Abstract
Developing lymphocytes somatically diversify their antigen-receptor loci through V(D)J recombination. The process is associated with allelic exclusion, which results in monoallelic expression of an antigen receptor locus. Various cis-regulatory elements control V(D)J recombination in a developmentally regulated manner, but their role in allelic exclusion is still unclear. At the immunoglobulin heavy chain locus (IgH), the Eμ enhancer plays a critical role in V(D)J recombination. We generated a mouse line with a replacement mutation in the constant region of the locus that duplicates the Eμ enhancer and allows premature expression of the γ3 heavy chain. Strikingly, IgM expression was completely and specifically excluded in cis from the mutant allele. This cis exclusion recapitulated the main features of allelic exclusion, including differential exclusion of variable genes. Notably, sense and antisense transcription within the distal variable domain and distal VH-DJH recombination were inhibited. cis exclusion was established and stably maintained despite an active endogenous Eμ enhancer. The data reveal the importance of the dynamic, developmental stage-dependent interplay between IgH locus enhancers and signaling in the induction and maintenance of allelic exclusion.
INTRODUCTION
Developing B and T lymphocytes have the capacity to somatically alter their genomes and diversify their antigen receptor loci through V(D)J recombination. This developmentally regulated process is initiated by the lymphoid-specific RAG1/2 complex, which recognizes conserved recombination signal sequences (RSSs) flanking the V, D, and J segments in the variable domain of antigen receptor (IgH, IgL, and TCR) loci (1, 2). V(D)J recombination correlates with chromatin modifications, germ line transcription of rearranging V, D, and J segments, and large-scale chromosome dynamics within nuclear compartments (3–5).
The mouse IgH locus contains ∼200 VH genes, which are subdivided into VH gene families. The most prominent are the distal VH genes, notably the large VHJ558 gene family, and the proximal VH genes. These are followed by a dozen D segments (∼60 kb), 4 JH segments (∼2 kb), and 8 constant genes (∼200 kb) (6, 7).
In B lymphocytes, V(D)J assembly starts at the IgH locus, where D segments are first recombined to JH segments on both alleles. Although they have the potential to undergo VH-DJH recombination on the two alleles, the vast majority of B lymphocytes are subject to allelic exclusion, i.e., a given B cell expresses only one IgH allele. A productive V(D)J rearrangement allows production of the μ heavy chain, which associates with surrogate light chains and signals an arrest of VH-DJH recombination on the second IgH allele. If the first VH-DJH rearrangement is not productive, then the second allele can undergo VH-DJH recombination (1, 8). Nonetheless, in rare B cells, productive rearrangements can occur on both alleles (i.e., allelic inclusion), but only one μ heavy chain from only one allele can associate with surrogate light chains (9).
Several lines of evidence support the notion that VH-DJH rearrangement is the regulated step in IgH allelic exclusion and that allelic exclusion is maintained by a feedback mechanism (1, 8). However, while feedback inhibition of VH-DJH recombination can explain the maintenance of allelic exclusion, the mechanisms that control its initiation are unknown, and various models have been proposed to account for the unequal availabilities of the two alleles for VH recombination, including stochastic choice and differential epigenetic marks (1, 8, 10). Moreover, it remains unclear how cis-acting elements, which regulate V(D)J recombination in a cell type- and developmental stage-specific manner, control the suppression of VH-DJH recombination during allelic exclusion.
In this context, various cis-regulatory elements, including enhancers and insulators, were identified at the IgH locus. The Eμ enhancer, located between the variable and constant domains, plays an important role in V(D)J recombination. Deletion or insulation of this element affects V(D)J recombination and sense and antisense transcription at specific sites of the IgH variable locus (11–14). The Eμ enhancer was also suggested to play an important role in allelic exclusion, as deletion of this element in a mouse model carrying a prerearranged V(D)J gene led to a substantial increase in B cell populations with allelic inclusion (15). Additionally, an “intergenic control region” (IGCR1) with insulator activity was identified between the VH and D clusters (16–19). Deletion of CTCF sites within this region perturbed germ line transcription and recombination of the proximal VH genes, the order and cell type specificity of V(D)J recombination, and feedback regulation and allelic exclusion of proximal VH-DJH recombination (19, 20). Another major cis-acting element is the 3′ regulatory region (3′RR), composed of four enhancers lying downstream of the IgH locus (21). Targeted deletion studies showed that the 3′RR affected IgM expression in resting B cells (22, 23), but its role in allelic exclusion is unknown.
Mature B cells have the unique ability to undergo an additional recombination-mediated diversification process through class switch recombination (CSR), which specifically targets the constant genes of the IgH locus. At the genomic level, CSR occurs between highly repetitive switch (S) sequences, located upstream of the constant genes. CSR thus enables activated B cells to switch from the expression of IgM to the expression of downstream isotypes (IgG, IgE, or IgA) (24). Determining whether these isotypes can replace IgM in promoting B cell development and allelic exclusion was approached by using transgenic mice or mice with an engineered endogenous IgH locus which express IgG1 or IgA, but this led to conflicting results (e.g., see references 25 to 29; discussed in references 27 and 28).
In this study, we analyzed a mouse line with a duplicated Eμ enhancer in the context of IgG3-driven B cell development. We show that duplication of Eμ leads to a phenomenon that we term cis exclusion, as distinct from allelic exclusion, by which the μ heavy chain gene from the mutant allele is stably and specifically excluded in cis.
MATERIALS AND METHODS
Mice.
The generation of the mutant mouse line is described in the supplemental material. B1-8 mice were provided by K. Rajewsky. The experiments on mice were carried out according to the CNRS ethical guidelines and were approved by the Regional (Midi-Pyrénées) Ethical Committee.
Flow cytometry analyses.
Sample preparation, staining, and antibodies and their sources are described in the supplemental material.
V(D)J rearrangement assays.
B cells from bone marrows were first sorted by using CD19-magnetic microbeads and LS columns (Miltenyi) and then labeled with anti-B220, anti-CD43, and anti-IgM, anti-IgG3, or anti-κ. The sorted pro-B (IgM− B220+ CD43high [wild type {WT}], IgG3− B220+ CD43high [A150], or κ− B220+ CD43high [WT and A150]) and pre-B (IgM− B220+ CD43low [WT], IgG3− B220+ CD43low [A150], or κ− B220+ CD43low [WT and A150]) cell fractions were harvested, and genomic DNAs were prepared by using a Puregene core kit A (Qiagen). Splenic B cells were negatively sorted by using CD43-magnetic microbeads and LS columns (Miltenyi). Genomic DNAs were resuspended and diluted for the PCR assay. The primers, normalization of amounts of DNA, and quantification were described previously (14, 30). Additional primers are listed in Table S3 in the supplemental material. The purity of the sorted populations was checked by fluorescence-activated cell sorter (FACS) analysis and by the rearrangement status of the κ locus.
Reverse transcription-PCR (RT-PCR).
B cell precursors from Rag2−/− bone marrows were sorted using CD19-magnetic microbeads (Miltenyi). Pro-B and pre-B cells were sorted as described above. Bone marrow IgMb+ and IgG3+ single expressers were sorted after staining with anti-B200, anti-IgMb, and anti-IgG3. Total RNA was reverse transcribed (Invitrogen) and subjected to semiquantitative PCR or real-time PCR. The primers, normalization, and quantification were described previously (14, 30). Additional primers are listed in Table S3 in the supplemental material.
Statistical analysis.
Results are expressed as means ± standard errors of the means (SEM) (GraphPad Prism), and overall differences between values from WT and mutant mice were evaluated by two-tailed unpaired Student's t test with Welch's correction. The difference between means is significant if the P value is <0.05, very significant if the P value is <0.01, and extremely significant if the P value is <0.001.
