Abstract
Elevated dopamine function and alterations in the medial temporal lobe structure and function (MTL) are two of the most robust findings in schizophrenia, but how interactions between these abnormalities underlie the onset of psychosis is unclear. Although several preclinical models of psychosis have been proposed, the methylazoxymethanol acetate (MAM) rodent model provides a mechanistic account linking these two clinical observations. The model proposes that psychosis develops as a result of a perturbation of MTL function, leading to elevated striatal dopamine dysfunction. We review a number of recent neuroimaging studies that examine components of the putative model in people with an ultra high risk (UHR) of psychosis. Whilst data from these studies are broadly consistent with the MAM model, that the potential for comparing various kinds of neurobiological data across animal and human studies imposes some limitations on what can be inferred from these data. Going forward, longitudinal studies are needed to explicitly test the model’s predictions in UHR populations.
Keywords: Neurobiology, Psychosis, Neuroimaging, Animal Research, Schizophrenia, Prodrome
The Neurobiology of Psychosis Onset
In recent years several animal models have been developed to advance research into the neurobiological mechanisms involved in the development of psychosis and emergence of symptoms associated with the disorder (see Box 1). The development of these preclinical models has been informed by clinical observations in patients with schizophrenia and psychosis. Two of the most robust and replicated clinical findings are elevated presynaptic dopamine function in the midbrain and striatum [1–3], and neuroanatomical and physiological alterations in the hippocampus and adjacent medial temporal lobe (MTL) structures [4–6]. However, these neurobiological changes have largely been identified through independent bodies of work, so how they interact during the development of psychosis is still unclear. Whilst dopamine dysfunction has historically been regarded as the primary factor underlying psychosis [7], recent work in experimental animals, using the methylating agent methylazoxymethanol acetate (MAM) has highlighted the role of a hippocampal-midbrain-striatal circuit, and introduced the concept that subcortical dopamine function is elevated as a consequence of changes in descending outputs from the MTL [8, 9]. The MAM animal model is appealing as it incorporates a disruption of brain development, which is thought to be fundamental to psychotic disorders [10–12]. Brain development is experimentally perturbed by the administration of methylazoxymethanol acetate to pregnant rats on gestational day 17 [12] (see Box 2 for details). An elaboration of this model can be extended to the psychopathology of psychosis, with the suggestion that the elevation in dopamine function leads to the formation of abnormal associations and that this underlies the generation of symptoms such as delusions [13, 14]. Ultimately, useful animal models of disease need to provide a framework in which to generate testable predictions for clinical research. Although just one of several animal models from which hypotheses can be derived, the MAM model provides a particularly promising framework for clinical research in psychosis (see Box 4).
Box 1. Rodent Developmental Models of Psychosis.
Prenatal immune activation
Based on the premise that prenatal infection acts as a “neurodevelopmental disease primer” for a number of chronic mental illnesses, including schizophrenia, these models use maternal gestational exposure to: human influenza virus, the viral mimic polyriboinosinic-polyribocytidilic acid (Poly I:C), the bacterial endotoxin lipopolysaccharide, the locally acting inflammatory agent turpentine, or selected inflammatory cytokines [18, 19].
Anatomy: Reduced thickness of the neocortex and hippocampus, Decreased myelination and axonal diameters in the hippocampus, No loss of oligodendrocytes
Pharmacology: Reductions of cortical Reelin immunoreactivity in the offspring
While this model may invite further research into the mechanisms involved in schizophrenia pathology, more conclusive data on the involvement of Reelin in schizophrenia and on the behavioral phenotype of the animal model are required before conclusions about the relevance of this model for schizophrenia can be made.
Neonatal hippocampal lesion
A neurodevelopmental model is generated by using adult rats with neonatal and adult ibotenic acid lesions of the ventral hippocampus, involving regions that directly project to the prefrontal cortex, i.e., ventral hippocampus and ventral subiculum and that correspond to the anterior hippocampus in humans [20].
Anatomy: Frontal lobe abnormalities, dopamine system dysregulation. Molecular changes in the PFC (decreased NAA levels, GAD67 mRNA, BDNF mRNA), Shorter and less branched basilar dendrites and reduced spine density at the mPFC
Neurophysiology: Increased mesolimbic/nigrostriatal dopamine transmission
Pharmacology: Amphetamine-induced hyperactivity, Apomorphine-induced stereotypies, Reduced catalepsy to haloperidol MK-801, PCP-induced hyperactivity
Behavior: Sensorimotor gating deficits, deficits in PPI and latent inhibition, impaired social behaviors and working memory problems
These models show the plausibility of neurodevelopmental damage having selected deleterious effects after a prolonged period of relative normalcy. However, lesion models have limited construct validity, as the schizophrenic brain does not manifest a “lesion” analogous to any of these models.
Chronic phencycidine (PCP)
These models involve the pharmacological blockade of NMDA receptors in adult animals, based on observations that noncompetitive NMDA antagonists, such as phencyclidine (PCP) and ketamine, exacerbate some psychotic symptoms in schizophrenic patients and have psychotomimetic effects in normal humans [21, 22].
Anatomy: Fewer PFC synapses, decreased parvalbumin (PV+) in hippocampus, Increased astroglia process density w/o change in glia number
Neurophysiology: Dysregulation of the firing patterns of mesolimbic and mesocortical dopaminergic neurons
Behavior: Sensorimotor gating deficits, reversal learning and extra-dimensional set-shifting, impaired social interactions, No perseverative responding
Unlike the etiological or neonatal lesion models, the PCP approach does not, however, address the developmental component of schizophrenia.
Methylazoxymethanol (MAM)
MAM administration to pregnant rats to disrupt embryonic brain development [8, 9, 12, 23].
Histology/Anatomy: Decreases in cortical thickness and increases in neuronal density (hippocampus, parahippocampal cortex, medial prefrontal cortex) and no differences in neocortical neuron number; Decreased parvalbumin expression in ventral hippocampus, medial and orbital prefrontal cortex.
Neurophysiology: Abnormalities in corticocortical synaptic transmission, Striatal hyperdopaminergia, Altered glutamatergic neurotransmission in the hippocampus, Disruption of evoked gamma rhythms
Pharmacology: Increased responsivity to psychostimulants (amphetamine, phencyclidine), rapid onset of antipsychotic drug effects on DA neurons
Behavior: Cognitive dysfunction, sensorimotor gating deficits, latent inhibition reversal learning, extradimensional set-shifting, prepulse inhibition, reduced social interaction, perseverative responding
Box 2. The MAM-treated Rat as a Pathophysiological Model of Schizophrenia.
Adult rats exposed to MAM (mg/kg) in utero at gestational day 17 show selective histopathology in mediodorsal thalamus, hippocampus, parahippocampal and prefrontal cortices [12] which may in part be due to decreased density of parvalbumin positive GABAergic interneurons throughout these regions [24]. In particular, reduced parvalbumin expression is seen in MAM treated rats in several regions associated with schizophrenia pathology such as the orbitofrontal and medial prefrontal cortex and hippocampus [25]. Reduced parvalbumin expression may impact on certain classes of cortical GABAaergic interneurons known to be decreased in schizophrenia [26].
Crucially, MAM-treated rats display elevated striatal dopaminergic activity, which is normalized by inactivating the subiculum, an output region of the MTL that projects to the nucleus accumbens via a polysynaptic pathway involving glutamatergic pyramidal neurons [8]. In healthy rats, activation of the subiculum increases subcortical dopamine activity [27]. In MAM-treated rats, over-activity in reciprocal signaling pathways between the MTL and striatum [8] due to a loss of γ-aminobutyric acid (GABA)ergic inhibition of pyramidal neurons in the MTL, leads to increased glutamate release in the striatum [28]. Increased activity in glutamate pyramidal neurons in the hippocampus leads to an increase in glutamate release in the striatum. This stimulates GABAergic neurons that project from the striatum to the ventral pallidum, thereby increasing inhibition of ventral pallidum GABAergic neurons, leading to the disinhibition of midbrain dopaminergic neurons and the increase in the release of dopamine from their terminals in the striatum. Dopaminergic neurons in the midbrain project back to the striatum and hippocampus, producing further disinhibition and forming a positive feedback loop [27]. The projections from the MTL to the striatum mainly terminate in its ventral (limbic) portion [29], which can, in turn, influence activity of dopamine neurons projecting to more dorsal (associative) striatal areas by a series of ‘spiralling loop’ connections with the midbrain [30, 31], and through MTL projections that overlap with those from prefrontal cortex in the striatum [32].
