Abstract
Lung cancer is one of the leading causes of cancer-related death around the world with the majority of diagnoses being non-small cell lung cancer (NSCLC). Given the poor survival rate and efficacy of current therapy for NSCLC, there is a need to identify and develop new therapeutic targets for treatment. We have observed significantly up-regulated levels of Fn14 in clinical samples of lung cancer relative to normal adjacent tissue. However, the functional role of Fn14 in these tumors is not understood yet. We used RT-PCR to establish the Fn14 expression profile in various NSCLC cell lines. Using isogenic variants of H460 NSCLC cell line with low, intermediate and high Fn14 expression as a cellular model, we determined that increased levels of integrin α6 in cells over-expressing Fn14 is suggestive of an important role of α6β1-fn14 interactions in motility of lung carcinoma and formation of metastases. Enhanced levels of Fn14 correlated with higher tumor cell migration and invasion in an MMP-1 dependent manner. Cells over-expressing Fn14 showed increased in vivo tumor formation with metastatic capacity to lymph nodes, lungs and liver. Thus, this research may be a step toward developing improved treatment strategies for NSCLC by improved detection and inhibition of metastases.
Keywords: Fn14, TNFRSF12A, non-small cell lung cancer, H460 cells, motility, tumor formation and metastasis, integrin α6
Lung cancer is the most common cancer in the world [1] and is the leading cause of cancer-related death around the world with the majority of diagnoses being non-small cell lung cancer (NSCLC) including both squamous carcinoma and adenocarcinoma. NSCLC accounts for over 85% of lung cancer cases with the 5-year survival rate only 14% [2,3]. Although tobacco smokers make up the majority of lung cancer cases, up to 20% of lung cancers are in never-smokers [2,4]. The American Cancer Society reported in 2008 that even after removing smoking-related lung cancer deaths, lung cancer in never-smokers is estimated to be the third leading cause of death in men and fifth leading cause of death in women [5]. Besides smoking, environmental exposure to pollutants in automobile exhaust, coal burning emissions, arsenic and other metals, asbestos and radon gas are known to cause lung cancer [6-8].
Treatments for NSCLC after mediastinum lymph node evaluation may involve partial or full lung removal for those with no evidence of lymph node involvement and patients with mediastinum lymph node connection undergo pre and post-operative chemotherapy, surgery and radiotherapy. Surgical treatment prolongs life at best by 10 months however; NSCLC is often metastatic leading to a poor prognosis for most patients diagnosed [9]. Whitest et al. recently observed that Fn14 (TNFRSF12A), a member of the tumor necrosis factor super-family of cell surface receptors, is significantly up-regulated in clinical samples of lung cancer relative to normal adjacent tissue what makes it an attractive target for use as a tumor marker and therapeutic target to improve the survival rates of NSCLC [3].
Fibroblast growth factor-inducible 14 (Fn14; gene TNFRSF12A) is a type 1 transmembrane protein comprised of 129 amino acids and the smallest known member of tumor necrosis factor super family of receptors [10] found on chromosome 16 [11]. Fn14 is most commonly known as the receptor for a multifunctional cytokine tumor necrosis factor-like weak inducer of apoptosis (TWEAK) which has been reported to induce a broad range of responses from apoptosis to cellular invasion, migration and survival [12]. Increased levels of Fn14 are often seen in tumor cells relative to low levels in normal cells [13]. Fn14 up-regulation has been observed in a variety of cancer cell lines such as breast, brain, bladder, skin, lung, ovarian, pancreatic, colon, prostate and cervical cancer cell lines [14] as well as in several solid tumors [15] including hepatocellular carcinoma [16], glioblastoma [17,18], gastrointestinal cancer [19], urothelial carcinoma [20], malignant ovarian tumors [21], prostate [22] and breast carcinoma [23]. Thus, increased expression of Fn14 might be indicative of cancer and suppression of its expression could be a possible therapeutic target to inhibit development and progression of various cancers. Increased expression of Fn14 has suggested its invasive properties in urothelial carcinoma [20]. Fn14 expression was detected in the majority of tumor types, including pancreatic cancer (60%), bone metastases (54%) and liver metastases in colorectal cancer (50%) [24]. High levels of Fn14 or TWEAK were observed in 60% of patients with esophageal cancer and 75% of patients with pancreatic cancer as well as in majority of human esophageal and pancreatic cell lines [13]. York et al. demonstrated the functional importance of Fn14/TWEAK pathway in pancreatic cancer progression [19]. Expression of TWEAK/Fn14 proves to be a marker for malignant ovarian tumors [21]. Huang et al. suggested that over expression of Fn14 may contribute to multiple malignant cellular phenotypes associated with prostate cancer progression [22]. Two research groups recently reported the up-regulation of Fn14 in NSCLC specimens [3,24]. However, the functional role of Fn14 in this type of tumors is not understood yet.
The mobility of malignant tumor cells depends on complex molecular interactions that regulate the structure, function, and interactions of cytoskeleton and extracellular matrix. Interims are a family of limerick, transmembrane proteins that mediate cell-cell and extracellular matrix adhesion and signals transuded by integrand play a role in many biological processes, including cell growth; differentiation, migration and apoptosis [25]. Increased levels of Fn14 are often indicative of enhanced motility of tumor cells. In addition, expression-correlated set of genes including Fn14 and interim β1 functions in molecular interaction networks promoting cellular migration via structural changes and signaling [26]. However, there is still very limited information available about the interactions between Fn14 and interims that might be involved in cancer cell motility and metastases.
In this study we demonstrate a functional role of Fn14 in carcinogenesis of non-small lung carcinoma. We show that Fn14 can induce the expression of interring α6 which is necessary to promote the migration, invasion and metastases formation of H460 non-small cell lung carcinoma cells. Moreover, we report that over-expression of Fn14 can increase the expression of various nuclear encoded genes known to be associated with malignant transformation of cells such as MMP-1. Furthermore, cells producing high levels of Fn14 showed significantly increased tumor formation, both in vitro and in vivo with increased metastatic capacity to lymph nodes, lungs and liver. Thus, Fn14 may serve as a tumor marker for lung carcinoma and as a novel therapeutic target for improving the survival rates of NSCLC.
Materials and methods
Cell culture and tissue collection
H460, non-small cell lung adenocarcinoma cell line was obtained from American Type Culture Collection (ATCC, Manassas, VA) for in vitro and in vivo studies. Cells were maintained in Dulbecco's modified eagle medium (DMEM; Gibson-BRL, Rockville, MD) and 10% fetal bovine serum supplemented with 50 μg/ml penicillin/streptomycin (Invitrogen, Carlsbad, CA) in a 5% carbon dioxide/95% environment at 37°C. All isogonics variants of H460 cancer cells were maintained in Dulbecoo's modified eagle media supplemented with 10% fetal bovine serum, 50 μg/ml penicillin/streptomycin and 2 μg/ml of selective antibiotic Blasticidine at 37°C and 5% carbon dioxide.
Lent virus transduction
Lent viral constructs were created to test the effect of Fn14 expression in H460 lung adenocarcinoma cells.
To generate H460 cells with stable Fn14 over expression, full length Fn14 cDNA clone along with PCR primers for amplification and modification of the resulting product for TOPO directional cloning were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and Biosynthesis (Lewisville, TX), respectively. The FN14 cDNA was PCR amplified from the original ATCC vector with Pixy polymerase to generate blunt-end PCR products for directional cloning into the expression pLenti6/V5-D-TOPO vector which was designed to facilitate rapid TOPO cloning and high level expression of PCR products in mammalian cells using ViraPower Lent viral Expression System (Invitrogen, Carlsbad, CA). PLenti6/V5-GW/lacZ was used as a positive control expression vector. This vector contains human cytomegalovirus (CMV) immediate early promoter for high-level constitutive expression of the gene of interest. Using the ViraPower Lent viral Expression System, we were able to create a replication-incompetent, HIV-1-based lent virus that was used to deliver and express Fn14 in H460 cells.