RESULTS
Replacement of the Iγ3 germ line promoter by a prerearranged V(D)J-Eμ cassette.
We previously showed that replacement of the Iγ3 germ line promoter with the Iγ1 promoter insulated ectopic Iγ1 but not endogenous Iγ1 from the 3′RR, suggesting a preferential interaction of the 3′RR with the most proximal promoter during CSR (31). Recent studies detected stable interactions between Eμ and 3′RR in Rag-deficient pro-B cells (18, 19, 32, 33). If the 3′RR indeed cooperates with Eμ in early B cell development, then ectopic, 3′RR-proximal Eμ may similarly insulate the endogenous Eμ enhancer and potentially affect IgH recombination/expression and B cell development. We resorted to a replacement approach whereby the Iγ3 promoter was replaced by a VHDJH2-JH3-JH4-Eμ unit, leaving intact the known cis elements required for V(D)J recombination and IgH expression and enabling premature expression of the γ3 heavy chain. The mutant line is called A150 henceforth (see Fig. S1 in the supplemental material).
B cell development under conditions of allelic inclusion, allelic exclusion, and isotypic competition.
We first analyzed the effect of premature expression of IgG3 on B cell development in various compartments of homozygous mice by using FACS. A striking and constant finding was that IgM-expressing B cells were barely detectable (<1%) in every compartment analyzed, while IgG3-expressing B cells formed the vast majority of B cell populations (see Fig. S2 in the supplemental material).
The absence of IgM expression in A150/A150 mice, together with previous findings that B cells expressing class-switched isotypes were poor competitors of IgM-expressing B cells in heterozygotes (28, 29), led us to investigate the effect of the mutation on IgM expression. In the A150 model, this unfolds into three lines of inquiry: the effect of the mutation on IgM expression from the mutant allele, potential allelic inclusion, and allelic exclusion.
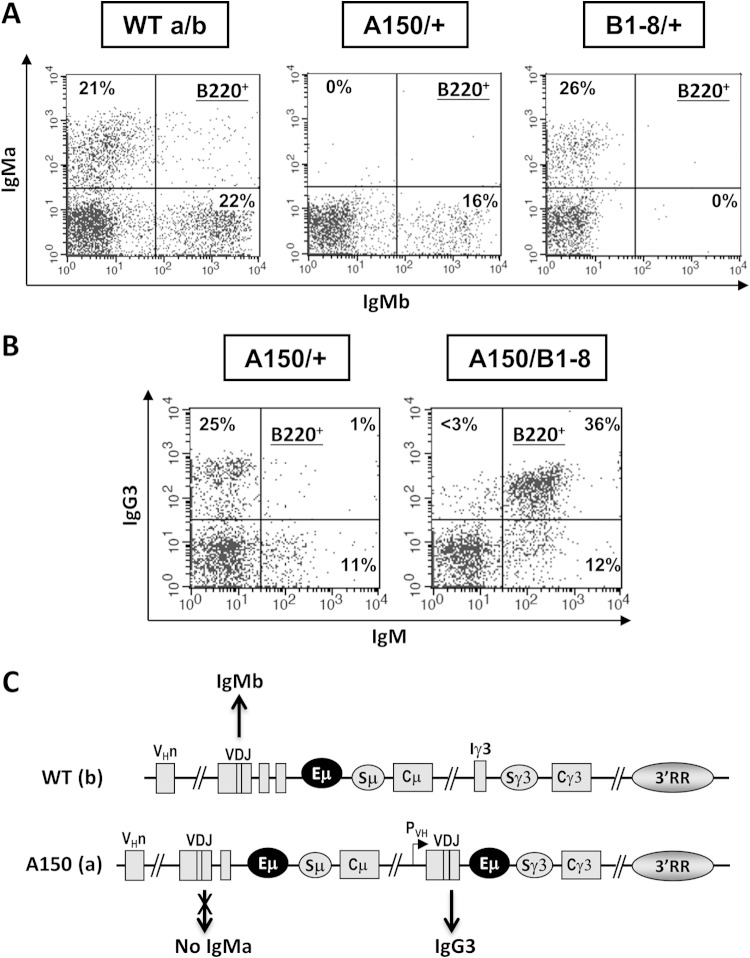
We used heterozygotes in which the WT allele was derived from a C57BL/6 mouse strain expressing the IgMb allotype. As an additional control, we used the B1-8 mouse line, which allows premature expression of IgMa and a nearly perfect allelic exclusion (34). In WT controls, IgMa+ and IgMb+ B cell populations were equally abundant in the bone marrow, whereas in B1-8/WT heterozygotes, the WT allele was completely excluded (Fig. 1A). Strikingly, surface IgMa was totally absent in the bone marrow of A150/WT mice (Fig. 1A and C), indicating a perfect exclusion of the mutant allele in regard to IgM expression.
FIG 1.
Flow cytometric analyses of allelic exclusion, allelic inclusion, and isotypic competition. (A) Single-cell suspensions from the bone marrows of mice with the indicated genotypes were stained with anti-B220 and monoclonal antibodies against the IgMa and IgMb allotypes and then gated on the B220+ population. Representative plots are shown (n = 5). (B) Cells from the bone marrows of mice with the indicated genotypes were stained with anti-B220, anti-IgM, and anti-IgG3 and then gated on the B220+ population. Representative plots are shown (n = 4). (C) Schematic recapitulating the FACS data for A150/+ mice. IgM is produced only from the wild-type allele. From the mutant allele, only IgG3 is expressed.
To investigate whether IgG3-expressing B cells can compete with IgM-expressing B cells, we looked at IgM and IgG3 surface expression patterns in the bone marrow of A150/WT and A150/B1-8 mice. In A150/WT mice, the WT allele must undergo productive V(D)J assembly in order to express IgM, whereas in A150/B1-8 mice, the B1-8-derived allele bears a prerearranged VDJ gene which enables premature expression of IgM (34). Hence, with these two models, we can correlate the isotypic competition status with allelic inclusion and/or V(D)J recombination.
The data show that B cells that prematurely express IgG3 can outcompete IgM-expressing B cells in A150/WT mice, since in the bone marrow, ∼25% of B cells express exclusively IgG3, and ∼11% express IgM only (Fig. 1B). In A150/B1-8 mice, in which both isotypes are prematurely expressed, the majority of B cells were subjected to allelic inclusion and coproduced IgM and IgG3 in the bone marrow and the spleen (Fig. 1B; see Fig. S2N in the supplemental material).
Interestingly, very few IgG3+ IgM+ double producers (<1%) were detected in the bone marrow of A150/WT mice, indicating that prematurely expressed IgG3 could mediate allelic exclusion (Fig. 1B) (see Discussion).
Together, the data show that the replacement mutation leads to a complete exclusion of IgM expression specifically from the mutant allele and that the prematurely expressed γ3 heavy chain can mediate robust allelic exclusion. In a context where the WT allele has to undergo V(D)J recombination, B cells that prematurely express IgG3 outcompete IgM-expressing B cells. Moreover, IgG3 and IgM can efficiently drive B cell development under conditions of allelic inclusion.
Reduction of proximal VH-DJH recombination and inhibition of distal VH-DJH recombination.
