Abstract
Lysophosphatidic acid (LPA) is a bioactive lipid mediator of inflammation via the LPA receptors 1–6. We and others have previously described pro-inflammatory and pro-fibrotic activities of LPA signaling in bleomycin- or lipopolysaccharide (LPS)-induced pulmonary fibrosis or lung injury models. In this study, we investigated if LPA signaling plays a role in the pathogenesis of systemic sepsis from an abdominal source. We report here that antagonism of the LPA receptor LPA1 with the small molecule ki16425 reduces the severity of abdominal inflammation and organ damage in the setting of peritoneal endotoxin exposure. Pretreatment of mice with intraperitoneal ki16425 eliminates LPS-induced peritoneal neutrophil chemokine and cytokine production, liver oxidative stress, liver injury, and cellular apoptosis in visceral organs. Mice pretreated with ki16425 are also protected from LPS-induced mortality. Tissue myeloperoxidase activity is not affected by LPA1 antagonism. We have shown that LPA1 is associated with LPS co-receptor CD14, the association is suppressed by ki16425. LPS-induced phosphorylation of PKCδ and p38 MAPK in liver cells and IL-6 production in Raw264 cells are likewise blunted by LPA1 antagonism. These studies indicate that the small molecule inhibitor of LPA1, ki16425, suppresses cytokine responses and inflammation in a peritoneal sepsis model by blunting downstream signaling through the LPA1-CD14-TLR4 receptor complex. This anti-inflammatory effect may represent a therapeutic strategy for the treatment of systemic inflammatory responses to infection of the abdominal cavity.
Introduction
Abdominal sepsis is a devastating disease that can rapidly progress to multiple organ failure, shock, and death. Abdominal sepsis carries a high mortality and represents a large public health burden (1–3). The predominant pathogens that cause abdominal sepsis are gram negative bacteria of the enterobacteriaceae family of gastrointestinal (GI) commensal and pathogenic bacteria such as Escherichia Coli, Salmonella species, and Enterobacter species among others (4, 5). These gram negative species all possess lipopolysaccharide (LPS, also known as endotoxin) as an integral component of their cell wall. Individuals afflicted with abdominal sepsis often suffer from endotoxemia and shock due to a robust inflammatory response that is triggered by LPS (6, 7). Several efforts have been attempted to reduce the inflammatory response to LPS as a protective therapy in patients with sepsis without convincing results (8).
Lysophosphatidic acid (LPA) is a naturally occurring inflammatory phospholipid, which contributes to the pathogenesis of many diseases including asthma, acute lung injury, and fibrosis (9–14). Biochemically, LPA ligates G protein-coupled surface receptors LPA1-6 with downstream activation of transcription factors including the nuclear factor kappa B (NF-κB) and second messengers such as p38 MAPK (15, 16). Among these receptors, LPA1 seems to be the most active in LPA signaling (17). There is a clear role for LPA1 in the pathobiology of lung injury and fibrosis (10, 12), as the activation of this signaling axis drives both pro-inflammatory and pro-fibrotic biology in pulmonary systems. In a murine model of lung fibrosis induced by bleomycin exposure, alveolar epithelia undergo increased apoptotic cell death while fibroblasts display enhanced survival and drive the fibrosis phenotype (18). Blocking the LPA signal decreases the post-inflammatory fibrosis in this bleomycin exposure model, as vascular leak and lung caspase-3 activity were both decreased in LPA1 knockout animals, which also demonstrate a survival advantage in the model (10). LPA1 knockdown or antagonism with small molecule drugs including the inhibitor ki16425 also reduces inflammatory cytokine signaling secondary to LPS exposure in the lungs (11). In those studies, LPA1 associates with the LPS co-receptor CD14 in response to LPS exposure to potentiate the endotoxin signaling through the toll like receptor (TLR)-4 pathway.
LPA signaling also contributes to inflammation in other organ systems and disease states (17). In a model of neuropathic pain, LPA is sufficient to cause abnormal pain behaviors (19), and these effects are reversed by the LPA1 blocker ki16425 (20). LPA signal blockade has also been described as beneficial in arthritic inflammation; ki16425 increased bone mineralization by blunting the proliferation of bone destructive osteoclasts while increasing cell numbers of bone creating osteoblasts (21). In this study, we present findings that in the setting of intraperitoneal LPS exposure, blockade of the LPA1 receptor with the ki16425 antagonist effectively blunts inflammation and inflammatory tissue damage by several measures and prolongs survival.
Methods and Materials
Sepsis model by intraperitoneal injection of LPS
C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME) and housed in a specific pathogen-free barrier facility maintained by the University of Pittsburgh Animal Resources Center. All the animal experiments were approved by IACUC of the University of Pittsburgh. Adult male, 8–10-week old mice, with an average weight of 20–25 g were anaesthetized with 3–5 ml/kg of anaesthesia mixture of ketamine and of xylazine. DMSO (5% in PBS) or ki16425 (20 mg/kg body weight, in 5% DMSO) was intraperitoneally (i.p.) injected for 1 h prior to i.p. injection of LPS (5 or 15 mg/kg body weight) or PBS alone. In our studies, a higher dose of LPS (15 mg/kg body weight) was used to cause mortality and a lower LPS dose (5 mg/kg body weight) was used for mechanistic studies as it was sufficient to induce sepsis and organ injury (22, 23). In case of i.p. LPS (15 mg/kg body weight), mice were closely monitored for 5 days. In case of i.p. LPS (5 mg/kg body weight), mice were sacrificed after 4 h, peritoneal lavage, plasma, liver and kidney tissues were collected. Liver and kidney tissues were fixed and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining was performed by Pathology Core Facility at University of Pittsburgh.
Cells and regents
Raw264 and HEK293 cells were cultured with DMEM medium containing 10 % fetal bovine serum (FBS) and antibiotics at 37°C in 5 % CO2 incubator. HepG2 cells were cultured in EMEM medium containing 10 % FBS and antibiotics. V5 antibody was from Life technologies (Grand Island, NY). Phospho-PKCδ and cleaved caspase 3 antibodies were from Cell Signaling (Danvers, MA). LPS, ki16425, and β-actin antibody were from Sigma (St. Louis, MO). Immunobilized protein A/G beads and phospho-p38 MAPK antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). All materials in highest grades used in the experiments are commercially available.
Mouse keratinocyte-derived cytokine (KC) and interleukin (IL)-6 measurements
Peritoneal lavage fluid was collected in 3 ml of PBS, followed by centrifugation at 500 g for 10 min to remove cell debris. KC and IL-6 levels were measured with ELISA kits for mouse KC or IL-6 according to the manufacture’s instruction (R&D system).
Oxidized protein detection
Liver and kidney tissue lysates were sonicated. Equal amounts of lysates (20 µg / 5 µl) were mixed with equal volume of 12% SDS, followed by addition of 10 µl of 1 × DNP solution. After 15 min incubation at room temperature, neutralizing solution was added. Protein oxidation was determined by immunoblotting with an anti-DNP antibody (EMD Millipore).
