Abstract
Distal sensory neuropathies are a hallmark of HIV infections and can result in persistent and disabling pain despite advances in antiretroviral therapies. HIV-sensory neuropathic (HIV-SN) pain may be amenable to cannabinoid treatment, but currently available agonist treatments are limited by untoward side effects and potential for abuse in this patient population. Fatty acid amide hydrolase (FAAH) inhibitors may offer an alternative approach by inhibiting the degradation of endocannabinoids with purportedly fewer untoward CNS side effects. In order to evaluate this potential approach in the management of HIV-SN pain, the recombinant HIV envelope protein gp120 was applied epineurally to the rat sciatic nerve to induce an HIV-SN-like pain syndrome. Two distinct FAAH inhibitory compounds, URB597 and PF-3845 were tested, and contrasted with standard antinociceptive gabapentin or vehicle treatment, for attenuation of tactile allodynia, cold allodynia, and mechanical hyperalgesia. Both FAAH inhibitors markedly reduced cold and tactile allodynia with limited anti-hyperalgesic effects. Peak antinociceptive effects produced by both agents were more modest than gabapentin in reducing tactile allodynia with similar potency ranges. URB597 produced comparable cold anti-allodynic effects to gabapentin, and the effects of both FAAH inhibitors were longer lasting than gabapentin. To assess the contribution of cannabinoid receptors in these antinociceptive effects, CB1 antagonist AM251 or CB2 antagonist SR144528 were tested in conjunction with FAAH inhibitors. Results suggested a contribution of both CB1- and CB2-mediated effects, particularly in reducing tactile allodynia. In summary, these findings support inhibition of endocannabinoid degradation as a promising target for management of disabling persistent HIV-SN pain syndromes.
Keywords: neuropathic pain, HIV neuropathy, allodynia, antinociception, endocannabinoid, gabapentin
Introduction
Sensory neuropathies have emerged as the most common and disabling neurological consequence of human immunodeficiency virus (HIV) infection. Commonly encountered symptoms of HIV sensory neuropathies (HIV-SN) include numbness and sensory loss, but frequently dominated by chronic neuropathic pain which can significantly diminish the quality of life and daily functioning in these patients (Freeman et al., 2014; Phillips et al., 2010; Robinson-Papp et al., 2010; Robertson et al., 2011; Schütz and Robinson-Papp, 2013; Verma et al., 2005). The pain is typically characterized by burning sensations, sharp stabbing, and paresthesias, predominantly affecting the distal innervation of the feet and hands. HIV-distal sensory polyneuropathies are attributable to both the disease itself and to some antiretroviral treatments which can exacerbate neurotoxicity (Ghosh et al., 2011). While combination antiretroviral therapy has markedly improved survival in HIV patients and reduced the incidence of neurological complications, HIV-SN prevalence remains high globally, estimated from 20% to over 50%, with nearly half of those experiencing severe pain (Ellis et al., 2010; Phillips et al., 2010). Symptomatic control of HIV-associated neuropathic pain is difficult to achieve using conventional analgesic therapies and further complicated by concerns with potential substance abuse disorders in this patient population (Phillips et al., 2010; Robinson-Papp et al., 2010). There is an urgent need to better understand the pathogenesis of HIV-SN, identify risk factors, develop effective preventative strategies, and improve symptom control among existing sufferers.
Gp120 is the external envelope protein of HIV which binds to the chemokine receptors CXCR4 and/or CCR5 on neurons. Peripheral application of gp120 produces neurotoxicity and nociceptive behavior in rodents (Herzberg et al., 2001; Keswani et al., 2003; Wallace et al., 2007a,b) suggesting that HIV-1 gp120 interactions with the peripheral nerve may be a causative factor in the generation of peripheral neuropathic pain in humans, and serve as a useful model for HIV-SN in rodents.
Cannabinoid (CB) receptor agonists have been shown to be effective in attenuation of pain-related behaviors in a wide variety of animal models (Hama and Sagen, 2007a; Hohmann, 2005; Pertwee, 2001; Rahn and Hohmann, 2009; Whiteside et al., 2007). The potent mixed CB agonist WIN 55,212-2 can reduce neuropathic pain symptoms in an HIV-SN model (Wallace et al., 2007a,b). In randomized clinical trial studies, efficacy of smoked cannabis in the management of painful HIV-SN has been reported in humans (Abrams et al., 2007; Ellis et al., 2009; Phillips et al., 2010). Alternative administration routes for cannabinoids in treatment HIV-SN pain are also under evaluation. Nevertheless, effective analgesic dosing is frequently associated with significant psychoactive side effects, potentially limiting their usefulness for prolonged pain management therapies. Instead, recent research has focused on targeting the endogenous cannabinoid system and endogenous fatty acid amides for the development of new analgesics (Ahn et al., 2011; Cravatt and Lichtman, 2003; Guindon et al., 2013). The endogenous cannabinoid system consists of endocannabinoid ligands, the enzymes that regulate their biosynthesis and catabolism, and two cannabinoid receptors (CB1 and CB2). The CB1 receptor mainly exists in the central nervous system and mediates potent analgesic as well as most of the adverse effects of cannabinoids (Martin et al., 1993; Ledent et al., 1999; Zimmer et al., 1999). Although the CB2 receptor is found primarily in peripheral immune cells (Galiegue et al., 1995), antinociceptive effects of CB2 agonists have been shown in models of acute, inflammatory and neuropathic pain (Anand et al., 2009; Elmes et al., 2005; Ibrahim et al., 2003; Quartilho et al., 2003; Sagar et al., 2010; Scott et al., 2004), and it has been suggested that CB2 receptors are upregulated in sensory neurons and the CNS following peripheral nerve injury (Beltramo et al., 2006; Hsieh et al., 2011; Wotherspoon et al., 2005).
Endocannabinoids serve as natural ligands for the CB receptors and TRP channels. Anandamide (AEA) and 2-arachidonoylglycerol (2-AG) are two central components of the endocannabinoid signaling networks (Ahn et al., 2008; Lambert and Fowler, 2005). Endocannabinoid levels are tightly controlled by enzymatic biosynthesis and degradation, particularly by fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), the primary catabolic enzymes of AEA and 2-AG, respectively (Blankman et al., 2007; Cravatt et al., 1996, 2001). Although AEA is rapidly hydrolyzed in vivo, pharmacological inhibition of FAAH using selective inhibitors can elevate and prolong AEA. FAAH inhibitors are being actively explored for their antinociceptive activity. The goal of the present study was to determine whether FAAH inhibition may be a promising alternative strategy in the therapeutic management of persistent HIV-SN pain.
1. Methods
1.1.Animals
Male Sprague-Dawley rats (Harlan, IN) weighing 200–250 g at the initiation of the study were housed in a temperature-controlled environment (67–73°C, 30–55% humidity) under a 12 h light/dark cycle; 2 animals per cage. Food and water were available ad libitum. Animals were acclimatized to housing facilities for at least 1 week prior to the start of the study. All animal procedures followed NIH guidelines and were approved by the University of Miami Animal Care and Use Committee.
1.2.Surgeries
Surgery was performed in an isolated facility intended for this purpose. Rats were anesthetized with isoflurane in O2 and the left sciatic nerve was exposed in the popliteal fossa without damaging the perineurium. Oxidized cellulose (Surgicel; Ethicon, Johnson & Johnson, NJ) was used as a carrier matrix to deliver proteins directly to the sciatic nerve. The oxidized cellulose was prepared in strips of about 8 mm long by 4 mm wide, cut obliquely to prevent unraveling. The strips were wrapped loosely around 4 mm of the sciatic nerve 2–3 mm proximal to the trifurcation, using care not to cause any nerve constriction. Strips were saturated with 50 µl of saline containing 300 ng gp120 (HIV-1 MN recombinant Baculovirus, product 1021-2, Immunodiagnostics, Bedford, MA), as described previously in our laboratory using this gp120 pain model (Herzberg and Sagen, 2001). Following nerve surgery, the muscles were sutured and the skin was closed with wound clips. Animals were held in recovery for observation for approximately 24 hours post-surgery and then returned to their home cages and checked daily. No animals died or were excluded from the study.
1.3.Behavioral tests
All the behavioral tests were done by the same person who was trained and unaware of experimental groups. Behavioral testing for antinociceptive drug treatments were initiated beginning 2 weeks post-gp120 administration. For the assessment of tactile and cold allodynia, the rats were placed on a metal mesh covered with a plastic dome (15×20×40 cm) for at least 20 minutes before testing. The thresholds for tactile allodynia were measured with a series of von Frey filaments. The filaments were applied to the plantar skin of the hind paw and bent slightly. Eight specific calibrated von Frey filaments were used via the up-down method to determine the withdrawal threshold (Chaplan et al., 1994). A positive response was defined as withdrawal and holding the paw up/or licking of the paw upon application of the stimulus, which was then followed by application of the next finer von Frey filament, while after a negative response, the next higher von Frey filament was applied. An upper limit of 15 g, which produces force equal to 15 g was selected, and the threshold was recorded as 15 if the strongest hair did not elicit a response. A pattern of six responses was used to calculate the 50% withdrawal threshold (g).
Cold allodynia was measured as the number of foot withdrawal responses after application of an acetone droplet (20 µl via blunted needle tip) to the plantar surface of the paw. Usually normal rats do not respond to acetone application, while neuropathic rats show pain-like responses such as foot shaking, biting, licking or jumping. Observation of at least one of these behaviors, which suggests the involvement of supraspinal processing, was considered a response. The test was repeated five times with an interval of approximately 3–5 min between each test. The response frequency to acetone was expressed as a percent response frequency ([number of paw withdrawals/number of trials] × 100).
