Abstract
Background
Sarcopenia is linked to functional impairments, loss of independence, and mortality. In the past few years, obesity has been established as being a risk factor for a decline in muscle mass and function. There are several molecular pathological mechanisms, which have been under discussion that might explain this relationship. However, most studies were conducted using male animals and for a short period of time.
Methods
In this study, the gender-specific effect of long-term, high-fat content feeding in Sprague–Dawley rats was examined. Development of the quadriceps muscle of the animals was monitored in vivo using magnetic resonance. The results of these measurements and of the biochemical analysis of the aged muscle were compared.
Results
Surprisingly, only male but not female rats showed a decline in muscle cross-sectional area at 16 months of age as a result of a chronic oversupply of dietary fats. This loss of muscle mass could not be explained by either de-regulation of the anabolic Akt pathway or by up-regulation of the main ubiquitin ligases of muscle, MAFbx and MuRF-1. However, fusion of satellite cells to myotubes was induced by the high-fat diet in male rats, possibly as a result of an increased need for compensatory regeneration processes. Caspase-3-dependent apoptosis induction, irrespective of diet, seems to be the major determinant of muscle decline during ageing in male but not female rats.
Conclusion
Taken together, activation of the apoptosis-inducing Caspase-3 seems to be the most important trigger for the age-related muscle loss. Male rats were more prone to the decline of muscle during ageing than female animals, which was further enforced by a long-term, high fat diet.
Keywords: Sarcopenic obesity, Lipotoxicity, Ageing skeletal muscle, Metabolism, Gender-specificity
Introduction
In the past few years, an age-dependent decline in muscle mass and strength and/or function has been classified as a distinct clinical phenotype referred to as sarcopenia.1 The cause of sarcopenia is multi-factorial and is therefore hard to discern, because many affected patients exhibit several of these morbidities at the same time.2 Possible underlying mechanisms are (i) disuse, (ii) altered endocrine function, (iii) chronic diseases, inflammation, (iv) insulin resistance, and (v) malnutrition.3–6 While young adults possess up to 50% of the total body weight as muscle mass, approximately half of this muscle tissue is lost during ageing. The decline in muscle is accompanied by an increase in fat mass, particularly visceral fat.7 Moreover, obesity has been shown to be associated with a functional decline in older adults.8,4 Consequently, the coincidence of sarcopenia and obesity results in super-additive functional impairments than that observed in either low muscle mass or high fat mass alone.9 However, there are conflicting epidemiological data regarding the gender-specific prevalence rates of sarcopenic obesity in humans, depending on the definition of obesity used, ranging from 4.4 to 84.0% in men and 3.6 to 94.0% in women.10
Hence, it has been speculated that there is a direct molecular mechanism linking fat and muscle.11 Consequently, excretion of pro-inflammatory cytokines like interleukine 6 (IL-6), tumour necrosis factor α (TNFα) and monocyte chemotactic protein-1 by adipocytes and—to an even greater extent—proadipocytes could play an important role.12 These ‘geriatric cytokines’ have been linked to decreased muscle mass and strength.13 In addition to clinical, observational studies, TNFα has been shown to induce apoptosis in muscle cells in vitro.14 Furthermore, an excess of fatty acids can directly induce apoptosis in several organs, including the liver, heart, and pancreas.15,16 Normally, fatty acids are mainly stored in adipocytes, but, in the case of a chronic overload with dietary triglycerides, they spill over and are enriched in other tissues. If there is no compensation by improved oxidative capacity, fatty acid derivates such as diacylglycerol, long chain acyl coenzyme A, and ceramide accumulate in the myofibres.17 Ultimately, this process leads to apoptosis induction in cultured myofibres.18 Moreover, the regeneration capacity of the myofibres is blunted because of exhaustion of muscular stem cells.19. As a result of lipotoxicity, these so-called satellite cells dys-differentiate into mesenchymal adipocyte-like default cells (MAD cells), which express typical genes of fat tissue such as peroxisome proliferator-activated receptor-γ.20 Consequently, loss of myonuclei cannot be compensated. In recent years, anabolic build-up of muscular structure protein has been shown to be deregulated by a high fat diet. Thereby, the energy sensing Akt pathway was altered, together with its downstream effectors S6K1 and 4E-BP1, which directly modulate protein translation.21 In addition to synthesis, proteasomal decay is a major contributor to net muscular protein turnover. The E3 ubiquitin ligases MURF1 and MAFbx, in particular, are responsible for the breakdown of proteins of the myotube and the reuse of the liberated amino acids for new protein synthesis or energy production.22 It should be noted that there are enormous differences in lipid storage between the two genders, leading to higher susceptibility for the lipotoxic effects reported in male subjects.23
We recently demonstrated that a chronically high fat diet (45 energy% provided by fat) resulted in a decreased cross-sectional area (CSA) of the quadriceps muscle of male Sprague–Dawley rats compared with littermates receiving standard rodent chow. The muscular degeneration was present on the occasion of the first magnetic resonance (MR) examination of the animals after 10 months of high-fat feeding.24 Therefore, in the present study, we used female Sprague–Dawley rats to reveal gender-specific effects of the high fat diet. Muscular development was non-invasively monitored using MR at four time points to elucidate the early onset of muscular atrophy.
Materials and methods
Animals and dietary procedure
Thirty-six male and forty-two female Sprague–Dawley rats were purchased (from Janvier Labs, Le Genest-Saint-Isle, France) at the age of 6 months and included in the study, without further selection or conditioning. The male rats were randomly subdivided into two groups receiving either a high-fat diet (HFD) [male HFD (mHFD); n = 18; 43 energy% of neutral fat, based on lard and corn oil] or a control diet (CD) [male CD (mCD); n = 18; 25 energy% of neutral fat] (Altromin, Lage, Germany). In contrast, female rats were alternatively assigned to the female HFD (fHFD; same composition as for the male rats) or the control group (female CD (fCD); 10 energy% of neutral fat) depending on body weight. While contents of protein and essential micronutrients were very similar in all diets, the higher fat content of the HFD was compensated for by a lower carbohydrate content to achieve comparable energy density. All rats were given ad libitum access to water and food. They were housed in groups of three rats per cage at a constant room temperature of 20°C and on a 12 h light–dark cycle until the end of the experiment. Only animals that survived the whole study period without obvious tumours were analysed. Therefore, all experiments presented in this publication were conducted with 6 mHFD, 12 mCD, 6 fHFD, and 5 fCD rats. All animal procedures were approved by the local animal rights committee in accordance with the German Law on Animal Protection.