Immunoprecipitation and western blotting
1000 µg of cell lysates were incubated with an antibody against LPA1, followed by protein A/G-agarose binding for 16 h at 4°C. Agarose beads were then washed with washing buffer containing 0.1% Triton-X100 for three times. LPA1 binding proteins were eluted by boiling. For western blotting, equal amounts of immunoprecipitation complex or equal amounts of protein (20 µg) from tissues or cells were subjected to 4–15% SDS/PAGE gels, followed by transferring to polyvinylidene difluoride membranes. Membrane was then blocked with 5 % (w/v) BSA in TBST (25 mM Tris-HCl, pH 7.4, 137 mM NaCl and 0.1 % Tween-20) for 1 h and incubated with antibodies (dilute 1:1000) in 5 % (w/v) BSA in TBST for overnight at 4 °C. The membranes were washed at least three times with TBST at 15 min intervals and then incubated with horseradish peroxidase-conjugated secondary antibodes (1: 3,000) for 1 h at room temperature. The membrane was developed with an enhanced chemiluminescence detection system.
MPO ELISA assay
Liver and kidney from i.p. LPS experimental C57BL/6 mice were harvested and homogenized by a mechanical homogenizer in 200 ul RIPA lysis buffer (20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM beta-glycerophosphate, 1 mM Na3VO4, 1 µg/ml leupeptin, 1mM PMSF, 28 mg/ml aprotinin), followed by sonication and centrifugation at 15,000 g for 20 min. Equal amounts of protein from each sample had been applied in duplicate to a MPO ELISA kit (Hycult Biotech Inc.) according the manufactory’s instruction.
Alanine transaminase (ALT) activity assay
To assess hepatic function and cellular injury following i.p. LPS, plasma ALT levels were measured using the Opera Clinical Chemistry System (Bayer).
Statistical analyses
All results in Fig. 2–7 were subjected to statistical analysis using one-way ANOVA and, where appropriate, analyzed by Student-Newman-Keul test. Data are expressed as means ± S.D. of samples (n=3–6) and level of significance was taken as p<0.05. Survival curve was analyzed using Graphpad prism 4.0.
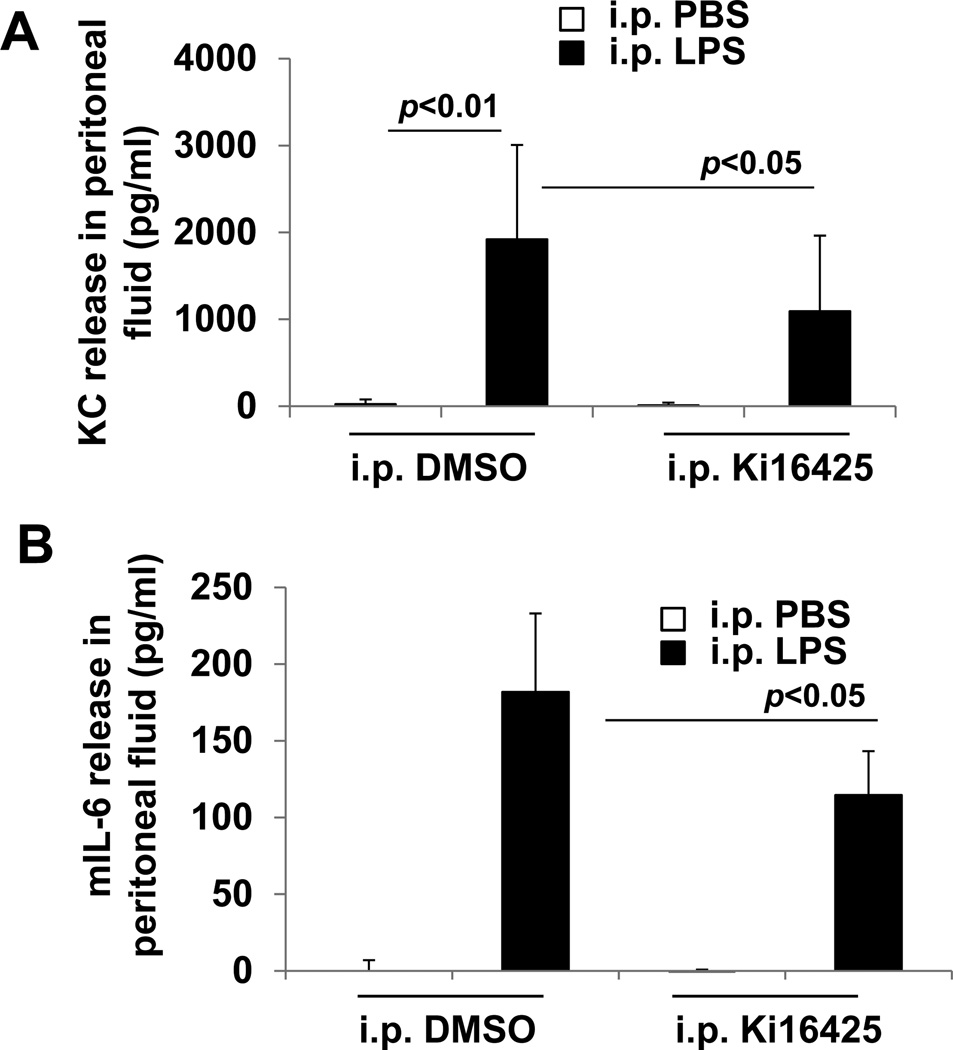
Fig. 2.
Ki16425 reduces the LPS-induced increase in peritoneal lavage levels of the KC and IL-6 in mice. Male C57BL/6 mice were given i.p. DMSO (5% in PBS) or i.p. ki16425 (20 mg/kg body weight) 1 h before LPS administration (i.p. 5 mg/kg body weight). Peritoneal lavage fluids were collected after 4 h and KC (A) and IL-6 (B) levels were measured by ELISA.
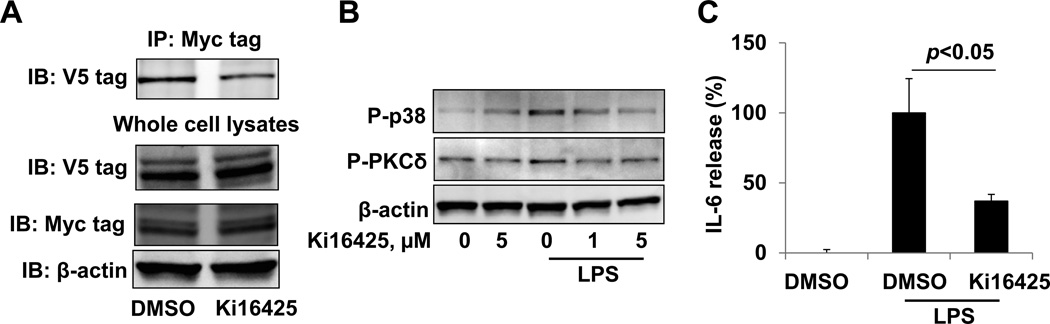
Fig. 7.
