Abstract
Insulin-producing β-cells within the pancreatic islet of Langerhans are responsible for maintaining glucose homeostasis; the loss or malfunction of β-cells results in diabetes mellitus. Recent advances in cell purification strategies and sequencing technologies as well as novel molecular tools have revealed that epigenetic modifications and long non-coding RNAs represent an integral part of the transcriptional mechanisms regulating pancreas development and β-cell function. Importantly, these findings have uncovered a new layer of gene regulation in the pancreas that can be exploited to enhance the restoration and/or repair of β-cells to treat diabetes.
Keywords: Pancreas, islet, β-cells, epigenetic, long noncoding RNAs
Pancreas development
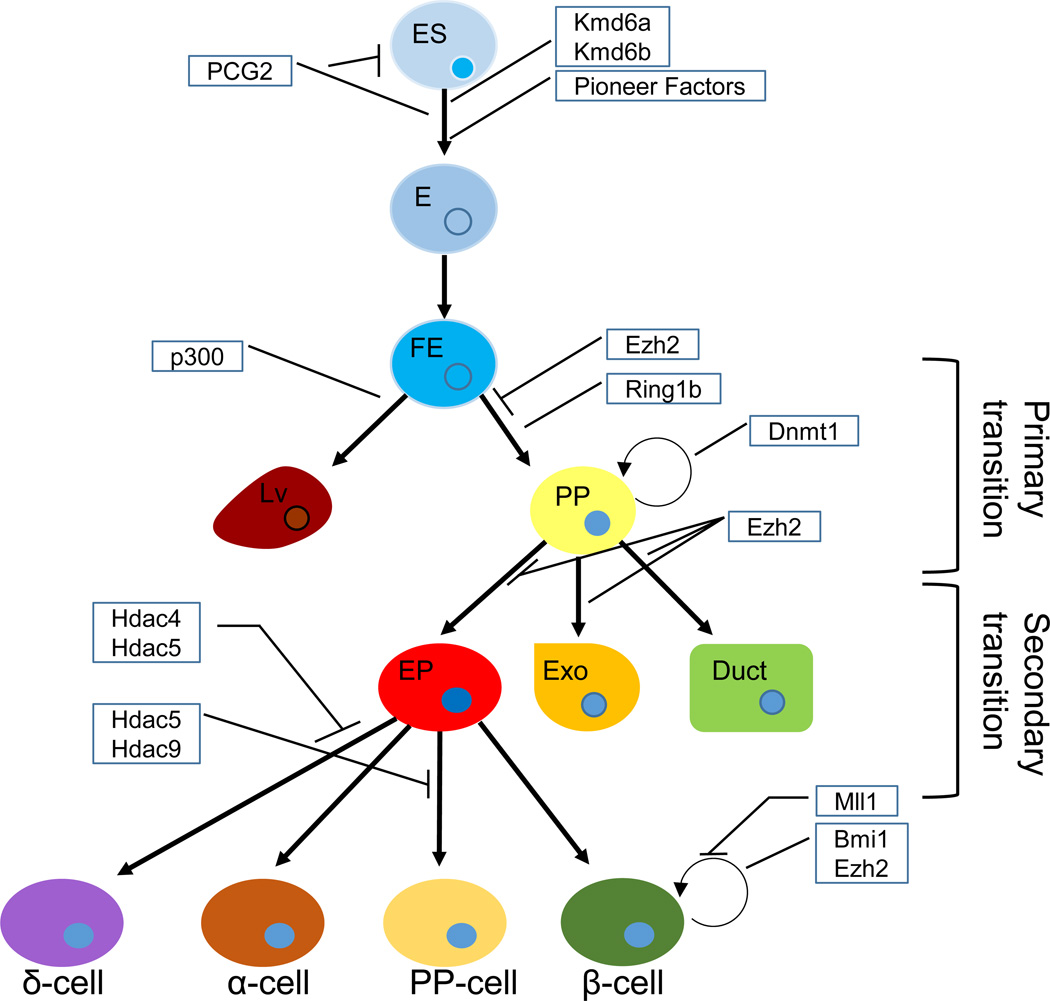
The pancreas is a bifunctional organ with exocrine and endocrine tissues regulating digestive processes and glucose homeostasis, respectively. These functionally distinct tissues arise from a common pancreatic progenitor pool in two major waves of differentiation termed the primary and secondary transitions (Figure 1) [1]. The primary transition refers to the specification of pancreas progenitors within the foregut endoderm and requires the essential transcription factors pancreatic and duodenal homeobox 1 (Pdx1) and pancreatic transcription factor 1a (Ptf1a). The secondary transition refers to the stage where pancreatic progenitors expand, and external signals direct cells toward endocrine versus exocrine fates. A critical phase of pancreatic islet differentiation is the activation of the basic helix-loop-helix transcription factor Neurogenin 3 (Neurog3) within a subset of pancreatic progenitor cells to specify the endocrine precursor pool; deletion of Neurog3 results in the absence of endocrine cell development [2]. In the adult pancreas, there are four endocrine cell types that constitute the mature islet, including insulin-producing β-cells, glucagon-producing α-cells, somatostatin-producing δ-cells and pancreatic polypeptide-producing PP cells. Cell fate determination of these four endocrine cell populations within the Neurog3+ cells depends on a number of additional transcription factors including Pdx1, Nkx2.2, Pax4, Pax6, Isl1, NeuroD1, Arx, and Nkx6.1 (reviewed in [3]). In this review we discuss recent advances in the characterization of epigenetic changes that take place in the specification of the pancreatic endocrine cells, with a special focus on β-cells (summarized in Figure 1). We also discuss how epigenetics can play a role in the etiology and treatment of pancreas-related diseases and speculate on the putative role of long non-coding RNAs in the maturation and function of β-cells. These studies are primarily based on genetic models of murine pancreas development (Table 1), isolated rodent and human islets, and in vitro differentiated embryonic stem (ES) cells.
Figure 1. Epigenetics and pancreas development.
Schematic representation of pancreas development with epigenetic modifiers involved in the process depicted in boxes. (ES) embryonic stem cell, (E) Endoderm, (FE) Foregut endoderm, (Lv) Liver, (PP) pancreas progenitors, (EP) Endocrine progenitors, (Exo) Exocrine cell.
Table 1.