Mechanical hyperalgesia was measured using an analgesiometer (Randall-Selitto test, Ugo Basile, Italy). This method allows for the determination of a threshold (in arbitrary units, as specified by the manufacturer) response to mechanical pressure. Rats are wrapped in a towel and an increasing force (48 g/s) is applied to the plantar surface of the hind paw until the rat reacts with vocalization or struggle or flight response. The vocalization threshold was measured 3–4 times in order to obtain two consecutive values that differed by no more than 10%. The apparatus terminated at 1000 g (25 in scale units) in the absence of a response.
On all testing days measurement of tactile and cold allodynia was followed by assessment of mechanical hypersensitivity.
1.4.Drug testing
FAAH inhibitor URB597 was purchased from Sigma Corporation (Sigma, St. Louis, USA). FAAH inhibitor PF-3845 was a kind gift from Professor Benjamin Cravatt (The Skaggs Institute for Chemical Biology Department of Chemical Physiology, Scripps Research Institute, La Jolla, CA) or purchased from Tocris (Cookson Inc, Bristol, UK). Gabapentin and CB1 antagonist AM251 were purchased from Tocris. CB2 antagonist SR144528 was a kind gift from Ironwood Pharmaceuticals, Inc.. Gabapentin (1.0–30.0 mg/kg) was dissolved in saline for i.p. injections. This dose range was selected based on previous findings in our laboratory showing antinociceptive effects in the absence of apparent untoward side effects in a neuropathic pain model (Hama and Sagen, 2007b). FAAH inhibitors URB597 and PF-3845 were dissolved in a vehicle consisting of Dulbecco’s Modified Eagle Medium (DMEM): Cremofor and saline in ratio of 1:1:8. URB597 was administered i.p. while PF-3845, which has shown oral bioavailability more amenable for clinical usage (Ahn et al., 2009), was administered p.o. Vehicle controls were administered by comparable routes for each drug (i.p. for URB597 control and p.o. for PF-3845 control). All solutions were warmed to room temperature prior to injection and prepared immediately before administration. Several doses of gabapentin (1, 3, 10 and 30 mg/kg, i.p.) were used for comparison and approximation of anti-nociceptive potency of the FAAH inhibitors. Since the goal of this study was to evaluate the maximum potential benefit of the FAAH inhibitors, and these agents reach a plateau in their ability to increase CNS levels of endocannabinoids, the dose producing maximum AEA elevations in previous studies in our lab and others was tested (10 mg/kg of PF-3845 and 3 mg/kg of URB597; Ahn et al., 2009; Bradshaw et al., 2009; Hama et al., 2014). In addition, both a higher and lower dose of both of these FAAH inhibitors (1 and 10 mg/kg URB597; 3 and 20 mg PF-3845) were included to determine for dose-ranging. All drugs were administered in a volume of 1 ml/kg 15 min prior to initiation of behavioral tests. The effect of different doses of gabapentin or saline vehicle was tested at 30, 60, 90 and 120 minutes after injection and the effects of URB597 and PF-3845 and their vehicles were evaluated hourly for 4 hours starting 15 minutes after injection, in order to cover the reported times for peak elevation of FAAs and antinociceptive effects. URB597 has been shown to produce a slow and reliable accumulation of AEA in the nervous system with a maximal effect at 2 hours post-injection (Fegley et al., 2005), while PF-3845 produces a more prolonged brain elevation of AEA, reaching maximal levels by approximately 3–4 hours (Ahn et al., 2009). In order to assess the contribution of CB receptors to antinociceptive effects of FAAH inhibitors, the CB1 receptor antagonist, AM251 (1 mg/kg, i.p.) or the CB2 receptor antagonist, SR144528 (1 mg/kg, i.p.), or 1:1:8 DMEM:cremofor:saline vehicle were injected immediately after injection of FAAH inhibitors to block onset of antinociceptive activity. A higher dose of each of the antagonists (3 mg/kg, i.p.) was also used in some animals in order to determine whether lack of antagonism in some cases might be due to insufficient antagonist dose. Since the antagonists are also inverse agonists, the effects of these administered alone were also tested.
Drug testing was done at 10–20 days following gp120 surgery when neuropathic pain symptoms are maximum and stable in this model. A within-subjects design was used to reduce the total number of animals needed for these experiments. For each study, treatments were counterbalanced across test days. In order to avoid carry over effects, drug (or vehicle) washout time between treatments was at least 3 days. In order to minimize potential bias, the experimenter was blinded to drug treatment.
Statistical analysis
Data are expressed as mean ± S.E.M (n = 8–12 per groups). Analysis of nociceptive data following treatments was performed using repeated measure two-way ANOVA. Bonferroni post-tests for multiple comparisons were carried out when p<0.05. Antagonist reversal data were analyzed using one-way ANOVA.
For dose-response comparisons, the drug treatment effects on were converted to a percent maximum possible effect (% MPE) for each drug as follows:
%MPE = [(Post-drug threshold – Pre-drug post-injury baseline) / (Cut-off threshold – Pre-drug post-injury baseline)] × 100
Cut-off threshold values were determined for each test as values representing no pain-related behavior; 15g for tactile allodynia test, 0% for cold allodynia responses and 36g for mechanical hyperalgesia responses. Mean MPE values were used to calculate 50% antinociceptive dose (A50) and 95% confidence intervals from the linear portion of the dose-response curves using a web-based program (Murray et al. 1981). The program can be found on the Web at: http://www.u.arizona.edu/~michaelo/. (Hama and Sagen 2011). Comparisons were made using one-way ANOVA.
2. Results
2.1.Antinociceptive effects of of gabapentin on gp120-induced neuropathic pain-related behavior
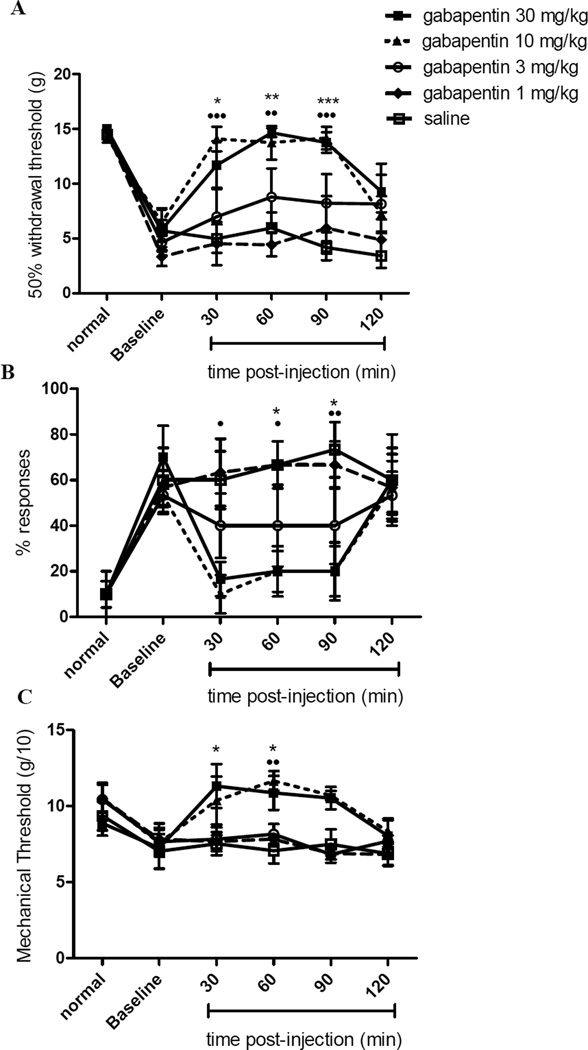
The effect of gabapentin on tactile (A) and cold (B) allodynia and mechanical hyperalgesia (C) were tested using von Frey, acetone, and Randall-Selitto test, respectively. Figure 1 shows the nociceptive responses of animals injected with gabapentin (1–30 mg/kg i.p.) or saline at various time intervals. Mean response thresholds in all tests before the gp120 surgery were similar in all groups (p>0.05). Gp120 administration around the sciatic nerve produced pain-related behavior in all three tests. Gabapentin treatment decreased the response thresholds to innocuous tactile and cold stimuli and noxious mechanical pressure (overall F (df 4, 5) = 19.85, 11.26, and 6.21, p<0.001 for von Frey, acetone, and Randall-Selitto tests, respectively). The lowest dose of gabapentin used (1 mg/kg) produced no apparent antinociceptive effects on any of the outcome measures compared to saline (p>0.05). The maximum antinociceptive effects were observed following administration of 10 or 30 mg/kg gabapentin (p<0.001 – 0.05 depending on dose, test, and time, compared with saline; see Fig. 1). The antinociceptive effects of 10 and 30 mg/kg of gabapentin were not significantly different from each other in any of the behavioral tests (p>0.05). Both of these doses nearly completely reversed allodynia and hyperalgesia to intact pre-gp120 response levels. The intermediate dose (3 mg/kg) appeared to produce a slight antinociceptive effect on tactile and cold allodynia, but this did not reach statistical significance. Maximum antinociceptive effects of gabapentin were observed at approximately one hour after administration, ranging from 30–90 min post-injection with the peak values between 30 and 90 minutes after injection, and declining towards pre-injection baselines by 120 min on all three tests.
Figure 1.
Effect of gabapentin and saline on tactile (A) and cold (B) allodynia and mechanical hyperalgesia (C) in the gp120 HIV-model of rat neuropathic pain. Pre-surgery responses (normal) and pre-drug baselines are indicated. Data are presented as mean ± SEM (n= 8 animals per treatment group). Asterisks and solid circles are differences between gabapentin 30 mg/kg and gabapentin 10 mg/kg respectively compared with saline treated rats. * and ●: p<0.05, ** and ●●: p<0.01, ***: p<0.001.