To create H460 cells with stably silenced Fn14 expression, two shrines directed against the Fn14 mRNA were designed using the Invitrogen's proprietary design software from siRNA sequences previously used in Fn14 transient transfect ion experiments (Invitrogen, Carlsbad, CA). Two strands of shRNA sequences targeting FN14 mRNA were synthesized (5′ – CACCGCAGGAGAGAGAAGTTCAC-CACGAATGGTGAACTTCTCTCTCTTGC – 3′ and 5′ – CACCGCCACTCATCATTCATTCATTTCGAAAAAT-GAATGAATGATGAGTGG – 3′), annealed and cloned into the entry pENTR/U6 vector which contains attL sites to facilitate transfer of the U6 RNAi cassette into the destination pLenti6/BLOCK-iT-DEST vector to generate an expression clone. To obtain pLenti6/BLOCK-iT expression clone, the LR clonuses reaction between entry and destination construct was performed using the Block-it Lent viral RNAi Expression kit (Invitrogen, Carlsbad, GA) according to manufacturer's instructions with some modifications. The expression clone was then packaged into the lent viral particles and used to stably transducer H460 cells with shRNA targets against Fn14 mRNA. PLenti6-GW/U6-laminshRNA plasmid was used as a positive control for lent virus production.
Quantitative Real-Time reverse transcriptase Polymer-ace Chain Reaction (RT-PCR)
Total RNA extraction from all isogonics variants of H460 cells was performed using RNAeasy Manikin (QIAGEN, Valencia, CA). Human Fn14 (Hs00171993_A1), ITGA6 (Hs01041011_m1) and GAPDH (Hs99999905_A1) primer/probes were obtained from Applied Bios stems (Branchburg, NJ). CDNA was synthesized from 500 ng of total RNA in a 50μl reaction with master mix containing 10×RT buffer, 5.5mM MgCl2, 2mM dNTPs, 2.5μM random hexamers, 2 units of RNase Inhibitor and 62.5 units of Multi Scribe Reverse Transcriptase. All Master Mix reagents were purchased from ABI (Applied Bios stems, Branchburg, NJ). Reactions were performed in MJ Thermo cycler PTC-200 (MJ Research, Watertown, MA) followed by these conditions: 25°C for 10 minutes, 48°C for 30 minutes and 95°C for 5 minutes. 10ng of cDNA was then used to amplify the human Fn14 and integrin α6 (ITGA6) sequence. The conditions for PCR reactions were: 10 minutes at 95°C followed by 15 seconds at 95°C, 1 minute at 60°C for 40 cycles by using ABI7000. PCR amplification of the human GAPDH was used to control quality of the cDNA. Non-template controls were included on each PCR plate. MRNA expression levels of Fn14 and ITGA6 were normalized to the GAPDH control. Amplification plots were generated and the Ct values (cycle number at which fluorescence reaches threshold) recorded.
Immunoprecipitation and western blot analysis
Cell lysates from all Fn14 isogonics' variants of H460 cells were prepared by harvesting the cells in RIPA lyses buffer containing 50mM Tris, pH 7.4, 150 mM NaCl, 1mM EDTA, 1% Triton N-100, 1% sodium deoxycholate and 0.1% sodium dodecyl sulfate and protease inhibitor mixture (Leupeptin, Apportioning, PMSF). Cell lysates were pre-cleared with protein A/G PLUS Agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA) and then the protein concentration was measured using Bio-Rad Protein Assay (Hercules, CA). Equal amounts of pre-cleared protein (1mg) were then incubated with 3μg of either rabbit monoclonal antibody against Fn14 (ITEM-1), mouse monoclonal antibody against human α3 integrin; clone P1B5, or rabbit monoclonal antibody against human α6 integrin; clone J1B5 at 4°C overnight. Ten protein A/G PLUS Agarose beads were added to each sample and incubated at 4°C for 1 hour to remove Fn14, α3 or α6 integrin. Beads were washed three times with cold PBS and 2× protein loading dye added to each bead pellet. To release the Fn14 or integrals from the beads to the loading dye, samples were denaturized at 95°C for 5 minutes and then separated by either 12% or 7.5% SDS-polyacrylamide gel electrophoresis followed by transfer to PVDF membranes. The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline, pH 8.0, with0.1% Tween 20 for 1 hour prior to an addition of primary western blot antibodies, followed by secondary anti-mouse or anti-rabbit horseradish peroxides-conjugated antibodies. Control mouse anti human β-acting primary antibody was obtained from Sigma-Aldrich (St. Louis, MO), rabbit anti-Fn14 (ITEM-1) monoclonal antibody from Abcam (Cambridge, MA), mouse anti-α3 integrin antibody (clone P1B5) from Millipore (EMD Millipore Corporation, Billerica, MA); rabbit anti-α6 integrin antibody (clone J1B5) and AA6α antibody for western blot detection of both α3 and α6 interims were greatly provided by Dr. Cress from the University of Arizona[27,28]. Secondary anti-mouse or anti-rabbit antibodies were obtained from Sigma-Aldrich (St. Louis, MO). Protein bands were identified by chemiluminescence and exposed on X-Omit AR film (Eastman Kodak Co., Rochester, NY).
Transwell migration assay
This assay was performed as described previously [29,30]. Briefly, eight micrometer pore size translucent transwell migration chambers (BD Biosciences', Bedford, MA) in 24-well plate were used for migration analysis. Briefly, 600μl of invasion buffer (DMEM media with 0.5% FBS, 0.1%BSA and without antibiotics) was added to each well, and 100ul of H460 isogonics' variants suspended in invasion buffer at concentration of 15×105/ml was placed on the top of the membrane. After overnight incubation of 18 hours at 37°C, 5% CO2, membranes were stained with 0.5% crystal violet in 20% methanol for 1 minute. The stain was rinsed of thoroughly with water, cells remaining on the top of the migration chamber were removed by lightly swabbing, and stained cells adhering to the bottom of the chamber were counted.
Invasion assay
Cell invasion was performed as described previously [30] using Matrigel™ pre-coated membrane filters (BD Biosciences, Bed fold, MA). A monolayer of cells (75% confluence) was suspended in 100 μl of Dulbecco's modified Eagle's medium containing 1mg/ml bovine serum albumin and 0.5% fetal bovine serum only or the same media but supplemented either with 5μM anti-MMP-1 blocking antibody (Ramped Systems, Minneapolis, MN) or 15μM irreversible inhibitor of NF-κB activation Bay11-7082 (EMD Millipore Corporation, Billerica, MA). Transwell inserts were then incubated for 24 hours at 37° C and 5% CO2. Non-invading cells were removed by wiping the upper side of the membrane, and invading cells that had crossed through the transwell barrier were fixed with methanol and stained with crystal violet. The number of invading cells was quantified by counting ten random fields (total magnification, × 200) per filter.
Image analysis of transwell migration assays
The 24-well BD Falcon cell culture companion plates for inserts (BD Biosciences, Bedford, MA) were analyzed for migration/invasion of H460 isogonics' cell line variants through the translucent transwell membrane (8.0um pore size, 1.0 × 105 pore density). Images of 14 fields per transwell were captured from each well using a 10× objective in combination with a 1.5× optima. Image capture was performed with an Olympus IMT-2 microscope (Olympus America Inc., Center Valley, PA), a Hamamatsu ORCA-100 grayscale CCD camera (Hamamatsu USA, Bridgewater, NJ), and a Lull motorized XY stage (Lull Electronic Products, Hawthorne, NY). The camera and stage was computer controlled by SimplePCI software version 6.2 (Compix Inc., Sewickley, PA). After the images were captured, the SimplePCI sofware created a binary image by performing an intensity threshold, than performed a size exclusion to avoid measuring the membrane pores, and the remaining binary image was measured for the area.
Soft agar colony forming assay
3% agar in sterile water was autoclaved and kept in water bath at 48°C. Te agar was then diluted in normal growth media (pre-warmed in 48°C water bath) to the final concentration of 0.6%. 4ml of 0.6% agar was then added as a bottom layer into the 6-well plates. After the solidification in a cell culture hood at room temperature, the top layer was prepared by mixing the 0.6% agar (48°C) and 20×104 of isogenic variants of H460 cells at 37°C in 1:1 ratio. After the top layer solidified at room temperature (30 minutes), the plates were transferred to the 37°C cell culture incubator and incubated for 14-21 days. During the incubation period, the cells were fed with 2ml of normal growing media twice a week. When the colonies were formed, the plates were stained with 1ml of 0.005% crystal violet for 1 hour. Ten colonies were count and representative pictures for each cell line variant taken.
Madrigal 3D growth assay
The integrin α6β1-dependent 3D assay was performed as described previously [31] with further modifications. First, the madrigal was warmed up at 37°C and then 300μl added to each well of 24-well plate. The plate was then placed into the hood for 2 hours till the madrigal solidified. Ten, 20×104 Fn14 isogenic variants of H460 cells suspended in 1ml of normal growing media were placed on the materiel. The plate was then incubated in cell culture incubator at 37°C, 5%CO2. After 24-48 hours the microscopic pictures were taken and results evaluated.
