Abstract
In the mouse retina, dopaminergic amacrine (DA) cells synthesize both dopamine and GABA. Both transmitters are released extrasynaptically and act on neighbouring and distant retinal neurons by volume transmission. In simultaneous recordings of dopamine and GABA release from isolated perikarya of DA cells, a proportion of the events of dopamine and GABA exocytosis were simultaneous, suggesting co-release. In addition, DA cells establish GABAergic synapses onto AII amacrine cells, the neurons that transfer rod bipolar signals to cone bipolars. GABAA but not dopamine receptors are clustered in the postsynaptic membrane. Therefore, dopamine, irrespective of its site of release—synaptic or extrasynaptic—exclusively acts by volume transmission. Dopamine is released upon illumination and sets the gain of retinal neurons for vision in bright light. The GABA released at DA cells' synapses probably prevents signals from the saturated rods from entering the cone pathway when the dark-adapted retina is exposed to bright illumination. The GABA released extrasynaptically by DA and other amacrine cells may set a ‘GABAergic tone’ in the inner plexiform layer and thus counteract the effects of a spillover of glutamate released at the bipolar cell synapses of adjacent OFF and ON strata, thus preserving segregation of signals between ON and OFF pathways.
Keywords: dopamine, GABA, extrasynaptic release, retina
1. Introduction
The retina is a region of the nervous system that has offered a unique access to the computations carried out by its neural networks because of its physical location, the regularity of its architecture, the distinctive morphology of its neurons and a knowledge of their inputs and outputs. It is for these reasons that we have an understanding of the precise function of extrasynaptic release of transmitters.
Upon light stimulation of the retina, a class of retinal neurons, the dopaminergic amacrine (DA) cells, release dopamine, a catecholamine modulator responsible for many of the events that lead to neural adaptation to light [1,2]. In cold-blooded animals, dopamine induces contraction of cones and movement of melanin granules in pigment epithelial cells [3,4]. In all studied vertebrates, dopamine decreases the conductance of the gap junctions between horizontal cells and thus reduces the size of their receptive field [5–7]; it also potentiates the activity of ionotropic glutamate receptors in both horizontal [8] and bipolar cells [9]; and, by acting on the gap junctions between AII amacrine cells, modifies both the spontaneous activity of ganglion cells and the centre–surround balance of their receptive field [10].
It was clear from these studies that dopamine is released upon illumination of the retina and controls the transition from scotopic (rod) to photopic (cone) vision by setting the gain of the retina for computation of bright light signals. It was also suggested that dopamine had to act by paracrine or volume transmission, because the dopamine receptors had a wider distribution in the retina than the processes of DA cells. As a result, dopamine has to travel through a distance of tens of micrometres from the closest processes of DA cells to reach the outer segments of photoreceptors and the pigment epithelium [2].
By contrast, however, to the wealth of information available on the pharmacological effects of dopamine on retinal neurons, little was known about the cellular mechanisms that control the release of this neurotransmitter in dark and light. To describe the precise anatomy of DA cells and record from them physiologically, we generated a line of transgenic mice in which dopaminergic neurons were labelled genetically by human placental alkaline phosphatase [11], developed a technique for single cell mRNA amplification and used cDNA screening to identify the transcripts that are components of DA cells' expression profile [12].
2. Structure and connectivity
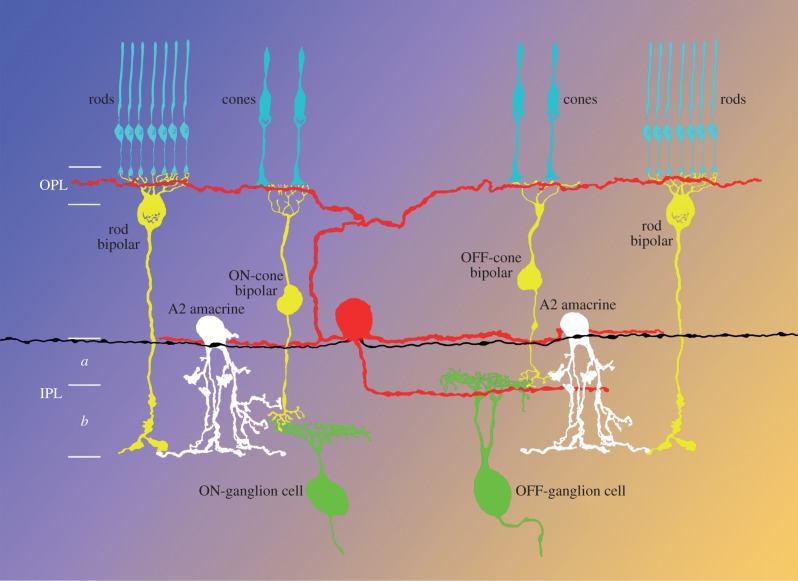
Anatomically, the mouse retina contains 575 ± 29 DA cells at a density of 44 ± 4 mm–2. Their cell body occupies the most vitreal tier of the inner nuclear layer and gives rise to three dendritic plexus, situated, respectively, in the outer plexiform layer (OPL) and strata 1 (S1) and 3 (S3) of the inner plexiform layer (IPL; figure 1). One to five axons originate from the cell body or the proximal dendrites and run an irregular course in S1, branching sparingly. The orientation of both dendritic and axonal branches is random, but rigorously tangential and confined to their respective layers (figure 2).
