Abstract
Background
Few studies have compared diets to determine if a program focused upon one dietary change results in collateral effects on other untargeted healthy diet components.
Objective
To evaluate a diet focused on increased fiber consumption versus the multi-component American Heart Association (AHA) dietary guidelines.
Design
Randomized controlled trial; June 2009 to January 2014. Trial registration: NCT00911885
Setting
Worcester, Massachusetts.
Patients
240 adults with metabolic syndrome.
Intervention
Subjects participated in individual and group sessions.
Measurements
Primary outcome was weight change at 12 months.
Results
At 12 months, mean (95% CI) change in weight in the high fiber group was −4.6 (−6.4, −2.9) pounds versus −6.0 (−7.7, −4.3) pounds in the AHA group; mean difference between groups 1.4 (−1.0, 3.8) pounds. During the trial, 12 participants (9.9%) dropped out of the fiber group, and 15 (12.6%) from the AHA group (p=0.55). In total, 8 participants developed diabetes (HbA1c≥6.5%) during the trial, 7 in the high fiber group and 1 in the AHA group (p=0.066).
Limitations
Generalizability is unknown. Maintenance of weight loss following cessation of group sessions at 12 months was not assessed. Definitive conclusions cannot be drawn regarding dietary equivalence as the study was powered for superiority.
Conclusions
The more complex AHA diet may result in up to 3.8 pounds more weight loss, however, a simplified approach to weight reduction emphasizing only increased fiber intake may result in a reasonable alternative for individuals with difficulty adhering to more complicated diet regimens.
Primary Funding Source
National Heart, Lung and Blood Institute.
INTRODUCTION
Healthy diet and lifestyle behaviors are the cornerstone of cardiometabolic health and strong evidence supports the efficacy of the American Heart Association (AHA) diet in preventing and treating metabolic syndrome.1,2 However, the numerous AHA dietary recommendations may create compliance challenges for individuals.3,4 The AHA dietary goals include: 1) consume vegetables and fruits; 2) eat whole-grain, high-fiber foods (>=30 g/day); 3) eat fish twice weekly; 4) consume lean animal and vegetable proteins; 5) reducing sugary beverages; 6) minimize sugar intake; 7) minimize sodium intake; 8) moderate to no alcohol intake; 9) 50-55% of calories from carbohydrate; 10) 15-20% calories from protein; 11) 30-35% calories from fat; 12) limit saturated fat to <7% of energy, trans fat to <1% of energy, and 13) cholesterol to <300 mg/day.
Few studies have explored weight loss outcomes as they relate to different dietary messages such as “eat more of this” (permissive) or “don’t eat that” (restrictive) and which, if any, of these messages result in collateral effects on other unadvised healthy diet components. Spring and colleagues5 found that a group encouraged to eat more fruits and vegetables, while reducing sedentary time, made greater improvements in all behaviors (including untargeted and unadvised aspects) compared to the group encouraged to reduce saturated fat and increase physical activity. The permissive dietary advice in Spring’s study, i.e., increase fruit and vegetables, had more collateral effects than the restrictive advice, i.e., reduce saturated fat intake.
Our study compared the efficacy of two approaches for dietary change in participants with metabolic syndrome: 1) a fiber-focused diet, and 2) the AHA diet.6 We hypothesized that the fiber-focused arm would be superior to the AHA intervention for weight loss, dietary quality, metabolic health, and adherence at 12 months.
METHODS
Design Overview
This randomized controlled trial evaluated a single-goal dietary recommendation to increase fiber intake (≥30g/day) to that of the multi-component AHA dietary guidelines for individuals with metabolic syndrome. Participants were randomized at baseline to either a high fiber diet (n=121) or the AHA diet (n=119). Research staff assessing outcomes were blinded to intervention assignment. The study’s Data Safety and Monitoring Board reviewed each adverse event, which was reported to the University of Massachusetts Medical School Institutional Review Board. No changes to either of the intervention protocols were required and were approved by the Institutional Review Board; all participants gave informed consent.
Setting and Participants
Participants were recruited in ten waves between June 2009 and January 2012 from Worcester, Massachusetts (Trial registration: NCT00911885; completed on June 1, 2009). Eligible participants were adults: 1) meeting criteria for the metabolic syndrome7; 2) a body mass index (BMI) 30-40 kg/m2; 3) ages 21 to 70 ; 4) physician’s approval to participate; and 5) non-smoking for ≥30 days. Exclusion criteria were: 1) clinically diagnosed diabetes, or a fasting blood sugar of ≥126 mg/dl; 2) an acute coronary event within the previous 6 months; 3) pregnant or lactating; 4) polycystic ovary syndrome; 5) plans to move out of the area during study; 6) a medical condition that precludes dietary recommendations; 7) major depression or suicidality; 8) participating in any weight loss program; 9) had bariatric surgery or using weight loss medication; and 10) an eating disorder.
Randomization and Interventions
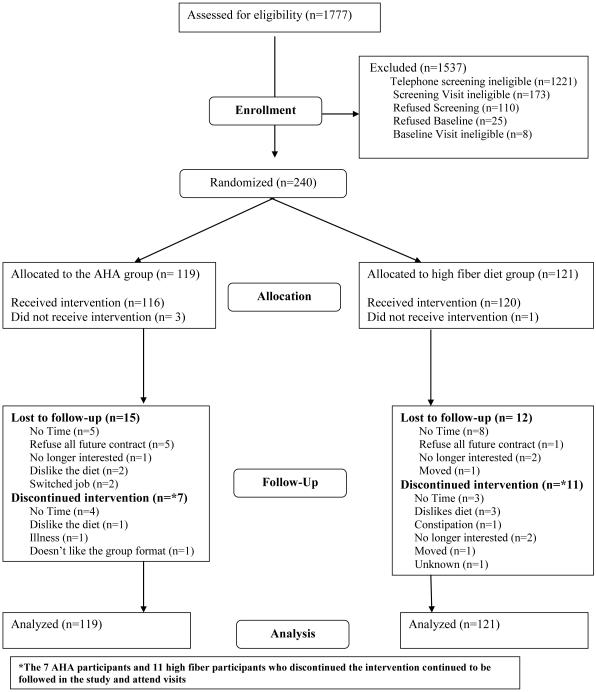
The CONSORT diagram in Figure 1 shows the number of participants recruited and randomized; and the number included in the analyses. Of the 1,777 individuals screened, 240 fulfilled the study criteria and were consented and enrolled in the trial. Participants were stratified by gender and BMI categories (25-29.9, and >=30 kg/m2). Within each strata, participants were randomized to the two interventions in randomly permuted blocks of size 6 via the RALLOC procedure using Stata8 to ensure that the distributions of gender and BMI were similar between the two interventions.
