Abstract
Although discovered as a placental protein present abundantly in the circulation of pregnant women, pregnancy-associated plasma protein-A (PAPP-A) is widely expressed in multiple tissues. PAPP-A is a highly specific metalloproteinase binding tightly to glycosaminoglycans present on the surface of cells. By cleaving a subset of insulin-like growth factor binding proteins (IGFBPs), PAPP-A thus functions within tissues as a growth-promoting enzyme, releasing bioactive IGF in close proximity to the IGF receptor. IGFBP-4 is believed to be the principal PAPP-A substrate, and the focus in this review is on PAPP-A enzymatic activity and its role in the PAPP-A-IGFBP-4-IGF axis, which is subject to regulation at several different levels. These include e.g., transcriptional control, competing reactions potentially sequestering IGF from IGFBP-4 and hence antagonizing PAPP-A-mediated IGF activation, and proteolytic inhibition of PAPP-A. The latter may involve the protein stanniocalcin-2 (STC2), recently found to potently inhibit PAPP-A activity by forming a covalent complex with PAPP-A. PAPP-A or complex-bound variants may escape from pathological tissues into the circulation. It is emphasized that the potential use of PAPP-A as a diagnostic or predictive biomarker in nonpregnant individuals requires precise knowledge of analyte identity and assay specificity in addition to an appropriate material for standardization. Finally, PAPP-A may serve as a therapeutic target to indirectly inhibit IGF signaling in tissues where this is driven by increased PAPP-A activity. By taking advantage of the intricate interaction between PAPP-A and IGFBP-4, highly specific and selective inhibition of PAPP-A is possible.
Keywords: Insulin-like growth factor (IGF), Insulin-like growth factor binding protein (IGFBP), Pappalysin, Pregnancy-associated plasma protein-A (PAPP-A), Proform of eosinophil major basic protein (proMBP), Stanniocalcin (STC)
The first 25 years of PAPP-A - a useful protein of unknown function
Human pregnancy-associated plasma protein-A (PAPP-A) was identified 40 years ago as a protein present abundantly in plasma of pregnant women (Lin et al. 1974). Following its discovery, several biological roles of PAPP-A were proposed, including a function as a proteinase inhibitor, inspired by similarities with α-2-macroglobulin (α2M) and pregnancy zone protein (PZP) (Sinosich 1990). However, no consensus was established regarding its biological role or subunit organization.
In 1993, it was found that what had previously been referred to as PAPP-A in pregnancy plasma is in fact a heterotetrameric disulfide bound 2:2 complex composed of two 200 kDa PAPP-A subunits and two proMBP subunits, denoted PAPP-A/proMBP (Oxvig et al. 1993). In SDS-PAGE, proMBP migrates as a smear of 50–90 kDa, only visible by Western blotting. Both types of subunits are heavily glycosylated, in particular proMBP which carries N- and O-linked glycans in addition to a glycosaminoglycan (GAG) moiety (Oxvig et al. 1994a; Oxvig et al. 1994b). The latter explains why the presence of proMBP had been overlooked in earlier studies. ProMBP (proform of eosinophil major basic protein) is known as the precursor of the 13 kDa MBP, present in the eosinophil leukocyte. During maturation of the eosinophil precursor cells, proMBP is cleaved, and mature cytotoxic MBP is stored in secretory granules (Popken-Harris et al. 1998). However, there is no evidence to suggest that proMBP of the PAPP-A/proMBP complex undergoes similar proteolytic processing.
One implication of the existence of the PAPP-A/proMBP complex is that preparations of polyclonal antibodies raised by immunization with antigen purified from pregnancy plasma or serum are polyspecific, recognizing both PAPP-A and proMBP (Oxvig et al. 1993). In addition, several reports have documented reactivity of commercial polyclonal antibodies towards other plasma proteins, probably typical contaminants of PAPP-A/proMBP preparations. These include haptoglobin (Bueler and Bersinger 1989), haptoglobin-related protein (Kuhajda et al. 1989), and pregnancy-specific β1-glycoprotein (SP1) (Bersinger et al. 1995). Therefore, the use of polyclonal antibodies should generally be avoided, in particular for tissue analysis, and the reactivity of monoclonal antibodies must be verified by documenting subunit specificity (Qin et al. 1997).
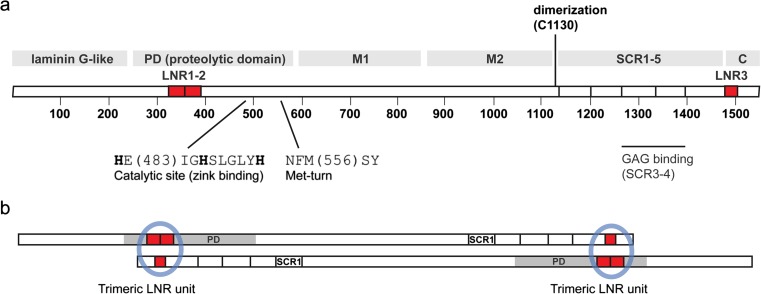
Cloning of cDNA (Kristensen et al. 1994) showed that the 1547-residue PAPP-A subunit contains a unique set of protein modules known from several different proteins (Fig. 1a). At the very N-terminus is a 250-residue laminin G-like module with weak similarity to, for example, the α-chain of laminins, neurexins, and sex hormone-binding globulin (Boldt et al. 2006). The function of this module is unknown. It is followed by a 350-residue module containing the so-called elongated zinc binding consensus sequence (Boldt et al. 2001), a defining feature of the large group of metzincin metalloproteinases, also including the MMPs and the ADAMs (Cerda-Costa and Gomis-Ruth 2014). PAPP-A does not share global similarity with any of the metzincins or other proteins, except for the paralog, PAPP-A2 (Overgaard et al. 2001). Within the metzincins, PAPP-A, PAPP-A2, and ulilysin (an archaeal proteinase of 29 kDa, containing only a proteinase domain (Tallant et al. 2006)) comprise the pappalysin family. The C-terminal fourth of the PAPP-A subunit harbors five short consensus repeat (SCR) modules, also known as complement control protein (CCP) modules, in addition to the C domain. SCR3 and SCR4 bind glycosaminoglycans (GAGs) and mediate PAPP-A cell surface binding (Laursen et al. 2002b; Weyer et al. 2004). No function has been assigned to the sequence stretch of >500 residues (M1 and M2, (Overgaard et al. 2003)), located between the proteolytic domain and SCR1. Finally, the PAPP-A subunit contains three Lin12-Notch repeat (LNR) modules, two of which (LNR1-2) are located within the proteolytic domain, and a third (LNR3), located in the C domain. Except for PAPP-A and PAPP-A2, the LNR module is known from the family of Notch receptors, where three modules are located in the extracellular portion, always next to one another (Boldt et al. 2004).
Fig. 1.
Schematic overview of the primary structure of PAPP-A. a The PAPP-A subunit is constituted by 1547 residues, 82 of which are cysteines. Protein modules are indicated with gray bars: The proteolytic domain (PD) of about 350 residues is preceded by a laminin G-like module of unknown function (Boldt et al. 2001). The PD contains the elongated zinc ion binding consensus sequence and a short sequence element responsible for formation of the Met-turn, both defining features of the metzincin superfamily of metalloproteinases (Boldt et al. 2001). Curiously, the second of the 22 exons of the PAPP-A gene encodes almost the entire laminin G-like module and approximately half of the PD (Overgaard et al. 2003). The PD is followed by two ill-defined regions, tentatively designated M1 and M2 based on disulfide structure, and then by five short consensus repeats (SCR1-5), also known as CCP modules (Overgaard et al. 2003). SCR3 and SCR4 bind glycosaminoglycans and are responsible for cell surface binding of PAPP-A (Laursen et al. 2002b). In addition, note that the PAPP-A subunit contains three Lin12-Notch repeat (LNR) modules shown in red, two of which (LNR1-2) are located within the PD and a third (LNR3) located in the C domain. The LNR modules determine proteolytic specificity of PAPP-A (Boldt et al. 2004). b PAPP-A exists as a 400 kDa dimer composed of two disulfide linked subunits. This simplified model highlights the antiparallel arrangement of the subunits and the two putative LNR units (blue circles), each formed from LNR1-2 in one subunit and LNR3 in the other (Weyer et al. 2007)
During human pregnancy, PAPP-A is primarily synthesized in the syncytiotrophoblast, while proMBP is synthesized in extravillous trophoblasts (X cells) (Bonno et al. 1994). The PAPP-A/proMBP complex forms in the extracellular environment (Glerup et al. 2005), but while almost all (99 %) of circulating PAPP-A (50 mg/L at term) is present in the form of the PAPP-A/proMBP complex in the second and third trimester, a variable fraction (up to 30 %) of PAPP-A exists as the uncomplexed dimer in the first trimester (Gyrup et al. 2007). PAPP-A mRNA can be detected in most tissues, but the level of PAPP-A synthesized in the human placenta greatly exceeds levels in any other tissue (Overgaard et al. 1999). Curiously, although the level of circulating PAPP-A is increased >10.000 fold in human pregnancy (Qin et al. 1997), this is not the case in pregnant mice, because PAPP-A is not synthesized in the murine placenta (Soe et al. 2002).
