Abstract
Despite improvements in the therapy of underlying heart disease sudden cardiac death (SCD) is a major cause of death worldwide. Disturbed Na and Ca handling is known to be a major predisposing factor for life-threatening tachyarrhythmias. In cardiomyocytes many ion channels and transporters, including voltage-gated Na and Ca channels, cardiac ryanodine receptors, Na/Ca-exchanger and SR Ca-ATPase are involved in this regulation. We have learned a lot about the pathophysiological relevance of disturbed ion channel function from monogenetic disorders. Changes in the gating of a single ion channel and/or the activity of an ion pump suffice to dramatically increase the propensity for arrhythmias even in structurally normal hearts. Nevertheless, patients with heart failure (HF) with acquired dysfunction in many ion channels and transporters exhibit profound dysregulation of Na and Ca handling and Ca/calmodulin dependent protein kinase, and are especially prone to arrhythmias. A deeper understanding of the underlying arrhythmic principles is mandatory if we are to improve their outcome. This review addresses basic tachy-arrhythmic mechanisms, the underlying ionic mechanisms and the consequences for ion homeostasis, and the situation in complex diseases like HF.
Keywords: sodium, calcium, calcium/calmodulin-dependent kinase II, alternans, arrhythmias
Introduction
Sudden cardiac death (SCD) refers to a sudden unexpected death from cardiac causes, and is a major cause of death worldwide.1 In patients with structural heart disease arrhythmias are the main cause of death.2 Despite improvements in primary and secondary prevention with substantial decline in mortality of coronary heart disease in recent years,1,3 SCD rates have declined to a much lesser extent.4 Implantable cardioverter-defibrillators (ICDs) can reduce cardiovascular mortality in high risk patients, but prevention of SCD is particularly challenging because the majority of cases occur in individuals without a prior diagnosis of cardiac disease or other clear risk factors for SCD. Thus, understanding the mechanisms of SCD is crucial for both identification of patients at risk and also for developing novel medical or interventional treatment.
SCD is descriptive of a clinical event, but is not a well-defined disease with one specific underlying mechanistic pathophysiology. In contrast to contractile failure, SCD is a consequence of the immediate and complete inability of the heart to maintain the circulation. The most likely SCD causes are lethal cardiac arrhythmias like ventricular tachycardia (VT) or ventricular fibrillation (VF), although bradyarrhythmias also play an important role.5 The imprecise definition may also include pulseless electrical activity e.g. as a consequence of large pulmonary embolism.
The underlying mechanisms of life-threatening ventricular tachyarrhythmias are manifold. They can occur as a consequence of isolated channelopathies in a structurally normal heart. However, more frequently, they are secondary to substantial dysregulation of intracellular Ca and Na handling that accompanies contractile dysfunction in HF. There is also a growing body of evidence that Ca/calmodulin-dependent kinase II (CaMKII) is involved. CaMKII regulates many Na and Ca handling proteins (and is also regulated by Ca) thereby influencing both contractile function and arrhythmogenesis. This review will discuss the mechanisms linking Ca and Na handling to arrhythmogenesis with special focus on CaMKII.
Excitation-contraction coupling
To understand arrhythmogenesis, basic knowledge about the fundamental principles of excitation-contraction coupling is required (Figure 1). Cardiac excitation is based on sarcolemmal ion fluxes that are tightly coupled to cytosolic Ca and contraction.6 Excitation is initiated by opening of voltage-gated Na channels and Na current influx (INa) leading to the rapid action potential (AP) upstroke (phase 0).6,7 Phase 0 is limited by fast INa inactivation and the activation of transient outward K channels (KV4.2, KV4.3, and KV1.4). These K channels generate transient outward K current (Ito) resulting in an early and partial repolarization i.e. the AP notch (phase 1), which is followed by a plateau phase (phase 2). During the latter, membrane conductance falls dramatically as inward and transient outward currents inactivate. Due to the time- and voltage-dependent gating properties of delayed and inward rectifier K channels K current fluxes are small and only capable of balancing the relatively small remaining inward Ca current (ICa) via L-type Ca channels (LTCC, Cav1.2). This is why the repolarization rate at plateau voltages is slow. Moreover, because of high membrane resistance, even small currents such as Na/Ca exchange current (INCX), Na/K-ATPase current (INaK) or ICa reactivation can destabilize the plateau membrane potential resulting in arrhythmias. ICa (which activates more slowly than INa) also progressively decays during the plateau. In ventricular cells, phase 3 repolarization ensues as K currents slowly activate (IKs, carried by Kv7.1, KCNQ1) or undergo voltage-dependent recovery from inactivation (IKr, carried by hERG, KCNH2), and IK1 (carried by Kir2.x channels) progressively contributes as the membrane repolarizes. IK1 also stabilizes diastolic Vm near −80 mV during phase 4. In pacemaker cells, the relative paucity of stabilizing IK18 together with the prominence of the hyperpolarization-activated non-specific cation current If (HCN channels) and Ca release driven INCX, are mainly responsible for diastolic depolarization leading to AP initiation and pacemaker function.6
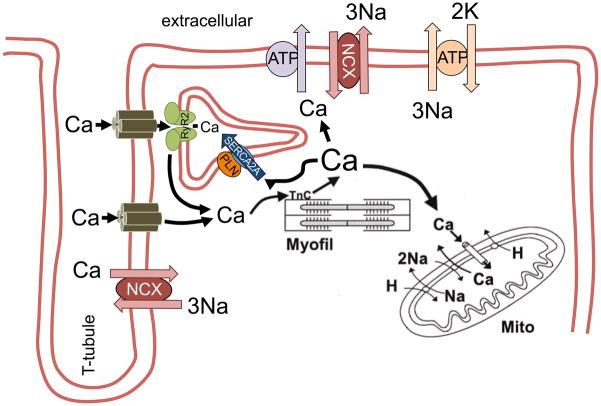
Figure 1.
Schematic diagram of cellular Ca fluxes during excitation-contraction coupling. Ca entry via L-type Ca channels triggers Ca-induced Ca release from the sarcoplasmic reticulum (SR), which results in myofilament activation. For relaxation, cytosolic Ca is transported into the SR via SR Ca-ATPase (SERCA2a) and into the extracellular space via sarcolemmal Na/Ca exchanger. Reproduced with permission from the AHA.184
The cardiomyocyte membrane (sarcolemma) has transverse invaginations called T-tubules (~200 nm diameter) that reach deep into the myocytes interior. T-tubules make close contact with enlarged junctional sarcoplasmic reticulum (SR) cisternae, with only a 14 nm wide dyadic cleft that separates the SR and T-tubular membranes. LTCCs are highly concentrated in the T- tubules at these junctional clefts and are in close proximity to cardiac ryanodine receptors (RyR2), the SR Ca release channel (Ca release microdomain). This LTCC-RyR2 proximity at the dyadic cleft means that Ca ions that enter via ICa cause local cleft [Ca] to be very high, sufficient to trigger the opening of RyR2 clusters responsible for SR Ca release during excitation-contraction coupling. This Ca-induced Ca release amplifies the sarcolemmal Ca influx and depending on species and heart rate the SR Ca release accounts for 70 to 92% of total Ca increase during systole. Total Ca is heavily buffered (~100:1), but free cytosolic [Ca] typically increases from 100 to 600 nmol/L, which leads to myofilament activation and contraction (systole). The [Ca]i rise is transient since ICa inactivation and RyR2 closure limit cytosolic Ca entry, and at the same time Ca removal pathways are activated. For Ca removal, the two major pathways are: SR Ca-ATPase (SERCA2a) and sarcolemmal Na/Ca exchange (NCX1), although a tiny fraction of Ca can be extruded by the sarcolemmal Ca-ATPase (PMCA) and mitochondrial Ca uniporter (MCU). During steady-state, the proportion of Ca transported into SR and out of the cell correspond exactly to the amount of Ca released from the SR and sarcolemmal Ca influx, respectively.
CaMKII
CaMKII is a serine/threonine kinase that can be activated by Ca/calmodulin binding to its regulatory domain. This activation results in the phosphorylation of target proteins including RyR2, NaV1.5, LTCC and phospholamban (the negative regulator of SERCA2a).9,10 Therefore, CaMKII is crucially involved in the regulation of excitation-contraction coupling.