Figure 1.
Diagram of the distribution of the processes of the DA cell (red). OPL, outer plexiform layer; IPL, inner plexiform layer; a, sublamina OFF; b, sublamina ON.
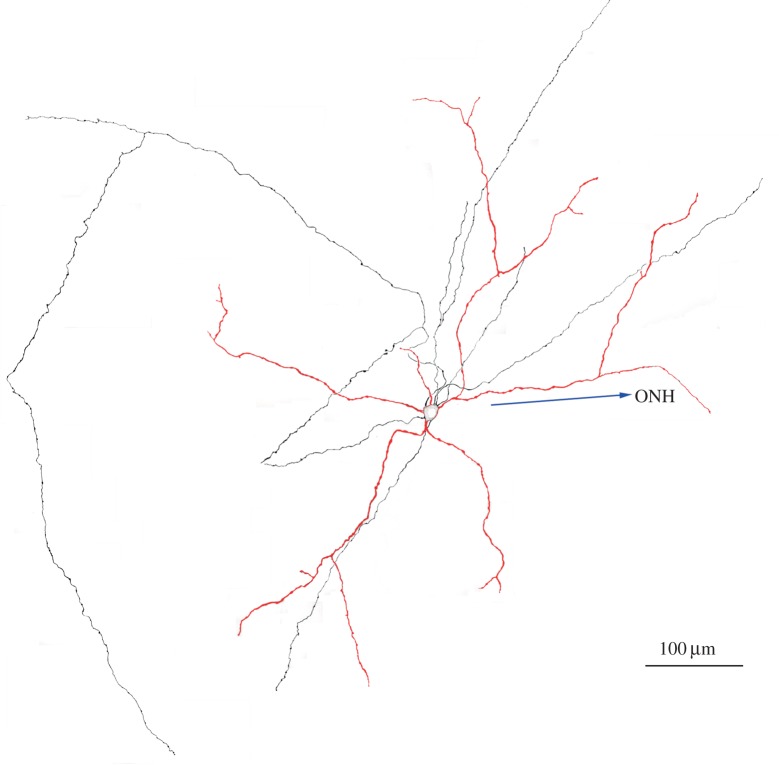
Figure 2.
A DA cell injected with neurobiotin was reconstructed from a montage of confocal images after treatment with a fluorescent antibody to the tracer. Only the cell body and the processes lying in stratum 1 of the inner plexiform layer are included in the figure. Dendrites and cell body are red, axons are black. The arrow points to the optic nerve head (from unpublished work in collaboration with B Lin and RH Masland).
DA cells receive excitatory glutamatergic input from ON-bipolar cells at reciprocal dyad synapses in stratum 3 of the IPL [13], and the source of their light responses is probably the ON-bipolars CB3n and/or CBb3 [14]. Recordings from DA cells have indeed confirmed that light excites a proportion of these neurons and causes a transient or sustained increase in the frequency of their spontaneous activity [15].
DA cells receive input from GABAergic amacrine cells in stratum 1 of the IPL [16–19]. Five types of amacrine cells, one narrow-field, one medium-field and three wide-field [20], are confined to S1 and they receive their input from three types of OFF-bipolars [14]. This GABAergic input reduces the spontaneous activity of DA cells in the dark [15].
DA cell processes in stratum 1 of the IPL make numerous synapses on the cell body of AII amacrine cells [21], the neurons inserted in series along the rod pathway. Originally interpreted by others and us as presynaptic dendrites, we have now demonstrated that they actually represent axonal endings of DA cells. At the site of these synapses, three markers: GABA, vesicular GABA transporter (VGAT) and vesicular monoamine transporter (VMAT2) are localized in the presynaptic ending, in perfect register with clusters of GABAA receptors in the postsynaptic membrane [22]. Interestingly, dopamine receptors do not form clusters in the postsynaptic membrane, thus confirming previous studies that reported the absence of either D1 [23,24] or D2/3 [25] receptors on the soma of AII amacrine cells.
Finally, the processes of DA cells in the outer plexiform layer only exceptionally establish synaptic contacts, but contain clusters of secretory organelles that are stained by antibodies to VMAT2 and VGAT [26].
3. Extrasynaptic release of dopamine and GABA
A most interesting observation was that DA cells possess a pacemaker activity and thus fire in vitro action potentials in a slow, rhythmic pattern [11,27], a property also found in dopaminergic neurons of the substantia nigra and ventral tegmental area of the midbrain [28–30]. We studied the constellation of voltage-gated channels responsible for this behaviour, as well as the effects of some neurotransmitters on the firing pattern, and formulated the hypothesis that the action potentials spread throughout the DA cell processes in the inner and outer plexiform layers and cause dopamine release.
