Abstract
There is increasing interest in non-rodent translational models for the study of human disease. The pig, in particular, serves as a useful animal model for the study of pathophysiological conditions relevant to the human intestine. This review assesses currently used porcine models of gastrointestinal physiology and disease and provides a rationale for the use of these models for future translational studies. The pig has proven its utility for the study of fundamental disease conditions such as ischemia/ reperfusion injury, stress-induced intestinal dysfunction, and short bowel syndrome. Pigs have also shown great promise for the study of intestinal barrier function, surgical tissue manipulation and intervention, as well as biomaterial implantation and tissue transplantation. Advantages of pig models highlighted by these studies include the physiological similarity to human intestine as well as to mechanisms of human disease. Emerging future directions for porcine models of human disease include the fields of transgenics and stem cell biology, with exciting implications for regenerative medicine.
Introduction
Digestive disease results in more than 230,000 deaths annually in the United States, with colorectal cancer as the leading cause of mortality in adults from digestive disease (1). Animal models are imperative for translational research targeted at improving human health. Because of the limitations of directly studying human disease in a clinical setting, animal models have been used extensively to expand basic science knowledge. Rodents, particularly mice, have been commonly used animal models of disease because of their relatively low cost, ease of maintenance, and rapid reproduction rate (2, 3). This has been facilitated by the creation of inbred strains that represent spontaneous models of disease. Examples include TNFΔARE and SAMP1/YitFc mice strains that exhibit Crohn’s disease-like ileitis as is seen in human patients (4, 5). They also make for effective models of cancer because of their high susceptibility to developing chemically induced malignancies (6). Furthermore, they are highly amenable to genetic manipulation (2, 7). The utilization of transgenic and knockout mice has provided invaluable insight into the impact of genetic mutations and specific genes on disease etiology and progression (8–10). However, murine models often lack key clinical signs or pathological changes representative of human gastrointestinal disease which are essential to improve translational studies and drug discovery (Table 1) (7, 11–19). Therefore, there is renewed interest in large animal models that more closely resemble human disease processes (20, 21) and provide a non-rodent model for drug discovery. Aside from physiological considerations, the larger size of pigs is advantageous for models requiring surgical manipulation, such as Thiry-Vella loops in which an isolated cannulated segment of intestine is studied in vivo (22), or where research involves tissue transplantation (23). Of other large animals used in biomedical research, dogs have been used extensively, particularly for the study of ischemia/ reperfusion injury. However, with increasing social pressures to limit use of dogs as experimental animals and the high mortality rate associated with some disease models, the use of dogs is declining (18, 23). However, dogs are particularly well suited and are increasingly utilized for the study of spontaneous naturally occurring diseases that also affect humans, such as cancer. In fact, clinical trials in veterinary oncology, have been used to inform drug efficacy and safety in humans (24).
Table 1.
Comparison of Porcine and Rodent Models of Digestive Disease
| Advantages | Disadvantages | |
|---|---|---|
| Porcine |
|
|
| Rodent |
|
|
The pig has a number of distinct advantages that has made this species a useful translational research animal model (Table 1). In particular, there are important anatomical and physiological similarities to human beings (19, 25). The pig has a comparably sized genome with extensive homology to humans. The pig genome has a 60% sequence homology to humans as compared to rodents with only 40% homology (26, 27). Additionally, as compared to mouse, rat, dog, cat or horse, the pig chromosomal structure is more like humans (28, 29). Pigs, like humans, are omnivores and share similar metabolic and intestinal physiological processes (19, 30, 31). For example, a comparison of the recommended daily allowances of vitamins and minerals in the human diet and the daily nutrient requirement of pigs reveal striking similarities between the two species in infancy, growth, reproduction, and lactation (19, 32). This likely contributes to their comparable mucosal barrier physiology and enteric microbiota, as well as susceptibility to select enteric pathogens (19, 33). The role of the intestinal microbiota in maintaining intestinal health has been highlighted in recent years, and disturbances in microbial composition have been associated with important human diseases such as diarrhea, neonatal necrotizing enterocolitis and obesity. Conversely, studies focusing on the relationship between the composition of the gut microbiota and disease have shown widely diverging results when comparing mice to humans (33–35). This has been addressed most recently by utilizing humanized germ-free mice transplanted with human microbiota (36–39). However, similarities in the intestinal microbial ecology between pigs and humans have made the pig a useful non-primate animal model for studies of dietary modulation of microbiota (40). Furthermore, a humanized germ-free pig model has also been established (33, 39). Under natural conditions though, both human and porcine gut microbiota consist mainly of Firmicutes and Bacteroidetes phyla although their overall gastrointestinal microbial diversity is affected by diet, age and environmental conditions (40–42). Additionally, pharmaceutical bioavailability and nutrient digestibility in pigs closely resemble that of humans (19, 25, 43). These characteristics have led to the use of pigs for the development of pig models of a number of gastrointestinal diseases including necrotizing enterocolitis (16, 44–48), acute mesenteric ischemia (18, 49–52), short bowel syndrome (43, 53–57), AIDS-associated Cryptosporidium infection (17, 58, 59), stress-induced intestinal dysfunction (60–63), cystic fibrosis (64, 65) and familial adenomatous polyposis (14). This review will highlight strengths and limitations of pig models of intestinal ischemia/reperfusion injury, stress-induced intestinal dysfunction and short bowel syndrome. In addition, we have reviewed information that will extend the discussion on animal models in the fields of transplantation, bioengineering, and transgenics.
Comparative gastrointestinal anatomy: Similarities and differences between humans and pigs
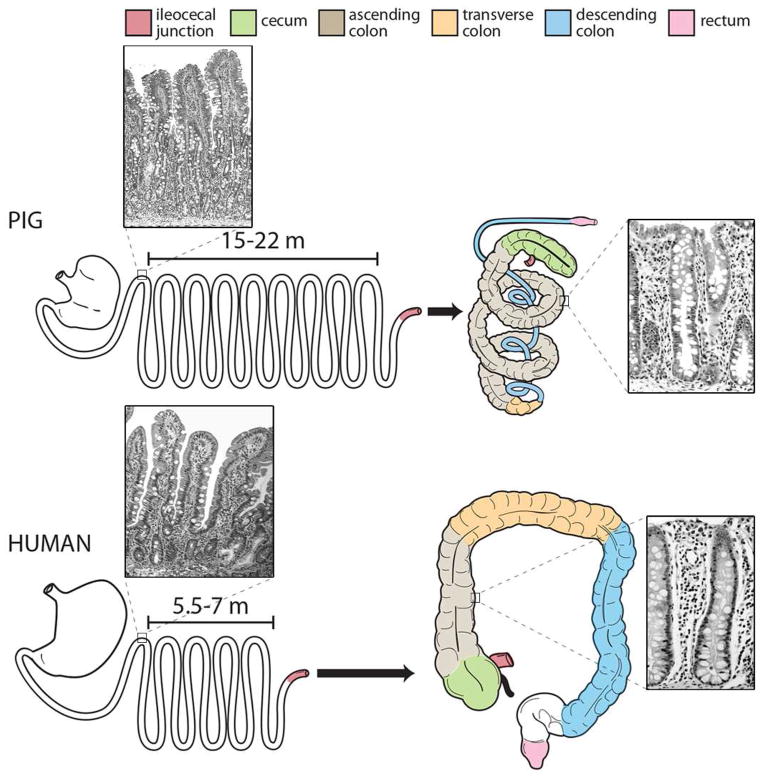
Pigs have significant anatomical and physiological similarity with human beings, with some key comparisons noted in Table 2 and Figure 1. The structure of the small intestine is very similar in humans and pigs, including macroscopic features such as the ratio of intestinal length per kilogram bodyweight (Table 2) (19). Other gross similarities include the presence of sacculations and taenia (bands of longitudinal muscle) extending along the majority of the colonic length in both human and porcine colons (30, 66). These anatomical similarities contribute to the comparable transit time and analogous digestive and absorptive processes reported for these species (67). Shared microscopic features also exist, including the structure of the villi and the types of cells that constitute the intestinal epithelium (Figure 1). For example, the villous projections within the small intestine of both species are finger shaped, whereas in rats they are flat leaf-like structures (30). Additionally, the cell lineages that constitute the intestinal epithelium, their phenotypic appearance, and their expression of unique protein biomarkers used for cellular identification are similar in pigs and humans (68). These include the ultrastructural appearance of stem, goblet, and enteroendocrine cells as well as absorptive enterocytes (68). A comparative analysis of sub-cellular features of porcine intestinal epithelial cells located within the crypt base, using transmission electron microscopy, revealed multiple irregularly shaped, small, columnar cells with basally located nuclei and scarce cytoplasm (68). This morphologic appearance is consistent with the description of crypt base columnar stem cells in humans (68, 69). Taken together, these features contribute to fundamental digestive processes such as mucosal transport and motility that are similar between pigs and humans (19). Additionally, pigs, like humans, are true omnivores, whereas other potential mammalian models such as dogs, cats, ruminants, rabbits and rodents have evolutionarily developed alternative digestive strategies (30, 70). Furthermore, pigs and humans have similar neonatal gut development and gastrointestinal immunologic responses to insult (30, 70, 71). As far as differences in gastrointestinal tract anatomy, the porcine cecum is relatively large and clearly delineated as compared to the human cecum, and the porcine colon is orientated in a spiral fashion (Figure 1) (30). Pigs also lack an appendix (30, 70). Other important considerations when using the pig as an animal model to study human development and disease is their rapid growth rate, their size once fully mature and differences in gut-associated lymphoid tissue (40, 72). For example, differences have been identified in the development, distribution, cellular composition and number of Peyer’s patches between pigs and humans. In general, Peyer’s patches are considered important in the gut to help discriminate between pathogenic and commensal bacteria however the significance of the differences between species remains unclear, especially in light of using the pig to model human intestine (72–74). Despite these differences, both pigs and humans are colon fermenters, unlike rodents that are cecal fermenters, and have similar colonic microbiota composition (32, 33).
Table 2.
Anatomical Comparison between Pigs and Humans (Adapted from Patterson et al. (19)
| Human | Pig | |
|---|---|---|
| Average birth weight (kg) | 3.4 | 1.4 |
| Average mature weight (kg) | 60–100 | 200–300 |
| Water composition at birth (%) | 82 | 82 |
| Water composition as adult (%) | 69 | 72 |
| Average lifespan (years) | 80 | 20 |
| Small Intestinal length as adult (m) | 5.5–7 | 15–22 |
| Large Intestinal length as adult (m) | 1.5 | 4–6 |
| Length of intestine per kilogram bodyweight (ratio) | ~0.1 | ~0.1 |
| Small Intestinal weight as adult (g) | 1040 | 2310 |
| Large Intestinal weight as adult (g) | 590 | 1970 |
Figure 1. Comparative Gastrointestinal Anatomy.
The porcine gastrointestinal anatomy (top) shares many similarities and some distinct differences to that of humans (bottom). The porcine intestinal length is greater than humans but the ratio of total length per kilogram of bodyweight for both pigs and humans is similar. The comparable portions of porcine and human colon are demonstrated with color. Images of histologic sections of porcine and human duodenum and colon are included for comparison.