CaMKII assembles into a dodecameric holoenzyme consisting of two stacked hexamers. One of the target proteins of CaMKII is the regulatory subunit of an adjacent CaMKII monomer. This auto-phosphorylation results in augmented activation that persists even after dissociation from Ca/CaM and provides an integrated response from the short-term fluctuations in intracellular Ca. It was also shown that reactive oxygen species (ROS)-dependent oxidation of CaMKII at two methionine residues at the regulatory domain (M281/282) results in autonomous activation similar to autophosphorylation.11 Thus, CaMKII can link increased ROS to their downstream effectors. Increased expression and activity of CaMKII12,13 has been linked to contractile dysfunction and arrhythmias in HF and upon conditions of increased ROS production.14–20
Mechanisms of electrical instability
Ventricular tachycardia or fibrillation are the result of cell membrane hyperexcitability, disturbed repolarization or defective conduction of the electrical wavefront across the myocardium.21 The presence of an electrically inactive scar tissue that often develops after myocardial infarction forces the wavefront to propagate around this line of block. The wavefront then enters the area behind the scar. If that wavefront reaches the back of the obstacle from multiple directions simultaneously (or near the same time) then the waves will annihilate (Figure 2A). However, if unidirectional block (often coupled with decremental conduction) exists around one side, the wavefront can reenter and re-excite tissue in front of the unidirectional block (Figure 2A). This can lead to stable macroscopic re-entry. Reentry can also occur around other functional obstacles (e.g. depolarized tissue), and can take the form of a rotor.21,22 Stable rotors lead to monomorphic VT. If the waves that propagate outward from a rotor develop wavebreaks, additional rotors are generated, which may lead to polymorphic VT and VF.22 This development is much more likely under conditions of altered excitation, repolarization or conduction due to disturbed ion channel function (Figure 3). A typical cause for this electric remodeling is structural remodeling associated with cardiac disease secondary to advanced age, increased oxidant stress, hypertension, diabetes, tissue injury or inflammation.21 However, the disturbed function of ion channels that underlies these changes in excitation, repolarization or conduction can also occur in structurally normal myocardium. Abnormal impulse conduction with consequent reentrant excitation can also be a consequence of Ca alternans (Figure 3). This is an example of how Ca dysregulation can cause reentry, which is the most common mechanism of electrical instability.
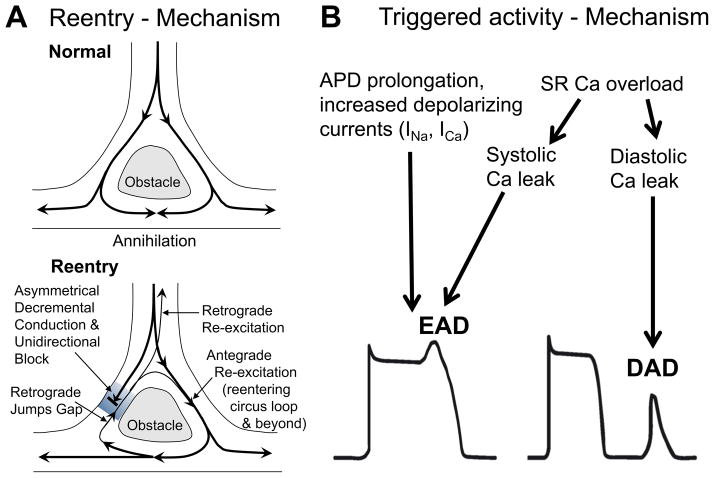
Figure 2.
Proarrhythmogenic mechanisms. A) Reentry. Upper panel shows normal conduction around an obstacle. The latter exhibits electrical properties that differ dramatically from the rest of the myocardium (slowed conduction and/or repolarization). The wavefront propagates around this line of block and reaches the back from multiple directions simultaneously resulting in wave annihilation. If, however, unidirectional block (often coupled with decremental conduction) exists around one side (lower panel), the wavefront can reenter and re-excite tissue in front of the unidirectional block. This can lead to stable reentry. B) Triggered activity. Increased depolarizing currents that result in prolongation of action potential duration (APD) can favour L-type Ca channel reactivation. Increased Ca entry via ICa can also lead to SR Ca overload and spontaneous SR Ca release during AP plateau phase. This may generate transient inward (ITi) current by Na/Ca exchange. Both Ca channel reactivation and ITi may depolarize the membrane during AP plateau phase, which can result in an early afterdepolarization (EAD). In addition, SR Ca overload may also result in diastolic SR Ca release. The consequent ITi may lead to depolarization from the resting membrane potential, which can result in a delayed afterdepolarization (DAD). Partially reproduced.31
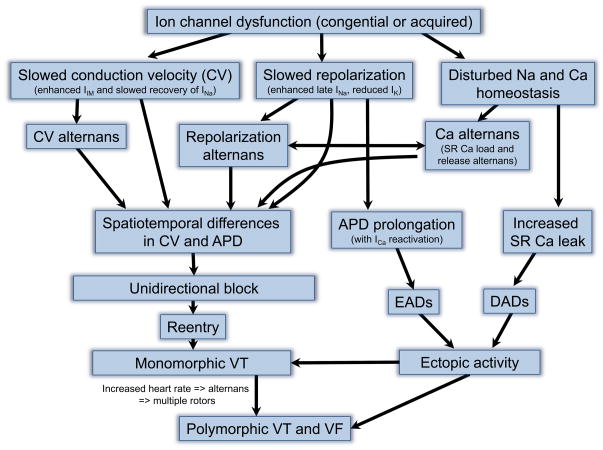
Figure 3.
Overview of proarrhythmic consequences of ion channel dysfunction. Congenital or acquired dysfunction of ion channels can result in 1) slowed conduction, 2) repolarization and/or 3) disturbed intracellular Na and Ca homeostatis. Slowed conduction velocity (CV; with or without CV alternans) or repolarization (with or without repolarization alternans) causes spatiotemporal differences in CV and action potential duration (APD) within the myocardium. These regional differences can lead to unidirectional block with reentry and rotor formation. The consequent monomorphic ventricular tachycardia (VT) may result in additional rotors by heart rate-dependent alternans causing polymorphic VT and ventricular fibrillation (VF). Disturbed Na and Ca homeostasis, on the other hand, may lead to Ca alternans, which is linked to repolarization alternans. Moreover, prolongation in APD duration causes EADs and increased Ca leak results in DADs, both of which underlie ectopic activity. The latter can also lead to monomorphic of polymorphic VT and VF.
Beside reentry, triggered activity is another important mechanism of electrical instability. The latter is the result of an imbalance of ion currents within a single cardiomyocyte. Such imbalances can be classified as either early or delayed depending on their occurrence during the action potential. Early afterdepolarizations (EADs) occur during the plateau phase of a cardiac action potential, while delayed afterdepolarizations (DADs) disturb the diastolic membrane potential after AP repolarization is complete (Figure 2B). Both EADs and DADs can reach the threshold inducing premature ventricular contractions, which can degenerate into monomorphic or polymorphic VTs.
Early afterdepolarization
EADs occur during the AP and are a consequence of increased inward currents or reduced repolarization reserve (reduced outward K currents).6 The plateau phase of the AP is especially vulnerable since repolarizing and depolarizing currents are small and nearly balanced. During this phase, small alterations in the amplitude of even one ionic current can result in the generation of an EADs. We have learned much about the fundamental mechanisms of triggered activity by studying familial disorders with prolonged repolarization. For example, congenital long QT syndrome 3 is characterized by profound AP prolongation due to increased persistent or late Na current (late INa),15,23 EADs are a major trigger for tachyarrythmias, but can also occur at low heart rates (where AP duration is intrinsically long). The long AP duration allows for reactivation of ICa at plateau potentials. The resulting inward current creates an EAD as a depolarization in the otherwise monotonically decaying plateau voltage.24,25 Amongst the long QT syndromes, the Timothy syndrome is a very rare form, caused by a mutation in CaV1.2 that prevents normal ICa inactivation. This disease is characterized by AP prolongation, increased QT intervals, life-threatening arrhythmias, and multisystem defects. Similar to other causes of AP prolongation, ICa reactivation is a major cause of EAD formation. Interestingly, pathological AP prolongation is a prominent feature of a proarrhythmic electrical remodeling in HF,26,27 but can also occur in response to drugs that inhibit K channels (especially IKr). This highlights the importance of this fundamental arrhythmic mechanism.
Beside these well-studied EADs arising during long AP plateaus via ICa reactivation, there is now compelling evidence that some EADs are initiated by SR Ca re-release during the AP that causes inward INCX. These EADs are more common during repolarization, and are sometimes called phase 3 EADs. The inward INCX may only cause a weak depolarization, but that can be amplified by re-activation of ICa,28,29 depending upon the voltage. That is, in atrial (and rodent ventricular) APs with INCX-prolonged late and plateau at voltages below −40 mV (where ICa reactivation does not occur), EADs may be driven by non-equilibrium reactivation of INa, which is facilitated by increased SR Ca release.30
Delayed afterdepolarization
DADs are a consequence of cytosolic and SR Ca overload.31 Both increased cytosolic and SR luminal Ca increase the diastolic open probability of cardiac RyR2.6 Ca sparks are elementary SR Ca release events that occur if a cluster of RyR2 open. During systolic Ca-induced Ca release, Ca sparks are synchronized within the cell and summate to form the Ca transient. Spontaneous Ca sparks are mainly responsible for diastolic SR Ca release and diastolic SR Ca leak via RyR2.32,33 Since RyR2 is in relatively close proximity to the NCX, this localized [Ca]i elevation drives inward INCX. Note that the stoichiometry of Na/Ca exchange (3Na+:1Ca2+) meaning that Ca extrusion is accompanied by a net inward movement of one positive charge via INCX which is almost entirely responsible for what used to be called transient inward current Iti.31 If this INCX is large enough, it can cause and appreciable DAD which can trigger an arrhythmic AP. Individual Ca sparks normally do not produce enough INCX to produce a measurable DAD because they are isolated unsynchronized events. However, at higher SR Ca content the Ca sparks are larger in amplitude and nearby RyR2 clusters are more sensitive to activation resulting in a cell-wide Ca wave with sufficient INCX to cause DADs and triggered APs. Sympathetic stimulation can drive up SR Ca content and increase the propensity for Ca waves and triggered beats.