Figure 1.
Study participation and follow-up rates
The primary endpoint was weight loss at 12 months, and a treatment goal for all participants was a weight reduction of 7% of baseline body weight, and the AHA recommendation of a 6-10% weight loss for patients with the metabolic syndrome.9 The AHA diet group was given individualized caloric goals to achieve weight loss. The high fiber group’s goal was to consume (≥30 g/day) of fiber. No caloric goals were given to these participants.
1) Intervention Format and Treatment Fidelity
The intervention consisted of two individual sessions and 12 group sessions described elsewhere.10 The number and length of the sessions was determined based on our experience in conducting dietary interventions with similar populations.11,12 Treatment fidelity was monitored by provider and auditor checklists. All sessions were audio-recorded, with a random selection of 10% of the sessions reviewed by an auditor.
2) High Fiber Diet
Participants randomized to the high fiber group received instructions on how to increase their fiber intake, and no physical activity recommendations were made.13,14 In order to avoid any potential bias, each session was structured to discuss a specific fiber theme with accompanying packets. Audited recordings showed that deviation was minimal i.e.: over 98% of the time, questions not relevant to fiber intake were redirected by the dietitian to include healthy options with fiber.
3) AHA Diet
Participants randomized to the AHA group received instructions for the AHA diet, including increasing fiber. Energy intake goals were calculated and provided to the participant by estimating the daily calories needed to maintain the participant's baseline weight, subtracted by 500-1,000 calories/day to achieve a 1-2 pound per week weight loss. Each participant was given a customized goal of saturated fat grams allowed per day (7% of estimated calories) and no physical activity recommendations were made.
Outcome measures
At baseline, 3-, 6-, and 12-month visits, body weight and height were measured using a calibrated balance scale. Participants wore light clothes and removed shoes for height and weight measurements. Three unannounced 24-hour recalls (24HRs) were conducted on randomly selected days within a 3-week period (two weekdays and one weekend) around the baseline, 6- and 12 month visits, and one recall at the 3-month visit, by dietitians blinded to participants’ treatment group and trained to use the University of Minnesota’s Nutrition Coordinating Center’s Nutrition Data System for Research software (versions used: NDS-R 2008-2012). The dietary recalls were conducted by telephone and participants were provided with 2-dimensional food portion models prior to the call. Dietary quality was measured by the Alternative Healthy Eating Index (AHEI).15,16 Physical activity was assessed in the same call as the 24-hour dietary assessment. We have used this validated methodology in several previous projects.17-21
At each visit, medication and dietary supplement use were documented using self-report and original container label information. Laboratory data consisted of fasting glucose, insulin, homeostasis model assessment of insulin resistance index (HOMA-IR), hemoglobin A1c (HbA1c), lipids, high sensitivity C-reactive protein (hsCRP), interleukin 6 (IL-6), tumor necrosis factor α (TNF-a)-receptor 2, and blood pressure. The concentration of hsCRP was determined using an immunoturbidimetric assay on the Roche P Modular system (Roche Diagnostics - Indianapolis, IN). IL-6 was measured by an ultra-sensitive ELISA assay from R&D Systems, Minneapolis, MN. TNF-a-receptor was measured by an ELISA assay from R&D Systems.
Statistical Analyses
The required number of participants for each group was estimated based on the primary outcome measure, change in body weight. Sample size was calculated using the method developed by Frison and Pocock.22 We assumed standard deviation of 31.5 lbs for body weight and an autocorrelation coefficient between pre and post treatment weight of 0.95. With 95 complete cases per group, the hypothesized difference in the change in body weight (3.5 lbs) between the groups could be detected with >80% power at a 5% significance level. Considering a possible attrition rate of 20%, the number of subjects in each group should be no less than 120 participants. Thus, a total of 240 participants were enrolled in the study.
All analyses were performed using SAS version 9.2 (SAS Institute, Cary, North Carolina). Comparisons between groups for baseline characteristics were conducted using t-tests for continuous variables or chi-squared tests for categorical variables. Linear mixed models (Proc Mixed) were used to evaluate intervention effects on change in body weight. Participant ID was included as a random effect, with time (baseline, 3-, 6-, and 12-month), group (high fiber vs. AHA ), and time-group interactions included as fixed effects. Similar analyses were conducted for other outcome variables, such as dietary quality and metabolic measures. For data that were right-skewed, which included HOMA-IR, fasting plasma glucose, fasting plasma insulin, triglycerides, hsCRP, IL-6, TNF-a-receptor 2, log gamma models (Proc Glimmix) were used.23 To determine if there were between group differences in adherence to the dietary goals, we compared groups using the proportion of participants that met the 13 AHA goals with logistic regression models for repeated measures fitted by generalized estimating equations and included terms for group, time, and group and time interaction.
Primary results presented under the mixed model analysis assume missing data follow a missing at random (MAR) framework. We conducted sensitivity analyses to explore how primary results might change under various informative missing scenarios, using the MI-based SAS Macro MIWithd by James Roger.24, 25 Under two of the MNAR(missing not at random) scenarios we considered, missing weights for participants who stopped their diets and left the trial were multiply imputed using a reference group defined as the group of 18 participants who stopped their diets but remained in the trial. All multiple imputation analyses were performed using 1,000 imputations and 1,000 iterations in the MCMC(Markov chain Monte Carlo) burn-in and 500 iterations between imputations.
RESULTS
The 1-year study completion rate was 89%; 12 participants (9.9%) dropped out of the high fiber group and 15 participants (12.6%) dropped out of the AHA group (p=0.55). The average age of participants was 52 years, BMI was 35 kg/m2, 72% were female, and 86% had at least a college education (see Table 1). Average caloric intake was 1880 kcal/day, and total dietary fiber intake was 19.1 g/day. No meaningful differences were found between the groups for baseline characteristics.
Table 1.
