Abstract
The early life experiences of an organism have the potential to alter their developmental trajectories. Perhaps one of the most powerful influences during this period is the parent-offspring relationship. Previous work in several mammalian species has demonstrated that parental care in early life and specifically maternal behavior can influence several adult outcomes in offspring, including affiliative and aggressive behavior, parental behavior, hypothalamic-pituitary-adrenal (HPA) functioning and risk of psychopathology. We have previously demonstrated that naturally occurring variation in the type and amount of care given to offspring in a biparental species, the prairie vole (Microtus ochrogaster), is related to social, anxiety-like, aggressive behaviors as well as HPA response to chronic and acute social stressors. Here we aim to determine the effects of early biparental care on HPA functioning and the interaction between early care and later reactivity to a forced swim test, an acute non-social stressor. Behavior during the swim test as well as several indicators of HPA activity, including plasma corticosterone (CORT), corticotropin releasing hormone immunoreactivity (CRH-ir), and vasopressin immunoreactivity (AVP-ir) were measured. Results here indicate an effect of early experience on AVP-ir but not CRH-ir or plasma CORT. There were no differences in CORT levels between high-contact (HC) and low-contact (LC) males or females for either control animals or after a swim stressor. CRH-ir was higher in the central amygdala following a swim test but was not influenced by early care. However, AVP-ir was not influenced by exposure to a swim stressor but was affected by early parental care in a sex-dependent manner. Female HC offspring had increased AVP-ir in the SON while HC male offspring had decreased AVP-ir in the PVN compared to their LC counterparts. The differential response of CRH and AVP to early experience and later stress, and the lack of an interaction between early care rearing and later adult stress, suggest an independence in response of some components of the HPA system. In addition, these findings expand our understanding of the relationship between naturally occurring variation in early biparental care and sexual dimorphisms in adult outcomes.
Keywords: Prairie vole, biparental care, natural variation, forced swim test, vasopressin, corticotropin releasing hormone
1. Introduction
Early life experiences have the potential to shape numerous outcomes in later life and its impact on response to stressors has been extensively studied in humans [1–4] as well as several animal models [5–9]. Models of early life experience in a variety of species have shown that suboptimal early rearing environments can lead to changes in the regulation of the hypothalamic-pituitary-adrenal (HPA) axis in response to later-life stressors. Naturally occurring infant abuse in rhesus monkeys in early life results in an increased cortisol and adrenocorticotropic hormone (ACTH) response in corticotropin-releasing hormone (CRH) stimulation [10] while offspring that experience increased rates of rejection in the first 2 months of life show increased cortisol responses to social separation at 6, 12, and 18 months of age in the cooperatively breeding marmoset [11]. Deprivations in the social environment in rodents, particularly in parental care, have similar consequences. Repeated long-term maternal separation in early life in rats leads to an increase in basal corticosterone (CORT) levels and increased CRH mRNA in the paraventricular nucleus of the hypothalamus (PVN) [12] as well as an increase in ACTH release following a brief restraint stress in adulthood [13]. Early weaning of offspring also leads to increased basal CORT and increased CRH mRNA after a stressor [14]. Taken together, these findings indicate that disruption of the early care environment has lasting implications for HPA activity, both at a basal level and in response to a stressor.
In response to an acute stressor, the PVN releases both CRH and vasopressin (AVP), which in turn stimulates corticotroph cells in the anterior pituitary to release ACTH into the peripheral blood supply. ACTH then acts on the adrenal glands, promoting the release of glucocorticoids, including CORT. Each point in this hormonal cascade has been found to be sensitive to increased amounts of tactile stimulation in the early environment of offspring. Early handling manipulations in which the offspring is briefly separated from the mother each day for approximately 2 weeks results in an increase in maternal care of pups upon their return to the nest [15–18]. This handling, and therefore increased maternal stimulation, leads to decreases in CRH production and secretion [13, 19], decreased basal levels of AVP in the median eminence [19], decreased ACTH release following restraint stress [19, 20], and a decreased CORT response to stressors [13, 21]. These same experiences in early life also impact the response of adult offspring to various stressors. For example, there are decreases in depressive-like behavior as measured by a forced swim test in animals experiencing early handling compared to those experiencing maternal separation [12, 22, 23].
Increased maternal stimulation also occurs in more naturalistic settings, such as communal rearing of offspring. Being reared in a communal nest, with multiple mothers and multiple litters, results in offspring that display decreases in anxiety-like behaviors in a novel environment [24], decreases in plasma CORT after social stress [25], and a faster habituation of the CORT response following repeated stress [26]. As with the handling paradigm in rats, communal rearing also produces changes in depressive-like behaviors in a sex-specific manner. The direction of these changes, however, is less clear than in the handling paradigm in rats, with reports of both increased “depression” behavior [27] and increased resilience to depressive-like behaviors [28]. Naturally occurring increases in maternal licking and grooming (LG) behavior in rats also have similar effects to early handling, where offspring reared by high-LG dams display decreased ACTH and CORT responses to restraint stress as well as increased glucocorticoid feedback sensitivity which allows the system to return to baseline faster [29]. These results in rats are due to epigenetic changes following varying early care rather than being strictly a genomic transmission of traits [30]. There is also evidence in California mice, a biparental rodent species, that increases in paternal grooming of offspring are associated with decreases in CORT levels in male offspring [31], similar to the effects of maternal grooming in rats.
The study of parental care in rodents and its influences on adult offspring outcomes has primarily focused on maternal behavior, likely because active care from the father is relatively rare in mammalian species. One exception is the prairie vole (Microtus ochrogaster), a socially monogamous rodent native to the midwestern United States that displays biparental care of offspring, the father typically displaying the same range of parental behaviors as the mother aside from lactation and nursing postures [32, 33]. This species has also repeatedly been shown to be sensitive to changes in early experiences, which results in differences in later parental behavior [34, 35], partner preference formation [35, 36], aggression [37, 38], and neuropeptide systems [39–41]. In addition, HPA functioning is sensitive to early handling [42] as well as early oxytocin (OT) manipulation [43]. Taken together, these studies provide ample evidence that several systems, including HPA functioning, are vulnerable to early life experiences in the prairie vole.
Here we examined the long-term effects of early life rearing environment on HPA functioning as well as the potential interaction between early rearing experience and later response to a non-social stressor in the prairie vole. In this species, the father is heavily involved in care of offspring, which provides an opportunity to study the effects of early biparental care. We have previously shown that this species displays naturally occurring variation in biparental care and that this variation is associated with an increase in pro-social behavior in high-contact (HC) adolescent males [44] as well as an increase in aggression in low-contact (LC) adult males and an increase in plasma CORT and vasopressin immunoreactivity (AVP-ir) in the supraoptic nucleus of the hypothalamus (SON) in HC adult females following chronic and acute social stress [45]. In addition, cross-fostering has shown that behavioral outcomes, in particular alloparenting, may be transmitted from parent to offspring through non-genomic routes while OT and AVP V1a receptor binding densities are transmitted through genetic means (Perkeybile et al, in review). In this study we investigated the relationship between the early rearing environment and adult responsiveness to a physical, non-social stressor, a forced swim test. We examined parental behavior received in the first days after birth, as well as behavior during the swim test. Plasma CORT levels were taken and CRH-ir and AVP-ir were quantified as indicators of HPA axis activity in response to the swim stressor. We predicted that LC offspring would display increased depressive-like behavior in the forced swim test compared to HC offspring. We also expected to see increases in plasma CORT levels, AVP-ir in the PVN and SON, and CRH-ir in the PVN and CeAmy in animals after a forced swim test compared to undisturbed controls, and for this post-swim test increase to be greater in LC compared to HC offspring. Because female prairie voles have previously shown increased HPA reactivity in response to stressors [45–47], we expected differences in these HPA outcome measures to be more pronounced in females compared to males. In particular, we anticipated that any increases in plasma CORT, AVP-ir or CRH-ir would be greatest in LC females.