Establishment of tumor xerographs
10×106 H460 cell line variants (LacZ, cDNA, shRNA) were suspended in 0.1 ml of sterile PBS and injected subcutaneously in the rear leg of male SCID mice. Te mice were obtained from the University of Arizona Cancer Center SCID house colony at the age of 9 weeks with weight average of 20 grams. Tumor diameters measured twice weekly at right angles (dshort and dlong) using electronic calipers were converted to volume by the formula, volume = (dshort)2 × (dlong)/2. Individual mice were sacrificed when the tumor volume reached 2000mm3 and the tumors were harvested (½ of tumor snap frozen and other ½ fixed in NBF).
This study was performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The IACUC protocol was approved by the University of Arizona Institutional Animal Care and Use Committee (Permit number 07-029). All procedures were performed to make every effort to minimize suffering.
Immunohistochemistry
After all SCID mice from both groups of our xenograft model (mice injected with H460 cells over-expressing Fn14 and mice injected with H460 cells with silenced Fn14) were sacrificed, tumor samples from each mice were obtained and fixed in NBF. Samples were stained with hematoxylin and eosin and a serial section was stained for Fn14, integrin α6 and PECAM using fluorescence immunohistochemistry with P4A8 (Biogen Idec, Inc., Cambridge, MA), AA6α (kindly provided by Dr. Cress, University of Arizona, Tucson, AZ) and PECAM (CD31) (BioLegend, San Diego, CA) antibodies respectively since we predicted that invading H460 cells over-expressing Fn14 will show most likely highest integrin α6 expression. Fn14 monoclonal antibody P4A8 was generated in an Fn14 knockout mouse [32] immunized with Fn14-mychis recombinant protein. Anti-Fn14 monoclonal antibodies were screened and selected for binding to Fn14-positive cell lines. Immunohistochemistry was done as previously described using 2.5 Ag/mL of the P4A8 antibody [17]. To visualize vascularization within tumor samples, serial sections were stained with PECAM (CD31) commonly used as an endothelial (vascular) cell marker. Co-localization of the proteins in tumor cells was visualized using quantum dot-based fluorescence detection with a fluorescence microscope through the Tissue Acquisition and Molecular Analysis Shared Service at the UA Cancer Center. A scoring system for chromospheres was used to capture the outcome: 0, negative; 1, weak; 2, moderate; 3, strong staining.
Statistical analysis
Data were collected and analyzed to obtain the mean and S.E.M for three independent experiments. Statistical significance between any two groups was determined by the two-tailed Student's t-test using Microsoft Excel, P values less than 0.05 were considered to be significant.
Results
Expression levels of Fn14 in cancer cells
Enhanced Fn14 expression levels are often found in various tumors and cancer cell lines. Quantitative real time PCR (RTPCR) analysis (Fig. 1A) confirmed up-regulation of Fn14 in several cancer cell lines including breast, prostate, colorectal, esophageal and NSCLC cells. Overall, the data indicate that Fn14 is expressed in all sixteen NSCLC cell lines with lower expression in H1155, H838, H1993, HCC15 and A549 cells, intermediate expression in H2122, H1975, H1792, H1568, H460, H1373, HCC193, HCC515, HCC461 and HCC44 cells and high expression in H226 cells (Fig. 1B)
Figure 1.
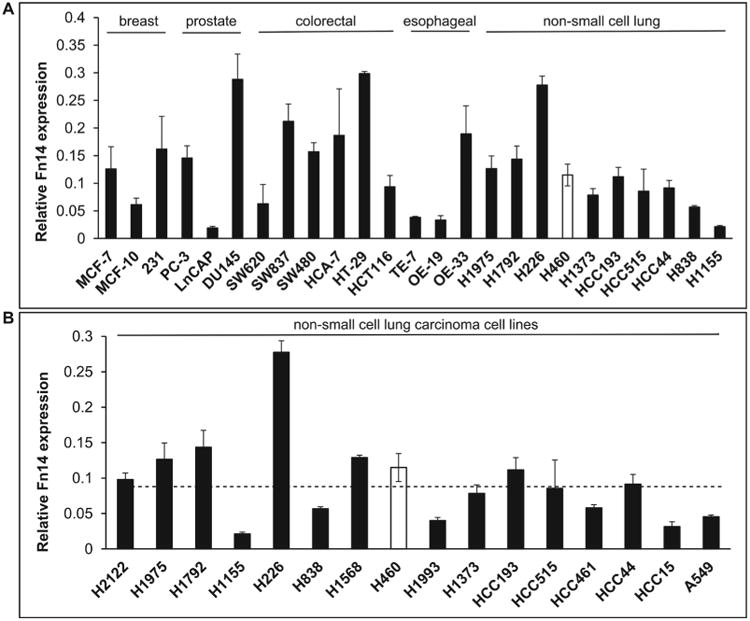
mRNA expression levels of Fn14 are elevated in various tumor cell lines. A) RT-PCR analysis of mRNA expression levels of Fn14 in breast, prostate, colorectal, esophageal and lung tumor cell lines. B) RT-PCR analysis of Fn14 expression in a panel of non-small cell lung carcinoma cells. Average Fn14 mRNA expression is shown with standard error bars. Averages represent Ct values after adjustment to GAPDH and are the result of a minimum of three independent replicates.
From the panel of sixteen NSCLC cell lines, we have chosen H460 cells with intermediate levels of Fn14 as a model to study the function of Fn14 in non-small lung carcinoma since H460 cells were never used before to investigate the functional role of Fn14 lung adenocarcinoma. To do so, we created H460 isogenic variants with altered Fn14 expression. As shown in Fig. 2, mRNA expression levels of Fn14 (Fig. 2A) and Fn14 protein levels (Fig. 2B) were stably altered in H460 cells relative to control by lent viral transduction of Fn14 cDNA or shRNA targeted against Fn14 mRNA. A variant in which LacZ was transuded served as a control for lent viral transduction and confirmed that viral transduction and blasticidin selection had no effect on Fn14 expression.
Figure 2.
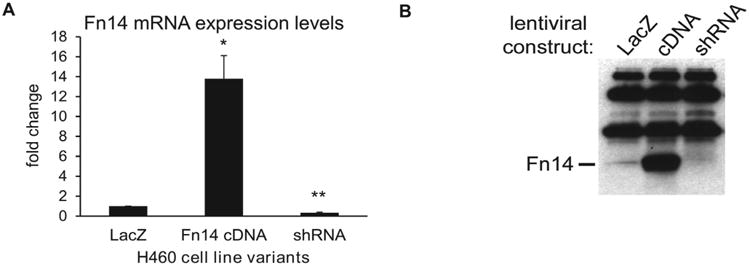
Fn14 expression is increased in NSCLC H460 cells produced by lentiviral infection with an Fn14 cDNA expression construct and decreased in cells produced by infection with a construct expressing shRNA targeting Fn14 mRNA. RT-PCR and Western blot confirmation of altered Fn14 expression in H460 cell line variants. A) Average Fn14 mRNA expression is shown with standard error bars. Asterisks indicate: *p<0.01; **p<0.001 (two-tailed unpaired Student's T-test). Averages represent Ct values after adjustment to GAPDH and are the result of a minimum of three independent replicates. B) The band representing Fn14 protein is indicated, extraneous bands represent the heavy and light chains of the antibody used to immune-precipitate Fn14.
Expression profile of integrin α6 in isogenic variants of H460
Numerous studies have implicated α6 integrin in cancer progression [33]. However, there is still little knowledge about the interactions between Fn14 and this integrin known for its potential role in facilitating cancer cell motility and metastases. Expression analysis using RTPCR revealed increased expression of integrin α6 in H460 cells with high Fn14 expression compared to the cells with low Fn14 expression (Fig. 3A). To evaluate protein levels of integrin α6 on the surface of H460 tumor cells producing various levels of Fn14, we have used AA6α rabbit polyclonal anti-α6 integrin antibody greatly provided by Dr. Cress from the University of Arizona [27]. This antibody is suitable for non-specific western blot detection of integrin α3 as well as integrin α6. Western blot analysis of all Fn14 H460 cell line variants showed high levels of this integrin combination (α3 and α6) in H460 cells over-expressing Fn14 and negligible levels in cells with silenced Fn14 expression (Fig. 3B). In effort to specifically detect the levels of integrin α6, we have precipitated α3 integrin from cell lysates of all isogenic variants of H460 cells using commercially available mouse monoclonal anti-integrin α3, clone P1B5, antibody and then AA6α antibody (detecting both integrin α3 and α6) was used for western blot analysis. Obtained data showed that neither of Fn14 isogenic variants expressed integrin α3 suggesting that data obtained from western blot analysis of cell lysates can be linked directly to the expression levels of integrin α6. To confirm this observation, we have used rat monoclonal anti-integrin α6 antibody, clone J1B5, kindly provided by Dr. Cress [27] to immune-precipitate integrin α6 from cell lysates of H460 cell line variants expressing various levels of Fn14. Ten, AA6α antibody was used for western blot detection of this integrin in these samples. As documented by Fig. 3B, increased levels of Fn14 were able to induce the expression of integrin α6 protein in H460 cells.
