Abstract
Objectives
Studies on ethnic disparities in glycaemic control have been contradictory, and compromised by excessively broad categories of ethnicity and inadequate adjustment for socioeconomic differences. We aimed to study the effect of ethnicity on glycaemic control in a large cohort of patients with type 2 diabetes.
Setting
We used nationwide data (mainly from primary care) from the Swedish National Diabetes Register (2002–2011) to identify patients with newly diagnosed (within 12 months) type 2 diabetes.
Participants
We included 131 935 patients (with 713 495 appointments), representing 10 ethnic groups, who were followed up to 10 years.
Primary and secondary outcome measures
Progress of glycated haemoglobin (HbA1c) for up to 10 years was examined. Mixed models were used to correlate ethnicity with HbA1c (mmol/mol). The effect of glycaemic disparities was examined by assessing the risk of developing albuminuria. The impact of ethnicity was compared to that of income, education and physical activity.
Results
Immigrants, particularly those of non-Western origin, received glucose-lowering therapy earlier, had 30% more appointments but displayed poorer glycaemic control (2–5 mmol/mol higher HbA1c than native Swedes). Probability of therapy failure was 28–111% higher for non-Western groups than for native Swedes. High-income Western groups remained below the target-level of HbA1c for 4–5 years, whereas non-Western populations never reached the target level. These disparities translated into 51–92% higher risk of developing albuminuria. The impact of ethnicity was greater than the effect of income and education, and equal to the effect of physical activity.
Conclusions
Despite earlier pharmacological treatment and more frequent appointments, immigrants of non-Western origin display poorer glycaemic control and this is mirrored in a higher risk of developing albuminuria.
Keywords: DIABETES & ENDOCRINOLOGY, EPIDEMIOLOGY
Strengths and limitations of this study.
To our knowledge, this is the largest study on ethnic differences in progress of glycaemic control. We included 131 935 patients (with 713 495 appointments) who were followed up to 10 years.
All major ethnic groups in the world were represented in this study cohort and they had fully equal access to, and use of, healthcare, regardless of ethnic and socioeconomic background. Indeed, we report that immigrants had higher consumption of healthcare. This contrasts against previous studies, which have been hampered by inappropriately broad categories of ethnicity, cross-sectional design, small samples, short follow-up and unequal access to or consumption of healthcare.
The longitudinal design, with at least annually updated information on all time-varying variables, allows for reliable estimates of the effect of ethnicity. We could control adequately for socioeconomic, demographic and health-related confounders.
The main findings (immigrants, particularly those of non-Western origin, display poor glycaemic control and have a considerably higher risk of developing albuminuria) indicate that much can be done to improve diabetes care for a large proportion of the diabetic population in Western countries.
When extrapolating our results, the fact that these ethnic differences could be expected in an equitable healthcare system needs to be taken into account. This should be taken into consideration in countries where access to healthcare is not equitable.
Introduction
Tight glucose control in type 2 diabetes has shown long-term beneficial effects on microvascular complications, cardiovascular disease and mortality.1–5 Studies of ethnic disparities in glycaemic control have been contradictory.6–9 They have, however, been hampered by inappropriately broad categories of ethnicity, cross-sectional design, small samples, short follow-up and unequal access to—or consumption of—healthcare. There are no reliable estimates of the true effect of ethnicity on glycaemic control.
This issue is important in Western societies, which are becoming more ethnically diverse due to accelerated migration from other areas of the world.10 The ethnic admixture of Western societies is currently far more diverse than the risk estimate tools of clinicians are prepared to handle. Immigrants may be at particular risk due to genetic susceptibility to insulin resistance,11 12 difficult transitional phases, and rapid changes in diet and lifestyle,13 as well as linguistic, cultural and financial barriers to obtaining proper healthcare.14 15
Sweden is an ethnically heterogeneous country in which all inhabitants enjoy access to every level of healthcare at a minimal cost.16 Immigrants are targeted in an effort to promote healthy lifestyles and consumption of healthcare.17 18 The great majority of Swedes with type 2 diabetes are included in the Swedish National Diabetes Register, which we used to analyse the impact of ethnicity on the progress of glycaemic control and on albuminuria as a marker for diabetic complications.
Methods
Data sources
Swedish authorities manage several nationwide registers, which may be linked through the unique personal identification number assigned to every Swede. The National Diabetes Register (NDR) has been described previously.19 It was launched in 1996 as a caregiver tool for local quality assurance purposes and as a feed-back tool in diabetes care.20 Data provided by nurses and physicians trained in register procedures, are obtained at visits to outpatient clinics of hospitals and primary care clinics. Clinical information and various measurements are updated at least once a year.
Patients with at least one entry in the NDR from 1 January 2002 to 31 December 2011 were included if they had been reported within 12 months of the date of diagnosis. Ninety-six per cent of the subjects had been diagnosed with type 2 diabetes on the basis of a clinical assessment. The remainder were included on the basis of the following definition: age 40 or older at the time of diagnosis and treated either with diet only, diet combined with oral hypoglycaemic agents (OHA), or a combination of OHA combined and insulin. This definition has been validated and used previously.21–23
Measures
Data on annual income in Swedish kronor, highest educational level regardless of country of domicile and country of birth (used as proxy for ethnicity/race), were obtained from the Longitudinal Integration Database for Health Insurance and Labour Market Studies, which is an official database administered by the Swedish National Board of Health and Welfare. Educational level was stratified into lower (9 years or less—the length of compulsory education in Sweden), intermediate (10–12 years—upper secondary) and higher (college or university). Income was stratified into quintiles (Q1 to Q5), with the highest (Q5) being the reference. Ethnic categories were based on an appraisal of ancestry and geography,24 with the exception of the fact that Nordic countries were classified separately in the present study for the purpose of examining whether immigrants from neighbouring countries also exhibited differences. Native Swedish patients served as the reference group. Sixty-two individuals were excluded because information about their country of birth was unavailable.
