Abstract
This study was performed to evaluate sexual transmission of Toxoplasma gondii in mice. RH strain tachyzoites were intraperitoneally inoculated into 10 Balb/C male mice and after 48 h, their semen were collected from epididymis and examined by giemsa staining and PCR. Twenty Balb/C female mice mated with four infected male mice four times and any mating time was 48 h whilst 20 female control mice mated with four uninfected male mice for 8 days. Female mate choice was assessed using a three-chambered cage. Four female mice were placed in a central chamber and in one side of it, two infected male mice were kept and in other side, two naïve male mice were placed. Due each quarter, every of the female movement was reported and then the female was replaced to middle chamber. Besides on the detection of DNA and whole parasite in semen, no abortion and death was seen in female mice. Pregnancy was seen only 4 out of 20 female mice which mated with infected males while 17 pregnancies were seen from 20 control female mice (P value = 0.0001). No statistical significant was seen in female mate choice between naïve male (45 movement) and infected male (36 movement). This study showed that toxoplasmosis could not transmit to female mice and their offspring due to mating and the parasite had not effect on female mate choice. It seems that infected male mice cannot entirely mate with females due to reduction of male weapon and body size, physiological vigor and energy.
Keywords: Toxoplasma gondii, Sexual transmission, Behavior, Mice
Introduction
Toxoplasma gondii is an intracellular protozoon that is distributed throughout the world. This parasite can infect each nucleated cells of birds and mammals, including humans. In human, Toxoplasma infection symptoms are usually mild and include manifestations such as fever, malaise and lymphadenopathy. However, the protozoan can cause congenital abnormalities such as abortion, chorioretinitis, jaundice and hydrocephaly (Montoya and Liesenfeld 2004). The disease in immunocompromissed persons is also severe and estimated that 23 % of HIV positive persons develop toxoplasmic encephalitis (Oksenhendler et al. 1994). Moreover, T. gondii infection in livestock animals such as sheep and goats is responsible for considerable economic losses due to abortion of fetus (Smith and Reduck 2000). The prevalence of toxoplasmosis in Fars Province, Southern Iran was shown to be 14.02 % in caprine (Asgari et al. 2007), 26.5 % in ovine (Asgari et al. 2009), 20.24 % in bovine (Asgari et al. 2010) and 36.1 % in chicken (Asgari et al. 2006).
Infection is frequently acquired by ingestion of undercooked or raw meat that contains tissue cysts, food or other material contaminated with eliminating oocysts in the feces resulting from the sexual phase in the ileum epithelium of cat (Dubey and Jones 2008). In addition, the transmission may occur through blood transfusion, organ transplant, drinking of milk (Pereira et al. 2010).
Spence et al. (1978) noticed presence of the protozoan in semen of rams. The presence of the parasite has also been confirmed by bioassays, immunohistochemistry and PCR methods in semen of male dogs and rams experimentally infected with T.gondii (Vyas 2013). Moreover, it was shown that artificial insemination with contaminated semen could lead to toxoplasmosis in ewes (Moraes et al. 2010).
The present study was undergone to assessing the role of Toxoplasma tachyzoites in sexual transmission due to mating and evaluation of behavioral changes on female mate choice.
Materials and methods
Animal
Totally 78 Inbred Balb/C mice (44 female and 834 male mice) were provided from Pasteur Institute, Tehran, Iran at 6–8 weeks aged and 22–25 g weight. Animals were kept at temperature of 22 °C and 40–50 % relative humidity in Laboratory Animal Center of Shiraz University of Medical Sciences in Shiraz, Southern Iran. The procedures of all trials and sacrifice were identical for all animals. During the experiments from May to June 2012, animals were housed in cages and maintained under controlled conditions (21 ± 2 °C, 65–70 % humidity and standard food and water ad libitum) during the experiments. The experiments were undertaken based on guidelines of laboratory animals in research and teaching book (Akins et al. 2004).
Parasite
Toxoplasma gondii RH strain was obtained from Tehran University of Medical Sciences, Tehran, Iran. Tachyzoites were provided by serial passages in the peritoneal cavity of the mice. Seventy-two hours after injection, mice were euthanized and the tachyzoites were collected by repeated flushing of the peritoneal cavity with phosphate-buffered saline (PBS; pH 7.4) and adjusted to a concentration of 2.5 × 107/mL.
