg−1 alendronate (ALN), 3 × a week for 27 weeks (n=12) and OVX+10 mg kg−1 Cathepsin-K inhibitor (CatKI) daily for 27 weeks. Data Set II consisted of 6-month-old New Zealand white female rabbits that were sham-operated (n=12), OVX (n=12) or OVX+0.05 mg kg−1 17β-estradiol (ERT) 3 × a week for 13 weeks (n=12). Samples from the cortical femur were polished and demineralized to make them suitable for atomic force microscopy (AFM) imaging. Type I collagen fibrils present in bundles or sheets, running parallel to each other, were combined into a class termed Parallel. Fibrils present outside of such structures, typically in images with an angular range of non-parallel fibrils, were combined into a class termed Oblique. The percentage of fibrils coded as Parallel for Sham animals in Data Sets I and II was 52% and 53%, respectively. The percentage of fibrils coded as Parallel for OVX animals in Data Sets I and II was 35% in both cases. ALN and ERT drug treatments reduced the change from 18 to 12%, whereas CatKI treatment reduced the change to 5%.
Introduction
Reduction in estrogen level affects over 75 million people worldwide by inducing Osteoporosis and Osteopenia, decreasing overall bone quality and increasing susceptibility to fracture.1,2 Clinical and scientific assessments have focused heavily on bone mineral density (BMD) as the primary assay of bone quality and drug treatment outcomes. However, BMD has demonstrated limitations for predicting bone fracture and understanding the roles of estrogen in bone quality, formation and resorption.3,4,5 In particular, BMD is unable to assess changes in Type I collagen structure that are keys to understanding bone toughness. The structural changes that occur in Type I collagen matrix under conditions of estrogen depletion and drug treatment remain largely unknown. Here we show that the Type I collagen microstructure is altered by estrogen depletion and that this change is largely prevented by treatment with a Cathepsin-K inhibitor (CatKI) and partially prevented with bisphosphonate or estradiol (ERT) treatment. These observations are particularly surprising for the bisphosphonate drug, which was developed to help maintain BMD and represents a new understanding of biochemical activity for this decade-old therapeutic. The ability of an inhibitor of the collagenase cathepsin-K to prevent estrogen-induced changes in Type I collagen microstructure is also previously unknown.
Results
Changes in Type I collagen microstructure and nanomorphology are conveniently measured using atomic force microscopy (AFM).6,7 Recent AFM studies indicated changes in the nanomorphology of the Type I collagen fibrils in ovariectomized (OVX) sheep8,9,10 and rats.11 In order to explore the effect of estrogen depletion and compare efficacy of CatKI and alendronate (ALN) drug treatments, the rabbit OVX model was developed by Merck Inc., as human CatKI's exhibit similar potency for the rabbit enzyme and the adult rabbit skeleton undergoes substantial cortical Haversian remodeling (unlike the rodent).12 Nine-month-old New Zealand white female rabbits (Data Set I) underwent the following treatments: sham operation+vehicle (n=11), ovariectomy+vehicle (OVX; n=12), OVX+200 μg kg−1 ALN, 3 × a week for 27 weeks (n=12) and OVX+10 mg kg−1 CatKI L-235 daily for 27 weeks. Treatments were initiated 3 days after surgery. Data Set II consisted of 6-month-old New Zealand white female rabbits that underwent the following treatments: sham operation+vehicle (n=12), ovariectomy+vehicle (OVX; n=12) or OVX+0.05 mg kg−1 ERT 3 × a week for 13 weeks (n=12). Treatments were initiated 17 days after surgery. Both studies were performed in prevention mode with drug dosing initiated soon after surgery. Sample sets were stored in 95% ethanol. Rabbit cortical femur samples were polished for 90 s, followed by sonication for 5 min. Demineralization was carried out for 90 min (9-month old) or 120 min (6-month old) using 0.5 M EDTA at pH 8.0. The samples were sonicated for 5 min before imaging. We confirmed that ethanol fixation did not change the overall distribution of collagen fibril D-spacings or microstructure by direct comparison with the analysis of fresh, frozen rabbit bone. AFM images were obtained using tapping mode in air using silicon cantilevers (tip radius 10 nm, force constant 40 N m−1 and resonance frequency 300 kHz). Details on sample preparation, imaging and image analysis protocols for Type I collagen in bone have been previously published.6 Images were obtained from locations from across the full 1.00 × 0.75 cm section of the femur mid-diaphysis (Supplementary Figure S1). All images were acquired in the plane parallel to the long bone axis and obtained from polished regions 100–300 μm below the bone surface. No variation in the collagen structure was noted as a function of the polishing depth employed. Image acquisition for the mid-diaphysis sections proceeded using the following procedure. First, 30 × 30-μm scans were obtained in six regions of the 1.00 × 0.75-cm bone imaging area. These were followed by 10 × 10-μm scans, and finally the 3.5 × 3.5 μm scans employed for image analysis. This approach ensured that the nano- to-micro-scale analysis of collagen fibrils was distributed across the 0.75 × 1.0-cm imaging area. An average of six 30 × 30-μm scans and thirteen 3.5 × 3.5-μm scans were obtained per animal. From these regions, the average of 75 fibrils per animal was obtained. The local microstructure about each fibril was examined and coded as either Parallel or Oblique. In a given image area, it is thus possible for both Parallel and Oblique fibrils to be present. Furthermore, the assignment is indicative of a local order between fibrils with a length scale of a few hundred nanometers. The assessment does not provide evidence, one way or the other, of overall micron- to millimeter-scale order as typically measured using X-ray diffraction methods.
Exemplar images highlighting the features observed in the local structural organization of the fibrils are illustrated in Figure 1. We noted two major qualitative features in the images: (1) Parallel regions of fibrils with subclasses of bundles (3–15 fibrils aligned in parallel with defined edges) and sheets (>20 fibrils aligned in a planar, parallel manner); (2) Oblique regions of fibrils exhibiting an angular range of fibril orientation. For all samples, the nanomorphology of individual fibrils, as quantified using the metric of the D-spacing, was measured, and each fibril was coded (blinded of the sample origin) for its local microstructure. Fibrils coded as present in bundles or sheets (Figure 1a) were grouped together as Parallel regions. A number of other features were also coded including fibril pairs and individual fibrils crossing bundles or sheets (Supplementary Figure S2). Roughly half of the fibrils observed were present in structures without the same degree of parallel alignment, exhibited a wide range of fiber–fiber angular dispersion and were classified as being present in the Oblique regions (Figure 1b). A total of 5673 individual fibrils were classified from 1081 3.5 × 3.5-μm images from a total of 94 animals across the seven experimental groups. The coding and frequency of Parallel and Oblique observations are summarized in Table 1. In all cases, the Parallel and Oblique codings captured ⩾95% of all measured fibrils. Additional exemplar images for the Sham, OVX, ALN, CatKI and ERT treatments are provided in Supplementary Figure S4.
Figure 1.
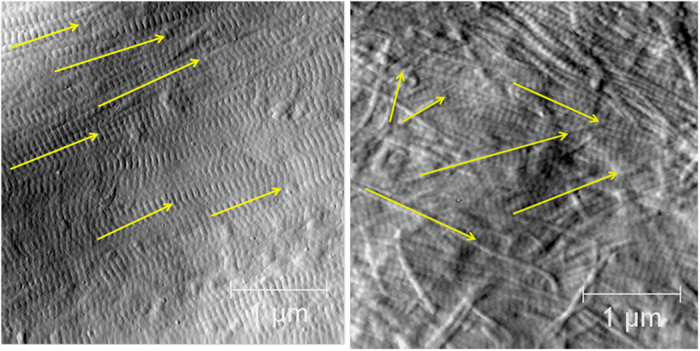
AFM images illustrating Parallel and Oblique regions of Type I collagen fibrils. (a) Parallel region showing multiple aligned fibrils (yellow arrows); (b) oblique region showing multiple fibrils with varying alignment (yellow arrows). 3.5 × 3.5 μm image.
