Abstract
In bilaterally symmetric animals, the precise assembly of neural circuitry at the midline is essential for coordination of the left and right sides of the body. Commissural axons must first be directed across the midline and then be prevented from re-crossing in order to ensure proper midline connectivity. Here, we review the attractants and repellents that direct axonal navigation at the ventral midline and the receptors on commissural neurons through which they signal. In addition, we discuss the mechanisms that commissural axons use to switch their responsiveness to midline-derived cues, so that they are initially responsive to midline attractants and subsequently responsive to midline repellents.
Introduction
During embryonic development, conserved families of attractive and repulsive cues steer axons by signaling through receptors that are expressed on axonal growth cones. Axons navigate a series of intermediate targets, or choice points, en route to their final synaptic targets. At each intermediate target, axons must switch their responsiveness to guidance cues, so that they are initially drawn to an intermediate target and subsequently repelled from it. The embryonic midline is an intermediate target for commissural axons in all bilaterally symmetric animals and precise navigation at the midline is essential to allow for left-right coordination of behaviors. In this review, we discuss studies of commissural axon guidance at the ventral midline of vertebrates and insects, paying particular attention to the insights they have provided into mechanisms growing axons use to modulate their responsiveness to cues as they navigate toward their final targets. Specifically, we discuss two populations of neurons: commissural interneurons in the spinal cord and in the Drosophila ventral nerve cord.
The cell bodies of spinal commissural neurons differentiate in the dorsal spinal cord and project their axons ventromedially toward the floor plate (Figure 1A).1 These axons subsequently exit the floor plate on the contralateral side and turn anteriorly toward the brain (Figure 1C).2 Pre- and post-crossing axonal segments can be differentially labeled using antibodies that recognize cell adhesion molecules that are expressed in spatially restricted patterns (Figures 1A and B). In addition, the spinal cord can be opened at the roof plate to create an “open-book” preparation and commissural neurons and their axons can be labeled and visualized with lipophilic dyes; this preparation is particularly useful for analysis of post-crossing axonal trajectories (Figure 1C).
Figure 1. Commissural interneurons in the embryonic spinal cord of mouse and ventral nerve cord of Drosophila.
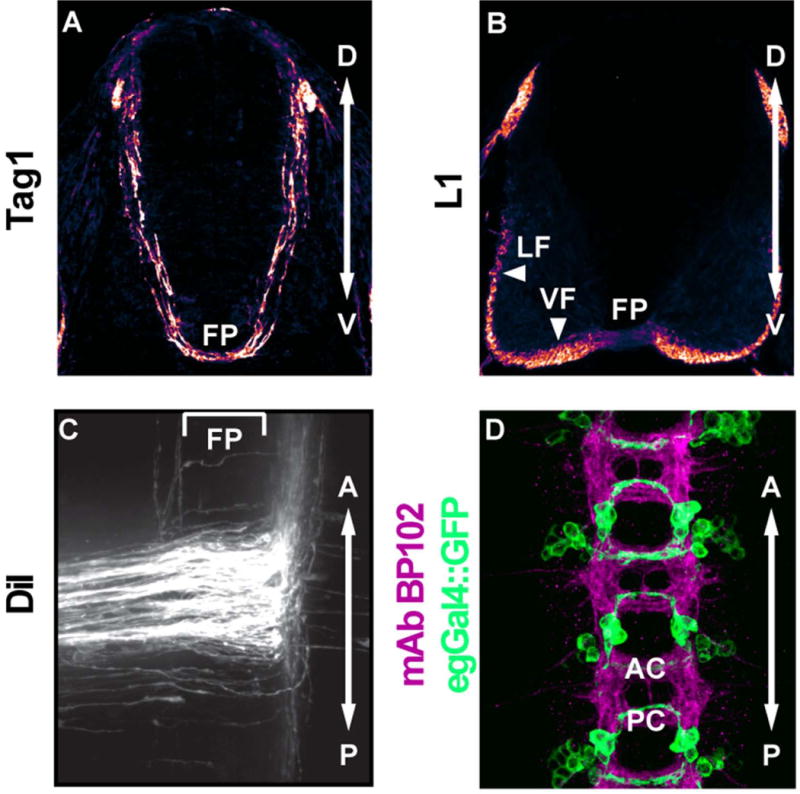
A) Transverse section of the mouse spinal cord at embryonic day 11.5. Pre-crossing spinal commissural neurons navigate ventromedially and express the cell adhesion molecule Tag1. B) Transverse section of the mouse spinal cord at embryonic day 11.5. Post-crossing commissural neurons express the cell adhesion marker L1. C) Open book preparation of the mouse spinal cord at embryonic day 11.5. Spinal commissural neurons are labeled by DiI injection into the dorsal spinal cord. The majority of post-crossing commissural axons turn anteriorly. The bracket indicates the position of the floor plate. D) Three segments of the Drosophila stage 16 embryonic ventral nerve cord. MAb BP102 (magenta) labels all axons in the central nervous system. egGal4 drives GFP (green) expression in a subset of commissural neurons. FP, floor plate. LF, lateral funiculus. VF, ventral funiculus. AC, anterior commissure. PC, posterior commissure. Panels A-C are reprinted from reference 67, with permission from Elsevier.
The ventral nerve cord of the Drosophila embryo has a segmentally repeated structure. Each abdominal hemisegment contains approximately 270 interneurons, most of which extend axons across the midline in either the anterior or posterior commissure.3 All axons in the central nervous system can be labeled by antibody staining (Figure 1D) and the fact that most abdominal interneurons project axons contralaterally3 facilitated forward genetic screens, which identified genes that play key roles in commissural axon guidance.4,5 In addition, subpopulations of neurons can be labeled with genetically encoded Gal4 elements, which can be used to direct the expression of axonal markers and other transgenes. This approach allows for quantitative comparison of axonal trajectories in wild-type and mutant backgrounds and also provides a powerful system in which to evaluate cell-specific and protein domain requirements in transgenic rescue experiments (Figure 1D).
Comparison of these two systems has revealed remarkable similarity in many of the core molecules and mechanisms that direct axon guidance at the midline. Here, we review and discuss recent insights into the molecular logic underlying the ability of axons to switch responsiveness at the midline choice point. A central recurrent theme of these studies is the diversity of mechanisms that have evolved to spatially and temporally restrict the activity of cell surface guidance receptors in order to ensure appropriate transitions in axon responsiveness. In addition, we highlight what we believe represent future directions and challenges for the field.
Growth toward the midline
As commissural axons approach the midline, they are preferentially responsive to midline attractants while suppressing their responsiveness to midline repellents. The first guidance cues to be implicated in commissural axon attraction were proteins of the Netrin family, which were initially identified for their roles in axon guidance and mesodermal cell migration in the nematode C. elegans.6,7 Netrins are secreted from the floor plate and ventral spinal cord, forming a ventral high to dorsal low gradient during the time when spinal commissural axons are growing toward the ventral midline.8–10 In vitro assays demonstrated that Netrin-1 elicits outgrowth of axons from spinal cord explants11 and induces attractive turning responses.8 Netrins are thought to signal both outgrowth and attraction through the receptor Deleted in Colorectal Cancer (DCC), which is expressed on commissural axons as they approach the midline.12 In the spinal cords of mouse embryos mutant for either Netrin-1 (the only Netrin expressed in the mouse spinal cord9,10) or Dcc, the ventral commissure is thin, but not absent; many axons stall before reaching the floor plate and commissural axons that normally project ventromedially in a tight bundle misproject laterally and are defasciculated.9,13 The observation that the ventral commissure is thinner in Netrin-1 mutants than in Dcc mutants14 and in vitro data indicating that Dcc mutant spinal cord explants retain some Netrin-responsiveness14,15 suggest that Netrin may promote midline crossing through both DCC-dependent and DCC-independent mechanisms.
One additional receptor through which Netrin might signal midline attraction is the DCC paralog, Neogenin (Neo), which is expressed on commissural axons.14 Mouse embryos mutant for Neo have no defects in commissural axon guidance, but in Dcc, Neo double mutants, the ventral commissure is thinner than in Dcc single mutants and comparably thin to the ventral commissure in netrin-1 mutants.14 Chickens have a single member of the DCC/Neo family, which has greater homology to mouse Neo than to mouse DCC. RNAi knockdown of this gene produces defects in commissural axon guidance in the chicken spinal cord reminiscent of the mouse Dcc mutant phenotype.16 However, Neo-dependent outgrowth and/or turning responses of spinal commissural neurons in response to Netrin have yet to be demonstrated. Netrin can bind to Neo,12, 17 but it does so with much lower affinity than Neo’s canonical repulsive ligand Repulsive Guidance Molecule (RGMa).18 RGMa mRNA is broadly expressed in the spinal cord,19 but its potential role in the guidance of spinal commissural axons has not been evaluated. Thus, it is not yet clear how Neo contributes to the establishment of the ventral commissure.
