Abstract
BACKGROUND
Among patients with stage I breast cancer, there is significant uncertainty concerning the optimal threshold at which to consider chemotherapy, and when considered, there is controversy regarding whether to consider non-intensive versus intensive regimens. The authors examined the types and costs of adjuvant chemotherapy received among patients with stage I breast cancer.
METHODS
The current study was a prospective cohort study including patients with stage I breast cancer who were treated at a National Comprehensive Cancer Network center from 2000 through 2009. Stage was defined according to the version of the American Joint Committee on Cancer Staging Manual applicable at the time of diagnosis. Stratifying by human epidermal growth factor receptor 2 (HER2), the authors examined the percentage of patients receiving intensive versus non-intensive chemotherapy regimens and the factors associated with type of chemotherapy administered using multivariable logistic regression. Costs of the most common regimens were estimated.
RESULTS
Of 8907 patients, 33% received adjuvant chemotherapy. Among those individuals, there was an increase in the use of intensive chemotherapy within the last decade, from 31% in 2000 through 2005 to 63% in 2008 through 2009 (including an increase in the use of the combination of docetaxel, carboplatin, and trastuzumab) among patients with HER2-positive disease and from 15% in 2000 through 2005 to 41% in 2008 through 2009 among patients with HER2-negative disease (32% of patients with hormone receptor-positive and 59% of patients with triple-negative disease). Among patients treated with non-intensive regimens, there was an increase in the use of the combination of docetaxel and cyclophosphamide noted, with a decrease in the use of the doxorubicin and cyclophosphamide combination. The choice of regimen varied significantly by institution. The major drivers of cost variation were the incorporation of biologics (eg, trastuzumab) and growth factors, with significant variation even within non-intensive and intensive regimens.
CONCLUSIONS
Over time, there was an increase in use of intensive regimens among Stage I breast cancer, with striking institutional and cost variations.
Keywords: chemotherapy, stage I, breast cancer, institutional variation, cost
INTRODUCTION
Stage I breast cancers represent nearly one-half of early breast cancer diagnoses and generally have an excellent prognosis.1,2 However, some patients have a sufficient risk of disease recurrence to drive a recommendation for adjuvant chemotherapy.3,4
The chemotherapy landscape for patients with early breast cancer has changed over time.5,6 In the early 2000’s, several trials demonstrated that multi-agent chemotherapy regimens such as combinations of anthracyclines and taxanes were more effective for the treatment of patients with early breast cancer compared with anthracycline-based regimens such as doxorubicin and cyclophosphamide (AC), although they increased the duration and cost of therapy and were associated with more toxicity.6,7 In approximately 2006, taxane-based regimens without anthracyclines also demonstrated efficacy, although these regimens also had other potential toxicities, such as febrile neutropenia and neurotoxicity.6-8 At the same time, multiple phase 3 trials suggested that adding trastuzumab to chemotherapy was associated with a 40% risk reduction in disease recurrence compared with chemotherapy alone.9 To our knowledge, patients with stage I disease have generally been excluded or underrepresented in such trials and therefore there is uncertainty regarding the preferred chemotherapy for these individuals.6,10
In the current study, we attempted to describe how medical oncologists have translated the above findings into routine practice. We focused on patients treated at a National Comprehensive Cancer Network (NCCN) center, and examined the type and costs of adjuvant chemotherapy and factors associated with its choice according to biologic subtype.
MATERIALS AND METHODS
Study Design and Data Source
The current study was a prospective cohort study performed using the NCCN breast cancer outcomes database. Patients were included if they received all or some of their treatment at a reporting center. Eight centers contributed data: City of Hope Cancer Research Hospital, The University of Texas MD Anderson Cancer Center, Fox Chase Cancer Center, Dana-Farber Cancer Institute, Roswell Park Cancer Institute, H. Lee Moffitt Cancer Center, University of Michigan Comprehensive Cancer Center, and Ohio State University.11
Institutional Review Boards (IRBs) from participating centers approved the study protocol. At centers in which the IRB required signed informed consent for data collection, only patients who provided consent were included; elsewhere, the IRB granted a waiver of signed informed consent.
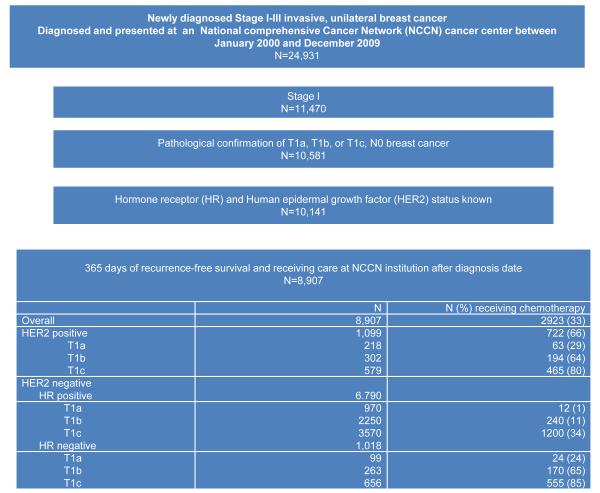
An analytic cohort of 8907 patients with stage I breast cancer was identified, 2923 of whom received chemotherapy (Fig. 1). Stage was defined according to the version of the American Joint Committee on Cancer (AJCC) Staging Manual applicable at the time of diagnosis. Patients treated with trastuzumab without chemotherapy (14 patients) were not included in the subgroup of patients treated with chemotherapy.
Figure 1.
Flow diagram of patient population. Note that patients treated with trastuzumab only were included in the non chemotherapy group (14 patients). HER2 indicates human epidermal growth factor receptor 2; HR, hormone receptor; NCCN, National Comprehensive Cancer Network.
Dependent Variables: Type of Chemotherapy Received
The database contains information regarding drug treatment as abstracted by chart review. Type of chemotherapy was grouped as intensive versus non-intensive, defined as shown in Table 1. Approximately 14% of patients were treated on trial.