Semen and infected male mice
The semen samples of ten infected male mice were collected from epididymis after 48 h after inoculation. The samples were examined by giemsa staining and PCR. The male designed for mating were selected 48 h onwards intraperitoneal inoculation.
DNA extraction
For extraction of DNA, approximately 50 μL of the semen was homogenized and was diluted with double-distilled water (1:10). Proteinase K (10 μL) and lysis buffer (50 mL of Tris–HCl, pH 7.6; 1 mM of EDTA, pH 8.0; 1 % Tween 20) was added to 400 μL of each sample and the samples were incubated for 24 h at 37 °C. The lysate was then extracted twice with phenol/chloroform/isoamyl before the DNA was precipitated with absolute ethanol. The precipitated DNA was resuspended in 100 μL of double-distilled water and stored at 4 °C until use.
Nested PCR
Nested primer sets (Bioneer, Korea) were used for amplifying fragments of the B1 gene as described by (Jones et al. 2000). The external primers were 5′-GGA ACT GCA TCC GTT CAT GAG-3′ and 5′-TCT TTA AAG CGT TCG TGG TC-3′ producing an amplified product of 193 bp.
All the PCR reactions were performed in a programmable thermocycler (Eppendorph, Mastercycler gradient). The first 25 μL of PCR reaction mixture contained outer primers at a final concentration of 50 pmol each, 2.5 mmol dNTPs, 1 μg of template, and 1.5 U recombinant Taq DNA polymerase (GENET BIO, Korea, A-type Prime TaqTM DNA polymerase), in 1× PCR reaction buffer (50 mmol/L KCl and 10 mmol/L Tris–HCl, 1.5 mmol/L MgCl2, and 0.1 % Triton X-100; Sinagen Co., Iran). The first step of amplification was 5 min of denaturation at 94 °C. This step was followed by 40 cycles, with each cycle consisting of 10 s at 94 °C, 10 s at the annealing temperature (57 °C) for each pair of primers, and 30 s at 72 °C. The final cycle was followed by an extension step of 10 min at 72 °C.
Nested reactions contained 1 μL first-round product, 10 mM Tris–HCl, pH 8.3 (at 25 °C), 50 pmol each, 2.5 mmol dNTPs, 1 μg of template, and 1.5 U recombinant Taq DNA polymerase. Internal primers were 5′-TGCATAGGTTGCAGTCACTG-3′ and 5′-GGCGACCAATCTGCGAATACACC-3′ producing an amplified product of 96 bp.
Nested PCRs were cycled 40 times using a denaturation step of 93 °C for 10 s, followed by annealing at 62.5 °C for 10 s and extension at 72 °C for 15 s.
The amplification products were detected by gel electrophoresis using 2 % agarose gel in 1× Tris–borate–EDTA buffer. DNA bands were visualized in the presence of ultraviolet light, following the staining with 0.5 % ethidium bromide.
Mating
Five sexually naive female and one sexually experienced infected male mouse were similarly housed in two cages. For competition, ten female mice and two infected male mice were also housed in one cage. The female mice were monitored by daily observation of vaginal plaque established after mating. The males were employed for mating 48 h after inoculation of the protozoan. The infected male mice were replaced within 48 h (four cycles) in the cages as they died after 5 day postinoculation. The pregnant mice were monitored, separated and maintained at individual cages. As control group, 20 sexually naive female and four naïve male animals were housed under identical conditions.
Sexual behavior
A three-chambered cage (height = 20, width = 30, length = 50 cm) was used to evaluate the sexual behavior of individual female mouse. The wall of chamber was a multi-pored wood and contained a one-way plexiglas door. Four female mice were placed in a central chamber and in one side of it, two infected male mice were kept and in other side, two naïve male mice were placed. The female mice could move freely from the central chamber to the side chambers through the one-way plexiglas door (3 × 3 cm in diameter). The frequency of entrances for the female mice at the sides was counted during a 6 h period.