Down Syndrome Cell Adhesion Molecule (DSCAM) has also been proposed to function as an attractive Netrin receptor in spinal commissural neurons. Both insect and vertebrate Netrin proteins can bind to DSCAM in a variety of in vitro assays.15,20,21 Disruption of DSCAM function by RNAi or expression of dominant negative forms of DSCAM causes many commissural axons to fail to reach the floor plate in rat and chicken spinal cords.15,21 However, analysis of mice with Dscam null mutations suggests that DSCAM is not required for Netrin-dependent midline attraction in mice.22 Dscam mutants have no defects in commissural guidance in the spinal cord and Dscam, Dcc double mutants have commissural guidance defects comparable to Dcc mutants. It is conceivable that in Dscam null mutants – but not in animals subject to acute Dscam knockdown – a compensatory mechanism emerges to allow for normal midline crossing; notably, DCC and Neogenin mRNA and protein levels are unchanged in Dscam mutants,22 indicating that if there is compensation, it can not be explained by up-regulation of other known Netrin receptors. A less likely possibility is that DSCAM plays essential roles in Netrin-dependent midline attraction in rat and chicken that are not conserved in the mouse. Alternatively, the RNAi phenotypes may represent an artifact of some sort, underscoring the ideas that knockdown data should be interpreted with caution and that genetic nulls should be analyzed whenever possible. The advent of new methods for genome modification that bypass the need for ES cell targeting should facilitate the analysis of null alleles in vertebrates other than mice.
Netrins and DCC play conserved roles to promote midline axon crossing. Flies have two Netrin genes, NetA and NetB, which are expressed transiently in midline neurons and persistently in midline glia during embryogenesis23,24 and only one ortholog of Dcc/Neo, Frazzled (Fra), which encodes a protein that is expressed on commissural axons in the ventral nerve cord.25 In embryos lacking both fly Netrin genes (NetAB) and in fra mutants, commissures are thin, but not absent, with posterior commissures more sensitive to loss of Netrin or Fra than anterior commissures.25,26 fra mutants display more severe commissural guidance defects than NetAB mutants, implying that Fra promotes midline crossing in part through a Netrin-independent mechanism (see below for further discussion).27,28 In fly embryos engineered so that the only Netrin protein is membrane-tethered, both the anterior and posterior commissures develop normally,26 suggesting that long-range diffusion of Netrins is not required for commissural axon attraction. In C. elegans, Netrin has been shown to polarize neurons in a DCC-dependent manner,29,30 but this output of Netrin/DCC signaling has yet to be implicated in midline crossing. In both insects and vertebrates, the extents to which defects in commissural axon guidance in Netrin and Dcc mutants reflect defects in outgrowth, attraction, polarization, and/or regulation of gene expression remain unknown. Identification and disruption of distinct cytoplasmic motifs or residues that are required for these diverse signaling outputs would allow the relative contributions of these pathways to be dissected in vivo.
DSCAM’s role in midline crossing and its potential function as a Netrin receptor have also been investigated in Drosophila. Dscam mutants are phenotypically normal with respect to midline axon crossing, but Dscam, fra double mutants have more severe midline crossing defects than either NetAB or fra mutants alone. Misexpression of DSCAM in ipsilateral neurons induces ectopic midline crossing, even in NetAB mutant embryos.20 These genetic data imply that DSCAM promotes midline axon crossing through a Netrin-independent mechanism, but they do not exclude the possibility that DSCAM also functions as an attractive Netrin receptor. Many axons cross the midline even in Dscam, fra double mutants, suggesting that midline attractive or lateral repulsive signaling pathways that guide commissural axons toward the midline remain to be identified. The fact that additional cues and receptors have not been isolated in mutagenesis screens that have approached genomic saturation4,5 suggests that these genes may have earlier roles in embryogenesis that preclude analysis of midline axon crossing phenotypes and/or that their functions may be redundantly encoded.
In vertebrates, parallel pathways that guide commissural axons toward the ventral midline have been studied in greater detail. The residual ability of Netrin-1-mutant floor plate tissue to elicit turning of commissural axons9,31 is partially blocked by cyclopamine, a pharmacological inhibitor of the Sonic Hedgehog (Shh) effector Smoothened (Smo), and turning assays performed on dissociated commissural neurons have provided direct evidence that Shh can act as a chemoattractive cue.31,32 Conditional deletion of Smo in commissural neurons in the dorsal spinal cord causes commissural axons to misproject laterally and defasciculate as they are growing toward the floor plate.31 Genetic ablation of the Shh receptor Boc, which is expressed in commissural neurons, produces a similar phenotype, and RNAi knockdown of Boc inhibits the turning of commissural neurons toward a source of Shh.33 Shh appears to signal chemoattraction without regulating gene expression, as neither pharmacological inhibition of transcription nor expression of a dominant repressor of Gli transcription factors blocks Shh-induced turning responses in vitro,32 but this question has not been investigated in vivo. Analysis of Gli2 conditional knockouts in spinal commissural neurons would test whether canonical Shh signaling impinges on midline crossing.
Netrin-1 mutant floor plate retains some ability to elicit attractive turning even in the presence of cyclopamine, suggesting that the floor plate might produce additional attractive guidance cues.31 Recently, Vascular Endothelial Growth Factor A (VEGF), which is expressed in the floor plate during commissural axon guidance, was identified as a chemoattractant for commissural neurons.34 In embryos that are floor plate haplodeficient for Vegf or in which the VEGF receptor Flk-1 has been conditionally deleted from spinal commissural neurons, commissural axons misproject laterally and are defasciculated as they grow toward the floor plate. Dissociated commissural neurons turn toward a source of VEGF in vitro, and this turning response is antagonized by the presence of function-blocking antibodies against Flk-1.
Interestingly, in vitro experiments have implicated Src family kinase (SFK) activation downstream of all three vertebrate midline attractive pathways: Netrin/DCC, Shh/Boc, and VEGF/Flk-1.32,34–37 Exposure to Netrin, Shh, or VEGF activates SFKs and pharmacological or genetic inhibition of SFK activity blunts the abilities of these cues to elicit turning responses in a variety of assays. These data suggest that SFK activation may be an intracellular signaling event on which multiple chemoattractive pathways converge. Commissural axon guidance has not been closely studied in mice deficient for one or more SFKs, but the large number of vertebrate SFKs and the abilities of SFKs to functionally compensate for each other in other contexts38 caution that it may be difficult to evaluate whether SFK activation is indeed a requisite step for midline chemoattraction in intact vertebrate embryos. To date, the question of whether SFK activity is required for midline axon crossing has only been investigated in vivo in flies, which have only two genes encoding SFKs. In Drosophila, reduction in SFK gene dosage causes ipsilateral axons to ectopically cross the midline and suppresses commissural axon guidance defects in genetic backgrounds in which midline attraction is disrupted.39 These phenotypes are consistent with a requirement for SFKs in midline repulsion, but not midline attraction, raising the possibility that flies and vertebrates use SFKs in opposite ways with respect to midline crossing. Alternatively, the vertebrate in vitro data implicating SFK activation in midline attraction may not reflect the in vivo functions of SFKs.
Finally, in the spinal cord, roof plate-derived repellents collaborate with floor plate-derived attractants to guide commissural axons toward the ventral midline. In the absence of a floor plate, commissural axons navigate normally through the dorsal part of the spinal cord before stalling,40–43 suggesting that cues from another source must guide these axons during the early part of their trajectories. Roof plate tissue repels axons from spinal cord explants and this activity can be mimicked by cell aggregates expressing members of the Bone Morphogenetic Protein (BMP) family, BMP7 and GDF7, which are expressed in the roof plate during commissural axon outgrowth.44 BMPs likely signal repulsion through BMP receptor IB (BMPRIB), as roof plates from Bmp7 of Gdf7 mutants lack the ability to repel commissural axons44,45 and spinal cord explants from BmprIb mutants are unresponsive to roof plate-induced repulsion.46 However, spinal cords from Bmp7, Gdf7, and BmprIb mutants display only modest defects in commissural axon guidance, with commissural axons occasionally invading the roof plate or taking an aberrant medial trajectory,45,46 suggesting that other factors – potentially the complement of floor plate-derived attractants – can compensate for the loss of roof plate repulsion.