Histone and DNA modifiers involved in pancreas development and phenotypes
| Modifier | Genotype | Experimental conditions |
Phenotype | Ref. |
|---|---|---|---|---|
| Polycomb repressive complex 2 | ||||
| Ezh2 | FoxA3Cre; Ezh2fl/fl | Conditional deletion in foregut endoderm | Ventral pancreatic bud expansion | [19] |
| Pdx1Cre; Ezh2fl/+ | Conditional heterozygous in pancreas progenitors | Increased number of endocrine progenitors and β-cells | [35] | |
| Pdx1Cre; Ezh2fl/fl | Conditional homozygous deletion in pancreas progenitors | Increased β-cells at birth; β-cell proliferation defects | [35] | |
| p48Cre; Ezh2fl/fl | Conditional deletion in pancreas progenitors | Pancreas regeneration defects after cerulein-induced pancreatitis | [43] | |
| RIPCre; Ezh2fl/fl | Conditional deletion in insulin producing cells | Decline in β-cell proliferation | [39] | |
| RIPrtTA; Ezh2OE | Conditional OE of Ezh2 in insulin expressing cells | Combined with TrxG knockdown in isolated islets from old mice increased proliferation of β-cells. | [45] | |
| Polycomb repressive complex 1 | ||||
| Bmi1 | Bmi1−/− | Global knockout | No defects in pancreas development (assessed at 6w); defects in pancreas regeneration after pancreatitis | [42] |
| Bmi1−/− | Global knockout | Severe defects in β-cell proliferation; reduced β-cell mass at 10w | [38] | |
| Bmi1+/− | Global heterozygote | Improved insulin sensitivity with reduced insulin secretion | [114] | |
| Ring1b | Pdx1Cre; Ring1bfl/fl | Conditional homozygous deletion in pancreas progenitors | Impaired glucose tolerance | [44] |
| RipCre; Ring1bfl/fl | Conditional deletion in insulin-producing cells | n/d | [44] | |
| Histone acetyltransferases | ||||
| P300 | p300+/− | Global heterozygote | Expansion of ventral pancreatic bud | [19] |
| Gcn5l2 | FoxA3Cre; Gcn5l2fl/fl | Conditional deletion in foregut endoderm | n/d | [19] |
| Class IIa Histone deacetylases | ||||
| Hdac4 | Hdac4−/− | Global knockout | Increased δ-cells in neonates | [37] |
| Hdac5 | Hdac 5−/− | Global knockout | Increased δ- and β-cells at e18.5 and P7 | [37] |
| Hdac9 | Hdac 9−/− | Global knockout | Increased β-cells at e18.5 and P8 | [37] |
| DNMTs | ||||
| DNMT1 | Pdx1Cre; Dnmt1fl/fl | Conditional deletion in pancreas progenitors | Pancreatic agenesis | [28] |
| RIPCre; Dnmt1fl/fl | Conditional deletion in insulin producing cells | β- to α-cell conversion, glucose intolerance | [47] | |
Epigenetic modifications during pancreas development
Endoderm differentiation
Cellular differentiation requires the establishment and maintenance of tissue specific patterns of gene expression in response to extracellular signaling. As with many developing tissues, epigenetic modifications within endodermal cells are dynamically positioned or removed to regulate gene expression in response to developmental cues. In particular, the promoters of lineage-determining factors are often enriched for epigenetic marks of both active and repressive chromatin (H3K4me3 and H3K27me3, respectively) in a bivalent “poised” state [4], which allows for rapid activation during development. In support of this idea, genomic analyses of endoderm differentiated from hESCs in vitro demonstrates that bivalent promoters are resolved to activate gene expression through depletion of H3K27me3, or repress gene expression through de novo H3K27 methylation [5, 6]. Consistently, H3K27me3 generated by the Polycomb repressive complex 2 (PRC2, see glossary) is necessary for the repression of pluripotency factors in the differentiating hESCs and for the appropriate specification of endoderm lineages [7]. Furthermore, the histone H3K27me2/3 demethylases, KDM6A and KDM6B, are upregulated after endoderm induction in hESCs [8], whereas their knockdown in hESCs significantly dysregulates WNT signaling and reduces the efficiency of endoderm specification [5, 8]. In vivo, definitive endoderm specification relies on extracellular signaling of Nodal/ActivinA via Smad2/3 phosphorylation to activate lineage specific transcription factors (for review see [9]). Based on in vitro differentiation studies, it has been proposed that the resolution of bivalent marks may be due to an interaction between KDM6B and SMAD2/3 causing loss of the H3K27me3 repressive mark in SMAD target genes [6, 10]. Consistently, the promoter of Eomes, a master regulator of endoderm induction [11, 12], is demethylated through the recruitment of Kdm6b and Tbx3 to a regulatory region upstream of the Eomes transcriptional start site (TSS). This facilitates enhancer-promoter association and demethylation of H3K27me3 to promote Eomes expression [13].
In addition to dynamic changes in histone methylation states, a subset of active endodermal genes also has a distinct signature of histone modifications at their enhancers [14]. The most common modification is the deposition of H2A.Z (associated with gene activation), suggesting a mechanism by which the enhancers of lineage-determinant genes are primed to be responsive to endodermal transcription factors. Lineage determination can also be specified by the action of pioneer factors, which modify the chromatin to allow access of other cell-fate specific transcription factors. Endoderm differentiation is known to be dependent on two pioneering transcription factors, Foxa2 and Gata4 (reviewed in [15]). Foxa2 and H2A.Z regulate nucleosome depletion and gene activation of endodermal genes [16], and Gata4 is known to facilitate the acetylation of H3K27 mediated by the histone acetyltransferase p300 [17]. This suggests a potential mechanism by which endoderm specification is initiated by pioneer factors.
These studies and others provide evidence that chromatin remodeling mediated by histone methyltransferases (HMTs), histone demethylases (HDMs) and other histone modifications (Box 1) play essential roles in endoderm specification. However, the timely regulation of chromatin modification, the binding of transcription factors and how these transcription factors are targeted to the appropriate chromatin domain remains elusive.
Box 1. Epigenetic modifications and development.
Cellular differentiation requires the establishment and maintenance of tissue specific patterns of gene expression in response to extracellular signaling to generate the variety of cell types present in an organism. According to the classic model of Waddington (reviewed in [90, 91]) this process is accompanied by epigenetic modifications that shape the structure of the chromatin, restrict differentiation potential and confer cellular specialization [92–94]. In agreement with this model, reprogramming of somatic cells required the depletion of epigenetic marks [95]. In addition, differentiation of ES or induced pluripotent stem (iPS) cells required epigenetic factors [96]. Cumulatively, this indicates the importance of epigenetics in cell fate specification.
Epigenetic modifications are dynamically positioned or removed in response to different developmental cues. The most common types of modifications include 1) methylation of DNA by DNA methyl transferases (DNMTs); 2) post-translational modification of histones, including acetylation (histone acetyltransferases (HATs) and deacetylases (HDACs), histone methylation (histone methyltransferases (HMT) and demethylases (HDM)), and histone ubiquitination (histone ubiquitin ligases); and 3) histone remodeling with the incorporation of histones variants such as H2A.Z and H3.3. ([97, 98].
Primary Transition
Signaling from the adjacent mesoderm induces the lineage-specific transcriptional program that specifies the ventral and dorsal pancreas within two distinct regions of the foregut endoderm. Recently, several in vivo studies have identified some of the key epigenetic changes associated with ventral pancreas induction. Within the prospective ventral pancreas endoderm, early mesodermal signals are believed to induce a bipotential precursor population for pancreas and liver [18]. The default fate of this ventral foregut endoderm region is activation of the pancreatic program; however fibroblast growth factor (FGF) and bone morphogenetic protein (BMP) signaling from the cardiac mesoderm and septum transversum actively block the pancreatic program to promote liver induction. Within this prospective liver domain, enhancer of zeste homolog 2 (Ezh2), a component of PRC2, represses Pdx1 expression to restrain ventral pancreas induction and allow for liver specification [19]. Moreover, a global reduction of p300-dependent histone acetylation, which is associated with gene activation, prevents liver specification and results in the expansion of pancreatic domain. This data suggests that the endoderm is pre-patterned to specify the ventral pancreatic lineage, and that liver specification is facilitated by epigenetic regulation. Interestingly, loss of Ezh2 does not affect dorsal pancreatic bud evagination and growth, suggesting that specification of the dorsal pancreas is independent of Ezh2. Although much is known about the source and identity of the signaling pathways and regulatory factors involved in dorsal pancreas induction [3], relatively little is known about the corresponding epigenetic events. However, in vitro hES cell differentiation studies have demonstrated that commitment to the pancreatic lineage requires stage-specific derepression of PcG-mediated repression through removal of H3K27me3 from the regulatory regions of genes encoding essential pancreatic transcription factors [5].
Once the pancreatic domains have been specified, FGF10 signaling from the mesenchyme promotes the expansion of Pdx1+ pancreatic progenitors [20]. During mitosis, many transcription factors and epigenetic marks are depleted from the chromatin, but cells must retain some memory of cell fate that will be passed to the progeny. Reactivation of the chromatin in a cell-type specific manner has been shown to be maintained by several mechanisms (reviewed in [21]) that include: 1) retention of epigenetic marks, such as H3K9me3 and H3K27me3 [22], recruitment of epigenetic modifiers, such as Ring1b [23], and placement of histone variants such as H2A.Z [24]; 2) the ability of pioneer transcription factor to bind mitotic chromatin, such as Foxa [25] and Gata factors [22], which could potentially explain the pancreatic bud arrest phenotype in the conditional ablation of Gata factors [26, 27]; and 3) by the methylation of DNA by DNMTs. In support of the importance of DNA methylation, pancreas development was greatly abrogated when Dnmt1 was deleted from pancreatic progenitors [28]. In the Dnmt1 mutant mice, pancreatic progenitor cells were arrested at G2/M and underwent apoptosis. Similar results have been reported in zebrafish [29]. These studies demonstrate that DNA methylation is essential for early pancreas development, however it is still not known whether this is due to dysregulation of key pancreatic transcription factors or general genome destabilization. The fact that the Dnmt1 mutant phenotype can be partially rescued by reducing the dosage of the transformation-related protein 53 (Trp53) gene supports the model of global genome instability [28, 29].