Box 4. MAM-model Based Predictions for Human Studies and Methodological Limitations.
The MAM model provides a framework for making testable predictions for clinical research studies in psychosis. For example, according to this model, people at high risk of psychosis, or in the early stages of a psychotic disorder, would be expected to show, relative to healthy controls:
-
Increased resting state perfusion and activation in the MTL
Limitation: Whilst MR and PET perfusion imaging provide an absolute measure of resting cerebral blood flow (rCBF), functional MRI, provides only a proximal and relative measure of neuronal activation. Thus predicting the polarity of a given effect is more difficult.
-
Increased glutamate levels in the MTL and striatum
Limitation: The MAM model predicts increased glutamate release in the pathways projecting from the ventral hippocampus (subiculum) to the ventral striatum. Measurement of glutamate concentrations in humans using MRS are difficult at such an anatomically localized level.
-
Reduced cortical and MTL GABA levels.
Limitation: Whilst GABA levels can be measured in the cortical areas using 1H-MRS, reliable measurement is more difficult in the MTL.
-
Increased dopamine release and neuronal activity in the midbrain and ventral striatum
Limitation: 18-Fluorodopa PET measures presynaptic dopamine syntheses but is not a direct measure of DA release in the synapse.
-
Altered associations between glutamate levels in MTL and striatum and dopamine function in the striatum and midbrain.
Limitation: The MAM model demonstrates a causal relationship between increased activity in ventral hippocampal pyramidal neurons, increased glutamate release and increased DA release in the VTA and striatum. Currently non-invasive neuroimaging in humans can only establish correlational associations between different neurotransmitter function/levels.
-
Altered functional relationships between the MTL, striatum and midbrain related to abnormal processing of novelty / motivational / emotional salience (i.e. attribution of salience to stimuli that would normally be non-salient).
Limitation: Testing this prediction requires complex effective connectivity modeling. The roles of GABA, Glu and DA signaling in such a model could only be inferred.
Human neuroimaging studies allow the measurement of brain structure, function and neurochemistry, all important elements of the MAM model. Moreover, recent multi-modal neuroimaging work has attempted to integrate different neuroimaging modalities to examine how these neurobiological factors interact. It should be considered however, that the potential for comparing various kinds of neurobiological data across animal and human studies has some limitations. In the context of the MAM model, and its predictions for clinical studies, human neuroimaging methods can only provide proximal measures of neuronal and neurotransmitter activity. For example, the electrophysiology techniques employed in studies of experimental animals provide a direct measure of neuronal activity that cannot be achieved with functional Magnetic Resonance Imaging (fMRI). Furthermore, microdialysis in freely moving animals allows for a dynamic measurement of neurochemistry that cannot be achieved with Positron Emission Tomography in humans. Similarly, Magnetic Resonance Spectroscopy (1H-MRS) can provide only a crude measure of neurotransmitter concentrations across large areas of tissue and cannot dissociate between metabolic and vesicular neurotransmitter concentrations, although glutamine levels are thought to be proportional to the vesicular glutamate fraction [15]. That said, the BOLD signal (measures with functional MRI) does reflect the neural response elicited by a stimulus [16] and is a ‘down-stream’ physiological measure of the neural activity directly measured by electrophysiological recordings. Furthermore, animal 1H-MRS allows the quantification of glutamate and GABA concentrations that can be verified with ex-vivo biochemical assays therefore providing a relevant measure of these neurotransmitters for preclinical research [17]. With these methodological caveats in mind, we review the human neuroimaging literature relevant to dysfunction in the putative hippocampal-midbrain-striatal circuit in schizophrenia and in individuals at ultra high risk (UHR) of developing the disorder, and discuss the extent to which the findings are consistent with the MAM model.
Are Data from Studies in Schizophrenia Consistent with the MAM Model?
Neuroimaging studies in patients who have already developed a psychotic disorder have examined several different elements of the putative model (Box 4 describes testable hypothesis for clinical studies derived from the MAM model).
Studies using positron emission tomography (PET) and single-photon emission computed tomography (SPECT) have shown that in schizophrenia subcortical dopamine synthesis and release are increased [7, 36–42]. Structural MRI studies have demonstrated reductions in MTL volume [4, 43–46], while fMRI studies have revealed altered MTL activity at rest [47–51], and altered MTL activation during cognitive tasks involving the processing of salient information in the domains of emotion [52–54], novelty [55], and reward processing [56]. Schizophrenia has also been associated with increased hippocampal glutamate levels [57, 58], although increased glutamate levels have also been identified in several regions other than the MTL [57, 59–61]. Moreover, both increased [62, 63] and decreased glutamate levels have been reported in cortical and striatal regions [64–66]. These inconsistencies may be related to between-study and within-study variation in the age, illness stage and the treatment history of the patients studied. One study has reported that GABA levels in the hippocampus are increased in schizophrenia [67], while a more recent study found no differences between patients with schizophrenia and healthy controls [66]. While these observations are broadly consistent with the MAM model, their interpretation is potentially confounded by the effects of illness and its treatment with antipsychotic medication [68–71].
The effects of antipsychotics may be particularly confounding, as these drugs act on central dopamine receptors [2] and even a small amount of treatment may have an effect: a single dose of antipsychotic medication can increase hippocampal perfusion in healthy volunteers [72]. Brain glutamate levels are also affected by antipsychotic medication [73], and may also vary with the stage of psychotic illness, with different findings in patients studied at illness onset compared to patients who have a long duration of illness [57].
Studies in Subjects at UHR for Psychosis
The MAM model is particularly relevant to the development of psychosis, rather than to the established disorder. Hence, experimental studies in people who are experiencing prodromal symptoms and are at high risk of becoming psychotic are especially useful in the context of assessing the validity of the MAM model. These individuals present to mental health services with a clinical syndrome characterized by ‘attenuated’ psychotic symptoms, and about a third will develop a psychotic disorder within 2 years [74]. They have thus been termed at ‘Ultra High Risk’ for psychosis. A further advantage in studying this group is that some of the factors that potentially confound the interpretation of data from chronic patients, such as antipsychotic medication, are minimized. Finally, longitudinal studies in UHR subjects provide a means of examining the human brain before and after the onset of psychosis in the same individual, which is an ideal paradigm of investigating factors relevant to the onset of psychosis. The present review has therefore particularly focused on data from this group.
Dopamine Dysfunction
PET studies have recently found that dopamine function is elevated in people at UHR for psychosis [75, 76], particularly in the subgroup that subsequently develops a psychotic disorder [77, 78]. This is evident in both the striatum and in the midbrain [77, 78] and a longitudinal PET study suggests that there is a progressive increase in striatal dopamine function as psychosis develops [79]. Striatal dopamine dysfunction in UHR cohorts is reported in the associative subdivision of the striatum, whereas no effects have been found on the ventral striatum [75–77, 80].
Medial Temporal Lobe Abnormalities
Several MRI studies using region of interest (ROI) or whole-brain voxel-based morphometric (VBM) methods have reported reduced hippocampal grey matter volume in UHR individuals relative to healthy controls [81–86]. Although not all studies have found reductions in hippocampal volume (e.g., [87]), a meta-analysis found that, overall, there was a significant reduction in MTL volume in UHR subjects [88]. There is also evidence that these reductions are greatest in the subgroup of UHR subjects who develop psychosis subsequent to scanning [88–90]. Within the MTL, reductions in volume have often been localized to the anterior part of the left parahippocampal gyrus [89, 90].