2.2.Effect of URB597 administration on gp120 induced neuropathic pain-related behavior
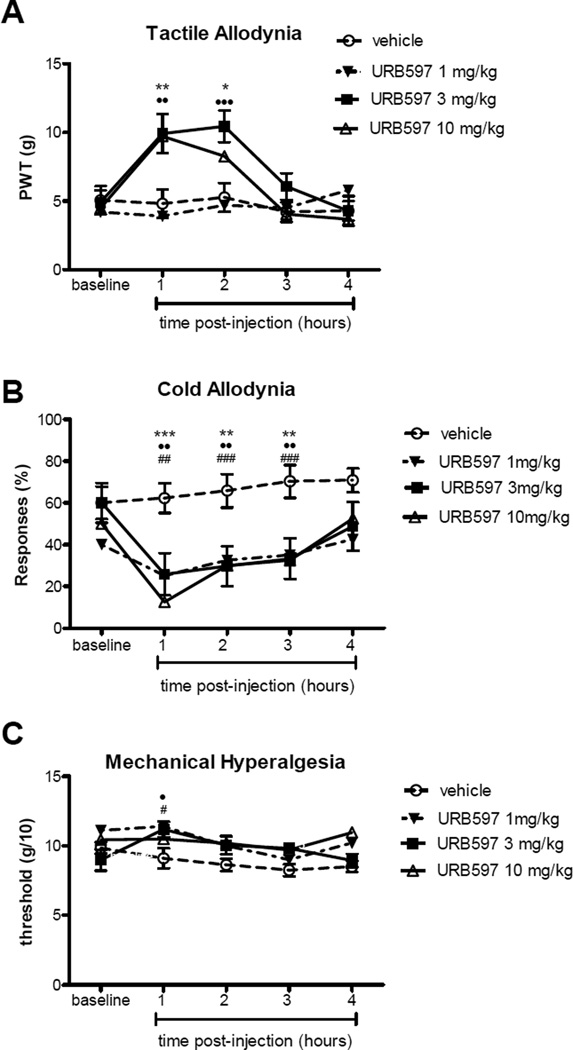
Figure 2 shows the effects of i.p injection of URB597 or its vehicle on tactile (A) and cold (B) allodynia and mechanical hyperalgesia (C). Pre-surgical responses are not repeated as the same animals were used as above. Pre-injection responses were obtained just prior to FAAH inhibitor administration and shown as the baseline values. I.p. injection of URB597 produced observable antinociceptive responses within first hour after injection. Significant differences were observed in rats treated with URB597 compared with rats treated with i.p. vehicle (overall F(df 3,4) = 11.273 (p<0.001), 9.23 (p<0.001) and 5.56 (p<0.001) for tactile allodynia, cold allodynia and mechanical hyperalgesia respectively. Anti-allodynic effects were sustained for 2 hours post-injection of URB597 at both 3 mg/kg and 10 mg/kg using the von Frey tactile allodynia assessment (Fig. 2A; p<0.05 and p<0.001, respectively) compared with vehicle treatment). Both the 3 mg/kg and 10 mg/kg doses of URB597 produced comparable tactile anti-allodynic effects (p>0.05 between the 2 treatment doses), and these were significantly greater than the low (1 mg/kg) dose of URB597 (p<0.05 ). Robust cold anti-allodynic effects were observed following treatment with all three doses of URB597 (Fig. 2B; p<0.01 compared with vehicle treatment). These antinociceptive effects were sustained for at least 3 hours at all three concentrations of URB597 using the acetone cold allodynia test. No statistically significant differences were found between any of the URB597 treatment doses using this test (p>0.05). The antinociceptive effects of URB597 on mechanical hyperalgesia were modest and shorter acting compared with the anti-allodynic effects, attaining statistical significance only during the first hour post-injection (Fig. 2C; p<0.05). By the second hour after injection of URB597, the response of the animals to the increased mechanical pressure in the Randall-Selitto test returned to its baseline level before injection (p>0.05).
Figure 2.
Effect of URB597 or vehicle on tactile (A) and cold (B) allodynia and mechanical hyperalgesia (C) in the gp120 HIV-model of rat neuropathic pain. The graphs show the baseline (before injection) values and up to 4 hours values following injection. Data are presented as mean±SEM (n= 8–12 animals per treatment group). Asterisks, solid circles, and hash marks are differences between URB597 10, 3, and 1 mg/kg, i.p. respectively compared with saline treated rats. * and ● and #: p<0.05, ** and ●● and ##: p<0.01, ***, ●●●, and ###: p<0.001.
2.3.Effect of PF-3845 administration on gp120 induced neuropathic pain-related behavior
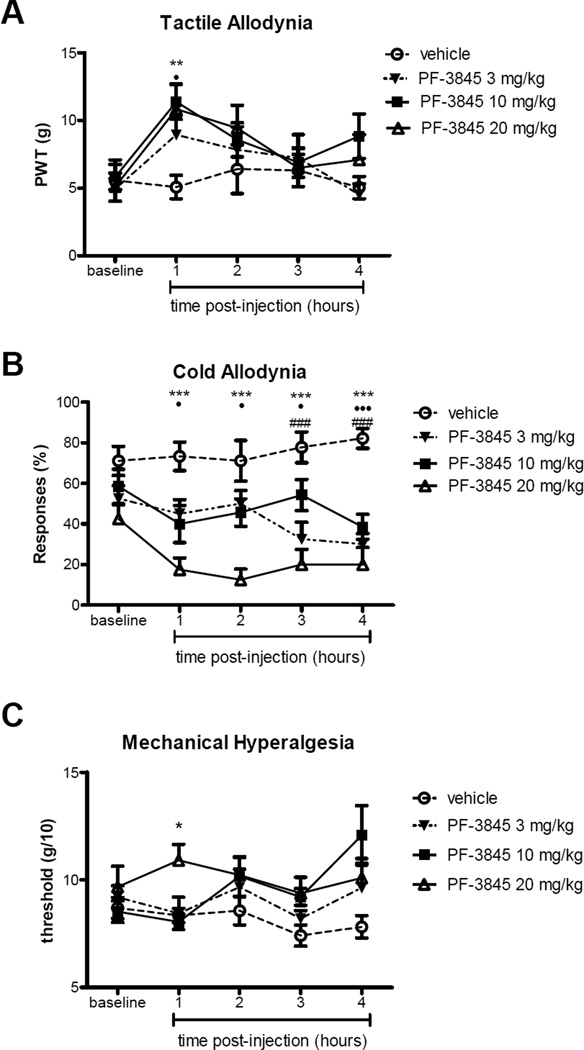
To examine the effect of an orally administered FAAH inhibitor with reported antinociceptive potency, PF-3845 treatment was assessed. The pre-injection baseline values in all groups for the three tests were similar to previous gabapentin and URB597 test groups, and no differences were observed in baseline values between the 2 groups prior to p.o. PF-3845 or vehicle treatment (Fig. 3; p>0.05). Antinociceptive effects of PF-3845 were observed for all 3 tests (overall F(df 3,4)=6.24 (p<0.001), 3.39 (p<0.05) and 2.98 (p<0.05) respectively). The time course of peak antinociceptive effects varied depending on outcome measures. Significant effects on tactile allodynia were observed at one hour after injection of PF-3845 (Fig. 3A; p<0.01 and p<0.05 for 20 mg/kg and 10 mg/kg PF-3845, respectively, compared with oral vehicle), but appeared to wane at later time points. However, effects on cold allodynia were observed at one hour post-PF-3845, and were sustained and robust at least through 4 hours following PF-3845 administration (Fig. 3B; p< 0.001 for all 3 doses of PF-3845 compared with vehicle at 4 hrs post-injection). In addition, the highest dose of PF-3845, 20 mg/kg, produced significantly greater anti-allodynic effects on cold responses than the lower doses (p<0.05 compared with 10 mg/kg PF-3845). Effects on noxious pressure threshold were marginal, only reaching statistical significance at 1 hr for the highest dose p<0.05.
Figure 3.
Time course of the effect of a single administration of PF-3845 or vehicle on tactile (A) and cold (B) allodynia and mechanical hyperalgesia (C) in the gp120 model of HIV neuropathic pain. Data are presented as mean ± SEM (n= 8–12 animals per treatment group). Asterisks, solid circles, and hash marks are differences between PF-3845 20, 10, and 3 mg/kg p.o., respectively compared with saline treated rats. * and ● and #: p<0.05, ** and ●● and ##: p<0.01, ***, ●●●, and ###: p<0.001.
