Abstract
Tobacco smoking is highly comorbid with heavy alcohol drinking, yet the interactions of tobacco smoking and alcohol drinking on brain catecholaminergic synaptic markers is unexplored. Here we evaluate the effects of alcohol drinking alone from comorbid alcohol drinking and tobacco smoking on DA and 5-HT transporter availability. Fourteen heavy alcohol drinking smokers (n=6) and nonsmokers (n=8) and 14 age-matched control smokers (n=6) and nonsmokers (n=8) were imaged with [123]β-CIT SPECT. Alcohol drinking smokers and nonsmokers consumed 134.3±100.3 and 196.5±139.9 drinks, respectively over the previous month and were imaged during acute withdrawal, e.g., within 5 days of their last drink. Striatal DA transporter availability was significantly higher (16%, P=0.04) in alcohol drinkers compared to controls. 5-HT transporter availability was also significantly higher in alcohol drinkers versus controls in the brainstem (25%, P=0.001) and the diencephalon (8%, P=0.01). This elevation was restricted to alcohol drinking nonsmokers with higher DA transporter availability in the striatum (26%, P=0.006), and higher 5-HT transporter availability in the diencephalon (26%, P=0.04) and brainstem (42%, P<.0002). There was a significant positive correlation between days since last drink and 5-HT transporter availability in the diencephalon (r=0.60, P=0.023) and brainstem (r=0.54, P=0.047), in the total group of alcohol drinkers and in the nonsmokers, but not the smokers. During the first week of abstinence, DA and 5-HT transporter availability is higher in alcohol drinking nonsmokers but not in alcohol drinking smokers. Smoking appears to suppress neuroadaptive changes in DA and 5-HT transporters during acute withdrawal from alcohol.
Keywords: Dopamine, Serotonin, Alcohol Withdrawal, Tobacco Smoking, Comorbidity, SPECT, beta-CIT
Introduction
Heavy alcohol drinking and tobacco smoking are highly associated. In the general population, it is estimated that 23% of people smoke cigarettes, up to 50% of individuals with alcohol abuse or dependence smoke(Grant et al. 2004), and as many as 30% of smokers are also alcohol-dependent (Miller and Gold 1998). Daily and occasional smokers are more likely than never smokers to be hazardous drinkers or to meet criteria for an alcohol use diagnosis (McKee et al. 2007). Smokers consume twice as much alcohol as nonsmokers (Carmody et al. 1985) and alcohol drinkers who also smoke use more cigarettes/day than non-alcohol-dependent smokers (Dawson 2000). The high comorbidity of smoking and drinking (Funk et al. 2006; Meyerhoff et al. 2006) may be due to several factors. First, social/peer pressures and availability of alcohol and cigarettes provide an environment that is conducive to drinking and smoking. Second, the effects of the drugs when used together may be additive or synergistic with regard to the reinforcing properties. Third, pharmacological effects or interactions of the drugs, such as changes in metabolism or cross-tolerance, may facilitate co-abuse. Fourth, genetic factors may contribute to this comorbidity. We hypothesize that in addition to these factors, there is an interaction of alcohol and tobacco smoking at the level of the dopamine (DA) and serotonin (5-HT) transporters.
A wealth of preclinical and clinical literature demonstrates the effects of both acute and chronic alcohol administration on the monoaminergic system [for reviews see (Chastain 2006; Vengeliene et al. 2008)]. Specifically, the mesolimbic dopamine system is the primary basis for the rewarding properties of most drugs of abuse, including alcohol (Di Chiara and Imperato 1988). While nicotine, the primary addictive chemical in tobacco smoke, exerts its initial effects at the nicotinic acetylcholine receptor, its effects on the mesolimbic dopamine system are also well-documented (Janhunen and Ahtee 2007). The serotonergic system is also implicated in both alcohol dependence (Vengeliene et al. 2008) and tobacco smoking (Seth et al. 2002). Dysfunctional 5-HT (Heinz et al. 2001) and DA system activity (Tupala and Tiihonen 2004) may represent a vulnerability to alcohol dependence.
DA and 5-HT transporter availability has been examined in vivo in alcohol drinkers. Chronic alcohol consumption has been found to result in lower (Laine et al. 1999; Mash et al. 1996; Repo et al. 1999; Tupala et al. 2001), higher (Mash et al. 1996; Tiihonen et al. 1995), or unchanged (Volkow et al. 1996) availability of DA transporters compared to control subjects. Specifically, chronic alcohol consumption is associated with lower DA transporter availability in human alcohol drinkers who were imaged up to 4 weeks after their last drink compared to control subjects. Interestingly, this lower DA transporter availability increases during acute withdrawal, e.g., during the first 5 days of abstinence in humans (Laine et al. 1999) and at 24 hours of abstinence in nonhuman primates (Mash et al. 1996).
5-HT transporter availability has been reported to be lower (Heinz et al. 1998b; Szabo et al. 2004) and similar (Brown et al. 2007) in recent and long term abstinent alcoholics compared to controls. In nonhuman primates, higher brainstem 5-HT transporters were associated with reduced sensitivity to alcohol intoxication, which is a marker of vulnerability to develop alcohol dependence (Heinz et al. 1998a), and brainstem 5-HT transporter availability was positively correlated with amount of alcohol consumption (Heinz et al. 2003). Taken together, these associated lines of research suggest an initial reduction in DA and 5-HT transporter availability during acute withdrawal, which increases over time in alcohol-dependent subjects compared to healthy subjects. Importantly, despite the high comorbidity of tobacco smoking with alcoholism, the majority of these studies have not controlled for smoking status.
The development and validation of [123]β-CIT (2 β-carbomethoxy-3 β-(4-iodophenyl)tropane) for use with single photon emission computed tomography (SPECT) has allowed the simultaneous examination of DA and 5-HT transporter availability in a region dependent manner (Seibyl et al. 1996; Seibyl et al. 1997). Here, we used [123]β-CIT SPECT to delineate the interactions of alcohol and comorbid alcohol and tobacco smoking on DA and 5-HT transporter availability during acute alcohol withdrawal (< 1 week) (Staley et al. 2005).