Glycaemic control was measured as HbA1c. Analyses were quality-assured nationwide by regular calibration with the HPLC Mono-S method, and then converted to mmol/mol (International Federation of Clinical Chemistry (IFCC)).25 Microalbuminuria was defined as two positive results for three urine samples obtained within 1 year, with positivity defined as an albumin:creatinine ratio of 3 to 30 mg/mmol (approximately 30–300 mg/g) or a urinary albumin clearance of 20–200 μg/min (20–300 mg/L). Macroalbuminuria was defined as an albumin:creatinine ratio of more than 30 mg/mmol (close to 300 or more mg/g) or a urinary albumin clearance of more than 200 μg/min (>300 mg/L). Body mass index (BMI) was calculated as weight in kilograms divided by height in metres squared. Systolic blood pressure was the mean value of two supine readings (Korotkoff 1–5) with a cuff of appropriate size and after at least 5 min of rest. Low-density lipoprotein, high-density lipoprotein and total cholesterol were measured in mmol/L. Physical activity was rated from 1 (never) to 5 (daily). Smoking was coded as present if the patient currently smoked. Use of lipid lowering medications was dichotomised. Glucose-lowering treatment was categorised as diet and lifestyle modifications, oral hypoglycaemic agents (OHAs), insulin or insulin and OHAs. Antihypertensive medication was dichotomised. Estimated-glomerular filtration rate (eGFR) was calculated by the Modification of Diet in Renal Disease equation.26 A history of cardiovascular disease (CVD) was defined as previous hospitalisation due to acute myocardial infarction or stroke (International Classification of Diseases (ICD)-10, I21, I60–I69; ICD-9, 410, 430–434, 436–438) according to the Swedish National Discharge Register, which is a reliable and validated method.27 28 All time-varying variables are updated in the NDR following each appointment.
Analyses and statistics
Baseline
The first observation was used to present characteristics at time of diagnosis (within 12 months). Continuous variables are reported as means, percentages and quintiles. T tests were used for continuous variables with native Swedes as the reference group. χ2 Tests were used for categorical variables. A p value <0.01 was considered statistically significant but multiple testing should be considered when interpreting the results.
Glycaemic control
Progress of glycaemic control was calculated as an unadjusted annual mean in relation to ethnicity. Adjusted figures were calculated using linear regression, to estimate differences in mmol/mol. Logistic regression was used to estimate the probability of achieving glycaemic control (ie, reaching target-level HbA1c <52 mmol/mol) during the second year after diagnosis.
To examine whether hypothesised differences in glycaemia are reflected on the risk of complications, we calculated the probability of developing albuminuria during the second year after diagnosis.
Mixed-effect models were used to account for repeated measurements on the same unit.29–31 HbA1c was centred around the mean.
Kaplan–Meier calculations were used to examine whether time to pharmacological treatment differed among ethnic groups. This was carried out to examine whether differences in HbA1c could be explained by disparities in use of medications.
Analyses were performed with SAS V.9.4 (SAS Institute, USA) and R (R Foundation for Statistical Computing).
Results
We included 131 935 individuals with newly diagnosed type 2 diabetes. A total of 713 495 appointments were registered. All major ethnic groups were represented in the study (see online supplementary figure A). A total of 82.7% of the study population consisted of native Swedes. Immigrants had more appointments per year. Non-Western immigrants in particular had almost 30% more appointments (table 1).
Table 1.
Characteristics of 131 935 individuals with newly diagnosed type 2 diabetes by ethnicity/race
| All | Sweden | Nordic countries | Europe (high income), North America and Oceania | Mediterranean Basin | Europe (low income), Russia and Central Asia | Middle East and North Africa | Sub-Saharan Africa | South Asia | East Asia | Latin America and Caribbean | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | 131 935 | 109 058 (82.66) | 6760 (5.12) | 3306 (2.51) | 511 (0.39) | 3870 (2.93) | 5031 (3.81) | 1127 (0.85) | 610 (0.46) | 802 (0.61) | 860 (0.65) |
| Males | 57 (75 083) | 57 (62 526) | 51 (3479) | 53 (1751) | 70 (360) | 54 (2075) | 62 (3142) | 59 (663)* | 56 (344)* | 37 (298) | 52 (445) |
| Age (years) | 62.8 (12.5) | 63.7 (12.2) | 63.8 (10.5)* | 64.4 (11.8) | 62.5 (10.6)* | 57.6 (11.7) | 52.3 (11.2) | 47.3 (11.2) | 46.0 (11.3) | 50.0 (12.8) | 54.5 (12.0) |
| Annual visits | 1.73 (2.14) | 1.67 (2.0) | 1.80 (2.22) | 1.96 (2.70) | 2.20 (3.24) | 2.05 (2.70) | 2.30 (3.34) | 2.22 (3.42) | 2.32 (3.40) | 1.91 (2.70) | 2.50 (3.20) |
