Abstract
Macrophages are key components of the innate immune response. These cells possess a diverse repertoire of receptors that allow them to respond to a host of external stimuli including cytokines, chemokines, and pathogen-associated molecules. Signals resulting from these stimuli activate a number of macrophage functional responses such as adhesion, migration, phagocytosis, proliferation, survival, cytokine release and production of reactive oxygen and nitrogen species. The cytoplasmic tyrosine kinase Src and its family members (SFKs) have been implicated in many intracellular signaling pathways in macrophages, initiated by a diverse set of receptors ranging from integrins to Toll-like receptors. However, it has been difficult to implicate any given member of the family in any specific pathway. SFKs appear to have overlapping and complementary functions in many pathways. Perhaps the function of these enzymes is to modulate the overall intracellular signaling network in macrophages, rather than operating as exclusive signaling switches for defined pathways. In general, SFKs may function more like rheostats, influencing the amplitude of many pathways.
Keywords: Tyrosine kinases, signal transduction, innate immunity, integrins, Toll-like receptors, ITIM, ITAM, adhesion, migration, Hck, Fgr, Lyn, Review
2. INTRODUCTION
2.1. Src family kinases – general approaches
c-Src was first described as the cellular counterpart of v-Src, the transforming gene found in Rous Sarcoma Virus, and has since been the focus of much research. Src and its other family members, referred to as Src Family Kinases (SFKs) throughout this review, have been implicated in many diverse signaling pathways in both immune (1) and non-immune cell types (2). This is apparent from the close to 25,000 PubMed hits one gets when researching these enzymes. Furthermore, a strong clinical interest has developed in SFKs based on the growing realization of their central roles in human cancer (3, 4).
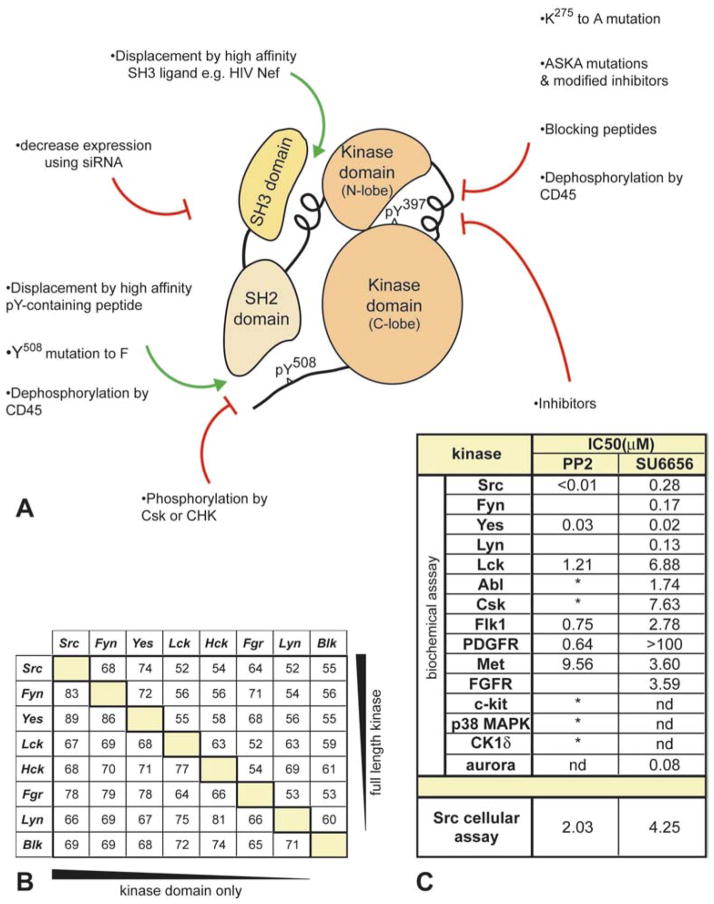
Src family members include c-Src, Fyn, c-Yes, Lck, Hck, c-Fgr, Lyn, Blk, and Yrk. Src, Fyn and Yes are ubiquitously expressed whereas Lck, Hck, Fgr, Lyn and Blk tend to be more restricted to cells of hematopoietic origin. Yrk has only been found in chickens. The SFKs are cytoplasmic tyrosine kinases, consisting of an N-terminal unique domain, a Src homology 3 (SH3) domain that can interact with polyproline-rich motifs, a Src homology 2 (SH2) domain that binds phosphotyrosine residues, and a tyrosine kinase domain (Figure 1A). Acylation of SFKs at their N-terminus (palmitoylation and/or myristoylation) allows their association with membranes. The crystal structure of several SFKs has been solved, in both active and inactive conformations, revealing complex and unexpected intramolecular regulation (reviewed in 5, 6). SFKs are held in an inactive conformation through phosphorylation of a C-terminal tyrosine residue, mediated by kinases such as C-terminal Src kinase (Csk) and Csk homologous kinase (Chk), which creates a binding site for the Src SH2 domain. A suboptimal Pro-X-X-Pro motif present in the linker between the SH2 and kinase domains binds to the SH3 domain, further stabilizing the inactive conformation. Activation of SFKs can occur either by proteins that interact with the SH3 domain, or by dephosphorylation of the C-terminal residue by phosphatases such as CD45. Subsequent autophosphorylation of a tyrosine residue in the kinase domain results in full kinase activation and phosphorylation of a variety of downstream substrates.
Figure 1.
The structure of SFKs, and approaches used to study their function. A. An outline of the structure of SFKs (without the N-terminal unique domain). Amino acid numbers refer to the SFK, Lyn. Intramolecular interactions hold SFKs in an inactive conformation; a polyproline-like sequence in the SH2-kinase linker is bound to the SH3 domain, and the tyrosine in the C-terminal tail is phosphorylated and bound to the SH2 domain. The activity of SFKs can be manipulated in several different ways both activating (green arrows) and inhibitory (red arrows). The table in B shows the percent similarities between human SFKs, comparing either the full length amino acid sequence (top-right) or the kinase domain only (bottom-left), as indicated by solid wedges on table sides. Sequences obtained from www.kinase.com were aligned using Clustal W (202). The table in C compares biochemical and cellular IC50 values for SU6656 and PP2 assayed in parallel (19 and R. A. Blake, unpublished observation). The cellular assay was performed as described in (203). The asterisk (*) indicates kinases that are also inhibited at concentrations of PP2 typically used by investigators as shown in other studies (22–24).
The SFKs share high overall amino acid similarity, especially between the kinase domains (Figure 1B), and in many in vitro overexpression systems they seem to have identical functions. However, there are subtle differences between the different family members that may distinguish their functions in vivo. In hematopoietic cells there are complex overlapping patterns of expression (1). The unique domains of SFKs share the least homology, and the function of this domain is the least understood. The acyl-modifications of the N-terminus differ between the family members which can alter their subcellular localization. Several family members are subject to alternative splicing (reviewed in 1) the role of which is not completely understood but could add further levels of complexity to SFK function.
Many substrates of SFKs have been identified (7). Analyzing the function of the different substrates links SFKs to pathways involved in growth, proliferation, transformation, differentiation, actin cytoskeletal reorganization, migration, and adhesion. In most cases SFKs are positive regulators of signaling pathways. However, uniquely in hematopoietic cells, SFKs, in particular Lyn kinase, also have important negative regulatory roles (8).
Despite the importance of SFKs in so many different signaling pathways, when mice lacking the different family members were generated, none showed very striking phenotypes implying that there is substantial functional overlap within this gene family. Study of Src-deficient mice, however, uncovered an interesting and unique function for the enzyme in osteoclast biology that could only partially be compensated for by other family members (2). Generation of mice that lack two or more family members has begun to reveal more about the function of SFKs. Deficiency of Src, Fyn and Yes results in embryonic lethality (9). Mice lacking both Hck and Fgr show some mild immunodeficiency (10). Analysis of Lyn-deficient mice revealed defects in B cells, along with the development of autoimmunity (11–14). The combined deficiency of Hck and Src greatly decreases survival, exacerbates the bone defect seen in Src-deficient mice, and increases the numbers of myeloid cells (15). A combined deficiency of Lyn and Hck also shows a much more profound phenotype than single mutant lyn−/− or hck−/− mice (T. Kawakami, J. Clin. Invest., in press (2008)). There do not appear to be compensatory increases in expression of other SFKs when family members are absent (16), but there may be increased expression of Src during macrophage differentiation or upon activation that could compensate for lack of other members such as Hck, Fgr or Lyn (17, 18).
Many different approaches have been attempted to define the general function of SFKs or the role of individual SFKs in intracellular signaling pathways (Figure 1A). Several small molecule inhibitors of Src have been described, the most well known being PP1 and PP2 and, more recently, SU6656 (19) and “Src inhibitor I” (20). PP3 is an inactive analog of PP2 often used as a negative control. A table in (21) lists IC50 values of many different compounds for several SFKs. However, comparison of IC50 values can only be made if the compounds are assayed under identical conditions for each kinase. Figure 1C lists biochemical IC50 values for SU6656, and PP2 when assayed together allowing a direct comparison between the two inhibitors (R. A. Blake, unpublished data). In general, the inhibitors hit all SFKs. The data suggests that SU6656, and PP2 to a lesser extent, are less potent against Lck but this could just reflect differences in the assay used for Lck. PP1 and PP2 are both good inhibitors of SFKs but they are structurally similar and also show similar off-target effects as each other. Caution should be used when concluding that SFKs are critical for a signaling pathway based on inhibition by one type of compound. The effect of several different compounds should be compared. It is well known that both PP1 and PP2 also inhibit signaling through the platelet-derived growth factor receptor (PDGFR), as well as through other protein kinases as indicated in Figure 1C (22–24).
Overexpression of SFKs in primary macrophages and cell lines has given some insight into SFK function. Constitutively active mutants of SFKs can be made by mutating the negative regulatory C-terminal tyrosine to phenylalanine (such as Y508 in mouse Lyn). Mutation of a lysine residue in the kinase domain to either alanine or methionine (K275 in mouse Lyn) results in a kinase dead allele. A combination of both Y508F and K275A mutations produces a strong dominant negative mutant. Mutations such as these have been used to investigate the function of the two different isoforms of Hck, p59Hck and p61Hck, which are produced from the same mRNA by using alternative ATG translational start sites. p59Hck is myristoylated and palmitoylated, and is localized to the plasma membrane. Overexpression of a constitutively active version of p59Hck causes reorganization of the actin cytoskeleton leading to plasma membrane protrusions (25). In contrast, p61Hck is only myristoylated and is localized to lysosomes. In macrophages, p61Hck also localizes to podosomes, which are actin-rich adhesive structures that are associated with protease activity. Overexpression of a constitutively active form of p61Hck leads to the formation of podosome clusters or rosettes (26, 27). Formation of podosomes in macrophages is dependent on the Wiscott-Aldrich syndrome protein (WASP) (28). The activity of WASP is in part regulated by tyrosine phosphorylation that is mediated by several SFKs including Hck (29).
Modulation of key normal cellular regulators of SFKs has been used in various ways to analyze SFK function (Figure 1). Mice deficient in Csk, an important negative regulator of SFKs, die early in embryogenesis (30). Using Csk-deficient embryonic stem cells to repopulate chimeric mice, a profound block in T and B cell development was observed, but Csk-deficient cells could support myeloid lineage development (31). A conditional deletion of Csk in granulocytes leads to dysregulated SFK activity and systemic inflammatory disease (32). A similar phenotype is observed in mice that express a constitutively active form of Hck, by mutation of the C-terminal tyrosine to phenylalanine thus making it insensitive to negative regulation by Csk, in place of wild type Hck (33). Conversely, overexpression of wild type or a membrane bound gain-of-function mutant of Csk in the RAW264.7 macrophage cell line inhibits phagocytosis, and can be rescued by Csk-insensitive mutants of Hck or Lyn (34). Chk is highly related to Csk and can also phosphorylate SFKs on their C-terminal tyrosine residue, but mice deficient in Chk have very minor defects (35). The receptor protein tyrosine phosphatase CD45 can dephosphorylate both the activating and inhibitory tyrosines of SFKs (36). However, deficiency of CD45, which in theory should result in poor activation of SFKs in hematopoietic cells, does not mimic loss of SFKs themselves – for example, CD45-deficient mice do not have the profound osteoclast defects seen in src−/− animals (36).
RNAi-mediated knockdown of SFKs and their regulators has been used to dissect the roles of these kinases in various signaling pathways. Adachi et al describe siRNA specific for Hck, Fgr or Lyn that were used to study complement-mediated phagocytosis (37) (see section 3.2.2), and siRNA specific for Csk has been used to assess SFK function downstream of Toll-like receptor (TLR) signaling in macrophages (38) (see section 3.3.1).
The use of analog-sensitive kinase alleles promises to be a useful technique for dissecting out the role of individual SFKs (39). Kinases containing functionally silent active site mutations are sensitized to inhibition by modified inhibitors that do not affect the wild type kinase. Replacement of the wild type kinase with the modified kinase allows for selective regulation by the modified inhibitors. This approach is particularly useful for families of highly related kinases, like the SFKs, where developing inhibitors specific for individual family members has been a challenge. Denzel et al describe the selective inhibition of a modified Lck with an analog of PP1 to assess T cell development in fetal thymic organ cultures (40).
Other tools for assessing SFK function have been described. Peptides based on short sequences taken from the kinase domain that are known to interact with regulatory molecules or downstream substrates become cell permeable when coupled to myristate. These peptides inhibit Lyn activity in vitro and reduce prostate tumor formation in vivo (41, 42). A myristate-coupled peptide based on the acidic region of the gp130 subunit of the IL-6 receptor that is known to interact with Hck was shown to prevent the interaction between gp130 and Hck, thus preventing IL-6-mediated stimulation of Hck kinase activity in the myeloma cell line 7TD1 (43).
2.2. Macrophages
Macrophages are the first responders of the innate immune system, both as tissue resident cells and as monocytes recruited from the blood into sites of inflammation, where they subsequently differentiate into macrophages within tissues. Macrophages can recognize a diversity of pathogens through a wide array of cell surface receptors that activate multiple intracellular signaling pathways leading to release of cytokines and chemokines such as Tumor Necrosis Factor alpha (TNF alpha), IL-6 and IL-12, phagocytosis of pathogens, production of reactive oxygen and nitrogen species, and processing/presentation of antigens. SFKs participate in signaling downstream of many of these receptors, which implicates them in the processes of macrophage proliferation, survival, apoptosis, phagocytosis, adhesion and migration.
One of the difficulties reviewing literature on macrophage biology is the great deal of heterogeneity between different types of tissue resident macrophages, and between inflammatory macrophages in different sites (reviewed in 44). Tissue resident macrophages include alveolar macrophages in the lung, Langerhans cells in the skin, Kupffer cells in the liver, several kinds of splenic macrophages, intestinal macrophages, microglial cells, tumor-associated macrophages (the presence of which is often associated with increased metastatic potential) and osteoclasts that have all developed specialized functions for their environment. In addition, different classes of activated macrophages can arise depending on the inflammatory stimulus, including classically activated cells that tend to be more proinflammatory, and alternatively activated macrophages that have more anti-inflammatory and wound healing properties. Primary macrophages studied in the lab are often either bone marrow-derived monocytes cultured in the presence of macrophage colony-stimulating factor (M-CSF) (Bone marrow-derived macrophages, or BMDMs) or macrophages collected from the peritoneum following an inflammatory stimulus such as injection of thioglycollate (Peritoneal-elicited macrophages, or PEMs).
All macrophage types express Hck, Fgr and Lyn kinases at roughly equivalent levels. Activated macrophages may also upregulate Src, and additional studies have reported Fyn and Yes expression at lower levels in some macrophage types (45). It is a challenge to tease apart the role of individual SFKs in macrophage biology for several reasons: SFKs act as both positive and negative regulators in many pathways as well as partially compensating for each other when missing. The heterogeneity of the different types of macrophage populations and available myeloid cell lines adds further complexity when trying to compare data between different studies.
3. SRC SIGNALING DOWNSTREAM OF MACROPHAGE CELL SURFACE RECEPTORS
There have been several good recent reviews on SFKs in leukocytes (1, 21, 46). In this review we will focus on the role SFKs play in signaling downstream from a variety of macrophage receptors, with a special emphasis on the discussion of these enzymes in integrin and Toll-like receptor (TLR) signal transduction where either new data or controversy exists.
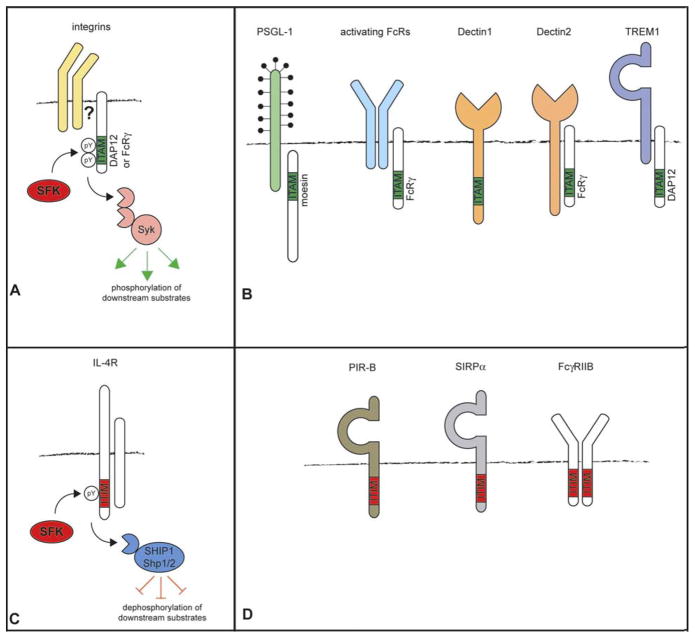
One common theme that is emerging in signaling downstream from many diverse cell surface receptors is the use of two motifs, the Immunoreceptor tyrosine-based activation motif (ITAM) and the Immunoreceptor tyrosine-based inhibitory motif (ITIM) (Figure 2). These motifs have been found in many diverse signaling molecules (reviewed in 47, 48). Both these motifs are thought to be phosphorylated by SFKs, resulting in either the recruitment and activation of Syk kinase to ITAMs, or the recruitment of protein tyrosine phosphatases, such as SH2-containing protein tyrosine phosphatase 1 (Shp1) and Shp2, or inositol phosphatases, such as SH2-containing inositol phosphatase (SHIP), to ITIMs (49–51). The use of these two signaling motifs will be discussed in the context of signaling downstream of several cell surface receptors (summarized in Figure 2).
