Abstract
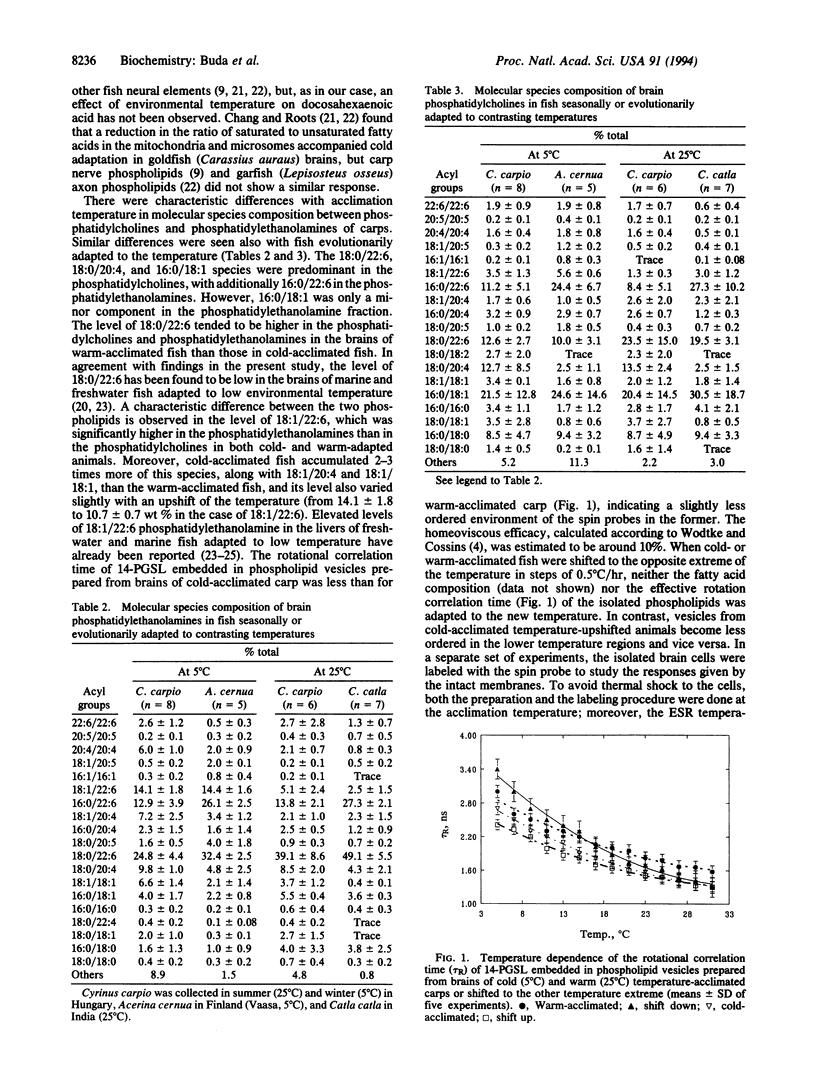
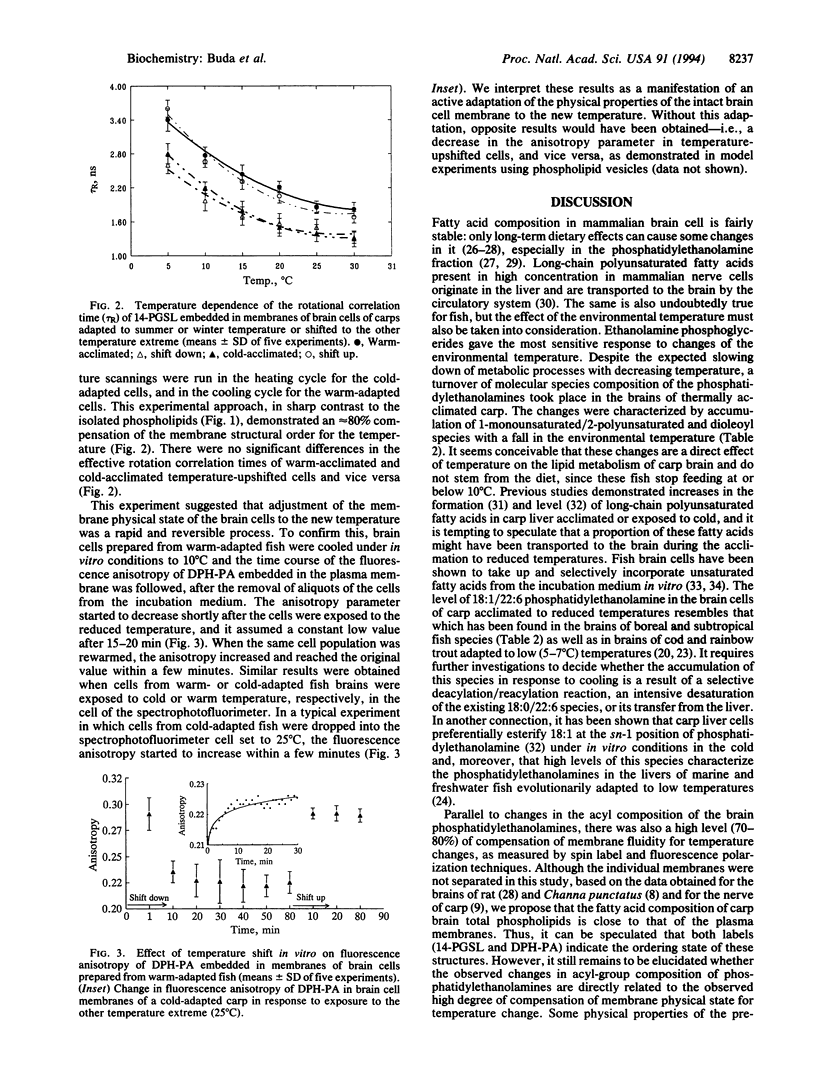
A comparison of the structural orders of membranes of a mixed brain-cell population isolated from Cyprinus carpio L. acclimated to either summer (23-25 degrees C) or winter (5 degrees C) revealed a high degree of compensation (80%) for temperature, as assayed by electron spin resonance spectroscopy. The cells rapidly forget their thermal history and adjust the physical properties of the membranes when shifted to the other extreme of temperature either in vivo or in vitro. Phospholipids separated from both types of animals exhibit only around 10% compensation. Arachidonic and docosahexaenoic acids are the major polyunsaturated fatty acids in the brains, but the fatty acid composition of the brain total phospholipids does not vary with adaptation to temperature. Separation of phosphatidylcholines and phosphatidylethanolamines into molecular species revealed a 2- to 3-fold accumulation of 18:1/22:6, 18:1/20:4, and 18:1/18:1 species in the latter; 18:0/22:6 showed an opposite tendency. Molecular species composition of phosphatidylcholines did not vary with the temperature. The same trends of changes were seen with brains of freshwater fish from subtropical (Catla catla L.) or boreal (Acerina cernua) regions. It is concluded that the gross amount of docosahexaenoic acid (22:6) plays only a minor role in adjusting the membrane physical properties to temperature. Factors other than lipids might be involved in the adaptation processes. Due to their specific molecular architecture, molecules such as 18:1/22:6, 18:1/20:4, or 18:1/18:1 phosphatidylethanolamine might prevent the contraction of membranes in the cold and may provide an environment for some other components involved in the temperature regulation of physical properties of nerve cell membranes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alsted A. L., Høy C. E. Fatty acid profiles of brain phospholipid subclasses of rats fed n - 3 polyunsaturated fatty acids of marine or vegetable origin. A two generation study. Biochim Biophys Acta. 1992 May 8;1125(3):237–244. doi: 10.1016/0005-2760(92)90051-v. [DOI] [PubMed] [Google Scholar]
- Barzanti V., Maranesi M., Solaini G., Turchetto E. Dietary lipid effects on microsome fatty acid composition of liver and brain, on liver glucose-6-phosphatase, and on brain 5'-nucleotidase activity in the rat. J Nutr Biochem. 1990 Jun;1(6):305–309. doi: 10.1016/0955-2863(90)90065-s. [DOI] [PubMed] [Google Scholar]