| BMI (kg/m2) | 30.4 (5.5) | 30.3 (5.5) | 31.2 (5.6) | 30.5 (5.3) | 30.1 (4.8)* | 31.3 (5.3) | 30.9 (5.2) | 28.8 (5.4) | 28.6 (5.2) | 27.3 (4.3) | 31.4 (5.5) |
| Waist circumference (cm) | 104.7 (13.4) | 105.2 (13.5) | 106.3 (13.5) | 104.2 (12.9) | 104.4 (11.8) | 105.0 (11.9) | 103.6 (11.5) | 100.2 (11.4) | 97.8 (11.8) | 92.4 (11.2) | 104.3 (11.7) |
| HbA1c (mmol/mol) | 52.2 (15.4) | 51.9 (15.1) | 52.3 (15.2)* | 52.2 (14.7)* | 52.8 (15.2)* | 54.3 (16.8) | 54.9 (17.2) | 58.0 (19.8) | 55.8 (16.7) | 55.9 (17.1) | 56.7 (19.8) |
| Systolic BP (mm Hg) | 137.5 (17.2) | 138.1 (17.1) | 139.5 (17.4) | 138.2 (17.4)* | 134.1 (16.2) | 135.2 (18.2) | 129.3 (16.4) | 126.9 (16.4) | 124.8 (14.4) | 126.7 (15.9) | 130.8 (15.4) |
| Chol/HDL ratio | 4.5 (1.5) | 4.4 (1.5) | 4.3 (1.5) | 4.5 (1.6)* | 4.6 (1.4)* | 4.9 (1.7) | 4.8 (1.5) | 4.6 (1.5) | 4.8 (1.4) | 4.4 (1.3)* | 4.7 (1.5) |
| LDL/HDL ratio | 2.6 (1.1) | 2.6 (1.1) | 2.5 (1.1) | 2.6 (1.1)* | 2.8 (1.1) | 2.9 (1.2) | 2.9 (1.1) | 2.9 (1.1) | 2.9 (1.0) | 2.6 (1.0)* | 2.7 (1.1) |
| eGFR (mL/min) | 83.4 (25.2) | 81.8 (24.0) | 83.0 (22.3) | 81.2 (21.8)* | 87.2 (25.0) | 90.4 (37.4) | 99.9 (24.9) | 124.9 (39.1) | 100.0 (23.9) | 100.9 (28.0) | 96.2 (26.6) |
| Smoker | 15 (19 657) | 14 (15 173) | 20 (1341) | 17 (556)* | 19 (99) | 23 (888) | 22 (1098) | 13 (149)* | 13 (77)* | 15 (122)* | 18 (154) |
| No physical activity | 12 (15 973) | 12 (12 935) | 13 (907)* | 12 (390)* | 15 (76)* | 13 (497)* | 16 (803) | 11 (128)* | 13 (82)* | 8 (66) | 10 (89)* |
| Daily physical activity | 26 (33 983) | 26 (28 207) | 24 (1651)* | 25 (842)* | 27 (139)* | 31 (1186)* | 22 (1116) | 23 (260)* | 23 (142)* | 29 (233)* | 24 (207)* |
| Income and education | |||||||||||
| Income Q1 (lowest) | 20 (24 925) | 16 (16 872) | 23 (1439) | 27 (852) | 23 (113) | 41 (1538) | 55 (2745) | 47 (526) | 35 (212) | 42 (333) | 35 (295) |
| Income Q5 (highest) | 20 (24 928) | 22 (22 068) | 15 (928) | 14 (452) | 14 (69) | 11 (398) | 10 (485) | 15 (165) | 17 (101) | 14 (113) | 18 (149) |
| <9 years education | 39 (51 890) | 40 (43 495) | 44 (3005) | 25 (814) | 41 (211) | 35 (1366)* | 37 (1883) | 33 (376) | 24 (148) | 42 (335)* | 30 (257) |
| 10–12 years education | 43 (56 146) | 43 (47 370) | 40 (2734)* | 48 (1593) | 42 (216) | 39 (1516)* | 29 (1444) | 35 (399) | 44 (267)* | 30 (243) | 42 (364)* |
| University/college education | 17 (22 140) | 16 (17 811) | 12 (790) | 24 (793) | 13 (68) | 15 (576)* | 25 (1275) | 22 (243) | 29 (176) | 24 (193) | 25 (215) |
| Glucose-lowering treatment | |||||||||||
| Diet | 53 (70 445) | 54 (59 371) | 53 (3602) | 53 (1763)* | 49 (251)* | 48 (1854)* | 44 (2225) | 38 (429) | 42 (255) | 40 (320) | 44 (375) |
| OHA | 37 (49 261) | 36 (39 662) | 38 (2599)* | 38 (1240)* | 43 (219) | 43 (1653) | 47 (2352) | 48 (537) | 47 (288) | 45 (364) | 40 (347) * |
| Insulin | 5 (6199) | 5 (5249) | 4 (251)* | 4 (148)* | 4 (21)* | 4 (143)* | 3 (164) | 7 (84) | 5 (30)* | 7 (59) | 6 (50)* |
| Insulin+OHA | 4 (5457) | 4 (4318) | 4 (284) | 4 (136)* | 4 (18)* | 5 (202)* | 5 (260) | 6 (69) | 6 (35)* | 7 (55) | 9 (80) |
| Complications | |||||||||||
| Albuminuria | 14 (17 988) | 14 (14 845) | 14 (920)* | 14 (453)* | 17 (88)* | 13 (502)* | 14 (701)* | 14 (157)* | 12 (75)* | 13 (106)* | 16 (141) |
| Previous CVD | 20 (26 166) | 21 (22 373) | 23 (1555) | 23 (759) | 19 (98)* | 16 (610)* | 11 (547) | 4 (46) | 8 (47) | 6 (48) | 10 (83) |
Sweden is the reference group for ethnicity.
*No statistically significant difference at the 0.01 level.
BMI, body mass index; BP, blood pressure; CVD, cardiovascular disease; HbA1c, glycated haemoglobin; OHA, oral hypoglycaemic agents; eGFR, estimated-glomerular filtration rate; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Characteristics at the time of diagnosis
Table 1 presents characteristics at the time of diagnosis. The proportion of males varied from 37% (East Asia) to 70% (Mediterranean Basin). Patients from high-income Western countries were as much as 18 years older at the time of diagnosis. Particularly low age at the time of diagnosis was found for South Asia (46), East Asia (50) and Sub-Saharan Africa (47.3). BMI was 2–3 kg/m2 lower among non-Western groups. Native Swedish patients had the lowest mean HbA1c (51.9 mmol/mol) of all groups. Low-income and non-Western groups had HbA1c ranging from 54 to 58 mmol/mol. Systolic blood pressure was lower among non-Western groups. Lipid profiles were less favourable among non-Western groups. There were no major ethnic differences in terms of physical activity. Smoking rates were higher among patients from the Middle East, North Africa and low-income Europe. A history of CVD was more common among Western groups, but non-Western groups were 10–12 years younger at the onset of CVD. The overall prevalence of albuminuria was approximately 14%, and there were no noteworthy ethnic differences. College or university education was more common among non-Western populations, but they were generally in lower income quintiles.