Exit from the midline
After commissural axons have reached the midline, they switch their responsiveness to midline cues, so that they can exit the midline and proceed toward their synaptic targets on the contralateral side of the embryo. During this phase of axon guidance, commissural axons are preferentially responsive to repellents expressed at the midline. The prototypical midline repulsive cues are Slit proteins, which signal repulsion through Roundabout (Robo) receptors. Slits and Robos were initially implicated in midline axon repulsion through forward genetic screens in Drosophila.4,5 Slit is expressed in midline glia throughout embryogenesis;47,48 Robo is expressed on axons and shows a striking localization to longitudinal connectives, but is largely excluded from commissural segments.47,48 In robo mutant fly embryos, ipsilateral axons ectopically cross the midline and both ipsilateral and commissural axons re-cross the midline.4,49 slit mutants have an even more dramatic phenotype, in which all axons collapse on the midline.48 Flies have three genes encoding Robo receptors and the observation that embryos mutant for both robo and robo2 are phenotypically indistinguishable from slit mutants with respect to midline crossing50,51 suggests that these two Robo receptors signal midline repulsion in response to Slit and that Slit-Robo signaling accounts for all midline repulsion in the fly. (Robo3 is not required for midline repulsion, but it plays an important role in mediolateral positioning of ipsilateral and post-crossing commissural axons).52,53
Subsequent analysis of mouse mutants has confirmed that Slit-Robo signaling plays a conserved role in midline repulsion. In mice, three Slit genes are expressed in the floor plate54–57 and function redundantly to repel post-crossing commissural axons. In mice lacking all three Slit genes (Slit 1/2/3), many commissural axons stall at the floor plate and some turn back toward the ipsilateral side.58 Mice express four Robo genes, three of which are involved in midline repulsion, while Robo4 is specifically expressed in the vascular system.59 Robo1 and Robo2 proteins are expressed at low levels on pre-crossing commissural axons and are up-regulated post-crossing.58 A study in chicken embryos suggests that insertion of Robo1 protein into the plasma membrane of post-crossing commissural axons depends on the vesicle fusion machinery component Rab Guanine Nucleotide Dissociation Inhibitor (RabGDI), which is, itself, preferentially expressed in commissural neurons post-crossing.60 Spinal commissural axons in Robo 1/2 double mutants stall at the floor plate, but these defects are not as frequent as in Slit 1/2/3 mutants, and Robo 1/2 mutants never display re-crossing errors,61 implying the existence of another repulsive Slit receptor. Robo3 seems to repel post-crossing commissural axons but also plays a key role in preventing premature Slit responsiveness in pre-crossing commissural axons (see below for further discussion).62,63
The absence of Slit signaling in the mouse does not lead to a complete loss of midline repulsion,58 suggesting that vertebrates require other midline repellents to collaborate with Slits to prevent ectopic midline crossing and to facilitate midline exit. The class 3 secreted Semaphorin, Sema3B, is expressed in the floor plate and ventral spinal cord during the period of commissural axon guidance64 and its co-receptors Neuropilin-2 (Nrp2) and Plexin-A1 (PlexA1) are expressed on commissural axons, with PlexA1 expression enriched on axons during and after crossing.65 In mouse embryos mutant for Sema3b, Nrp2, or PlexA1, many commissural axons fail to exit the midline.64–66 In addition, PlexA1 can function as a repulsive receptor for Slit, as Slit2 binds to PlexA1 and can induce growth cone collapse of Robo1/2 mutant spinal commissural neurons in a PlexA1-dependent manner,66 suggesting that PlexA1 may be the additional Slit receptor implied by the difference in the strengths of the Robo 1/2 and Slit 1/2/3 phenotypes. Compound mutants in which Slit and Sema3B signaling are perturbed in combination have yet to be analyzed, so it is not clear whether additional repulsive signaling pathways facilitate floor plate exit and prevent inappropriate midline crossing.
Regulation of responsiveness to midline cues
How do commissural neurons regulate their sensitivity to midline cues so that they are preferentially responsive to midline attractants pre-crossing, but preferentially responsive to midline repellents post-crossing? The persistence of Netrin-1 and Shh expression in the floor plate past the time when commissural axons have crossed the midline8,10,67 suggests that commissural neurons might actively silence their attraction to midline cues once they have reached the floor plate. The idea that commissural neurons may repress their attraction to Netrin in response to Slit exposure has emerged from a series of in vitro experiments.68 Dissociated Xenopus spinal neurons turn toward a source of Netrin in culture and even though these neurons are not repelled by Slit, exposure to Slit blunts their attraction to Netrin. In this context, Slit triggers a physical interaction between the cytoplasmic domains of DCC and Robo1 and Slit’s ability to silence responsiveness to Netrin depends on DCC-Robo1 binding. The cytoplasmic motifs in these receptors that mediate the interaction are required for the silencing response and artificially restoring these receptors’ abilities to interact with each other also restores Slit’s ability to block attraction to Netrin. This model has yet to be tested in vivo.
Like Netrin, Shh continues to be expressed in the floor plate throughout spinal cord development, but commissural axons switch the polarity of their responsiveness to Shh after they reach the floor plate. After exiting the midline, most spinal commissural axons turn anteriorly. However, disruption of Shh signaling through a variety of pharmacological and genetic approaches causes post-crossing commissural axons to choose an anterior or posterior trajectory at random.67,69,70 Shh mRNA and protein are expressed in the spinal cord in a posterior high to anterior low gradient,67,69 suggesting that Shh might signal repulsion in post-crossing commissural neurons. When dissociated spinal commissural neurons are cultured and exposed to a gradient of Shh, the polarity of their response depends on their age.67 Neurons that have been cultured for a short time are attracted to Shh, while neurons that have been cultured for longer are repelled by Shh, consistent with the idea that as they are growing toward the midline, commissural neurons are attracted to floor plate-derived Shh, but after crossing the midline, they are repelled by the high concentration of Shh in the posterior spinal cord. This switch is mediated by 14-3-3 adaptor proteins, which are preferentially expressed on post-crossing commissural axons and whose expression increases over time in cultured commissural neurons (Figure 2). In vitro, Shh-dependent repulsion in aged neurons can be blocked by pharmacological manipulations that antagonize 14-3-3 and can be mimicked in young neurons by premature expression of 14-3-3 or by manipulations that induce 14-3-3 activity independent of Shh. In the spinal cord, treatment with 14-3-3 inhibitors randomizes anterior-posterior turning after midline crossing, but has no effect on midline attraction, suggesting that 14-3-3 is specifically required for post-crossing commissural axon guidance in response to Shh. As 14-3-3 mRNA expression patterns have not yet been described in pre- and post-crossing commissural neurons, it is not clear at what level 14-3-3 expression is regulated to switch on Shh repulsion.
Figure 2. Commissural axons switch the polarity of their response to Shh.
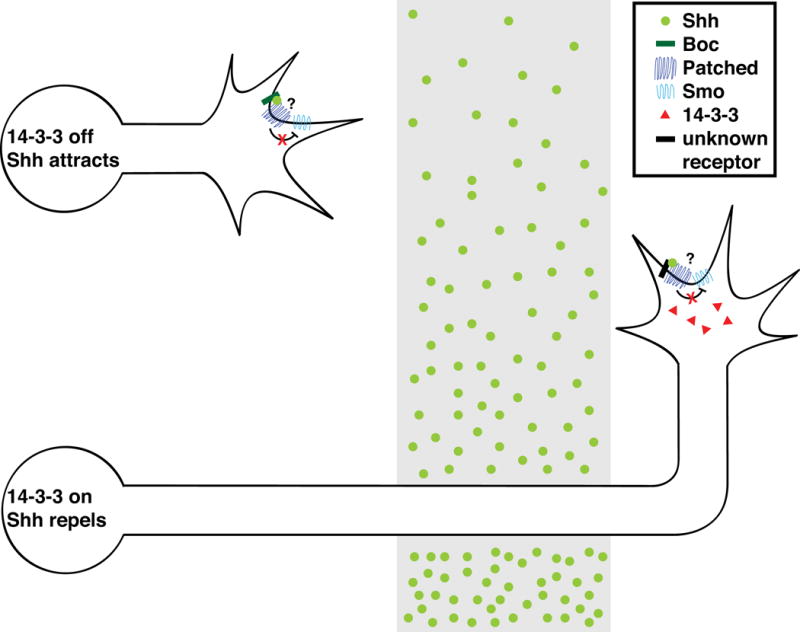
Pre-crossing spinal commissural neurons signal attraction to midline-derived Shh through the receptor Boc. These neurons turn anteriorly after they have crossed the midline, in response to a posterior high to anterior low gradient of Shh, but the relevant Shh receptor is not known. 14-3-3 is specifically expressed in post-crossing commissural neurons and is required for Shh-dependent repulsion, but not attraction. Both attractive and repulsive Shh signaling depend on Smo, but it is not clear whether the Shh co-receptor Patched, which relieves repression of Smo to permit Shh signaling in other contexts, is required for Shh-dependent attraction or repulsion.
