Abstract
The associations between prenatal cocaine exposure (PCE) and adolescent behavior, cognitive development, and physical growth were examined in 219 15-year-olds who have participated in a longitudinal study since their fourth gestational month. During the first trimester, 42% of the women used cocaine, with use declining across pregnancy. At the 15-year follow-up, the caregivers were, on average, 43 years old, had 13 years of education, and 50% were African American. First trimester PCE was not associated with global cognitive development or with measures of learning and memory. First trimester PCE was significantly related to adolescentreported delinquent behavior, poorer problem solving and abstract reasoning, and reduced weight, height, and head circumference at 15 years. These results were significant after other factors that affect these domains were controlled in regression analyses. In addition, exposure to violence partially mediated the effect of PCE on delinquent behavior. These adolescent domains are important because they are predictors of poorer adult functioning.
Keywords: prenatal cocaine exposure, adolescence, growth, behavior problems, delinquency
1. Introduction
This report addresses three domains of adolescent development: behavior, cognition, and physical growth, each of which has been reported to be associated with prenatal cocaine exposure (PCE). These domains are important areas of adolescent development as they may portend early adult functioning.
We have found persistent associations between PCE and child temperament and behavior problems at 1, 3, 7, and 10 years of age (Richardson et al., 2008, 2009, 2011; Richardson, Goldschmidt et al., 2013), which is consistent with reviews suggesting that the behavior domain is most likely to be affected (Ackerman et al., 2010; Behnke et al., 2013; Buckingham-Howes et al., 2013). Others have also reported that PCE is associated with child or adolescent behavior problems, particularly externalizing behaviors such as aggression and delinquency (Bada et al., 2011, 2012; Bennett et al., 2007, 2013; Delaney-Black et al., 2000; Fisher et al., 2011; McLaughlin et al., 2011; Min, Minnes, Lang et al., 2014; Min, Minnes, Yoon et al., 2014; Minnes et al., 2010; Nordstrom Bailey et al., 2005; Sood et al., 2005; Whitaker et al., 2011). However, not all reports have confirmed these findings (Accornero et al., 2002, 2006; Allen et al., 2014; Bada et al., 2008; Bennett et al., 2002; Bridgett et al., 2011; Gerteis et al., 2011; Greenwald et al., 2011; Linares et al., 2006; Savage et al., 2005; Warner, Behnke, Hou et al., 2006). This is an important domain to investigate because adolescent behavior problems predict poorer outcomes in adulthood, including substance abuse, risky sex, psychiatric disorders, and less successful adjustment (Doherty et al., 2008; Fergusson et al., 2007; Ramrakha et al., 2007).
A second domain that has implications for adult functioning is child and adolescent cognitive development. We found a significant association between PCE and short-term memory (Richardson et al., 2009), and other researchers have reported deficits in specific areas such as verbal reasoning/memory (Bennett et al., 2002), executive function (Minnes et al., 2014; Warner, Behnke, Eyler et al., 2006), cognitive processing/learning (Bridgett et al., 2011; Mayes et al., 2005, 2007), visual spatial/math skills (Singer et al., 2004, 2008), visual motor integration (Arendt et al., 2004; Schroder et al., 2004), and attention, inhibitory control, and impulsivity (Accornero et al., 2007; Bandstra et al., 2001; Bendersky et al., 2003; Carmody et al., 2011; Noland et al., 2005; Rose-Jacobs et al., 2009). However, there are also reports that show no negative effects of PCE on specific areas of cognition (Betancourt et al., 2011; Eyler et al., 2009; Hurt et al., 2009; Li et al., 2009; Rose-Jacobs et al., 2011), and most groups, including ours, do not find a relation between PCE and global cognitive development (Accornero et al., 2007; Bandstra et al., 2002; Frank et al., 2005; Hurt et al., 1997, 2001; Messinger et al., 2004; Morrow et al., 2004, 2006; Singer et al., 2004).
In terms of the third domain of growth, it is unclear whether the association between PCE and early childhood growth deficits persists into adolescence. We found significant associations between PCE and growth deficits at follow-ups through 10 years of age (Richardson et al., 1999, 2008, 2009, 2011; Richardson, Goldschmidt et al., 2013). Some other studies have also reported that PCE has a detrimental effect on childhood growth (Covington et al., 2002; Minnes et al., 2006; Rivkin et al., 2008; Shankaran et al., 2011). However, others have reported that it has no effect at younger ages (Arendt et al., 2004; Bada et al., 2012; Frank et al., 2002; Lumeng et al., 2007; Warner, Behnke, Eyler et al., 2006), and two studies reported that PCE was associated with increased body mass index and obesity in some subgroups of children (LaGasse et al., 2011; Shankaran et al., 2010). To our knowledge, there have been no reports on the effects of PCE on adolescent growth.
It is also important to consider the effects of the postnatal environment on development. Offspring with PCE are at greater risk of being raised in an adverse environment as a consequence of their PCE-associated deficits and of having a mother with a problematic lifestyle. Women who use drugs are less capable of providing a good home environment, more likely to use other drugs, more transient, have less social support, and have more psychiatric problems (Stratton et al., 1996), factors that predict poorer offspring outcomes (Greenberg & Crnic, 1988; Sameroff et al., 1987; Sroufe & Rutter, 1984; Werner & Smith, 1992). Some of the inconsistent findings described above could be due to differential consideration of environmental variables. Further, childhood exposure to violence and abuse are associated with both PCE and behavior problems (Bada et al., 2011; Delaney-Black et al., 2011; Frank et al., 2011; Richardson, Goldschmidt et al., 2013; Schwab-Stone et al., 2013) and, therefore, could function as mediators of the effects of PCE.
The purpose of these analyses was to investigate the effects of PCE on adolescent behavior, cognition, and growth. We carefully measured postnatal environmental exposures, and considered their effects in the analyses. Based on the literature and our previous findings, we hypothesized that there would be detrimental effects of PCE on behavior and growth. However, because of the inconsistency in the literature, we did not hypothesize an expected effect of PCE on cognition.
2. Methods
2.1. Study Design
Women 18 years or older who attended the prenatal clinic at Magee-Womens Hospital (MWH) in Pittsburgh, PA from March 1988 through December 1992 were eligible to participate. Written consent was obtained according to the guidelines of the University of Pittsburgh’s Institutional Review Board and the Research Review and Human Experimentation Committee of MWH. A Certificate of Confidentiality was obtained from the Department of Health and Human Services to assure participants that their responses could not be subpoenaed.
Women were initially approached for interview during their fourth or fifth prenatal month by trained research staff. Women were not enrolled if they came in for their first prenatal visit after the fifth month or if they did not speak English. No information was obtained from the medical charts about a woman’s drug use before she was asked to participate in the study. Ninety percent of the women approached agreed to be interviewed. Medical chart reviews were conducted to assess whether refusal to participate was associated with drug use. A random sample of those women who were approached but refused to participate was selected: Only 5% had a history of drug use during the current pregnancy.
At the initial assessment, women were interviewed about their use of cocaine, crack, alcohol, marijuana, tobacco and other drugs for the year prior to pregnancy and for the first trimester. The core data set consisted of information about sociodemographic characteristics, life events (Dohrenwend et al., 1978), social support (Berkman & Syme, 1979), and psychiatric symptomatology (Center for Epidemiologic Studies – Depression Scale [CES-D], Radloff, 1977; Spielberger State-Trait Anxiety Inventory [STAI], Spielberger et al., 1970).
All women who reported using any cocaine or crack during the first trimester were enrolled, along with the next woman interviewed who reported no cocaine or crack use during pregnancy or in the year before pregnancy. Of the women initially interviewed, 320 (18%) met the inclusion criteria and were enrolled in the study. Women selected for the study were interviewed at seven months about their substance use during the second trimester and the core data set was repeated. The women were interviewed again at 24 to 48 hours postpartum, when they were asked about third trimester substance use and the core data set. All newborns received comprehensive physical examinations, generally within 24 to 48 hours of delivery, by study nurse clinicians who were unaware of prenatal exposure status. The mothers and offspring were assessed at 1, 3, 7, 10, and 15 years postpartum. At all assessment phases, the mothers were interviewed with the core data set, including questions about substance use over the past year.