2.4.Dose-response comparisons of gabapentin and FAAH inhibitors on gp120 induced neuropathic pain-related behavior
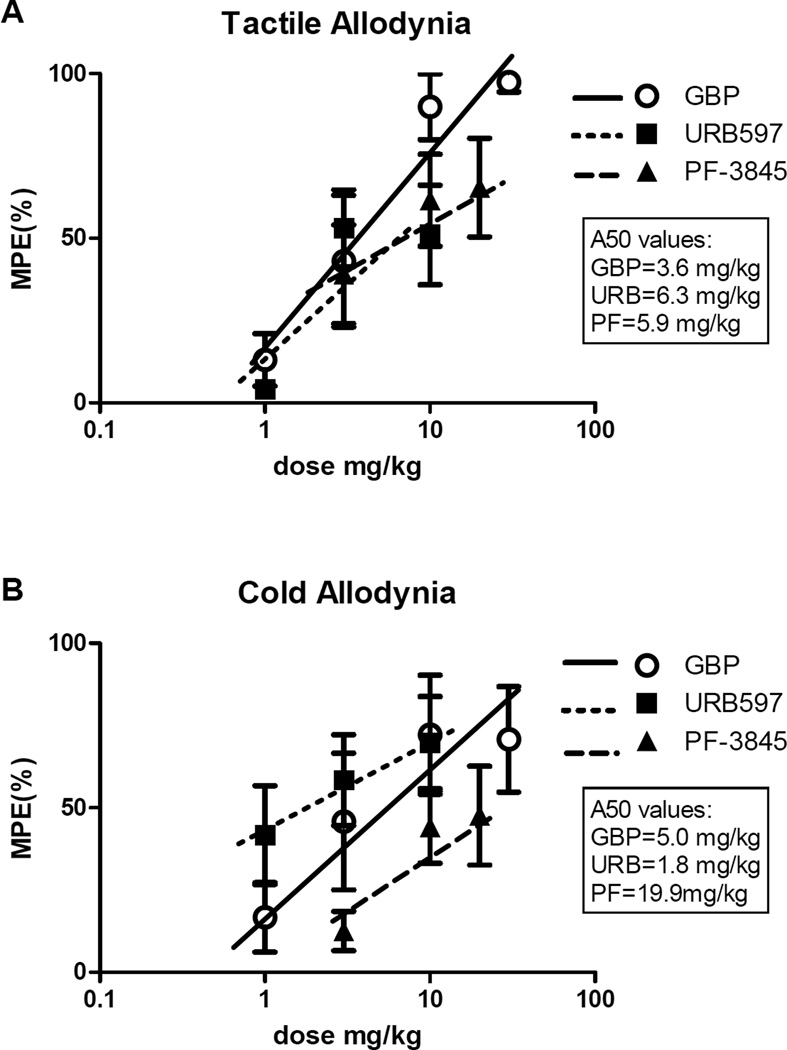
For assessing relative antinociceptive potencies of the FAAH inhibitors compared with more standard gabapentin treatment, dose-response curves were constructed at the 60 min post-injection time points. Fig. 4 shows the MPEs and A50s for the drug treatment groups as assessed for tactile (Fig. 4A) and cold (Fig. 4B) allodynia (effects on mechanical hyperalgesia are not shown due to marginal effects on this outcome measure. The MPE for gabapentin suggested higher achieveable potency of this agent in reducing tactile allodynia compared with the FAAH inhbitors, although the dose-response differences in antinociceptive effects of URB597 or PF-3845 compared with gabapentin did not reach statistical significance (p>0.05). Similarly, MPEs for cold allodynia were comparable for URB597 and gabapentin (p>0.05 between all 3 drug treatment groups). Calculated A50 values for gabapentin treatment were 3.6 mg/kg (C.L. 1.4–9.6), 6.3 mg/kg (C.L. 2.6–10.6.) for URB597, and 5.9 mg/kg (C.L. 0.4–8.0) for PF-3845 for effects on tactile allodynia. For effects on cold allodynia, the calculated A50s were 5.0 mg/kg (C.L. 2.0–6.7), 1.8 mg/kg (C.L. 0.8–6.9), and 19.9 mg/kg (C.L. 18.5–21.4) for gabapentin, URB597, and PF-3845, respectively. Values for mechanical allodynia were not calculated as there was less than 50% effect of any of the tested drugs using this test.
Figure 4.
Comparison of the maximal possible effects of gabapentin, URB597 and PF-3845 at 60 minutes post administration in the gp120 model for tactile allodynia (A) and cold allodynia (B (n=8 rats). The 50% antinociceptive dose (A50) is indicated for each drug in plot inset.
2.5 Effect of CB1 and CB2 receptor antagonist on antinociceptive effects of FAAH inhibitors
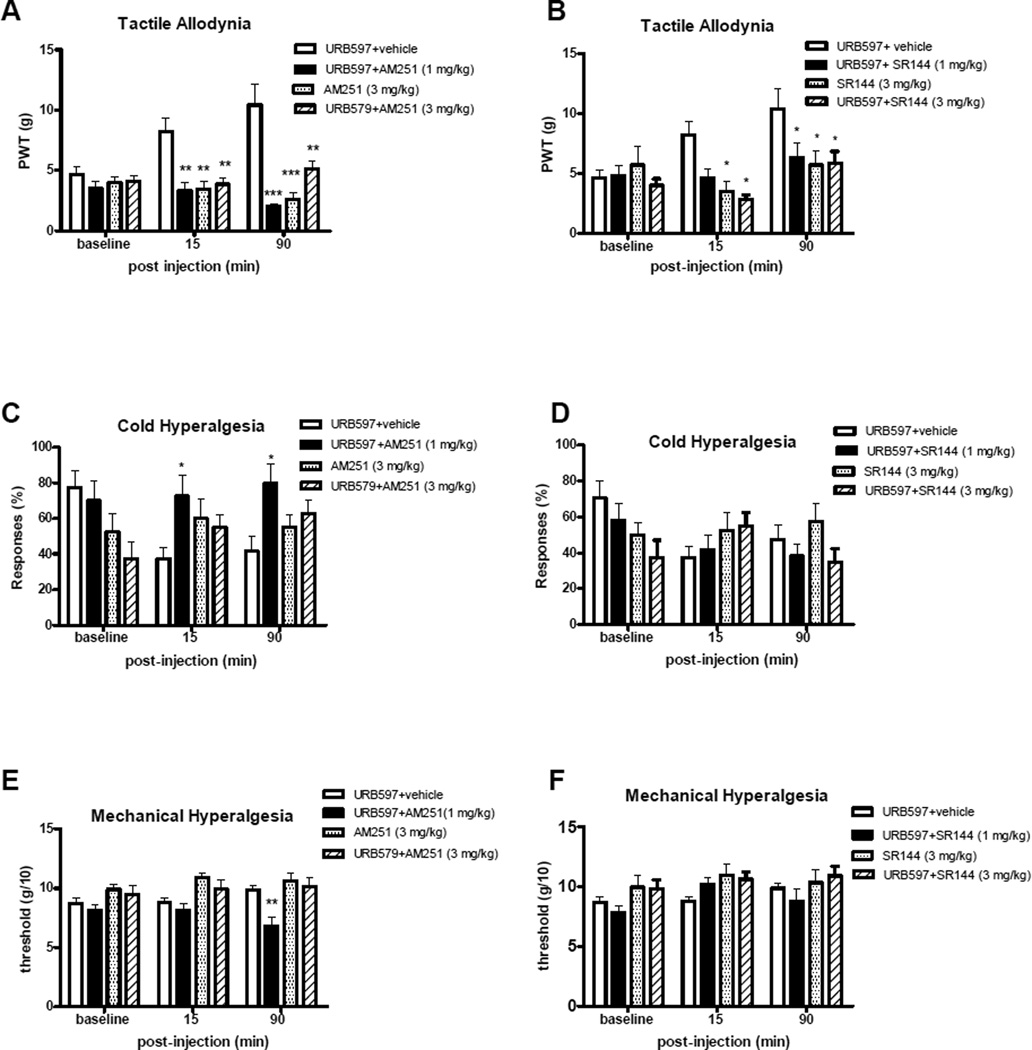
To explore which subtype(s) of cannabinoid receptor may mediate the antiallodynic and antihyperalgesic effects of URB597, CB1 antagonist AM251 or CB2 antagonist SR144528 was injected concomitantly with the URB597. As shown in Fig. 5A, the tactile anti-allodynic effect of URB597 was completely blocked by the CB1 antagonist AM251 at 15 – 90 min post-injection (Fig. 4A; p<0.01 and 0.001 compared with vehicle-treated animals receiving URB597, as assessed by von Frey responses). Both the 1 mg/kg and 3 mg/kg doses of the AM251 produced reduced the anti-allodynic effects of URB597 with co-administration. The AM251 alone (3 mg/kg) did not produce alterations in tactile responses, either increasing or decreasing tactile allodynia. Similarly, cold anti-allodynic effects of URB597 were reversed by AM251 treatment (1 mg/kg; Fig. 5C; p<0.05 compared with vehicle at 15–90 min post-injection). The higher dose of AM251 (3 mg/kg) was not as robust in reversing cold anti-allodynic effects of URB597 as the 1 mg/kg dose, only showing a non-significant trend toward partial reversal (p>0.05). Paradoxically, this higher dose of the CB1 antagonist alone (without URB597) appeared to produce some increased cold allodynia, although this was not statistically significant. Mechanical anti-hyperalgesia was also reversed by 1 mg/kg AM251, but this was only apparent by 90 min post-injection (Fig. 5E; p<0.01 compared with vehicle); no effects of AM251 (3 mg/kg) either alone or in combination with URB597 on mechanical hyperalgesia were observed. The CB2 antagonist SR144528 also attenuated the URB597 effects on tactile allodynia at both doses of the antagonist (Fig. 5B, p<0.05 compared with vehicle). SR144528 alone did not attenuate tactile allodynia. In contrast to effects on tactile allodynia, SR144528 treatment did not produce any significant effects on cold allodynia or mechanical hypearalges (Fig. 5D,F, p>0.05 compared with vehicle).
Figure 5.
Effect of CB1 and CB2 receptor antagonists on the antinociceptive effect of i.p. URB597. Sensory behaviors (tactile allodynia: A and B; cold allodynia: C and D; mechanical hyperalgesia: E and F were evaluated 15 and 90 min after co-adminstration of URB597 and CB1 antagonist AM251 (A,C,and E) or CB2 antagonist SR144528 (B,D, and F), or vehicle. Higher doses of CB1 and CB2 antagonists were assessed by themselves and in combination with URB597. Data are expressed as mean ± SEM (n = 8–11 animals per treatment group). Asterisks show the differences compared with vehicle injected group. *: p<0.05, **: p<0.01, ***: p<0.001.