Maternal serum levels of PAPP-A have long been known to be depressed in the first trimester of Down’s syndrome pregnancies (Wald et al. 1992), which is widely used in prenatal diagnosis. Other chromosomal abnormalities and adverse pregnancy outcomes, e.g., intrauterine growth retardation and preeclampsia, are also associated with depressed levels of PAPP-A (Kirkegaard et al. 2010), as is low weight at birth (Smith et al. 2002).
Proteolytic activity of PAPP-A
Although predicted from the amino acid sequence to be a metalloproteinase, the proteolytic activity of PAPP-A was demonstrated experimentally only in 1999. PAPP-A was shown to be secreted from fibroblasts and osteoblasts, and to be responsible for the previously observed proteolytic activity towards insulin-like growth factor binding protein (IGFBP)-4 (Lawrence et al. 1999). PAPP-A was subsequently shown to be the proteinase responsible for cleavage of IGFBP-4 in ovarian follicular fluid (Conover et al. 1999), and to be secreted from granulosa cells (Conover et al. 2001), vascular smooth muscle cells (Bayes-Genis et al. 2001b), and endometrial stromal cells (Giudice et al. 2002). It was later shown that IGFBP-2 (Monget et al. 2003) and IGFBP-5 (Laursen et al. 2001) are also PAPP-A substrates. Importantly, while these IGFBPs are also cleaved by other proteinases, physiological cleavage of IGFBP-4 may be limited to PAPP-A (Laursen et al. 2007; Ning et al. 2008). Compared to PAPP-A, PAPP-A2 has currently been much less studied. IGFBP-3 and IGFBP-5 are known substrates of PAPP-A2 (Overgaard et al. 2001), and during human pregnancy, circulating IGFBP-5 undergoes cleavage, for which PAPP-A2 is responsible (Yan et al. 2010). The specificities of PAPP-A and PAPP-A2 towards the six different IGFBPs are summarized in Table 1.
Table 1.
Abilities of the pappalysins (PAPP-A and PAPP-A2) to proteolytically cleave the six IGFBPs
| Substrate | PAPP-A | PAPP-A2 | Effect of IGF on cleavage | Reference |
|---|---|---|---|---|
| IGFBP-1 | no | no | – | – |
| IGFBP-2 | YES | no | Enhanced | 1 |
| IGFBP-3 | no | YES | Enhanced | 2 |
| IGFBP-4 | YES | no | Enhances (IGF is required) | 3, 4 |
| IGFBP-5 | YES | YES | Decreased (PA)/Enhanced (PA2) | 2, 4 |
| IGFBP-6 | no | no | – | – |
PAPP-A cleaves its three IGFBP substrates at single sites; IGFBP-4 is cleaved at Met-135/Lys-136 located in the linking domain (Laursen et al. 2002a). In general, cleavage allows dissociation of bound bioactive IGF, as individual N- and C-domains of IGFBPs have reduced affinity for the IGFs (Laursen et al. 2007). The PAPP-A cleavage reaction of IGFBP-4 is quite unusual in several regards:
Unlike most protein substrates, none of the residues in close proximity to the cleavage site are important for the proteolytic reaction. Rather, several basic amino acids of IGFBP-4, located up to 16 residues N-terminal to the scissile bond, are important for substrate binding or cleavage (Laursen et al. 2002a). Accordingly, a synthetic peptide spanning the cleavage site must be longer than 20 amino acids to function as a PAPP-A substrate (Gyrup et al. 2007; Laursen et al. 2002a).
Cleavage of IGFBP-4 requires that it binds IGF-I or -II (Laursen et al. 2001; Qin et al. 2000). PAPP-A activity towards the IGFBP-4/IGF complex has high kinetic efficiency (specificity constant with bound IGF-II: kcat/KM = 1.4 μM−1 s−1), while cleavage of IGFBP-4 in the absence of IGF is negligible (Gyrup and Oxvig 2007). This strict IGF dependency is a unique property of PAPP-A and IGFBP-4. In fact, cleavage of IGFBP-5 by PAPP-A is reduced approximately three fold upon IGF binding (Gyrup and Oxvig 2007; Laursen et al. 2001). Curiously, although PAPP-A2 cleavage of IGFBP-3 or IGFBP-5 occurs efficiently in the absence of IGF, the reaction is slightly enhanced by IGF (Gaidamauskas et al. 2013).
The three LNR modules of the PAPP-A subunit each bind a calcium ion and are involved in determination of proteolytic specificity. When calcium binding is disrupted by substitution of single amino acids in either of the LNR modules, activity towards IGFBP-4 is lost completely, while cleavage of IGFBP-5 is unaffected (Boldt et al. 2004). In agreement with this, PAPP-A truncated before LNR3 is unable to cleave IGFBP-4, but fully active towards IGFBP-5. However, co-transfection of cells with cDNA encoding this truncated variant and cDNA encoding a proteolytically inactivated variant of PAPP-A, which carries a single amino acid substitution in the active site (Glu-483-Ala), causes activity of the truncated variant towards IGFBP-4 to be restored (Weyer et al. 2007). This experiment suggests a model in which the subunits of the wild-type PAPP-A dimer are arranged in an antiparallel manner and two putative LNR units are formed in trans (Fig. 1b). Based on surface plasmon resonance analysis, IGFBP-4 interacts directly with residues of the LNR3 module, thus defining a classical proteinase substrate-binding exosite (Weyer et al. 2007). Finally, the unusually long 63-residue sequence stretch between the zinc binding site and the Met-turn (Boldt et al. 2001) contains several acidic residues, which are strictly required for proteolysis of both IGFBP-4 and IGFBP-5 (Gaidamauskas et al. 2013). Together, experimental data thus suggests an unusually complex mode of proteinase-substrate interaction between PAPP-A and IGFBP-4.
While all MMPs are inhibited by tissue inhibitors of metalloproteinases (TIMPs) (Nagase et al. 2006), none of these inhibit PAPP-A or PAPP-A2. Rather, proMBP inhibits PAPP-A by irreversible formation of the 2:2 PAPP-A/proMBP complex (Overgaard et al. 2004; Overgaard et al. 2000). Complex formation involves rearrangement of specific disulfide bonds within and between the PAPP-A and proMBP subunits (Glerup et al. 2005). ProMBP thus binds via cysteine residues of PAPP-A which are located close to the active site, rendering PAPP-A inactive towards all of its substrates. The linear GAG chain present on Ser-62 of proMBP is not required for formation of the PAPP-A/proMBP complex, but upon complex formation with surface-bound PAPP-A, the proMBP GAG causes detachment of PAPP-A by displacing GAG of cell-surface proteoglycans, which bind PAPP-A via SCR3 and SCR4 (Glerup et al. 2007; Laursen et al. 2002b). Except for stanniocalcins (STCs), found recently to inhibit PAPP-A activity (cf. below), no other protein is known to modulate pappalysin activity.
Although all known pappalysin substrates are IGFBPs, and no synthetic low-molecular-weight substrates have been identified, other protein substrates may obviously exist. However, the inability of PAPP-A to show any activity in standard gelatinolytic proteinase assays (unpublished findings), suggests that PAPP-A is a highly selective enzyme, unlikely to participate in, for example, extracellular matrix degradation in spite of its relationship with the MMPs. PAPP-A can undergo inactivating autoproteolytic cleavage at a specific site within LNR2, but under physiological conditions, this appears to be limited (Boldt et al. 2001).
The role of PAPP-A in the IGF system: proteolytic release of active IGF in close proximity to the IGF1 receptor
The six IGFBPs all bind IGFs with higher affinity than the IGF1 receptor (IGF1R) (Forbes et al. 2012). Therefore, reduced IGF affinity of the IGFBPs, which may result from limited proteolytic cleavage, will cause increased IGF signaling (Bunn and Fowlkes 2003; Firth and Baxter 2002). Although this is straightforward to demonstrate in vitro, analysis of such a relationship in vivo is challenging. The focus here is entirely on PAPP-A.