Magnetic resonance examination
Magnetic resonance examinations including morphometric imaging and MR spectroscopy were performed at 16 and 21 months of age for both genders using an identical protocol for all animals. The female rats were additionally analysed at 7 and 12 months of age. The body weight of the animals was measured shortly before the MR examination for optimal dosage of the narcotic medication. Intraperitoneal injection of pentobarbital (Narcoren®, Merial, Hallbergmoos, Germany) was used for inducing anaesthesia. During the MR examination, the animals were provided with oxygen via a mask, and cooling was prevented with a blanket. MR was performed on a 1.5 T clinical scanner (Magnetom Avanto, Siemens Healthcare, Erlangen, Germany) using an 8-channel array coil designed for human knee examinations. To ensure the reproducibility of measurements, the hind-limbs were extended backwards. T1-weighted spin echo (SE) sequences were applied to image the quadriceps muscles of both hind-limbs in longitudinal and transverse orientation (voxel size: 0.4 × 0.4 × 2.0 mm). T1-weighted SE images of both hind-limbs, perpendicular to the long axis of the muscle, were used for determination of the maximum CSA of the quadriceps.24 MR spectra were post-processed using the software package offered by the vendor: processing included subtraction of the water signal (when applicable), Fourier transformation, Hanning filtering with a width of 700 ms, zero filling a measured vector from a length of 1024 to a length of 2048, base line correction (6th polynome order), curve fitting, and manual phase correction for all spectra. Relative lipid content was calculated as ‘lipid fraction’ = [magnetic resonance spectroscopy (MRS)lipids/(MRSlipids + MRSwater)] * 100 with MRSwater being the area under the water signal in the spectrum recorded without water suppression, and MRSlipids as the integral signal taking into account all lipid signal contributions between 0.9 and 1.6 ppm and between 1.9 and 2.6 ppm.
Ex vivo examinations
Between 21 (male) and 22 (female) months of age, all surviving animals were sacrificed and the M. vastus lateralis was isolated and stored frozen at −80°C.
For lysis, 50 mg of the muscle was sonicated in 500 μL ice-cold radio-immuno-precipitation assay buffer [RIPA buffer: 20 mM Tris–HCl, 150 mM NaCl, 1% (vol/vol) NP-40, 1% (wt/vol) sodium deoxycholate, 1 mM EDTA, 1 mM EGTA, 2.5 mM sodium pyrophosphate 1 mM β-glycerophosphate, 1 mM sodium vanadate, 1 µg/mL leupeptin; pH 7.5), and the debris was removed by centrifugation (10 000 g for 2 min). For each sample, 30 µg total protein from the remaining supernatants was used for SDS-PAGE analysis. After activation of the stain-free gels (BioRad, Hercules, CA, USA) with ultaviolet, total protein content on the western blot was used for normalization of the densitometric data. For immuno-detection, the following primary antibodies were used: Ser473P-Akt / total Akt, total S6K1 (p70 ribosomal protein S6 kinase 1), Ser65P-4E-BP1 / total 4E-BP1 (eukaryotic initiation factor 4E binding protein 1), Ser240/244P-rpS6 / total rpS6 (ribosomal protein S6), Atrogin/MAFbx (Muscle atrophy F box), MURF-1 (Muscle RING Finger-1), and Caspase 3 (all Santa Cruz Biotechnology Inc., Dallas, TX, USA). After incubation with the secondary antibodies (anti-mouse horseradish peroxidase-conjugated (HRP), anti-rabbit HRP-conjugated; both from Santa Cruz Biotechnology Inc.) for 2 h luminescence was measured with a gel-imaging system (BioRad). For densitometric evaluation of all female rats one arbitrary chosen animal was run on every gel.
A part of the vastus lateralis was partially fixed in 10% neutral buffered formaldehyde, embedded in paraffin, and cut in 5 µm sections. In haematoxylin-eosin (HE) stained sections, 400 ± 10% myofibres of each animal were analysed. All fibres were counted as being centrally nucleated that contained at least one nucleus that was not associated with the sarcolemma. For the determination of the muscle fibres, CSA200 ± 10% myofibres per animal were manually outlined using the analySIS® Image Processing software (Soft Imaging System GmbH, Muenster, Germany).
Statistical analysis
Statistical analysis was performed using SPSS 22.0 (IBM SPSS Statistics, Armonk, NY, USA). All data are given as mean ± standard deviation (SD). For all MRI data, results for the right and left legs were averaged. Kolmogorov–Smirnov test was used to test the normal distribution of data, and homogeneity of variances was confirmed by Levene's test. Depending on the results of these tests, either two-sided, unpaired t-testing, Welch test or the Mann–Whitney U test were applied for analysis of the data. P < 0.05 was regarded as statistically significant. The male and female rats were not statistically compared, because there were some differences between these two sub-studies (e.g. content of fat in the control diet). Pearson's product–moment correlation coefficient was used to analyse the linear correlation between two variables.