In the intact heart the INCX produced by a Ca wave in a single myocyte is insufficient to trigger an appreciable DAD or AP because all of the neighboring cells can effectively clamp that single cell at the diastolic voltage. However, when these Ca waves and consequent INCX are synchronized regionally among many cells (by the prior AP and similar RyR2 recovery kinetics), that region can initiate triggered beats as premature ventricular contractions for the whole heart.34,35 The heart is relatively protected from these triggered arrhythmias initiated via EADs and DADs, but under pathological conditions due to either genetic ion channel/transporter mutations or acquired diseases like HF both the cellular propensity for EADs and DADs and their ability to cause whole heart premature ventricular contractions can be greatly increased.34–37
Cardiac alternans
Cardiac alternans is a known risk factor for cardiac arrhythmias and SCD.38–40 The transition from monomorphic VT to polymorphic VT or VF requires breaks in the wave propagating from a stable rotor. During VT, cardiomyocyte Ca cycles at high rates. The time for intracellular Ca removal is drastically shortened and can elevate diastolic [Ca]i and limit SR Ca load.41 This can also encroach upon the recovery time of the ICa-induced SR Ca release.42,43,64,65 Normally, [Ca]i is tightly controlled, but Ca dysregulation can predispose to afterdepolarizations and cardiac alternans.44–47 Cardiac alternans are alternating beats with large/small amplitude Ca transient and long/short APD (Figure 4A). Most common in large mammals are electromechanically (or Vm-Ca) concordant alternans in which the large Ca transient and contraction is associated with the longer APD. However, Vm-Ca discordant alternans can also occur. Alternans can also be spatially concordant, where all regions of the ventricle are in phase with each other, or spatially discordant where different regions are out of phase with each other. As we will see the Ca and Vm changes are usually functionally linked, but both can occur independently.44
Figure 4.
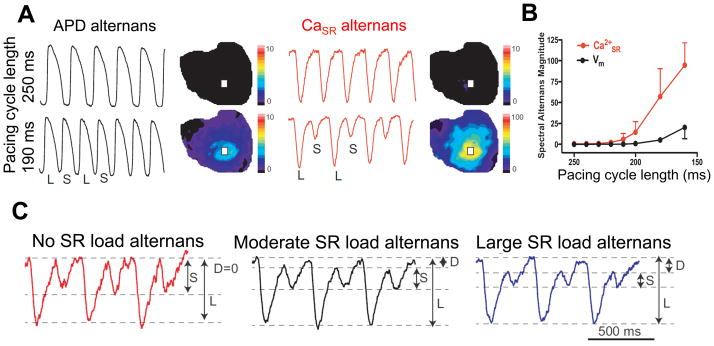
Characteristics of cardiac alternans. A) Simultaneous recordings of AP and intra-SR free [Ca] in isolated Langendorff-perfused rabbit hearts by optical mapping. At shorter pacing intervals (190 ms), alternans of the AP duration as well as SR Ca release alternans was observed. B) SR Ca release alternans can occur at heart rates where APD alternans is not yet detectable. C) Ca release alternans can be amplified by alternating changes in SR Ca load, i.e. SR Ca load alternans. Data reproduced with permission.43
Repolarization alternans
Alternation in APD is called repolarization alternans. Since this type of alternans involves membrane potential, it can be clinically observed as T-wave alternans (TWA).48 TWA has been shown to be associated with cardiac arrhythmias and SCD.49–51 TWA is not restricted to a specific underlying disease but can be observed in HF, Brugada syndrome, and long QT syndrome.52
TWA can result from changes in sarcolemmal ion current recovery (typically of INa or ICa) manifested by a steep slope of cellular APD restitution.53 This type of TWA typically occurs at high heart rates with reduced diastolic intervals.48,54,55 The dependence of APD and conduction velocity (CV) on the preceding diastolic interval are called APD restitution and CV restitution, respectively. Shorter diastolic intervals result in shorter APD and slower CV.45 Both APD and CV restitution critically depend on the speed of recovery from inactivation of sarcolemmal ion channels. Nevertheless, other mechanisms, for instance SR Ca release, can also contribute to APD restitution changes. Intracellular Ca released from the SR inactivates ICa. A large SR Ca release could, therefore, result in a more pronounced Ca-dependent inactivation of ICa, which would influence APD restitution.
CV restitution, on the other hand, mainly depends on Na channel recovery.56 This is due to the fact that conduction velocity depends on AP upstroke velocity, and the latter is determined by the magnitude of INa. Na channel recovery is usually very fast, thus, CV restitution occurs only at very high heart rates, as would be the influence of Na channel recovery on APD restitution. However, under conditions of slowed Na channel recovery, the impact on CV restitution (and also APD restitution) may already occur at much lower heart rates. This is important, since slowed Na channel recovery has been shown to be a feature of many cardiac diseases like HF,14,57 or ischemia56,58,59 (where CaMKII may be involved)14 and Brugada syndrome.60
As mentioned above, APD restitution depends on sarcolemmal ion channel recovery. Amongst them, L-type Ca channels are very important. ICa is the major inward current during the AP plateau.6 As stated above AP plateau is the most vulnerable phase of the AP. This explains, why inhibition of ICa has been shown to reduce the slope of the APD restitution curve. In addition, the recovery of Ca channels is slower compared to, for instance, Na channels. Therefore, Ca channels influence APD restitution already at lower heart rates. Interestingly, since ICa also determines SR Ca load, and vice versa, APD alternans and Ca alternans are functionally linked.
Repolarization alternans can be either spatially concordant or discordant.48,61 It was shown that Na channel recovery is critically involved in spatially discordant alternans.61,62 Since spatially discordant alternans is accompanied by changes in conduction velocity, both T wave and QRS are affected resulting in T-wave and QRS alternans (Figure 6). Repolarization alternans can be proarrhythmic by two mechanisms. First, spatially discordant alternans results in spatial dispersion of repolarization providing a substrate for reentry.48 Second, at high heart rates alternans can promote conduction block, which may trigger rotor formation. Alternans-dependent arrhythmias are, therefore, most likely generated under conditions of increased heart rate like during exercise or occur secondary to a stable rotor-dependent ventricular tachycardia. In fact, alternans may mechanistically explain why a stable ventricular tachycardia may degenerate into a multiple wavelet-driven polymorphic ventricular tachycardia or VF.63,64
Figure 6.
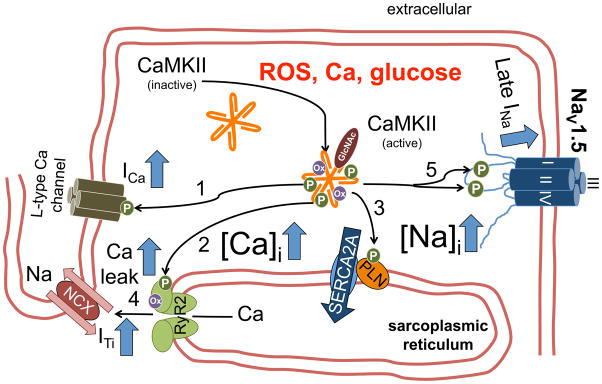
CaMKII-dependent mechanisms of triggered activity. CaMKII is activated by pathophysiologically relevant stimuli, i.e. increased reactive oxygen species (ROS), increased intracellular Ca, hyperglycemia. CaMKII-dependent phosphorylation of L-type Ca channels (1) may increase ICa window current predisposing to EADs. Increased CaMKII-dependent RyR2 phosphorylation (2) results in increased diastolic Ca leak. CaMKII-dependent phospholamban (PLN) phosphorylation (3) maintains SR Ca content, which may also stimulate diastolic RyR2 openings from the luminal side. Diastolic SR Ca release triggers transient inward current (ITi, 4) and DADs. Increased CaMKII-dependent phosphorylation of NaV1.5 (5) leads to enhanced late INa, which predisposes for both EADs and DADs (Figure 5).
Importantly, if APD is substantially prolonged, which is a typical feature of HF 26,27 or long QT syndrome, 65 ion channel recovery is already impaired at lower heart rates. Even without AP prolongation, if Na channel recovery is impaired like in HF14,57, ischemia56,58,59 and Brugada syndrome60 alternans-dependent arrhythmias can occur at much lower heart rates.