TABLE 1.
Type of Chemotherapy Regimen for Patients With Stage I Breast Cancer, 2000 to 2009a
| HER2 Positive |
HER2 Negative |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR Positive |
HR Negative |
|||||||||||
| T1a N=63 |
T1b N=194 |
T1c N=465 |
Total N=722 |
T1a N=12 |
T1b N=240 |
T1c N=1200 |
T1a N=24 |
T1b N=170 |
T1c N=555 |
Total N=2201 |
||
| Intensive | Anthracycline-containing + taxane ±trastuzumab, % |
13 | 24 | 40 | 34 | 0 | 16 | 18 | 29 | 20 | 38 | 23 |
| AC+paclitaxel | 10 | 15 | 31 | 25 | 0 | 6 | 8 | 13 | 10 | 25 | 12 | |
| TAC or AC+docetaxel | 0 | 2 | 3 | 2 | 0 | 2 | 1 | 0 | 0 | 1 | 1 | |
| FAC or CAF+paclitaxel | 2 | 3 | 3 | 3 | 0 | 5 | 5 | 13 | 5 | 6 | 5 | |
| FAC or CAF+docetaxel | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Other anthracycline+ paclitaxel |
2 | 3 | 3 | 3 | 0 | 1 | 3 | 4 | 4 | 4 | 3 | |
| Other anthracycline+ docetaxel |
0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 2 | 2 | 1 | |
| Docetaxel-carboplatin ± trastuzumab (%) |
29 | 16 | 11 | 14 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | |
| Other ±trastuzumab (%) | 3 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total intensive, % | 44 | 41 | 51 | 48 | 0 | 18 | 18 | 29 | 21 | 39 | 24 | |
| Non-intensive | Anthracycline-containing ± trastuzumab (%) |
35 | 38 | 37 | 37 | 58 | 60 | 63 | 58 | 57 | 47 | 58 |
| AC | 29 | 33 | 29 | 30 | 50 | 43 | 47 | 46 | 46 | 36 | 44 | |
| FAC or CAF | 5 | 3 | 5 | 4 | 8 | 10 | 12 | 7 | 7 | 7 | 10 | |
| Other anthracycline | 2 | 2 | 3 | 2 | 0 | 7 | 5 | 4 | 4 | 4 | 5 | |
| Docetaxel-cyclophosphamide ±trastuzumab, % |
5 | 5 | 3 | 4 | 33 | 14 | 10 | 4 | 14 | 6 | 10 | |
| Other ±trastuzumab, % | 16 | 16 | 9 | 12 | 8 | 8 | 9 | 8 | 8 | 8 | 9 | |
| CMF | 0 | 2 | 1 | 1 | 0 | 5 | 4 | 0 | 5 | 5 | 4 | |
| Paclitaxel | 16 | 13 | 7 | 10 | 0 | 2 | 3 | 8 | 3 | 3 | 3 | |
| Other | 0 | 1 | 1 | 1 | 8 | 2 | 2 | 0 | 1 | 1 | 1 | |
| Total non-intensive, % | 56 | 59 | 49 | 52 | 100 | 82 | 82 | 71 | 79 | 61 | 76 | |
| Chemotherapy on clinical trial | 19 | 15 | 18 | 17 | 0 | 12 | 13 | 4 | 13 | 12 | 13 | |
Abbreviations: AC, doxorubicin and cyclophosphamide; CAF, cyclophosphamide, doxorubicin, and 5-fluorouracil; CMF, cyclophosphamide, methotrexate, and 5-fluorouracil; FAC, 5-fluorouracil, doxorubicin, and cyclophosphamide; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; TAC, docetaxel, doxorubicin, and cyclophosphamide.
Approximately 97% of “other” regimens were non-intensive.
Independent Variables
Information regarding tumor size, tumor grade, lymphovascular invasion (LVI), hormone receptor (HR), Oncotype Dx recurrence scores (Genomic Health, Inc) (RS), and human epidermal growth factor receptor 2 (HER2) status was abstracted from pathology reports. HR was considered positive if the estrogen receptor and/or progesterone receptor were positive. For HER2 classification, the fluorescence in situ hybridization result was used, if available. If only immunohistochemistry was available, scores of 3+, “high positive,” or “positive NOS [not otherwise specified]” were considered HER2 positive, whereas scores of 2+, 1+, 0, or “negative” were considered HER2 negative; 63 patients (1%) were “positive NOS.” Oncotype Dx RS were categorized as low (1-17), intermediate (18-30), or high (31-100).Tumor grade was categorized as high (according to histologic grade or, if not available, by nuclear grade) or low-intermediate. Age at diagnosis was abstracted by chart review. Data regarding race/ethnicity were derived from patient surveys collected at the time of the initial presentation to the NCCN center, whereas information regarding comorbidities was collected either via surveys or chart review. The comorbidity score was derived from the systems of Charlson et al and Katz et al.12,13 Of the entire cohort, cardiac comorbidities were only present at baseline in 2.4% of patients and therefore this was not explored as an independent variable.
Costs
Drug costs per regimen included costs of chemotherapy drugs, trastuzumab, and growth factor support. Antiemetics, transfusions, and other supportive therapies were not included. For the primary cost estimates analyses, we used the following formula: total cost per regimen = total mg per cycle × price per mg (US$) × 1.06 × the number of cycles per regimen, applying the dosing proposed by the NCCN guidelines10 and the 2006 Medicare Part B Drug Average Sales Price.14 The costs were estimated for a 50-year-old woman with a body surface area of 1.7, weight of 70 kg, and serum creatinine level of 1 mg/dL. We repeated these analyses with the 2009 and 2014 Medicare Part B Drug Average Sales Price.14
Statistical Analysis
All the analyses were stratified by HER2 status. The receipt of chemotherapy and of a particular regimen over time was summarized descriptively. For patients with HER2-negative disease, we further stratified the analysis by HR status.
