Abstract
Background
Proton-pump inhibitors (PPIs) are among the most frequently prescribed medications. Community-acquired pneumonia (CAP) is a common cause of morbidity, mortality and healthcare spending. Some studies suggest an increased risk of CAP among PPI users. We conducted a systematic review and meta-analysis to determine the association between outpatient PPI therapy and risk of CAP in adults.
Methods
We conducted systematic searches of MEDLINE, EMBASE, CINAHL, Cochrane Central Register of Controlled Trials, Scopus and Web of Science on February 3, 2014. Case-control studies, case-crossover, cohort studies and randomized controlled trials reporting outpatient PPI exposure and CAP diagnosis for patients ≥18 years old were eligible. Our primary outcome was the association between CAP and PPI therapy. A secondary outcome examined the risk of hospitalization for CAP and subgroup analyses evaluated the association between PPI use and CAP among patients of different age groups, by different PPI doses, and by different durations of PPI therapy.
Results
Systematic review of 33 studies was performed, of which 26 studies were included in the meta-analysis. These 26 studies included 226,769 cases of CAP among 6,351,656 participants. We observed a pooled risk of CAP with ambulatory PPI therapy of 1.49 (95% CI 1.16, 1.92; I2 99.2%). This risk was increased during the first month of therapy (OR 2.10; 95% CI 1.39, 3.16), regardless of PPI dose or patient age. PPI therapy also increased risk for hospitalization for CAP (OR 1.61; 95% CI: 1.12, 2.31).
Discussion
Outpatient PPI use is associated with a 1.5-fold increased risk of CAP, with the highest risk within the first 30 days after initiation of therapy. Providers should be aware of this risk when considering PPI use, especially in cases where alternative regimens may be available or the benefits of PPI use are uncertain.
Introduction
Community-acquired pneumonia (CAP) is a common diagnosis associated with substantial morbidity and healthcare expenditure. In 2006 alone, 4.2 million ambulatory care visits for CAP occurred in the United States [1]. Medicare data from 2007–2008 indicated a 30-day mortality ranging from 3.8 to 8.5% depending on severity of disease [2]. Annual healthcare costs incurred by patients with CAP are estimated to be approximately $13 billion among Medicare fee-for service patients [2]. Implementation of guidelines for antibiotic selection [3, 4] and administration of pneumococcal vaccination [5, 6] have been shown to reduce CAP incidence, morbidity and mortality. Identification and avoidance of medications associated with an increased risk of CAP could further reduce CAP incidence.
Proton pump inhibitors (PPIs) are among the most widely prescribed medications. In 2011, omeprazole was the sixth most commonly prescribed medication in the United States with nearly 60 million prescriptions [7]. PPIs have become a 10 billion dollar industry with over 15 million Americans taking these medications, not including over-the-counter usage [8]. Although evidence and guidelines support the use of PPIs for gastroesophageal reflux disease (GERD) [9] and select cases of duodenal and gastric ulcers [10], evaluation of PPI therapy in the ambulatory setting suggests that as few as 35% of patients taking PPIs have an appropriate indication documented [11, 12]. A spectrum of side effects are associated with PPI therapy, including deficiencies of critical vitamins and minerals, Clostridium difficile-associated diarrhea infection and hip fracture [13]. CAP has been hypothesized as an additional untoward consequence of PPI therapy [14].
We conducted a systematic review and meta-analysis of observational and randomized studies to determine whether outpatient PPI therapy is associated with increased risk of CAP among adults as compared to no PPI therapy. To identify particularly high-risk patients, we conducted additional analyses to determine the CAP risk stratified by PPI dose, duration of PPI therapy, and age of participant.
Methods
Our systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)[15] and Meta-analysis of Observational Studies in Epidemiology (MOOSE)[16] (PRISMA checklist reported in S1 Table). A written review protocol was not drafted.
Data Sources and Search Strategy
We performed systematic searches of MEDLINE (via PubMed), EMBASE, CINAHL, Cochrane Central Register of Controlled Trials (CENTRAL), Scopus, Web of Science and ClinicalTrials.gov on February 3, 2014. Our search strings included controlled vocabulary and related keywords for two concepts: pneumonia and acid suppressants (S2 Table). We did not limit our search based on study design, publication year, language or inclusion of human participants. Authors of potentially eligible abstracts, posters or manuscripts were contacted via email to clarify study information and obtain additional data. Approval for use of unpublished data in our analysis and certification of data validity was documented electronically.
Study Selection
We included in our review studies reporting data to allow the calculation of a measure of risk of CAP among adult participants with and without exposure to outpatient PPI therapy. We followed guidance from the Cochrane Collaboration to include both observational and randomized studies to maximize study, participant and event inclusion into our analysis and to thereby allow the conduct of sensitivity and subgroup analyses [17, 18]. We excluded case reports and case series. We screened systematic reviews for the inclusion of data not published elsewhere and for references not otherwise captured by our search strategy. We excluded studies in which <95% of participants were over 18 years old. We also excluded studies if PPIs were exclusively administered intravenously, in the inpatient setting, peri-procedurally, or as part of Helicobacter pylori therapy. CAP cases were identified by the definitions utilized in each included study. We excluded studies in which CAP preceded PPI exposure or in which the temporal relationship was ambiguous.
Data Extraction
Two authors independently screened studies for inclusion and a third author adjudicated discordant assessments. Title/abstract and full text screening were conducted in a similar fashion; however, specific exclusion reasons were documented only during full text screening. Upon selection of the final group of studies, two authors independently extracted qualitative and quantitative data using a standardized data extraction form adjudicated by a third author. To assess the methodological quality of observational studies, we used a modified version of the Newcastle-Ottawa Scale [19] (S3 Table). We applied this validated tool to characterize participant selection, comparability of populations, and outcome assessment.
Analyses
The primary outcome of this meta-analysis was incident CAP during treatment with outpatient PPI therapy. Sensitivity analyses examined our primary outcome among studies in which PPI therapy was the single form of gastric acid suppression, studies with our strict definition of CAP that included radiographic confirmation, and studies with lower risk of bias (defined as low risk on ≥4 out of 7 criteria for cohort studies and ≥6 out of 8 criteria for case-control studies using the modified Newcastle-Ottawa Scale). Secondary analyses evaluated the risk of CAP with H2-receptor antagonist (H2RA) therapy and risk of hospitalization for CAP with PPI therapy. Lastly, we examined three subgroups of participant populations in order to more specifically characterize the risk of CAP associated with PPI therapy: those treated with different PPI doses (high dose > 1 defined daily dose [DDD] and low dose PPI ≤ 1 DDD), durations of PPI therapy prior to CAP diagnosis (<1 month, 1–6 months, >6 months), and age categories (<65 years old or ≥65 years old). All subgroup analyses were defined a priori.
Statistical Analysis
We report measures of association extracted from the included studies: odds ratios (ORs), relative risks (RRs) and hazard ratios (HRs), each with a 95% confidence interval (CI). For studies that did not report a measure of association between PPI exposure and CAP but did include the required information, we calculated an unadjusted odds ratio using the Peto method [20].
We generated a funnel plot to visually assess reporting bias and used Egger’s test to assess asymmetry of the funnel plot. In our qualitative analysis, we critically examined the study population, study design, internal and external validity, and exposure and outcome ascertainment and definitions.
To determine the proportion of variability in the effect estimates due to heterogeneity, we calculated the I2 statistic [21]. We adhered to the accepted cutoff of 50% to define significant heterogeneity and additionally calculated the associated p-value (via χ2 test) [21]. We generated forest plots for each of our exposure-outcome comparisons of interest. Because of significant clinical, methodological and statistical heterogeneity across the included studies, we used a random-effects based model to calculate the weighted mean, variance of the summary effect, and associated 95% confidence interval and p-value. Since pneumonia is a rare clinical event, all effect estimates were assumed to approximate the relative risk and were pooled in meta-analyses. All statistical analyses were conducted using STATA v12 [22].