Ca alternans
From the surface electrical measurements of an electrocardiogram it is impossible to state if APD alternans is accompanied by Ca alternans, or whether one leads to the other. However, extensive work over the past years has shown that Ca and APD alternans usually co-exist, are mechanistically linked and that Ca-driven alternans appear to be more clinically relevant in the setting of heart disease.53
Alternans start to occur as pacing rate is increased, and Ca alternans occur even when myocytes are voltage clamped with identical Vm waveforms, consistent with Ca-driven alternans.44,45,66
In the absence of voltage clamp those Ca alternans also cause APD alternans, and there are logical reasons why a larger vs. smaller Ca transient would alter APD. The two most prominent [Ca]i-dependent currents are ICa and INCX. A large Ca transient would a) strengthen Ca-dependent inactivation of ICa which would shorten APD, and b) drive increased inward INCX, which would prolong APD. In mammals with prominent AP plateaus, concordant Vm-Ca alternans usually predominate, suggesting that the INCX effects are more powerful contributors to alternans. However, some conditions could shift this balance to favor a predominant role for ICa inactivation and discordant Vm-Ca alternans. As stimulation rate increases alternans start at a certain threshold and the alternans ratio (large:small Ca transient amplitude or APD) increases progressively. This can be seen as alternans in the amount of SR Ca release in direct measurements of intra-SR free [Ca] ([Ca]SR), in both isolated myocytes and whole heart (Figure 4A).42,43
Several factors contribute to alternans, and they seem to follow a sequence. The first step seems to be an encroachment on RyR2 recovery so that SR Ca release decreases, despite unaltered APD, ICa and [Ca]SR.42,43,67
This is because RyR2 restitution is much slower than normal ICa restitution. The partial failure of SR Ca release at this first small beat allows improved RyR2 recovery at the next large beat, but then the cycle repeats. SR Ca release alternans can appear at heart rates where the consequent APD alternans are not yet detectable (Figure 4B). As Ca release alternans grow they are amplified by alternating changes in SR Ca load, i.e. SR Ca load alternans (Figure 4C). The small release limits Ca-dependent inactivation of ICa, thereby increasing Ca influx and load for the next (large) beat. The small Ca transient also drives less Ca extrusion via NCX, which limits Ca loss at the small beat. Then at the large beat there is greater Ca-dependent ICa inactivation and greater Ca efflux via NCX, which reduces cell and SR Ca load, setting the scene for very stable alternans, up to 6–7 Hz (150 ms cycle length) in rabbit hearts (Figure 4C). The steep non-linear dependence of SR Ca release on diastolic SR Ca load41,68 enhances the impact of the SR load alternans on Ca release alternans.46 At even higher stimulation rates, shorter diastolic intervals or more depolarized diastolic voltage, one can encroach on ICa (or even INa) restitution, which may also exacerbate a sort of Ca-driven alternans (smaller ICa causes smaller Ca transient and APD). But here the lines become blurred with the electrical, restitution-driven alternans involving APD and CV restitution, as discussed above.
A number of factors can influence the pacing rate at which alternans is observed. Inhibition of glycolysis, mitochondrial energetic limitations, decreased SERCA function and redox modification of RyR2 can all favor alternans.69–72 All of these factors may be relevant in pathophysiological conditions like HF, thus enhancing the propensity for alternans and arrhythmogenic sequelae in these conditions.
Similar to repolarization alternans, Ca alternans can be spatially in phase (concordant) or out of phase, i.e. discordant, in different regions of a single myocytes73–75 or hearts, and spatially discordant alternans have been associated with lethal VTs and VF in patients.48,76
Arrhythmias in HF and ischemia/reperfusion
Despite improvements in HF therapy and ischemia/reperfusion associated myo-cardial damage, it has proven difficult to develop antiarrhythmic treatments and prevent SCD. Conventional ion channel blockers that are used as antiarrhythmic drugs are known to induce arrhythmias in structural heart disease.
There is an increasing body of evidence that HF and ischemic heart disease are accompanied by alterations in intracellular Na and Ca handling. Both conditions are characterized by an increased generation of ROS,77–79 which may contribute to disturbed Na and Ca handling.80 Changes in intracellular Na and Ca handling are associated with electrical instability and many of these alterations are linked to both contractile dysfunction and arrhythmias.
Late Na current
As stated above, APD is increased in HF.27 Beside reduction of K current expression, increased magnitude of late INa has been shown to contribute to AP prolongation in HF,81 and after myocardial infaction.82 Late INa is generated by dysfunctional inactivation of cardiac voltage-gated Na channels NaV1.5.83 The detailed molecular mechanism is not fully understood.84
Although late INa has a small amplitude compared with peak INa it persists for hundreds of milliseconds during the cardiac AP,85 providing a source for increasing [Na]i.14,15 Increased [Na]i is a well-known feature of HF, ischemia/reperfusion or other conditions of increased ROS production and can contribute to contractile dysfunction and arrhythmias.15,18,23,86–90
The late INa-dependent prolongation of the AP plateau renders the membrane potential vulnerable for EADs (Figure 5).15 A similar arrhythmic mechanism can be found in familial long QT syndrome 3 (LQT3). Mutations in the gene encoding for NaV1.5 (SCN5A) have been shown to increase late INa leading to AP prolongation and EADs.91,92 Transmural differences in late INa might also increase dispersion of repolarization,93 which underlies the development of torsade de pointes tachyarrhy-thmias. In addition, late INa is involved in cardiac alternans: ischemia-induced spatially discordant repolarization alternans has been shown to be prevented by inhibition of late INa.94
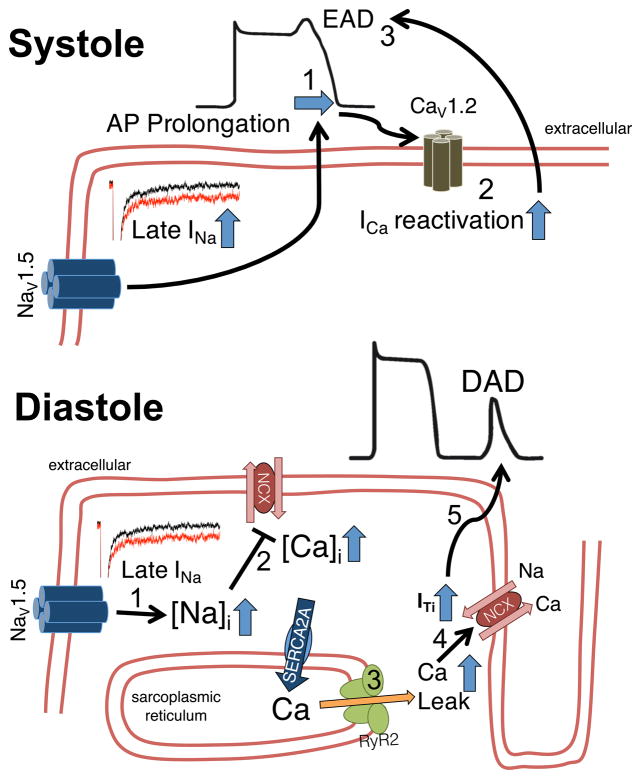
Figure 5.
Proarrhythmogenic mechanisms of enhanced late INa. In systole (upper panel), enhanced late INa leads to AP prolongation (1). The longer AP plateau phase increases the likelihood of ICa reactivation (2), which may lead to early afterdepolarizations (EAD, 3). Lower panel: The increased amount of Na influx also results in increased intracellular Na (1), which impairs Ca elimination (2) by the Na/Ca exchanger (either less forward or even increased reverse mode activity). In diastole, the increased intracellular Ca facilitates SR Ca leak (3), which could lead to transient inward current (ITi) by the Na/Ca exchanger (4). The latter can result in delayed afterdepolarizations (DAD, 5).