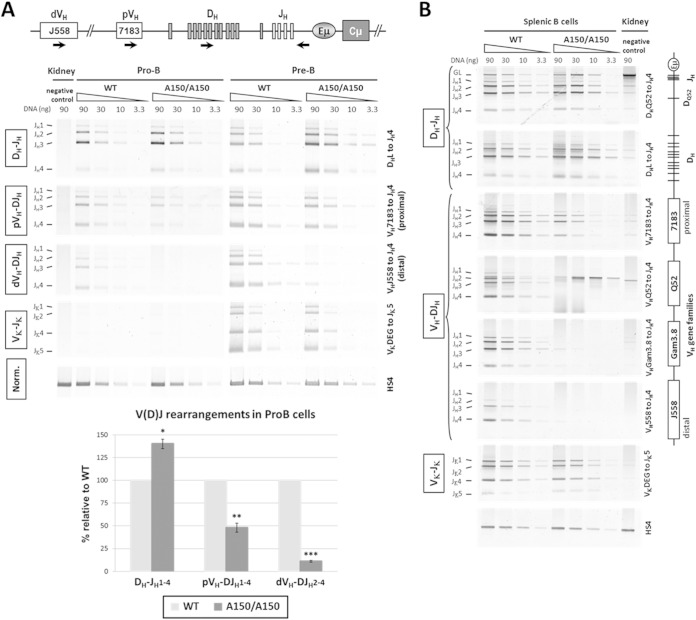
The complete exclusion of IgM expression from the A150 mutant allele led us to suspect the involvement of mechanisms that act in cis, specifically the duplication of the Eμ enhancer, which may affect V(D)J recombination. In order to understand the molecular basis of cis exclusion, we performed a V(D)J recombination assay on sorted pro-B, pre-B, and splenic cells from homozygous A150 mice. This allowed us to investigate more precisely whether and at which step the mutation affects V(D)J assembly, independently of the V(D)J recombination events and allelic competition that occur in heterozygous mice.
We used a primer that pairs downstream of the JH4 segment and primers that bind different VH and D gene segment families. We found a slight accumulation of DJH recombination intermediates in A150 pro-B cells. Interestingly, proximal VH-DJH recombination was reduced and distal VH-DJH recombination almost inhibited in pro-B cells, and only low levels of distal VH-DJH recombination were detected in pre-B cells (Fig. 2A). Vκ-Jκ recombination was unaffected, indicating that the mutation did not interfere with the developmental stage-dependent retargeting of the RAG complex to the κ locus (Fig. 2A). In the spleen, while randomization of the repertoire was obvious in WT controls, VH-DJH rearrangements involving VH gene segment families located upstream of the proximal VH7183 family were essentially absent in A150 spleens (Fig. 2B).
FIG 2.
V(D)J rearrangement analyses. (A) Genomic DNAs were prepared from sorted pro-B and pre-B cells and were subjected to semiquantitative PCR to amplify D-JH, VH-DJH, and Vκ-Jκ rearrangements, using primers that bind the indicated segments and primers that pair 3′ of JH4 (for the IgH locus) or 3′ of Jκ5 (for the κ locus). Kidney DNA was used as a negative control. A PCR analysis of the HS4 enhancer from the 3′RR was used for normalization of DNA input. The PCR for VκJκ rearrangement also allowed us to check the purity of the sorted populations (n = 3 for each experiment, with a pool of >5 mice). Quantification of the recombination events at the IgH locus of pro-B cells is displayed in the histogram. (B) Genomic DNAs were prepared from sorted splenic B cells and subjected to PCR as described for panel A (n = 2). The relative positions of the VH gene families are indicated on the right. The signals detected for the VHQ52 gene segment in the A150 lanes were derived from the inserted VH gene. Data are presented as means ± SEM. ***, P < 0.001; **, P < 0.01; *, P < 0.05.
Thus, the mutation leads to a mild accumulation of DJH alleles, a reduced amount of proximal VH-DJH recombination, and a nearly complete inhibition of distal VH-DJH recombination. We concluded that cis exclusion of IgM in A150 mice is mediated, at least in part, by reduced proximal VH-DJH recombination and a profound impairment of distal VH-DJH recombination.
Duplication of the Eμ enhancer severely affects distant germ line transcription.
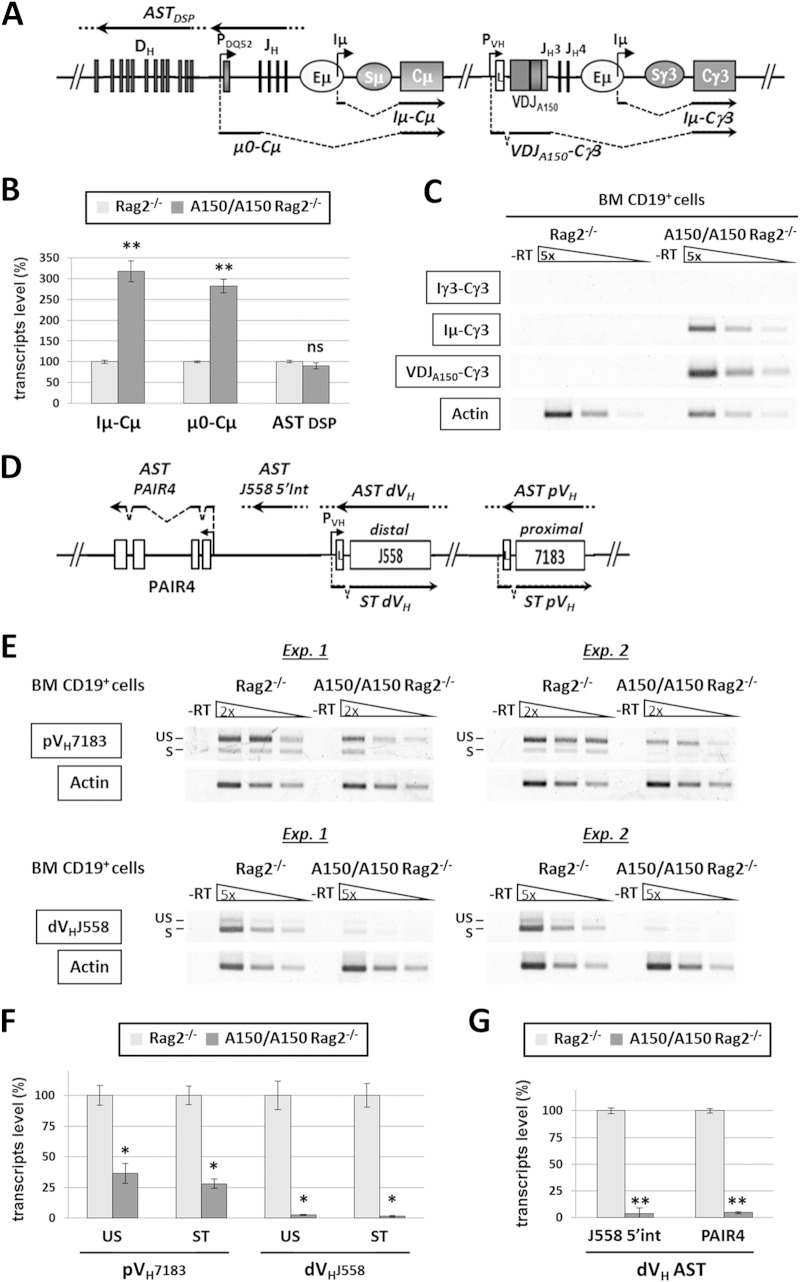
Given the impaired VH-DJH recombination seen in A150 mice, and since sense and antisense germ line transcription precedes VH-DJH recombination (35, 36), we investigated the effect of Eμ duplication on germ line transcription. To this end, the A150 mutation was brought into the Rag2-deficient background, which precludes V(D)J recombination. Total RNA was extracted from bone marrow CD19+ cells and analyzed by semiquantitative or real-time RT-PCR.