Glycaemic control—crude figures
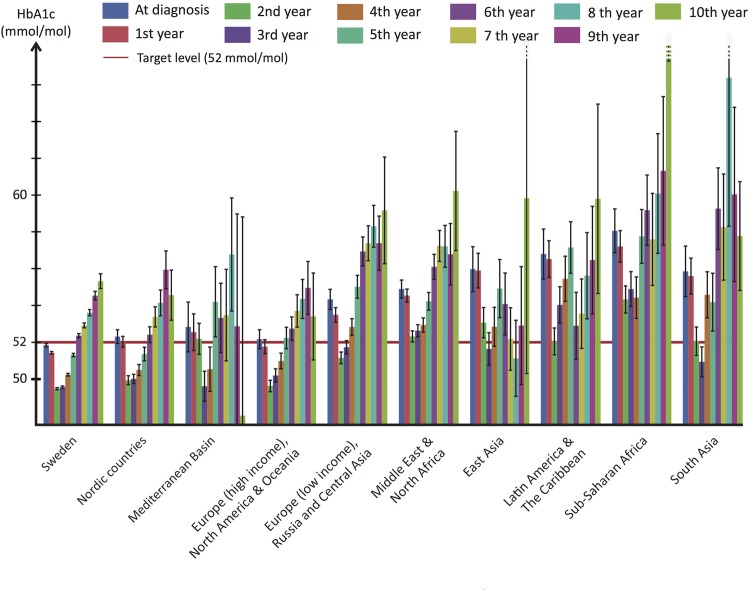
HbA1c declined during the first 1–3 years of follow-up and then increased for all ethnic groups (figure 1). However, there were conspicuous and consistent ethnic differences in HbA1c levels throughout the study. Swedish patients (the reference category) had the lowest mean HbA1c at every point in time while patients from high-income Western countries had only slightly higher levels. Patients from low-income European countries, Russia and Central Asia, as well as all non-Western populations, had substantially higher HbA1c levels throughout the study. On average, Swedish and other Nordic populations remained below the target level until the fifth year. Mediterranean Basin, high-income European countries, North American and Oceanic populations, remained below the target level until the fourth year, whereas low-income European countries, Russian and Central Asian populations remained below only until the second year. The remaining five ethnic groups did not reach the target level at any point.
Figure 1.
Progress of glycaemic control from time of diagnosis by ethnicity/race. Annual mean glycated haemoglobin from time of diagnosis by ethnicity/race. The red horizontal line in the background depicts the national target level (52 mmol/mol) set for type 2 diabetes.
Glycaemic control-adjusted figures
Since there was an interaction (p<0.0001) between ethnicity and glucose-lowering treatment, we stratified the analysis by type of treatment. Table 2 presents β coefficients (ie, predicted difference in mmol/mol HbA1c) by ethnicity. To convey a sense of the impact of ethnicity as compared to other factors, coefficients for physical activity are also presented.
Table 2.
Prediction of HbA1c (mmol/mol) by type of glucose-lowering treatment
| Diet and lifestyle | OHA | Insulin (±OHA) | |
|---|---|---|---|
| Ethnicity/race | |||
| Sweden | Reference | Reference | Reference |
| East Asia | 3.79 (2.59 to 5) | 1.84 (0.7 to 2.97) | 2.13 (−0.36 to 4.62) |
| Europe (high-income), North America and Oceania | 0.14 (−0.37 to 0.65) | 0.19 (−0.41 to 0.79) | 1.86 (0.42 to 3.3) |
| Europe (low-income), Russia and Central Asia | 1.58 (1.05 to 2.11) | 1.2 (0.66 to 1.74) | 1.89 (0.63 to 3.16) |
| Latin America and The Caribbean | 1.89 (0.76 to 3.02) | 2.42 (1.3 to 3.54) | 4.84 (2.6 to 7.07) |
| Mediterranean Basin | 0.57 (−0.74 to 1.88) | 0.61 (−0.84 to 2.05) | 1.17 (−2.7 to 5.04) |
| Middle East and North Africa | 1.85 (1.37 to 2.34) | 0.93 (0.46 to 1.41) | 2.79 (1.58 to 4.01) |
| Nordic countries | −0.01 (−0.36 to 0.35) | 0.19 (−0.22 to 0.6) | 0.81 (−0.19 to 1.8) |
| South Asia | 4.21 (2.85 to 5.56) | 1.93 (0.6 to 3.25) | 1.91 (−1.12 to 4.94) |
| Sub-Saharan Africa | 3.26 (2.22 to 4.3) | 3.61 (2.57 to 4.64) | 1.58 (−0.47 to 3.63) |
| Physical activity | |||
| Daily physical activity | Reference | Reference | Reference |
| 3–5 times/week | 0.07 (0.01 to 0.14) | 0.11 (0.01 to 0.22) | 0.19 (−0.06 to 0.45) |
| 1–2 times/week | 0.26 (0.19 to 0.34) | 0.51 (0.4 to 0.62) | 0.74 (0.46 to 1.01) |
| Less than once/week | 0.48 (0.39 to 0.57) | 1.03 (0.9 to 1.17) | 1.38 (1.06 to 1.7) |
| No physical activity | 0.76 (0.66 to 0.86) | 1.20 (1.06 to 1.35) | 1.32 (1.00 to 1.65) |
Figures are β coefficients (95% CI) that predict the change in HbA1c (mmol/mol).
The effect of physical activity is presented for comparison.
Example of interpretation: after accounting for included covariates, East Asian ethnicity predicts 3.79 mmol/mol higher HbA1c among persons on diet and lifestyle modifications.
Model adjustments: age, sex, age at onset of diabetes, duration of diabetes, quadratic effect of duration of diabetes, BMI, smoking status, history of cardiovascular disease, physical activity, income, education, lipid lowering medication and eGFR.
BMI, body mass index; eGFR, estimated-glomerular filtration rate; HbA1c, glycated haemoglobin; OHA, oral hypoglycaemic agents.
Being from Sub-Saharan Africa, South Asia, East Asia, the Middle East and North Africa, Latin America or low-income European countries, Russia and Central Asia predicted substantially higher HbA1c. East Asian ethnicity predicted 1.8–3.8 mmol/mol higher HbA1c, depending on the stratum. Similarly, low-income European countries, Russia and Central Asia predicted 1.2–1.9 mmol/mol higher HbA1c. Latin America and the Caribbean predicted 1.9–4.8 mmol/mol higher HbA1c. South Asian origin predicted 1.9–4.2 mmol/mol higher HbA1c. Sub-Saharan Africa predicted 1.6–3.6 mmol/mol higher HbA1c. In comparison, no physical activity predicted 0.8–1.3 mmol/mol higher HbA1c, as compared to daily physical activity.