On a cellular level, there is controversy within the literature of the presence or absence of the Paneth cell within the pig (75–77); a fully differentiated cell type that secretes antibacterial substances and has a proposed role in supporting the adjacent crypt base columnar cells within the small intestinal crypt base of other species, including humans (78–80). However, based on recent work, a cell sharing ultrastructural qualities attributed to the Paneth cell, including large size, elongated flattened nucleus and located adjacent to the crypt base columnar cells has been identified within porcine small intestine (68). These cells lack certain morphological features historically used to identify Paneth cells such as electron dense apically situated granules. Furthermore, no commercially available antibodies have thus far demonstrated cross reactivity to lysozyme, a well-accepted and commonly used biomarker for Paneth cells (68). However, further research is required to conclusively prove whether the cells that reside next to the crypt base columnar cells in pigs share the same functional role as the Paneth cell in other species. Never the less these differences appear to have relatively little effect on overall physiological function, which is critical when selecting an animal model for human digestive disease. In addition, there are important similarities between other cell populations thought to be critical to maintenance of the stem cell niche. For example, antibodies raised against human-derived proteins utilized as specific biomarkers for the identification of stem, progenitor, absorptive enterocyte, enteroendocrine and goblet cells cross react with porcine intestinal tissue, and demonstrate the same expression pattern observed in humans (68). These include antibodies against both SOX9 and HOPX that identify stem and progenitor cells. It should be noted, that antibody based tests are limited by available functional antibodies and none have specifically identified the crypt based columnar population. However, it has been shown that pigs do express crypt base columnar cell gene biomarkers (68). Work is needed to further develop the pig as a model to study intestinal stem cells however, considering what is established, the pig serves as a valuable model for studies of human intestinal injury, repair and efficacy of novel therapeutics (Table 3).
Table 3.
Direct Clinical Application of Porcine Models
| Clinical Impact | Research Support Using Porcine Models |
|---|---|
| • Understanding role of early life stress on onset and exacerbation of clinical symptoms of human gastrointestinal diseases | Early life stress induces a marked elevation of serum and intestinal corticotropin releasing factor (61) that plays a critical role in intestinal permeability and diarrhea Administration of CRF receptor antagonists and mast cell stabilizers to pigs prevented stress-induced increases in intestinal permeability (60, 61, 63, 86) |
| • Treatment of reperfusion Injury | Activation of xanthine oxidase plays less important role than initially postulated in inciting injury Injury is mediated by neutrophil release of oxygen metabolites and transmigration through epithelial tight junctions creating physical damage (121) |
| • Treatment of NEC patients | Oral probiotics reduce the incidence and severity of NEC in very low birth weight infants (148) |
| • Dietary considerations following massive bowel resection | GLP-2 hastens intestinal adaptation; FDA drug approval (43, 56, 57, 163–165) |
| • Successful intestinal transplantation | Simultaneous liver transplant does not decrease the risk of bowel graft rejection and is no longer recommended in patients with SBS with normal liver function (23) |
| • Cause and treatment of gastrointestinal dysfunction associated with cystic fibrosis | Pig model of CF accurately displays clinical manifestations of human disease permitting study of pathogenesis of intestinal obstruction (11, 12, 175, 192) |
| • Diagnosis of Familial Adenomatous Polyposis | Porcine transgenic model accurately represents disease manifestation, including location and histologic appearance of lesions (14) |
Psychological stress-induced intestinal dysfunction
Stress plays a central role in the onset and exacerbation of clinical symptoms in several gastrointestinal diseases of humans. Progress in the field of stress-related gastrointestinal disease has been hampered by the lack of relevant animal models of stress that recapitulate the chronic and complex biology of stress and the clinically relevant outcomes (e.g., diarrhea and weight loss) that are observed in humans. Much of the work in this field has utilized rodent models of short term/acute stress (81, 82). These models have yielded important insight into the mechanisms by which stress initiates gastrointestinal disease; however, there are obvious developmental and biological differences between rodents and humans that limit their translational implications. Porcine models are ideal for studying stress-related gastrointestinal disease because: (1) compared with rodents, the porcine gastrointestinal tract is most similar to humans with regard to development, anatomy, and function (83); (2) pigs possess a highly developed central and peripheral nervous system in order to perceive and integrate complex stress signaling processes in the brain and peripheral nervous systems (84); and (3) pigs, similar to humans, exhibit pathophysiological hallmarks of disease (intestinal inflammation) and relevant clinical signs (e.g. diarrhea and reduced weight gain) when subjected to stress.
Altered function of the enteric nervous system (ENS) plays a central, pathophysiological role in stress-related gastrointestinal diseases as it modulates stress-induced changes in motility, inflammation, intestinal permeability, and visceral hypersensitivity; therefore, the ENS is currently a therapeutic target. The porcine ENS has been shown to have similarities to human ENS that may offer advantages over commonly used rodent models (84). For example, intrinsic primary afferents in the human and porcine enteric nervous systems differ significant from rodents and other small laboratory animals in their distribution electrophysiological behavior, and synaptic properties (84, 85). These interspecies differences are important when considering animal models of human disease because it is imperative that therapeutic treatments for stress associated intestinal disorders such as IBS are based on results from animals with extensive similarity in their gastrointestinal tracts (84).
A novel porcine model of stress-induced gastrointestinal dysfunction that exhibits remarkable similarity to the pathophysiology of stress-related gastrointestinal disease in humans is the porcine early weaning stress model. In the porcine early weaning stress model, pigs are weaned from the sow at an early age (prior to 21 days of age), as compared to the natural weaning process which occurs gradually over months. The early weaning model has been valuable in studying the mechanisms of early life stress-induced gastrointestinal disease (60–63) while at the same time understanding the impact of stressor on gastrointestinal health in veterinary medicine. Early weaning stress is a common stress in pig production and involves an initial early life stress event followed by consistent, heterotypic stressors associated with changes in management and social conditions until they have reached market weight. This condition models aspects of heterotypic chronic stress in humans. Early weaning in pigs induces a stress response remarkably similar to humans and is characterized by elevated serum and intestinal corticotropin releasing factor (CRF) and subsequent modulation of intestinal mast cells that has been shown to play a critical role in intestinal permeability and diarrhea in pigs and humans (60, 61, 63, 86). In addition to the CRF-mast cell axis, other stress-induced neurochemicals may be involved in the intestinal pathophysiology associated with stress. For example, it has been shown that adrenocorticotropic hormone (ACTH) and norepinephrine (NE) increased the attachment of enterohemorrhagic Escherichia coli O157:H7 (EHEC) to isolate ex vivo porcine intestinal mucosa (87, 88).
Cholinergic and noradrenergic stimulation in isolated ex vivo preparations of porcine colonic mucosa was also shown to stimulate secretory IgA from the porcine intestine which in turn could modulate mucosal immunity during the stress response (89). Together, these findings suggest that mediators released during the stress response, such as NE and ACTH, may directly enhance the binding of bacteria, stimulate IgA secretion potentially modulating the intestinal stress response.
Ischemia/ reperfusion injury
Intestinal ischemic injury occurs when the blood supply to a segment of intestine is compromised. Paradoxically, tissue damage may continue when blood flow is reestablished and this is referred to as reperfusion injury. Injury attributed to ischemia/reperfusion injury is associated with many disease states including: intestinal volvulus (90), acute mesenteric ischemia (91), intestinal transplantation (92) and systemic disease that lowers tissue perfusion such as hemorrhagic shock or cardiopulmonary disease (93, 94). In general, an excess of 50% of patients suffering from intestinal ischemia/reperfusion succumb to sepsis associated with a breakdown in intestinal barrier function (95). In addition, ischemia is thought to contribute to necrotizing enterocolitis (NEC), the most common life-threatening gastrointestinal emergency in neonatal patients (96–98). Ultimately, mortality depends on the duration, location, and length of bowel affected by impaired perfusion. The type and degree of injury to the intestinal mucosa resulting from a pathologic decrease in intestinal blood flow is influenced by the microvascular architecture within the villi. Experimental models of ischemia have shown that the morphological signs of injury begin at the villus tip and progress toward the base of the villus (15). The villus circulation comprises blood entering the villus via a centrally-located arteriole, which arborizes at the tip of the villus and converges into venules located beside the arteriole and on the periphery of the villus (99). This sets up a counter-current exchange whereby oxygen diffuses from the arterioles toward low oxygen tension venous blood flowing in the opposite direction within the venules. Diffusion is facilitated by the relatively low rate of blood flow within villi. This in turn makes the tip of the villous relatively hypoxic even under normal conditions, which is exacerbated when blood flow is reduced or obstructed. However, the degree to which ischemia promotes mucosal injury is dependent upon the anatomy of the villous microvasculature, which affects the efficiency of counter-current exchange. Importantly, this microvascular architecture varies between species. The pig and human villous microvascular architecture are very similar, arborizing at the tip of the villous into a fountain-like pattern and converging into one or two venules located beside the arteriole (99, 100). The vascular architecture in rodents varies from that found in humans and is thought to influence the functional differences that have been appreciated between the mucosa of these species and may contribute to differences in mucosal susceptibility or response to injury (101–104). The specific microscopic architectural features of pigs and humans that are shared including villus structure and vascular anatomy likely contribute to interspecies similarities in ischemic damage (18, 101). Additionally, since many forms of intestinal ischemic injury involve decreased tissue perfusion and the villi are the first and most dramatically damaged in ischemic injury, the pig may more accurately model this disease process in humans (15, 105).
In clinical cases, the degree of damage varies according to the type of ischemic injury, and it is therefore not possible to fully understand all of the features of ischemia/ reperfusion injury with a single model. In light of this, porcine studies elucidating mechanisms of ischemia/ reperfusion injury relevant to human disease have assessed mesenteric ischemia, volvulus, and hemorrhagic shock to understand the relative contribution of ischemia and reperfusion to mucosal injury (18, 22, 23, 49–52, 106–108). Mesenteric ischemia was found to cause the most severe time-dependent injury, which was marginally exacerbated by reperfusion, and only at select time points (2-hours of ischemia, 1-hour reperfusion) (15). Alternatively, low-flow ischemia induced by hemorrhagic shock in pigs caused minimal ischemic injury, with no evidence of reperfusion injury (15). This is in marked contrast to rodent and feline studies exploring a range of low-flow states, all of which induced minimal ischemic injury, but were accompanied by marked reperfusion injury (109–113).
A great deal of the research on ischemia/ reperfusion injury has focused on the reperfusion phase, with the hope that basic findings would translate to the clinical ability of blocking injury by treating prior to reperfusion. Mechanisms of reperfusion injury have largely been documented in rodent and feline models, with the seminal discovery of xanthine oxidase as a central enzyme in the development of reactive oxygen metabolites (ROM) (15, 109, 110, 114–116). Studies in these laboratory animals have shown that small intestinal ischemia is followed by a robust reperfusion injury as a result of mucosal xanthine oxidase-induced oxidant release. However, there is doubt as to the clinical relevance of these findings in humans, which lack mucosal xanthine oxidase at birth, and intestinal enzyme expression remains relatively low throughout development to adulthood (15, 117). This pattern of xanthine oxidase expression is very similar in pigs, whereas rodents maintain levels 4–5 fold higher (15). Therefore information gleaned from rodent and feline models that attribute reperfusion injury to activation of xanthine oxidase may not be readily extrapolated to humans or may be relevant only to particular types of injury (10, 109, 111, 118–120). Another critical element shown to induce and perpetuate reperfusion injury is the neutrophil. Neutrophils are activated by chemoattractants induced by reactive oxygen metabolites, after which they greatly amplify reperfusion injury (121). The key neutrophil population involved in reperfusion injury in rodents is the resident mucosal population, as opposed to neutrophils recruited from the circulation (122). However, pigs have a relatively small population of mucosal neutrophils, as do humans, in contrast to the large population of resident mucosal neutrophils in rodents and cats (15). Collectively, these findings may explain the relative lack of reperfusion injury in the pig under a variety of ischemic conditions, including mesenteric ischemia, volvulus, and shock. These considerations are relevant to effective translation of basic science research to the treatment of human ischemia/ reperfusion injury (104, 123, 124).