Shh-dependent repulsion appears to be Smo-dependent, as conditional deletion of Smo from spinal commissural neurons leads to randomization of anterior-posterior turning.67 In chickens, mRNA for Hedgehog-interacting protein (Hhip), an inhibitor of Shh signaling, is transiently expressed in commissural neurons once their axons have reached the midline and RNAi knockdown of Hhip causes both midline stalling and aberrant posterior turns of post-crossing commissural axons.69 These defects have been interpreted as evidence that Hhip is a receptor through which Shh signals repulsion in post-crossing commissural neurons.69,71 However, Hhip has not been shown to signal in response to Shh in any context and is instead thought to antagonize Hh signaling by sequestering Hh proteins and restricting their diffusion.72–74 A requirement for Hhip in mediating Shh-dependent repulsion has yet to be established through turning or collapse assays, and mice mutant for Hhip do not display anterior-posterior turning defects.67 The observation that Hhip knockdown causes midline stalling69 suggests the alternative possibility that Hhip may be transiently expressed in commissural neurons to blunt attraction to floor plate-derived Shh as commissural axons are exiting the midline. Functional studies assessing the potential contributions of the Shh receptors Boc, Cdo, and Gas1 in the guidance of post-crossing commissural axons have not been reported. Gas1 repels enteric axons from gut-derived Shh,75 raising the possibility that Gas1 may signal Shh-dependent axon repulsion in other contexts.
In addition, considerable evidence has emerged indicating that commissural neurons actively inhibit their responsiveness to midline repellents while they are growing toward the midline. Vertebrates limit Robo repulsion in pre-crossing commissural axons through Robo3 (Figure 3). In mouse embryos mutant for Robo3, all spinal commissural axons fail to cross the midline.62 While floor plate tissue elicits outgrowth of axons from wild-type spinal cord explants, Robo3 mutant axons fail to grow out of explants when exposed to wild-type floor plate tissue. Blockade of Slit activity with a soluble Robo2 ectodomain restores the ability of Robo3 mutant explants to respond to floor plate-derived outgrowth signals. Likewise, a combination of Netrin-1 and Slit2 induces axonal outgrowth from wild-type, but not Robo3 mutant explants, suggesting that the endogenous function of Robo3 in pre-crossing commissural axons is to prevent precocious Slit responsiveness.62 Genetic data support the idea that the failure of Robo3 mutant commissural axons to reach the floor plate in vivo is due excessive Slit repulsion through Robo1 and Robo2, as reduction in Robo1, Robo2, or Slit gene dosage partially rescues Robo3 mutants.61,62 However, even the complete loss of Robo1 and Robo2 fails to fully rescue midline crossing defects in Robo3 mutants, suggesting that Robo3 promotes midline crossing in part through Robo1- and Robo2-independent mechanisms.61 Robo3, Slit 1/2/3 compound mutants have not been analyzed, so it is not yet clear whether this mechanism is Slit-dependent. In light of the observation that Robo1 and Robo2 are expressed at very low levels on pre-crossing commissural axons,58 these genetic data imply that the activity of this small pool of Robo1 and Robo2 must be antagonized to prevent premature repulsion. A recent study suggests that Robo3 may promote midline attraction in addition to antagonizing midline repulsion.76 Robo3 mutant spinal cord explants display a reduced outgrowth response when exposed to Netrin-1 and, although Robo3 does not directly bind to Netrin, it does form a complex with DCC. Rescue experiments with a form of DCC that cannot bind to Robo3 would test whether Netrin-DCC attraction depends on this Robo3-DCC complex.
Figure 3. Robo3 regulates Slit responsiveness of commissural axons.
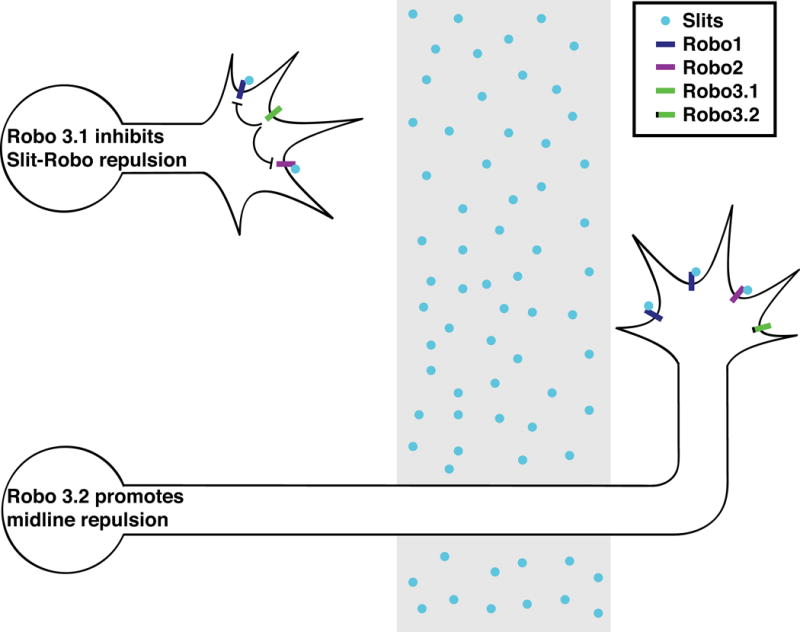
In pre-crossing spinal commissural neurons, Robo3.1 inhibits Slit repulsion through Robo1 and Robo2. After crossing, Robo3.1 is no longer expressed and Robo3.2 collaborates with Robo1 and Robo2 to signal midline repulsion.
Robo3 is alternatively spliced, yielding two variants that differ in their cytoplasmic domains.63 One splice variant, Robo3.1, is specifically expressed on pre-crossing commissural axons, while the other, Robo3.2, is specifically expressed on post-crossing commissural axons. Isoform-specific rescue and RNAi experiments suggest that Robo3.1 is required to facilitate midline crossing, while Robo3.2 contributes to midline repulsion in post-crossing commissural axons. Notably, Robo3.2 knockdown in a Robo1, Robo2 background causes occasional re-crossing of commissural axons, a phenotype observed in Slit 1/2/3 mutants, but not in Robo1, Robo2 mutants. It has been speculated that perhaps Robo3.1 acts as a Slit sink, preventing Robo1 and Robo2 from binding to Slit, but lacking the ability to signal repulsion, while Robo3.2 functions as a classical Robo receptor, signaling repulsion in response to Slit.62,63 However, this possibility seems unlikely in light of reports that mammalian Robo3 proteins do not bind Slit,76–79 leaving the questions of how Robo3.1 antagonizes Robo1 and Robo2 activity and how Robo3.2 signals midline repulsion unresolved. In addition, the mechanisms regulating the alternative splicing of Robo3 remain unknown. Thus, many aspects of Robo3 function in commissural neurons both before and after midline crossing warrant further exploration.
In addition to limiting their responsiveness to Slits, pre-crossing commissural neurons suppress their responsiveness to Sema3B, in part through degrading PlexA1 by proteolysis (Figure 4).65 As commissural axons are growing toward the midline, they express only a low level of PlexA1, but PlexA1 expression is up-regulated on commissural axons after they have reached the midline. Spinal commissural neurons display increased PlexA1 expression upon exposure to floor plate-conditioned media, suggesting that the floor plate produces soluble factors that promote PlexA1 expression. PlexA1 is a substrate for calpain cleavage and blunting calpain activity either by RNAi knockdown or with pharmacological inhibitors causes spinal commissural neurons, which are ordinarily unresponsive to Sema3B, to undergo growth cone collapse when exposed to Sema3B. When mouse spinal cords are treated with calpain inhibitors, commissural axons stall at the floor plate, consistent with a role for calpain proteolysis in sensitizing commissural neurons to floor plate-derived repellents. Experiments with a calpain-insensitive variant of PlexA1, which would be predicted to be active pre-crossing and therefore to prematurely signal midline repulsion, could validate the model that calpain proteolysis of PlexA1 is indeed responsible for limiting Sema3B responsiveness in pre-crossing commissural neurons.
Figure 4. GDNF modulates Sema3B responsiveness by regulating PlexA1 proteolysis.