Probability of failure to achieve the target level during the second year
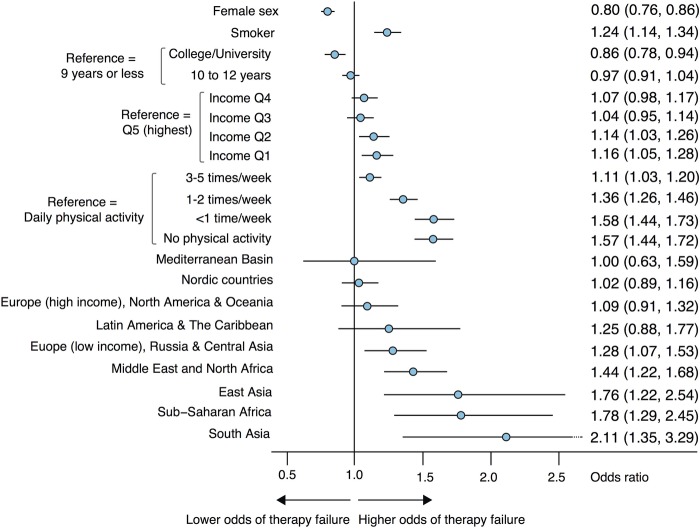
Low-income European countries, Middle East and North Africa, East Asia, Sub-Saharan Africa and South Asia displayed substantially higher odds of not achieving the target-level for HbA1c (figure 2). ORs (95% CI) for East Asia, Sub-Saharan Africa and South Asia were 1.76 (1.22, 2.54), 1.78 (1.29, 2.45) and 2.11 (1.35, 3.29), respectively. The effects of income and education were significant, although less pronounced. Not being physically active was associated with 57% higher odds of failure, as compared to daily physical activity.
Figure 2.
Probability (OR) of achieving glycaemic control (<52 mmol/mol) during the second year after diagnosis. Adjusted for ethnicity, sex, age, body mass index, income, education, smoking status, physical activity and type of glucose-lowering treatment.
Probability of having albuminuria during the second year
Immigrants had 6% to 92% higher odds of having albuminuria (figure 3). The risk of albuminuria was particularly high (51% to 92% higher risk) in non-Western and low-income groups.
Figure 3.
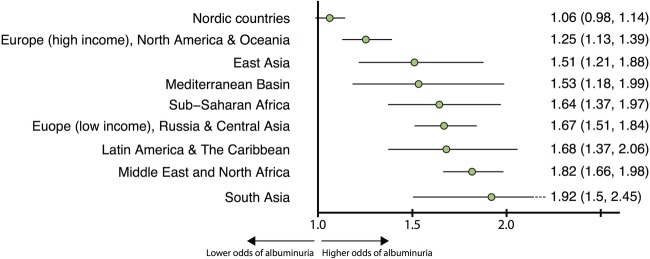
Probability (OR) of having albuminuria during the second year after diagnosis. Adjusted for age, sex, systolic blood pressure and estimated glomerular filtration rate.
Time to start of pharmacological treatment
Median time to start of pharmacological treatment was shorter for non-Western populations compared with Western populations (see online supplementary figure B).
Discussion
Our study provides firm evidence that ethnicity is a strong predictor of glycaemic control, and on a par with physical activity. We also show ethnic differences in glycaemia for all major ethnic groups and how these disparities are mirrored in another important risk marker, albuminuria. We believe that our results call for more individualised management and increased efforts to eliminate ethnic inequalities.
Glucose control is a cornerstone of diabetes care. Previous studies on ethnic differences in glycaemic control might be compromised in several ways: unequal access to—or use of—healthcare, inappropriately broad categories of ethnicity, cross-sectional design, small samples and short follow-up, are frequent flaws that might explain contradictory results.6–8
We examined 131 935 newly diagnosed patients with type 2 diabetes, including 713 495 observations during a decade of monitoring. Our cohort contains the majority of all new cases of type 2 diabetes in Sweden during the study period. All major ethnic groups were adequately represented. All participants had full access to every level of healthcare at a minimal cost. The Swedish social welfare system fully covers all necessary healthcare for individuals who do not have an income. Thus, neither access to nor consumption of healthcare is likely to have confounded our results. Swedish authorities frequently target immigrant and disadvantaged groups in ways to increase their use of healthcare and promote health behaviours. This was reflected, in our study, by the fact that immigrants had 30% more appointments at their clinic.
We noted marked ethnic differences in HbA1c both at the time of diagnosis and during follow-up. Immigrants consistently exhibited poorer glycaemic control. High-income Western groups remained below the target-level of HbA1c for 4–5 years after diagnosis, whereas low-income European countries, Russia and Central Asian patients maintained the target level for an average of only 3 years. Non-Western populations had substantially higher HbA1c throughout the study and never reached the guideline target level. Adjusted figures showed 2–5 mmol/mol higher HbA1c among non-Western groups. These disparities translated into a 28–111% higher risk of not achieving target level of HbA1c and a 51–92% higher risk of developing albuminuria among non-Western groups compared with native Swedes. After the end of follow-up, 40–45% of individuals from high-income Western countries were in glycaemic control, compared to 5%, 25% and 30% for Sub-Saharan Africa, South Asia, and the Middle East and North Africa, respectively. These differences could not be explained by disparities in instituting glucose-lowering medications use, or access to healthcare.
A recent Scottish study compared glycaemic control in Pakistanis, Indians, Chinese and African-Caribbeans with white Scottish individuals. Unadjusted figures revealed that, compared to the white Scottish group, all other groups had significantly greater proportions of people with suboptimal glycaemic control (defined as HbA1c>58 mmol/mol). Adjusted ORs for suboptimal glycaemic control were significantly higher among Pakistanis (OR 1.85 (95% CI 1.68 to 2.04)) and Indians (1.62 (95% CI 1.38 to 1.89)).9 This is in line with our results, and we provide additional details and ethnic groups to this comparison.