- Bell M. V., Tocher D. R. Molecular species composition of the major phospholipids in brain and retina from rainbow trout (Salmo gairdneri). Occurrence of high levels of di-(n-3)polyunsaturated fatty acid species. Biochem J. 1989 Dec 15;264(3):909–915. doi: 10.1042/bj2640909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M. C., Roots B. I. The effect of temperature- and oxygen-acclimation on phospholipids of goldfish (Carassius auratus L.) brain mitochondria. Neurochem Res. 1985 Sep;10(9):1231–1246. doi: 10.1007/BF00964842. [DOI] [PubMed] [Google Scholar]
- Chang M. C., Roots B. I. The effects of temperature- and oxygen-acclimation on phospholipids of goldfish (Carassius auratus L.) brain microsomes. Neurochem Res. 1985 Mar;10(3):355–375. doi: 10.1007/BF00964605. [DOI] [PubMed] [Google Scholar]
- Cossins A. R., Prosser C. L. Evolutionary adaptation of membranes to temperature. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2040–2043. doi: 10.1073/pnas.75.4.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossins A. R., Prosser C. L. Variable homeoviscous responses of different brain membranes of thermally-acclimated goldfish. Biochim Biophys Acta. 1982 May 7;687(2):303–309. doi: 10.1016/0005-2736(82)90559-4. [DOI] [PubMed] [Google Scholar]
- Dey I., Buda C., Wiik T., Halver J. E., Farkas T. Molecular and structural composition of phospholipid membranes in livers of marine and freshwater fish in relation to temperature. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7498–7502. doi: 10.1073/pnas.90.16.7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Farkas T., Csengeri I. Biosynthesis of fatty acids by the carp, Cyprinus carpio L., in relation to environmental temperature. Lipids. 1976 May;11(5):401–407. doi: 10.1007/BF02532847. [DOI] [PubMed] [Google Scholar]
- Fine J. B., Sprecher H. Unidimensional thin-layer chromatography of phospholipids on boric acid-impregnated plates. J Lipid Res. 1982 May;23(4):660–663. [PubMed] [Google Scholar]
- Hargreaves K. M., Clandinin M. T. Dietary control of diacylphosphatidylethanolamine species in brain. Biochim Biophys Acta. 1988 Sep 2;962(1):98–104. doi: 10.1016/0005-2760(88)90100-2. [DOI] [PubMed] [Google Scholar]
- Jost P., Libertini L. J., Hebert V. C., Griffith O. H. Lipid spin labels in lecithin multilayers. A study of motion along fatty acid chains. J Mol Biol. 1971 Jul 14;59(1):77–98. doi: 10.1016/0022-2836(71)90414-1. [DOI] [PubMed] [Google Scholar]
- Kitagawa S., Matsubayashi M., Kotani K., Usui K., Kametani F. Asymmetry of membrane fluidity in the lipid bilayer of blood platelets: fluorescence study with diphenylhexatriene and analogs. J Membr Biol. 1991 Feb;119(3):221–227. doi: 10.1007/BF01868727. [DOI] [PubMed] [Google Scholar]