Figure 2.
Use of IT AM and ITIM-based mechanisms to signal downstream of macrophage receptors. A. Macrophage integrins depend on the ITAM-containing molecules DAP12 (and to a lesser extent FcR gamma) to initiate downstream outside-in signaling reactions. In this model, SFKs phosphorylate the ITAM-containing adapters, allowing recruitment of Syk kinase by binding to the Syk SH2 domains, leading to Syk activation and downstream phosphorylation responses. Integrins recruit either DAP12 or FcR gamma by an as yet undetermined mechanism. B. Examples of other macrophage receptors using ITAM-based mechanisms to activate downstream signaling pathways. Receptors such as the Fc receptor, Dectin-2 and TREM1 associate with ITAM-containing adapter molecules through charged interactions in their transmembrane domains. Dectin-1 contains an ITAM-like sequence in its cytoplasmic tail, and PSGL-1 associates with the ITAM-containing ERM protein, moesin. C. Macrophage receptors such as the IL-4 alpha chain use ITIM-based mechanisms to down-modulate signaling pathways. Phosphorylation of the ITIM by SFKs leads to recruitment of protein and lipid phosphatases such as Shp1, Shp2 and SHIP1 that dephosphorylate signaling molecules and inhibit downstream pathways. D. Examples of other macrophage receptors that utilize ITIM-containing domains for inhibitory signaling.
3.1. Macrophage development
3.1.1. Receptor tyrosine kinases
M-CSF is required for macrophage differentiation. Mice lacking functional M-CSF (Csf1op/Csf1op mice) are deficient in macrophages in most tissues (52, 53). M-CSF is recognized by the tyrosine kinase receptor M-CSFR or CSF1-R, also known as the proto-oncogene c-fms (reviewed in 54). SFKs have been shown to play a role downstream of the M-CSFR. Upon ligand binding, the receptor dimerizes and autophosphorylates several tyrosine residues in the cytoplasmic domain of the receptor which leads to recruitment of signaling proteins through their SH2 domains. Src associates with the M-CSFR when overexpressed in fibroblasts (55). The interaction between Src and the M-CSFR has also been demonstrated in myeloid cell lines. Tyrosine residue Y559 of the M-CSFR is critical for Src association; in the myeloid cell line M1, association of SFKs with a receptor containing a Y559F mutation is significantly reduced (56). In the myeloid progenitor cell line 32D, SFKs are required for M-CSFR signaling downstream to the kinase, Akt, especially in cells that express a mutant M-CSFR lacking the binding site for phosphatidylinositol-3-kinase (PI3K) (57). More recently, expression of chimeric receptors in BMDMs identified Y559 as a key residue for linking downstream signals to survival and proliferation (58) and actin cytoskeletal reorganization through PI3K and Cbl (59).
Despite a clear role for SFKs downstream of the M-CSFR, macrophage development is normal in mice lacking single or multiple members of the Src family (10, 12, 13, 15). In addition, macrophage development is normal when SFKs are deregulated. In chimeric mice, generated using Csk-deficient embryonic stem cells, macrophages develop normally without this key negative regulator of SFKs. Furthermore, overexpression of constitutively active Hck does not affect macrophage development (31, 33). These observations suggest that SFKs are not absolutely required for signaling downstream of the M-CSFR for macrophage development. Alternatively, it is possible that compensation with other family members provides enough SFK activity to facilitate M-CSFR signaling during macrophage development. For example, Src is upregulated in macrophages from triple mutant hck−/−fgr−/−lyn−/− mice (17), although BMDMs proliferate normally in quadruple mutant src−/−hck−/−fgr−/−lyn−/− mice (C. A. L. unpublished observation). BMDMs from Lyn-deficient mice exhibit hyperproliferation in response to M-CSF. Mice with deregulated Lyn, either by gene knockout or expression of a gain-of-function mutant of Lyn, show an expansion of the myeloid compartment, but this could reflect homeostatic proliferation due to the absence of B cells (12). Nevertheless, interpretation of these knockout studies is complicated by the possibility of compensation between signaling pathways in SFK-deficient cells. For example, down regulation of inhibitory signaling may allow SFK-deficient cells to respond relatively normally to M-CSF. Indeed, expression of the inhibitory molecule Cbl is markedly decreased in hck−/−fgr−/−lyn−/− macrophages (60). Conditional knockouts of Src family kinases will help to pick apart these developmental signaling pathways, but investigators have been slow to develop these mutants, perhaps due to the viable phenotypes of conventional knockouts.
3.1.2. Cytokine receptors
In various cell lines, SFKs have been shown to play a role in signaling downstream of cytokine receptors, contributing to signal transducer and activator of transcription (STAT) phosphorylation along with Janus family kinases (JAKs) (reviewed in 61, 62–64). However, few studies have assessed the role of SFKs in cytokine signaling in primary macrophages where they could impact the activation or differentiation status in response to different cytokines.
Granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-3 are required for myeloid differentiation and survival. The receptors for these cytokines are related and consist of a cytokine specific alpha subunit and a common beta subunit (gp130). IL-3 treatment of a 32D myeloid cell line (that depends on IL-3 for survival) results in activation of the SFKs, Src, Hck, Fgr and Lyn (65, 66). In these cells, Hck is able to interact directly and constitutively with the common beta subunit of the IL-3 receptor, and this association increases upon tyrosine phosphorylation of the receptor (67). Hck also interacts with STAT3 when co-expressed in Rat-2 fibroblasts (68). Similarly, other studies found that Src, when activated in response to IL-3, also associates with STAT3, leading to increased DNA binding activity. In this study, Src-mediated STAT3 activation was required for myeloid cell proliferation (65). STAT5 can also be activated downstream of IL-3, or by Bcr-Abl transformation; expression of Hck is required for the Bcr-Abl-mediated STAT5 activation (69).
GM-CSF can increase Lyn kinase activity in myeloid cell lines (70). However, in contrast to other SFKs, Lyn appears to have a negative regulatory role in signaling downstream of the GM-CSF receptor as BMDMs from Lyn-deficient mice hyperproliferate in response to GM-CSF (12). In this pathway, it is postulated that activation of Lyn following GM-CSF treatment leads to phosphorylation of ITIM-containing receptors in macrophages, such as paired Ig-like receptor B (PIR-B) or signal regulatory protein alpha (SIRP alpha), which in turn recruit the phosphatase Shp1 to downmodulate signaling responses (Figure 2D). This hypothesis is supported by the similarity of GM-CSF responses between Lyn-deficient and Shp 1-deficient cells (71). However, direct modulation of PIR-B or SIRP alpha phosphorylation by GM-CSF has not been demonstrated in primary macrophages.
IL-4 binding to its receptor, which consists of an IL-4 alpha subunit and a common gamma chain, induces alternative activation in macrophages resulting in anti-inflammatory responses. Kasiwada et al identified an ITIM in the cytoplasmic region of the IL-4 alpha subunit (Figure 2C) (72). Expression of a mutant IL-4 alpha subunit, in which the tyrosine contained within the ITIM is mutated to phenylalanine, causes 32D cells to hyperproliferate in response to IL-4 when compared to expression of wild type IL-4 alpha. Phosphorylation of STAT6 is also increased. IL-4 stimulation results in SHIP association with the receptor. However, phosphorylation of the ITIM by SFKs was not addressed in this study.
3.2. Macrophage adhesion and migration
3.2.1. G-protein coupled receptors
In many cell types, SFKs have a role in the phosphorylation of G beta gamma subunits, suggesting that they should be key regulators of G-protein coupled receptors (GPCRs) (reviewed in 73). Again, as with studies of cytokine signaling, most of these examples come from experiments using cell lines and relatively few investigators have examined the contribution of SFKs to GPCR-mediated chemokine signaling in primary macrophages.
Of the SFKs, Lyn has been implicated most frequently in chemokine receptor signaling. Lyn activation occurs in primary human macrophages treated with macrophage inflammatory protein 1 alpha (MIP1 alpha), MIP1 beta and RANTES, the major ligands for the GPCR CCR5. CCR5 is also an important coreceptor for HIV infection of macrophages (74). HIV infection of macrophages leads to Lyn activation. In macrophages lacking CCR5, no activation of Lyn is seen in response to MIP1 beta or HIV-1 gp120. Activation of Lyn is required for downstream stimulation of the mitogen activated protein kinases (MAPK) extracellular signal-regulated kinase (ERK) 1 and 2, and TNF alpha production triggered by gp120. All of these observations support a positive role for Lyn in transducing signals from CCR5.
Lyn activation is also seen in response to the chemokine stromal cell-derived factor 1 alpha (SDF1 alpha) in myeloid cell lines and in hematopoietic progenitors. SDF1 alpha is recognized by the GPCR, CCR4; in this pathway, Lyn functions upstream of PI3K activation. Indeed, Lyn-deficient bone marrow mononuclear cells display impaired chemotaxis responses to SDF1 alpha, supporting the primary role of this SFK in chemokine signaling downstream of CCR4 (75).
Monocyte chemoattractant protein-1 (MCP-1) is important for monocyte recruitment to sites of inflammation via its receptor, CCR2. MCP-1 stimulation of the monocyte cell line, THP1, and human peripheral blood mononuclear cells leads to Src kinase activation. Treatment of these cells with the Src inhibitor PP2 prevents MCP-1 stimulated migration (76) implicating Src as a major positive signaling component of the CCR2 pathway.
In contrast to the above observations, the SFKs Hck and Fgr have been shown to play important inhibitory roles in chemokine signaling in neutrophils and dendritic cells (77). hck−/−fgr−/− double mutant neutrophils and dendritic cells show increased signaling and functional responses to stimulation by MIP1 alpha and MIP2, which signal through CCR1 and CXCR2, respectively. This negative regulation requires the ability of these kinases to phosphorylate the ITIM-containing receptor PIR-B. However, a negative regulatory role for these particular SFKs in macrophage GPCR signaling has not been reported. As a further complication, these two same kinases seem to be positively involved in signaling pathways downstream of the peptide formyl-Met-Leu-Phe (fMLP) stimulation (through the fMLP receptor) in neutrophils (78). Clearly, SFKs have divergent roles in the overall modulation of GPCR signaling in hematopoietic cells.
3.2.2. Integrins
Integrins are heterodimers of alpha and beta transmembrane subunits that provide an important link between the macrophage and its environment. The primary integrins expressed on macrophages are alpha 4 beta 1, alpha 5 beta 1, alpha V beta 5, alpha M beta 2 (or Mac1) and alpha L beta 2 (or leukocyte function-associated antigen 1 (LFA1)), and their ligands include a wide variety of extracellular matrix proteins such as fibronectin and fibrinogen, and adhesive molecules on endothelial cells such as intercellular adhesion molecules (ICAMs) and vascular cell adhesion molecule (VCAM). Mac1 is also known as the complement receptor CR3, and binds to the inflammatory mediator iC3b. Integrin signaling is important for migration and adhesion, but also for differentiation, survival and effector functions of leukocytes (79–81).
In resting cells, integrins adopt an inactive, folded conformation. Signaling via other receptors on macrophages such as chemokine or cytokine receptors induces integrin inside-out signaling which leads to tail separation in the cytoplasmic region of the integrins. Structural changes are relayed through the transmembrane domain and result in an unfolding of the extracellular region of the integrin, producing a high affinity binding site for the ligand. Ligand binding initiates outside-in signaling (82).
A clear role for SFKs in outside-in integrin signaling has been established in many cell types, including macrophages. Whether these kinases also function in the inside-out pathway leading to integrin unfolding and activation is less clear. Outside-in integrin signaling can be easily studied in neutrophils and platelets because these cell types manifest good functional readouts for integrin-mediated activation. A requirement for SFKs in integrin-dependent signaling in these cell types has been clearly demonstrated (reviewed in 81, 83). In macrophages, the functional consequences of integrin engagement are more difficult to study, yet it is clear that SFKs play a central role in integrin outside-in signaling in these cells as well. The SFKs Hck and Fgr are activated when macrophages are plated on fibronectin or by crosslinking their alpha 5 or beta 1 integrins, and tyrosine phosphorylation of paxillin, cortactin, Pyk2 and Syk is observed (84). This phosphorylation is lost when macrophages lacking both Hck and Fgr are plated on fibronectin. hck−/−fgr−/− macrophages also manifest delayed spreading, an abnormal actin cytoskeletal structure, and reduced in vitro migration in both a wound healing assay and in a Boyden chamber. These defects are not observed in macrophages from single mutant mice lacking only one of the kinases (84). Macrophages lacking c-Cbl exhibit a similar phenotype, which correlates with reduced c-Cbl tyrosine phosphorylation in hck−/−fgr−/− macrophages (85). This suggests that Cbl plays a positive functional role in macrophage integrin signaling downstream of SFK activation. Cbl may link integrin signaling to the activation of PI3K, since phospho-Cbl associates with the p85 subunit of PI3K and translocates the enzyme to the membrane in adherent macrophages (86).
Macrophages lacking all three SFKs, Hck, Fgr and Lyn, show a more profound effect and are unable to spread or firmly adhere when plated on fibronectin (60). The presence of these SFKs is required for the integrin beta 1- and beta 3-induced tyrosine phosphorylation of c-Cbl and increased membrane-associated PI3K activity (86). Integrin beta 1-mediated activation of MAPKs and NF kappa B is not affected in hck−/−fgr−/−lyn−/− macrophages (60). Macrophages lacking all three SFKs show defective migration in an in vivo thioglycollate peritonitis model, a defect that is not observed in single or double SFK mutant mice. Reducing Hck, Fgr and Lyn protein using siRNA in U937 macrophages also leads to reduced c-Cbl tyrosine phosphorylation, phagocytosis and respiratory burst in response to complement-opsonized zymosan although this could be mediated by both beta 2 integrins and Dectin-1 (37).
Macrophages deficient in Lyn have uncovered an important negative regulatory role for this SFK in integrin signaling pathways. Lyn-deficient macrophages have a hyperadhesive phenotype that correlates with a modestly reduced migratory capacity in vitro. Similarly, neutrophils from Lyn-deficient mice show clear evidence of increased integrin-mediated functional responses (ROS production and degranulation) following adhesion. Lyn functions as a negative regulator by phosphorylating ITIM-containing immunoreceptors, such as SIRP alpha and PIR-B, that recruit the phosphatase Shp1 to turn off signaling (88, 89). Significantly reduced tyrosine phosphorylation of SIRP alpha and PIR-B is observed in adherent Lyn-deficient macrophages.
The importance of SFKs in integrin signaling is further highlighted by modulating SFK regulators such as CD45 and Csk. The receptor tyrosine phosphatase CD45 can dephosphorylate both the activating and inhibitory tyrosines in SFKs. Macrophages that lack CD45 show hyperphosphorylation and hyperactivation of both Hck and Lyn upon integrin binding, and are unable to maintain stable adhesion (90). Loss of Csk in granulocytes, resulting in SFK activation, leads to hyperadhesion and reduced migration (32). Adhesion and migration of macrophages appears to be controlled by a fine balance of positive and negative signals downstream of integrins mediated by SFKs.
The SFK Fgr can also act as a negative regulator of integrin signaling, but in a kinase-independent manner. Macrophages that lack Fgr spread much better on the beta 2 integrin ligand ICAM-1 than wild-type or Hck-deficient macrophages, whereas no difference is observed on beta 1 ligands. Using the macrophage cell line BAC1.2F5, which is deficient in Fgr, expression of either wild-type or kinase dead Fgr prevents spreading on ICAM-1. The SH2 domain of Fgr was shown to interact with Y324 of Syk and inhibit Syk activity, which could account for reduced spreading (91). Complement-mediated phagocytosis is also more efficient both in the BAC1.2F5 cell line which lacks Fgr, and in Fgr-deficient macrophages. Expression of both wild-type or kinase dead Fgr in these cells leads to reduced CR3-mediated phagocytosis. The authors noted that this effect was opposite to that seen in neutrophils lacking Fgr. Fgr was found to associate with the ITIM-containing immunoreceptor SIRP alpha therefore recruiting the phosphatase Shp1 (92). The potential mechanism by which Fgr acts as a negative regulator of integrin signaling is unclear.
How are Src kinases becoming activated by integrins? It has been suggested that integrin clustering stabilizes active Src, perhaps by colocalization with phosphatases that can dephosphorylate the negative regulatory site on the C-terminal tail of SFKs (87, 93). Clustering could be mediated by recruitment of signaling proteins to lipid rafts, which are detergent-insoluble membrane regions rich in cholesterol and sphingolipids. Many components of immunoreceptor signaling are enriched in lipid rafts, including SFKs, and disruption of rafts impairs signal transduction (discussed in (94)), however, the role of lipid rafts in integrin-dependent signaling is less well understood. Studies from neutrophils and platelets suggest that rafts may not be important (97). A direct interaction between integrin beta subunit cytoplasmic tails and Src has been shown in Chinese hamster ovary (CHO) cells engineered to overexpress both Src and integrins (95). In this model, clustering of ligand bound integrins may function to bring receptor-associated SFKs in close proximity to each other, where they can transphosphorylate and therefore transactivate. The relative contributions of these models of SFK activation to signaling downstream of integrins remains to be seen.
Studies of neutrophil and platelet integrin signaling have identified similarities between integrin-dependent signals and signaling through classical immunoreceptors (reviewed in 96, 97). Similar signaling molecules are involved in both pathways including Src family and Syk kinases, the adapter protein SH2-containing leukocyte protein of 76kDa (SLP76) and the guanine-nucleotide exchange factor, Vav. In classical immunoreceptor signaling, receptors recruit activated Syk or ZAP70 kinases through phosphorylated ITAMs contained within the receptor itself or an accessory molecule. SFKs are responsible for phosphorylating the ITAM (50). It appears that integrin signaling also depends on SFK-mediated ITAM phosphorylation to recruit and activate Syk and initiate downstream signaling (Figure 2A). Macrophages lacking the ITAM-containing adapter protein DAP12 are unable to induce ERK1/2 phosphorylation when plated onto Valmark plastic dishes, a response shown to be beta 2 integrin-dependent (90). The defect is restored by expression of wild type DAP12, but not by a mutant of DAP12 that can no longer be phosphorylated by SFKs, in which the two tyrosine residues contained within the ITAM are mutated to phenylalanine (98). A similar observation was made in osteoclasts, where signaling through the integrin alpha V beta 3 is critical for their function. c-Src-mediated phosphorylation of the ITAM-containing adapters DAP12 and the Fc receptor gamma-chain (FcR gamma) is required for Syk activation downstream of alpha V beta 3 activation (87). Although the mechanism linking integrins to ITAM-containing adapter molecules such as DAP12 is unclear, it is clear that integrin outside-in signal transduction should be included in the list of ITAM-mediated signaling reactions initiated by SFKs (Figure 2).