Multivariable logistic regression models examined patient characteristics associated with the type of chemotherapy received. To understand what currently drives medical decisions, these models were restricted to patients whose adjuvant chemotherapy was not given within a clinical trial and to those diagnosed in 2006 and thereafter, which coincided with the publication of the US Oncology Adjuvant Trial 9735 comparing AC with the combination of docetaxel and cyclophosphamide (TC) as adjuvant therapy for patients with breast cancer and after the adjuvant trastuzumab trial presentations, including the Breast Cancer International Research Group (BCIRG) 006 trial of the combination of docetaxel, carboplatin, and trastuzumab (TCH), AC followed by paclitaxel and trastuzumab (ACTH), or chemotherapy without trastuzumab.7,8
Separate models were fit for the HER2-negative and HER2-positive cohorts. We used logistic regression models to examine whether the patient received an intensive chemotherapy regimen versus a non-intensive regimen. Among patients with HER2-positive disease, we examined patient characteristics associated with the type of intensive chemotherapy regimen (TCH vs ACTH). Among patients with HER2-negative disease, we examined patient characteristics associated with the type of non-intensive chemotherapy regimen (TC vs AC). All variables were included in the models. The association between Oncotype Dx RS and type of chemotherapy is only relevant for the subgroup of patients with HER2-negative/HR-positive disease. Of the subgroup of 485 patients included in the multivariable model with HER2-negative/HR-positive tumors, 55% (265 patients) had an available Oncotype Dx RS result. Among these patients, we explored the univariate association between Oncotype Dx RS and type of chemotherapy.
Cost per common regimen was summarized descriptively. All P values presented are 2-sided, with statistical significance defined as P <.05. Statistical analyses were conducted using Stata statistical software (version 13.1; StataCorp LP, College Station, Tex).
RESULTS
Use of Chemotherapy From 2000 to 2009
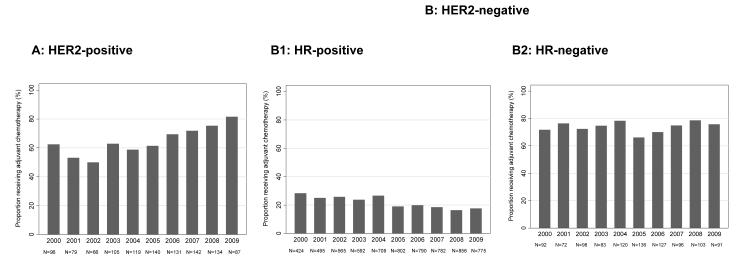
Among 8907 patients with stage I breast cancer, 33% received chemotherapy, with striking differences in chemotherapy use observed by subtype of disease; a higher percentage of patients with HER2-positive disease (66%) and triple-negative breast cancer (TNBC) (HER2-negative/HR-negative disease) (74%) received chemotherapy when compared with patients with HER2-negative/HR-positive disease (21%) (Fig. 1).Time trends in chemotherapy use differed by subtype (Fig. 2).
Figure 2.
Chemotherapy with or without trastuzumab received between 2000 and 2009 (A) the percentage of patients with human epidermal growth factor receptor 2 (HER2)-positive disease receiving chemotherapy and (B) patients with HER2-negative disease, subdivided into (B1) the percentage of patients with HER2-negative, hormone receptor (HR)-positive disease receiving chemotherapy and (B2) the percentage of patients with HER2-negative/HR-negative disease receiving chemotherapy.
Use of Intensive Versus Non-intensive Chemotherapy Regimens From 2000 Through 2009
Table 2 presents the baseline clinicopathological characteristics of those patients treated with chemotherapy.
TABLE 2.
Baseline Characteristics for Patients With Stage I Breast Cancer Who Received Adjuvant Chemotherapy, 2000 to 2009
| HER2 Positive |
HER2 Negative |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR Positive |