Ki16425 attenuates LPA1 / CD14 association and LPS-induced signaling and cytokine release. A. Cell lysates from CD14-V5 and LPA1-Myc over-expressed HEK293 cells were incubated with DMSO or ki16425 (5 µM) for 1 h. Cell lysates were then subjected to immunoprecipitation with a Myc tag antibody, followed by a V5 tag immunoblotting. Input lysates were analyzed with V5 tag, Myc tag, and β-actin immunoblotting. B. HepG2 cells were treated with ki16425 prior to LPS (1 µg/ml, 30 min) treatment. Cell lysates were analyzed by phospho (P)-PKCδ, P-p38 MAPK, and β-actin immunoblotting. C. Raw264 cells were treated with DMSO or Ki16425 (5 µM) for 30 min prior to LPS treatment (0.2 µg/ml, 3 h). IL-6 release in medium was measured by ELISA.
Results
Ki16425 preserves survival in a mouse model of peritoneal endotoxin exposure
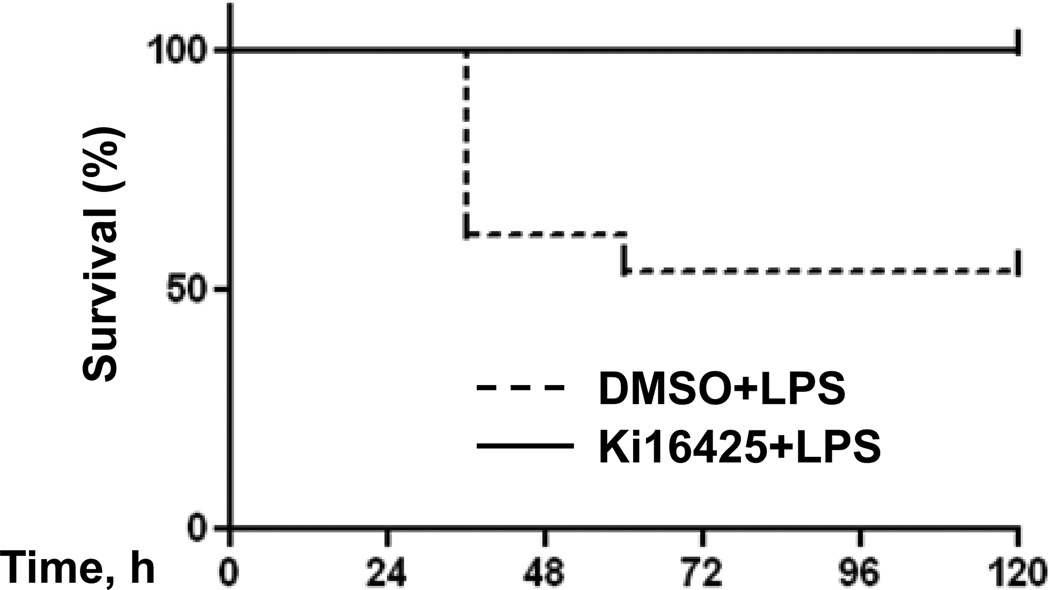
Since the LPA1 antagonist ki16425 effectively blunts inflammation due to LPS exposure in mouse pulmonary systems (11), we decided to investigate if the inflammation generated by peritoneal LPS would be likewise attenuated. Mice were pretreated with intraperitoneal (i.p) ki16425 or vehicle (DMSO) 1 h before being subjected to lethal endotoxaemia (LPS, 15 mg/kg body weight, i.p.). Figure 1 shows that the vehicle-treated animals display 50% morality in this model within 72 h of treatment, while no mortality was observed among the ki16425 treated group. There was no mortality in 5 days among animals treated with i.p. PBS as an LPS control (data not shown).
Fig. 1.
Ki16425 preserves survival of LPS-treated mice. Male C57BL/6 mice at 8 to 10 weeks of age were given i.p. DMSO (5% in PBS) (solid line, n = 11) or i.p. ki16425 (dashed line, n = 13) (20 mg/kg body weight) 1 h before LPS administration (i.p. 15 mg/kg body weight). Survival of the mice was monitored at intervals of 12 h for 96 h. The overall difference in survival rate between the two groups was significant (p<0.01).
LPS-induced inflammatory and apoptotic markers are reduced with LPA1 antagonism in the abdominal cavity
To explore the mechanism of the findings described above, we assayed the peritoneal lavage fluid and visceral organs for inflammatory markers and evidence of apoptosis. First, we assayed peritoneal lavage fluid for the neutrophil chemokine KC (also known as CXCL1) and IL-6, and find that LPS treatment (5 mg/kg body weight) robustly induces the expression of both KC and IL-6 within 4 h. In the setting of ki16425 treatment, KC and IL-6 levels are significantly reduced compared to vehicle treated animals (Fig 2).
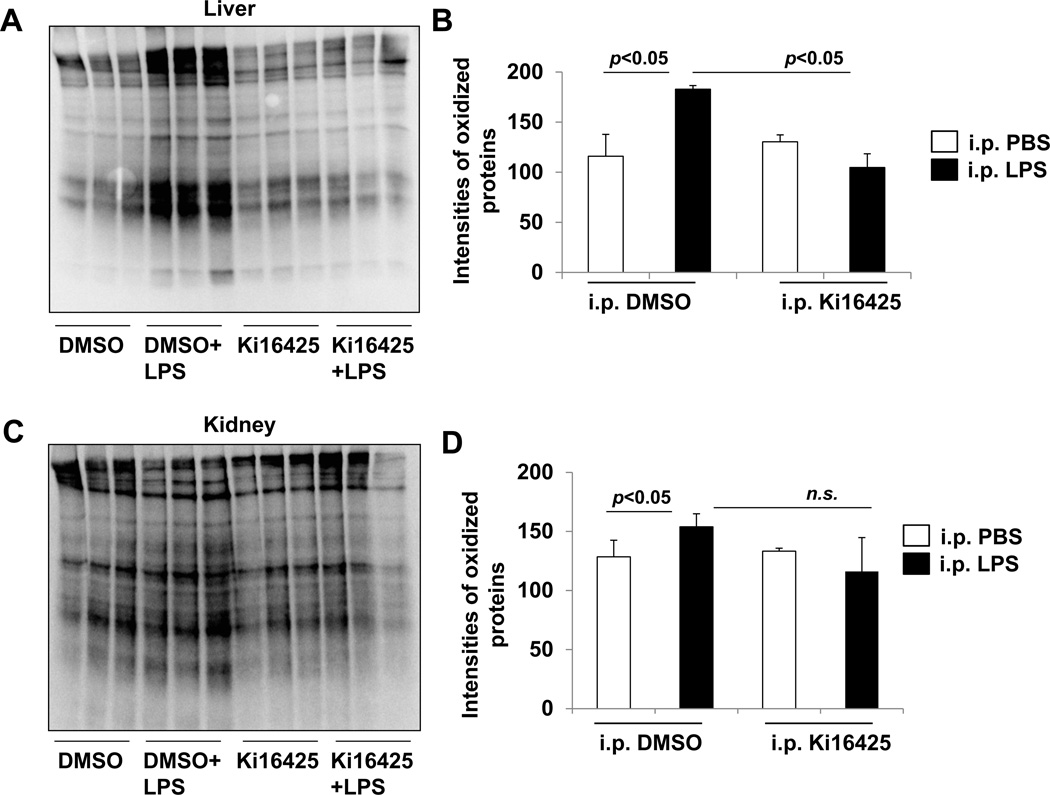
Protein oxidation is an important component of inflammatory tissue damage due to the elicitation of harmful reactive oxygen species. Therefore we evaluated if the antagonism of LPA1 affected protein oxidation in the liver and kidney tissues of these LPS-exposed animals. Immunoblots of tissue homogenates reveal that protein oxidation in the livers of LPS-exposed animals is markedly increased compared to non-LPS treated mice. Ki16425 treatment significantly attenuated the LPS-induced protein oxidation (Fig 3A, B). In kidney tissues, we observe a small but significant increase in oxidized proteins after intraperitoneal LPS exposure in the vehicle (DMSO) treated group (Fig 3C, D). In mice treated with ki16425, the quantity of protein oxidation in LPS exposed animals is decreased, but no statistically significant compared to the vehicle-treated LPS exposed animals (Fig 3C, D).
