Abstract
Agriculture industry workers are at a higher risk for chronic bronchitis and obstructive pulmonary diseases, and current therapeutics are not entirely effective. We previously found that the specialized pro-resolving lipid mediator (SPM) maresin-1 (MaR1) reduced pro-inflammatory cytokine release and intracellular adhesion molecule-1 (ICAM-1) expression in bronchial epithelial cells exposed to extracts of organic dust (DE) derived from swine confinement facilities in vitro. The objective of this study was to determine whether MaR1 is effective at limiting lung inflammation associated with acute and repetitive exposures to DE in an established murine model of inhalant dust exposures. C57Bl/6 mice were treated with MaR1 or vehicle control and intranasally instilled with DE once or daily for 3 weeks. Bronchial alveolar lavage fluid was analyzed for total and differential cell counts and pro-inflammatory cytokine levels, and lung tissues were assessed for histopathology and ICAM-1 expression. In both single and repetitive DE exposure studies, MaR1 significantly decreased bronchoalveolar lavage neutrophil infiltration, IL-6, TNF-α, and CXCL1 levels without altering repetitive DE-induced bronchiolar/alveolar inflammation or lymphoid aggregate formation. Lung tissue ICAM-1 expression was also reduced in both single and repetitive exposure studies. These data suggest that MaR1 might contribute to an effective strategy to reduce airway inflammatory diseases induced by agricultural-related organic dust environmental exposures.
Keywords: maresin-1, specialized pro-resolving mediators, airway inflammation, organic dust exposures, omega-3 fatty acids
Introduction
In agricultural industries, organic dust exposure triggers significant airway inflammation. A one-time or acute exposure to these organic dust environments can result in acute lung injury, asthma-like symptoms, or organic dust toxic syndrome (1-3). Long-term exposures such as those experienced by individuals employed in concentrated animal feeding operations (CAFOs) lead to chronic bronchitis and obstructive pulmonary diseases (4-8). Acute exposures to organic dusts such as those found in swine CAFOs result in pro-inflammatory cytokine release, including IL-6 and TNF-α, and neutrophil chemoattractants, coinciding with the influx of neutrophils in humans and rodents (9,10). Similarly, repeated organic dust exposures in humans and animal models demonstrate a persistence of airway inflammatory indices, albeit dampened, with evidence of lung histopathologic changes. It is recognized that persons with chronic exposures are at a heightened risk for lung function decline and chronic respiratory diseases, including chronic bronchitis and obstructive pulmonary disease (11-13). Building ventilation is thought to be a primary means of reducing respiratory health risks, although these engineering interventions are often not sufficient for protection against dust-induced airway inflammation and disease (3). While ventilator masks are effective at preventing disease, this method of prevention is inconsistently utilized by the susceptible populations (14). Furthermore, currently available therapeutics, including corticosteroids and β2 adrenergic receptor agonists, do not adequately treat or alleviate disease (8). More effective preventative and treatment strategies are required to improve the health of affected individuals.
The health benefits of diets high in ω-3 fatty acids are increasingly recognized (15-19), although the mechanisms underlying these benefits are not well understood. Recently, ω-3 fatty acid-derived specialized pro-resolving mediators (SPM) have been linked to mechanisms underlying the anti-inflammatory benefits of ω-3 fatty acids (20-23). For example, the SPM resolvin E1 was found to lower inflammatory cytokines during acute lung injury, to limit lymphocyte recruitment, and to reduce airway hyper-responsiveness in a murine asthma model (24,25). In preclinical investigations, treatment with the SPM resolvin D1 diminished lipopolysaccharide-induced lung inflammation, as well as cigarette smoke-induced lung inflammation in mice (26,27). In the case of the SPM maresin-1 (MaR1), reduced neutrophil infiltration and increased macrophage phagocytic capacities were demonstrated in a murine peritonitis model, and pulmonary edema, neutrophil influx, and pro-inflammatory mediator production were reduced in MaR1-treated mice in a lipopolysaccharide-induced acute lung injury model (28,29). In a murine model of acute lung injury, MaR1 was found to decrease lung neutrophils, edema, and pro-inflammatory mediators (30). Furthermore, we found that MaR1 is effective in reducing pro-inflammatory responses associated with organic dust exposures in vitro. Namely, MaR1 treatment reduced protein kinase C (PKC)-ε and PKC-α activities, serum response element binding activities, and pro-inflammatory cytokine release in airway epithelial cells and ex vivo murine lung slice cultures exposed to organic dust extracts derived from swine confinement facilities (DE) (31) .
Based on these data, we hypothesized that treatment with MaR1 would diminish the airway inflammatory consequences induced by organic dusts in vivo. To test this hypothesis, we utilized an established murine model of single and repetitive DE treatment-induced airway inflammation (13). In this model, we assessed the effect of MaR1 pretreatment prior to single and repetitive DE exposures on cellular infiltration, pro-inflammatory cytokine release, expression of the adhesion molecule ICAM-1 in airway epithelial cells, and lung histopathology. MaR1 reduced DE-induced pro-inflammatory cytokine release, neutrophil influx, and epithelial cell ICAM-1 expression. Although, it did not significantly alter DE-induced lymphoid aggregate formation and peribronchiolar/perivascular inflammation in the lung parenchyma. The results of these studies suggest a potential role for SPM like MaR1 in modulating airway inflammation associated with agriculture exposures. SPM may represent novel candidates for preventative or treatment strategies for individuals working in agricultural industries.
Materials and Methods
Materials
7(S)-Maresin-1 (7S,14R-dihydroxy-4Z,8E,10Z,12Z,16Z,19Z-docosahexaenoic acid) and 7(R)-Maresin-1 (7R,14S-dihydroxy-4Z,8E,10E,12Z,16Z,19Z-docosahexaenoic acid) were purchased from Cayman Chemical (Ann Arbor, MI, USA).
Preparation of DE
DE was prepared as previously described (32). Briefly, settled dust from local swine confinement facilities was collected. Dusts were suspended in Hank’s Buffered Saline Solution (HBSS) at 1 g dust per 10 ml HBSS, and incubated at room temperature for 1 hr. The aqueous extract was centrifuged twice at 4250g for 20 minutes each and the resulting supernatant was sterile filtered (0.22 μm), which also removes coarse particles. The final solution, representing 100% aqueous DE, was frozen into aliquots and stored at −20 C. In these investigations, we have utilized two different lots of DE (collected on different dates) as well as different preparations; no lot-to-lot or preparation-induced variability was detected.
Animal Care and Housing
Male C57Bl/6 mice of 6-8 weeks in age were obtained from Jackson Laboratories (Bar Harbor, ME, USA) and housed under pathogen-free conditions in group-housing cages. Mice were fed ad libitum a standard mouse chow diet and water. The University of Nebraska Medical Center Animal Care Facilities supervised the health and diet of mice, and all experiments performed were regulated and approved by the University of Nebraska Medical Center Institutional Animal Care and Use committee.
In Vivo DE Exposure Model
In vivo murine studies were performed using a standardized established model of organic dust exposure that has been previously characterized. (13, 33, 34) For single (1-time) exposure studies, mice were given ethanol vehicle (2 × 10−6 % ethanol in PBS), 0.1, or 1 ng MaR1 as a 50 l intraperitoneal (i.p.) injection at 18 hours before and 30 minutes prior to a single intranasal (i.n.) instillation consisting of 50 l of sterile saline solution or 12.5% DE. In an additional set of experiments, mice were given 1 ng MaR1 in a single 50 μl i.n. instillation prior to DE treatment as above. For repetitive exposure studies, mice were given ethanol vehicle (2 × 10−5 % ethanol in PBS) or 10 ng MaR1 i.p. 30 minutes prior to every i.n. instillation of saline solution or 12.5% DE for 15 consecutive weekdays. In all studies, individual groups consisted of a minimum of 5 mice each. At 5 hours following final DE instillation when cytokine release in the BALF peaks (13), mice were euthanized and BALF was obtained using 3 × 1-ml aliquots of phosphate-buffered saline (PBS). Lungs were subsequently inflated with formalin at a pressure of 20 cmH2O for 24 hours with formalin submersion to preserve pulmonary architecture.