The data show that there were increased levels of the endogenous μ0 and Iμ sense transcripts, derived from the DQ52 promoter and the Eμ enhancer, respectively. In contrast, antisense transcripts across the DH cluster were unaffected (Fig. 3A and B). Iμ-Cγ3 germ line transcripts and mature VDJA150-Cγ3 transcripts, derived from the ectopic Eμ and PVH promoters, respectively, were readily detected in Rag2-deficient A150 pro-B cells (Fig. 3A and C). Interestingly, within the proximal VH domain, both sense and antisense transcript levels were reduced but were readily detectable. In contrast, both sense and antisense transcripts were barely detectable within the distal VH domain (Fig. 3D to G).
FIG 3.
Analysis of germ line transcription in A150 B cell precursors. (A) Schematic showing the germ line and V(D)JA150 transcripts analyzed at the D-Cμ domain and at the mutated γ3 constant gene of the mutant IgH locus. (B) CD19+ cells were sorted from the bone marrows (BM) of Rag2−/− and A150/A150 Rag2−/− mice, and quantitative RT-PCR was performed on total RNA. The Gapdh and Ywhaz transcripts were used for normalization. Iμ, μ0, and DSP transcript levels were quantified by setting the corresponding transcript levels in Rag2−/− controls as 100% (n = 4). (C) Analysis of germ line and coding transcripts at the ectopic transcription unit by semiquantitative RT-PCR. Actin transcripts were used for normalization (n = 4). (D) Schematic indicating the relative positions of the transcripts analyzed within the variable region. AST, antisense transcripts; ST, spliced transcripts (sense). (E) Analysis of proximal (pVH7183) and distal (dVHJ558) germ line transcripts by semiquantitative RT-PCR. Results of two independent experiments are shown. Actin transcripts were used for normalization (n = 4). S, spliced transcripts; US, unspliced (antisense/primary sense) transcripts. (F) Quantification of sense and antisense transcript levels as assayed by semiquantitative RT-PCR in panel E. (G) Quantification of intergenic antisense transcripts within the VHJ558 cluster and of PAX5-activated intergenic repeat 4 antisense transcripts by quantitative RT-PCR. The Gapdh and Ywhaz transcripts were used for normalization (n = 4). Data are presented as means ± SEM. **, P < 0.01; *, P < 0.05; ns, not significant.
The data show that both noncoding and coding transcripts, derived from the ectopic Eμ enhancer and the PVH promoter, respectively, are already produced in Rag2-deficient pro-B cells. Importantly, the replacement mutation leads to a downregulation of germ line transcription within the proximal VH domain and to an almost complete inhibition of germ line transcription within the distal VH domain.
Reduced transcription of the endogenous μ heavy chain gene during cis exclusion.
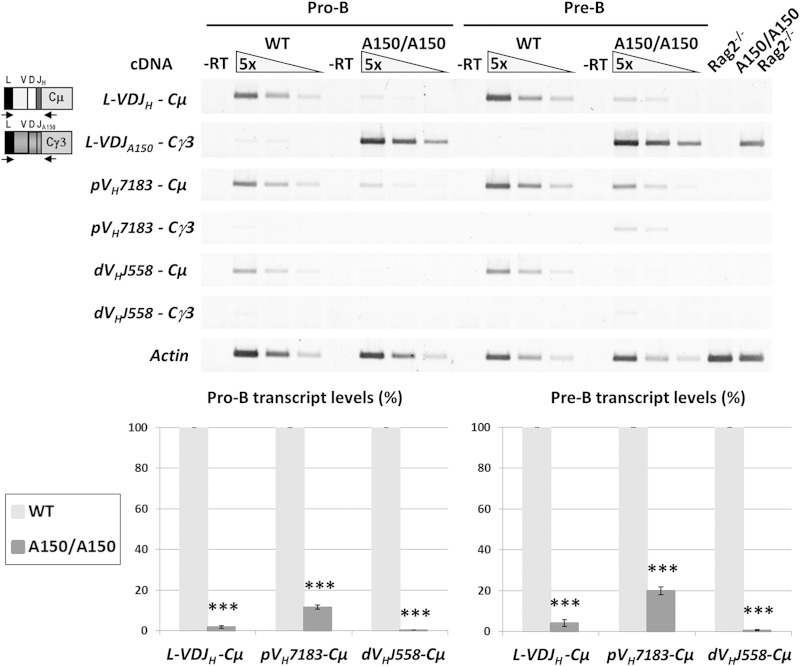
In order to investigate the potential transcriptional basis of IgM cis exclusion, we analyzed mature transcripts produced by the rearranged mutant allele. Total RNAs were prepared from sorted pro-B and pre-B cells and analyzed by semiquantitative RT-PCR.
By using primers that bind the leader sequence of the inserted VH segment and the Cγ3-1 exon, γ3 transcripts were readily detected in A150 pro-B and pre-B cells (Fig. 4). With a Cμ1 reverse primer, μ transcripts were detected in WT pro-B and pre-B cells. In contrast, very low levels of these transcripts were detected in A150 pro-B and pre-B cells. Proximal VH-containing γ3 transcripts were undetectable in WT controls, and only a faint signal was detected in A150 pre-B cells (Fig. 4). Interestingly, proximal VH-containing μ transcripts were detected in both WT and A150 mice, but their levels were decreased ∼10 times and ∼5 times in mutant pro-B and pre-B cells, respectively (Fig. 4). Consistent with the inhibition of distal VH-DJH recombination, distal VH-containing μ transcripts were barely detectable (Fig. 4).
FIG 4.
Analysis of μ and γ3 heavy chain transcripts in A150 pro-B and pre-B cells. Pro-B and pre-B cells were sorted from the bone marrows of WT and A150 mice, and RT-PCR was performed on total RNA. A specific primer that binds the leader sequence of the inserted VH segment (schematics on the left), degenerate primers that bind the VH7183 or VHJ558 gene family, and specific Cμ1 or Cγ3-1 reverse primers were used to amplify the mature VDJ-Cμ and VDJ-Cγ3 transcripts. The actin transcripts were used for normalization of single-stranded DNA input. Single-stranded cDNAs from Rag2−/− and A150/A150 Rag2−/− mice were included as controls. The corresponding histograms are shown (n = 2 for each experiment, with a pool of >3 mice). ***, P < 0.001.
We concluded that cis exclusion of IgM correlates with a markedly decreased transcription of the endogenous proximal VH-containing μ genes and with a virtual absence of distal VH-containing μ gene expression.
Restored transcription of rearranged genes upon ectopic V(D)J recombination and deletion of the endogenous Eμ enhancer.
Competition experiments showed that ∼1/10 of heterozygous A150/WT B cells expressed IgM only (Fig. 1). This observation, together with the fact that IgMa expression was totally excluded from the mutant allele and the γ3 heavy chain gene was already expressed in B cell precursors, led us to explore the mechanisms that switch off IgG3 production in the IgMb+ population. Among several possibilities (see Discussion and the supplemental material), we considered a deletional process that may involve either V(D)J recombination or CSR in pro-B/pre-B precursors of the IgMb+ population. Indeed, early transcription of the ectopic JH3 and JH4 RSSs and of Sγ3 (Fig. 3A) may provide accessible substrates for at least low levels of V(D)J recombination and Sμ/Sγ3 CSR, respectively. The data show that the loss of IgG3 expression in the IgMb+ population is due mainly to an ectopic V(D)J recombination which targets ectopic JH3 or JH4 RSSs (see Fig. S3 and S4 in the supplemental material).