2.2 Sample Characteristics
Of the 320 women selected for the study, 17 became ineligible for participation because of abortion/miscarriage/infant death (N=5), home delivery (N=1), or moving out of the area (N=11). Of the remaining 303 eligible women, 1 was lost to follow-up and 2 refused further participation. Thus, delivery assessments were completed on 300 mothers. Four pairs of twins and 1 child with Trisomy 21 were excluded from further follow-up, yielding a birth cohort of 295 mothers and infants.
At the 15-year follow-up, 219 subjects out of the 295 in the birth cohort were seen (74% of the birth cohort). Seven women refused the 15-year phase only, 12 refused any further contact, 31 were lost to follow-up, 17 moved, 3 offspring were in foster care and could not be located, and 6 offspring had died. Subjects who participated in the 15-year phase (N = 219) did not differ from those who did not participate (N = 76) on the following: prenatal alcohol, tobacco, or other illicit drug exposure; maternal race, age, income, marital status, work status; parity, pregnancy, labor, or delivery complications; or infant birth weight, length, head circumference, or gestational age. The subjects who did not participate had fewer years of education at delivery (11.6 vs. 12.0 years, p < 0.05), were more likely to be marijuana users during the first trimester (46% vs. 30%, p < 0.01), and were less likely to be cocaine users during the second trimester (1% vs. 10%, p < 0.05) than those who did participate.
At the 15-year follow-up, 19% of the offspring were not in maternal custody, in which case the current caregiver was interviewed. The mean age of the women was 43 years (range = 33 – 75), their mean level of education was 13.1 years (range = 9 – 20), 50% were African American, 58% were single, 53% had a man living in the household, the median family income was $2000/month (range = $0 – $12,083), and 70% worked and/or attended school.
The mean age of the offspring at the 15-year assessment was 15.6 years (median = 15.3; SD = 0.8; range = 15 – 18). Ninety-two percent were seen before 17 years of age. Fifty-two percent were males. The average weight was 154.4 pounds (SD = 43; range = 90 – 340), the average height was 66.2 inches (SD = 3.5; range = 57 – 76), and the average head circumference was 561 mm (SD = 18; range = 521 – 620). The mean Wechsler Intelligence Scale for Children-III (WISC-III) (Wechsler, 1991) composite score was 87.9 (SD = 17.4; range = 47 – 129). The mean grade in school was 9.4 (median = 9.0, SD = 0.9, range = 7 – 12).
2.3. Variables
2.3.1. Maternal Cocaine and Other Substance Use
Maternal cocaine and crack, tobacco, alcohol, marijuana, and other illicit drug use were assessed during confidential interviews by research staff at each assessment phase. Cocaine and crack use were reported in lines, rocks, or grams, or in cost at 15 years if the woman could not report quantity. For these analyses, cocaine use was dichotomized into any use vs. no use for the each of the three trimesters and for the 15-year phase. The alcohol and marijuana variables were average number of drinks or joints per day, respectively, and were log transformed to reduce skewness. Tobacco use was analyzed as number of cigarettes per day. The alcohol, marijuana, and tobacco measures were used as continuous variables in the analyses. See Day and Robles (1989) and Richardson et al. (2008) for detailed information about calculation of the first trimester substance use variables.
2.3.2. Offspring Measures
Behavior
The Self-Reported Delinquency Scale (SRD) (Loeber et al., 1998), completed by the adolescents, is based on the National Youth Survey self-reported delinquency questionnaire (Elliott et al., 1985). The instrument assesses antisocial behaviors such as purposefully breaking or damaging things, stealing, hitting, fighting, and running away from home; the subscales are damage, theft, violence, and status offenses. The Revised Dimensions of Temperament Survey (DOTS-R) (Windle & Lerner, 1986) is a self-assessment of temperament, or behavioral style, and consists of activity, approach/withdrawal, flexibility/rigidity, mood, rhythmicity, task orientation, distractibility, and persistence subscales.
Cognitive
The following battery was administered to the 15-year-olds in private testing rooms by experienced assessment staff trained to reliability. Periodic reliability checks were conducted to maintain consistent administration and scoring, and examiners were blind to maternal prenatal and current drug use. The Wechsler Intelligence Scale for Children Third Edition (WISC-III) (Wechsler, 1991) vocabulary and block design short form was used to estimate IQ. This two-subtest short form has been reported to have very good reliability and validity (Sattler, 2001). The supplementary Mazes subtest was also administered as an additional measure of problem solving. The Children’s Category Test (CCT) (Level 2; ages 9 – 16) (Boll, 1993) requires subjects to deduce the principle underlying each subtest, providing a measure of abstract reasoning and executive functioning. The Children’s Memory Scale (CMS) (Cohen, 1997) is a downward extension of the Wechsler Memory Scale and is suitable for ages 5 to 16 years. Verbal and visual memory, short- and long-term memory, and recall, recognition, and working memory were evaluated.
Growth
At 15 years, the offspring’s weight, height, and head circumference were measured by the child assessment staff who were blind to prenatal and current drug use. A history of illnesses, injuries, and hospitalizations since the previous phase was obtained from the mother. Pubertal development was assessed with the Pubertal Development Scale (PDS) (Petersen et al., 1988), a self-report scale that assesses perception of the adolescent’s development relative to peers. The variable was coded as 1 (develop much earlier relative to others their own age) to 5 (develop much later relative to others their own age).
2.3.3. Additional Measures
Mothers were interviewed at the 15-year phase about their sociodemographic, household, and psychological characteristics, and they completed the Home Observation for Measurement of the Environment – Short Form (HOME-SF: Baker & Mott, 1989), which assesses the quality of cognitive stimulation and emotional support. The adolescents completed the following selfreport scales: “My Parents” (Steinberg et al., 1992), consisting of the involvement subscale, reflecting their view of the parent as loving, responsive, and involved, and the supervision subscale, assessing their view of parental monitoring and limit setting; the Childhood Trauma Questionnaire (CTQ) (Bernstein & Fink, 1998), a measure of physical and emotional abuse and neglect, and sexual abuse; and the Screen for Adolescent Violence Exposure (SAVE) (Hastings & Kelly, 1997) that assesses lifetime exposure to violence in the neighborhood, at school, and at home. Examples of SAVE items include: “I have heard about someone getting shot”, “I have seen someone get badly hurt”, “Grown-ups hit me”, “Someone has pulled a knife on me”, and “I have seen someone get killed”. We adapted the SAVE, changing the Likert-scale ratings into dichotomous responses (yes = directly experienced or witnessed; no = no experience or only seen on TV or heard on radio). The variable used was total exposure to direct violence (e.g., I got stabbed, I got shot, grown-ups hit me).
2.4. Statistical Analyses
The frequency distribution of each outcome variable was examined and statistical analyses were chosen accordingly. Two variables were transformed for the analyses: offspring’s weight was log transformed to reduce the positive skewness of its distribution, and the DOTS-R mood subscale was dichotomized to lower quartile (negative mood) versus all others due to its highly asymmetric distribution. Multiple stepwise regression with forward stepping was applied to the continuous outcome variables and logistic regression was applied to the dichotomous variables. The relations between PCE and the outcome variables were first examined descriptively by comparing the means and box plots of the outcome variables between exposed and non-exposed offspring. Caregiver sociodemographic and psychosocial characteristics, offspring characteristics, current substance use, and other prenatal substance use were considered as covariates. This was based on the literature and prior analyses of these data and on the associations of these variables with the outcomes or PCE in initial bivariable analyses. Table 1 shows the covariates that were considered for inclusion in the analyses. All regressions were run separately by trimester to assess the effects of exposure during each time period.
Table 1.