Results
Search Results and Study Characteristics
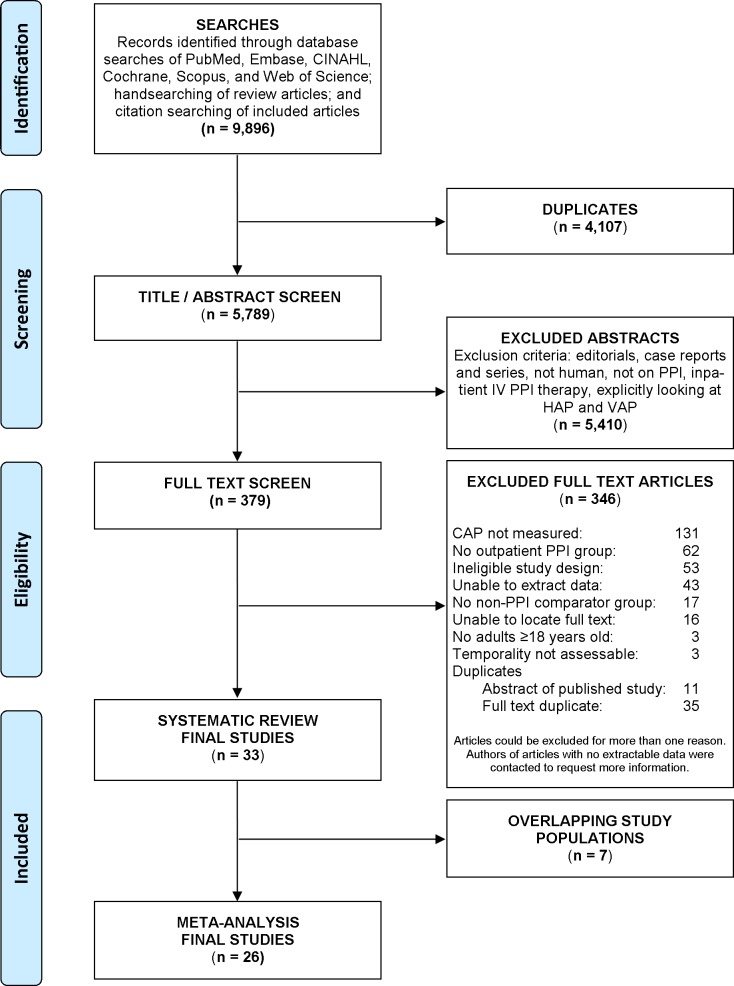
Our systematic search identified 5789 unique titles and abstracts. Of these, 5410 studies were excluded based on predetermined eligibility criteria during title/abstract review. The remaining 379 studies underwent full text screening and 33 ultimately met criteria for inclusion in our systematic review [23–55]. Due to overlapping study populations, 26 of these 33 studies were included in our meta-analysis [23–25, 27, 28, 31–40, 43–52, 54]. Of the 346 excluded full texts, the most common reason for exclusion was a lack of CAP assessment (n = 131), followed by absence of an appropriate PPI exposure group (n = 62), and ineligible study design (n = 53). Reasons for exclusion are detailed in the PRISMA flow diagram (Fig 1) [56].
Fig 1. Flow Diagram of Study Selection, adapted from PRISMA Group 2009 Flow Diagram.
Studies could be excluded for more than one reason, therefore the sum of exclusion reasons exceeds total studies. Examples of studies lacking extractable data included abstracts without full results (such as abstracts reporting an odds ratio without exposure or outcome definitions) or papers summarizing outcomes qualitatively in the text (such as manuscripts reporting adverse event categories that grouped pneumonia among other events).
Systematic Review
A qualitative synthesis of the 33 studies meeting our inclusion criteria is summarized in Table 1 and S4 Table. The majority of studies were of case-control design (n = 18); 10 were cohort studies, 4 randomized controlled trials (RCTs), and 1 case-crossover study. Studies were conducted across the world with the United States and the United Kingdom contributing data to the most studies (11 and 10 studies, respectively). Data collection spanned over 2 decades (1987–2011).
Table 1. Qualitative analysis of 32 Studies under Systematic Review.
| Author Year, Study Design | Location, Years of Conduct | Source of Cases | CAP setting | CAP Definition | # Cases / # Participants | Acid-suppression | Methods of Ascertainment | Ref. |
|---|---|---|---|---|---|---|---|---|
| Almirall 2008, Case-Control | Spain, 1999–2000 | Patients >14 years old at 64 primary care centers | Inpatient or Outpatient | Medical record review with prescription of antibiotics and new radiological findings suggestive of infiltrate | 1336 / 2662 | PPI | PPI: Questionnaire; CAP: Medical, radiographic records | 23 |
| Chen 2013, Cohort | Taiwan, 1998–2008 | CKD patients in Taiwan Health Insurance Research Database | Inpatient | Diagnostic codes (ICD-9) | 619 / 8076 | PPI | PPI: Prescription records; CAP: Diagnostic codes (ICD-9) | 24 |
| Dublin 2010, Case-Control | Washington, USA, 2000–2003 | Adults 65–94 years old in Group Health Integrated healthcare delivery system | Inpatient or Outpatient | Diagnostic codes (ICD-9), confirmed by review of radiology and hospital records | 1125 / 3360 | PPI | PPI: Pharmacy database; CAP: Diagnostic codes (ICD-9), medical records | 25 |
| Ernst 2012, Case-Control | United Kingdom, 1997–2009 | Anti-parkinsonian drug users 40–89 years old in the General Practice Research Database | Inpatient | Diagnostic codes (ICD-10) | 1835 / 17923 | PPI | PPI: Prescription records; CAP: Diagnostic codes (ICD-10) | 26 |
| Filion 2013, Cohort | Canada, UK, & USA, 1997–2010 | New NSAID users ≥40 years old in eight research databases | Inpatient | Diagnostic codes (ICD-10) | 5135 / 4238504 | PPI | PPI: Pharmacy and prescription records; CAP: Diagnostic codes (ICD-10) | 27 |
| Gau 2010, Case- Control | Ohio, USA, 2004, 2006 | Rural community hospital admissions ≥65 years old | Inpatient | Discharge diagnosis with radiographic confirmation | 194 / 1146 | PPI | PPI: Medication reconciliation, physician or nursing home notation; CAP: Diagnostic codes, medical records | 28 |
| Gulmez 2007, Case- Control | Funen, Denmark, 2000–2004 | Government Patient Registries | Inpatient | Diagnostic codes (ICD-8 & ICD-10) | 7642 / 41818 | PPI | PPI: Pharmacy database; CAP: Diagnostic codes (ICD-8 & ICD-10) | 29 |
| Hennessy 2007, Case-Control | United Kingdom, 1987–2002 | Adults ≥65 years old in the General Practice Research Database | Inpatient | Diagnostic codes (Read) | 12044 / 60220 | PPI | PPI: Prescription records; CAP: Diagnostic codes (Read) | 30 |
| Hermos 2012, Case-Control | USA, 1996–2007 | New England Veterans Healthcare System | Inpatient or Outpatient | Diagnostic code (ICD-9) and pharmacy record of respiratory antibiotic prescription | 1544 / 16984 | PPI | PPI: Pharmacy database; CAP: Diagnostic codes (ICD-9), pharmacy records | 31 |
| Jena 2013, Cohort | USA, 1997–2007 | Adults ≥30 years old in six employer-based insurance plans | Inpatient or Outpatient | Diagnostic codes (ICD-9) | 16827 / 54490 | PPI | PPI: Prescription drug claims database; CAP: Diagnostic codes (ICD-9) | 32 |
| Juthani-Mehta 2013, Cohort | Pennsylvania & Tennessee, USA, 1998–2008 | Adults 70–79 years old in the Health, Aging, and Body Composition Study | Inpatient | Medical record review of radiography, respiratory symptoms, physical examination, and diagnostic codes (ICD-9) | 193 / 1441 | PPI | PPI: Patient pill bottles; CAP: Diagnostic codes (ICD-9), medical records | 33 |
| Laheij 2003, Cohort | Netherlands, 2002 | Outpatient endoscopy service and surrounding community | Inpatient or Outpatient | Patient report | 6 / 405 | PPI or H2RA | PPI: Self-report via questionnaire; CAP: Self-report via questionnaire | 34 |
| Laheij 2004, Case- Control | Netherlands, 1995–2002 | Integrated Primary Care Information (IPCI) project | Inpatient or Outpatient | Medical record review of radiography, microbiology or respiratory symptoms | 475 / 5165 | PPI | PPI: Prescription records, IPCI database; CAP: Medical record, IPCI database | 35 |
| Liu 2012, Case-Crossover | Taiwan, 1998–2007 | Adults ≥18 years old with history of stroke and pneumonia hospitalization | Inpatient | Diagnostic codes (ICD-9) | 13832 / 13832 | PPI | PPI: Insurance database; CAP: Diagnostic codes (ICD-9) | 36 |