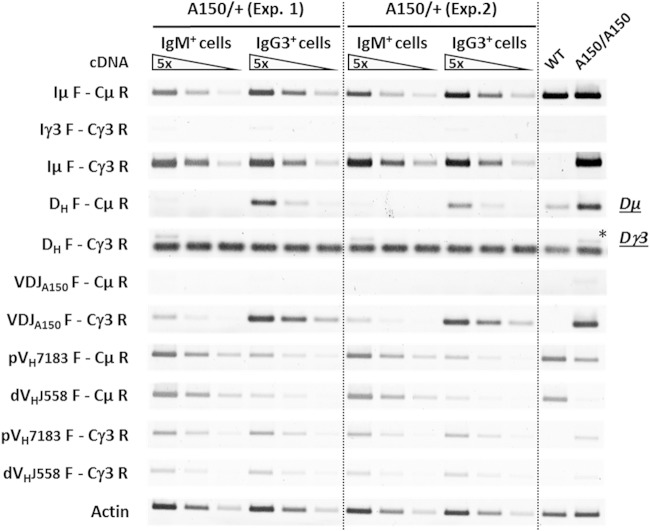
Upon D-JH recombination, Dμ transcripts derived from the promoter of the recombined D segment are produced (37, 38). We postulated that if ectopic D-JH recombination occurred in pro-B precursors, one might detect Dγ3 transcripts in the IgMb+ population. While Dμ transcripts were detected in both populations, albeit at severely decreased levels, as expected, in the IgMb+ fraction, Dγ3 transcripts were detected exclusively in the IgMb+ population (Fig. 5), confirming the occurrence of ectopic D-JH recombination on at least a fraction of A150 alleles and hinting at the possibility that another fraction had undergone ectopic VH-DJH recombination. In this case, proximal VH- and, potentially, distal VH-containing γ3 transcripts should be detected.
FIG 5.
Transcription analysis upon ectopic V(D)J recombination in heterozygotes. IgM+ and IgG3+ populations were sorted from the bone marrows of A150/WT mice, and RT-PCR was performed on total RNA. Specific primers were used to amplify the indicated spliced transcripts. Dμ and Dγ3 transcripts, resulting from endogenous DJH and ectopic DJH recombination intermediates, respectively, are indicated. Controls included the corresponding transcripts from WT and A150/A150 mice. The actin transcripts were used for normalization of single-stranded DNA input. The data shown are the results of two independent sorting procedures and experiments (pools of 4 mice).
In the IgG3+ population, abundant mature VDJA150-Cγ3 transcripts were detected. In contrast, lower transcript levels were found in the IgMb+ population, indicating that a subset of this population has an intact VDJA150 unit (Fig. 5; see Fig. S3 and S4 in the supplemental material). The proximal VH- and distal VH-containing μ transcripts were detected in both populations but were less abundant in the IgG3+ population (Fig. 5). Interestingly, roughly equal levels of proximal VH-containing γ3 transcripts were detected in the IgMb+ and IgG3+ populations, suggesting that ectopic proximal VH-DJH recombination and expression occurred on subsets of A150 alleles of the two populations (Fig. 5). Strikingly, while very low levels of distal VH-containing γ3 transcripts were detected in A150/A150 controls (Fig. 4 and 5), they were now readily detectable in both the IgMb+ and IgG3+ populations, indicating that a fraction of these populations had undergone ectopic distal VH-DJH recombination and accumulated higher levels of μ transcripts.
Cloning and sequencing of proximal VH- and distal VH-containing γ3 cDNAs from the IgG3+ population revealed an overwhelming majority (>95%) of productive rearrangements. In contrast, the majority of proximal VH-containing γ3 (∼59%) and distal VH-containing γ3 (∼76%) cDNA sequences from the IgMb+ population revealed nonproductive rearrangements. Within the limits of our data set, no evidence for recombination with cryptic RSSs was found (see Fig. S5 and Table S1 in the supplemental material).
The data show that the loss of IgG3 expression in the relatively small IgMb+ population of A150/WT bone marrow results mainly from an ectopic V(D)J recombination on the A150 allele. Importantly, the transcripts that were severely decreased (proximal VH-containing γ3 transcripts) or virtually inhibited (distal VH-containing γ3 transcripts) in homozygous A150 B cells appeared to be restored upon ectopic V(D)J recombination and associated deletion of endogenous Eμ.
DISCUSSION
A striking finding of this study is that the replacement mutation leads to a complete and stable cis exclusion of the μ gene specifically on the mutant allele. This pattern arose during ontogeny, was also established in the bone marrow, and was maintained in the lymphoid compartments of the adult mouse. In no instance did we detect IgM expression, or IgM and IgG3 coexpression, from the mutant allele. Only in heterozygous mice was IgM detected, but in this context, IgM was produced by the wild-type allele in WT/A150 mice and by the B1-8 allele in B1-8/A150 mice.
In B1-8/A150 mice, in stark contrast to results for WT/A150 mice, the majority of B cells were subject to allelic inclusion, and the ratio of double producers to single producers was even higher in the spleen, suggesting that two different isotypes with allelic inclusion can drive B cell development and may confer a selective advantage over single producers. The possibility of B cell development under conditions of allelic inclusion of two different μ heavy chains was reported previously (34). Our findings extend this notion to different isotypes, with potentially different specificities.
Interestingly, we found that about 1/10 of WT/A150 bone marrow B cells were IgM single producers. This is more remarkable if one takes into account that only 1/3 of translational reading frames allow the production of μ heavy chains from the WT allele. How, then, are IgM-only-expressing B cells generated, while the γ3 heavy chain is presumably constitutively expressed? A mechanism such as an induction of apoptosis of B cells that coproduce IgM and IgG3 is unlikely; the phenotype of B1-8/A150 mice argues against it. Alternatively, signals transmitted through the μ pre-B cell receptor (pre-BCR) may silence γ3 heavy chain gene expression on the alternate allele at the transcriptional level. This is unlikely, because of the biallelic nature of IgH gene transcription (39–41). Additionally, signaling through the pre-BCR downregulates the expression of surrogate light chains but not that of IgH genes (42). Finally, if the μ pre-BCR silenced γ3 heavy chain gene expression at the transcriptional level, it would be apparent in B1-8/A150 mice, which is clearly not the case.
Instead, the data point to a deletional process. Several mechanisms can be invoked, including V(D)J recombination, VH replacement, and CSR. We found no evidence for VH replacement, and only very low (if any) levels of CSR were detected, making it difficult to quantify its real contribution. In contrast, the vast majority of deletional events involved an ectopic V(D)J recombination. Thus, the inserted unit in the IgH constant region provides sufficient elements for chromatin opening, transcription, targeting, and activity of the RAG complex (43). This suggests that the ectopic JH segments are brought into close proximity of endogenous D segments for ectopic V(D)J recombination to occur, implying that the endogenous and ectopic Eμ enhancers are positioned nearby (see below).
For WT/A150 mice, we cannot, at first glance, ascertain whether cis exclusion results from allelic exclusion or from a combination of both allelic exclusion and γ3 heavy chain-mediated signaling. In homozygotes, cis exclusion may also result from γ3 heavy chain-mediated feedback inhibition. However, we think that cis exclusion is likely to result from mechanisms that act in cis. Indeed, it would be difficult to figure out why (i) in heterozygous mice, γ3 heavy chain-mediated signaling would lead to such perfect exclusion of the μ allele precisely on the mutant chromosome but not on the WT allele; and (ii) in homozygous mice, none of the productive endogenous rearrangements [i.e., not involving Cμ deletion through ectopic V(D)J recombination] led to IgM expression. These remarks rather point to an effect which likely results from Eμ duplication and a potential perturbation of Eμ interactions.
