Abstract
URF13 is a mitochondrially encoded, integral membrane protein found only in maize carrying the cms-T cytoplasm. URF13 is associated with cytoplasmic male sterility, Texas type, and causes susceptibility to the fungal pathogens Bipolaris maydis race T and Phyllosticta maydis. URF13 is predicted to contain three transmembrane alpha-helices and is a receptor for the pathotoxins (T-toxins) produced by B. maydis race T and P. maydis. Binding of T-toxin to URF13 leads to membrane permeability. Cross-linking of URF13 oligomers with N,N'-dicyclohexylcarbodiimide (DCCD) protects Escherichia coli cells expressing URF13 and cms-T mitochondria from the permeability caused by T-toxin or methomyl. Using mutated forms of URF13 expressed in E. coli cells, we determined the molecular mechanism of DCCD protection. We separately changed Lys-37 in helix II to isoleucine (K37I-URF13) and Lys-32 in the helix I/helix II loop region to alanine (K32A-URF13). DCCD treatment of K37I-URF13-expressing cells did not protect the cells from permeability caused by T-toxin or methomyl. DCCD cross-linking was greatly reduced in K37I-URF13 and in D39V-URF13-expressing cells, but it was unaffected in K32A-URF13-expressing cells. Binding of methomyl or T-toxin decreases DCCD cross-linking of URF13 oligomers expressed in either E. coli or cms-T mitochondria. We conclude that Asp-39 in helix II is cross-linked by DCCD to Lys-37 in helix II of an adjacent URF13 molecule and that this cross-linking protects against toxin-mediated permeabilization. Our results also indicate that helices II form a central core in URF13 oligomers.
Full text
PDF

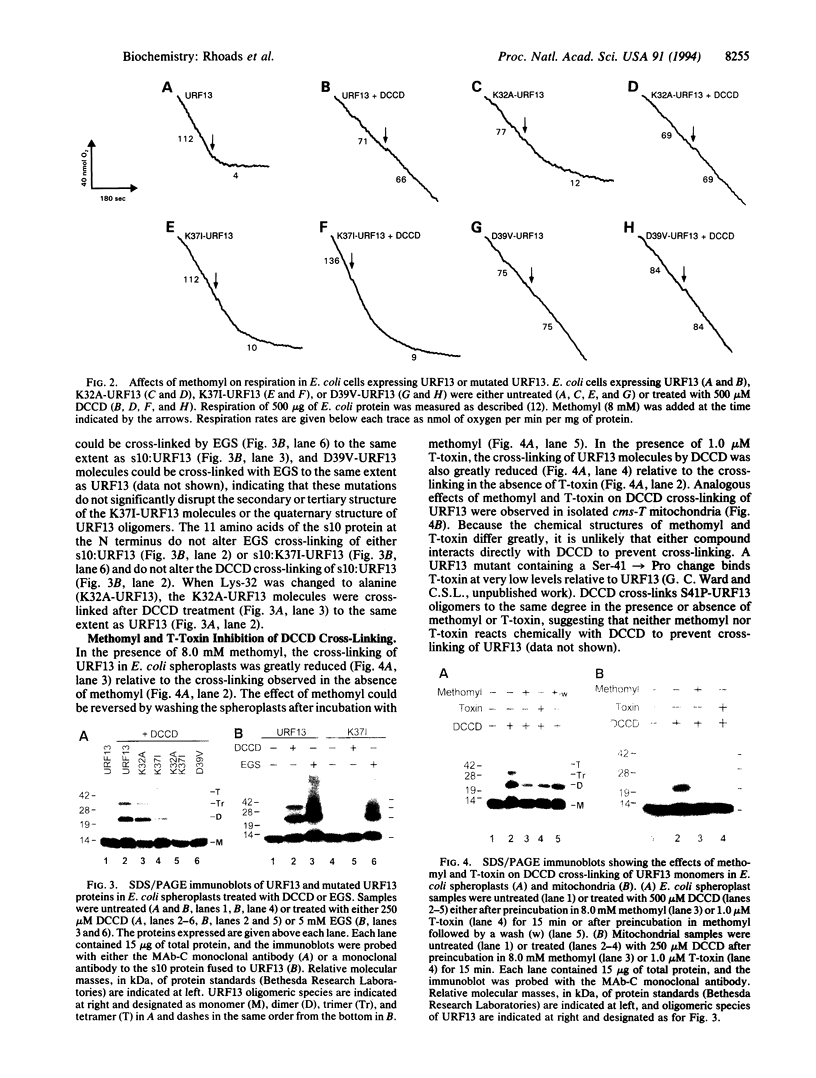
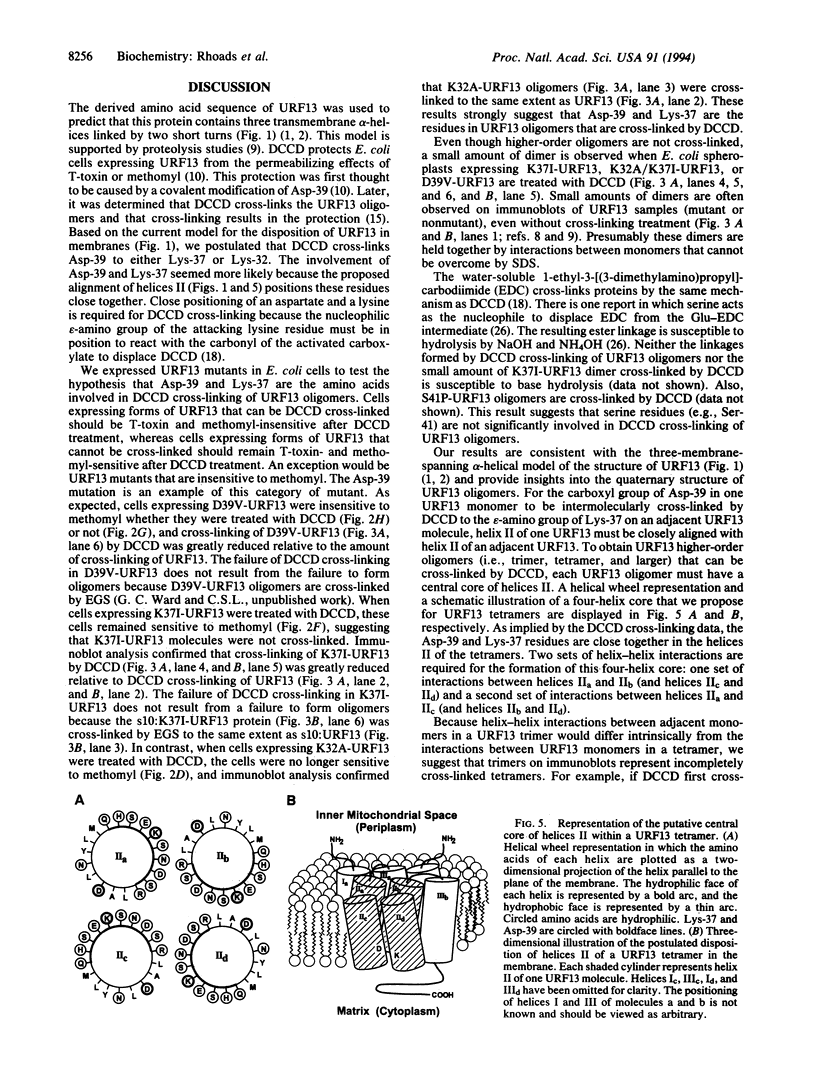

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaustein R. O., Koehler T. M., Collier R. J., Finkelstein A. Anthrax toxin: channel-forming activity of protective antigen in planar phospholipid bilayers. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2209–2213. doi: 10.1073/pnas.86.7.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun C. J., Siedow J. N., Levings C. S., 3rd Fungal toxins bind to the URF13 protein in maize mitochondria and Escherichia coli. Plant Cell. 1990 Feb;2(2):153–161. doi: 10.1105/tpc.2.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun C. J., Siedow J. N., Williams M. E., Levings C. S., 3rd Mutations in the maize mitochondrial T-urf13 gene eliminate sensitivity to a fungal pathotoxin. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4435–4439. doi: 10.1073/pnas.86.12.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallmann H. G., Flynn T. G., Dunn S. D. Determination of the 1-ethyl-3-[(3-dimethylamino)propyl]-carbodiimide- induced cross-link between the beta and epsilon subunits of Escherichia coli F1-ATPase. J Biol Chem. 1992 Sep 15;267(26):18953–18960. [PubMed] [Google Scholar]