Figure 3.
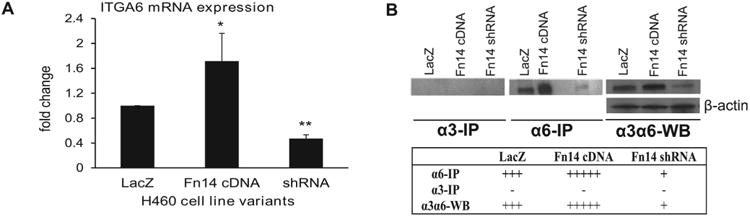
Fn14 induces expression of integrin α6. A) RT-PCR analysis of mRNA levels of integrin α6 in Fn14 isogenic variants of H460 cells. Integrin α6 (ITGA6) expression was normalized to GAPDH control. The results are obtained from a minimum of three independent experiments. Asterisks indicate: *p<0.05; **p<0.0005 (two-tailed unpaired Student's T-test). B) Immune-precipitation using anti-α3 antibody showing that none of the H460 variants with altered Fn14 expression produced integrand α3 protein. Immune-precipitation using anti-α6 antibody and western blot analysis (using AA6α antibody) of cell lysates of H460 isogenic variants showing increased levels of integrin α6 in cells over-expressing Fn14.
Fn14-driven tumor cell motility is MMP-1 and integrin α6 dependent
One of the behaviors of cells that are associated with tumors is their ability to migrate and invade. To examine whether the H460 cells over-expressing Fn14 would be associated with higher levels of cellular migration and invasiveness compared to H460 cells expressing very low levels of Fn14. We conducted transwell migration and invasion assays to determine the migratory and invasive potential of H460 cells with various Fn14 levels. Cells expressing high levels of Fn14 demonstrated significantly increased tumor cell migration through uncoated transwell inserts as well as invasion through madrigals coated inserts (Fig. 4A) compared to cells expressing very low levels of Fn14. Since Fn14 is a receptor, we next tested if increased migration was ligand-dependent by addition of the Fn14-specifc ligand TWEAK. Addition of TWEAK did appear to increase cell migration, but with no significance between all Fn14 isogenic variants (data not shown). In addition, the invasion through madrigal was MMP-1-dependent since anti-MMP-1 blocking antibody significantly decreased tumor cell invasion of H460 cells expressing high levels of Fn14 but had no significant effect on H460 cells with knocked down Fn14 expression (Fig. 4B). Given the role integrants play in cell migration and based on the results we achieved by examining tumor cell invasion on a madrigal surface, we have hypothesized that integrin α6 may explain Fn14-driven tumor cell invasion. To measure the requirement for integrin α6 in NSCLC tumor cell invasion, we used a 3-dimensional madrigal matrix to evaluate the formation of integrin α6-dependent anastomosing structures in H460 cells with altered Fn14 expression. As shown in Fig. 4C, cells with forced Fn14 expression formed anastomosing structures on the madrigal surface, while Fn14-silenced cells did not.
Figure 4.
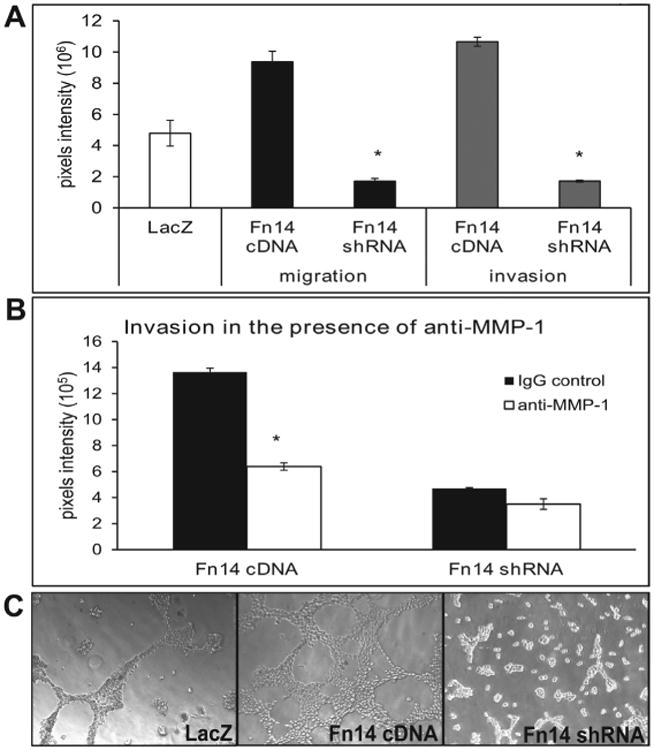
Expression of Fn14 affects H460 tumor cell migration and invasion. A) High expression levels of Fn14 increased cell migration through uncoated transwells as well as invasion through madrigal coated inserts, while loss of expression decreased migration relative to control cells. The data are shown as pixel intensities of cells invaded through the uncoated insert or madrigals with standard error bars and are the result of at least three independent experiments. Asterisk indicate p< 0.01 (two-tailed unpaired Student's T-test). B) Invasion of H460 cells expressing high levels of Fn14 was significantly decreased in the presence of 5μM anti-MMP-1 antibody while invasion of H460 cells with low Fn14 expression was not f by anti-MMP-1 functional blocking antibody. The data are shown as pixel intensities of cells that invaded through the madrigal with standard error bars and are the result of at least three independent experiments. Asterisk indicate p< 0.01 (two-tailed unpaired Student's T-test) relative to Gig treated cells. C) Fn14 over-expressing cells had made integrin alpha 6-dependent anastomosing structures while Fn14-silnced cells were largely unorganized. The data are shown as representative microscopic pictures from at least three independent experiments.
The role of Fn14 in an in vitro and in vivo tumor formation and metastases
Activation of NF-κB and Rac1 by Fn14 may have effects on cellular phenotype beyond migration and invasion. To test additional changes in tumor cell behavior, we compared the H460 variants' capacity for self-renewal in a soft agar colony formation assay. We have observed significantly increased number of colonies formed by H460 cells expressing high levels of Fn14 relative to cells with silenced Fn14 (Fig. 5).
Figure 5.
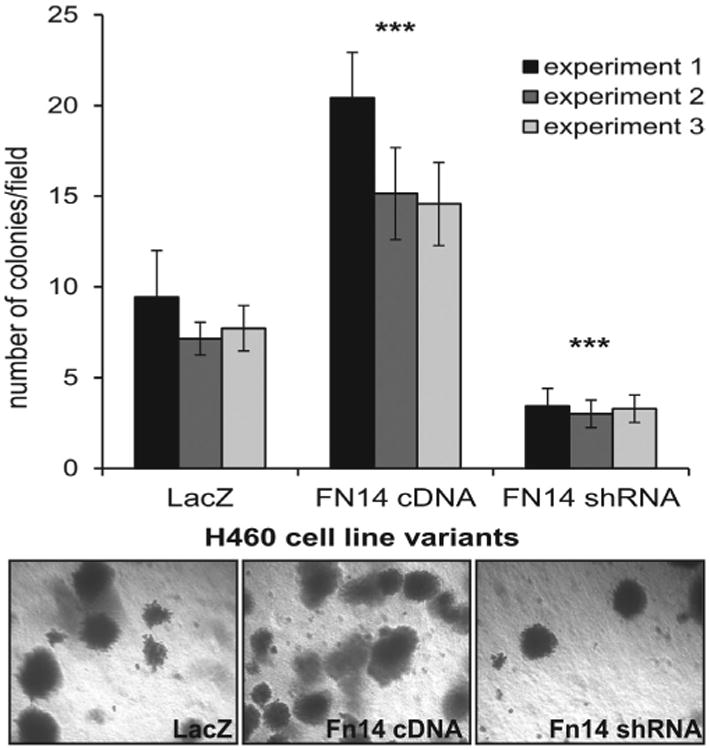
In vitro colony formation was increased in cells expressing high levels of Fn14. H460 cells with low Fn14 expression (Fn14 shRNA) form significantly fewer colonies than H460 cells with high Fn14 expression (Fn14 cDNA), while control cells with endogenous Fn14 expression display intermediate colony forming ability (LacZ). Colony formation was tested in three independent experiments for each cell line variant; average colonies/field are shown; error bars, standard deviation; a two-tailed t-test with variances assumed equal was performed on the three cell lines within each experiment, all nine statistical tests yielded a p<0.001.