Results
Development of cross-sectional area and body weight during ageing
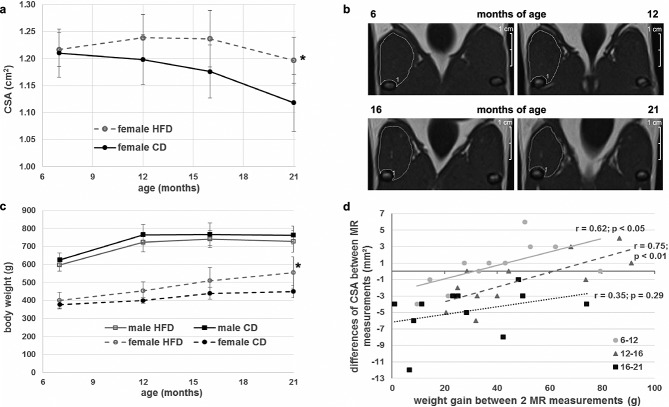
Recently, we reported that male Sprague–Dawley rats receiving an HFD for 10 months have a significantly lower quadriceps CSA than littermates receiving a control diet.24 Therefore, we raised the question of whether the same holds true for female Sprague–Dawley rats. Baseline (7 months of age) and subsequent (12 months and 16 months of age) MR examinations revealed no significant effect of the HFD [female HFD (fHFD] compared with the control group (fCD) (P = 0.74; P = 0.12; P = 0.68, respectively). On the other hand, at 21 months of age, the CSA was even elevated in the fHFD group compared with the fCD (fCD: 1.12 cm2; fHFD: 1.20 cm2; P < 0.05). This higher CSA of the animals receiving HFD could not be explained by accumulation of intramuscular fat because the lipid fraction measured by MR was comparable between both dietary groups at all analysed time points (See Supporting Information, Figure S11a). The fHFD animals exhibited a relatively stable quadriceps CSA until 16 months of age. In contrast, the fCD group had its maximum mean CSA at the age of 6 months, which decreased slightly in the following year (Figure1A). The female animals revealed a highly significant decline in CSA between 16 and 21 months of age, irrespective of diet (multivariate test: age effect P < 0.001; age * diet interaction P = 0.35). MR images of one representative fHFD animal at all four time points are depicted in Figure1B. Remarkably, in a recent publication, we showed that mHFD rats had a significant lower CSA than the mCD group at 16 months of age, while the muscular decline shown by these two groups exhibited no difference during the last 5 months of the study.24 However, the muscular atrophy was about four times higher in male than in female rats (22% vs. 5%, P < 0.01), with a higher variance within both dietary groups (Figure S1B). Therefore, we posed the question of how these observed muscular changes during lifetime could be connected with the development of total body weight. Male rats showed a very similar weight development irrespective of their diet. In contrast, female rats who received an HFD gained more weight during their lifetime than those maintained on standard diet (Figure1C). The weight difference became significant at 21 months of age (450 g fCD vs. 555 g fHFD; P < 0.05). Therefore, we hypothesize that the gain in muscle seen in female rats was caused by the training effect of increased body weight.25 For analysis of the interrelationship between muscle CSA and total body weight, all female rats—irrespective of diet—were considered together as one group. In so doing, a close positive correlation was found between weight gain and increment of the CSA, as well between 7 and 12 months of age (r = 0.62; P < 0.05) as between 12 and 16 months of age (r = 0.75; P < 0.01). However, this anabolic effect was strictly blunted in the older animals (16–21 months of age) exhibiting a net decrease in muscle diameter of all female rats (r = 0.35; P = 0.29) (Figure1D).
Figure 1.
Weight and cross-sectional area (CSA) development of the rats. (A) Mean CSA of the female rats during the study. The first measurement at 7 months of age was performed before the rats were divided into the control (black circles) and high-fat diet (HFD) (grey open circles) groups. At 21 months of age, the female HFD (fHFD) group showed a significantly higher CSA than the female control diet (fCD) group. (B) Representative images of the quadriceps muscle of one fHFD rat at 7, 12, 16, and 21 months of age. (C) Development of body weight of male CD (black squares and solid line), male HFD (grey, open squares, and solid lines), fCD (black circles and dashed line) and fHFD (grey open circles and dashed line). While the body mass of the male animals reached a plateau at 12 months, the weight of the female rats increased throughout the whole lifetime. Only the fHFD group at 21 months of age was significantly heavier than the age-matched fCD group. (D) Correlation between total body weight gain and CSA changes between two magnetic resonance (MR) measurements of all female animals. Between 7–12 and 12–16 months of age, CSA was significantly correlated with increased body mass. This relationship was abolished between 16 and 21 months of age. Only animals that survived the whole study period were analysed. All data are presented as mean ± SD. * Significant difference between dietary groups within gender.
Expression and activity of regulators of anabolic muscular protein expression
We wanted to elucidate a possible direct effect of excess dietary fatty acids on anabolism. One major contributor to muscular protein build up is Akt, which leads to inactivation of the translational repressor 4E-BP1 and stimulation of translational activity of rP S6 by phosphorylation. However, this pathway is influenced by other multiple factors such as AMPK or availability of essential amino acids.22 Therefore, we decided to analyse several members of this pathway and their phosphorylation status, in order to obtain a more comprehensive overview of muscular transcription. In Figure2A, exemplary western blots of Akt, 4E-BP1, S6K1, and rP S6 of the female rats are shown. Densitometric analysis of all female rats is summarized in Figure2B. There were no significant changes detectable between the dietary groups. The HFD neither changed the total protein content nor changed the phosphorylation status of the proteins analysed.
Figure 2.
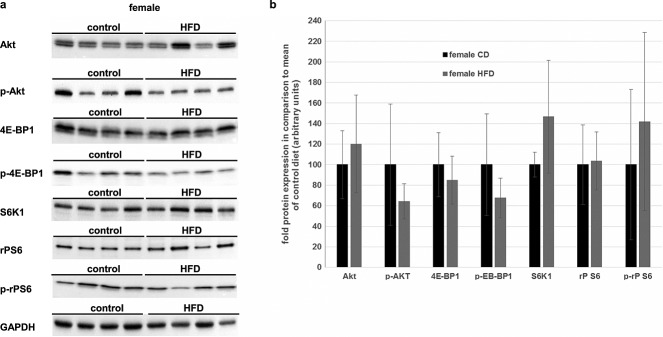
Effects of a long-term high-fat diet (HFD) on regulators of muscular protein biosynthesis in female rats. (A) Protein-lysates of the M. vastus lateralis dexter of female rats were used for immunoblot analysis of total cellular protein level of Akt, 4E-BP1, S6K1, rP S6, and GAPDH (glyceraldehyde 3-phosphate dehydrogenase). Additionally, the phosphorylated forms (Ser473P-Akt, Ser65P-4E-BP1, and Ser240/244P-rpS6) were measured. For comparison, GAPDH, as a house-keeper, is added. Representative blots are shown. (B) Densitometric analysis of the immunoblots of all female animals that survived the whole study period (female CD: n = 5, black bars; female HFD: = 6, grey bars). There were no significant changes detectable between the two dietary groups. Intensity of the bands was normalized to total protein content on the western blot membrane using the Stain-Free™ gel system (BioRad). All data are presented as mean ± SD.