Secondary transition: Endocrine specification
Beginning around embryonic day (e) e12.5 in mouse, the pancreatic epithelium undergoes a major wave of cell differentiation. The three lineages of the pancreas, endocrine, exocrine and ductal cells, are allocated in a Notch-dependent manner (Figure 1). First, Notch allows the expansion of the pancreatic progenitor domain by repressing precocious differentiation. Second, a dynamic Notch gradient determines the allocation of the different pancreatic lineages [30]. Studies from many different groups have begun to elucidate the complex signaling pathways involved in these processes (reviewed in [31, 32]). Although it has been shown that the Notch signaling pathway interacts with chromatin modifiers in other tissues and organisms [33], the mechanism by which Notch signaling modifies chromatin structure during pancreas development is unknown and warrants future studies.
Differentiation of endocrine cells requires the expression of the endocrine master regulator Neurog3 by a mechanism that has been proposed to involve lateral inhibition of Notch [34]. Genome-wide ChIP-seq analysis of H3K27me3 in sorted Neurog3+ cells showed increased PRC2-mediated methylation compared to sorted pancreatic progenitors [35]. However, in endocrine cells differentiated from hESCs there appeared to be a global decrease of H3K27me3 [5]. These epigenetic differences between the in vivo and in vitro differentiation of endocrine cells could be due to the presence of different inductive signals in the two systems and/or the use of alternative mechanisms of gene repression. However, both in vitro and in vivo, H3K27me3 marks are lost from the promoters of endocrine-specific transcription factors, suggesting the importance of HMT and HDM in endocrine-specific gene regulation.
It has also been shown that Ezh2 suppresses the normal extent of endocrine cell induction; conditional homozygous and heterozygous deletion of Ezh2 in Pdx1+ pancreatic progenitors results in increased numbers of Neurog3+ cells without changes in proliferation [35]. This suggests that the elevated Neurog3+ cell numbers may be due to increased specification at the expense of other lineages, although this has not been directly tested. Interestingly, conditional ablation of Ezh2 in neuronal progenitor cells also results in the upregulation of Neurog3 and enhanced neuronal differentiation [36], suggesting that a common mechanism exists in neurons and endocrine cells to regulate cellular differentiation.
Endocrine cells
The fundamental role of pancreatic islet endocrine cells is to secrete hormones to maintain glucose homeostasis. The temporal regulation of Neurog3 expression, together with a combination of endocrine specific transcription factors, determines the specification of the different endocrine cell types in the islet. Loss- and gain-of-function studies have shown that HDACs are required for the establishment of the appropriate ratio of β- and δ-cells. Mice lacking Hdac5 and Hdac9 showed an increased β-cell mass and mice lacking Hdac4 and Hdac9 contained more δ cells [37].
β-cell mass has also been shown to be dependent on the levels of Ezh2. When a single allele of Ezh2 is lost, there is augmented β-cell mass and improved glucose clearance in adult mice. However, complete loss of Ezh2 resulted in reduced β-cell mass in the adult and mild hyperglycemia. Similar outcomes were reported in mouse pancreatic explants and in pancreatic cells differentiated from hESCs when chemically treated with inhibitors of Ezh2 [35]. These findings suggest that the Ezh2-mediated repression of endocrine cell fate is conserved between mouse and human and Ezh2 levels could be manipulated in vitro to improve the yield of β-cells. Moreover, mutations in Ezh2 did not appear to affect the other endocrine populations suggesting that this could be a β-cell specific mechanism.
Histone methylation and ubiquitination have also been shown to regulate β-cell proliferation through transcriptional regulation of the Ink4a locus [38, 39]. Two cell cycle inhibitors Cdkn2a and Cdkn2b are encoded within this locus, of which Cdkn2a has been shown to be associated with the decline of β-cell proliferation observed in adult β-cells [40]. Ezh2 and Bmi1, members of the PRC2 and PRC1 complex, respectively, maintain Cdkn2a repression in young β-cells, but their expression is lost in adult β-cells, leading to a reduction of repressive epigenetic marks and transcriptional activation. Loss of Ezh2 expression has been shown to be associated with the age-dependent reduction of extracellular signaling mediated by platelet-derived growth factor (Pdgf) due to decreased expression of the Pdgf receptor [41]. Accordingly, global genetic deletion of either Ezh2 or Bmi1 resulted in reduced β-cell proliferation, decreased β-cell mass and glucose intolerance [38, 39]. Furthermore, re-establishment of Pdgf-mediated signaling in mice and isolated human islets restore Ezh2 expression and β-cell proliferation in adult islets [41]. However, mice with conditional deletion of either Ezh2 or Bmi1 do not display defects in pancreas development, although these mice were unable to recover from chemically-induced pancreatitis [42, 43]. The inconsistency between these studies may be explained by the different ages analyzed, strain background, and/or the subtle nature of the endocrine defects that were observed in the original studies. Another study exploring the role of PRC1 components in mice demonstrated that the loss of Ring1b in pancreatic progenitors did not result in upregulation of Cdkn2a, nor lead to a reduction of β-cell mass. However, deletion of Ring1b did result in the aberrant expression of neural genes and β-cell dysfunction as measured by defects in glucose clearance. Interestingly, deletion of Ring1b in β-cells using the RIP:Cre allele did not result in any observable phenotype, suggesting that once Ring1b repression is established, it is maintained independently of Ring1b. Maintenance of repression was shown to be dependent on H3K9me3 [44]. Although no phenotypes were observed in adult Ring1b mutant mice, it would be interesting to further examine how these animals compensate in more demanding metabolic conditions, such as high fat diet or pregnancy. It is possible that similar defects in β-cell replication in vivo will be observed once the challenged β-cells are induced to proliferate. Alternatively, different components of PRC1 may be required for distinct aspects of β-cell maturation and function.
With aging, not only are the repressive marks mediated by PRC1 and PRC2 lost in β-cells, but there is also an accumulation of H3K4me3 marks at the Ink4a locus causing transcriptional activation of Cdkn2a [38]. This permissive chromatin status of the Ink4a locus appears to be reverted during β-cell regeneration in a mouse model of diabetes [38]. Restoration of Ezh2 activity is sufficient to induce proliferation of β-cells in young mice; however, in adult mice, deletion of Mll1, a member of the Trithorax group (TrxG) complex, is also required [45]. Cumulatively, there is evidence to suggest that β-cell proliferation is regulated by epigenetic modifications in the Ink4a locus mediated by the PRC1, PRC2, and TrxG complexes. The reinforcement of Cdkn2a expression in β-cells, first by losing H3K27m3 and later by accumulating H3K4me3 over time could be a defense mechanism to prevent unusually elevated numbers of β-cells that, in a normal situation, could lead to fatal hypoglycemia. Interestingly, reversibility of this “braking mechanism” could open the door to novel treatments of diseases associated with β-cell deficits, such as diabetes.
DNA methylation in β-cells has also been shown to be essential for the maintenance of β-cell identity. Conditional deletion of Dnmt1 in immature β-cells results in ectopic expression of Arx and the cell fate conversion of β-cells into α-cells, causing glucose intolerance [47]. Furthermore, it has been shown that the homeobox factor Nkx2.2 recruits a repressive complex that includes Hdac1 and Dnmt3a specifically to the Arx promoter in β-cells; disruption of this complex resulted in ectopic Arx expression in β-cells, leading to β- to α-cell conversion and diabetes [48].