To establish the role of Fn14 in an in vivo tumor formation and metastases, we evaluated the effect of Fn14 and integrin α6 on tumor growth in a SCID mouse tumor xerograph model. We observed significantly larger tumors in the group of mice injected with H460 cells expressing high levels of Fn14 compared to the group of mice injected with cells expressing low Fn14 levels (Fig. 6A). Mouse xerograph model for NSCLC tumor cell migration, invasion and angiogenesis was carried out long enough for potential metastases to occur. Since, integrin α6β1 heterodyne is known for its role in tumor cell migration and metastatic processes; we sought to evaluate the tumors generated from H460 cells expressing various levels of Fn14. Our data show a striking difference in tumor cell motility between the cells with altered Fn14 levels. Moreover, increased levels of integrin α6 were detected using immunohistochemistry in tumors from the mice injected with H460 cells over-expressing Fn14 compared to low levels of integrin α6 in tumors generated from H460 cells with knocked down expression of Fn14. Metastases in lungs, liver and lymph nodes (Fig. 6B) were found in mice initially injected with H460 tumor cells over-expressing Fn14 compared to mice initially injected with H460 tumor cells expressing very low levels of Fn14. This dramatic difference in tumor cell motility confirmed our in vitro data and indicates the ability to create in vivo model for NSCLC migration, invasion and metastases. Furthermore, the tumors produced in mice injected with H460 tumor cells expressing very low levels of Fn14 were significantly smaller and necrotic with viable cells only on the periphery of the tumor (Fig. 6C) and contained significantly fewer blood vessels while tumors from mice injected with cells over-expressing Fn14 were larger, less necrotic with viable cells throughout the tumor (Fig. 6D) and showed more vasculature (Supplemental Fig. 1A). Moreover, the Fn14 was able to significantly increase the expression of well-known antigenic factor IL-8 (Supplemental Figure 1B).
Figure 6.
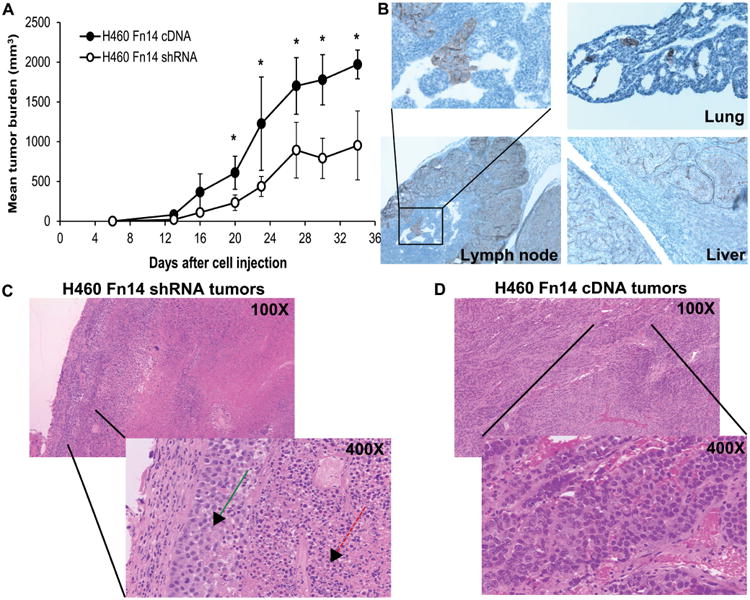
Fn14 expression regulates the in vivo tumor growth of H460 NSCLC cells. A) Ten million H460 cells with altered Fn14 expression was injected subcutaneously into the flanks of four SCID mice per variant. Twenty days after cell injection, mice injected with H460 Fn14 cDNA cells (higher Fn14 expression) showed significantly increased tumor growth than H460 shRNA cells (low Fn14 expression). The data represent a mean tumor burden with standard error bars and asterisk indicates p< 0.05 (two-tailed unpaired Student's T-test). B) No tumor cells were observed migrating in xerograph tumors from Fn14-silenced cells while clusters of cells where frequently seen migrating in tumors from Fn14-expressing cells. Immunohistochemistry for integrin α6 from serial sections of Fn14 over-expressing tumor showed that tumor cells have invaded into the lymph nodes, liver and lungs of mice that were injected subcutaneously with H460 cells over-expressing Fn14. Hamper stain showing bulk C) Fn14 shRNA – green arrow shows the viable cells on periphery of the tumor while the red arrow indicated the necrotic cells inside of the tumor; and D) Fn14 cDNA tumors, higher magnification view, objective lens magnification is indicated.
Discussion
In this study, we explored the functional role of Fn14 in lung adenocarcinoma using H460 cell line as a model. We determined that Fn14 facilitates in vitro tumor cell migration, invasion, and colony forming by up-regulation of integrin α6β1 and MMP-1. Moreover, we document the ability of Fn14 to facilitate in vivo tumor formation, angiogenesis, and metastases.
Fn14 (TNFRSF12A) is known as a member of the tumor necrosis factor super-family of cell surface receptors capable of regulating proliferation, cellular adhesion and migration, invasion, apoptosis, differentiation and survival [3, 13, 34]. Previously, it was documented that high expression of Fn14 receptor in primary breast tumors from patients who did not received chemotherapy correlated strongly with metastases. On the other hand, low Fn14 expression correlated with lymph node-negative disease and well differentiated tumors, what is indicative of good prognosis [15]. Another group reported that in breast adenocarcinomas, certain proteins including Fn14 associated with the endoplasmic reticulum stress phenotype are candidate markers of brain metastases [35]. Recently, it was suggested that Fn14 protein is a valuable marker of breast carcinoma progression and might be a good prognostic marker for breast carcinoma patients [23]. Moreover, Asrani et al. recently reported that Fn14 is able to promote breast cancer cell migration, invasion and MMP-9 expression [36]. Huang et al. reported that Fn14 over-expression promotes androgen-independent prostate cancer progression what correlates with poor treatment outcomes [22]. Lastly, another group found increased expression of Fn14 in histological sections of human prostate adenocarcinoma and two human prostate cancer cell lines with higher expression in the androgen-independent PC-3 cells relative to androgen-sensitive LNCaP prostate cancer cells [37]. These observations confirm our findings of higher Fn14 expression in DU145 and PC-3 prostate cancer cells relative to lower expression in LNCaP cells. Higher Fn14 expression was also documented in human endometrial cancer specimens compared with that in normal endometrial specimens [34]. Gu et al. recently found expression of Fn14 in human malignant ovarian tumors, but not in normal ovarian tissues or benign epithelial ovarian tumors. Moreover, they suggested that Fn14 could act as ovarian tumor suppressor through the activation of macrophages and can be detected as a malignant ovarian tumor marker [21]. The functional importance of Fn14/TWEAK pathway was demonstrated in pancreatic cancer progression by showing a significant inhibition of tumor cell growth after anti-Fn14 blocking antibody treatment in vitro as well as in vivo using a marine gastrointestinal cancer model [19]. Fn14 was found to be over-expressed in gastric tumor tissues compared to normal tissue; and altered Fn14 expression levels affected gastric cancer cellular growth and were correlated with survival of patients with gastric cancer [38]. Furthermore, Fn14 expression was detected in ∼60% of tested melanoma cell lines. Fn14 expression was low in normal skin, but elevated in 93% of primary melanoma specimens and in 58% of melanoma metastases tested suggesting Fn14 as a therapeutic target in melanoma [39]. Heterogeneously expressed Fn14 in cancerous and non-cancerous structures correlates negatively with the grade and survival of renal cell carcinoma patients [40]. Fn14 was also found to be over-expressed in migrating gloom cells in vitro and in glioblastoma clinical specimens in vivo suggesting that targeted therapy against Fn14 as an adjuvant to surgery may improve management of invasive gloom cells and advance the outcome of this devastating cancer [41]. Moreover, both TWEAK and its receptor Fn14 were increased in neuroblastoma cell lines and high-stage primary tumors and found to function as important regulators of primary neuroblastoma growth, invasion and survival [42]. There is only one report which is showing that there are elevated expression levels of Fn14 in non-small cell lung cancer correlated with activated EGFR that can promote NSCLC cell migration and invasion [3]. However, there are still missing information about a functional role of Fn14 and mechanism of lung adenocarcinoma motility.