Fig. 3.
Ki16425 attenuates LPS-induced accumulation of oxidized proteins in visceral organs of mice. Male C57BL/6 mice (n=6/group) were given i.p. DMSO (5% in PBS) or i.p. ki16425 (20 mg/kg body weight) 1 h before LPS administration (i.p. 5 mg/kg body weight). Liver (A, B) and kidney (C, D) tissues were collected after 4 h and protein oxidation was determined by immunoblotting. Intensities of immunoblots were analyzed with Image J software.
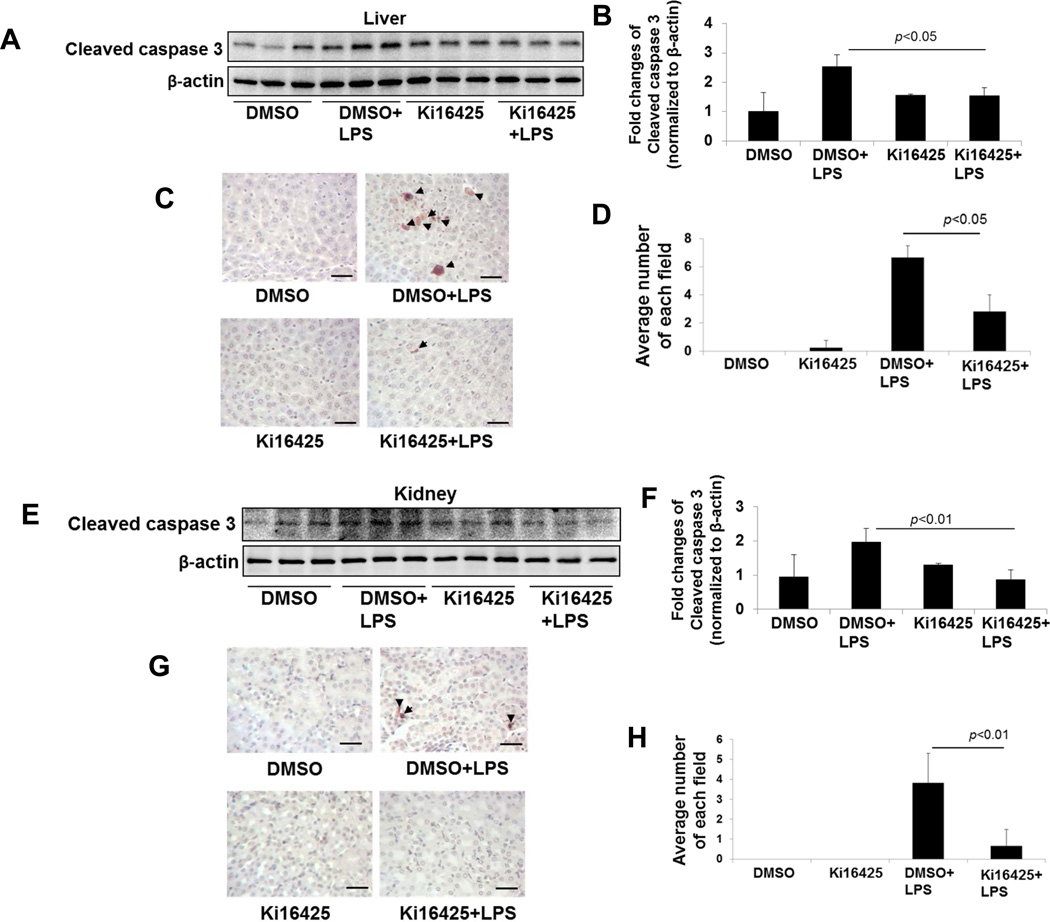
We next evaluated if the changes in protein oxidation noted in the liver was related to cell death by apoptosis. In the lungs, ki16425 protects the epithelia from LPS-induced apoptosis with tissue reductions in the apoptotic marker cleaved caspase-3 (18). We evaluated tissue homogenates from DMSO or ki16425-treated mice for the caspase-3 cleavage product after abdominal LPS challenge by immunoblotting. LPS caused a substantial increase in caspase-3 cleavage in both liver (Fig. 4A, B) and kidney (Fig. 4E, F) tissues in this model, and this effect was significantly blocked by treatment with ki16425. Further, TUNEL staining shows that ki16425 attenuated LPS-induced apoptosis in both liver (Fig. 4C, D) and kidney (Fig. 4G, H) tissues. Taken together, these results indicate that the pharmacologic antagonism of LPA1 by ki16425 reduces inflammatory chemokine production, oxidative stress, and cell death in the setting of abdominal LPS exposure.
Fig. 4.
Ki16425 reduces the LPS-induced increase in caspase 3 cleavage in mice. Male C57BL/6 mice were given i.p. DMSO (5% in PBS) or i.p. ki16425 (20 mg/kg body weight) 1 h before LPS administration (i.p. 5 mg/kg body weight). Liver (A, B) and kidney (E, F) tissues were collected after 4 h and cleaved caspase 3 levels were determined by immunoblotting. Intensities of immunoblots were analyzed with Image J software. Cellular apoptosis was examined by TUNEL staining (C, liver; G, Kidney). Arrows show TUNEL positive cells. Scale bar: 100 µm. (D, H) Number of TUENL positive cells in each filed (n=3–6).