2. Methods and Materials
2.1 Subjects
Subjects were laboratory-bred prairie voles (Microtus ochrogaster), descendants of a stock wild-caught near Champaign, IL. Animals were housed on a 14:10 light dark cycle with lights on a 0600. Food (Purina high-fiber rabbit chow) and water were available in the home cage ad libitum. Breeder pairs and pre-weaning offspring were housed in large polycarbonate cages (44 × 22 × 16 cm) and were given cotton nestlets for bedding. Offspring were weaned on postnatal (PND) 20 and housed with an age-matched same-sex unrelated animal in a small polycarbonate cage (27 × 16 × 16 cm) until testing. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the University of California, Davis.
2.2 Parental care quantification and ranking
Following methods previously described [44], the type and amount of parental care given to offspring was observed for two separate litters for 40 different breeder pairs. Each parent was observed for 20 minutes in the morning (0800–1200) and 20 minutes in the afternoon (1300–1700) twice from PND 1–3 for a total of 4 maternal care and 4 paternal care observations per litter for two separate litters. Observations were done with the animals in their home cage; animals were not disturbed during the observations. Behaviors were recorded in real time by a trained observer using Behavior Tracker software (www.behaviortracker.com). Behaviors recorded were based on an ethogram presented in Stone & Bales, 2010 and included maternal and paternal huddling, non-huddling contact, licking/grooming, retrievals, and non-pup directed behaviors, such as nest building, eating and drinking. Maternal nursing postures were also recorded, including neutral, lateral, and active nursing. Parents were distinguished from one another based on individual characteristics such as fur color and markings, body size, or the presence of pups visibly attached to the nipple.
In order to rank breeder pairs in relation to one another, total contact times for pup-directed behavior were summed across each observation for the mother and the father. A mean was then calculated for the mother and for the father, and these two means were then summed to produce an average total contact score for the breeder pair. These scores for all 40 breeder pairs were then rank-ordered and split into quartiles. The top quartile breeder pairs produced high-contact (HC) offspring while the bottom quartile breeder pairs produced low-contact (LC) offspring for subsequent testing. Breeder pairs that fell in the middle quartiles were not used for this study.
2.3 Early parental care of subject offspring
Parental care observations were conducted upon the birth of subsequent litters to determine early life care directed toward subject offspring. Within 24 hours of birth, offspring of breeders ranked as HC or LC were briefly removed from the home cage, at which time they were weighed, sexes were determined, and they were dyed for immediate identification using Nyanzol dye and toe clipped for long-term identification – ear tagging was not possible due to the age of the pups. Offspring were also randomly assigned to adult behavioral testing conditions at this time. Only one animal per litter per sex was included in each condition. If necessary, litters were culled to 4 offspring, 2 males and 2 females (HC males, n = 30; LC males, n = 28; HC females, n = 31; LC females, n = 26). Only those litters with at least 3 pups were included in the study. Time in which pups were out of the nest was kept under 15 minutes. Focal observations were conducted on each pup on PND 1–2 for 5 minutes in the morning and 5 minutes in the afternoon (4 total observations per subject) to characterize the type and amount of parental care each offspring received in the first days following birth. Observations were done in real time by a trained observer blind to parental ranking using Behavior Tracker. All observations were conducted while the animals were in their home cage and animals were not disturbed. Behaviors recorded include maternal and paternal huddling, non-huddling contact, licking/grooming, retrievals, nest building, and autogrooming. Maternal nursing postures were also recorded, including neutral, lateral, and active nursing.
2.4 Behavioral testing
Offspring were weaned on PND 20, weighed, and were then pair-housed with a same-sex age-matched unrelated animal who was not a subject in this study. On PND 48 half of the subject offspring underwent behavioral testing (n = 36; 19 high-contact; 17 low-contact) while the other half remained undisturbed as controls (n = 40; 22 high-contact; 18 low-contact), followed by blood collection and brain removal. All behavioral testing occurred between 1400–1500.
2.4.1 Forced swim
Following 4 weeks of pair housing, 36 animals (HC males, n = 9; LC males, n = 9; HC females, n = 10; LC females, n = 8) were exposed to a 3-minute forced swim test, a non-social stressor. Animals were placed in a large polycarbonate cage (20 × 25 × 45 cm) filled with lukewarm water to a depth that does not allow animals to climb out the top but also prevents them from touching the bottom, thereby forcing them to swim. This test elicits a reliable HPA response and has previously been used as a mild stressor in the laboratory [20, 48]. Behavior in the chamber was recorded and later scored by an observer blind to condition. Behaviors recorded included swimming (animal is calming swimming around chamber; includes diving), floating (animal is immobile, not swimming or struggling) and struggling (animal is persistently swimming against the sides or corners of the tank). Following behavioral testing, animals were removed from the swim chamber and returned to their home cage.
2.5 Blood collection and brain extraction
Two hours after behavioral testing a blood sample was collected and brains were extracted. This time point was chosen in an attempt to measure peak CRH-ir and AVP-ir as well as to examine a potentially extended plasma CORT response following a stressor. Samples were taken from undisturbed control animals at the same time (HC males, n = 11; LC males, n = 9; HC females, n = 11; LC females, n = 9). Animals were euthanized via cervical dislocation and rapid decapitation under deep anesthesia. Trunk blood was collected and immediately kept on ice. Samples were collected within 4 minutes of disturbing the home cage to avoid increases in plasma CORT due to disturbance. Brains were removed rapidly and fixed via passive perfusion in a 4% paraformaldehyde/acrolein solution.
2.6 Corticosterone radioimmunoassay
Following collection, blood samples were centrifuged at 4° C for 12 minutes and plasma was extracted and stored at −20° C until assayed. Plasma CORT was assayed using a radioimmunoassay (MP Biomedicals, Irvine, CA, USA) previously validated for use in the prairie vole [48]. Non-extracted samples were diluted 1:2,000 so that all values fell on the standard curve. Intra-assay coefficients of variation (CV) averaged 1.50%. There is no inter-assay CV to report as all samples were run in one assay.
2.7 Immunohistochemistry
Brains were sliced at 40 μm and stored in cryoprotectant at −20°C until assayed. Free-floating sections were washed in 0.01M KPBS and then pre-treated in 1% sodium borohydride for 20 minutes, followed by KPBS washes. Sections were incubated for 15 minutes in 0.014% phenylhydrazine, washed in KPBS, and then incubated in blocking solution (1% bovine serum albumin, 1% normal goat serum, and 0.3% Triton-X in KPBS) at room temperature for 1 hour. After another KPBS wash, sections were incubated in a primary antibody solution that contained blocking solution and rabbit anti-CRH antibody (1:40,000 dilution for sections containing PVN, 1:80,000 dilution for sections containing CeAmy; provided by Dr. Ann-Judith Silverman) or guinea pig anti-AVP antibody (1:50,000 dilution; Bachem, Torrance, CA, USA) for one hour at room temperature, then 65 hours at 4° C.