Staining for Differential Cell Counts from BALF
BALF was centrifuged at 1500 rpm for 5 minutes to pellet cells. Following red blood cell lysis, total BALF cells numbers were enumerated by hemocytometer and cytospins of BALF cells were prepared using a Wescor CYTOPRO (Logan, Utah, USA). Slides were stained using Siemens Diff-Quik Stain Set (Newark, DE, USA). Differential cell counts were then quantified under light microscopy.
BALF Cytokines/Chemokines
The first 1 ml of BALF following centrifugation to remove cells was utilized for analyses. TNF-α, IL-6, CXCL1 and CXCL2 levels were quantitated using enzyme linked immunosorbant assays according to manufacturer’s directions (R&D Systems, Minneapolis, MN).
Tissue Histology
Inflated, formalin-fixed whole lungs were paraffin-embedded, microtome sectioned at 5 microns, and stained with H&E utilizing standard processing, sectioning and staining techniques at the University of Nebraska Medical Center Tissue Sciences Facility. Whole lung microtome sections were reviewed and semi-quantitatively scored for the degree and distribution of inflammation by a lung pathologist blinded to treatment conditions, and scores (0-3) were determined with the highest score representing maximal inflammation as previously described (13).
Immunohistochemistry
To determine epithelial cell ICAM-1 expression, unstained slides of sectioned (4-5 μm) lung were cleared with Protocol Safeclear II (Fisher Scientific, Kalamazoo, MI, USA) and rehydrated in an ethanol gradient. Antigen retrieval was performed using 2N HCl. Slides were blocked for 1 hour in a PBS-tween containing 5% w/v nonfat dry milk solution. Rat anti-mouse ICAM-1 antibody (Clone YN1/1.7.4; Biolegend, San Diego, CA, USA) was used a dilution of 1:75. Slides were incubated in primary antibody at 4° C overnight. Following 3 consecutive 5 minute washes in PBS-tween, slides were incubated for 1 hour in 1:750 (goat) anti-mouse secondary antibody conjugated to biotin (Biolegend, San Diego, CA, USA) at room temperature. The Vector Labs ABC elite reagent kit and the Vector Labs ImmPACT DAB kit (Burlingame, CA, USA) were employed for visualization. Cells were counterstained with hematoxylin and blued with 0.1% sodium bicarbonate. Tissues were dehydrated, cleared, cover-slipped, and photographed under light microscopy.
Statistical Analyses
Statistical analyses were performed using Graphpad Prism software (La Jolla, CA, USA) and data are expressed as mean +/− the standard error of the mean. The ANOVA method was used for determining significance amongst all groups in an experiment, with Tukey’s method for post-hoc comparisons amongst groups to correct for the additional error rates that occurs with multiple groups/comparisons. A p value of less than or equal to 0.05 was considered to be a significant difference between two groups.
Results
MaR1 pretreatment reduces neutrophil influx and pro-inflammatory cytokine release following single exposures to DE
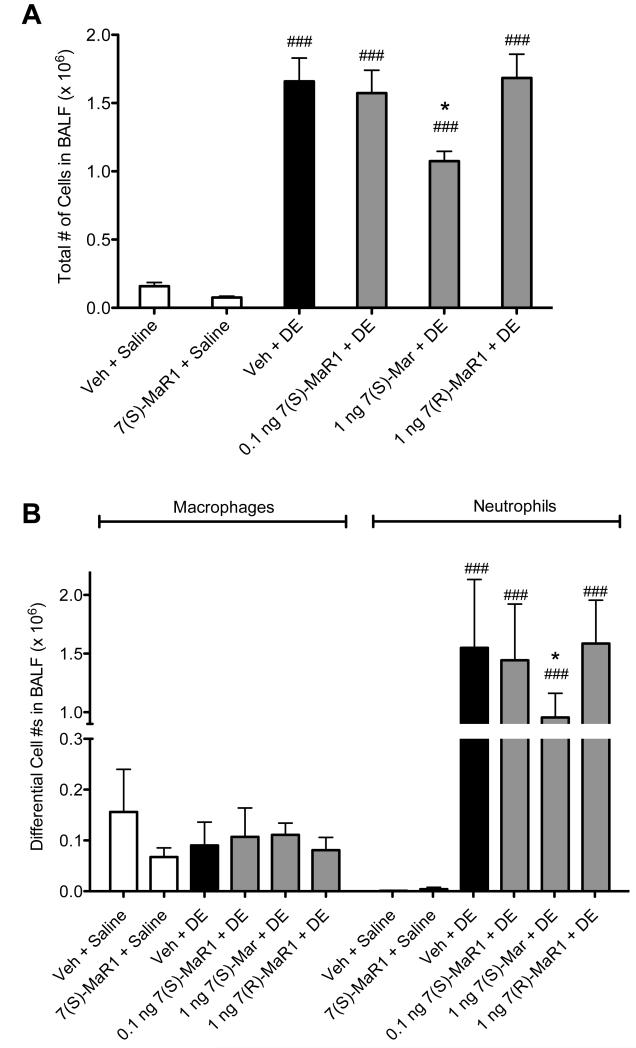
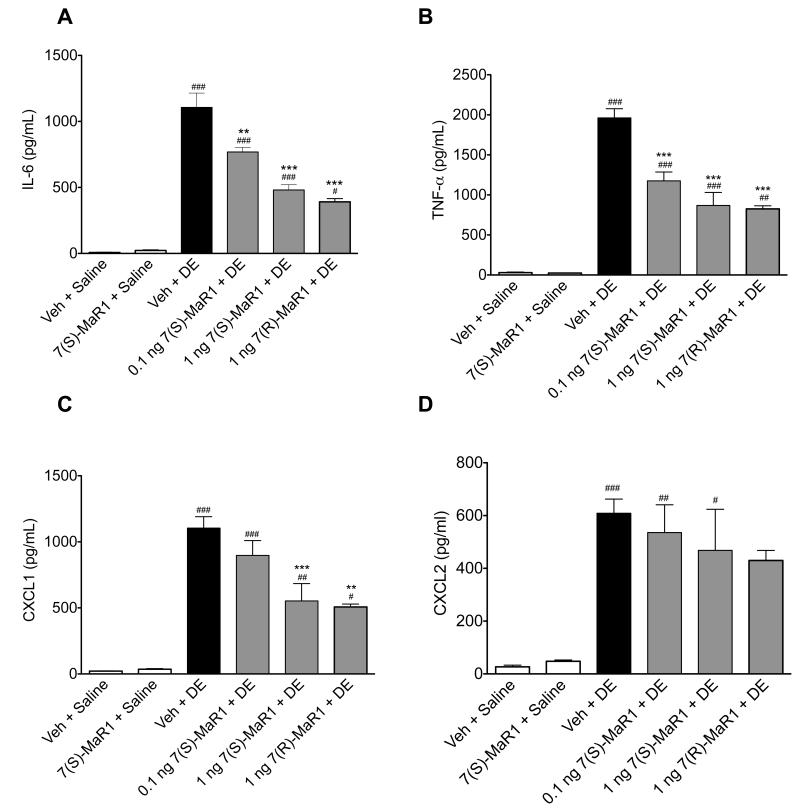
We have previously reported that a one-time exposure to DE in mice leads to marked neutrophil influx along with significant increases in TNF-α, IL-6, CXCL1 and CXCL2 in BALF (13). Pretreatment with 1 ng MaR1 prior to DE caused a reduction in total cell infiltration (Figure 1A) corresponding to a significant decrease in neutrophils compared to mice receiving vehicle + DE (Figure 1B, p < 0.05). Dose response studies with MaR1 at lower (0.1 ng) and higher (10 -1000 ng; data not shown) doses were conducted, and 1 ng MaR1 was the optimal dose in these acute exposure studies. While no toxicity was seen at higher MaR1 doses, it is of note that higher dose ranges at times resulted in increased inflammatory mediator production compared to vehicle + DE groups (data not shown), indicating a biphasic response. MaR1 has two different isoforms (7[S] and 7[R]) and isoform-specific effects have been described (35). Therefore, we tested the efficacy of both isoforms in our single exposure investigations. It is notable that at the 1 ng dose, the 7(R)- isoform of MaR1 did not reduce DE-induced neutrophil influx as the 7(S)-MaR1 isoform did (Figure 1B). Both isoforms of MaR1 significantly reduced the levels of DE-induced TNF-α, IL-6, and CXCL1 in the BALF (Figure 2A-C). Neither MaR1 treatment had a significant effect on DE-induced CXCL2 production (Figure 2D). There was no significant difference between 1 ng 7(R)-MaR1 and 1 ng 7(S)-MaR1 when comparing DE-induced cytokine release; both were similarly effective at reducing TNF- α, IL-6, and CXCL1 levels in mice receiving a single instillation of DE (Figure 2).