Beside AP prolongation, late INa-dependent Na overload leads to intracellular Ca accumulation either by reduced Ca exit due to limitations to Ca extrusion by NCX or by additional Ca entry via reverse mode NCX.15 Intracellular Ca accumulation is associated with increased diastolic SR Ca leak and DADs (Figure 5). Moreover, it contributes to diastolic dysfunction in HF and under conditions of increased ROS production,15,89 and it may even lead to cellular hypercontracture.95
AP-clamp experiments revealed that late INa-dependent Na entry also substantially contributes to arrhythmias in LQT3 syndrome.96 Consistent with this evidence, blocking late INa and Ca entry via NCX have been shown to inhibit afterdepolarizations.23,97
What are the mechanisms that lead to increased late INa? ROS have been shown to increase [Na]i, prolong APD and induce EADs, and ROS-enhanced late INa may be involved.15,23,90 Ahern and colleagues showed that nitrosylation of NaV1.5 increased late INa under physiologic and pathophysiologic conditions.98 Over recent years, however, a body of evidence suggests that CaMKII associates with and phosphorylates NaV1.5 leading to increased late Na current, and some ROS and nitric oxide effects may be mediated via CaMKII activation (Figure 6).14,15,57,99–103
Since late INa is strongly linked to cardiac arrythmogenesis, strategies aimed at inhibiting late INa may have strong anti-arrhythmic potential and may possibly be used to prevent SCD. The clinically approved anti-anginal drug ranolazine was found to inhibit late INa.104,105 Within the therapeutic range, which varies between 2 and 8 μmol/L, ranolazine also inhibits ICa by 30%, which could limit Ca entry,105 and IKr, which can prolong APD. Depending of the actual ionic conditions, the effects of ranolazine on APD may vary. Inhibition of late INa by ranolazine has been shown, for instance, to reduce ROS-dependent AP prolongation, as well as intracellular Na and Ca accumulation, diastolic dysfunction and arrhythmias.15,23,88,106,107 Ranolazine has been demonstrated to inhibit afterdepolarizations, to reduce transmural dispersion of repolarization and to inhibit spatially discordant repolarization alternans in various models of enhanced late INa.94,104,105,108–110 It also prevented pacing-induced reentry and multifocal ventricular fibrillation.111 In experimental models of systolic HF, ranolazine treatment also improved systolic function112 possibly by reducing late INa-dependent diastolic SR Ca leak.18
The clinical safety and efficacy of ranolazine are well investigated. A large clinical outcome trial investigating the efficacy of ranolazine to reduce cardiovascular death, myocardial infarction or recurrent ischemia in more than 6000 patients with acute coronary syndrome (MERLIN-TIMI-36) was not able to show an improvement in outcome.113 Nevertheless, subgroup analysis revealed that in placebo treated patients prolonged QTc was a significant independent predictor for SCD, while this was not the case in patients treated with ranolazine,114 suggesting a potential beneficial effect in the prevention of SCD. Investigation of the seven-day Holter monitoring data acquired in the MERLIN-TIMI-36 trial revealed a reduction in the incidence of ventricular tachycardia in the ranolazine treated patients.115 Ranolazine also reduced VT burden and ICD shocks in a small observational study of patients with refractory VT and previous ICD shocks.116 In a small study of patients with long QT syndrome 3, ranolazine shortened the QTc interval in a concentration-dependent manner.117 Moreover, in patients with atrial fibrillation (AF) a dose-finding randomized controlled trial (RAFFAELLO) showed a decreased rate of in overall AF recurrence.118
CaMKII
CaMKII is a central regulator of many Na and Ca (and K) channels and transporters. It is well established that transgenic overexpression of CaMKII results in the development of HF and arrhythmias,14,119 and that CaMKII inhibition prevented afterdepolarizations and arrhythmias upon β-adrenergic stimulation.17
Increased CaMKII autophoshorylation was observed in rabbit chronic atrioventricular block models of left ventricular dysfunction, acquired long QT syndrome and incessant ventricular tachycardia.120,121 In addition, CaMKII inhibition prevents the development of structural heart disease or arrhythmias upon myocardial infarction,122 increased ROS formation,15 pressure overload,123 or pacing-induced incessant VT.121 Calcineurin overexpressing mice with increased CaMKII activity show contractile dysfunction and arrhythmias.124 Also here, CaMKII inhibition improved contractile function and suppressed arrhythmias.124
Oxidized CaMKII has been shown to be involved in ROS-induced enhancement of late INa (Figure 6) leading to intracellular Na and Ca accumulation, contractile dysfunction and triggered activity.15,125 Increased oxidative activation of CaMKII due to enhanced NADPH oxidase 2 may also be important for angiotensin II-dependent arrhythmogenesis.126,127 Moreover, oxidized CaMKII plays a role in diabetes-dependent increased mortality after myocardial infarction possibly by sinus node dysfunction.128 A novel CaMKII activation pathway by O-linked glycosylation at serine 279 was demonstrated during acute hyperglycemia. This activation confers similar autonomous activity as autophosphorylation or oxidation.129 O-linked glycosylation upon diabetic hyperglycemia was shown to result in increased diastolic SR Ca leak and be pro-arrhythmogenic.129 CaMKII can be also nitrosylated at another site in the regulatory domain to cause autonomous activation.130 Nitrosylation-dependent CaMKII activation has been implicated in increased SR Ca leak in cardiac myocytes.131,132 Neurohumoral activation that occurs in HF has been shown to activate CaMKII. Both β-adrenergic stimulation and angiotensin II exposure can activate CaMKII either by increased cytosolic Ca or ROS.11,122, 126,133–136
The mechanisms of CaMKII dependent arrhythmias are complex, since CaMKII regulates a variety of ion channels and transporters (Figure 6). CaMKII was first identified to increase peak L-type Ca current and slow ICa inactivation,137–139 which may predispose to EADs.140–142
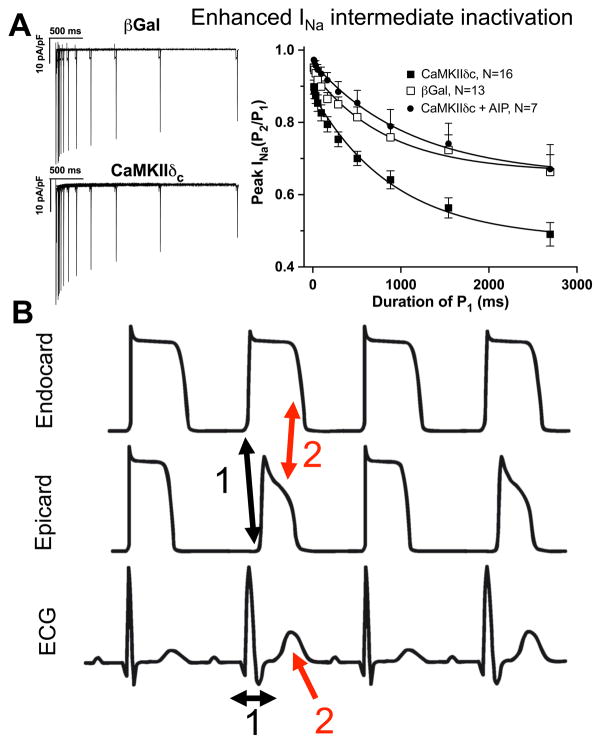
CaMKII is also known to associate with and phosphorylate cardiac NaV1.5.14,15,57,99–103 Beside increased late INa that may contribute to AP prolongation, increased EAD and DAD formation and repolarization alternans, CaMKII-dependent phosphorylation also enhanced INa intermediate inactivation and slowed recovery from inactivation.14,57,102,103 Both effects reduce the steady-state availability of INa, which could lead to repolarization alternans at high heart rates. Reduced Na channel availability can also increase transmural dispersion of repolarization and slow intra-ventricular conduction (Figure 7).14 This peculiar phenotype of CaMKII-dependent gain in Na channel function (enhanced late INa) and loss of Na channel function (reduced INa availability) may sound incongruous. However, similar changes in INa gating have been observed in a SCN5A mutation (1795InsD) associated with features of both Brugada and long QT syndrome.143,144 Thus, increased CaMKII activity in HF may lead to an acquired from of combined long QT and Brugada syndrome.
Figure 7.
CaMKII-dependent regulation of INa gating and alternans. A) CaMKII-dependent phosphorylation of NaV1.5 has been shown to enhance INa intermediate inactivation (reproduced from Wagner et al14 with permission; whole-cell patch clamp in rabbit ventricular myocytes). As a result, the number of available Na channels is reduced especially at shorter diastolic intervals. B) Consequences of enhanced INa intermediate inactivation: CV and repolarization alternans. Reduced Na channel availability results in slowed intramural conduction and slowed AP upstroke velocity (1) evident as broader QRS complex on surface ecg (1). In addition, K channel expression is larger in epicardium. Therefore, repolarization is faster in epicardium, especially if Na current is reduced (2). This leads to increased transmural dispersion of repolarization evident as larger and wider T wave on surface ecg (2). Interestingly, Na channels in intermediate inactive state cannot be activated (and become refractory) during the excitation. Thus, these channels are available for the consecutive excitation. Consequently, conduction velocity and AP upstroke velocity will be larger, AP duration longer for the consecutive excitation: the typical pattern of CV and repolarization alternans.
There is an overwhelming body of evidence that CaMKII regulates RyR2 and SERCA2a. While RyR2 is directly phosphorylated at serine 2814,145 the activity of SERCA2a is indirectly influenced by phosphorylation of phospholamban (PLN) at threonine 17. The latter results in relief of PLN-dependent SERCA2a inhibition and activation of SERCA2a.146 In HF, increased diastolic Ca leak through RyR2 occurs in the face of a reduced SR Ca reuptake due to a smaller SERCA2a/PLN expression ratio. This results in a reduced SR Ca content, which is an important determinant for the impaired Ca transient amplitude of failing cardiomyocytes.6,147,148 In HF CaMKII-dependent diastolic SR Ca leak is a major cause for DADs by activating forward mode NCX.149,150
Zhang et al.151 tried to rescue cardiac function in CaMKIIδC transgenic with reduced SR Ca load by PLN ablation. While this improved SR Ca load and Ca transients, this occurred at the expense of increased CaMKII-dependent SR Ca leak, mitochondrial Ca loading and myocyte death. Thus despite improved myocyte function, cardiac function, HF progression and mortality were worsened by the PLN ablation.
More insights into the role of CaMKII-dependent SR Ca leak for contractile dysfunction and arrhythmias in HF comes from animal models with increased afterload or myocardial infarction. It was shown that afterload induced HF development and arrhythmias were inhibited in knock-in mice harboring only a mutant RyR2 (S2814A) that cannot be phosphorylated by CaMKII at that site.152,153 This knock-in however, did not prevent HF post-myocardial infarction.152 In contrast, recent data show similar CaMKII-dependent SR Ca leak in isolated human ventricular cardiomyocytes from ischemic and dilated cardiomyopathy.154
Also, KI mice with a CaMKII-phosphomimetic mutation (S2814D) exhibited increased SR Ca leak and developed sustained ventricular tachycardia and SCD upon β-adrenergic stimulation, programmed electrical stimulation and increased afterload.153 This sort of CaMKII-dependent SR Ca leak has also been observed upon β-adrenergic stimulation,155–157 and by activation of NADPH oxidase resulting in DADs.126 While much attention has been on ventricular muscle, it was also shown that CaMKII-dependent SR Ca leak is crucially involved in atrial arrhythmo-genesis.158–161 For ROS-induced SR Ca leak, however, a direct oxidation of the RyR2 has also been proposed.15,162–164 Thus, CaMKII inhibition may be a novel strategy to prevent SCD.