| Long 2013, Case-Control | USA, 1997–2009 | IBD patients <64 years old in the Life Link Health Plan Claims Database | Inpatient or Outpatient | Diagnostic codes (ICD-9) with antibiotic prescription or hospital admission | 4856 / 23784 | PPI | PPI: Outpatient pharmacy claims; CAP: Diagnostic codes (ICD-9), pharmacy records | 37 |
| Mastronarde 2009, RCT | USA, 2004–2008 | Adults with poorly controlled asthma enrolled in ALA- ACRC | Outpatient | Not reported | 1 / 402 | PPI | PPI: Randomized per protocol; CAP: Adverse event reporting | 38 |
| Meijvis 2011, Case-Control | Netherlands, 2004–2010 | Two teaching hospitals | Inpatient | New infiltrate on a chest radiograph with at least two clinical or laboratory findings consistent with pneumonia | 430 / 2150 | PPI | PPI: Pharmacy database; CAP: Medical admission records | 39 |
| Morris 2013, Cohort | USA, 2009–2011 | Kidney transplant recipients at 3 hospitals | Inpatient or Outpatient | Positive sputum culture with medical record review for clinical correlation | 4 / 211 | PPI | PPI: Prescription records; CAP: Medical records, sputum culture | 40 |
| Muellerova 2012, Case-Control | United Kingdom, 1996–2005 | COPD patients ≥ 45 years old in General Practice Research Database | Inpatient or Outpatient | Diagnostic codes (Read) | 1469 / 8814 | PPI | PPI: Prescription records; CAP: Diagnostic codes (Read) | 41 |
| Myles 2009, Case-Control | United Kingdom, 2001–2002 | Patients >40 years old in The Health Improvement Network (THIN) | Inpatient or Outpatient | Diagnostic codes (Read) | 3709 / 25883 | PPI | PPI: Pharmacy records; CAP: Diagnostic codes (Read) | 42 |
| Nielsen 2012, Case-Control | Denmark, 1997–2009 | Patients ≥15 years old in the Danish National Registry of Patients | Inpatient | Diagnostic codes (ICD-8 & ICD-10) | 70914 / 780054 | PPI | PPI: Prescription codes; CAP: Diagnostic codes (ICD-8 & ICD-10) | 43 |
| Pasina 2011, Cohort | Italy, 2008 | Patients ≥65 years old admitted at 38 internal medicine wards | Inpatient | Diagnostic codes (ICD-9) | 28 / 1332 | PPI | PPI: Prescription codes; CAP: Diagnostic codes (ICD-9) | 44 |
| Quagliarello 2005, Cohort | New Haven, CT, USA, 2001–2003 | Nursing home residents >65 years old | Inpatient or Outpatient | Compatible symptoms with radiographic findings | 112 / 613 | PPI or H2RA | PPI: Nursing home records; CAP: Medical & radiographic records | 45 |
| Ramsay 2013, Cohort | Australia, 2007–2011 | Adults ≥ 65 years old, eligible for DVA services | Inpatient | Diagnostic codes (ICD-10) | 6775 / 105467 | PPI | PPI: Prescription records; CAP: Diagnostic codes (ICD-10) | 46 |
| Rodriguez 2009, Case-Control | United Kingdom, 2000–2005 | Patients 20–79 years old in The Health Improvement Network (THIN) | Inpatient or Outpatient | Diagnostic codes (Read) | 7297 / 17920 | PPI | PPI: Pharmacy records; CAP: Diagnostic codes (Read) | 47 |
| Roughead 2009, Cohort | Australia, 2002–2006 | Patients ≥65 years old with full Veterans' Affairs benefits | Inpatient | Diagnostic codes (ICD-10) | 13876 / 185533 | PPI | PPI: Pharmacy records; CAP: Diagnostic codes (ICD-10) | 48 |
| Sarkar 2008, Case-Control | United Kingdom, 1987–2002 | Patients ≥18 years old in General Practice Research Database | Inpatient or Outpatient | Diagnostic codes (Read or Oxford) | 80066 / 879947 | PPI | PPI: Prescription records; CAP: Diagnostic codes (Read or Oxford) | 49 |
| Scheiman 2011, RCT | Europe, Australia, Asia, Africa, Americas, 2007–2008 | Aspirin users ≥18 years old with history or risk of peptic ulcer | Inpatient or Outpatient | Not reported | 9 / 2426 | PPI | PPI: Randomized per study protocol; CAP: Adverse event reporting | 50 |
| Sugano 2011, RCT | Japan, 2007–2008 | Long-term low-dose aspirin users with history of ulcer | Inpatient | Not reported | 1 / 461 | PPI | PPI: Randomized per study protocol; CAP: Adverse event reporting | 51 |
| Sugano 2012, RCT | Japan, 2007–2009 | Long-term NSAID users with history of ulcer | Inpatient | Not reported | 6 / 366 | PPI | PPI: Randomized per study protocol; CAP: Adverse event reporting | 52 |
| van de Garde 2006 (Thorax), Case-Control | United Kingdom, 1987–2001 | Diabetic patients ≥ 18 years old in General Practice Research Database | Inpatient or Outpatient | Diagnostic codes (Read) | 4719 / 20041 | PPI or H2RA | PPI: Prescription records; CAP: Diagnostic codes (Read) | 53 |
| van de Garde 2006 (ERJ), Case-Control | Netherlands, 1995–2000 | Adults >18 years old in the PHARMO record linkage system | Inpatient | Diagnostic codes (ICD-9) | 1108 / 4925 | PPI | PPI: Pharmacy database; CAP: Diagnostic codes (ICD-9) | 54 |
| van de Garde 2007 (J HTN), Case-Control | United Kingdom, 1987–2001 | Diabetic patients ≥ 18 years old in General Practice Research Database | Inpatient or Outpatient | Diagnostic codes (Read) | 4719 / 20041 | PPI or H2RA | PPI: Prescription records; CAP: Diagnostic codes (Read) | 55 |
ALA, American Lung Association; ACRC, Asthma Clinical Research Centers; CAP, community-acquired pneumonia; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CT, Connecticut; DVA, Department of Veterans’ Affairs; H2RA, histamine-2 receptor-antagonist; IBD, inflammatory bowel disease; IPCI, Integrated Primary Care Information; NSAID, non-steroidal anti-inflammatory drug; PPI, proton pump inhibitor; RCT, randomized controlled trial; Ref., study reference number; USA, United States of America
For the majority of studies, PPI exposure could include any available drug in the class. However, two RCTs administered esomeprazole only [38, 50] and two RCTs administered lansoprazole only [51, 52]. Four studies included exposed participants who were treated with either PPI or H2-receptor antagonist therapy (H2RA). Most studies used pharmacy or prescription records to identify PPI exposure (n = 24, 73%) and diagnostic codes to identify CAP cases (n = 23, 70%).