Analysis of data
The data were analyzed using SPSS software (version 11.5, Chicago, IL, USA) by non-parametric test of Mann–Whitney. A P value less than 0.05 was considered statistically significant.
Results
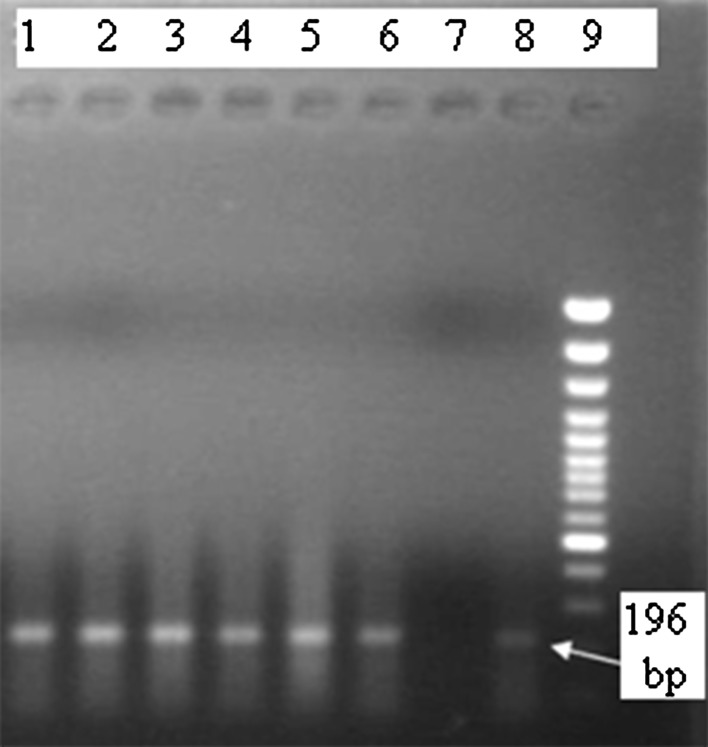
All semen samples collected from epididymis of infected male mice following 48 h after inoculation were positive by giemsa staining and PCR methods (Fig. 1). No abortion and death were visible in female mice mated with infected male animals. Pregnancy was seen in only 4 out of 20 female mice after mating with infected males. Seventeen pregnancies were observed among 20 control female mice (Fischer test, P value = 0.0001). A total of 81 female movements were reported in the cages that in 45 of them, females tended to mate naïve male mice while 36 of them tended to mate infected male animals. Fifteen out of 96 selections did not show any movement. The statistical analysis of the data on mate selection and attraction of mice was not statistically significant.
Fig. 1.
Agarose gel electrophoresis of nested PCR products: Lane 9 molecular marker, lane 8 Toxoplasma gondii (positive control), Lane 7 negative control, Lanes 1–6 semen samples of infected male mice
Discussion
Toxoplasma in semen of experimentally infected rams was first reported by Spence et al. (1978). Later, contaminated semen samples from dogs, rats and rams experimentally infected to the parasite were also confirmed by bioassay and PCR methods (Arantes et al. 2009; Dass et al. 2011; Lopes et al. 2009). Its tachyzoites were detected in testis and epididymis of experimentally infected intermediate hosts using immunohistochemistry test (Lopes et al. 2009). Infection to the parasite has also been reported in ewes after use of contaminated semen in artificial insemination (Moraes et al. 2010).
In the present study, tachyzoites of the parasite were seen in semen samples of infected males. Moreover, the PCR results provided evidences on existence of DNA of the parasite. All infected male mice could not entirely mate with females but few pregnancies were seen in female mice housed with infected male animals. The tachyzoites did not have the potential in disease transmission to their offsprings. In our study, the viability and infectivity of these tachyzoites were not evaluated.
Teale et al. (1982) reported contamination of semen in experimentally infected rams after inoculation of the parasite in sheep. As the tachyzoite of the protozoa is not resistant to the acidic pH of stomach, there is controversy on acquired infection of the protozoan. Dubey (2002) reported when cats were fed with tachyzoites, they could shed large numbers of oocysts. Asgari et al. (2011) findings revealed when tachyzoites in milk underwent heating up to 37 °C for 30 min, they still could be a possible route of transmission for the parasite to human. The result of another study showed that Toxoplasma could affect reproductive factors in male rats (Terpsidis et al. 2009). Lopes et al. (2009) denoted to evidences of infection transmission in experimentally infected rams (Lopes et al. 2009).