Following this incubation, sections were rinsed in KPBS and then incubated for 1 hour at room temperature in biotinylated goat anti-rabbit (CRH; Vector Laboratories, Burlingame, CA, USA) or biotinylated goat anti-guinea pig (AVP; Vector Laboratories) IgG in blocking solution (1:600 dilution). Sections were then washed in KPBS and incubated in avidin-biotin peroxidase solution (Vectastain Elite ABC Kit; Vector Laboratories; 5 μl solution A, 5 μl solution B, 0.4% Triton-X, and 1% bovine serum albumin in KPBS) for 1 hour at room temperature. Sections were next washed in KPBS and then in 0.175M sodium acetate before staining was developed using a diaminobenzidine solution (DAB Peroxidase Substrate Kit; Vector Laboratories), followed by rinsing with sodium acetate, then rinsing with KPBS. Sections were then mounted onto glass slides and allowed to dry overnight. Slides were then dehydrated with ethanol, cleared with Histoclear (National Diagnostics, Atlanta, GA, USA), and coverslips mounted with Histomount (National Diagnostics).
2.8 Microscopy and Quantification of Immunoreactivity
Images of the PVN, SON and CeAmy were captured with a Zeiss Axioimager using an Axiocam MRC camera at 10X magnification. Analysis was done using NIH Image J software. When analyzing both AVP-ir and CRH-ir in the PVN, hand counts of labeled cell bodies were conducted for a single section of tissue at a caudal point in the PVN that takes a characteristic shape and fiber projection pattern described previously [49, 50]. This section is approximate to Figure 37 in the Franklin and Pxinos (2008) [51] mouse brain atlas. Cell counts for the SON (AVP-ir only) were conducted at the same level used for the PVN analysis. Because stained fiber density was very high and stained cells were so faint, cell counting was not possible in the CeAmy when analyzing CRH-ir and only density measurements were collected for this region, as described below.
Density measurements were taken for AVP-ir in the PVN and SON and for CRH-ir in the PVN and CeAmy. The percentage of immunoreactive staining for each region was determined using the threshold function to separate stained cell bodies and fiber projections from background. For all measurements, a standardized sampling area was used for each tissue section to ensure that any differences seen were not the result of changes in defining borders for each region. This method has been used previously for both AVP-ir and CRH-ir [42, 50, 52]. All density measurements were taken bilaterally and the two measurements were then averaged to produce a single percentage of staining for each region. In the PVN and SON, measurements were taken from the same single section as was used for cell body counts. Three density measurements were collected within each section of PVN: one including the region of both cells and fibers (sampling area: 40 × 70 μm), and two measuring projecting fibers (vertical sampling area: 50 × 90 μm; horizontal sampling area: 40 × 20 μm). Two areas were used to ensure the fornix was not included in the sampling area and the density of the fiber projections was the sum of the two measurement areas. A single box was used in the SON region that encompassed both cell bodies and fiber projections (sampling area: 90 × 40 μm). The image was rotated approximately 45° in Image J prior to taking the density measurements to avoid inclusion of the optic tract in the sampling area. A rounded sampling area was used for the CeAmy (sampling area diameter: 125 μm). For this region, density measurements were taken bilaterally for 4 sections, corresponding to Figures 41–43 in the Franklin and Paxinos (2008) mouse brain atlas. In some cases, only 3 sections were available for analysis. Percentages were then averaged across all sections to produce a single percentage of immunoreactive staining for the region.
2.9 Data analysis
Analyses were conducted using SAS 9.2. All significance levels were set at p < 0.05 and residuals were checked for normality and transformed via a square root transformation when necessary. Early parental care was compared between HC and LC offspring using an analysis of variance (ANOVA) with Breeder Pair included as a random variable. Body weights were analyzed with independent t-tests for birth (PND 0) and weaning (PND 20) weights and with a 2-way Group × Condition ANOVA for weights at adult testing (PND 48). Behavior in a forced swim test was analyzed using an ANOVA. Physiological measures, including plasma corticosterone and CRH and AVP immunoreactivity, were analyzed with 2-way Group × Condition ANOVAs. Because the interest was in the effects of early experience on adult physiology and behavior for each sex, analyses of stress physiology and behavior in a forced swim test were analyzed separately for both sexes.
3. Results
3.1 Early parental care and weights of subject offspring
When quantifying the type and amount of parental behavior directed toward each offspring in the first few days following birth, breeders ranked as HC spent more time overall caring for offspring. Total contact with offspring was significantly higher in HC compared to LC pairs (F(1,72) = 23.35, p = 0.0001). Weights from all offspring were collected at birth, at weaning, and at testing. There were no differences in offspring weights between HC and LC groups or between male and female offspring at any of these time points.
3.2 Forced swim test
Behaviors were recorded during a short forced swim test, a reliable physical stressor in the prairie vole, to examine stress coping behavior. There were no differences in any of the behaviors measured between HC and LC offspring for either males or females (see Table 1).
Table 1.
Forced Swim Test. Behavior in a 3-minute forced swim test (sec); mean (SE).
| Swim | Struggle | Float | Dive | |
|---|---|---|---|---|
| HC Male Offspring | 116.67 (12.11) | 54.22 (11.28) | 6.22 (1.78) | 0.56 (0.50) |
| LC Male Offspring | 134.00 (7.19) | 35.75 (5.66) | 8.38 (4.27) | 0.25 (0.30) |
|
| ||||
| HC Female Offspring | 131.30 (8.96) | 43.10 (8.50) | 2.90 (1.19) | 0.60 (0.40) |
| LC Female Offspring | 138.25 (5.41) | 38.75 (5.50) | 1.13 (0.64) | 0.00 (0.00) |
3.3 Plasma CORT, CRH-ir, and AVP-ir
Plasma CORT levels were examined two hours following the completion of a swim test to investigate HPA response to a non-social stressor following varying early care. There were no differences in CORT levels between HC and LC control or swim-stressed offspring for either males or females (see Table 2).
Table 2.
Plasma Corticosterone. Plasma CORT measures (ng/mL); means (SE).
| Control | Swim Stressor | |
|---|---|---|
| HC Male Offspring | 1056.23 (162.89) | 972.97 (59.57) |
| LC Male Offspring | 1102.68 (234.63) | 1415.46 (206.74) |
|
| ||
| HC Female Offspring | 1272.73 (259.14) | 1094.99 (161.40) |
| LC Female Offspring | 1124.58 (134.83) | 1406.68 (260.82) |
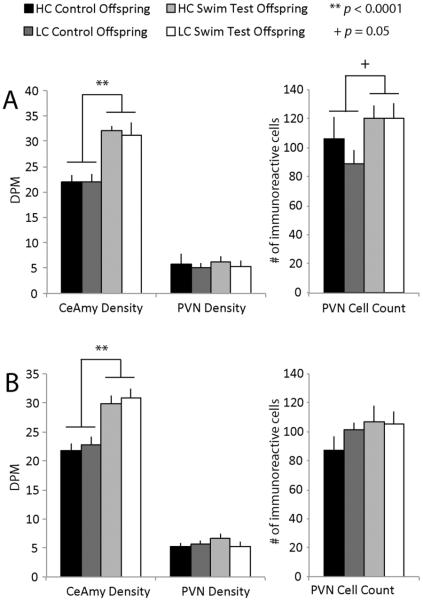
Differences in CRH-ir were observed in the CeAmy and PVN between control and stress conditions but not between early rearing environment, while differences in AVP-ir were observed in the SON and PVN between HC and LC offspring but not between control and stress conditions. For CRH-ir, there was a main effect of testing condition, where the density of CRH-ir staining in the CeAmy was higher after a swim stressor in both female (F(2,37) = 38.26, p < 0.0001; Figure 1A) and male (F(2,35) = 32.85, p < 0.0001; Figure 1B) compared to controls, but there was no main effect of early rearing environment group and no group × condition interaction. There was also a trend in female offspring for a greater number of CRH-labeled cells in the PVN following a swim stressor (F(2,36) = 4.10, p = 0.0511).