Thus far we have discussed how epigenetic changes during pancreas development modify gene expression at discrete loci, however significant advances in sequencing technologies and computational approaches have allowed large-scale integrative analyses of gene expression, chromatin modifications and DNA methylation (Table 2) to generate a global snapshot of the human β-cell epigenome and transcriptome [49–52]. Maps of open chromatin in human islets reveal the existence of regulatory regions at TSSs, as expected, but have also revealed the presence of open chromatin in intergenic regions. Notably, the open chromatin at intergenic regions appears to be more islet specific than at TSSs, suggesting that these distal regulatory regions may confer tissue specific gene expression patterns [50, 51, 53]. These cis-regulatory regions are associated in clusters of open regulatory elements, termed COREs [50], some of which have enhancer characteristics (based on histone modifications) and are enriched for β-cell–related transcription factor sequence recognition motifs [53, 54]. These findings implicate a subset of transcriptionally active chromatin domains in the regulation of gene expression, as is seen in other cell types [55, 56]; but this has not been formally tested in islets. However, many of the SNPs associated with type 2 diabetes map to open chromatin regions outside of coding genes and promoters [51–53, 57], suggesting that these regions are important for β-cell function (Box 2). Genome-wide profiles of histone modifications in hESC-derived β-cells also suggest that inappropriate chromatin remodeling during the in vitro differentiation process may contribute to the impaired metabolic functions associated with these β-like cells [5].
Table 2.
Datasets available for the study of pancreas development and β-cell function.
| ES/iPS | FE | PP | EP | β-cells | α- cells |
ε-cells | Islet | T2D | Exo | Accession numbers |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| ChIP-Seq | |||||||||||
| H3K4me1 | hES [57] | H [51–53] | (57) MTAB-1990 (51) GEOD-23784 (52) MTAB-189/191 (53) MTAB-1919 |
||||||||
| H3K4me2 | H [52] | (52) MTAB-189/191 | |||||||||
| H3K4me3 | M [67] hES[5] |
M [67] hES[5] |
H [64] M [67] hES[5] |
H [64] | H [49, 51, 52, 115] M [67] hES[5] |
H [64] | (67) MTAB-906/877 (5) MTAB-1086 (64) GEOD-50386 (49) MTAB-1294 (115) GEOD-51310/51311/51312 |
||||
| H3K36me3 | H [49, 115] | M [67] | |||||||||
| H3K27me3 | M[67] hES[5] |
M[35] hES[5] |
M[35, 67] hES[5] |
M[35] | H[64] M[67] hES[5] |
H[64] | H[52] M[54, 67] hES[5] |
H[64] M[67] |
(35) GEOD-56617 (54) GEOD-30298 |
||
| H3K27ac | H[53, 115] | ||||||||||
| H3K9me3 | M [54] | ||||||||||
| H3K79me2 | H[51] | ||||||||||
| H2A.Z | H[53] | ||||||||||
| RNApolII | H[49] | ||||||||||
| CTCF | H[51, 53] | ||||||||||
| Ring1b | M[44] | M[44] | (44) MTAB-1402 | ||||||||
| Islet TF | hES[57] | H[53, 116] M[54, 116] |
(116) MTAB-1143 | ||||||||
| DNA methylation | H[117] | H[109–112, 117] | H[109–111] | (109) GEOD-21232 (110) journal access (111) GEOD-38642 (112) GEOD-62640 (117) GEOD-52238 |
|||||||
| RNA-seq | M[67] | M[67] | H[49, 64, 118] M[49, 67, 119] |
H[64, 118] M[119] |
M[120] | H[49, 115, 121] M [77] |
H[121] | H[49, 64, 118] | (118) MTAB-463/465 (119) GEOD-54973 (121) GEOD-50398 (77) SRA056174 |
||
| DNase I | H[51] | ||||||||||
| FAIRE | H[50] | ||||||||||
(ES) embryonic stem cell, (iPS) induced pluripotent stem cells, (FE) Foregut endoderm, (PP) pancreas progenitors, (EP) Endocrine progenitors, (T2D) Type 2 diabetic islets, (Exo) Exocrine cell, (hES) Human embryonic stem cells, (H) Human, (M) Mouse. Accession numbers to dataset repositories at the European Bioinformatic Institute or the DNA Data Bank of Japan are indicated with each corresponding reference and only referred to once.
Box 2. Type 2 diabetes, epigenetics and long non coding RNAs.
Type 2 diabetes (T2D) results from the loss of glucose homeostasis due to insulin resistance and/or beta cell dysfunction associated with genetic factors, lifestyle and aging. Genome wide association studies have identified over 70 loci that are robustly associated with T2D risk [99]; however, this can only explain about 10–15% of T2D cases. Interestingly, most of the causal SNPs fall outside of coding genes, suggesting that epigenetics and non-coding sequences, including regulatory regions and ncRNAs, are important for the pathophysiology of T2D. In support of this idea, studies in human populations affected by periods of famine showed that children born under starvation conditions were at higher risk of poor metabolic control [100] and T2D [101] later in life, suggesting a role for heritable factors that are independent of DNA sequence. Additional studies showed defects in DNA methylation at the IGF2 locus [102], however these changes were measured in blood cells and do not directly address the presence of epigenetic modifications specific to the islet. Animal models of prenatal under-nutrition showed that rats exposed to intrauterine growth retardation (IUGR) develop T2D in the adulthood [103] and this phenomenon correlated with missexpression of the essential islet transcription factor Pdx1 as a result of epigenetic modifications in its promoter [104], similar to what it is observed in islets from T2D patients [105]. Also, progeny from rats fed a low protein diet during pregnancy and lactation showed epigenetic modifications at the Hnf4α locus in islet-derived DNA that prevents enhancer-promoter interaction and increases the risk of T2D [106]. Moreover, mice exposed to high fat diet showed differences in islet DNA methylation in loci associated with T2D [107]. Isolated human islets exposed to long chain saturated fatty acids also showed global changes in DNA methylation and gene expression, including in some genes which were dysregulated in islets from T2D subjects [108]. These examples suggest that the environment can affect gene expression through epigenetics, and could represent potential targets for drug therapy to prevent or revert diabetic pathologies. Recently, the scientific community has begun to perform integrative analysis of allelic variance traits associated with T2D using global DNA methylation and gene expression analysis from islets isolated from T2D and non-diabetic donors [109–112]. With this approach, several candidate loci have been identified in pathways related to beta cell function and T2D to warrant future detailed functional studies.
The role of lncRNAs in T2D has just begun to be appreciated, as discussed in the main text, but the potential implications are exciting. As proof of principle, it has been recently shown that MEG3, a lncRNA that contains multiple microRNAs, is hypermethylated and dowregulated in islets from T2D donors. Interestingly, some of the mRNAs targeted by these MEG3 microRNAs are aberrantly overexpressed in T2D suggesting that MEG3 may be relevant in regulating beta cell function [113]. Furthermore, the lncRNA HI-LNC25 was identified in isolated islets and shown to regulate the expression of GLIS3, a transcription factor that contains T2D risk variants [49].
Although, we are still in the early days of understanding how epigenetics and lncRNAs modulate the function of β-cells, there is much evidence to suggest that chromatin modifications, chromatin structure and ncRNAs could be an integral part of gene regulation with the potential to be targets of novel therapies designed to treat T2D.