It seems that sexual transmission of disease to females is based on tendency of them to mate with infected males. Sexual behavior of animals in disease transmission has also been investigated by some researchers (Milinski and Hamilton 2001). Toxoplasmosis can affect behavior and neurophysiology of infected hosts (Flegr 2000; Torrey and Yolken 2003). The findings in some studies revealed cognitive deficits in mice after infection with T.gondii (Hodkova et al. 2007; Webster 2007).
Kankov et al. (2011) showed that Toxoplasma infection affect the level of serum testosterone in mice and human while the changes in testosterone level may influence the probability of infection to Toxoplasma in other animals. Mougeot et al. (2005) showed that testosterone-treated males of red grouse had significantly more infection to Trichostrongylus tenuis (a common nematode parasite in this host) than the control group. They had more weight loss than the controls. These testosterone-treated males had bigger sexual appendages, higher pairing and breeding success than the controls. The sexual traits may reliably be advertising the inherent ability of individuals to resist parasite infection (Hamilton and Zuk 1982).
As females can benefit from mating of males with fewer parasites, their offspring would not be affected by transmission (Møller et al. 1999). Parasites may genetically influence the offsprings (Agrawal 2006; Gandon and Otto 2007). Potentially and genetically more resistant offsprings may be produced by preferential mating with genetically dissimilar ones (Hedrick 2002; Howard and Lively 2004; Milinski 2006).
Infection to coccidian can affect mating in wild turkeys as uninfected females demonstrate preference for males with higher-quality ornaments (Buchholz, 2003). Polak et al. (2007) demonstrated that mites on Drosophila nigrospiracula can drive sexual selection by physical inhibition of host copulation. As resistance to mite infection in natural populations is heritable, it emphasizes the evolutionary potential of parasite-mediated sexual selection (Polak et al. 2007).
Results of one study on male mice infected with intestinal nematode of Heligmosomoides polygyrus indicated that female mice preferentially mated with uninfected males although the male infection status did not influence female reproductive success (Ehman and Scott 2002).
In another study, the parasite could alter mate choice in rats whereby infected males were considered more attractive by females (Dass et al. 2011). Dass et al. (2011) reported this change on mating selection for uninfected females would not increase sexual transmission of T.gondii.
In our study, no statistically significant were seen in infection to the parasite and mating preference of mice. Even sexual pheromones may pose inhibitory activities; it seems that infection to Toxoplasma affects male genitalia, body size, physiological vigor and energy which changes in male physical appearance influence the sexual selection.
Acknowledgments
The authors would like to thank the Office of Vice-Chancellor for Research of Tehran University of Medical Sciences and Tehran University for financial support of this project. We also thank the staff of the Animal Laboratory Centre, Shiraz University of Medical Sciences for cooperation in experimental studies.