Indeed, we recorded by amperometry dopamine oxidation currents from genetically identified, isolated cell bodies of DA cells using a carbon fibre electrode in contact with the cell membrane [31,32]. We found that solitary perikarya of dopaminergic neurons spontaneously released packets of 103–105 dopamine molecules at irregular intervals and low frequency. Because presynaptic active zones are absent in the cell body of DA cells, this release was by necessity extrasynaptic. The release events could be induced by depolarization of the cell membrane and were dependent upon the entry of extracellular Ca2+: thus, they were caused by exocytosis. This spontaneous release was triggered by the action potentials that DA cells generate in a rhythmic fashion upon removal of all synaptic influences. Thus, extrasynaptic dopamine release by DA cells was controlled by their pacemaker activity.
The immunocytochemical evidence that the synapses between DA cells and AII amacrines were GABAergic suggested to us that the dopaminergic neurons of the retina represent another instance of co-localization of dopamine with other low molecular mass transmitters that act on ionotropic postsynaptic receptors and therefore convey faster synaptic signals to the postsynaptic cell [33,34]. Midbrain dopaminergic neurons make excitatory glutamatergic synapses onto the projection neurons of the accumbens [35–38]. GABA or its critical biosynthetic enzyme glutamic acid decarboxylase (GAD) are present in a subpopulation of cells of the substantia nigra [39,40] and co-transmission of dopamine and GABA was observed in the periglomerular cells of the olfactory bulb [41]. We therefore investigated whether, in addition to dopamine, isolated DA cell perikarya released GABA extrasynaptically in the absence of presynaptic active zones [42].
DA cell perikarya express GABAA receptors on their surface, including the alpha4 subunit, which is exclusively extrasynaptic [43]. Furthermore, we showed that isolated perikarya respond to application of exogenous GABA with a chloride current mediated by GABAA receptors [44]. We therefore exploited the presence of these receptors on DA cells to measure by patch-clamp the Cl− current activated by the release of their own transmitter. When intracellular free Ca2+ was raised to 100 nM to evoke somatic release, the cells generated transient current events that were suppressed by antagonists of the GABAA receptors and thus appeared to represent episodes of GABA release. Further experiments supported the conclusion that upon GABA exocytosis, the transmitter diffused from the site of release into the surrounding solution and activated nearby native GABAA receptor channels randomly distributed over the plasma membrane of the DA cell. In fact: (i) DA cell perikarya contain organelles stained by antibodies to GABA [22], GAD [45–47], synaptobrevin 2 (VAMP2 [26]) and VGAT [48]. (ii) Examination of the surface of isolated DA cells by confocal microscopy, after staining with antibodies to both synapsin and gephyrin, ruled out the possibility that GABAergic endings presynaptic to DA cell bodies had survived the procedure of retinal dissociation. (iii) The frequency of the GABAergic currents depended on the concentration of intracellular free Ca2+ in the patch pipette and increased upon membrane depolarization.
The release was extrasynaptic because presynaptic active zones are absent in the perikarya of DA cells. Furthermore, it was caused by exocytosis, rather than by a transporter-mediated process, because (i) the events were transient on the millisecond time scale; (ii) the release of GABA was abolished by the treatment with bafilomycin A1, a blocker of the vesicular proton pump; and (iii) it was dependent on the intracellular Ca2+ concentration.
To establish whether such a mechanism could account for the transient waveform of the observed GABAergic currents, we built a mathematical model of the total current over the area of the plasma membrane surrounding the release site as a function of time and distance. This model predicted the amplitude and rise time of the observed current transients when the size of the source of GABA was set at 27 000 to 40 000 molecules, a quantal content of the same order of magnitude as that measured by amperometry in the case of large events of dopamine exocytosis (40 000 molecules [32]). This suggests that the GABA-containing organelles responsible for the current events detected in our experiments were larger than conventional 40 nm synaptic vesicles and could correspond to the tubules, cisterns, dense core vesicles and secretory granules that are commonly observed in electron micrographs of the cytoplasm of the perikarya of DA cells (see below). These considerations do not rule out the possibility that small GABAergic vesicles also release their contents at the cell surface, but small quanta of transmitter were below the resolution of our experimental probe.
Release of GABA was stimulated by depolarization of the cell membrane: among the stimuli applied, sustained (1 s) square pulses were the most effective in increasing the frequency of the discharge and trains of action potentials the least effective. These results are explained by the different effectiveness of the various stimuli in causing a surge of intracellular Ca2+ to the level required to trigger GABA release. With depolarizing stimuli, the critical features that distinguished extrasynaptic from synaptic GABA release were (i) long latency, (ii) low frequency and (iii) long duration of the discharge: most probably, these properties reflected a slow recruitment of organelles that were few in number and situated at a considerable distance from one another and from the cell membrane, as seen in specimens stained with anti-synapsin antibodies. Furthermore, with all modalities of stimulation, the events of GABA release exhibited considerable variability in their amplitude, probably a function of the distance of the activated GABAA receptors from the site of exocytosis, heterogeneity in the diameter of the organelles associated with exocytosis and/or variability in their transmitter contents.