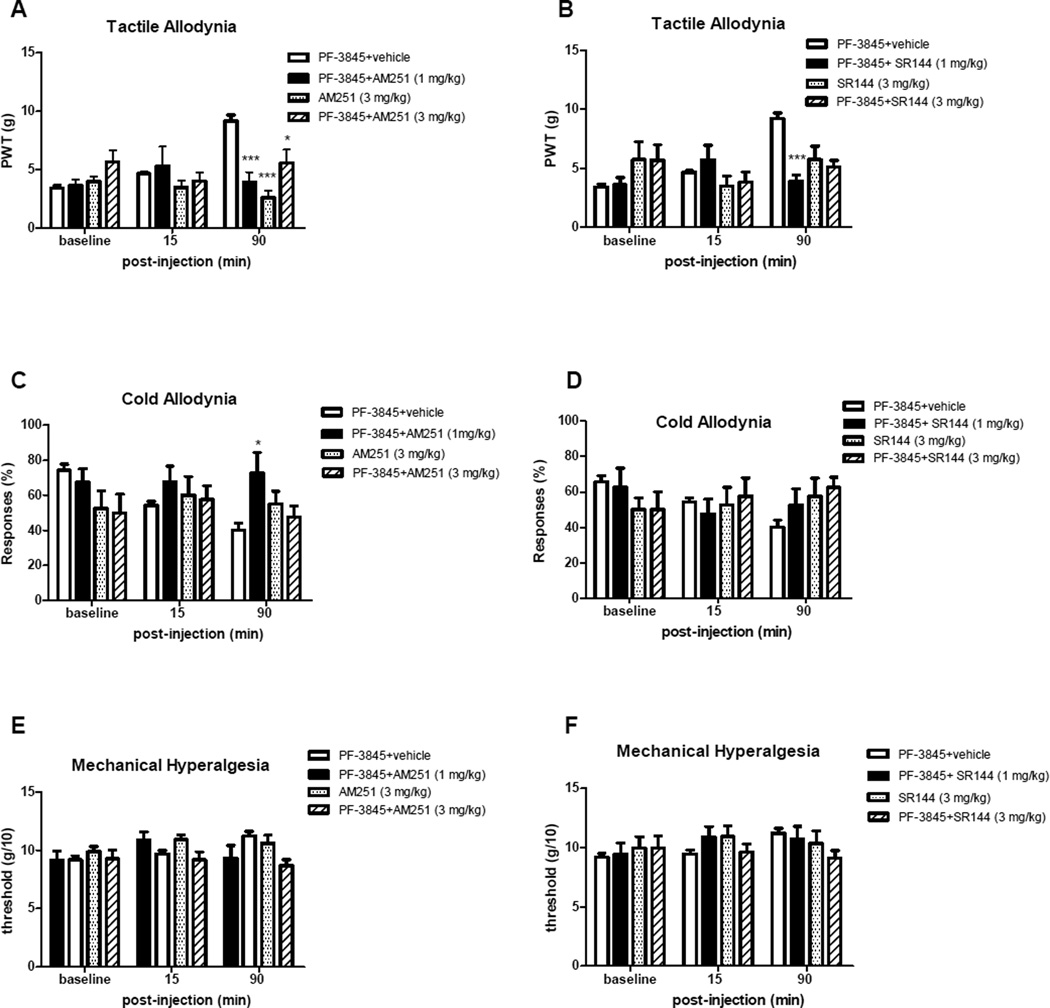
The effects of AM251 and SR144528 on the pain behavior reducing effects of PF-3845 are shown on Figure 5. Injection of either AM251 or SR144528 (1 mg/kg) significantly blocked the antiallodynic effect of PF3854 on responses to von Frey filament as observed at 90 min after injection (Figs. 6A and 6B; p<0.001 compared with vehicle). Neither antagonist produced significant antinociceptive effects in the absence of FAAH inhibitors. Treatment with CB1 antagonist AM251 (1 mg/kg; Fig. 6C), but not CB2 antagonist SR144528 (Fig. 6D), attenuated the antiallodynic effects of PF-3845 on cold responses (p<0.05 for PF-3845 + AM251 compared with vehicle at 90 min). No other significant effects on cold allodynia or mechanical hyperalgesia were observed in this group for CB antagonist alone or in combination with PF3845 were observed (Figs. 6C–F).
Figure 6.
Effect of CB1 and CB2 receptor antagonists on the antinociceptive effect of p.o. PF-3845. Sensory behaviors (tactile allodynia: A and B; cold allodynia: C and D; mechanical hyperalgesia: E and F were evaluated 15 and 90 min after co-administration of PF-3845 and CB1 antagonist AM251 (A,C, and E) or CB2 antagonist SR144528 (B,D, and F), or vehicle. Higher doses of CB1 and CB2 antagonists were also used by themselves and in combination with PF3845. Data are expressed as mean ± SEM (n = 8 animals per treatment group). Asterisks show the differences compared with vehicle injected group. *: p<0.05; **: p<0.01, ***: p<0.001.
3. Discussion
The management of persistent neuropathic pain associated with HIV continues to be a major therapeutic challenge motivating the search for improved treatment options. This study has characterized the antinociceptive effects of the systemic administration of two different selective FAAH inhibitors, URB597 and PF-3845, in an experimental model of HIV neuropathic pain. The overall results indicated that the pain relieving effects of FAAH inhibitors URB597 and PF-3845 are comparable to standard antinociceptive gabapentin treatment in the rat gp120 model, albeit with slightly longer duration. In addition, findings from this study suggest a role for both CB1 and CB2 receptor activation in reducing HIV-SN pain-related behavior.
Gabapentin was selected as a positive control as it is currently among the top prescribed medications for treating clinical neuropathic pain of various etiologies, and also was shown to effectively in reduce gp120-induced mechanical hypersensitivity (Wallace et al., 2007a,b). Gabapentin has been reported to significantly reduce pain in patients with HIV-SN in a placebo-controlled study (Hahn et al., 2004). However, except for slight improvement in hyperalgesia in some patients, placebo-controlled trials with pregabalin in these patients did not show significant pain improvement (Simpson et al., 2010, 2014). This failure was attributed in part to the complexity and variability of HIV-SN and the high placebo effects in the patients, but also reveals some limitations in translating robust preclinical findings to successful clinical outcomes.
Although HIV neuropathic pain has been a difficult clinical challenge, refractory to most currently available pharmacologic options, anecdotal reports and promising randomized clinical trials using smoked cannabis (Abrams et al., 2007; Ellis et al., 2009; Phillips et al., 2010) provide the underlying impetus for the current study. The potent mixed cannabinoid agonist WIN 55,212-2 can attenuate mechanical hypersensitivity in the gp120 model (Wallace et al., 2007a,b). Cannabinoids have long been known to exhibit antinociceptive activity in animal models of pain through both spinal and supraspinal mechanisms (Martin et al., 1993; Martin et al., 1999; Pertwee, 2001). There is considerable evidence in animal models supporting the effectiveness of cannabinoids in alleviating the experimental persistent pain induced of various etiologies, including inflammation, peripheral nerve injury and disease, and spinal cord injury (Hama and Sagen, 2007a, 2011; Herzberg et al., 1997; Li et al., 1999; Martin et al., 1999; Wallace et al., 2007). Promising preclinical and clinical studies suggest that activation of CB receptors may be particularly effective in alleviating HIV-SN pain. However, the long-term clinical utility of CB1 agonists for persistent pain may be limited by untoward CNS side effects. The use of FAAH inhibitors is a novel means of pharmacologically increasing endocannabinoid levels, while possibly avoiding the undesirable side effects produced by exogenous cannabinoids. FAAH controls the degradation of endogenous AEA as well as several other FAAs, including palmitoyl ethanolamide (PEA) and oleoyl ethanolamide (OEA). FAAH inhibitors have been reported to reduce pain behaviors in several animal models in the absence of cannabinoid-like untoward side effects (Ahn et al., 2009, 2011; Chang et al., 2006; Guindon et al., 2013; Jayamanne et al., 2006; Jhaveri et al., 2006; Kinsey et al., 2009; Russo et al., 2007). Although CNS levels of FAAs were not assayed in the current study, recent findings in our laboratory showed consistent elevations of FAAs, including AEA, in both brain and spinal cord at the doses and time courses of URB597 and PF-3485 in the current study (Hama et al., 2014).
URB597 is selective covalent inhibitor of FAAH that elevates AEA and other FAAs in brain and spinal cord after systemic administration in rodents and primates (Ahn et al., 2009; Hama et al., 2014; Justinova et al., 2008; Kinsey et al., 2009; Russo et al., 2007). It has antihyperalgesic effects in various rodent pain models (Ahn et al., 2008; Guindon et al., 2013; Jahaveri et al., 2006; Jayamanne et al., 2006; Kinsey et al., 2009; Naidu et al., 2010; Russo et al., 2007). The antinociceptive effects of URB597 in mouse and rat inflammatory pain models have been consistent, although effects on neuropathic pain appear more complex. While systemic administration of URB597 can effectively reduce neuropathic pain symptoms in mouse peripheral nerve injury models, it was reportedly ineffective in reducing tactile allodynia following sciatic nerve ligation in rats (Jayamanne et al., 2006). Recent findings in our lab also indicated that systemic administration of URB597 is ineffective in reducing neuropathic pain behaviors in a rat spinal cord injury model despite elevated AEA levels in brain and spinal cord tissue (Hama et al., 2014). In contrast, both URB597 and a brain-impermeant FAAH inhibitor (URB937) have been recently reported to reverse neuropathic pain symptoms in a rat chemotherapy-induced neuropathy model (Guindon et al., 2013). In the current study, gp120 sciatic nerve exposure produces neuropathic pain-like symptoms, but is also thought to have an inflammatory component, as is elevates peripheral nerve and spinal cord inflammatory mediators (Herzberg and Sagen, 2001). Thus, anti-allodynic effects of URB597 in this and other models may be mediated in part via reducing the inflammatory component of the pain processes.