MTL function is also altered in UHR populations. In an fMRI study using a verbal memory task, UHR subjects showed reduced activation in the left parahippocampal gyrus during word encoding, and altered hippocampal engagement bilaterally during correct word recognition [91]. Furthermore, in a longitudinal fMRI study, clinical and functional improvement in UHR subjects was associated with a longitudinal normalization of altered activation in the right parahippocampal gyrus during a working memory task [92]. Increased hippocampal activation during a verbal fluency task has also been reported in UHR subjects that developed psychosis relative to those that did not [78]. Increases in activation in MTL regions, particularly in the amygdala, have been reported in UHR cohorts during abnormal emotional salience attribution, in terms of hyperactivation of emotional brain regions to otherwise neutral stimuli [93]. Interestingly, such hyperactivation may predict levels of psychotic symptoms and global functioning [94]. Schobel and colleagues [95] found that resting regional cerebral blood volume (CBV) was increased in the CA1 region of the hippocampus in UHR subjects who subsequently developed psychosis. A longitudinal follow-up in this cohort showed that the onset of psychosis was associated with a progressive increase in CBV that extended from the CA1 region into the subiculum [95].
Glutamate and GABA dysfunction
1H-MR spectroscopy (MRS) in UHR individuals suggests that glutamate levels in the thalamus are lower than in healthy controls [96], and are associated with poor clinical and functional outcomes [97]. Independent work has reported that both UHR and first episode subjects have higher levels of glutamate in the caudate nucleus than controls [80], and that UHR subjects that subsequently developed psychosis had higher striatal glutamate levels than UHR subjects who did not become psychotic [98]. A study that examined a medial prefrontal region failed to find altered glutamate or glutamine in UHR or first episode subjects, but did find reductions in chronic patients [99].
Currently, there are no published neuroimaging studies reporting GABA concentrations in UHR subjects. These are, however, of great interest, as the MAM model proposes that excessive glutamatergic activity in the MTL is secondary to GABA dysfunction.
Multimodal Imaging Studies in UHR Subjects
Multimodal neuroimaging studies provide a particularly useful source of data for examining putative interactions within the MAM model between MTL activity, glutamate and dopamine function. A number of recent studies have thus acquired different types of neuroimaging data from the same UHR subjects (summarized in Table 1).
TABLE 1.
Multimodal imaging studies in people at Ultra High Risk of psychosis.
| Ref. | Modalities | Findings | HC | UHR | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| n | Age | M/F | n | Age (SD) | M/F | ||||||
| Roiser et al., 2013 | fMRI (salience attribution task) & 18F-DOPA PET | UHR: negative correlation between striatal dopamine synthesis capacity and hippocampal activation to irrelevant stimulus features. HC: opposite correlation | 18 | 26.5 (6) | 10/8 | 18 | 25.7 (4.3) | 7/11 | |||
| Schobel et al., 2013 | Perfusion MRI & sMRI | UHR: Hippocampal hypermetabolism at baseline predicted hippocampal atrophy, which occurred during progression to psychosis | - | - | - |
|
|
|
|||
| Allen et al., 2012 | fMRI (episodic memory task) & 18F-DOPA PET | UHR: positive correlation between hippocampal activation during memory task and 18F-DOPA uptake. HC: opposite correlation | 14 | 25.7 (4.1) | 9/5 | 20 | 26.3 (5.1) | 10/10 | |||
| Fusar-Poli et al., 2011 | fMRI (VF task) & 18F-DOPA PET | UHR: positive correlation between striatal dopamine synthesis capacity and activation in the IFC. HC: no correlation | 14 | 25.5 (3.6) | 10/4 | 20 | 26.7 (5) | 11/9 | |||
| Fusar-Poli et al., 2011 | fMRI (VF task) & 1H-MRS | UHR: positive association between thalamic glutamate levels and activation in hippocampus and in temporal cortex; negative association between thalamic glutamate levels and activation in prefrontal cortex. HC: opposite correlation in the prefrontal and temporal cortex and in the hippocampus. | 17 | 25.5 (3.6) | 10/7 | 24 | 26.7 (5) | 23/1 | |||
| Valli et al., 2011 | fMRI (episodic memory task) & 1H-MRS | HC: positive correlation between MTL activation during episodic encoding and MTL glutamate. UHR: no correlation | 14 | 25.6 (3.7) | 6/8 | 22 | 25.72 (4.9) | 12 / 10 | |||
| Fusar-Poli et al., 2010 | fMRI (WM task) & 18F-DOPA PET | UHR: negative correlation between striatal dopamine synthesis capacity and prefrontal activation. HC: opposite correlation | 14 | 25.5 (3.6) | - | 20 | 26.6 (5) | - | |||
| Stone et al., 2010 | 1H-MRS & 18F-DOPA PET | UHR: negative relationship between hippocampal glutamate levels and striatal dopamine synthesis capacity. HC: no correlation | 12 | - | - | 16 | - | - | |||
| Stone et al., 2009 | 1H-MRS & sMRI | UHR: level of thalamic glutamate positively correlated with GMV in the MTL and insula. HC: no correlation | 27 | 25 (4) | 14/13 | 27 | 25(5) | 19/8 | |||
Note: CBV, Cerebral Blood Volume; FG, Frontal Gyrus; fMRI, functional magnetic resonance imaging; Glx, glutamate plus glutamine; GMV, Gray Matter Volume; HC, Healthy Controls; IFG, Inferior Frontal Gyrus; MMN, Mismatch Negativity; MRS, magnetic resonance spectroscopy; MTL, Medial Temporal Gyrus; NP, No Psychosis; P; Psychosis; R, Right; sMRI, structural magnetic resonance imaging; UHR, Ultra High Risk; VF, verbal fluency; WM, working memory; WMV, White Matter Volume.
Glutamate and Grey Matter Volume
Stone and colleagues [96] investigated the relationship between regional glutamate levels and grey matter volume by combining 1H-MRS and volumetric MRI. In UHR subjects, the degree to which thalamic glutamate levels were reduced was directly correlated with the magnitude of the reduction in grey matter volume in the MTL. No such relationship was evident in the controls. This suggests that thalamic glutamatergic dysfunction in UHR individuals is associated with cortical structural abnormalities.
Glutamate MRS and fMRI
Animal studies have shown that hippocampal glutamate is critically involved in memory encoding [100]. Combining fMRI data acquired during memory encoding and 1H-MRS glutamate measures, Valli and colleagues [101] found that in control subjects, MTL activation was positively correlated with hippocampal glutamate levels, but that this relationship was not evident in UHR subjects. This suggests that in UHR subjects there may be a breakdown in the normal relationship between hippocampal glutamate levels and MTL activation. Another fMRI study in UHR subjects examined the relationship between thalamic glutamate levels and activation during a verbal fluency task [102]. The relationship between thalamic glutamate levels and both MTL and PFC activation was significantly altered in UHR subjects compared to controls. Further work suggests that the relationship between thalamic glutamate levels and PFC function is particularly perturbed in UHR subjects with poor functional outcomes [103].
Glutamate and Dopamine
The MAM model proposes that striatal hyperdopaminergia is driven by upstream changes in hippocampal glutamate function. Using 1H-MRS and 18F-DOPA PET data from the same individuals, Stone and colleagues [104] found a negative relationship between MTL glutamate and striatal dopaminergic function in UHR subjects that was absent in controls, and was most marked in the UHR subjects that subsequently developed psychosis.
Dopamine and fMRI
Allen and colleagues [105] used a verbal memory task to examine the relationship between MTL activation and striatal dopaminergic function, combining fMRI and 18F-DOPA PET in the same subjects. The relationship between striatal dopamine function (in the limbic subdivision) and MTL activation during both verbal encoding and recognition in UHR subjects was significantly different to that in controls. In controls, there was a negative correlation between activation averaged across the subiculum and hippocampus during correct recognition trials, and dopamine levels in the limbic striatum: this correlation was absent in the UHR group.