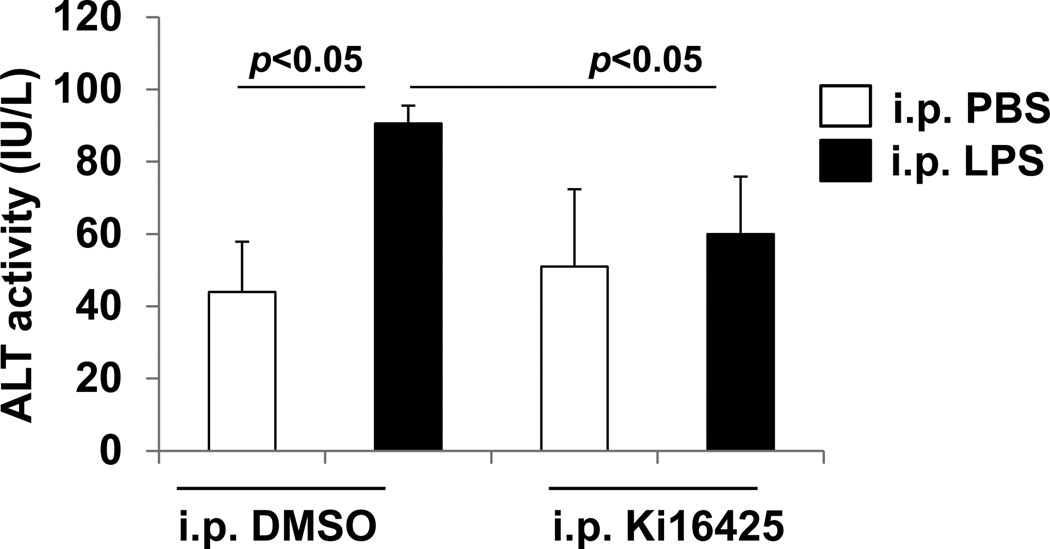
Ki16425 prevents liver dysfunction after LPS exposure
Inflammation and cell death often lead to organ dysfunction. The most commonly analyzed clinical plasma markers for liver injury are the transaminases alanine aminotransferase (ALT). We evaluated plasma of mice exposed to LPS with or without pretreatment with ki16425 for ALT by conventional immunoassay. LPS exposure caused a significant increase in plasma ALT in vehicle treated mice, which was completely suppressed by ki16425 antagonism of LPA1 (Fig 5). These results indicate that part of ki16425’s protection from abdominal sepsis mortality may be through the prevention of liver damage.
Fig. 5.
Ki16425 attenuates the LPS-induced increase in plasma ALT activity in mice. Male C57BL/6 mice were given i.p. DMSO (5% in PBS) or i.p. ki16425 (20 mg/kg body weight) 1 h before LPS administration (i.p. 5 mg/kg body weight). Plasma was collected and ALT activity was determined by immunoassay.
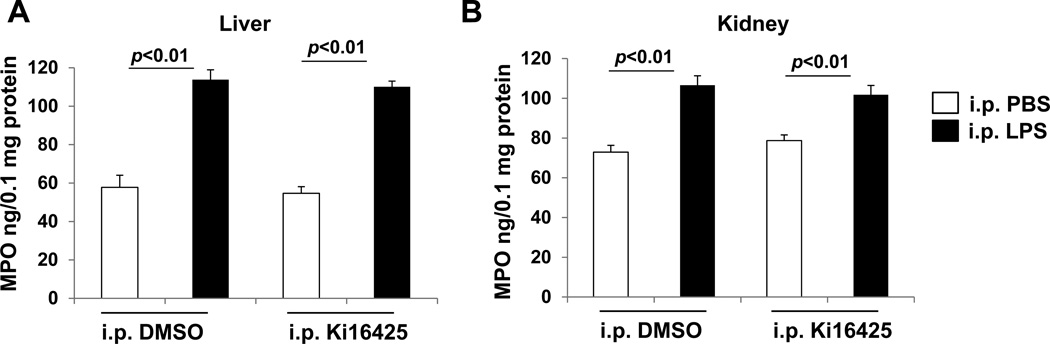
Myeloperoxidase activity after LPS exposure is unaffected by LPA1 blockade
Since myeloperoxidase (MPO) is a neutrophil-derived enzyme which can lead to the generation of reactive oxygen species, we analyzed tissue homogenates for MPO levels with an enzyme assay for peroxidase activity. We observe that LPS treatment causes a doubling of MPO levels in liver tissues with or without ki16425, and that there is a significant but less substantial increase in MPO levels in kidney tissues that is also unchanged with ki16425 (Fig 6). We therefore conclude that despite the decreases we observe in neutrophil chemokines and oxidative stress, there is a similar quantity of the neutrophil-derived myeloperoxidase enzyme in LPS-induced peritoneal inflammation regardless of LPA1 antagonism.
Fig. 6.
Ki16425 has no effect on LPS-induced increase in MPO levels in mice. Male C57BL/6 mice were given i.p. DMSO (5% in PBS) or i.p. ki16425 (20 mg/kg body weight) 1 h before LPS administration (i.p. 5 mg/kg body weight). Liver (A) and kidney (B) tissues were collected after 4 h and MPO levels were measured by ELISA.
Ki16425 reduces LPA1 association with CD14 and LPS-induced signaling and cytokine production
We sought to determine a mechanism for the protection from inflammation, cell death, organ dysfunction, and mortality that we observe with ki16425 treatment. We have previously described an increase in the association of LPA1 with the LPS co-receptor CD14 after LPA treatment (11). To determine if ki16425 treatment affects the interaction between LPA1 and CD14, Myc tagged LPA1 and V5 tagged CD14 were over-expressed in HEK293 cells, a human kidney cell line with high transfection efficiency. Cell lysates were incubated with ki16425 for 1 h, followed by co-immunoprecipitation with a Myc tag antibody and immunoblotting with a V5 tag antibody. As shown in Fig. 7A, treatment with ki16425 causes a diminution of the association of CD14-V5 and LPA1-Myc. We have shown that inhibition of LPA1 by ki16425 reduced LPS-induced phosphorylation of I-κB, p38 MAPK, and Erk1/2, and cytokine release in mouse lung epithelial cells [11]. To examine if similar physiology may be active in the abdominal LPS model, we evaluated the effect of ki16425 on LPS-induced signaling and cytokine release in the hepatocyte cells HepG2 and macrophage cell line Raw264, as cell death or inflammatory responses of these cells contribute to the pathogenesis of liver failure (24–26). LPS-induced phosphorylation of PKCδ and p38 MAPK were attenuated by ki16425 in HepG2 cells (Fig. 7B). LPS-induced IL-6 production is suppressed with ki16425 to less than half the levels generated by vehicle treated Raw264 cells (Fig 7C). These findings imply that blockade of LPA1 by ki16425 decreases the LPA1 potentiation of LPS signaling by reducing LPA1 association with CD14. Ki16425 thus blunts the downstream inflammatory cytokine production implicated in endotoxin-dependent tissue damage.