A total of 6,546,396 study participants were included in our qualitative synthesis. Studies included participants with a breadth of comorbidities which are described in S4 Table; those studies examining CAP within specific disease populations are noted in Table 1. Examples include studies restricting to persons taking anti-Parkinsonian medications [26], to those with newly initiated NSAID therapy [27], to persons with a stroke history [36], or to persons with prior kidney transplantation [40]. total of 262,906 cases of CAP were reported. One study identified CAP cases managed exclusively in the outpatient setting [38]; all other studies presented inpatient or a mix of inpatient and outpatient CAP cases. Individual study definitions of CAP are outlined in S4 Table.
Assessment of Methodological Quality
The included observational studies generally demonstrated good methodologic quality, as assessed by a modified Newcastle-Ottawa Scale (S1 Fig) [19]. Case-control studies appropriately conducted and clearly reported exposure and outcome ascertainment. Only one study documented the non-response rate [31]. Cohort studies demonstrated greater risk of bias than case-control studies, particularly with regards to assessment of CAP and follow-up of participants. Among the cohort studies, three showed particularly high risk for bias with respect to our association of interest [34, 40, 44]. The Cochrane Collaboration’s tool [57] was used to assess sequence generation, allocation concealment, and blinding in the included RCTs; all satisfied the requirements for a low risk of bias in these areas. The tool’s assessments of incomplete outcome reporting and selective outcome reporting, however, are not relevant to the studies included in this review because none of these included CAP as a primary outcome. To address the potentially high risk of bias associated with RCTs not designed to assess the association between PPI exposure and CAP, a sensitivity analysis was conducted excluding RCTs from the meta-analysis.
Our funnel plot (S2 Fig) showed clustering of studies below our summary effect estimate. Egger’s test confirmed asymmetry of the funnel plot (0.27; 95% CI 0.14, 0.40; p<0.001). This may reflect a publication bias attenuating our results.
Meta-Analysis
Our meta-analysis pools data from 26 of the 33 studies included in the systematic review. These 26 studies include 6,351,656 (97.0%) participants and 226,769 (86.2%) cases of CAP. Seven studies were not included in the meta-analysis due to substantially overlapping participant populations [26, 29, 30, 41, 42, 53, 55].
Community-Acquired Pneumonia
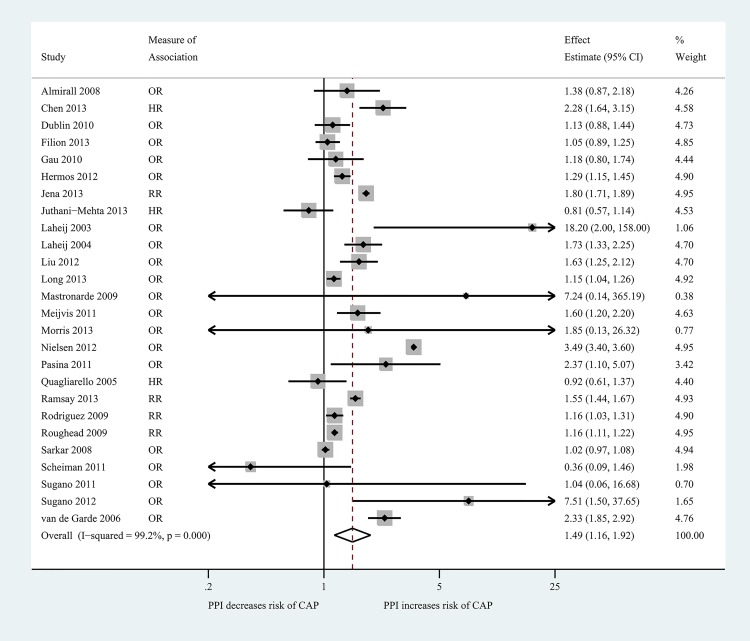
Of the 26 studies included in our primary meta-analysis, 15 reported a statistically significant increased risk of CAP with PPI use [24, 31, 32, 34–37, 39, 43, 44, 46–48, 52, 54] and 11 reported no statistically significant association [23, 25, 27, 28, 33, 38, 40, 45, 49–51] (Fig 2; S3 Fig). The 15 studies demonstrating significantly increased risk included 138,593 of the total reported 226,769 cases of CAP (61.1%). Four studies reported unadjusted ORs [23, 33, 34, 54]. The Peto method was used to calculate an unadjusted OR for 7 studies [38, 40, 43, 44, 50–52]. Collectively, our meta-analysis identified a significantly increased risk of CAP with outpatient PPI use, with a pooled RR of 1.49 (95% CI 1.16, 1.92). The I2 statistic for this summary measure was 99.2% (p<0.001), indicating significant heterogeneity across studies.
Fig 2. Summary Forest Plot of Overall Risk of Community-Acquired Pneumonia with Outpatient Proton Pump Inhibitor Use, subdivided by study design and effect estimate.
Solid diamond represents effect estimate. Shaded box size is proportional to the weight of the study in the meta-analysis. Confidence intervals are denoted by horizontal lines, with arrows where confidence interval extends beyond figure. Vertical dashed line represents the null effect. The open diamond is centered at the summary effect estimate and proportional to the confidence interval.
Sensitivity Analyses
When limiting our analysis to studies that measured PPI exposure separately from other gastric acid suppressant drugs, we found that the association between PPI exposure and CAP remained similar to our primary analysis and heterogeneity remained high (RR 1.48; 95% CI 1.14, 1.92; I2 99.2%) [23–25, 27, 28, 31–33, 35–40, 43, 44, 46–52, 54]. When limiting our analysis to the 6 studies meeting our strict CAP definition which included radiographic confirmation [23, 25, 28, 33, 35, 39, 45], the increased risk of CAP with PPI therapy was attenuated and heterogeneity was reduced (RR 1.22; 95% CI 0.99, 1.52; I 2 65.8%). When we excluded RCTs, point estimates and confidence intervals were nearly identical to those from the primary analysis (RR 1.49; 95% CI 1.15, 1.93; I2 99.3%). Our results were also robust to sensitivity analyses restricting to the 13 studies identified as having lower risk of bias and to studies reporting adjusted measures of association (S5 Table).
Secondary Analyses
Risk of Hospitalization for CAP with PPI Therapy
We pooled 16 studies in order to evaluate the secondary outcome of hospital admission for CAP among PPI users compared to non-PPI users [23, 24, 27, 28, 33, 36, 39, 43, 46–52, 54]. The pooled relative risk of hospitalization with CAP was 1.61-fold higher among PPI users as compared to non-PPI users (95% CI: 1.12, 2.31; I2 99.3%).
Risk of CAP with H2RA Therapy
In order to evaluate CAP risk with H2RA therapy, we pooled 8 studies that reported this risk separately from the risk associated with PPI therapy [23, 25, 27, 28, 35, 47, 49, 51]. Combined, these studies included 4837 cases of CAP. When pooled, these studies demonstrated no significant CAP risk with H2RA therapy as compared to no acid-suppression therapy (RR 1.00; 95% CI 0.90, 1.12; I2 33.7%).