Figure 1.
CRH-ir in the CeAmy and PVN. (A) Density of CRH-ir fibers and cells in the CeAmy was significantly elevated following a swim stressor in female offspring (F(2,37) = 38.26, p < 0.0001). The number of CRH-immunopositive cells also tended to be elevated after a swim stressor in female offspring (F(2,36) = 4.10, p = 0.0511), regardless of early rearing environment. (B) Density of CRH-ir fibers and cells in the CeAmy were also greater after a swim stressor in male offspring (F(2,35) = 32.85, p < 0.0001).
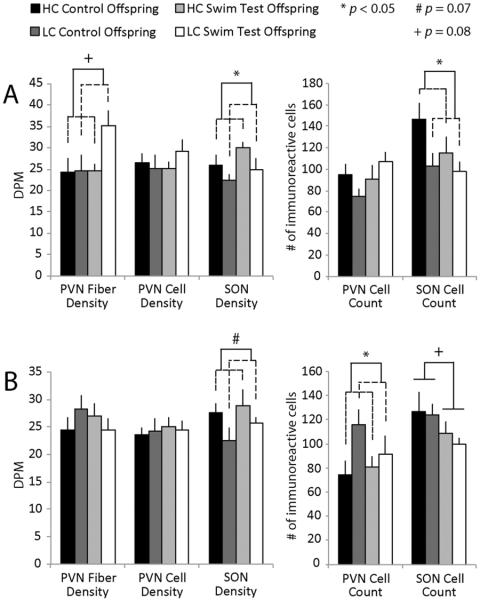
When examining AVP-ir in the SON, there was a main effect for early rearing environment group for both staining density and the number of stained cells. Females of HC parents had a greater density of AVP-ir staining (F(2,36) = 4.20, p = 0.0486; Figure 2A) as well as a greater number of AVP-labeled cells in the SON (F(2,36) = 4.76, p = 0.0364) compared to LC females. There was no main effect of testing condition or a group × condition interaction for either measure. There was also a trend for HC males to have a greater density of AVP-ir staining in the SON compared to LC males (F(2,34) = 3.42, p = 0.0738; Figure 2B). In the PVN, there was a main effect of early rearing environment group, with LC males having a greater number of AVP-labeled cells compared to HC males (F(2,34) = 4.54, p = 0.0412), but no main effect of condition and no group × condition interaction. There was a trend for a greater density of AVP-ir staining of PVN fiber projections in LC compared to HC females (F(2,36) = 3.09, p = 0.0882).
Figure 2.
AVP-ir in PVN and SON. (A) Female offspring of HC parents had significantly greater cell number and density of AVP-immunoreactive staining in the SON compared to LC females (cell count: (F(2,36) = 4.76, p = 0.0364) and; density: (F(2,36) = 4.20, p = 0.0486), while LC female offspring tended to have greater AVP-ir staining density in the PVN fibers (F(2,36) = 3.09, p = 0.0882). (B) Male offspring of LC parents had a greater number of AVP-positive cells in the PVN (F(2,34) = 4.54, p = 0.0412) while HC males tended to have greater AVP-ir density in the SON (F(2,34) = 3.42, p = 0.0738). In addition, there tended to be a decrease in the number of AVP-labeled cells in male offspring, regardless of early care, following a swim stressor (F(2,34) = 3.11, p = 0.0875).
4. Discussion
This study supports the assertion that early life experiences of an individual have the potential to alter neuropeptide production, however, we found no evidence that they impact response to a non-social stressor. Several animal models of varying early experience show that decreased tactile stimulation results in increased HPA activity at all levels following acute non-social stressors, including increased CRH mRNA and immunoreactivity in the hypothalamus [13, 19], increased ACTH release from the pituitary [19, 20, 29], and increased plasma CORT levels [21, 25, 29]. Here we have demonstrated that naturally occurring variation in early biparental care resulted in differences in AVP-ir in the PVN and SON but did not directly alter behavior or other components of the HPA axis. Male offspring of LC parents had increased AVP-ir in the PVN while HC female offspring had increased AVP-ir in the SON, regardless of testing condition. The early rearing environment was not, however, related to changes in CRH-ir or plasma CORT following an acute non-social stressor. The lack of any interaction between early rearing experience and later stress exposure for either AVP or CRH suggests a potential dissociation between the two peptides during HPA activation.
Responses to an acute stressor are heavily mediated by CRH neurons in the PVN and CeAmy. CRH neurons in the CeAmy project to the locus coeruleus (LC). Release of CRH activates neurons in the LC, increasing their firing rate and therefore increasing the release of norepinephrine (NE) [53–55]. NE then acts on CRH neurons located in the PVN, beginning the hormonal cascade associated with activation of the HPA axis [56, 57]. The increase in CRH-ir density in the CeAmy in both sexes following an acute non-social stressor, regardless of early rearing experience, indicates that the forced swim test used here was an adequate stimulus to initiate a physiological stress response, as expected. Early handling studies have shown decreases in CRH mRNA in both the CeAmy and PVN in handled compared to non-handled offspring [19] as well as decreased NE in the PVN [20]. This suggests that increased early life tactile stimulation decreases CRH activity in this circuit and leads to a decrease in HPA activity following a stressor. Support for this idea comes from the rat model of varying maternal care. Adult offspring of high LG dams have decreased CRH receptor density in the LC [58] as well as decreased CRH mRNA in the PVN and a decreased HPA response to an acute stressor [29]. These results suggest a mechanism by which increased tactile stimulation in early life down-regulates CRH activity in adulthood in response to a stressor in both the PVN and CeAmy. Our lack of a difference in CRH-ir in either the PVN or CeAmy following varying early rearing is therefore somewhat surprising. It may be that the relatively high amount of CRH mRNA in the prairie vole, particularly in the PVN [59] limits the amount of increase in activity possible for LC offspring, although we might still expect a decrease in CRH activity in HC offspring. Alternately, the differences in early tactile experience between HC and LC groups may have been too subtle to create measurable differences in CRH-ir, particularly because time alone in the nest is relatively low even for LC offspring because of the presence of both the mother and father as caregivers.