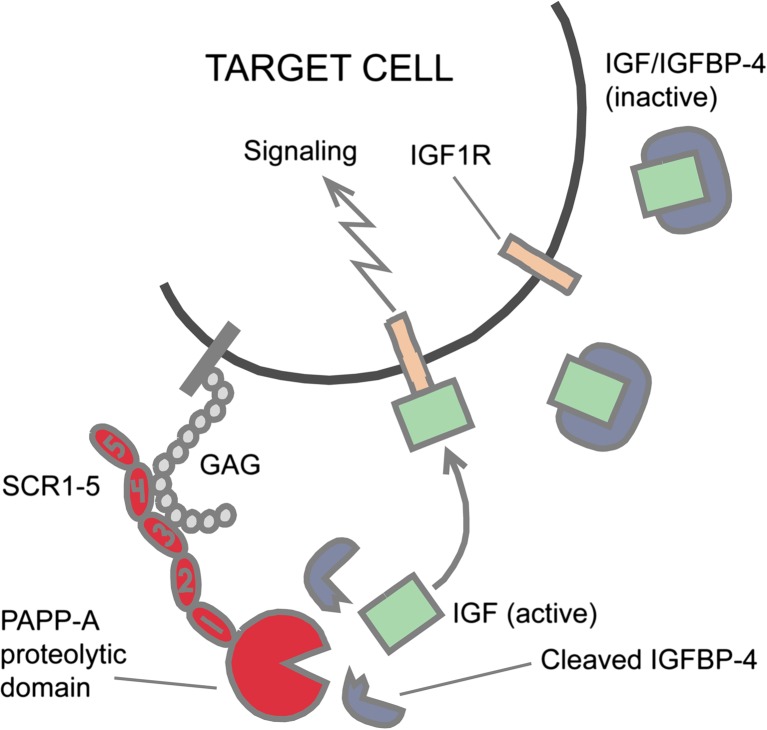
Even though its name indicates a biological function of PAPP-A in the circulation, there is currently no data to suggest that. By means of modules SCR3 and SCR4, the dimeric PAPP-A will bind cell surface molecules displaying GAGs. Cell-surface bound PAPP-A is proteolytically active (Laursen et al. 2002a), and it is reasonable to assume that in tissues, the majority of PAPP-A is surface bound. Therefore, cleavage of IGFBP-4 occurs in close proximity to the IGF1R, increasing the chance that released IGF will cause receptor stimulation (Laursen et al. 2002a). This simple model is depicted in Fig. 2. In vivo, the scenario is complicated by many factors, such as the presence of other IGFBPs that might sequester IGF. In the presence of IGFBP-5, which has a 10-fold higher affinity for the IGFs than IGFBP-4, IGF will only become bound to IGFBP-4 following release from IGFBP-5. Therefore, for IGF to be bound by IGFBP-4 and transported to the cell surface, other mechanisms must operate in the extracellular environment to locally eliminate the IGF binding capacity of any IGFBP-5 present. Many factors thus define the molecular network of proteins that potentially control the pericellular concentration of bioactive IGF (Laursen et al. 2007).
Fig. 2.
The role of PAPP-A and IGFBP-4 in the IGF system. Binding of PAPP-A to proteoglycans on the cell surface (Laursen et al. 2002b) causes proteolytic cleavage of IGFBP-4 to occur in close proximity of the IGF receptor, increasing the probability that released IGF leads to receptor signaling (Laursen et al. 2007). Surface binding is mediated by PAPP-A modules SCR3 and SCR4 (cf. Fig. 1). Other IGFBPs which are not PAPP-A substrates may be present. The involved components may come from the same or from neighboring cells. Curiously, this model does not apply to PAPP-A2, which shows no cell surface binding (Laursen et al. 2002b)
PAPP-A knockout mice are proportional dwarfs, reduced 40 % in size (Conover et al. 2004a), a phenotype which is remarkably similar to the phenotype of IGF-II knockout mice (DeChiara et al. 1990), and which can be rescued by disruption of IGF-II imprinting (Bale and Conover 2005). These data provided the first in vivo evidence that PAPP-A is an important component of the IGF system. It was later reported that IGFBP-4 knockout mice are smaller (10–15 %) than wild-type littermates (Ning et al. 2006). Although at first this might seem contradictory, it does support the idea that IGFBP-4 has a critical function in the final delivery of IGF to its receptor (Laursen et al. 2007) (cf. above). This interpretation is also supported by the earlier finding that systemic administration of IGFBP-4 increases the local bioavailability of IGF in bone (Miyakoshi et al. 2001), and later by analysis of crosses between PAPP-A null and IGFBP-4 null mice (Ning et al. 2008). The authors concluded that IGFBP-4 cleavage is required to activate most, if not all, IGF-II mediated growth-promoting activity.
In agreement with the existence of a PAPP-A → IGFBP-4 → IGF axis, a strong anabolic effect of PAPP-A in mice has been demonstrated by transgenic expression in osteoblasts, which caused a marked increase in the rate of bone formation without affecting bone resorption (Qin et al. 2006). Similarly, targeted overexpression of PAPP-A by using a muscle-specific promoter significantly increased the skeletal muscle weight and muscle fiber area (Rehage et al. 2007). In contrast, tissue specific effects of reduced IGF signaling have been reported in the PAPP-A knockout mouse, which shows reduced ovarian function and fertility (Nyegaard et al. 2010), and resistance to age-dependent thymic involution (Vallejo et al. 2009). And finally, like several other genes of the IGF axis (Junnila et al. 2013), the absence of PAPP-A causes a markedly (35 %) increased lifespan in mice (Conover and Bale 2007).
Transcriptional regulation of PAPP-A has been studied in vitro and in vivo. Based on available data it can be concluded that PAPP-A is regulated in inflammatory injury responses and tissue remodeling (Conover 2012). Principally dermal fibroblasts, osteoblasts, coronary artery endothelium and smooth muscle cells have been studied (Conover et al. 2006; Conover et al. 2004b; Conover et al. 2008; Ortiz et al. 2003; Resch et al. 2004). Invariably, tumor necrosis factor (TNF)α and interleukin (IL)-1β promote PAPP-A expression, and IL-4 and transforming growth factor (TGF)β induce PAPP-A expression in osteoblasts. Known inhibitors of PAPP-A transcription include interferon (INF)-γ in fibroblasts and resveratrol in smooth muscle cells. It is interesting that PAPP-A expression is increased in several models of tissue injury. These include a model of healing human skin (Chen et al. 2003), models of vascular injury in pigs (Bayes-Genis et al. 2001b) and mice (Resch et al. 2006), and a murine model of skeletal muscle injury (Rehage et al. 2007).
It should be emphasized that functions of PAPP-A unrelated to IGF signaling are certainly possible. Any hypothetical biological role of PAPP-A outside of the IGF system falls within one of the following three categories: 1) Proteolytic cleavage by PAPP-A of substrates other than IGFBPs to affect e.g., signaling pathways. There is currently no evidence of such substrates, as mentioned above, 2) Activities of PAPP-A which are not dependent on its proteolytic activity. Modulation of the early developmental rate in zebrafish by PAPP-A (Kjaer-Sorensen et al. 2013) was the first reported example of a proteinase-independent function. Similarly, zebrafish PAPP-A2 has recently been reported to promote Notch signaling independently of its proteolytic activity (Kjaer-Sorensen et al. 2014), or 3) Proteolytic cleavage of the IGFBPs to regulate IGF-independent functions of these proteins. Many different biological activities are ascribed to specific IGFBPs (Baxter 2014), but there is currently no example of the involvement of PAPP-A in modulating such activities. However, data indicate that in zebrafish, PAPP-A2 modulates BMP signaling through cleavage of IGFBP-3 (Kjaer-Sorensen et al. 2014).
Possible pathophysiological roles and therapeutic targeting of PAPP-A
PAPP-A was first linked to atherosclerosis when abundant expression was demonstrated in unstable human plaques (Bayes-Genis et al. 2001a). It was later shown that the absence of PAPP-A in mice caused a dramatic reduction in plaque development in an ApoE knockout model of atherosclerosis (Bale et al. 2014; Harrington et al. 2007). Although a role of PAPP-A as an extracellular matrix degrading enzyme within the plaque is unlikely (cf. above), the exact role of PAPP-A in promoting bioactivity of IGF and the consequences of increased IGF signaling in the vasculature is a matter of debate (Consuegra-Sanchez et al. 2009). Diabetic nephropathy is another pathological condition that may develop as a consequence of increased PAPP-A activity. In humans, diabetic nephropathy is associated with an elevated level of glomerular PAPP-A expression, and the absence of PAPP-A in mice confers resistance to the development of pathological hallmarks of diabetic nephropathy (Mader et al. 2013).
The association and possible involvement of PAPP-A in cancer development and/or growth is increasingly suggested in the literature. It involves several different tissues and includes at least ovarian cancer (Boldt and Conover 2011; Callahan et al. 2003; Kalli et al. 2004; Suzuki et al. 2003; Tanaka et al. 2004), renal cancer (Dalgin et al. 2007), breast cancer (Loddo et al. 2014; Mansfield et al. 2014; Ryan et al. 2009), lung cancer (Bulut et al. 2009; Pan et al. 2012; Salim et al. 2013), gastric cancer (Nagarajan et al. 2012), and pleural mesothelioma (Huang et al. 2013). As indicated above, earlier reports may be in error because conclusions were drawn following the use of polyspecific PAPP-A antibodies (see e.g., (Kuhajda and Eggleston 1985) and (Kuhajda et al. 1989)).
It should also be noted that PAPP-A knockout mice show a striking resistance to the development of spontaneous cancer (Conover and Bale 2007; Conover et al. 2010), although this is possibly explained by the lack of thymic involution and hence a lower degree of decline in immune competence in older knockout mice (Vallejo et al. 2009). Mechanistic details of PAPP-A function in tumors are generally lacking, but data suggests that p53 can be involved in oncogenic regulation of PAPP-A transcription (Chander et al. 2011). However, regardless of underlying mechanism, altered expression of PAPP-A clearly has the potential to cause dysregulated IGF signaling, which has been linked extensively to human cancer (Pollak 2012; Samani et al. 2007).
For these reasons, PAPP-A may comprise a desirable therapeutic target to indirectly inhibit IGF signaling. In cancer therapy, direct inhibition of IGF signaling by IGF1R targeting has been attempted extensively with variable success and several obvious disadvantages because it lacks specificity (Heidegger et al. 2011; Pollak 2012; Yee 2012). Targeting of PAPP-A in selected tumors where increased IGF signaling may be driven by locally increased PAPP-A activity could therefore represents an alternative strategy with fewer potential side effects. By targeting the unique substrate binding exosite of PAPP-A, specific and selective inhibition can be obtained in vitro (Mikkelsen et al. 2008), and in vivo proof-of-concept for this strategy has been provided by using a murine xenograft model (Mikkelsen et al. 2014). In this model, targeting of PAPP-A reduced tumor growth, but a similar strategy of indirect targeting of IGF signaling may be relevant to control growth selectively of other tissues. These include not only cardiovascular tissues, but also e.g., growth of visceral fat (Conover et al. 2013).