Discussion
In the current study we employ a mouse model of abdominal sepsis to evaluate if the LPA signaling pathway significantly potentiates inflammatory signaling in response to LPS in the abdominal cavity. Our findings suggest that, consistent with the report we have published in pulmonary pathology, LPA1 blockade significantly protects animals from inflammatory injury, organ dysfunction, and mortality after LPS exposure. The small molecule ki16425 is a specific and potent inhibitor of the LPA1-3 receptors, and has been used to elucidate the activity and therapeutic potential of LPA signaling in several studies (20, 21, 27–29). This study reveals a novel role for LPA1 signaling in the potentiation of the LPS response in peritonitis. The most striking finding in our study is the survival benefit we see with ki16425 treatment, which is completely protective for mortality from peritoneal sepsis at the concentrations of drug and LPS we used. Interestingly, the effects of ki16425 reduce most but not all markers of inflammation we examined in this model with robust reductions in KC, liver oxidative stress, liver and kidney cell apoptosis, and plasma ALT. Our findings contrast somewhat to other models of LPS peritonitis, where authors show improvement of septic parameters and liver injury with administration of exogenous LPA (18:1) (14, 29). In our study we do not administer LPA, but use the inhibitor ki16425 for receptor blockade, as endogenous LPA contains different fatty acid chains such as 18:0, 18:2, 20:4, may have different effects compared to exogenous LPA. LPA can also signal through the PPARγ receptor in some systems, and while the work of Murch et al (29) report blockade of beneficial LPA activity with ki16425 in a rat model, they do not report if ki16425 affects LPS-induced injury in the absence of LPA. Indeed, multi-pronged signals by lipid mediators via receptors with multiple alternative ligands can make interpretation of physiology and therapeutic benefit challenging in model systems.
The endotoxin related increase in MPO levels in liver and kidney is unchanged with ki16425, suggesting that ki16425 does not block neutrophil influx to these tissues. MPO may be differentially activated, or other enzymes such as macrophage NADPH oxidases or the inducible nitric oxide synthase may mediate the observed oxidative stress. We also report that kidney protein oxidation is mildly elevated with LPS exposure without significant reduction by ki16425, suggesting that the protective effect of ki16425 in kidney may be independent on suppression of protein oxidation.
The liver dysfunction exhibited by these mice is evident by the increase in plasma ALT, and this is nearly completely suppressed by ki16425. LPA biology has not been extensively studied in liver systems. Phosphorylation of PKCδ is known to regulate apoptosis (30–32) and phosphorylation of p38 MAPK is involved in cytokine release (33, 34). Our results show that ki16425 attenuates PKCδ and p38 MAPK phosphorylation with LPS exposure, which may in turn reduce downstream cell death signaling and transcriptional activation. These in vitro and in vivo experiments show that ki16425 attenuates LPS-mediated pro-inflammatory responses and protects against endotoxin-induced cell death in hepatocytes through a newly described mechanism. Here we show that ki16425 abrogates the association between LPA1 and CD14, a co-receptor of LPS. Liver macrophages are a likely source of injurious inflammatory mediators like IL-6 and KC (26). Our findings in the Raw264 (macrophage cell line) cells indicate that ki16425 diminishes robust LPS-induced inflammatory responses in Raw264, suggesting that ki16426 confers an anti-inflammatory and anti-apoptotic effect in both liver epithelia and macrophages. We believe therefore that LPA receptor antagonism may be a valid target for future studies in inflammatory disorders and may represent an attractive therapeutic strategy for endotoxin-mediated disease.
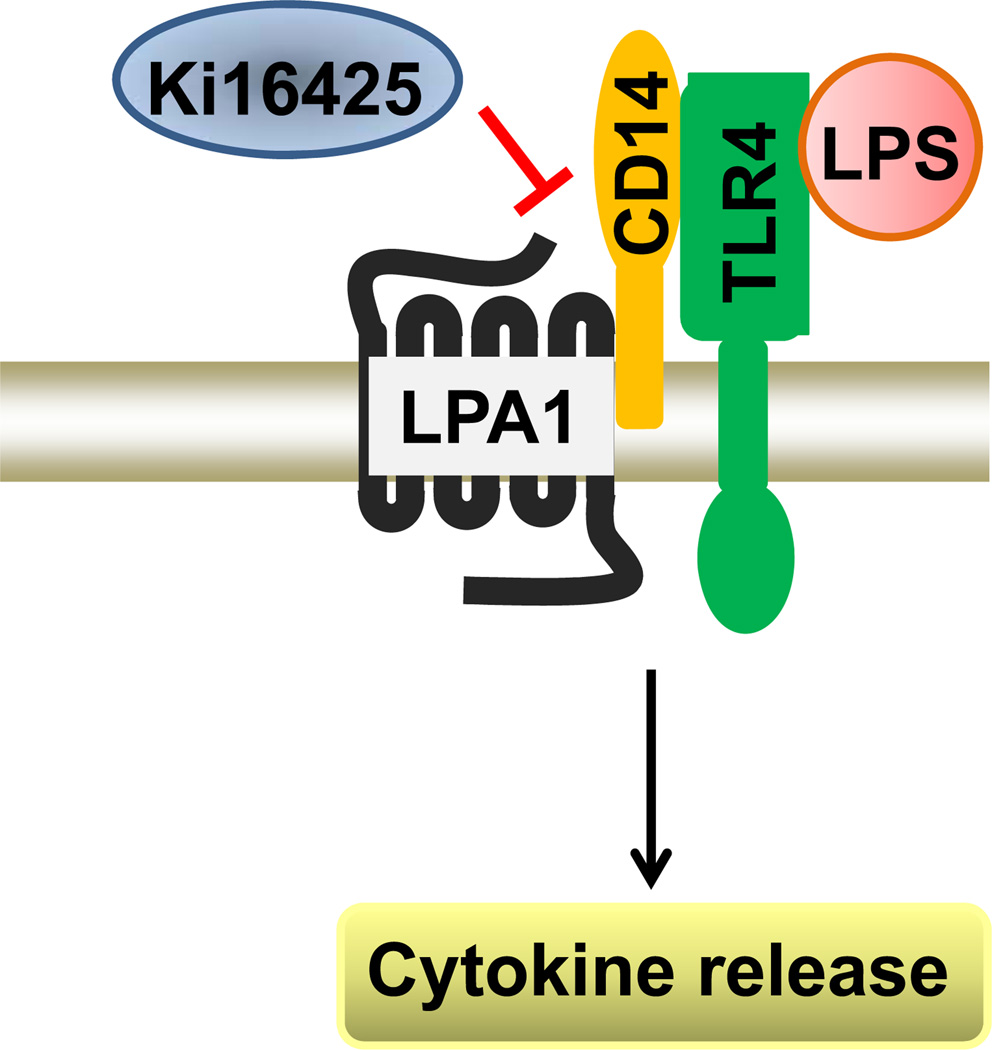
Fig. 8.