In addition to the CRH release in response to an acute stressor, there is also an increase in AVP production and release into the hypophyseal portal blood system, and these two peptides work in tandem to promote the activity of the HPA axis [60]. Similar to the outcomes seen in measures of CRH, early life experiences can also alter the AVP system. As described previously, early handling of rat pups increases GC negative feedback sensitivity [61], which then results in a decrease in CRH mRNA and immunoreactivity in the CeAmy and PVN as well as decreased basal levels of CRH in the median eminence. This same decrease in basal median eminence levels is also seen for AVP following early handling [19]. Here we found a decrease in AVP-ir in the PVN of HC male offspring, fitting with previous results in rat handling paradigms. This decrease was not, however, seen in female offspring – females instead displayed an increase in AVP-ir in the SON after HC rearing. For both males and females, these differences in AVP-ir were seen only between early rearing environment groups and not between control and swim stress conditions. This differential response of CRH and AVP, where CRH-ir was altered only by exposure to a stressor while AVP was impacted only by early rearing experience, demonstrates an interesting and somewhat unexpected independence between the two peptides. Because response to an acute stressor is mediated by both CRH and AVP release from the PVN, as previously discussed, we anticipated that both CRH-ir and AVP-ir would increase as the HPA system is mobilized in response to the forced swim stressor. Instead, our results show that, at least for AVP, this was not the case. Given this lack of a difference in AVP-ir following a stressor, this AVP being measured is likely not being released as a component of the stress response.
Aside from its synergistic actions with CRH in promoting HPA activity, AVP is also heavily involved in the regulation of social behavior. AVP involvement has been documented in several social behaviors, including aggression [38, 62, 63], scent marking behavior [64], pair bonding [62, 65], and parental care [66, 67]. In females, the AVP system plays a role in maternal behavior and aggression [68–72], as well as pair bonding in the prairie vole [65]. AVP and the closely related neuropeptide OT are synthesized in the PVN and SON of the hypothalamus. Additional regions have been found to display AVP-ir, such as the bed nucleus of the stria terminalis [73], medial amygdala, locus coeruleus, and additional hypothalamic nuclei [74], while several other hypothalamic nuclei show OT-ir [50], indicating that the production of these neuropeptides is not limited to the PVN and SON.
In the prairie vole, OT produced in the SON projects in part to the nucleus accumbens [75], an area involved in partner preference formation as well as alloparental behavior. While AVP projections from the SON have not been extensively mapped in the prairie vole, it may be that there are also similar central AVP projections from the SON. The AVP-ir measured here may, in fact, be projecting centrally to influence social behavior and be sensitive to early-life experience in a sex-dependent manner. It would be worthwhile to follow up on this current finding to include measures of social behavior, in particular parental behavior and partner preference formation, as well as detailed mapping of the central AVP projections from both the SON and the PVN.
As previously discussed, HPA function and associated behavioral responses to non-social stressors are vulnerable to early experiences. In particular, decreased early tactile stimulation in several different experimental paradigms in rats results in decreased GC feedback sensitivity in response to a GC challenge [29, 61], while increases in anxiety- and depressive-like behaviors in response to the same early experiences are often reported [12, 15, 22, 76]. If this prairie vole model of naturally occurring variation in early parental care was creating similar outcomes in offspring as the rat model is, we would expect to see decreased immobility as well as decreased CORT levels in HC compared to LC offspring in response to a forced swim stressor. This was, however, not the case. Previous work in this model of variation in early care has shown a rise in plasma CORT after chronic social isolation housing in HC compared to LC female offspring [45]. The lack of a difference in plasma CORT between HC and LC females after a forced swim test here suggests a differential response in females to social versus non-social stressors or perhaps to chronic compared to acute stressors following varying early life experiences.
In a typically functioning HPA axis, CORT feeds back primarily on the PVN to initiate a shutdown of the hormonal cascade response. This feedback begins within minutes of the onset of the stressor (see Myers et al, 2012 [77] for a review of feedback mechanisms). Previous work has characterized the typical HPA response to a swim stressor in the prairie vole, where CORT peaks 15–30 minutes after the onset of the stressor and begins to decline after 60 minutes [48]. While the collection time point used here (120 minutes post-stressor) occurs in this period of decline, there is evidence of a lowered glucocorticoid feedback sensitivity of the HPA axis in low LG rat offspring that leads to an extended period of elevated plasma CORT following a stressor. Decreased glucocorticoid receptor (GR) concentrations in the hippocampus are associated with impairments in negative feedback mechanisms [78–80]. Hippocampal GR are sensitive to early experience and can be increased in the rat by both early handling [81, 82] and by increased early maternal LG [29]. This alteration in hippocampal GR concentrations and, in turn, the GC negative feedback system suggests one potential mechanism through which early life experiences can lead to an extended HPA response to stressors. While we did not directly test GC feedback sensitivity or assess hippocampal GR concentrations in this study, our results indicate that the HPA feedback loop is likely less or entirely unaffected by variation in early care in the prairie vole.
Sexually dimorphic responses to both social and non-social stressors have been previously widely reported [83–85]. The duration of stressor exposure also results in differential responses between males and females. For example, male rats respond to a single session of restraint stress and habituate with repeated sessions while females do not display as pronounced a response to a single restrain yet fail to habituate to repeated restraint [86]. In a chronic mild stressor paradigm, female rats were found to be more reactive on a number of behavioral and physiological parameters while still showing greater resilience to an additional novel stressor exposure than were males [87]. In the prairie vole, chronic social isolation results in increased plasma CORT and ACTH in females but not males [47] and females also have decreases in AVP-ir in the PVN after long-term isolation [52]. We have also demonstrated similar sex-dependent responses to social stressors that were associated with early-life experience [45]. Here we also see a sexually dimorphic production of AVP-ir in hypothalamic nuclei that was dependent on early rearing experience but not adult non-social stress exposure. Female offspring of HC parents have increased levels of AVP-ir in the SON while HC males have a decreased number of AVP-labeled cells in the PVN compared to LC offspring. These findings provide further evidence of sexual dimorphisms in HPA activity and support the idea of sex-dependent sensitivities to early rearing experiences in prairie voles.
In conclusion, the results of this study demonstrate an expected increase in CRH-ir following a non-social stressor that was not sex- or early experience-dependent. However, there was an interesting sex-dependent AVP response in hypothalamic nuclei that was associated with the early rearing experience but was independent of adult stressor exposure. These findings provide additional evidence of how naturally occurring variations in early life experiences are related to adult HPA functioning, in particular AVP-ir, in a sex-dependent manner. The lack of differences in other measured components of the HPA response between HC and LC offspring may be due to the relative subtlety of variation in early rearing experience measured here. Continuing research in naturally occurring variation in behavior and physiology and the mechanisms for this variation, particularly in highly social species such as the prairie vole, will add to our understanding of the link between the early environment and later outcomes, including individual differences and sexual dimorphisms in vulnerabilities to various psychopathologies.
Highlights.
The relationship between early experience and non-social stress response is examined.
CRH-ir was increased after a swim stressor but was not impacted by early experience.
AVP-ir was impacted by early care but not by a swim stressor in a sex-dependent way.
High-contact females had increased AVP-ir in the SON compared to low-contact females.
High-contact males had decreased AVP-ir in the PVN compared to low-contact males.
Acknowledgements
The authors thank Meredith Lee and Julie VanWesterhuyzen for technical assistance and Drs. Cindy Clayton and Rhonda Oates-O'Brien for veterinary care.
Role of the funding sources This work was supported by HD060117 to K.L.B. and by the University of California, Davis. Funding sources had no role in the design of experiments, in the collection, analysis, and interpretation of data, in the writing of this report, or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest The authors report that they have no conflicts of interest.