To study a functional role of Fn14 in NSCLC, we have created three isogenic cell line variants with altered Fn14 expression: H460 cells over-expressing Fn14 (Fn14cDNA), cells with silenced Fn14 expression (shRNA) and LacZ cells as a control for lent viral production. Using these isogenic variants, we determined that suppressed/increased expression of Fn14 in tumor cells affects their capacity for migration in a TWEAK independent manner. We have found that the higher the expression of Fn14 within the NSCLC cells is, the more advanced the migratory abilities are observed. These observations correlate with similar findings of Whitest et al. who found that Fn14 depletion reduced in vitro migration and invasive capacity of HCC827 and H1975 NSCLC cell lines. The ectopic expression of Fn14 in A549 NSCLC cells enhanced their migratory abilities approximately 2-fold [3]
Integrants are important for facilitating cellular adhesion, motility and metastases. However, there is only limited information available about the specific interactions betweenFn14 and integrants that might be involved in cancer cell motility and metastases. It has been previously shown that expression-correlated set of genes including Fn14 and integrin β1 functions in molecular interaction networks promoting cellular migration via structural changes and signaling [26]. There is an evidence of α6β1 involvement in migration and invasiveness of various tumor cells. For example, α6β1 is implicated in glioblastoma cell migration, invasiveness and gloom progression [43]. This integrin is also known to mediate adhesion and migration of highly invasive and metastatic breast carcinomas [44], hematoma cells [45] and pancreatic carcinoma [46]. Other studies indicate that α6β1and α3β1 are maintained in the majority of prostate carcinomas and remain associated with invasive carcinoma, the latter being predominant [47,48]. Moreover, a potential role for integrin α6β1 in directing prostate tumor cell invasion on nerves during per neural invasion was suggested [49]. Our present study reports for the first time the interactions between Fn14 levels and integrin α6β1 necessary to promote the migration and invasion of non-small cell lung carcinoma using a H460 cells. Obtained data suggest that Fn14 can alter the in vitro and in vivo motile characteristics of non-small cell lung carcinoma H460 cells in a α6β1 dependent manner.
Integrin α6β1 is known to be involved in madrigal morphogenesis and cell spreading [31]. When cells are plated on basement membrane matrix (madrigal), collagen, or other substrates, they often form integrin α6-dependent anastomosing cellular network, which is an in vitro model of angiogenesis and tumor cell invasion [31]. In our study, we used formation of α6-dependent structures to measure the requirement for integrin α6 induced by Fn14 in NSCLC tumor cell invasion. H460 cells over-expressing Fn14 formed anastomosing structures on the madrigal surface, while Fn14-silenced cells did not. Thus, this is a first report of adhesion strengthening α6-dependent cords on madrigal induced by Fn14.
Furthermore, we showed that increased levels of Fn14 induced two other well-known targets of NF-κB signaling pathway, integrin α6 and matrix metalloprotease 1 (MMP-1). However, whether the induction of integrin α6 and MMP-1 by Fn14 is responsible for increased NSCLC tumor cell migration and invasion has not been studied yet. Integrin α6 is expressed on the cell surface as a hetero-dimer with integrin β1. Integrin α6β1 is the receptor for laminin and involved in tumor cell migration [47]. In our tumor xerograph model, we observed increased invasiveness of NSCLC cells expressing Fn14 mediated through the integrin α6. The cells invaded through membranes, including those lining lymph vessels, lungs and liver. This invasive phenotype requires migration, but also the ability to degrade the extracellular matrix that constitutes membranes. MMP-1 is a secreted protein that functions to degrade collagen in the extra-cellular matrix and is associated with tumor cell invasion [50]. Our in vitro data indicate involvement of MMP-1 in invasiveness of H460 cells over-expressing Fn14 since we observed a striking difference in invasive capabilities of these cells that showed significantly higher invasion to the lymph vessels, lungs and liver while Fn14-silenced cells failed to invade surrounding tissue. Previously, it was shown that MMP-1 expression is associated with poor prognosis in esophageal adenocarcinoma [51]. Recently, it was shown that the high plasma levels of MMP-1 were associated with advanced-stage of lung cancer and significantly lower overall survival rate of these patients. MMP-1 protein was found to be extraordinarily over-expressed in tumor tissues of lung cancer patients and was associated with the cancer progression including tumor size, staging and lymphatic invasion in patients suffering from lung cancers [52]
In a summary, we report for the first time a functional role of altered Fn14 expression in non-small cell lung carcinoma H460 cells. We demonstrate that increased expression of Fn14 is associated with tumor cell migration, invasion, angiogenesis, tumor formation and metastases. NSCLC tumor cells expressing high levels of Fn14 showed increased in vitro migration through uncoated transwell inserts. Moreover, increased levels of Fn14 correlate with enhanced in vitro tumor cell invasion through the madrigal in an MMP-1-dependent manner. Fn14 was able to induce expression of integrin α6 what is suggestive of a novel mechanism of lung carcinoma migration and invasion through α6β1-Fn14 interactions. Thus, this research may be a step toward developing improved treatment strategies for non-small cell lung cancer by improved detection and inhibition of metastasis.
Supplementary Material
Supplemental Figure 1. Increased levels of vascularization visible in Fn14 cDNA tumors relative to Fn14 shRNA tumors. A) Hamper and immunohistochemistry for PECAM, an endothelial (vascular) cell marker, from serial sections of Fn14 over-expressing tumors are shown relative to almost no vascularization present in tumors formed from H460 cells expressing negligible levels of Fn14 which were significantly necrotic. B) Quantitative RTPCR histogram showing the mRNA expression levels of agiogenic factor IL-8 in tumors produced by mice injected with altered levels of Fn14. The data are presented as a mean± SEM with p<.05.
Acknowledgments
We would like to thank Dr. Anne Cress for providing anti-α6 interim antibody clone J1B5 and AA6α antibody for Western blot and IHC analyses. We also thank Dr. Jana for his valuable insights, critique of the manuscript and his assistance in preparing this manuscript. Immunohistochemistry was performed by the university of Arizona Cancer Center's Tissue Acquisition Cellular/Molecular Analysis Shared Service; RTPCR data were generated by the University of Arizona Cancer Center Genomics Shared Service; and mouse xerograph experiments were performed by the University of Arizona Experimental Mouse Shared Service; all services are supported by NCI grant 5P30CA023074. This work was support by the NCI CA023074-26 and a pilot grant to GW (ES06694).
Footnotes
Supplementary information is available in the online version of the paper.
References
- 1.Bray F, Sankila R, Ferlay J, Parkin DM. Estimates of cancer incidence and mortality in Europe in 1995. Eur J Cancer. 2002;38:99–166. doi: 10.1016/s0959-8049(01)00350-1. http://dx.doi.org/10.1016/S0959-8049(01)00350-1. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman PC, Mauer AM, Vokes EE. Lung cancer. Lancet. 2000;355:479–485. doi: 10.1016/S0140-6736(00)82038-3. http://dx.doi.org/10.1016/S0140-6736(00)82038-3. [DOI] [PubMed] [Google Scholar]
- 3.Whitsett TG, Cheng E, Inge L, Asrani K, Jameson NM, et al. Elevated Expression of Fn14 in Non-Small Cell Lung Cancer Correlates with Activated EGFR and Promotes Tumor Cell Migration and Invasion. American Journal of Pathology. 2012;181:111–120. doi: 10.1016/j.ajpath.2012.03.026. http://dx.doi.org/10.1016/j.ajpath.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yano T, Miura N, Takenaka T, Haro A, Okazaki H, et al. Never-smoking nonsmall cell lung cancer as a separate entity – Clinic pathologic features and survival. Cancer. 2008;113:1012–1018. doi: 10.1002/cncr.23679. http://dx.doi.org/10.1002/cncr.23679. [DOI] [PubMed] [Google Scholar]
- 5.AMERICAN CANCER SOCIETY. American Cancer Society: New York 2008; pp v.