HR Negative |
||||||||||
| T1a N=63 |
T1b N=194 |
T1c N=465 |
Total N=722 |
T1a N=12 |
T1b N=240 |
T1c N=1200 |
T1a N=24 |
T1b N=170 |
T1c N=555 |
Total N=2201 |
|
| Age at diagnosis, y % | |||||||||||
| <50 | 57 | 40 | 46 | 45 | 75 | 59 | 52 | 58 | 34 | 43 | 49 |
| 50 to 59 | 25 | 36 | 33 | 33 | 17 | 31 | 34 | 33 | 39 | 34 | 34 |
| 60 to 69 | 16 | 23 | 17 | 19 | 8 | 9 | 12 | 4 | 23 | 20 | 14 |
| ≥70 | 2 | 2 | 4 | 3 | 0 | 2 | 2 | 4 | 4 | 3 | 2 |
| Race/ethnicity, % | |||||||||||
| Non-Hispanic white | 67 | 79 | 82 | 80 | 75 | 83 | 84 | 75 | 84 | 78 | 82 |
| Non-Hispanic black | 10 | 5 | 6 | 6 | 17 | 4 | 5 | 17 | 8 | 11 | 7 |
| Hispanic | 11 | 8 | 6 | 7 | 8 | 8 | 6 | 4 | 3 | 6 | 6 |
| Unknown/other | 14 | 9 | 6 | 7 | 0 | 6 | 5 | 4 | 5 | 5 | 5 |
| Comorbidity score, % | |||||||||||
| 0 | 94 | 82 | 83 | 83 | 83 | 85 | 82 | 92 | 79 | 81 | 82 |
| ≥1 | 6 | 18 | 17 | 16 | 17 | 15 | 18 | 8 | 21 | 19 | 18 |
| Year of diagnosis, % | |||||||||||
| 2000 | 2 | 5 | 11 | 8 | 8 | 6 | 9 | 0 | 7 | 10 | 8 |
| 2001 | 8 | 6 | 5 | 6 | 0 | 7 | 9 | 0 | 10 | 7 | 8 |
| 2002 | 3 | 4 | 5 | 5 | 8 | 5 | 11 | 13 | 8 | 10 | 10 |
| 2003 | 5 | 11 | 9 | 9 | 17 | 9 | 10 | 8 | 10 | 8 | 9 |
| 2004 | 0 | 9 | 11 | 10 | 8 | 10 | 14 | 8 | 9 | 14 | 13 |
| 2005 | 17 | 11 | 11 | 12 | 8 | 12 | 10 | 17 | 9 | 13 | 11 |
| 2006 | 14 | 9 | 14 | 13 | 8 | 16 | 10 | 17 | 14 | 11 | 11 |
| 2007 | 16 | 18 | 12 | 14 | 8 | 10 | 10 | 8 | 9 | 10 | 10 |
| 2008 | 14 | 18 | 12 | 14 | 17 | 11 | 9 | 13 | 12 | 10 | 10 |
| 2009 | 21 | 9 | 9 | 10 | 17 | 14 | 8 | 17 | 12 | 8 | 9 |
| Tumor size, cm | |||||||||||
| Median | 0.4 | 0.8 | 1.5 | 1.3 | 0.4 | 0.9 | 1.5 | 0.5 | 0.9 | 1.5 | 1.1 |
| IQR | 0.3-0.5 | 0.7-1.0 | 1.3-1.8 | 0.9-1.6 | 0.4-0.5 | 0.8-1.0 | 1.3-1.8 | 0.4-0.5 | 0.8-1.0 | 1.4-1.8 | 1.5-1.7 |
| HR status, % | |||||||||||
| Negative | 49 | 45 | 34 | 39 | 0 | 0 | 0 | 100 | 100 | 100 | 34 |
| Positive | 51 | 55 | 66 | 61 | 100 | 100 | 100 | 0 | 0 | 0 | 66 |
| Grade, % | |||||||||||
| Low/intermediate | 24 | 37 | 33 | 33 | 67 | 61 | 58 | 21 | 14 | 11 | 43 |
| High | 73 | 61 | 66 | 66 | 33 | 37 | 41 | 79 | 86 | 88 | 56 |
| Unknown | 3 | 2 | 1 | 1 | 0 | 2 | 1 | 0 | 0 | 1 | 1 |
| Lymphovascular invasion (%) | |||||||||||
| No | 87 | 80 | 75 | 78 | 92 | 81 | 77 | 83 | 90 | 83 | 80 |
| Yes | 11 | 20 | 24 | 22 | 8 | 17 | 22 | 13 | 10 | 16 | 19 |
| Unknown | 2 | 1 | 1 | 1 | 0 | 2 | 1 | 4 | 0 | 1 | 1 |
Abbreviations: HER2, human epidermal growth factor receptor 2; HR, hormone receptor; IQR, interquartile range.
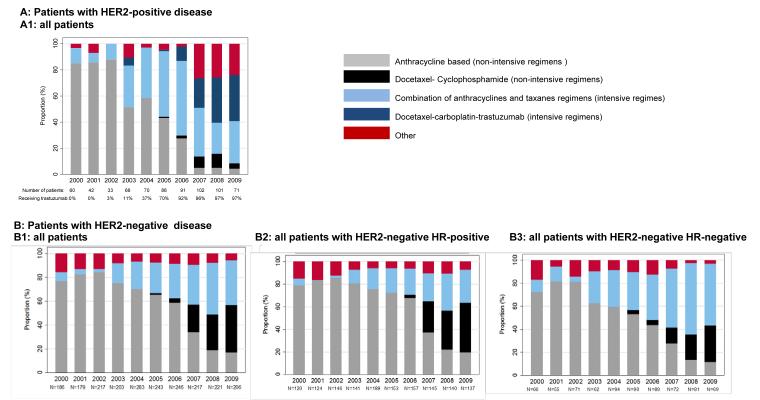
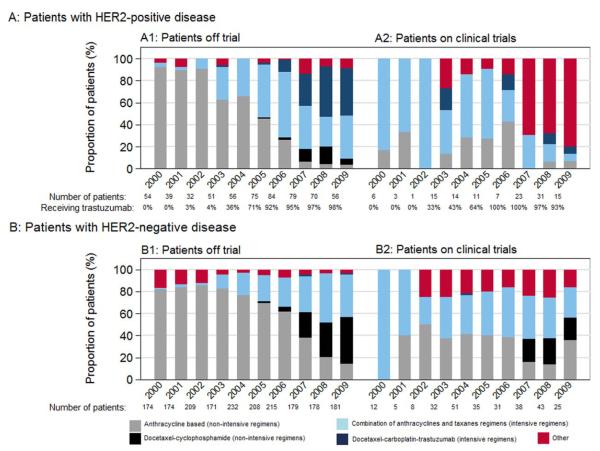
Among 2923 patients who received chemotherapy, 30% (865 patients) were treated with intensive regimens from 2000 through 2009. Figure 3 represents treatment patterns over the last decade and Figure 4 represents treatments both off and on trial.
Figure 3.
Type of chemotherapy with or without trastuzumab received between 2000 and 2009 among (A) patients with human epidermal growth factor receptor 2 (HER2)-positive disease, with the percentage of all patients receiving each chemotherapy (722 patients) and (B) patients with HER2-negative disease, subdivided into (B1) the percentage of all patients receiving each chemotherapy regimen (2201 patients), (B2) the percentage of patients with HER2-negative/hormone receptor (HR)-positive disease receiving each chemotherapy regimen (1425 patients), and (B3) the percentage of patients with HER2-negative/HR-negative disease receiving each chemotherapy regimen (749 patients).
Figure 4.