References
- [1].Gunnar MR, Brodersen L, Nachmias M, Buss K, Rigatuso J. Stress reactivity and attachment security. Developmental Psychobiology. 1996;29:191–204. doi: 10.1002/(SICI)1098-2302(199604)29:3<191::AID-DEV1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- [2].Kajantie E, Raikkonen K. Early life predictors of the physiological stress response later in life. Neuroscience and Biobehavioral Reviews. 2010;35:23–32. doi: 10.1016/j.neubiorev.2009.11.013. [DOI] [PubMed] [Google Scholar]
- [3].Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- [4].Heim C, Newport DJ, Mletzko T, Miller AH, Hemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- [5].Levine S. Infantile experience and resistance to physiological stress. Science. 1957;126:405. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- [6].Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biological Psychiatry. 2000;48:1164–74. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- [7].Capitanio JP, Mendoza SP, Mason WA, Maninger N. Rearing environment and hypothalamic-pituitary-adrenal regulation in young rhesus monkeys (Macaca mulatta) Developmental Psychobiology. 2005;46:318–30. doi: 10.1002/dev.20067. [DOI] [PubMed] [Google Scholar]
- [8].Meaney MJ, Szyf M, Seckl JR. Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health. Trends in Molecular Medicine. 2007;13:269–77. doi: 10.1016/j.molmed.2007.05.003. [DOI] [PubMed] [Google Scholar]
- [9].Sanchez MM. The impact of early adverse care on HPA axis development: Nonhuman primate models. Hormones and Behavior. 2006;50:623–31. doi: 10.1016/j.yhbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- [10].Sanchez MM, McCormack K, Grand AP, Fulks R, Graff A, Maestripieri D. Effects of sex and early maternal abuse on adrenocorticotropin hormone and cortisol responses to the corticotropin-releasing hormone challenge during the first 3 years of life in group-living rhesus monkeys. Dev. Psychopathol. 2010;22:45–53. doi: 10.1017/S0954579409990253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Birnie AK, Taylor JH, Cavanaugh J, French JA. Quality of maternal and paternal care predicts later stress reactivity in the cooperatively-breeding marmoset (Callithrix geoffroyi) Psychoneuroendocrinology. 2013;38:3003–14. doi: 10.1016/j.psyneuen.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Aisa B, Tordera R, Lasheras B, Del Rio J, Ramirez MJ. Effects of maternal separation on hypothalamic-pituitary-adrenal responses, cognition and vulnerability to stress in adult female rats. Neuroscience. 2008;154:1218–26. doi: 10.1016/j.neuroscience.2008.05.011. [DOI] [PubMed] [Google Scholar]
- [13].Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) messenger-RNA, median-eminence CRF content and stress-induced release in adult rats. Molecular Brain Research. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- [14].Kikusui T, Nakamura K, Kakuma Y, Mori Y. Early weaning augments neuroendocrine stress responses in mice. Behav. Brain Res. 2006;175:96–103. doi: 10.1016/j.bbr.2006.08.007. [DOI] [PubMed] [Google Scholar]
- [15].Boccia ML, Pedersen CA. Brief vs. long maternal separations in infancy: contrasting relationships with adult maternal behavior and lactation levels of aggression and anxiety. Psychoneuroendocrinology. 2001;26:657–72. doi: 10.1016/s0306-4530(01)00019-1. [DOI] [PubMed] [Google Scholar]
- [16].Tang AC, Akers KG, Reeb BC, Romeo RD, McEwen BS. Programming social, cognitive, and neuroendocrine development by early exposure to novelty. Proc Natl Acad Sci U S A. 2006;103:15716–21. doi: 10.1073/pnas.0607374103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Smotherman WP, Bell RW. Maternal mediation of early experience. In: Smotherman WP, Bell RW, editors. Maternal Influence and Early Behavior. Spectrum Publishing; New York: 1980. [Google Scholar]
- [18].Smotherman WP, Brown CP, Levine S. Maternal responsiveness following differential pup treatment and mother-pup interactions. Horm Behav. 1977;8:242–53. doi: 10.1016/0018-506x(77)90041-1. [DOI] [PubMed] [Google Scholar]
- [19].Viau V, Sharma S, Plotsky PM, Meaney MJ. Increased plasma ACTH responses to stress in nonhandled compared with handled rats require basal levels of corticosterone and are associated with increased levels of ACTH secretagogues in the median eminence. Journal of Neuroscience. 1993;13:1097–105. doi: 10.1523/JNEUROSCI.13-03-01097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Liu D, Caldji C, Sharma S, Plotsky PM, Meaney MJ. Influence of neonatal rearing conditions on stress-induced adrenocorticotropin responses and norepinepherine release in the hypothalamic paraventricular nucleus. J. Neuroendocrinol. 2000;12:5–12. doi: 10.1046/j.1365-2826.2000.00422.x. [DOI] [PubMed] [Google Scholar]
- [21].Levine S, Haltmeyer GC, Kargs GG, Denenberg VH. Physiological and behavioral effects of infantile stimulation. Physiol. Behav. 1967;2:5. [Google Scholar]
- [22].Aisa B, Tordera R, Lasheras B, Del Rio J, Ramirez MJ. Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology. 2007;32:256–66. doi: 10.1016/j.psyneuen.2006.12.013. [DOI] [PubMed] [Google Scholar]
- [23].Mourlon V, Baudin A, Blanc O, Lauber A, Giros B, Naudon L, et al. Maternal deprivation induces depressive-like behaviours only in female rats. Behav. Brain Res. 2010;213:278–87. doi: 10.1016/j.bbr.2010.05.017. [DOI] [PubMed] [Google Scholar]
- [24].Curley JP, Davidson S, Bateson P, Champagne FA. Social enrichment during postnatal development induces transgenerational effects on emotional and reproductive behavior in mice. Front. Behav. Neurosci. 2009;3 doi: 10.3389/neuro.08.025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Branchi I, D'Andrea I, Cirulli F, Lipp HP, Alleva E. Shaping brain development: Mouse communal nesting blunts adult neuroendocrine and behavioral response to social stress and modifies chronic antidepressant treatment outcome. Psychoneuroendocrinology. 2010;35:743–51. doi: 10.1016/j.psyneuen.2009.10.016. [DOI] [PubMed] [Google Scholar]
- [26].Cirulli F, Berry A, Bonsignore LT, Capone F, D'Andrea I, Aloe L, et al. Early life influences on emotional reactivity: Evidence that social enrichment has greater effects than handling on anxiety-like behaviors, neuroendocrine responses to stress and central BDNF levels. Neuroscience and Biobehavioral Reviews. 2010;34:808–20. doi: 10.1016/j.neubiorev.2010.02.008. [DOI] [PubMed] [Google Scholar]
- [27].Branchi I, D'Andrea I, Sietzema J, Fiore M, Di Fausto V, Aloe L, et al. Early social enrichment augments adult hippocampal BDNF levels and survival of BRDU-positive cells while increasing anxiety- and “depression”-like behavior. Journal of Neuroscience Research. 2006;83:965–73. doi: 10.1002/jnr.20789. [DOI] [PubMed] [Google Scholar]
- [28].D'Andrea I, Gracci F, Alleva E, Branchi I. Early social enrichment provided by communal nest increases resilience to depression-like behavior more in female than in male mice. Behav. Brain Res. 2010;215:71–6. doi: 10.1016/j.bbr.2010.06.030. [DOI] [PubMed] [Google Scholar]