Increased circulating levels of PAPP-A in nonpregnant individuals may reflect excessive synthesis in pathological tissue. Since the discovery of PAPP-A in human atherosclerotic plaques and the finding of increased circulating levels in patients with acute coronary syndromes (Bayes-Genis et al. 2001a), its biomarker potential in cardiovascular disease has been extensively explored (Kalousova et al. 2014; Li et al. 2013). Similarly, reports of increased circulating levels in cancer patients have also appeared (Bulut et al. 2009). Even though commercial immunoassays are typically based on monoclonal antibodies, their specificity is rarely documented. It is important to rule out that a given antibody is not specific for known or unknown proteins, which bind to PAPP-A, and to confirm that the antibody binds to an epitope of PAPP-A, which is not altered upon binding of e.g., proMBP (or other PAPP-A binding proteins, cf. below). Another problem often neglected is the bias introduced when patients have been given heparin, although it directly follows from the finding that PAPP-A binds to the surface of cells and can be detached with GAG, e.g., heparin (Laursen et al. 2002b). Heparin treatment of cardiovascular patients therefore potentially causes release into the circulation of PAPP-A, resulting in artificially increased levels, which may not be directly associated with changes in the pathological tissue monitored. Finally, the lack of defined standard materials for calibration is also a problem that makes comparison of studies difficult.
Stanniocalcin-2 - a novel member of the IGF system
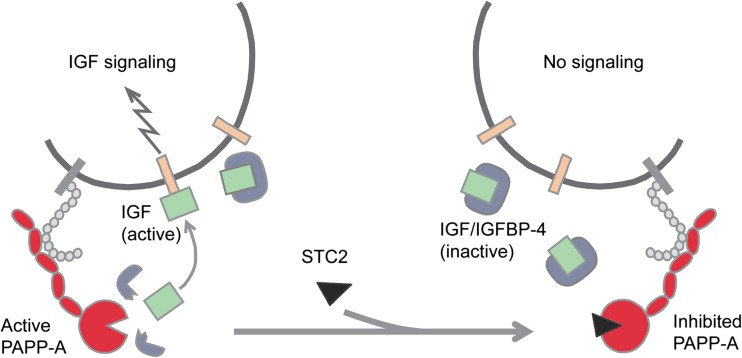
Recently, the protein stanniocalcin-2 (STC2) was identified as a powerful inhibitor of PAPP-A proteolytic activity (Jepsen et al. 2014). It has been known for several years that STC2 knockout mice are 15 % larger than wild-type littermates (Chang et al. 2008), and that transgenic overexpression of STC2 causes a reduction in size of up to 45 % (Gagliardi et al. 2005). However, the mechanism behind these opposite phenotypes has remained obscure until recently, when it was found that STC2 is able to form a covalent complex with PAPP-A, completely abrogating its proteolytic activity towards IGFBP-4. Mouse embryonic fibroblasts (MEFs) normally secrete active PAPP-A, but medium conditioned by MEFs originating from STC2 transgenic mice contains the inhibited PAPP-A-STC2 complex. Furthermore, transgenic mice overexpressing a variant of STC2, in which a single amino acid has been substituted to eliminate its ability to inhibit PAPP-A, grow like wild-type mice (Jepsen et al. 2014). This means that STC2 has the potential to control PAPP-A activity in tissues by changing the balance between active and inhibited PAPP-A: A change in the direction of increased PAPP-A-STC2 complex causes reduced local IGF signaling (Fig. 3). It should be stressed that neither PAPP-A knockout mice (Conover et al. 2004a), transgenic STC2 mice (Gagliardi et al. 2005), or STC2 knockout mice (Chang et al. 2008) show any alterations in circulating IGF-I, emphasizing that STC2, PAPP-A, and IGFBP-4 regulate IGF signaling locally. The operation of such local regulatory mechanism is also highlighted by the earlier finding that liver-specific knockout of IGF-I in mice causes a dramatic reduction (by 75 %) in the concentration of circulating IGF-I, while it has no effect on growth (Sjogren et al. 1999; Yakar et al. 1999).
Fig. 3.
Model showing the effect of stanniocalcin-2 (STC2) on PAPP-A-mediated IGF signaling. STC2 binds irreversibly (covalently) to PAPP-A and completely abrogates the ability of PAPP-A to cleave IGFBP-4 (Jepsen et al. 2014). Transgenic overexpression of STC2 (Gagliardi et al. 2005) results in mice with the same dwarf phenotype as PAPP-A knockout mice, suggesting that all of PAPP-A is inhibited (Jepsen et al. 2014). In contrast, knockout of STC2 causes increased growth (Chang et al. 2008), suggesting that in these mice, all of PAPP-A is active. We hypothesize that normally and under pathological conditions, the level of STC2 has the potential to determine the balance between active and inactive PAPP-A, thus comprising the first molecule of an STC2 → PAPP-A → IGFBP-4 → IGF axis
STC2 and its only homolog, stanniocalcin-1 (STC1), have been studied extensively, but not previously connected with the IGF system (Yeung et al. 2012). STC1 was first discovered by purification from the corpuscles of Stannius, present exclusively in teleost fish (Wagner et al. 1986). It is well established that STC1 regulates plasma Ca2+ concentration in fish by inhibiting gill uptake of calcium from the environment (Fenwick and So 1974; Wagner and Dimattia 2006). However, such hypocalcemic function of STC1 is thought to have been lost in mammals, in which the hormone calcitonin functions similarly (Chang et al. 2008). Rather than being expressed from a single organ, both of the stanniocalcins are widely expressed in mammals and reported to be involved in several different biological processes (see (Yeung et al. 2012) for an overview). In vitro analysis has shown that STC1 also potently inhibits PAPP-A (unpublished). Obviously, further studies are required to reveal to what extent reported STC1/2 functions may result from interaction with PAPP-A. The STCs have the potential to effectively influence PAPP-A activity, but it is still unknown whether other STC functions may be affected by PAPP-A binding.
Finally, it is interesting that multiple articles link either up- or down-regulation of the stanniocalcins to human cancer (Chang et al. 2003; Yeung et al. 2012). No clear picture can be drawn at this time, but it is tempting to hypothesize that PAPP-A and STC1/2 can be part of the same regulatory system in pathological tissues where they are co-expressed. For example, 1) loss of BRCA1 tumor suppressor function in breast cancer causes STC1 expression to become undetectable (Welcsh et al. 2002), 2) STC1 is down-regulated in cervical cancer tissue, and STC1 knockdown in a cervical cancer derived cells causes increased cell growth and invasiveness, while STC1 overexpression inhibited proliferation and invasion (Guo et al. 2013), and 3) late relapse of breast cancer correlates strongly with high expression of STC1 and STC2, which therefore act as survival factors (Joensuu et al. 2008).
Challenges and directions for the future
Problems waiting to be solved and possibilities to be explored are numerous. The greatest challenge is to clarify further details of how the concentration of bioavailable IGF is determined by molecules of the network surrounding the PAPP-A-IGFBP-4-IGF axis. In particular, this involves delineating the precise mechanisms of autocrine signaling and intercellular communication in many tissues under normal and pathological conditions. As mentioned, IGFBP-5 may prevent IGF from becoming available to IGFBP-4 and hence reduce activation by PAPP-A, or the presence of STC2 may completely block PAPP-A activity.
Second, efforts should be aimed at understanding the normal and pathophysiological role of the STCs in the IGF system. This includes defining STC activities which are unrelated to but may function synergistically with IGF signaling. Third, the use of PAPP-A as a target for therapeutic intervention in, for example, cancer and cardiovascular disease should be explored. Its potential is currently supported by several promising animal models. Fourth, the biomarker potential of PAPP-A and related molecules that may escape into the circulation awaits careful investigation. This should include analysis of antibody specificity, epitope stability and the presence or absence of PAPP-A epitopes in various complexes of PAPP-A. It should also include the development of characterized standard materials for calibration, prepared according to the exact nature of the desired analytes.
Lastly, PAPP-A may be involved in regulatory pathways outside the IGF system, which should always be kept in mind.