Recently, we were able to demonstrate that the metabolic de-regulation because of an excess of fat could lead to a downshift of the Akt pathway in male Wistar rats.21 Therefore, the male Sprague–Dawley rats were analysed using the same parameters and standardizations as described earlier (Figure3A). Like the females, the males showed no significant changes between the dietary groups except for a higher phosphorylation of Akt. However, this effect was quite minor and did not result in enhanced phosphorylation of its downstream effectors (Figure3B).
Figure 3.
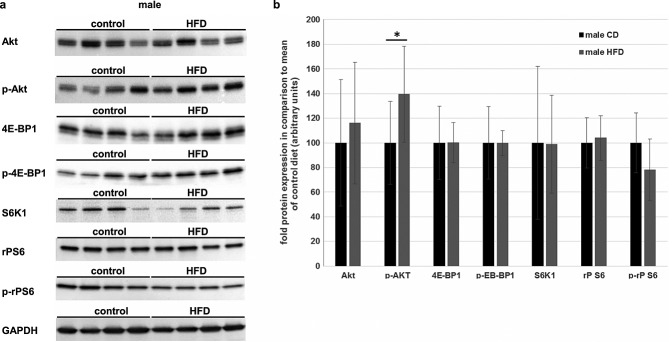
Effects of a long-term high-fat diet (HFD) on regulators of muscular protein biosynthesis in male rats. (A) M. vastus lateralis dexter of male rats was lysed and analysed by immunoblotting. Representative blots of total cellular protein level of Akt, 4E-BP1, S6K1, rP S6, and GAPDH and additionally of the phosphorylated forms (Ser473P-Akt, Ser65P-4E-BP1, and Ser240/244P-rpS6) are shown. (B) Densitometric analysis of the immunoblots of all male animals that survived the whole study period (male control diet (CD): n = 12, black bars; male HFD: n = 7, grey bars). The total protein contents of all analysed regulators did not differ between the CD and the HFD group. Ser473P-Akt was significantly elevated in the HFD group. However, Ser65P-4E-BP1 and Ser240/244P-rpS6, which are the downstream effectors of P-Akt, revealed no difference between the dietary groups Total protein content on the western blot membrane, measured by the Stain-Free™ gel system (BioRad), was used for normalization. All data are presented as mean ± SD. * Significant difference between dietary groups.
Regulation of proteasomal muscle protein decay, myonuclei regeneration, and apoptosis
Apart from the anabolic protein build-up, muscular turnover is influenced by the two E3 ubiquitin ligases MuRF-1 and MAFbx.26 Accordingly, we analysed these two proteins with western blotting (Figure4A). MuRF-1 was only slightly expressed, and there were no significant changes detectable between control and HFD groups for male or female animals (Figure4B). In female rats, MAFbx exhibits only low levels, with no differences apparent between the diets. One male rat showed a strong induction of MAFbx expression compared with its littermates. However, the rest of the mHFD group showed very similar results to the mCD group (Figure4B).
Figure 4.
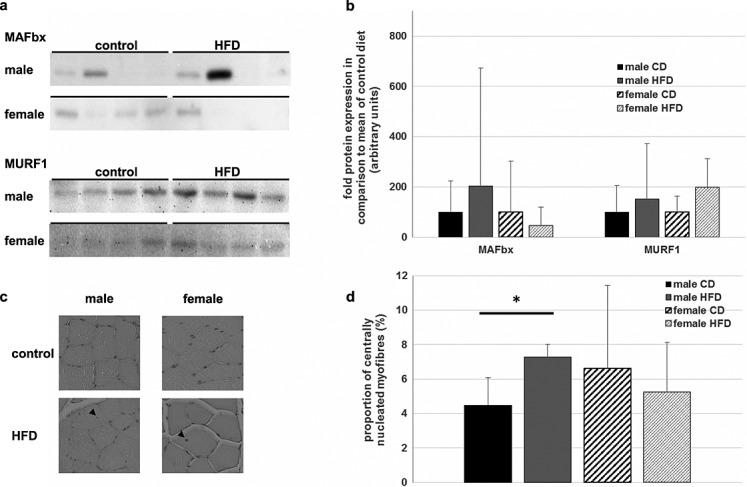
Influence of chronically high-fat diet (HFD) on proteasomal protein decay and muscular regeneration. (A) Expression of the two ubiquitin ligases MAFbx and MuRF-1 in the M. vastus lateralis was visualized with immunoblotting. Most of the animals showed no or only little expression of MAFbx, albeit some rats showed a strong induction of this ligase. MuRF-1 protein levels differed only slightly in females, while male rats showed greater inter-individual variance. (B) Mean expressions of MAFbx and MURF-1 were determined by densitometric analysis. The control group of each gender was arbitrary set as 100. There were no significant differences detectable between the high-fat and the control diet group. Mean normalized protein level + SD is shown in the diagram. (C) Paraffin-embedded sections of the quadriceps muscle were haematoxylin-eosin stained. About 400 fibres of each animal were evaluated and those with at least one nucleus, which was not located at the sarcolemma, were counted as centrally nucleated. Exemplary pictures of each gender and dietary group are shown, and small arrows mark centrally located nuclei. (D) Statistical analysis of histology of all animals (male CD: n = 4; male HFD: n = 8; female CD: n = 4; female HFD: n = 5). Mean percent of centrally nucleated fibres per total number of myofibres + SD is depicted. While male rats receiving an HFD had a significantly greater number of regenerated fibres, there were no differences between the dietary groups amongst the female rats. * Significant difference between dietary groups.
Muscular regeneration and anabolic build-up are also enhanced by the fusion of precursor cells— satellite cells—with existing or newly formed myofibres. This process leads to myofibres with centrally located nuclei that are even detectable 14 days after fusion.27 Consequently, we analysed haematoxylin-eosin stained sections of the rats tibialis anterior muscle for signs of regeneration processes (Figure4C). Four hundred ± 10% myofibres were counted, and the amount of centrally nucleated fibres per total number of analysed myofibres was calculated (Figure4D). In so doing, mHFD animals displayed a significantly higher portion of centrally nucleated fibres compared with the mCD (4.5% mCD vs. 7.3% mHFD; P < 0.05). In contrast, there were no significant differences between the two dietary groups of female rats (6.6% fCD vs. 4.0% fHFD; P = 0.3).