Materials and Methods
Subjects
14 heavy alcohol drinkers (35.0 ± 12.0 y; age range 21–54; 5 women, 9 men; 1 Hispanic, 4 African American, 9 Caucasian) and 14 healthy controls (38.3 ± 10.0 y; age range 21–54; 7 women, 7 men; 2 African American, 12 Caucasian) participated in the study. In each group there were 8 nonsmokers and 6 smokers (Table 1). For simplicity we refer to the groups as alcohol smokers, alcohol nonsmokers, control smokers and control nonsmokers throughout the paper. A negative breathalyzer reading was required prior to initiating the intake appointment or brain imaging scans. Eligibility was determined as follows. Alcohol drinkers consumed at least 25 drinks per month based on self-report obtained using the Timeline FollowBack Interview (Sobell and Sobell 1993). Control subjects, e.g., nondrinkers, were required to drink less than 10 drinks per year and could not have a history of alcohol abuse or dependence. Smoking status was verified by plasma cotinine levels >150 ng/mL on the day of intake. Nonsmokers were required to have smoked less than 100 cigarettes in their lifetime and none in the previous 2 years, with plasma cotinine levels <50 ng/mL on the day of intake. In addition, all subjects had a medical examination by a study physician to exclude any major medical issues or neurological disorders. This included a physical examination, electrocardiogram, serum chemistries, thyroid function studies, complete blood count, urinalysis, and urine toxicology screening. Subjects were given structured interviews using the SCID to rule out any Axis I Disorder except Alcohol Abuse or Dependence or Nicotine Dependence. All subjects had no history of significant medical illness or major head trauma. All subjects had no use of any psychotropic medications or herbal products during the 6 months prior to their participation. All women of childbearing age were required to have a negative pregnancy test during the screening process and prior to radiotracer injection on each study day. All healthy women were included; we did not exclude subjects for menstrual cycle irregularities or hormonal birth control. Menstrual cycle phase data was not collected. After complete description of the study to the subjects, written informed consent was obtained.
Table 1.
Demographics and clinical characteristics of control and alcohol drinker nonsmokers and smokers.
| Total | Nonsmokers | Smokers | ||||
|---|---|---|---|---|---|---|
| Controls (n=14) |
Drinkers (n=14) |
Controls (n=8) |
Drinkers (n=8) |
Controls (n=6) |
Drinkers (n=6) |
|
| Age | 38.4±9.9 | 35.0±12.0 | 38.8±8.3 | 34.0±11.9 | 37.8±12.6 | 36.5±13.1 |
| Sex | 8M; 6F | 9M; 5F | 4M; 4F | 3M; 5F | 4M; 2F | 6M; 0F |
| Average drinks/month | - | 196.5±139.9 | - | 134.3±100.3 | ||
| Years Drinking | - | 17.9±11.0 | - | 20.0±12.8 | ||
| Days since last drink (range) | - | 1–5 | - | 1–2 | ||
| ADS | - | 3.6±2.7 | - | *7.0±2.7 | ||
| Cigarettes/day | - | - | 24.2±4.9 | 20.0±6.3 | ||
| Years Smoking | - | - | 22.2±9.7 | 17.4±12.6 | ||
| FTND | - | - | 5.8±2.5 | 3.8±1.8 | ||
Data presented are means ± standard deviation unless otherwise noted. There are no significant differences in any of the variables between groups. The CIWAAr was also given to all heavy alcohol drinking subjects on scan days. The scores were not elevated, and ranged from 0–1, thus are not shown.
Information only obtained for 3 of the 6 subjects.
Alcohol drinkers were given a variety of assessments to determine demographic and other behavioral information. Assessments of alcohol drinking behavior included the Timeline Followback (Sobell and Sobell 1993), Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised (CIWA-Ar) (Sullivan et al. 1989), Alcohol Dependence Scale (ADS) (Skinner and Hom 1984) and assessments of mood included the Beck Depression Inventory (BDI) (Beck et al. 1961) and the Center for Epidemiologic Studies Depression Scale (CES-D) (Radloff 1977).
[123]β-CIT SPECT and magnetic resonance imaging scans
The radiotracer [123]β-CIT was synthesized from the tributylstanyl precursor, with a radiochemical purity > 97%, as previously described (Baldwin et al. 1993). On Day 1 of the study, subjects were pretreated with stable iodine approximately 30 min prior to radiotracer injection to limit thyroid uptake of 123I. [123]β-CIT was administered by bolus intravenous injection to alcohol drinkers 220.5 ± 23.3 MBq and control subjects 223.3 ± 8.7 MBq. Subjects returned the next day and were scanned 21–24 hours after radiotracer injection. Prior to imaging, 5 external fiducial markers of 123I were placed on the subjects head. One 24-minute emission scan and one 15-min simultaneous transmission and emission scan were obtained on a Picker Prism 3000 three-headed camera (Phillips, Cleveland, Ohio) equipped with a low-energy, ultra-high resolution fanbeam collimator (photopeak window 159 keV ± 10%, matrix 128 × 128) with a uniform sensitivity across the field of view. A 57Co-distributed source was measured with each experiment to control for daily variation in camera sensitivity. The axial resolution (full width at half maximum) of the camera was 12.2 mm, measured with a 123I line source in water in a cylindrical phantom. Blood was drawn on Day 1 prior to radiotracer injection and approximately 21 hours after injection to determine radiotracer metabolism and protein binding, or fP.
MRI was performed on a 1.5 Tesla GE Signa device (TR=25 milliseconds, TE=5 milliseconds, number of excitations=2, matrix=256×256 pixels, and field of view=24 cm).
Image analysis and outcome measure
[123]β-CIT labels DA and 5-HT transporters in a region specific manner. Specifically, [123]β-CIT labels DA transporters in the striatum and 5-HT transporters in the diencephalon and brainstem (Laruelle et al. 1993). SPECT images were analyzed with an MRI-based region-of-interest (ROI) approach as previously described (Staley et al. 2001; Staley et al. 2006). Briefly, emission data were reconstructed and a nonuniform attenuation correction was applied. Then, the MRI was coregistered to the emission scan to provide an anatomical guide for the placement of two-dimensional ROIs using MEDx software (Medical Numerics, Inc.). The chosen ROIs are those known to contain DA transporters, e.g., caudate and putamen that are averaged into one striatal region, 5-HT transporters, e.g., diencephalon and brainstem, and the cerebellum, which is used a background region. Two raters conducted the analysis. Variability between the raters was less than 12 % across regions of interest. The mean of the two raters is reported.
The primary outcome measure used was BPP, which is proportional to the binding potential (binding potential, in mL/g, equal to receptor number divided by receptor affinity) defined as follows: (specific – nondisplaceable uptake) / total parent in plasma. It is computed as (region of interest activity – cerebellum) / total parent in plasma. The cerebellum, which has no detectable levels of DA transporters and minimal levels of 5-HT transporters, is used as the background region. To control for differences in radiotracer metabolism and protein binding, the measures of total plasma parent concentration, fP (protein binding), and free plasma parent concentration, defined as total parent concentration * fP, were compared between groups.