Figure 1. Total and differential cell counts in BALF following single DE exposure in mice receiving pretreatment with MaR1 given intraperitoneally.
Mice were pretreated with 0, 0.1, or 1 ng MaR1 given i.p. at 18 hrs and 30 minutes prior to a single intranasal treatment of DE. At five hours following DE exposure, BALF was collected. Mean total cell counts (A) and neutrophils and macrophages (B) are shown with standard error bars. ### p < 0.001 compared to vehicle + saline group; * p < 0.05 compared to vehicle + DE treatment group; N ≥ 5 mice per group.
Figure 2. Pro-inflammatory cytokine levels in BALF following single DE exposure in mice receiving pretreatment with MaR1 given intraperitoneally.
Mice were pretreated with 0, 0.1, or 1 ng MaR1 given i.p. at 18 hrs and 30 minutes prior to a single intranasal dose of DE. At five hours following DE exposure, BALF were retrieved and analyzed for IL-6 (A), TNF-α (B), CXCL1 (C), and CXCL2 (D) cytokine levels. ### p < 0.001 compared to vehicle + saline group; ## p < 0.01 compared to vehicle + saline group; # p < 0.05 compared to vehicle + saline group; *** p < 0.001 compared to vehicle + DE treatment group; ** p < 0.01 compared to vehicle + DE treatment group. Data are represented as mean values with standard error bars. N ≥ 5 mice per group.
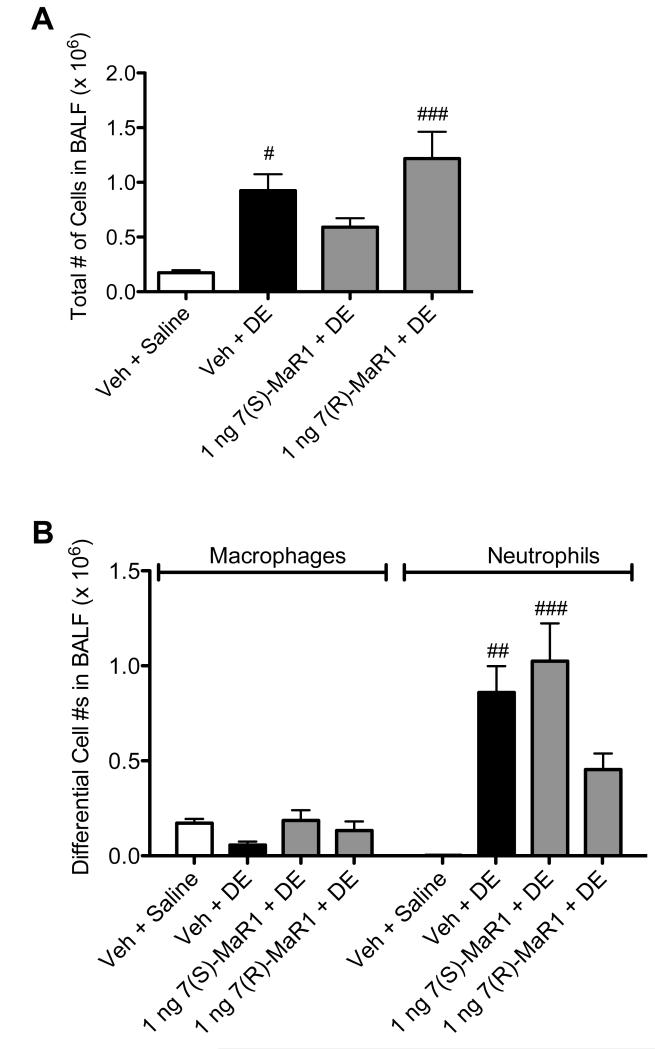
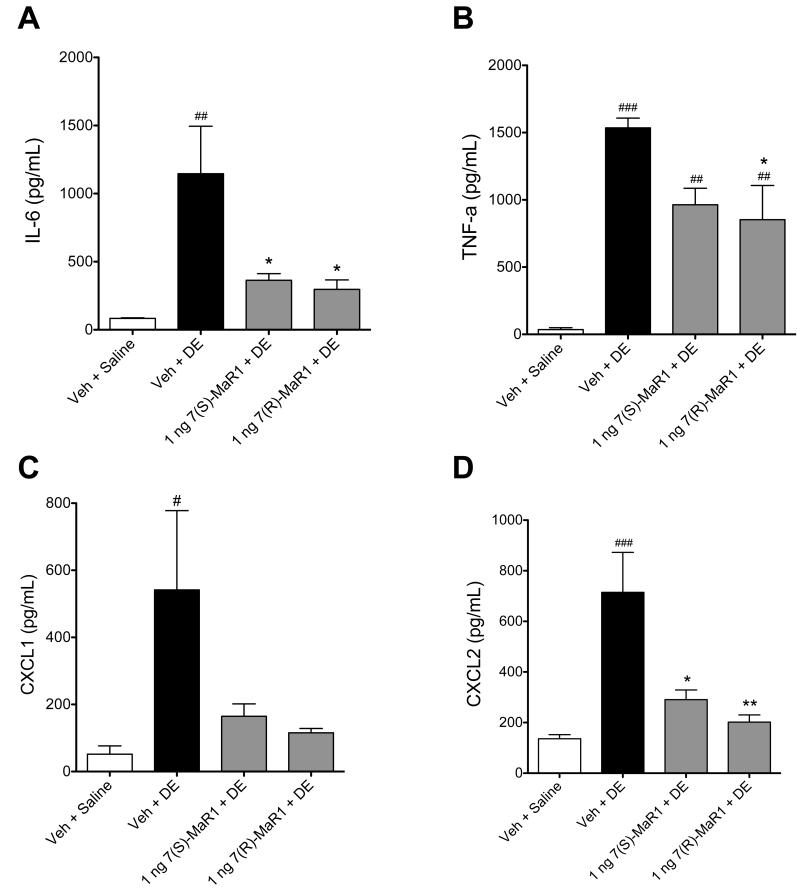
In separate experiments, mice were given MaR1 via i.n. instillation 30 minutes prior to treatment with DE to determine whether route of administration would impact the effect of MaR1 on DE-induced airway inflammation. As shown in Figure 3A, treatment with i.n. 7(S)-MaR1 blunted DE-induced total cell influx. This lower total cell infiltration correlated with a trend towards reduction in neutrophil influx, which was not significantly increased over saline controls (Figure 3B, p < 0.05). These findings were similar to the observations found with i.p. MaR1 treatment (Figure 1), whereby total and neutrophil infiltration were increased as compared to saline controls, but diminished when compared to vehicle + DE treated mice. Pretreatment with i.n. 7(S)- and 7(R)-MaR1 both reduced DE-induced IL-6 and CXCL2, and TNF-α production was significantly lower in 7(R)-MaR1 + DE treated mice compared to DE-treated mice (Figure 4A, B, D). In 7(S)- or 7(R)-MaR1 + DE-treated mice, CXCL1 production was not significantly increased over saline-instilled controls (Figure 4C). Together, these data suggest a role for MaR1 in reducing the airway inflammatory responses following single DE exposures. Our data also indicate that 7(S) and 7(R) isoforms of MaR1, as well as MaR1 administration route may modify effects in limiting airway inflammation associated with a single exposure to DE.