Selected baseline characteristics of participants between AHA and fiber groups
| Variable | Overall | AHA (n=119) |
Fiber (n=121) |
|
|---|---|---|---|---|
| Age (y)* | 52.2 (10.1) | 52.5 (9.9) | 52.0 (10.3) | |
| 20-40 | 27 (11.3%) | 12 (10.1%) | 15 (12.4%) | |
| 41-50 | 66 (27.5%) | 35 (29.4%) | 31 (25.6%) | |
| 51-60 | 95 (39.6%) | 46 (38.7%) | 49 (40.5%) | |
| 61-70 | 52 (21.7%) | 26 (21.9%) | 26 (21.5%) | |
|
| ||||
| Gender | ||||
| Male | 67 (27.9%) | 34 (28.6%) | 33 (27.3%) | |
| Female | 173 (72.1%) | 85 (71.4%) | 88 (72.7%) | |
|
| ||||
| Race/ethnicity | ||||
| White | 208 (87.4%) | 106 (89.8%) | 102 (85%) | |
| Other | 30 (12.6%) | 12 (10.2%) | 18 (15%) | |
|
| ||||
| Marital status | ||||
| Married | 162 (68.1%) | 83 (70.3%) | 79 (65.8%) | |
| Not Married | 78 (31.9%) | 35 (29.7%) | 41 (40%) | |
|
| ||||
| Education | ||||
| High school or less | 33 (13.9%) | 13 (11.1%) | 20 (16.7%) | |
| College | 86 (36.3%) | 38 (32.5%) | 48 (40%) | |
| Post College | 118 (49.8%) | 66 (56.4%) | 52 (43.3%) | |
|
| ||||
| BMI (kg/m2) * | 35.0 (2.9) | 34.9 (3.1) | 35.0 (2.8) | |
| 30-35 | 118 (49.2%) | 61 (51.3%) | 57 (47.1%) | |
| 35.1-40 | 122 (50.8%) | 58 (48.7%) | 64 (52.9%) | |
|
| ||||
| Working Status | ||||
| Employed | 186 (78.5%) | 93 (78.8%) | 93 (78.2%) | |
| Unemployed | 51 (21.5%) | 25 (21.2%) | 26 (21.9%) | |
|
| ||||
| Income | $0-$30,000 | 24 (10%) | 12 (10.1%) | 12 (9.9%) |
| $30,000-$50,000 | 43 (17.9%) | 19 (16%) | 224 (19.8%) | |
| $50,000-$75,000 | 40 (16.7%) | 20 (16.8%) | 20 (16.5%) | |
| More than $75,000 | 79 (32.9%) | 43 (36.1%) | 36 (29.8%) | |
| Unclear | 54 (22.5%) | 25 (21%) | 29 (24%) | |
|
| ||||
| Systolic BP (mmHg)* | 135.9 (9.8) | 135.2 (10.6) | 136.6 (9.0) | |
|
| ||||
| Diastolic BP (mmHg)* | 80.4 (8.8) | 80.0 (8.6) | 80.8 (8.9) | |
|
| ||||
| HDL (mg/dL) * | 47.7 (10.0) | 46.5 (8.9) | 48.9 (10.9) | |
|
| ||||
| Triglycerides (mg/dL) † | 135.5 (90.5) | 139.0 (86.0) | 125.0 (90.0) | |
|
| ||||
| Fasting blood glucose (mg/dL) † | 98.0 (15.0) | 96.0 (17.0) | 99.0 (13.0) | |
|
| ||||
| HbAlc (%)* | 5.7 (0.4) | 5.7 (0.4) | 5.7 (0.4) | |
|
| ||||
| Insulin (uIU/mL) † | 11.0 (12.0) | 11.0 (11.0) | 11.5 (11.0) | |
|
| ||||
| HOMA-IR† | 2.8 (2.6) | 2.9 (2.3) | 2.8 (2.4) | |
|
| ||||
| Dietary intake* | ||||
|
| ||||
| Dietary quality score (AHEI) | 36.9 (10.9) | 37.2 (11.1) | 36.6 (10.8) | |
|
| ||||
| Energy, kcal/d | 1880.1 (641.5) | 1957.7 ( 708.5) |
1803.7 (560.5) | |
|
| ||||
| Energy from fat, % | 33.0 (5.8) | 33.1 (5.6) | 32.9 (6.0) | |
|
| ||||
| Energy from saturated fat, % | 11.5 (2.7) | 11.6 (2.8) | 11.4 (2.6) | |
|
| ||||
| Energy from carbohydrate, % | 47.2 (7.2) | 47.2 (7.4) | 47.3 (7.0) | |
|
| ||||
| Energy from protein, % | 17.4 (4.2) | 17.1 (4.1) | 17.8 (4.3) | |
|
| ||||
| Total fiber, g/d | 19.1 (6.9) | 19.5 (7.3) | 18.8 (6.5) | |
|
| ||||
|
Total physical
activity(min/week)* |
211.3 (107.1) | 217.2 (110.3) | 205.5 (104.1) | |
|
| ||||
|
Leisure-time physical
activity(min/week)* |
18.9 (25.2) | 18.9 (25.0) | 18.9 (25.6) | |
| Metabolic syndrome criteria | ||||
| Waist circumference: men>=40; women>=35 inches |
229 (95.4%) | 112 (94.1%) | 117 (96.7%) | |
| Fasting glucose >=100 mg/dL and <126mg/dL |
156 (65%) | 77 (64.7%) | 79 (65.35) | |
| Systolic blood pressure >=130 or diastolic blood pressure >=85 mmHg |
239 (100%) | 119 (100%) | 120 (100%) | |
| HDL: men<40 mg/dL; women: <50mg/dL |
138 (57.5%) | 70 (58.8%) | 68 (56.2%) | |
| Triglycerides >=150 mg/dL | 153 (63.8%) | 77 (64.7%) | 76 (62.8%) | |
| At least 3 yes above | 240 (100%) | 119 (100%) | 121 (100%) | |
Note. BMI=Body Mass Index; BP=Blood Pressure; HDL= High-density lipoprotein, HbA1c = hemoglobin A1c; HOMA-IR = homeostasis model assessment of insulin resistance; AHEI=Alternative Healthy Eating Index
Mean(SD)
Median(IQR)
Body weight and waist circumference (Table 2 and Figure 2)
Table 2.