Ki16425 attenuates LPS signal. LPS induces signal through its receptor TLR4 and co-receptor CD14. Ki16425 reduces LPS signal through inhibition of LPA1/CD14 association.
Background
LPA receptors contribute to the pathogenesis of asthma, acute lung injury, and fibrosis, however, the role of LPA receptors in sepsis is still unclear.
Translational significance
Our results suggest that LPA1 antagonist ki16425 reduces LPA1 interaction with CD14, thus reducing LPS-induced sepsis.
Acknowledgements
This study was supported by the US National Institutes of Health (R01 HL01916 and R01HL112791 to Y.Z.) and American Heart Association awards 12SDG9050005 (J.Z.). All the authors have read the journal’s policy on conflicts of interest and have no conflicts of interest to declare. All the authors have read the journal’s authorship agreement and that the manuscript has been reviewed by and approved by all named authors.
Abbreviate
- LPA
lysophosphatidic acid
- LPS
lipopolysacharride
- TLR4
toll like receptor 4
- MPO
myeoloperoxidase
- ALT
Alanine transaminase
- i.p.
intraperitoneal
- FBS
fetal bovine serum
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labeling
- GI
gastrointestinal
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levy MM, Artigas A, Phillips GS, Rhodes A, Beale R, Osborn T, et al. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. The Lancet Infectious diseases. 2012 Dec;12(12):919–924. doi: 10.1016/S1473-3099(12)70239-6. PubMed PMID: 23103175. [DOI] [PubMed] [Google Scholar]
- 2.Weledji EP, Ngowe MN. The challenge of intra-abdominal sepsis. International journal of surgery. 2013;11(4):290–295. doi: 10.1016/j.ijsu.2013.02.021. PubMed PMID: 23473994. [DOI] [PubMed] [Google Scholar]
- 3.Ragetly GR, Bennett RA, Ragetly CA. Septic peritonitis: etiology, pathophysiology, and diagnosis. Compendium. 2011 Oct;33(10):E1–E6. quiz E7. PubMed PMID: 22012841. [PubMed] [Google Scholar]
- 4.Walker AP, Krepel CJ, Gohr CM, Edmiston CE. Microflora of abdominal sepsis by locus of infection. Journal of clinical microbiology. 1994 Feb;32(2):557–558. doi: 10.1128/jcm.32.2.557-558.1994. PubMed PMID: 8150975. Pubmed Central PMCID: 263077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galofre J, Moreno A, Mensa J, Miro JM, Gatell JM, Almela M, et al. Analysis of factors influencing the outcome and development of septic metastasis or relapse in Salmonella bacteremia. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1994 Jun;18(6):873–878. doi: 10.1093/clinids/18.6.873. PubMed PMID: 8086546. [DOI] [PubMed] [Google Scholar]
- 6.Salomao R, Brunialti MK, Rapozo MM, Baggio-Zappia GL, Galanos C, Freudenberg M. Bacterial sensing, cell signaling, and modulation of the immune response during sepsis. Shock. 2012 Aug;38(3):227–242. doi: 10.1097/SHK.0b013e318262c4b0. PubMed PMID: 22777111. [DOI] [PubMed] [Google Scholar]
- 7.Buttenschoen K, Radermacher P, Bracht H. Endotoxin elimination in sepsis: physiology and therapeutic application. Langenbeck's archives of surgery / Deutsche Gesellschaft fur Chirurgie. 2010 Aug;395(6):597–605. doi: 10.1007/s00423-010-0658-6. PubMed PMID: 20582603. [DOI] [PubMed] [Google Scholar]
- 8.Angus DC. The search for effective therapy for sepsis: back to the drawing board? Jama. 2011 Dec 21;306(23):2614–2615. doi: 10.1001/jama.2011.1853. PubMed PMID: 22187284. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Y, Natarajan V. Lysophosphatidic acid (LPA) and its receptors: role in airway inflammation and remodeling. Biochimica et biophysica acta. 2013 Jan;1831(1):86–92. doi: 10.1016/j.bbalip.2012.06.014. PubMed PMID: 22809994. Pubmed Central PMCID: 3491109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nature medicine. 2008 Jan;14(1):45–54. doi: 10.1038/nm1685. PubMed PMID: 18066075. [DOI] [PubMed] [Google Scholar]
- 11.Zhao J, He D, Su Y, Berdyshev E, Chun J, Natarajan V, et al. Lysophosphatidic acid receptor 1 modulates lipopolysaccharide-induced inflammation in alveolar epithelial cells and murine lungs. American journal of physiology Lung cellular and molecular physiology. 2011 Oct;301(4):L547–L556. doi: 10.1152/ajplung.00058.2011. PubMed PMID: 21821728. Pubmed Central PMCID: 3191756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang LS, Fu P, Patel P, Harijith A, Sun T, Zhao Y, et al. Lysophosphatidic acid receptor-2 deficiency confers protection against bleomycin-induced lung injury and fibrosis in mice. American journal of respiratory cell and molecular biology. 2013 Dec;49(6):912–922. doi: 10.1165/rcmb.2013-0070OC. PubMed PMID: 23808384. Pubmed Central PMCID: 3931116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He D, Su Y, Usatyuk PV, Spannhake EW, Kogut P, Solway J, et al. Lysophosphatidic acid enhances pulmonary epithelial barrier integrity and protects endotoxin-induced epithelial barrier disruption and lung injury. The Journal of biological chemistry. 2009 Sep 4;284(36):24123–24132. doi: 10.1074/jbc.M109.007393. PubMed PMID: 19586906. Pubmed Central PMCID: 2782006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan H, Zingarelli B, Harris V, Tempel GE, Halushka PV, Cook JA. Lysophosphatidic acid inhibits bacterial endotoxin-induced pro-inflammatory response: potential anti-inflammatory signaling pathways. Molecular medicine. 2008 Jul-Aug;14(7–8):422–428. doi: 10.2119/2007-00106.Fan. PubMed PMID: 18431464: Pubmed Central PMCID: 2323339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noguchi K, Herr D, Mutoh T, Chun J. Lysophosphatidic acid (LPA) and its receptors. Current opinion in pharmacology. 2009 Feb;9(1):15–23. doi: 10.1016/j.coph.2008.11.010. PubMed PMID: 19119080. [DOI] [PubMed] [Google Scholar]
- 16.Mutoh T, Chun J. Lysophospholipid activation of G protein-coupled receptors. Subcellular biochemistry. 2008;49:269–297. doi: 10.1007/978-1-4020-8831-5_10. PubMed PMID: 18751915. [DOI] [PubMed] [Google Scholar]