3.2.3. Selectins
P-selectin glycoprotein ligand 1 (PSGL-1) is a selectin ligand important for the tethering and rolling of monocytes through its interaction with endothelial-expressed selectins. This allows subsequent firm adhesion and transmigration mediated by integrins (99). Upon interaction with selectins, PSGL-1 transduces downstream signals. Increased tyrosine phosphorylation is observed in neutrophils treated with either anti-PSGL-1 antibody or plated on P-selectin, resulting in ERK activation and IL-8 secretion (100). Treatment of monocytes with soluble E-selectin (which also binds to PSGL-1) leads to increased tyrosine phosphorylation, which is inhibited by pretreatment with PP2. Furthermore, monocyte chemotaxis in response to soluble E-selectin is inhibited by PP2 suggesting an involvement of SFKs (101). Treatment of U937 cells with anti-PSGL-1 antibody results in increased tyrosine phosphorylation of both Syk and the ezrin-radixin-moesin (ERM) actin-binding protein moesin (102). An association between Syk expressed in a rabbit reticulocyte system and glutathione S-transferase (GST) fusion proteins of both PSGL-1 and moesin is mediated through an ITAM-like domain present in moesin (Figure 2B). Syk is clearly required for rolling of leukocytes on E-selectin-coated surfaces as demonstrated by studies using syk−/− cells in an autoperfused flow chamber (103). SFKs could potentially play a role in PSGL-1 signaling by phosphorylating the ITAM in moesin but this has not been investigated.
3.3. Macrophage innate immune function
3.3.1. Toll-like receptors
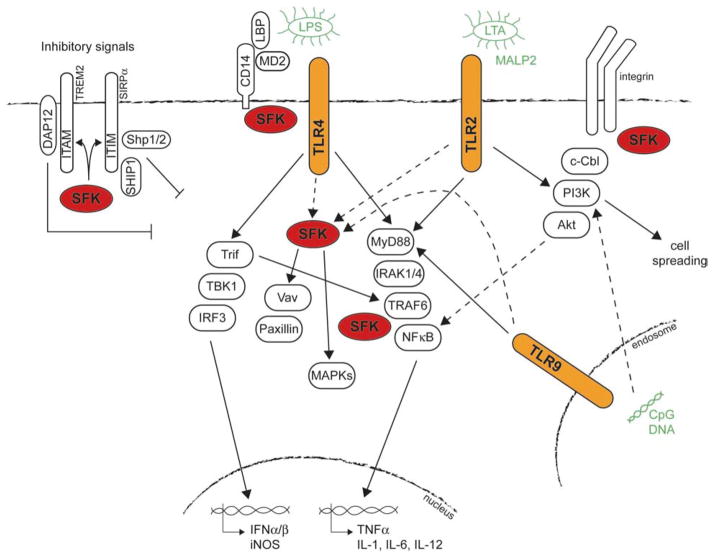
Macrophages express many members of the Toll-like receptor (TLR) family, which recognize a diverse array of pathogen-associated molecules (reviewed in 104). Ligand binding to TLRs leads to recruitment of adapter molecules such as MyD88 or TIR (Toll-IL-1 receptor) domain-containing adapter inducing interferon beta (TRIF) through homophilic interactions between TIR domains. These adapter molecules bring in other downstream signaling proteins such as TNF receptor-associated factor 6 (TRAF6) and IL-1 receptor-associated kinases (IRAKs), activation of which results in stimulation of the NF kappa B pathway, and the MAPK kinases p38, ERK and Jun N-terminal kinase (JNK) (Figure 3). Activation of the TLR pathway in macrophages can be measured by increased cytokine production including TNF alpha, IL-1, IL-6 and IL-12 and production of reactive oxygen (ROS) and nitrogen species. Treatment of macrophages with lipopolysaccharide (LPS), a major component of the cell wall of Gram-negative bacteria, and a ligand for TLR4, has long been known to lead to a rapid induction of tyrosine phosphorylation (105). Treatment of macrophages with other TLR ligands such as lipoteichoic acid (LTA), a component of Gram-positive bacterial cell walls and CpG DNA, a motif abundant in bacterial genomes, which are ligands for TLR2/6 and 9 respectively, also induce tyrosine phosphorylation (106–108). Src kinases Lyn and Hck are activated following treatment of macrophages with LPS (18, 109–111) and CpG (112). Furthermore, treatment of macrophages with ligands for several different TLRs leads to tyrosine phosphorylation of Src kinase substrates, such as Vav, Pyk2, Cbl, Syk and paxillin (38, 106–108, 112–115). Many of these studies demonstrated that increases in phosphorylation are inhibited if cells are pretreated with inhibitors specific for SFKs such as PP1 or PP2. LPS treatment also leads to tyrosine phosphorylation of TLR2 and 4 themselves, which is thought to be mediated by SFKs. COS cells transiently transfected with the coreceptors CD14 and MD2 along with either TLR2 or 4 show increased tyrosine phosphorylation following LPS stimulation that is sensitive to inhibition by PP1 or PP2 (116). In addition, TLR9 is tyrosine phosphorylated upon CpG stimulation. SFK-mediated activation of Syk may contribute to the tyrosine phosphorylation of both TLR4 and 9 (112, 117). Looking further downstream, pretreatment of macrophages with PP1 inhibits both LPS-induced Ras activation, TNF alpha secretion and nitric oxide (NO) release (17, 118–120) and CpG-induced TNF alpha and IL-6 production, inducible nitric oxide synthase (iNOS) accumulation and NF kappa B activation (107, 112). However, because PP1 and PP2 can inhibit p38 MAPK (Figure 1C), many of these results may be due to off-target effects of the inhibitors.
Figure 3.
Involvement of SFKs in TLR signaling in macrophages. Signaling downstream of TLRs 2, 4 and 9 in response to their respective ligands leads to activation of MAP kinases, production of cytokines, and actin cytoskeletal changes. Studies mainly using SFK inhibitors such as PP1, PP2 and SU6656 have shown that SFKs are involved in many of these responses, however experiments with SFK-deficient mice have produced different results (indicating no differences or even paradoxical increases in TLR type signaling) hence the controversy regarding the role of SFKs in TLR signaling. There are several ways in which SFKs may modulate TLR signaling, both positively and negatively, rather than being directly involved. CD14 binds to SFKs, which could recruit the SFKs to TLR4 during LPS stimulation and therefore be involved in proximal TLR signaling events, such as phosphorylation of substrates such as Vav and paxillin. TLR2 and 9 do not require co-receptors, but may recruit SFKs by clustering with other receptors such as integrins that are known to signal through SFKs. SFKs have also been shown to act more distal to TLRs, possibly interacting with downstream molecules such as TRAF6. Furthermore, through phosphorylation of ITAMs or ITIMs, SFKs can provide signals that down-modulate signaling through TLRs. Solid arrows indicate well-defined pathways, while dotted arrows indicate potential roles by which SFKs may modulate specific TLR pathways.
SFKs have also been implicated in TLR signaling pathways by modulating their expression. Overexpression of an activated mutant of Hck in the macrophage cell line BAC1.2F5 leads to increased TNF alpha production following LPS treatment, whereas antisense oligonucleotides that reduce Hck expression reduce LPS-induced TNF alpha production (121). RNAi knockdown of Hck and Lyn in the human monocyte cell line THP1 blocks CpG-induced TNF alpha production (112). LPS treatment of the macrophage cell line J774A.1 engineered to overexpress Csk leads to reduced production of TNF alpha, iNOS, IL-1 alpha and IL-6 (122). Csk is tyrosine phosphorylated following LPS stimulation of macrophages, but the role of Csk in TLR-mediated cytokine production is unclear. RNAi knockdown of Csk in RAW264.7 macrophages, which should lead to increased SFK activity and therefore increased cytokine production, actually leads to reduced LPS-induced cytokine production (38). This could be mediated by a reduction in Fgr protein levels observed in macrophages expressing lower levels of Csk. Treatment of macrophages with TLR ligands LPS or CpG DNA causes increased spreading and adhesion that is inhibited by RNAi knockdown of Hck and Lyn, or treatment with PP1 or PP2 (108, 112, 114, 115). Scholz et al showed that Hck phosphorylates Cbl upon LPS treatment, which leads to recruitment of PI3K (114), a signaling pathway previously implicated in cell spreading downstream of beta 1 integrins (60). Together, these types of experiments have lead to the general notion that SFKs play a central role in various aspects of TLR signaling in macrophages.
Surprisingly, none of the above observations are supported by studies using primary macrophages from SFK knockout mice. Macrophages that lack the three most highly expressed SFKs, Hck, Fgr and Lyn, respond normally or, in the case of PEMs, are even hyperresponsive to LPS treatment, despite reduced cellular tyrosine phosphorylation in both resting and stimulated cells (16). While no compensatory changes in other SFKs were reported in initial studies with hck−/−fgr−/−lyn−/− macrophages, subsequent experiments have suggested that Src kinase activity itself is increased in the triple mutant cells (C. A. L. unpublished observations). Indeed, there are two reports that stimulation of RAW264.7 macrophages or primary PEMs with LPS leads to upregulation of Src, suggesting that this kinase may compensate for deficiency of Hck, Fgr and Lyn in TLR signaling responses (17, 120). However, limited studies examining bone marrow derived macrophages from quadruple mutant src−/−hck−/−fgr−/−lyn−/− have failed to reveal major defects in LPS induced NO production or cytokine release (C. A. L. unpublished observations). Thus the overall role of SFKs in TLR signaling pathways remains somewhat nebulous.
The hyperresponsiveness of hck−/−fgr−/−lyn−/− PEMs brings up two interesting questions. Firstly, the fact that responses of BMDMs and PEMs are different suggests that there are signaling differences between these two types of macrophages. The pattern of tyrosine phosphorylated proteins produced following LPS stimulation of these two types of macrophages is clearly distinct (16). Furthermore, LPS-stimulation of Kupffer cells leads to TNF alpha production and this is unaffected by PP1 pretreatment (123). A comparison of different macrophage subsets may shed more light on this confusing area. Secondly, the mechanism of SFK-mediated negative regulation of TLR signaling is unclear. The hyperresponsiveness to LPS seen in PEMs could be due to lack of Lyn, and therefore reduced phosphorylation of ITIM-containing molecules and failure to recruit phosphatases such as Shp1 and 2, and SHIP1 that can down modulate various signaling pathways. In support of this, LPS-stimulation of RAW264.7 macrophages that overexpress Shp1 show reduced TNF alpha and iNOS production (124), and reduced expression of either Shp2 or SHIP1 using siRNA leads to increased LPS responses although this may be phosphatase-independent (125, 126). Alternatively, ITAM-containing proteins such as DAP12 can also have a negative regulatory role in signaling downstream of TLRs (127). This function of DAP12 is dependent on tyrosine phosphorylation of its ITAM, thus implicating a negative role for SFKs. Further experiments are needed to distinguish between these possibilities.
How does TLR signaling activate SFKs? Given our present understanding of TLR signaling from MyD88 and related adapters to TRAF6 activation, the involvement of IRAK1 and 4, and ultimately the phosphorylation of I kappa B kinase (IKK), it is unclear where to place SFKs in this pathway (Figure 3). The glycosylphosphatidylinositol (GPI)-linked cell surface receptor CD14, which is known to associate with many SFKs, functions as an important co-receptor in TLR signaling and may be the receptor that is involved in activation of tyrosine kinase pathways downstream of TLRs (see section 3.4). Surprisingly, tyrosine phosphorylation resulting from CpG stimulation of macrophages occurs in the absence of either TLR9 or MyD88 implying that SFKs are involved in a distinct pathway that appears to be important for actin cytoskeletal rearrangements (112). This pathway involves PI3K and Akt, and is required for downstream activation of NF kappa B. Tyrosine phosphorylation of MAPKs in response to LPS is lost in macrophages lacking TLR4, but is only delayed in MyD88-deficient macrophages (128) suggesting that SFKs can act downstream of multiple TLR adapter proteins. Kawai et al showed few other changes in total cellular tyrosine phosphorylation in response to LPS, but the basal level of phosphorylation was high in both wild type and MyD88-deficient macrophages in these experiments. SFK-mediated phosphorylation of paxillin occurs in macrophages treated with either MALP2 (a TLR2 ligand) or LPS (106). TLR2 signaling is dependent on MyD88, whereas TLR4 can use other adapter proteins and this is reflected in the response of MyD88-deficient macrophages to the different TLR ligands. Paxillin phosphorylation is lost in MyD88-deficient macrophages treated with MALP2, but unaffected in LPS treated cells (106). Surprisingly, LPS-stimulated tyrosine phosphorylation of Vav is dependent on MyD88 (129). LPS stimulation of iNOS occurs by a MyD88-independent, TRIF-dependent pathway that is sensitive to inhibition by PP1 and SU6656. Expression of constitutively active TRIF also induces iNOS, and this can be inhibited with PP1 and SU6656, which places SFKs downstream of TLR adapters (120). Hazeki et al showed that PP2 does not inhibit MALP2 stimulation of IRAK activation, suggesting that SFKs act downstream of IRAK activation (106). Of course, given the potential off target effects of these inhibitors, it is possible that other downstream kinases are being inhibited in these experiments.
Overexpression studies in HEK293 cells have demonstrated that the SH3 domain of Src can associate with TRAF6, a key molecule involved in downstream signaling pathways from TLRs (130) suggesting that TRAF6 could be the connection to SFKs. However, Mukundan et al report not being able to see an association between Src and TRAF6 in monocytes or macrophages (131). Interactions between Src and TRAF6 have been observed downstream of the IL-1 receptor, which uses a similar signaling pathway to TLR4 utilizing MyD88, in other cell types including hepatocytes and osteoclasts (130, 132, 133). Src has also been shown to associate with IKK when overexpressed in HEK293 cells (134). In summary, despite abundant experiments implicating SFKs in different TLR pathways, an overall picture of how these kinases fit into the complex signaling pathways downstream of pattern recognition receptors remains very unclear. The potential involvement of SFKs at multiple steps in the TLR signaling pathway is summarized in Figure 3.
Perhaps an alternative view to the role of SFKs in TLR responses is to invoke these kinases in the regulation of other pathways that are indirectly or secondarily activated by TLR agonists. Rather than functioning in a membrane or TLR proximal step, SFKs may be more important in modulating downstream responses. Such crosstalk is observed in macrophages that undergo oxidative stress and are then primed to respond to lower doses of LPS, which allows for more robust inflammatory responses under stress conditions. At low doses of LPS, PP2 does not inhibit activation of p38 or NF kappa B, however, pretreatment of macrophages with H2O2 in order to induce oxidative stress causes low dose LPS signaling to become SFK-dependent (135, 136). This is also observed in an in vivo model (see section 4.2). Crosstalk between TLRs and other cell surface receptors such as integrins, perhaps mediated by clustering, could explain SFK involvement downstream of TLR signaling. Plating THP1 cells onto fibrinogen leads to Mac1-dependent NF kappa B activation that is mediated by Mac1 association with IRAK1, and activation of TRAF6 and transforming growth factor beta activated kinase 1 (TAK1) (137). Perhaps SFKs are required for the transient cytoskeletal changes that occur to allow spreading, or initiate migration in response to TLR stimulation and these changes are required for other signals to be propagated. Production of TNF alpha as a result of TLR signaling could also lead to subsequent integrin activation and cell spreading which are known to require SFKs. Binding of Porphyromonas gingivalis fimbriae to TLR2 initiates a Mac1-dependent spreading response that is dependent on PI3K, however the role of SFKs was not assessed in this study (138). Ligands may activate several pathways downstream of TLRs that can be modulated by SFKs. Leu et al noted that over a long time course of LPS treatment, there are several waves of protein tyrosine phosphorylation (17). Perhaps a more detailed kinetic analysis of TLR-agonist-induced tyrosine phosphorylation of SFKs and their substrates would provide further insight into whether SFKs function as critical components of specific signaling steps downstream of TLRs versus functioning as overall modulators of related intracellular pathways that indirectly affect TLR signaling.
3.3.2. Fc receptors
Macrophages express Fc receptors for IgA, IgE and IgG on their cell surface. Of these, most research has addressed signaling by IgG FcRs (which in the mouse includes the activating receptors Fc gamma RI, Fc gamma RIII and Fc gamma RIV; and the inhibitory receptor Fc gamma RUB which will be discussed in section 3.3.3). Crosslinking of Fc gamma Rs by immune complexes, or binding of antibody-opsonized particles on the surface of macrophages leads to the activation of SFKs (139, 140). This results in phosphorylation of ITAM-containing proteins such as the Fc receptor common gamma-chain (known as FcR gamma, Figure 2), which then recruits Syk and initiates a cascade of downstream signals that result in phagocytosis and cellular activation (such as iNOS upregulation, oxidative burst and cytokine production). Both SFKs and Syk are recruited to actin-rich phagocytic cups during IgG-dependent phagocytosis (45). Fc gamma R signaling has been studied in macrophages from mice lacking SFKs. BMDMs lacking both Hck and Fgr show wild type levels of signaling and phagocytosis (10). Macrophages deficient in Lyn show modest proximal signaling defects but phagocytosis is normal (141). hck−/−fgr−/−lyn−/− macrophages can phagocytose particles, but with delayed kinetics. A similar defect is seen in src−/−hck−/−fgr−/−lyn−/− macrophages and furthermore treatment of macrophages with PP1 does not completely block Fc gamma R-mediated phagocytosis. In hck−/−fgr−/−lyn−/− macrophages, actin cup formation is delayed and early proximal signaling such as gamma-chain and Syk phosphorylation, as well as PI3K and ERK activation are impaired, which leads to a defective respiratory burst following Fc gamma R engagement. Similarly, PP1 inhibits the Fc gamma R-mediated respiratory burst in both U937 cells and primary human macrophages (142, 143). This data suggests that SFKs are important but not essential for proximal signaling and phagocytosis downstream of Fc gamma Rs, but are required for activation of NADPH oxidase leading to respiratory burst. In contrast, the downstream kinase Syk is absolutely required for phagocytosis (144, 145). In the absence of SFKs, Syk can autophosphorylate inefficiently, but its activity appears to be enough for phagocytosis to occur. This probably occurs due to a low level ability of Syk to phosphorylate the FcR gamma ITAM, which then recruits more Syk molecules to the phagocytic cup to a point that a threshold level of signaling is reached that is sufficient for phagocytosis. Hence, in the Fc gamma R pathway, SFKs may function mainly to accelerate phagocytosis by efficiently phosphorylating ITAMs in FcR gamma, leading to rapid Syk recruitment and activation. The ability of SFKs to turn up the “gain” of Fc gamma R signaling, by rapid ITAM phosphorylation, may be especially important at low levels of Fc gamma R crosslinking, for example when a macrophage encounters a pathogen that is only weakly opsonized by IgG. Hence, SFKs may be important to allow macrophages to recognize IgG-opsonized pathogens early in the immune response. However, a direct comparison of the phagocytic phenotypes of hck−/−fgr−/−lyn−/− versus syk−/− macrophages reveals that SFKs are doing more in the Fc gamma R pathway than just phosphorylating ITAMs. While hck−/−fgr−/−lyn−/− macrophages show slow actin cup formation and delayed phagocytosis, syk−/− cells show normal actin cup formation but have a complete block in the subsequent steps of phagocytic vesicle closure. Thus the block in phagocytosis in these two cell types is different, with SFKs playing a role in both actin polymerization dynamics and ITAM phosphorylation (141).