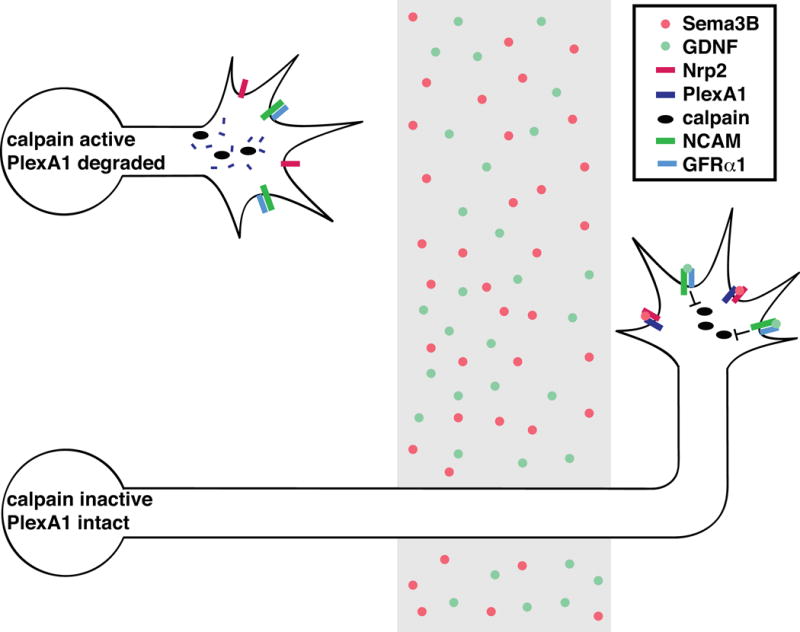
As spinal commissural axons are growing toward the midline, calpain cleaves the Sema3B receptor PlexA1 to reduce sensitivity to Sema3B. When these neurons reach the midline, GDNF signals through NCAM and its co-receptor GFRα1 to reduce calpain activity. Sema3B then signals repulsion through Nrp2 and PlexA1.
Exposure to floor plate-conditioned media antagonizes calpain activity in dorsal spinal cord tissue,65 implying the existence of soluble floor plate-derived factors that block calpain activity. Glial cell line-derived neurotrophic factor (GDNF) is expressed in the floor plate during commissural axon guidance and it mimics the abilities of floor plate-conditioned medium to sensitize commissural neurons to Sema3B-induced growth cone collapse and to reduce both calpain activity and the abundance of PlexA1 proteolytic fragments in the spinal cord.80 Medium conditioned by floor plate tissue from Gdnf mutant mice has reduced ability to sensitize commissural neurons to Sema3B-induced growth cone collapse. In embryos mutant for Gdnf or its receptor Neural Cell Adhesion Molecule (NCAM), spinal commissural axons frequently stall in the floor plate, consistent with GDNF’s proposed function in promoting midline repulsion. Other floor plate-derived factors that sensitize commissural neurons to Sema3B repulsion have been identified, including Shh70 and Neuronal Cell Adhesion Molecule (NrCAM), which is cleaved to release a soluble ectodomain;65 NrCAM inhibits PlexA1 expression,65 but it is not clear whether either of these soluble factors regulates calpain activity.
In flies, there is no evidence that commissural neurons modulate their responsiveness to midline attractants. However, like vertebrates, flies inhibit Slit-Robo repulsion in pre-crossing commissural neurons, but through a different mechanism (Figure 5). The endosomal protein Commissureless (Comm) binds Robo and prevents its trafficking to the growth cone, instead targeting it for lysosomal degradation.81,82 Expression of comm mRNA is tightly spatiotemporally controlled so that comm is specifically expressed in commissural neurons as they are sending their axons across the midline, but not before or after, and comm is rarely expressed in ipsilateral neurons.81 This pulse of comm expression in commissural neurons reduces their responsiveness to Slit during midline crossing. comm mutants have a dramatic phenotype in which no axons cross the midline4,83 and analysis of robo, comm double mutants indicates that robo is epistatic to comm.4 Surprisingly, embryos in which the endogenous robo gene is replaced with a mutant version that cannot be sorted by Comm are phenotypically normal,84 suggesting that Comm can regulate Slit-Robo repulsion through an additional mechanism.
Figure 5. Comm regulates Slit responsiveness by inhibiting trafficking of Robo to the growth cone.
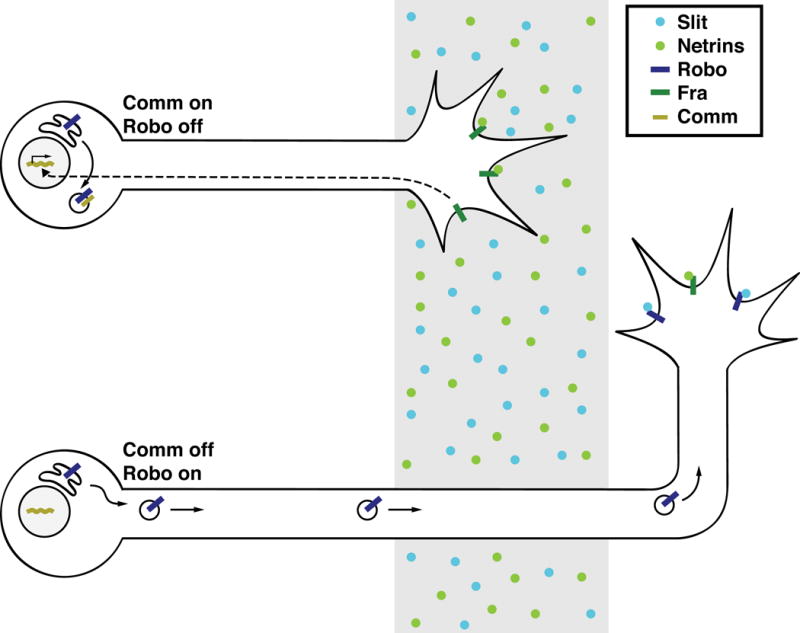
In Drosophila, as commissural neurons grow toward the midline, they express the endosomal protein Comm, which targets newly synthesized Robo for lysosomal degradation. Fra regulates comm transcription independent of its canonical ligands, Netrins. After crossing, Comm expression is extinguished and Robo is trafficked to the growth cone, where it signals repulsion in response to Slit.
comm expression is regulated, in part, by Fra. In fra mutants, comm expression is reduced in commissural neurons, but this output of Fra is Netrin-independent, as comm expression is unaffected in NetAB mutants.28 It is not clear whether Fra’s ability to regulate comm is ligand-dependent or at what level it is regulated to produce the appropriate temporal pattern of comm expression. Fra appears to regulate comm transcription rather than the stability of comm mRNA, as comm pre-mRNA is reduced in fra mutants.28 DCC and Neogenin, are proteolytically processed, releasing their intracellular domains, which are capable of nuclear translocation.85–88 Moreover, these intracellular domains function as transcriptional activators in reporter assays in vitro,86,87 raising the possibility that Fra may regulate comm expression through direct transcriptional activation. As fra mutants have much milder midline crossing defects than comm mutants, other mechanisms must exist to regulate comm.
Conclusion
In the past two decades, many cues and receptors that attract commissural axons toward and repel them away from the ventral midline have been identified, and loss of function genetic data suggest that additional cues and receptors that regulate commissural axon pathfinding still await discovery. For example, additional factors that promote the growth of commissural axons toward the midline in the Drosophila embryo have yet to be identified. (Hedgehog and VEGF are not likely to signal midline attraction in the fly, as the only Drosophila hedgehog gene does not display prominent midline expression89,90 and flies do not have a VEGF ortholog.) These predicted additional midline attractive cues and receptors may not have strong single mutant phenotypes. Indeed, examples of molecules whose contribution to commissural axon guidance can only be observed in genetic backgrounds in which the process is partially perturbed have already been described, such as Dscam in Drosophila and Neogenin in mice. As additional cues and receptors that promote midline crossing are identified in the fly, it will be interesting to determine to what extent they represent conserved midline guidance mechanisms.
Both intrinsic (i.e. 14-3-3) and extrinsic (i.e. GDNF) factors that enable axons to modulate their responsiveness to midline cues have been identified, but our understanding of the cellular mechanisms that allow axons to switch their responsiveness to cues is incomplete. Of particular interest, the mechanism(s) through which Robo3 antagonizes Slit repulsion through Robo1 and Robo2 remains a mystery. Regulation of receptor expression appears to be a common mechanism through which axonal sensitivity to cues can be gated. Recent reports that axon guidance receptors themselves can regulate both transcription28,86,87 and translation91 raise the intriguing possibility that guidance receptors may be able to directly regulate their own expression or expression of other receptors.
Acknowledgments
We thank Tim Evans for help with the figures, Frédéric Charron and Patricia Yam for sharing images, and Celine Santiago for comments on the manuscript. The research in the Bashaw lab is supported by NSF grant IOS-1355181 and NIH grant R01NS054739. ANF was supported by NIH grant T32GM008216.
Contributor Information
Alexandra Neuhaus-Follini, Department of Neuroscience, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104.