- Davidson V. L., Brunden K. R., Cramer W. A., Cohen F. S. Studies on the mechanism of action of channel-forming colicins using artificial membranes. J Membr Biol. 1984;79(2):105–118. doi: 10.1007/BF01872115. [DOI] [PubMed] [Google Scholar]
- Dewey R. E., Levings C. S., 3rd, Timothy D. H. Novel recombinations in the maize mitochondrial genome produce a unique transcriptional unit in the Texas male-sterile cytoplasm. Cell. 1986 Feb 14;44(3):439–449. doi: 10.1016/0092-8674(86)90465-4. [DOI] [PubMed] [Google Scholar]
- Dewey R. E., Siedow J. N., Timothy D. H., Levings C. S., 3rd A 13-kilodalton maize mitochondrial protein in E. coli confers sensitivity to Bipolaris maydis toxin. Science. 1988 Jan 15;239(4837):293–295. doi: 10.1126/science.3276005. [DOI] [PubMed] [Google Scholar]
- Dewey R. E., Timothy D. H., Levings C. S. A mitochondrial protein associated with cytoplasmic male sterility in the T cytoplasm of maize. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5374–5378. doi: 10.1073/pnas.84.15.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack E., Lin C., Yang H., Horner H. T. T-URF 13 Protein from Mitochondria of Texas Male-Sterile Maize (Zea mays L.) : Its Purification and Submitochondrial Localization, and Immunogold Labeling in Anther Tapetum during Microsporogenesis. Plant Physiol. 1991 Mar;95(3):861–870. doi: 10.1104/pp.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch D. H., Romero-Mira M., Ehrlich B. E., Finkelstein A., DasGupta B. R., Simpson L. L. Channels formed by botulinum, tetanus, and diphtheria toxins in planar lipid bilayers: relevance to translocation of proteins across membranes. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1692–1696. doi: 10.1073/pnas.82.6.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden M. J., Sze H. Effects of Helminthosporium maydis Race T Toxin on Electron Transport in Susceptible Corn Mitochondria and Prevention of Toxin Actions by Dicyclohexylcarbodiimide. Plant Physiol. 1989 Dec;91(4):1296–1302. doi: 10.1104/pp.91.4.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden M. J., Sze H. Helminthosporium maydis T Toxin Increased Membrane Permeability to Ca in Susceptible Corn Mitochondria. Plant Physiol. 1984 May;75(1):235–237. doi: 10.1104/pp.75.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspi C. I., Siedow J. N. Cross-linking of the cms-T maize mitochondrial pore-forming protein URF13 by N,N'-dicyclohexylcarbodiimide and its effect on URF13 sensitivity to fungal toxins. J Biol Chem. 1993 Mar 15;268(8):5828–5833. [PubMed] [Google Scholar]
- Korth K. L., Kaspi C. I., Siedow J. N., Levings C. S., 3rd URF13, a maize mitochondrial pore-forming protein, is oligomeric and has a mixed orientation in Escherichia coli plasma membranes. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10865–10869. doi: 10.1073/pnas.88.23.10865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson E., Howlett B., Jagendorf A. Artificial reductant enhancement of the Lowry method for protein determination. Anal Biochem. 1986 Jun;155(2):243–248. doi: 10.1016/0003-2697(86)90432-x. [DOI] [PubMed] [Google Scholar]