Sample characteristics of variables considered for inclusion in the analyses
| No cocaine use 1st trimester | Cocaine use 1st trimester | p valuea | |
|---|---|---|---|
| First Trimester Maternal Characteristics | n = 124 | n = 95 | |
| African American (%) | 43.5 | 57.9 | 0.04 |
| Age (years) (mean, SD) | 24.1 (4.7) | 26.4 (5.2) | 0.001 |
| Single (%) | 71.8 | 87.4 | 0.005 |
| Education (years) (mean, SD) | 12.2 (1.4) | 11.8 (1.2) | 0.05 |
| Family Income ($/month) (mean, SD) | 773 (615) | 604 (691) | 0.01 |
| Work/Attend School (%) | 52.4 | 35.8 | 0.01 |
| Cigarettes/Day (mean, SD) | 6.1 (9.4) | 10.3 (9.5) | 0.001 |
| Drinks/Day (mean, SD) | 0.28 (.7) | 2.02 (2.8) | 0.000 |
| Joints/Day (mean, SD) | 0.06 (.2) | 0.33 (.9) | 0.004 |
| Other Illicit Drugs (excluding cocaine) (%) | 3.2 | 8.4 | 0.09 |
| Height (inches) (mean, SD) | 64.4 (2.8) | 64.5 (2.7) | ns |
| 15-Year Caregiver Characteristics | |||
| Age (years) (mean, SD) | 41.3 (6.4) | 44.2 (7.3) | 0.002 |
| Single (%) | 47.6 | 72.6 | 0.0002 |
| Education (years) (mean, SD) | 13.1 (1.5) | 13.0 (1.7) | ns |
| Family income ($/month) (mean, SD) | 2854 (2233) | 1926 (1344) | 0.0002 |
| Work/Attend school (%) | 68.5 | 72.3 | ns |
| Cigarettes/Day (mean, SD) | 5.8 (8.6) | 7.7 (8.2) | ns |
| Drinks/Day (mean, SD) | 0.5 (1.2) | 1.1 (1.8) | 0.01 |
| Joints/Day (mean, SD) | 0.09 (.7) | 0.08 (.3) | ns |
| Cocaine (% use) | 1.6 | 13.7 | 0.0005 |
| Other Drugs (excluding cocaine) (%) | 0.8 | 6.3 | 0.02 |
| Depression (CES-D) (mean, SD) | 39.9 (10.4) | 41.3 (10.3) | ns |
| Hostility (STAI) (mean, SD) | 15.9 (4.5) | 17.1 (5.1) | ns |
| Home environment (HOME) (mean, SD) | 11.2 (2.7) | 10.4 (2.6) | 0.02 |
| Household size (#) (mean, SD) | 4.1 (1.4) | 3.9 (1.7) | ns |
| Life events (mean, SD) | 3.8 (3.0) | 4.5 (2.9) | 0.02 |
| Social support (# people to turn to) (mean, SD) | 4.4 (2.3) | 4.1 (2.2) | ns |
| 15-Year Offspring Characteristics | |||
| Age (years) (mean, SD) | 15.8 (.9) | 15.4 (.7) | 0.002 |
| Gender (% male) | 50.8 | 53.7 | ns |
| Custody status (% non-maternal) | 10.5 | 29.5 | 0.0004 |
| Child abuse/neglect (CTQ) (mean, SD) | 2.0 (.7) | 2.2 (.9) | ns |
| Exposure to violence (SAVE) (mean, SD) | 0.64 (1.0) | 1.04 (1.2) | 0.008 |
| # of hospitalizations (mean, SD) | 0.2 (.6) | 0.4 (.8) | ns |
| # of illnesses (mean, SD) | 0.7 (1) | 0.7 (1) | ns |
| # of injuries (mean, SD) | 0.5 (.8) | 0.4 (.7) | ns |
| WISC-III estimated IQ (mean, SD) | 88.5 (18.1) | 87.2 (16.6) | ns |
| Pubertal development | 2.8 (.8) | 2.9 (.7) | ns |
| Parental involvement | 29.4 (4.2) | 28.9 (4.3) | ns |
| Parental supervision | 20.7 (3.9) | 20.2 (4.1) | ns |
Based on t-test or Mann-Whitney for continuous variables and on Chi-square test for dichotomous variables.
The tolerance of each covariate in the final model was examined to assure that the estimated regression slopes were not unstable due to multicollinearity. Residuals and the modified Cook’s statistic (Cook & Weisberg, 1982) were used to identify possible outliers and influential points. There were no outliers or influential points for the DOTS-R, WISC-III, or head circumference. There was one influential case each for the CCT, weight, and height, one outlier for the SRD theft subscale, two influential points each for the damage, theft, and violence subscales, and two influential points for the CMS. The significant relations with PCE reported here are those that remained stable after removal of the outliers or influential cases.
Mediating analyses were conducted to determine whether exposure to violence and child abuse influenced the relation between PCE and 15-year behavior because of earlier findings that these variables are related to both PCE and adolescent behavior (Bada et al., 2011; Delaney-Black et al., 2011; Frank et al., 2011; Richardson, Goldschmidt et al., 2013; Schwab-Stone et al., 2013). We also tested whether birth weight mediated the relation between PCE and 15-year growth because of our previous findings that PCE was associated with decreased birth weight (Richardson et al., 1999). Mediation was evaluated through path analysis using the product of coefficients (Sobel, 1987) and by examining the significance of the direct effect after inclusion of the mediating effect in the model. If the direct effect remained significant, we considered the mediation to be partial. The path parameters were estimated using Lisrel 8.5 (Jöreskog & Sörbom, 2001).
3. Results
3.1. Descriptive Analyses
During the first, second, and third trimesters, 42.4%, 7.5%, and 10.3% of the women used cocaine, respectively, and 18.6%, 4.5%, and 6.2% used ≥ 1 line/day, respectively. The mean level of cocaine use for the women who used during the first trimester was approximately 8 lines/day. Of these women, 50% reported snorting powder cocaine only, while the rest smoked crack (see Richardson et al., 2008 for detailed information about calculation of the cocaine/crack amounts). During the second and third trimesters, the mean level of use among the users was approximately 6 lines/day and 5 lines/day, respectively. Of the women who used during the third trimester, 20% reported snorting powder cocaine only and the rest smoked crack. All women who used second and third trimester had used first trimester. Cocaine use increased from later pregnancy levels during the early postpartum years (15% and 13% at 1 and 3 years), but had decreased to 7% by 15 years. At 15 years, only 1.8% of the women used ≥ 1 line/day and, among the users, the mean level of use was approximately 4.4 lines/day.
At enrollment, women who used cocaine during the first trimester were more likely to be African-American, older, single, less educated, to have lower family incomes, and to be less likely to be working and/or attending school than were the non-users (Table 1). In the first trimester, cocaine users used significantly more tobacco cigarettes, alcohol, and marijuana than did the non-users. At 15 years, most of these characteristics continued to differ; more caregivers were single, family income was lower, and current use of alcohol, cocaine, and other illicit drugs was greater among first trimester cocaine users compared to non-users. In addition, at 15 years, life events, non-maternal custody, and lifetime exposure to violence were higher and quality of the home environment was lower among the offspring of first trimester users compared to those of non-users (Table 1).
3.2. Regression Analyses
Behavior
At 15 years, those with first trimester cocaine exposure had significantly more self-reported delinquent behavior problems on the SRD (Table 2). The adolescents who were exposed to cocaine during the first trimester reported more damage, theft, and status offenses than those who were not exposed during the first trimester. There was no association between PCE and the SRD violence subscale, and there was no relation between second or third trimester PCE and any of the SRD subscales. Adolescents who were exposed to first trimester cocaine use also reported marginally significantly poorer mood (less happy) on the DOTS-R than those who were not exposed (Table 2). Third trimester exposure was significantly related to poorer selfreported mood and to less persistence (Table 2). There were no significant associations between PCE and the other DOTS-R subscales (activity, approach/withdrawal, flexibility/rigidity, rhythmicity, task orientation, distractibility).
Table 2.