How relevant is cis exclusion to genuine allelic exclusion? Remarkably, cis exclusion recapitulates on the very same allele the main known features of allelic exclusion regarding VH gene family restriction. Indeed, various studies have shown that allelic exclusion of proximal VH genes is less stringent than that of distal VH genes, especially for the most proximal VH genes (e.g., see references 19, 44, and 45). Consistent with these findings, we found that distal VH-DJH recombination was virtually inhibited and proximal VH-DJH recombination was only reduced. Moreover, the differential recombination of proximal versus distal VH genes correlated well with the levels of their corresponding sense and antisense germ line transcripts. This correlation between germ line transcription and cis exclusion is also relevant to allelic exclusion. The developmentally regulated sense and antisense transcription of the VH region occurs following D-JH recombination, likely to provide accessible substrates for VH-DJH recombination (35, 36). The correlation that we found between VH recombination and germ line transcription during cis exclusion is consistent with a model in which sense and antisense transcription within the VH region is downregulated by allelic exclusion, not by the recombination event itself (36). In support of this notion, distal VH transcription was already inhibited on the cis-excluded allele in the absence of detectable distal VH-DJH recombination. The latter finding strongly suggests that the control of germ line transcription is the primary event during allelic exclusion.
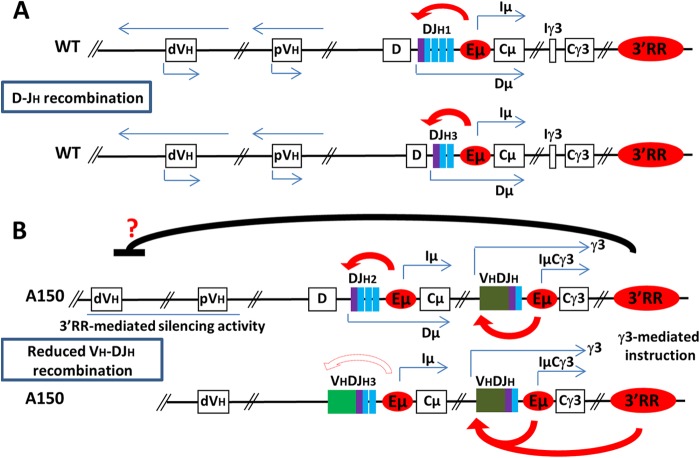
With regard to VH germ line transcription, an important issue is to explain why sense and antisense transcription is affected within the VH domain. It is plausible that, similar to findings for μ-transgenic mice (46), the development of A150 mutant B cells is accelerated such that there is not enough time for the opening of the variable region's chromatin. We cannot formally exclude this possibility, although we note that in A150 mice, D-JH rearrangements occurred (with even a slight accumulation of DJH intermediates) at the right developmental stage (pro-B stage), in contrast to μ-transgenic mice, in which D-JH rearrangements occurred mostly at the pre-B stage (46). We rather favor the view that impairment of VH germ line transcription in A150 mice results from altered interactions between cis-regulatory elements. In this context, it was shown that distal VH transcription was Eμ independent, while transcription of the most proximal VH genes was affected by Eμ (11–14, 18, 47). Our interpretation is that these defects largely (but not exclusively) reflect a transcriptional silencing activity which is mediated by the other master cis-acting element of the IgH locus, namely, the 3′RR. This is consistent with our recent finding that in the absence of the 3′RR, germ line transcription is upregulated along the VH domain and distal VH-DJH recombination is enhanced, which led us to propose that a productive rearrangement on one allele instructs the 3′RR to inhibit germ line transcription within the VH region on the second allele (F. Z. Braikia and A. A. Khamlichi, submitted for publication) (see the model in Fig. 6). Within the time window when the 3′RR mediates its silencing activity within the VH domain, the Eμ enhancer mainly focuses on DJH transcription (14).
FIG 6.
Model for the transcriptional control of allelic exclusion. The model shown represents a speculative view of how allelic exclusion is regulated through an interplay between the Eμ enhancer, the 3′RR, and signaling. For the sake of clarity, only the interactions between the Eμ enhancer and the 3′RR are highlighted. Other cis-regulatory elements (such as IGCR1) which also play an important role in allelic exclusion are not shown. (A) In this model, a productive rearrangement on one allele instructs the 3′RR on the second allele to mediate a transcriptional silencing activity within the VH region, leading to downregulation of sense and antisense transcription and VH-DJH recombination. (B) In A150 mice, the prerearranged VDJ gene in the constant region allows for premature production of the γ3 heavy chain, which instructs the 3′RR to mediate its silencing activity within the VH region, leading to an impairment of VH-DJH recombination. The 3′RR preferentially interacts with the proximal, ectopic Eμ enhancer to boost γ3 gene expression. In the absence of endogenous Eμ/3′RR cooperation, rearranged μ gene transcription is too low to allow robust signaling (see the text for details). pVH, proximal VH cluster; dVH, distal VH cluster.
Thus, duplication of the Eμ enhancer appears not to affect the 3′RR-mediated silencing activity within the distal VH domain. In contrast, cooperation between the endogenous and ectopic Eμ enhancers may explain the increased levels of Iμ and μ0 germ line transcripts, implying that the two enhancers lie in close proximity prior to D-JH recombination. The occurrence of ectopic D-JH recombination involving synapsis and ligation of an endogenous D segment and a remote ectopic JH segment provides an indirect support of this interpretation. This is also consistent with the finding that the 3′RR is dispensable for germ line transcription within the D-Cμ domain (Braikia and Khamlichi, submitted).
Why is transcription of μ genes reduced on the cis-excluded allele? Our findings suggest that duplication of the Eμ enhancer does not necessarily lead to upregulation of μ gene expression. A likely explanation is that following VH-DJH recombination, the 3′RR preferentially interacts with the most proximal, ectopic Eμ enhancer (Fig. 6). Interestingly, when the Iγ3 germ line promoter was replaced with the Iγ1 germ line promoter, transcription from ectopic Iγ1 but not from the endogenous 3′RR-proximal Iγ1 was reduced (31). Although this finding is indirect, as that study was performed on splenic B cells, it suggests that when upstream duplicated targets are available, the 3′RR prominently interacts with the most proximal target.
Significantly, any ectopic V(D)J recombination on the A150 allele deletes the endogenous Eμ enhancer. Ectopic Eμ, in cooperation with the 3′RR, would thus mediate a high-level expression of ectopically rearranged proximal VH- and distal VH-containing γ3 genes. The relatively higher levels of their corresponding transcripts, though they occur in only a fraction of heterozygous B cells, provide a physiological support of this model. Moreover, we recently showed that upon completion of V(D)J recombination, the 3′RR shifts from a transcriptional silencer to an enhancer of both Eμ- and PVH-derived transcription (Braikia and Khamlichi, submitted). Thus, the Eμ enhancer likely requires the cooperation of the 3′RR for high-level expression of rearranged heavy chain genes, which would be essential for the strength of signaling required for the maintenance of allelic exclusion.
In conclusion, the cis exclusion reported here recapitulates essential features of allelic exclusion and thus provides important insights into the transcriptional and recombinational mechanisms that underlie genuine allelic exclusion. Distal VH-DJH recombination is virtually inhibited, while proximal VH-DJH recombination is only reduced. This correlates perfectly with an inhibition of distal VH transcription and a reduced proximal VH transcription. The developmental stage-dependent dynamics of Eμ-3′RR interaction (though by no means only this) may play a critical role in allelic exclusion, in that, as instructed by a productive rearrangement, the 3′RR downregulates germ line transcription in the variable region of the excluded allele. On the productive allele, the 3′RR cooperates with the Eμ enhancer to achieve high expression levels to yield a robust signal for the maintenance of allelic exclusion. Thus, our study reveals that an intricate, developmental stage-dependent interplay between cis-acting elements and signaling plays an important role in the initiation and maintenance of allelic exclusion.