Statistical Analysis
All outcomes were tested for normality using Kolmogorov-Smirnov test statistics and normal probability plots. DA and 5-HT transporter availability and V2 values (cerebellum uptake/total parent) were approximately normal. Radiotracer metabolism and protein binding values, e.g., fP, free parent, and total parent, were approximately normal after log transformation and thus, the log transformed values were used for analysis. Each outcome was evaluated using two-way ANOVA models where group (alcohol drinkers vs. controls) and smoking status (smokers vs. nonsmokers) were included as between-subject explanatory factors. Group comparisons within each level of smoking status were made to interpret significant interactions. These latter two comparisons were adjusted for Type I error using the Bonferroni correction. In the above models, age and gender were considered as potential covariates but were dropped for parsimony as neither of these variables was significant. Potential associations between brain and alcohol measures were assessed using correlation analysis. Analyses were performed using SAS, version 9.1 (Cary, NC) and all tests were considered statistically significant at P<0.05.
Results
Group Characteristics
Twenty-eight subjects composed of 14 controls and 14 alcohol drinkers with 8 nonsmokers and 6 smokers per group participated in the study (Table 1). Alcohol drinkers were imaged between 1 and 5 days after their last drink. Alcohol smokers and nonsmokers did not differ significantly in average number of drinks per month, years drinking or the ADS. Alcohol smokers and control smokers did not differ significantly in numbers of cigarettes smoked per day, years smoked or FTND. Control subjects denied drinking alcohol within the month prior to the scan, no control subject reported drinking more than 8 drinks in a one-year period, and control subjects denied use of other illicit or psychotropic drugs.
DA transporter availability
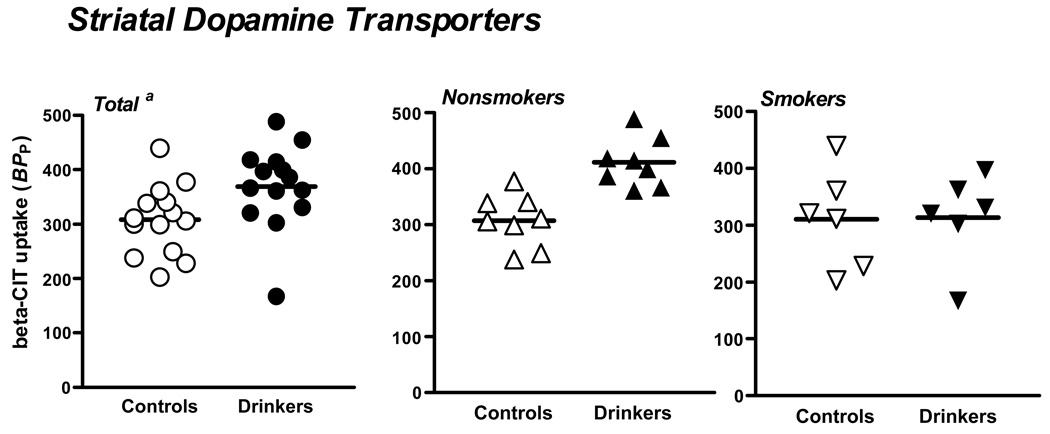
Overall striatal DA transporter levels were significantly higher in alcohol drinkers versus controls (F(1,24)=4.9, P=.04). Additionally, the group by smoking interaction was significant (F(1,24)=4.4, P=.05)) which was driven by greater DA transporter availability among alcohol nonsmokers versus control nonsmokers (F(1,24)=10.9, adjusted P=.006), with no difference between alcohol smokers and control smokers (F(1,24)=.01, adjusted P=1.0) (See Figure 1).
Figure 1.
Striatal [123I]β-CIT binding in controls and alcohol drinkers in the total group and by smoking status. a. The bar represents the mean in each group. Percent difference was calculated as [(alcohol drinker – control)/alcohol drinker x 100]. In the total group, there was a 16% difference (P=0.04), in the nonsmokers a 26% difference (P=0.006), and in the smokers a 1% difference (P=1.0).
5-HT transporter availability
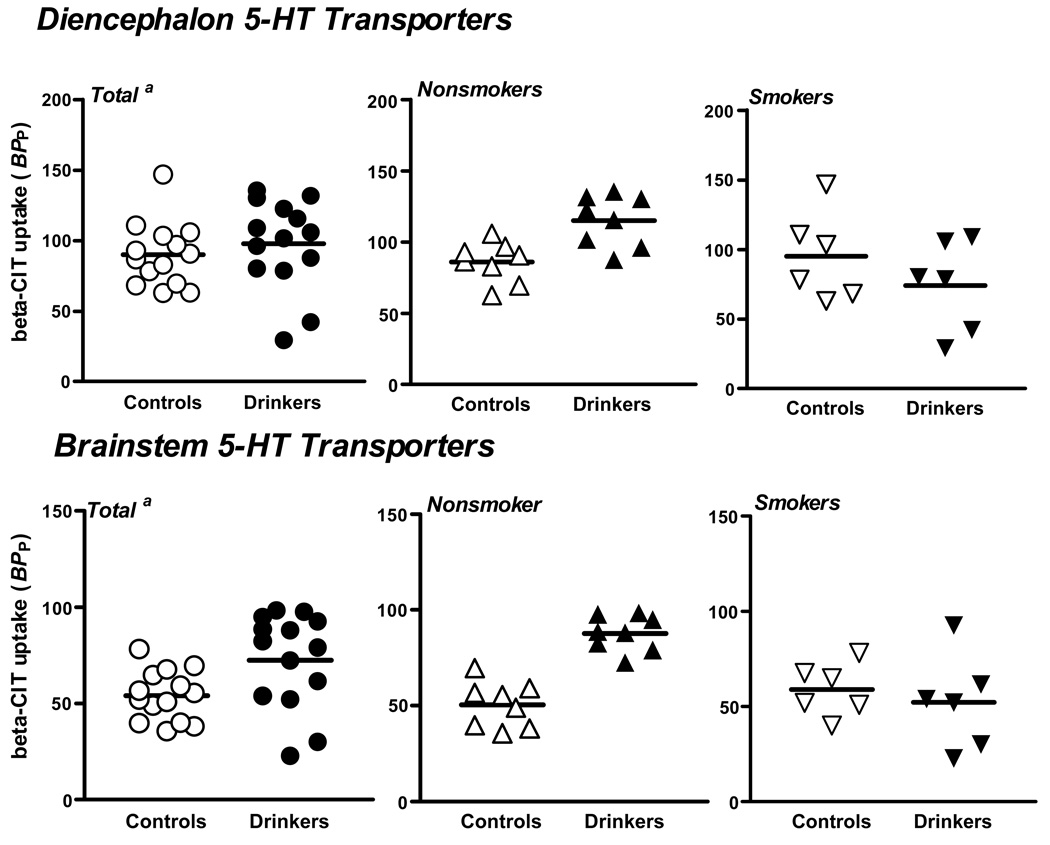
A significant group by smoking status interaction was observed when assessing 5-HT transporter availability in both the diencephalon (F(1,24)=7.7, P=.01) and brainstem (F(1,24)=14.1, P=.001). Specifically, alcohol nonsmokers had significantly greater diencephalon (F(1,24)=6.2, adjusted P=.04) and brainstem (F(1,24)=23.5, adjusted P<.0002) 5-HT transporter availability compared to control nonsmokers, whereas diencephalon (F(1,24)=2.3, adjusted P=.30) and brainstem (F(1,24)=0.58, adjusted P=.90) 5-HT transporter availability did not differ significantly between alcohol smokers and control smokers (See Figure 2).