Therefore, we wondered if the decreased muscle CSA in the male HFD group was caused by loss of muscle fibres or by atrophy of the CSA of the muscle fibres. Four hundred ± 10% myofibres per animal were manually outlined to measure the mean fibre CSA (male CD: n = 4; male HFD: n = 8; female CD: n = 4; female HFD: n = 5). The fibre CSA of the high fat diet groups tended to be lower in both genders but these differences were not statistically significant (male CD: 5378 µm2 vs. male HFD: 4383 µm2, P = 0.38; female CD: 3974 µm2 vs. female HFD: 2799 µm2, P = 0.08; Figure S2). Hence, it could be speculated that a chronical high fat diet rather leads to loss of muscle fibres—possibly through apoptotic processes.
Fatty acids have been shown to induce apoptosis in the skeletal muscle of obese rats.28 Therefore, we analysed the amount of cleaved Caspase 3, as a marker of apoptosis. While all female rats showed only very low levels of cleaved Caspase 3, there were some male rats that showed strong induction of Caspase 3 activation (Figure5A). The HFD did not lead to an increase of this pro-apoptotic process in either gender when compared with the CD group (Figure5B). However, activation of Caspase 3 was the only factor analysed in the male rats that correlated with the decline in quadriceps CSA between 16 and 21 months of age (τ = 0.44; P < 0.05). We were not able to detect a correlation between loss of muscle mass between 16 and 22 months of age and the biochemical and histological parameters measured in this project in female rats.
Figure 5.
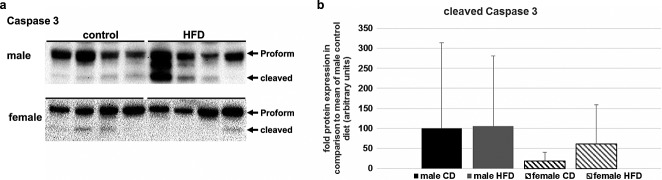
Apoptose induction in skeletal muscle of aged rats. (A) Total protein level of Pro-Caspase 3 and cleaved Caspase 3 was measured using immunoblotting. Female rats had no or very low amounts of activated Caspase 3. In contrast, nearly all male rats exhibited a certain level of cleaved Caspase 3, and some animals showed strong apoptosis induction. (B) Summary of the densitometric analysis of the cleaved Caspase 3. Male rats showed considerable apoptose induction, whereas females had low levels of cleaved Caspase 3. Differences between dietary groups were not significant for both genders. Data are presented as mean + SD.
Discussion
In a very recent study, we were able to establish that a diet with an elevated amount of fat could lead to loss of muscle mass, even in the absence of obesity.24 Following this initial morphometric MR analyses in male Sprague–Dawley rats, the present study was intended to focus on potential molecular pathology. In addition, the study was extended to female rats to elucidate gender-specific adaption to an excess of dietary fat. We found that feeding female rats with chronically high fat even elevates quadriceps CSA compared with animals receiving standard rodent chow. This result is in sharp contrast to muscle loss in male rats because of an excess of dietary fatty acids.21,29 We hypothesize that this gender-dependent effect could be explained by the training effect of the higher body mass, because weight and CSA gain were correlated until 16 months of age. Consistent with that finding, 12 weeks of high fat feeding has been shown to increase muscle fibre area in young mice.18 In human subjects, several studies were also able to detect a larger diameter of type II fibres in obese adults.30,31 This gain in muscle mass due to elevated body weight has been linked to the ‘obesity paradox’, which predicts a better outcome for obese people with chronic diseases compared with those with a normal weight.32,33 However, anabolic resistance increases throughout the later stages of life 34 blunting the training effect after 18 months of age in the female rats with our model. In contrast, the male Sprague–Dawley rats used in this study showed similar total body weight, irrespective of diet, abolishing the anabolic stimuli seen in the female rats. Concordant with this, male rats without a static phase of obesity showed an elevated basal fractional protein synthesis rate compared with their lean littermates.29 In addition, there are strong gender-dependent differences in the regional distribution and physiology of fat storage in rats, as well as humans.23,35 Female rats receiving an HFD exhibit higher weight gain but lower insulin resistance and a better capacity to counteract the fat-induced muscular damage compared with their male littermates.36 Therefore, the lack of a static phase of obesity in female rodents seems to offer protection against lipotoxicity by preventing a spill-over of fatty acids from adipocytes to other tissues.37
The old rats in our model did not exhibit significant changes in the anabolic Akt pathway, apart from an increased stimulation of Akt in male rats. Nevertheless, this elevated phosphorylation did not lead to activation of its downstream effectors. Taken together, advanced age and a static body weight seem to level differences in the Akt pathway evoked by diet and/or obesity.
Even though the CSA of both genders significantly declined between 16 and 21 months of age, males lost about four times more muscle than the females. On the other hand, decline in muscle area at this stage of life was independent of diet. Therefore, we speculate that, in mature rats, ageing processes override the effects of HFD. Consequently, male subjects are more prone to losing much muscle mass than females, as has also been demonstrated for human seniors.38,39
Muscle size is controlled by proteasomal degradation processes mediated by the two ubiquitin ligases MAFbx and MuRF1, in rodents as well as humans.40,41 Both ligases have been shown to increase during ageing, leading to atrophy.26 While MuRF1 primarily acts on the proteasomal decay of muscular structure proteins, MAFbx regulates protein transcription by degrading MyoD and eIF3-f.42,43 In contrast to a study with overweight Wistar rats, we were not able to detect a significant elevation in MuRF1 protein content because of the HFD.28 However, while the diets used in our study contained similar amounts of protein, the overfed rats used by Sishi et al. received only 60% of the protein received by the control group. Therefore, the activation of MuRF1 in this study could possibly be because of dietary amino acid deficiency.28 MafBX did not seem to influence muscle loss, because neither its cellular level nor the phosphorylation status of its downstream effector rpS6 were altered by the HFD. However, in our study, the concentrations of MafBX and MuRF1 were only measured at 21 months of age. Therefore, we could not exclude that these regulators of the proteasomal decay are upregulated during ageing as proposed by others.44 Consistent with that, there were no significant changes detectable between the mean fibre CSA of the dietary groups in both genders. Taken together, muscular protein build-up and degradation is apparently balanced after chronic fat overload in our animal model.