Table 1. Frequency of Type I collagen fibrils observed in Parallel and Oblique microstructures.
| Treatment | No. of fibrils | No. of animals | Frequency in parallel microstructures (%, s.e.) | Frequency in oblique microstructures (%, s.e.) | MicrostructureFrequency difference |
|---|---|---|---|---|---|
| Data Set I | |||||
| Sham | 825 | 11 | 52 (5) | 45 (5) | 7(10) |
| OVX | 808 | 12 | 35 (5) | 63 (5) | −28 (10) |
| ALN | 971 | 12 | 40 (5) | 57 (5) | −17 (10) |
| CatKI | 1001 | 13 | 47 (5) | 49 (5) | −2 (10) |
| Data Set II | |||||
| Sham | 579 | 12 | 53 (5) | 42 (5) | 11 (10) |
| OVX | 728 | 12 | 35 (5) | 62 (5) | −27 (10) |
| ERT | 761 | 12 | 41 (4) | 58 (4) | −17 (10) |
Abbreviations: ALN, alendronate; CatKI, Cathepsin-K inhibitor; ERT, estradiol; OVX, ovariectomized.
To assess the significance of the differences in proportion of fibrils that are in Parallel and Oblique regions by the treatment group, we employed a logistic regression model using generalized estimating equations (GEEs),13 with correlations between any two pairs of observations within the same animal being constant, estimated from the data. The proportion of each type of fibril was compared for each treatment versus sham using a binary outcome, Yij, where Yij=1 if the fibril was in a given microenvironment (that is, Parallel or Oblique) and Yij=0 if the fibril was not, with a logit link function. All s.e.'s were computed using the robust Huber–White estimates. The GEE model that was employed is shown below:
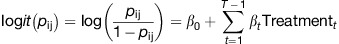 |
where pij=probability (Yij=1) for fibril I in animal j, β0 is the intercept and βt fits the effect of treatment t, t=1 to T, with treatment T being the reference category.
Post hoc comparisons of collagen fibril orientations were then carried out between treatment groups, using a Bonferroni correction for multiple comparisons with statistical significance assigned for P-values below 0.05.
Compared with Sham, the OVX-treated animals in Data Set I had a 17% decrease (P-value=0.0045) in the proportion of fibrils occurring in Parallel structures and an 18% increase (P-value=0.0026) in the proportion of fibrils occurring in Oblique structures. ALN treatment was previously observed to function as an antiresorptive leading to a BMD higher than that in the vehicle-treated group.12 In this study, ALN treatment resulted in a frequency of collagen fibril observations intermediate between Sham and OVX values, although the differences in proportions of fibrils in Parallel and Oblique regions in these treatments were not significant. The bone resorption inhibitor CatKI also gave intermediate values for the frequency of collagen fibril observations and showed even less shift from the initial Sham values, with none of the comparisons versus other treatments being significant. Statistically, the changes in the proportion of fibrils in Parallel and Oblique regions for the drug-treated sample were not significantly different from the Sham samples. These data indicate that the drugs prevent the OVX-induced change in the collagen microstructure in addition to their traditionally understood roles of preventing decreases in BMD. For the OVX treatment in Data Set II, an 18% decrease (P-value=0.0188) was observed in the proportion of fibril occurrence in Parallel structures and a 20% increase (P-value=0.0048) was observed in the proportion of fibril occurrence in Oblique structures compared with Sham. ERT treatment resulted in a partial prevention of change in the collagen microstructure, although the difference in ERT versus Sham in proportion of Parallel region fibrils was not significant (P=0.0961), whereas the difference in the proportion of Oblique fibrils was significant (0.0185). The magnitude of observed changes was similar to ALN treatment and less effective compared with CatKI treatment in preventing microstructure change. Analysis of the D-spacing variation for all seven samples showed a range of values between ∼58 and 70 nm, consistent with previous observations of D-spacing distribution.6,8,14 However, no shift of the D-spacing values was observed for these samples, unlike the previous comparison for Sham and OVX sheep samples (Supplementary Figure S3).8,9
Discussion