Change (95% CI)) from baseline for primary and secondary outcomes during the dietary trial, Worcester, Massachusetts, 2009-2014.
| 3-months | 6-months | 12-months | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| AHA | High Fiber | Between group difference |
AHA | High Fiber | Between group difference |
AHA | High Fiber | Between group difference |
|
| Anthropometric | |||||||||
| Weight (lbs) | −4.4 (−6.1, −2.7) | −3.5 (−5.2, −1.8) | 0.9 (−1.5, 3.3) | −6.1 (−7.8, −4.3) | −5.0 (−6.8, −3.3) | 1.0 (−1.4, 3.5) | −6.0 (−7.7, −4.3) | −4.6 (−6.4, −2.9) | 1.4 (−1.0, 3.8) |
| Waist circumference (inches) |
−0.8 (−1.1, −0.5) | −0.3 (−0.6, 0.0) | 0.5 (0.1, 1.0) | −0.9 (−1.3, −0.6) | −0.4 (−0.7, −0.0) | 0.6 (0.1, 1.0) | −0.4 (−0.7, −0.1) | 0.1 (−0.2, 0.5) | 0.5 (0.1, 1.0) |
| BMI (kg/m2) | −0.7 (−0.9, −0.4) | −0.6 (−0.8, −0.3) | 0.1 (−0.3, 0.5) | −1.0 (−1.2, −0.7) | −0.8 (−1.1, −0.5) | 0.1 (−0.3, 0.5) | −1.0 (−1.2, −0.7) | −0.8 (−1.0, −0.5) | 0.2 (−0.2, 0.6) |
| Daily Dietary Intake | |||||||||
| AHEI score | 3.3 (0.9, 5.8) | 4.5 (2.1, 6.9) | 1.2 (−2.2, 4.6) | 6.0 (3.5, 8.5) | 8.0 (5.5, 10.4) | 1.9 (−1.6, 5.5) | 5.4 (2.9, 8.0) | 5.2 (2.6, 7.8) | −0.2 (−3.9, 3.4) |
| Dietary fiber (g) | 2.7 (0.5, 4.8) | 7.1 (5.0, 9.2) | 4.4 (1.5, 7.4) | 0.9 (−1.2, 3.1) | 6.4 (4.3, 8.5) | 5.5 (2.4, 8.5) | 1.3 (−0.9, 3.5) | 4.7 (2.5, 6.9) | 3.4 (0.3, 6.5) |
| Cereal fiber (g) | 3.2 (2.1, 4.3) | 3.3 (2.2, 4.4) | 0.1 (−1.5, 1.6) | 1.8 (0.7, 3.0) | 2.8 (1.7, 3.9) | 1.0 (−0.6, 2.6) | 1.6 (0.5, 2.8) | 1.4 (0.2, 2.5) | −0.3 (−1.9, 1.4) |
| Insoluble fiber (g) | 2.7 (1.0, 4.4) | 5.5 (3.8, 7.1) | 2.7 (0.4, 5.1) | 1.3 (−0.4, 3.1) | 5.0 (3.3, 6.7) | 3.6 (1.2, 6.0) | 1.4 (−0.4, 3.1) | 3.8 (2.1, 5.5) | 2.4 (−0.1, 4.9) |
| Soluble fiber (g) | −0.1 (−0.7, 0.6) | 1.7 (1.0, 2.3) | 1.7 (0.8, 2.6) | 0.0 (−0.6, 0.7) | 1.4 (0.7, 2.0) | 1.4 (0.4, 2.3) | 0.3 (−0.4, 1.0) | 0.9 (0.3, 1.6) | 0.6 (−0.3, 1.6) |
| Fruit (servings) | −0.0 (−0.3, 0.3) | 0.3 (−0.0, 0.6) | 0.3 (−0.1, 0.7) | 0.1 (−0.2, 0.4) | 0.4 (0.1, 0.7) | 0.4 (−0.1, 0.8) | 0.1 (−0.2, 0.4) | 0.3 (0.0, 0.6) | 0.2 (−0.2, 0.7) |
| Vegetable (servings) | 0.8 (0.4, 1.2) | 0.2 (−0.2, 0.6) | −0.6 (−1.1, −0.0) | 0.6 (0.2, 1.0) | 0.4 (0.0, 0.8) | −0.2 (−0.8, 0.3) | 0.6 (0.2, 1.0) | 0.4 (0.0, 0.8) | −0.2 (−0.8, 0.4) |
| Total energy (kcal) | −407.2 (− 514.9, −299.4) |
−200.5 (−307.0, −94.0) |
206.6 (55.1, 358.1) |
−421.2 (−531.5, −311.0) |
−130.0 (−239.3, −20.7) |
291.2 (135.9, 446.5) |
−464.6 (−578.0, −351.2) |
−200.0 (−313.2, −86.9) |
264.6 (104.4, 424.7) |
| % Carbohydrate | −0.7 (−2.4, 1.0) | 3.3 (1.6, 5.0) | 4.0 (1.6, 6.4) | −0.5 (−2.3, 1.3) | 1.8 (0.0, 3.5) | 2.2 (−0.2, 4.7) | 0.7 (−1.1, 2.5) | 1.6 (−0.2, 3.4) | 0.9 (−1.6, 3.5) |
| % Protein | 3.1 (2.0, 4.2) | 0.5 (−0.5, 1.6) | −2.5 (−4.1, −1.0) | 2.1 (1.0, 3.2) | 0.3 (−0.8, 1.4) | −1.8 (−3.4, −0.3) | 2.1 (1.0, 3.3) | 1.2 (0.1, 2.3) | −0.9 (−2.5, 0.7) |
| % Fat | −2.0 (−3.5, −0.5) | −3.2 (−4.7, −1.8) | −1.3 (−3.3, 0.8) | −1.2 (−2.7, 0.3) | −1.8 (−3.3, −0.3) | −0.5 (−2.7, 1.6) | −2.0 (−3.6, −0.5) | −3.1 (−4.6, −1.5) | −1.1 (−3.3, 1.1) |
| % Saturated fat | −2.3 (−3.0, −1.6) | −2.4 (−3.1, −1.7) | −0.1 (−1.1, 0.9) | −1.6 (−2.3, −0.9) | −1.6 (−2.3, −0.9) | 0.0 (−1.0, 1.0) | −1.9 (−2.6, −1.2) | −1.8 (−2.5, −1.1) | 0.1 (−0.9, 1.1) |