Type of chemotherapy with or without trastuzumab received between 2000 and 2009 among (A) patients with human epidermal growth factor receptor 2 (HER2)-positive disease, with the percentage of patients receiving each chemotherapy, subdivided into (A1) the percentage of patients receiving each chemotherapy off trial (596 patients) and (A2) the percentage of patients receiving each chemotherapy on trial (126 patients) and (B) among patients with HER2-negative disease, subdivided into (B1) the percentage of patients receiving each chemotherapy regimen off trial (1921 patients) and (B2) the percentage of patients receiving each chemotherapy regimen on trial (280 patients).
Among patients with HER2-positive disease who received chemotherapy, 48% were treated with an intensive chemotherapy regimen, including 44% of patients with T1a disease, 41% of patients with T1b disease, and 51% of patients with T1c disease. Over time, there was an increase in the use of intensive regimens, from 31% in 2000 through 2005 to 63% in 2008 through 2009. Trastuzumab was administered to 61% of patients with HER2-positive disease, with >90% of those patients diagnosed during or after 2006 receiving trastuzumab.
Among patients with HER2-negative disease who were treated with chemotherapy, 24% received an intensive regimen (19% of patients with T1a and T1b disease and 25% of patients with T1c disease). Particularly among patients with TNBC, we found 29% of patients with T1a disease, 21% of patients with T1b disease, and 39% of patients with T1c disease were treated with intensive chemotherapy regimens. Over time, there was an increase in the use of intensive chemotherapy, from 15% in 2000 through 2005 to 41% in 2008 through 2009. Patients with HR-negative and HR-positive disease contributed differently to this trend; by 2008 through 2009, only 32% of patients with HR-positive disease who were treated with chemotherapy received intensive regimens, but 59% of patients with TNBC received an anthracycline and taxane combination.
Use of Non–Anthracycline-Containing Regimens
In 2000, 87% of patients received anthracycline-based regimens. However, by 2008 through 2009, 55% of patients with HER2-positive disease received TCH and, among patients with HER2-negative disease, TC was used by 59% of patients receiving non-intensive chemotherapy regimens.
Factors Associated With the Choice of Chemotherapy
The baseline characteristics of patients treated between 2006 and 2009 off trial were similar to those of the overall cohort (data not shown). There were small differences in the comorbidity score noted among the patients with HER2-positive disease (18% of those treated off trial vs 10% of those treated on trial had ≥1 comorbidities). Table 3 represents the results of the multivariable model, which examined characteristics associated with the type of chemotherapy received.
TABLE 3.
Multivariable Logistic Regression Analysis to Investigate Characteristics Associated With Chemotherapy Received Among Patients With Stage I Breast Cancer Diagnosed Between 2006 and 2009 and Treated Off the Clinical Trial
| HER2 Positive |
HER2 Negative |
|||||||
|---|---|---|---|---|---|---|---|---|
| Intensive Versus Non-intensive N=289 |
TCH Versus ACTH N=210 |
Intensive Versus Non-intensive N=753 |
TC Versus AC N=447 |
|||||
|
|
||||||||
| Outcome | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI |
| Age at diagnosis, y | 0.98 | 0.95-1.01 | 1.02 | 0.99-1.06 | 0.95 | 0.93-0.97 | 1.03 | 1.01-1.06 |
| HR positive (vs negative) | 0.67 | 0.34-1.33 | 1.01 | 0.49-2.09 | 0.25 | 0.16-0.37 | 0.78 | 0.40-1.54 |
| Tumor size, cm | 2.03 | 1.04-3.96 | 0.25 | 0.12-0.51 | 2.57 | 1.67-3.94 | 1.12 | 0.60-2.11 |
| Race/ethnicity (other vs non-Hispanic white) |
0.53 | 0.25-1.15 | 1.25 | 0.55-2.84 | 1.30 | 0.86-1.96 | 1.56 | 0.77-3.17 |
| Comorbidity score (≥1 vs 0) | 0.65 | 0.31-1.37 | 1.94 | 0.85-4.42 | 1.09 | 0.71-1.67 | 1.25 | 0.64-2.45 |
| Year of diagnosis | 1.43 | 1.06-1.92 | 1.83 | 1.34-2.51 | 1.34 | 1.15-1.56 | 3.53 | 2.71-4.59 |
| High grade (yes vs no) | 1.22 | 0.62-2.40 | 2.87 | 1.34-6.13 | 1.88 | 1.26-2.81 | 0.76 | 0.42-1.36 |
| Lymphovascular invasion (yes vs no) | 1.47 | 0.63-3.41 | 0.92 | 0.38-2.24 | 2.05 | 1.30-3.23 | 0.98 | 0.48-2.00 |
| Center | ||||||||
| A | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| B | 0.05 | 0.01-0.32 | 0.29 | 0.04-1.93 | 0.25 | 0.10-0.61 | 0.15 | 0.04-0.54 |
| C | 0.65 | 0.11-3.97 | 0.30 | 0.07-1.28 | 1.00 | 0.42-2.35 | 1.93 | 0.48-7.77 |
| D | 1.10 | 0.20-6.12 | 0.70 | 0.20-2.41 | 1.55 | 0.75-3.21 | 0.20 | 0.06-0.67 |
| E | 0.09 | 0.01-0.62 | 0.14 | 0.01-1.70 | 1.11 | 0.46-2.66 | 2.36 | 0.51-10.8 |
| F | 0.12 | 0.02-0.64 | 1.01 | 0.21-4.95 | 0.90 | 0.36-2.29 | 0.52 | 0.12-2.18 |
| G | 0.11 | 0.02-0.63 | 1.32 | 0.31-5.57 | 0.17 | 0.06-0.46 | 0.07 | 0.02-0.29 |
| H | 0.20 | 0.03-1.46 | 0.78 | 0.12-5.01 | 0.25 | 0.10-0.64 | 1.11 | 0.29-4.20 |
Abbreviations: 95% CI, 95% confidence interval; AC, doxorubicin and cyclophosphamide; ACTH, doxorubicin, cyclophosphamide, paclitaxel, and trastuzumab; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; OR, odds ratio; TC, docetaxel and cyclophosphamide; TCH, docetaxel, carboplatin, and trastuzumab.