Subgroup Analyses
Subgroup analyses are summarized in Table 2. The 8 studies reporting risk of CAP with low dose PPI therapy (≤ 1 DDD), as compared to no PPI therapy [23, 28, 39, 47, 49–52], identified a pooled RR of 1.31 (95% CI 1.04, 1.66), which was similar to findings among those taking high dose PPI therapy (RR 1.33; 95% CI 1.05, 1.69) [23, 28, 35, 39, 47, 49, 50]. Treatment with a PPI for less than 1 month was associated with the highest risk of CAP (OR 2.10; 95% CI 1.39, 3.16), as compared to no PPI therapy [23, 35, 39, 47, 49, 51]. The magnitude of CAP risk decreased and lost statistical significance as duration of PPI therapy increased (OR 1.51; 95% CI 0.92, 2.49 for 1–6 months [23, 35, 49, 51, 52] and OR 1.37; 95% CI 0.85, 2.20 for >6months) [23, 35, 49, 51, 52]. Participants aged ≥ 65 years demonstrated significantly increased risk of CAP with PPI therapy as compared to those not taking PPIs (OR 1.33; 95% CI 1.13, 1.58; I2 85.4%) [23–25, 28, 31, 45, 46, 48, 50–52]. Similar findings were observed among participants <65 years old (OR 1.34; 95% CI 1.04, 1.71; I2 60.1%) [23, 24, 31, 37, 50–52].
Table 2. Summary of Subgroup Analyses.
| Subgroup | # of Studies | Pooled Effect Estimate | 95% CI | I2 (%) | p-value for Heterogeneity | Reference | |
|---|---|---|---|---|---|---|---|
| PPI Dose* | High | 7 | 1.33 | 1.05–1.69 | 34.0 | 0.168 | 23,28,35,39,47,49,50 |
| Low | 8 | 1.31 | 1.04–1.66 | 71.4 | 0.001 | 23,28,39,47,49,50,51,52 | |
| PPI Duration† | <1 month | 6 | 2.10 | 1.39–3.16 | 72.5 | 0.003 | 23,35,39,47,49,51 |
| 1–6 months | 5 | 1.51 | 0.92–2.49 | 63.3 | 0.028 | 23,35,49,51,52 | |
| >6 months | 5 | 1.37 | 0.85–2.20 | 74.1 | 0.004 | 23,35,49,51,52 | |
| Age | <65 years | 7 | 1.34 | 1.04–1.71 | 60.1 | 0.020 | 23,24,31,37,50,51,52 |
| >65 years | 11 | 1.33 | 1.13–1.58 | 85.4 | <0.001 | 23,24,25,28,31,45,46,48,50,51,52 | |
* Dose is categorized as Low for doses ≤ 1 defined daily dose, High for doses > 1 defined daily dose
† Duration refers to duration of time taking PPI prior to community-acquired pneumonia diagnosis
Discussion
This systematic review of 33 studies and meta-analysis of 26 studies demonstrated a 1.5-fold increased risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy. PPI therapy was also associated with an increased risk for hospitalization with CAP (1.6-fold). No association was observed between H2RA use and CAP among studies examining participants taking H2RA therapy alone. CAP risk did not vary by PPI dose or participant age but more than doubled among those treated for less than 1 month, as compared to those not taking PPIs. Our findings were robust to sensitivity analyses restricting to studies exploring only PPI therapy (excluding H2RA therapy), studies utilizing a CAP definition requiring radiographic confirmation, and studies found to have lower risk of bias during qualitative analysis.
Several pathogenic mechanisms have been proposed to explain the association between PPI use and incidence of CAP. Decreased gastric acidity is associated with alteration of gut flora [58–61]. Micro-aspiration of the altered gut flora is one hypothesized mechanism for the increased CAP risk observed in the setting of elevated gut pH. More recently, proton pumps have been localized to the upper and lower respiratory tract [62, 63], suggesting that pH dysregulation may additionally alter respiratory flora, thereby directly inducing infection [64]. Our findings are consistent with the theory that PPI therapy may lead to CAP both through acute pH dysregulation and alteration of the gut microbiome. CAP risk was greatest during the first month of therapy, which is the time period during which the aero-digestive microbiome may be in greatest flux [65]. This association between CAP risk and short duration of PPI therapy, in conjunction with a lack of dose and duration effect, suggest that the acute changes occurring with onset of PPI therapy may be responsible for CAP risk. Our findings cannot define a causal relationship but do highlight an important association that requires further investigation. CAP risk was absent among participants taking H2RAs, which have weaker acid suppressant activity than do PPIs, however our study was not constructed to fully investigate this question.
Given the widespread usage of PPI therapy, often without an appropriate indication, the excess risk of CAP among PPI users could translate into a substantial burden on the healthcare system. Moreover, the increased risk of hospitalization for CAP underscores the potential clinical and financial impact of this adverse effect. Careful consideration of the risks, benefits and alternative treatment options should occur with all PPI prescriptions. Our observation that CAP risk was increased with PPI therapy, regardless of PPI dose or participant age, implies that alternate therapies, when appropriate, may be a strategy for reducing CAP risk. Secondary analysis showing an absence of CAP risk among participants taking H2RAs further supports the reduced risk profile of alternate therapies.
Our systematic review and meta-analysis could be subject to several sources of bias. Confounding by indication may have augmented our findings if the incidence of CAP is higher among persons taking PPIs due to the nature of their disease state, unrelated to the PPI therapy. GERD, a common indication for PPI therapy [12, 65], may directly increase risk of CAP by increasing microaspiration of gut flora. Several studies included in our review utilized different methods to reduce this source of confounding. Four studies restricted study enrollment to participants with a non-GERD indication for PPI [27, 40, 51, 52], one study employed a case-crossover design so that participants in both the exposed and unexposed groups possessed the same comorbidities [36], and two studies compared current PPI users to former PPI users to limit variability in comorbidities between cases and controls [31, 35]. Jena, et al., further explored the concept of confounding by using prescription drug and medical claims data to demonstrate statistically significant associations between PPI use and conditions without a readily apparent pathophysiologic link to PPI use, such as osteoarthritis, chest pain, and urinary tract infection. The authors raise the important point that the observed relationship between PPI use and CAP may be influenced by unmeasured confounders. This warrants further investigation, including replication of the findings using clinically-driven criteria for disease outcomes and efforts to elucidate specific confounders that may be driving any associations. Importantly, the absence of a known pathophysiologic mechanism for a causal relationship does not preclude the existence of one.
Reporting bias is an additional source of bias inherent to systematic reviews and meta-analyses. Our search was designed to detect a broad array of study designs respiratory diagnoses, yet only identified four RCTs reporting pneumonia as an adverse event. The four RCTs included in our review contribute few patients to the primary analysis, were not designed specifically to address our study question, and relied upon adverse event reporting to assess the incidence of pneumonia. Still, our findings were essentially unchanged in multiple sensitivity analyses, including an analysis that excluded RCTs.
Publishing bias may have also influenced our findings. Though our funnel plot was limited by the number of included studies [56, 66], it suggested a publication bias toward a protective or null effect of PPI therapy. If present, the magnitude of the association we report may actually be smaller than the true effect.
Our study has limitations. Several studies reported data on overlapping participant populations. We selected only the most inclusive studies but cannot guarantee inclusion of all eligible participants in our meta-analysis. The included studies reported one of three different measures of association: odds ratio, hazard ratio, or relative risk. Because CAP is a rare outcome, the odds ratio and hazard ratio are each assumed to approximate the relative risk. We observed the greatest risk for CAP in the setting of PPI use for less than 1 month, which may have a biological explanation but may also, at least partly, reflect a protopathic bias due to initiation of PPI therapy around the time of the development of respiratory symptoms.
Tests for heterogeneity demonstrated variability of findings across studies; however consistent demonstration of increased CAP risk with PPI therapy and the findings from our qualitative analysis suggested that pooling of the 26 identified studies for meta-analysis was reasonable despite differences in methodology. The impact of these differences was assessed in subgroup, secondary, and sensitivity analyses. In many cases, studies demonstrated significantly less heterogeneity when grouped for these additional analyses. The subgroup analysis of the association between high-dose PPI exposure and CAP demonstrated the least heterogeneity (I2 34.0%). Almost all of these analyses supported our primary findings. Among sensitivity analyses, only the analysis examining the strictest CAP definition failed to show a significant association between PPI exposure and CAP. With only 7 included studies, representing 3,865 CAP cases among 16,537 participants, this sensitivity analysis was likely underpowered to detect a significant difference.