| White:Red meat | 0.3 (−0.1, 0.6) | 0.4 (0.0, 0.7) | 0.1 (−0.4, 0.5) | 0.3 (−0.0, 0.7) | 0.4 (0.1, 0.8) | 0.1 (−0.4, 0.6) | 0.3 (0.0, 0.7) | 0.3 (−0.0, 0.7) | −0.0 (−0.5, 0.5) |
| Blood pressure | |||||||||
| Systolic (mmHg) | −2.5 (−4.5, −0.6) | −1.5 (−3.5, 0.5) | 1.0 (−1.7, 3.8) | −4.2 (−6.2, −2.3) | −2.8 (−4.8, −0.8) | 1.5 (−1.4, 4.3) | −3.1 (−5.1, −1.1) | −3.5 (−5.5, −1.5) | −0.4 (−3.2, 2.4) |
| Diastolic (mmHg) | −1.7 (−3.3, −0.1) | −0.8 (−2.4, 0.9) | 0.9 (−1.4, 3.2) | −1.3 (−2.9, 0.4) | −1.1 (−2.8, 0.6) | 0.2 (−2.2, 2.5) | −2.2 (−3.8, −0.5) | −2.5 (−4.2, −0.9) | −0.4 (−2.7, 2.0) |
| Metabolic | |||||||||
| Fasting glucose†
(mg/dL) |
1.2 (−0.7, 2.9) | −1.7 (−3.9, 0.5) | −2.9 (−5.8, 0.0) | 2.4 (0.2, 4.5) | 1.9 (−0.2, 4.0) | −0.4 (−3.5, 2.7) | 2.5 (0.4, 4.5) | 0.7 (−1.6, 2.9) | −1.8 (−4.8, 1.4) |
| HbA1c (%) | NA | NA | NA | 0.0 (−0.0, 0.0) | 0.0 (−0.0, 0.0) | 0.0 (−0.1, 0.1) | −0.0 (−0.0, 0.0) | 0.0 (−0.0, 0.1) | 0.0 (−0.0, 0.1) |
| Fasting insulin†
(μU/ml) |
NA | NA | NA | −2.7 (−4.3, −1.2) | −0.7 (−2.6, 1.0) | 1.8 (−0.2, 4.3) | −1.6 (−3.3, −0.1) | −2.2 (−4.0, −0.6) | −0.7 (−2.4, 1.4) |
| HOMA-IR† | NA | NA | NA | −0.6 (−1.0, −0.2) | −0.1 (−0.6, 0.4) | 0.5 (−0.1, 1.1) | −0.3 (−0.7, 0.1) | −0.5 (−1.0, −0.1) | −0.2 (−0.7, 0.3) |
| Blood lipids | |||||||||
| HDL (mg/dL) | −1.4 (−2.5, −0.2) | −1.5 (−2.7, −0.4) | −0.1 (−1.8, 1.5) | −1.4 (−2.5, −0.2) | −0.6 (−1.8, 0.6) | 0.8 (−0.9, 2.4) | −0.5 (−1.7, 0.6) | −0.8 (−2.0, 0.3) | −0.3 (−1.9, 1.4) |
| LDL (mg/dL) | −1.8 (−6.1, 2.4) | 0.2 (−4.1, 4.4) | 2.0 (−4.0, 8.0) | −1.5 (−5.9, 2.9) | −3.2 (−7.5, 1.2) | −1.7 (−7.9, 4.5) | 1.7 (−2.6, 6.1) | −1.1 (−5.4, 3.3) | −2.8 (−9.0, 3.4) |
| Total cholesterol (mg/dL) |
−5.9 (−10.8, −1.1) | −1.8 (−6.7, 3.1) | 4.1 (−2.8, 11.0) | −3.2 (−8.2, 1.8) | −4.9 (−9.9, 0.2) | −1.7 (−8.7, 5.4) | −1.5 (−6.4, 3.5) | −4.0 (−9.0, 1.0) | −2.6 (−9.6, 4.5) |
| Triglyceride† (mg/dL) | −9.4 (−18.9, −0.5) | −0.7 (−10.1, 8.1) | 8.0 (−3.7, 20.9) | −1.0 (−12.2, 9.3) | −4.7 (−14.0, 3.9) | −3.7 (−15.7, 9.7) | −11.4 (−21.4, −2.0) | −7.6 (−16.8, 1.1) | 3.2 (−8.6, 16.1) |
| Inflammation | |||||||||
| Hs-CRP † (mg/L) | NA | NA | NA | −0.4 (−0.8, −0.1) | −0.3 (−0.7, 0.1) | 0.2 (−0.3, 0.8) | 0.0 (−0.4, 0.4) | −0.1 (−0.6, 0.3) | −0.1 (−0.7, 0.5) |
| TNF-α receptor 2 †
(pg/mL) |
NA | NA | NA | −80.1 (−154.2, −8.0) | −22.6 (−107.2, 59.3) | 55.5 (−51.6, 167.1) | −59.7 (−152.9, 30.4) | −90.0 (−173.2, −9.4) | −32.9 (−148.8, 88.6) |
| IL-6† (pg/mL) | NA | NA | NA | 0.0 (−0.3, 0.3) | 0.3 (0.1, 0.5) | 0.3 (−0.1, 0.6) | −0.1 (−0.4, 0.2) | 0.0 (−0.2, 0.2) | 0.1 (−0.2, 0.5) |
Note. BMI=Body Mass Index; AHEI=Alternative Healthy Eating Index; HbA1c = hemoglobin A1c; HOMA-IR = homeostasis model assessment of insulin resistance; HDL= High-density lipoprotein; LDL= low-density lipoprotein; hsCRP=high sensitivity C-reactive protein, IL-6= Interleukin 6, TNF-a=Tumor necrosis factor α.
Adjusted means and 95% confidence intervals are presented.
Differences between groups are equal to High Fiber group minus AHA group.
indicates the use of a log gamma model for the analysis of the variables.