- 6.Boffetta P. Epidemiology of environmental and occupational cancer. Ontogeny. 2004;23:6392–6403. doi: 10.1038/sj.onc.1207715. http://dx.doi.org/10.1038/sj.onc.1207715. [DOI] [PubMed] [Google Scholar]
- 7.Boffetta P. Human cancer from environmental pollutants: The epidemiological evidence. Mutation Research-Genetic Toxicology and Environmental Mutagenesis. 2006;608:157–162. doi: 10.1016/j.mrgentox.2006.02.015. http://dx.doi.org/10.1016/j.mrgentox.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Ferreccio C, Sancha AM. Arsenic exposure and its impact on health in Chile. Journal of Health Population and Nutrition. 2006;24:164–175. [PubMed] [Google Scholar]
- 9.Minna JD, Roth JA, Gazdar AF. Focus on lung cancer. Cancer Cell. 2002;1:49–52. doi: 10.1016/s1535-6108(02)00027-2. http://dx.doi.org/10.1016/S1535-6108(02)00027-2. [DOI] [PubMed] [Google Scholar]
- 10.Winkles JA. The Tweak – Fn14 cytokine-receptor axis: discovery, biology and therapeutic targeting. Nature Reviews Drug Discovery. 2008;7:411–425. doi: 10.1038/nrd2488. http://dx.doi.org/10.1038/nrd2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu LX, Zhang HH, Mei YF, Zhao YP, Zhang ZY. Role of tumor necrosis factor-like weak inducer of apoptosis (TWEAK)/fibroblast growth factor-inducible 14 (Fn14) axis in rheumatic diseases. Chinese Medical Journal. 2012;125:3898–3904. [PubMed] [Google Scholar]
- 12.Wiley SR, Winkles JA. TWEAK, a member of the TNF super family, is a multifunctional cytokine that binds the Tweak/Fn14 receptor. Cytokine & Growth Factor Reviews. 2003;14:241–249. doi: 10.1016/s1359-6101(03)00019-4. http://dx.doi.org/10.1016/S1359-6101(03)00019-4. [DOI] [PubMed] [Google Scholar]
- 13.Michaelson JS, Burkly LC. Therapeutic Targeting of TWEAK/Fn14 in Cancer: Exploiting the Intrinsic Tumor Cell Killing Capacity of the Pathway. Death Receptors and Cognate Legends' in Cancer. 2009;49:145–160. doi: 10.1007/400_2008_18. http://dx.doi.org/10.1007/400_2008_18. [DOI] [PubMed] [Google Scholar]
- 14.Zhou H, Marks JW, Hittelman WN, Yagita H, Cheung LH, et al. Development and Characterization of a Potent Immunoconjugate Targeting the Fn14 Receptor on Solid Tumor Cells. Molecular Cancer Terapeutics. 2011;10:1276–1288. doi: 10.1158/1535-7163.MCT-11-0161. http://dx.doi.org/10.1158/1535-7163.MCT-11-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willis AL, Tran NL, Chatigny JM, Charlton N, Vu H, et al. The fibroblast growth factor-inducible 14 receptor is highly expressed in HER2-positive breast tumors and regulates breast cancer cell invasive capacity. Molecular Cancer Research. 2008;6:725–734. doi: 10.1158/1541-7786.MCR-08-0005. http://dx.doi.org/10.1158/1541-7786.MCR-08-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng SL, Guo Y, Factor VM, Thorgeirsson SS, Bell DW, et al. The Fn14 immediate-early response gene is induced during liver regeneration and highly expressed in both human and marine hepatocellular carcinomas. AM J Pathol. 2000;156:1253–1261. doi: 10.1016/S0002-9440(10)64996-6. http://dx.doi.org/10.1016/S0002-9440(10)64996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tran NL, Mcdonough WS, Donohue PJ, Winkles JA, Berens TJ, et al. The human Fn14 receptor gene is up-regulated in migrating glioma cells in vitro and over-expressed in advanced glial tumors. American Journal of Pathology. 2003;162:1313–1321. doi: 10.1016/S0002-9440(10)63927-2. http://dx.doi.org/10.1016/S0002-9440(10)63927-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tran NL, Mcdonough WS, Savitch BA, Fortin SP, Winkles JA, et al. Increased fibroblast growth factor-inducible 14 expression levels promote glioma cell invasion via Rac1 and nuclear factor-kappaB and correlate with poor patient outcome. Cancer Res. 2006;66:9535–9542. doi: 10.1158/0008-5472.CAN-06-0418. http://dx.doi.org/10.1158/0008-5472.CAN-06-0418. [DOI] [PubMed] [Google Scholar]
- 19.Yoriki R, Akashi S, Sho M, Nomi T, Yamato I, et al. Therapeutic potential of the TWEAK/Fn14 pathway in intractable gastrointestinal cancer. Experimental and Therapeutic Medicine. 2011;2:103–108. doi: 10.3892/etm.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimada K, Fujii T, Tsujikawa K, Anai S, Fujimoto K, et al. ALKBH3 contributes to survival and angiogenesis of human urothelial carcinoma cells through NADPH oxidase and tweak/Fn14/VEGF signals. Cling Cancer Res. 2012;18:5247–5255. doi: 10.1158/1078-0432.CCR-12-0955. http://dx.doi.org/10.1158/1078-0432.CCR-12-0955. [DOI] [PubMed] [Google Scholar]
- 21.Gu LY, Dai L, Cao C, Zhu J, Ding CW, et al. Functional Expression of Tweak and the Receptor Fn14 in Human Malignant Ovarian Tumors: Possible Implication for Ovarian Tumor Intervention. Plops One. 2013;8 doi: 10.1371/journal.pone.0057436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang MG, Narita S, Tsuchiya N, Ma ZY, Nu-Makura K, et al. Overexpression of Fn14 promotes androgen-independent prostate cancer progression through MMP-9 and correlates with poor treatment outcome. Carcinogenesis. 2011;32:1589–1596. doi: 10.1093/carcin/bgr182. http://dx.doi.org/10.1093/carcin/bgr182. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Liu Y, Wei MJ, Mi XY, Wang EH. Clinical correlations and prognostic relevance of Fn14 expression in breast carcinoma. Histol Histopathology. 2013;28:859–864. doi: 10.14670/HH-28.859. [DOI] [PubMed] [Google Scholar]
- 24.Culp PA, Choi D, Zhang YK, Yin J, Seto P, et al. Antibodies to Tweak Receptor Inhibit Human Tumor Growth through Dual Mechanisms. Clinical Cancer Research. 2010;16:497–508. doi: 10.1158/1078-0432.CCR-09-1929. http://dx.doi.org/10.1158/1078-0432.CCR-09-1929. [DOI] [PubMed] [Google Scholar]
- 25.Lee JW, Juliano R. Mutagenic signal transduction by in-tegrin- and growth factor receptor-mediated pathways. Mol Cells. 2004;17:188–202. [PubMed] [Google Scholar]
- 26.Kohn KW, Zeeberg BR, Reinhold WC, Sunshine M, Luna A, et al. Gene expression profiles of the NCI-60 human tumor cell lines define molecular interaction networks governing cell migration processes. Plops One. 2012;7:e35716. doi: 10.1371/journal.pone.0035716. http://dx.doi.org/10.1371/journal.pone.0035716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pawar SC, Demetriou MC, Nagle RB, Bowden GT, Cress AE. Integrin alpha6 cleavage: a novel modifcation to modulate cell migration. Exp Cell Res. 2007;313:1080–1089. doi: 10.1016/j.yexcr.2007.01.006. http://dx.doi.org/10.1016/j.yexcr.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Damsky CH, Librach C, Lim KH, Fitzgerald ML, Mcmaster MT, et al. Integrin switching regulates normal trophoblast invasion. Development. 1994;120:3657–3666. doi: 10.1242/dev.120.12.3657. [DOI] [PubMed] [Google Scholar]