The risk of albuminuria was assessed in order to determine whether ethnic differences in glycaemic control were reflected in the development of diabetes-related complications. Poor glycaemic control is a main cause of albuminuria and renal lesions in diabetes, making albuminuria a suitable marker for complications.32 33
Studies have shown that African-Americans and Hispanics have a higher prevalence of albuminuria compared with Caucasians.34 Jolly et al35 reported that this was also true for Asians. Our study describes the adjusted risk of albuminuria in all major ethnic groups; immigrants, particularly those of non-Western origin, have a substantially higher risk of developing albuminuria. This predicts a high future risk of developing cardiovascular disease. It also suggests that ethnic differences in HbA1c reflect actual differences in glucose-levels. Above all, it underlines the need for ethnic-specific screening and management.
This study shows that South Asians have the greatest risk of developing albuminuria. Previous studies have shown that South Asians exhibit poor risk factor control and have an exceptionally high waist-hip-ratio;36 37 they are at greater risk of developing diabetes;38 39 they develop diabetes earlier in life;40 41 and they exhibit poor glycaemic control.9 42 A recent study demonstrated that the cut-offs for overweight and obesity in South Asians are substantially lower than cut-offs for white Europeans.43 Clinicians should be aware of this vulnerability and intensify risk factor control in South Asians.
The groups with the poorest glycaemic control and greatest risk of albuminuria in our study were Asia, Sub-Saharan Africa, the Middle East and North Africa, low-income Europe, Russia and Central Asia. They represent a large and growing proportion of the population in high-income areas such as North America and the European Union. We believe our results can be generalised to economically developed Western countries. Clinicians and healthcare planners should be aware of the challenges posed by immigrants and adjust the management accordingly. Effective strategies to reduce these health disparities remain elusive and need to be addressed. The problem might be further complicated by a potential interaction between ethnicity and the effectiveness of glucose-lowering medications. Although our study was not designed to explore these associations, we show that there was an effect-modification of ethnicity on glucose-lowering therapy. A previous study revealed ethnic differences in the efficacy of insulin,44 but there are considerable gaps in the knowledge that is currently available on this topic.
Limitations
It is important to consider the implications of the use of HbA1c when making ethnic comparisons. HbA1c is not a direct measure of glycaemia and might be influenced by factors that are independent of blood glucose levels. Studies have shown that African-Americans average approximately 0.20 percentage points higher HbA1c than Caucasian Americans.45 46 However, the design of these studies differs from ours. Furthermore, African-Americans descend largely from West Africa. Africans exhibit the greatest genetic diversity of all human populations.47 A recent study examined two African populations and showed that their HbA1c differed; lower levels were found for the East African population.48 The African population in our study descends largely from East Africa. Thus, we believe that our results for Sub-Saharan Africans and the remaining ethnicities should be interpreted as reflecting differences in glycaemic control. This is underscored by the fact that, in our study, Sub-Saharan Africa, as well as the remaining non-Western populations, had considerably higher risk of developing albuminuria, a glycaemia-related complication.
We created ethnic categories by grouping geographically adjacent countries. One could argue that this does not measure ethnicity per se. Regrettably, there is no consensus regarding criteria for defining an ethnic group. Many populations, both within and between countries, share characteristics. Moreover, ethnicity is a changeable concept; neighbouring populations tend to adapt to and adopt each other, which blurs differences. Some individuals identify themselves with several ethnicities.49 We defined rather large ethnic categories by assessing each countries ethnic composition, economic development, history and religion.50 51 This ought to encircle ethnically homogenous populations.
Conclusions
Despite earlier start of glucose-lowering therapies, full access to healthcare at a minimal cost and more appointments, immigrants—particularly those of non-Western origin—with type 2 diabetes, have substantially higher HbA1c, greater risk of therapy failure and higher probability of developing albuminuria, than native Swedes. The impact of ethnicity on glycaemic control is greater than the effect of income and educational level, and on a par with the effect of physical activity. Thus, ethnicity is integral to glycaemic control and needs to be carefully considered if diabetes care is to improve.
Acknowledgments
The authors would like to thank the various regional NDR coordinators, as well as contributing nurses, physicians and patients. The Swedish Diabetes Association and the Swedish Society of Diabetology support the NDR. The authors would also like to thank George Lappas (University of Gothenburg) and Szilard Nemes (University of Gothenburg) for their valuable statistical guidance.
Footnotes
Contributors: ARa, A-MS, ARo, BZ, BE and SG contributed to the conception and design of the study. ARa performed statistical analyses and wrote the first draft of the article. ARa, A-MS, ARo, BZ, BE and SG contributed to the interpretation of data. ARa, A-MS, ARo, BZ, BE and SG contributed to critical revision of the article for important intellectual content.
Funding: This study was funded by the Swedish National Diabetes Register. The Swedish Association of Local Authorities and Regions funds the NDR. Additional support: the Swedish Heart and Lung Foundation, and the Swedish Research Council (SIMSAM) (grant numbers 2013-5187 and 2013-4236).
Competing interests: None declared.
Ethics approval: The regional ethical review board at the University of Gothenburg, Gothenburg, Sweden.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The Swedish Data Protection Authority regulates access to the NDR, and any other public database, through the Personal Data Act. Access to data requires approval from The Regional Ethical Review Board, the National Board of Health and Welfare, Statistics Sweden and the Swedish National Diabetes Register. The authors are happy to provide detailed information regarding this.