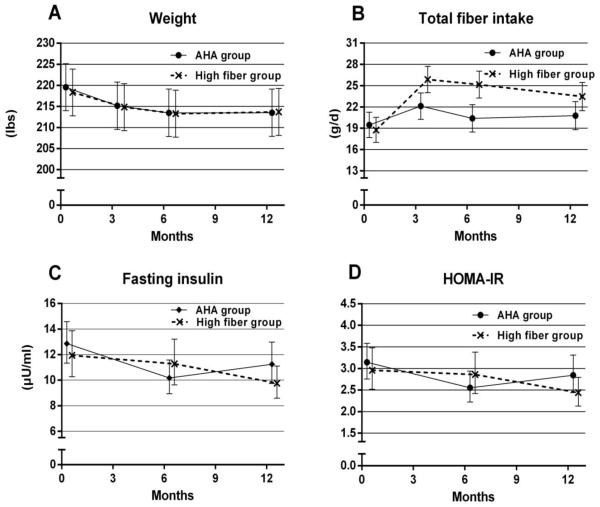
Figure 2.
Weight, dietary fiber, fasting insulin and HOMA-IR over time during the dietary trial
Adjusted means were presented, bars indicate 95% confidence interval of the estimate
At 3 months, mean (95% CI) change in weight in the high fiber group was −3.5 (−5.2, −1.8) pounds as compared to −4.4 (−6.1, 2.7) in the AHA group; mean difference between groups 0.9 (−1.5, 3.3) pounds. Weight loss was maintained in both groups at 6 and 12 months. At 12 months, weight loss for the high fiber group −4.6 [95% CI −6.4, −2.9] and for the AHA group was −6.0 [95% CI −7.7, −4.3]; mean difference between groups 1.4 (−1.0, 3.8). The sensitivity analysis conducted to explore the robustness of these results to informative missingness provided similar estimates of the between group differences in weight loss at 12 months but with wider confidence bounds (Appendix Table 1). Reduction in waist circumference at 12 months was greater in the AHA as compared to the high fiber group (mean group difference 0.5 inches (95% CI: 0.1, 1.0).
Dietary intake (Table 2)
Participants in both treatment groups decreased their total caloric intake over the 1-year study duration [−200.0 (−313.2, −86.9) kcal/day for high fiber group versus −464.6 (−578.0, −351.2) kcal/day for the AHA group, mean group difference 264.6 (104.4, 424.7); the reduction in total caloric intake was greater in the AHA group and there was a difference between the groups at 3, 6, and 12 months. At 3 months, the high fiber group increased their total dietary fiber [7.1 (5.0, 9.2) versus 2.7 (0.5, 4.8) g/day) with mean group difference 4.4 (1.5, 7.4), insoluble [5.5 (3.8, 7.1) versus 2.7 (1.0, 4.4) g/day] mean group difference 2.7 (0.4, 5.1) and soluble fiber [1.7 (1.0, 2.3) versus −0.1 (−0.7, 0.6) g/day] mean group difference 1.7 (0.8, 2.6) compared with the AHA group. There was a significant difference between the groups regarding % of calories from carbohydrates at the 3-month follow-up visit [3.3 (1.6, 5.0) for the high fiber group versus −0.7 (−2.4, 1.0) the AHA group] mean group difference 4.0 (1.6, 6.4). Percent of calories from protein was also significant at the 3-month follow-up visit and at the 6-month visit as well.
Blood pressure and metabolic measures (Table 2)
Both diastolic and systolic blood pressure decreased during the trial with no difference between the two groups. Changes in fasting insulin, HOMA-IR, HbA1c, total cholesterol, LDL, HDL, triglycerides, hsCRP, IL6, and TNF-a- receptor were also not different between two groups.
Medication use and physical activity
At baseline, 44.5% were taking antihypertensive medication in the AHA group compared with 47.9% in the high fiber group, while 39.5% were on lipid-lowering medication in the AHA group, compared with 39.7% in the high fiber group. No changes in the use of these two medications during the trial were observed. Two patients in the fiber arm added medications; one added glipizide 2.5 mg 10 days prior to their final measurements, and the other added metformin 850 mg 3 months prior to final measurements. Data from these 2 participants did not affect final group results. There were no differences for total and leisure-time physical activity over time within and between groups (data not shown).
Session attendance and adherence
For both groups, mean attendance was 7.9 sessions (SD=3.9) out of 14 sessions. The proportion of participants meeting the AHA dietary goals increased (appendix Table 2), and no meaningful group differences were observed.
Adverse events
A total of 16 adverse events (no diabetes) were reported. It was determined that the causes of these adverse events were not “treatment-related.” Adverse events by group were summarized in Table 3 of the appendix. In total, 8 participants met the criteria for diabetes (HbA1c≥6.5) during the trial, 7 in the high fiber group and 1 in the AHA group (p=0.066) (Table 4 in appendix).
DISCUSSION
No clear differences were found between the two treatment groups, suggesting that a dietary intervention focusing on a targeted fiber goal may be able to achieve clinically meaningful weight loss similar to the widely applied, but more intense AHA dietary guidelines. The present study also offers insight similar to Spring and colleagues’ 5 findings, that dietary instructions targeting one area of diet may have collateral positive effects on other non-targeted dietary behaviors.
However, the ways in which the dynamic of a diet are changed by the addition or removal of a nutrient to the diet are not completely understood and deserve further study. For example, increases in sugar intake were observed at the population level during the time when low-fat diets were highly recommended.26 Also, compared with the multi-component AHA diet, a dietary message that focuses on one dietary component such as dietary fiber, is permissive, i.e., it encourages an increase in a healthy behavior, versus restrictive which advises individuals to limit an unhealthy behavior. Our findings are also consistent with several meta-analyses that found little to no difference in diet studies comparing low-fat, low-carb, and/or Mediterranean diets on weight loss.27
Both intervention arms showed improvement in insulin resistance and fasting insulin during the trial. We were encouraged to see the decline of fasting insulin and HOMA-IR in the high fiber group at 12 months, possibly due to the longer-term effect of dietary fiber. Long-term improvements in insulin resistance and fasting insulin have significant clinical implications for patients with metabolic syndrome.7 National survey data indicate that the current average daily intake of dietary fiber is only 16 grams,28-32 and the AHA published a national age-stratified data in 2013 showing that only 7.1% adults aged 40-59 year consume ≥30 grams fiber/day.33 Increasing dietary fiber 34 may accompany other healthy dietary changes, as our study demonstrated, with the potential to significantly improve metabolic health and positively impact public health.