The physical size of the pig as a model of human disease also enhances the feasibility of creating surgical models that recreate clinically relevant disease, including acute mesenteric ischemia (AMI) (18, 49, 108). Approximating the human size is also crucial in the development and testing of imaging modalities for early diagnosis of AMI (91, 125). This is likely why research focused on improving early diagnosis of AMI commonly utilizes porcine models to evaluate the efficacy of imaging modalities such as radiography, angiography, computed tomography and magnetic resonance imaging (50–52, 106, 126).
Mucosal repair
The slow progress in translating basic science findings on reperfusion injury to clinical trials has been reviewed elsewhere (123, 124). More information on mechanisms of mucosal repair is critical. Three important stages of intestinal epithelial repair have been identified. First, the immediate post injury or acute stage of repair is when barrier function is re-established by villous contraction and cellular restitution. The process of epithelial restitution has been re-examined in porcine models of ischemic injury, and found to include a novel component that had received little attention in cell models: tight junction repair (127–129). Specifically, initial studies on post-ischemic epithelial repair in pigs revealed a lack of barrier function recovery until tight junctions within restituting epithelium had closed (128). Thus, the term restitution now integrates the concepts of villus contraction, epithelial crawling, and tight junction re-organization. This proliferation independent process ensures that the basement membrane is covered, epithelial continuity is established and inter-cellular tight junctions are re-assembled (127, 130). During the subsequent or sub-acute period, cellular proliferation, differentiation and the onset of inflammation predominate (22). Finally, during the chronic stage of mucosal repair the normal mucosal architecture is re-established, the small intestinal villi for example, in addition to varying degrees of fibrosis that depend on the initial degree of injury and inflammation (131). Each of these processes has been dissected in depth, particularly the latter which continues to be a strong thrust of porcine studies (132–134). In terms of translation of such studies, the potential for pharmacologically hastening tight junction repair has been demonstrated in porcine post-ischemic bowel and awaits human trials (135).
Evaluation of mucosal repair following ischemic injury in pigs has also revealed a different time course of neutrophil infiltration as compared to murine and feline studies. In particular, ischemia in rodents and cats showed peak infiltration of neutrophils within 1-hour of reperfusion injury (116, 136, 137), whereas in porcine studies, peak neutrophil infiltration occurred 6-hours following ischemic injury (121). The importance of this finding was that rather than exacerbating injury, this delayed neutrophil infiltration may hamper restitution, particularly tight junction repair, as neutrophils coursed through the paracellular spaces of restituting epithelium. Of translational significance, inhibiting neutrophil adhesion, or treating with superoxide dismutase could restore optimal repair (121). Porcine studies have also shown that neutrophils induce physical damage to the epithelial tight junctions as they transmigrate across ischemic-injured mucosa (121). Other porcine studies have focused on understanding mechanisms of tight junction repair following ischemic injury, including clinically relevant pharmacological interventions (22, 135, 138, 139).
Necrotizing enterocolitis
NEC is an abdominal emergency that afflicts approximately 10% of infants born with low birth weight, and has a mortality rate of approximately 30% (16, 98). Severe, irreversible damage to the intestine often necessitates extensive surgical resection with resultant short bowel syndrome (SBS). This syndrome is associated with debilitating malnutrition, diarrhea, abdominal pain and fatigue (98, 140). The piglet is the only animal model that develops NEC under the same conditions that predispose human infants, namely prematurity, bacterial colonization and enteral feeding (16, 45, 141). Pigs also demonstrate pathognomonic signs of human disease including abdominal distension, food intolerance and regurgitation as well as mucosal necrosis in the distal small intestine and colon and pneumatosis intestinalis (40, 45, 142, 143). One of the most important features of NEC shared by neonates and piglet models is that disease is only seen in premature individuals (48). Importantly, ‘total parenteral nutrition’ (TPN), which is associated with onset of NEC in preterm infants, can be administered to piglets. Piglets not only share predisposing factors and clinical signs of NEC but also the histologic indicators of disease. This has led to extensive work utilizing the pre-term piglet model for treatment and drug discovery to improve the care of affected infants (44–47, 141, 143–146). For example, the severity and degree of mucosal atrophy were reduced in a piglet model when probiotics consisting of Bifidobacterium animalis and four Lactobacillus species were administered post parturition (47). Notably, the positive findings in porcine studies have been applied to human subjects and have confirmed the decreased incidence of NEC resulting from probiotic therapy (40, 147, 148).
Short bowel syndrome
Although the preterm piglet is an ideal model of SBS in NEC patients, variations between infant, juvenile and adult growth rates as well as developmental physiology preclude extrapolation of knowledge based on neonatal models of SBS to older patients. Here again, pigs provide a translational advantage because developmental and age-related changes in the gut are similar between pigs and humans (43, 149–151). Short bowel syndrome as a result of extensive resection of ischemic-injured or Crohn’s disease-affected bowel is the most common cause of intestinal failure in adults (140). Following extensive intestinal resection, the mucosal surface area is decreased, impairing nutrient and water absorption. This may result in diarrhea, fluid and electrolyte abnormalities, and malabsorption and weight loss (140). To offset SBS, the intestinal remnant undergoes an adaptive response that includes villus hyperplasia, increased crypt depth, and increased brush border enzyme activity that results in increased mucosal surface area with improved fluid and nutrient absorption. Although, morphological and functional changes characteristic of intestinal adaptation have been reported in rodents, the pig provides a more suitable model to study specific nutritional requirements critical to the treatment of SBS patients (31, 32, 43, 56, 57, 152–156). Total parenteral nutrition is currently the treatment of choice in humans and as was previously noted, can be administered to pigs (140). However, treatment with TPN is fraught with complications in SBS patients. These include venous thrombosis, infection and organ failure as well as sleep disruption, anxiety and depression (140). These clinical obstacles have driven extensive research into ways of minimizing TPN use by identifying effective mitogenic agents that hasten intestinal adaptation and improve the absorptive capacity of the remaining intestine. Murine models have successfully demonstrated the mitogenic effects of Glucagon-like peptide 2 (GLP-2) therapy; however, the FDA required the use of non-rodent models for pharmaceutical testing prior to human clinical trials. Porcine studies effectively demonstrated the rapid intestinal growth and maturation, increased intestinal brush border enzyme expression, decreased apoptosis and proteolysis, increased nutrient absorption and intestinal blood flow following treatment with GLP-2 (145, 157–162). GLP-2 has now emerged as a potential therapeutic option for some patients and is currently used in humans (163–165). Further work has focused on the appropriate time frame for administration of therapies aimed at enhancing the adaptive response (55). For example, a porcine model was utilized to specifically identify changes in the absolute numbers of epithelial cells within distinct mucosal populations and the time course of those changes in remnant bowel following extensive resection to give insight into potential targeted therapies (55). That study found that despite an early increase in proliferative and enterocyte cell number, following bowel resection, the cells were immature and incapable of normal absorptive function. It was therefore concluded that therapeutic interventions should be aimed at increasing cellular differentiation into mature cell types, not just general cellular proliferation to hasten return of normal absorptive function (55).
Transplantation studies
Ultimately, when extensive and irreversible injury to the intestine occurs and when TPN therapy fails to effectively maintain SBS patients, intestinal transplantation is indicated (140). Unfortunately, there is an unacceptably high mortality following intestinal transplantation. For example, death occurs in 40% of patients within five years of receiving an allograft (166). Acute rejection, chronic allograft dysfunction and ischemia/ reperfusion injury within the transplanted bowel are the most severe and common complications associated with bowel transplantation (23, 92). The limited options for replacing resected intestine make allotransplantation, xenotransplantation and tissue engineering research integral to improving available therapies for SBS patients. The pig represents an ideal model for each of these types of studies because of their shared size and specific physiologic, immunologic and organ developmental similarities with humans that translate into answering questions of practicality and generalized efficacy (23, 167–170). For instance, the current reference standard for detection of acute cellular rejection of allografts is a histological grading system (171) similar to the grading system used to identify allograft rejection in pigs used in transplantation research (23). The grading scale ranges from mild rejection, as determined by the presence of greater than 6 apoptotic cells out of 10 crypts and a mild to moderate inflammatory infiltrate of predominantly mononuclear cells, to severe rejection characterized by crypt destruction, mucosal erosions and severe inflammatory infiltration (23).
Although rodent models of allotransplantation have contributed to improvements within the field, the increased gross size of the pig permits investigation of alternative surgical therapies that mimic those possible in humans, combined with low mortality in experimental animals as compared to rodents (23, 172, 173). Examples of porcine models of transplantations include: orthotopic (graft in gastrointestinal continuity), heterotopic (graft not in gastrointestinal continuity), segmental (Thiry-Vella loop), whole small bowel or combined small bowel with colon (23, 170). Therefore, porcine experimental work directly facilitates the development of procedures for use in clinical patients (23, 170, 173).
Despite improvements in the success of alloengraftment surgery in recent years, particularly in specialized centers, recipients must cope with lifelong immunosuppressive therapies and the co-morbidities and mortality associated with this therapy (140, 169). The limitation of rodent models for these translational studies is associated with the major immunological differences that exist between species. In recent years, significant advances have been made in the development of ‘humanized’ mice to study human biological processes. However, these developments do not preclude the need for a large, more biologically complex system, to verify findings (38). Although there are immunological differences between humans and pigs, specific similarities and recent advances in the development of genetically modified animals has made the pig the most likely potential source for xenografts for humans (25, 168, 174). The demand for human organ donations significantly overwhelms the supply with over 100,000 recipient candidates awaiting organ transplants in the U.S. (175). The first transgenic pig was generated in 1985 and the first swine model derived specifically for the betterment of human health was in the field of xenotransplantation (175, 176). Pigs are considered the preferred donor species because their organ size is compatible with humans and because using non-human primate species poses difficult ethical concerns and infectious diseases (168, 177, 178). The development of genetically modified pigs expressing human complement pathway regulatory proteins (hCPRPs) or that have the α1,3-galactosyltransferase gene “knocked-out” (GalT-KO), has overcome the initial barriers posed by acute graft rejection (177–179). With these genetic modifications to inhibit recipient immunologic responses, porcine to non-human primate heart and kidney transplants have been successful (180).
Bioengineering offers an alternative source of tissue for intestinal replacement and avoids the complications associated with ischemia/ reperfusion injury of the donor tissue and life-long immunosuppressive therapy for the transplant recipient. Creating bioengineered tissues is an exciting prospect for regenerative medicine in the future, but advancements have been hindered by the anatomic and physiologic complexity of the intestine. Compared to other areas of intestinal research, studies on intestinal tissue engineering to replace segments of native intestine are limited (174, 181, 182). A porcine model was successfully used to generate tissue-engineered small intestine from autologous tissue, thereby re-creating conditions required for successful clinical use (174). Organoid units (isolated intestinal crypt cultures with epithelial stem cells and intact surrounding mesenchymal elements) were derived from resected small intestine, placed on biodegradable polyglycolic acid tubes, and implanted into the omentum or mesentery of the autologous animal. Intestinal tissue composed of all anatomic layers, including mucosa adjacent to an innervated muscularis, was shown to have developed (174). The far-reaching therapeutic potential of this research could serve as the basis for tissue replacement for a multitude of diseases. Ultimately, large animals and particularly the pig are biologically complex systems that serve a critical role in addressing the safety of a therapeutic regimen and verifying mechanistic findings from rodent models.