Figure 3. Total and differential cell counts in BALF following single DE exposure in mice receiving pretreatment with MaR1 given intranasally.
Mice were pretreated with 0, or 1 ng MaR1 given i.n. at 18 hrs and 30 minutes prior to a single intranasal dose of DE. At five hours following DE exposure, BALF was recovered. Total cell counts were enumerated (A), as well as differential counts of neutrophils and macrophages (B). ### p < 0.001 compared to vehicle + saline group; ## p < 0.01 compared to vehicle + saline group; # p < 0.05 compared to vehicle + saline group. Data are represented as mean values with standard error bars. N ≥ 5 mice per group.
Figure 4. Pro-inflammatory cytokine levels in BALF following single DE exposure in mice receiving pretreatment with MaR1 given intranasally.
Mice were pretreated with 0 or 1 ng MaR1 given i.n. at 18 hrs and 30 minutes prior to a single intranasal dose of DE. At five hours following DE exposure, BALF were retrieved and analyzed for IL-6 (A), TNF-α (B), CXCL1 (C), and CXCL2 (D) cytokine levels. ### p < 0.001 compared to vehicle + saline group; ## p < 0.01 compared to vehicle + saline group; # p < 0.05 compared to vehicle + saline group; ** p < 0.01 versus DE treatment group; * p < 0.05 versus DE treatment group. Data are represented as mean values with standard error bars. N ≥ 5 mice per group.
MaR1 pretreatment reduces neutrophil influx and pro-inflammatory cytokine release following repetitive exposure to DE
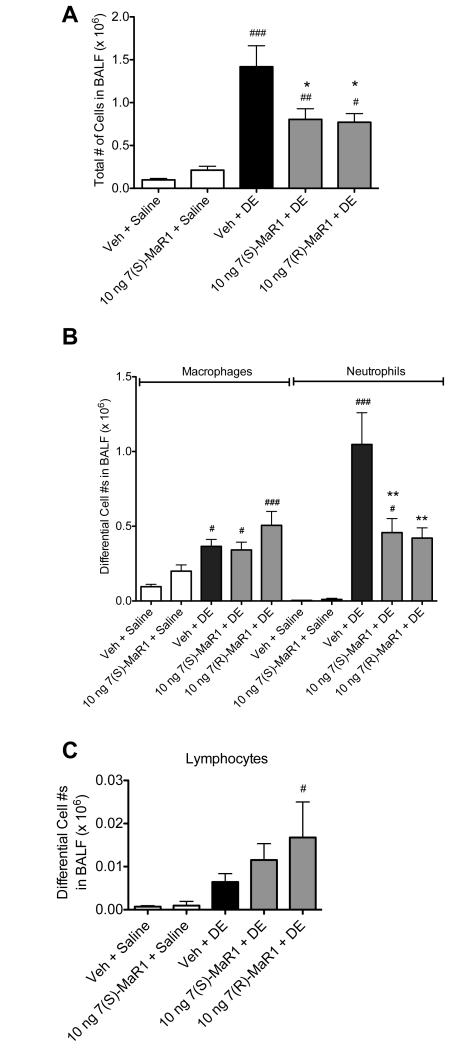
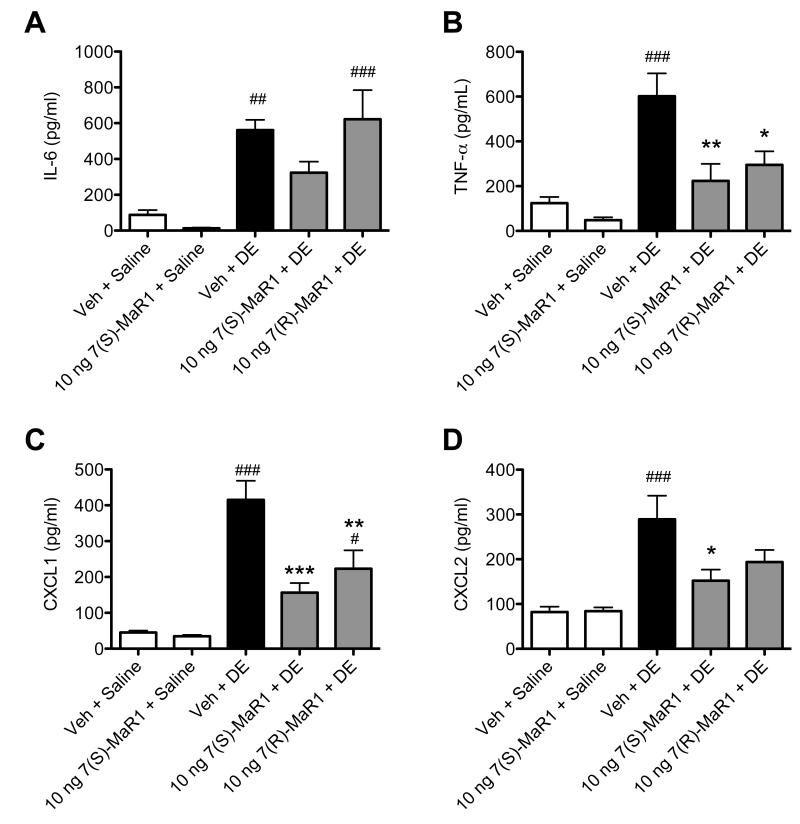
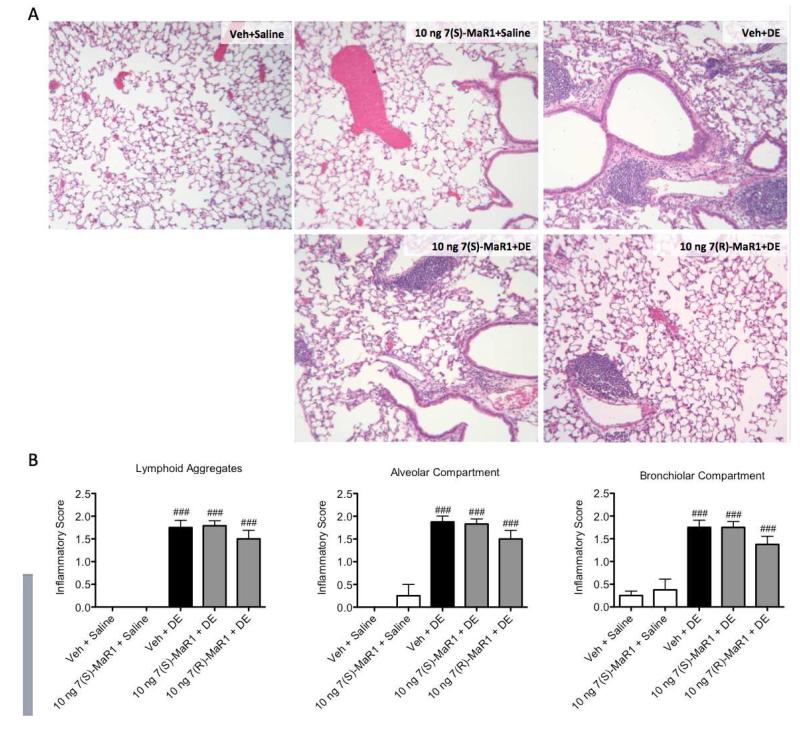
Repetitive exposure to DE in mice leads to neutrophil influx in the BALF, pro-inflammatory cytokine production, and peribronchial/vascular inflammation with lymphoid aggregate formation (13). In these studies, we sought to determine whether MaR1 treatment would reduce these established inflammatory consequences following repetitive DE exposures. For 15 consecutive weekdays, mice were given 0 or 10 ng MaR1 by i.p. inoculation followed by i.n. instillation of DE. BALF and lung tissues were harvested five hours following the final DE exposures and assayed for markers of inflammation. MaR1 treatment decreased DE-induced total cell and neutrophil influx significantly compared to mice receiving vehicle + DE (Figure 5). DE-induced TNF-α and CXCL1 BALF levels were decreased in animals receiving 7(S)- and 7(R)-MaR1 pretreatment as compared to vehicle control (Figure 6B, C), and DE-induced CXCL2 levels were reduced by 7(S)-MaR1 (Figure 6D). Although, there was no significant difference in alveolar or bronchiolar compartment inflammation or lymphoid aggregate formation in the 7(S)- or 7(R)-MaR1+DE-treated mice as compared to vehicle + DE control animals (Figure 7). It is notable that at lower and higher doses (0.1 – 1 ng, 100 – 200 ng), MaR1 was not as effective as the 10 ng dose at altering the cellular influx and cytokine/chemokine production associated with the repetitive DE exposure (data not shown). These data suggest that MaR1 may be effective in reducing airway neutrophil and pro-inflammatory mediator release associated with DE exposures, but does not substantially attenuate DE-induced lung parenchymal histopathologic inflammatory changes.