Dedifferentiation and trans-differentiation
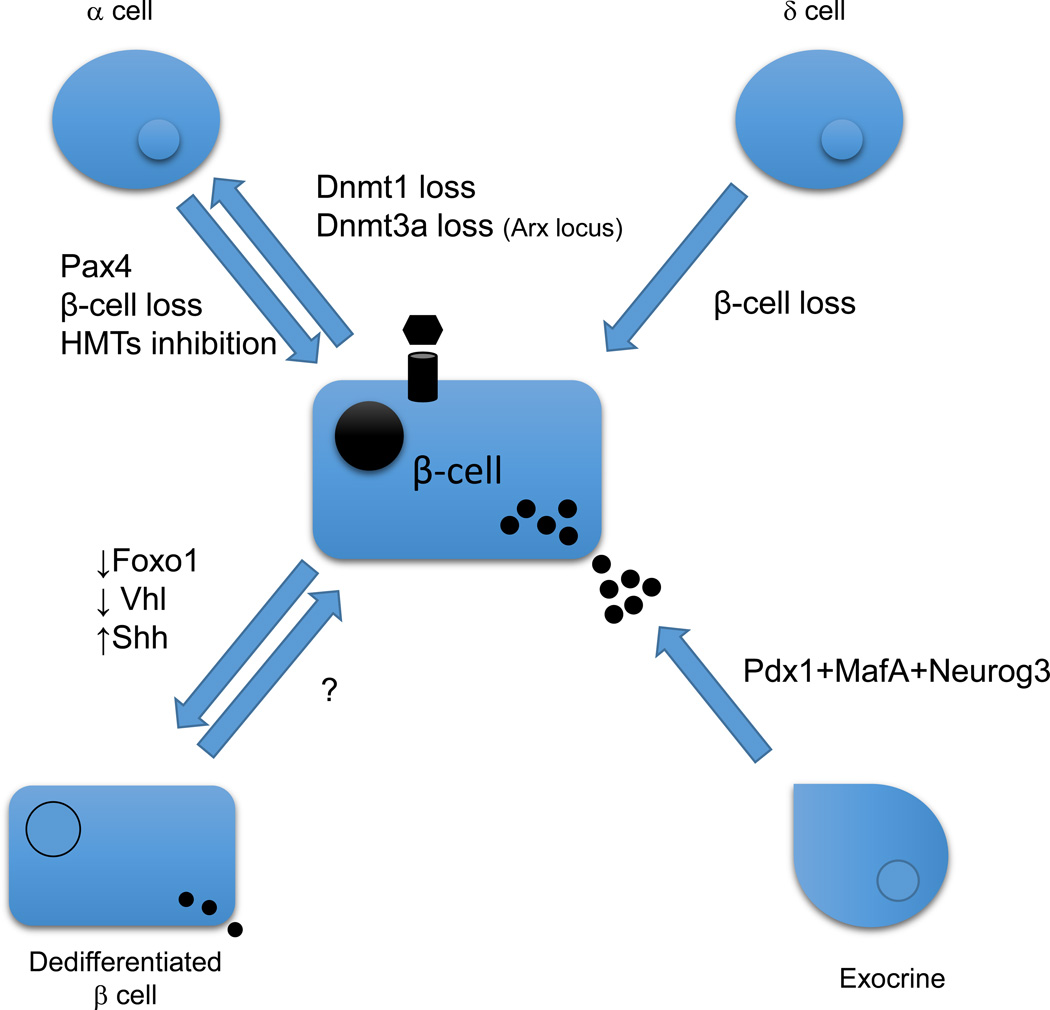
Recently, two novel concepts related to cell plasticity, dedifferentiation and transdifferentiation, have influenced the β-cell biology field. The genetic or environmental origins of these two processes are represented in Figure 2. Dedifferentiation has been described in several genetic mouse models, including conditional deletion of von Hippel-Lindau (Vhl) [58] and Foxo genes [59], and the genetic reactivation of Shh signaling in β-cells [60]. In these conditions, β-cells appear to revert to a more dedifferentiated progenitor program of gene expression. Based on these findings and complementary data from mouse models of type 2 diabetes, it has been proposed that dedifferentiation may be a mechanism employed by the β-cell to elude cell death in conditions of glycemic stress. Furthermore, there is speculation that the dedifferentiated state is also associated with a more plastic epigenetic map, which would explain the increased cell fate changes observed in diabetic or stressed β-cells [59]. If this is true, epigenetic marks may represent new targets for treating type 2 diabetes. Furthermore, β-cells in a dedifferentiated state may be more receptive to induced proliferation that could restore euglycemia in diabetic patients.
Figure 2. De-differentiation and trans-differentiation of β-cells.
Recent examples of environmental and genetic factors that cause β-cell dedifferentiation and transdifferentiation.
Transdifferentiation has also emerged as a novel mechanism to generate new β-cells. Several studies have demonstrated the ability to reprogram cells within pancreas, intestine, liver and other tissues through the mis-expression of pancreatic transcription factors and/or modification of epigenetic marks. For example, overexpression of MafA, Pdx1 and Neurog3 in exocrine cells [61], Pdx1 overexpression in liver cells [62], and Pax4 overexpression in α-cells [63] converts the respective cell types into β-like cells. Cell fate conversion of α- to β-cells in vitro has also been demonstrated by inhibiting HMTs in isolated human islets [64]. Moreover, near complete ablation of β-cells with diphtheria toxin induces transdifferentiation of α-and δ-cells into β-cells, suggesting that circulating factors or paracrine signals can instruct the differentiation [65, 66]. Cell fate conversions have mostly been demonstrated between cell types that share developmental programs and common epigenetic characteristics [64, 67]. Interestingly, most of the trans-differentiation events occurred only in subpopulations of cells, suggesting that not every cell is responsive to the conversion stimuli and that some cells may retain a more permissive epigenetic state. Similar arguments are being considered to explain the low efficiency of iPS generation with the Yamanaka factors [68].
Long non-coding RNAs and pancreas development
Over the last decade, genome-wide analyses have revealed that most of the human genome is transcribed, including a large number of long non-coding RNAs (lncRNAs) longer than 200 nucleotides and with no protein-coding potential [69–71]. It is important to note that lncRNAs represents a broad definition of ncRNAs and includes several classes of functional RNAs, including enhancer RNAs (eRNAs) and intergenic-, sense- and antisense- lncRNAs. Although it is not clear what fraction of the currently annotated lncRNAs are functional, there is increasing evidence of the involvement of lncRNAs in cell biology and human disease (see [72, 73] for review). Among the diverse cellular functions associated with lncRNAs, a recurrent theme is their ability to interact and recruit chromatin modifiers including histone modifiers and DNMTs [74]. However, despite the identification of increased numbers of lncRNAs, there is still very little evidence of their function in pancreas development and/or β-cell function [75, 76]. One reason that could account for this lack of functional information is the fact that lncRNA expression is extremely tissue- and stage-specific. Thus, their functional characterization will require tissue-specific analysis at different stages of development. Several recent reports have uncovered the existence of lncRNAs in the pancreatic islet. An integrative analysis of epigenetic modifications and gene expression identified many lncRNAs enriched in the islet of Langerhans [49]. This study showed that islet-enriched lncRNAs were conserved in mouse and human and developmentally regulated. Furthermore, it demonstrated that some lncRNAs were dysregulated in islets from type 2 diabetic patients. Another group reached similar conclusions by performing transcriptome reconstruction of isolated β-cells and mouse islets [77], and another study identified lncRNAs specific for β- and α-cells in human islets [64]. The potential importance of these islet-specific lncRNAs has been supported by genome-wide association studies (GWAS) to identify genetic variants underlying susceptibility to type 2 diabetes and metabolic disorders [78, 79]. Interestingly, although a few SNP candidates map to coding genes, a large proportion of the SNPs fall into non-coding regions, some of which are close to or within islet-specific lncRNAs [49] and cis-regulatory elements [53]. Overall, these studies suggest that lncRNAs may be part of the regulatory network of gene expression that regulates β-cell development and function; however, detailed functional analysis will be needed to begin to understand the biology of these novel non-coding genes.
Several of the known functions of lncRNAs may also be relevant to pancreas biology. For example, mammalian chromatin modifiers lack site-specific DNA response elements, and targeting of these complexes has been shown to be mediated by lncRNAs. Consistent with this, recent reports demonstrate that PRC2 can be targeted to nascent transcripts independent of the nucleotide sequence [80, 81]. Given that some enhancers can produce eRNAs, it is possible that histone modifiers are recruited to islet-specific eRNAs to regulate β-cell maturation and function. In addition, lncRNAs might play a role in the recruitment of cohesins and the stabilization of enhancer-promoter interactions [82–84]. Moreover, clusters of open chromatin elements and chromatin looping have been described in islets, and it is possible that lncRNAs are also involved in this process [85]. These are just some examples among many of how lncRNAs may regulate islet specific gene expression [86]. Overall, lncRNAs have been shown be an integral part of gene regulation in many cellular systems, but their role in pancreas development and β-cell function has just recently begun to be appreciated. It is likely that in the near future, novel lncRNAs will be functionally characterized to further elucidate their regulation of pancreas development and β-cell function.