OVX is a common model used to study the effects of estrogen depletion.8,9,15,16,17 Pennypacker et al.12 applied the OVX model to study osteopenia in rabbits using the samples described herein and demonstrated osteoclastic bone resorption resulting from estrogen depletion. On the basis of the strong positive correlations between the experimental strength parameters and the tissue bone mineral content from animals from the same study, it was demonstrated that there were no obvious treatment-related changes in the material properties of vertebrae and femurs in the OVX rabbits treated with odanacatib or ALN for 27 weeks. Here, we directly examined the effects of estrogen deficiency and two different bone resorption inhibitors at the microfibril structure level and observed changes in the relative frequency of fibril occurrence in local Parallel and Oblique microstructures. The link between increased resorption and the changes reported here to Type I collagen fibril microstructure remains unknown; however, related studies in the literature provide clues to possible mechanisms. In the mouse model, OVX reduces proteoglycan levels.11 Furthermore, biglycan and decorin knockout mice have abnormal collagen fibril morphologies.12 We hypothesize that reduced proteoglycan levels perturb the interactions between adjacent fibrils, resulting in a change to the microstructure. Cathepsin-K, a collagenase secreted by osteoclasts, cleaves proteoglycans from the fibril exterior before degrading the primary fibril structure.18 OVX results in increased osteoclast activity coupled to increased cathepsin-K activity. This is directly countered by the ERT therapy resulting in partial prevention of the collagen fibril microstructure changes. Strikingly, application of CatKI, which can prevent proteoglycan cleavage directly at the level of enzyme activity, gives almost complete prevention of fibril microstructure change. By way of contrast, treatment with a bisphosphonate is known to induce a change to osteoclast cell morphology and eventual cell death.19 Thus, ALN reduces the amount of bone resorption, but also indirectly lowers the activity of cathepsin-K enzyme, which is consistent with observation that ALN treatment partially prevents changes to Type I collagen microstructure. Recently, Reznikov et al.20 reported that the human lamellar bone consists of two different materials that differ substantially in terms of collagen fibril order. The connection between our fibril-level local organization classification (Parallel versus Oblique) and their observation of ‘ordered' and ‘disordered' materials is not clear at this time; however, changes in the relative quantities of the ‘ordered' and the ‘disordered' materials as a function of estrogen depletion and drug treatment are also a possible explanation of our data. These changes could also be related to the impacts of proteoglycan levels as discussed above.
In summary, a quantitative analysis of Type I collagen fibril organization indicates a significant decrease in fibrils appearing in Parallel structures such as bundles and sheets (18%) and a concomitant increase in fibrils appearing in Oblique structures, on OVX treatment of 6- and 9-month-old rabbits. ALN and ERT drug treatments partially prevent the microstructure changes (12% decrease in Parallel structures), and CatKI treatment almost completely prevents the structural change (5% decrease in Parallel structures). The change in Type I collagen microstructure induced by OVX is a previously unrecognized aspect of the impact of estrogen depletion on bone quality. In addition, the amelioration of these microstructure changes is a previously unrecognized mechanism of drug activity.
Materials and Methods
All rabbit femur samples were obtained from Merck Research Laboratory, West Point, PA, USA, and treatment of these animals was previously described in detail.12 Sample preparation and the AFM imaging methods employed in this work have also been described in detail.6 All imaging was carried out in air using a PicoPlus 5500 AFM (Agilent, Santa Clara, CA, USA) employing tapping mode with VistaProbes T300R probes (NanoScience, Phoenix, AZ, USA; nominal radius 10 nm, force constant 40 N m−1, resonance frequency 300 kHz). Line scan rates were set at 2 Hz or lower at 512 lines per frame. Image analysis and measurements were performed using the SPIP software (Image Metrology, Horsholm, Denmark).
Supplementary Material
Acknowledgments
This work was supported in part by an IISP grant from Merck Inc. to MMBH. We thank MD Morris and CM Les for many fruitful discussions on the impact of estrogen depletion and bisphosphonate treatment on bone. We thank HG Bone for fruitful discussions including suggesting the CatKI experiment, bringing to the attention of the UM group the unpublished Sham, OVX, CatKI, ALN and ERT studies of Pennypacker et al.12 and introducing the UM group to the Bone Biology group at Merck Inc.
Footnotes
LTD and BLP work for Merck Inc. The remaining authors declare no conflict of interest.