Figure 5. Total and differential cell counts in BALF following repetitive DE exposures in mice receiving pretreatment with MaR1.
Mice were pretreated with 0 or 10 ng MaR1 given i.p. 30 minutes prior to an intranasal dose of DE, given daily for 15 consecutive weekdays. At five hours following the final MaR1/DE exposure, BALF were retrieved. Total cell counts were enumerated (A), as well as differential counts of neutrophils and macrophages (B) and lymphocytes (C). ### p < 0.001 compared to vehicle + saline group; ## p < 0.01 compared to vehicle + saline group; # p < 0.05 compared to vehicle + saline group; *** p < 0.001 versus DE treatment group. Data are represented as mean values with standard error bars. N ≥ 5 mice for control groups and N≥ 10 mice for treatment groups.
Figure 6. Pro-inflammatory cytokine levels in BALF following repetitive DE exposures in mice receiving pretreatment with MaR1.
Mice were pretreated with 0 or 10 ng MaR1 given i.p. 30 minutes prior to an intranasal dose of DE, given daily for 15 consecutive weekdays. At five hours following the final MaR1/DE exposure, BALF were retrieved and analyzed for IL-6 (A), TNF-α (B), CXCL1 (C), and CXCL2 (D) cytokine levels. ### p < 0.001 compared to vehicle + saline group; ## p < 0.01 compared to vehicle + saline group; # p < 0.05 compared to vehicle + saline group; *** p < 0.001 versus Veh + DE treatment group; ** p < 0.01 versus Veh + DE treatment group; * p < 0.05 versus Veh + DE treatment group. Data are represented as mean values with standard error bars. N ≥ 5 mice for control groups and N≥ 10 mice for treatment groups.
Figure 7. Lung histopathology following repetitive DE exposures in mice receiving pretreatment with MaR1.
Mice were pretreated with 0 or 10 ng MaR1 given i.p. 30 minutes prior to an intranasal dose of DE, given daily for 15 consecutive weekdays. At five hours following the final MaR1/DE exposure, lungs were formalin-fixed and inflated for paraffin embedding and sectioning. Lungs were stained with hematoxylin and eosin (A), then scored by a lung pathologist for markers of chronic inflammation (B). ### p < 0.001 compared to vehicle + saline group. Data are represented as mean values with standard error bars. N ≥ 4 mouse lungs used in analyses for control groups and N ≥ 8 mice for treatment groups.
MaR1 pretreatment reduces DE-induced epithelial cell ICAM-1 expression following single and repetitive DE instillation
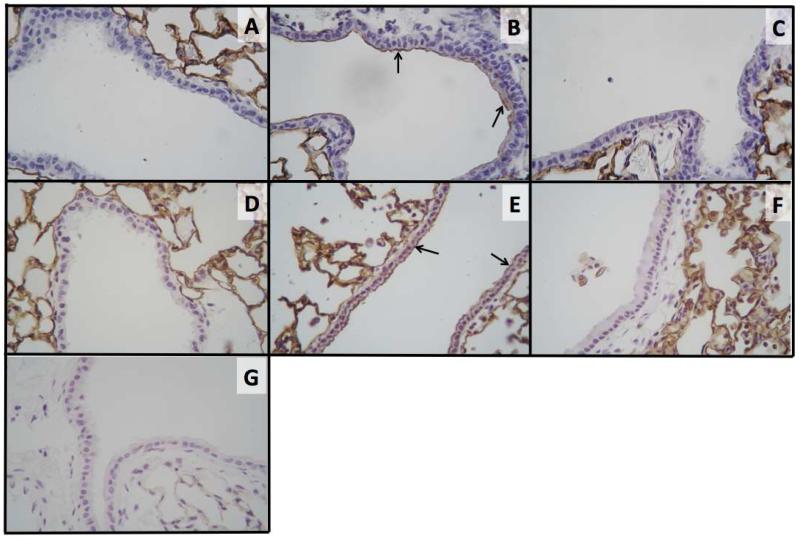
We have previously shown that DE treatment upregulates bronchial epithelial cell ICAM-1 surface expression and increases neutrophil adhesion in vitro (36). In these studies, we have found that pretreatment with 1 ng 7(S)-MaR1 given i.p. prior to a single instillation of DE reduces DE-induced ICAM-1 expression on epithelial cells by immunohistochemistry staining techniques (Figure 8A-C). In addition, mice receiving 10 ng 7(S)-MaR1 in combination with repetitive DE exposures have reduced ICAM-1 expression in airway epithelial cells compared to mice receiving vehicle + DE (Figure 8D-F). Findings of reduced ICAM-1 surface expression were similar with 7(R)-MaR1 + DE single and repetitive treatments (data not shown). Together, these data show that MaR1 reduces DE-induced epithelial cell ICAM-1 expression that may, in part, explain the reduction in DE-induced neutrophilia found with MaR1 treatment.
Figure 8. ICAM-1 expression in airway epithelial cells following single or repetitive DE exposures in mice receiving pretreatment with MaR1.
In single DE exposure studies, mice were pretreated with 0 or 1 ng MaR1 given i.p. at 18 hrs and 30 minutes prior to a single intranasal dose of DE. In repetitive DE exposure studies, mice were pretreated with 0 or 10 ng MaR1 given i.p. 30 minutes prior to an intranasal dose of DE, given daily for 15 consecutive weekdays. At five hours following final DE exposure, lungs were formalin-fixed and inflated for paraffin embedding and sectioning.
Immunohistochemistry was performed to assess ICAM-1 expression levels in airway epithelial cells following DE treatment with or without MaR1 pretreatment. Treatments: (A) single vehicle + saline, (B) single vehicle + DE, (C) single 1 ng 7(S)-MaR1 + DE, (D) repetitive vehicle + saline, (E) repetitive vehicle + DE, (F) repetitive 10 ng 7(S)-MaR1 + DE, (G) Secondary antibody only control. N ≥ 3 mouse lungs used in analyses per group. Arrows indicate representative points of staining (ICAM-1 expression).
Discussion
Through these studies, we have found a potential role for the SPM MaR1 in limiting the airway inflammatory consequences following organic dust exposures. Utilizing an animal model of organic dust exposure-induced lung disease, we have demonstrated that MaR1 treatment significantly reduces the pro-inflammatory cytokine/chemokine release associated with single and repetitive exposures to DE. The findings of these investigations are summarized in Supplementary Summary Table I. In our studies, i.p. administration of MaR1 resulted in significantly reduced neutrophil influx following both a one-time and repetitive DE exposure. Furthermore, DE-induced ICAM-1 expression was reduced in the epithelium of the airways with MaR1 treatment. Collectively, these data suggest SPM such as MaR1 may hold potential for limiting the adverse effects of acute and repetitive organic dust-induced airway inflammation.