- [29].Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–62. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- [30].Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–8. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- [31].Frazier CRM, Trainor BC, Cravens CJ, Whitney TK, Marler CA. Paternal behavior influences development of aggression and vasopressin expression in male California mouse offspring. Hormones and Behavior. 2006;50:699–707. doi: 10.1016/j.yhbeh.2006.06.035. [DOI] [PubMed] [Google Scholar]
- [32].Oliveras D, Novak M. A comparison of paternal behavior in the meadow vole Microtus pennsylvanicus, the pine vole M. pinetorum and the prairie vole M. ochrogaster. Animal Behaviour. 1986;34:519–26. [Google Scholar]
- [33].Solomon NG. Comparison of parental behavior in male and female prairie voles (Microtus ochrogaster) Canadian Journal of Zoology-Revue Canadienne De Zoologie. 1993;71:434–7. [Google Scholar]
- [34].Bales KL, Pfeifer LA, Carter CS. Sex differences and developmental effects of manipulations of oxytocin on alloparenting and anxiety in prairie voles. Developmental Psychobiology. 2004;44:123–31. doi: 10.1002/dev.10165. [DOI] [PubMed] [Google Scholar]
- [35].Bales KL, van Westerhuyzen JA, Lewis-Reese AD, Grotte ND, Lanter JA, Carter CS. Oxytocin has dose-dependent developmental effects on pair-bonding and alloparental care in female prairie voles. Hormones and Behavior. 2007;52:274–9. doi: 10.1016/j.yhbeh.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bales KL, Carter CS. Developmental exposure to oxytocin facilitates partner preferences in male prairie voles (Microtus ochrogaster) Behavioral Neuroscience. 2003;117:854–9. doi: 10.1037/0735-7044.117.4.854. [DOI] [PubMed] [Google Scholar]
- [37].Bales KL, Carter CS. Sex differences and developmental effects of oxytocin on aggression and social behavior in prairie voles (Microtus ochrogaster) Hormones and Behavior. 2003;44:178–84. doi: 10.1016/s0018-506x(03)00154-5. [DOI] [PubMed] [Google Scholar]
- [38].Stribley JM, Carter CS. Developmental exposure to vasopressin increases aggression in adult prairie voles. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:12601–4. doi: 10.1073/pnas.96.22.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bales KL, Plotsky PM, Young LJ, Lim MM, Grotte N, Ferrer E, et al. Neonatal oxytocin manipulations have long-lasting, sexually dimorphic effects on vasopressin receptors. Neuroscience. 2007;144:38–45. doi: 10.1016/j.neuroscience.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kramer KM, Choe C, Carter CS, Cushing BS. Developmental effects of oxytocin on neural activation and neuropeptide release in response to social stimuli. Hormones and Behavior. 2006;49:206–14. doi: 10.1016/j.yhbeh.2005.07.001. [DOI] [PubMed] [Google Scholar]
- [41].Yamamoto Y, Cushing BS, Kramer KM, Epperson PD, Hoffman GE, Carter CS. Neonatal manipulations of oxytocin alter expression of oxytocin and vasopressin immunoreactive cells in the paraventricular nucleus of the hypothalamus in a gender-specific manner. Neuroscience. 2004;125:947–55. doi: 10.1016/j.neuroscience.2004.02.028. [DOI] [PubMed] [Google Scholar]
- [42].Bales KL, Boone E, Epperson P, Hoffman G, Carter CS. Are behavioral effects of early experience mediated by oxytocin? Front Psychiatry. 2011;2:24. doi: 10.3389/fpsyt.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kramer KM, Cushing BS, Carter CS. Developmental effects of oxytocin on stress response: single versus repeated exposure. Physiol. Behav. 2003;79:775–82. doi: 10.1016/s0031-9384(03)00175-6. [DOI] [PubMed] [Google Scholar]
- [44].Perkeybile AM, Griffin LL, Bales KL. Natural variation in early parental care correlates with social behaviors in adolescent prairie voles (Microtus ochrogaster) Front. Behav. Neurosci. 2013;7:21. doi: 10.3389/fnbeh.2013.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Perkeybile AM, Bales KL. Early rearing experience is related to altered aggression and vasopressin production following chronic social isolation in the prairie vole. Behav Brain Res. 2015;283:37–46. doi: 10.1016/j.bbr.2015.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Grippo AJ, Cushing BS, Carter CS. Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosomatic Medicine. 2007;69:149–57. doi: 10.1097/PSY.0b013e31802f054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, et al. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007;32:966–80. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Taymans SE, DeVries AC, DeVries MB, Nelson RJ, Friedman TC, Castro M, et al. The hypothalamic-pituitary-adrenal axis of prairie voles (Microtus ochrogaster): Evidence for target tissue glucocorticoid resistance. General and Comparative Endocrinology. 1997;106:48–61. doi: 10.1006/gcen.1996.6849. [DOI] [PubMed] [Google Scholar]
- [49].Kenkel WM, Paredes J, Yee JR, Pournajafi-Nazarloo H, Bales KL, Carter CS. Neuroendocrine and behavioural responses to exposure to an infant in male prairie voles. J Neuroendocrinol. 2012;24:874–86. doi: 10.1111/j.1365-2826.2012.02301.x. [DOI] [PubMed] [Google Scholar]
- [50].Wang ZX, Zhou L, Hulihan TJ, Insel TR. Immunoreactivity of central vasopressin and oxytocin pathways in microtine rodents: A quantitative comparative study. Journal of Comparative Neurology. 1996;366:726–37. doi: 10.1002/(SICI)1096-9861(19960318)366:4<726::AID-CNE11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- [51].Franklin KBJ, Paxinos G. The Mouse Brain in Stereotxic Coordinates. 3rd ed. Academic Press; New York: 2008. [Google Scholar]
- [52].Ruscio MG, Sweeny T, Hazelton J, Suppatkul P, Carter CS. Social environment regulates corticotropin releasing factor, corticosterone and vasopressin in juvenile prairie voles. Hormones and Behavior. 2007;51:54–61. doi: 10.1016/j.yhbeh.2006.08.004. [DOI] [PubMed] [Google Scholar]
- [53].Van Bockstaele EJ, Colago EEO, Valentino RJ. Amygdaloid corticotropin-releasing factor targets locus coeruleus dendrites: Substrate for the co-ordination of emotional and cognitive limbs of the stress response. J. Neuroendocrinol. 1998;10:743–57. doi: 10.1046/j.1365-2826.1998.00254.x. [DOI] [PubMed] [Google Scholar]
- [54].Gray TS, Bingaman EW. The amygdala: Corticotropin-releasing factor, steroids, and stress. Critical Reviews in Neurobiology. 1996;10:155–68. doi: 10.1615/critrevneurobiol.v10.i2.10. [DOI] [PubMed] [Google Scholar]
- [55].Valentino RJ, Curtis AL, Page ME, Pavcovich LA, Florin-Lechner SM. Advances in pharmacology. Vol. 42. San Diego, Calif.: 1998. Activation of the locus ceruleus brain noradrenergic system during stress: circuitry, consequences, and regulation; pp. 781–4. [DOI] [PubMed] [Google Scholar]
- [56].Ziegler DR, Cass WA, Herman JP. Excitatory influence of the locus coeruleus in hypothalamic-pituitary-adrenocortical axis responses to stress. J. Neuroendocrinol. 1999;11:361–9. doi: 10.1046/j.1365-2826.1999.00337.x. [DOI] [PubMed] [Google Scholar]