Among patients with HER2-positive disease, characteristics that were found to factor into the use of intensive versus non-intensive chemotherapy regimens included year of diagnosis, NCCN center, and tumor size. These analyses confirmed an increase in the use of intensive regimens over time (odds ratio [OR], 1.43 per year of diagnosis; 95% confidence interval [95% CI], 1.06-1.92). As expected, patients with larger tumors had a higher probability of receiving an intensive regimen (OR, 2.03 per cm; 95% CI, 1.04-3.96). We observed dramatic institutional variability (using center A as a reference, the OR of receiving intensive vs non-intensive therapy ranged between 0.05-1.10 [P<.001], with the unadjusted institutional use of intensive regimens ranging from 42%-90%). These same characteristics were found to be associated with the choice of TCH versus ACTH. There was an increase in the use of TCH over time (OR, 1.83 per year of diagnosis; 95% CI, 1.34-2.51). Patients with larger tumors were less likely to be treated with TCH versus ACTH (OR, 0.25 per cm; 95% CI, 0.12-0.51). Finally, the unadjusted institutional use of TCH regimens ranged from 25% to 53%.
For patients with HER2-negative disease, variables that were found to factor into the choice of intensive regimens included the same significant predictors as for patients with HER2-positive disease (year, NCCN center [the institutional use of intensive regimens ranged from 14%-50%], and tumor size), as well as age, HR status, grade, and LVI. For patients treated with non-intensive chemotherapy regimens, the choice between the TC versus AC regimens was significantly influenced by age, year, and reporting center. There was an increase in the use of TC over time (OR, 3.53 per year of diagnosis; 95% CI, 2.71-4.59), with significant center variability (compared with center A, the OR of receiving TC vs AC chemotherapy ranged from 0.07-2.36 [P<.001], with the unadjusted institutional use of TC ranging from 18%-79%). Among patients with HR-positive disease, exploratory analyses suggested that Oncotype Dx RS may be associated with treatment received; in particular, patients with higher scores were significantly more likely to receive intensive regimens (Table 4). However, we were unable to determine whether the association was independent of other factors due to collinearity between the Oncotype Dx RS and tumor grade.
TABLE 4.
Cross-tabulation of Oncotype Dx scores and Pathologic stage, and Unadjusted Association With Type of Chemotherapy Among Patients Diagnosed Between 2006 and 2009 Who Were Treated Off Clinical Trial With HER2-Negative and HR-Positive Disease With Oncotype Dx Scores
| T1a, No. (%) |
T1b, No. (%) |
T1c, No. (%) |
Total, No. (%) |
% receiving Intensive Versus non-intensivea |
% receiving TC Versus ACb |
|
|---|---|---|---|---|---|---|
|
|
||||||
| N=3 | N=75 | N=189 | N=268 | |||
| Oncotype Dx recurrence score | ||||||
| Low | 0 | 10 (13) | 24 (13) | 34 (13) | 21 | 58 |
| Intermediate | 3 (100) | 45 (60) | 113 (60) | 161 (61) | 16 | 53 |
| High | 0 | 20 (27) | 50 (27) | 70 (26) | 40 | 39 |
Abbreviations: AC, doxorubicin and cyclophosphamide; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; TC, docetaxel and cyclophosphamide.
There was collinearity between Oncotype Dx recurrence score and grade (high-grade disease was present, respectively, for 26%, 35%, and 66% of patients with low, intermediate, and high scores).
P value for trend, <.001.
P value for trend, .10.
Costs
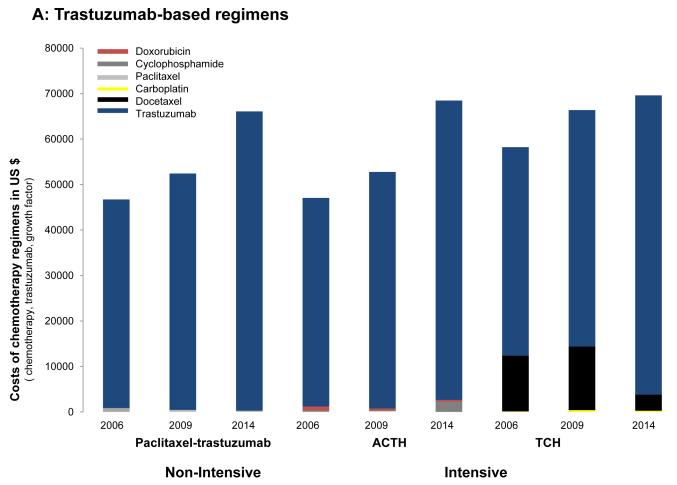
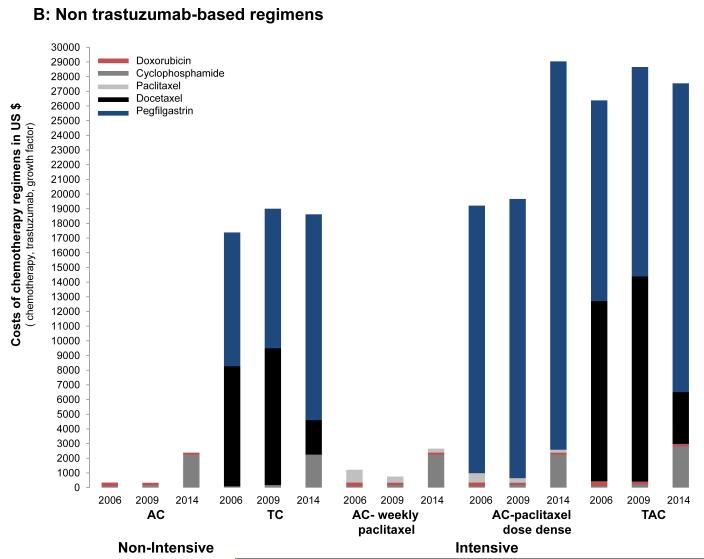
We found significant cost variability (Fig. 5). In 2006, the drug cost of trastuzumab-based regimens was primarily driven by the use of trastuzumab (1 year of trastuzumab treatment exceeded $45,000). The cost of using a non-intensive strategy such as paclitaxel with trastuzumab was $46,178, whereas the costs of intensive regimens such as TCH or ACTH were $58,115 and $47,062, respectively. When comparing the drug cost of non–trastuzumab-based regimens, the differences were mainly driven by the use of pegfilgastrim and docetaxel. Among non-intensive strategies, 4 cycles of AC cost $346 and 4 cycles of TC cost $17,383. Among intensive strategies, 8 cycles of dose-dense doxorubicin, cyclophosphamide, paclitaxel (ACHT) ACT cost $19,213 and 6 cycles of the combination of doxorubicin, cyclophosphamide, and docetaxel cost $26,378.