Recent studies in the bioactive lipids field have revealed a role for the SPM derivatives of omega-3 fatty acids in resolving airway inflammation. Interestingly, it has been reported that diets high in poly-unsaturated fatty acids may significantly decrease mortality risk while improving clinical outcomes in patients experiencing acute lung injury or acute respiratory distress syndrome (37). We have recently demonstrated that treatment with the omega-3 fatty acid docosahexaenoic acid for one week prior to a single DE exposure significantly reduced neutrophil influx and pro-inflammatory cytokine production (38). Yet, no previous studies have reported a role for SPM in organic dust-induced inflammation or identified the specific effects that MaR1 may influence during these lung inflammatory processes. MaR1 is has been shown to be produced by neutrophil-platelet aggregates particularly during early stages of an inflammatory response, as well as by macrophages that are activated at sites of inflammation (28, 30). Neutrophil influx is a key marker of DE-induced airway and inflammation, and we have shown macrophages to be important during the resolution of organic dust-mediated effects (39). Additionally, our studies using a human bronchial epithelial cell line (BEAS-2B) and precision-cut mouse lung slices revealed MaR1 also acts on bronchial epithelial cells to limit pro-inflammatory responses to DE (31). These studies together indicate the potential utility of this dietary lipid derivative in modulating lung inflammation, while illustrating the promise of using SPM such as MaR1 as treatments for lung inflammatory disease.
Our findings indicate that MaR1 pretreatment effectively reduces the DE-induced pro-inflammatory cytokine/chemokine release seen with single and repetitive exposures to DE. Along with altering cytokine/chemokine production, MaR1 pretreatment correlated with blunted epithelial cell ICAM-1 expression and a moderate reduction in neutrophil influx into the airways in both our single and repetitive DE exposure models. These results corroborate with results found in murine models of peritonitis, colitis, and lipopolysaccharide-induced acute lung injury where MaR1 treatment reduced neutrophil influx and ICAM-1 expression (28, 29, 35, 40). The induction of ICAM-1 expression on airway epithelial cells during inflammatory insults is important for neutrophil functioning within the inflammatory lung environment (41, 42). The airway epithelium serves as a first responder during DE exposure by releasing pro-inflammatory cytokines and chemokines to recruit neutrophils into the airway while upregulating ICAM-1 on their surfaces to facilitate neutrophil target cell interaction (32, 41). Our results indicate that MaR1 is acting at multiple levels to affect this neutrophil functioning, by limiting neutrophil chemoattractants CXCL1 and/or CXCL2, reducing neutrophil influx, and lowering DE-stimulated epithelial cell ICAM-1 surface expression. These actions facilitated a reduction in the inflammatory environment of the lung and may be important measures in the prevention of adverse consequences associated with organic dust exposures.
While MaR1 appears to have significant effects on the neutrophil-mediated components of DE-induced airway inflammation, MaR1 did not have a significant effect on the peribronchiolar/perivascular inflammation or lymphoid aggregates that characterize the histopathology associated with our repetitive exposure model (13). MaR1 may not be dramatically affecting the recruitment and activation of lymphocytes associated with these inflammatory responses. We did however see a modest but significant increase in lymphocyte influx in the BALF of mice receiving 7(R)-MaR1 treatment along with repetitive DE exposure (Figure 5C). Investigations relating to the role of MaR1 in lymphocyte functioning during inflammatory responses is thus warranted. While MaR1 has been shown to affect neutrophils as well as macrophage phenotype and functioning during inflammation (26, 40), to our knowledge there is no documentation of MaR1 directly affecting lymphocyte response. Future studies investigating the role of MaR1 in these cell types will be valuable for deducing the role of this lipid mediator in modulating inflammation resolution.
A major feature of repetitive DE-induced airway inflammation is the presence of marked alveolar and bronchiolar inflammation along with the presence of lymphoid aggregates. MaR1 treatment during repetitive DE exposures did not significantly alter any of these histopathological features, even after increasing the dose of MaR1 given during the repetitive exposure studies up to 200 ng daily (data not shown). While we did not see significant changes in repetitive DE-induced histopathology with MaR1 treatment, MaR1 may be more effective in accelerating the resolution phase following cessation of the DE exposures rather than limiting the persistent inflammation associated with ongoing repetitive exposures. Macrophages appear to be vital for regulating and limiting the exaggerated inflammatory response associated with repetitive DE exposures (39), and MaR1 has been shown to induce an M2 macrophage phenotype that is important for inflammation resolution (26, 40). It is therefore plausible that MaR1 may act at the macrophage level following repetitive DE exposures to facilitate resolution. Future studies investigating this hypothesis may be warranted to provide insight into the role of MaR1 in the resolution of airway inflammation.
To further understand how MaR1 modulates airway inflammation during DE treatment, we examined the importance of administration route of MaR1 prior to i.n. DE treatment in our single DE exposure murine model. In one experimental design, MaR1 was given i.p. at 18 hrs and 30 minutes prior to DE, while in a second design mice received MaR1 via i.n. instillation. An interesting difference in MaR1 administration route involved the mediator’s efficacy in reducing the murine IL-8 cognates CXCL1 and CXCL2. In single exposure studies, mice pretreated with MaR1 via i.p. administration, CXCL1 levels were decreased following DE exposure without impacting CXCL2, while mice pretreated with MaR1 via i.n. instillation did show significantly decreased CXCL2 (and CXCL1 levels were not significantly higher than saline controls). Thus, route of MaR1 administration may be important for elucidating the mediator’s full range of effects in the single exposure scenario. While CXCL1 and CXCL2 bind CXCR2 and are important neutrophil chemoattractants that are thought to have somewhat redundant affects, recently published data suggests these chemokines have different roles in regulating neutrophil recruitment, with both chemokines operating in the same pathway or event sequence (43). Reducing only one of these two neutrophil chemoattractants may thus still significantly alter the ability of neutrophils to migrate to the lungs.
The SPM MaR1 can be synthesized as two separate isomer formations, 7(S)-MaR1 and 7(R)-MaR1, with 7(R)-MaR1 having been confirmed to be endogenously produced by macrophages in vivo during the resolution of murine peritonitis (28, 35). Because the 7(S)- and 7(R)- isomers of MaR1 have been shown to give differential effects (35), we assessed for selective effects with the two separate isomers of MaR1. In our model of acute DE exposure, we found that 7(S)-MaR1 pre-treatment resulted in decreased neutrophil recruitment to the lungs when given either i.p. or i.n. as compared to 7(R)-MaR1, while both isomers were similarly effective at reducing BALF pro-inflammatory cytokine levels and airway epithelial cell ICAM-1 expression. Although, when we assessed MaR1 isoform differences in our repetitive exposure model, we found that at the 10 ng MaR1 dose, the 7(S)- and 7(R)-MaR1 isomers had similar efficacies in reducing neutrophil infiltration and pro-inflammatory cytokine release. These data suggest isomer-specific actions of MaR1 are present in the lungs particularly during acute inflammatory responses to DE. Of note, we did not investigate how utilizing a combined 7(S)- and 7(R)-MaR1 therapy might alter DE-induced airway inflammation, although these investigations may be useful in better understanding MaR1 mechanisms of action.
With regards to mechanisms of action, other SPM have been found to bind and signal through G protein-coupled receptors to propagate their anti-inflammatory, pro-resolving effects (44). While there has been no specific receptor identified for MaR1 as of yet, it is highly plausible that MaR1 acts in a similar manner. Thus, the differing efficacies of 7(S)- and 7(R)-MaR1 may be due to different binding efficiencies or characteristics of their interactions with cell surface receptors. Furthermore, certain SPM have been shown to bind to more than one receptor (44), and biphasic responses/efficacies were found in our own investigations (data not shown), as well as in other reported studies (45). Thus, it is possible that different binding efficacies at multiple receptors could explain some of the isomer-specific or biphasic efficacies that are seen.