References
- Bale LK, Chakraborty S, Conover CA. Inducible reduction in pregnancy-associated plasma protein-A gene expression inhibits established atherosclerotic plaque progression in mice. Endocrinology. 2014;155:1184–1187. doi: 10.1210/en.2013-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale LK, Conover CA. Disruption of insulin-like growth factor-II imprinting during embryonic development rescues the dwarf phenotype of mice null for pregnancy-associated plasma protein-A. J Endocrinol. 2005;186:325–331. doi: 10.1677/joe.1.06259. [DOI] [PubMed] [Google Scholar]
- Baxter RC. IGF binding proteins in cancer: mechanistic and clinical insights. Nat Rev Cancer. 2014;14:329–341. doi: 10.1038/nrc3720. [DOI] [PubMed] [Google Scholar]
- Bayes-Genis A, Conover CA, Overgaard MT, Bailey KR, Christiansen M, Holmes DR, Jr, Virmani R, Oxvig C, Schwartz RS. Pregnancy-associated plasma protein A as a marker of acute coronary syndromes. N Engl J Med. 2001;345:1022–1029. doi: 10.1056/NEJMoa003147. [DOI] [PubMed] [Google Scholar]
- Bayes-Genis A, Schwartz RS, Lewis DA, Overgaard MT, Christiansen M, Oxvig C, Ashai K, Holmes DR, Jr, Conover CA. Insulin-like growth factor binding protein-4 protease produced by smooth muscle cells increases in the coronary artery after angioplasty. Arterioscler Thromb Vasc Biol. 2001;21:335–341. doi: 10.1161/01.atv.21.3.335. [DOI] [PubMed] [Google Scholar]
- Bersinger NA, Zakher A, Huber U, Pescia G, Schneider H. A sensitive enzyme immunoassay for pregnancy-associated plasma protein A (PAPP-A): a possible first trimester method of screening for Down syndrome and other trisomies. Arch Gynecol Obstet. 1995;256:185–192. doi: 10.1007/BF00634490. [DOI] [PubMed] [Google Scholar]
- Boldt HB, Conover CA. Overexpression of pregnancy-associated plasma protein-A in ovarian cancer cells promotes tumor growth in vivo. Endocrinology. 2011;152:1470–1478. doi: 10.1210/en.2010-1095. [DOI] [PubMed] [Google Scholar]
- Boldt HB, Glerup S, Overgaard MT, Sottrup-Jensen L, Oxvig C. Definition, expression, and characterization of a protein domain in the N-terminus of pregnancy-associated plasma protein-A distantly related to the family of laminin G-like modules. Protein Expr Purif. 2006;48:261–273. doi: 10.1016/j.pep.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Boldt HB, Kjaer-Sorensen K, Overgaard MT, Weyer K, Poulsen CB, Sottrup-Jensen L, Conover CA, Giudice LC, Oxvig C. The Lin12-notch repeats of pregnancy-associated plasma protein-A bind calcium and determine its proteolytic specificity. J Biol Chem. 2004;279:38525–38531. doi: 10.1074/jbc.M405222200. [DOI] [PubMed] [Google Scholar]
- Boldt HB, Overgaard MT, Laursen LS, Weyer K, Sottrup-Jensen L, Oxvig C. Mutational analysis of the proteolytic domain of pregnancy-associated plasma protein-A (PAPP-A): classification as a metzincin. Biochem J. 2001;358:359–367. doi: 10.1042/0264-6021:3580359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonno M, Oxvig C, Kephart GM, Wagner JM, Kristensen T, Sottrup-Jensen L, Gleich GJ. Localization of pregnancy-associated plasma protein-A and colocalization of pregnancy-associated plasma protein-A messenger ribonucleic acid and eosinophil granule major basic protein messenger ribonucleic acid in placenta. Lab Invest. 1994;71:560–566. [PubMed] [Google Scholar]
- Bueler MR, Bersinger NA. Antiserum to pregnancy-associated plasma protein A (PAPP-A) recognizes human haptoglobin. Br J Obstet Gynaecol. 1989;96:867–869. doi: 10.1111/j.1471-0528.1989.tb03330.x. [DOI] [PubMed] [Google Scholar]
- Bulut I, Coskun A, Ciftci A, Cetinkaya E, Altiay G, Caglar T, Gulcan E. Relationship between pregnancy-associated plasma protein-A and lung cancer. Am J Med Sci. 2009;337:241–244. doi: 10.1097/MAJ.0b013e31818967a3. [DOI] [PubMed] [Google Scholar]
- Bunn RC, Fowlkes JL. Insulin-like growth factor binding protein proteolysis. Trends Endocrinol Metab. 2003;14:176–181. doi: 10.1016/s1043-2760(03)00049-3. [DOI] [PubMed] [Google Scholar]
- Callahan G, Denison SR, Phillips LA, Shridhar V, Smith DI. Characterization of the common fragile site FRA9E and its potential role in ovarian cancer. Oncogene. 2003;22:590–601. doi: 10.1038/sj.onc.1206171. [DOI] [PubMed] [Google Scholar]
- Cerda-Costa N, Gomis-Ruth FX. Architecture and function of metallopeptidase catalytic domains. Protein Sci. 2014;23:123–144. doi: 10.1002/pro.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chander H, Halpern M, Resnick-Silverman L, Manfredi JJ, Germain D. Skp2B overexpression alters a prohibitin-p53 axis and the transcription of PAPP-A, the protease of insulin-like growth factor binding protein 4. PLoS One. 2011;6:e22456. doi: 10.1371/journal.pone.0022456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AC, Hook J, Lemckert FA, McDonald MM, Nguyen MA, Hardeman EC, Little DG, Gunning PW, Reddel RR. The murine stanniocalcin 2 gene is a negative regulator of postnatal growth. Endocrinology. 2008;149:2403–2410. doi: 10.1210/en.2007-1219. [DOI] [PubMed] [Google Scholar]
- Chang AC, Jellinek DA, Reddel RR. Mammalian stanniocalcins and cancer. Endocr Relat Cancer. 2003;10:359–373. doi: 10.1677/erc.0.0100359. [DOI] [PubMed] [Google Scholar]
- Chen BK, Leiferman KM, Pittelkow MR, Overgaard MT, Oxvig C, Conover CA. Localization and regulation of pregnancy-associated plasma protein a expression in healing human skin. J Clin Endocrinol Metab. 2003;88:4465–4471. doi: 10.1210/jc.2003-030193. [DOI] [PubMed] [Google Scholar]
- Conover CA. Key questions and answers about pregnancy-associated plasma protein-A. Trends Endocrinol Metab. 2012;23:242–249. doi: 10.1016/j.tem.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover CA, Bale LK. Loss of pregnancy-associated plasma protein A extends lifespan in mice. Aging Cell. 2007;6:727–729. doi: 10.1111/j.1474-9726.2007.00328.x. [DOI] [PubMed] [Google Scholar]
- Conover CA, Bale LK, Harrington SC, Resch ZT, Overgaard MT, Oxvig C. Cytokine stimulation of pregnancy-associated plasma protein A expression in human coronary artery smooth muscle cells: inhibition by resveratrol. Am J Physiol Cell Physiol. 2006;290:C183–C188. doi: 10.1152/ajpcell.00199.2005. [DOI] [PubMed] [Google Scholar]
- Conover CA, Bale LK, Mader JR, Mason MA, Keenan KP, Marler RJ. Longevity and age-related pathology of mice deficient in pregnancy-associated plasma protein-A. J Gerontol A Biol Sci Med Sci. 2010;65:590–599. doi: 10.1093/gerona/glq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover CA, Bale LK, Overgaard MT, Johnstone EW, Laursen UH, Fuchtbauer EM, Oxvig C, van Deursen J. Metalloproteinase pregnancy-associated plasma protein A is a critical growth regulatory factor during fetal development. Development. 2004;131:1187–1194. doi: 10.1242/dev.00997. [DOI] [PubMed] [Google Scholar]
- Conover CA, Chen BK, Resch ZT. Regulation of pregnancy-associated plasma protein-A expression in cultured human osteoblasts. Bone. 2004;34:297–302. doi: 10.1016/j.bone.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Conover CA, Faessen GF, Ilg KE, Chandrasekher YA, Christiansen M, Overgaard MT, Oxvig C, Giudice LC. Pregnancy-associated plasma protein-a is the insulin-like growth factor binding protein-4 protease secreted by human ovarian granulosa cells and is a marker of dominant follicle selection and the corpus luteum. Endocrinology. 2001;142:2155. doi: 10.1210/endo.142.5.8286. [DOI] [PubMed] [Google Scholar]
- Conover CA, Harrington SC, Bale LK. Differential regulation of pregnancy associated plasma protein-A in human coronary artery endothelial cells and smooth muscle cells. Growth Horm IGF Res. 2008;18:213–220. doi: 10.1016/j.ghir.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover CA, Harstad SL, Tchkonia T, Kirkland JL. Preferential impact of pregnancy-associated plasma protein-A deficiency on visceral fat in mice on high-fat diet. Am J Physiol Endocrinol Metab. 2013;305:E1145–E1153. doi: 10.1152/ajpendo.00405.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover CA, Oxvig C, Overgaard MT, Christiansen M, Giudice LC. Evidence that the insulin-like growth factor binding protein-4 protease in human ovarian follicular fluid is pregnancy associated plasma protein-A. J Clin Endocrinol Metab. 1999;84:4742–4745. doi: 10.1210/jcem.84.12.6342. [DOI] [PubMed] [Google Scholar]