Figure 5.
Cost of chemotherapy regimens (including chemotherapy, trastuzumab, and growth factor support) in 2006, 2009 and 2014 for (A) trastuzumab-based regimens and (B) non–trastuzumab-based regimens. AC indicates doxorubicin and cyclophosphamide; ACTH, doxorubicin, cyclophosphamide, paclitaxel, and trastuzumab; TAC, docetaxel, doxorubicin, and cyclophosphamide; TC, docetaxel and cyclophosphamide; TCH, docetaxel, carboplatin, and trastuzumab.
The estimates for 2009 were similar; however, with docetaxel at that point available as a generic drug, there were no longer significant price differences noted among trastuzumab-based regimens and among non–trastuzumab-based regimens; rather, these differences were now mainly driven by the use of growth factor support. For example, there was an 8-fold to 12-fold price difference when comparing regimens such as AC or AC followed by weekly paclitaxel with regimens such as TC, dose-dense ACT, or the combination of doxorubicin, cyclophosphamide, and docetaxel.
DISCUSSION
Previously, we demonstrated a strong increase in chemotherapy use among patients with T1abN0 breast cancers who are treated at NCCN institutions.4 In the current study, we extended this work to include patients with T1cN0 tumors and analyze factors associated with the choice of intensive versus non-intensive regimens and the costs of different chemotherapy regimens. Among nearly 9000 women presenting with stage I breast cancer, we documented an increased use of intensive chemotherapy over the last decade and a substantial decline in the use of anthracycline-based regimens. We observed striking institutional variations with regard to the choice of chemotherapy, variations that were of a greater magnitude than clinicopathological factors. We also found a substantial variation in costs, particularly among non–trastuzumab-based regimens, which varied by >75-fold, with the use of growth factor support being the major driver of this variation.
We noted an increase in the use of intensive regimens over time, most notably among patients with TNBC and HER2-positive disease.
As expected, we found that, among patients with TNBC, a higher percentage of patients with T1c tumors received intensive regimens compared with those with T1ab tumors (29% of patients with T1a disease, 21% of patients with T1b disease, and 39% of patients with T1c disease); we also found that over time there was an increase in the use of intensive regimens among this patient population (59% of patients with TNBC in 2008 through 2009).Prior meta-analyses demonstrated that the incorporation of taxanes into an anthracycline regimen results in a 16% relative reduction in the risk of disease recurrence, which translated into an absolute gain in recurrence-free survival of 4.6%.6,15 However, the majority of adjuvant clinical trials either excluded women with stage I disease entirely or only included a small percentage of such women.6 Therefore, the absolute benefit derived from the use of more intensive regimens among this patient population is based largely on extrapolation,6 raising the question of the appropriate threshold at which to incorporate taxanes in patients with high-risk biology but lower-risk anatomic stage, given the added toxicities and costs incurred.
Among patients with HER2-positive disease, by the period between 2008 and 2009, the majority of patients were also treated with intensive regimens (63%), and the majority received TCH. The overwhelming efficacy of anti-HER2 therapies has raised hopes that for patients with lymph node-negative tumors, less chemotherapy may be appropriate if combined with trastuzumab. Recent prospective data evaluating a non-intensive regimen, paclitaxel with trastuzumab, in approximately 400 patients with lymph node-negative, HER2-positive breast cancer demonstrated a 3-year disease-free survival estimate of 98%.16 Given the favorable toxicity profile of the combination of paclitaxel and trastuzumab, these data highlight the large number of women who could be spared potential side effects if the combination of paclitaxel and trastuzumab is adopted as standard of care in the treatment of stage I HER2-positive breast cancer in lieu of ACTH or TCH.
In the current study, we also observed a significant decline in the use of anthracycline-based chemotherapy within NCCN institutions over time. This is consistent with data from Giordano et al, which showed declines in anthracycline use across the United States.5
Not surprisingly, apart from the time trend described, treatment choices were found to be influenced by tumor characteristics (HR, size, grade, and LVI; exploratory analyses also suggested that among patients with HER2-negative/HR-positive tumors, Oncotype Dx RS may impact treatment choice). In addition, we also identified a very strong institutional influence in treatment choice. For example, the institutional use of intensive regimens ranged from 42% to 90% in patients with HER2-positive tumors and from 14% to 50% in patients with HER2-negative tumors and the institutional use of TC ranged from 18% to 79%. The majority of guidelines regarding adjuvant chemotherapy endorse several possible regimens.10,17 The data from the current study suggest that when reasonable treatment uncertainty exists, institutional culture tends to drive patterns of care.