References
- Agrawal AF. Similarity selection and the evolution of sex: revisiting the red queen. PLoS Biol. 2006;4:1364–1371. doi: 10.1371/journal.pbio.0040265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akins CK, Panicker S, Cunningham CL. Laboratory animals in research and teaching: ethics, care, and methods. Washington, DC: APA; 2004. [Google Scholar]
- Arantes TP, Lopes WDZ, Ferreira RM, Pieroni JSP, Pinto VMR, Sakamoto CA, Costa AJD. Toxoplasma gondii: evidence for the transmission by semen in dogs. Exp Parasitol. 2009;123:190–194. doi: 10.1016/j.exppara.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Asgari Q, Farzaneh A, Kalantari M, Mohajeri FA, Moazeni M, et al. Seroprevalence of free-ranging chicken toxoplasmosis in sub-urban regions of Shiraz. Iran Int J Poult Sci. 2006;5:262–264. doi: 10.3923/ijps.2006.262.264. [DOI] [Google Scholar]
- Asgari Q, Moazzeni M, Akrami FM, Kalantri M, Zarifi M, et al. Seroprevalence of Toxoplasma gondii among caprines in Fars Province, Southern Iran. J Vet Parasitol. 2007;21:153–155. [Google Scholar]
- Asgari Q, Mehrabani D, Moazzeni M, Akrami-Mohajeri F, Kalantari M, Motazedian MH, Hatam GR. The seroprevalence of ovine toxoplasmosis in Fars Province, Southern Iran. Asian J Anim Vet Adv. 2009;4:332–336. doi: 10.3923/ajava.2009.332.336. [DOI] [Google Scholar]
- Asgari Q, Mehrabani D, Moazeni M, Akrami-Mohajeri F, Kalantari M, et al. The seroprevalence of bovine toxoplasmosis in Fars Province, Southern Iran. Asian J Anim Vet Adv. 2010;5:210–216. doi: 10.3923/ajava.2010.210.216. [DOI] [Google Scholar]
- Asgari Q, Mehrabani D, Motazedian MH, Kalantari M, Nouroozi J, Adnani Sadati SJ. The viability and infectivity of Toxoplasma gondii tachyzoites in dairy products undergoing food processing. Asian J Anim Sci. 2011;5:202–207. doi: 10.3923/ajas.2011.202.207. [DOI] [Google Scholar]
- Buchholz R. Effects of parasitic infection on mate sampling by female wild turkeys (Meleagris gallopavo): should infected females be more or less choosy? Behav Ecol. 2003;15(4):687–694. doi: 10.1093/beheco/arh066. [DOI] [Google Scholar]
- Dass SA, Vasudevan A, Dutta D, Soh LJ, Sapolsky RM, Vyas A. Protozoan parasite Toxoplasma gondii manipulates mate choice in rats by enhancing attractiveness of males. PLoS ONE. 2011;6:e27229. doi: 10.1371/journal.pone.0027229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey JP. Tachyzoite-induced life cycle of Toxoplasma gondii in cats. J Parasitol. 2002;88(4):713–717. doi: 10.1645/0022-3395(2002)088[0713:TILCOT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Jones JL. Toxoplasma gondii infection in humans and animals in the United States. Int J Parasitol. 2008;38:1257–1278. doi: 10.1016/j.ijpara.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Ehman KD, Scott ME. Female mice mate preferentially with non-parasitized males. Parasitology. 2002;125:461–466. doi: 10.1017/S003118200200224X. [DOI] [PubMed] [Google Scholar]
- Flegr J. Effects of Toxoplasma on human behavior. Schizophr Bull. 2000;33:757–760. doi: 10.1093/schbul/sbl074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandon S, Otto SP. The evolution of sex and recombination in response to abiotic or coevolutionary fluctuations in epistasis. Genetics. 2007;175:1835–1853. doi: 10.1534/genetics.106.066399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton WD, Zuk M. Heritable true fitness and bright birds—a role for parasites. Science. 1982;218:384–387. doi: 10.1126/science.7123238. [DOI] [PubMed] [Google Scholar]
- Hedrick PW. Pathogen resistance and genetic variation at MHC loci. Evolution. 2002;56:1902–1908. doi: 10.1111/j.0014-3820.2002.tb00116.x. [DOI] [PubMed] [Google Scholar]
- Hodkova H, Kodym P, Flegr J. Poorer results of mice with latent toxoplasmosis in learning tests: impaired learning processes or then novelty discrimination mechanism? Parasitology. 2007;134:1329–1337. doi: 10.1017/S0031182007002673. [DOI] [PubMed] [Google Scholar]