Supplementary Material
ACKNOWLEDGMENTS
We thank K. Rajewsky for kindly providing B1-8 mice. We thank the IPBS animal facility staff and F. L'Faqihi and V. Duplan-Eche at the Purpan CPTP platform for their excellent work.
C.L. and Z.O. were supported by fellowships from the MREN and the MREN, FRM, and EMBO-STF, respectively. This work was supported by the Fondation ARC (grant PJA 20141201647), the Agence Nationale de la Recherche, the Institut National du Cancer, the Ligue contre le Cancer-Comité de Haute-Garonne, and the Cancéropôle Grand-Sud-Ouest.
N.P. and C.L. performed research; Z.O. and M.L.B. generated the A150 mouse line; M.M. managed the mouse lines; N.P., C.L., and A.A.K. analyzed data; and A.A.K. designed research and wrote the paper.
We declare that we have no conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.00294-15.
REFERENCES
- 1.Jung D, Giallourakis C, Mostoslavsky R, Alt FW. 2006. Mechanism and control of V(D)J recombination at the immunoglobulin heavy chain locus. Annu Rev Immunol 24:541–570. doi: 10.1146/annurev.immunol.23.021704.115830. [DOI] [PubMed] [Google Scholar]
- 2.Schatz DG, Ji Y. 2011. Recombination centres and the orchestration of V(D)J recombination. Nat Rev Immunol 11:251–263. doi: 10.1038/nri2941. [DOI] [PubMed] [Google Scholar]
- 3.Bossen C, Mansson R, Murre C. 2012. Chromatin topology and the regulation of antigen receptor assembly. Annu Rev Immunol 30:337–356. doi: 10.1146/annurev-immunol-020711-075003. [DOI] [PubMed] [Google Scholar]
- 4.Seitan VC, Krangel MS, Merkenschlager M. 2012. Cohesin, CTCF and lymphocyte antigen receptor locus rearrangement. Trends Immunol 33:153–159. doi: 10.1016/j.it.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stubbington MJ, Corcoran AE. 2013. Non-coding transcription and large-scale nuclear organisation of immunoglobulin recombination. Curr Opin Genet Dev 23:81–88. doi: 10.1016/j.gde.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Johnston CM, Wood AL, Bolland DJ, Corcoran AE. 2006. Complete sequence assembly and characterization of the C57BL/6 mouse Ig heavy chain V region. J Immunol 176:4221–4234. doi: 10.4049/jimmunol.176.7.4221. [DOI] [PubMed] [Google Scholar]
- 7.Retter I, Chevillard C, Scharfe M, Conrad A, Hafner M, Im TH, Ludewig M, Nordsiek G, Severitt S, Thies S, Mauhar A, Blocker H, Muller W, Riblet R. 2007. Sequence and characterization of the Ig heavy chain constant and partial variable region of the mouse strain 129S1. J Immunol 179:2419–2427. doi: 10.4049/jimmunol.179.4.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vettermann C, Schlissel MS. 2010. Allelic exclusion of immunoglobulin genes: models and mechanisms. Immunol Rev 237:22–42. doi: 10.1111/j.1600-065X.2010.00935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ten Boekel E, Melchers F, Rolink AG. 1998. Precursor B cells showing H chain allelic inclusion display allelic exclusion at the level of pre-B cell receptor surface expression. Immunity 8:199–207. doi: 10.1016/S1074-7613(00)80472-0. [DOI] [PubMed] [Google Scholar]
- 10.Bergman Y, Cedar H. 2010. Epigenetic control of recombination in the immune system. Semin Immunol 22:323–329. doi: 10.1016/j.smim.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perlot T, Alt FW, Bassing CH, Suh H, Pinaud E. 2005. Elucidation of IgH intronic enhancer functions via germ-line deletion. Proc Natl Acad Sci U S A 102:14362–14367. doi: 10.1073/pnas.0507090102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Afshar R, Pierce S, Bolland DJ, Corcoran A, Oltz EM. 2006. Regulation of IgH gene assembly: role of the intronic enhancer and 5′DQ52 region in targeting DHJH recombination. J Immunol 176:2439–2447. doi: 10.4049/jimmunol.176.4.2439. [DOI] [PubMed] [Google Scholar]
- 13.Chakraborty T, Perlot T, Subrahmanyam R, Jani A, Goff PH, Zhang Y, Ivanova I, Alt FW, Sen R. 2009. A 220-nucleotide deletion of the intronic enhancer reveals an epigenetic hierarchy in immunoglobulin heavy chain locus activation. J Exp Med 206:1019–1027. doi: 10.1084/jem.20081621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puget N, Hirasawa R, Nguyen Hu NS, Laviolette-Malirat N, Feil R, Khamlichi AA. 2015. Insertion of an imprinted insulator into the IgH locus reveals developmentally regulated, transcription-dependent control of V(D)J recombination. Mol Cell Biol 35:529–543. doi: 10.1128/MCB.00235-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li F, Eckhardt LA. 2009. A role for the IgH intronic enhancer E mu in enforcing allelic exclusion. J Exp Med 206:153–167. doi: 10.1084/jem.20081202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Degner SC, Wong TP, Jankevicius G, Feeney AJ. 2009. Cutting edge: developmental stage-specific recruitment of cohesin to CTCF sites throughout immunoglobulin loci during B lymphocyte development. J Immunol 182:44–48. doi: 10.4049/jimmunol.182.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Featherstone K, Wood AL, Bowen AJ, Corcoran AE. 2010. The mouse immunoglobulin heavy chain V-D intergenic sequence contains insulators that may regulate ordered V(D)J recombination. J Biol Chem 285:9327–9338. doi: 10.1074/jbc.M109.098251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Degner SC, Verma-Gaur J, Wong TP, Bossen C, Iverson GM, Torkamani A, Vettermann C, Lin YC, Ju Z, Schulz D, Murre CS, Birshtein BK, Schork NJ, Schlissel MS, Riblet R, Murre C, Feeney AJ. 2011. CCCTC-binding factor (CTCF) and cohesin influence the genomic architecture of the Igh locus and antisense transcription in pro-B cells. Proc Natl Acad Sci U S A 108:9566–9571. doi: 10.1073/pnas.1019391108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo C, Yoon HS, Franklin A, Jain S, Ebert A, Cheng HL, Hansen E, Despo O, Bossen C, Vettermann C, Bates JG, Richards N, Myers D, Patel H, Gallagher M, Schlissel MS, Murre C, Busslinger M, Giallourakis CC, Alt FW. 2011. CTCF-binding elements mediate control of V(D)J recombination. Nature 477:424–430. doi: 10.1038/nature10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin SG, Guo C, Su A, Zhang Y, Alt FW. 2015. CTCF-binding elements 1 and 2 in the IgH intergenic control region cooperatively regulate V(D)J recombination. Proc Natl Acad Sci U S A 112:1815–1820. doi: 10.1073/pnas.1424936112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khamlichi AA, Pinaud E, Decourt C, Chauveau C, Cogné M. 2000. The 3′ IgH regulatory region: a complex structure in a search for a function. Adv Immunol 75:317–345. doi: 10.1016/S0065-2776(00)75008-5. [DOI] [PubMed] [Google Scholar]
- 22.Pinaud E, Khamlichi AA, Le Morvan C, Drouet M, Nalesso V, Le Bert M, Cogné M. 2001. Localization of the 3′ IgH locus elements that effect long-distance regulation of class switch recombination. Immunity 15:187–199. doi: 10.1016/S1074-7613(01)00181-9. [DOI] [PubMed] [Google Scholar]