Generalizability is limited as the sample consisted mostly of white women who were well-educated. Baseline fiber and dietary quality were already above the national average in our sample. We are unaware of data to support whether the same results could be hypothesized for other groups with a higher burden of adverse cardiometabolic health.
Drawbacks to the fiber-focused message may include missed information about other important dietary metrics. However, our study demonstrated that untargeted aspects of diet improved in the high fiber group (e.g., white: red meat ratio), possibly due to substituting high fiber foods for less healthy foods. The exact amount of information to deliver in a dietary intervention remains an elusive question. The challenge is to identify the ideal amount of information to change behavior without overwhelming the participant.35 Finally, we note that a diagnosis of diabetes using HbA1c≥6.5% was not defined during the trial until 2012.36 We, therefore, had 6 subjects with diabetes at baseline.
Though the primary goal of our study was not met (that the high fiber arm would achieve superior weight loss), we found that a single component dietary intervention can achieve clinically meaningful weight loss similar to that of the multi-component AHA diet. We were also encouraged by the improvements in blood pressure, dietary quality, and insulin resistance, all integral in the prevention of diabetes, cardiovascular disease, and management of the metabolic syndrome.
Supplementary Material
ACKNOWLEDGMENTS
We express our thanks to the participants for their contributions to the study. We also want to thank Ms. Penny Rosenzweig, MS, RD, and Ms. Judy Palken, MS, RD, for delivering the nutritional interventions; Ms. Vijayalakshmi Patil, MS, for coordinating nutritional classes; Ms. Nancy Mecone, BSN and Ms. Annabella Aguirre, MT, ACSP, for clinical measurements and blood draws; and Mr. Don Northway and Ms. Kristie Capurso for subject recruitment; Finally, we thank for the study Data Safety and Monitoring Board members: Drs. Cara B. Ebbeling, Edward Stanek, III, and James Chesebro, for their unyielding support during the trial.
References
- 1.Zivkovic AM, German JB, Sanyal AJ. Comparative review of diets for the metabolic syndrome: implications for nonalcoholic fatty liver disease. Am J Clin Nutr. 2007;86:285–300. doi: 10.1093/ajcn/86.2.285. [DOI] [PubMed] [Google Scholar]
- 2.Feldeisen SE, Tucker KL. Nutritional strategies in the prevention and treatment of metabolic syndrome. Appl Physiol Nutr Metab. 2007;32:46–60. doi: 10.1139/h06-101. [DOI] [PubMed] [Google Scholar]
- 3.Ockene IS, Hayman LL, Pasternak RC, Schron E, Dunbar-Jacob J. Task force #4--adherence issues and behavior changes: achieving a long-term solution. Journal of the American College of Cardiology. 2002;40:630–640. doi: 10.1016/s0735-1097(02)02078-8. [DOI] [PubMed] [Google Scholar]
- 4.Pearson TA, Palaniappan LP, Artinian NT, Carnethon MR, Criqui MH, Daniels SR, Fonarow GC, Fortmann SP, Franklin BA, Galloway JM, Goff DC, Jr., Heath GW, Frank AT, Kris-Etherton PM, Labarthe DR, Murabito JM, Sacco RL, Sasson C, Turner MB. American heart association guide for improving cardiovascular health at the community level, 2013 update: a scientific statement for public health practitioners, healthcare providers, and health policy makers. Circulation. 2013;127:1730–1753. doi: 10.1161/CIR.0b013e31828f8a94. [DOI] [PubMed] [Google Scholar]
- 5.Spring B, Schneider K, McFadden HG, Vaughn J, Kozak AT, Smith M, Moller AC, Epstein LH, Demott A, Hedeker D, Siddique J, Lloyd-Jones DM. Multiple behavior changes in diet and activity: a randomized controlled trial using mobile technology. Arch Intern Med. 2012;172:789–796. doi: 10.1001/archinternmed.2012.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B, Karanja N, Lefevre M, Rudel L, Sacks F, Van Horn L, Winston M, Wylie-Rosett J. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 7.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr., Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 8.Ryan P. sxd1.1: Update to random allocation of treatment to blocks. Stata Technical Bulletin. :36–37. 501999. [Google Scholar]
- 9.Grundy SM, Hansen B, Smith SC, Jr., Cleeman JI, Kahn RA. Clinical management of metabolic syndrome: report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Circulation. 2004;109:551–556. doi: 10.1161/01.CIR.0000112379.88385.67. [DOI] [PubMed] [Google Scholar]
- 10.Merriam PA, Persuitte G, Olendzki BC, Schneider K, Pagoto SL, Palken JL, Ockene IS, Ma Y. Dietary intervention targeting increased fiber consumption for metabolic syndrome. J Acad Nutr Diet. 2012;112:621–623. doi: 10.1016/j.jand.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ockene IS, Tellez TL, Rosal MC, Reed GW, Mordes J, Merriam PA, Olendzki BC, Handelman G, Nicolosi R, Ma Y. Outcomes of a Latino community-based intervention for the prevention of diabetes: the Lawrence Latino Diabetes Prevention Project. Am J Public Health. 2012;102:336–342. doi: 10.2105/AJPH.2011.300357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pagoto S, Kantor L, Bodenlos J, Gitkind M, Ma Y. Adoption of the Diabetes Prevention Program in a hospital-based weight loss clinic. Health Psychology. 2007;27(1):S91–98. doi: 10.1037/0278-6133.27.1.S91. [DOI] [PubMed] [Google Scholar]
- 13.Park Y, Hunter DJ, Spiegelman D, Bergkvist L, Berrino F, van den Brandt PA, Buring JE, Colditz GA, Freudenheim JL, Fuchs CS, Giovannucci E, Goldbohm RA, Graham S, Harnack L, Hartman AM, Jacobs DR, Jr., Kato I, Krogh V, Leitzmann MF, McCullough ML, Miller AB, Pietinen P, Rohan TE, Schatzkin A, Willett WC, Wolk A, Zeleniuch-Jacquotte A, Zhang SM, Smith-Warner SA. Dietary fiber intake and risk of colorectal cancer: a pooled analysis of prospective cohort studies. JAMA : the journal of the American Medical Association. 2005;294:2849–2857. doi: 10.1001/jama.294.22.2849. [DOI] [PubMed] [Google Scholar]