Transgenic pigs
The impetus for advancement in the field of transgenic pig development has been due to limitations in rodent models when their small size, short life span or inadequate representation of a disease phenotype prevents successful translational research (12). The majority of transgenic pig work has been in the field of xenotransplantation, as previously discussed. However, in recent years there have been significant improvements in the technology used to create genetically modified pigs to model human diseases (11, 12, 175). Comprehensive tables listing the available genetically modified porcine models for use in biomedicine and of human disease can be found in Whyte et. al. and Fan et. al. (11, 175). One of the best examples with regard to intestinal disease is the development of the porcine model of cystic fibrosis (CF) (64). Mouse models lack typical disease manifestations, including the intestinal obstruction phenotype that is observed in human patients (175, 180). Transgenically modified CFTR−/− and CFTRΔF508 pigs have proven superior by displaying the same clinical signs as seen in humans, including intestinal, bile duct, and pancreatic duct obstructions, as well as liver lesions, low levels of IGF-1 and the hallmark phenotype of lung disease (65, 180).
Previously, CF was the only intestinal disease with a representative porcine model (64, 183). However, gene-targeted pigs with mutations in the adenomatous polyposis coli (APC) gene have recently been established (14). Colorectal cancer is one of the most frequent causes of cancer worldwide and is the leading cause of gastrointestinal related mortality (1, 184). For this reason, much emphasis has been placed on the study of familial adenomatous polyposis (FAP) in humans. Murine Apc mutants have been used in this research but have significant limitations including differing location and histologic appearance of lesions as compared to humans (14, 185). In particular, in humans, colonic adenomas develop during childhood, whereas gastric polyps and duodenal adenomas occur during adulthood (14). In contrast, murine models develop polyps in the duodenum and small bowel, and these polyps lack dysplastic cells in the superficial mucosa (14). The porcine model of FAP, on the other hand, shares the anatomical location and histologic appearance of human disease (14). With regard to the diagnosis and treatment of FAP, the porcine model illustrates the advantage of large animals to facilitate advances in pharmaceutical, endoscopic, and surgical interventions. The completed pig genome project will provide the platform for further transgenic work to create pertinent pig models of human disease, particularly where rodent models fail to recapitulate clinical signs of disease (180).
Future promise for translational research
The need for large animal models to improve translational research is well accepted (20, 21). The limited availability of human-derived tissues requires that information gained from animal models is extrapolated to humans. The fact that mice and other rodents are the most commonly used preclinical models has been implicated in the “pipeline problem” (20, 186). One of the reasons for this problem is that rodent injury models fail to recapitulate human disease. For instance, murine injury models used in the study of intestinal epithelial stem cells (IESCs) have utilized chemical or mechanically induced mucosal injury or whole body irradiation to create damage (172, 187–190). None of these are common sources of mucosal injury in human clinical cases. Therefore, a great deal of additional research is needed before stem cell therapy can become a clinical reality. For example, a study examining the engraftment potential of IESCs following mucosal injury in Thiry-Vella loops was hampered by high mortality rates and the authors indicated the need for a large animal model in which Thiry-Vella loops are well tolerated (172). Until recently, a large animal model to study intestinal stem cell biology remained elusive. Currently, the cross reactivity of commercially available antibodies to identify specific cell types in pig intestinal tissue has been demonstrated (14, 55, 59, 68, 174, 191). Validation of tools for histologic, protein and mRNA based analysis of porcine IESCs have also been completed (68). Additionally, this study describes a method for successful long-term culture of porcine crypts into fully differentiated enteroids (isolated intestinal crypt cultures with epithelial stem cells without intact surrounding mesenchymal elements). Compared to the availability of reagents and tools for the study of rodent tissue, those for pig remains limited. However, recent work provides a platform for translational research, including for stem cell engraftment studies that are currently limited by the small size and high mortality rates of rodents (172). The pig as a model to study IESCs in disease affords the opportunity to bridge the gap between lab bench discovery and bedside application.
Figure 2. Ischemia Reperfusion Injury and Recovery.
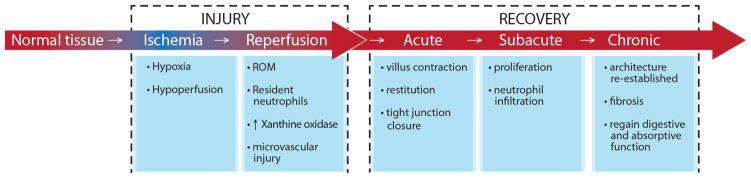
Intestinal ischemic injury occurs when the blood supply to a segment of intestine is compromised. Paradoxically, tissue damage may continue when blood flow is reestablished, a process call reperfusion injury. Following the initial injury induced by ischemia and reperfusion there are three important stages of intestinal epithelial repair. There is an immediate post injury or acute repair, a subsequent sub-acute period when cell proliferation occurs and a final, chronic stage of mucosal repair when normal architecture is re-established. ROM = Reactive Oxygen Metabolites
Abbreviations
- SOX9
Sex Determining Region Y-box 9
- HOPX
Homeodomain-only protein X
- ENS
Enteric Nervous System
- IBS
Irritable Bowel Syndrome
- CRF
Corticotropin Releasing Factor
- ACTH
Adrenocorticotrophic hormone
- NE
Norepinephrine
- NEC
Neonatal necrotizing enterocolitis
- ROM
Reactive Oxygen Metabolites
- AMI
Acute Mesenteric Ischemia
- SBS
Short Bowel Syndrome
- TPN
Total Parenteral Nutrition
- GLP-2
Glucagon-like Peptide 2
- hCPRPs
Human Complement Pathway Regulatory Proteins
- Gal T-KO
α1,3-galactosyl transferase gene knock-out
- CF
Cystic Fibrosis
- FAP
Familial Adenomatous Polyposis
- IESCs
Intestinal Epithelial Stem Cells
Footnotes
Conflicts of Interest: All authors have read the journal’s policy on disclosure of potential conflicts of interest and have none to declare.
Authorship agreement: All authors have read the journal’s authorship agreement.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the united states: 2012 update. Gastroenterology. 2012;143:1179–87. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung SK, Lee AY, Chung SS. Mouse models for human diseases. Hong Kong Med J. 1997;3:201–9. [PubMed] [Google Scholar]
- 3.Low MJ. Mouse models in gastroenterology research. Gastroenterology. 2012;143:1410–2. doi: 10.1053/j.gastro.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Pizarro TT, Pastorelli L, Bamias G, et al. SAMP1/YitFc mouse strain: A spontaneous model of crohn’s disease-like ileitis. Inflamm Bowel Dis. 2011;17:2566–84. doi: 10.1002/ibd.21638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner SJ, Schmidt A, Effenberger MJ, Gruber L, Danier J, Haller D. Semisynthetic diet ameliorates crohn’s disease-like ileitis in TNFDeltaARE/WT mice through antigen-independent mechanisms of gluten. Inflamm Bowel Dis. 2013;19:1285–94. doi: 10.1097/MIB.0b013e318281f573. [DOI] [PubMed] [Google Scholar]
- 6.Heindryckx F, Colle I, Van Vlierberghe H. Experimental mouse models for hepatocellular carcinoma research. Int J Exp Pathol. 2009;90:367–86. doi: 10.1111/j.1365-2613.2009.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guilbault C, Saeed Z, Downey GP, Radzioch D. Cystic fibrosis mouse models. Am J Respir Cell Mol Biol. 2007;36:1–7. doi: 10.1165/rcmb.2006-0184TR. [DOI] [PubMed] [Google Scholar]
- 8.Mouse Genome Sequencing Consortium. Waterston RH, Lindblad-Toh K, Birney E, et al. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420:520–62. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- 9.Lee HT, Kim M, Kim JY, et al. Critical role of interleukin-17A in murine intestinal ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2013;304:G12–25. doi: 10.1152/ajpgi.00201.2012. [DOI] [PubMed] [Google Scholar]
- 10.Deshmukh DR, Mirochnitchenko O, Ghole VS, et al. Intestinal ischemia and reperfusion injury in transgenic mice overexpressing copper-zinc superoxide dismutase. Am J Physiol. 1997;273:C1130–5. doi: 10.1152/ajpcell.1997.273.4.C1130. [DOI] [PubMed] [Google Scholar]
- 11.Fan N, Lai L. Genetically modified pig models for human diseases. J Genet Genomics. 2013;40:67–73. doi: 10.1016/j.jgg.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Aigner B, Renner S, Kessler B, et al. Transgenic pigs as models for translational biomedical research. J Mol Med (Berl) 2010;88:653–64. doi: 10.1007/s00109-010-0610-9. [DOI] [PubMed] [Google Scholar]
- 13.Bendixen E, Danielsen M, Larsen K, Bendixen C. Advances in porcine genomics and proteomics--a toolbox for developing the pig as a model organism for molecular biomedical research. Brief Funct Genomics. 2010;9:208–19. doi: 10.1093/bfgp/elq004. [DOI] [PubMed] [Google Scholar]
- 14.Flisikowska T, Merkl C, Landmann M, et al. A porcine model of familial adenomatous polyposis. Gastroenterology. 2012;143:1173–5. doi: 10.1053/j.gastro.2012.07.110. [DOI] [PubMed] [Google Scholar]
- 15.Blikslager AT, Roberts MC, Rhoads JM, Argenzio RA. Is reperfusion injury an important cause of mucosal damage after porcine intestinal ischemia? Surgery. 1997;121:526–34. doi: 10.1016/s0039-6060(97)90107-0. [DOI] [PubMed] [Google Scholar]
- 16.Azcarate-Peril MA, Foster DM, Cadenas MB, et al. Acute necrotizing enterocolitis of preterm piglets is characterized by dysbiosis of ileal mucosa-associated bacteria. Gut Microbes. 2011;2:234–43. doi: 10.4161/gmic.2.4.16332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Argenzio RA, Liacos JA, Levy ML, Meuten DJ, Lecce JG, Powell DW. Villous atrophy, crypt hyperplasia, cellular infiltration, and impaired glucose-na absorption in enteric cryptosporidiosis of pigs. Gastroenterology. 1990;98:1129–40. doi: 10.1016/0016-5085(90)90325-u. [DOI] [PubMed] [Google Scholar]