Greg J. Bashaw, Email: gbashaw@mail.med.upenn.edu, Department of Neuroscience, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104
References
- 1.Dodd J, Morton SB, Karagogeos D, Yamamoto M, Jessell TM. Spatial regulation of axonal glycoprotein expression on subsets of embryonic spinal neurons. Neuron. 1988;1:105–116. doi: 10.1016/0896-6273(88)90194-8. [DOI] [PubMed] [Google Scholar]
- 2.Bovolenta P, Dodd J. Guidance of commissural growth cones at the floor plate in embryonic rat spinal cord. Development. 1990;109:435–447. doi: 10.1242/dev.109.2.435. [DOI] [PubMed] [Google Scholar]
- 3.Rickert C, Kunz T, Harris KL, Whitington PM, Technau GM. Morphological characterization of the entire interneuron population reveals principles of neuromere organization in the ventral nerve cord of Drosophila. J Neurosci. 2011;31:15870–15883. doi: 10.1523/JNEUROSCI.4009-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seeger M, Tear G, Ferres-Marco D, Goodman CS. Mutations affecting growth cone guidance in Drosophila: genes necessary for guidance toward or away from the midline. Neuron. 1993;10:409–426. doi: 10.1016/0896-6273(93)90330-t. [DOI] [PubMed] [Google Scholar]
- 5.Hummel T, Schimmelpfeng K, Klämbt C. Commisure formation in the embryonic CNS of Drosophila. Dev Biol. 1999;209:381–398. doi: 10.1006/dbio.1999.9235. [DOI] [PubMed] [Google Scholar]
- 6.Hedgecock EM, Culotti JG, Hall DH. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron. 1990;4:61–85. doi: 10.1016/0896-6273(90)90444-k. [DOI] [PubMed] [Google Scholar]
- 7.Ishii N, Wadsworth WG, Stern BD, Culotti JG, Hedgecock EM. UNC-6, a laminin-related protein, guides cell and pioneer axon migrations in C. elegans. Neuron. 1992;9:873–881. doi: 10.1016/0896-6273(92)90240-e. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994;78:425–435. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- 9.Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy TE, Wang H, Marshall W, Tessier-Lavigne M. Axon guidance by diffusible chemoattractants: a gradient of netrin protein in the developing spinal cord. J Neurosci. 2006;26:8866–8874. doi: 10.1523/JNEUROSCI.5191-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serafini T, Kennedy TE, Galko MJ, Mirzayan C, Jessell TM, Tessier-Lavigne M. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78:409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- 12.Keino-Masu K, Masu M, Hinck L, Leonardo ED, Chan SS, Culotti JG, Tessier-Lavigne M. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell. 1996;87:175–185. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- 13.Fazeli A, Dickinson SL, Hermiston ML, Tighe RV, Steen RG, Small CG, Stoeckli ET, Keino-Masu K, Masu M, Rayburn H, et al. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature. 1997;386:796–804. doi: 10.1038/386796a0. [DOI] [PubMed] [Google Scholar]
- 14.Xu K, Wu Z, Renier N, Antipenko A, Tzvetkova-Robev D, Xu Y, Minchenko M, Nardi-Dei V, Rajashankar KR, Himanen J, et al. Structures of netrin-1 bound to two receptors provide insight into its axon guidance mechanism. Science. 2014;344:1275–1279. doi: 10.1126/science.1255149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ly A, Nikolaev A, Suresh G, Zheng Y, Tessier-Lavigne M, Stein E. DSCAM is a netrin receptor that collaborates with DCC in mediating turning responses to netrin-1. Cell. 2008;133:1241–1254. doi: 10.1016/j.cell.2008.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phan KD, Croteau LP, Kam JW, Kania A, Cloutier JF, Butler SJ. Neogenin may functionally substitute for Dcc in chicken. PLoS One. 2011;6:322072. doi: 10.1371/journal.pone.0022072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H, Copeland NG, Gilbert DJ, Jenkins NA, Tessier-Lavigne M. Netrin-3, a mouse homolog of human NTN2L, is highly expressed in sensory ganglia and shows differential binding to netrin receptors. J Neurosci. 1999;19:4938–4947. doi: 10.1523/JNEUROSCI.19-12-04938.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajagopalan S, Deitinghoff L, Davis D, Conrad S, Skutella T, Chedotal A, Mueller BK, Strittmatter SM. Neogenin mediates the action of repulsive guidance molecule. Nat Cell Biol. 2004;6:756–762. doi: 10.1038/ncb1156. [DOI] [PubMed] [Google Scholar]
- 19.Niederkofler V, Salie R, Sigrist M, Arber S. Repulsive guidance molecule (RGM) gene function is required for neural tube closure but not retinal topography in the mouse visual system. J Neurosci. 2004;24:808–818. doi: 10.1523/JNEUROSCI.4610-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrews GL, Tanglao S, Farmer WT, Morin S, Brotman S, Berberoglu MA, Price H, Fernandez GC, Mastick GS, Charron F, et al. Dscam guides embryonic axons by Netrin-dependent and -independent functions. Development. 2008;135:3839–3848. doi: 10.1242/dev.023739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu G, Li W, Wang L, Kar A, Guan KL, Rao Y, Wu JY. DSCAM functions as a netrin receptor in commissural axon pathfinding. Proc Natl Acad Sci U S A. 2009;106:2951–2956. doi: 10.1073/pnas.0811083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmesino E, Haddick PC, Tessier-Lavigne M, Kania A. Genetic analysis of DSCAM’s role as a Netrin-1 receptor in vertebrates. J Neurosci. 2012;32:411–416. doi: 10.1523/JNEUROSCI.3563-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris R, Sabatelli LM, Seeger MA. Guidance cues at the Drosophila CNS midline: identification and characterization of two Drosophila Netrin/UNC-6 homologs. Neuron. 1996;17:217–228. doi: 10.1016/s0896-6273(00)80154-3. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell KJ, Doyle JL, Serafini T, Kennedy TE, Tessier-Lavigne M, Goodman CS, Dickson BJ. Genetic analysis of Netrin genes in Drosophila: Netrins guide CNS commissural axons and peripheral motor axons. Neuron. 1996;17:203–215. doi: 10.1016/s0896-6273(00)80153-1. [DOI] [PubMed] [Google Scholar]
- 25.Kolodziej PA, Timpe LC, Mitchell KJ, Fried SR, Goodman CS, Jan LY, Jan YN. frazzled encodes a Drosophila member of the DCC immunoglobulin subfamily and is required for CNS and motor axon guidance. Cell. 1996;87:197–204. doi: 10.1016/s0092-8674(00)81338-0. [DOI] [PubMed] [Google Scholar]
- 26.Brankatschk M, Dickson BJ. Netrins guide Drosophila commissural axons at short range. Nat Neurosci. 2006;9:188–194. doi: 10.1038/nn1625. [DOI] [PubMed] [Google Scholar]
- 27.Garbe DS, O’Donnell M, Bashaw GJ. Cytoplasmic domain requirements for Frazzled-mediated attractive axon turning at the Drosophila midline. Development. 2007;134:4325–4334. doi: 10.1242/dev.012872. [DOI] [PubMed] [Google Scholar]
- 28.Yang L, Garbe DS, Bashaw GJ. A frazzled/DCC-dependent transcriptional switch regulates midline axon guidance. Science. 2009;324:944–947. doi: 10.1126/science.1171320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adler CE, Fetter RD, Bargmann CI. UNC-6/Netrin induces neuronal asymmetry and defines the site of axon formation. Nat Neurosci. 2006;9:511–518. doi: 10.1038/nn1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu Z, Li H, Wadsworth WG. The roles of multiple UNC-40 (DCC) receptor-mediated signals in determining neuronal asymmetry induced by the UNC-6 (netrin) ligand. Genetics. 2009;183:941–949. doi: 10.1534/genetics.109.108654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen sonic hedgehog is an axonal chemattractant that collaborates with netrin-1 in midline axon guidance. Cell. 2003;113:11–23. doi: 10.1016/s0092-8674(03)00199-5. [DOI] [PubMed] [Google Scholar]