In the United States alone, >100,000 women are diagnosed with stage I breast cancer each year.18 In an era of increasing scrutiny of the cost-effectiveness of therapy and attention to toxicity and quality of life, the current study data point to large gaps of knowledge with which to guide the care of patients with stage I breast cancer. For example, moving from 4 cycles of AC administered every 3 weeks to dose-dense ACT results in a 50-fold increase in costs, primarily due to the need for growth factor support. This trend was observed in a substantial number of patients with HER2-negative breast cancer; however, to the best of our knowledge, the incremental benefit in this patient population, particularly among patients with T1ab tumors, remains poorly described. In the setting of HER2-positive disease, multiple new biologic agents (eg, pertuzumab and trastuzumab emtansine) are being developed. Although undoubtedly there is great potential for these agents to improve cure rates, they will add cost, and the question of cost-effectiveness relative to current regimens will be a key question in the setting of stage I disease, in which the prognosis is already excellent.
We acknowledge several limitations of the current study. It was limited to patients who presented to academic centers, and thus may not capture changes in treatment strategies in the community, in which the majority of cancer care is delivered. Nevertheless, many of the trends observed have similarly been observed in population-based data.5 We classified regimens on an ever/never basis and did not address dose or scheduling. The reasons for treatment selection and changes were not available. The database does not capture detailed toxicity data nor did patients report outcomes. The classification of HER2 and HR status was based on the standards used in each center applicable at the time of diagnosis. In particular, the possibility of some differences in the interpretation of HR status over time exists, particularly in cases with low-level (<10%) expression. Because of a small sample size among patients with HER2-negative/HR-positive disease, we could not examine the independent influence of Oncotype Dx RS on the type of chemotherapy received. Caution should also be used when citing overall patterns because changes in practice occurred during the study period (introduction of Oncotype Dx RS, use of trastuzumab). Finally, the cost calculation was based only on drug costs, not on capturing metrics such as the number of visits or infusion time.
The results of the current study demonstrate an intensification of treatment and an increase in the use of taxane-based regimens, with a concomitant decrease in the use of anthracyclines among patients with stage I breast cancer. These results also demonstrated a high institutional and cost variability. These results highlight the need for dedicated studies among the growing number of patients with small lymph node-negative breast cancer.
Supplementary Material
Acknowledgments
FUNDING SUPPORT
Supported by a grant from the Fundacao Para a Ciencia e Tecnologia (The Foundation for Science and Technology) (HMSP-ICS/0004/201), the National Cancer Institute Specialized Program of Research Excellence in Breast Cancer (grant NIH P50 CA089393), the National Comprehensive Cancer Network, and the Conquer Cancer Foundation.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Dr. Lin received grants from Genentech, GlaxoSmithKline, Novartis, and Array BioPharma for work performed outside of the current study. Dr. Winer received a grant from Genentech for work performed outside of the current study.
REFERENCES
- 1.Chia SK, Speers CH, Bryce CJ, Hayes MM, Olivotto IA. Ten-year outcomes in a population-based cohort of node-negative, lymphatic, and vascular invasion-negative early breast cancers without adjuvant systemic therapies. J Clin Oncol. 2004;22:1630–1637. doi: 10.1200/JCO.2004.09.070. [DOI] [PubMed] [Google Scholar]
- 2.Rosen PP, Groshen S, Kinne DW, Norton L. Factors influencing prognosis in node-negative breast carcinoma: analysis of 767 T1N0M0/T2N0M0 patients with long-term follow-up. J Clin Oncol. 1993;11:2090–2100. doi: 10.1200/JCO.1993.11.11.2090. [DOI] [PubMed] [Google Scholar]
- 3.Hanrahan EO, Gonzalez-Angulo AM, Giordano SH, et al. Overall survival and cause-specific mortality of patients with stage T1a,bN0M0 breast carcinoma. J Clin Oncol. 2007;25:4952–4960. doi: 10.1200/JCO.2006.08.0499. [DOI] [PubMed] [Google Scholar]
- 4.Vaz-Luis I, Ottesen RA, Hughes ME, et al. Outcomes by tumor subtype and treatment pattern in women with small, node-negative breast cancer: a multi-institutional study. J Clin Oncol. 2014;32:2142–2150. doi: 10.1200/JCO.2013.53.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giordano SH, Lin YL, Kuo YF, Hortobagyi GN, Goodwin JS. Decline in the use of anthracyclines for breast cancer. J Clin Oncol. 2012;30:2232–2239. doi: 10.1200/JCO.2011.40.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Peto R, Davies C, Godwin J, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432–444. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones SE, Savin MA, Holmes FA, et al. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol. 2006;24:5381–5387. doi: 10.1200/JCO.2006.06.5391. [DOI] [PubMed] [Google Scholar]
- 8.Slamon D, Eiermann W, Robert N, et al. Breast Cancer International Research Group Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moja L, Tagliabue L, Balduzzi S, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev. 2012;4:CD006243. doi: 10.1002/14651858.CD006243.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network [Accessed February 16, 2015];Breast Cancer version I. 2015 Available at: nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 11.Punglia RS, Hughes ME, Edge SB, et al. Factors associated with guideline-concordant use of radiotherapy after mastectomy in the National Comprehensive Cancer Network. Int J Radiat Oncol Biol Phys. 2008;72:1434–1440. doi: 10.1016/j.ijrobp.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 13.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Medicare & Medicaid Services [Accessed February 16, 2015];2006, 2009, 2014 ASP Drug pricing files. Available at: https://www.cms.gov/Medicare/Medicare-Feefor-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/ [PubMed]
- 15.Hayes DF, Thor AD, Dressler LG, et al. Cancer and Leukemia Group B (CALGB) Investigators HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357:1496–1506. doi: 10.1056/NEJMoa071167. [DOI] [PubMed] [Google Scholar]
- 16.Tolaney SM, Barry WT, Dang CT, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med. 2015;372:134–141. doi: 10.1056/NEJMoa1406281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldhirsch A, Winer EP, Coates AS, et al. Panel members Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Cancer Institute Surveillance, Epidemiology, and End Results Program [Accessed February 16, 2015];SEER Stat Fact Sheets: Breast Cancer. Available at: http://seer.cancer.gov/statfacts/html/breast.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.