Following injury of muscle fibres by trauma or eccentric contractions, satellite cells were unable to repair the damage.45 This could result from either fusion of these progenitor cells to form new myofibres or by enhancement of the number of nuclei of existing myofibres. Newly added myonuclei could be detected histologically, as they are often located centrally in the myofibre.27 However, several studies have suggested that the regenerative capacity of these satellite cells declines during ageing.19 In addition, chronic overload of fatty acids leads to dys-differentiation of the progenitor cells to MAD cells. These MAD cells express typical marker gens of adipocytes and increase the amount of inter-muscular adipose tissue.20 In our histological experiments, the HFD even amplified the number of centrally nucleated fibres, which points to a stable regeneration capacity of the satellite cells. On the other hand, there has to be an inductor for satellite cell activation. In the last few years, it has been shown that obesity leads to elevated blood levels of several pro-inflammatory proteins, such as IL-6, TNFα, and MCP-1.12 TNFα excreted by adipose tissue, in particular, appears to induce apoptosis in muscle cells.14 In addition, accumulation of fatty acids and their derivatives in non-adipose tissues results in metabolic imbalances and ultimately cell death.16 Acute elevation of fatty acids results in apoptosis induction in vivo as well as in cultured myofibres.18,46 However, we did not find elevation of activated Caspase 3 as an apoptosis marker because of chronical fatty acid overload. This finding is supported by another study that also failed to demonstrate apoptosis induction because of an HFD.18 Therefore, we could not link the increased regeneration processes of the high-fat fed animals to apoptotic loss of nuclei. Perhaps this may be explained by the fact that centrally nucleated fibres exhibit the accumulated regeneration process for a longer time, while acute apoptosis induction is a highly variable process in terms of time. On the other hand, oxidative stress due to fatty acid overload might lower gene transcription and translation,47 leading to activation of satellite cells as compensation. However, we did not detect an anabolic response, like up-regulation of the protein levels of members of the Akt pathway nor a hypertrophy of the muscle fibre CSA, which would explain the increased number of centrally nucleated fibres in the male HFD group.
Surprisingly, in male rats, we found an approximately two times higher level of cleaved Caspase 3 than in the female animals, irrespective of the fat content of the diet. Consistent with this finding, male rats showed a good correlation between the level of cleaved Caspase 3 and the decline in CSA between 16 and 21 months of age, which was not apparent in the female rats. Therefore, it could be speculated that male rats are more prone to apoptosis induction. In line with this, other studies have also reported a high impact of apoptosis on the decline in muscle mass during ageing.48,49
This study has some limitations, which have to be addressed in future investigations. Firstly, the control diet for the male and female rats differed in terms of fat content (25 energy% vs. 10 energy% of neutral fat). In addition, the male rats were not measured with MR imaging during early adulthood. Hence, it is not possible to conclude that the HFD prevents anabolic build-up of muscle or whether it leads to premature atrophy during ageing in male rats. Based on this information, the optimal month for the re-evaluation of the impact of an excess of dietary fats on the Akt pathway should be determined. The protective effect of the HFD on female rats is possibly caused by the lack of a menopause in this animal model. Several publications point to the relationship between development of sarcopenia and hormonal changes because of menopause in human subjects.50–52 Therefore, it would be of great interest to investigate whether induction of menopause in female rats would alter the result for the muscular development.
Conclusion
Loss of muscle CSA because of a chronically HFD seems to be limited to male rats. In contrast, female rats showed even a higher CSA in those animals receiving more dietary fat, possibly as a result of the training effect of greater body weight. Neither the anabolic Akt pathway nor the ubiquitin-dependent protein degradation could be linked to diet-induced muscle decline. However, we were able to demonstrate an elevated tendency for regeneration processes caused by the HFD in male rats. The lack of deregulation could be explained by the overwhelming age-dependent apoptosis induction in male rats that was independent of the dietary regimen.
Acknowledgments
This study was supported by the Bayerische Forschungsstiftung in the context of the Bavarian cluster project ‘Amyotrophia (Sarcopenia) and Osteoporosis—Consequence of limited regeneration in age (FORMOsA)’.
We would like to thank Rita Brunner-Ploss, Christine Kokott, and Christine Hechtl for the technical assistance and helpful discussions.
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle (von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle 2010; 1: 7–8).
Conflict of interest
None declared.
Supporting Information
Supporting info item
Supporting info item
References
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing. 2010;39:412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biolo G, Cederholm T, Muscaritoli M. Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases: from sarcopenic obesity to cachexia. Clin Nutr. 2014;33:737–48. doi: 10.1016/j.clnu.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. 2000;904:437–48. doi: 10.1111/j.1749-6632.2000.tb06498.x. [DOI] [PubMed] [Google Scholar]
- Bouchard DR, Dionne IJ, Brochu M. Sarcopenic/obesity and physical capacity in older men and women: data from the Nutrition as a Determinant of Successful Aging (NuAge)—the Quebec longitudinal study. Obesity. 2009;17:2082–8. doi: 10.1038/oby.2009.109. [DOI] [PubMed] [Google Scholar]
- Volkert D. The role of nutrition in the prevention of sarcopenia. Wien Med Wochenschr. 2011;161:409–15. doi: 10.1007/s10354-011-0910-x. [DOI] [PubMed] [Google Scholar]
- Cruz-Jentoft AJ, Landi F, Schneider SM, Zúñiga C, Arai H, Boirie Y, et al. 2014. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing;afu115.