Several previous meta-analyses have sought to evaluate the relationship between PPI therapy and CAP. Giuliano, et al, concluded that patients prescribed PPIs had an increased risk for CAP, particularly at high PPI doses and soon after initiation of PPI therapy [67]. Eom, et al., found that patients prescribed any acid suppressant therapy were at increased risk of community-acquired or hospital-acquired pneumonia [68]. Our meta-analysis enhances the existing literature by adding data from 19 studies that were not included in these prior investigations. These 19 studies were identified through a combination of our broad, updated database search and our extensive pursuit of unpublished data. The inclusion of unpublished data also strengthened our subgroup and sensitivity analyses.
In conclusion, we conducted a thorough systematic review and rigorous meta-analysis of studies reporting the risk of CAP with ambulatory PPI therapy. We identified a 1.5-fold increased risk for CAP with outpatient PPI therapy, with additional analyses revealing even greater risk during the first month of therapy and for CAP hospitalization. Our findings add to currently available literature through the breadth of our search, inclusion of unpublished data and updated research, and extent of analysis. Given the morbidity and mortality associated with CAP and the extent of PPI use in the United States, identification of any risk associated with PPI use is critical for risk stratification and modification where possible. Future studies should employ a rigorous definition of CAP and ascertain PPI exposure at various doses and durations to further explore the association between PPI exposure and CAP within specific populations. Studies are also needed to clarify any pathophysiologic mechanisms underlying the observed association. We recommend careful consideration of the risks and benefits when initiating PPI therapy and heightened awareness regarding this risk factor for adults presenting with pneumonia.
Supporting Information
(DTA)
Risk of bias was assessed using a modified Newcastle-Ottawa Scale as outlined in S3 Table. The risk associated with each criterion on the scale corresponds to a circle in this figure. Low risk is represented by a green circle, medium risk yellow, and high risk red.
(PDF)
This funnel plot displays standard error of the effect estimate as a measure of study size on the vertical axis and estimated effect of PPI therapy on CAP diagnosis on the horizontal axis. Dashed lines represent pseudo-95% confidence interval lines, drawn around the summary fixed-effect estimate of the effect of PPI therapy on CAP diagnosis.
(PDF)
(PDF)
(PDF)
We performed systematic searches of MEDLINE (via PubMed), EMBASE, CINAHL, Cochrane Central Register of Controlled Trials (CENTRAL), Scopus, Web of Science and ClinicalTrials.gov on February 3, 2014. All search strings included controlled vocabulary and related keywords for two concepts: pneumonia and acid suppressants. A representative search string used for MEDLINE (via PubMed) is presented here. Concept terms were combined via the Boolean operator “AND” for database searches.
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
Contributors: The authors would like to acknowledge Dr. Robert Wise (Johns Hopkins University) who helped formulate our research question through his expertise in the area of interest. We thank informationists Carrie Price (Johns Hopkins University) and Robert Wright (Johns Hopkins University) who facilitated search string development and database querying. We also thank the Systematic Review and Meta-Analysis course instructors and teaching assistants at the Bloomberg School of Public Health, who provided critical support and professional guidance throughout the research process.
Prior Presentation: These data were presented, in part, at IDWeek by Dr. Crowell in San Francisco, CA on October 4th, 2013.
Data Availability
All relevant data are within the paper and its supporting information files.
Funding Statement
No funding was provided specifically for the conduct of this review. Dr. Lambert is supported by NIH Grant T32 HL007534 31A1, Dr. Drummond by NIH Grant K23 HL103192 04 and Dr. Crowell by NIH Grant T32 AI007291 23. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. File TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122(2):130–41. Epub 2010/03/06. 10.3810/pgm.2010.03.2130 . [DOI] [PubMed] [Google Scholar]
- 2. Yu H, Rubin J, Dunning S, Li S, Sato R. Clinical and economic burden of community-acquired pneumonia in the Medicare fee-for-service population. Journal of the American Geriatrics Society. 2012;60(11):2137–43. Epub 2012/11/01. 10.1111/j.1532-5415.2012.04208.x . [DOI] [PubMed] [Google Scholar]
- 3. Fung HB, Monteagudo-Chu MO. Community-acquired pneumonia in the elderly. Am J Geriatr Pharmacother. 2010;8(1):47–62. Epub 2010/03/17. 10.1016/j.amjopharm.2010.01.003 . [DOI] [PubMed] [Google Scholar]
- 4. Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2007;44 Suppl 2:S27–72. Epub 2007/02/06. 10.1086/511159 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnstone J, Marrie TJ, Eurich DT, Majumdar SR. Effect of pneumococcal vaccination in hospitalized adults with community-acquired pneumonia. Archives of internal medicine. 2007;167(18):1938–43. Epub 2007/10/10. 10.1001/archinte.167.18.1938 . [DOI] [PubMed] [Google Scholar]
- 6. Koivula I, Sten M, Leinonen M, Makela PH. Clinical efficacy of pneumococcal vaccine in the elderly: a randomized, single-blind population-based trial. Am J Med. 1997;103(4):281–90. Epub 1997/11/14. . [DOI] [PubMed] [Google Scholar]
- 7.IMS Institute for Healthcare Informatics. The Use of Medicines in the United States: Review of 2011. 2012.
- 8.IMS Institute for Healthcare Informatics. Medicine Use and Shifting Costs of Healthcare: A Review of the Use of Medicines in the U.S. in 2013. 2014.
- 9. Kahrilas PJ, Shaheen NJ, Vaezi MF, Hiltz SW, Black E, Modlin IM, et al. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135(4):1383–91, 91 e1–5. 10.1053/j.gastro.2008.08.045 . [DOI] [PubMed] [Google Scholar]
- 10. Pierson TC, Kieffer TL, Ruff CT, Buck C, Gange SJ, Siliciano RF. Intrinsic stability of episomal circles formed during human immunodeficiency virus type 1 replication. Journal of virology. 2002;76(8):4138–44. Epub 2002/03/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heidelbaugh JJ, Goldberg KL, Inadomi JM. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. The American journal of managed care. 2010;16(9):e228–34. . [PubMed] [Google Scholar]
- 12. Jacobson BC, Ferris TG, Shea TL, Mahlis EM, Lee TH, Wang TC. Who is using chronic acid suppression therapy and why? Am J Gastroenterol. 2003;98(1):51–8. 10.1111/j.1572-0241.2003.07186.x . [DOI] [PubMed] [Google Scholar]
- 13. Heidelbaugh JJ, Goldberg KL, Inadomi JM. Overutilization of proton pump inhibitors: a review of cost-effectiveness and risk [corrected]. The American journal of gastroenterology. 2009;104 Suppl 2:S27–32. Epub 2009/03/06. 10.1038/ajg.2009.49 . [DOI] [PubMed] [Google Scholar]
- 14. Restrepo MI, Mortensen EM, Anzueto A. Common medications that increase the risk for developing community-acquired pneumonia. Curr Opin Infect Dis. 2010;23(2):145–51. Epub 2010/01/16. 10.1097/QCO.0b013e328336eac1 . [DOI] [PubMed] [Google Scholar]
- 15. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Annals of internal medicine. 2009;151(4):W65–94. . [DOI] [PubMed] [Google Scholar]
- 16. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA: the journal of the American Medical Association. 2000;283(15):2008–12. . [DOI] [PubMed] [Google Scholar]
- 17. Anglemyer A, Horvath HT, Bero L. Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials. The Cochrane database of systematic reviews. 2014;4:MR000034 10.1002/14651858.MR000034.pub2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shrier I, Boivin JF, Steele RJ, Platt RW, Furlan A, Kakuma R, et al. Should meta-analyses of interventions include observational studies in addition to randomized controlled trials? A critical examination of underlying principles. American journal of epidemiology. 2007;166(10):1203–9. 10.1093/aje/kwm189 . [DOI] [PubMed] [Google Scholar]
- 19.Wells GA SB, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [cited 2013 February 17]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 20. Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Progress in cardiovascular diseases. 1985;27(5):335–71. . [DOI] [PubMed] [Google Scholar]
- 21. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.StataCorp. 2012. Stata Statistical Software: Release 12. College Station TSL.