The role of SFKs in Fc gamma R-mediated phagocytosis has also been studied by overexpression of Csk. Expression of either wild type or constitutively membrane-bound forms of Csk, which should inhibit SFK activity, blocks phagocytosis in RAW macrophages (34). Given the conclusion that SFKs are not absolutely required for Fc gamma R phagocytosis in primary macrophages, this data may suggest that Csk has other targets in addition to SFKs. Alternatively, differences between phagocytic signaling in the RAW macrophage cell line versus primary BMDMs may account for these differences.
Independently of other SFKs, Fgr has also been shown to exert a negative regulatory effect on Fc gamma R-mediated (and CR3-mediated) phagocytosis by binding to the ITIM-containing molecule SIRP alpha and increasing the association of SIRP alpha with Shp1, however this effect is independent of the kinase activity of Fgr (92). The mechanism of this potential negative regulatory function for Fgr in the IgG-mediated phagocytic pathway remains very unclear.
Fc alpha Rs and Fc epsilon Rs also signal through the ITAM-containing FcR gamma subunit; crosslinking of either of these receptors leads to increased cellular tyrosine phosphorylation. PP1 and PP2 inhibit the tyrosine phosphorylation and respiratory burst resulting from crosslinking Fc alpha Rs in human monocyte/macrophages (146). Depending on the ligand, Fc alpha Rs can induce positive or negative effects on immune cell function and the extent of ITAM phosphorylation appears to be a critical distinction (147). The role of SFKs downstream of the Fc epsilon R has not been studied in macrophages, although studies in mast cells, which respond robustly to Fc epsilon R engagement, have demonstrated an important role for Lyn and Fyn in downstream pathways from this receptor (148).
3.3.3. Activating and inhibitory immunoreceptors
Macrophages express many C-type lectin receptors on their cell surface (reviewed in 149). Dectin-1 binds beta-glucan and is the primary receptor on macrophages for phagocytosis of various fungi and yeast particles. Its cytoplasmic tail contains an ITAM-like sequence that is required for signaling and the phagocytic function of the receptor (Figure 2B) (150). Both SFK and Syk are activated when macrophages are fed zymosan. Syk activation is blocked when macrophages are pretreated with PP2, and although internalization of zymosan still occurs, ROS production is also blocked (151). SFKs appear to be playing a similar role to that in Fc gamma R-mediated phagocytosis i.e. they are important but non-essential for phagocytosis, but are required for oxidative burst. Dectin-2 is also expressed on the surface of activated macrophages where it recognizes the hyphal forms of several different yeasts. The short cytoplasmic domain of Dectin-2 lacks any signaling motifs, but the receptor was found to associate with the FcR gamma chain, and signal through an ITAM-like pathway (Figure 2B) (152). Dectin-2 signaling has not been studied in macrophages deficient in SFKs, but PP2 inhibits the tyrosine phosphorylation of FcR gamma, the internalization of ligand-bound dectin-2 and production of the cytokines IL-1ra and TNF alpha in RAW macrophages treated with C. albicans hyphae, thus suggesting an important role for SFKs.
The family of triggering receptors expressed on myeloid cells (TREMs) includes TREM1, 2 and 3 (reviewed by 153). TREM1 and 2 are known to associate with the ITAM-containing adapter DAP12 in order to couple to downstream signaling pathways (Figure 2B). TREM1 acts synergistically with receptors such as TLRs to enhance inflammatory responses. Crosslinking of TREM1 results in increased cellular tyrosine phosphorylation (154). TREM2 has the opposite effect; engagement of this receptor limits macrophage activation in response to TLRs and FcR ligands (155, 156). Given that SFKs can phosphorylate the ITAM of DAP12, exactly how DAP12 mediates both activation and inhibition of signaling downstream of TREM1 and 2 respectively is under investigation.
Macrophages express several inhibitory immunoreceptors that contain an ITIM in their cytoplasmic domains and limit inflammatory responses, including SIRP alpha, PIR-B, myeloid-associated Ig-like receptor (MAIR-I) and Fc gamma RIIB (Figure 2D) (157). The ability of SFKs to phosphorylate ITIMs, which leads to recruitment of protein phosphatases e.g. Shp1 and Shp2, and lipid phosphatases e.g. SHIP1 and downregulation of signaling pathways, suggests that SFKs have an important role in negative signaling in addition to positive signaling. In Lyn-deficient macrophages, adhesion-dependent tyrosine phosphorylation of both SIRP alpha and PIR-B is reduced (88). This leads to more rapid cell spreading and increased adhesion of lyn−/− macrophages (and granulocytes) to surfaces that engage their beta 2 integrins. This result correlates with the hyperadhesive phenotype seen in Shp1-deficient macrophages (158). Similarly, phosphorylation of ITIM-containing receptors by Lyn following GM-CSF has been suggested to explain the hyperproliferative response of lyn−/− macrophages to this cytokine (71).
3.4. Other macrophage receptors
CD40 is a member of the TNF receptor superfamily that, together with its ligand CD154, enhances the inflammatory responses of a variety of cell types including macrophages. Stimulation of CD40 on monocytes with soluble crosslinked CD154 induces cellular tyrosine phosphorylation and leads to the production of inflammatory cytokines. Pretreatment with the SFK inhibitor PP2 prior to stimulation inhibits the production of IL-1 beta and TNF alpha, thus implicating SFKs in signaling downstream of CD40 (131). Increased phosphorylation and activation of SFKs is observed upon CD40 stimulation, which is required for subsequent ERK activation. Binding of TRAF6 to CD40 is required for downstream signaling to ERK1 and 2. Although a TRAF6 binding protein can disrupt the association of TRAF6 with CD40 and block downstream signaling, it does not interfere with Src activation, placing Src upstream of TRAF6. Src has been shown to interact directly with TRAF6 in overexpression studies (130, 133), but this has not been observed in primary macrophages. SFKs have also been implicated in cell survival pathways downstream of TNF family receptors such as receptor activator of NF kappa B (RANK) in osteoclasts; expression of a truncated Src that lacks the kinase domain in osteoclasts of src−/− mice reduces Akt activation, decreases osteoclast survival and exacerbates the osteopetrotic phenotype of src−/− mice (159).
GPI-linked receptors lack cytoplasmic domains and therefore must couple with other receptors to signal. For example, CD14 is a co-receptor for LPS along with TLR4, LPS-binding protein and MD-2. The SFK Lyn, but not Hck and Fgr, co-precipitates with CD14 in human monocytes, while LPS stimulation leads to increased kinase activity of CD14-associated Lyn (110). The mechanism of the interaction is not clear; perhaps clustering in lipid rafts is required. Evidence suggests that the GPI-linked receptor, urokinase plasminogen activator receptor (uPA-R or CD87) links to integrins to influence cellular responses (160). The protease activity of the ligand is activated upon binding to the receptor but signaling through the receptor is initiated in myeloid cells treated with either activated or non-activated ligand, resulting in increased tyrosine phosphorylation, cell adhesion and chemotaxis. uPA-R has been reported to be present in a large complex containing the SFKs Hck, Fgr, Lyn and Fyn, along with beta 2 integrins Mac1 and LFA1 in monocytes (161). Similarly, activation of the SFK Hck occurs following urokinase treatment of U937 cells (162). Clustering of proteins within lipid raft domains may allow receptors access to a variety of downstream signaling molecules.
Scavenger receptors are transmembrane proteins that recognize a wide variety of ligands including oxidized low-density lipoproteins (LDL), apoptotic cells and pathogens. There are several subclasses of receptors that share little sequence homology (163). There are several examples of SFK involvement downstream of these receptors. The ligand acetyl low-density lipoprotein binds to Scavenger receptor A (SR-A) on THP1 cells and induces increased cellular tyrosine phosphorylation and activation of Lyn. SR-A and Lyn were found to be constitutively associated in these cells (164). CD36 is a Class B scavenger receptor and its ligand is fibrillar beta-amyloid. Binding of ligand to peritoneal macrophages results in increased cellular tyrosine phosphorylation, activation of ERK1/2, production of ROS and secretion of chemokines such as MCP-1 (165). These responses are blocked by pretreatment with PP1, and reduced in macrophages lacking either Fyn or Lyn suggesting SFKs are involved in signaling downstream of CD36. In contrast to SR-A, Lyn and CD36 co-immunoprecipitate upon ligand engagement (165, 166). The fibrillar form of apolipoprotein C-II results in similar macrophage responses that are mediated by CD36 binding and Lyn-dependent ERK activation (167). Scavenger receptors are also involved in the uptake of apoptotic cells, along with many other cell surface receptors including integrins and Fc receptors (reviewed in 168). Hu et al reported that peritoneal macrophages are much more efficient at phagocytosis of apoptotic cells than resident alveolar macrophages (169). No differences are observed between the two types of macrophages in their rates of phagocytosis of IgG-opsonized sheep red blood cells. Phagocytosis of apoptotic cells by alveolar macrophages is sensitive to inhibition by PP2 whereas peritoneal macrophages are not, indicating that multiple signaling pathways are involved with different requirements for SFKs (170).
Antigen presenting cells (APCs), such as macrophages and dendritic cells, express major histocompatibility complex (MHC) Class II molecules on their surface to present peptide antigens to CD4 T cells. A well known series of signaling events occurs downstream of the T cell receptor in the T cell upon binding of peptide-MHC complexes (50). Less well understood is the signaling that occurs in the APC (171). Cross linking MHC class II molecules on the cell surface of macrophages also leads to an increase in tyrosine phosphorylation and upregulation of cytokine release, TNF alpha and IL-1 beta (172–174). Pre-treatment of cells with the SFK inhibitor PP1 blocks both tyrosine phophosphorylation and cytokine release. The cytoplasmic tails of MHCII molecules are short and are indispensable for signaling; studies in B cells have shown that the transmembrane domain is required for these signals to occur (175). In B cells, ITAM containing molecules Ig alpha and beta have been shown to be required for signal transduction by directly associating with the Class II molecules (176). Perhaps a similar mechanism is used by macrophages through engagement of DAP12 or FcR gamma molecules. The MHCII molecules may be recruited to or present in lipid rafts in order to signal through SFKs (177, 178).
4. ROLE OF SRC FAMILY KINASES IN MACROPHAGES IN VIVO
4.1. Migration and infection models
The role of macrophages in in vivo disease models is often studied using the Csf1op/Csf1op mouse that lacks M-CSF, thus reducing macrophage development. Some macrophages do develop in these mice, perhaps because of regulation by GM-CSF or additional ligands that can bind to the M-CSFR (such as the recently described novel cytokine FPT025: see ref 179). Since the phenotype of mice lacking the MCSF-R is more severe than that seen in Csf1op/Csf1op animals it is likely that additional cytokines are recognized by the receptor (180). The macrophages that do develop in the Csf1op/Csf1op mice mostly function normally, except for reduced cytokine production. In many infection or inflammation models, it is hard to separate the role of macrophages from neutrophils although selective macrophage ablation using liposome-mediated delivery of the bisphosphonate Clodronate has been used to study macrophage-specific functions (181). There is no mouse model that lacks SFKs in macrophages alone, thus making efforts to directly address the function of these kinases in this cell type in vivo difficult. It is also difficult to separate positive and negative regulatory signaling pathways in these models. It is clear that more specific models need to be generated in order to ascertain the role of SFKs in macrophage biology during disease. Below, we highlight some macrophage-dependent disease models where the role of SFKs has been addressed.
Macrophage migration in vivo is commonly assessed using the thioglycollate peritonitis model; macrophage recruitment into the peritoneum is observed around 24 hours and peaks within 72 hours following injection. Single mutant mice that lack just Hck, Fgr, or Lyn alone, or double mutant mice that lack both Hck and Fgr, display no defects in macrophage migration in the peritonitis model. However, hck−/−fgr−/−lyn−/− mice show a 2–3 fold reduction in macrophage recruitment (60). This is distinct from neutrophils that do not appear to require SFKs for migration (182).
In an infection model using Listeria monocytogenes, where initial responses to infection are known to be mediated by the innate immune response including macrophages, mice lacking Hck and Fgr are more susceptible to Listeria infection, with a reduced ability to kill pathogens that leads to reduced survival (10). The response of hck−/−fgr−/−lyn−/− mice to Listeria infection is unknown.
Low titer encephalomyocarditis virus (EMC-D) infection-induced diabetes is a macrophage-dependent disease model (183). Selective infection of pancreatic beta-cells with the virus results in initial recruitment of macrophages, and production of inflammatory mediators such as TNF alpha, IL-1 beta and iNOS by activated macrophages, which ultimately destroy the beta-cells, resulting in diabetes. Depletion of macrophages in this model by treatment with anti-Mac1 antibodies prevents development of diabetes (183). Choi et al found that Hck is activated in PEMs from EMC-D-infected mice (184). Treatment with the SFK inhibitor PP2 reduces Hck activation, reduces expression of inflammatory cytokines and decreases the incidence of diabetes. In addition, transfer of macrophages from mice infected with EMC-D into mice pretreated with a subdiabetogenic dose of streptozotocin, which induces minor damage to the pancreas enough to cause recruitment of macrophages, is sufficient to transfer diabetes. Infection of mice lacking SFKs, in particular Hck, with low dose EMC-D has not been reported.
4.2. Endotoxic shock models
Endotoxic shock in mice is modeled by either intraperitoneal or intravenous injection of bacterial LPS, after which systemic production of inflammatory cytokines can be followed. In the intraperitoneal model, pretreatment of mice with PP2 prior to LPS injection results in significantly reduced levels of TNF alpha, suggesting that SFKs play a role in TLR-mediated cytokine production in vivo (17). Based on in vitro data with hck−/−fgr−/−lyn−/− macrophages, one would expect that LPS administration to mice lacking SFK activity (either via knockouts or treatment with PP2) would result in normal or even increased TNF alpha production. Indeed, mice deficient in both Hck and Fgr do manifest normal serum TNF alpha responses following in vivo administration of LPS (185), while LPS injection experiments with triple mutant hck−/−fgr−/−lyn−/− animals have not been performed. Interestingly, both animals treated with PP2 or lacking Hck and Fgr kinases have reduced mortality in the endotoxin model, despite the differences in serum TNF alpha levels (185, 186). Since mortality in this model is mediated mainly by inflammatory cell migration into tissues (predominantly the liver and kidney), it is possible that resistance to LPS in hck−/−fgr−/− mice is due to reduced infiltration of inflammatory cells (neutrophils and macrophages) into tissues as a result of impaired integrin outside-in signaling. In these studies the hck−/−fgr−/− mice did display reduced release of liver and kidney enzymes, consistent with reduced tissue damage, following LPS injection. One is left to explain the results with SFK inhibitors in LPS endotoxic shock models by hypothesizing that the compounds must be affecting other signaling pathways, such as p38 MAPKs, to reduce cytokine responses and protect animals from LPS toxicity. Mice lacking just one SFK respond like wild type mice to the high dose LPS, indicating compensation by other family members (185). Conversely, mice expressing a constitutively active Hck are more susceptible to LPS-mediated endotoxic shock, and show increased levels of serum TNF alpha following LPS injection (33). Whether this is due to the enhanced ability of macrophages carrying an activated version of Hck to migrate into tissues, thus causing more tissue injury following LPS administration, or due to a primary role for SFK in the TLR4 signaling pathway remains unclear. There was no difference between the response of wild type or hck−/−fgr−/− mice treated with low dose LPS following d-galactosamine sensitization, which impairs liver metabolism (185).
Intranasal administration of LPS has been used as a model of endotoxin-mediated acute lung injury. In this model, delivery of LPS to the lung leads to robust inflammatory cell infiltration, cytokine production and pulmonary edema. Pretreatment with Src inhibitors SU6656 or PP2 prevents the increase in TNF alpha and IL-6 seen in the lungs and serum of untreated mice (186). In addition, expression of dominant negative Src in the lungs by adenoviral infection can inhibit LPS-induced cytokine production. In a pulmonary endotoxic shock model, when mice are subjected to oxidative stress, such as ischemia-reperfusion, the subsequent response to low dose LPS is enhanced (187). These mice show robust pulmonary inflammation and mortality. In this model, Hck is activated in alveolar macrophages from mice that undergo ischemia-reperfusion, which is prevented by PP2 treatment. A modest reduction in subsequent LPS-mediated lung injury is also observed in the PP2 treated mice. In both these models, LPS could be acting on a combination of cell types to induce inflammatory responses, including alveolar macrophages and lung epithelial cells. It is interesting to note that mice expressing a constitutively activated form of Hck show spontaneous lung disease, with increased monocyte infiltration, and intranasal treatment with LPS in these mice caused greatly increased cytokine production (33). In addition, mice with a conditional deletion of Csk driven by granulocyte elastase-Cre spontaneously develop lung inflammation suggesting that hyperactivation of SFKs can directly induce lung injury (32). However, in mice expressing activated SFKs in macrophage/neutrophil lineages, it is quite possible that these cells have enhanced migratory responses and are thus entering tissues, such as the alveolar space, more readily in response to tonic inflammatory stimuli. Thus, the mechanisms by which SFKs act in these inflammatory models (either within the TLR pathway or the integrin pathway) remain to be defined.