- Howard RS, Lively CM. Good vs complementary genes for parasite resistance and the evolution of mate choice. BMC Evol Biol. 2004;4:48. doi: 10.1186/1471-2148-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CD, Okhravi N, Adamson P, Tasker Sh, Lightman S. Comparison of PCR detection methods for B1, P30, and 18S rDNA genes of T. gondii in aqueous humor. IOVS. 2000;41(3):634–644. [PubMed] [Google Scholar]
- Kankov Š, Kodym P, Flegr J. Direct evidence of Toxoplasma-induced changes in serum testosterone in mice. Exp Parasitol. 2011;128:181–183. doi: 10.1016/j.exppara.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Lopes WD, Costa AJ, Souza FA, Rodrigues JD, Costa GH, Soares VE, Silva GS. Semen variables of sheep (Ovis aries) experimentally infected with Toxoplasma gondii. Anim Rep Sci. 2009;111(2–4):312–319. doi: 10.1016/j.anireprosci.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Milinski M. The major histocompatibility complex, sexual selection, and mate choice. Annu Rev Ecol Evol Syst. 2006;37:159–186. doi: 10.1146/annurev.ecolsys.37.091305.110242. [DOI] [Google Scholar]
- Milinski M, Hmilton B (2001) Sexual and parasite. Behav Ecol 12:264–266
- Møller AP, Christe P, Lux E. Parasitism, host immune function, and sexual selection. Q Rev Biol. 1999;74:3–20. doi: 10.1086/392949. [DOI] [PubMed] [Google Scholar]
- Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- Moraes EPBX, Freitas AC, Gomes-Filho MA, Guerra MMP, Silva MAR, Pereira MF, Braga VA, Mota RA. Characterization of reproductive disorders in ewes given an intrauterine dose of Toxoplasma gondii tachyzoites during the intrauterine insemination. Anim Rep Sci. 2010;122:36–41. doi: 10.1016/j.anireprosci.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Mougeot F, Redpath SM, Piertney SB. Elevated spring testosterone increases parasite intensity in male red grouse. Behav Ecol. 2005;17(1):117–125. doi: 10.1093/beheco/arj005. [DOI] [Google Scholar]
- Oksenhendler E, Charreau I, Tournerie C, Azihary M, Carbon C, Aboulker JP. Toxoplasma gondii infection in advanced HIV infection. AIDS. 1994;8(4):483–487. doi: 10.1097/00002030-199404000-00010. [DOI] [PubMed] [Google Scholar]
- Pereira KS, Franco RM, Leal DA. Transmission of toxoplasmosis (Toxoplasmagondii) by foods. Adv Food Nutr Res. 2010;60:1–19. doi: 10.1016/S1043-4526(10)60001-0. [DOI] [PubMed] [Google Scholar]
- Polak M, Luong LT, Starmer WT. Parasites physically block host copulation: a potent mechanism of parasite-mediated sexual selection. Behav Ecol. 2007;18(5):952–957. doi: 10.1093/beheco/arm066. [DOI] [Google Scholar]
- Smith JE, Reduck NR. Toxoplasmagondii strain variation and pathogenicity. In: Cary JW, Linz JE, Bhatnaga B, editors. Microbial foodborne diseases: mechanisms of pathogenesis and toxin synthesis. Lancaster: Technnomic; 2000. [Google Scholar]
- Spence JB, Beattie CP, Faulkner J, Henry L, Watson WA. Toxoplasma gondii in the semen of rams. Vet Rec. 1978;102(2):38–39. doi: 10.1136/vr.102.2.38. [DOI] [PubMed] [Google Scholar]
- Teale AJ, Blewett DA, Miller JK. Experimentally induced toxoplasmosis in young rams: the clinical syndrome and semen secretion of Toxoplasma. Vet Rec. 1982;111(3):53–55. doi: 10.1136/vr.111.3.53. [DOI] [PubMed] [Google Scholar]
- Terpsidis KI, Papazahariadou MG, Taitzoglou IA, Papaioannou NG, Georgiadis MP, Theodoridis I. Toxoplasma gondii: reproductive parameters in experimentally infected male rats. Exp Parasitol. 2009;121:238–241. doi: 10.1016/j.exppara.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Yolken RH. Toxoplasma gondii and schizophrenia. Emerg Infec Dis. 2003;9:1375–1380. doi: 10.3201/eid0911.030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A. Parasite-augmented mate choice and reduction in innate fear in rats infected by Toxoplasma gondii. J Exper Biol. 2013;216:120–126. doi: 10.1242/jeb.072983. [DOI] [PubMed] [Google Scholar]
- Webster JP. The effect of Toxoplasma gondii on animal behavior: playing cat and mouse. Schizophr Bull. 2007;33(3):752–756. doi: 10.1093/schbul/sbl073. [DOI] [PMC free article] [PubMed] [Google Scholar]