PF-3845 is a selective covalent inhibitor of FAAH which carbamylates the active serine site of FAAH. Improved properties of PF-3845 include its oral bioavailability and extended longevity of action (Ahn et al., 2009; Booker et al., 2012). While both URB597 and PF-3845 increase brain anandamide levels, the duration of brain FAA elevation is considerably longer following PF-3845 treatment (Ahn et al., 2009). Findings in the current study are also suggestive of prolonged antinociceptive effects of PF-3845, particularly in reducing cold allodynia, although animals were not evaluated beyond the 4 hours after drug administration.
Results of this study suggest that FAAH inhibitors can produce comparable anti-allodynic effects to gabapentin in the gp120 HIV neuropathic pain model, as indicated by dose-response comparisons and A50 ranges. In particular, URB597 was equally or more effective as gabapentin in reducing cold allodynia in this model. The anti-allodynic effects of URB597 appeared to reach their maximum potential in the 3 mg/kg dose range, with no further improvement at higher doses, possibly due to a ceiling effect on endogenous FAA levels if FAAH is maximally inhibited. In contrast, PF-3485 appeared less effective than URB597 in reducing cold allodynia, and its effectiveness was further increased with higher doses up to 20 mg/kg. The relatively lower potency of PF-3485 may be due to lower bioavailability via the oral dosing route, although previous findings in our group has demonstrated that this dose and route results in high levels of FAAs in brain and spinal cord (Hama et al., 2014). Both URB597 and PF-3845 produced more moderate maximal effects on tactile allodynia than gabapentin, but with similar potencies. Gabapentin was also more effective than the FAAH inhibitors in reducing mechanical hyperalgesia in the gp120 model, although none of the agents tested were robust in this behavioral measure. Of note, the FAAH inhibitors produced prolonged antinociception (e.g. 3–4 hours for cold allodynia) in comparison with gabapentin, which reversed to pre-injection baselines by 2 hours following administration.
There are numerous studies in rat models of peripheral neuropathic pain that demonstrate significant suppression of thermal and mechanical hypersensitivity with non-selective CB receptor agonists, which is attenuated with selective CB1 receptor antagonists (Bridges et al., 2001; Fox et al., 2001; Herzberg et al., 1997; Ulugol et al., 2004). Activation of the CB2 receptor has also been suggested as a potential therapeutic target, and CB2-selective agonists display antinociceptive activity in rodent models of persistent inflammatory and neuropathic pain (Anand et al., 2009; Whiteside et al., 2007). CB2 receptors are thought to be primarily peripherally localized, but can be upregulated in the spinal cord following peripheral nerve injuries (Anand et al., 2009; Beltramo et al., 2006). A role for both CB1 and CB2 receptors in mediating antinociceptive effects of FAAH inhibitors is suggested by the blockade of anti-allodynic effects in CB1 (−/−) or CB2 (−/−) mice (Kinsey et al., 2009, 2010). To investigate the mechanism of action of the FAAH inhibitors used in the present study, the effects of selective CB1 or CB2 antagonists were assessed. Findings supported a prominent role for CB1 receptors in mediating the antinociceptive effects of both FAAH inhibitors on both tactile and cold allodynia induced by gp120. Higher doses of CB1 antagonist AM251 did not further reverse the anti-allodynic effects of either URB597 or PF-3845. In addition, CB2 receptors appeared to play a role in some of the anti-allodynic effects of both URB597 and PF-3845, since effects on tactile allodynia were nearly equally blocked by either CB1 antagonist AM251 or CB2 antagonist SR144528. In contrast, attenuation of cold allodynia by FAAH inhibitors in this model was attenuated by the CB1, but not the CB2 antagonist, even when higher doses of CB2 antagonist SR144 was evaluated in some animals. These findings suggest that the two cannabinoid receptors play differential roles in mediating the antinociceptive actions of FAAH blockade in the gp120 HIV pain model. Interestingly, the higher dose of AM251 showed a tendency (albeit non-significant) to reduce cold allodynia on its own in some cases. This may be indicative of the emergence of off-target or mixed agonist-antagonist effects of this agent at higher doses.
Thus, it is likely that systemically administered FAAH inhibitors can block symptoms of HIV-SN pain in this gp120 model via both CB1 and CB2 receptor activation. Since a peripherally restricted FAAH inhibitor has been shown to attenuate inflammatory and neuropathic pain behavior, a role for peripheral endocannabinoids in pain modulation has also been suggested (Clapper et al., 2010; Guindon et al., 2013). Although URB597 most likely acts at CNS sites to reduce nociception in the current study, where CB1 receptors predominate, the contribution of peripheral targets cannot be excluded since it was systemically administered. Thus FAAH inhibitors in the current study may reduce gp120 allodynia via central and/or peripheral CB receptors. FAAs such as AEA also activate the TRPV1 receptor, albeit with lower affinity than cannabinoid receptors, but TRPV1 receptors do not appear to play a predominant role in the antiallodynic effects of PF-3845 (Booker et al, 2012). PEA as well as novel endogenous N-acyl amides can activate the TRPV1 receptor (Borelli et al., 2014; Raboune et al., 2014). In this experiment we did not test the role of TRPV1 receptors so we cannot exclude the role of these receptors in gp120 HIV pain model. In addition, potential non-CB mediated roles of PEA and OEA could contribute to the antinociceptive effects observed.
It has been suggested that pharmacotherapies targeting the endocannabinoid catabolic enzymes are less likely to produce tolerance than direct acting CB1 receptor agonists (Falenski et al., 2010). This is another potential advantage of FAAH inhibitors in the treatment of persistent pain. Nevertheless, this is somewhat controversial, with some reports showing that normal CB1 receptor function is maintained without CB1 agonist cross-tolerance following repeated treatment with FAAH inhibitors (Schlosburg et al., 2010, 2014) and others showing reduced effectiveness of FAAH inhibitors on inflammatory pain behaviors following repeated administration (Okine et al., 2012). This may be dose or model dependent, and would be interesting to explore for HIV-SN pain in future studies.
Cannabinergic agents may offer promise in clinical pain management both on their own and as adjuncts to conventional therapeutic agents. Inhibitors of endocannabinoid-degrading enzymes such FAAH may function to selectively enhance CB-mediated neurotransmission only in nervous system, where endocannabinoids are synthesized and released on demand, thereby preventing the induction of side effects associated with more global activation (Cravatt and Lichtman, 2003). Thus FAAH inhibitors may be good candidates for alleviation of pain in conditions which are resistant to prolonged treatment with conventional analgesics and in patient populations where the emetic effects of opioids are poorly tolerated such as AIDs patients. The current results support this approach for the treatment of persistent HIV-SN pain and suggest that inhibition of endocannabinoid degradation is a promising target for management of this disabling pain syndrome.
Highlights.
Fatty acid amide hydrolase inhibitors reduced nociception in rat HIV neuropathy model
Two distinct FAAH inhibitors attenuated cold and tactile allodynia
Antinociceptive potency of FAAH inhibitors were comparable to gabapentin, but more prolonged
Anti-allodynic effects were partially mediated by both CB1 and CB2 receptors
Acknowledgments
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [NS51667].We thank Ironwood Pharmaceuticals for providing the SR144528. We thank Dr. Benjamin Cravatt (The Skaggs Institute for Chemical Biology Department of Chemical Physiology, Scripps Research Institute) for the kind gift of FAAH inhibitor PF-3845 and Drs. Kay Ahn and Doug Johnson (Pfizer, Inc.) for the precursor to PF-3845. We thank Mr. Luke McIntosh and Ms. Deborah Hopman for assistance in behavioral testing.