- Consuegra-Sanchez L, Fredericks S, Kaski JC. Pregnancy-associated plasma protein-A (PAPP-A) and cardiovascular risk. Atherosclerosis. 2009;203:346–352. doi: 10.1016/j.atherosclerosis.2008.07.042. [DOI] [PubMed] [Google Scholar]
- Dalgin GS, Holloway DT, Liou LS, DeLisi C. Identification and characterization of renal cell carcinoma gene markers. Cancer Inform. 2007;3:65–92. [PMC free article] [PubMed] [Google Scholar]
- DeChiara TM, Efstratiadis A, Robertson EJ. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature. 1990;345:78–80. doi: 10.1038/345078a0. [DOI] [PubMed] [Google Scholar]
- Fenwick JC, So YP. A perfusion study of the effect of stanniectomy on the net influx of calcium 45 across an isolated eel gill (1) J Exp Zool. 1974;188:125–131. doi: 10.1002/jez.1401880112. [DOI] [PubMed] [Google Scholar]
- Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- Forbes BE, McCarthy P, Norton RS. Insulin-like growth factor binding proteins: a structural perspective. Front Endocrinol (Lausanne) 2012;3:38. doi: 10.3389/fendo.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardi AD, Kuo EY, Raulic S, Wagner GF, DiMattia GE. Human stanniocalcin-2 exhibits potent growth-suppressive properties in transgenic mice independently of growth hormone and IGFs. Am J Physiol Endocrinol Metab. 2005;288:E92–E105. doi: 10.1152/ajpendo.00268.2004. [DOI] [PubMed] [Google Scholar]
- Gaidamauskas E, Gyrup C, Boldt HB, Schack VR, Overgaard MT, Laursen LS, Oxvig C. IGF dependent modulation of IGF binding protein (IGFBP) proteolysis by pregnancy-associated plasma protein-A (PAPP-A): multiple PAPP-A-IGFBP interaction sites. Biochim Biophys Acta. 2013;1830:2701–2709. doi: 10.1016/j.bbagen.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Giudice LC, Conover CA, Bale L, Faessen GH, Ilg K, Sun I, Imani B, Suen LF, Irwin JC, Christiansen M, Overgaard MT, Oxvig C. Identification and regulation of the IGFBP-4 protease and its physiological inhibitor in human trophoblasts and endometrial stroma: evidence for paracrine regulation of IGF-II bioavailability in the placental bed during human implantation. J Clin Endocrinol Metab. 2002;87:2359–2366. doi: 10.1210/jcem.87.5.8448. [DOI] [PubMed] [Google Scholar]
- Glerup S, Boldt HB, Overgaard MT, Sottrup-Jensen L, Giudice LC, Oxvig C. Proteinase inhibition by proform of eosinophil major basic protein (pro-MBP) is a multistep process of intra- and intermolecular disulfide rearrangements. J Biol Chem. 2005;280:9823–9832. doi: 10.1074/jbc.M413228200. [DOI] [PubMed] [Google Scholar]
- Glerup S, Kloverpris S, Laursen LS, Dagnaes-Hansen F, Thiel S, Conover CA, Oxvig C. Cell surface detachment of pregnancy-associated plasma protein-A requires the formation of intermolecular proteinase-inhibitor disulfide bonds and glycosaminoglycan covalently bound to the inhibitor. J Biol Chem. 2007;282:1769–1778. doi: 10.1074/jbc.M608454200. [DOI] [PubMed] [Google Scholar]
- Guo F, Li Y, Wang J, Li Y, Li Y, Li G. Stanniocalcin1 (STC1) inhibits cell proliferation and invasion of cervical cancer cells. PLoS One. 2013;8:e53989. doi: 10.1371/journal.pone.0053989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyrup C, Christiansen M, Oxvig C. Quantification of proteolytically active pregnancy-associated plasma protein-A with an assay based on quenched fluorescence. Clin Chem. 2007;53:947–954. doi: 10.1373/clinchem.2006.080614. [DOI] [PubMed] [Google Scholar]
- Gyrup C, Oxvig C. Quantitative analysis of insulin-like growth factor-modulated proteolysis of insulin-like growth factor binding protein −4 and −5 by pregnancy-associated plasma protein-A. Biochemistry. 2007;46:1972–1980. doi: 10.1021/bi062229i. [DOI] [PubMed] [Google Scholar]
- Harrington SC, Simari RD, Conover CA. Genetic deletion of pregnancy-associated plasma protein-A is associated with resistance to atherosclerotic lesion development in apolipoprotein E-deficient mice challenged with a high-fat diet. Circ Res. 2007;100:1696–1702. doi: 10.1161/CIRCRESAHA.106.146183. [DOI] [PubMed] [Google Scholar]
- Heidegger I, Pircher A, Klocker H, Massoner P. Targeting the insulin-like growth factor network in cancer therapy. Cancer Biol Ther. 2011;11:701–707. doi: 10.4161/cbt.11.8.14689. [DOI] [PubMed] [Google Scholar]
- Huang J, Tabata S, Kakiuchi S, Van The T, Goto H, Hanibuchi M, Nishioka Y. Identification of pregnancy-associated plasma protein A as a migration-promoting gene in malignant pleural mesothelioma cells: a potential therapeutic target. Oncotarget. 2013;4:1172–1184. doi: 10.18632/oncotarget.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen MR, Kloverpris S, Mikkelsen JH, Pedersen JH, Fuchtbauer EM, Laursen LS, Oxvig C. Stanniocalcin-2 inhibits mammalian growth by proteolytic inhibition of the insulin-like growth factor axis. J Biol Chem. 2014 doi: 10.1074/jbc.M114.611665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joensuu K, Heikkila P, Andersson LC. Tumor dormancy: elevated expression of stanniocalcins in late relapsing breast cancer. Cancer Lett. 2008;265:76–83. doi: 10.1016/j.canlet.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Junnila RK, List EO, Berryman DE, Murrey JW, Kopchick JJ. The GH/IGF-1 axis in ageing and longevity. Nat Rev Endocrinol. 2013;9:366–376. doi: 10.1038/nrendo.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalli KR, Chen BK, Bale LK, Gernand E, Overgaard MT, Oxvig C, Cliby WA, Conover CA. Pregnancy-associated plasma protein-A (PAPP-A) expression and insulin-like growth factor binding protein-4 protease activity in normal and malignant ovarian surface epithelial cells. Int J Cancer. 2004;110:633–640. doi: 10.1002/ijc.20185. [DOI] [PubMed] [Google Scholar]
- Kalousova M, Zima T, Krane V, Marz W, Wanner C, Tesar V, Drechsler C, German D, Dialysis Study I Pregnancy-associated plasma protein A associates with cardiovascular events in diabetic hemodialysis patients. Atherosclerosis. 2014;236:263–269. doi: 10.1016/j.atherosclerosis.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Kirkegaard I, Uldbjerg N, Oxvig C. Biology of pregnancy-associated plasma protein-A in relation to prenatal diagnostics: an overview. Acta Obstet Gynecol Scand. 2010;89:1118–1125. doi: 10.3109/00016349.2010.505639. [DOI] [PubMed] [Google Scholar]
- Kjaer-Sorensen K, Engholm DH, Jepsen MR, Morch MG, Weyer K, Hefting LL, Skov LL, Laursen LS, Oxvig C (2014) Pregnancy-associated plasma protein-A2 modulates development of cranial cartilage and angiogenesis in zebrafish embryos. J Cell Sci [DOI] [PubMed]
- Kjaer-Sorensen K, Engholm DH, Kamei H, Morch MG, Kristensen AO, Zhou J, Conover CA, Duan C, Oxvig C. Pregnancy-associated plasma protein A (PAPP-A) modulates the early developmental rate in zebrafish independently of its proteolytic activity. J Biol Chem. 2013;288:9982–9992. doi: 10.1074/jbc.M112.426304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen T, Oxvig C, Sand O, Moller NP, Sottrup-Jensen L. Amino acid sequence of human pregnancy-associated plasma protein-A derived from cloned cDNA. Biochemistry. 1994;33:1592–1598. doi: 10.1021/bi00172a040. [DOI] [PubMed] [Google Scholar]
- Kuhajda FP, Eggleston JC. Pregnancy-associated plasma protein A. A clinically significant predictor of early recurrence in stage I breast carcinoma is independent of estrogen receptor status. Am J Pathol. 1985;121:342–348. [PMC free article] [PubMed] [Google Scholar]
- Kuhajda FP, Katumuluwa AI, Pasternack GR. Expression of haptoglobin-related protein and its potential role as a tumor antigen. Proc Natl Acad Sci U S A. 1989;86:1188–1192. doi: 10.1073/pnas.86.4.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen LS, Kjaer-Sorensen K, Andersen MH, Oxvig C. Regulation of insulin-like growth factor (IGF) bioactivity by sequential proteolytic cleavage of IGF binding protein −4 and −5. Mol Endocrinol. 2007;21:1246–1257. doi: 10.1210/me.2006-0522. [DOI] [PubMed] [Google Scholar]