Using a salience attribution task, Roiser and colleagues report that UHR subjects attributed inappropriate importance to unrewarded stimuli [106], and that this was associated with altered activation in the ventral striatum and an altered relationship between hippocampal responses and striatal dopamine function [106]. These findings are broadly consistent with the model proposed by Kapur and colleagues, which suggests that salience processing is perturbed prior to the onset of psychosis, and is driven by abnormal striatal dopamine function [34].
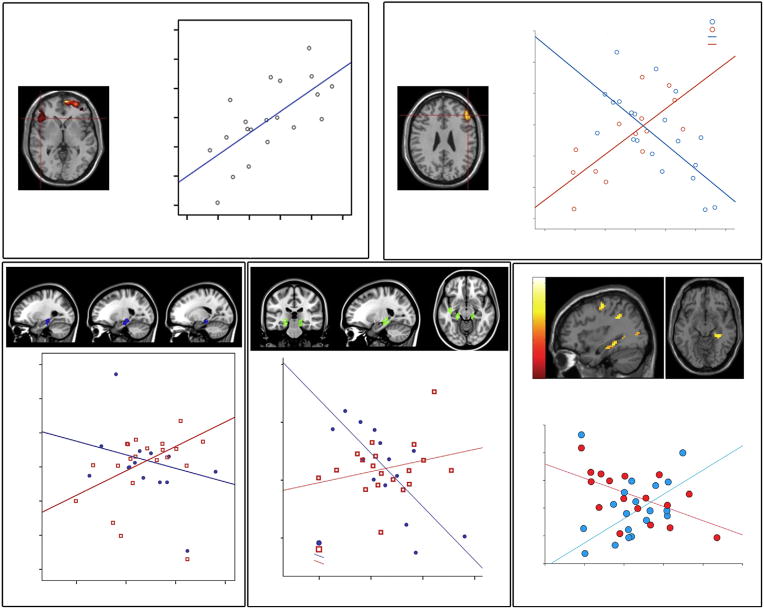
Fusar-Poli and colleagues combined fMRI and 18F-DOPA PET to examine the relationship between prefrontal cortical activation (using a working memory task) and striatal dopamine function in people at UHR of psychosis [107]. In UHR subjects, dorsolateral PFC activation was negatively correlated with presynaptic dopamine function in the associative striatum, whereas in controls the correlation was positive. A similar study using 18F-DOPA PET and fMRI in conjunction with a verbal fluency task found that in UHR subjects, the ventral PFC response was positively correlated with the level of striatal dopamine function, a relationship that was absent in controls [108]. Collectively, these findings suggest that subcortical dopamine dysfunction in UHR subjects is related to alterations in both medial temporal and prefrontal function. This is consistent with the notion that descending inputs from cortical regions may drive elevated dopamine function in psychosis [8, 9]. Furthermore, while the MTL and PFC each have a well-established role in cognitive processes, the precise ways in which these regions interact to support these functions is not fully understood. Research in rodents shows that a projection of neurons extending from the CA1 region of the hippocampus and subiculum to the PFC is critically involved in aspects of cognition related to executive function [109]. The implications for the MAM model of putative MTL-PFC dysregulation in psychosis are currently unclear. Figure 1 displays MTL/PFC – dopamine correlations in UHR subjects across a range of cognitive tasks.
Figure 1.
Altered relationship between cortical activation and subcortical dopamine function in subjects at Ultra High Risk of Psychosis (UHR). (A) Functional activation in the left inferior frontal gyrus (IFG) during verbal fluency is positively correlated with presynaptic dopaminergic activity in the associative striatum [108]. (B) Functional activation in the right IFG during working memory is positively correlated in healthy controls but negatively correlated in UHR subjects [107]. (C) Functional activation in the medial temporal lobe (MTL) during verbal encoding is positively correlated with subcortical dopamine levels in UHR subjects but not in healthy controls [105]. (D) MTL activation during verbal recognition was negatively correlated with dopamine levels in the healthy control group but not in the UHR group [105]. (E) Abnormal interaction between functional activation in right hippocampus and subcortical dopamine function during the processing of reward salience [106]. Ki = 18F-fluorodopa influx constants. All figures adapted with permission from the author’s original work.
Longitudinal Multimodal Studies
Schobel and colleagues [110] reported that UHR subjects showed increased hippocampal perfusion in the CA1 subfield of the hippocampus at presentation, and that this was associated with a longitudinal reduction in hippocampal volume during the progression to psychosis, especially in the CA1 field and the subiculum/ventral hippocampus. Although this study did not examine interactions with striatal dopamine levels or in vivo measures of glutamate function, in a series of related experiments in mice, the authors found that similar changes in hippocampal CBV and volume could be induced by ketamine, and were dependent on local glutamate release. Furthermore, these volumetric changes were associated with a local reduction in parvalbumin positive GABA neurons. Excessive glutamate concentrations around neurons can result in excitotoxicity through the influx of calcium ions [111]. The notion that increased glutamate levels might lead to reduction in grey matter volume is consistent with data from neuroimaging studies that have combined MRS and MRI in first-episode patients and individuals at UHR [61, 96].
To What Extent Do the Human and Animal Data Converge?
The data reviewed above suggest that a number of findings from neuroimaging studies in schizophrenia patients are broadly consistent with the MAM model. Studies in UHR populations have produced similar results, revealing altered interactions between regions and neurotransmitter systems implicated in the MAM model.
A prediction central to the MAM model is increased dopamine function in the ventral (limbic) striatum, although MAM rats also display hyperactivity in the lateral VTA, which projects to the associative striatum [31]. Independent groups have confirmed striatal dopamine dysfunction in schizophrenia patients and people at UHR but mainly in the associative (dorsal) striatum, with a lack of effects in its ventral portion [75–77, 80]. These findings in humans represent an inconsistency between the predictions derived from the MAM model and clinical observations. One multimodal imaging study reports an association between MTL functional activation and dopamine levels in the ventral striatum [105] and it has been established that MTL projections to the ventral striatum can influence activity of dopamine neurons in the associative striatum via connections with the midbrain [30, 31]. Nevertheless, an important question still remains about dopaminergic dysfunction in different striatal subdivisions and further research is warranted to resolve the disparity between MAM model predictions and clinical observations regarding dopamine dysfunction.
Whilst evidence of increased caudate glutamate levels [80], and of altered relationship between MTL glutamate levels and striatal dopamine in UHR populations, are in line with the MAM model, there is also evidence that glutamate levels are reduced in the thalamus [96]. Although the MAM model does not make specific predictions about glutamate activity in this region, the thalamus is a key component of the circuit that links the MTL and PFC to the striatum and the midbrain [112]. According to the MAM model, cortical glutamate levels are increased due to a reduction in GABAergic inhibition of local pyramidal neurons [14]. However, MRS studies in patients with schizophrenia have reported both increased and decreased cortical glutamate levels. Decreases have been described in the medial prefrontal cortex [57], whereas increases have been reported in the hippocampus [67]. Most studies in unmedicated first episode psychosis patients have found elevated glutamate and glutamine levels in the hippocampus, anterior cingulate and thalamus [58, 60]. This potentially confusing set of findings may partly reflect a variation in the nature of alterations in glutamate levels according to the stage of psychotic illness [57]. Longitudinal MRS studies could help to resolve this issue, but there are few such studies in the literature [61, 97]. Nevertheless, across a variety of regions, glutamate levels in UHR and psychotic subjects have often been found to be increased. The reduction in thalamic glutamate levels could be related to increased cortical glutamate levels; overactivity in thalamic pyramidal neurons (perhaps due to NMDA receptor dysfunction on local GABAergic interneurons) may result in a depletion of local glutamate levels, but an increase in glutamate release from the dense projections of the thalamic neurons to cortical regions. This would be consistent with evidence of both reduced thalamic and increased cortical glutamate levels in the same UHR subjects [96].