Abbreviations
- HIV
human immunodeficiency virus
- HIV-SN
HIV sensory neuropathy
- CB
cannabinoid
- FAA
fatty acid amide
- FAAH
fatty acid amide hydrolase
- AEA
anandamide
- 2-AG
2-arachidonoylglycerol
- MAGL
monoacylglycerol lipase
- PEA
palmitoyl ethanolamide
- OEA
oleoyl ethanolamide
- URB597
Cyclohexylcarbamic acid 3′-carbamoyl-biphenyl-3-yl ester
- PF-3845
N-3-pyridinyl-4-[[3-[[5-(trifluoromethyl)-2-pyridinyl]oxy]phenyl]methyl]-1-piperidinecarboxamide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams DI, Jay CA, Shade SB, Vizoso H, Reda H, Press S, Kelly ME, Rowbotham MC, Petersen KL. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515–521. doi: 10.1212/01.wnl.0000253187.66183.9c. [DOI] [PubMed] [Google Scholar]
- Ahn K, McKinney MK, Cravatt BF. Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chem Rev. 2008;108:1687–1707. doi: 10.1021/cr0782067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn K, Johnson DS, Mileni M, Beidler D, Long JZ, McKinney MK, Weerapana E, Sadagopan N, Liimatta M, Smith SE, Lazerwith S, Stiff C, Kamtekar S, Bhattacharya K, Zhang Y, Swaney S, Van Becelaere K, Stevens RC, Cravatt BF. Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain. Chem Biol. 2009;16:411–420. doi: 10.1016/j.chembiol.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn K, Smith SE, Liimatta MB, Beidler D, Sadagopan N, Dudley DT, Young T, Wren P, Zhang Y, Swaney S, Van Becelaere K, Blankman JL, Nomura DK, Bhattachar SN, Stiff C, Nomanbhoy TK, Weerapana E, Johnson DS, Cravatt BF. Mechanistic and pharmacological characterization of PF-04457845: A highly potent and selective fatty acid amide hydrolase inhibitor that reduces inflammatory and noninflammatory pain. J Pharmacol Exp Ther. 2011;338:114–124. doi: 10.1124/jpet.111.180257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand P, Whiteside G, Fowler CJ, Hohmann AG. Targeting CB2 receptors and the endocannabinoid system for the treatment of pain. Brain Res Rev. 2009;60:255–266. doi: 10.1016/j.brainresrev.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltramo M, Bernardini N, Bertorelli R, Campanella M, Nicolussi E, Fredduzzi S, Reggiani A. CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms. Eur J Neurosci. 2006;23:1530–1538. doi: 10.1111/j.1460-9568.2006.04684.x. [DOI] [PubMed] [Google Scholar]
- Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booker L, Kinsey SG, Abdullah RA, Blankman JL, Long JZ, Ezzili C, Boger DL, Cravatt BF, Lichtman AH. The FAAH Inhibitor PF-3845 acts in the nervous system to reverse lipopolysaccharide-induced tactile allodynia in mice. Br J Pharmacol. 2012;165:2485–2496. doi: 10.1111/j.1476-5381.2011.01445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borelli F, Romano B, Petrosino S, Pagano E, Capasso R, Coppola D, Battista G, Orlando P, Di Marzo V, Izzo AA. Palmitoylethanolamide, a naturally-occurring lipid, is an orally effective intestinal anti-inflammatory agent. Br J Pharmacol. doi: 10.1111/bph.12907. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw HB, Rimmerman N, Hu SS, Benton VM, Stuart JM, Masuda K, Cravatt BF, O’Dell DK, Walker JM. the endocannabinoid anandamide is a precursor for the signaling lipid N-arachidonoyl glycine by two distinct pathways. BMC Biochem. 2009;10:14. doi: 10.1186/1471-2091-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges D, Ahmad K, Rice AS. The synthetic cannabinoid WIN55,212-2 attenuates hyperalgesia and allodynia in a rat model of neuropathic pain. Br J Pharmacol. 2001;133:586–594. doi: 10.1038/sj.bjp.0704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Luo L, Palmer JA, Sutton S, Wilson SJ, Barbier AJ, Breitenbucher JG, Chaplan SR, Webb M. Inhibition of fatty acid amide hydrolase produces analgesia by multiple mechanisms. Br J Pharmacol. 2006;148:102–113. doi: 10.1038/sj.bjp.0706699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Clapper JR, Moreno-Sanz G, Russo R, Guijarro A, Vacondio F, Duranti A, Tontini A, Sanchini S, Sciolino NR, Spradley JM, Hohmann AG, Calignano A, Mor M, Tarzia G, Piomelli D. Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism. Nat Neurosci. 2010;13:1265–1270. doi: 10.1038/nn.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Lichtman AH. Fatty acid amide hydrolase: an emerging therapeutic target in the endocannabinoid system. Curr Opin Chem Biol. 2003;7:469–475. doi: 10.1016/s1367-5931(03)00079-6. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Rosario D, Clifford DB, McArthur JC, Simpson D, Alexander T, Gelman BB, Vaida F, Collier A, Marra CM, Ances B, Atkinson JH, Dworkin RH, Morgello S, Grant I. Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: the CHARTER Study. Arch Neurol. 2010;67:552–558. doi: 10.1001/archneurol.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, Toperoff W, Vaida F, van den Brande G, Gonzales J, Gouaux B, Bentley H, Atkinson JH. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34:672–680. doi: 10.1038/npp.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmes SJ, Winyard LA, Medhurst SJ, Clayton NM, Wilson AW, Kendall DA, Chapman V. Activation of CB1 and CB2 receptors attenuates the induction and maintenance of inflammatory pain in the rat. Pain. 2005;118:327–335. doi: 10.1016/j.pain.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Falenski KW, Thorpe AJ, Schlosburg JE, Cravatt BF, Abdullah RA. FAAH_/_ mice display differential tolerance, dependence, and cannabinoid receptor adaptation after δ9-tetrahydrocannabinol and anandamide administration. Neuropsychopharmacology. 2010;35:1775–1787. doi: 10.1038/npp.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3'-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther. 2005;313:352–358. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]
- Fox A, Kesingland A, Gentry C, McNair K, Patel S, Urban L, James I. The role of central and peripheral cannabinoid1 receptors in the antihyperalgesic activity of cannabinoids in a model of neuropathic pain. Pain. 2001;92:91–100. doi: 10.1016/s0304-3959(00)00474-7. [DOI] [PubMed] [Google Scholar]
- Freeman R, Baron R, Bouhassira D, Cabrera J, Emir B. Sensory profiles of patients with neuropathic pain based on the neuropathic pain symptoms and signs. Pain. 2014;155:367–376. doi: 10.1016/j.pain.2013.10.023. [DOI] [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Chandran AB, Jansen JP. Epidemiology of HIV-related neuropathy - A systematic literature review. AIDS Res Hum Retroviruses. 2011 doi: 10.1089/AID.2011.0116. [DOI] [PubMed] [Google Scholar]
- Guindon J, Lai J, Takacs SM, Bradshaw HB, Hohmann AG. Alterations in endocannabinoid tone following chemotherapy-induced peripheral neuropathy: effects of endocannabinoid deactivation inhibitors targeting fatty-acid amide hydrolase and monoacylglycerol lipase in comparison to reference analgesics following cisplatin treatment. Pharmacol Res. 2013;67:94–109. doi: 10.1016/j.phrs.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn K, Arendt G, Braun JS, von Giesen H-J, Husstedt IW, Maschke M, Straube E, Schielke E. A placebo-controlled trial of gabapentin for painful HIV-associated sensory neuropathies. J Neurol. 2004;251:1260–1266. doi: 10.1007/s00415-004-0529-6. [DOI] [PubMed] [Google Scholar]
- Hama AT, Germano P, Varghese M, Cravatt BF, Milne GT, Pearson JP, Sagen J. Fatty acid amide hydrolase (FAAH) inhibitors exert pharmacological effects, but lack analgesic efficacy in rats with neuropathic spinal cord injury pain. PLOS One. 2014;9(5):e96396, 1–12. doi: 10.1371/journal.pone.0096396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama A, Sagen J. Antinociceptive effect of cannabinoid agonist WIN 55,212-2 in rats with a spinal cord injury. Exp Neurol. 2007a;204:454–457. doi: 10.1016/j.expneurol.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama A, Sagen J. Behavioral characterization and effect of clinical drugs in a rat model of pain following spinal cord compression. Brain Res. 2007b;1185:117–1128. doi: 10.1016/j.brainres.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Hama A, Sagen J. Activation of spinal and supraspinal cannabinoid-1 receptors leads to antinociception in a rat model of neuropathic spinal cord injury pain. Brain Res. 2011;1412:44–54. doi: 10.1016/j.brainres.2011.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg U, Sagen J. Peripheral nerve exposure to HIV viral envelope protein gp120 induces neuropathic pain and spinal gliosis. J Neuroimmunol. 2001;116:29–39. doi: 10.1016/s0165-5728(01)00288-0. [DOI] [PubMed] [Google Scholar]
- Herzberg U, Eliav E, Bennett GJ, Kopin IJ. The analgesic effects of R(+)-WIN 55,212-2 mesylate, a high affinity cannabinoid agonist, in a rat model of neuropathic pain. Neurosci Lett. 1997;221:157–160. doi: 10.1016/s0304-3940(96)13308-5. [DOI] [PubMed] [Google Scholar]