4. Co-release of dopamine and GABA
In all instances of co-localization or co-release, different vesicular transporters, VMAT2 for dopamine, VGAT for GABA and the various VGLUT isoforms for glutamate, load the transmitters into membrane-bounded secretory organelles [33,49], either 40–50 nm synaptic vesicles or 80–90 nm large dense core vesicles (LDCV [50,51]). The question therefore arose whether in DA cells the two transmitters were stored within the same or separate organelles. To solve this problem, we used two approaches: (i) we combined patch-clamp and amperometry in simultaneous recordings of dopamine and GABA release from the same isolated DA cell body and (ii) we used immunocytochemistry with both confocal and electron microscopes to investigate the co-localization of VMAT2 and VGAT in cryosections of the mouse retina [48].
We observed that a proportion of the GABAergic and amperometric dopamine oxidation events appeared to coincide in time, and we generated a perievent time histogram by measuring the time intervals between GABAergic events and the immediately preceding and following dopaminergic events. The histogram peaked at a ±4 ms time window and showed that 16% of the amperometric events coincided with GABAergic events, because no peak would have occurred if association between the two types of currents had been completely random. The coincidence of GABA and amperometric events, however, could have been due to chance. To rule out this possibility, we first observed that the histograms of inter-event time intervals for dopamine and GABA release could be described with a single exponential fit: therefore, the release of both transmitters was based on a Poisson process. Then, under the null hypothesis of independence between the two types of events, we carried out a Monte Carlo simulation. In 104 trials, in no instance were we able to reproduce the number of coincidences observed experimentally. We therefore concluded that the coincidence of dopaminergic and GABAergic events was not due to chance and reflected release of the two types of transmitters from the same organelle.
To identify GABAergic and dopaminergic organelles in the cytoplasm of DA cell bodies, we obtained confocal images of cryostat sections of formaldehyde-fixed retinas after triple staining with antibodies to tyrosine hydroxylase, VGAT and VMAT2 and focused our attention to the perikarya of DA cells. We observed that three different populations of organelles were scattered throughout the cytoplasm of DA cells: one stained by the antibody to VGAT alone, one by the antibody to VMAT2 alone and one stained by both antibodies. We digitized the intensity of all pixels and used odds ratio statistics to measure the strength of the association between the two transporters. We could therefore rule out that the co-localization of VGAT and VMAT2 in a number of the cytoplasmic organelles of DA cells was due to biased sampling or chance superimposition and concluded (i) that a proportion of the secretory organelles in the cytoplasm of DA cells bodies contain both GABA and dopamine and (ii) that both transmitters are released simultaneously by exocytosis upon depolarization of the cell membrane.
What is the identity of the somatic organelles that release GABA and dopamine? Electron micrographs show that the DA cell cytoplasm contains secretory granules, 0.3–0.5 µm in diameter, and 100 nm LDCVs, in addition to a plethora of polymorphous membranous compartments that can be variously described as vesicles, tubules and cisterns. Our confocal images showed that the organelles that contained VMAT2, VGAT or varying amounts of both transporters exhibited apparent sizes of 0.3–1.4 µm and, therefore, probably corresponded to the granules and LDCVs present in the electron micrographs. It must be emphasized, however, that our identification of the immunoreactive organelles was biased towards structures larger than a single pixel. Indeed, the broad spectrum of sizes of the transmitter quanta and the fact that both their GABAergic and dopaminergic populations were skewed towards smaller events indicate that the secretory organelles were highly heterogeneous and had to include a proportion of the small membranous profiles visible with the electron microscope.
There is also evidence that synaptic vesicles in DA cells may contain both dopamine and GABA: as noted above, confocal microscopy had shown co-localization of VMAT2, VGAT and GABA in the axonal varicosities of DA cells [22], and we observed in electron micrographs of retinal cryosections that both synaptic vesicles and LDCVs were apparently labelled by antibodies to both transporters.
The relative contents of dopamine and GABA seem to vary dramatically among different secretory organelles. This is partly caused by the different sensitivity of the methods used to measure the size of the released quanta: amperometry is in fact very sensitive compared to recording current events after release, diffusion and binding of GABA to its receptors on the cell surface. Nevertheless, this variability must also reflect differences in the relative organelle concentration of the two transmitters. Indeed, some organelles seem to contain a single vesicular transporter, either VGAT or VMAT2, and others contain both, as demonstrated by immunocytochemistry. We have suggested previously [48] that the presence of variable numbers of VMAT2 and VGAT molecules within the membrane of the synaptic vesicles may result from stochastic sorting of the two transporters during recycling from the cell membrane. In a similar manner, the sorting of both VMAT2 and VGAT to the various secretory organelles in the cell body may be determined stochastically at the moment of their budding from the trans-Golgi network or the somatic cell membrane. A stochastic transporter sorting during recycling may also explain the discrepancies in the literature concerning co-localization and co-release of two low molecular mass transmitters in the central and peripheral neurons, where it is controversial whether the two transmitters are contained within the same or separate synaptic vesicles [52–55].
5. Dopaminergic amacrine cells: retina's jack of all trades
When the dark-adapted retina is illuminated with bright light, DA cells are depolarized by ON-bipolar cells and release both dopamine and GABA.