Figure 2.
Diencephalon and brainstem [123I]β-CIT binding in controls and alcohol drinkers in the total group and by smoking status. a. In the total group, there was an 8% difference (P=0.01), in the nonsmokers a 26% difference (P=0.04), and in the smokers a −28% difference (P=0.30). b. In the total group, there was a 25% difference (P=0.001), in the nonsmokers a 42% difference (P<0.0002), and in the smokers a −13% difference (P=0.90).
V2 values (cerebellum uptake/total parent) were compared between groups to ensure that group differences were not due to differences in nondisplaceable uptake. Again, a significant interaction was observed between group and smoking status (F(1,24)=11.3, P=.003), which was driven by significantly higher V2 levels among alcohol nonsmokers compared to control nonsmokers (F(1,24)=22.8, adjusted P<.0002), with no group differences observed among smokers (F(1,24)=0.1, adjusted P=1.0). Higher V2 levels could be attributed to the small but detectable number of 5-HT transporters in the vermis of the cerebellum (Backstrom et al. 1989; Laruelle et al. 1988). Importantly, cerebellar V2 levels were not lower in the alcohol nonsmokers as would be expected if there was significant atrophy of the cerebellum, indicating that the higher numbers of DA and 5-HT transporters in other brain areas are not due to group differences in nondisplaceable uptake.
Radiotracer metabolism and protein binding
Radiotracer metabolism and protein binding values were also examined between groups. Significantly lower levels of both total parent (F(1,24)=8.5, P=.008) and free parent, defined as fP * total parent (F(1,24)=6.3, P=.02), were observed in alcohol drinkers compared to controls. While there were no significant main or interactive effects of smoking on each of these variables (all P>.28), the observed group differences were primarily driven by significantly lower total (F(1,24)=9.3, adjusted P=.012) and free (F(1,24)=7.4, adjusted P=.02) parent levels among alcohol nonsmokers versus control nonsmokers. This finding suggests that alcohol nonsmokers had faster metabolism of [123I]β-CIT. By using the outcome measure BPP we correct for group differences in radiotracer metabolism.
Correlations between DA and 5-HT transporters and alcohol use history
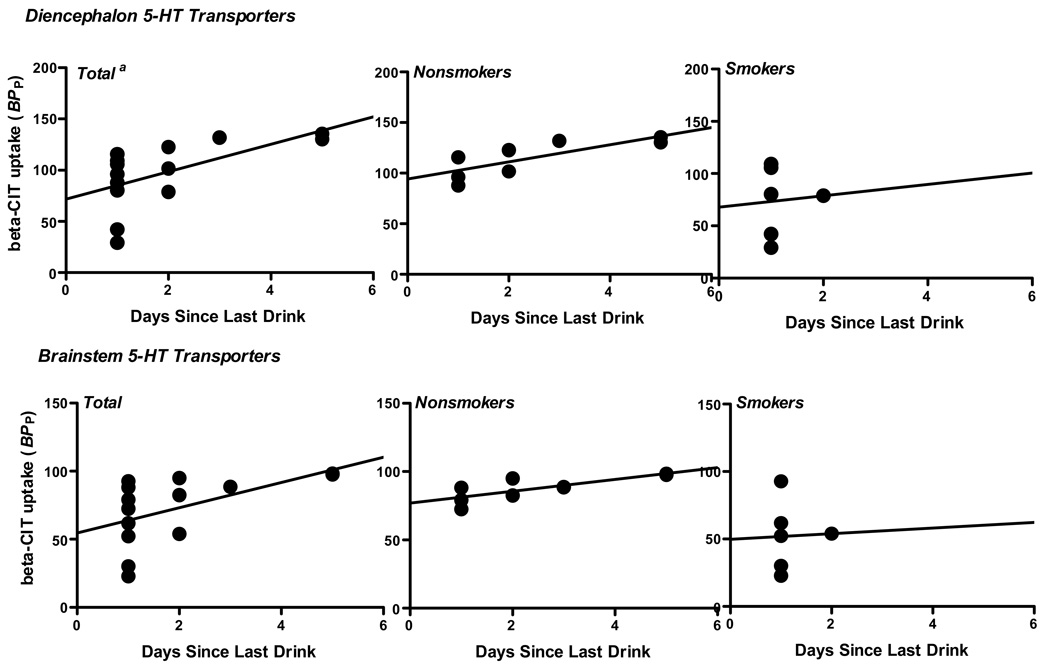
Pearson correlations were performed between striatal DA and brainstem and diencephalon 5-HT transporter availability and alcohol measures including days since last drink, average drinks per month, years of drinking, and scores on the ADS, BDI and CES-D. There was a significant positive correlation between days since last drink and diencephalon (r=.60, P=.02) and brainstem (r=.54, P=.05) 5-HT availability (See Figure 3). However, stratified analysis revealed these associations present in nonsmokers (r=.79, P=.02 and r=.79, P=.02 for diencephalon and brainstem, respectively) but not smokers (r=.07, P=.90 and r=.03, P=.95, respectively.) There were no significant correlations between striatal DA and brainstem and diencephalon 5-HT transporter availability and other alcohol or depression measures.
Figure 3.
Diencephalon and brainstem [123I]β-CIT binding in alcohol drinkers in the total group and by smoking status as a function of days since last drink. a. Significant linear regression coefficients were found in the total group and in the nonsmokers, but not the smokers for both brain regions.
Discussion
In the present study, we report significantly higher striatal DA transporters in alcoholic nonsmokers but not alcoholic smokers during acute withdrawal. A similar pattern was noted also for 5-HT transporters with higher diencephalon and brainstem availability in the alcoholic nonsmokers but not alcoholic smokers. The significant positive correlation between diencephalon and brainstem 5-HT transporter availability and days since last drink in the alcoholic nonsmokers suggests that the higher availability was due to a compensatory increase in response to acute withdrawal from alcohol.