Catecholaminergic polymorphic ventricular tachycardia
Increased SR Ca leak is causally involved in catecholaminergic polymorphic ventricular tachycardia (CPVT), a rare familial condition characterized by ventricular arrhythmias that are associated with exercise or catecholaminergic stimulation in a structurally normal heart.165 CPVT occurs at young ages. For example the majority of 101 patients with CPVT had symptoms before the age of 21.166 Importantly, CPVT accounts for 15% of all sudden unexplained deaths in young people. CPVT is linked to mutations in RyR2 and the intra-SR Ca binding and RyR2-associated proteins calsequestrin (CASQ2) and triadin (TRND).167–171 For a substantial fraction of patients, however, the disease-causing gene has remained elusive to date. As a result of the irregular SR Ca release172 electrogenic NCX is activated causing DADs. Clinically, patients exhibit monomorphic ventricular premature beats and more severe bidirectional ventricular tachycardia, which can degenerate into polymorphic VT and VF. Interestingly, pharmacological CaMKII inhibition has recently been shown to inhibit stress-induced arrhythmias and triggered activity in a mouse model of CPVT (RyR2 R4496C+/−).173 The opposite, transgenic CaMKII overexpression in RyR2 R4496C+/− mice resulted in increase SR Ca leak, DADs, arrhythmias upon β-adrenergic stimulation, and increased mortality possibly due to SCD.174 Since disturbed CaMKII-dependent regulation of SR Ca release is a frequent feature of HF, exercise-induced arrhythmias in HF may be related to the same underlying mechanism. For more information about this important genetic arrhythmogenic disease the reader is referred to these more comprehensive reviews.175–177
Epigenetic modifications related to SCD
Epigenetic regulation of gene expression is increasingly recognized as important contributor arrhythmias.178 This is an emerging area includes microRNAs, DNA methylation and histone modifications (e.g. acetylation/deacetylation). Apropos to the Ca/CaMKII focus here, it is known that CaMKII can importantly influence the nuclear export of class II histone deacetylases (e.g. HDAC4 and HDAC5) and that that can modulate transcription of key proteins involved in hypertrophic signaling and arrhythmias.179–181 CaMKII can also directly phosphorylate histone H3 and contribute to hypertrophic changes in gene expression.182,183 Thus, CaMKII seems to be integrally involved in epigenetic mechanisms of cardiac hypertrophy, and some of tha consequent changes in protein expression (of ion channels and Ca regulatory proteins) may also contribute to enhanced propensity for arrhythmias.
Conclusions
Cardiac myocyte Na and Ca fluxes and concentrations are tightly controlled, but also change dynamically as part of normal physiological modulation of cardiac electrical, contractile and energetic state. There is very tight coupling between Na, Ca, electrical, mechanical and energetic properties in the heart, only some of which we touched upon here. Many arrhythmias are a result of dysregulation of this complex system. To understand how deranged Na and Ca homeostasis comes about in pathophysiological states and how it contributes to electrical and contractile dysfunction such as HF, arrhythmias and SCD is a challenge. But this understanding is essential for progress in novel therapeutic approaches to these major clinical problems.
Supplementary Material
Acknowledgments
Citation of financial support
S.W. and L.S.M. are funded by Deutsche Forschungsgemeinschaft (DFG) through an International Research Training Group GRK 1816. L.S.M. is funded by the Fondation Leducq Transatlantic Network on “Redox and Nitrosative Regulation of Cardiac Remodeling”. L.S.M., and S.W. are funded by the DZHK (Deutsches Zentrum für Herz-Kreislauf-Forschung - German Centre for Cardiovascular Research). D.M.B. is funded by NIH grants (R37-HL30077, P01-HL080101 and R01-HL081562).
Non-standard Abbreviations and Acronyms
- AP
action potential
- APD
action potential duration
- CaMKII
Ca/Calmodulin-dependent protein kinase
- II CPVT
catecholaminergic polymorphic ventricular tachycardia
- CV
conduction velocity
- DAD
delyed afterdepolarization
- EAD
early afterdepolarization
- HF
heart failure
- ICD
implantable cardioverter-defibrillators
- If
hyperpolarization-activated non-specific cation current
- IK1
inward rectifying K current
- IKr
rapid activating delayed rectifying K current
- IKs
slowly activating delayed rectifiying K current
- INa
Na current
- INaK
Na/K-ATPase current
- INCX
Na/Ca exchange current
- ITI
transient inward current
- Ito
transient outward potassium current
- LQT
long QT syndrome
- LTCC
L-type Ca channels
- MCU
mitochondrial Ca uniporter
- MERLIN-TIMI-36 trial
Metabolic Efficiency With Ranolazine for Less Ischemia in Non ST-Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36 trial
- NCX1
Na/Ca-exchanger 1
- PLN
phospholamban
- PMCA
sarcolemmal Ca ATPase
- RAFFAELLO trial
Ranolazine in Atrial Fibrillation Following An ELectricaL CardiOversion trial
- ROS
reactive oxygen species
- RyR2
cardiac ryanodine receptor 2
- SCD
sudden cardiac death
- SERCA2a
SR Ca ATPase 2a
- SR
sarcoplasmic reticulum
- TWA
T wave alternans
- VF
ventricular fibrillation
- Vm
membrane potential
- VT
ventricular tachycardia
Footnotes
Disclosures
L.S.M receives research grants from Gilead and is involved in clinical trials with Gilead and Menarini. L.S.M received speaker’s honoraria from Berlin-Chemie.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2014 update: A report from the american heart association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lehnart SE, Ackerman MJ, Benson DW, Jr, Brugada R, Clancy CE, Donahue JK, George AL, Jr, Grant AO, Groft SC, January CT, Lathrop DA, Lederer WJ, Makielski JC, Mohler PJ, Moss A, Nerbonne JM, Olson TM, Przywara DA, Towbin JA, Wang LH, Marks AR. Inherited arrhythmias: A national heart, lung, and blood institute and office of rare diseases workshop consensus report about the diagnosis, phenotyping, molecular mechanisms, and therapeutic approaches for primary cardiomyopathies of gene mutations affecting ion channel function. Circulation. 2007;116:2325–2345. doi: 10.1161/CIRCULATIONAHA.107.711689. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH, Capewell S. Explaining the decrease in u.S. Deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356:2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 4.Dudas K, Lappas G, Stewart S, Rosengren A. Trends in out-of-hospital deaths due to coronary heart disease in sweden (1991 to 2006) Circulation. 2011;123:46–52. doi: 10.1161/CIRCULATIONAHA.110.964999. [DOI] [PubMed] [Google Scholar]
- 5.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345:1473–1482. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 6.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 7.Grandi E, Pasqualini FS, Bers DM. A novel computational model of the human ventricular action potential and ca transient. J Mol Cell Cardiol. 2010;48:112–121. doi: 10.1016/j.yjmcc.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho HS, Takano M, Noma A. The electrophysiological properties of spontaneously beating pacemaker cells isolated from mouse sinoatrial node. J Physiol. 2003;550:169–180. doi: 10.1113/jphysiol.2003.040501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swaminathan PD, Purohit A, Hund TJ, Anderson ME. Calmodulin-dependent protein kinase II: Linking heart failure and arrhythmias. Circ Res. 2012;110:1661–1677. doi: 10.1161/CIRCRESAHA.111.243956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson ME, Brown JH, Bers DM. Camkii in myocardial hypertrophy and heart failure. J Mol Cell Cardiol. 2011;51:468–473. doi: 10.1016/j.yjmcc.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O’Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirchhefer U, Schmitz W, Scholz H, Neumann J. Activity of camp-dependent protein kinase and Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human hearts. Cardiovasc Res. 1999;42(1):254–261. doi: 10.1016/s0008-6363(98)00296-x. [DOI] [PubMed] [Google Scholar]