- Sakuma K, Yamaguchi A. Sarcopenic obesity and endocrinal adaptation with age. Int J Endocrinol. 2013 doi: 10.1155/2013/204164. 204164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts Instrumental Activities of Daily Living disability in the elderly. Obes Res. 2004;12:1995–2004. doi: 10.1038/oby.2004.250. [DOI] [PubMed] [Google Scholar]
- Rolland Y, Lauwers-Cances V, Cristini C. Janssen I, Morley JE, et al. Difficulties with physical function associated with obesity, sarcopenia, and sarcopenic-obesity in community-dwelling elderly women: the EPIDOS (EPIDemiologie de l'OSteoporose) Study. Am J Clin Nutr. 2009;89:1895–900. doi: 10.3945/ajcn.2008.26950. Kan GA van, [DOI] [PubMed] [Google Scholar]
- Batsis JA, Barre LK, Mackenzie TA, Pratt SI, Lopez-Jimenez F, Bartels SJ. Variation in the prevalence of sarcopenia and sarcopenic obesity in older adults associated with different research definitions: dual-energy X-ray absorptiometry data from the National Health and Nutrition Examination Survey 1999–2004. J Am Geriatr Soc. 2013;61:974–80. doi: 10.1111/jgs.12260. [DOI] [PubMed] [Google Scholar]
- Kirkland JL, Tchkonia T, Pirtskhalava T, Han J, Karagiannides I. Adipogenesis and aging: does aging make fat go MAD? Exp Gerontol. 2002;37:757–67. doi: 10.1016/s0531-5565(02)00014-1. [DOI] [PubMed] [Google Scholar]
- Schrager MA, Metter EJ, Simonsick E, Ble A, Bandinelli S, Lauretani F, et al. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol Bethesda Md 1985. 2007;102:919–25. doi: 10.1152/japplphysiol.00627.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaap LA, Pluijm SMF, Deeg DJH, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119:526.e9–526.e17. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- Marzetti E, Carter CS, Wohlgemuth SE, Lees HA, Giovannini S, Anderson B, et al. Changes in IL-15 expression and death-receptor apoptotic signaling in rat gastrocnemius muscle with aging and life-long calorie restriction. Mech Ageing Dev. 2009;130:272–80. doi: 10.1016/j.mad.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusminski CM, Shetty S, Orci L, Unger RH, Scherer PE. Diabetes and apoptosis: lipotoxicity. Apoptosis. 2009;14:1484–95. doi: 10.1007/s10495-009-0352-8. [DOI] [PubMed] [Google Scholar]
- Van Herpen NA, Schrauwen-Hinderling VB. Lipid accumulation in non-adipose tissue and lipotoxicity. Physiol Behav. 2008;94:231–41. doi: 10.1016/j.physbeh.2007.11.049. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001;86:5755–61. doi: 10.1210/jcem.86.12.8075. [DOI] [PubMed] [Google Scholar]
- Turpin SM, Ryall JG, Southgate R, Darby I, Hevener AL, Febbraio MA, et al. Examination of lipotoxicity in skeletal muscle of high-fat fed and ob/ob mice. J Physiol. 2009;587:1593–605. doi: 10.1113/jphysiol.2008.166033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadi F, Ponsot E. The biology of satellite cells and telomeres in human skeletal muscle: effects of aging and physical activity. Scand. J Med Sci Sports. 2010;20:39–48. doi: 10.1111/j.1600-0838.2009.00966.x. [DOI] [PubMed] [Google Scholar]
- Sepe A, Tchkonia T, Thomou T, Zamboni M, Kirkland JL. Aging and regional differences in fat cell progenitors – a mini-review. Gerontology. 2010;57:66–75. doi: 10.1159/000279755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollheimer LC, Buettner R, Pongratz G, Brunner-Ploss R, Hechtl C, Banas M, et al. Sarcopenia in the aging high-fat fed rat: a pilot study for modeling sarcopenic obesity in rodents. Biogerontology. 2012;13:609–20. doi: 10.1007/s10522-012-9405-4. [DOI] [PubMed] [Google Scholar]
- Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem J. 2012;441:1–21. doi: 10.1042/BJ20110892. [DOI] [PubMed] [Google Scholar]
- Bloor ID, Symonds ME. Sexual dimorphism in white and brown adipose tissue with obesity and inflammation. Horm Behav. 2014;66:95–103. doi: 10.1016/j.yhbeh.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Fellner C, Schick F, Kob R, Hechtl C, Vorbuchner M, Büttner R, et al. Diet-induced and age-related changes in the quadriceps muscle: MRI and MRS in a rat model of sarcopenia. Gerontology. 2014;60:530–38. doi: 10.1159/000360289. [DOI] [PubMed] [Google Scholar]
- Morse WI, Soeldner JS. The non-adipose body mass of obese women: evidence of increased muscularity. Can Med Assoc J. 1964;90:723. [PMC free article] [PubMed] [Google Scholar]
- Clavel S, Coldefy A-S, Kurkdjian E, Salles J, Margaritis I, Derijard B. Atrophy-related ubiquitin ligases, atrogin-1 and MuRF1 are up-regulated in aged rat Tibialis Anterior muscle. Mech Ageing Dev. 2006;127:794–801. doi: 10.1016/j.mad.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Ciciliot S, Schiaffino S. Regeneration of mammalian skeletal muscle: basic mechanisms and clinical implications. Curr Pharm Des. 2010;16:906–14. doi: 10.2174/138161210790883453. [DOI] [PubMed] [Google Scholar]
- Sishi B, Loos B, Ellis B, Smith W, du Toit EF, Engelbrecht A-M. Diet-induced obesity alters signalling pathways and induces atrophy and apoptosis in skeletal muscle in a prediabetic rat model. Exp Physiol. 2011;96:179–93. doi: 10.1113/expphysiol.2010.054189. [DOI] [PubMed] [Google Scholar]
- Nilsson MI, Dobson JP, Greene NP, Wiggs MP, Shimkus KL, Wudeck EV, et al. Abnormal protein turnover and anabolic resistance to exercise in sarcopenic obesity. FASEB J. 2013;27:3905–16. doi: 10.1096/fj.12-224006. [DOI] [PubMed] [Google Scholar]
- Malenfant P, Joanisse DR, Thériault R, Goodpaster BH, Kelley DE, Simoneau J-A. Fat content in individual muscle fibers of lean and obese subjects. Int J Obes. 2001;25:1316–21. doi: 10.1038/sj.ijo.0801733. [DOI] [PubMed] [Google Scholar]