- [57].Itoi K, Sugimoto N. The Brainstem Noradrenergic Systems in Stress, Anxiety and Depression. J. Neuroendocrinol. 2010;22:355–61. doi: 10.1111/j.1365-2826.2010.01988.x. [DOI] [PubMed] [Google Scholar]
- [58].Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:5335–40. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Lim MM, Tsivkovskaia NO, Bai Y, Young LJ, Ryabinin AE. Distribution of corticotropin-releasing factor and urocortin 1 in the vole brain. Brain Behavior and Evolution. 2006;68:229–40. doi: 10.1159/000094360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lightman SL. The neuroendocrinology of stress: a never ending story. J Neuroendocrinol. 2008;20:880–4. doi: 10.1111/j.1365-2826.2008.01711.x. [DOI] [PubMed] [Google Scholar]
- [61].Meaney MJ, Aitken DH, Viau V, Sharma S, Sarrieau A. Neonatal handling alters adrenocortical negative feedback sensitivity and hippocampal type II glucocorticoid receptor binding in the rat. Neuroendocrinology. 1989;50:597–604. doi: 10.1159/000125287. [DOI] [PubMed] [Google Scholar]
- [62].Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–8. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- [63].Ferris CF, Potegal M. Vasopressin receptor blockade in the anterior hypothalamus suppresses aggression in hamsters. Physiol. Behav. 1988;44:235–9. doi: 10.1016/0031-9384(88)90144-8. [DOI] [PubMed] [Google Scholar]
- [64].Ferris CF, Albers HE, Wesolowski SM, Goldman BD, Luman SE. Vasopressin injected into the hypothalamus triggers a stereotypic behavior in golden-hamsters. Science. 1984;224:521–3. doi: 10.1126/science.6538700. [DOI] [PubMed] [Google Scholar]
- [65].Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behavioral Neuroscience. 1999;113:1071–9. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- [66].Parker KJ, Lee TM. Central vasopressin administration regulates the onset of facultative paternal behavior in Microtus pennsylvanicus (Meadow voles) Hormones and Behavior. 2001;39:285–94. doi: 10.1006/hbeh.2001.1655. [DOI] [PubMed] [Google Scholar]
- [67].Wang ZX, Ferris CF, Devries GJ. Role of septal vasopressin innervation in paternal behavior in prairie voles (Microtus ochrogaster) Proceedings of the National Academy of Sciences of the United States of America. 1994;91:400–4. doi: 10.1073/pnas.91.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Bester-Meredith JK, Marler CA. Naturally occurring variation in vasopressin immunoreactivity is associated with maternal behavior in female Peromyscus mice. Brain Behavior and Evolution. 2012;80:244–53. doi: 10.1159/000341899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Bosch OJ, Neumann ID. Vasopressin released within the central amygdala promotes maternal aggression. European Journal of Neuroscience. 2010;31:883–91. doi: 10.1111/j.1460-9568.2010.07115.x. [DOI] [PubMed] [Google Scholar]
- [70].Bosch OJ, Pfortsch J, Beiderbeck DI, Landgraf R, Neumann ID. Maternal behaviour is associated with vasopressin release in the medial preoptic area and bed nucleus of the stria terminalis in the rat. J. Neuroendocrinol. 2010;22:420–9. doi: 10.1111/j.1365-2826.2010.01984.x. [DOI] [PubMed] [Google Scholar]
- [71].Bosch OJ, Neumann ID. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Horm Behav. 2012;61:293–303. doi: 10.1016/j.yhbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- [72].Nephew BC, Byrnes EM, Bridges RS. Vasopressin mediates enhanced offspring protection in multiparous rats. Neuropharmacology. 2010;58:102–6. doi: 10.1016/j.neuropharm.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].van Leeuwen F, Caffe R. Vasopressin-immunoreactive cell bodies in the bed nucleus of the stria terminalis of the rat. Cell Tissue Res. 1983;228:525–34. doi: 10.1007/BF00211473. [DOI] [PubMed] [Google Scholar]
- [74].Caffe AR, Vanleeuwen FW. Vasopressin-immunoreactive cells in the dorsomedial hypothalamic region, medial amygdaloid nucleus and locus coeruleus of the rat. Cell and Tissue Research. 1983;233:23–33. doi: 10.1007/BF00222229. [DOI] [PubMed] [Google Scholar]
- [75].Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, et al. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009;162:892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG. Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long-Evans rats. Pharmacology Biochemistry and Behavior. 2002;73:131–40. doi: 10.1016/s0091-3057(02)00781-5. [DOI] [PubMed] [Google Scholar]
- [77].Myers B, McKlveen JM, Herman JP. Neural regulation of the stress response: The many faces of feedback. Cellular and Molecular Neurobiology. 2012;32:683–94. doi: 10.1007/s10571-012-9801-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocrine Reviews. 1991;12:118–34. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- [79].Sapolsky RM, Krey LC, McEwen BS. Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proceedings of the National Academy of Sciences of the United States of America-Biological Sciences. 1984;81:6174–7. doi: 10.1073/pnas.81.19.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Sapolsky RM, Meaney MJ, McEwen BS. The development of the glucocorticoid receptor system in the rat limbic brain .3. Negative-feedback regulation. Developmental Brain Research. 1985;18:169–73. doi: 10.1016/0165-3806(85)90261-5. [DOI] [PubMed] [Google Scholar]
- [81].Meaney MJ, Aitken DH. The effects of early postnatal handling on hippocampal glucocorticoid receptor concentrations - Temporal parameters. Developmental Brain Research. 1985;22:301–4. doi: 10.1016/0165-3806(85)90183-x. [DOI] [PubMed] [Google Scholar]
- [82].Odonnell D, Larocque S, Seckl JR, Meaney MJ. Postnatal handling alters glucocorticoid, but not mineralocorticoid messenger-RNA expression in the hippocampus of adult rats. Molecular Brain Research. 1994;26:242–8. doi: 10.1016/0169-328x(94)90096-5. [DOI] [PubMed] [Google Scholar]
- [83].Bourke CH, Neigh GN. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Hormones and Behavior. 2011;60:112–20. doi: 10.1016/j.yhbeh.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Luine V. Sex Differences in Chronic Stress Effects on Memory in Rats. Stress-the International Journal on the Biology of Stress. 2002;5:205–16. doi: 10.1080/1025389021000010549. [DOI] [PubMed] [Google Scholar]
- [85].Palanza P. Animal models of anxiety and depression: how are females different? Neuroscience and Biobehavioral Reviews. 2001;25:219–33. doi: 10.1016/s0149-7634(01)00010-0. [DOI] [PubMed] [Google Scholar]
- [86].Kennett GA, Chaouloff F, Marcou M, Curzon G. Female rats are more vulnerable than males in an animal model of depression: The possible role of serotonin. Brain Research. 1986;382:416–21. doi: 10.1016/0006-8993(86)91355-7. [DOI] [PubMed] [Google Scholar]
- [87].Dalla C, Antoniou K, Drossopoulou G, Xagoraris M, Kokras N, Sfikakis A, et al. Chronic mild stress impact: Are females more vulnerable? Neuroscience. 2005;135:703–14. doi: 10.1016/j.neuroscience.2005.06.068. [DOI] [PubMed] [Google Scholar]