References
- 1.Holman RR, Paul SK, Bethel MA et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–89. 10.1056/NEJMoa0806470 [DOI] [PubMed] [Google Scholar]
- 2.Patel A, MacMahon S, Chalmers J et al. , ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–72. 10.1056/NEJMicm066227 [DOI] [PubMed] [Google Scholar]
- 3.Sarwar N, Gao P, Seshasai SR et al. , Emerging Risk Factors C. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215–22. 10.1016/S0140-6736(10)60484-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seshasai SR, Kaptoge S, Thompson A et al. , Emerging Risk Factors C. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011;364:829–41. 10.1056/NEJMoa1008862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lind M, Bounias I, Olsson M et al. Glycaemic control and incidence of heart failure in 20,985 patients with type 1 diabetes: an observational study. Lancet 2011;378:140–6. 10.1016/S0140-6736(11)60471-6 [DOI] [PubMed] [Google Scholar]
- 6.Fan T, Koro CE, Fedder DO et al. Ethnic disparities and trends in glycemic control among adults with type 2 diabetes in the U.S. from 1988 to 2002. Diabetes Care 2006;29:1924–5. 10.2337/dc05-2238 [DOI] [PubMed] [Google Scholar]
- 7.Harris MI, Eastman RC, Cowie CC et al. Racial and ethnic differences in glycemic control of adults with type 2 diabetes. Diabetes Care 1999;22:403–8. 10.2337/diacare.22.3.403 [DOI] [PubMed] [Google Scholar]
- 8.Davis TM, Cull CA, Holman RR et al. Relationship between ethnicity and glycemic control, lipid profiles, and blood pressure during the first 9 years of type 2 diabetes: U.K. Prospective Diabetes Study (UKPDS 55). Diabetes Care 2001;24:1167–74. 10.2337/diacare.24.7.1167 [DOI] [PubMed] [Google Scholar]
- 9.Negandhi PH, Ghouri N, Colhoun HM et al. Ethnic differences in glycaemic control in people with type 2 diabetes mellitus living in Scotland. PLoS ONE 2013;8:e83292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Information UDOP. International migration and development. United Nations Press Release 2013. 10.1371/journal.pone.0083292 [DOI]
- 11.Ramachandran A, Ma RCW, Snehalatha C. Diabetes in Asia. Lancet 2010;375:408–18. 10.1016/S0140-6736(09)60937-5 [DOI] [PubMed] [Google Scholar]
- 12.Shai I, Jiang R, Manson JE et al. Ethnicity, obesity, and risk of type 2 diabetes in women: a 20-year follow-up study. Diabetes Care 2006;29:1585–90. 10.2337/dc06-0057 [DOI] [PubMed] [Google Scholar]
- 13.Richard Sicree JSPZ. The global burden of diabetes and impaired glucose tolerance. International Diabetes Federation, 2013. [Google Scholar]
- 14.Lutsey PL, Pereira MA, Bertoni AG et al. Interactions between race/ethnicity and anthropometry in risk of incident diabetes: the multi-ethnic study of atherosclerosis. Am J Epidemiol 2010;172:197–204. 10.1093/aje/kwq100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narayan KM, Boyle JP, Thompson TJ et al. Lifetime risk for diabetes mellitus in the United States. JAMA 2003;290:1884–90. 10.1001/jama.290.14.1884 [DOI] [PubMed] [Google Scholar]
- 16.Davis K, Ballreich J. Equitable access to care—how the United States ranks internationally. N Engl J Med 2014;371:1567–70. 10.1056/NEJMp1406707 [DOI] [PubMed] [Google Scholar]
- 17.Billing A. Health assessment of newly immigrated individuals. http://www.folkhalsomyndigheten.se/pagefiles/12920/halsoundersokningar-nyanlanda-invandrare.pdf
- 18.Haglind P, ed. Tobacco prevention among immigrants. 2nd edn. Institute of Population Health, 2012. http://www.folkhalsomyndigheten.se/documents/livsvillkor-levnadsvanor/andts/tobak/uppdrag/nationella-tobaksuppdraget/samverkansprojektet/slutrapport-tobaksprevention-invandrargrupper.pdf [Google Scholar]
- 19.Lind M, Svensson A-M, Kosiborod M et al. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med 2014;371: 1972–82. 10.1056/NEJMoa1408214 [DOI] [PubMed] [Google Scholar]
- 20.Gudbjornsdottir S, Cederholm J, Nilsson PM et al. The National Diabetes Register in Sweden: an implementation of the St. Vincent Declaration for Quality Improvement in Diabetes Care. Diabetes Care 2003;26:1270–6. 10.2337/diacare.26.4.1270 [DOI] [PubMed] [Google Scholar]
- 21.Ekström N, Schiöler L, Svensson A-M et al. Effectiveness and safety of metformin in 51 675 patients with type 2 diabetes and different levels of renal function: a cohort study from the Swedish National Diabetes Register. BMJ Open 2012;2:e001076–6. 10.1136/bmjopen-2012-001076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cederholm J, Gudbjörnsdottir S, Eliasson B et al. Blood pressure and risk of cardiovascular diseases in type 2 diabetes: further findings from the Swedish National Diabetes Register (NDR-BP II). J Hypertens 2012;30:2020–30. 10.1097/HJH.0b013e3283577bdf [DOI] [PubMed] [Google Scholar]
- 23.Lind M, Olsson M, Rosengren A et al. The relationship between glycaemic control and heart failure in 83,021 patients with type 2 diabetes. Diabetologia 2012;55:2946–53. 10.1007/s00125-012-2681-3 [DOI] [PubMed] [Google Scholar]
- 24.Rotimi CN, Jorde LB. Ancestry and disease in the age of genomic medicine. N Engl J Med 2010;363:1551–8. 10.1056/NEJMra0911564 [DOI] [PubMed] [Google Scholar]
- 25.Hoelzel W, Weykamp C, Jeppsson J-O et al. IFCC reference system for measurement of hemoglobin A1c in human blood and the national standardization schemes in the United States, Japan, and Sweden: a method-comparison study. Clin Chem 2004;50:166–74. 10.1373/clinchem.2003.024802 [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, Bosch JP, Lewis JB et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461–70. 10.7326/0003-4819-130-6-199903160-00002 [DOI] [PubMed] [Google Scholar]
- 27.Ludvigsson JF, Andersson E, Ekbom A et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450 10.1186/1471-2458-11-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ingelsson E, Arnlöv J, Sundström J et al. The validity of a diagnosis of heart failure in a hospital discharge register. Eur J Heart Fail 2005;7:787–91. 10.1016/j.ejheart.2004.12.007 [DOI] [PubMed] [Google Scholar]