- Gavin TP, Stallings HWS, Zwetsloot KA, Westerkamp LM, Ryan NA, Moore RA, et al. Lower capillary density but no difference in VEGF expression in obese vs. lean young skeletal muscle in humans. J Appl Physiol. 2005;98:315–21. doi: 10.1152/japplphysiol.00353.2004. [DOI] [PubMed] [Google Scholar]
- Casas-Vara A, Santolaria F, Fernández-Bereciartúa A, González-Reimers E, García-Ochoa A, Martínez-Riera A. The obesity paradox in elderly patients with heart failure: analysis of nutritional status. Nutrition. 2012;28:616–22. doi: 10.1016/j.nut.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Kalantar-Zadeh K, Streja E, Kovesdy CP, Oreopoulos A, Noori N, Jing J, et al. The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clin Proc. 2010;85:991–1001. doi: 10.4065/mcp.2010.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbré F, Gomez-Cabrera MC, Nascimento AL, Sanchis-Gomar F, Martinez-Bello VE, Tresguerres JAF, et al. Age associated low mitochondrial biogenesis may be explained by lack of response of PGC-1α to exercise training. AGE. 2012;34:669–79. doi: 10.1007/s11357-011-9264-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson C, Niklasson M, Eriksson E, Björntorp P, Holmäng A. Imprinting of female offspring with testosterone results in insulin resistance and changes in body fat distribution at adult age in rats. J Clin Invest. 1998;101:74. doi: 10.1172/JCI1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Pérez Y, Amengual-Cladera E, Català-Niell A, Thomàs-Moyà E, Gianotti M, Proenza AM, et al. Gender dimorphism in high-fat-diet-induced insulin resistance in skeletal muscle of aged rats. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2008;22:539–48. doi: 10.1159/000185538. [DOI] [PubMed] [Google Scholar]
- Nadal-Casellas A, Proenza AM, Lladó I, Gianotti M. Sex-dependent differences in rat hepatic lipid accumulation and insulin sensitivity in response to diet-induced obesity. Biochem. Cell Biol. 2012;90:164–72. doi: 10.1139/o11-069. [DOI] [PubMed] [Google Scholar]
- Yamada M, Moriguch Y, Mitani T, Aoyama T, Arai H. Age-dependent changes in skeletal muscle mass and visceral fat area in Japanese adults from 40 to 79 years-of-age. Geriatr Gerontol Int. 2014;14:8–14. doi: 10.1111/ggi.12209. [DOI] [PubMed] [Google Scholar]
- Speakman JR, Westerterp KR. Associations between energy demands, physical activity, and body composition in adult humans between 18 and 96 y of age. Am J Clin Nutr. 2010;92:826–34. doi: 10.3945/ajcn.2009.28540. [DOI] [PubMed] [Google Scholar]
- Garvey SM, Dugle JE, Kennedy AD, McDunn JE, Kline W, Guo L, et al. Metabolomic profiling reveals severe skeletal muscle group-specific perturbations of metabolism in aged FBN rats. Biogerontology. 2014;15:217–32. doi: 10.1007/s10522-014-9492-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma K, Watanabe K, Hotta N, Koike T, Ishida K, Katayama K, et al. The adaptive responses in several mediators linked with hypertrophy and atrophy of skeletal muscle after lower limb unloading in humans. Acta Physiol. 2009;197:151–9. doi: 10.1111/j.1748-1716.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- Cohen S, Brault JJ, Gygi SP, Glass DJ, Valenzuela DM, Gartner C, et al. During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. J Cell Biol. 2009;185:1083–95. doi: 10.1083/jcb.200901052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagirand-Cantaloube J, Cornille K, Csibi A, Batonnet-Pichon S, Leibovitch MP, Leibovitch SA. Inhibition of Atrogin-1/MAFbx mediated MyoD proteolysis prevents skeletal muscle atrophy in vivo. PLoS ONE. 2009;4:e4973. doi: 10.1371/journal.pone.0004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwee DT, Baehr LM, Philp A, Baar K, Bodine SC. Maintenance of muscle mass and load-induced growth in Muscle RING Finger 1 null mice with age. Aging Cell. 2014;13:92–101. doi: 10.1111/acel.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christov C, Chretien F, Abou-Khalil R, Bassez G, Vallet G, Authier F-J, et al. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell. 2007;18:1397–409. doi: 10.1091/mbc.E06-08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpin SM, Lancaster GI, Darby I, Febbraio MA, Watt MJ. Apoptosis in skeletal muscle myotubes is induced by ceramides and is positively related to insulin resistance. Am J Physiol Endocrinol Metab. 2006;291:E1341–50. doi: 10.1152/ajpendo.00095.2006. [DOI] [PubMed] [Google Scholar]
- Powers SK, Wiggs MP, Duarte JA, Zergeroglu AM, Demirel HA. Mitochondrial signaling contributes to disuse muscle atrophy. Am J Physiol Endocrinol Metab. 2012;303:E31–9. doi: 10.1152/ajpendo.00609.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirks AJ, Leeuwenburgh C. The role of apoptosis in age-related skeletal muscle atrophy. Sports Med Auckl NZ. 2005;35:473–83. doi: 10.2165/00007256-200535060-00002. [DOI] [PubMed] [Google Scholar]
- Whitman SA, Wacker MJ, Richmond SR, Godard MP. Contributions of the ubiquitin–proteasome pathway and apoptosis to human skeletal muscle wasting with age. Pflüg Arch. 2005;450:437–46. doi: 10.1007/s00424-005-1473-8. [DOI] [PubMed] [Google Scholar]
- Lee J-Y, Lee D-C. Muscle strength and quality are associated with severity of menopausal symptoms in peri- and post-menopausal women. Maturitas. 2013;76:88–94. doi: 10.1016/j.maturitas.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Kallman DA, Plato CC, Tobin JD. The role of muscle loss in the age-related decline of grip strength: cross-sectional and longitudinal perspectives. J Gerontol. 1990;45:M82–8. doi: 10.1093/geronj/45.3.m82. [DOI] [PubMed] [Google Scholar]
- Samson MM, Meeuwsen IB, Crowe A, Dessens JA, Duursma SA, Verhaar HJ. Relationships between physical performance measures, age, height and body weight in healthy adults. Age Ageing. 2000;29:235–42. doi: 10.1093/ageing/29.3.235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item
Supporting info item