- 32.Yam PT, Langlois SD, Morin S, Charron F. Sonic hedgehog guides axons through a noncanonical, Src-family-kinase-dependent signaling pathway. Neuron. 2009;62:349–362. doi: 10.1016/j.neuron.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 33.Okada A, Charron F, Morin S, Shin DS, Wong K, Fabre PJ, Tessier-Lavigne M, McConnell SK. Boc is a receptor for sonic hedgehog in the guidance of commissural axons. Nature. 2006;444:369–373. doi: 10.1038/nature05246. [DOI] [PubMed] [Google Scholar]
- 34.Ruiz de Almodovar C, Fabre PJ, Knevels E, Coulon C, Segura I, Haddick PC, Aerts L, Delattin N, Strasser G, Oh WJ, et al. VEGF mediates commissural axon chemoattraction through its receptor Flk1. Neuron. 2011;70:966–978. doi: 10.1016/j.neuron.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li W, Lee J, Vikis HG, Lee SH, Liu G, Aurandt J, Shen TL, Fearon ER, Guan JL, Han M, et al. Activation of FAK and Src are receptor-proximal events required for Netrin signaling. Nat Neurosci. 2004;7:1213–1221. doi: 10.1038/nn1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu G, Beggs H, Jürgensen C, Park HT, Tang H, Gorski J, Jones KR, Reichardt LF, Wu J, Rao Y. Netrin requires focal adhesion kinase and Src family kinases for axon outgrowth and attraction. Nat Neurosci. 2004;7:1222–1232. doi: 10.1038/nn1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meriane M, Tcherkezian J, Webber CA, Danek EI, Triki I, McFarlane S, Bloch-Gallego E, Lamarche-Vane N. Phosphorylation of DCC by Fyn mediates Netrin-1 signaling in growth cone guidance. J Cell Biol. 2004;167:687–698. doi: 10.1083/jcb.200405053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stein PL, Vogel H, Soriano P. Combined deficiencies of Src, Fyn, and Yes tyrosine kinases in mutant mice. Genes Dev. 1994;8:1999–2007. doi: 10.1101/gad.8.17.1999. [DOI] [PubMed] [Google Scholar]
- 39.O’Donnell MP, Bashaw GJ. Src inhibits midline axon crossing independent of Frazzled/Deleted in Colorectal Carcinoma (DCC) receptor tyrosine phosphorylation. J Neurosci. 2013;33:305–314. doi: 10.1523/JNEUROSCI.2756-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bovolenta P, Dodd J. Perturbation of neuronal differentiation and axon guidance in the spinal cord of mouse embryos lacking a floor plate: analysis of Danforth’s short-tail mutation. Development. 1991;113:625–639. doi: 10.1242/dev.113.2.625. [DOI] [PubMed] [Google Scholar]
- 41.Hatta K, Kimmel CB, Ho RK, Walker C. The cyclops mutation blocks specification of the floor plate of the zebrafish central nervous system. Nature. 1991;350:339–341. doi: 10.1038/350339a0. [DOI] [PubMed] [Google Scholar]
- 42.Placzek M, Yamada T, Tessier-Lavigne M, Jessell T, Dodd J. Control of dorsoventral pattern in vertebrate neural development: induction and polarizing properties of the floor plate. Development. 1991;(Suppl 2):105–122. [PubMed] [Google Scholar]
- 43.Yamada T, Placzek M, Tanaka H, Dodd J, Jessell TM. Control of cell pattern in the developing nervous system: polarizing activity of the floor plate and notochord. Cell. 1991;64:635–647. doi: 10.1016/0092-8674(91)90247-v. [DOI] [PubMed] [Google Scholar]
- 44.Augsburger A, Schuchardt A, Hoskins S, Dodd J, Butler S. BMPs as mediators of roof plate repulsion of commissural neurons. Neuron. 1999;24:127–141. doi: 10.1016/s0896-6273(00)80827-2. [DOI] [PubMed] [Google Scholar]
- 45.Butler SJ, Dodd J. A role for BMP heterodimers in roof plate-mediated repulsion of commissural axons. Neuron. 2003;38:389–401. doi: 10.1016/s0896-6273(03)00254-x. [DOI] [PubMed] [Google Scholar]
- 46.Yamauchi K, Phan KD, Butler SJ. BMP type I receptor complexes have distinct activities mediating cell fate and axon guidance decisions. Development. 2008;135:1119–1128. doi: 10.1242/dev.012989. [DOI] [PubMed] [Google Scholar]
- 47.Rothberg JM, Jacobs JR, Goodman CS, Artavanis-Tsakonas S. slit: an extracellular protein necessary for development of midline glia and commissural axon pathways contains both EGF and LRR domains. Genes Dev. 1990;4:2169–2187. doi: 10.1101/gad.4.12a.2169. [DOI] [PubMed] [Google Scholar]
- 48.Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the robo receptor in Drosophila. Cell. 1999;96:785–794. doi: 10.1016/s0092-8674(00)80589-9. [DOI] [PubMed] [Google Scholar]
- 49.Kidd T, Brose K, Mitchell KJ, Fetter RD, Tessier-Lavigne M, Goodman CS, Tear G. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell. 1998;92:205–215. doi: 10.1016/s0092-8674(00)80915-0. [DOI] [PubMed] [Google Scholar]
- 50.Rajagopalan S, Nicolas E, Vivancos V, Berger J, Dickson BJ. Crossing the midline: roles and regulation of Robo receptors. Neuron. 2000;28:767–777. doi: 10.1016/s0896-6273(00)00152-5. [DOI] [PubMed] [Google Scholar]
- 51.Simpson JH, Kidd T, Bland KS, Goodman CS. Short-range and long-range guidance by Slit and its Robo receptors: Robo and Robo2 play distinct roles in midline guidance. Neuron. 2000;28:753–766. doi: 10.1016/s0896-6273(00)00151-3. [DOI] [PubMed] [Google Scholar]
- 52.Rajagopalan S, Vivancos V, Nicolas E, Dickson BJ. Selecting a longitudinal pathway: Robo receptors specify the lateral positioning of axons in the Drosophila CNS. Cell. 2000;103:1033–1045. doi: 10.1016/s0092-8674(00)00207-5. [DOI] [PubMed] [Google Scholar]
- 53.Simpson JH, Bland KS, Fetter RD, Goodman CS. Short-range and long-range guidance by Slit and its Robo receptors: a combinatorial code of Robo receptors controls lateral positioning. Cell. 2000;103:1019–1032. doi: 10.1016/s0092-8674(00)00206-3. [DOI] [PubMed] [Google Scholar]
- 54.Holmes GP, Negus K, Burridge L, Raman S, Algar E, Yamada T, Little MH. Distinct but overlapping expression patterns of two vertebrate slit homologs implies functional roles in CNS development and organogenesis. Mech Dev. 1998;79:57–72. doi: 10.1016/s0925-4773(98)00174-9. [DOI] [PubMed] [Google Scholar]
- 55.Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell. 1999;96:795–806. doi: 10.1016/s0092-8674(00)80590-5. [DOI] [PubMed] [Google Scholar]
- 56.Li HS, Chen JH, Wu W, Fagaly T, Zhou L, Yuan W, Dupuis S, Jiang ZH, Nash W, Gick C, et al. Vertebrate slit, a secreted ligand for the transmembrane protein roundabout, is a repellent for olfactory bulb axons. Cell. 1999;96:807–818. doi: 10.1016/s0092-8674(00)80591-7. [DOI] [PubMed] [Google Scholar]
- 57.Yuan W, Zhou L, Chen JH, Wu JY, Rao Y, Ornitz DM. The mouse SLIT family: secreted ligands for ROBO expressed in patterns that suggest a role in morphogenesis and axon guidance. Dev Biol. 1999;212:290–306. doi: 10.1006/dbio.1999.9371. [DOI] [PubMed] [Google Scholar]
- 58.Long H, Sabatier C, Ma L, Plump A, Yuan W, Ornitz DM, Tamada A, Murakami F, Goodman CS, Tessier-Lavigne M. Conserved roles for Slit and Robo proteins in midline commissural axon guidance. Neuron. 2004;42:213–223. doi: 10.1016/s0896-6273(04)00179-5. [DOI] [PubMed] [Google Scholar]
- 59.Park KW, Morrison CM, Sorenson LK, Jones CA, Rao Y, Chien CB, Wu JY, Urness LD, Li DY. Robo4 is a vascular-specific receptor that inhibits endothelial migration. Dev Biol. 2003;261:251–267. doi: 10.1016/s0012-1606(03)00258-6. [DOI] [PubMed] [Google Scholar]
- 60.Philipp M, Niederkofler V, Debrunner M, Alther T, Kunz B, Stoeckli ET. RabGDI controls axonal midline crossing by regulating Robo1 surface expression. Neural Dev. 2012;7:36. doi: 10.1186/1749-8104-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jaworski A, Long H, Tessier-Lavigne M. Collaborative and specialized functions of Robo1 and Robo2 in spinal commissural axon guidance. J Neurosci. 2010;30:9445–9453. doi: 10.1523/JNEUROSCI.6290-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sabatier C, Plump AS, Ma L, Brose K, Tamada A, Murakami F, Lee EY, Tessier-Lavigne M. The divergent Robo family protein rig-1/Robo3 is a negative regulator of slit responsiveness required for midline crossing by commissural axons. Cell. 2004;117:157–169. doi: 10.1016/s0092-8674(04)00303-4. [DOI] [PubMed] [Google Scholar]