-
(i)
The synapses made by DA cells onto AII amacrine cells are GABAergic, since GABAA but not dopamine receptors are clustered in the postsynaptic active zone. By inhibiting their postsynaptic target, these synapses may prevent the signals of the saturated rods from entering the cone pathway when the dark-adapted retina is suddenly exposed to bright illumination. Indeed, GABAergic inhibition by DA cells may cause the silent pause or hyperpolarization of the AII amacrine cell when the stimulus reaches photopic range during an intensity series [56].
-
(ii)
Dopamine—regardless of its site of release, synaptic or extrasynaptic—acts by volume transmission on metabotropic receptors diffusely distributed over the surface of most cells of the retina and thus sets the gain of the retinal circuits for vision in bright light.
-
(iii)
Since the physiological action at the synapse is exclusively determined by the nature of the cluster of ionotropic receptors within the postsynaptic membrane, irrespective of the dual transmitter contents of the presynaptic vesicles, in the retina there is no such thing as a ‘dopaminergic synapse’.
-
(iv)
The GABA released extrasynaptically by DA cells may exert a local feedback on the parent neuron to dampen the frequency of its spontaneous firing. In addition, DA cells are not the only neurons in the retina that release GABA extrasynaptically, because in the course of our studies we repeatedly measured spontaneous GABAergic currents from isolated cell bodies of non-dopaminergic cells in our short-term cultures of dissociated retinas. Considering that about half of the 26 types of amacrine cells are GABAergic [57], paracrine secretion may sustain a non-negligible tone of GABA in the intercellular spaces of the IPL, in spite of the uptake of this transmitter by Müller glial cells [58]. Thus, the ubiquitous presence of extracellular GABA may control the excitability of the neural processes in the IPL by acting on metabotropic GABAB receptors, which are widely expressed on amacrine and ganglion cells [59], as well as on synaptic and extrasynaptic ionotropic GABAA and GABAC receptors. In fact, tonic GABAergic currents have been reported in bipolar cell terminals [60,61] and starburst amacrine cells [62]. The IPL is unique in the nervous system because in its five, 10 μm thick strata are segregated the synapsing processes of over 50 different types of neurons, each encoding different parameters of the light stimuli: the glutamatergic synapses connecting OFF-cone bipolars to OFF-ganglion cells in stratum 2 are adjacent to the glutamatergic synapses between ON-cone bipolars and ON-ganglion cells in stratum 3. It is known that blocking GABAC receptors unmasks a sizeable OFF excitation in ON-ganglion cells whose dendrites ramify close to the ON–OFF border [63]. Thus, the presence of GABA in the intercellular spaces of the IPL may be essential in counteracting the spillover of signals from the OFF and ON sublaminae, and thus contribute to Roska and Werblin's ‘vertical inhibition’ between strata.
-
(v)
DA cells contain a circadian clock that anticipates predictable variations in retinal illumination [64].
-
(vi)
In addition to dopamine and GABA, DA cells synthesize a large repertory of additional molecules: these include ferritin, the neuropeptide CART, the cytokine IFN-α, the chemokine MCP-1 and the hormone insulin [12], suggesting that a large portion of the DA cell proteome is still uncharacterized.
Ethics statement
All procedures involving mice in the original articles discussed in this review were in accordance with National Institutes of Health USA guidelines and approved by the Institutional Animal Care and Use Committee of Harvard Medical School.
Funding statement
The work reviewed in this article was supported by the National Institutes of Health grant no. EY01344 (E.R.).
Authors' contributions
All authors contributed to the writing of this review.
Competing interests
We have no competing interests.
References
- 1.Witkovsky P, Dearry A. 1991. Functional roles of dopamine in the vertebrate retina. Progr. Retinal Res. 11, 247–292. ( 10.1016/0278-4327(91)90031-V) [DOI] [Google Scholar]
- 2.Witkovsky P. 2004. Dopamine and retinal function. Doc. Ophthalmol. 108, 17–40. ( 10.1023/B:DOOP.0000019487.88486.0a) [DOI] [PubMed] [Google Scholar]
- 3.Dearry A, Burnside B. 1986. Dopaminergic regulation of cone retinomotor movement in isolated teleost retinas. I. Induction of cone contraction is mediated by D2 receptors. J. Neurochem. 46, 1006–1021. ( 10.1111/j.1471-4159.1986.tb00612.x) [DOI] [PubMed] [Google Scholar]