Regression analyses of 15-year outcomesa
| Outcome variable | Total R2 | Significant predictors | Raw beta | Standardized regression coefficient | p value | ||
|---|---|---|---|---|---|---|---|
| BEHAVIOR | |||||||
| SRD: Damage | 0.11 | First trimester cocaine | 0.35 | 0.17 | < 0.01 | ||
| Current maternal cigarette use | 0.02 | 0.17 | < 0.01 | ||||
| Maternal education | 0.09 | 0.14 | < 0.05 | ||||
| First trimester marijuana | 0.45 | 0.12 | < 0.05 | ||||
| SRD: Theft | 0.12 | First trimester marijuana | 1.40 | 0.22 | < 0.001 | ||
| Parental involvement | −0.06 | −0.18 | < 0.01 | ||||
| First trimester cocaine | 0.37 | 0.12 | <0.05 | ||||
| SRD: Status Offenses | 0.12 | First trimester cocaine | 0.67 | 0.23 | < 0.001 | ||
| Parental involvement | −0.06 | −0.18 | < 0.01 | ||||
| Current maternal cigarette use | 0.02 | 0.14 | < 0.05 | ||||
| DOTS-R mood | Parental involvement | −0.12 | 0.88 (0.82 − 0.95)b | < .01 | |||
| Third trimester cocaine | 1.17 | 3.21 (1.25 − 8.21)b | < .05 | ||||
| First trimester cocaine | 0.55 | 1.73 (.92 − 3.28)b | < .10 | ||||
| DOTS-R persistence | 0.07 | Gender | 0.60 | 0.19 | < 0.01 | ||
| Parental involvement | 0.06 | 0.15 | < 0.05 | ||||
| Third trimester cocaine | −0.82 | −0.15 | < 0.05 | ||||
| COGNITIVE | |||||||
| Children’s Category Test | 0.15 | Race | 4.80 | 0.26 | < 0.001 | ||
| Maternal education | 1.65 | 0.22 | < 0.01 | ||||
| First trimester cocaine | −3.60 | −0.17 | < 0.05 | ||||
| WISC Mazes | 0.08 | First trimester cocaine | −1.23 | −0.19 | < 0.01 | ||
| Current maternal cigarette use | −0.06 | −0.16 | < 0.05 | ||||
| Home environment | −0.16 | −0.14 | < 0.05 | ||||
| GROWTH | |||||||
| Weightc | 0.13 | Genderd | 0.12 | 0.25 | < 0.001 | ||
| Pubertal development | −0.07 | −0.22 | < 0.001 | ||||
| First trimester cocaine | −0.10 | −0.20 | < 0.001 | ||||
| Height | 0.60 | Gender | 4.80 | 0.69 | < 0.001 | ||
| Maternal height | 0.40 | 0.32 | < 0.001 | ||||
| Pubertal development | −0.53 | −0.12 | < 0.01 | ||||
| Age | 0.46 | 0.11 | < 0.01 | ||||
| First trimester cocaine | −0.69 | −0.10 | < 0.05 | ||||
| Current maternal marijuana use | −1.45 | −0.09 | < 0.05 | ||||
| Head circumference | 0.16 | Gender | 12.27 | 0.35 | < .001 | ||
| Pubertal development | −4.77 | −0.21 | < .01 | ||||
| Current maternal cigarette use | −0.36 | −0.18 | < .05 | ||||
| First trimester cocaine | −5.28 | −0.15 | < .05 | ||||
Results are presented only for those outcomes where prenatal cocaine exposure was significant. Predictors are listed in order of standardized regression coefficient, an indication of the magnitude of the effect. Multiple regression analyses were conducted for all outcomes except the DOTS-R mood subscale, which was dichotomized into the lowest quartile vs. all others and a logistic regression was performed.
Adjusted odds ratio and 95% confidence interval
Log transformed
0 = Female, 1 = Male
Cognitive
First trimester cocaine exposure predicted significantly poorer performance on the CCT and the Mazes subtest of the WISC-III (Table 2). There was no association between first trimester exposure and the other cognitive outcomes, nor any effects of second or third trimester cocaine exposure on the cognitive outcomes.
Growth
First trimester cocaine use was significantly associated with reduced weight, height, and head circumference at 15 years of age (Table 2). The adjusted mean growth parameters were significantly different between offspring with PCE and those with no PCE during the first trimester: Adolescents exposed to cocaine during the first trimester of pregnancy weighed 18.7 pounds less (p < .01), were 0.57 inches shorter (p < .05), and their head circumference was 5.3 millimeters smaller (p < .05) than the unexposed adolescents, after adjusting for significant covariates. There were no significant associations between second and third trimester exposure and growth.
3.3. Mediating Analyses
Child abuse was not associated with PCE and therefore could not serve as a mediator. Lifetime exposure to violence partially mediated the effects of PCE on the adolescent-reported SRD outcomes of status offenses and damage, and completely mediated the relation between PCE and theft. In other words, for status offenses and damage, the direct effects of PCE on these outcome variables remained significant after inclusion of exposure to violence in the model. For theft, PCE was no longer a significant predictor once exposure to violence was added to the model. The Sobel test of mediation for status offenses, damage, and theft was 2.2 (p < 0.05), 2.3 (p < 0.05), and 2.5 (p < 0.01), respectively. Nineteen percent, 25%, and 43% of the total effect of PCE on status offenses, damage, and theft, respectively, was explained by the indirect pathway through exposure to violence. Figure 1 illustrates the mediation model for the SRD damage score. The significant DOTS-R subscales were not mediated by exposure to violence.
Figure 1.
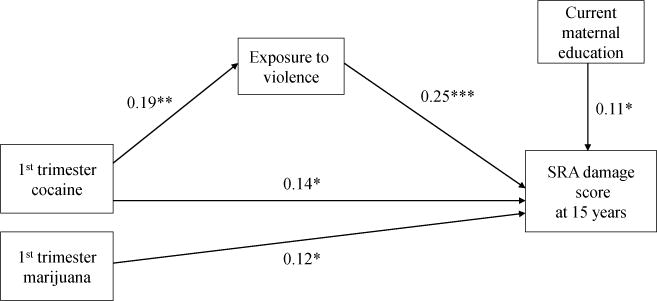
Exposure to violence partially mediates the relation between first trimester cocaine exposure and self-reported delinquency at 15 yearsa
aStandardized coefficients, p<.05, ** p<.01, ***p<.001
We also tested whether the effect of PCE on birth weight (Richardson et al., 1999) mediated the effect of PCE on 15-year weight. At 15 years, birth weight did not mediate the effects of PCE on current weight (Sobel z = −1.0, p = 0.2). With inclusion of birth weight in the model, PCE continued to be significantly related to offspring 15-year weight.
4. Discussion
We have extended the exploration of the effects of prenatal cocaine exposure to define the outcomes of exposed offspring at 15 years of age. We found a significant association between first trimester cocaine exposure and self-reported delinquency, which is consonant with the previous research reviewed earlier that found problems in the externalizing domain. The SRD was a new measure added at the 15-year follow-up to obtain the adolescent’s report of his/her own behavior, which at this age is a more accurate indicator of delinquent behavior than maternal report (Loeber et al., 1989; Verhulst & van der Ende, 1992). The association between PCE and adolescent delinquent behavior, as well as that with substance use that we reported earlier (Richardson, Larkby et al., 2013), is consistent with reports by other investigators (Allen et al., 2014; Delaney-Black et al., 2011; Frank et al., 2011; Lambert et al., 2013; Min, Minnes, Lang et al., 2014; Min, Minnes, Yoon et al., 2014; Minnes et al., 2014). In addition, in their recent review, Buckingham-Howes et al. (2013) concluded that the effects of PCE on child behavior persist into adolescence. As we noted earlier, these adolescent behaviors are a significant concern because they predict maladaptive adult functioning. The association with PCE highlights one possible avenue for prevention and intervention efforts.