4.3. Human immunodeficiency virus models
Nef is an important determinant for human immunodeficiency virus (HIV) pathogenesis, affecting viral load and disease progression. Nef causes increased viral replication in both macrophages and lymphocytes. Expression of nef as a transgene under the CD4 promoter leads to an acquired immunodeficiency syndrome (AIDS)-like disease (188). Nef contains a proline-rich region that can bind SH3 domains, and its association with Hck resulting in kinase activation has been well documented (Figure 1) (189). Of the target cell types infected with HIV, the interaction between Nef and Hck occurs in macrophages but not in CD4 T cells. Expression of Nef in RAW264.7 or THP1 macrophage cell lines leads to ERK activation, which can be blocked by expression of dominant negative Hck (190). Hanna et al showed that the proline-rich region of Nef is critical for its ability to cause symptoms in transgenic mice (191). To address the role of Hck in Nef-mediated disease, mice deficient in Hck were crossed with a strain of mice expressing a mutated HIV genome that mirrors the disease seen in Nef transgenic mice. There is no difference in pathological changes between wild type and hck−/− mice, but hck−/− mice show a significant delay in mortality (191). Perhaps crossing the Nef transgenic with mice deficient in multiple SFKs would show a more profound effect on disease. Using an inducible Nef construct in a myeloid cell line, Suzu et al suggested that Nef prevents M-CSFR signaling to Hck, resulting in reduced proliferation and differentiation of macrophages (192).
4.4. Src family kinases and leukemia
A role for SFKs in various types of myeloid leukemia has been demonstrated through the use of Src inhibitors in models of these diseases. Chronic myeloid leukemia (CML) is caused by the Philadelphia chromosome translocation that results in a Bcr-Abl fusion protein with increased kinase activity. Abl kinase inhibitors such as imatinib have been used successfully to treat CML in patients. SFKs do not seem to have a role in initial stages of the disease as hck−/−fgr−/−lyn−/− mice can still develop CML (193). Unfortunately, imatinib resistant CML is often observed, due to either mutations in Abl that prevent interaction with the inhibitor, or increases in SFK expression, such as Lyn (194). Bcr-Abl is known to activate SFKs, Hck and Lyn. Dual specificity inhibitors, which target both Abl and Src kinases are now being used clinically with significant efficacy, implicating SFKs in disease progression (195).
Bcr-Abl fusions are also etiologic in forms of acute lymphocytic leukemia (ALL), which can be modeled in mice by retroviral transduction of bone marrow with viruses encoding the Bcr-Abl protein. Surprisingly, hck−/−fgr−/−lyn−/− mice fail to develop ALL in this disease model (193), presumably because of expression of Fgr and Lyn in B-lymphocytes. Pharmacologic blockade of SFKs reduces disease progression in the Bcr-Abl infected mice (196).
Acute myeloid leukemia (AML) can be caused by a variety of different mutations, one of the most common is internal tandem duplications of the receptor tyrosine kinase FLT3 (FLT3/ITD) leading to its constitutive activation. 32D cells expressing FLT3/ITD become IL-3 independent. FLT3/ITD associates with Lyn, causing its phosphorylation. Inhibition of SFKs either by PP2 treatment or siRNA suppresses the growth of FLT3/ITD expressing 32D cells. PP2 treatment of mice injected subcutaneously with FLT3/ITD expressing 32D cells blocks tumor formation (197). Thus, in these models, it appears that SFK do not play a major role in the initiation of tumor formation but have a significant effect on tumor progression. Similar results have been suggested for other tumor types involving SFKs (4).
4.5. Other disease models
Scavenger receptors on macrophages such as CD36 and MSR-A recognize and internalize oxidized LDL to become foam cells, which can accumulate in arterial walls producing fatty streaks, a precursor to atherosclerotic lesions. Macrophages play an important role in the metabolism of serum lipids as evidenced by the fact that the incidence of atherosclerotic lesions is reduced in susceptible mice such as apolipoprotein E-deficient animals that are also macrophage-deficient (198). A recent study demonstrated that Lyn associates with CD36, and found inhibition of SFKs reduced CD36-dependent foam cell formation (166). Lyn can also associate with MSR-A (164). Miki et al investigated the incidence of atherosclerosis in Lyn-deficient mice. No differences in development of atherosclerotic lesions are observed between wild type or lyn−/− mice fed either a normal, or a high fat diet, despite the fact that lyn−/− mice have significantly higher serum cholesterol levels. This may be partly explained by the observation that lyn−/− PEMs showed reduced proliferative responses to oxidized LDL and reduced CD40-dependent MCP-1 production in vitro (199).
An in vivo model of Alzheimers Disease, which is induced by injection of fibrillar beta-amyloid into the brain resulting in recruitment of microglial cells, is dependent on signaling through CD36. Mice deficient in Lyn showed a reduction in microglial recruitment. Chemotaxis of lyn−/− microglia to MCP-1 in vitro is not defective, but lyn−/− microglia secrete less MCP-1 in response to fibrillar beta-amyloid treatment (165).
5. PERSPECTIVE
In this review, we discuss the role of SFKs in signaling pathways downstream of the major classes of macrophage cell surface receptors involved in development, adhesion, migration and immune cell function. These kinases have been implicated in many pathways through the use of various tools such as small molecule inhibitors, siRNA inhibition, expression of regulatory proteins (CD45 and Csk) and genetically deficient mice. These different approaches have led to some controversy, such as the role of SFKs in TLR signaling. Indeed, in the TLR pathway it seems that SFKs can influence the signaling response at steps everywhere from the membrane to the activation of NF kappa B. Moreover, in this pathway as well as in other pathways (such as Fc receptor-dependent phagocytosis) there are apparently contradictory results involving positive and negative regulatory functions of SFKs such as Fgr. Indeed, there are also suggestions in the literature that Src kinase itself may have functional roles in osteoclasts that do not depend on kinase activity (200), suggesting that SFKs may even have protein adapter or scaffolding functions. The study of SFK-deficient mice has only partially helped in addressing these issues. There is clearly some redundancy in the role of the different family members but also unique functions that are not compensated for when individual kinases are lost. The most clear and reproducible unique function among the SFKs appears to be the central role of Lyn kinase in negative regulation of various signaling pathways mediated by ITIMs. One general conclusion that arises from the study of SFK-deficient mice is that these enzymes have a primary role in ITAM-mediated signaling pathways, either from immunoreceptors or other receptor types that have co-opted the immunoreceptor signaling paradigm. However, there are several examples where SFKs are not absolutely required for ITAM-mediated signaling – the best example being Fc gamma R-mediated phagocytosis which can progress in the absence of SFKs. This contrasts with the absolute requirement for Syk in immunoreceptor pathways. Finally, though often not supported by studies in knockout mice, there is just too much data to ignore the potential roles of SFKs in multiple other pathways that do not rely on ITAM- or ITIM-like mechanisms.
Overall, it is difficult to assign a specific function to this family of kinases in macrophages (and other cell types). We are left with many individual and seemingly different functional roles in divergent pathways. Perhaps a better way to conceptualize the role of SFKs is to view them as signal modulators in many pathways, rather than being absolutely required in any specific class of intracellular signaling. A good analogy is to think of these enzymes as molecular rheostats, which can increase or decrease the gain or strength of signaling along any given pathway. This contrasts with the role of other kinases, such as Syk or IRAKs, which are absolutely required for signaling along defined pathways, such as immunoreceptors or TLRs, respectively. These latter kinases are more like molecular switches, turning signaling on and off. The importance of modulating signaling over a large dynamic range, rather than just turning it on or off, is clear when we look at processes such as T-cell development where it is clearly defined that too little or too much signaling from the T cell receptor has important consequences (201). It is logical to assume that fine-tuned modulation of other pathways, such as Fc receptor, TLR or integrin signaling in macrophages, can be just as important to achieve the appropriate level of cellular activation.
There are, however, many remaining questions concerning the role of SFKs in macrophages, in particular the different roles of SFKs in different types of macrophages is only just being appreciated. Clearly, the issue of macrophage heterogeneity is a major contributor to the complexity of SFK function in this cell lineage. Indeed, it is interesting to speculate that a differential dependence on SFK-mediated signaling may be a major contributor to heterogeneity in the functional responses of these cells. Certainly, the use of lineage specific knockouts of these enzymes will help us address the roles of SFKs in different macrophage types (and in macrophages in general).
Finally, it is important to consider whether therapeutic targeting of SFKs should remain an active goal for the treatment of inflammatory, autoimmune and neoplastic diseases. The ongoing success of dual specificity inhibitors in the treatment of imatinib resistant CML would argue that SFK blockade could be very useful. Perhaps we will see renewed efforts to design SFK inhibitors as secondary agents to supplement treatments using inhibitors against other kinases such as growth factor receptors. However, it is difficult to understand how inhibiting kinases that have such divergent functions will be possible without unwanted side effects. As research efforts continue to uncover the biological roles of SFKs in macrophages, a clear enough picture may emerge to facilitate the development of specific SFK inhibitors for specific macrophage-mediated diseases. It may be that we need only to partially inhibit SFK function, and therefore modulate the overall signaling network, to treat chronic macrophage diseases such as atherosclerosis.
Acknowledgments
This work was supported by NIH grants AI068150 and AI065495 to C.A.L. and T32 CA009043 to C.L.A. Thanks to Robert Blake for critical reading of the manuscript.
Abbreviations
- AIDS
acquired immunodeficiency syndrome
- ALL
acute lymphocytic leukemia
- AML
acute myeloid leukemia
- APC
antigen presenting cell
- BMDMs
bone marrow-derived macrophages
- Chk
Csk homologous kinase
- CHO
Chinese hamster ovary
- CML
chronic myeloid leukemia
- Csk
C-terminal Src kinase
- EMC-D
encephalomyocarditis virus
- ERK
extracellular signal-regulated kinase
- ERM
ezrin-radixin-moesin
- FcR gamma
Fc receptor gamma-chain
- FLT3/ITD
internal tandem duplications of FLT3
- fMLP
formyl-Met-Leu-Phe
- GST
glutathione S-transferase
- GPI
glycosylphosphatidylinositol
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- HIV
human immunodeficiency virus
- ICAM
intercellular adhesion molecule
- IKK
I kappa B kinase
- iNOS
inducible nitric oxide synthase
- ITAM
immunoreceptor tyrosine-based activation motif
- ITIM
immunoreceptor tyrosine-based inhibitory motif
- JAK
Janus family kinase
- JNK
Jun N-terminal kinase
- LDL
low-density lipoprotein
- LFA1
leukocyte function-associated antigen 1
- LPS
lipopolysaccharide
- LTA
lipoteichoic acid
- M-CSF
macrophage colony-stimulating factor
- M-CSFR
macrophage colony-stimulating factor receptor
- MIP1 alpha
macrophage inflammatory protein 1 alpha
- MSR-A
macrophage scavenger receptor A
- MHC
major histocompatibility complex
- MAPK
mitogen activated protein kinase
- MAIR-I
myeloid-associated Ig-like receptor
- NO
nitric oxide
- PEMs
peritoneal-elicited macrophages
- PI3K
phosphatidylinositol 3-kinase
- PIR-B
paired Ig-like receptor B
- PDGFR
platelet-derived growth factor receptor
- PSGL-1
P selectin glycoprotein ligand 1
- RANK
receptor activator of NF kappa B
- ROS
reactive oxygen species
- SFK
Src family kinase
- SH2
Src homology domain 2
- SH3
Src homology domain 3
- Shpl
SH2-containing protein tyrosine phosphatase 1
- SHIP
SH2-containing inositol phosphatase
- SIRP alpha
signal regulatory protein alpha
- STAT
signal transducer and activator of transcription
- SLP76
SH2-containing leukocyte protein of 76kDa
- SDF1 alpha
stromal cell-derived factor 1 alpha
- TIR
Toll-IL-1 receptor
- TLR
Toll-like receptor
- TNF alpha
tumor necrosis factor alpha
- TRAF6
TNF receptor-associated factor 6
- TAK1
transforming growth factor beta activated kinase 1
- TREM
triggering receptor expressed on myeloid cells
- TRIF
TIR domain-containing adapter inducing interferon beta
- VCAM
vascular cell adhesion molecule
- WASP
Wiscott-Aldrich syndrome protein
References
- 1.Lowell CA. Src-family kinases: rheostats of immune cell signaling. Mol Immunol. 2004;41:631–43. doi: 10.1016/j.molimm.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Miyazaki T, Tanaka S, Sanjay A, Baron R. The role of c-Src kinase in the regulation of osteoclast function. Mod Rheumatol. 2006;16:68–74. doi: 10.1007/s10165-006-0460-z. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez RH, Kantarjian HM, Cortes JE. The role of Src in solid and hematologic malignancies: development of new-generation Src inhibitors. Cancer. 2006;107:1918–29. doi: 10.1002/cncr.22215. [DOI] [PubMed] [Google Scholar]
- 4.Chen T, George JA, Taylor CC. Src tyrosine kinase as a chemotherapeutic target: is there a clinical case? Anticancer Drugs. 2006;17:123–31. doi: 10.1097/00001813-200602000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Superti-Furga G, Gonfloni S. A crystal milestone: the structure of regulated Src. Bioessays. 1997;19:447–50. doi: 10.1002/bies.950190602. [DOI] [PubMed] [Google Scholar]
- 6.Sicheri F, Kuriyan J. Structures of Src-family tyrosine kinases. Curr Opin Struct Biol. 1997;7:777–85. doi: 10.1016/s0959-440x(97)80146-7. [DOI] [PubMed] [Google Scholar]
- 7.Brown MT, Cooper JA. Regulation, substrates and functions of src. Biochim Biophys Acta. 1996;1287:121–49. doi: 10.1016/0304-419x(96)00003-0. [DOI] [PubMed] [Google Scholar]
- 8.Hibbs ML, Harder KW. The duplicitous nature of the Lyn tyrosine kinase in growth factor signaling. Growth Factors. 2006;24:137–49. doi: 10.1080/08977190600581327. [DOI] [PubMed] [Google Scholar]
- 9.Stein PL, Vogel H, Soriano P. Combined deficiencies of Src, Fyn, and Yes tyrosine kinases in mutant mice. Genes Dev. 1994;8:1999–2007. doi: 10.1101/gad.8.17.1999. [DOI] [PubMed] [Google Scholar]
- 10.Lowell CA, Soriano P, Varmus HE. Functional overlap in the src gene family: inactivation of hck and fgr impairs natural immunity. Genes Dev. 1994;8:387–98. doi: 10.1101/gad.8.4.387. [DOI] [PubMed] [Google Scholar]
- 11.Chan VW, Meng F, Soriano P, DeFranco AL, Lowell CA. Characterization of the B lymphocyte populations in Lyn-deficient mice and the role of Lyn in signal initiation and down-regulation. Immunity. 1997;7:69–81. doi: 10.1016/s1074-7613(00)80511-7. [DOI] [PubMed] [Google Scholar]
- 12.Harder KW, Parsons LM, Armes J, Evans N, Kountouri N, Clark R, Quilici C, Grail D, Hodgson GS, Dunn AR, Hibbs ML. Gain- and loss-of-function Lyn mutant mice define a critical inhibitory role for Lyn in the myeloid lineage. Immunity. 2001;15:603–15. doi: 10.1016/s1074-7613(01)00208-4. [DOI] [PubMed] [Google Scholar]