- 4.Dearry A, Burnside B. 1989. Light-induced dopamine release from teleost retinas acts as a light-adaptive signal to the retinal pigment epithelium. J. Neurochem. 53, 870–878. ( 10.1111/j.1471-4159.1989.tb11785.x) [DOI] [PubMed] [Google Scholar]
- 5.Piccolino M, Neyton J, Gerschenfeld HM. 1984. Decrease of gap junction permeability induced by dopamine and cyclic adenosine 3′: 5′-monophosphate in horizontal cells of turtle retina. J. Neurosci. 4, 2477–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lasater EM, Dowling JE. 1985. Dopamine decreases conductance of the electrical junctions between cultured retinal horizontal cells. Proc. Natl Acad. Sci. USA 82, 3025–3029. ( 10.1073/pnas.82.9.3025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeVries SH, Schwartz EA. 1989. Modulation of an electrical synapse between solitary pairs of catfish horizontal cells by dopamine and second messengers. J. Physiol. 414, 351–375. ( 10.1113/jphysiol.1989.sp017692) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knapp AG, Dowling JE. 1987. Dopamine enhances excitatory amino-acid gated conductances in cultured retinal horizontal cells. Nature 325, 437–439. ( 10.1038/325437a0) [DOI] [PubMed] [Google Scholar]
- 9.Maguire G, Werblin F. 1994. Dopamine enhances a glutamate-gated ionic current in OFF bipolar cells of the tiger salamander retina. J. Neurosci. 14, 6094–6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daw NW, Jensen RJ, Brunken WJ. 1990. Rod pathways in mammalian retinae. Trends Neurosci. 13, 110–115. ( 10.1016/0166-2236(90)90187-F) [DOI] [PubMed] [Google Scholar]
- 11.Gustincich S, Feigenspan A, Wu D-K, Koopman LJ, Raviola E. 1997. Control of dopamine release in the retina: a transgenic approach to neural networks. Neuron 18, 723–736. ( 10.1016/S0896-6273(00)80313-X) [DOI] [PubMed] [Google Scholar]
- 12.Gustincich S, et al. 2004. Gene discovery in genetically labeled single dopaminergic neurons of the retina. Proc. Natl Acad. Sci. USA 101, 5069–5074. ( 10.1073/pnas.0400913101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Contini M, Lin B, Kobayashi K, Okano H, Masland RH, Raviola E. 2010. Synaptic input of ON-bipolar cells onto the dopaminergic neurons of the mouse retina. J. Comp. Neurol. 518, 2035–2050. ( 10.1002/cne.22320) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacNeil MA, Heussy JK, Dacheux RE, Raviola E, Masland RH. 2004. The population of bipolar cells in the rabbit retina. J. Comp. Neurol. 472, 73–86. ( 10.1002/cne.20063) [DOI] [PubMed] [Google Scholar]
- 15.Zhang D-Q, Zhou T-R, McMahon DG. 2007. Functional heterogeneity of retinal dopaminergic neurons underlying their multiple roles in vision. J. Neurosci. 27, 692–699. ( 10.1523/JNEUROSCI.4478-06.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pourcho RG. 1982. Dopaminergic amacrine cells in the cat retina . Brain Res. 252, 101–109. ( 10.1016/0006-8993(82)90982-9) [DOI] [PubMed] [Google Scholar]
- 17.Pourcho RG, Goebel DJ. 1985. A combined Golgi and autoradiographic study of (3H)glycine-accumulating amacrine cells in the cat retina. J. Comp. Neurol. 33, 473–480. ( 10.1002/cne.902330406) [DOI] [PubMed] [Google Scholar]
- 18.Kolb H, Cuenca N, Wang HH, Dekorver L. 1990. The synaptic organization of the dopaminergic amacrine cell in the cat retina. J. Neurocytol. 19, 343–366. ( 10.1007/BF01188404) [DOI] [PubMed] [Google Scholar]
- 19.Kolb H, Cuenca N, Dekorver L. 1991. Postembedding immunocytochemistry for GABA and glycine reveals the synaptic relationships of the dopaminergic amacrine cell of the cat retina. J. Comp. Neurol. 310, 267–284. ( 10.1002/cne.903100210) [DOI] [PubMed] [Google Scholar]
- 20.MacNeil MA, Heussy JK, Dacheux RF, Raviola E, Masland RH. 1999. The shapes and numbers of amacrine cells: matching of photofilled with Golgi-stained cells in the rabbit retina and comparison with other mammalian species. J. Comp. Neurol. 413, 305–326. () [DOI] [PubMed] [Google Scholar]
- 21.Strettoi E, Raviola E, Dacheux RF. 1992. Synaptic connections of the narrow-field, bistratified rod amacrine cell (AII) in the rabbit retina. J. Comp. Neurol. 325, 152–168. ( 10.1002/cne.903250203) [DOI] [PubMed] [Google Scholar]
- 22.Contini M, Raviola E. 2003. GABAergic synapses made by a retinal dopaminergic neuron. Proc. Natl Acad. Sci. USA 100, 1358–1363. ( 10.1073/pnas.0337681100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veruki ML, Wässle H. 1996. Immunohistochemical localization of dopamine D1 receptors in rat retina. Eur. J. Neurosci. 8, 2286–2297. ( 10.1111/j.1460-9568.1996.tb01192.x) [DOI] [PubMed] [Google Scholar]
- 24.Nguyen-Legros J, Simon A, Caillé I, Bloch B. 1997. Immunocytochemical localization of dopamine D1 receptors in the retina of mammals. Vis. Neurosci. 14, 545–551. ( 10.1017/S0952523800012207) [DOI] [PubMed] [Google Scholar]