- 23. Almirall J, Bolibar I, Serra-Prat M, Roig J, Hospital I, Carandell E, et al. New evidence of risk factors for community-acquired pneumonia: a population-based study. European Respiratory Journal. 2008;31(6):1274–84. Epub 2008/01/25. 10.1183/09031936.00095807 . [DOI] [PubMed] [Google Scholar]
- 24.Chen CH, Lin HC, Lin HL, Lin YT, Chou JM, Hsu SP, et al. Proton pump inhibitor usage and the associated risk of pneumonia in patients with chronic kidney disease. Journal of microbiology, immunology, and infection = Wei mian yu gan ran za zhi. 2013. 10.1016/j.jmii.2013.10.004 . [DOI] [PubMed]
- 25. Dublin S, Walker RL, Jackson ML, Nelson JC, Weiss NS, Jackson LA. Use of proton pump inhibitors and H2 blockers and risk of pneumonia in older adults: a population-based case-control study. Pharmacoepidemiol Drug Saf. 2010;19(8):792–802. Epub 2010/07/14. 10.1002/pds.1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ernst P, Renoux C, Dell'Aniello S, Suissa S. Pramipexole use and the risk of pneumonia. BMC neurology. 2012;12:113 Epub 2012/10/02. 10.1186/1471-2377-12-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Filion KB, Chateau D, Targownik LE, Gershon A, Durand M, Tamim H, et al. Proton pump inhibitors and the risk of hospitalisation for community-acquired pneumonia: replicated cohort studies with meta-analysis. Gut. 2013. Epub 2013/07/17. 10.1136/gutjnl-2013-304738 . [DOI] [PMC free article] [PubMed]
- 28. Gau JT, Acharya U, Khan S, Heh V, Mody L, Kao TC. Pharmacotherapy and the risk for community-acquired pneumonia. BMC Geriatr. 2010;10:45 Epub 2010/07/08. 10.1186/1471-2318-10-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gulmez SE, Holm A, Frederiksen H, Jensen TG, Pedersen C, Hallas J. Use of proton pump inhibitors and the risk of community-acquired pneumonia—A population-based case-control study. Archives of Internal Medicine. 2007;167(9):950–5. . [DOI] [PubMed] [Google Scholar]
- 30. Hennessy S, Bilker WB, Leonard CE, Chittams J, Palumbo CM, Karlawish JH, et al. Observed association between antidepressant use and pneumonia risk was confounded by comorbidity measures. Journal of clinical epidemiology. 2007;60(9):911–8. Epub 2007/08/11. 10.1016/j.jclinepi.2006.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hermos JA, Young MM, Fonda JR, Gagnon DR, Fiore LD, Lawler EV. Risk of community-acquired pneumonia in veteran patients to whom proton pump inhibitors were dispensed. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2012;54(1):33–42. Epub 2011/11/22. 10.1093/cid/cir767 . [DOI] [PubMed] [Google Scholar]
- 32. Jena AB, Sun E, Goldman DP. Confounding in the association of proton pump inhibitor use with risk of community-acquired pneumonia. Journal of general internal medicine. 2013;28(2):223–30. Epub 2012/09/08. 10.1007/s11606-012-2211-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Juthani-Mehta M, De Rekeneire N, Allore H, Chen S, O'Leary JR, Bauer DC, et al. Modifiable risk factors for pneumonia requiring hospitalization of community-dwelling older adults: the Health, Aging, and Body Composition Study. Journal of the American Geriatrics Society. 2013;61(7):1111–8. 10.1111/jgs.12325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Laheij RJ, Van Ijzendoorn MC, Janssen MJ, Jansen JB. Gastric acid-suppressive therapy and community-acquired respiratory infections. Aliment Pharmacol Ther. 2003;18(8):847–51. Epub 2003/10/11. . [DOI] [PubMed] [Google Scholar]
- 35. Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA: the journal of the American Medical Association. 2004;292(16):1955–60. Epub 2004/10/28. 10.1001/jama.292.16.1955 . [DOI] [PubMed] [Google Scholar]
- 36. Liu CL, Shau WY, Wu CS, Lai MS. Angiotensin-converting enzyme inhibitor/angiotensin II receptor blockers and pneumonia risk among stroke patients. J Hypertens. 2012;30(11):2223–9. Epub 2012/08/30. 10.1097/HJH.0b013e328357a87a . [DOI] [PubMed] [Google Scholar]
- 37. Long MD, Martin C, Sandler RS, Kappelman MD. Increased risk of pneumonia among patients with inflammatory bowel disease. Am J Gastroenterol. 2013;108(2):240–8. Epub 2013/01/09. 10.1038/ajg.2012.406 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mastronarde JG, Anthonisen NR, Castro M, Holbrook JT, Leone FT, Teague WG, et al. Efficacy of esomeprazole for treatment of poorly controlled asthma. The New England journal of medicine. 2009;360(15):1487–99. Epub 2009/04/10. 10.1056/NEJMoa0806290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Meijvis SCA, Cornips MCA, Voorn GP, Souverein PC, Endeman H, Biesma DH, et al. Microbial evaluation of proton-pump inhibitors and the risk of pneumonia. European Respiratory Journal. 2011;38(5):1165–72. 10.1183/09031936.00020811 [DOI] [PubMed] [Google Scholar]
- 40. Morris G, Hardinger K, Tsapepas D, Tichy E. A comparison of histamine receptor antagonists versus proton pump inhibitor stress ulcer prophylaxis in kidney transplant recipients. American Journal of Transplantation. 2013;13:550. [DOI] [PubMed] [Google Scholar]
- 41. Muellerova H, Chigbo C, Hagan GW, Woodhead MA, Miravitlles M, Davis KJ, et al. The natural history of community-acquired pneumonia in COPD patients: A population database analysis. Respiratory medicine. 2012;106(8):1124–33. Epub 2012/05/25. 10.1016/j.rmed.2012.04.008 . [DOI] [PubMed] [Google Scholar]
- 42. Myles PR, Hubbard RB, McKeever TM, Pogson Z, Smith CJ, Gibson JE. Risk of community-acquired pneumonia and the use of statins, ace inhibitors and gastric acid suppressants: a population-based case-control study. Pharmacoepidemiol Drug Saf. 2009;18(4):269–75. Epub 2009/02/25. 10.1002/pds.1715 . [DOI] [PubMed] [Google Scholar]
- 43. Nielsen AG, Nielsen RB, Riis AH, Johnsen SP, Sorensen HT, Thomsen RW. The impact of statin use on pneumonia risk and outcome: a combined population-based case-control and cohort study. Critical care. 2012;16(4):R122 Epub 2012/07/14. 10.1186/cc11418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pasina L, Nobili A, Tettamanti M, Salerno F, Corrao S, Marengoni A, et al. Prevalence and appropriateness of drug prescriptions for peptic ulcer and gastro-esophageal reflux disease in a cohort of hospitalized elderly. European journal of internal medicine. 2011;22(2):205–10. Epub 2011/03/16. 10.1016/j.ejim.2010.11.009 . [DOI] [PubMed] [Google Scholar]
- 45. Quagliarello V, Ginter S, Han L, Van Ness P, Allore H, Tinetti M. Modifiable risk factors for nursing home-acquired pneumonia. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2005;40(1):1–6. 10.1086/426023 . [DOI] [PubMed] [Google Scholar]
- 46. Ramsay EN, Pratt NL, Ryan P, Roughead EE. Proton pump inhibitors and the risk of pneumonia: a comparison of cohort and self-controlled case series designs. BMC medical research methodology. 2013;13(1):82 Epub 2013/06/27. 10.1186/1471-2288-13-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rodriguez LA, Ruigomez A, Wallander MA, Johansson S. Acid-suppressive drugs and community-acquired pneumonia. Epidemiology. 2009;20(6):800–6. Epub 2009/10/03. 10.1097/EDE.0b013e3181b5f27d . [DOI] [PubMed] [Google Scholar]