- Lear J. D., Wasserman Z. R., DeGrado W. F. Synthetic amphiphilic peptide models for protein ion channels. Science. 1988 May 27;240(4856):1177–1181. doi: 10.1126/science.2453923. [DOI] [PubMed] [Google Scholar]
- Levings C. S., 3rd, Siedow J. N. Molecular basis of disease susceptibility in the Texas cytoplasm of maize. Plant Mol Biol. 1992 May;19(1):135–147. doi: 10.1007/BF00015611. [DOI] [PubMed] [Google Scholar]
- Levings C. S., 3rd The Texas cytoplasm of maize: cytoplasmic male sterility and disease susceptibility. Science. 1990 Nov 16;250(4983):942–947. doi: 10.1126/science.250.4983.942. [DOI] [PubMed] [Google Scholar]
- Matthews D. E., Gregory P., Gracen V. E. Helminthosporium maydis Race T Toxin Induces Leakage of NAD from T Cytoplasm Corn Mitochondria. Plant Physiol. 1979 Jun;63(6):1149–1153. doi: 10.1104/pp.63.6.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. J., Koeppe D. E. Southern corn leaf blight: susceptible and resistant mitochondria. Science. 1971 Jul 2;173(3991):67–69. doi: 10.1126/science.173.3991.67. [DOI] [PubMed] [Google Scholar]
- Mosser G., Mallouh V., Brisson A. A 9 A two-dimensional projected structure of cholera toxin B-subunit-GM1 complexes determined by electron crystallography. J Mol Biol. 1992 Jul 5;226(1):23–28. doi: 10.1016/0022-2836(92)90120-9. [DOI] [PubMed] [Google Scholar]
- Nałecz M. J., Casey R. P., Azzi A. Use of N,N'-dicyclohexylcarbodiimide to study membrane-bound enzymes. Methods Enzymol. 1986;125:86–108. doi: 10.1016/s0076-6879(86)25009-0. [DOI] [PubMed] [Google Scholar]
- Ojcius D. M., Young J. D. Cytolytic pore-forming proteins and peptides: is there a common structural motif? Trends Biochem Sci. 1991 Jun;16(6):225–229. doi: 10.1016/0968-0004(91)90090-i. [DOI] [PubMed] [Google Scholar]
- Oka T., Murata Y., Nakanishi T., Yoshizumi H., Hayashida H., Ohtsuki Y., Toyoshima K., Hakura A. Similarity, in molecular structure and function, between the plant toxin purothionin and the mammalian pore-forming proteins. Mol Biol Evol. 1992 Jul;9(4):707–715. doi: 10.1093/oxfordjournals.molbev.a040754. [DOI] [PubMed] [Google Scholar]
- Presnell S. R., Cohen F. E. Topological distribution of four-alpha-helix bundles. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6592–6596. doi: 10.1073/pnas.86.17.6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witholt B., Boekhout M., Brock M., Kingma J., Heerikhuizen H. V., Leij L. D. An efficient and reproducible procedure for the formation of spheroplasts from variously grown Escherichia coli. Anal Biochem. 1976 Jul;74(1):160–170. doi: 10.1016/0003-2697(76)90320-1. [DOI] [PubMed] [Google Scholar]