- 18.Block T, Isaksson HS, Acosta S, Bjorck M, Brodin D, Nilsson TK. Altered mRNA expression due to acute mesenteric ischaemia in a porcine model. Eur J Vasc Endovasc Surg. 2011;41:281–7. doi: 10.1016/j.ejvs.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Patterson JK, Lei XG, Miller DD. The pig as an experimental model for elucidating the mechanisms governing dietary influence on mineral absorption. Exp Biol Med (Maywood) 2008;233:651–64. doi: 10.3181/0709-MR-262. [DOI] [PubMed] [Google Scholar]
- 20.National Institute of Health. NIH symposium; improving animal models for regenerative medicine; May 23–24, 2012; Bethesda, MD. Bethesda, MD: 2012. [Google Scholar]
- 21.National Institute of Diabetes and Digestive and Kidney Diseases. Opportunities and challenges in digestive diseases research: Recommendations of the national commission on digestive diseases. [Internet] 2009 [Google Scholar]
- 22.Blikslager AT, Rhoads JM, Bristol DG, Roberts MC, Argenzio RA. Glutamine and transforming growth factor-alpha stimulate extracellular regulated kinases and enhance recovery of villous surface area in porcine ischemic-injured intestine. Surgery. 1999;125:186–94. [PubMed] [Google Scholar]
- 23.Yandza T, Tauc M, Saint-Paul MC, Ouaissi M, Gugenheim J, Hebuterne X. The pig as a preclinical model for intestinal ischemia-reperfusion and transplantation studies. J Surg Res. 2012;178:807–19. doi: 10.1016/j.jss.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 24.Burton J, Khanna C. The role of clinical trials in veterinary oncology. Vet Clin North Am Small Anim Pract. 2014;44:977–87. doi: 10.1016/j.cvsm.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Douglas WR. Of pigs and men and research: A review of applications and analogies of the pig, sus scrofa, in human medical research. Space Life Sci. 1972;3:226–34. doi: 10.1007/BF00928167. [DOI] [PubMed] [Google Scholar]
- 26.Thomas JW, Touchman JW, Blakesley RW, et al. Comparative analyses of multi-species sequences from targeted genomic regions. Nature. 2003;424:788–93. doi: 10.1038/nature01858. [DOI] [PubMed] [Google Scholar]
- 27.Humphray SJ, Scott CE, Clark R, et al. A high utility integrated map of the pig genome. Genome Biol. 2007;8:R139. doi: 10.1186/gb-2007-8-7-r139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy WJ, Larkin DM, Everts-van der Wind A, et al. Dynamics of mammalian chromosome evolution inferred from multispecies comparative maps. Science. 2005;309:613–7. doi: 10.1126/science.1111387. [DOI] [PubMed] [Google Scholar]
- 29.Meyers SN, Rogatcheva MB, Larkin DM, et al. Piggy-BACing the human genome II. A high-resolution, physically anchored, comparative map of the porcine autosomes. Genomics. 2005;86:739–52. doi: 10.1016/j.ygeno.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 30.Kararli TT. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos. 1995;16:351–80. doi: 10.1002/bdd.2510160502. [DOI] [PubMed] [Google Scholar]
- 31.Deglaire A, Moughan PJ. Animal models for determining amino acid digestibility in humans - a review. Br J Nutr. 2012;108:S273–81. doi: 10.1017/S0007114512002346. [DOI] [PubMed] [Google Scholar]
- 32.Miller ER, Ullrey DE. The pig as a model for human nutrition. Annu Rev Nutr. 1987;7:361–82. doi: 10.1146/annurev.nu.07.070187.002045. [DOI] [PubMed] [Google Scholar]
- 33.Pang X, Hua X, Yang Q, et al. Inter-species transplantation of gut microbiota from human to pigs. ISME J. 2007:1156–62. doi: 10.1038/ismej.2007.23. [DOI] [PubMed] [Google Scholar]
- 34.Schwiertz A, Taras D, Schafer K, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 2010;18:190–5. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 35.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 36.Bereswill S, Fischer A, Plickert R, et al. Novel murine infection models provide deep insights into the “menage a trois” of campylobacter jejuni, microbiota and host innate immunity. PLoS One. 2011;6:e20953. doi: 10.1371/journal.pone.0020953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brehm MA, Shultz LD, Greiner DL. Humanized mouse models to study human diseases. Curr Opin Endocrinol Diabetes Obes. 2010;17:120–5. doi: 10.1097/MED.0b013e328337282f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheer N, Snaith M, Wolf CR, Seibler J. Generation and utility of genetically humanized mouse models. Drug Discov Today. 2013;18:1200–11. doi: 10.1016/j.drudis.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Gootenberg DB, Turnbaugh PJ. Companion animals symposium: Humanized animal models of the microbiome. J Anim Sci. 2011;89:1531–7. doi: 10.2527/jas.2010-3371. [DOI] [PubMed] [Google Scholar]
- 40.Heinritz SN, Mosenthin R, Weiss E. Use of pigs as a potential model for research into dietary modulation of the human gut microbiota. Nutr Res Rev. 2013;26:19–209. doi: 10.1017/S0954422413000152. [DOI] [PubMed] [Google Scholar]
- 41.Leser TD, Amenuvor JZ, Jensen TK, Lindecrona RH, Boye M, Moller K. Culture-independent analysis of gut bacteria: The pig gastrointestinal tract microbiota revisited. Appl Environ Microbiol. 2002;68:673–90. doi: 10.1128/AEM.68.2.673-690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamendella R, Domingo JW, Ghosh S, Martinson J, Oerther DB. Comparative fecal metagenomics unveils unique functional capacity of the swine gut. BMC Microbiol. 2011;11:103. doi: 10.1186/1471-2180-11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bines JE, Taylor RG, Justice F, et al. Influence of diet complexity on intestinal adaptation following massive small bowel resection in a preclinical model. J Gastroenterol Hepatol. 2002;17:1170–9. doi: 10.1046/j.1440-1746.2002.02872.x. [DOI] [PubMed] [Google Scholar]
- 44.Thymann T, Moller HK, Stoll B, et al. Carbohydrate maldigestion induces necrotizing enterocolitis in preterm pigs. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1115–25. doi: 10.1152/ajpgi.00261.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sangild PT, Siggers RH, Schmidt M, et al. Diet- and colonization-dependent intestinal dysfunction predisposes to necrotizing enterocolitis in preterm pigs. Gastroenterology. 2006;130:1776–92. doi: 10.1053/j.gastro.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 46.Van Haver ER, Sangild PT, Oste M, Siggers JL, Weyns AL, Van Ginneken CJ. Diet-dependent mucosal colonization and interleukin-1beta responses in preterm pigs susceptible to necrotizing enterocolitis. J Pediatr Gastroenterol Nutr. 2009;49:90–8. doi: 10.1097/MPG.0b013e31818de393. [DOI] [PubMed] [Google Scholar]
- 47.Siggers RH, Siggers J, Boye M, et al. Early administration of probiotics alters bacterial colonization and limits diet-induced gut dysfunction and severity of necrotizing enterocolitis in preterm pigs. J Nutr. 2008;138:1437–44. doi: 10.1093/jn/138.8.1437. [DOI] [PubMed] [Google Scholar]
- 48.Sangild PT, Thymann T, Schmidt M, Stoll B, Burrin DG, Buddington RK. Invited review: The preterm pig as a model in pediatric gastroenterology. J Anim Sci. 2013;91:4713–29. doi: 10.2527/jas.2013-6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang ZH, Qiang JW, Feng XY, Li RK, Sun RX, Ye XG. Acute mesenteric ischemia induced by ligation of porcine superior mesenteric vein: Multidetector CT evaluations. Acad Radiol. 2010;17:1146–52. doi: 10.1016/j.acra.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 50.Bruhn RS, Distelmaier MS, Hellmann-Sokolis M, Naami A, Kuhl CK, Hohl C. Early detection of acute mesenteric ischemia using diffusion-weighted 3.0-T magnetic resonance imaging in a porcine model. Invest Radiol. 2013;48:231–7. doi: 10.1097/RLI.0b013e3182809143. [DOI] [PubMed] [Google Scholar]
- 51.Schwartz CA, Haage P, Hohl C. Experimental early detection of acute mesenteric ischemia with functional MRI (DWI) and parallel imaging. Rofo. 2012;184:520–6. doi: 10.1055/s-0031-1299414. [DOI] [PubMed] [Google Scholar]
- 52.Rosow DE, Sahani D, Strobel O, et al. Imaging of acute mesenteric ischemia using multidetector CT and CT angiography in a porcine model. J Gastrointest Surg. 2005;9:1262–75. doi: 10.1016/j.gassur.2005.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pereira-Fantini PM, Thomas SL, Taylor RG, et al. Colostrum supplementation restores insulin-like growth factor-1 levels and alters muscle morphology following massive small bowel resection. JPEN J Parenter Enteral Nutr. 2008;32:266–75. doi: 10.1177/0148607108316197. [DOI] [PubMed] [Google Scholar]
- 54.Pereira-Fantini PM, Nagy ES, Thomas SL, et al. GLP-2 administration results in increased proliferation but paradoxically an adverse outcome in a juvenile piglet model of short bowel syndrome. J Pediatr Gastroenterol Nutr. 2008;46:20–8. doi: 10.1097/01.mpg.0000304449.46434.06. [DOI] [PubMed] [Google Scholar]
- 55.Pereira-Fantini PM, Thomas SL, Wilson G, Taylor RG, Sourial M, Bines JE. Short- and long-term effects of small bowel resection: A unique histological study in a piglet model of short bowel syndrome. Histochem Cell Biol. 2011;135:195–202. doi: 10.1007/s00418-011-0778-2. [DOI] [PubMed] [Google Scholar]
- 56.Nagy ES, Paris MC, Taylor RG, et al. Colostrum protein concentrate enhances intestinal adaptation after massive small bowel resection in juvenile pigs. J Pediatr Gastroenterol Nutr. 2004;39:487–92. doi: 10.1097/00005176-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 57.Paris MC, Fuller PJ, Carstensen B, et al. Plasma GLP-2 levels and intestinal markers in the juvenile pig during intestinal adaptation: Effects of different diet regimens. Dig Dis Sci. 2004;49:1688–95. doi: 10.1023/b:ddas.0000043388.52260.2f. [DOI] [PubMed] [Google Scholar]
- 58.Gookin JL, Chiang S, Allen J, et al. NF-kappaB-mediated expression of iNOS promotes epithelial defense against infection by cryptosporidium parvum in neonatal piglets. Am J Physiol Gastrointest Liver Physiol. 2006;290:G164–74. doi: 10.1152/ajpgi.00460.2004. [DOI] [PubMed] [Google Scholar]
- 59.Foster DM, Stauffer SH, Stone MR, Gookin JL. Proteasome inhibition of pathologic shedding of enterocytes to defend barrier function requires X-linked inhibitor of apoptosis protein and nuclear factor kappaB. Gastroenterology. 2012;143:133–44. doi: 10.1053/j.gastro.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 60.Smith F, Clark JE, Overman BL, et al. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am J Physiol Gastrointest Liver Physiol. 2010;298:G352–63. doi: 10.1152/ajpgi.00081.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moeser AJ, Lascelles DX, Blikslager AT. Early weaning stress predisposes adult pigs to stress-induced colonic barrier dysfunction: A novel model for stress-induced gastrointestinal disorders in humans. Gastroenterology. 2006;130:A89. [Google Scholar]