- 48. Roughead EE, Ramsay EN, Pratt NL, Ryan P, Gilbert AL. Proton-pump inhibitors and the risk of antibiotic use and hospitalisation for pneumonia. The Medical journal of Australia. 2009;190(3):114–6. Epub 2009/02/11. . [DOI] [PubMed] [Google Scholar]
- 49. Sarkar M, Hennessy S, Yang YX. Proton-pump inhibitor use and the risk for community-acquired pneumonia. Annals of internal medicine. 2008;149(6):391–8. Epub 2008/09/17. . [DOI] [PubMed] [Google Scholar]
- 50. Scheiman JM, Devereaux PJ, Herlitz J, Katelaris PH, Lanas A, Veldhuyzen van Zanten S, et al. Prevention of peptic ulcers with esomeprazole in patients at risk of ulcer development treated with low-dose acetylsalicylic acid: a randomised, controlled trial (OBERON). Heart. 2011;97(10):797–802. Epub 2011/03/19. 10.1136/hrt.2010.217547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sugano K, Matsumoto Y, Itabashi T, Abe S, Sakaki N, Ashida K, et al. Lansoprazole for secondary prevention of gastric or duodenal ulcers associated with long-term low-dose aspirin therapy: results of a prospective, multicenter, double-blind, randomized, double-dummy, active-controlled trial. Journal of gastroenterology. 2011;46(6):724–35. Epub 2011/04/19. 10.1007/s00535-011-0397-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sugano K, Kontani T, Katsuo S, Takei Y, Sakaki N, Ashida K, et al. Lansoprazole for secondary prevention of gastric or duodenal ulcers associated with long-term non-steroidal anti-inflammatory drug (NSAID) therapy: results of a prospective, multicenter, double-blind, randomized, double-dummy, active-controlled trial. Journal of gastroenterology. 2012;47(5):540–52. Epub 2012/03/06. 10.1007/s00535-012-0541-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. van de Garde EMW, Hak E, Souverein PC, Hoes AW, van den Bosch JMM, Leufkens HGM. Statin treatment and reduced risk of pneumonia in patients with diabetes. Thorax. 2006;61(11):957–61. Epub 2006/07/01. 10.1136/thx.2006.062885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. van de Garde EM, Souverein PC, van den Bosch JM, Deneer VH, Leufkens HG. Angiotensin-converting enzyme inhibitor use and pneumonia risk in a general population. The European respiratory journal. 2006;27(6):1217–22. Epub 2006/02/04. 10.1183/09031936.06.00110005 . [DOI] [PubMed] [Google Scholar]
- 55. van de Garde EMW, Souverein PC, Hak E, Deneer VHM, van den Bosch JMM, Leufkens HGM. Angiotensin-converting enzyme inhibitor use and protection against pneumonia in patients with diabetes. Journal of hypertension. 2007;25(1):235–9. Epub 2006/12/05. 10.1097/HJH.0b013e328010520a . [DOI] [PubMed] [Google Scholar]
- 56. Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta-analysis. Statistics in medicine. 2001;20(4):641–54. 10.1002/sim.698 . [DOI] [PubMed] [Google Scholar]
- 57.Higgins J GS. Cochrane Handbook for Systematic Reviews of Interventions: The Cochrane Collaboration; 2011 [cited 2013 February 17]. Available from: www.cochrane-handbook.org.
- 58. Dellipiani AW, Girdwood RH. Bacterial Changes in the Small Intestine in Malabsorptive States and in Pernicious Anaemia. Clinical science. 1964;26:359–74. . [PubMed] [Google Scholar]
- 59. Borriello SP, Reed PJ, Dolby JM, Barclay FE, Webster AD. Microbial and metabolic profile of achlorhydric stomach: comparison of pernicious anaemia and hypogammaglobulinaemia. Journal of clinical pathology. 1985;38(8):946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sanduleanu S, Jonkers D, De Bruine A, Hameeteman W, Stockbrugger RW. Non-Helicobacter pylori bacterial flora during acid-suppressive therapy: differential findings in gastric juice and gastric mucosa. Aliment Pharmacol Ther. 2001;15(3):379–88. . [DOI] [PubMed] [Google Scholar]
- 61. Williams C, McColl KE. Review article: proton pump inhibitors and bacterial overgrowth. Aliment Pharmacol Ther. 2006;23(1):3–10. 10.1111/j.1365-2036.2006.02707.x . [DOI] [PubMed] [Google Scholar]
- 62. Altman KW, Haines GK 3rd, Hammer ND, Radosevich JA. The H+/K+-ATPase (proton) pump is expressed in human laryngeal submucosal glands. The Laryngoscope. 2003;113(11):1927–30. . [DOI] [PubMed] [Google Scholar]
- 63. Altman KW, Waltonen JD, Tarjan G, Radosevich JA, Haines GK 3rd. Human lung mucous glands manifest evidence of the H+/K+-ATPase proton pump. The Annals of otology, rhinology, and laryngology. 2007;116(3):229–34. . [DOI] [PubMed] [Google Scholar]
- 64. Fohl AL, Regal RE. Proton pump inhibitor-associated pneumonia: Not a breath of fresh air after all? World journal of gastrointestinal pharmacology and therapeutics. 2011;2(3):17–26. 10.4292/wjgpt.v2.i3.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chey WD, Mody RR, Wu EQ, Chen L, Kothari S, Persson B, et al. Treatment patterns and symptom control in patients with GERD: US community-based survey. Current medical research and opinion. 2009;25(8):1869–78. 10.1185/03007990903035745 . [DOI] [PubMed] [Google Scholar]
- 66. Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. Bmj. 2006;333(7568):597–600. 10.1136/bmj.333.7568.597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Giuliano C, Wilhelm SM, Kale-Pradhan PB. Are proton pump inhibitors associated with the development of community-acquired pneumonia? A meta-analysis. Expert review of clinical pharmacology. 2012;5(3):337–44. Epub 2012/06/16. 10.1586/ecp.12.20 . [DOI] [PubMed] [Google Scholar]
- 68. Eom CS, Jeon CY, Lim JW, Cho EG, Park SM, Lee KS. Use of acid-suppressive drugs and risk of pneumonia: a systematic review and meta-analysis. CMAJ: Canadian Medical Association journal = journal de l'Association medicale canadienne. 2011;183(3):310–9. Epub 2010/12/22. 10.1503/cmaj.092129 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DTA)
Risk of bias was assessed using a modified Newcastle-Ottawa Scale as outlined in S3 Table. The risk associated with each criterion on the scale corresponds to a circle in this figure. Low risk is represented by a green circle, medium risk yellow, and high risk red.
(PDF)
This funnel plot displays standard error of the effect estimate as a measure of study size on the vertical axis and estimated effect of PPI therapy on CAP diagnosis on the horizontal axis. Dashed lines represent pseudo-95% confidence interval lines, drawn around the summary fixed-effect estimate of the effect of PPI therapy on CAP diagnosis.
(PDF)
(PDF)
(PDF)
We performed systematic searches of MEDLINE (via PubMed), EMBASE, CINAHL, Cochrane Central Register of Controlled Trials (CENTRAL), Scopus, Web of Science and ClinicalTrials.gov on February 3, 2014. All search strings included controlled vocabulary and related keywords for two concepts: pneumonia and acid suppressants. A representative search string used for MEDLINE (via PubMed) is presented here. Concept terms were combined via the Boolean operator “AND” for database searches.
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its supporting information files.