- 25.Derouiche A, Asan E. 1999. The dopamine D2 receptor subfamily in rat retina: ultrastructural immunogold and in situ hybridization studies. Eur. J. Neurosci. 11, 1391–1402. ( 10.1046/j.1460-9568.1999.00557.x) [DOI] [PubMed] [Google Scholar]
- 26.Witkovsky P, Gábriel R, Krizaj D. 2008. Anatomical and neurochemical characterization of dopaminergic interplexiform processes in mouse and rat retinas. J. Comp. Neurol. 510, 158–174. ( 10.1002/cne.21784) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feigenspan A, Gustincich S, Bean BP, Raviola E. 1998. Spontaneous activity of solitary dopaminergic cells of the retina. J. Neurosci. 18, 6776–6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grace AA, Onn SP. 1989. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J. Neurosci. 9, 3463–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yung WH, Hausser MA, Jack JJ. 1991. Electrophysiology of dopaminergic and non-dopaminergic neurones of the guinea-pig substantia nigra pars compacta in vitro. J. Physiol. 436, 643–667. ( 10.1113/jphysiol.1991.sp018571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puopolo M, Raviola E, Bean BP. 2007. Roles of subthreshold calcium current and sodium current in spontaneous firing of mouse midbrain dopamine neurons. J. Neurosci. 27, 645–656. ( 10.1523/JNEUROSCI.4341-06.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hochstetler SE, Puopolo M, Gustincich S, Raviola E, Wightman RM. 2000. Real-time amperometric measurements of zeptomole quantities of dopamine released from neurons. Anal. Chem. 72, 489–496. ( 10.1021/ac991119x) [DOI] [PubMed] [Google Scholar]
- 32.Puopolo M, Hochstetler SE, Gustincich S, Wightman RM, Raviola E. 2001. Extrasynaptic release of dopamine in a retinal neuron: activity dependence and transmitter modulation. Neuron 30, 211–225. ( 10.1016/S0896-6273(01)00274-4) [DOI] [PubMed] [Google Scholar]
- 33.Seal RP, Edwards RH. 2006. Functional implications of neurotransmitter co-release: glutamate and GABA share the load. Curr. Opin. Pharmacol. 6, 114–119. ( 10.1016/j.coph.2005.12.001) [DOI] [PubMed] [Google Scholar]
- 34.Hnasko TS, Edwards RH. 2012. Neurotransmitter corelease: mechanism and physiological role. Annu. Rev. Physiol. 74, 225–243. ( 10.1146/annurev-physiol-020911-153315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sulzer D, Joyce MP, Lin L, Geldwert D, Haber SN, Hattori T, Rayport S. 1998. Dopamine neurons make glutamatergic synapses in vitro. J. Neurosci. 18, 4588–4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joyce MP, Rayport S. 2000. Mesoaccumbens dopamine neuron synapses reconstructed in vitro are glutamatergic. Neuroscience 99, 445–456. ( 10.1016/S0306-4522(00)00219-0) [DOI] [PubMed] [Google Scholar]
- 37.Chuhma N, Zhang H, Masson J, Zhuang X, Sulzer D, Hen R, Rayport S. 2004. Dopamine neurons mediate a fast excitatory signal via their glutamatergic synapses. J. Neurosci. 24, 972–981. ( 10.1523/JNEUROSCI.4317-03.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. 2010. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J. Neurosci. 30, 8229–8233. ( 10.1523/JNEUROSCI.1754-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campbell KJ, Takada M, Hattori T. 1991. Co-localization of tyrosine hydroxylase and glutamate decarboxylase in a subpopulation of single nigrotectal projection neurons. Brain Res. 558, 239–244. ( 10.1016/0006-8993(91)90774-P) [DOI] [PubMed] [Google Scholar]
- 40.González-Hernández T, Barroso-Chinea P, Acevedo A, Salido E, Rodríguez M. 2001. Colocalization of tyrosine hydroxylase and GAD65 mRNA in mesostriatal neurons. Eur. J. Neurosci. 13, 57–67. ( 10.1046/j.1460-9568.2001.01371.x) [DOI] [PubMed] [Google Scholar]
- 41.Maher BJ, Westbrook GL. 2008. Co-transmission of dopamine and GABA in periglomerular cells. J. Neurophysiol. 99, 1559–1564. ( 10.1152/jn.00636.2007) [DOI] [PubMed] [Google Scholar]
- 42.Hirasawa H, Puopolo M, Raviola E. 2009. Extrasynaptic release of GABA by retinal dopaminergic neurons. J. Neurophysiol. 102, 146–158. ( 10.1152/jn.00130.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gustincich S, Feigenspan A, Sieghart W, Raviola E. 1999. Composition of the GABA(A) receptors of retinal dopaminergic neurons. J. Neurosci. 19, 7812–7822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feigenspan A, Gustincich S, Raviola E. 2000. Pharmacology of GABA(A) receptors of retinal dopaminergic neurons. J. Neurophysiol. 84, 1697–1707. [DOI] [PubMed] [Google Scholar]
- 45.Kosaka T, Kosaka K, Hataguchi Y, Nagatsu I, Wu J-Y, Ottersen OP, Storm-Mathisen J, Hama K. 1987. Catecholaminergic neurons containing GABA-like and/or glutamic acid decarboxylase-like immunoreactivities in various brain regions of the rat. Exp. Brain Res. 66, 191–210. ( 10.1007/BF00236215) [DOI] [PubMed] [Google Scholar]