- 13.Hoch B, Meyer R, Hetzer R, Krause EG, Karczewski P Max Delbrèuck Center for Molecular Medicine B-BG. Identification and expression of δ-isoforms of the multifunctional Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human myocardium. Circ Res. 1999;84(6):713–721. doi: 10.1161/01.res.84.6.713. [DOI] [PubMed] [Google Scholar]
- 14.Wagner S, Dybkova N, Rasenack E, Jacobshagen C, Fabritz L, Kirchhof P, Maier S, Zhang T, Hasenfuss G, Brown J, Bers D, Maier L. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest. 2006;116:3127–3138. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagner S, Ruff HM, Weber SL, Bellmann S, Sowa T, Schulte T, Anderson ME, Grandi E, Bers DM, Backs J, Belardinelli L, Maier LS. Reactive oxygen species-activated Ca/calmodulin kinase IIδ is required for late INa augmentation leading to cellular Na and Ca overload. Circ Res. 2011;108:555–565. doi: 10.1161/CIRCRESAHA.110.221911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toischer K, Rokita AG, Unsold B, Zhu W, Kararigas G, Sossalla S, Reuter SP, Becker A, Teucher N, Seidler T, Grebe C, Preuss L, Gupta SN, Schmidt K, Lehnart SE, Kruger M, Linke WA, Backs J, Regitz-Zagrosek V, Schafer K, Field LJ, Maier LS, Hasenfuss G. Differential cardiac remodeling in preload versus afterload. Circulation. 2010;122:993–1003. doi: 10.1161/CIRCULATIONAHA.110.943431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sag CM, Wadsack DP, Khabbazzadeh S, Abesser M, Grefe C, Neumann K, Opiela MK, Backs J, Olson EN, Brown JH, Neef S, Maier SK, Maier LS. Calcium/calmodulin-dependent protein kinase II contributes to cardiac arrhythmogenesis in heart failure. Circulation Heart failure. 2009;2:664–675. doi: 10.1161/CIRCHEARTFAILURE.109.865279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sag CM, Mallwitz A, Wagner S, Hartmann N, Schotola H, Fischer TH, Ungeheuer N, Herting J, Shah AM, Maier LS, Sossalla S, Unsold B. Enhanced late INa induces proarrhythmogenic SR Ca leak in a CaMKII-dependent manner. J Mol Cell Cardiol. 2014;76:94–105. doi: 10.1016/j.yjmcc.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Sag CM, Kohler AC, Anderson ME, Backs J, Maier LS. Camkii-dependent SR Ca leak contributes to doxorubicin-induced impaired Ca handling in isolated cardiac myocytes. J Mol Cell Cardiol. 2011;51:749–759. doi: 10.1016/j.yjmcc.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sag CM, Wolff HA, Neumann K, Opiela MK, Zhang J, Steuer F, Sowa T, Gupta S, Schirmer M, Hunlich M, Rave-Frank M, Hess CF, Anderson ME, Shah AM, Christiansen H, Maier LS. Ionizing radiation regulates cardiac Ca handling via increased ROS and activated CaMKII. Basic Res Cardiol. 2013;108:385. doi: 10.1007/s00395-013-0385-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandit SV, Jalife J. Rotors and the dynamics of cardiac fibrillation. Circ Res. 2013;112:849–862. doi: 10.1161/CIRCRESAHA.111.300158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss JN, Qu Z, Chen PS, Lin SF, Karagueuzian HS, Hayashi H, Garfinkel A, Karma A. The dynamics of cardiac fibrillation. Circulation. 2005;112:1232–1240. doi: 10.1161/CIRCULATIONAHA.104.529545. [DOI] [PubMed] [Google Scholar]
- 23.Song Y, Shryock JC, Wagner S, Maier LS, Belardinelli L. Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther. 2006;318:214–222. doi: 10.1124/jpet.106.101832. [DOI] [PubMed] [Google Scholar]
- 24.January CT, Riddle JM. Early afterdepolarizations: Mechanism of induction and block. A role for l-type Ca2+ current. Circ Res. 1989;64:977–990. doi: 10.1161/01.res.64.5.977. [DOI] [PubMed] [Google Scholar]
- 25.Antoons G, Volders PG, Stankovicova T, Bito V, Stengl M, Vos MA, Sipido KR. Window ca2+ current and its modulation by ca2+ release in hypertrophied cardiac myocytes from dogs with chronic atrioventricular block. J Physiol. 2007;579:147–160. doi: 10.1113/jphysiol.2006.124222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kääb S, Nuss HB, Chiamvimonvat N, O’Rourke B, Pak PH, Kass DA, Marban E, Tomaselli GF. Ionic mechanism of action potential prolongation in ventricular myocytes from dogs with pacing-induced heart failure. Circ Res. 1996;78:262–273. doi: 10.1161/01.res.78.2.262. [DOI] [PubMed] [Google Scholar]
- 27.Beuckelmann DJ, Näbauer M, Erdmann E. Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ Res. 1993;73:379–385. doi: 10.1161/01.res.73.2.379. [DOI] [PubMed] [Google Scholar]
- 28.Burashnikov A, Antzelevitch C. Acceleration-induced action potential prolongation and early afterdepolarizations. J Cardiovasc Electrophysiol. 1998;9:934–948. doi: 10.1111/j.1540-8167.1998.tb00134.x. [DOI] [PubMed] [Google Scholar]
- 29.Volders PG, Vos MA, Szabo B, Sipido KR, de Groot SH, Gorgels AP, Wellens HJ, Lazzara R. Progress in the understanding of cardiac early afterdepolarizations and torsades de pointes: Time to revise current concepts. Cardiovasc Res. 2000;46:376–392. doi: 10.1016/s0008-6363(00)00022-5. [DOI] [PubMed] [Google Scholar]
- 30.Edwards AG, Grandi E, Hake JE, Patel S, Li P, Miyamoto S, Omens JH, Heller Brown J, Bers DM, McCulloch AD. Nonequilibrium reactivation of Na+ current drives early afterdepolarizations in mouse ventricle. Circ Arrhythm Electrophysiol. 2014;7:1205–1213. doi: 10.1161/CIRCEP.113.001666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bers DM. Excitation-contraction coupling and cardiac contractile force. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2001. [Google Scholar]
- 32.Bassani RA, Bers DM. Rate of diastolic ca release from the sarcoplasmic reticulum of intact rabbit and rat ventricular myocytes. Biophys J. 1995;68:2015–2022. doi: 10.1016/S0006-3495(95)80378-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bers DM. Cardiac sarcoplasmic reticulum calcium leak: Basis and roles in cardiac dysfunction. Annu Rev Physiol. 2014;76:107–127. doi: 10.1146/annurev-physiol-020911-153308. [DOI] [PubMed] [Google Scholar]
- 34.Xie Y, Sato D, Garfinkel A, Qu Z, Weiss JN. So little source, so much sink: Requirements for afterdepolarizations to propagate in tissue. Biophys J. 2010;99:1408–1415. doi: 10.1016/j.bpj.2010.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Myles RC, Wang L, Kang C, Bers DM, Ripplinger CM. Local β-adrenergic stimulation overcomes source-sink mismatch to generate focal arrhythmia. Circ Res. 2012;110:1454–1464. doi: 10.1161/CIRCRESAHA.111.262345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: Roles of sodium-calcium exchange, inward rectifier potassium current, and residual β-adrenergic responsiveness. Circ Res. 2001;88:1159–1167. doi: 10.1161/hh1101.091193. [DOI] [PubMed] [Google Scholar]
- 37.Myles RC, Wang L, Bers DM, Ripplinger CM. Decreased inward rectifying K+ current and increased ryanodine receptor sensitivity synergistically contribute to sustained focal arrhythmia in the intact rabbit heart. J Physiol. 2014 doi: 10.1113/jphysiol.2014.279638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker ML, Rosenbaum DS. Repolarization alternans: Implications for the mechanism and prevention of sudden cardiac death. Cardiovasc Res. 2003;57:599–614. doi: 10.1016/s0008-6363(02)00737-x. [DOI] [PubMed] [Google Scholar]
- 39.Comtois P, Nattel S. Atrial repolarization alternans as a path to atrial fibrillation. J Cardiovasc Electrophysiol. 2012;23:1013–1015. doi: 10.1111/j.1540-8167.2012.02391.x. [DOI] [PubMed] [Google Scholar]
- 40.Verrier RL, Klingenheben T, Malik M, El-Sherif N, Exner DV, Hohnloser SH, Ikeda T, Martinez JP, Narayan SM, Nieminen T, Rosenbaum DS. Microvolt t-wave alternans testing has a role in arrhythmia risk stratification. J Am Coll Cardiol. 2012;59:1572–1573. doi: 10.1016/j.jacc.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 41.Bassani JW, Yuan W, Bers DM. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol. 1995;268:C1313–1319. doi: 10.1152/ajpcell.1995.268.5.C1313. [DOI] [PubMed] [Google Scholar]
- 42.Picht E, DeSantiago J, Blatter LA, Bers DM. Cardiac alternans do not rely on diastolic sarcoplasmic reticulum calcium content fluctuations. Circ Res. 2006;99:740–748. doi: 10.1161/01.RES.0000244002.88813.91. [DOI] [PubMed] [Google Scholar]