- 62.Moeser AJ, Ryan KA, Nighot PK, Blikslager AT. Gastrointestinal dysfunction induced by early weaning is attenuated by delayed weaning and mast cell blockade in pigs. Am J Physiol Gastrointest Liver Physiol. 2007;293:G413–21. doi: 10.1152/ajpgi.00304.2006. [DOI] [PubMed] [Google Scholar]
- 63.Moeser AJ, Klok CV, Ryan KA, et al. Stress signaling pathways activated by weaning mediate intestinal dysfunction in the pig. Am J Physiol Gastrointest Liver Physiol. 2007;292:G173–81. doi: 10.1152/ajpgi.00197.2006. [DOI] [PubMed] [Google Scholar]
- 64.Welsh MJ, Rogers CS, Stoltz DA, Meyerholz DK, Prather RS. Development of a porcine model of cystic fibrosis. Trans Am Clin Climatol Assoc. 2009;120:149–62. [PMC free article] [PubMed] [Google Scholar]
- 65.Rogan MP, Reznikov LR, Pezzulo AA, et al. Pigs and humans with cystic fibrosis have reduced insulin-like growth factor 1 (IGF1) levels at birth. Proc Natl Acad Sci U S A. 2010;107:20571–5. doi: 10.1073/pnas.1015281107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stevens CE, Argenzio RA, Roberts MC. Comparative physiology of the mammalian colon and suggestions for animal models of human disorders. Clin Gastroenterol. 1986;15:763–85. [PubMed] [Google Scholar]
- 67.Graham H, Aman P. The pig as a model in dietary fibre digestion studies. Scand J Gastroenterol Suppl. 1987;129:55–61. doi: 10.3109/00365528709095851. [DOI] [PubMed] [Google Scholar]
- 68.Gonzalez LM, Williamson I, Piedrahita JA, Blikslager AT, Magness ST. Cell lineage identification and stem cell culture in a porcine model for the study of intestinal epithelial regeneration. PLoS One. 2013;8:e66465. doi: 10.1371/journal.pone.0066465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barker N, van Oudenaarden AF, Clevers H. Identifying the stem cell of the intestinal crypt: Strategies and pitfalls. Cell stem cell. 2012;11:452–60. doi: 10.1016/j.stem.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 70.Swindle MM, Smith AC. Comparative anatomy and physiology of the pig. Scand J Lab Anim Sci Suppl I. 1998;25:11–21. [Google Scholar]
- 71.Dekaney CM, Bazer FW, Jaeger LA. Mucosal morphogenesis and cytodifferentiation in fetal porcine small intestine. Anat Rec. 1997;249:517–23. doi: 10.1002/(SICI)1097-0185(199712)249:4<517::AID-AR12>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 72.Sinkora M, Stepanova K, Butler JE, et al. Ileal peyer’s patches are not necessary for systemic B cell development and maintenance and do not contribute significantly to the overall B cell pool in swine. J Immunol. 2011;187:5150–61. doi: 10.4049/jimmunol.1101879. [DOI] [PubMed] [Google Scholar]
- 73.Butler JE, Sinkora M. The enigma of the lower gut-associated lymphoid tissue (GALT) J Leukoc Biol. 2013;94:259–70. doi: 10.1189/jlb.0313120. [DOI] [PubMed] [Google Scholar]
- 74.Jung C, Hugot JP, Barreau F. Peyer’s patches: The immune sensors of the intestine. Int J Inflam. 2010;2010:12p. doi: 10.4061/2010/823710. ages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Myer MS. Paneth cells in the pig-a controversial issue. J S Afr Vet Assoc. 1982;53:69. [PubMed] [Google Scholar]
- 76.Myer MS. The presence of paneth cells confirmed in the pig. Onderstepoort J Vet Res. 1982;49:131–2. [PubMed] [Google Scholar]
- 77.Trautmann A. Fundamentals of the histology of domestic animals. Ithaca, N.Y: Comstock Pub. Associates; 1952. 1884–1952. [Google Scholar]
- 78.Satoh Y, Yamano M, Matsuda M, Ono K. Ultrastructure of paneth cells in the intestine of various mammals. J Electron Microsc Tech. 1990;16:69–80. doi: 10.1002/jemt.1060160109. [DOI] [PubMed] [Google Scholar]
- 79.Sato T, van Es JH, Snippert HJ, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–8. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Farin HF, Van Es JH, Clevers H. Redundant sources of wnt regulate intestinal stem cells and promote formation of paneth cells. Gastroenterology. 2012;143:1518–1529. doi: 10.1053/j.gastro.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 81.Santos J, Saunders PR, Hanssen NP, et al. Corticotropin-releasing hormone mimics stress-induced colonic epithelial pathophysiology in the rat. Am J Physiol. 1999;277:G391–9. doi: 10.1152/ajpgi.1999.277.2.G391. [DOI] [PubMed] [Google Scholar]
- 82.Gourcerol G, Wu SV, Yuan PQ, et al. Activation of corticotropin-releasing factor receptor 2 mediates the colonic motor coping response to acute stress in rodents. Gastroenterology. 2011;140:1586–96. doi: 10.1053/j.gastro.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Swine in the laboratory: Surgery, anesthesia, imaging, and experimental techniques [Internet] Boca Raton: CRC Press; 2007. [Google Scholar]
- 84.Brown DR, Timmermans JP. Lessons from the porcine enteric nervous system. Neurogastroenterol Motil. 2004;16:50–4. doi: 10.1111/j.1743-3150.2004.00475.x. [DOI] [PubMed] [Google Scholar]
- 85.Brown DR, O’Grady SM. Regulation of ion transport in the porcine intestinal tract by enteric neurotransmitters and hormones. Comp Biochem Physiol A Physiol. 1997;118:309–17. doi: 10.1016/s0300-9629(96)00311-8. [DOI] [PubMed] [Google Scholar]
- 86.Overman EL, Rivier JE, Moeser AJ. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-alpha. PLoS One. 2012;7:e39935. doi: 10.1371/journal.pone.0039935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schreiber KL, Brown DR. Adrenocorticotrophic hormone modulates escherichia coli O157:H7 adherence to porcine colonic mucosa. Stress. 2005;8:185–90. doi: 10.1080/10253890500188732. [DOI] [PubMed] [Google Scholar]
- 88.Chen C, Lyte M, Stevens MP, Vulchanova L, Brown DR. Mucosally-directed adrenergic nerves and sympathomimetic drugs enhance non-intimate adherence of escherichia coli O157:H7 to porcine cecum and colon. Eur J Pharmacol. 2006;539:116–24. doi: 10.1016/j.ejphar.2006.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schmidt LD, Xie Y, Lyte M, Vulchanova L, Brown DR. Autonomic neurotransmitters modulate immunoglobulin A secretion in porcine colonic mucosa. J Neuroimmunol. 2007;185:20–8. doi: 10.1016/j.jneuroim.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Puleo F, Arvanitakis M, Van Gossum A, Preiser JC. Gut failure in the ICU. Semin Respir Crit Care Med. 2011;32:626–38. doi: 10.1055/s-0031-1287871. [DOI] [PubMed] [Google Scholar]
- 91.Yasuhara H. Acute mesenteric ischemia: The challenge of gastroenterology. Surg Today. 2005;35:185–95. doi: 10.1007/s00595-004-2924-0. [DOI] [PubMed] [Google Scholar]
- 92.Lenaerts K, Ceulemans LJ, Hundscheid IH, Grootjans J, Dejong CH, Olde Damink SW. New insights in intestinal ischemia-reperfusion injury: Implications for intestinal transplantation. Curr Opin Organ Transplant. 2013;18:298–303. doi: 10.1097/MOT.0b013e32835ef1eb. [DOI] [PubMed] [Google Scholar]
- 93.Mallick IH, Yang W, Winslet MC, Seifalian AM. Ischemia-reperfusion injury of the intestine and protective strategies against injury. Dig Dis Sci. 2004;49:1359–77. doi: 10.1023/b:ddas.0000042232.98927.91. [DOI] [PubMed] [Google Scholar]
- 94.Trompeter M, Brazda T, Remy CT, Vestring T, Reimer P. Non-occlusive mesenteric ischemia: Etiology, diagnosis, and interventional therapy. Eur Radiol. 2002;12:1179–87. doi: 10.1007/s00330-001-1220-2. [DOI] [PubMed] [Google Scholar]
- 95.Reginelli A, Genovese E, Cappabianca S, Iacobellis F, Berritto D, Fonio P, et al. Intestinal ischemia: US-CT findings correlations. Crit Ultrasound J. 2013;5:S7. doi: 10.1186/2036-7902-5-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364:255–64. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nankervis CA, Giannone PJ, Reber KM. The neonatal intestinal vasculature: Contributing factors to necrotizing enterocolitis. Semin Perinatol. 2008;32:83–91. doi: 10.1053/j.semperi.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 98.Gregory KE, Deforge CE, Natale KM, Phillips M, Van Marter LJ. Necrotizing enterocolitis in the premature infant: Neonatal nursing assessment, disease pathogenesis, and clinical presentation. Adv Neonatal Care. 2011;11:155–64. doi: 10.1097/ANC.0b013e31821baaf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Casley-Smith GB., JR . In: Physiology of the intestinal circulation. APS, Granger DN, Granger DN, Shepherd AP, editors. New York: Raven Press; 1984. pp. 9–31. [Google Scholar]
- 100.Bellamy JE, Latshaw WK, Nielsen NO. The vascular architecture of the porcine small intestine. Can J Comp Med. 1973;37:56–62. [PMC free article] [PubMed] [Google Scholar]
- 101.Bijlsma PB, Peeters RA, Groot JA, Dekker PR, Taminiau JAJM, Van Der Meer R. Differential in vivo and in vitro intestinal permeability to lactulose and mannitol in animals and humans: A hypothesis. Gastroenterology. 1995;108:687–96. doi: 10.1016/0016-5085(95)90440-9. [DOI] [PubMed] [Google Scholar]
- 102.Nejdfors P, Ekelund M, Jeppsson B, Westrom BR. Mucosal in vitro permeability in the intestinal tract of the pig, the rat, and man: Species- and region-related differences. Scand J Gastroenterol. 2000;35:501–7. doi: 10.1080/003655200750023769. [DOI] [PubMed] [Google Scholar]
- 103.Chen YM, Zhang JS, Duan XL. Changes of microvascular architecture, ultrastructure and permeability of rat jejunal villi at different ages. World J Gastroenterol. 2003;9:795–9. doi: 10.3748/wjg.v9.i4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Delahunty T, Hollander D. A comparison of intestinal permeability between humans and three common laboratory animals. Comp Biochem Physiol A Comp Physiol. 1987;86:565–7. doi: 10.1016/0300-9629(87)90542-1. [DOI] [PubMed] [Google Scholar]
- 105.Udassin R, Vromen A, Haskel Y. The time sequence of injury and recovery following transient reversible intestinal ischemia. J Surg Res. 1994;56:221–5. doi: 10.1006/jsre.1994.1035. [DOI] [PubMed] [Google Scholar]
- 106.Klein HM, Klosterhalfen B, Kinzel S, et al. CT and MRI of experimentally induced mesenteric ischemia in a porcine model. J Comput Assist Tomogr. 1996;20:254–61. doi: 10.1097/00004728-199603000-00016. [DOI] [PubMed] [Google Scholar]