- 13.Hibbs ML, Tarlinton DM, Armes J, Grail D, Hodgson G, Maglitto R, Stacker SA, Dunn AR. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995;83:301–11. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 14.Nishizumi H, Taniuchi I, Yamanashi Y, Kitamura D, Ilic D, Mori S, Watanabe T, Yamamoto T. Impaired proliferation of peripheral B cells and indication of autoimmune disease in lyn-deficient mice. Immunity. 1995;3:549–60. doi: 10.1016/1074-7613(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 15.Lowell CA, Niwa M, Soriano P, Varmus HE. Deficiency of the Hck and Src tyrosine kinases results in extreme levels of extramedullary hematopoiesis. Blood. 1996;87:1780–92. [PubMed] [Google Scholar]
- 16.Meng F, Lowell CA. Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of Src-family kinases Hck, Fgr, and Lyn. J Exp Med. 1997;185:1661–70. doi: 10.1084/jem.185.9.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leu TH, Charoenfuprasert S, Yen CK, Fan CW, Maa MC. Lipopolysaccharide-induced c-Src expression plays a role in nitric oxide and TNFalpha secretion in macrophages. Mol Immunol. 2006;43:308–16. doi: 10.1016/j.molimm.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Boulet I, Ralph S, Stanley E, Lock P, Dunn AR, Green SP, Phillips WA. Lipopolysaccharide- and interferon-gamma-induced expression of hck and lyn tyrosine kinases in murine bone marrow-derived macrophages. Oncogene. 1992;7:703–10. [PubMed] [Google Scholar]
- 19.Blake RA, Broome MA, Liu X, Wu J, Gishizky M, Sun L, Courtneidge SA. SU6656, a selective src family kinase inhibitor, used to probe growth factor signaling. Mol Cell Biol. 2000;20:9018–27. doi: 10.1128/mcb.20.23.9018-9027.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian G, Cory M, Smith AA, Knight WB. Structural determinants for potent, selective dual site inhibition of human pp60c-src by 4-anilinoquinazolines. Biochemistry. 2001;40:7084–91. doi: 10.1021/bi0100586. [DOI] [PubMed] [Google Scholar]
- 21.Okutani D, Lodyga M, Han B, Liu M. Src protein tyrosine kinase family and acute inflammatory responses. Am J Physiol Lung Cell Mol Physiol. 2006;291:L129–41. doi: 10.1152/ajplung.00261.2005. [DOI] [PubMed] [Google Scholar]
- 22.Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem J. 2003;371:199–204. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tatton L, Morley GM, Chopra R, Khwaja A. The Src-selective kinase inhibitor PP1 also inhibits Kit and Bcr-Abl tyrosine kinases. J Biol Chem. 2003;278:4847–53. doi: 10.1074/jbc.M209321200. [DOI] [PubMed] [Google Scholar]
- 25.Carreno S, Caron E, Cougoule C, Emorine LJ, Maridonneau-Parini I. p59Hck isoform induces F-actin reorganization to form protrusions of the plasma membrane in a Cdc42- and Rac-dependent manner. J Biol Chem. 2002;277:21007–16. doi: 10.1074/jbc.M201212200. [DOI] [PubMed] [Google Scholar]
- 26.Poincloux R, Vincent C, Labrousse A, Castandet J, Rigo M, Cougoule C, Bordier C, Le Cabec V, Maridonneau-Parini I. Re-arrangements of podosome structures are observed when Hck is activated in myeloid cells. Eur J Cell Biol. 2006;85:327–32. doi: 10.1016/j.ejcb.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Cougoule C, Carreno S, Castandet J, Labrousse A, Astarie-Dequeker C, Poincloux R, Le Cabec V, Maridonneau-Parini I. Activation of the lysosome-associated p61Hck isoform triggers the biogenesis of podosomes. Traffic. 2005;6:682–94. doi: 10.1111/j.1600-0854.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 28.Tsuboi S. Requirement for a complex of Wiskott-Aldrich syndrome protein (WASP) with WASP interacting protein in podosome formation in macrophages. J Immunol. 2007;178:2987–95. doi: 10.4049/jimmunol.178.5.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cory GO, Garg R, Cramer R, Ridley AJ. Phosphorylation of tyrosine 291 enhances the ability of WASp to stimulate actin polymerization and filopodium formation. J Biol Chem. 2002;277:45115–21. doi: 10.1074/jbc.M203346200. [DOI] [PubMed] [Google Scholar]
- 30.Imamoto A, Soriano P. Disruption of the csk gene, encoding a negative regulator of Src family tyrosine kinases, leads to neural tube defects and embryonic lethality in mice. Cell. 1993;73:1117–24. doi: 10.1016/0092-8674(93)90641-3. [DOI] [PubMed] [Google Scholar]
- 31.Gross JA, Appleby MW, Chien S, Nada S, Bartelmez SH, Okada M, Aizawa S, Perlmutter RM. Control of lymphopoiesis by p50csk, a regulatory protein tyrosine kinase. J Exp Med. 1995;181:463–73. doi: 10.1084/jem.181.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas RM, Schmedt C, Novelli M, Choi BK, Skok J, Tarakhovsky A, Roes J. C-terminal SRC kinase controls acute inflammation and granulocyte adhesion. Immunity. 2004;20:181–91. doi: 10.1016/s1074-7613(04)00023-8. [DOI] [PubMed] [Google Scholar]
- 33.Ernst M, Inglese M, Scholz GM, Harder KW, Clay FJ, Bozinovski S, Waring P, Darwiche R, Kay T, Sly P, Collins R, Turner D, Hibbs ML, Anderson GP, Dunn AR. Constitutive activation of the SRC family kinase Hck results in spontaneous pulmonary inflammation and an enhanced innate immune response. J Exp Med. 2002;196:589–604. doi: 10.1084/jem.20020873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki T, Kono H, Hirose N, Okada M, Yamamoto T, Yamamoto K, Honda Z. Differential involvement of Src family kinases in Fc gamma receptor-mediated phagocytosis. J Immunol. 2000;165:473–82. doi: 10.4049/jimmunol.165.1.473. [DOI] [PubMed] [Google Scholar]
- 35.Lee BC, Avraham S, Imamoto A, Avraham HK. Identification of the nonreceptor tyrosine kinase MATK/CHK as an essential regulator of immune cells using Matk/CHK-deficient mice. Blood. 2006;108:904–7. doi: 10.1182/blood-2005-12-4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hermiston ML, Xu Z, Weiss A. CD45: a critical regulator of signaling thresholds in immune cells. Annu Rev Immunol. 2003;21:107–37. doi: 10.1146/annurev.immunol.21.120601.140946. [DOI] [PubMed] [Google Scholar]
- 37.Adachi R, Suzuki K. Lyn, one of the Src-family tyrosine kinases expressed in phagocytes, plays an important role in beta2 integrin-signalling pathways in opsonized zymosan-activated macrophage-like U937 cells. Cell Biochem Funct. 2006;25:323–333. doi: 10.1002/cbf.1393. [DOI] [PubMed] [Google Scholar]
- 38.Aki D, Mashima R, Saeki K, Minoda Y, Yamauchi M, Yoshimura A. Modulation of TLR signalling by the C-terminal Src kinase (Csk) in macrophages. Genes Cells. 2005;10:357–68. doi: 10.1111/j.1365-2443.2005.00839.x. [DOI] [PubMed] [Google Scholar]
- 39.Bishop AC, Buzko O, Shokat KM. Magic bullets for protein kinases. Trends Cell Biol. 2001;11:167–72. doi: 10.1016/s0962-8924(01)01928-6. [DOI] [PubMed] [Google Scholar]
- 40.Denzel A, Hare KJ, Zhang C, Shokat K, Jenkinson EJ, Anderson G, Hayday A. Cutting edge: a chemical genetic system for the analysis of kinases regulating T cell development. J Immunol. 2003;171:519–23. doi: 10.4049/jimmunol.171.2.519. [DOI] [PubMed] [Google Scholar]
- 41.Goldenberg-Furmanov M, Stein I, Pikarsky E, Rubin H, Kasem S, Wygoda M, Weinstein I, Reuveni H, Ben-Sasson SA. Lyn is a target gene for prostate cancer: sequence-based inhibition induces regression of human tumor xenografts. Cancer Res. 2004;64:1058–66. doi: 10.1158/0008-5472.can-03-2420. [DOI] [PubMed] [Google Scholar]
- 42.Niv MY, Rubin H, Cohen J, Tsirulnikov L, Licht T, Peretzman-Shemer A, Cna’an E, Tartakovsky A, Stein I, Albeck S, Weinstein I, Goldenberg-Furmanov M, Tobi D, Cohen E, Laster M, Ben-Sasson SA, Reuveni H. Sequence-based design of kinase inhibitors applicable for therapeutics and target identification. J Biol Chem. 2004;279:1242–55. doi: 10.1074/jbc.M306723200. [DOI] [PubMed] [Google Scholar]
- 43.Hausherr A, Tavares R, Schaffer M, Obermeier A, Miksch C, Mitina O, Ellwart J, Hallek M, Krause G. Inhibition of IL-6-dependent growth of myeloma cells by an acidic peptide repressing the gp130-mediated activation of Src family kinases. Oncogene. 2007 doi: 10.1038/sj.onc.1210306. [DOI] [PubMed] [Google Scholar]
- 44.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 45.Majeed M, Caveggion E, Lowell CA, Berton G. Role of Src kinases and Syk in Fcgamma receptor-mediated phagocytosis and phagosome-Iysosome fusion. J Leukoc Biol. 2001;70:801–11. [PubMed] [Google Scholar]
- 46.Korade-Mirnics Z, Corey SJ. Src kinase-mediated signaling in leukocytes. J Leukoc Biol. 2000;68:603–13. [PubMed] [Google Scholar]
- 47.Barrow AD, Trowsdale J. You say ITAM and I say ITIM, let’s call the whole thing off: the ambiguity of immunoreceptor signalling. Eur J Immunol. 2006;36:1646–53. doi: 10.1002/eji.200636195. [DOI] [PubMed] [Google Scholar]
- 48.Fodor S, Jakus Z, Mocsai A. ITAM-based signaling beyond the adaptive immune response. Immunol Lett. 2006;104:29–37. doi: 10.1016/j.imlet.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Isakov N. Immunoreceptor tyrosine-based activation motif (ITAM), a unique module linking antigen and Fc receptors to their signaling cascades. J Leukoc Biol. 1997;61:6–6. doi: 10.1002/jlb.61.1.6. [DOI] [PubMed] [Google Scholar]
- 50.Palacios EH, Weiss A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene. 2004;23:7990–8000. doi: 10.1038/sj.onc.1208074. [DOI] [PubMed] [Google Scholar]
- 51.Malbec O, Fong DC, Turner M, Tybulewicz VL, Cambier JC, Fridman WH, Daeron M. Fc epsilon receptor I-associated lyn-dependent phosphorylation of Fc gamma receptor IIB during negative regulation of mast cell activation. J Immunol. 1998;160:1647–58. [PubMed] [Google Scholar]
- 52.Wiktor-Jedrzejczak WW, Ahmed A, Szczylik C, Skelly RR. Hematological characterization of congenital osteopetrosis in op/op mouse. Possible mechanism for abnormal macrophage differentiation. J Exp Med. 1982;156:1516–27. doi: 10.1084/jem.156.5.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cecchini MG, Dominguez MG, Mocci S, Wetterwald A, Felix R, Fleisch H, Chisholm O, Hofstetter W, Pollard JW, Stanley ER. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development. 1994;120:1357–72. doi: 10.1242/dev.120.6.1357. [DOI] [PubMed] [Google Scholar]
- 54.Hamilton JA. CSF-1 signal transduction. J Leukoc Biol. 1997;62:145–55. doi: 10.1002/jlb.62.2.145. [DOI] [PubMed] [Google Scholar]
- 55.Courtneidge SA, Dhand R, Pilat D, Twamley GM, Waterfield MD, Roussel MF. Activation of Src family kinases by colony stimulating factor-1, and their association with its receptor. Embo J. 1993;12:943–50. doi: 10.1002/j.1460-2075.1993.tb05735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marks DC, Csar XF, Wilson NJ, Novak U, Ward AC, Kanagasundarum V, Hoffmann BW, Hamilton JA. Expression of a Y559F mutant CSF-1 receptor in M1 myeloid cells: a role for Src kinases in CSF-1 receptor-mediated differentiation. Mol Cell Biol Res Commun. 1999;1:144–52. doi: 10.1006/mcbr.1999.0123. [DOI] [PubMed] [Google Scholar]
- 57.Lee AW, States DJ. Both src-dependent and -independent mechanisms mediate phosphatidylinositol 3-kinase regulation of colony-stimulating factor 1-activated mitogen-activated protein kinases in myeloid progenitors. Mol Cell Biol. 2000;20:6779–98. doi: 10.1128/mcb.20.18.6779-6798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takeshita S, Faccio R, Chappel J, Zheng L, Feng X, Weber JD, Teitelbaum SL, Ross FP. c-FMS tyrosine 559 is a major mediator of M-CSF-induced proliferation of primary macrophages. J Biol Chem. 2007;282:18980–90. doi: 10.1074/jbc.M610938200. [DOI] [PubMed] [Google Scholar]
- 59.Faccio R, Takeshita S, Colaianni G, Chappel J, Zallone A, Teitelbaum SL, Ross FP. M-CSF regulates the cytoskeleton via recruitment of a multimeric signaling complex to c-FMS Y559/697/721. J Biol Chem. 2007;282:18991–9. doi: 10.1074/jbc.M610937200. [DOI] [PubMed] [Google Scholar]
- 60.Meng F, Lowell CA. A beta 1 integrin signaling pathway involving Src-family kinases, Cbl and PI-3 kinase is required for macrophage spreading and migration. Embo J. 1998;17:4391–403. doi: 10.1093/emboj/17.15.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taniguchi T. Cytokine signaling through nonreceptor protein tyrosine kinases. Science. 1995;268:251–5. doi: 10.1126/science.7716517. [DOI] [PubMed] [Google Scholar]
- 62.Hayakawa F, Naoe T. SFK-STAT pathway: an alternative and important way to malignancies. Ann N Y Acad Sci. 2006;1086:213–22. doi: 10.1196/annals.1377.002. [DOI] [PubMed] [Google Scholar]
- 63.Silva CM. Role of STATs as downstream signal transducers in Src family kinase-mediated tumorigenesis. Oncogene. 2004;23:8017–23. doi: 10.1038/sj.onc.1208159. [DOI] [PubMed] [Google Scholar]
- 64.Reddy EP, Korapati A, Chaturvedi P, Rane S. IL-3 signaling and the role of Src kinases, JAKs and STATs: a covert liaison unveiled. Oncogene. 2000;19:2532–47. doi: 10.1038/sj.onc.1203594. [DOI] [PubMed] [Google Scholar]
- 65.Chaturvedi P, Reddy MV, Reddy EP. Src kinases and not JAKs activate STATs during IL-3 induced myeloid cell proliferation. Oncogene. 1998;16:1749–58. doi: 10.1038/sj.onc.1201972. [DOI] [PubMed] [Google Scholar]
- 66.Anderson SM, Jorgensen B. Activation of src-related tyrosine kinases by IL-3. J Immunol. 1995;155:1660–70. [PubMed] [Google Scholar]
- 67.Burton EA, Hunter S, Wu SC, Anderson SM. Binding of src-like kinases to the beta-subunit of the interleukin-3 receptor. J Biol Chem. 1997;272:16189–95. doi: 10.1074/jbc.272.26.16189. [DOI] [PubMed] [Google Scholar]
- 68.Schreiner SJ, Schiavone AP, Smithgall TE. Activation of STAT3 by the Src family kinase Hck requires a functional SH3 domain. J Biol Chem. 2002;277:45680–7. doi: 10.1074/jbc.M204255200. [DOI] [PubMed] [Google Scholar]
- 69.Klejman A, Schreiner SJ, Nieborowska-Skorska M, Slupianek A, Wilson M, Smithgall TE, Skorski T. The Src family kinase Hck couples BCR/ABL to STAT5 activation in myeloid leukemia cells. Embo J. 2002;21:5766–74. doi: 10.1093/emboj/cdf562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Corey S, Eguinoa A, Puyana-Theall K, Bolen JB, Cantley L, Mollinedo F, Jackson TR, Hawkins PT, Stephens LR. Granulocyte macrophage-colony stimulating factor stimulates both association and activation of phosphoinositide 3OH-kinase and src-related tyrosine kinase(s) in human myeloid derived cells. Embo J. 1993;12:2681–90. doi: 10.1002/j.1460-2075.1993.tb05929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harder KW, Quilici C, Naik E, Inglese M, Kountouri N, Turner A, Zlatic K, Tarlinton DM, Hibbs ML. Perturbed myelo/erythropoiesis in Lyn-deficient mice is similar to that in mice lacking the inhibitory phosphatases SHP-1 and SHIP-1. Blood. 2004;104:3901–10. doi: 10.1182/blood-2003-12-4396. [DOI] [PubMed] [Google Scholar]
- 72.Kashiwada M, Giallourakis CC, Pan PY, Rothman PB. Immunoreceptor tyrosine-based inhibitory motif of the IL-4 receptor associates with SH2-containing phosphatases and regulates IL-4-induced proliferation. J Immunol. 2001;167:6382–7. doi: 10.4049/jimmunol.167.11.6382. [DOI] [PubMed] [Google Scholar]
- 73.Luttrell DK, Luttrell LM. Not so strange bedfellows: G-protein-coupled receptors and Src family kinases. Oncogene. 2004;23:7969–78. doi: 10.1038/sj.onc.1208162. [DOI] [PubMed] [Google Scholar]
- 74.Tomkowicz B, Lee C, Ravyn V, Cheung R, Ptasznik A, Collman RG. The Src kinase Lyn is required for CCR5 signaling in response to MIP-1beta and R5 HIV-1 gp120 in human macrophages. Blood. 2006;108:1145–50. doi: 10.1182/blood-2005-12-012815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ptasznik A, Urbanowska E, Chinta S, Costa MA, Katz BA, Stanislaus MA, Demir G, Linnekin D, Pan ZK, Gewirtz AM. Crosstalk between BCR/ABL oncoprotein and CXCR4 signaling through a Src family kinase in human leukemia cells. J Exp Med. 2002;196:667–78. doi: 10.1084/jem.20020519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arefieva TI, Kukhtina NB, Antonova OA, Krasnikova TL. MCP-1-stimulated chemotaxis of monocytic and endothelial cells is dependent on activation of different signaling cascades. Cytokine. 2005;31:439–46. doi: 10.1016/j.cyto.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 77.Zhang H, Meng F, Chu CL, Takai T, Lowell CA. The Src family kinases Hck and Fgr negatively regulate neutrophil and dendritic cell chemokine signaling via PIR-B. Immunity. 2005;22:235–46. doi: 10.1016/j.immuni.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 78.Fumagalli L, Zhang H, Baruzzi A, Lowell CA, Berton G. The Src family kinases Hck and Fgr regulate neutrophil responses to N-formyl-methionyl-leucyl-phenylalanine. J Immunol. 2007;178:3874–85. doi: 10.4049/jimmunol.178.6.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shi C, Simon DI. Integrin signals, transcription factors, and monocyte differentiation. Trends Cardiovasc Med. 2006;16:146–52. doi: 10.1016/j.tcm.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 80.Mayadas TN, Cullere X. Neutrophil beta2 integrins: moderators of life or death decisions. Trends Immunol. 2005;26:388–95. doi: 10.1016/j.it.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 81.Lowell CA, Berton G. Integrin signal transduction in myeloid leukocytes. J Leukoc Biol. 1999;65:313–20. doi: 10.1002/jlb.65.3.313. [DOI] [PubMed] [Google Scholar]