- 29.Diggle PJ HPLK-YZS. Analysis of longitudinal data. 2nd edn. Oxford University Press, 2002. [Google Scholar]
- 30.Egede LE, Gebregziabher M, Hunt KJ et al. Regional, geographic, and racial/ethnic variation in glycemic control in a national sample of veterans with diabetes. Diabetes Care 2011;34:938–43. 10.2337/dc10-1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinstock RS, Teresi JA, Goland R et al. Glycemic control and health disparities in older ethnically diverse underserved adults with diabetes: five-year results from the Informatics for Diabetes Education and Telemedicine (IDEATel) study. Diabetes Care 2011;34:274–9. 10.2337/dc10-1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mogensen CE, Poulsen PL. Microalbuminuria, glycemic control, and blood pressure predicting outcome in diabetes type 1 and type 2. Kidney Int Suppl 2004;66:S40–1. 10.1111/j.1523-1755.2004.09210.x [DOI] [PubMed] [Google Scholar]
- 33.Levin SR, Coburn JW, Abraira C et al. Effect of intensive glycemic control on microalbuminuria in type 2 diabetes. Veterans Affairs Cooperative Study on Glycemic Control and Complications in Type 2 Diabetes Feasibility Trial Investigators. Diabetes Care 2000;23:1478–85. 10.2337/diacare.23.10.1478 [DOI] [PubMed] [Google Scholar]
- 34.Sinha SK, Shaheen M, Rajavashisth TB et al. Association of race/ethnicity, inflammation, and albuminuria in patients with diabetes and early chronic kidney disease. Diabetes Care 2014;37:1060–8. 10.2337/dc13-0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jolly SE, Burrows NR, Chen S-C et al. Racial and ethnic differences in albuminuria in individuals with estimated GFR greater than 60 mL/min/1.73 m(2): results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 2010;55:S15–22. 10.1053/j.ajkd.2009.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lean ME, Han TS, Bush H et al. Ethnic differences in anthropometric and lifestyle measures related to coronary heart disease risk between South Asian, Italian and general-population British women living in the west of Scotland. Int J Obes Relat Metab Disord 2001;25:1800–5. 10.1038/sj.ijo.0801823 [DOI] [PubMed] [Google Scholar]
- 37.Forouhi NG, Sattar N, Tillin T et al. Do known risk factors explain the higher coronary heart disease mortality in South Asian compared with European men? Prospective follow-up of the Southall and Brent studies, UK. Diabetologia 2006;49:2580–8. 10.1007/s00125-006-0393-2 [DOI] [PubMed] [Google Scholar]
- 38.Danaei G, Finucane MM, Lu Y et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 2011;378:31–40. 10.1016/S0140-6736(11)60679-X [DOI] [PubMed] [Google Scholar]
- 39.Bellary S, O'Hare JP, Raymond NT et al. Premature cardiovascular events and mortality in south Asians with type 2 diabetes in the United Kingdom Asian Diabetes Study—effect of ethnicity on risk. Curr Med Res Opin 2010;26:1873–9. 10.1185/03007995.2010.490468 [DOI] [PubMed] [Google Scholar]
- 40.Mukhopadhyay B, Forouhi NG, Fisher BM et al. A comparison of glycaemic and metabolic control over time among South Asian and European patients with type 2 diabetes: results from follow-up in a routine diabetes clinic. Diabet Med 2006;23:94–8. 10.1111/j.1464-5491.2005.01735.x [DOI] [PubMed] [Google Scholar]
- 41.Ethnicity and cardiovascular disease. The incidence of myocardial infarction in white, South Asian, and Afro-Caribbean patients with type 2 diabetes (U.K. Prospective Diabetes Study 32). Diabetes Care 1998;21:1271–7. 10.2337/diacare.21.8.1271 [DOI] [PubMed] [Google Scholar]
- 42.Fischbacher CM, Bhopal R, Steiner M et al. Is there equity of service delivery and intermediate outcomes in South Asians with type 2 diabetes? Analysis of DARTS database and summary of UK publications. J Public Health (Oxf) 2009;31:239–49. 10.1093/pubmed/fdp003 [DOI] [PubMed] [Google Scholar]
- 43.Ntuk UE, Gill JMR, Mackay DF et al. Ethnic-specific obesity cutoffs for diabetes risk: cross-sectional study of 490,288 UK biobank participants. Diabetes Care 2014;37:2500–7. 10.2337/dc13-2966 [DOI] [PubMed] [Google Scholar]
- 44.Davidson JA, Wolffenbuttel BH, Arakaki RF et al. Impact of race/ethnicity on efficacy and safety of two starter insulin regimens in patients with type 2 diabetes: a posthoc analysis of the DURABLE trial. Ethn Dis 2013;23:393–400. [PubMed] [Google Scholar]
- 45.Ziemer DC, Kolm P, Weintraub WS et al. Glucose-independent, black-white differences in hemoglobin A1c levels: a cross-sectional analysis of 2 studies. Ann Intern Med 2010;152:770–7. 10.7326/0003-4819-152-12-201006150-00004 [DOI] [PubMed] [Google Scholar]
- 46.Herman WH, Ma Y, Uwaifo G et al. Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care 2007;30:2453–7. 10.2337/dc06-2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tishkoff SA, Reed FA, Friedlaender FR et al. The genetic structure and history of Africans and African Americans. Science 2009;324:1035–44. 10.1126/science.1172257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hare MJL, Magliano DJ, Zimmet PZ et al. Glucose-independent ethnic differences in HbA1c in people without known diabetes. Diabetes Care 2013;36:1534–40. 10.2337/dc12-1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Braun L, Fausto-Sterling A, Fullwiley D et al. Racial categories in medical practice: how useful are they? PLoS Med 2007;4:e271 10.1371/journal.pmed.0040271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.The Central Intelligence Agency. The World Fact Book. https://www.cia.gov/library/publications/the-world-factbook/ (accessed 7 Mar 2015).
- 51.Bank TW. Classification of Countries. http://www.worldbank.org/en/country (accessed 7 Mar 2015).