- 23.Vincent-Fabert C, Fiancette R, Pinaud E, Truffinet V, Cogné N, Cogné M, Denizot Y. 2010. Genomic deletion of the whole IgH 3′ regulatory region (hs3a, hs1,2, hs3b, and hs4) dramatically affects class switch recombination and Ig secretion to all isotypes. Blood 116:1895–1898. doi: 10.1182/blood-2010-01-264689. [DOI] [PubMed] [Google Scholar]
- 24.Stavnezer J, Guikema JE, Schrader CE. 2008. Mechanism and regulation of class switch recombination. Annu Rev Immunol 26:261–292. doi: 10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamura K, Kudo A, Ebihara T, Kamino K, Araki K, Kumahara Y, Watanabe T. 1986. Cell-type-specific and regulated expression of a human gamma 1 heavy-chain immunoglobulin gene in transgenic mice. Proc Natl Acad Sci U S A 83:2152–2156. doi: 10.1073/pnas.83.7.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roth PE, Doglio L, Manz JT, Kim JY, Lo D, Storb U. 1993. Immunoglobulin gamma 2b transgenes inhibit heavy chain gene rearrangement, but cannot promote B cell development. J Exp Med 178:2007–2021. doi: 10.1084/jem.178.6.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pogue SL, Goodnow CC. 2000. Gene dose-dependent maturation and receptor editing of B cells expressing immunoglobulin (Ig)G1 or IgM/IgG1 tail antigen receptors. J Exp Med 191:1031–1044. doi: 10.1084/jem.191.6.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waisman A, Kraus M, Seagal J, Ghosh S, Melamed D, Song J, Sasaki Y, Classen S, Lutz C, Brombacher F, Nitschke L, Rajewsky K. 2007. IgG1 B cell receptor signaling is inhibited by CD22 and promotes the development of B cells whose survival is less dependent on Ig alpha/beta. J Exp Med 204:747–758. doi: 10.1084/jem.20062024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duchez S, Amin R, Cogné N, Delpy L, Sirac C, Pascal V, Corthésy B, Cogné M. 2010. Premature replacement of mu with alpha immunoglobulin chains impairs lymphopoiesis and mucosal homing but promotes plasma cell maturation. Proc Natl Acad Sci U S A 107:3064–3069. doi: 10.1073/pnas.0912393107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haddad D, Oruc Z, Puget N, Laviolette-Malirat N, Philippe M, Carrion C, Le Bert M, Khamlichi AA. 2011. Sense transcription through the S region is essential for immunoglobulin class switch recombination. EMBO J 30:1608–1620. doi: 10.1038/emboj.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oruc Z, Boumédiène A, Le Bert M, Khamlichi AA. 2007. Replacement of Igamma3 germ-line promoter by Igamma1 inhibits class-switch recombination to IgG3. Proc Natl Acad Sci U S A 104:20484–20489. doi: 10.1073/pnas.0608364104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo C, Gerasimova T, Hao H, Ivanova I, Chakraborty T, Selimyan R, Oltz EM, Sen R. 2011. Two forms of loops generate the chromatin conformation of the immunoglobulin heavy-chain gene locus. Cell 147:332–343. doi: 10.1016/j.cell.2011.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medvedovic J, Ebert A, Tagoh H, Tamir IM, Schwickert TA, Novatchkova M, Sun Q, Huis In't Veld PJ, Guo C, Yoon HS, Denizot Y, Holwerda SJ, de Laat W, Cogne M, Shi Y, Alt FW, Busslinger M. 2013. Flexible long-range loops in the VH gene region of the Igh locus facilitate the generation of a diverse antibody repertoire. Immunity 39:229–244. doi: 10.1016/j.immuni.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sonoda E, Pewzner-Jung Y, Schwers S, Taki S, Jung S, Eilat D, Rajewsky K. 1997. B cell development under the condition of allelic inclusion. Immunity 6:225–233. doi: 10.1016/S1074-7613(00)80325-8. [DOI] [PubMed] [Google Scholar]
- 35.Yancopoulos GD, Alt FW. 1985. Developmentally controlled and tissue-specific expression of unrearranged VH gene segments. Cell 40:271–281. doi: 10.1016/0092-8674(85)90141-2. [DOI] [PubMed] [Google Scholar]
- 36.Bolland DJ, Wood AL, Johnston CM, Bunting SF, Morgan G, Chakalova L, Fraser PJ, Corcoran AE. 2004. Antisense intergenic transcription in V(D)J recombination. Nat Immunol 5:630–637. doi: 10.1038/ni1068. [DOI] [PubMed] [Google Scholar]
- 37.Reth MG, Alt FW. 1984. Novel immunoglobulin heavy chains are produced from DJH gene segment rearrangements in lymphoid cells. Nature 312:418–423. doi: 10.1038/312418a0. [DOI] [PubMed] [Google Scholar]
- 38.Alessandrini A, Desiderio SV. 1991. Coordination of immunoglobulin DJH transcription and D-to-JH rearrangement by promoter-enhancer approximation. Mol Cell Biol 11:2096–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delpy L, Sirac C, Le Morvan C, Cogné M. 2004. Transcription-dependent somatic hypermutation occurs at similar levels on functional and nonfunctional rearranged IgH alleles. J Immunol 173:1842–1848. doi: 10.4049/jimmunol.173.3.1842. [DOI] [PubMed] [Google Scholar]
- 40.Daly J, Licence S, Nanou A, Morgan G, Martensson IL. 2007. Transcription of productive and nonproductive VDJ-recombined alleles after IgH allelic exclusion. EMBO J 26:4273–4282. doi: 10.1038/sj.emboj.7601846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eberle AB, Herrmann K, Jack HM, Muhlemann O. 2009. Equal transcription rates of productively and nonproductively rearranged immunoglobulin mu heavy chain alleles in a pro-B cell line. RNA 15:1021–1028. doi: 10.1261/rna.1516409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melchers F. 2005. The pre-B-cell receptor: selector of fitting immunoglobulin heavy chains for the B-cell repertoire. Nat Rev Immunol 5:578–584. doi: 10.1038/nri1649. [DOI] [PubMed] [Google Scholar]
- 43.Ji Y, Little AJ, Banerjee JK, Hao B, Oltz EM, Krangel MS, Schatz DG. 2010. Promoters, enhancers, and transcription target RAG1 binding during V(D)J recombination. J Exp Med 207:2809–2816. doi: 10.1084/jem.20101136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Costa TE, Suh H, Nussenzweig MC. 1992. Chromosomal position of rearranging gene segments influences allelic exclusion in transgenic mice. Proc Natl Acad Sci U S A 89:2205–2208. doi: 10.1073/pnas.89.6.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roldán E, Fuxa M, Chong W, Martinez D, Novatchkova M, Busslinger M, Skok JA. 2005. Locus “decontraction” and centromeric recruitment contribute to allelic exclusion of the immunoglobulin heavy-chain gene. Nat Immunol 6:31–41. doi: 10.1038/ni1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang Y, Bosma MJ, Bosma GC. 1999. Extended duration of DH-JH rearrangement in immunoglobulin heavy chain transgenic mice: implications for regulation of allelic exclusion. J Exp Med 189:1295–1305. doi: 10.1084/jem.189.8.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bolland DJ, Wood AL, Afshar R, Featherstone K, Oltz EM, Corcoran AE. 2007. Antisense intergenic transcription precedes Igh D-to-J recombination and is controlled by the intronic enhancer Emu. Mol Cell Biol 27:5523–5533. doi: 10.1128/MCB.02407-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.