- Laursen LS, Overgaard MT, Nielsen CG, Boldt HB, Hopmann KH, Conover CA, Sottrup-Jensen L, Giudice LC, Oxvig C. Substrate specificity of the metalloproteinase pregnancy-associated plasma protein-A (PAPP-A) assessed by mutagenesis and analysis of synthetic peptides: substrate residues distant from the scissile bond are critical for proteolysis. Biochem J. 2002;367:31–40. doi: 10.1042/BJ20020831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen LS, Overgaard MT, Soe R, Boldt HB, Sottrup-Jensen L, Giudice LC, Conover CA, Oxvig C. Pregnancy-associated plasma protein-A (PAPP-A) cleaves insulin-like growth factor binding protein (IGFBP)-5 independent of IGF: implications for the mechanism of IGFBP-4 proteolysis by PAPP-A. FEBS Lett. 2001;504:36–40. doi: 10.1016/s0014-5793(01)02760-0. [DOI] [PubMed] [Google Scholar]
- Laursen LS, Overgaard MT, Weyer K, Boldt HB, Ebbesen P, Christiansen M, Sottrup-Jensen L, Giudice LC, Oxvig C. Cell surface targeting of pregnancy-associated plasma protein A proteolytic activity. Reversible adhesion is mediated by two neighboring short consensus repeats. J Biol Chem. 2002;277:47225–47234. doi: 10.1074/jbc.M209155200. [DOI] [PubMed] [Google Scholar]
- Lawrence JB, Oxvig C, Overgaard MT, Sottrup-Jensen L, Gleich GJ, Hays LG, Yates JR, 3rd, Conover CA. The insulin-like growth factor (IGF)-dependent IGF binding protein-4 protease secreted by human fibroblasts is pregnancy-associated plasma protein-A. Proc Natl Acad Sci U S A. 1999;96:3149–3153. doi: 10.1073/pnas.96.6.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhou C, Zhou X, Song L, Hui R. PAPP-A in cardiac and non-cardiac conditions. Clin Chim Acta. 2013;417:67–72. doi: 10.1016/j.cca.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Lin TM, Halbert SP, Spellacy WN. Measurement of pregnancy-associated plasma proteins during human gestation. J Clin Invest. 1974;54:576–582. doi: 10.1172/JCI107794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loddo M, Andryszkiewicz J, Rodriguez-Acebes S, Stoeber K, Jones A, Dafou D, Apostolidou S, Wollenschlaeger A, Widschwendter M, Sainsbury R, Tudzarova S, Williams GH. Pregnancy-associated plasma protein A regulates mitosis and is epigenetically silenced in breast cancer. J Pathol. 2014;233:344–356. doi: 10.1002/path.4393. [DOI] [PubMed] [Google Scholar]
- Mader JR, Resch ZT, McLean GR, Mikkelsen JH, Oxvig C, Marler RJ, Conover CA. Mice deficient in PAPP-A show resistance to the development of diabetic nephropathy. J Endocrinol. 2013;219:51–58. doi: 10.1530/JOE-13-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield AS, Visscher DW, Hart SN, Wang C, Goetz MP, Oxvig C, Conover CA. Pregnancy-associated plasma protein-A expression in human breast cancer. Growth Horm IGF Res. 2014;24:264–267. doi: 10.1016/j.ghir.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen JH, Gyrup C, Kristensen P, Overgaard MT, Poulsen CB, Laursen LS, Oxvig C. Inhibition of the proteolytic activity of pregnancy-associated plasma protein-A by targeting substrate exosite binding. J Biol Chem. 2008;283:16772–16780. doi: 10.1074/jbc.M802429200. [DOI] [PubMed] [Google Scholar]
- Mikkelsen JH, Resch ZT, Kalra B, Savjani G, Kumar A, Conover CA, Oxvig C. Indirect targeting of IGF receptor signaling in vivo by substrate-selective inhibition of PAPP-A proteolytic activity. Oncotarget. 2014;5:1014–1025. doi: 10.18632/oncotarget.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakoshi N, Qin X, Kasukawa Y, Richman C, Srivastava AK, Baylink DJ, Mohan S. Systemic administration of insulin-like growth factor (IGF)-binding protein-4 (IGFBP-4) increases bone formation parameters in mice by increasing IGF bioavailability via an IGFBP-4 protease-dependent mechanism. Endocrinology. 2001;142:2641–2648. doi: 10.1210/endo.142.6.8192. [DOI] [PubMed] [Google Scholar]
- Monget P, Mazerbourg S, Delpuech T, Maurel MC, Maniere S, Zapf J, Lalmanach G, Oxvig C, Overgaard MT. Pregnancy-associated plasma protein-A is involved in insulin-like growth factor binding protein-2 (IGFBP-2) proteolytic degradation in bovine and porcine preovulatory follicles: identification of cleavage site and characterization of IGFBP-2 degradation. Biol Reprod. 2003;68:77–86. doi: 10.1095/biolreprod.102.007609. [DOI] [PubMed] [Google Scholar]
- Nagarajan N, Bertrand D, Hillmer AM, Zang ZJ, Yao F, Jacques PE, Teo AS, Cutcutache I, Zhang Z, Lee WH, Sia YY, Gao S, Ariyaratne PN, Ho A, Woo XY, Veeravali L, Ong CK, Deng N, Desai KV, Khor CC, Hibberd ML, Shahab A, Rao J, Wu M, Teh M, Zhu F, Chin SY, Pang B, So JB, Bourque G, Soong R, Sung WK, Tean Teh B, Rozen S, Ruan X, Yeoh KG, Tan PB, Ruan Y. Whole-genome reconstruction and mutational signatures in gastric cancer. Genome Biol. 2012;13:R115. doi: 10.1186/gb-2012-13-12-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ning Y, Schuller AG, Bradshaw S, Rotwein P, Ludwig T, Frystyk J, Pintar JE. Diminished growth and enhanced glucose metabolism in triple knockout mice containing mutations of insulin-like growth factor binding protein −3, −4, and −5. Mol Endocrinol. 2006;20:2173–2186. doi: 10.1210/me.2005-0196. [DOI] [PubMed] [Google Scholar]
- Ning Y, Schuller AG, Conover CA, Pintar JE. Insulin-like growth factor (IGF) binding protein-4 is both a positive and negative regulator of IGF activity in vivo. Mol Endocrinol. 2008;22:1213–1225. doi: 10.1210/me.2007-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyegaard M, Overgaard MT, Su YQ, Hamilton AE, Kwintkiewicz J, Hsieh M, Nayak NR, Conti M, Conover CA, Giudice LC. Lack of functional pregnancy-associated plasma protein-A (PAPPA) compromises mouse ovarian steroidogenesis and female fertility. Biol Reprod. 2010;82:1129–1138. doi: 10.1095/biolreprod.109.079517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz CO, Chen BK, Bale LK, Overgaard MT, Oxvig C, Conover CA. Transforming growth factor-beta regulation of the insulin-like growth factor binding protein-4 protease system in cultured human osteoblasts. J Bone Miner Res. 2003;18:1066–1072. doi: 10.1359/jbmr.2003.18.6.1066. [DOI] [PubMed] [Google Scholar]
- Overgaard MT, Boldt HB, Laursen LS, Sottrup-Jensen L, Conover CA, Oxvig C. Pregnancy-associated plasma protein-A2 (PAPP-A2), a novel insulin-like growth factor-binding protein-5 proteinase. J Biol Chem. 2001;276:21849–21853. doi: 10.1074/jbc.M102191200. [DOI] [PubMed] [Google Scholar]
- Overgaard MT, Glerup S, Boldt HB, Rodacker V, Olsen IM, Christiansen M, Sottrup-Jensen L, Giudice LC, Oxvig C. Inhibition of proteolysis by the proform of eosinophil major basic protein (proMBP) requires covalent binding to its target proteinase. FEBS Lett. 2004;560:147–152. doi: 10.1016/S0014-5793(04)00095-X. [DOI] [PubMed] [Google Scholar]
- Overgaard MT, Haaning J, Boldt HB, Olsen IM, Laursen LS, Christiansen M, Gleich GJ, Sottrup-Jensen L, Conover CA, Oxvig C. Expression of recombinant human pregnancy-associated plasma protein-A and identification of the proform of eosinophil major basic protein as its physiological inhibitor. J Biol Chem. 2000;275:31128–31133. doi: 10.1074/jbc.M001384200. [DOI] [PubMed] [Google Scholar]
- Overgaard MT, Oxvig C, Christiansen M, Lawrence JB, Conover CA, Gleich GJ, Sottrup-Jensen L, Haaning J. Messenger ribonucleic acid levels of pregnancy-associated plasma protein-A and the proform of eosinophil major basic protein: expression in human reproductive and nonreproductive tissues. Biol Reprod. 1999;61:1083–1089. doi: 10.1095/biolreprod61.4.1083. [DOI] [PubMed] [Google Scholar]
- Overgaard MT, Sorensen ES, Stachowiak D, Boldt HB, Kristensen L, Sottrup-Jensen L, Oxvig C. Complex of pregnancy-associated plasma protein-A and the proform of eosinophil major basic protein. Disulfide structure and carbohydrate attachment. J Biol Chem. 2003;278:2106–2117. doi: 10.1074/jbc.M208777200. [DOI] [PubMed] [Google Scholar]
- Oxvig C, Haaning J, Hojrup P, Sottrup-Jensen L. Location and nature of carbohydrate groups in proform of human major basic protein isolated from pregnancy serum. Biochem Mol Biol Int. 1994;33:329–336. [PubMed] [Google Scholar]
- Oxvig C, Sand O, Kristensen T, Gleich GJ, Sottrup-Jensen L. Circulating human pregnancy-associated plasma protein-A is disulfide-bridged to the proform of eosinophil major basic protein. J Biol Chem. 1993;268:12243–12246. [PubMed] [Google Scholar]