Given that V2 is an indirect measure of cerebellar atrophy and is significantly higher in alcohol nonsmokers compared to controls it is unlikely that the higher DA and 5-HT transporter availability is due to atrophy of the reference region, e.g., the cerebellum. While it is unexpected to have higher numbers of V2 in the reference region, this is not surprising given that low, but detectable levels of 5-HT transporters have been measured in the vermis of the cerebellum (Backstrom et al. 1989; Laruelle et al. 1988). While studies in control subjects demonstrate no significant measurable levels of 5-HT transporters in the cerebellum (Staley et al. 2001), after alcohol exposure and withdrawal the measurable numbers may become more significant. This theory needs to be validated in a future study with a more selective paradigm.
This study differs from studies showing a decrease in DA transporter availability in recently abstinent alcoholics in vivo up to 4 weeks of abstinence (Laine et al. 1999), postmortem, up to 10 hr after the last drink (Tupala et al. 2001) and after chronic alcohol and 1 day of withdrawal in vervet monkeys (Mash et al. 1996), but is consistent with results indicating increased DA transporter availability in alcohol-preferring versus non-preferring monkeys (Mash et al. 1996). Notably, there was a decrease in DA transporter availability after chronic alcohol compared to controls, which then increased during acute withdrawal (Laine et al. 1999; Mash et al. 1996) and in some subjects approached or surpassed the level of DA transporter availability in control subjects (Laine et al. 1999).
The main differences between previous studies examining DA transporter availability in alcoholics and controls and the current study are 1) the systematic examination of tobacco smoking in the current study and 2) the severity of alcohol dependence. First, higher availability of DA transporters in alcohol nonsmokers but not smokers compared to controls suggests that smoking suppresses the alcohol-induced increase in DA transporter availability. The previous in vivo study (Laine et al. 1999) did not control for smoking, thus it is not clear whether there is a similar effect of comorbid tobacco smoking in chronic severe alcohol dependence. Second, subjects in the current study exhibit a range of alcohol consumption, from heavy drinkers to subjects that meet dependence (e.g., a range of 25–428 drinks/month), and did not require detoxification or inpatient hospitalization prior to withdrawal. Thus, this study may highlight a difference in DA transporter availability between alcoholics and heavy drinkers. It is possible that alcoholics have an altered “set point” in DA transporter availability compared to both healthy controls and to heavy alcohol drinkers (Koob 2003). Lower DA transporters in alcohol drinkers have been hypothesized to be due to a downregulation of DA transporters in response to chronic alcohol consumption. In this study the subjects had been drinking for an average of 19 years (range 6–40 years) suggesting that the chronicity of drinking alone does not lead to lower DA transporters, but the amount of alcohol consumed is also a key part. It is possible that a subsyndromal level of alcohol drinking leads to a compensatory increase in DA transporter availability, measured during acute withdrawal. Indeed, it is interesting that Mash and colleagues (1996) similarly found increased DA transporters in alcohol-preferring monkeys that had not chronically consumed large quantities of alcohol, and notably were nicotine-naive. Thus, a higher DA transporter availability is likely linked to lower DA tone, and may indicate a vulnerability to alcohol dependence. Taken together, these studies suggest that moderate to heavy drinking leads to higher DA transporter availability that is suppressed by tobacco smoking. More severe alcohol dependence may be associated with lower DA transporters and may also be modulated by tobacco smoking. Studies designed to address this question in more severe alcoholic populations need to be done within the first week of alcohol withdrawal.
These results extend the findings from previous studies that demonstrated lower 5-HT transporter availability in alcoholics abstinent for > 3 weeks (Heinz et al. 1998b; Szabo et al. 2004). Specifically, lower midbrain and brainstem 5-HT transporter availability was found in alcohol-dependent men after 3–5 weeks (Heinz et al. 1998b) and at least 2 years (Szabo et al. 2004) of abstinence compared to healthy controls, which was correlated with depression and anxiety during withdrawal (Heinz et al. 1998b). A more recent study reported no difference in 5-HT transporter availability between alcoholics (smokers and nonsmokers), who were abstinent an average of 14 days, and control nonsmokers using [11C]DASB (Brown et al. 2007), which is a more selective ligand for 5-HT transporters than [123I]β-CIT. In the present study we observed higher 5-HT transporter availability in both the diencephalons and brainstem of alcoholic nonsmokers versus control nonsmokers that was not evident in alcoholic nonsmokers. The significant positive correlation between diencephalon and brainstem 5-HT transporter availability and days since last drink, suggests that during acute withdrawal, there is a compensatory increase in 5-HT transporters in nonsmokers but not smokers, suggesting that smoking suppresses the alcohol-induced increase in 5-HT transporter availability over the first week of abstinence. Of note, there are limitations to [123]β-CIT SPECT measurement of 5-HT transporter availability, e.g., the radiotracer may be influenced by endogenous 5-HT (Heinz et al. 2004) and there are difficulties in quantification due to the low signal in the neocortex (Kuikka et al. 1995; vanDyck et al. 2000). However, the main finding of the current study lies in the modulation of 5-HT transporter availability by comorbid tobacco smoking and heavy drinking. Previous in vivo 5-HT transporter studies attempted to control for smoking in the alcoholic samples; however the studies did not include sufficient control smokers to systematically assess the effects of smoking between all groups. Specifically, the studies included either no control smokers (Brown et al. 2007) or two control smokers (Heinz et al. 1998b; Szabo et al. 2004).
There are several reasons that DA and 5-HT transporter availability may differ between alcoholic smokers and nonsmokers. First, genetic differences may play a role such that alcoholics who smoke may be genetically distinct from alcoholics who do not smoke, and there is evidence to suggest that there is a common genetic component that contributes to alcohol and nicotine dependence (True et al. 1999). There may be a unique genetic contribution of the DA transporter gene to striatal DA transporter availability in alcoholics that is also correlated with alcohol withdrawal severity (Heinz et al. 2000a), and a possible link exists between reduced 5-HT transporter availability with the serotonin genotype in alcoholics (Heinz et al. 2000b). Second, there may be neurochemical differences between alcohol drinkers that smoke and do not smoke. Tobacco smoke contains up to 4000 chemicals including nicotine, and monoamine oxidase inhibitors (MAOIs) that alter the activity of DA and 5-HT. Specifically, chronic MAO inhibition increases extracellular (Lamensdorf et al. 1996) and tissue (Ilani et al. 2000) DA levels. Consistently, low platelet MAO activity has been reported in both alcoholics (Hallman et al. 1991; von Knorring et al. 1985; von Knorring et al. 1987) and tobacco smokers (Von Knorring and Oreland 1985). We propose that chronic MAO inhibition from tobacco smoking increases DA release, and together with the increased DA release from heavy alcohol drinking, leads to a downregulation of the DA transporter in alcoholic smokers compared to the alcoholic nonsmokers. Notably, a previous study found no direct effect of tobacco smoking on the DA transporter (Staley et al. 2001), which implies there is an interaction of tobacco smoking and alcohol drinking at the DA transporter.