Concluding Remarks
Past studies of pancreas development and β-cell function have largely focused on coding genes. In recent years, great advances in cell purification strategies, in vitro differentiation of β-cells from hESCs, and sequencing technologies have provided the scientific community with large datasets of islet-specific transcription factor binding sites, epigenetic modifications and gene expression profiles during different time points of mouse and human pancreas development (summarized in Table 2). The integrated analyses of these datasets have uncovered the existence of chromatin domains and lncRNAs with mostly unknown functions, but with potential major implications for β-cell development and/or function (Box2). Our current knowledge of pancreas development, the numerous genetic tools available [87] and the capacity to reproduce pancreas development in vitro [88, 89] warrant future studies exploring how chromatin structure, discrete chromatin transcriptional domains, and lncRNAs can be related to β-cell fate specification and function.
Highlights.
Epigenetic modifications are required for the appropriate specification β-cells
A global epigenome snapshot of the islet has been generated
Islet-specific long non-coding RNAs have been identified
Table compiling available Chip-Seq and RNA-seq datasets in the pancreas
Acknowledgements
We apologize to the authors whose research and/or original publications could not be cited or discussed due to space limitations.
We would like to thank members of the Sussel lab for useful discussion and critical evaluation of the manuscript. Support was received from the NIH U01 DK087711 and R01 DK082590 (L.S.), Foundation for Diabetes Research (L.S.), the Russell Berrie Foundation (L.A.) and Juvenile Diabetes Foundation (L.A.).
Glossary
- DNMTs
DNA methyltransferases. Catalyze the methylation of DNA, which, in mammals, occurs mainly on cytosines in CpG dinucleotides.
- EZH2
Enhancer of zeste 2. A histone methyltransferase member of the polycomb repressive complex 2 that mediates the methylation of histone 3 on lysine 4 (H3K4me3); associated with transcriptional repression.
- HATs
Histone acetyltransferases. Acetylate lysine residues of histones. Neutralize the negative charge of DNA relaxing the chromatin structure and promoting transcription.
- HDACs
Histone deacetylases. Remove acetyl groups from lysine residues on histones, enabling chromatin to compact and causing transcriptional repression.
- HDMs
Histone demethylases. Remove methyl groups from histones.
- HMTs
Histone methyltransferases. Add methyl groups to lysine or arginine residues of histones.
- KDM6
Family of histone demethylases that includes KDM6a and KDM6b. Remove methyl groups from lysine 27 di- and tri-methylated on histone 3 (H3K27me2/3)
- PRC2
Polycomb repressive complex 2. Complex of proteins with histone methyltranferase activity. Primarily trimethylates histone 3 on lysine K27 (H3K27me3) and represses transcription.
- PRC1
Polycomb repressive complex 1. Monoubiquitylates histone H2A on lysine 119 (H2AK119ub1) and represses transcription.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pictet R, Rutter WJ. Development of the embryonic endocrine pancreas. In: Steiner DF, Frenkel N, editors. Handbook of physiology. Vol. 1. Washington, DC: Williams and Wilkins; 1972. pp. 25–66. [Google Scholar]
- 2.Gradwohl G, et al. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci U S A. 2000;97(4):1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan FC, Wright C. Pancreas organogenesis: from bud to plexus to gland. Dev Dyn. 2011;240(3):530–565. doi: 10.1002/dvdy.22584. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein BE, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125(2):315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 5.Xie R, et al. Dynamic chromatin remodeling mediated by polycomb proteins orchestrates pancreatic differentiation of human embryonic stem cells. Cell Stem Cell. 2013;12(2):224–237. doi: 10.1016/j.stem.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim SW, et al. Chromatin and transcriptional signatures for Nodal signaling during endoderm formation in hESCs. Dev Biol. 2011;357(2):492–504. doi: 10.1016/j.ydbio.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Margueron R, Reinberg D. The Polycomb complex PRC2 and its mark in life. Nature. 2011;469(7330):343–349. doi: 10.1038/nature09784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang W, Wang J, Zhang Y. Histone H3K27me3 demethylases KDM6A and KDM6B modulate definitive endoderm differentiation from human ESCs by regulating WNT signaling pathway. Cell Res. 2013;23(1):122–130. doi: 10.1038/cr.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCracken KW, Wells JM. Molecular pathways controlling pancreas induction. Semin Cell Dev Biol. 2012;23(6):656–662. doi: 10.1016/j.semcdb.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahle O, Kumar A, Kuehn MR. Nodal signaling recruits the histone demethylase Jmjd3 to counteract polycomb-mediated repression at target genes. Sci Signal. 2010;3(127):ra48. doi: 10.1126/scisignal.2000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teo AK, et al. Pluripotency factors regulate definitive endoderm specification through eomesodermin. Genes Dev. 2011;25(3):238–250. doi: 10.1101/gad.607311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnold SJ, et al. Pivotal roles for eomesodermin during axis formation, epithelium-to-mesenchyme transition and endoderm specification in the mouse. Development. 2008;135(3):501–511. doi: 10.1242/dev.014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kartikasari AE, et al. The histone demethylase Jmjd3 sequentially associates with the transcription factors Tbx3 and Eomes to drive endoderm differentiation. EMBO J. 2013;32(10):1393–1408. doi: 10.1038/emboj.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loh KM, et al. Efficient endoderm induction from human pluripotent stem cells by logically directing signals controlling lineage bifurcations. Cell Stem Cell. 2014;14(2):237–252. doi: 10.1016/j.stem.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaret KS, Carroll JS. Pioneer transcription factors: establishing competence for gene expression. Genes Dev. 2011;25(21):2227–2241. doi: 10.1101/gad.176826.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, et al. Foxa2 and H2A.Z mediate nucleosome depletion during embryonic stem cell differentiation. Cell. 2012;151(7):1608–1616. doi: 10.1016/j.cell.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He A, et al. Dynamic GATA4 enhancers shape the chromatin landscape central to heart development and disease. Nat Commun. 2014;5:4907. doi: 10.1038/ncomms5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deutsch G, et al. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development. 2001;128(6):871–881. doi: 10.1242/dev.128.6.871. [DOI] [PubMed] [Google Scholar]
- 19.Xu CR, et al. Chromatin "prepattern" and histone modifiers in a fate choice for liver and pancreas. Science. 2011;332(6032):963–966. doi: 10.1126/science.1202845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobberup S, et al. Conditional control of the differentiation competence of pancreatic endocrine and ductal cells by Fgf10. Mech Dev. 2010;127(3–4):220–234. doi: 10.1016/j.mod.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kadauke S, Blobel GA. Mitotic bookmarking by transcription factors. Epigenetics Chromatin. 2013;6(1):6. doi: 10.1186/1756-8935-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kadauke S, et al. Tissue-specific mitotic bookmarking by hematopoietic transcription factor GATA1. Cell. 2012;150(4):725–737. doi: 10.1016/j.cell.2012.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Follmer NE, Wani AH, Francis NJ. A polycomb group protein is retained at specific sites on chromatin in mitosis. PLoS Genet. 2012;8(12):e1003135. doi: 10.1371/journal.pgen.1003135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly TK, et al. H2A.Z maintenance during mitosis reveals nucleosome shifting on mitotically silenced genes. Mol Cell. 2010;39(6):901–911. doi: 10.1016/j.molcel.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caravaca JM, et al. Bookmarking by specific and nonspecific binding of FoxA1 pioneer factor to mitotic chromosomes. Genes Dev. 2013;27(3):251–260. doi: 10.1101/gad.206458.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xuan S, et al. Pancreas-specific deletion of mouse Gata4 and Gata6 causes pancreatic agenesis. J Clin Invest. 2012;122(10):3516–3528. doi: 10.1172/JCI63352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carrasco M, et al. GATA4 and GATA6 control mouse pancreas organogenesis. J Clin Invest. 2012;122(10):3504–3515. doi: 10.1172/JCI63240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Georgia S, Kanji M, Bhushan A. DNMT1 represses p53 to maintain progenitor cell survival during pancreatic organogenesis. Genes Dev. 2013;27(4):372–377. doi: 10.1101/gad.207001.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson RM, et al. Loss of Dnmt1 catalytic activity reveals multiple roles for DNA methylation during pancreas development and regeneration. Dev Biol. 2009;334(1):213–223. doi: 10.1016/j.ydbio.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Afelik S, Jensen J. Notch signaling in the pancreas: patterning and cell fate specification. Wiley Interdiscip Rev Dev Biol. 2013;2(4):531–544. doi: 10.1002/wdev.99. [DOI] [PubMed] [Google Scholar]