- 82.Ginsberg MH, Partridge A, Shattil SJ. Integrin regulation. Curr Opin Cell Biol. 2005;17:509–16. doi: 10.1016/j.ceb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 83.Berton G, Lowell CA. Integrin signalling in neutrophils and macrophages. Cell Signal. 1999;11:621–35. doi: 10.1016/s0898-6568(99)00003-0. [DOI] [PubMed] [Google Scholar]
- 84.Suen PW, Ilic D, Caveggion E, Berton G, Damsky CH, Lowell CA. Impaired integrin-mediated signal transduction, altered cytoskeletal structure and reduced motility in Hck/Fgr deficient macrophages. J Cell Sci. 1999;112:4067–78. doi: 10.1242/jcs.112.22.4067. [DOI] [PubMed] [Google Scholar]
- 85.Caveggion E, Continolo S, Pixley FJ, Stanley ER, Bowtell DD, Lowell CA, Berton G. Expression and tyrosine phosphorylation of Cbl regulates macrophage chemokinetic and chemotactic movement. J Cell Physiol. 2003;195:276–89. doi: 10.1002/jcp.10236. [DOI] [PubMed] [Google Scholar]
- 86.Ojaniemi M, Martin SS, Dolfi F, Olefsky JM, Vuori K. The proto-oncogene product p120(cbl) links c-Src and phosphatidylinositol 3′-kinase to the integrin signaling pathway. J Biol Chem. 1997;272:3780–7. doi: 10.1074/jbc.272.6.3780. [DOI] [PubMed] [Google Scholar]
- 87.Zou W, Kitaura H, Reeve J, Long F, Tybulewicz VL, Shattil SJ, Ginsberg MH, Ross FP, Teitelbaum SL. Syk, c-Src, the alpha v beta3 integrin, and ITAM immunoreceptors, in concert, regulate osteoclastic bone resorption. J Cell Biol. 2007;176:877–88. doi: 10.1083/jcb.200611083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pereira S, Lowell C. The Lyn tyrosine kinase negatively regulates neutrophil integrin signaling. J Immunol. 2003;171:1319–27. doi: 10.4049/jimmunol.171.3.1319. [DOI] [PubMed] [Google Scholar]
- 89.Pereira S, Zhang H, Takai T, Lowell CA. The inhibitory receptor PIR-B negatively regulates neutrophil and macrophage integrin signaling. J Immunol. 2004;173:5757–65. doi: 10.4049/jimmunol.173.9.5757. [DOI] [PubMed] [Google Scholar]
- 90.Roach T, Slater S, Koval M, White L, Cahir McFarland ED, Okumura M, Thomas M, Brown E. CD45 regulates Src family member kinase activity associated with macrophage integrin-mediated adhesion. Curr Biol. 1997;7:408–17. doi: 10.1016/s0960-9822(06)00188-6. [DOI] [PubMed] [Google Scholar]
- 91.Vines CM, Potter JW, Xu Y, Geahlen RL, Costello PS, Tybulewicz VL, Lowell CA, Chang PW, Gresham HD, Willman CL. Inhibition of beta 2 integrin receptor and Syk kinase signaling in monocytes by the Src family kinase Fgr. Immunity. 2001;15:507–19. doi: 10.1016/s1074-7613(01)00221-7. [DOI] [PubMed] [Google Scholar]
- 92.Gresham HD, Dale BM, Potter JW, Chang PW, Vines CM, Lowell CA, Lagenaur CF, Willman CL. Negative regulation of phagocytosis in murine macrophages by the Src kinase family member, Fgr. J Exp Med. 2000;191:515–28. doi: 10.1084/jem.191.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Obergfell A, Eto K, Mocsai A, Buensuceso C, Moores SL, Brugge JS, Lowell CA, Shattil SJ. Coordinate interactions of Csk, Src, and Syk kinases with [alpha]IIb[beta]3 initiate integrin signaling to the cytoskeleton. J Cell Biol. 2002;157:265–75. doi: 10.1083/jcb.200112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shaw AS. Lipid rafts: now you see them, now you don’t. Nat Immunol. 2006;7:1139–42. doi: 10.1038/ni1405. [DOI] [PubMed] [Google Scholar]
- 95.Arias-Salgado EG, Lizano S, Sarkar S, Brugge JS, Ginsberg MH, Shattil SJ. Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc Natl Acad Sci U S A. 2003;100:13298–302. doi: 10.1073/pnas.2336149100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abram CL, Lowell CA. The expanding role for ITAM-based signaling pathways in immune cells. Sci STKE. 2007;2007:re2. doi: 10.1126/stke.3772007re2. [DOI] [PubMed] [Google Scholar]
- 97.Abram CL, Lowell CA. Convergence of immunoreceptor and integrin signaling. Immunological Reviews. 2007;218:29–44. doi: 10.1111/j.1600-065X.2007.00531.x. [DOI] [PubMed] [Google Scholar]
- 98.Mocsai A, Abram CL, Jakus Z, Hu Y, Lanier LL, Lowell CA. Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs. Nat Immunol. 2006;7:1326–33. doi: 10.1038/ni1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kuijper PH, Gallardo Torres HI, Houben LA, Lammers JW, Zwaginga JJ, Koenderman L. P-selectin and MAC-1 mediate monocyte rolling and adhesion to ECM-bound platelets under flow conditions. J Leukoc Biol. 1998;64:467–73. doi: 10.1002/jlb.64.4.467. [DOI] [PubMed] [Google Scholar]
- 100.Hidari KI, Weyrich AS, Zimmerman GA, McEver RP. Engagement of P-selectin glycoprotein ligand-1 enhances tyrosine phosphorylation and activates mitogen-activated protein kinases in human neutrophils. J Biol Chem. 1997;272:28750–6. doi: 10.1074/jbc.272.45.28750. [DOI] [PubMed] [Google Scholar]
- 101.Kumar P, Hosaka S, Koch AE. Soluble E-selectin induces monocyte chemotaxis through Src family tyrosine kinases. J Biol Chem. 2001;276:21039–45. doi: 10.1074/jbc.M009099200. [DOI] [PubMed] [Google Scholar]
- 102.Urzainqui A, Serrador JM, Viedma F, Yanez-Mo M, Rodriguez A, Corbi AL, Alonso-Lebrero JL, Luque A, Deckert M, Vazquez J, Sanchez-Madrid F. ITAM-based interaction of ERM proteins with Syk mediates signaling by the leukocyte adhesion receptor PSGL-1. Immunity. 2002;17:401–12. doi: 10.1016/s1074-7613(02)00420-x. [DOI] [PubMed] [Google Scholar]
- 103.Zarbock A, Lowell CA, Ley K. Spleen Tyrosine Kinase Syk Is Necessary for E-Selectin-induced alpha L beta 2 integrin-mediated rolling on intercellular adhesion molecule-1. Immunity. 2007;26:773–83. doi: 10.1016/j.immuni.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 105.Weinstein SL, Gold MR, DeFranco AL. Bacterial lipopolysaccharide stimulates protein tyrosine phosphorylation in macrophages. Proc Natl Acad Sci U S A. 1991;88:4148–52. doi: 10.1073/pnas.88.10.4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hazeki K, Masuda N, Funami K, Sukenobu N, Matsumoto M, Akira S, Takeda K, Seya T, Hazeki O. Toll-like receptor-mediated tyrosine phosphorylation of paxillin via MyD88-dependent and -independent pathways. Eur J Immunol. 2003;33:740–7. doi: 10.1002/eji.200323375. [DOI] [PubMed] [Google Scholar]
- 107.Stovall SH, Yi AK, Meals EA, Talati AJ, Godambe SA, English BK. Role of vavl- and src-related tyrosine kinases in macrophage activation by CpG DNA. J Biol Chem. 2004;279:13809–16. doi: 10.1074/jbc.M311434200. [DOI] [PubMed] [Google Scholar]
- 108.Achuthan A, Elsegood C, Masendycz P, Hamilton JA, Scholz GM. CpG DNA enhances macrophage cell spreading by promoting the Src-family kinase-mediated phosphorylation of paxillin. Cell Signal. 2006;18:2252–61. doi: 10.1016/j.cellsig.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 109.Beaty CD, Franklin TL, Uehara Y, Wilson CB. Lipopolysaccharide-induced cytokine production in human monocytes: role of tyrosine phosphorylation in transmembrane signal transduction. Eur J Immunol. 1994;24:1278–84. doi: 10.1002/eji.1830240606. [DOI] [PubMed] [Google Scholar]
- 110.Stefanova I, Corcoran ML, Horak EM, Wahl LM, Bolen JB, Horak ID. Lipopolysaccharide induces activation of CD14-associated protein tyrosine kinase p53/561yn. J Biol Chem. 1993;268:20725–8. [PubMed] [Google Scholar]
- 111.Herrera-Velit P, Reiner NE. Bacterial lipopolysaccharide induces the association and coordinate activation of p53/561yn and phosphatidylinositol 3-kinase in human monocytes. J Immunol. 1996;156:1157–65. [PubMed] [Google Scholar]
- 112.Sanjuan MA, Rao N, Lai KT, Gu Y, Sun S, Fuchs A, Fung-Leung WP, Colonna M, Karlsson L. CpG-induced tyrosine phosphorylation occurs via a TLR9-independent mechanism and is required for cytokine secretion. J Cell Biol. 2006;172:1057–68. doi: 10.1083/jcb.200508058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Orlicek SL, Hanke JH, English BK. The src family-selective tyrosine kinase inhibitor PP1 blocks LPS and IFN-gamma-mediated TNF and iNOS production in murine macrophages. Shock. 1999;12:350–4. doi: 10.1097/00024382-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 114.Scholz G, Cartledge K, Dunn AR. Hck enhances the adherence of lipopolysaccharide-stimulated macrophages via Cbl and phosphatidylinositol 3-kinase. J Biol Chem. 2000;275:14615–23. doi: 10.1074/jbc.275.19.14615. [DOI] [PubMed] [Google Scholar]
- 115.Williams LM, Ridley AJ. Lipopolysaccharide induces actin reorganization and tyrosine phosphorylation of Pyk2 and paxillin in monocytes and macrophages. J Immunol. 2000;164:2028–36. doi: 10.4049/jimmunol.164.4.2028. [DOI] [PubMed] [Google Scholar]
- 116.Medvedev AE, Piao W, Shoenfelt J, Rhee SH, Chen H, Basu S, Wahl LM, Fenton MJ, Vogel SN. Role of TLR4 tyrosine phosphorylation in signal transduction and endotoxin tolerance. J Biol Chem. 2007;282:16042–16053. doi: 10.1074/jbc.M606781200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chaudhary A, Fresquez TM, Naranjo MJ. Tyrosine kinase Syk associates with toll-like receptor 4 and regulates signaling in human monocytic cells. Immunol Cell Biol. 2007;85:249–256. doi: 10.1038/sj.icb7100030. [DOI] [PubMed] [Google Scholar]
- 118.David MD, Cochrane CL, Duncan SK, Schrader JW. Pure lipopolysaccharide or synthetic lipid A induces activation of p21Ras in primary macrophages through a pathway dependent on Src family kinases and PI3K. J Immunol. 2005;175:8236–41. doi: 10.4049/jimmunol.175.12.8236. [DOI] [PubMed] [Google Scholar]
- 119.Fan H, Teti G, Ashton S, Guyton K, Tempel GE, Halushka PV, Cook JA. Involvement of G(i) proteins and Src tyrosine kinase in TNFalpha production induced by lipopolysaccharide, group B Streptococci and Staphylococcus aureus. Cytokine. 2003;22:126–33. doi: 10.1016/s1043-4666(03)00122-4. [DOI] [PubMed] [Google Scholar]
- 120.Lee JY, Lowell CA, Lemay DG, Youn HS, Rhee SH, Sohn KH, Jang B, Ye J, Chung JH, Hwang DH. The regulation of the expression of inducible nitric oxide synthase by Src-family tyrosine kinases mediated through MyD88-independent signaling pathways of Toll-like receptor 4. Biochem Pharmacol. 2005;70:1231–40. doi: 10.1016/j.bcp.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 121.English BK, Ihle JN, Myracle A, Yi T. Hck tyrosine kinase activity modulates tumor necrosis factor production by murine macrophages. J Exp Med. 1993;178:1017–22. doi: 10.1084/jem.178.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Iwabuchi K, Hatakeyama S, Takahashi A, Ato M, Okada M, Kajino Y, Kajino K, Ogasawara K, Takami K, Nakagawa H, Onoe K. Csk overexpression reduces several monokines and nitric oxide productions but enhances prostaglandin E2 production in response to lipopolysaccharide in the macrophage cell line J774A.1. Eur J Immunol. 1997;27:742–9. doi: 10.1002/eji.1830270324. [DOI] [PubMed] [Google Scholar]
- 123.Overland G, Stuestol JF, Dahle MK, Myhre AE, Netea MG, Verweij P, Yndestad A, Aukrust P, Kullberg BJ, Warris A, Wang JE, Aasen AO. Cytokine responses to fungal pathogens in Kupffer Cells are Toll-like receptor 4 independent and mediated by tyrosine kinases. Scand J Immunol. 2005;62:148–54. doi: 10.1111/j.1365-3083.2005.01653.x. [DOI] [PubMed] [Google Scholar]
- 124.Hardin AO, Meals EA, Yi T, Knapp KM, English BK. SHP-1 inhibits LPS-mediated TNF and iNOS production in murine macrophages. Biochem Biophys Res Commun. 2006;342:547–55. doi: 10.1016/j.bbrc.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 125.An H, Zhao W, Hou J, Zhang Y, Xie Y, Zheng Y, Xu H, Qian C, Zhou J, Yu Y, Liu S, Feng G, Cao X. SHP-2 phosphatase negatively regulates the TRIF adaptor protein-dependent type I interferon and proinflammatory cytokine production. Immunity. 2006;25:919–28. doi: 10.1016/j.immuni.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 126.An H, Xu H, Zhang M, Zhou J, Feng T, Qian C, Qi R, Cao X. Src homology 2 domain-containing inositol-5-phosphatase 1 (SHIP1) negatively regulates TLR4-mediated LPS response primarily through a phosphatase activity- and PI-3K-independent mechanism. Blood. 2005;105:4685–92. doi: 10.1182/blood-2005-01-0191. [DOI] [PubMed] [Google Scholar]
- 127.Hamerman JA, Tchao NK, Lowell CA, Lanier LL. Enhanced Toll-like receptor responses in the absence of signaling adaptor DAP12. Nat Immunol. 2005;6:579–86. doi: 10.1038/ni1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–22. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 129.Miletic AV, Graham DB, Montgrain V, Fujikawa K, Kloeppel T, Brim K, Weaver B, Schreiber R, Xavier R, Swat W. Vav proteins control MyD88-dependent oxidative burst. Blood. 2007;109:3360–8. doi: 10.1182/blood-2006-07-033662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang KZ, Wara-Aswapati N, Boch JA, Yoshida Y, Hu CD, Galson DL, Auron PE. TRAF6 activation of PI 3-kinase-dependent cytoskeletal changes is cooperative with Ras and is mediated by an interaction with cytoplasmic Src. J Cell Sci. 2006;119:1579–91. doi: 10.1242/jcs.02889. [DOI] [PubMed] [Google Scholar]
- 131.Mukundan L, Bishop GA, Head KZ, Zhang L, Wahl LM, Suttles J. TNF receptor-associated factor 6 is an essential mediator of CD40-activated proinflammatory pathways in monocytes and macrophages. J Immunol. 2005;174:1081–90. doi: 10.4049/jimmunol.174.2.1081. [DOI] [PubMed] [Google Scholar]
- 132.Nakamura I, Kadono Y, Takayanagi H, Jimi E, Miyazaki T, Oda H, Nakamura K, Tanaka S, Rodan GA, Duong le T. IL-1 regulates cytoskeletal organization in osteoclasts via TNF receptor-associated factor 6/c-Src complex. J Immunol. 2002;168:5103–9. doi: 10.4049/jimmunol.168.10.5103. [DOI] [PubMed] [Google Scholar]
- 133.Funakoshi-Tago M, Tago K, Sonoda Y, Tominaga S, Kasahara T. TRAF6 and C-SRC induce synergistic AP-1 activation via P13-kinase-AKT-JNK pathway. Eur J Biochem. 2003;270:1257–68. doi: 10.1046/j.1432-1033.2003.03487.x. [DOI] [PubMed] [Google Scholar]
- 134.Funakoshi-Tago M, Tago K, Andoh K, Sonoda Y, Tominaga S, Kasahara T. Functional role of c-Src in IL-1-induced NF-kappa B activation: c-Src is a component of the IKK complex. J Biochem (Tokyo) 2005;137:189–97. doi: 10.1093/jb/mvi018. [DOI] [PubMed] [Google Scholar]
- 135.Khadaroo RG, Kapus A, Powers KA, Cybulsky MI, Marshall JC, Rotstein OD. Oxidative stress reprograms lipopolysaccharide signaling via Src kinase-dependent pathway in RAW 264.7 macrophage cell line. J Biol Chem. 2003;278:47834–41. doi: 10.1074/jbc.M302660200. [DOI] [PubMed] [Google Scholar]
- 136.Khadaroo RG, Parodo J, Powers KA, Papia G, Marshall JC, Kapus A, Rotstein OD. Oxidant-induced priming of the macrophage involves activation of p38 mitogen-activated protein kinase through an Src-dependent pathway. Surgery. 2003;134:242–6. doi: 10.1067/msy.2003.228. [DOI] [PubMed] [Google Scholar]
- 137.Shi C, Zhang X, Chen Z, Robinson MK, Simon DI. Leukocyte integrin Mac-1 recruits toll/interleukin-1 receptor superfamily signaling intermediates to modulate NF-kappaB activity. Circ Res. 2001;89:859–65. doi: 10.1161/hh2201.099166. [DOI] [PubMed] [Google Scholar]
- 138.Harokopakis E, Albzreh MH, Martin MH, Hajishengallis G. TLR2 transmodulates monocyte adhesion and transmigration via Racl- and PI3K-mediated inside-out signaling in response to Porphyromonas gingivalis fimbriae. J Immunol. 2006;176:7645–56. doi: 10.4049/jimmunol.176.12.7645. [DOI] [PubMed] [Google Scholar]
- 139.Wang AV, Scholl PR, Geha RS. Physical and functional association of the high affinity immunoglobulin G receptor (Fc gamma RI) with the kinases Hck and Lyn. J Exp Med. 1994;180:1165–70. doi: 10.1084/jem.180.3.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ghazizadeh S, Bolen JB, Fleit HB. Physical and functional association of Src-related protein tyrosine kinases with Fc gamma RII in monocytic THP-1 cells. J Biol Chem. 1994;269:8878–84. [PubMed] [Google Scholar]
- 141.Fitzer-Attas CJ, Lowry M, Crowley MT, Finn AJ, Meng F, DeFranco AL, Lowell CA. Fcgamma receptor-mediated phagocytosis in macrophages lacking the Src family tyrosine kinases Hck, Fgr, and Lyn. J Exp Med. 2000;191:669–82. doi: 10.1084/jem.191.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Erdreich-Epstein A, Liu M, Kant AM, Izadi KD, Nolta JA, Durden DL. Cbl functions downstream of Src kinases in Fc gamma RI signaling in primary human macrophages. J Leukoc Biol. 1999;65:523–34. doi: 10.1002/jlb.65.4.523. [DOI] [PubMed] [Google Scholar]