- Oxvig C, Sand O, Kristensen T, Kristensen L, Sottrup-Jensen L. Isolation and characterization of circulating complex between human pregnancy-associated plasma protein-A and proform of eosinophil major basic protein. Biochim Biophys Acta. 1994;1201:415–423. doi: 10.1016/0304-4165(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Pan H, Hanada S, Zhao J, Mao L, Ma MZ. Protein secretion is required for pregnancy-associated plasma protein-A to promote lung cancer growth in vivo. PLoS One. 2012;7:e48799. doi: 10.1371/journal.pone.0048799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12:159–169. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- Popken-Harris P, Checkel J, Loegering D, Madden B, Springett M, Kephart G, Gleich GJ. Regulation and processing of a precursor form of eosinophil granule major basic protein (ProMBP) in differentiating eosinophils. Blood. 1998;92:623–631. [PubMed] [Google Scholar]
- Qin QP, Christiansen M, Oxvig C, Pettersson K, Sottrup-Jensen L, Koch C, Norgaard-Pedersen B. Double-monoclonal immunofluorometric assays for pregnancy-associated plasma protein A/proeosinophil major basic protein (PAPP-A/proMBP) complex in first-trimester maternal serum screening for Down syndrome. Clin Chem. 1997;43:2323–2332. [PubMed] [Google Scholar]
- Qin X, Byun D, Lau KH, Baylink DJ, Mohan S. Evidence that the interaction between insulin-like growth factor (IGF)-II and IGF binding protein (IGFBP)-4 is essential for the action of the IGF-II-dependent IGFBP-4 protease. Arch Biochem Biophys. 2000;379:209–216. doi: 10.1006/abbi.2000.1872. [DOI] [PubMed] [Google Scholar]
- Qin X, Wergedal JE, Rehage M, Tran K, Newton J, Lam P, Baylink DJ, Mohan S. Pregnancy-associated plasma protein-A increases osteoblast proliferation in vitro and bone formation in vivo. Endocrinology. 2006;147:5653–5661. doi: 10.1210/en.2006-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehage M, Mohan S, Wergedal JE, Bonafede B, Tran K, Hou D, Phang D, Kumar A, Qin X. Transgenic overexpression of pregnancy-associated plasma protein-A increases the somatic growth and skeletal muscle mass in mice. Endocrinology. 2007;148:6176–6185. doi: 10.1210/en.2007-0274. [DOI] [PubMed] [Google Scholar]
- Resch ZT, Chen BK, Bale LK, Oxvig C, Overgaard MT, Conover CA. Pregnancy-associated plasma protein a gene expression as a target of inflammatory cytokines. Endocrinology. 2004;145:1124–1129. doi: 10.1210/en.2003-1313. [DOI] [PubMed] [Google Scholar]
- Resch ZT, Simari RD, Conover CA. Targeted disruption of the pregnancy-associated plasma protein-A gene is associated with diminished smooth muscle cell response to insulin-like growth factor-I and resistance to neointimal hyperplasia after vascular injury. Endocrinology. 2006;147:5634–5640. doi: 10.1210/en.2006-0493. [DOI] [PubMed] [Google Scholar]
- Ryan AJ, Napoletano S, Fitzpatrick PA, Currid CA, O’Sullivan NC, Harmey JH. Expression of a protease-resistant insulin-like growth factor-binding protein-4 inhibits tumour growth in a murine model of breast cancer. Br J Cancer. 2009;101:278–286. doi: 10.1038/sj.bjc.6605141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim H, Arvanitis A, de Petris L, Kanter L, Haag P, Zovko A, Ozata DM, Lui WO, Lundholm L, Zhivotovsky B, Lewensohn R, Viktorsson K. miRNA-214 is related to invasiveness of human non-small cell lung cancer and directly regulates alpha protein kinase 2 expression. Genes Chromosomes Cancer. 2013;52:895–911. doi: 10.1002/gcc.22085. [DOI] [PubMed] [Google Scholar]
- Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- Sinosich MJ. Molecular characterization of pregnancy-associated plasma protein-A by electrophoresis. Electrophoresis. 1990;11:70–78. doi: 10.1002/elps.1150110115. [DOI] [PubMed] [Google Scholar]
- Sjogren K, Liu JL, Blad K, Skrtic S, Vidal O, Wallenius V, LeRoith D, Tornell J, Isaksson OG, Jansson JO, Ohlsson C. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci U S A. 1999;96:7088–7092. doi: 10.1073/pnas.96.12.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GC, Stenhouse EJ, Crossley JA, Aitken DA, Cameron AD, Connor JM. Early-pregnancy origins of low birth weight. Nature. 2002;417:916. doi: 10.1038/417916a. [DOI] [PubMed] [Google Scholar]
- Soe R, Overgaard MT, Thomsen AR, Laursen LS, Olsen IM, Sottrup-Jensen L, Haaning J, Giudice LC, Conover CA, Oxvig C. Expression of recombinant murine pregnancy-associated plasma protein-A (PAPP-A) and a novel variant (PAPP-Ai) with differential proteolytic activity. Eur J Biochem. 2002;269:2247–2256. doi: 10.1046/j.1432-1033.2002.02883.x. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Kobayashi H, Tanaka Y, Hirashima Y, Kanayama N, Takei Y, Saga Y, Suzuki M, Itoh H, Terao T. Bikunin target genes in ovarian cancer cells identified by microarray analysis. J Biol Chem. 2003;278:14640–14646. doi: 10.1074/jbc.M300239200. [DOI] [PubMed] [Google Scholar]
- Tallant C, Garcia-Castellanos R, Seco J, Baumann U, Gomis-Ruth FX. Molecular analysis of ulilysin, the structural prototype of a new family of metzincin metalloproteases. J Biol Chem. 2006;281:17920–17928. doi: 10.1074/jbc.M600907200. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Kobayashi H, Suzuki M, Hirashima Y, Kanayama N, Terao T. Genetic downregulation of pregnancy-associated plasma protein-A (PAPP-A) by bikunin reduces IGF-I-dependent Akt and ERK1/2 activation and subsequently reduces ovarian cancer cell growth, invasion and metastasis. Int J Cancer. 2004;109:336–347. doi: 10.1002/ijc.11700. [DOI] [PubMed] [Google Scholar]
- Vallejo AN, Michel JJ, Bale LK, Lemster BH, Borghesi L, Conover CA. Resistance to age-dependent thymic atrophy in long-lived mice that are deficient in pregnancy-associated plasma protein A. Proc Natl Acad Sci U S A. 2009;106:11252–11257. doi: 10.1073/pnas.0807025106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GF, Dimattia GE. The stanniocalcin family of proteins. J Exp Zool A Comp Exp Biol. 2006;305:769–780. doi: 10.1002/jez.a.313. [DOI] [PubMed] [Google Scholar]
- Wagner GF, Hampong M, Park CM, Copp DH. Purification, characterization, and bioassay of teleocalcin, a glycoprotein from salmon corpuscles of Stannius. Gen Comp Endocrinol. 1986;63:481–491. doi: 10.1016/0016-6480(86)90149-8. [DOI] [PubMed] [Google Scholar]
- Wald N, Stone R, Cuckle HS, Grudzinskas JG, Barkai G, Brambati B, Teisner B, Fuhrmann W. First trimester concentrations of pregnancy associated plasma protein A and placental protein 14 in Down’s syndrome. BMJ. 1992;305:28. doi: 10.1136/bmj.305.6844.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welcsh PL, Lee MK, Gonzalez-Hernandez RM, Black DJ, Mahadevappa M, Swisher EM, Warrington JA, King MC. BRCA1 transcriptionally regulates genes involved in breast tumorigenesis. Proc Natl Acad Sci U S A. 2002;99:7560–7565. doi: 10.1073/pnas.062181799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyer K, Boldt HB, Poulsen CB, Kjaer-Sorensen K, Gyrup C, Oxvig C. A substrate specificity-determining unit of three Lin12-Notch repeat modules is formed in trans within the pappalysin-1 dimer and requires a sequence stretch C-terminal to the third module. J Biol Chem. 2007;282:10988–10999. doi: 10.1074/jbc.M607903200. [DOI] [PubMed] [Google Scholar]
- Weyer K, Overgaard MT, Laursen LS, Nielsen CG, Schmitz A, Christiansen M, Sottrup-Jensen L, Giudice LC, Oxvig C. Cell surface adhesion of pregnancy-associated plasma protein-A is mediated by four clusters of basic residues located in its third and fourth CCP module. Eur J Biochem. 2004;271:1525–1535. doi: 10.1111/j.1432-1033.2004.04061.x. [DOI] [PubMed] [Google Scholar]
- Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, LeRoith D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci U S A. 1999;96:7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Baxter RC, Firth SM. Involvement of pregnancy-associated plasma protein-A2 in insulin-like growth factor (IGF) binding protein-5 proteolysis during pregnancy: a potential mechanism for increasing IGF bioavailability. J Clin Endocrinol Metab. 2010;95:1412–1420. doi: 10.1210/jc.2009-2277. [DOI] [PubMed] [Google Scholar]
- Yee D. Insulin-like growth factor receptor inhibitors: baby or the bathwater? J Natl Cancer Inst. 2012;104:975–981. doi: 10.1093/jnci/djs258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung BH, Law AY, Wong CK. Evolution and roles of stanniocalcin. Mol Cell Endocrinol. 2012;349:272–280. doi: 10.1016/j.mce.2011.11.007. [DOI] [PubMed] [Google Scholar]