- Hohmann AG. A cannabinoid pharmacotherapy for chemotherapy-evoked painful peripheral neuropathy. Pain. 2005;118:3–5. doi: 10.1016/j.pain.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Hsieh GC, Pai M, Chandran P, Hooker BA, Zhu CZ, Salyers AK, Wensink EJ, Zhan C, Carroll WA, Dart MJ, Yao BB, Honore P, Meyer MD. Central and peripheral sites of action for CB receptor mediated analgesic activity in chronic inflammatory and neuropathic pain models in rats. Br J Pharmacol. 2011;162:428–440. doi: 10.1111/j.1476-5381.2010.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MM, Deng H, Zvonok A, Cockayne DA, Kwan J, Mata HP, Vanderah TW, Lai J, Porreca F, Makriyannis A, Malan TP., Jr. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci U S A. 2003;100:10529–10533. doi: 10.1073/pnas.1834309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayamanne A, Greenwood R, Mitchell VA, Aslan S, Piomelli D, Vaughan CW. Actions of the FAAH inhibitor URB597 in neuropathic and inflammatory chronic pain models. Br J Pharmacol. 2006;147:281–288. doi: 10.1038/sj.bjp.0706510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri MD, Richardson D, Kendall DA, Barrett DA, Chapman V. Analgesic effects of fatty acid amide hydrolase inhibition in a rat model of neuropathic pain. J Neurosci. 2006;26:13318–13327. doi: 10.1523/JNEUROSCI.3326-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Mangieri RA, Bortolato M, Chefer SI, Mukhin AG, Clapper JR, King AR, Redhi GH, Yasar S, Piomelli D, Goldberg SR. Fatty acid amide hydrolase inhibition heightens anadamide signaling without producing reinforcing effects in primates. Biol Psychiat. 2008;64:930–937. doi: 10.1016/j.biopsych.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keswani SC, Polley M, Pardo CA, Griffin JW, McArthur JC, Hoke A. Schwann cell chemokine receptors mediate HIV-1 gp120 toxicity to sensory neurons. Ann Neurol. 2003;54:287–296. doi: 10.1002/ana.10645. [DOI] [PubMed] [Google Scholar]
- Kinsey SG, Long JZ, Cravatt BF, Lichtman AH. Fatty acid amide hydrolase and monoacylglycerol lipase inhibitors produce anti-allodynic effects in mice through distinct cannabinoid receptor mechanisms. J Pain. 2010;11:1420–1428. doi: 10.1016/j.jpain.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, Long JZ, O'Neal ST, Abdullah RA, Poklis JL, Boger DL, Cravatt BF, Lichtman AH. Blockade of endocannabinoid-degrading enzymes attenuates neuropathic pain. J Pharmacol Exp Ther. 2009;330:902–910. doi: 10.1124/jpet.109.155465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert DM, Fowler CJ. The endocannabinoid system: drug targets, lead compounds, and potential therapeutic applications. J Med Chem. 2005;48:5059–5087. doi: 10.1021/jm058183t. [DOI] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Bohme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Li J, Daughters RS, Bullis C, Bengiamin R, Stucky MW, Brennan J, Simone DA. The cannabinoid receptor agonist WIN 55,212-2 mesylate blocks the development of hyperalgesia produced by capsaicin in rats. Pain. 1999;81:25–33. doi: 10.1016/s0304-3959(98)00263-2. [DOI] [PubMed] [Google Scholar]
- Martin WJ, Lai NK, Patrick SL, Tsou K, Walker JM. Antinociceptive actions of cannabinoids following intraventricular administration in rats. Brain Res. 1993;629:300–304. doi: 10.1016/0006-8993(93)91334-o. [DOI] [PubMed] [Google Scholar]
- Martin WJ, Loo CM, Basbaum AI. Spinal cannabinoids are anti-allodynic in rats with persistent inflammation. Pain. 1999;82:199–205. doi: 10.1016/S0304-3959(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Murray RB, Gmerek DE, Cowan A, Tallarida RJ. Use of programmable protocol timer and data logger in the monitoring of animal behavior. Pharmacol Biochem Behav. 1981;15:135–140. doi: 10.1016/0091-3057(81)90352-x. [DOI] [PubMed] [Google Scholar]
- Naidu PS, Kinsey SG, Guo TL, Cravatt BF, Lichtman AH. Regulation of inflammatory pain by inhibition of fatty acid amide hydrolase. J Pharmacol Exp Ther. 2010;334:182–190. doi: 10.1124/jpet.109.164806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okine BN, Norris LM, Woodhams S, Burston J, Patel A, Alexander SP, Barrett DA, Kendall DA, Bennett AM, Chapman V. Lack of effect of chronic pre-treatment with the FAAH inhibitor URB597 on inflammatory pain behaviour: evidence for plastic changes in the endocannabinoid system. Br J Pharmacol. 2012;167:627–640. doi: 10.1111/j.1476-5381.2012.02028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Cannabinoid receptors and pain. Prog Neurobiol. 2001;63:569–611. doi: 10.1016/s0301-0082(00)00031-9. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Cherry CL, Cox S, Marshall SJ, Rice AS. Pharmacological treatment of painful HIV-associated sensory neuropathy: a systematic review and meta-analysis of randomised controlled trials. PLoS One. 2010;5:e14433. doi: 10.1371/journal.pone.0014433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartilho A, Mata HP, Ibrahim MM, Vanderah TW, Porreca F, Makriyannis A, Malan TP., Jr. Inhibition of inflammatory hyperalgesia by activation of peripheral CB2 cannabinoid receptors. Anesthesiology. 2003;99:955–960. doi: 10.1097/00000542-200310000-00031. [DOI] [PubMed] [Google Scholar]
- Raboune S, Stuart JM, Leishman E, TAkacs SM, Rhodes B, Basnet A, Jameyfield E, McHugh D, Widlanski T, Bradshaw HB. Novel endogenous N-acyl amides activate TRPV1-4 receptors, BV-2 microglia, and are regulated in brain in an acute model of inflammation. Front Cell Neurosci. 2014;8(195):1–11. doi: 10.3389/fncel.2014.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn EJ, Hohmann AG. Cannabinoids as pharmacotherapies for neuropathic pain: from the bench to the bedside. Neurotherapeutics. 2009;6:713–737. doi: 10.1016/j.nurt.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson K, Kumwenda J, Supparatpinyo K, Jiang JH, Evans S, Campbell TB, Price RW, Murphy R, Hall C, Marra CM, Marcus C, Berzins B, Masih R, Santos B, Silva MT, Kumarasamy N, Walawander A, Nair A, Tripathy S, Kanyama C, Hosseinipour M, Montano S, La Rosa A, Amod F, Sanne I, Firnhaber C, Hakim J, Brouwers PA. multinational study of neurological performance in antiretroviral therapy-naive HIV-1-infected persons in diverse resource-constrained settings. J Neurovirol. 2011;17:438–447. doi: 10.1007/s13365-011-0044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-Papp J, Morgello S, Vaida F, Fitzsimons C, Simpson DM, Elliott KJ, Al-Lozi M, Gelman BB, Clifford D, Marra CM, McCutchan JA, Atkinson JH, Dworkin RH, Grant I, Ellis R. Association of self-reported painful symptoms with clinical and neurophysiologic signs in HIV-associated sensory neuropathy. Pain. 2010;151:732–736. doi: 10.1016/j.pain.2010.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo R, Loverme J, La Rana G, Compton TR, Parrott J, Duranti A, Tontini A, Mor M, Tarzia G, Calignano A, Piomelli D. The fatty acid amide hydrolase inhibitor URB597 (cyclohexylcarbamic acid 3'-carbamoylbiphenyl-3-yl ester) reduces neuropathic pain after oral administration in mice. J Pharmacol Exp Ther. 2007;322:236–242. doi: 10.1124/jpet.107.119941. [DOI] [PubMed] [Google Scholar]
- Sagar DR, Jhaveri MD, Richardson D, Gray RA, de Lago E, Fernández-Ruiz J, Barrett DA, Kendall DA, Chapman V. Endocannabinoid regulation of spinal nociceptive processing in a model of neuropathic pain. Eur J Neurosci. 2010;31:1414–1422. doi: 10.1111/j.1460-9568.2010.07162.x. [DOI] [PubMed] [Google Scholar]
- Schlosburg JE, Kinsey SG, Ignatowska-Jankowska B, Ramesh D, Abdullah RA, Tao Q, Booker L, Long JZ, Selley DE, Cravatt BF, Lichtman AH. Prolonged monoacylglycerol lipase blockade causes equivalent cannabinoid receptor type 1 receptor-mediated adaptations in fatty acid amide hydrolase wild-type and knockout mice. J Pharmacol Exp Ther. 2014;350:196–204. doi: 10.1124/jpet.114.212753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütz SJ, Robbinson-Papp J. HIV-related neuropathy: current perspectives. HIV/AIDS Res Palliative Care. 2013;5:243–251. doi: 10.2147/HIV.S36674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DA, Wright CE, Angus JA. Evidence that CB-1 and CB-2 cannabinoid receptors mediate antinociception in neuropathic pain in the rat. Pain. 2004;109:124–131. doi: 10.1016/j.pain.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Simpson DM, Schifitto G, Clifford DB, Murphey TK, Durso-De Cruz E, Glue P, Whalen E, Emir B, Scott GN, Freeman R. HIV Neuropathy Study Group. Pregabalin for painful HIV neuropathy: a randomized, double-blind, placebo-controlled trial. Neurology. 2010;74:413–420. doi: 10.1212/WNL.0b013e3181ccc6ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson DM, Rice ASC, Emir B, Landen J, Semel D, Chew ML, Sporn J. A randomized, double-blind, placebo-controlled trial and open-label extension study to evaluate the efficacy and safety of pregabalin in the treatment of neuropathic pain associated with human immunodeficiency virus neuropathy. Pain. 2014 doi: 10.1016/j.pain.2014.05.027. (in press). [DOI] [PubMed] [Google Scholar]
- Ulugol A, Karadag HC, Ipci Y, Tamer M, Dokmeci I. The effect of WIN 55,212-2, a cannabinoid agonist, on tactile allodynia in diabetic rats. Neurosci Lett. 2004;371:167–170. doi: 10.1016/j.neulet.2004.08.061. [DOI] [PubMed] [Google Scholar]
- Verma S, Estanislao L, Simpson D. HIV-associated neuropathic pain: epidemiology, pathophysiology and management. CNS Drugs. 2005;19:325–334. doi: 10.2165/00023210-200519040-00005. [DOI] [PubMed] [Google Scholar]
- Wallace VC, Blackbeard J, Pheby T, Segerdahl AR, Davies M, Hasnie F, Hall S, McMahon SB, Rice AS. Pharmacological, behavioural and mechanistic analysis of HIV-1 gp120 induced painful neuropathy. Pain. 2007a;133:47–63. doi: 10.1016/j.pain.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace VC, Blackbeard J, Segerdahl AR, Hasnie F, Pheby T, McMahon SB, Rice AS. Characterization of rodent models of HIV-gp120 and anti-retroviral-associated neuropathic pain. Pain. 2007b;130:2688–2702. doi: 10.1093/brain/awm195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside GT, Lee GP, Valenzano KJ. The role of the cannabinoid CB2 receptor in pain transmission and therapeutic potential of small molecule CB2 receptor agonists. Curr Med Chem. 2007;14:917–936. doi: 10.2174/092986707780363023. [DOI] [PubMed] [Google Scholar]
- Wotherspoon G, Fox A, McIntyre P, Colley S, Bevan S, Winter J. Peripheral nerve injury induces cannabinoid receptor 2 protein expression in rat sensory neurons. Neuroscience. 2005;135:235–245. doi: 10.1016/j.neuroscience.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci U S A. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]