References
- Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006; 17: 1726–1733. [DOI] [PubMed] [Google Scholar]
- Viguet-Carrin S, Garnero P, Delmas PD. The role of collagen in bone strength. Osteoporos Int 2006; 17: 319–336. [DOI] [PubMed] [Google Scholar]
- Burr DB. The contribution of the organic matrix to bone's material properties. Bone 2002; 31: 8–11. [DOI] [PubMed] [Google Scholar]
- Cranney A, Guyatt G, Griffith L, Wells G, Tugwell P, Rosen C et al. Summary of meta-analyses of therapies for postmenopausal osteoporosis. Endocr Rev 2002; 23: 570–578. [DOI] [PubMed] [Google Scholar]
- Sornay-Rendu E, Delmas PD. Advances in osteoporosis diagnosis: the use of clinical risk factors. Mediographica 2008; 30: 350–554. [Google Scholar]
- Erickson B, Fang M, Wallace JM, Orr BG, Les CM, Banaszak Holl MM. Nanoscale structure of type I collagen fibrils: quantitative measurement of D-spacing. Biotechnol J 2013; 8: 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M, Goldstein EL, Turner AS, Les CM, Orr BG, Fisher GJ et al. Type I collagen D-spacing in fibril bundles of dermis, tendon, and bone: bridging between nano- and micro-level tissue hierarchy. ACS Nano 2012; 6: 9503–9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JM, Erickson B, Les CM, Orr BG, Banaszak Holl MM. Distribution of type I collagen morphologies in bone: relation to estrogen depletion in bone. Bone 2010; 46: 1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M, Liroff KG, Turner AS, Les CM, Orr BG, Banaszak Holl MM. Estrogen depletion results in nanoscale morphology changes in dermal collagen. J Invest Dermatol 2012; 132: 1791–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M, Banaszak Holl MM. Variation in type I collagen fibril nanomorphology: the significance and origin. BoneKey 2013; 2: 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafantari H, Kounadi E, Fatourous M, Milonakis M, Tzaphlidou M. Structural alterations in rat skin and bone collagen fibrils induced by ovariectomy. Bone 2000; 26: 349–353. [DOI] [PubMed] [Google Scholar]
- Pennypacker BL, Duong LT, Cusick TE, Masarachia PJ, Gentile MA, Gauthier JY et al. Cathepsin K inhibitors prevent bone loss in estrogen-deficient rabbits. J Bone Miner Res 2011; 26: 252–262. [DOI] [PubMed] [Google Scholar]
- Diggle PJ, Heagerty P, Liang K-Y, Zeger SL. Analysis of Longitudinal Data Oxford University Press2002, . [Google Scholar]
- Wallace JM, Chen Q, Fang M, Erickson B, Orr BG, Banaszak Holl MM. Type I collagen exists as a distribution of nanoscale morphologies in teeth, bones, and tendons. Langmuir 2010; 26: 7349–7354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost HM, Jee WSS. On the rat model of human osteopenias and osteoporosis. Bone Miner 1992; 18: 227–236. [DOI] [PubMed] [Google Scholar]
- Kalu DN. The ovariectomized rat model of postmenopausal bond loss. Bone Miner 1991; 15: 175–191. [DOI] [PubMed] [Google Scholar]
- Smith SY, Jolette J, Turner CH. Skeletal health: primate model of postmenopausal osteoporosis. Am J Primatol 2009; 71: 752–765. [DOI] [PubMed] [Google Scholar]
- Panwar P, Du X, Sharma V, Lamour G, Castro M, Li HB et al. Effects of cysteine proteases on the structural and mechanical properties of collagen fibers. J Biol Chem 2013; 288: 5940–5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers MJ, Crockett JC, Coxon FP, Monkkonen J. Biochemical and molecular mechanisms of action of bisphosphonates. Bone 2011; 49: 34–41. [DOI] [PubMed] [Google Scholar]
- Reznikov N, Shahar R, Weiner S. Three-dimensional structure of human lamellar bone: the presence of two different materials and new insights into the hierarchical organization. Bone 2014; 59: 93–104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