The MAM model also predicts that cortical GABA levels should be decreased in psychosis due to loss and dysfunction of inhibitory GABAergic interneurons. There have only been a small number of MRS studies of GABA in patients with schizophrenia, but these have found increases in cortical GABA levels [67]. This has been interpreted as reflecting a compensatory increase in firing by unaffected GABAergic interneurons [113]. As with MRS studies of glutamate, disease stage may influence the nature of the findings: for example, GABA levels in the basal ganglia appear to be reduced in patients in the early stage of psychosis, whereas increased GABA levels in the anterior cingulate cortex and the parieto-occipital cortex have been reported in chronic patients [15]. Antipsychotic medication may also affect MRS measures of GABA [15]. To date, no studies have examined GABAergic function in medication naïve patients with psychosis, or in UHR subjects. Similarly, how GABA levels relate to glutamate levels in the same individual has yet to be investigated.
It is important to bear in mind that studies in UHR and psychotic subjects have also identified neurobiological findings in other regions and pathways that are not directly related to the MAM model. Thus, structural and functional alterations in UHR and psychotic subjects are not restricted to a circuit involving the MTL, striatum and midbrain: rather, the onset of psychosis had also been associated with alterations in the structure, function and connectivity of the prefrontal, anterior cingulate, lateral temporal and cerebellar cortices [82, 89–91, 114, 115]. Similarly, the model does not postulate a mechanistic role for other neurobiological factors that are potentially relevant to psychosis, such as the endocannabinoid system [116] and neuroinflammation [117, 118] (Box 5).
Box 5. Outstanding Questions.
What Does the Model Not Explain About the Onset of Psychosis?
The MAM model provides a testable neurobiological framework in which to formulate hypotheses about the development of psychosis in humans. However, there are some factors that are implicated in the development of psychosis that it does not incorporate. Psychosis has a strong genetic component ([119]), but the role of specific risk genes in the model has yet to be determined.
Work in experimental animals suggests that stress can influence brain GABA function in the MTL [120–122]. MAM-treated rats are anxious and hyper-responsive to stress [123], and peripubertal administration of benzodiazepines prevents MAM-induced pathology, blocking the elevation in dopamine function normally seen in MAM-treated animals [124]. Stress could also lead to changes in the MTL through its effects on cortisol levels. Cortisol levels are altered in UHR subjects [125–127], and are associated with reduced MTL volume in first episode psychosis [128].
Alterations in the PFC could influence the MAM model circuit in a number of ways. Research in rodents shows that neurons in the CA1 region of the hippocampus and the subiculum project directly to the PFC [109]. It is possible that the hippocampal–PFC–striatal projections regulate dopamine levels at rest, but in the presence of salient stimuli, the direct connection between the hippocampus and striatum by-passes the PFC [129, 130]. The PFC is also one of the few cortical areas that has direct projections to dopaminergic neurons in the midbrain [131].
Concluding Remarks
There is a substantial body of evidence from a range of studies in patients with psychosis and individuals at UHR for the disorder that supports the MAM model. Much of the human data has come from neuroimaging studies, including unimodal studies of a particular component of the model and multimodal studies that have examined more than one component.
Multimodal studies can be particularly informative as they allow an assessment of the putative interactions between different components that are thought to be critical to the model. Longitudinal multimodal studies also allow investigation of the chronology of these alterations. Overall, the literature indicates that a hippocampal-midbrain-striatal circuit is abnormal in psychosis, and that this involves alterations in MTL structure and activity, and changes in glutamate and dopamine function. However, caution is needed when comparing various kinds of data across rodent and human studies, as these are not measuring precisely the same neurophysiological and neurochemical processes.
Crucially, further work is required to clarify the chronology of these alterations in humans, and their etiology. Longitudinal multimodal studies in high-risk subjects, and studies that integrate neurobiological findings with genetic and environmental risk factors, are crucially needed to address these issues. In addition, the model provides a basis for evaluating the impact of novel experimental and clinical interventions, such as the administration of compounds that act on GABA or glutamate function in people at high risk of psychosis.
Box 3. Link Between Pathophysiology And Behavior.
Lisman and Grace propose that activation of the hippocampal-midbrain loop begins when the hippocampus receives new information not already stored in long-term memory [14]. The resulting novelty signal is conveyed through the hippocampal subiculum, nucleus accumbens, and ventral pallidum to the ventral tegmental area (VTA) where it contributes to novelty-dependent firing of dopaminergic cells. In the ascending arm of the loop, dopamine (DA) is released within the hippocampus enhancing Long Term Potentiation (LTP, a form of synaptic plasticity important for learning [13]).
Functional Magnetic Resonance Imaging (fMRI) studies in healthy human subjects suggest that VTA activation is driven by absolute rather than relative novelty [33] as well as other types of salient stimuli. Thus, the human VTA, when activated with the hippocampus, contributes to enhanced learning in the context of absolute novelty. However, as psychosis develops, increased striatal dopamine release may perturb the hippocampal-VTA loop and disrupt the normal attribution of salience, such that non-novel or unrewarding stimuli become salient. This is thought to underlie the development of the inappropriate associations that underlie psychotic symptoms, particularly delusions [34]. Disruption of dopaminergic signaling in the same network may also alter PFC function and the cognitive impairments widely seen in schizophrenia patients [35].
Acknowledgments
This work was supported by a NARSAD Young Investigator Grant (#21200, G.M.).
References
- 1.Howes OD, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Archives of general psychiatry. 2012;69:776–786. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. The American journal of psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- 3.Howes OD, et al. Mechanisms underlying psychosis and antipsychotic treatment response in schizophrenia: insights from PET and SPECT imaging. Current pharmaceutical design. 2009;15:2550–2559. doi: 10.2174/138161209788957528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Honea R, et al. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. The American journal of psychiatry. 2005;162:2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- 5.Arnold SE. The medial temporal lobe in schizophrenia. The Journal of neuropsychiatry and clinical neurosciences. 1997;9:460–470. doi: 10.1176/jnp.9.3.460. [DOI] [PubMed] [Google Scholar]
- 6.Tamminga CA, et al. The hippocampal formation in schizophrenia. The American journal of psychiatry. 2010;167:1178–1193. doi: 10.1176/appi.ajp.2010.09081187. [DOI] [PubMed] [Google Scholar]
- 7.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophrenia bulletin. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lodge DJ, Grace AA. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends in pharmacological sciences. 2011;32:507–513. doi: 10.1016/j.tips.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flagstad P, et al. Disruption of neurogenesis on gestational day 17 in the rat causes behavioral changes relevant to positive and negative schizophrenia symptoms and alters amphetamine-induced dopamine release in nucleus accumbens. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004;29:2052–2064. doi: 10.1038/sj.npp.1300516. [DOI] [PubMed] [Google Scholar]
- 11.Grace AA, Moore H. Regulation of information flow in the nucleus accumbens: A model for the pathophysiology of schizophrenia. In: Lenzenweger MF, Dworkin RH, editors. Origins and development of schizophrenia: Advances in experimental psychopathology. American Psychological Association Press; 1998. pp. 123–157. [Google Scholar]