- 143.Park RK, Erdreich-Epstein A, Liu M, Izadi KD, Durden DL. High affinity IgG receptor activation of Src family kinases is required for modulation of the Shc-Grb2-Sos complex and the downstream activation of the nicotinamide adenine dinucleotide phosphate (reduced) oxidase. J Immunol. 1999;163:6023–34. [PubMed] [Google Scholar]
- 144.Crowley MT, Costello PS, Fitzer-Attas CJ, Turner M, Meng F, Lowell C, Tybulewicz VL, DeFranco AL. A critical role for Syk in signal transduction and phagocytosis mediated by Fcgamma receptors on macrophages. J Exp Med. 1997;186:1027–39. doi: 10.1084/jem.186.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kiefer F, Brumell J, Al-Alawi N, Latour S, Cheng A, Veillette A, Grinstein S, Pawson T. The Syk protein tyrosine kinase is essential for Fcgamma receptor signaling in macrophages and neutrophils. Mol Cell Biol. 1998;18:4209–20. doi: 10.1128/mcb.18.7.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Park RK, Izadi KD, Deo YM, Durden DL. Role of Src in the modulation of multiple adaptor proteins in FcalphaRI oxidant signaling. Blood. 1999;94:2112–20. [PubMed] [Google Scholar]
- 147.Pasquier B, Launay P, Kanamaru Y, Moura IC, Pfirsch S, Ruffle C, Henin D, Benhamou M, Pretolani M, Blank U, Monteiro RC. Identification of FcalphaRI as an inhibitory receptor that controls inflammation: dual role of FcRgamma ITAM. Immunity. 2005;22:31–42. doi: 10.1016/j.immuni.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 148.Furumoto Y, Gomez G, Gonzalez-Espinosa C, Kovarova M, Odom S, Ryan JJ, Rivera J. The role of Src family kinases in mast cell effector function. Novartis Found Symp. 2005;271:39–47. discussion 47–53, 95–9. [PubMed] [Google Scholar]
- 149.Robinson MJ, Sancho D, Slack EC, LeibundGut-Landmann S, Reis e Sousa C. Myeloid C-type lectins in innate immunity. Nat Immunol. 2006;7:1258–65. doi: 10.1038/ni1417. [DOI] [PubMed] [Google Scholar]
- 150.Fuller GL, Williams JA, Tomlinson MG, Eble JA, Hanna SL, Pohlmann S, Suzuki-Inoue K, Ozaki Y, Watson SP, Pearce AC. The C-type lectin receptors CLEC-2 and Dectin-1, but not DC-SIGN, signal via a novel YXXL-dependent signaling cascade. J Biol Chem. 2007;282:12397–409. doi: 10.1074/jbc.M609558200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Underhill DM, Rossnagle E, Lowell CA, Simmons RM. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood. 2005;106:2543–50. doi: 10.1182/blood-2005-03-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sato K, Yang XL, Yudate T, Chung JS, Wu J, Luby-Phelps K, Kimberly RP, Underhill D, Cruz PD, Jr, Ariizumi K. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor gamma chain to induce innate immune responses. J Biol Chem. 2006;281:38854–66. doi: 10.1074/jbc.M606542200. [DOI] [PubMed] [Google Scholar]
- 153.Klesney-Tait J, Turnbull IR, Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006;7:1266–73. doi: 10.1038/ni1411. [DOI] [PubMed] [Google Scholar]
- 154.Bouchon A, Dietrich J, Colonna M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol. 2000;164:4991–5. doi: 10.4049/jimmunol.164.10.4991. [DOI] [PubMed] [Google Scholar]
- 155.Turnbull IR, Gilfillan S, Cella M, Aoshi T, Miller M, Piccio L, Hernandez M, Colonna M. Cutting edge: TREM-2 attenuates macrophage activation. J Immunol. 2006;177:3520–4. doi: 10.4049/jimmunol.177.6.3520. [DOI] [PubMed] [Google Scholar]
- 156.Hamerman JA, Jarjoura JR, Humphrey MB, Nakamura MC, Seaman WE, Lanier LL. Cutting edge: inhibition of TLR and FcR responses in macrophages by triggering receptor expressed on myeloid cells (TREM)-2 and DAP12. J Immunol. 2006;177:2051–5. doi: 10.4049/jimmunol.177.4.2051. [DOI] [PubMed] [Google Scholar]
- 157.Gergely J, Pecht I, Sarmay G. Immunoreceptor tyrosine-based inhibition motif-bearing receptors regulate the immunoreceptor tyrosine-based activation motif-induced activation of immune competent cells. Immunol Lett. 1999;68:3–15. doi: 10.1016/s0165-2478(99)00024-3. [DOI] [PubMed] [Google Scholar]
- 158.Roach TI, Slater SE, White LS, Zhang X, Majerus PW, Brown EJ, Thomas ML. The protein tyrosine phosphatase SHP-1 regulates integrin-mediated adhesion of macrophages. Curr Biol. 1998;8:1035–8. doi: 10.1016/s0960-9822(07)00426-5. [DOI] [PubMed] [Google Scholar]
- 159.Xing L, Venegas AM, Chen A, Garrett-Beal L, Boyce BF, Varmus HE, Schwartzberg PL. Genetic evidence for a role for Src family kinases in TNF family receptor signaling and cell survival. Genes Dev. 2001;15:241–53. doi: 10.1101/gad.840301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Reuning U, Magdolen V, Hapke S, Schmitt M. Molecular and functional interdependence of the urokinase-type plasminogen activator system with integrins. Biol Chem. 2003;384:1119–31. doi: 10.1515/BC.2003.125. [DOI] [PubMed] [Google Scholar]
- 161.Bohuslav J, Horejsi V, Hansmann C, Stockl J, Weidle UH, Majdic O, Bartke I, Knapp W, Stockinger H. Urokinase plasminogen activator receptor, beta 2-integrins, and Src-kinases within a single receptor complex of human monocytes. J Exp Med. 1995;181:1381–90. doi: 10.1084/jem.181.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chiaradonna F, Fontana L, Iavarone C, Carriero MV, Scholz G, Barone MV, Stoppelli MP. Urokinase receptor-dependent and -independent p56/59(hck) activation state is a molecular switch between myelomonocytic cell motility and adherence. Embo J. 1999;18:3013–23. doi: 10.1093/emboj/18.11.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Murphy JE, Tedbury PR, Homer-Vanniasinkam S, Walker JH, Ponnambalam S. Biochemistry and cell biology of mammalian scavenger receptors. Atherosclerosis. 2005;182:1–15. doi: 10.1016/j.atherosclerosis.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 164.Miki S, Tsukada S, Nakamura Y, Aimoto S, Hojo H, Sato B, Yamamoto M, Miki Y. Functional and possible physical association of scavenger receptor with cytoplasmic tyrosine kinase Lyn in monocytic THP-1-derived macrophages. FEBS Lett. 1996;399:241–4. doi: 10.1016/s0014-5793(96)01332-4. [DOI] [PubMed] [Google Scholar]
- 165.Moore KJ, El Khoury J, Medeiros LA, Terada K, Geula C, Luster AD, Freeman MW. A CD36-initiated signaling cascade mediates inflammatory effects of beta-amyloid. J Biol Chem. 2002;277:47373–9. doi: 10.1074/jbc.M208788200. [DOI] [PubMed] [Google Scholar]
- 166.Rahaman SO, Lennon DJ, Febbraio M, Podrez EA, Hazen SL, Silverstein RL. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 2006;4:211–21. doi: 10.1016/j.cmet.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Medeiros LA, Khan T, El Khoury JB, Pham CL, Hatters DM, Howlett GJ, Lopez R, O’Brien KD, Moore KJ. Fibrillar amyloid protein present in atheroma activates CD36 signal transduction. J Biol Chem. 2004;279:10643–8. doi: 10.1074/jbc.M311735200. [DOI] [PubMed] [Google Scholar]
- 168.Gregory CD, Devitt A. The macrophage and the apoptotic cell: an innate immune interaction viewed simplistically? Immunology. 2004;113:1–14. doi: 10.1111/j.1365-2567.2004.01959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hu B, Sonstein J, Christensen PJ, Punturieri A, Curtis JL. Deficient in vitro and in vivo phagocytosis of apoptotic T cells by resident murine alveolar macrophages. J Immunol. 2000;165:2124–33. doi: 10.4049/jimmunol.165.4.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hu B, Punturieri A, Todt J, Sonstein J, Polak T, Curtis JL. Recognition and phagocytosis of apoptotic T cells by resident murine tissue macrophages require multiple signal transduction events. J Leukoc Biol. 2002;71:881–9. [PMC free article] [PubMed] [Google Scholar]
- 171.Wade WF, Davoust J, Salamero J, Andre P, Watts TH, Cambier JC. Structural compartmentalization of MHC class II signaling function. Immunol Today. 1993;14:539–46. doi: 10.1016/0167-5699(93)90184-M. [DOI] [PubMed] [Google Scholar]
- 172.Trede NS, Geha RS, Chatila T. Transcriptional activation of IL-1 beta and tumor necrosis factor-alpha genes by MHC class II ligands. J Immunol. 1991;146:2310–5. [PubMed] [Google Scholar]
- 173.Mehindate K, Thibodeau J, Dohlsten M, Kalland T, Sekaly RP, Mourad W. Cross-linking of major histocompatibility complex class II molecules by staphylococcal enterotoxin A superantigen is a requirement for inflammatory cytokine gene expression. J Exp Med. 1995;182:1573–7. doi: 10.1084/jem.182.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Morio T, Geha RS, Chatila TA. Engagement of MHC class II molecules by staphylococcal superantigens activates src-type protein tyrosine kinases. Eur J Immunol. 1994;24:651–8. doi: 10.1002/eji.1830240325. [DOI] [PubMed] [Google Scholar]
- 175.Andre P, Cambier JC, Wade TK, Raetz T, Wade WF. Distinct structural compartmentalization of the signal transducing functions of major histocompatibility complex class II (Ia) molecules. J Exp Med. 1994;179:763–8. doi: 10.1084/jem.179.2.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lang P, Stolpa JC, Freiberg BA, Crawford F, Kappler J, Kupfer A, Cambier JC. TCR-induced transmembrane signaling by peptide/MHC class II via associated Ig-alpha/beta dimers. Science. 2001;291:1537–40. doi: 10.1126/science.291.5508.1537. [DOI] [PubMed] [Google Scholar]
- 177.Huby RD, Dearman RJ, Kimber I. Intracellular phosphotyrosine induction by major histocompatibility complex class II requires co-aggregation with membrane rafts. J Biol Chem. 1999;274:22591–6. doi: 10.1074/jbc.274.32.22591. [DOI] [PubMed] [Google Scholar]
- 178.Bouillon M, El Fakhry Y, Girouard J, Khalil H, Thibodeau J, Mourad W. Lipid raft-dependent and -independent signaling through HLA-DR molecules. J Biol Chem. 2003;278:7099–107. doi: 10.1074/jbc.M211566200. [DOI] [PubMed] [Google Scholar]
- 179.Lin H, Huang MM, Leo C, Ji M, Wu G, Zhou A, Halenbeck R, Qin M, Linnemann T, Behrens D, Giese S, Bosch E, Justin K, Pham A, Lee E, Hestir K, Zhang H, Wong J, Wang Y, Chu K, Doberstein S, Williams L, Hollenbaugh D. A novel cytokine, FPT025, regulates myeloid growth and differentiation via the M-CSF receptor. Blood (ASH Annual Meeting Abstracts) 2006;108(191A):Abstract 634. [Google Scholar]
- 180.Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, Sylvestre V, Stanley ER. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111–20. doi: 10.1182/blood.v99.1.111. [DOI] [PubMed] [Google Scholar]
- 181.van Rooijen N, van Kesteren-Hendrikx E. “In vivo” depletion of macrophages by liposome-mediated “suicide”. Methods Enzymol. 2003;373:3–16. doi: 10.1016/s0076-6879(03)73001-8. [DOI] [PubMed] [Google Scholar]
- 182.Mocsai A, Zhou M, Meng F, Tybulewicz VL, Lowell CA. Syk is required for integrin signaling in neutrophils. Immunity. 2002;16:547–58. doi: 10.1016/s1074-7613(02)00303-5. [DOI] [PubMed] [Google Scholar]
- 183.Baek HS, Yoon JW. Direct involvement of macrophages in destruction of beta-cells leading to development of diabetes in virus-infected mice. Diabetes. 1991;40:1586–97. doi: 10.2337/diab.40.12.1586. [DOI] [PubMed] [Google Scholar]
- 184.Choi KS, Jun HS, Kim HN, Park HJ, Eom YW, Noh HL, Kwon H, Kim HM, Yoon JW. Role of Hck in the pathogenesis of encephalomyocarditis virus-induced diabetes in mice. J Virol. 2001;75:1949–57. doi: 10.1128/JVI.75.4.1949-1957.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lowell CA, Berton G. Resistance to endotoxic shock and reduced neutrophil migration in mice deficient for the Src-family kinases Hck and Fgr. Proc Natl Acad Sci USA. 1998;95:7580–4. doi: 10.1073/pnas.95.13.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Severgnini M, Takahashi S, Tu P, Perides G, Homer RJ, Jhung JW, Bhavsar D, Cochran BH, Simon AR. Inhibition of the Src and Jak kinases protects against lipopolysaccharide-induced acute lung injury. Am J Respir Crit Care Med. 2005;171:858–67. doi: 10.1164/rccm.200407-981OC. [DOI] [PubMed] [Google Scholar]
- 187.Khadaroo RG, He R, Parodo J, Powers KA, Marshall JC, Kapus A, Rotstein OD. The role of the Src family of tyrosine kinases after oxidant-induced lung injury in vivo. Surgery. 2004;136:483–8. doi: 10.1016/j.surg.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 188.Hanna Z, Kay DG, Rebai N, Guimond A, Jothy S, Jolicoeur P. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell. 1998;95:163–75. doi: 10.1016/s0092-8674(00)81748-1. [DOI] [PubMed] [Google Scholar]
- 189.Moarefi I, LaFevre-Bernt M, Sicheri F, Huse M, Lee CH, Kuriyan J, Miller WT. Activation of the Src-family tyrosine kinase Hck by SH3 domain displacement. Nature. 1997;385:650–3. doi: 10.1038/385650a0. [DOI] [PubMed] [Google Scholar]
- 190.Biggs TE, Cooke SJ, Barton CH, Harris MP, Saksela K, Mann DA. Induction of activator protein 1 (AP-1) in macrophages by human immunodeficiency virus type-1 NEF is a cell-type-specific response that requires both hck and MAPK signaling events. J Mol Biol. 1999;290:21–35. doi: 10.1006/jmbi.1999.2849. [DOI] [PubMed] [Google Scholar]
- 191.Hanna Z, Weng X, Kay DG, Poudrier J, Lowell C, Jolicoeur P. The pathogenicity of human immunodeficiency virus (HIV) type 1 Nef in CD4C/HIV transgenic mice is abolished by mutation of its SH3-binding domain, and disease development is delayed in the absence of Hck. J Virol. 2001;75:9378–92. doi: 10.1128/JVI.75.19.9378-9392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Suzu S, Harada H, Matsumoto T, Okada S. HIV-1 Nef interferes with M-CSF receptor signaling through Hck activation and inhibits M-CSF bioactivities. Blood. 2005;105:3230–7. doi: 10.1182/blood-2004-06-2084. [DOI] [PubMed] [Google Scholar]
- 193.Hu Y, Liu Y, Pelletier S, Buchdunger E, Warmuth M, Fabbro D, Hallek M, Van Etten RA, Li S. Requirement of Src kinases Lyn, Hck and Fgr for BCR-ABL1-induced B-lymphoblastic leukemia but not chronic myeloid leukemia. Nat Genet. 2004;36:453–61. doi: 10.1038/ng1343. [DOI] [PubMed] [Google Scholar]
- 194.Donato NJ, Wu JY, Stapley J, Gallick G, Lin H, Arlinghaus R, Talpaz M. BCR-ABL independence and LYN kinase overexpression in chronic myelogenous leukemia cells selected for resistance to STI571. Blood. 2003;101:690–8. doi: 10.1182/blood.V101.2.690. [DOI] [PubMed] [Google Scholar]
- 195.Martinelli G, Soverini S, Rosti G, Baccarani M. Dual tyrosine kinase inhibitors in chronic myeloid leukemia. Leukemia. 2005;19:1872–9. doi: 10.1038/sj.leu.2403950. [DOI] [PubMed] [Google Scholar]
- 196.Hu Y, Swerdlow S, Duffy TM, Weinmann R, Lee FY, Li S. Targeting multiple kinase pathways in leukemic progenitors and stem cells is essential for improved treatment of Ph+ leukemia in mice. Proc Natl Acad Sci U S A. 2006;103:16870–5. doi: 10.1073/pnas.0606509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Okamoto M, Hayakawa F, Miyata Y, Watamoto K, Emi N, Abe A, Kiyoi H, Towatari M, Naoe T. Lyn is an important component of the signal transduction pathway specific to FLT3/ITD and can be a therapeutic target in the treatment of AML with FLT3/ITD. Leukemia. 2007;21:403–10. doi: 10.1038/sj.leu.2404547. [DOI] [PubMed] [Google Scholar]
- 198.Smith JD, Trogan E, Ginsberg M, Grigaux C, Tian J, Miyata M. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc Natl Acad Sci USA. 1995;92:8264–8. doi: 10.1073/pnas.92.18.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Miki S, Horikawa K, Nishizumi H, Suemura M, Sato B, Yamamoto M, Takatsu K, Yamamoto T, Miki Y. Reduction of atherosclerosis despite hypercholesterolemia in lyn-deficient mice fed a high-fat diet. Genes Cells. 2001;6:37–42. doi: 10.1046/j.1365-2443.2001.00396.x. [DOI] [PubMed] [Google Scholar]
- 200.Schwartzberg PL, Xing L, Hoffmann O, Lowell CA, Garrett L, Boyce BF, Varmus HE. Rescue of osteoclast function by transgenic expression of kinase-deficient Src in src−/− mutant mice. Genes Dev. 1997;11:2835–44. doi: 10.1101/gad.11.21.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hayes SM, Love PE. Strength of signal: a fundamental mechanism for cell fate specification. Immunol Rev. 2006;209:170–5. doi: 10.1111/j.0105-2896.2006.00356.x. [DOI] [PubMed] [Google Scholar]
- 202.Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003;31:3497–500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Blake RA. Cellular screening assays using fluorescence microscopy. Curr Opin Pharmacol. 2001;1:533–9. doi: 10.1016/s1471-4892(01)00092-3. [DOI] [PubMed] [Google Scholar]