There are several limitations to the study. First, due to the small sample size, the results should be interpreted with caution and they require replication. Specifically, there is some between-subject variability in the tobacco-smoker groups, most notably in DA transporter availability. However, the data were analyzed in the presence and absence of the subjects with the lowest DA and 5-HT transporter availability and significance of the results was maintained. Second, while it is known that there are sex differences in the availability of both 5-HT (Staley et al. 2001) and DA (Lavalaye et al. 2000; Mozley et al. 2001; Staley et al. 2001) transporter availability, there was no effect of sex in the current study. These previous studies were conducted in healthy subjects. One issue with controlling for menstrual cycle phase or hormone fluctuations in studies with problem drinkers and smokers is that alcohol (Emanuele et al. 2002) and tobacco smoking (Brown et al. 1988; Windham et al. 1999) can disrupt the menstrual cycle making it difficult to control for the menstrual cycle in this population. We did not control for hormonal fluctuations or menstrual cycle phase in the current study and this will have to be evaluated in a future study. Third, there are limitations to [123I]beta-CIT SPECT. Specifically, the radiotracer is not selective, but measures both striatal DA and brainstem and diencephalon 5-HT transporters. Additionally, [123I]beta-CIT is not sensitive to the measurement of DA transporters in the cortex, which were recently found to be higher in postmortem brain of alcohol-dependent individuals vs. controls (Tupala et al. 2006).
In summary, high striatal DA and diencephalon and brainstem 5-HT transporter availability in alcohol nonsmokers during acute abstinence may reflect a neuroadaptive response to acute alcohol withdrawal, and/or a vulnerability or transition to more severe alcohol dependence. This effect is suppressed by tobacco smoking and may have implications for the design of treatment plans for alcoholics who also smoke. While additional studies are needed, it may be speculated that by suppressing the neuroadaptive change in DA and 5-HT transporters during acute abstinence, tobacco smoking may alleviate some of the withdrawal symptoms from alcohol. This suggests that alcoholic smokers desiring to quit drinking and smoking simultaneously should be encouraged to use nicotine replacement strategies or nicotinic agonist medication to help manage alcohol withdrawal symptoms.
Table 2.
DA and 5-HT Transporter Availability by Alcohol Drinking and Smoking Status:BPP Mean (SD)
| Nonsmokers |
Smokers |
|||||||
|---|---|---|---|---|---|---|---|---|
| Controls (n=8) |
Drinkers (n=8) |
% Difference |
P-Value | Controls (n=6) |
Drinkers (n=6) |
% Difference |
P-Value | |
| DA Transporter | ||||||||
| Striatum | 306 (47) | 411 (43) | 26% | .006 | 311 (87) | 313 (80) | 1% | 1.0 |
| 5-HT Transporter | ||||||||
| Diencephalon | 85 (13) | 115 (18) | 26% | .04 | 95 (32) | 74 (33) | −28% | .30 |
| Brainstem | 51 (11) | 88 (9) | 42% | <.0002 | 59 (14) | 52 (25) | −13% | .90 |
Acknowledgments
This work was supported by the Department of Veterans Affairs (via support for the Alcohol Research Center), U.S. Veterans Affairs VISN 1 Mental Illness Research Education and Clinical Center (MIRECC), National Institute of Alcohol and Alcoholism (KO1AA00288; RO1 AA-11321; K05 AA-14906-01; I-P50 AA-12870-03), and National Institute of Drug Abuse (KO1DA02065; KO2DA21863).
Footnotes
Disclosures/ Conflict of Interest: Dr. Krystal serves as a consultant to Astra-Zeneca, Bristol-Myers Squibb, Cypress Bioscience, Inc., Eli Lilly and Co., Forest Laboratories, Glaxo-SmithKline, Houston Pharma, Janssen Research Foundation, Lohocla Research Corporation, Merz Pharmaceuticals, Organon Pharmaceuticals/Division of Schering-Plough Research Institute, Pfizer Pharmaceuticals, Schering Corporation acting through Schering-Plough Research Institute Division, Shire Pharmaceuticals, Takeda Industries, Tetragenex Pharmaceuticals, Transcept Pharmaceuticals, UCB Pharma, and US Micron. Dr. Krystal has patents pending for glutamatergic agents for psychiatric disorders and oral ketamine for depression. No other authors report any conflicts of interest.