- 12.Moore H, et al. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biological psychiatry. 2006;60:253–264. doi: 10.1016/j.biopsych.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lisman J, et al. A neoHebbian framework for episodic memory; role of dopamine-dependent late LTP. Trends in neurosciences. 2011;34:536–547. doi: 10.1016/j.tins.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Port JD, Agarwal N. MR spectroscopy in schizophrenia. Journal of magnetic resonance imaging : JMRI. 2011;34:1251–1261. doi: 10.1002/jmri.22787. [DOI] [PubMed] [Google Scholar]
- 16.Logothetis NK. The neural basis of the blood-oxygen-level-dependent functional magnetic resonance imaging signal. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2002;357:1003–1037. doi: 10.1098/rstb.2002.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waschkies CF, et al. Neuropharmacological and neurobiological relevance of in vivo (1)H-MRS of GABA and glutamate for preclinical drug discovery in mental disorders. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:2331–2339. doi: 10.1038/npp.2014.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nyffeler M, et al. Maternal immune activation during pregnancy increases limbic GABAA receptor immunoreactivity in the adult offspring: implications for schizophrenia. Neuroscience. 2006;143:51–62. doi: 10.1016/j.neuroscience.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 19.Jaaro-Peled H, et al. Review of pathological hallmarks of schizophrenia: comparison of genetic models with patients and nongenetic models. Schizophrenia bulletin. 2010;36:301–313. doi: 10.1093/schbul/sbp133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipska BK, Weinberger DR. To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2000;23:223–239. doi: 10.1016/S0893-133X(00)00137-8. [DOI] [PubMed] [Google Scholar]
- 21.Krystal JH, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Archives of general psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 22.Lahti AC, et al. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1995;13:9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- 23.Lodge DJ, Grace AA. Gestational methylazoxymethanol acetate administration: a developmental disruption model of schizophrenia. Behavioural brain research. 2009;204:306–312. doi: 10.1016/j.bbr.2009.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lodge DJ, et al. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gastambide F, et al. Selective remediation of reversal learning deficits in the neurodevelopmental MAM model of schizophrenia by a novel mGlu5 positive allosteric modulator. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:1057–1066. doi: 10.1038/npp.2011.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akbarian S, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Archives of general psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 27.Hammad H, Wagner JJ. Dopamine-mediated disinhibition in the CA1 region of rat hippocampus via D3 receptor activation. The Journal of pharmacology and experimental therapeutics. 2006;316:113–120. doi: 10.1124/jpet.105.091579. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez-Burgos G, Lewis DA. NMDA receptor hypofunction, parvalbumin-positive neurons, and cortical gamma oscillations in schizophrenia. Schizophrenia bulletin. 2012;38:950–957. doi: 10.1093/schbul/sbs010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen MX, et al. Connectivity-based segregation of the human striatum predicts personality characteristics. Nature neuroscience. 2009;12:32–34. doi: 10.1038/nn.2228. [DOI] [PubMed] [Google Scholar]
- 30.Haber SN. The primate basal ganglia: parallel and integrative networks. Journal of chemical neuroanatomy. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Lodge DJ, Grace AA. Divergent activation of ventromedial and ventrolateral dopamine systems in animal models of amphetamine sensitization and schizophrenia. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2012;15:69–76. doi: 10.1017/S1461145711000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mailly P, et al. The rat prefrontostriatal system analyzed in 3D: evidence for multiple interacting functional units. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:5718–5727. doi: 10.1523/JNEUROSCI.5248-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bunzeck N, Duzel E. Absolute coding of stimulus novelty in the human substantia nigra/VTA. Neuron. 2006;51:369–379. doi: 10.1016/j.neuron.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 34.Kapur S, et al. From dopamine to salience to psychosis--linking biology, pharmacology and phenomenology of psychosis. Schizophrenia research. 2005;79:59–68. doi: 10.1016/j.schres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Progress in neurobiology. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Mackay AV, et al. Increased brain dopamine and dopamine receptors in schizophrenia. Archives of general psychiatry. 1982;39:991–997. doi: 10.1001/archpsyc.1982.04290090001001. [DOI] [PubMed] [Google Scholar]
- 37.Zakzanis KK, Hansen KT. Dopamine D2 densities and the schizophrenic brain. Schizophrenia research. 1998;32:201–206. doi: 10.1016/s0920-9964(98)00041-3. [DOI] [PubMed] [Google Scholar]
- 38.Abi-Dargham A. Do we still believe in the dopamine hypothesis? New data bring new evidence. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2004;7(Suppl 1):S1–5. doi: 10.1017/S1461145704004110. [DOI] [PubMed] [Google Scholar]
- 39.Hietala J, et al. Measurement of striatal D2 dopamine receptor density and affinity with [11C]-raclopride in vivo: a test-retest analysis. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1999;19:210–217. doi: 10.1097/00004647-199902000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Lindstrom LH, et al. Increased dopamine synthesis rate in medial prefrontal cortex and striatum in schizophrenia indicated by L-(beta-11C) DOPA and PET. Biological psychiatry. 1999;46:681–688. doi: 10.1016/s0006-3223(99)00109-2. [DOI] [PubMed] [Google Scholar]
- 41.Laruelle M, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davis KL, et al. Dopamine in schizophrenia: a review and reconceptualization. The American journal of psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- 43.Shenton ME, et al. Amygdala-hippocampal shape differences in schizophrenia: the application of 3D shape models to volumetric MR data. Psychiatry research. 2002;115:15–35. doi: 10.1016/s0925-4927(02)00025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lawrie SM, Abukmeil SS. Brain abnormality in schizophrenia. A systematic and quantitative review of volumetric magnetic resonance imaging studies. The British journal of psychiatry : the journal of mental science. 1998;172:110–120. doi: 10.1192/bjp.172.2.110. [DOI] [PubMed] [Google Scholar]
- 45.Steen RG, et al. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. The British journal of psychiatry : the journal of mental science. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- 46.Wright IC, et al. Meta-analysis of regional brain volumes in schizophrenia. The American journal of psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 47.Andreasen NC, et al. Hypofrontality in schizophrenia: distributed dysfunctional circuits in neuroleptic-naive patients. Lancet. 1997;349:1730–1734. doi: 10.1016/s0140-6736(96)08258-x. [DOI] [PubMed] [Google Scholar]
- 48.Malaspina D, et al. Resting neural activity distinguishes subgroups of schizophrenia patients. Biological psychiatry. 2004;56:931–937. doi: 10.1016/j.biopsych.2004.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scheef L, et al. Resting-state perfusion in nonmedicated schizophrenic patients: a continuous arterial spin-labeling 3.0-T MR study. Radiology. 2010;256:253–260. doi: 10.1148/radiol.10091224. [DOI] [PubMed] [Google Scholar]
- 50.Pinkham A, et al. Resting quantitative cerebral blood flow in schizophrenia measured by pulsed arterial spin labeling perfusion MRI. Psychiatry research. 2011;194:64–72. doi: 10.1016/j.pscychresns.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Horn H, et al. Structural and metabolic changes in language areas linked to formal thought disorder. The British journal of psychiatry : the journal of mental science. 2009;194:130–138. doi: 10.1192/bjp.bp.107.045633. [DOI] [PubMed] [Google Scholar]