In terms of internalizing behavior problems, we found a marginally significant effect of PCE on negative mood. At 10 years, we reported that were no direct effects of PCE on the Children’s Depression Inventory (Richardson, Goldschmidt et al., 2013). At 15 years, we used the DOTS-R, which covers a broader range of temperamental characteristics. The few other reports of internalizing behaviors in this age group also found no effects of PCE (Bada et al., 2011; Min, Minnes, Yoon et al., 2014). This is an understudied area deserving of more attention (Buckingham-Howes et al., 2013), and it will be of interest to study the association between PCE and depression in young adulthood when symptoms will likely have increased.
We also found that first trimester PCE significantly predicted poorer performance on the Children’s Category Test and the Mazes subtest of the WISC-III, an indication that PCE is associated with a reduced capacity for problem solving and abstract reasoning. These adolescent outcomes were not found to be affected by PCE in other reports (Betancourt et al., 2011; Li et al., 2009; Rose-Jacobs et al., 2011). However, there are few reports in the literature, so this association remains to be determined. We did not find an effect of PCE on overall IQ, consistent with most other reports, nor on learning and memory.
As we have reported at earlier ages (Richardson et al., 2008, 2009, 2011; Richardson, Goldschmidt et al., 2013), offspring with PCE were significantly smaller at age 15 even after adjusting for factors such as gender, pubertal development, and maternal height. Each of the growth measures (weight, height, and head circumference) was affected by first trimester exposure. This pattern of symmetrical growth retardation is consistent with early pregnancy exposure (Bayer et al., 1993; Villar & Belizan, 1982). We also tested whether the effects of PCE on birth weight (Richardson et al., 1999) mediated the effects of PCE on size at 15 years and it did not. Thus, in our cohort, the offspring with PCE have been consistently smaller since birth and remain smaller through puberty.
There are some limitations to this study. The sample was drawn from a prenatal clinic that serves low income women and, therefore, generalizations cannot be made to samples with different socioeconomic characteristics. The women who used cocaine prenatally were different from women who did not use in several important ways, such as other substance use, race, and socioeconomic characteristics. We controlled for these differences in the analyses, but it is possible that other confounding variables could explain the differences between the offspring who were and were not exposed. We also did not have biological measures of cocaine exposure during gestation. However, as we and others have shown (Lester et al., 2001; Richardson et al., 1999), carefully conducted and detailed interviews identify more users than do urine screens, and are a cost-effective way to document PCE, especially during early pregnancy. Additional limitations include the use of a two-subtest version of the WISC-III to estimate IQ and the potential lack of power to detect second and third trimester effects.
There are, however, significant advantages to this cohort. The follow-up rates for this sample have been very good; 74% of the birth cohort was seen at 15 years, which is a difficult task when following a low income substance-using sample. We found very few differences between the subjects who were and were not assessed at 15 years. Therefore, it is unlikely that the significant findings could be attributed to attrition bias. We have comprehensive and careful measures of cocaine that allow us to evaluate patterns of exposure by trimester. Similarly, we have comprehensive assessments of alcohol, tobacco, marijuana, and other illicit drug use during pregnancy, and of the sociodemographic and environmental characteristics of the mother-child dyad, which allows us to control for the effects of these exposures. We also have an equal representation of African American and Caucasian women in the sample, which was representative of the prenatal clinic from which they were recruited.
In summary, we have shown a significant negative effect of PCE across the three domains of behavior, cognitive functioning, and physical growth, expressed as delinquent behavior, problem solving and abstract reasoning, and reductions in weight, height, and head circumference. These effects of PCE are consistent with earlier findings from this cohort, with other reports in the field, and with the animal literature (Ross et al., 2015). Thus, we have demonstrated continued deficits related to PCE through age 15. This cohort is being followed into young adulthood, when these adolescent outcomes may be predictive of maladaptive functioning during the next developmental time period.
Supplementary Material
Highlights.
The association between prenatal cocaine exposure (PCE) and adolescent development has not been well-studied.
Offspring and their mothers were followed from the fourth gestational month through 15 years of age.
PCE was associated with adolescent self-reported delinquent behavior, poorer problem solving and abstract reasoning, and physical growth, controlling for other predictors of the outcomes.
Adolescent outcomes are important because they are predictors of adult functioning.
Acknowledgments
This research was supported by the National Institute on Drug Abuse grants DA05460 and DA008916 (G. Richardson, Principal Investigator).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Accornero V, Amado AJ, Morrow CE, Xue L, Anthony JC, Bandstra ES. Impact of prenatal cocaine exposure on attention and response inhibition as assessed by continuous performance tests. J Dev Behav Pediatr. 2007;28:195–205. doi: 10.1097/01.DBP.0000268560.72580.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accornero V, Anthony JC, Morrow CE, Xue L, Bandstra ES. Prenatal cocaine exposure: an examination of childhood externalizing and internalizing behavior problems at age 7 years. Epidemiologia e Psichiatria Sociale. 2006;15:20–9. [PMC free article] [PubMed] [Google Scholar]
- Accornero V, Morrow C, Bandstra E, Johnson A, Anthony J. Behavioral outcome of preschoolers exposed prenatally to cocaine: role of maternal behavioral health. J Pediatr Psychol. 2002;27:259–69. doi: 10.1093/jpepsy/27.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman J, Riggins T, Black M. A review of the effects of prenatal cocaine exposure among school-aged children. Pediatrics. 2010;125:554–65. doi: 10.1542/peds.2009-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JWP, Bennett DS, Carmody DP, Wang Y, Lewis M. Adolescent risk-taking as a function of prenatal cocaine exposure and biological sex. Neurotoxicol Teratol. 2014;41:65–70. doi: 10.1016/j.ntt.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt RE, Short EJ, Singer LT, Minnes S, Hewitt J, Flynn S, Carlson L, Min MO, Klein N, Flannery D. Children prenatally exposed to cocaine: developmental outcomes and environmental risks at seven years of age. J Dev Behav Pediatr. 2004;25:83–90. doi: 10.1097/00004703-200404000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bada HS, Bann CM, Bauer CR, Shankaran S, Lester B, LaGasse L, Hammond J, Whitaker T, Das A, Tan S, Higgins R. Preadolescent behavior problems after prenatal cocaine exposure: relationship between teacher and caretaker ratings (Maternal Lifestyle Study) Neurotoxicol Teratol. 2011;33:78–87. doi: 10.1016/j.ntt.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bada H, Bann C, Whitaker T, Bauer C, Shankaran S, LaGasse L, Lester B, Hammond J, Higgins R. Protective factors can mitigate behavior problems after prenatal cocaine and other drug exposures. Pediatrics. 2012;130:e1479–88. doi: 10.1542/peds.2011-3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bada H, Langer J, Twomey J, Bursi C, LaGasse L, Bauer C, Shankaran S, Lester B, Higgins R, Maza P. Importance of stability of early living arrangements on behavior outcomes of children with and without prenatal drug exposure. J Dev Behav Pediatr. 2008;29:173–82. doi: 10.1097/DBP.0b013e3181644a79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PC, Mott FL. National Longitudinal Study of Youth Child Handbook. Columbus OH: Center for Human Resource Research, Ohio State University; 1989. [Google Scholar]