References
- Backstrom I, Bergstrom M, Marcusson J. High affinity [3H]paroxetine binding to serotonin uptake sites in human brain tissue. Brain Res. 1989;486:261–268. doi: 10.1016/0006-8993(89)90511-8. [DOI] [PubMed] [Google Scholar]
- Baldwin R, Zea-Ponce Y, Zoghbi S, Laurelle M, Al-Tikriti M, Sybirska E, et al. Evaluation of the monoamine uptake site ligand [123I]methyl 3β-(4-iodophenyl)-tropane-2β-carboxylate ([123I]β-CIT) in non-human primates: pharmacokinetics, biodistribution and SPECT brain imaging coregistered with MRI. Nucl Med Biol. 1993;20:597–606. doi: 10.1016/0969-8051(93)90028-s. [DOI] [PubMed] [Google Scholar]
- Beck S, Ward C, Mendelsohn M, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Brown AK, George DT, Fujita M, Liow JS, Ichise M, Hibbeln J, et al. PET [11C]DASB imaging of serotonin transporters in patients with alcoholism. Alcohol Clin Exp Res. 2007;31:28–32. doi: 10.1111/j.1530-0277.2006.00261.x. [DOI] [PubMed] [Google Scholar]
- Brown S, Vessey M, Stratton I. The influence of method of contraception and cigarette smoking on menstrual patterns. British Journal of Obstetrics and Gynecology. 1988;95:905–910. doi: 10.1111/j.1471-0528.1988.tb06578.x. [DOI] [PubMed] [Google Scholar]
- Carmody T, Brischetto C, Matarazzo J, O’Donnell R, Connor W. Co-occurrent use of cigarettes, alcohol, and coffee in healthy, community-living men and women. Health Psychol. 1985;4:323–335. doi: 10.1037//0278-6133.4.4.323. [DOI] [PubMed] [Google Scholar]
- Chastain G. Alcohol, neurotransmitter systems, and behavior. J Gen Psychol. 2006;133:329–335. doi: 10.3200/GENP.133.4.329-335. [DOI] [PubMed] [Google Scholar]
- Dawson D. Drinking as a risk factor for sustained smoking. Drug and Alcohol Dependence. 2000;59:235–249. doi: 10.1016/s0376-8716(99)00130-1. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuele MA, Wezeman F, Emanuele NV. Alcohol’s effects on female reproductive function. Alcohol Res Health. 2002;26:274–281. [PMC free article] [PubMed] [Google Scholar]
- Funk D, Marinelli PW, Le AD. Biological processes underlying co-use of alcohol and nicotine: neuronal mechanisms, cross-tolerance, and genetic factors. Alcohol Res Health. 2006;29:186–192. [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Hallman J, von Knorring L, Edman G, Oreland L. Personality traits and platelet monoamine oxidase activity in alcoholic women. Addict Behav. 1991;16:533–541. doi: 10.1016/0306-4603(91)90061-l. [DOI] [PubMed] [Google Scholar]
- Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, et al. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology. 2000a;22:133–139. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Heinz A, Higley JD, Gorey JG, Saunders RC, Jones DW, Hommer D, et al. In vivo association between alcohol intoxication, aggression, and serotonin transporter availability in nonhuman primates. Am J Psychiatry. 1998a;155:1023–1028. doi: 10.1176/ajp.155.8.1023. [DOI] [PubMed] [Google Scholar]
- Heinz A, Jones DW, Gorey JG, Bennet A, Suomi SJ, Weinberger DR, et al. Serotonin transporter availability correlates with alcohol intake in non-human primates. Mol Psychiatry. 2003;8:231–234. doi: 10.1038/sj.mp.4001214. [DOI] [PubMed] [Google Scholar]
- Heinz A, Jones DW, Mazzanti C, Goldman D, Ragan P, Hommer D, et al. A relationship between serotonin transporter genotype and in vivo protein expression and alcohol neurotoxicity. Biol Psychiatry. 2000b;47:643–649. doi: 10.1016/s0006-3223(99)00171-7. [DOI] [PubMed] [Google Scholar]
- Heinz A, Jones DW, Zajicek K, Gorey JG, Juckel G, Higley JD et al. Depletion and restoration of endogenous monoamines affects beta-CIT binding to serotonin but not dopamine transporters in non-human primates. J Neural Transm. 2004;(Suppl):29–38. doi: 10.1007/978-3-7091-0579-5_4. [DOI] [PubMed] [Google Scholar]
- Heinz A, Mann K, Weinberger DR, Goldman D. Serotonergic dysfunction, negative mood states, and response to alcohol. Alcohol Clin Exp Res. 2001;25:487–495. [PubMed] [Google Scholar]
- Heinz A, Ragan P, Jones DW, Hommer D, Williams W, Knable MB, et al. Reduced central serotonin transporters in alcoholism. Am J Psychiatry. 1998b;155:1544–1549. doi: 10.1176/ajp.155.11.1544. [DOI] [PubMed] [Google Scholar]
- Ilani T, Lamensdorf I, Finberg JP. Selective monoamine oxidase subtype inhibition and striatal extracellular dopamine in the guinea-pig. Br J Pharmacol. 2000;130:1992–1998. doi: 10.1038/sj.bjp.0703493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janhunen S, Ahtee L. Differential nicotinic regulation of the nigrostriatal and mesolimbic dopaminergic pathways: implications for drug development. Neurosci Biobehav Rev. 2007;31:287–314. doi: 10.1016/j.neubiorev.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Kuikka J, Tihonen J, Bergstrom K, Karhu J, Hartikainen P, Viinamaki H, et al. Imaging of serotonin and dopamine transporters in the living human brain. Eur. J. Nucl. Med. 1995;22:346–350. doi: 10.1007/BF00941852. [DOI] [PubMed] [Google Scholar]
- Laine TP, Ahonen A, Torniainen P, Heikkila J, Pyhtinen J, Rasanen P, et al. Dopamine transporters increase in human brain after alcohol withdrawal. Mol Psychiatry. 1999;4:189–191. doi: 10.1038/sj.mp.4000514. 104–5. [DOI] [PubMed] [Google Scholar]
- Lamensdorf I, Youdim MB, Finberg JP. Effect of long-term treatment with selective monoamine oxidase A and B inhibitors on dopamine release from rat striatum in vivo. J Neurochem. 1996;67:1532–1539. doi: 10.1046/j.1471-4159.1996.67041532.x. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Baldwin R, Malison R, Zea-Ponce Y, Zoghbi S, Al-Tikriti M, et al. SPECT imaging of dopamine and serotonin transporters with [123I]β-CIT: pharmacological characterization of brain uptake in nonhuman primates. Synapse. 1993;13:295–309. doi: 10.1002/syn.890130402. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Vanisberg M, Maloteaux J. Regional and subcellular localization in human brain of [3H]paroxetine binding, a marker of serotonin uptake sites. Biol Psychiatry. 1988;24:299–309. doi: 10.1016/0006-3223(88)90198-9. [DOI] [PubMed] [Google Scholar]
- Lavalaye J, Booij J, Reneman L, Habraken J, Royen Ev. Effect of age and gender on dopamine transporter imaging with [123I]FP-CIT SPET in healthy volunteers. Eur J Nucl Med. 2000;27:867–869. doi: 10.1007/s002590000279. [DOI] [PubMed] [Google Scholar]
- Mash DC, Staley JK, Doepel FM, Young SN, Ervin FR, Palmour RM. Altered dopamine transporter densities in alcohol-preferring vervet monkeys. Neuroreport. 1996;7:457–462. doi: 10.1097/00001756-199601310-00020. [DOI] [PubMed] [Google Scholar]