- 17.Lin ME, Herr DR, Chun J. Lysophosphatidic acid (LPA) receptors: signaling properties and disease relevance. Prostaglandins & other lipid mediators. 2010 Apr;91(3–4):130–138. doi: 10.1016/j.prostaglandins.2009.02.002. PubMed PMID: 20331961. Pubmed Central PMCID: 2845529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Funke M, Zhao Z, Xu Y, Chun J, Tager AM. The lysophosphatidic acid receptor LPA1 promotes epithelial cell apoptosis after lung injury. American journal of respiratory cell and molecular biology. 2012 Mar;46(3):355–364. doi: 10.1165/rcmb.2010-0155OC. PubMed PMID: 22021336. Pubmed Central PMCID: 3326436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inoue M, Rashid MH, Fujita R, Contos JJ, Chun J, Ueda H. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nature medicine. 2004 Jul;10(7):712–718. doi: 10.1038/nm1060. PubMed PMID: 15195086. [DOI] [PubMed] [Google Scholar]
- 20.Ma L, Matsumoto M, Xie W, Inoue M, Ueda H. Evidence for lysophosphatidic acid 1 receptor signaling in the early phase of neuropathic pain mechanisms in experiments using Ki-16425, a lysophosphatidic acid 1 receptor antagonist. Journal of neurochemistry. 2009 Apr;109(2):603–610. doi: 10.1111/j.1471-4159.2009.05987.x. PubMed PMID: 19222705. [DOI] [PubMed] [Google Scholar]
- 21.Orosa B, Garcia S, Martinez P, Gonzalez A, Gomez-Reino JJ, Conde C. Lysophosphatidic acid receptor inhibition as a new multipronged treatment for rheumatoid arthritis. Annals of the rheumatic diseases. 2014 Jan;73(1):298–305. doi: 10.1136/annrheumdis-2012-202832. PubMed PMID: 23486415. [DOI] [PubMed] [Google Scholar]
- 22.Faggioni R, Cattley RC, Guo J, Flores S, Brown H, Qi M, et al. IL-18-binding protein protects against lipopolysaccharide- induced lethality and prevents the development of Fas/Fas ligand-mediated models of liver disease in mice. Journal of immunology. 2001 Nov 15;167(10):5913–5920. doi: 10.4049/jimmunol.167.10.5913. PubMed PMID: 11698468. [DOI] [PubMed] [Google Scholar]
- 23.Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007 Apr 1;55(5):453–462. doi: 10.1002/glia.20467. PubMed PMID: 17203472. Pubmed Central PMCID: 2871685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu B, Yang YJ, Han YL, Lu SZ, Li B, Liu Q, et al. Validation of residual SYNTAX score with second-generation drug-eluting stents: one-year results from the prospective multicentre SEEDS study. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2014 May;10(1):65–73. doi: 10.4244/EIJV10I1A12. PubMed PMID: 24603020. [DOI] [PubMed] [Google Scholar]
- 25.Kosai K, Matsumoto K, Funakoshi H, Nakamura T. Hepatocyte growth factor prevents endotoxin-induced lethal hepatic failure in mice. Hepatology. 1999 Jul;30(1):151–159. doi: 10.1002/hep.510300102. PubMed PMID: 10385651. [DOI] [PubMed] [Google Scholar]
- 26.Sandahl TD, Gronbaek H, Moller HJ, Stoy S, Thomsen KL, Dige AK, et al. Hepatic macrophage activation and the LPS pathway in patients with alcoholic hepatitis: a prospective cohort study. The American journal of gastroenterology. 2014 Nov;109(11):1749–1756. doi: 10.1038/ajg.2014.262. PubMed PMID: 25155228. [DOI] [PubMed] [Google Scholar]
- 27.Orosa B, Martinez P, Gonzalez A, Guede D, Caeiro JR, Gomez-Reino JJ, et al. Effect of lysophosphatidic acid receptor inhibition on bone changes in ovariectomized mice. Journal of bone and mineral metabolism. 2014 Jul 4; doi: 10.1007/s00774-014-0607-5. PubMed PMID: 24994065. [DOI] [PubMed] [Google Scholar]
- 28.Pradere JP, Klein J, Gres S, Guigne C, Neau E, Valet P, et al. LPA1 receptor activation promotes renal interstitial fibrosis. Journal of the American Society of Nephrology : JASN. 2007 Dec;18(12):3110–3118. doi: 10.1681/ASN.2007020196. PubMed PMID: 18003779. [DOI] [PubMed] [Google Scholar]
- 29.Murch O, Collin M, Thiemermann C. Lysophosphatidic acid reduces the organ injury caused by endotoxemia-a role for G-protein-coupled receptors and peroxisome proliferatoractivated receptor-gamma. Shock. 2007 Jan;27(1):48–54. doi: 10.1097/01.shk.0000235086.63723.7e. PubMed PMID: 17172980. [DOI] [PubMed] [Google Scholar]
- 30.Chichger H, Grinnell KL, Casserly B, Chung CS, Braza J, Lomas-Neira J, et al. Genetic disruption of protein kinase Cdelta reduces endotoxin-induced lung injury. American journal of physiology Lung cellular and molecular physiology. 2012 Nov 15;303(10):L880–L888. doi: 10.1152/ajplung.00169.2012. PubMed PMID: 22983354. Pubmed Central PMCID: 3517673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park CH, Choi BH, Jeong MW, Kim S, Kim W, Song YS, et al. Protein kinase Cdelta regulates vaccinia-related kinase 1 in DNA damage-induced apoptosis. Molecular biology of the cell. 2011 Apr 15;22(8):1398–1408. doi: 10.1091/mbc.E10-08-0717. PubMed PMID: 21346188: Pubmed Central PMCID: 3078082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larroque-Cardoso P, Swiader A, Ingueneau C, Negre-Salvayre A, Elbaz M, Reyland ME, et al. Role of protein kinase C delta in ER stress and apoptosis induced by oxidized LDL in human vascular smooth muscle cells. Cell death & disease. 2013;4:e520. doi: 10.1038/cddis.2013.47. PubMed PMID: 23449456. Pubmed Central PMCID: 3734829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernandes AF, Bian Q, Jiang JK, Thomas CJ, Taylor A, Pereira P, et al. Proteasome inactivation promotes p38 mitogen-activated protein kinase-dependent phosphatidylinositol 3-kinase activation and increases interleukin-8 production in retinal pigment epithelial cells. Molecular biology of the cell. 2009 Aug;20(16):3690–3699. doi: 10.1091/mbc.E08-10-1068. PubMed PMID: 19570915. Pubmed Central PMCID: 2777929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saatian B, Zhao Y, He D, Georas SN, Watkins T, Spannhake EW, et al. Transcriptional regulation of lysophosphatidic acid-induced interleukin-8 expression and secretion by p38 MAPK and JNK in human bronchial epithelial cells. The Biochemical journal. 2006 Feb 1;393(Pt 3):657–668. doi: 10.1042/BJ20050791. PubMed PMID: 16197369. Pubmed Central PMCID: 1360718. [DOI] [PMC free article] [PubMed] [Google Scholar]