- Bandstra E, Morrow C, Anthony J, Accornero V, Fried P. Longitudinal investigation of task persistence and sustained attention in children with prenatal cocaine exposure. Neurotoxicol Teratol. 2001;23:545–59. doi: 10.1016/s0892-0362(01)00181-7. [DOI] [PubMed] [Google Scholar]
- Bandstra E, Morrow C, Vogel A, Fifer R, Ofir A, Dausa A, Xue L, Anthony J. Longitudinal influence of prenatal cocaine exposure on child language functioning. Neurotoxicol Teratol. 2002;24:297–308. doi: 10.1016/s0892-0362(02)00192-7. [DOI] [PubMed] [Google Scholar]
- Bayer S, Altman J, Russo R, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- Behnke M, Smith VC, Committee on Substance Abuse, Committee on Fetus and Newborn Prenatal substance abuse: short- and long-term effects on the exposed fetus. Pediatrics. 2013;131:e1009–24. doi: 10.1542/peds.2012-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendersky M, Gambini G, Lastella A, Bennett DS, Lewis M. Inhibitory motor control at five years as a function of prenatal cocaine exposure. J Dev Behav Pediatr. 2003;24:345–51. doi: 10.1097/00004703-200310000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D, Bendersky M, Lewis M. Children’s intellectual and emotional-behavioral adjustment at 4 years as a function of cocaine exposure, maternal characteristics, and environmental risk. Dev Psychol. 2002;38:648–58. doi: 10.1037//0012-1649.38.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D, Bendersky M, Lewis M. Preadolescent health risk behavior as a function of prenatal cocaine exposure and gender. J Dev Behav Pediatr. 2007;28:467–72. doi: 10.1097/DBP.0b013e31811320d8. [DOI] [PubMed] [Google Scholar]
- Bennett D, Marini V, Berzenski S, Carmody D, Lewis M. Externalizing problems in late childhood as a function of prenatal cocaine exposure and environmental risk. J Pediatr Psychol. 2013;38:296–308. doi: 10.1093/jpepsy/jss117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman LF, Syme SL. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am J Epidemiol. 1979;109:186–204. doi: 10.1093/oxfordjournals.aje.a112674. [DOI] [PubMed] [Google Scholar]
- Bernstein D, Fink L. Childhood Trauma Questionnaire: a retrospective self-report. San Antonio TX: Harcourt Brace and Company; 1998. [Google Scholar]
- Betancourt LM, Yang W, Brodsky NL, Gallagher PR, Malmud EK, Giannetta JM, Farah MJ, Hurt H. Adolescents with and without gestational cocaine exposure: longitudinal analysis of inhibitory control, memory and receptive language. Neurotoxicol Teratol. 2011;33:36–46. doi: 10.1016/j.ntt.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll T. Children’s Category Test. San Antonio TX: The Psychological Corporation; 1993. [Google Scholar]
- Bridgett DJ, Mayes LC. Development of inhibitory control among prenatally cocaine exposed and non-cocaine exposed youths from late childhood to early adolescence: the effects of gender and risk and subsequent aggressive behavior. Neurotoxicol Teratol. 2011;33:47–60. doi: 10.1016/j.ntt.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham-Howes S, Berger SS, Scaletti LA, Black MM. Systematic review of prenatal cocaine exposure and adolescent development. Pediatrics. 2013;131:e1917–36. doi: 10.1542/peds.2012-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody DP, Bennett DS, Lewis M. The effects of prenatal cocaine exposure and gender on inhibitory control and attention. Neurotoxicol Teratol. 2013;33:61–8. doi: 10.1016/j.ntt.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. Children’s Memory Scale. New York: Psychological Corporation; 1997. [Google Scholar]
- Cook RD, Weisberg S. Residuals and influence in regression. New York: Chapman and Hall; 1982. [Google Scholar]
- Covington C, Nordstrom-Klee B, Ager J, Sokol R, Delaney-Black V. Birth to age 7 growth of children prenatally exposed to drugs. A prospective study Neurotoxicol Teratol. 2002;24:489–96. doi: 10.1016/s0892-0362(02)00233-7. [DOI] [PubMed] [Google Scholar]
- Day N, Robles N. Methodological issues in the measurement of substance use. Ann NY Acad Sci. 1989;562:8–13. doi: 10.1111/j.1749-6632.1989.tb21002.x. [DOI] [PubMed] [Google Scholar]
- Delaney-Black V, Chiodo LM, Hannigan JH, Greenwald MK, Janisse J, Patterson G, Huestis MA, Partridge RT, Ager J, Sokol RJ. Prenatal and postnatal cocaine expsoure predict teen cocaine use. Neurotoxicol Teratol. 2011;33:110–9. doi: 10.1016/j.ntt.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney-Black V, Covington C, Templin T, Ager J, Nordstrom-Klee B, Martier S, Leddick L, Czerwinski RH, Sokol R. Teacher-assessed behavior of children prenatally exposed to cocaine. Pediatrics. 2000;106:782–91. doi: 10.1542/peds.106.4.782. [DOI] [PubMed] [Google Scholar]
- Doherty EE, Green KM, Ensminger ME. Investigating the long-term influence of adolescent delinquency on drug use initiation. Drug Alcohol Depen. 2008;93:72–84. doi: 10.1016/j.drugalcdep.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrenwend BS, Krasnoff K, Askenasy AR, Dohrenwend P. Exemplification of a method for scaling life events: the PERI life events scale. J Health Soc Behavior. 1978;19:205–29. [PubMed] [Google Scholar]
- Elliott DS, Huizinga D, Ageton SS. Explaining delinquency and drug use. Beverly Hills CA: Sage Publications; 1985. [Google Scholar]
- Eyler FD, Warner TD, Behnke M, Hou W, Wobie K, Garvan CW. Executive functioning at ages 5 and 7 years in children with prenatal cocaine exposure. Dev Neurosci. 2009;31:121–36. doi: 10.1159/000207500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Ridder EM. Conduct and attentional problems in childhood and adolescence and later substance use, abuse and dependence. Drug Alcohol Depen. 2007;88S:S14–S26. doi: 10.1016/j.drugalcdep.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Lester BM, Dgarmo DS, LaGasse LL, Lin H, Shankaran S, Bada HS, Bauer CR, Hammond J, Whitaker T, Higgins R. The combined effects of prenatal drug exposure and early adversity on neurobehavioral disinhibition in childhood and adolescence. Dev Psychopathol. 2011;23:777–88. doi: 10.1017/S0954579411000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DA, Rose-Jacobs R, Beeghly M, Augustyn M, Bellinger D, Cabral H, Heeren T. Level of prenatal cocaine exposure and scores on the Bayley Scales of Infant Development: modifying effects of caregiver, early intervention, and birth weight. Pediatrics. 2002;110:1143–52. doi: 10.1542/peds.110.6.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DA, Rose-Jacobs R, Beeghly M, Wilbur M, Bellinger D, Cabral H. Level of prenatal cocaine exposure and 48-month IQ: importance of preschool enrichment. Neurotoxicol Teratol. 2005;27:15–28. doi: 10.1016/j.ntt.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Frank DA, Rose-Jacobs R, Crooks D, Cabral HJ, Gerteis J, Hacker KA, Martin B, Weinstein ZB, Heeren T. Adolescent initiation of licit and illicit substance use: impact of intrauterine exposures and post-natal exposure to violence. Neurotoxicol Teratol. 2011;33:100–9. doi: 10.1016/j.ntt.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerteis J, Chartrand M, Martian B, Cabral H, Rose-Jacobs R, Crooks D, Frank D. Are there effects of intrauterine cocaine exposure on delinquency during early adolescence? A preliminary report J Dev Behav Pediatr. 2011;32:393–401. doi: 10.1097/DBP.0b013e318218d9f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M, Crnic K. Longitudinal predictors of developmental status and social interaction in premature and full-term infants at age two. Child Dev. 1988;59:554–70. doi: 10.1111/j.1467-8624.1988.tb03216.x. [DOI] [PubMed] [Google Scholar]