- 31.Serup P. Signaling pathways regulating murine pancreatic development. Semin Cell Dev Biol. 2012;23(6):663–672. doi: 10.1016/j.semcdb.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Shih HP, Wang A, Sander M. Pancreas organogenesis: from lineage determination to morphogenesis. Annu Rev Cell Dev Biol. 2013;29:81–105. doi: 10.1146/annurev-cellbio-101512-122405. [DOI] [PubMed] [Google Scholar]
- 33.Schwanbeck R. The Role of Epigenetic Mechanisms in Notch Signaling During Development. J Cell Physiol. 2014 doi: 10.1002/jcp.24851. [DOI] [PubMed] [Google Scholar]
- 34.Magenheim J, et al. Ngn3(+) endocrine progenitor cells control the fate and morphogenesis of pancreatic ductal epithelium. Dev Biol. 2011;359(1):26–36. doi: 10.1016/j.ydbio.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu CR, et al. Dynamics of genomic H3K27me3 domains and role of EZH2 during pancreatic endocrine specification. EMBO J. 2014 doi: 10.15252/embj.201488671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirabayashi Y, et al. Polycomb limits the neurogenic competence of neural precursor cells to promote astrogenic fate transition. Neuron. 2009;63(5):600–613. doi: 10.1016/j.neuron.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 37.Lenoir O, et al. Specific control of pancreatic endocrine beta- and delta-cell mass by class IIa histone deacetylases HDAC4, HDAC5, and HDAC9. Diabetes. 2011;60(11):2861–2871. doi: 10.2337/db11-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dhawan S, Tschen SI, Bhushan A. Bmi-1 regulates the Ink4a/Arf locus to control pancreatic beta-cell proliferation. Genes Dev. 2009;23(8):906–911. doi: 10.1101/gad.1742609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen H, et al. Polycomb protein Ezh2 regulates pancreatic beta-cell Ink4a/Arf expression and regeneration in diabetes mellitus. Genes Dev. 2009;23(8):975–985. doi: 10.1101/gad.1742509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krishnamurthy J, et al. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature. 2006;443(7110):453–457. doi: 10.1038/nature05092. [DOI] [PubMed] [Google Scholar]
- 41.Chen H, et al. PDGF signalling controls age-dependent proliferation in pancreatic beta-cells. Nature. 2011;478(7369):349–355. doi: 10.1038/nature10502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fukuda A, Morris JPt, Hebrok M. Bmi1 is required for regeneration of the exocrine pancreas in mice. Gastroenterology. 2012;143(3):821–831. doi: 10.1053/j.gastro.2012.05.009. e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mallen-St Clair J, et al. EZH2 couples pancreatic regeneration to neoplastic progression. Genes Dev. 2012;26(5):439–444. doi: 10.1101/gad.181800.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Arensbergen J, et al. Ring1b bookmarks genes in pancreatic embryonic progenitors for repression in adult beta cells. Genes Dev. 2013;27(1):52–63. doi: 10.1101/gad.206094.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou JX, et al. Combined modulation of polycomb and trithorax genes rejuvenates beta cell replication. J Clin Invest. 2013;123(11):4849–4858. doi: 10.1172/JCI69468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia-Ocana A, Stewart AF. "RAS"ling beta cells to proliferate for diabetes: why do we need MEN? J Clin Invest. 2014;124(9):3698–3700. doi: 10.1172/JCI77764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dhawan S, et al. Pancreatic beta cell identity is maintained by DNA methylation-mediated repression of Arx. Dev Cell. 2011;20(4):419–429. doi: 10.1016/j.devcel.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Papizan JB, et al. Nkx2.2 repressor complex regulates islet beta-cell specification and prevents beta-to-alpha-cell reprogramming. Genes Dev. 2011;25(21):2291–2305. doi: 10.1101/gad.173039.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moran I, et al. Human beta cell transcriptome analysis uncovers lncRNAs that are tissue-specific, dynamically regulated, and abnormally expressed in type 2 diabetes. Cell Metab. 2012;16(4):435–448. doi: 10.1016/j.cmet.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gaulton KJ, et al. A map of open chromatin in human pancreatic islets. Nat Genet. 2010;42(3):255–259. doi: 10.1038/ng.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stitzel ML, et al. Global epigenomic analysis of primary human pancreatic islets provides insights into type 2 diabetes susceptibility loci. Cell Metab. 2010;12(5):443–455. doi: 10.1016/j.cmet.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhandare R, et al. Genome-wide analysis of histone modifications in human pancreatic islets. Genome Res. 2010;20(4):428–433. doi: 10.1101/gr.102038.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pasquali L, et al. Pancreatic islet enhancer clusters enriched in type 2 diabetes risk-associated variants. Nat Genet. 2014;46(2):136–143. doi: 10.1038/ng.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tennant BR, et al. Identification and analysis of murine pancreatic islet enhancers. Diabetologia. 2013;56(3):542–552. doi: 10.1007/s00125-012-2797-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dowen JM, et al. Control of cell identity genes occurs in insulated neighborhoods in Mammalian chromosomes. Cell. 2014;159(2):374–387. doi: 10.1016/j.cell.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y, et al. Chromatin connectivity maps reveal dynamic promoter-enhancer long-range associations. Nature. 2013;504(7479):306–310. doi: 10.1038/nature12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weedon MN, et al. Recessive mutations in a distal PTF1A enhancer cause isolated pancreatic agenesis. Nat Genet. 2014;46(1):61–64. doi: 10.1038/ng.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Puri S, Akiyama H, Hebrok M. VHL-mediated disruption of Sox9 activity compromises beta-cell identity and results in diabetes mellitus. Genes Dev. 2013;27(23):2563–2575. doi: 10.1101/gad.227785.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Talchai C, et al. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell. 2012;150(6):1223–1234. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Landsman L, Parent A, Hebrok M. Elevated Hedgehog/Gli signaling causes beta-cell dedifferentiation in mice. Proc Natl Acad Sci U S A. 2011;108(41):17010–17015. doi: 10.1073/pnas.1105404108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou Q, et al. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455(7213):627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferber S, et al. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med. 2000;6(5):568–572. doi: 10.1038/75050. [DOI] [PubMed] [Google Scholar]
- 63.Collombat P, et al. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell. 2009;138(3):449–462. doi: 10.1016/j.cell.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bramswig NC, et al. Epigenomic plasticity enables human pancreatic alpha to beta cell reprogramming. J Clin Invest. 2013;123(3):1275–1284. doi: 10.1172/JCI66514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thorel F, et al. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464(7292):1149–1154. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chera S, et al. Diabetes recovery by age-dependent conversion of pancreatic delta-cells into insulin producers. Nature. 2014;514(7523):503–507. doi: 10.1038/nature13633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Arensbergen J, et al. Derepression of Polycomb targets during pancreatic organogenesis allows insulin-producing beta-cells to adopt a neural gene activity program. Genome Res. 2010;20(6):722–732. doi: 10.1101/gr.101709.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Watanabe A, Yamada Y, Yamanaka S. Epigenetic regulation in pluripotent stem cells: a key to breaking the epigenetic barrier. Philos Trans R Soc Lond B Biol Sci. 2013;368(1609):20120292. doi: 10.1098/rstb.2012.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cabili MN, et al. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011;25(18):1915–1927. doi: 10.1101/gad.17446611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carninci P, et al. The transcriptional landscape of the mammalian genome. Science. 2005;309(5740):1559–1563. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 71.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136(4):629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 72.Wahlestedt C. Targeting long non-coding RNA to therapeutically upregulate gene expression. Nat Rev Drug Discov. 2013;12(6):433–446. doi: 10.1038/nrd4018. [DOI] [PubMed] [Google Scholar]