Taken together, these findings identify the potential of pro-resolving mediators such as MaR1 in limiting airway inflammation associated with acute and repetitive exposures to agricultural dusts. Pretreatment of MaR1 prior to DE exposure reduced lung neutrophil influx and pro-inflammatory cytokine production, while reducing ICAM-1 expression in airway epithelial cells. However, MaR1 did not impact the histopathological markers of chronic inflammation associated with repetitive DE exposure. These findings are highly relevant, because acute and repetitive exposures to organic dusts lead to harmful inflammatory cascades resulting in chronic disease; novel preventative and therapeutic modalities for treating these diseases are required. Clinical investigations indicate that modest decreases (of less than 50%) of certain cytokine levels in BALF/sputum correlate with disease severity and/or risk of death in diseases like chronic obstructive pulmonary disease and acute respiratory distress syndrome (46, 47). Thus, while MaR1 did not completely abrogate the lung inflammatory response to DE, the decreased inflammatory mediator production and neutrophil influx seen in our studies is likely to be biologically significant. While MaR1 did not alter the histologic measures of inflammation and lymphoid aggregate formation following repetitive DE exposure, its anti-inflammatory effect on BALF cytokine levels and neutrophil influx suggest it may be beneficial in an adjunctive therapeutic capacity. Furthermore, while MaR1 did not alter the histological features of repetitive DE-induced lung inflammation, it is possible that other SPM may better target this aspect of the airway inflammatory response to DE. Thus, future investigations are warranted to further understand the role of MaR1 and other SPM during acute and chronic organic dust exposures and how these lipid mediators might be exploited for use in treatment strategies for airway disease resulting from agricultural dust exposures.
Supplementary Material
Supplemental Summary Table I. Summary of single and repetitive DE exposure model +/− MaR1 results. In vehicle column, arrows indicate significant changes compared to vehicle + saline group. In 7(S)-MaR1 and 7(R)-MaR1 columns, arrows indicate significant changes compared to vehicle + saline group. n.s.d.: no significant difference.
Background: Agricultural organic dust exposures trigger harmful airway inflammation, and agriculture workers are at risk for developing debilitating airway inflammatory diseases. Omega-3 fatty acid-derived specialized pro-resolving lipid mediators like maresin-1 promote the endogenous resolution of inflammation. These attributes make lipid mediators like maresin-1 attractive for use in preventing and treating inflammatory lung diseases.
Translational Significance: Our findings indicate a role for maresin-1 in reducing the severity of airway inflammation caused by organic dust exposures. These findings may contribute to novel strategies for preventing and treating airway inflammatory diseases in agriculture workers.
Acknowledgments
These investigations were supported by funding from R010H008539 to DJR, F32ES022913 to TMN, R01ES0193525 to JAP, R01OH010162 to TAW, and the Central States Center for Agricultural Safety and Health (U54OH010162). All authors have read the journal’s authorship agreement and reviewed/edited and approved the manuscript.
List of Abbreviations
- SPM
specialized pro-resolving lipid mediator
- MaR1
maresin-1
- ICAM-1
intracellular adhesion molecule-1
- DE
extracts of organic dust
- CAFO
concentrated animal feeding operation
- PKC
protein kinase C
- BALF
bronchoalveolar lavage fluid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have read the journal’s authorship agreement and policy on disclosure of potential conflicts of interest; there are no conflicts of interest to report.
References
- 1.Respiratory health hazards in agriculture. Am J Respir Crit Care Med. 1998;158(5 Pt 2):S1–S76. doi: 10.1164/ajrccm.158.supplement_1.rccm1585s1. [DOI] [PubMed] [Google Scholar]
- 2.Von Essen S, Donham K. Illness and injury in animal confinement workers. Occup Med. 1999;14(2):337–350. [PubMed] [Google Scholar]
- 3.Kirkhorn SR, Garry VF. Agricultural lung diseases. Environ Health Perspect. 2000;108(Suppl 4):705–712. doi: 10.1289/ehp.00108s4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eduard W, Pearce N, Douwes J. Chronic bronchitis, COPD, and lung function in farmers: the role of biological agents. Chest. 2009;136(3):716–725. doi: 10.1378/chest.08-2192. [DOI] [PubMed] [Google Scholar]
- 5.Langley RL. Consequences of respiratory exposures in the farm environment. N C Med J. 2011;72(6):477–480. [PubMed] [Google Scholar]
- 6.Iversen M, Dahl R. Working in swine-confinement buildings causes an accelerated decline in FEV1: a 7-yr follow-up of Danish farmers. Eur Respir J. 2000;16(3):404–408. doi: 10.1034/j.1399-3003.2000.016003404.x. [DOI] [PubMed] [Google Scholar]
- 7.Monso E, Riu E, Radon K, et al. Chronic obstructive pulmonary disease in never-smoking animal farmers working inside confinement buildings. Am J Ind Med. 2004;46(4):357–362. doi: 10.1002/ajim.20077. [DOI] [PubMed] [Google Scholar]
- 8.Szczyrek M, Krawczyk P, Milanowski J, Jastrzebska I, Zwolak A, Daniluk J. Chronic obstructive pulmonary disease in farmers and agricultural workers - an overview. Ann Agric Environ Med. 2011;18(2):310–313. [PubMed] [Google Scholar]
- 9.Wang Z, Larsson K, Palmberg L, Malmberg P, Larsson P, Larsson L. Inhalation of swine dust induces cytokine release in the upper and lower airways. Eur Respir J. 1997;10(2):381–387. doi: 10.1183/09031936.97.10020381. [DOI] [PubMed] [Google Scholar]
- 10.Deetz DC, Jagielo PJ, Quinn TJ, Thorne PS, Bleuer SA, Schwartz DA. The kinetics of grain dust-induced inflammation of the lower respiratory tract. Am J Respir Crit Care Med. 1997;155(1):254–259. doi: 10.1164/ajrccm.155.1.9001321. [DOI] [PubMed] [Google Scholar]
- 11.Von Essen S, Romberger D. The respiratory inflammatory response to the swine confinement building environment: the adaptation to respiratory exposures in the chronically exposed worker. J Agric Saf Health. 2003;9(3):185–196. doi: 10.13031/2013.13684. [DOI] [PubMed] [Google Scholar]
- 12.Palmberg L, Larssson BM, Malmberg P, Larsson K. Airway responses of healthy farmers and nonfarmers to exposure in a swine confinement building. Scand J Work Environ Health. 2002;28(4):256–263. doi: 10.5271/sjweh.673. [DOI] [PubMed] [Google Scholar]
- 13.Poole JA, Wyatt TA, Oldenburg PJ, et al. Intranasal organic dust exposure-induced airway adaptation response marked by persistent lung inflammation and pathology in mice. Am J Physiol Lung Cell Mol Physiol. 2009;296(6):L1085–95. doi: 10.1152/ajplung.90622.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell DC, Schenker MB. Protection against breathing dust: behavior over time in Californian farmers. J Agric Saf Health. 2008;14(2):189–203. doi: 10.13031/2013.24350. [DOI] [PubMed] [Google Scholar]
- 15.Singer P, Shapiro H, Theilla M, Anbar R, Singer J, Cohen J. Anti-inflammatory properties of omega-3 fatty acids in critical illness: novel mechanisms and an integrative perspective. Intensive Care Med. 2008;34(9):1580–1592. doi: 10.1007/s00134-008-1142-4. [DOI] [PubMed] [Google Scholar]
- 16.Deckelbaum RJ, Torrejon C. The omega-3 fatty acid nutritional landscape: health benefits and sources. J Nutr. 2012;142(3):587S–591S. doi: 10.3945/jn.111.148080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Lorgeril M, Salen P. New insights into the health effects of dietary saturated and omega-6 and omega-3 polyunsaturated fatty acids. BMC Med. 2012;10 doi: 10.1186/1741-7015-10-50. 50-7015-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campoy C, Escolano-Margarit MV, Anjos T, Szajewska H, Uauy R. Omega 3 fatty acids on child growth, visual acuity and neurodevelopment. Br J Nutr. 2012;107(Suppl 2):S85–106. doi: 10.1017/S0007114512001493. [DOI] [PubMed] [Google Scholar]