- 14.Chuwa EWL. Dietary fibre. British Journal of Surgery. 2006;93:3. doi: 10.1002/bjs.5249. [DOI] [PubMed] [Google Scholar]
- 15.McCullough ML, Willett WC. Evaluating adherence to recommended diets in adults: the Alternate Healthy Eating Index. Public Health Nutr. 2006;9:152–157. doi: 10.1079/phn2005938. [DOI] [PubMed] [Google Scholar]
- 16.McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, Spiegelman D, Hunter DJ, Colditz GA, Willett WC. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr. 2002;76:1261–1271. doi: 10.1093/ajcn/76.6.1261. [DOI] [PubMed] [Google Scholar]
- 17.Merriam PA, Ockene IS, Hebert JR, Rosal MC, Matthews CE. Seasonal variation of blood cholesterol levels: study methodology. J Biol Rhythms. 1999;14:330–339. doi: 10.1177/074873099129000669. [DOI] [PubMed] [Google Scholar]
- 18.Matthews CE, Freedson PS, Stanek EJ, Hebert JR, Merriam PA, Rosal MC, Ebbeling CB, Ockene IS. Seasonal Variation of Household, Occupational, and leisure-time Physical Activity: Longitudinal Analyses from the Seasonal Variation of Cholesterol Study. American journal of epidemiology. 2001;153:172–183. doi: 10.1093/aje/153.2.172. [DOI] [PubMed] [Google Scholar]
- 19.Matthews CE, Hebert JR, Freedson PS, Stanek EJ, 3rd, Merriam PA, Ebbeling CB, Ockene IS. Sources of variance in daily physical activity levels in the seasonal variation of blood cholesterol study. American journal of epidemiology. 2001;153:987–995. doi: 10.1093/aje/153.10.987. [DOI] [PubMed] [Google Scholar]
- 20.Ma Y, Olendzki B, Li W, Hafner A, Chiriboga D, Hebert J, Campbell M, Sarnie M, Ockene IS. Seasonal Variation in Food Intake, Physical Activity, and Body Weight in a Free-living Population. Eur J Clin Nutr. 2006;60:519–528. doi: 10.1038/sj.ejcn.1602346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matthews CE, Freedson P, Hebert J, Stanek E, Ockene I, Merriam P. Comparison of physical activity assessment methods in the Seasonal Variation of Blood Cholesterol Levels Study. Medicine and science in sports and exercise. 2000;32:976–984. doi: 10.1097/00005768-200005000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Frison L, Pocock SJ. Repeated measures in clinical trials: analysis using mean summary statistics and its implications for design. Statistics in medicine. 1992;11:1685–1704. doi: 10.1002/sim.4780111304. [DOI] [PubMed] [Google Scholar]
- 23.Feng C, Wang H, Lu N, Tu XM. Log transformation: application and interpretation in biomedical research. Statistics in medicine. 2013;32:230–239. doi: 10.1002/sim.5486. [DOI] [PubMed] [Google Scholar]
- 24.Roger J. SAS code for sensitivity analysis using mutiple imputation. 2014 http://www.missingdata.org.uk/. Accessed August 28, 2014.
- 25.Carpenter JR, Roger JH, Kenward MG. Analysis of longitudinal trials with protocol deviation: a framework for relevant, accessible assumptions, and inference via multiple imputation. Journal of biopharmaceutical statistics. 2013;23:1352–1371. doi: 10.1080/10543406.2013.834911. [DOI] [PubMed] [Google Scholar]
- 26.Yang Q, Zhang Z, Gregg EW, Flanders WD, Merritt R, Hu FB. Added Sugar Intake and Cardiovascular Diseases Mortality Among US Adults. JAMA internal medicine. 2014;174:516–524. doi: 10.1001/jamainternmed.2013.13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pagoto S, Appelhans B. A Call for the End to the Diet Debates. JAMA : the journal of the American Medical Association. 2013;310:687–688. doi: 10.1001/jama.2013.8601. [DOI] [PubMed] [Google Scholar]
- 28.Ajani UA, Ford ES, Mokdad AH. Dietary fiber and C-reactive protein: findings from national health and nutrition examination survey data. J Nutr. 2004;134:1181–1185. doi: 10.1093/jn/134.5.1181. [DOI] [PubMed] [Google Scholar]
- 29.King DE, Egan BM, Geesey ME. Relation of dietary fat and fiber to elevation of C-reactive protein. Am J Cardiol. 2003;92:1335–1339. doi: 10.1016/j.amjcard.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 30.Block G, Subar AF. Estimates of nutrient intake from a food frequency questionnaire: the 1987 National Health Interview Survey. J Am Diet Assoc. 1992;92:969–977. [PubMed] [Google Scholar]
- 31.Ma Y, Griffith J, Chasan-Taber L, Olendzki B, Jackson E, Stanek E, III, Li W, Pagoto S, Hafner A, Ockene I. Association between dietary fiber and serum C-reactive protein. Am J Clin Nutr. 2006;83:760–766. doi: 10.1093/ajcn/83.4.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma Y, Hébert J, Li W, Bertone-Johnson E, Olendzki B, Ockene I, Pagoto S, Rosal M, Ockene J, Tinker L, Griffith J, Liu S. Association between dietary fiber and markers of systemic inflammation in the Women's Health Initiative Observational Study. Nutrition. 2008 Oct;24(10):941–9. doi: 10.1016/j.nut.2008.04.005. Epub 2008 Jun 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marlett JA, McBurney MI, Slavin JL. Position of the American Dietetic Association: health implications of dietary fiber. J Am Diet Assoc. 2002;102:993–1000. doi: 10.1016/s0002-8223(02)90228-2. [DOI] [PubMed] [Google Scholar]
- 35.Lappalainen R, Sairanen E, Jarvela E, Rantala S, Korpela R, Puttonen S, Kujala UM, Myllymaki T, Peuhkuri K, Mattila E, Kaipainen K, Ahtinen A, Karhunen L, Pihlajamaki J, Jarnefelt H, Laitinen J, Kutinlahti E, Saarelma O, Ermes M, Kolehmainen M. The effectiveness and applicability of different lifestyle interventions for enhancing wellbeing: the study design for a randomized controlled trial for persons with metabolic syndrome risk factors and psychological distress. BMC public health. 2014;14:310. doi: 10.1186/1471-2458-14-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.American Diabetes A Standards of medical care in diabetes--2012. Diabetes care. 2012;35(Suppl 1):S11–63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.