- 73.Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marchese FP, Huarte M. Long non-coding RNAs and chromatin modifiers: their place in the epigenetic code. Epigenetics. 2014;9(1):21–26. doi: 10.4161/epi.27472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pullen TJ, Rutter GA. Roles of lncRNAs in pancreatic beta cell identity and diabetes susceptibility. Front Genet. 2014;5:193. doi: 10.3389/fgene.2014.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kameswaran V, Kaestner KH. The Missing lnc(RNA) between the pancreatic beta-cell and diabetes. Front Genet. 2014;5:200. doi: 10.3389/fgene.2014.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ku GM, et al. Research resource: RNA-Seq reveals unique features of the pancreatic beta-cell transcriptome. Mol Endocrinol. 2012;26(10):1783–1792. doi: 10.1210/me.2012-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Scott RA, et al. Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat Genet. 2012;44(9):991–1005. doi: 10.1038/ng.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morris AP, et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet. 2012;44(9):981–990. doi: 10.1038/ng.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Davidovich C, et al. Promiscuous RNA binding by Polycomb repressive complex 2. Nat Struct Mol Biol. 2013;20(11):1250–1257. doi: 10.1038/nsmb.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kaneko S, et al. PRC2 binds active promoters and contacts nascent RNAs in embryonic stem cells. Nat Struct Mol Biol. 2013;20(11):1258–1264. doi: 10.1038/nsmb.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Orom UA, et al. Long noncoding RNAs with enhancer-like function in human cells. Cell. 2010;143(1):46–58. doi: 10.1016/j.cell.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim TK, et al. Widespread transcription at neuronal activity-regulated enhancers. Nature. 2010;465(7295):182–187. doi: 10.1038/nature09033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li W, et al. Functional roles of enhancer RNAs for oestrogen-dependent transcriptional activation. Nature. 2013;498(7455):516–520. doi: 10.1038/nature12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hacisuleyman E, et al. Topological organization of multichromosomal regions by the long intergenic noncoding RNA Firre. Nat Struct Mol Biol. 2014;21(2):198–206. doi: 10.1038/nsmb.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kretz M, et al. Control of somatic tissue differentiation by the long non-coding RNA TINCR. Nature. 2013;493(7431):231–235. doi: 10.1038/nature11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Magnuson MA, Osipovich AB. Pancreas-specific Cre driver lines and considerations for their prudent use. Cell Metab. 2013;18(1):9–20. doi: 10.1016/j.cmet.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kroon E, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 89.Pagliuca FW, et al. Generation of functional human pancreatic beta cells in vitro. Cell. 2014;159(2):428–439. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Holliday R. Epigenetics: a historical overview. Epigenetics. 2006;1(2):76–80. doi: 10.4161/epi.1.2.2762. [DOI] [PubMed] [Google Scholar]
- 91.Felsenfeld G. A brief history of epigenetics. Cold Spring Harb Perspect Biol. 2014;6(1) doi: 10.1101/cshperspect.a018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 2007;128(4):635–638. doi: 10.1016/j.cell.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 93.Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447(7143):425–432. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- 94.Zhu J, et al. Genome-wide chromatin state transitions associated with developmental and environmental cues. Cell. 2013;152(3):642–654. doi: 10.1016/j.cell.2012.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Papp B, Plath K. Epigenetics of reprogramming to induced pluripotency. Cell. 2013;152(6):1324–1343. doi: 10.1016/j.cell.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liang G, Zhang Y. Embryonic stem cell and induced pluripotent stem cell: an epigenetic perspective. Cell Res. 2013;23(1):49–69. doi: 10.1038/cr.2012.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen T, Dent SY. Chromatin modifiers and remodellers: regulators of cellular differentiation. Nat Rev Genet. 2014;15(2):93–106. doi: 10.1038/nrg3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Petty E, Pillus L. Balancing chromatin remodeling and histone modifications in transcription. Trends Genet. 2013;29(11):621–629. doi: 10.1016/j.tig.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Replication, D.I.G. et al. Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nat Genet. 2014;46(3):234–244. doi: 10.1038/ng.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295(7):349–353. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- 101.Thurner S, et al. Quantification of excess risk for diabetes for those born in times of hunger, in an entire population of a nation, across a century. Proc Natl Acad Sci U S A. 2013;110(12):4703–4707. doi: 10.1073/pnas.1215626110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Heijmans BT, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008;105(44):17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Simmons RA, Templeton LJ, Gertz SJ. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes. 2001;50(10):2279–2286. doi: 10.2337/diabetes.50.10.2279. [DOI] [PubMed] [Google Scholar]
- 104.Park JH, et al. Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J Clin Invest. 2008;118(6):2316–2324. doi: 10.1172/JCI33655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang BT, et al. Insulin promoter DNA methylation correlates negatively with insulin gene expression and positively with HbA(1c) levels in human pancreatic islets. Diabetologia. 2011;54(2):360–367. doi: 10.1007/s00125-010-1967-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sandovici I, et al. Maternal diet and aging alter the epigenetic control of a promoter-enhancer interaction at the Hnf4a gene in rat pancreatic islets. Proc Natl Acad Sci U S A. 2011;108(13):5449–5454. doi: 10.1073/pnas.1019007108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Multhaup ML, et al. Mouse-human experimental epigenetic analysis unmasks dietary targets and genetic liability for diabetic phenotypes. Cell Metab. 2015;21(1):138–149. doi: 10.1016/j.cmet.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hall E, et al. Effects of palmitate on genome-wide mRNA expression and DNA methylation patterns in human pancreatic islets. BMC Med. 2014;12:103. doi: 10.1186/1741-7015-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Volkmar M, et al. DNA methylation profiling identifies epigenetic dysregulation in pancreatic islets from type 2 diabetic patients. EMBO J. 2012;31(6):1405–1426. doi: 10.1038/emboj.2011.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dayeh T, et al. Genome-wide DNA methylation analysis of human pancreatic islets from type 2 diabetic and non-diabetic donors identifies candidate genes that influence insulin secretion. PLoS Genet. 2014;10(3):e1004160. doi: 10.1371/journal.pgen.1004160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Taneera J, et al. A systems genetics approach identifies genes and pathways for type 2 diabetes in human islets. Cell Metab. 2012;16(1):122–134. doi: 10.1016/j.cmet.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 112.Olsson AH, et al. Genome-Wide Associations between Genetic and Epigenetic Variation Influence mRNA Expression and Insulin Secretion in Human Pancreatic Islets. PLoS Genet. 2014;10(11):e1004735. doi: 10.1371/journal.pgen.1004735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kameswaran V, et al. Epigenetic Regulation of the DLK1-MEG3 MicroRNA Cluster in Human Type 2 Diabetic Islets. Cell Metabolism. 2014;19(1):135–145. doi: 10.1016/j.cmet.2013.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cannon CE, et al. The Polycomb protein, Bmi1, regulates insulin sensitivity. Mol Metab. 2014;3(8):794–802. doi: 10.1016/j.molmet.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Parker SC, et al. Chromatin stretch enhancer states drive cell-specific gene regulation and harbor human disease risk variants. Proc Natl Acad Sci U S A. 2013;110(44):17921–17926. doi: 10.1073/pnas.1317023110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Khoo C, et al. Research resource: the pdx1 cistrome of pancreatic islets. Mol Endocrinol. 2012;26(3):521–533. doi: 10.1210/me.2011-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nissenbaum J, et al. Global indiscriminate methylation in cell-specific gene promoters following reprogramming into human induced pluripotent stem cells. Stem Cell Reports. 2013;1(6):509–517. doi: 10.1016/j.stemcr.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dorrell C, et al. Transcriptomes of the major human pancreatic cell types. Diabetologia. 2011;54(11):2832–2844. doi: 10.1007/s00125-011-2283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Benner C, et al. The transcriptional landscape of mouse beta cells compared to human beta cells reveals notable species differences in long non-coding RNA and protein-coding gene expression. BMC Genomics. 2014;15:620. doi: 10.1186/1471-2164-15-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Arnes L, et al. Ghrelin expression in the mouse pancreas defines a unique multipotent progenitor population. PLoS One. 2012;7(12):e52026. doi: 10.1371/journal.pone.0052026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou Y, et al. TCF7L2 is a master regulator of insulin production and processing. Hum Mol Genet. 2014 doi: 10.1093/hmg/ddu359. [DOI] [PMC free article] [PubMed] [Google Scholar]