- Kuhry J. G., Duportail G., Bronner C., Laustriat G. Plasma membrane fluidity measurements on whole living cells by fluorescence anisotropy of trimethylammoniumdiphenylhexatriene. Biochim Biophys Acta. 1985 Apr 22;845(1):60–67. doi: 10.1016/0167-4889(85)90055-2. [DOI] [PubMed] [Google Scholar]
- Lee J. A., Cossins A. R. Temperature adaptation of biological membranes: differential homoeoviscous responses in brush-border and basolateral membranes of carp intestinal mucosa. Biochim Biophys Acta. 1990 Jul 24;1026(2):195–203. doi: 10.1016/0005-2736(90)90064-u. [DOI] [PubMed] [Google Scholar]
- Rouser G., Fkeischer S., Yamamoto A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970 May;5(5):494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- Scott B. L., Bazan N. G. Membrane docosahexaenoate is supplied to the developing brain and retina by the liver. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2903–2907. doi: 10.1073/pnas.86.8.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinensky M. Homeoviscous adaptation--a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Feb;71(2):522–525. doi: 10.1073/pnas.71.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahin Q. S., Blum M., Carafoli E. The fatty acid composition of subcellular membranes of rat liver, heart, and brain: diet-induced modifications. Eur J Biochem. 1981 Dec;121(1):5–13. doi: 10.1111/j.1432-1033.1981.tb06421.x. [DOI] [PubMed] [Google Scholar]
- Takamura H., Narita H., Urade R., Kito M. Quantitative analysis of polyenoic phospholipid molecular species by high performance liquid chromatography. Lipids. 1986 May;21(5):356–361. doi: 10.1007/BF02535701. [DOI] [PubMed] [Google Scholar]
- Tocher D. R., Bell J. G., Sargent J. R. Incorporation of [3H]arachidonic and [14C]eicosapentaenoic acids into glycerophospholipids and their metabolism via lipoxygenases in isolated brain cells from rainbow trout Oncorhynchus mykiss. J Neurochem. 1991 Dec;57(6):2078–2085. doi: 10.1111/j.1471-4159.1991.tb06425.x. [DOI] [PubMed] [Google Scholar]
- Tocher D. R., Sargent J. R. Direct effects of temperature on phospholipid and polyunsaturated fatty acid metabolism in isolated brain cells from rainbow trout, Oncorhynchus mykiss. Comp Biochem Physiol B. 1992 Mar;101(3):353–359. doi: 10.1016/0305-0491(92)90012-g. [DOI] [PubMed] [Google Scholar]
- Wodtke E., Cossins A. R. Rapid cold-induced changes of membrane order and delta 9-desaturase activity in endoplasmic reticulum of carp liver: a time-course study of thermal acclimation. Biochim Biophys Acta. 1991 May 7;1064(2):343–350. doi: 10.1016/0005-2736(91)90321-x. [DOI] [PubMed] [Google Scholar]