- 107.Jacobi SK, Moeser AJ, Corl BA, Harrell RJ, Blikslager AT, Odle J. Dietary long-chain PUFA enhance acute repair of ischemia-injured intestine of suckling pigs. J Nutr. 2012;142:1266–71. doi: 10.3945/jn.111.150995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Acosta S, Nilsson TK, Malina J, Malina M. L-lactate after embolization of the superior mesenteric artery. J Surg Res. 2007;143:320–8. doi: 10.1016/j.jss.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 109.Granger DN, Hollwarth ME, Parks DA. Ischemia-reperfusion injury: Role of oxygen-derived free radicals. Acta Physiol Scand Suppl. 1986;548:47–63. [PubMed] [Google Scholar]
- 110.Smith SM, Grisham MB, Manci EA, Granger DN, Kvietys PR. Gastric mucosal injury in the rat. role of iron and xanthine oxidase. Gastroenterology. 1987;92:950–6. doi: 10.1016/0016-5085(87)90969-3. [DOI] [PubMed] [Google Scholar]
- 111.Parks DA, Granger DN. Contributions of ischemia and reperfusion to mucosal lesion formation. Am J Physiol. 1986;250:G749–53. doi: 10.1152/ajpgi.1986.250.6.G749. [DOI] [PubMed] [Google Scholar]
- 112.Haglind E, Haglund U, Lundgren O, Stenberg B. Mucosal lesions of the small intestine after intestinal vascular obstruction in the rat. Acta Chir Scand. 1985;151:147–50. [PubMed] [Google Scholar]
- 113.Boros M, Takaichi S, Hatanaka K. Ischemic time-dependent microvascular changes and reperfusion injury in the rat small intestine. J Surg Res. 1995;59:311–20. doi: 10.1006/jsre.1995.1170. [DOI] [PubMed] [Google Scholar]
- 114.Granger DN, McCord JM, Parks DA, Hollwarth ME. Xanthine oxidase inhibitors attenuate ischemia-induced vascular permeability changes in the cat intestine. Gastroenterology. 1986;90:80–4. doi: 10.1016/0016-5085(86)90078-8. [DOI] [PubMed] [Google Scholar]
- 115.Parks DA, Bulkley GB, Granger DN, Hamilton SR, McCord JM. Ischemic injury in the cat small intestine: Role of superoxide radicals. Gastroenterology. 1982;82:9–15. [PubMed] [Google Scholar]
- 116.Grisham MB, Hernandez LA, Granger DN. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am J Physiol. 1986;251:G567–74. doi: 10.1152/ajpgi.1986.251.4.G567. [DOI] [PubMed] [Google Scholar]
- 117.Crissinger KD, Grisham MB, Granger DN. Developmental biology of oxidant-producing enzymes and antioxidants in the piglet intestine. Pediatr Res. 1989;25:612–6. doi: 10.1203/00006450-198906000-00012. [DOI] [PubMed] [Google Scholar]
- 118.Suzuki M, Inauen W, Kvietys PR, et al. Superoxide mediates reperfusion-induced leukocyte-endothelial cell interactions. Am J Physiol. 1989;257:H1740–5. doi: 10.1152/ajpheart.1989.257.5.H1740. [DOI] [PubMed] [Google Scholar]
- 119.Zimmerman BJ, Grisham MB, Granger DN. Role of oxidants in ischemia/reperfusion-induced granulocyte infiltration. Am J Physiol. 1990;258:G185–90. doi: 10.1152/ajpgi.1990.258.2.G185. [DOI] [PubMed] [Google Scholar]
- 120.Lee H, Ko EH, Lai M, et al. Delineating the relationships among the formation of reactive oxygen species, cell membrane instability and innate autoimmunity in intestinal reperfusion injury. Mol Immunol. 2014;58:151–9. doi: 10.1016/j.molimm.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gayle J, Jones SL, Argenzio RA, Blikslager AT. Neutrophils increase paracellular permeability of restituted ischemic-injured porcine ileum. Surgery. 2002;132:461–70. doi: 10.1067/msy.2002.125320. [DOI] [PubMed] [Google Scholar]
- 122.Kubes P, Hunter J, Granger DN. Ischemia/reperfusion-induced feline intestinal dysfunction: Importance of granulocyte recruitment. Gastroenterology. 1992;103:807–12. doi: 10.1016/0016-5085(92)90010-v. [DOI] [PubMed] [Google Scholar]
- 123.Lehr HA, Menger MD, Granger DN. Ischemia-reperfusion injury: Enthusiasm in laboratory research but dilemma in clinical trials? Circulation. 1994;90:1580. [PubMed] [Google Scholar]
- 124.Derikx JP, Matthijsen RA, de Bruine AP, van Dam RM, Buurman WA, Dejong CH. A new model to study intestinal ischemia-reperfusion damage in man. J Surg Res. 2011;166:222–6. doi: 10.1016/j.jss.2009.09.046. [DOI] [PubMed] [Google Scholar]
- 125.Acosta S, Block T, Bjornsson S, Resch T, Bjorck M, Nilsson T. Diagnostic pitfalls at admission in patients with acute superior mesenteric artery occlusion. J Emerg Med. 2012;42:635–41. doi: 10.1016/j.jemermed.2011.03.036. [DOI] [PubMed] [Google Scholar]
- 126.Paral J, Ferko A, Plodr M, et al. Laparoscopic diagnostics of acute bowel ischemia using ultraviolet light and fluorescein dye: An experimental study. Surg Laparosc Endosc Percutan Tech. 2007;17:291–5. doi: 10.1097/SLE.0b013e3180dc9376. [DOI] [PubMed] [Google Scholar]
- 127.Blikslager AT, Moeser AJ, Gookin JL, Jones SL, Odle J. Restoration of barrier function in injured intestinal mucosa. Physiol Rev. 2007;87:545–64. doi: 10.1152/physrev.00012.2006. [DOI] [PubMed] [Google Scholar]
- 128.Blikslager AT, Roberts MC, Argenzio RA. Prostaglandin-induced recovery of barrier function in porcine ileum is triggered by chloride secretion. Am J Physiol. 1999;276:G28–36. doi: 10.1152/ajpgi.1999.276.1.G28. [DOI] [PubMed] [Google Scholar]
- 129.Gookin JL, Galanko JA, Blikslager AT, Argenzio RA. PG-mediated closure of paracellular pathway and not restitution is the primary determinant of barrier recovery in acutely injured porcine ileum. Am J Physiol Gastrointest Liver Physiol. 2003;285:G967–79. doi: 10.1152/ajpgi.00532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Park PO, Haglund U. Regeneration of small bowel mucosa after intestinal ischemia. Crit Care Med. 1992;20:135–9. doi: 10.1097/00003246-199201000-00026. [DOI] [PubMed] [Google Scholar]
- 131.Pucilowska JB, Williams KL, Lund PK. Fibrogenesis. IV. fibrosis and inflammatory bowel disease: Cellular mediators and animal models. Am J Physiol Gastrointest Liver Physiol. 2000;279:G653–9. doi: 10.1152/ajpgi.2000.279.4.G653. [DOI] [PubMed] [Google Scholar]
- 132.Moeser AJ, Haskell MM, Shifflett DE, Little D, Schultz BD, Blikslager AT. ClC-2 chloride secretion mediates prostaglandin-induced recovery of barrier function in ischemia-injured porcine ileum. Gastroenterology. 2004;127:802–15. doi: 10.1053/j.gastro.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 133.Little D, Dean RA, Young KM, et al. PI3K signaling is required for prostaglandin-induced mucosal recovery in ischemia-injured porcine ileum. Am J Physiol Gastrointest Liver Physiol. 2003;284:G46–56. doi: 10.1152/ajpgi.00121.2002. [DOI] [PubMed] [Google Scholar]
- 134.Moeser AJ, Nighot PK, Ryan KA, Wooten JG, Blikslager AT. Prostaglandin-mediated inhibition of na+/H+ exchanger isoform 2 stimulates recovery of barrier function in ischemia-injured intestine. Am J Physiol Gastrointest Liver Physiol. 2006;291:G885–94. doi: 10.1152/ajpgi.00380.2005. [DOI] [PubMed] [Google Scholar]
- 135.Moeser AJ, Nighot PK, Engelke KJ, Ueno R, Blikslager AT. Recovery of mucosal barrier function in ischemic porcine ileum and colon is stimulated by a novel agonist of the ClC-2 chloride channel, lubiprostone. Am J Physiol Gastrointest Liver Physiol. 2007;292:G647–56. doi: 10.1152/ajpgi.00183.2006. [DOI] [PubMed] [Google Scholar]
- 136.Hernandez LA, Grisham MB, Twohig B, Arfors KE, Harlan JM, Granger DN. Role of neutrophils in ischemia-reperfusion-induced microvascular injury. Am J Physiol. 1987;253:H699–703. doi: 10.1152/ajpheart.1987.253.3.H699. [DOI] [PubMed] [Google Scholar]
- 137.Granger DN, Benoit JN, Suzuki M, Grisham MB. Leukocyte adherence to venular endothelium during ischemia-reperfusion. Am J Physiol. 1989;257:G683–8. doi: 10.1152/ajpgi.1989.257.5.G683. [DOI] [PubMed] [Google Scholar]
- 138.Rhoads JM, Argenzio RA, Chen W, et al. L-glutamine stimulates intestinal cell proliferation and activates mitogen-activated protein kinases. Am J Physiol. 1997;272:G943–53. doi: 10.1152/ajpgi.1997.272.5.G943. [DOI] [PubMed] [Google Scholar]
- 139.Ahdieh N, Blikslager AT, Bhat BG, Coleman RA, Argenzio RA, Rhoads JM. L-glutamine and transforming growth factor-alpha enhance recovery of monoacylglycerol acyltransferase and diacylglycerol acyltransferase activity in porcine postischemic ileum. Pediatr Res. 1998;43:227–33. doi: 10.1203/00006450-199802000-00012. [DOI] [PubMed] [Google Scholar]
- 140.Gotthardt DN, Gauss A, Zech U, et al. Indications for intestinal transplantation: Recognizing the scope and limits of total parenteral nutrition. Clin Transplant. 2013;27:49–55. doi: 10.1111/ctr.12161. [DOI] [PubMed] [Google Scholar]
- 141.Cilieborg MS, Thymann T, Siggers R, et al. The incidence of necrotizing enterocolitis is increased following probiotic administration to preterm pigs. J Nutr. 2011;141:223–30. doi: 10.3945/jn.110.128561. [DOI] [PubMed] [Google Scholar]
- 142.Lennon D, Zanganeh T, Borum PR. Development of the piglet neonatal intensive care unit for translational research. Lab Anim (NY) 2011;40:253–8. doi: 10.1038/laban0811-253. [DOI] [PubMed] [Google Scholar]
- 143.Sangild PT, Petersen YM, Schmidt M, et al. Preterm birth affects the intestinal response to parenteral and enteral nutrition in newborn pigs. J Nutr. 2002;132:3786–94. doi: 10.1093/jn/132.9.2673. [DOI] [PubMed] [Google Scholar]
- 144.Cilieborg MS, Schmidt M, Skovgaard K, et al. Fetal lipopolysaccharide exposure modulates diet-dependent gut maturation and sensitivity to necrotising enterocolitis in pre-term pigs. Br J Nutr. 2011;106:852–61. doi: 10.1017/S000711451100047X. [DOI] [PubMed] [Google Scholar]
- 145.Vegge A, Thymann T, Lund P, et al. Glucagon-like peptide-2 induces rapid digestive adaptation following intestinal resection in preterm neonates. Am J Physiol Gastrointest Liver Physiol. 2013;305:G277–85. doi: 10.1152/ajpgi.00064.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Siggers RH, Siggers J, Thymann T, Boye M, Sangild PT. Nutritional modulation of the gut microbiota and immune system in preterm neonates susceptible to necrotizing enterocolitis. J Nutr Biochem. 2011;22:511–21. doi: 10.1016/j.jnutbio.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 147.Hoyos AB. Reduced incidence of necrotizing enterocolitis associated with enteral administration of lactobacillus acidophilus and bifidobacterium infantis to neonates in an intensive care unit. Int J Infect Dis. 1999;3:197–202. doi: 10.1016/s1201-9712(99)90024-3. [DOI] [PubMed] [Google Scholar]
- 148.Lin HC, Su BH, Chen AC, et al. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2005;115:1–4. doi: 10.1542/peds.2004-1463. [DOI] [PubMed] [Google Scholar]
- 149.Flores CA, Wells MA, Morrill M, Bustamante SA, Koldovsky O. Diet, age and intestinal bile acids in pigs. Nutrition. 1992;8:418–20. [PubMed] [Google Scholar]