- McKee SA, Falba T, O’Malley SS, Sindelar J, O’Connor PG. Smoking status as a clinical indicator for alcohol misuse in US adults. Arch Intern Med. 2007;167:716–721. doi: 10.1001/archinte.167.7.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhoff DJ, Tizabi Y, Staley JK, Durazzo TC, Glass JM, Nixon SJ. Smoking comorbidity in alcoholism: neurobiological and neurocognitive consequences. Alcohol Clin Exp Res. 2006;30:253–264. doi: 10.1111/j.1530-0277.2006.00034.x. [DOI] [PubMed] [Google Scholar]
- Miller N, Gold M. Comorbid cigarette and alcohol addiction: epidemiology and treatment. J Addict Dis. 1998;17:55–66. doi: 10.1300/J069v17n01_06. [DOI] [PubMed] [Google Scholar]
- Mozley LH, Gur RC, Mozley PD, Gur RE. Striatal dopamine transporters and cognitive functioning in healthy men and women. Am J Psychiatry. 2001;158:1492–1499. doi: 10.1176/appi.ajp.158.9.1492. [DOI] [PubMed] [Google Scholar]
- Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychol Meas. 1977;1:385–401. [Google Scholar]
- Repo E, Kuikka JT, Bergstrom KA, Karhu J, Hiltunen J, Tiihonen J. Dopamine transporter and D2-receptor density in late-onset alcoholism. Psychopharmacology (Berl) 1999;147:314–318. doi: 10.1007/s002130051173. [DOI] [PubMed] [Google Scholar]
- Seibyl JP, Laruelle M, van Dyck CH, Wallace E, Baldwin RM, Zoghbi S, et al. Reproducibility of iodine-123-beta-CIT SPECT brain measurement of dopamine transporters. J Nucl Med. 1996;37:222–228. [PubMed] [Google Scholar]
- Seibyl JP, Marek K, Sheff K, Baldwin RM, Zoghbi S, Zea-Ponce Y, et al. Test/retest reproducibility of iodine-123-betaCIT SPECT brain measurement of dopamine transporters in Parkinson’s patients. J Nucl Med. 1997;38:1453–1459. [PubMed] [Google Scholar]
- Seth P, Cheeta S, Tucci S, File SE. Nicotinic--serotonergic interactions in brain and behaviour. Pharmacol Biochem Behav. 2002;71:795–805. doi: 10.1016/s0091-3057(01)00715-8. [DOI] [PubMed] [Google Scholar]
- Skinner H, Hom J. Toronto: Addiction Research Foundation; 1984. Alcohol dependence scale (ADS) User’s Guide. [Google Scholar]
- Sobell L, Sobell M. Timeline Followback: A technique for assessing self-reported ethanol consumption. In: Allen J, Litten R, editors. Techniques to assess alcohol consumption. New Jersey: Humana Press; 1993. [Google Scholar]
- Staley J, Krishnan-Sarin S, Zoghbi S, GTamagnan, Fujita M, Seibyl J, et al. Sex differences in [123I]beta-CIT SPECT measures of dopamine and serotonin transporter availability in healthy smokers and nonsmokers. Synapse. 2001;41:275–284. doi: 10.1002/syn.1084. [DOI] [PubMed] [Google Scholar]
- Staley JK, Gottschalk C, Petrakis IL, Gueorguieva R, O’Malley S, Baldwin R, et al. Cortical gamma-aminobutyric acid type A-benzodiazepine receptors in recovery from alcohol dependence: relationship to features of alcohol dependence and cigarette smoking. Arch Gen Psychiatry. 2005;62:877–888. doi: 10.1001/archpsyc.62.8.877. [DOI] [PubMed] [Google Scholar]
- Staley JK, Sanacora G, Tamagnan G, Maciejewski PK, Malison RT, Berman RM, et al. Sex differences in diencephalon serotonin transporter availability in major depression. Biol Psychiatry. 2006;59:40–47. doi: 10.1016/j.biopsych.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Sullivan J, Sykora K, Schneiderman J, Naranjo C, Sellers E. Assessment of alcohol withdrawal: therevised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br J Addiction. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Szabo Z, Owonikoko T, Peyrot M, Varga J, Mathews WB, Ravert HT, et al. Positron emission tomography imaging of the serotonin transporter in subjects with a history of alcoholism. Biol Psychiatry. 2004;55:766–771. doi: 10.1016/j.biopsych.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Kuikka J, Bergstrom K, Hakola P, Karhu J, Ryynanen OP, et al. Altered striatal dopamine re-uptake site densities in habitually violent and non-violent alcoholics. Nat Med. 1995;1:654–657. doi: 10.1038/nm0795-654. [DOI] [PubMed] [Google Scholar]
- True WR, Xian H, Scherrer JF, Madden PA, Bucholz KK, Heath AC, et al. Common genetic vulnerability for nicotine and alcohol dependence in men. Arch Gen Psychiatry. 1999;56:655–661. doi: 10.1001/archpsyc.56.7.655. [DOI] [PubMed] [Google Scholar]
- Tupala E, Halonen P, Tiihonen J. Visualization of the cortical dopamine transporter in type 1 and 2 alcoholics with human whole hemisphere autoradiography. Eur Neuropsychopharmacol. 2006;16:552–560. doi: 10.1016/j.euroneuro.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Tupala E, Kuikka JT, Hall H, Bergstrom K, Sarkioja T, Rasanen P, et al. Measurement of the striatal dopamine transporter density and heterogeneity in type 1 alcoholics using human whole hemisphere autoradiography. Neuroimage. 2001;14:87–94. doi: 10.1006/nimg.2001.0793. [DOI] [PubMed] [Google Scholar]
- Tupala E, Tiihonen J. Dopamine and alcoholism: neurobiological basis of ethanol abuse. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1221–1247. doi: 10.1016/j.pnpbp.2004.06.022. [DOI] [PubMed] [Google Scholar]
- vanDyck C, Malison R, Seibyl J, Laruelle M, Klumpp H, Zoghbi S, et al. Age-related decline in central serotonin transporter availability with [123I]β-CIT SPECT. Neurobiology of Aging. 2000;21:497–501. doi: 10.1016/s0197-4580(00)00152-4. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, et al. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res. 1996;20:1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- von Knorring AL, Bohman M, von Knorring L, Oreland L. Platelet MAO activity as a biological marker in subgroups of alcoholism. Acta Psychiatr Scand. 1985;72:51–58. doi: 10.1111/j.1600-0447.1985.tb02570.x. [DOI] [PubMed] [Google Scholar]
- Von Knorring L, Oreland L. Personality traits and platelet monoamine oxidase in tobacco smokers. Psychol Med. 1985;15:327–334. doi: 10.1017/s0033291700023606. [DOI] [PubMed] [Google Scholar]
- von Knorring L, Oreland L, von Knorring AL. Personality traits and platelet MAO activity in alcohol and drug abusing teenage boys. Acta Psychiatr Scand. 1987;75:307–314. doi: 10.1111/j.1600-0447.1987.tb02793.x. [DOI] [PubMed] [Google Scholar]
- Windham G, Elkin E, Swan S, Waller K, Fenster L. Cigarette smoking and effects on menstrual function. Obstetrics and Gynecology. 1999;93:59–65. doi: 10.1016/s0029-7844(98)00317-2. [DOI] [PubMed] [Google Scholar]