- 63.Chen Z, Gore BB, Long H, Ma L, Tessier-Lavigne M. Alternative splicing of the Robo3 axon guidance receptor governs the midline switch from attraction to repulsion. Neuron. 2008;58:325–332. doi: 10.1016/j.neuron.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 64.Zou Y, Stoeckli E, Chen H, Tessier-Lavigne M. Squeezing axons out of the gray matter: a role for slit and semaphorin proteins from midline and ventral spinal cord. Cell. 2000;102:363–375. doi: 10.1016/s0092-8674(00)00041-6. [DOI] [PubMed] [Google Scholar]
- 65.Nawabi H, Briançon-Marjollet A, Clark C, Sanyas I, Takamatsu H, Okuno T, Kumanogoh A, Bozon M, Takeshima K, Yoshida Y, et al. A midline switch of receptor processing regulates commissural axon guidance in vertebrates. Genes Dev. 2010;24:396–410. doi: 10.1101/gad.542510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Delloye-Bourgeois C, Jacquier A, Charoy C, Reynaud F, Nawabi H, Thoinet K, Kindbeiter K, Yoshida Y, Zagar Y, Kong Y, et al. PlexinA1 is a new Slit receptor and mediates axon guidance function of Slit C-terminal fragments. Nat Neurosci. 2015;18:36–45. doi: 10.1038/nn.3893. [DOI] [PubMed] [Google Scholar]
- 67.Yam PT, Kent CB, Morin S, Farmer WT, Alchini R, Lepelletier L, Colman DR, Tessier-Lavigne M, Fournier AE, Charron F. 14-3-3 proteins regulate a cell-intrinsic switch from sonic hedgehog-mediated commissural axon attraction to repulsion after midline crossing. Neuron. 2012;76:735–749. doi: 10.1016/j.neuron.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 68.Stein E, Tessier-Lavigne M. Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science. 2001;291:1928–1938. doi: 10.1126/science.1058445. [DOI] [PubMed] [Google Scholar]
- 69.Bourikas D, Pekarik V, Baeriswyl T, Grunditz A, Sadhu R, Nardó M, Stoeckli ET. Sonic hedgehog guides commissural axons along the longitudinal axis of the spinal cord. Nat Neurosci. 2005;8:297–304. doi: 10.1038/nn1396. [DOI] [PubMed] [Google Scholar]
- 70.Parra LM, Zou Y. Sonic hedgehog induces response of commissural axons to Semaphorin repulsion during midline crossing. Nat Neurosci. 2010;13:29–35. doi: 10.1038/nn.2457. [DOI] [PubMed] [Google Scholar]
- 71.Wilson NH, Stoeckli ET. Sonic hedgehog regulates its own receptor on postcrossing commissural axons in a glypican1-dependent manner. Neuron. 2013;79:478–491. doi: 10.1016/j.neuron.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 72.Chuang PT, McMahon AP. Vertebrate Hedgehog signalling modulated by induction of a Hedgehog-binding protein. Nature. 1999;397:617–621. doi: 10.1038/17611. [DOI] [PubMed] [Google Scholar]
- 73.Chuang PT, Kawcak T, McMahon AP. Feedback control of mammalian Hedgehog signaling by the Hedgehog-binding protein, Hip1, modulates Fgf signaling during branching morphogenesis of the lung. Genes Dev. 2003;17:342–347. doi: 10.1101/gad.1026303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jeong J, McMahon AP. Growth and pattern of the mammalian neural tube are governed by partially overlapping feedback activities of hedgehog antagonists patched 1 and Hhip1. Development. 2005;132:143–154. doi: 10.1242/dev.01566. [DOI] [PubMed] [Google Scholar]
- 75.Jin S, Martinelli DC, Zheng X, Tessier-Lavigne M, Fan CM. Gas1 is a receptor for sonic hedgehog to repel enteric axons. Proc Natl Acad Sci USA. 2015;112:E73–80. doi: 10.1073/pnas.1418629112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zelina P, Blockus H, Zagar Y, Péres A, Friocourt F, Wu Z, Rama N, Fouquet C, Hohenester E, Tessier-Lavigne M, et al. Signaling switch of the axon guidance receptor Robo3 during vertebrate evolution. Neuron. 2014;84:1258–1272. doi: 10.1016/j.neuron.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 77.Camurri L, Mambetisaeva E, Davies D, Parnavelas J, Sundaresan V, Andrews W. Evidence for the existence of two Robo3 isoforms with divergent biochemical properties. Mol Cell Neurosci. 2005;30:485–493. doi: 10.1016/j.mcn.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 78.Mambetisaeva ET, Andrews W, Camurri L, Annan A, Sundaresan V. Robo family of proteins exhibit differential expression in mouse spinal cord and Robo-Slit interaction is required for midline crossing in vertebrate spinal cord. Dev Dyn. 2005;233:41–51. doi: 10.1002/dvdy.20324. [DOI] [PubMed] [Google Scholar]
- 79.Li L, Liu S, Lei Y, Cheng Y, Yao C, Zhen X. Robo3.1A suppresses slit-mediated repulsion by triggering degradation of Robo2. J Neurosci Res. 2014;92:835–846. doi: 10.1002/jnr.23364. [DOI] [PubMed] [Google Scholar]
- 80.Charoy C, Nawabi H, Reynaud F, Derrington E, Bozon M, Wright K, Falk J, Helmbacher F, Kindbeiter K, Castellani V. gdnf activates midline repulsion by Semaphorin3B via NCAM during commissural axon guidance. Neuron. 2012;75:1051–1066. doi: 10.1016/j.neuron.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 81.Keleman K, Rajagopalan S, Cleppien D, Teis D, Paiha K, Huber LA, Technau GM, Dickson BJ. Comm sorts robo to control axon guidance at the Drosophila midline. Cell. 2002;110:415–427. doi: 10.1016/s0092-8674(02)00901-7. [DOI] [PubMed] [Google Scholar]
- 82.Keleman K, Ribiero C, Dickson BJ. Comm function in commissural axon guidance: cell autonomous sorting of Robo in vivo. Nat Neurosci. 2005;8:156–163. doi: 10.1038/nn1388. [DOI] [PubMed] [Google Scholar]
- 83.Tear G, Harris R, Sutaria S, Kilomanski K, Goodman CS, Seeger MA. commissureless controls growth cone guidance across the CNS midline in Drosophila and encodes a novel membrane protein. Neuron. 1996;16:501–514. doi: 10.1016/s0896-6273(00)80070-7. [DOI] [PubMed] [Google Scholar]
- 84.Gilestro GF. Redundant mechanisms for regulation of midline crossing in Drosophila. PLoS One. 2008;3:e3798. doi: 10.1371/journal.pone.0003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Galko MJ, Tessier-Lavigne M. Function of an axonal chemoattractant modulated by metalloprotease activity. Science. 2000;289:1365–1367. doi: 10.1126/science.289.5483.1365. [DOI] [PubMed] [Google Scholar]
- 86.Taniguchi Y, Kim SH, Sisodia SS. Presenilin-dependent “gamma-secretase” processing of deleted in colorectal cancer (DCC) J Biol Chem. 2003;278:30425–30428. doi: 10.1074/jbc.C300239200. [DOI] [PubMed] [Google Scholar]
- 87.Goldschneider D, Rama N, Guix C, Mehlen P. The neogenin intracellular domain regulates gene transcription via nuclear translocation. Mol Cell Biol. 2008;28:4068–4079. doi: 10.1128/MCB.02114-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bai G, Chivatakarn O, Bonanomi D, Lettieri K, Franco L, Xia C, Stein E, Ma L, Lewcock JW, Pfaff SL. Presenilin-dependent receptor processing is required for axon guidance. Cell. 2011;144:106–118. doi: 10.1016/j.cell.2010.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mohler J, Vani K. Molecular organization and embryonic expression of the hedgehog gene involved in cell-cell communication in segmental patterning of Drosophila. Development. 1992;115:957–971. doi: 10.1242/dev.115.4.957. [DOI] [PubMed] [Google Scholar]
- 90.Tabata T, Eaton S, Kornberg TB. The Drosophila hedgehog gene is expressed specifically in posterior compartment cells and is a target of engrailed regulation. Genes Dev. 1992;6:2635–2645. doi: 10.1101/gad.6.12b.2635. [DOI] [PubMed] [Google Scholar]
- 91.Tcherkezian J, Brittis PA, Thomas F, Roux PP, Flanagan JG. Transmembrane receptor DCC associates with protein synthesis machinery and regulates translation. Cell. 2010;141:632–644. doi: 10.1016/j.cell.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