- Greenwald M, Chiodo L, Hannigan J, Sokol R, Janisse J, Delaney-Black V. Teens with heavy prenatal cocaine exposure respond to experimental social provocation with escape not aggression. Neurotoxicol Teratol. 2011;33:198–204. doi: 10.1016/j.ntt.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings TL, Kelly ML. Development and validation of the Screen for Adolescent Violence Exposure (SAVE) J Abn Child Psych. 1997;25:511–20. doi: 10.1023/a:1022641916705. [DOI] [PubMed] [Google Scholar]
- Hurt H, Betancourt LM, Malmud EK, Shera DM, Giannetta JM, Brodsky NL, Farah MJ. Children with and without gestational cocaine exposure: a neurocognitive systems analysis. Neurotoxicol Teratol. 2009;31:334–41. doi: 10.1016/j.ntt.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt H, Malmud E, Betancourt L, Braitman L, Brodsky N, Giannetta J. Children with in utero cocaine exposure do not differ from control subjects on intelligence testing. Arch Pediatr Adolesc Med. 1997;151:1237–41. doi: 10.1001/archpedi.1997.02170490063011. [DOI] [PubMed] [Google Scholar]
- Hurt H, Malmud E, Betancourt LM, Brodsky NL, Giannetta JM. A prospective comparison of developmental outcome of children with in utero cocaine exposure and controls using the Battelle Developmental Inventory. J Dev Behav Pediatr. 2001;22:27–34. doi: 10.1097/00004703-200102000-00005. [DOI] [PubMed] [Google Scholar]
- Jöreskog K, Sörbom D. LISREL 8.5 for Windows [Computer software] Lincolnwood IL: Scientific Software International, Inc; 2001. [Google Scholar]
- LaGasse LL, Gaskins RB, Bada HS, Shankaran S, Liu J, Lester BM, Bauer CR, Higgins RD, Das A, Roberts M. Prenatal cocaine exposure and childhood obesity at nine years. Neurotoxicol Teratol. 2011;33:188–197. doi: 10.1016/j.ntt.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert B, Bann C, Bauer C, Shankaran S, Bada H, Lester B, Whitaker T, LaGasse L, Hammond J, Higgins R. Risk-taking behavior among adolescents with prenatal drug exposure and extrauterine environmental adversity. J Dev Behav Pediatr. 2013;34:669–79. doi: 10.1097/01.DBP.0000437726.16588.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester B, ElSohly M, Wright L, Smeriglio V, Verter J, Bauer C, Shankaran S, Bada HS, Walls HH, Huestis MA, Finnegan LP, Maza PL. The Maternal Lifestyle Study: drug use by meconium toxicology and maternal self-report. Pediatrics. 2001;107:309–17. doi: 10.1542/peds.107.2.309. [DOI] [PubMed] [Google Scholar]
- Li Z, Coles CD, Lynch ME, Hamann S, Peltier S, LaConte S, Hu X. Prenatal cocaine exposure alters emotional arousal regulation and its effects on working memory. Neurotoxicol Teratol. 2009;31:342–8. doi: 10.1016/j.ntt.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares TJ, Singer LT, Kirchner L, Short EJ, Min MO, Hussey P, Minnes S. Mental health outcomes of cocaine-exposed children at 6 years of age. J Pediatr Psychol. 2006;31:85–97. doi: 10.1093/jpepsy/jsj020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber R, Farrington DP, Stouthamer-Loeber M, Van Kammen WB. Antisocial behavior and mental health problems Explanatory factors in childhood and adolescence. Mahwah NJ: Lawrence Erlbaum Associates, Publishers; 1998. [Google Scholar]
- Loeber R, Stouthamer-Loeber M, Van Kammen WB, Farrington DP. Development of a new measure of self-reported antisocial behavior for young children: prevalence and reliability. In: Klein MW, editor. Cross National Research and Self-Reported Crime and Delinquency. Dordrecht, Netherlands: Kluwer-Nijhoff; 1989. [Google Scholar]
- Lumeng JC, Cabral HJ, Gannon K, Heeren T, Frank DA. Prenatal exposures to cocaine and alcohol and physical growth patterns to age 8 years. Neurotoxicol Teratol. 2007;29:446–57. doi: 10.1016/j.ntt.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes LC, Molfese DL, Key APF, Hunter NC. Event-related potentials in cocaineexposed children during a Stroop task. Neurotoxicol Teratol. 2005;27:797–813. doi: 10.1016/j.ntt.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Mayes LC, Snyder PJ, Langlois E, Hunter N. Visuospatial working memory in schoolaged children exposed in utero to cocaine. Child Neuropsychol. 2007;13:205–18. doi: 10.1080/09297040600888753. [DOI] [PubMed] [Google Scholar]
- McLaughlin AA, Minnes S, Singer LT, Min M, Short EJ, Scott TL, Satayathum S. Caregiver and self-report of mental health symptoms in 9-year old children with prenatal cocaine exposure. Neurotoxicol Teratol. 2011;33:582–91. doi: 10.1016/j.ntt.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messinger DS, Bauer CR, Das A, Seifer R, Lester BM, LaGasse LL, Wright LL, Shankaran S, Bada HS, Smeriglio VL, Langer JC, Beeghly M, Poole WK. The Maternal Lifestyle Study: cognitive, motor, and behavioral outcomes of cocaine-exposed and opiateexposed infants through three years of age. Pediatrics. 2004;113:1677–85. doi: 10.1542/peds.113.6.1677. [DOI] [PubMed] [Google Scholar]
- Min MO, Minnes S, Lang A, Weishampel P, Short EJ, Yoon S, Singer LT. Externalizing behavior and substance use related problems at 15 years in prenatally cocaine exposed adolescents. J Adolescence. 2014;37:269–79. doi: 10.1016/j.adolescence.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min MO, Minnes S, Yoon S, Short EJ, Singer LT. Self-reported adolescent behavioral adjustment: effects of prenatal cocaine exposure. J Adolesc Health. 2014;55:167–74. doi: 10.1016/j.jadohealth.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnes S, Robin NH, Alt AA, Kirchner HL, Satayathum S, Salbert BA, Ellison L, Singer LT. Dysmorphic and anthropometric outcomes in 6-year-old prenatally cocaine-exposed children. Neurotoxicol Teratol. 2006;28:28–38. doi: 10.1016/j.ntt.2005.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnes S, Singer LT, Kirchner HL, Short E, Lewis B, Satayathum S, Queh D. The effects of prenatal cocaine exposure on problem behavior in children 4–10 years. Neurotoxicol Teratol. 2010;32:443–51. doi: 10.1016/j.ntt.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnes S, Singer LT, Min MO, Lang AM, Ben-Harush A, Short E, Wu M. Comparison of 12-year-old children with prenatal exposure to cocaine and non-exposed controls on caregiver ratings of executive function. J Youth Adolescence. 2014;43:53–69. doi: 10.1007/s10964-013-9927-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow CE, Culbertson JL, Accornero VH, Xue L, Anthony JC, Bandstra ES. Learning disabilities and intellectual functioning in school-aged children with prenatal cocaine exposure. Dev Neuropsychol. 2006;30:905–31. doi: 10.1207/s15326942dn3003_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow CE, Vogel AL, Anthony JC, Ofir AY, Dausa AT, Bandstra ES. Expressive and receptive language functioning in preschool children with prenatal cocaine exposure. J Pediatr Psychol. 2004;29:543–54. doi: 10.1093/jpepsy/jsh056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noland JS, Singer LT, Short E, Minnes S, Arendt RE, Kirchner HL, Bearer C. Prenatal drug exposure and selective attention in preschoolers. Neurotoxicol Teratol. 2005;27:429–38. doi: 10.1016/j.ntt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Nordstrom Bailey B, Sood BG, Sokol RJ, Ager J, Janisse J, Hannigan JH, Covington C, Delaney-Black V. Gender and alcohol moderate prenatal cocaine effects on teacher-report of child behavior. Neurotoxicol Teratol. 2005;27:181–9. doi: 10.1016/j.ntt.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Petersen A, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adol. 1988;17:117–33. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psych Meas. 1977;1:385–401. [Google Scholar]