- 52.Holt DJ, et al. Increased medial temporal lobe activation during the passive viewing of emotional and neutral facial expressions in schizophrenia. Schizophrenia research. 2006;82:153–162. doi: 10.1016/j.schres.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 53.Holt DJ, et al. Sustained activation of the hippocampus in response to fearful faces in schizophrenia. Biological psychiatry. 2005;57:1011–1019. doi: 10.1016/j.biopsych.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 54.Hall J, et al. Overactivation of fear systems to neutral faces in schizophrenia. Biological psychiatry. 2008;64:70–73. doi: 10.1016/j.biopsych.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 55.Tamminga CA, et al. Hippocampal novelty activations in schizophrenia: disease and medication effects. Schizophrenia research. 2012;138:157–163. doi: 10.1016/j.schres.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 56.Heinz A, Schlagenhauf F. Dopaminergic dysfunction in schizophrenia: salience attribution revisited. Schizophrenia bulletin. 2010;36:472–485. doi: 10.1093/schbul/sbq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marsman A, et al. Glutamate in schizophrenia: a focused review and meta-analysis of (1)H-MRS studies. Schizophrenia bulletin. 2013;39:120–129. doi: 10.1093/schbul/sbr069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kraguljac NV, et al. Increased hippocampal glutamate and volumetric deficits in unmedicated patients with schizophrenia. JAMA psychiatry. 2013;70:1294–1302. doi: 10.1001/jamapsychiatry.2013.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bustillo JR, et al. Glutamate as a marker of cognitive function in schizophrenia: a proton spectroscopic imaging study at 4 Tesla. Biological psychiatry. 2011;69:19–27. doi: 10.1016/j.biopsych.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Theberge J, et al. Glutamate and glutamine measured with 4.0 T proton MRS in never-treated patients with schizophrenia and healthy volunteers. The American journal of psychiatry. 2002;159:1944–1946. doi: 10.1176/appi.ajp.159.11.1944. [DOI] [PubMed] [Google Scholar]
- 61.Theberge J, et al. Longitudinal grey-matter and glutamatergic losses in first-episode schizophrenia. The British journal of psychiatry : the journal of mental science. 2007;191:325–334. doi: 10.1192/bjp.bp.106.033670. [DOI] [PubMed] [Google Scholar]
- 62.Choe BY, et al. Observation of metabolic changes in chronic schizophrenia after neuroleptic treatment by in vivo hydrogen magnetic resonance spectroscopy. Investigative radiology. 1996;31:345–352. doi: 10.1097/00004424-199606000-00006. [DOI] [PubMed] [Google Scholar]
- 63.Ongur D, et al. Elevated gamma-aminobutyric acid levels in chronic schizophrenia. Biological psychiatry. 2010;68:667–670. doi: 10.1016/j.biopsych.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goto N, et al. Reduction of brain gamma-aminobutyric acid (GABA) concentrations in early-stage schizophrenia patients: 3T Proton MRS study. Schizophrenia research. 2009;112:192–193. doi: 10.1016/j.schres.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 65.Yoon JH, et al. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:3777–3781. doi: 10.1523/JNEUROSCI.6158-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stan AD, et al. Magnetic resonance spectroscopy and tissue protein concentrations together suggest lower glutamate signaling in dentate gyrus in schizophrenia. Molecular psychiatry. 2014 doi: 10.1038/mp.2014.54. [DOI] [PubMed] [Google Scholar]
- 67.Kegeles LS, et al. Hippocampal pathology in schizophrenia: magnetic resonance imaging and spectroscopy studies. Psychiatry research. 2000;98:163–175. doi: 10.1016/s0925-4927(00)00044-5. [DOI] [PubMed] [Google Scholar]
- 68.Fusar-Poli P, et al. Progressive brain changes in schizophrenia related to antipsychotic treatment? A meta-analysis of longitudinal MRI studies. Neuroscience and biobehavioral reviews. 2013;37:1680–1691. doi: 10.1016/j.neubiorev.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Navari S, Dazzan P. Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. Psychological medicine. 2009;39:1763–1777. doi: 10.1017/S0033291709005315. [DOI] [PubMed] [Google Scholar]
- 70.Ho BC, et al. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Archives of general psychiatry. 2011;68:128–137. doi: 10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abbott CC, et al. Antipsychotic drug effects in schizophrenia: a review of longitudinal FMRI investigations and neural interpretations. Current medicinal chemistry. 2013;20:428–437. doi: 10.2174/0929867311320030014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Handley R, et al. Acute effects of single-dose aripiprazole and haloperidol on resting cerebral blood flow (rCBF) in the human brain. Human brain mapping. 2013;34:272–282. doi: 10.1002/hbm.21436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de la Fuente-Sandoval C, et al. Glutamate levels in the associative striatum before and after 4 weeks of antipsychotic treatment in first-episode psychosis: a longitudinal proton magnetic resonance spectroscopy study. JAMA psychiatry. 2013;70:1057–1066. doi: 10.1001/jamapsychiatry.2013.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fusar-Poli P, et al. Predicting Psychosis Meta-analysis of Transition Outcomes in Individuals at High Clinical Risk. Archives of general psychiatry. 2012;69:220–229. doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- 75.Howes OD, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Archives of general psychiatry. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- 76.Egerton A, et al. Presynaptic Striatal Dopamine Dysfunction in People at Ultra-high Risk for Psychosis: Findings in a Second Cohort. Biological psychiatry. 2013;74:106–112. doi: 10.1016/j.biopsych.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 77.Howes OD, et al. Dopamine Synthesis Capacity Before Onset of Psychosis: A Prospective [(18)F]-DOPA PET Imaging Study. Am J Psychiat. 2011;168:1311–1317. doi: 10.1176/appi.ajp.2011.11010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Allen P, et al. Transition to Psychosis Associated With Prefrontal and Subcortical Dysfunction in Ultra High-Risk Individuals. Schizophrenia bulletin. 2012;38:1268–1276. doi: 10.1093/schbul/sbr194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Howes O, et al. Progressive increase in striatal dopamine synthesis capacity as patients develop psychosis: a PET study. Molecular psychiatry. 2011;16:885–886. doi: 10.1038/mp.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de la Fuente-Sandoval C, et al. Higher levels of glutamate in the associative-striatum of subjects with prodromal symptoms of schizophrenia and patients with first-episode psychosis. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:1781–1791. doi: 10.1038/npp.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Phillips LJ, et al. Non-reduction in hippocampal volume is associated with higher risk of psychosis. Schizophrenia research. 2002;58:145–158. doi: 10.1016/s0920-9964(01)00392-9. [DOI] [PubMed] [Google Scholar]
- 82.Borgwardt SJ, et al. Regional gray matter volume abnormalities in the at risk mental state. Biological psychiatry. 2007;61:1148–1156. doi: 10.1016/j.biopsych.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 83.Witthaus H, et al. Hippocampal subdivision and amygdalar volumes in patients in an at-risk mental state for schizophrenia. J Psychiatr Neurosci. 2010;35:33–40. doi: 10.1503/jpn.090013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wood SJ, et al. Hippocampal pathology in individuals at ultra-high risk for psychosis: A multi-modal magnetic resonance study. NeuroImage. 2010;52:62–68. doi: 10.1016/j.neuroimage.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 85.Wood SJ, et al. Hippocampal and anterior cingulate morphology in subjects at ultra-high-risk for psychosis: the role of family history of psychotic illness. Schizophrenia research. 2005;75:295–301. doi: 10.1016/j.schres.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 86.Hurlemann R, et al. Interrelated neuropsychological and anatomical evidence of hippocampal pathology in the at-risk mental state. Psychological medicine. 2008;38:843–851. doi: 10.1017/S0033291708003279. [DOI] [PubMed] [Google Scholar]
- 87.Velakoulis D, et al. Hippocampal and amygdala volumes according to psychosis stage and diagnosis - A magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Archives of general psychiatry. 2006;63:139–149. doi: 10.1001/archpsyc.63.2.139. [DOI] [PubMed] [Google Scholar]
- 88.Smieskova R, et al. Neuroimaging predictors of transition to psychosis A systematic review and meta-analysis. Neuroscience and biobehavioral reviews. 2010;34:1207–1222. doi: 10.1016/j.neubiorev.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 89.Pantelis C, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 90.Mechelli A, et al. Neuroanatomical abnormalities that predate the onset of psychosis: a multicenter study. Archives of general psychiatry. 2011;68:489–495. doi: 10.1001/archgenpsychiatry.2011.42. [DOI] [PubMed] [Google Scholar]
- 91.Allen P, et al. Altered prefrontal and hippocampal function during verbal encoding and recognition in people with prodromal symptoms of psychosis. Schizophrenia bulletin. 2011;37:746–756. doi: 10.1093/schbul/sbp113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fusar-Poli P, et al. Altered brain function directly related to structural abnormalities in people at ultra high risk of psychosis: longitudinal VBM-fMRI study. Journal of psychiatric research. 2011;45:190–198. doi: 10.1016/j.jpsychires.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 93.Seiferth NY, et al. Increased neural response related to neutral faces in individuals at risk for psychosis. NeuroImage. 2008;40:289–297. doi: 10.1016/j.neuroimage.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 94.Modinos G, et al. Overactivation of Emotional Brain Systems Predicts Severity of Positive Symptoms and Global Functioning in Prodromal and First-Episode Psychosis. International Early Psychosis Association (IEPA) 2014 [Google Scholar]