- 46.Wässle H, Chun MH. 1988. Dopaminergic and indoleamine-accumulating amacrine cells express GABA-like immunoreactivity in the cat retina. J. Neurosci. 8, 3383–3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wulle I, Wagner HJ. 1990. GABA and tyrosine hydroxylase immunocytochemistry reveal different patterns of colocalization in retinal neurons of various vertebrates. J. Comp. Neurol. 296, 173–178. ( 10.1002/cne.902960111) [DOI] [PubMed] [Google Scholar]
- 48.Hirasawa H, Betensky RA, Raviola E. 2012. Corelease of dopamine and GABA by a retinal dopaminergic neuron. J. Neurosci. 32, 13 281–13 291. ( 10.1523/JNEUROSCI.2213-12.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edwards RH. 2007. The neurotransmitter cycle and quantal size. Neuron 55, 835–858. ( 10.1016/j.neuron.2007.09.001) [DOI] [PubMed] [Google Scholar]
- 50.Nirenberg MJ, Chan J, Liu Y, Edwards RH, Pickel VM. 1996. Ultrastructural localization of the vesicular monoamine transporter-2 in midbrain dopaminergic neurons: potential sites for somatodendritic storage and release of dopamine. J. Neurosci. 16, 4135–4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nirenberg MJ, Chan J, Liu Y, Edwards RH, Pickel VM. 1997. Vesicular monoamine transporter-2: immunogold localization in striatal axons and terminals. Synapse 26, 194–198. () [DOI] [PubMed] [Google Scholar]
- 52.Li WC, Soffe SR, Roberts A. 2004. Glutamate and acetylcholine corelease at developing synapses. Proc. Natl Acad. Sci. USA 101, 15 488–15 493. ( 10.1073/pnas.0404864101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boulland JL, Jenstad M, Boekel AJ, Wouterlood FG, Edwards RH, Storm-Mathisen J, Chaudhry FA. 2009. Vesicular glutamate and GABA transporters sort to distinct sets of vesicles in a population of presynaptic terminals. Cereb. Cortex 19, 241–248. ( 10.1093/cercor/bhn077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fattorini G, Verderio C, Melone M, Giovedì S, Benfenati F, Matteoli M, Conti F. 2009. VGLUT1 and VGAT are sorted to the same population of synaptic vesicles in subsets of cortical axon terminals. J. Neurochem. 110, 1538–1546. ( 10.1111/j.1471-4159.2009.06251.x) [DOI] [PubMed] [Google Scholar]
- 55.Lee S, Kim K, Zhou ZJ. 2010. Role of ACh-GABA cotransmission in detecting image motion and motion direction. Neuron 68, 1159–1172. ( 10.1016/j.neuron.2010.11.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xin D, Bloomfield SA. 1999. Comparison of the responses of AII amacrine cells in the dark- and light-adapted rabbit retina. Vis. Neurosci. 16, 653–665. ( 10.1017/S0952523899164058) [DOI] [PubMed] [Google Scholar]
- 57.Strettoi E, Masland RH. 1996. The number of unidentified amacrine cells in the mammalian retina. Proc. Natl Acad. Sci. USA 93, 14 906–14 911. ( 10.1073/pnas.93.25.14906) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Biedermann B, Bringmann A, Reichenbach A. 2002. High-affinity GABA uptake in retinal glial (Müller) cells of the guinea pig: electrophysiological characterization, immunohistochemical localization, and modeling of efficiency. Glia 39, 217–228. ( 10.1002/glia.10097) [DOI] [PubMed] [Google Scholar]
- 59.Yang XL. 2004. Characterization of receptors for glutamate and GABA in retinal neurons. Prog. Neurobiol. 73, 127–150. ( 10.1016/j.pneurobio.2004.04.002) [DOI] [PubMed] [Google Scholar]
- 60.Hull C, Li GL, von Gersdorff H. 2006. GABA transporters regulate a standing GABA(C) receptor-mediated current at a retinal presynaptic terminal. J. Neurosci. 26, 6979–6984. ( 10.1523/JNEUROSCI.1386-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palmer MJ. 2006. Functional segregation of synaptic GABA(A) and GABA(C) receptors in goldfish bipolar cell terminals. J. Physiol. 577, 45–53. ( 10.1113/jphysiol.2006.119560) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang CT, Blankenship AG, Anishchenko A, Elstrott J, Fikhman M, Nakanishi S, Feller MB. 2007. GABA(A) receptor-mediated signaling alters the structure of spontaneous activity in the developing retina. J. Neurosci. 27, 9130–9140. ( 10.1523/JNEUROSCI.1293-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roska B, Werblin F. 2001. Vertical interactions across ten parallel, stacked representations in the mammalian retina. Nature 410, 583–587. ( 10.1038/35069068) [DOI] [PubMed] [Google Scholar]
- 64.Dorenbos R, Contini M, Hirasawa H, Gustincich S, Raviola E. 2007. Expression of circadian clock genes in retinal dopaminergic cells. Vis. Neurosci. 24, 573–580. ( 10.1017/S0952523807070538) [DOI] [PubMed] [Google Scholar]