- 29.Jandova J, Beyer TE, Meuillet EJ, Watts GS. The matrix protein CCN1/CYR61 is required for alpha nu beta(5)-mediated cancer cell migration. Cell Biochemistry and Function. 2012;30:687–695. doi: 10.1002/cbf.2853. http://dx.doi.org/10.1002/cbf.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jandova J, Shi MJ, Norman KG, Stricklin GP, Sligh JE. Somatic alterations in mitochondrial Dna produce changes in cell growth and metabolism supporting a tumorigenic phenotype. Biochemical ET Biophysical Act-Molecular Basis of Disease. 2012;1822:293–300. doi: 10.1016/j.bbadis.2011.11.010. http://dx.doi.org/10.1016/j.bbadis.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang XA, Kazarov AR, Yang XW, Bontrager AL, Stipp CS, et al. Function of the tetraspanin CD151-alpha 6 beta 1 integrin complex during cellular morphogenesis. Molecular Biology of the Cell. 2002;13:1–11. doi: 10.1091/mbc.01-10-0481. http://dx.doi.org/10.1091/mbc.01-10-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jakubowski A, Ambrose C, Parr M, Lincecum JM, Wang MZ, et al. Tweak induces liver progenitor cell proliferation. Journal of Clinical Investigation. 2005;115:2330–2340. doi: 10.1172/JCI23486. http://dx.doi.org/10.1172/JCI23486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mercurio AM, Bachelder RE, Rabinovitz I, O'Connor KL, Tani T, et al. The metastatic odyssey: the in-terrain connection. Surge Once Cling N AM. 2001;10:313–328. viii–ix. [PubMed] [Google Scholar]
- 34.Wang DF, Fung JNT, Tuo Y, Hu LN, Chen C. TWEAK/Fn14 promote apoptosis of human endometrial cancer cells via capsize pathway. Cancer Letters. 2010;294:91–100. doi: 10.1016/j.canlet.2010.01.027. http://dx.doi.org/10.1016/j.canlet.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 35.Sanz-Pamplona R, Aragues R, Driouch K, Martin B, Oliva B, et al. Expression of Endoplasmic Reticulum Stress Proteins is a Candidate Marker of Brain Metastasis in both ErbB-2(+) and ErbB-2(-) Primary Breast Tumors. American Journal of Pathology. 2011;179:564–579. doi: 10.1016/j.ajpath.2011.04.037. http://dx.doi.org/10.1016/j.ajpath.2011.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asrani K, Keri RA, Galisteo R, Brown SAN, Morgan SJ, et al. The HER2- and Heregulin beta 1 (HRG)-Inducible Tnfr Superfamily Member Fn14 Promotes HRG-Driven Breast Cancer Cell Migration, Invasion, and MMP9 Expression. Molecular Cancer Research. 2013;11:393–404. doi: 10.1158/1541-7786.MCR-12-0542. http://dx.doi.org/10.1158/1541-7786.MCR-12-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanz AB, Sanchez-Nino MD, Carrasco S, Man-Zarbeitia F, Ruiz-Andres O, et al. Inflammatory Cytokines and Survival Factors from Serum Modulate Tweak-Induced Apoptosis in PC-3 Prostate Cancer Cells. Plops One. 2012;7 doi: 10.1371/journal.pone.0047440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwon OH, Park SJ, Kang TW, Kim M, Kim JH, et al. Elevated fibroblast growth factor-inducible 14 expression promotes gastric cancer growth via nuclear factor-kappab and is associated with poor patient outcome. Cancer Letts. 2012;314:73–81. doi: 10.1016/j.canlet.2011.09.016. http://dx.doi.org/10.1016/j.canlet.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 39.Zhou H, Ekmekcioglu S, Marks JW, Mohanne-Dali KA, Asrani K, et al. The Tweak Receptor Fn14 is a Terapeutic Target in Melanoma: Immunotoxins Targeting Fn14 Receptor for Malignant Melanoma Treatment. Journal of Investigative Dermatology. 2013;133:1052–1062. doi: 10.1038/jid.2012.402. http://dx.doi.org/10.1038/jid.2012.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pelekanou V, Notas G, Theodoropoulou K, Kampa M, Takos D, et al. Detection of the TNFSF Members BAFF, APRIL, Tweak and their Receptors in Normal Kidney and Renal Cell Carcinomas: Correlation with Clinical and Histological Development of the Disease. Journal of Pathology. 2011;224:S5–S5. doi: 10.3233/ACP-2011-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tran NL, Mcdonough WS, Savitch BA, Sawyer TF, Winkles JA, et al. The tumor necrosis factor-like weak inducer of apoptosis (TWEAK)-fibroblast growth factor-inducible 14 (Fn14) signaling system regulates glioma cell survival via NFkappaB pathway activation and BCL-XL/BCL-W expression. J Boil Chem. 2005;280:3483–3492. doi: 10.1074/jbc.M409906200. http://dx.doi.org/10.1074/jbc.M409906200. [DOI] [PubMed] [Google Scholar]
- 42.Pettersen I, Baryawno N, Abel F, Bakkelund WH, Zykova SN, et al. Expression of TWEAK/Fn14 in neuroblastoma: Implications in tumorigenesis. International Journal of Oncology. 2013;42:1239–1248. doi: 10.3892/ijo.2013.1800. [DOI] [PubMed] [Google Scholar]
- 43.Delamarre E, Taboubi S, Mathieu S, Berenguer C, Rigot V, et al. Expression of Integrin alpha 6 beta 1 Enhances Tumor genesis in Glioma Cells. American Journal of Pathology. 2009;175:844–855. doi: 10.2353/ajpath.2009.080920. http://dx.doi.org/10.2353/ajpath.2009.080920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaw LM, Chao C, Wewer UM, Mercurio AM. Function of the integrin alpha 6 beta 1 in metastatic breast carcinoma cells assessed by expression of a dominant negative receptor. Cancer Research. 1996;56:959–963. [PubMed] [Google Scholar]
- 45.Dai JY, Dou KF, Wang CH, Zhao P, Lau WB, et al. The interaction of HAb18G/CD147 with integrin alpha6beta1 and its implications for the invasion potential of human hematoma cells. BMC Cancer. 2009;9:337. doi: 10.1186/1471-2407-9-337. http://dx.doi.org/10.1186/1471-2407-9-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sawai H, Okada Y, Funahashi H, Matsuo Y, Takahashi H, et al. Interleukin-1alpha enhances the aggressive behavior of pancreatic cancer cells by regulating the alpha6beta1-integrin and urokinase plasminogen activator receptor expression. BMC Cell Biol. 2006;7:8. doi: 10.1186/1471-2121-7-8. http://dx.doi.org/10.1186/1471-2121-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cress AE, Rabinovitz I, Zhu W, Nagle RB. The alpha 6 beta 1 and alpha 6 beta 4 integrants in human prostate cancer progression. Cancer Meat stasis Rev. 1995;14:219–228. doi: 10.1007/BF00690293. http://dx.doi.org/10.1007/BF00690293. [DOI] [PubMed] [Google Scholar]
- 48.King TE, Pawar SC, Majuta L, Sroka IC, Wynn D, et al. The Role of Alpha 6 Integrin in Prostate Cancer Migration and Bone Pain in a Novel Xerograph Model. Plops One. 2008;3 doi: 10.1371/journal.pone.0003535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sroka IC, Anderson TA, Mcdaniel KM, Nagle RB, Gretzer MB, et al. The laminin binding integrin alpha6beta1 in prostate cancer per neural invasion. J Cell Physiology. 2010;224:283–288. doi: 10.1002/jcp.22149. http://dx.doi.org/10.1002/jcp.22149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. http://dx.doi.org/10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 51.Mori M, Mimori K, Sadanaga N, Inoue H, Tanaka Y, et al. Prognostic impact of tissue inhibitor of matrix metalloproteinase-1 in esophageal carcinoma. Into J Cancer. 2000;88:575–578. doi: 10.1002/1097-0215(20001115)88:4<575::aid-ijc9>3.0.co;2-c. http://dx.doi.org/10.1002/1097-0215(20001115)88:4<575∷AID-IJC9>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 52.Li M, Xiao T, Zhang Y, Feng L, Lin DM, et al. Prognostic significance of matrix metalloproteinase-1 levels in peripheral plasma and tumor tissues of lung cancer patients. Lung Cancer. 2010;69:341–347. doi: 10.1016/j.lungcan.2009.12.007. http://dx.doi.org/10.1016/j.lung-can.2009.12.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Increased levels of vascularization visible in Fn14 cDNA tumors relative to Fn14 shRNA tumors. A) Hamper and immunohistochemistry for PECAM, an endothelial (vascular) cell marker, from serial sections of Fn14 over-expressing tumors are shown relative to almost no vascularization present in tumors formed from H460 cells expressing negligible levels of Fn14 which were significantly necrotic. B) Quantitative RTPCR histogram showing the mRNA expression levels of agiogenic factor IL-8 in tumors produced by mice injected with altered levels of Fn14. The data are presented as a mean± SEM with p<.05.