- 95.Schobel SA, et al. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Archives of general psychiatry. 2009;66:938–946. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stone JM, et al. Glutamate dysfunction in people with prodromal symptoms of psychosis: relationship to gray matter volume. Biological psychiatry. 2009;66:533–539. doi: 10.1016/j.biopsych.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 97.Egerton A, et al. Relationship Between Brain Glutamate Levels and Clinical Outcome in Individuals at Ultra High Risk of Psychosis. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.de la Fuente-Sandoval C, et al. Striatal glutamate and the conversion to psychosis: a prospective 1H-MRS imaging study. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2013;16:471–475. doi: 10.1017/S1461145712000314. [DOI] [PubMed] [Google Scholar]
- 99.Natsubori T, et al. Reduced Frontal Glutamate + Glutamine and N-Acetylaspartate Levels in Patients With Chronic Schizophrenia but not in Those at Clinical High Risk for Psychosis or With First-Episode Schizophrenia. Schizophrenia bulletin. 2013 doi: 10.1093/schbul/sbt124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Day M, et al. Glutamate-receptor-mediated encoding and retrieval of paired-associate learning. Nature. 2003;424:205–209. doi: 10.1038/nature01769. [DOI] [PubMed] [Google Scholar]
- 101.Valli I, et al. Altered medial temporal activation related to local glutamate levels in subjects with prodromal signs of psychosis. Biological psychiatry. 2011;69:97–99. doi: 10.1016/j.biopsych.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 102.Fusar-Poli P, et al. Thalamic glutamate levels as a predictor of cortical response during executive functioning in subjects at high risk for psychosis. Archives of general psychiatry. 2011;68:881–890. doi: 10.1001/archgenpsychiatry.2011.46. [DOI] [PubMed] [Google Scholar]
- 103.Allen P, et al. Functional outcome in people at high risk for psychosis predicted by thalamic glutamate levels and prefronto-striatal activation. Schizophrenia bulletin. doi: 10.1093/schbul/sbu115. In revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stone JM, et al. Altered relationship between hippocampal glutamate levels and striatal dopamine function in subjects at ultra high risk of psychosis. Biological psychiatry. 2010;68:599–602. doi: 10.1016/j.biopsych.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 105.Allen P, et al. Abnormal relationship between medial temporal lobe and subcortical dopamine function in people with an ultra high risk for psychosis. Schizophrenia bulletin. 2012;38:1040–1049. doi: 10.1093/schbul/sbr017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roiser JP, et al. Neural and behavioral correlates of aberrant salience in individuals at risk for psychosis. Schizophrenia bulletin. 2013;39:1328–1336. doi: 10.1093/schbul/sbs147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fusar-Poli P, et al. Abnormal frontostriatal interactions in people with prodromal signs of psychosis: a multimodal imaging study. Archives of general psychiatry. 2010;67:683–691. doi: 10.1001/archgenpsychiatry.2010.77. [DOI] [PubMed] [Google Scholar]
- 108.Fusar-Poli P, et al. Abnormal prefrontal activation directly related to pre-synaptic striatal dopamine dysfunction in people at clinical high risk for psychosis. Molecular psychiatry. 2011;16:67–75. doi: 10.1038/mp.2009.108. [DOI] [PubMed] [Google Scholar]
- 109.Godsil BP, et al. The hippocampal-prefrontal pathway: the weak link in psychiatric disorders? European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2013;23:1165–1181. doi: 10.1016/j.euroneuro.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 110.Schobel SA, et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78:81–93. doi: 10.1016/j.neuron.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Choi DW. Calcium and excitotoxic neuronal injury. Annals of the New York Academy of Sciences. 1994;747:162–171. doi: 10.1111/j.1749-6632.1994.tb44407.x. [DOI] [PubMed] [Google Scholar]
- 112.Alexander GE, et al. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual review of neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 113.Volk DW, et al. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Archives of general psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 114.Yaakub SN, et al. Preserved working memory and altered brain activation in persons at risk for psychosis. The American journal of psychiatry. 2013;170:1297–1307. doi: 10.1176/appi.ajp.2013.12081135. [DOI] [PubMed] [Google Scholar]
- 115.Brune M, et al. An fMRI study of “theory of mind” in at-risk states of psychosis: comparison with manifest schizophrenia and healthy controls. NeuroImage. 2011;55:329–337. doi: 10.1016/j.neuroimage.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 116.van Winkel R, Kuepper R. Epidemiological, neurobiological, and genetic clues to the mechanisms linking cannabis use to risk for nonaffective psychosis. Annual review of clinical psychology. 2014;10:767–791. doi: 10.1146/annurev-clinpsy-032813-153631. [DOI] [PubMed] [Google Scholar]
- 117.Mondelli V, et al. Stress and inflammation reduce brain-derived neurotrophic factor expression in first-episode psychosis: a pathway to smaller hippocampal volume. The Journal of clinical psychiatry. 2011;72:1677–1684. doi: 10.4088/JCP.10m06745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mondelli V, Howes O. Inflammation: its role in schizophrenia and the potential anti-inflammatory effects of antipsychotics. Psychopharmacology. 2014;231:317–318. doi: 10.1007/s00213-013-3383-3. [DOI] [PubMed] [Google Scholar]
- 119.Giusti-Rodriguez P, Sullivan PF. The genomics of schizophrenia: update and implications. The Journal of clinical investigation. 2013;123:4557–4563. doi: 10.1172/JCI66031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Callaghan BL, Richardson R. Maternal separation results in early emergence of adult-like fear and extinction learning in infant rats. Behavioral neuroscience. 2011;125:20–28. doi: 10.1037/a0022008. [DOI] [PubMed] [Google Scholar]
- 121.Gao J, et al. Glutamate and GABA imbalance promotes neuronal apoptosis in hippocampus after stress. Medical science monitor : international medical journal of experimental and clinical research. 2014;20:499–512. doi: 10.12659/MSM.890589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Caldji C, et al. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2000;22:219–229. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- 123.Zimmerman EC, et al. Abnormal stress responsivity in a rodent developmental disruption model of schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:2131–2139. doi: 10.1038/npp.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Du Y, Grace AA. Peripubertal diazepam administration prevents the emergence of dopamine system hyperresponsivity in the MAM developmental disruption model of schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:1881–1888. doi: 10.1038/npp.2013.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Day F, et al. Blunted Cortisol Awakening Response in People at Ultra High Risk of Developing Psychosis. Schizophrenia research. 2014 doi: 10.1016/j.schres.2014.06.041. [DOI] [PubMed] [Google Scholar]
- 126.Thompson KN, et al. HPA axis functioning associated with transition to psychosis: combined DEX/CRH test. Journal of psychiatric research. 2007;41:446–450. doi: 10.1016/j.jpsychires.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 127.Walker EF, et al. Cortisol levels and risk for psychosis: initial findings from the North American prodrome longitudinal study. Biological psychiatry. 2013;74:410–417. doi: 10.1016/j.biopsych.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mondelli V, et al. Higher cortisol levels are associated with smaller left hippocampal volume in first-episode psychosis. Schizophrenia research. 2010;119:75–78. doi: 10.1016/j.schres.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Belujon P, et al. Disruption of prefrontal cortical-hippocampal balance in a developmental model of schizophrenia: reversal by sulpiride. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2013;16:507–512. doi: 10.1017/S146114571200106X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Belujon P, et al. Role of the prefrontal cortex in altered hippocampal-accumbens synaptic plasticity in a developmental animal model of schizophrenia. Cerebral cortex. 2014;24:968–977. doi: 10.1093/cercor/bhs380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Watabe-Uchida M, et al. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]