- 150.Montgomery RK, Mulberg AE, Grand RJ. Development of the human gastrointestinal tract: Twenty years of progress. Gastroenterology. 1999;116:702–31. doi: 10.1016/s0016-5085(99)70193-9. [DOI] [PubMed] [Google Scholar]
- 151.Zabielski R, Le Huerou-Luron I, Guilloteau P. Development of gastrointestinal and pancreatic functions in mammalians (mainly bovine and porcine species): Influence of age and ingested food. Reprod Nutr Dev. 1999;39:5–26. doi: 10.1051/rnd:19990101. [DOI] [PubMed] [Google Scholar]
- 152.Wolvekamp MC, Heineman E, Taylor RG, Fuller PJ. Towards understanding the process of intestinal adaptation. Dig Dis. 1996;14:59–72. doi: 10.1159/000171539. [DOI] [PubMed] [Google Scholar]
- 153.Thompson JS, Quigley EM, Adrian TE. Smooth muscle adaptation after intestinal transection and resection. Dig Dis Sci. 1996;41:1760–7. doi: 10.1007/BF02088742. [DOI] [PubMed] [Google Scholar]
- 154.Bicakci U, Tuncel OK, Bilgici B, et al. The comparison of the intestinal adaptation effects of subcutaneous and oral insulin in a rats with short bowel syndrome. Afr J Paediatr Surg. 2013;10:91–4. doi: 10.4103/0189-6725.115030. [DOI] [PubMed] [Google Scholar]
- 155.Moughan PJ, Birtles MJ, Cranwell PD, Smith WC, Pedraza M. The piglet as a model animal for studying aspects of digestion and absorption in milk-fed human infants. World Rev Nutr Diet. 1992;67:40–113. doi: 10.1159/000419461. [DOI] [PubMed] [Google Scholar]
- 156.Benhamou PH, Canarelli JP, Richard S, et al. Human recombinant growth hormone increases small bowel lengthening after massive small bowel resection in piglets. J Pediatr Surg. 1997;32:1332–6. doi: 10.1016/s0022-3468(97)90315-8. [DOI] [PubMed] [Google Scholar]
- 157.Burrin DG, Stoll B, Jiang R, et al. GLP-2 stimulates intestinal growth in premature TPN-fed pigs by suppressing proteolysis and apoptosis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1249–56. doi: 10.1152/ajpgi.2000.279.6.G1249. [DOI] [PubMed] [Google Scholar]
- 158.Guan X, Karpen HE, Stephens J, et al. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology. 2006;130:150–64. doi: 10.1053/j.gastro.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 159.Guan X, Stoll B, Lu X, et al. GLP-2-mediated up-regulation of intestinal blood flow and glucose uptake is nitric oxide-dependent in TPN-fed piglets 1. Gastroenterology. 2003;125:136–47. doi: 10.1016/s0016-5085(03)00667-x. [DOI] [PubMed] [Google Scholar]
- 160.Petersen YM, Elnif J, Schmidt M, Sangild PT. Glucagon-like peptide 2 enhances maltase-glucoamylase and sucrase-isomaltase gene expression and activity in parenterally fed premature neonatal piglets. Pediatr Res. 2002;52:498–503. doi: 10.1203/00006450-200210000-00007. [DOI] [PubMed] [Google Scholar]
- 161.Petersen YM, Hartmann B, Holst JJ, Le Huerou-Luron I, Bjornvad CR, Sangild PT. Introduction of enteral food increases plasma GLP-2 and decreases GLP-2 receptor mRNA abundance during pig development. J Nutr. 2003;133:1781–6. doi: 10.1093/jn/133.6.1781. [DOI] [PubMed] [Google Scholar]
- 162.Sangild PT, Tappenden KA, Malo C, et al. Glucagon-like peptide 2 stimulates intestinal nutrient absorption in parenterally fed newborn pigs. J Pediatr Gastroenterol Nutr. 2006;43:160–7. doi: 10.1097/01.mpg.0000228122.82723.1b. [DOI] [PubMed] [Google Scholar]
- 163.Jeppesen PB, Hartmann B, Thulesen J, et al. Glucagon-like peptide 2 improves nutrient absorption and nutritional status in short-bowel patients with no colon. Gastroenterology. 2001;120:806–15. doi: 10.1053/gast.2001.22555. [DOI] [PubMed] [Google Scholar]
- 164.Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B, O’Keefe SJ. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut. 2011;60:902–14. doi: 10.1136/gut.2010.218271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jeppesen PB, Pertkiewicz M, Messing B, et al. Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology. 2012;143:1473–1481. doi: 10.1053/j.gastro.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 166.Abu-Elmagd KM, Kosmach-Park B, Costa G, et al. Long-term survival, nutritional autonomy, and quality of life after intestinal and multivisceral transplantation. Ann Surg. 2012;256:494–508. doi: 10.1097/SLA.0b013e318265f310. [DOI] [PubMed] [Google Scholar]
- 167.Rothkotter HJ. Anatomical particularities of the porcine immune system--a physician’s view. Dev Comp Immunol. 2009;33:267–72. doi: 10.1016/j.dci.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 168.Ibrahim Z, Busch J, Awwad M, Wagner R, Wells K, Cooper DK. Selected physiologic compatibilities and incompatibilities between human and porcine organ systems. Xenotransplantation. 2006;13:488–99. doi: 10.1111/j.1399-3089.2006.00346.x. [DOI] [PubMed] [Google Scholar]
- 169.Kirk AD. Crossing the bridge: Large animal models in translational transplantation research. Immunol Rev. 2003;196:176–96. doi: 10.1046/j.1600-065x.2003.00081.x. [DOI] [PubMed] [Google Scholar]
- 170.Weih S, Kessler M, Fonouni H, et al. Review of various techniques of small bowel transplantation in pigs. J Surg Res. 2011;171:709–18. doi: 10.1016/j.jss.2011.07.052. [DOI] [PubMed] [Google Scholar]
- 171.Ruiz P, Bagni A, Brown R, et al. Histological criteria for the identification of acute cellular rejection in human small bowel allografts: Results of the pathology workshop at the VIII international small bowel transplant symposium. Transplant Proc. 2004;36:335–7. doi: 10.1016/j.transproceed.2004.01.079. [DOI] [PubMed] [Google Scholar]
- 172.Avansino JR, Chen DC, Woolman JD, Hoagland VD, Stelzner M. Engraftment of mucosal stem cells into murine jejunum is dependent on optimal dose of cells. J Surg Res. 2006;132:74–9. doi: 10.1016/j.jss.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 173.Kobayashi E, Hishikawa S, Teratani T, Lefor AT. The pig as a model for translational research: Overview of porcine animal models at jichi medical university. Transplant Res. 2012;1:8. doi: 10.1186/2047-1440-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sala FG, Kunisaki SM, Ochoa ER, Vacanti J, Grikscheit TC. Tissue-engineered small intestine and stomach form from autologous tissue in a preclinical large animal model. J Surg Res. 2009;156:205–12. doi: 10.1016/j.jss.2009.03.062. [DOI] [PubMed] [Google Scholar]
- 175.Whyte JJ, Prather RS. Genetic modifications of pigs for medicine and agriculture. Mol Reprod Dev. 2011;78:879–91. doi: 10.1002/mrd.21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hammer RE, Pursel VG, Rexroad CE, Jr, et al. Production of transgenic rabbits, sheep and pigs by microinjection. Nature. 1985;315:680–3. doi: 10.1038/315680a0. [DOI] [PubMed] [Google Scholar]
- 177.Pierson RN, 3rd, Dorling A, Ayares D, et al. Current status of xenotransplantation and prospects for clinical application. Xenotransplantation. 2009;16:263–80. doi: 10.1111/j.1399-3089.2009.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Klymiuk N, Aigner B, Brem G, Wolf E. Genetic modification of pigs as organ donors for xenotransplantation. Mol Reprod Dev. 2010;77:209–21. doi: 10.1002/mrd.21127. [DOI] [PubMed] [Google Scholar]
- 179.Le Bas-Bernardet S, Anegon I, Blancho G. Progress and prospects: Genetic engineering in xenotransplantation. Gene Ther. 2008;15:1247–56. doi: 10.1038/gt.2008.119. [DOI] [PubMed] [Google Scholar]
- 180.Walters EM, Wolf E, Whyte JJ, et al. Completion of the swine genome will simplify the production of swine as a large animal biomedical model. BMC Med Genomics. 2012;5:55. doi: 10.1186/1755-8794-5-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bitar KN, Raghavan S. Intestinal tissue engineering: Current concepts and future vision of regenerative medicine in the gut. Neurogastroenterol Motil. 2012;24:7–19. doi: 10.1111/j.1365-2982.2011.01843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Spurrier RG, Grikscheit TC. Tissue engineering the small intestine. Clin Gastroenterol Hepatol. 2013;11:354–8. doi: 10.1016/j.cgh.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 183.Rogers CS, Hao Y, Rokhlina T, et al. Production of CFTR-null and CFTR-DeltaF508 heterozygous pigs by adeno-associated virus-mediated gene targeting and somatic cell nuclear transfer. J Clin Invest. 2008;118:1571–7. doi: 10.1172/JCI34773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rizk P, Barker N. Gut stem cells in tissue renewal and disease: Methods, markers, and myths. Wiley Interdiscip Rev Syst Biol Med. 2012;4:475–96. doi: 10.1002/wsbm.1176. [DOI] [PubMed] [Google Scholar]
- 185.Boivin GP, Washington K, Yang K, et al. Pathology of mouse models of intestinal cancer: Consensus report and recommendations. Gastroenterology. 2003;124:762–77. doi: 10.1053/gast.2003.50094. [DOI] [PubMed] [Google Scholar]
- 186.Phillips KA, Van Bebber S, Issa AM. Diagnostics and biomarker development: Priming the pipeline. Nat Rev Drug Discov. 2006;5:463–9. doi: 10.1038/nrd2033. [DOI] [PubMed] [Google Scholar]
- 187.Van Landeghem L, Santoro MA, Krebs AE, et al. Activation of two distinct Sox9-EGFP-expressing intestinal stem cell populations during crypt regeneration after irradiation. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1111–32. doi: 10.1152/ajpgi.00519.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yui S, Nakamura T, Sato T, et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5(+) stem cell. Nat Med. 2012;18:618–23. doi: 10.1038/nm.2695. [DOI] [PubMed] [Google Scholar]
- 189.Chen DC, Agopian VG, Avansino JR, Lee JK, Farley SM, Stelzner M. Optical tissue window: A novel model for optimizing engraftment of intestinal stem cell organoids. J Surg Res. 2006;134:52–60. doi: 10.1016/j.jss.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 190.Dekaney CM, Gulati AS, Garrison AP, Helmrath MA, Henning SJ. Regeneration of intestinal stem/progenitor cells following doxorubicin treatment of mice. Am J Physiol Gastrointest Liver Physiol. 2009;297:G461–70. doi: 10.1152/ajpgi.90446.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Willing BP, Van Kessel AG. Enterocyte proliferation and apoptosis in the caudal small intestine is influenced by the composition of colonizing commensal bacteria in the neonatal gnotobiotic pig. J Anim Sci. 2007;85:3256–66. doi: 10.2527/jas.2007-0320. [DOI] [PubMed] [Google Scholar]
- 192.Stoltz DA, Rokhlina T, Ernst SE, et al. Intestinal CFTR expression alleviates meconium ileus in cystic fibrosis pigs. J Clin Invest. 2013;123:2685–93. doi: 10.1172/JCI68867. [DOI] [PMC free article] [PubMed] [Google Scholar]