- 19.Baum SJ, Kris-Etherton PM, Willett WC, et al. Fatty acids in cardiovascular health and disease: a comprehensive update. J Clin Lipidol. 2012;6(3):216–234. doi: 10.1016/j.jacl.2012.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji RR, Xu ZZ, Strichartz G, Serhan CN. Emerging roles of resolvins in the resolution of inflammation and pain. Trends Neurosci. 2011;34(11):599–609. doi: 10.1016/j.tins.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwab JM, Serhan CN. Lipoxins and new lipid mediators in the resolution of inflammation. Curr Opin Pharmacol. 2006;6(4):414–420. doi: 10.1016/j.coph.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Serhan CN. Controlling the resolution of acute inflammation: a new genus of dual anti-inflammatory and proresolving mediators. J Periodontol. 2008;79(8 Suppl):1520–1526. doi: 10.1902/jop.2008.080231. [DOI] [PubMed] [Google Scholar]
- 23.Weylandt KH, Chiu CY, Gomolka B, Waechter SF, Wiedenmann B. Omega-3 fatty acids and their lipid mediators: towards an understanding of resolvin and protectin formation. Prostaglandins Other Lipid Mediat. 2012;97(3-4):73–82. doi: 10.1016/j.prostaglandins.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Seki H, Fukunaga K, Arita M, et al. The anti-inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injury. J Immunol. 2010;184(2):836–843. doi: 10.4049/jimmunol.0901809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aoki H, Hisada T, Ishizuka T, et al. Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochem Biophys Res Commun. 2008;367(2):509–515. doi: 10.1016/j.bbrc.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Hsiao HM, Sapinoro RE, Thatcher TH, et al. A novel anti-inflammatory and pro-resolving role for resolvin d1 in acute cigarette smoke-induced lung inflammation. PLoS One. 2013;8(3):e58258. doi: 10.1371/journal.pone.0058258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao Z, Dong J, Wu W, et al. Resolvin D1 attenuates inflammation in lipopolysaccharide-induced acute lung injury through a process involving the PPARgamma/NF-kappaB pathway. Respir Res. 2012;13:110. doi: 10.1186/1465-9921-13-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serhan CN, Yang R, Martinod K, et al. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med. 2009;206(1):15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gong J, Wu ZY, Qi H, et al. Maresin 1 mitigates lipopolysaccharide-induced acute lung injury in mice. Br J Pharmacol. 2014 doi: 10.1111/bph.12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdulnour RE, Dalli J, Colby JK, et al. Maresin 1 biosynthesis during platelet-neutrophil interactions is organ-protective. Proc Natl Acad Sci U S A. 2014;111(46):16526–16531. doi: 10.1073/pnas.1407123111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nordgren TM, Heires AJ, Wyatt TA, et al. Maresin-1 reduces the pro-inflammatory response of bronchial epithelial cells to organic dust. Respir Res. 2013;14(1) doi: 10.1186/1465-9921-14-51. 51-9921-14-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romberger DJ, Bodlak V, Von Essen SG, Mathisen T, Wyatt TA. Hog barn dust extract stimulates IL-8 and IL-6 release in human bronchial epithelial cells via PKC activation. J Appl Physiol. 2002;93(1):289–296. doi: 10.1152/japplphysiol.00815.2001. [DOI] [PubMed] [Google Scholar]
- 33.Poole JA, Alexis NE, Parks C, et al. Repetitive organic dust exposure in vitro impairs macrophage differentiation and function. J Allergy Clin Immunol. 2008;122(2):375–82. 382, e1–4. doi: 10.1016/j.jaci.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poole JA, Gleason AM, Bauer C, et al. αβ T cells and a mixed Th1/ Th17 response are important in organic dust-induced airway disease. Ann Allergy Asthma Immunol. 2012 doi: 10.1016/j.anai.2012.06.015. http://dx.doi.org/10.1016/j.anai.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serhan CN, Dalli J, Karamnov S, et al. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012;26(4):1755–1765. doi: 10.1096/fj.11-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mathisen T, Von Essen SG, Wyatt TA, Romberger DJ. Hog barn dust extract augments lymphocyte adhesion to human airway epithelial cells. J Appl Physiol. 2004;96(5):1738–1744. doi: 10.1152/japplphysiol.00384.2003. [DOI] [PubMed] [Google Scholar]
- 37.Pontes-Arruda A, Demichele S, Seth A, Singer P. The use of an inflammation-modulating diet in patients with acute lung injury or acute respiratory distress syndrome: a meta-analysis of outcome data. JPEN J Parenter Enteral Nutr. 2008;32(6):596–605. doi: 10.1177/0148607108324203. [DOI] [PubMed] [Google Scholar]
- 38.Nordgren TM, Friemel TD, Heires AJ, Poole JA, Wyatt TA, Romberger DJ. The omega-3 Fatty Acid docosahexaenoic Acid attenuates organic dust-induced airway inflammation. Nutrients. 2014;6(12):5434–5452. doi: 10.3390/nu6125434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poole JA, Gleason AM, Bauer C, et al. CD11c+/CD11b+ Cells are Critical for Organic Dust-Elicited Murine Lung Inflammation. Am J Respir Cell Mol Biol. 2012 doi: 10.1165/rcmb.2012-0095OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marcon R, Bento AF, Dutra RC, Bicca MA, Leite DF, Calixto JB. Maresin 1, a proresolving lipid mediator derived from omega-3 polyunsaturated fatty acids, exerts protective actions in murine models of colitis. J Immunol. 2013;191(8):4288–4298. doi: 10.4049/jimmunol.1202743. [DOI] [PubMed] [Google Scholar]
- 41.Tosi MF, Stark JM, Smith CW, Hamedani A, Gruenert DC, Infeld MD. Induction of ICAM-1 expression on human airway epithelial cells by inflammatory cytokines: effects on neutrophil-epithelial cell adhesion. Am J Respir Cell Mol Biol. 1992;7(2):214–221. doi: 10.1165/ajrcmb/7.2.214. [DOI] [PubMed] [Google Scholar]
- 42.Bloemen PG, van den Tweel MC, Henricks PA, et al. Expression and modulation of adhesion molecules on human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1993;9(6):586–593. doi: 10.1165/ajrcmb/9.6.586. [DOI] [PubMed] [Google Scholar]
- 43.De Filippo K, Dudeck A, Hasenberg M, et al. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood. 2013;121(24):4930–4937. doi: 10.1182/blood-2013-02-486217. [DOI] [PubMed] [Google Scholar]
- 44.Serhan CN, Krishnamoorthy S, Recchiuti A, Chiang N. Novel anti-inflammatory--pro-resolving mediators and their receptors. Curr Top Med Chem. 2011;11(6):629–647. doi: 10.2174/1568026611109060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rogerio AP, Haworth O, Croze R, et al. Resolvin D1 and aspirin-triggered resolvin D1 promote resolution of allergic airways responses. J Immunol. 2012;189(4):1983–1991. doi: 10.4049/jimmunol.1101665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goodman RB, Strieter RM, Martin DP, et al. Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;154(3 Pt 1):602–611. doi: 10.1164/ajrccm.154.3.8810593. [DOI] [PubMed] [Google Scholar]
- 47.Hacievliyagil SS, Gunen H, Mutlu LC, Karabulut AB, Temel I. Association between cytokines in induced sputum and severity of chronic obstructive pulmonary disease. Respir Med. 2006;100(5):846–854. doi: 10.1016/j.rmed.2005.08.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Summary Table I. Summary of single and repetitive DE exposure model +/− MaR1 results. In vehicle column, arrows indicate significant changes compared to vehicle + saline group. In 7(S)-MaR1 and 7(R)-MaR1 columns, arrows indicate significant changes compared to vehicle + saline group. n.s.d.: no significant difference.