- Ramrakha S, Bell ML, Paul C, Dickson N, Moffitt TE, Caspi A. Childhood behavior problems linked to sexual risk taking in young adulthood: a birth cohort study. J Am Acad Child Psychol. 2007;46:1272–9. doi: 10.1097/chi.0b013e3180f6340e. [DOI] [PubMed] [Google Scholar]
- Richardson GA, Goldschmidt L, Larkby C, Day NL. Effects of prenatal cocaine exposure on child behavior and growth at 10 years of age. Neurotoxicol Teratol. 2013;40:1–8. doi: 10.1016/j.ntt.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GA, Goldschmidt L, Leech S, Willford J. Prenatal cocaine exposure: effects on mother- and teacher-rated behavior problems and growth in school-age children. Neurotoxicol Teratol. 2011;33:69–77. doi: 10.1016/j.ntt.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GA, Goldschmidt L, Willford J. The effects of prenatal cocaine use on infant development. Neurotoxicol Teratol. 2008;30:96–106. doi: 10.1016/j.ntt.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GA, Goldschmidt L, Willford J. Continued effects of prenatal cocaine use: preschool development. Neurotoxicol Teratol. 2009;31:325–33. doi: 10.1016/j.ntt.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GA, Hamel S, Goldschmidt L, Day N. Growth of infants prenatally exposed to cocaine/crack: comparison of a prenatal care & a no prenatal care sample. Pediatrics. 1999;104:e18. doi: 10.1542/peds.104.2.e18. [DOI] [PubMed] [Google Scholar]
- Richardson GA, Larkby C, Goldschmidt L, Day NL. Adolescent initiation of drug use: effects of prenatal cocaine exposure. J Am Acad Child Psy. 2013;52:37–46. doi: 10.1016/j.jaac.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivkin MJ, Davis PE, Lemaster JL, Cabral HJ, Warfield SK, Mulkern RV, Robson CD, Rose-Jacobs R, Frank DA. Volumetric MRI study of brain in children with intrauterine exposure to cocaine, alcohol, tobacco, and marijuana. Pediatrics. 2008;121:741–50. doi: 10.1542/peds.2007-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose-Jacobs R, Soenksen S, Appugleise DP, Cabral HJ, Richardson MA, Beeghly M, Heeren TC, Frank DA. Early adolescent executive functioning, intrauterine exposures and own drug use. Neurotoxicol Teratol. 2011;33:379–92. doi: 10.1016/j.ntt.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose-Jacobs R, Waber D, Beeghly M, Cabral H, Appugleise D, Heeren T, Marani J, Frank DA. Intrauterine cocaine exposure and executive functioning in middle childhood. Neurotoxicol Teratol. 2009;31:159–68. doi: 10.1016/j.ntt.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross EJ, Graham DL, Money KM, Stanwood GD. Developmental consequences of fetal exposure to drugs: what we know and what we still must learn. Neuropsychopharmacology Reviews. 2015;40:61–87. doi: 10.1038/npp.2014.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sameroff A, Seifer R, Barocas R, Zax M, Greenspan S. Intelligence quotient scores of 4-year-old children: social environmental risk factors. Pediatrics. 1987;79:343–50. [PubMed] [Google Scholar]
- Sattler JM. Assessment of children: cognitive applications. Fourth. San Diego CA: Jerome M Sattler Publisher; 2001. [Google Scholar]
- Savage J, Brodsky NL, Malmud E, Giannetta JM, Hurt H. Attentional functioning and impulse control in cocaine-exposed and control children at age ten years. J Dev Behav Pediatr. 2005;26:42–7. [PubMed] [Google Scholar]
- Schroder M, Snyder P, Sielski I, Mayes L. Impaired performance of children exposed in utero to cocaine on a novel test of visuospatial working memory. Brain Cognition. 2004;55:409–12. doi: 10.1016/j.bandc.2004.02.062. [DOI] [PubMed] [Google Scholar]
- Schwab-Stone M, Koposov R, Vermeiren R, Ruchkin V. Cross-cultural findings on community violence exposure and internalizing psychopathology: comparing adolescents in the United States, Russia, and Belgium. Child Psychiatry Hum Dev. 2013;44:516–24. doi: 10.1007/s10578-012-0344-8. [DOI] [PubMed] [Google Scholar]
- Shankaran S, Bann C, Bauer C, Lester B, Bada H, Das A, Higgins R, Poole K, LaGasse L, Hammond J, Woldt E. Prenatal cocaine exposure and body mass index and blood pressure at 9 years of age. J Hypertens. 2010;28:1166–1175. [PMC free article] [PubMed] [Google Scholar]
- Shankaran S, Das A, Bauer CR, Bada HS, Lester BM, Wright LL, Higgins RD, Poole WK. Prenatal cocaine exposure and small-for-gestational-age status: effects on growth at 6 years of age. Neurotoxicol Teratol. 2011;33:575–81. doi: 10.1016/j.ntt.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer LT, Minnes S, Short E, Arendt R, Farkas K, Lewis B, Klein N, Russ S, Min MO, Kirchner HL. Cognitive outcomes of preschool children with prenatal cocaine exposure. J Am Med Assoc. 2004;291:2448–56. doi: 10.1001/jama.291.20.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer LT, Nelson S, Short E, Min MO, Lewis B, Russ S, Minnes S. Prenatal cocaine exposure: drug and environmental effects at 9 years. J Pediatr. 2008;153:105–11. doi: 10.1016/j.jpeds.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel M. Direct and indirect effects in linear structural equation models. Sociol Method Res. 1987;16:155–76. [Google Scholar]
- Sood BG, Nordstrom Bailey B, Covington C, Sokol RJ, Ager J, Janisse J, Hannigan JH, Delaney-Black V. Gender and alcohol moderate caregiver reported child behavior after prenatal cocaine. Neurotoxicol Teratol. 2005;27:191–201. doi: 10.1016/j.ntt.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R. Manual for the State-Trait Anxiety Inventory. Palo Alto CA: Consulting Psychologists Press, Inc; 1970. [Google Scholar]
- Sroufe L, Rutter M. The domain of developmental psychopathology. Child Dev. 1984;55:17–29. [PubMed] [Google Scholar]
- Steinberg L, Lamborn S, Dornbusch S, Darling N. Impact of parenting practices on adolescent achievement: authoritative parenting, school-involvement, and encouragement to success. Child Dev. 1992;63:1266–81. doi: 10.1111/j.1467-8624.1992.tb01694.x. [DOI] [PubMed] [Google Scholar]
- Stratton KR, Howe CJ, Battaglia FC. eds Fetal Alcohol Syndrome: diagnosis, epidemiology, prevention, and treatment. Washington DC: National Academy Press; 1996. [Google Scholar]
- Verhulst FC, van der Ende J. Agreement between parents’ reports and adolescents’ selfreports of problem behavior. J Child Psychol Psychiat. 1992;33:1011–23. doi: 10.1111/j.1469-7610.1992.tb00922.x. [DOI] [PubMed] [Google Scholar]
- Villar J, Belizan J. The timing factor in the pathophysiology of the intrauterine growth retardation syndrome. Obstet Gynecol Surv. 1982;37:499–506. doi: 10.1097/00006254-198208000-00001. [DOI] [PubMed] [Google Scholar]
- Warner TD, Behnke M, Eyler FD, Padgett K, Leonard C, Hou W, Garvan CW, Schmalfuss IM, Blackband SJ. Diffusion tensor imaging of frontal white matter and executive functioning in cocaine-exposed children. Pediatrics. 2006;118:2014–24. doi: 10.1542/peds.2006-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner TD, Behnke M, Hou W, Garvan C, Wobie K, Eyler F. Predicting caregiverreported behavior problems in cocaine-exposed children at 3 years. J Dev Behav Pediatr. 2006;27:83–92. doi: 10.1097/00004703-200604000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children – Third Edition Manual. San Antonio TX: The Psychological Corporation; 1991. [Google Scholar]
- Werner E, Smith R. Overcoming the odds: high risk children from birth to adulthood. Ithaca NY: Cornell University Press; 1992. [Google Scholar]
- Whitaker T, Bada H, Bann C, Shankaran S, LaGasse L, Lester B, Bauer C, Hammond J, Higgins R. Serial pediatric symptom checklist screening in children with prenatal drug exposure. J Dev Behav Pediatr. 2011;32:206–15. doi: 10.1097/DBP.0b013e318208ee3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle M, Lerner R. Reassessing the dimension of temperament individuality across the life span: the Revised Dimensions of Temperament Survey (DOTS-R) J Adolesc Res. 1986;1:213–30. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