- 43.Wang L, Myles RC, De Jesus NM, Ohlendorf AK, Bers DM, Ripplinger CM. Optical mapping of sarcoplasmic reticulum Ca2+ in the intact heart: Ryanodine receptor refractoriness during alternans and fibrillation. Circ Res. 2014;114:1410–1421. doi: 10.1161/CIRCRESAHA.114.302505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chudin E, Goldhaber J, Garfinkel A, Weiss J, Kogan B. Intracellular Ca2+ dynamics and the stability of ventricular tachycardia. Biophys J. 1999;77:2930–2941. doi: 10.1016/S0006-3495(99)77126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goldhaber JI, Xie LH, Duong T, Motter C, Khuu K, Weiss JN. Action potential duration restitution and alternans in rabbit ventricular myocytes: The key role of intracellular calcium cycling. Circ Res. 2005;96:459–466. doi: 10.1161/01.RES.0000156891.66893.83. [DOI] [PubMed] [Google Scholar]
- 46.Diaz ME, O’Neill SC, Eisner DA. Sarcoplasmic reticulum calcium content fluctuation is the key to cardiac alternans. Circ Res. 2004;94:650–656. doi: 10.1161/01.RES.0000119923.64774.72. [DOI] [PubMed] [Google Scholar]
- 47.Alvarez-Lacalle E, Cantalapiedra IR, Penaranda A, Cinca J, Hove-Madsen L, Echebarria B. Dependency of calcium alternans on ryanodine receptor refractoriness. PloS one. 2013;8:e55042. doi: 10.1371/journal.pone.0055042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pastore JM, Girouard SD, Laurita KR, Akar FG, Rosenbaum DS. Mechanism linking t-wave alternans to the genesis of cardiac fibrillation. Circulation. 1999;99:1385–1394. doi: 10.1161/01.cir.99.10.1385. [DOI] [PubMed] [Google Scholar]
- 49.Rosenbaum DS, Jackson LE, Smith JM, Garan H, Ruskin JN, Cohen RJ. Electrical alternans and vulnerability to ventricular arrhythmias. N Engl J Med. 1994;330:235–241. doi: 10.1056/NEJM199401273300402. [DOI] [PubMed] [Google Scholar]
- 50.Armoundas AA, Tomaselli GF, Esperer HD. Pathophysiological basis and clinical application of t-wave alternans. J Am Coll Cardiol. 2002;40:207–217. doi: 10.1016/s0735-1097(02)01960-5. [DOI] [PubMed] [Google Scholar]
- 51.Narayan SM. T-wave alternans and the susceptibility to ventricular arrhythmias. J Am Coll Cardiol. 2006;47:269–281. doi: 10.1016/j.jacc.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 52.Qu Z, Xie Y, Garfinkel A, Weiss JN. T-wave alternans and arrhythmogenesis in cardiac diseases. Frontiers in physiology. 2010;1:154. doi: 10.3389/fphys.2010.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pruvot EJ, Katra RP, Rosenbaum DS, Laurita KR. Role of calcium cycling versus restitution in the mechanism of repolarization alternans. Circ Res. 2004;94:1083–1090. doi: 10.1161/01.RES.0000125629.72053.95. [DOI] [PubMed] [Google Scholar]
- 54.Mironov S, Jalife J, Tolkacheva EG. Role of conduction velocity restitution and short-term memory in the development of action potential duration alternans in isolated rabbit hearts. Circulation. 2008;118:17–25. doi: 10.1161/CIRCULATIONAHA.107.737254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Christini DJ, Riccio ML, Culianu CA, Fox JJ, Karma A, Gilmour RF., Jr Control of electrical alternans in canine cardiac purkinje fibers. Physical review letters. 2006;96:104101. doi: 10.1103/PhysRevLett.96.104101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qu Z, Karagueuzian HS, Garfinkel A, Weiss JN. Effects of Na+ channel and cell coupling abnormalities on vulnerability to reentry: A simulation study. Am J Physiol Heart Circ Physiol. 2004;286:H1310–1321. doi: 10.1152/ajpheart.00561.2003. [DOI] [PubMed] [Google Scholar]
- 57.Koval OM, Snyder JS, Wolf RM, Pavlovicz RE, Glynn P, Curran J, Leymaster ND, Dun W, Wright PJ, Cardona N, Qian L, Mitchell CC, Boyden PA, Binkley PF, Li C, Anderson ME, Mohler PJ, Hund TJ. Ca2+/calmodulin-dependent protein kinase II-based regulation of voltage-gated Na+ channel in cardiac disease. Circulation. 2012;126:2084–2094. doi: 10.1161/CIRCULATIONAHA.112.105320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pu J, Boyden PA. Alterations of na+ currents in myocytes from epicardial border zone of the infarcted heart. A possible ionic mechanism for reduced excitability and postrepolarization refractoriness. Circ Res. 1997;81:110–119. doi: 10.1161/01.res.81.1.110. [DOI] [PubMed] [Google Scholar]
- 59.Joyner RW, Ramza BM, Osaka T, Tan RC. Cellular mechanisms of delayed recovery of excitability in ventricular tissue. Am J Physiol. 1991;260:H225–233. doi: 10.1152/ajpheart.1991.260.1.H225. [DOI] [PubMed] [Google Scholar]
- 60.Wang DW, Makita N, Kitabatake A, Balser JR, George AL., Jr Enhanced Na+ channel intermediate inactivation in brugada syndrome. Circ Res. 2000;87:E37–43. doi: 10.1161/01.res.87.8.e37. [DOI] [PubMed] [Google Scholar]
- 61.Hayashi H, Shiferaw Y, Sato D, Nihei M, Lin SF, Chen PS, Garfinkel A, Weiss JN, Qu Z. Dynamic origin of spatially discordant alternans in cardiac tissue. Biophys J. 2007;92:448–460. doi: 10.1529/biophysj.106.091009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cao JM, Qu Z, Kim YH, Wu TJ, Garfinkel A, Weiss JN, Karagueuzian HS, Chen PS. Spatiotemporal heterogeneity in the induction of ventricular fibrillation by rapid pacing: Importance of cardiac restitution properties. Circ Res. 1999;84:1318–1331. doi: 10.1161/01.res.84.11.1318. [DOI] [PubMed] [Google Scholar]
- 63.Wu TJ, Lin SF, Baher A, Qu Z, Garfinkel A, Weiss JN, Ting CT, Chen PS. Mother rotors and the mechanisms of d600-induced type 2 ventricular fibrillation. Circulation. 2004;110:2110–2118. doi: 10.1161/01.CIR.0000143834.51102.91. [DOI] [PubMed] [Google Scholar]
- 64.Garfinkel A, Kim YH, Voroshilovsky O, Qu Z, Kil JR, Lee MH, Karagueuzian HS, Weiss JN, Chen PS. Preventing ventricular fibrillation by flattening cardiac restitution. Proc Natl Acad Sci U S A. 2000;97:6061–6066. doi: 10.1073/pnas.090492697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Q, Chen Q, Li H, Towbin JA. Molecular genetics of long QT syndrome from genes to patients. Current opinion in cardiology. 1997;12:310–320. doi: 10.1097/00001573-199705000-00013. [DOI] [PubMed] [Google Scholar]
- 66.Qu Z, Nivala M, Weiss JN. Calcium alternans in cardiac myocytes: Order from disorder. J Mol Cell Cardiol. 2013;58:100–109. doi: 10.1016/j.yjmcc.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kornyeyev D, Petrosky AD, Zepeda B, Ferreiro M, Knollmann B, Escobar AL. Calsequestrin 2 deletion shortens the refractoriness of Ca2+ release and reduces rate-dependent Ca2+-alternans in intact mouse hearts. J Mol Cell Cardiol. 2012;52:21–31. doi: 10.1016/j.yjmcc.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shannon TR, Ginsburg KS, Bers DM. Potentiation of fractional sarcoplasmic reticulum calcium release by total and free intra-sarcoplasmic reticulum calcium concentration. Biophysical Journal. 2000;78:334–343. doi: 10.1016/S0006-3495(00)76596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hüser J, Wang YG, Sheehan KA, Cifuentes F, Lipsius SL, Blatter LA. Functional coupling between glycolysis and excitation-contraction coupling underlies alternans in cat heart cells. J Physiol. 2000;524(Pt 3):795–806. doi: 10.1111/j.1469-7793.2000.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Florea SM, Blatter LA. The role of mitochondria for the regulation of cardiac alternans. Frontiers in physiology. 2010;1:141. doi: 10.3389/fphys.2010.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cutler MJ, Wan X, Laurita KR, Hajjar RJ, Rosenbaum DS. Targeted SERCA2a gene expression identifies molecular mechanism and therapeutic target for arrhythmogenic cardiac alternans. Circ Arrhythm Electrophysiol. 2009;2:686–694. doi: 10.1161/CIRCEP.109.863118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Belevych AE, Terentyev D, Viatchenko-Karpinski S, Terentyeva R, Sridhar A, Nishijima Y, Wilson LD, Cardounel AJ, Laurita KR, Carnes CA, Billman GE, Gyorke S. Redox modification of ryanodine receptors underlies calcium alternans in a canine model of sudden cardiac death. Cardiovasc Res. 2009;84:387–395. doi: 10.1093/cvr/cvp246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aistrup GL, Kelly JE, Kapur S, Kowalczyk M, Sysman-Wolpin I, Kadish AH, Wasserstrom JA. Pacing-induced heterogeneities in intracellular Ca2+ signaling, cardiac alternans, and ventricular arrhythmias in intact rat heart. Circ Res. 2006;99:e65–73. doi: 10.1161/01.RES.0000244087.36230.bf. [DOI] [PubMed] [Google Scholar]
- 74.Aistrup GL, Shiferaw Y, Kapur S, Kadish AH, Wasserstrom JA. Mechanisms underlying the formation and dynamics of subcellular calcium alternans in the intact rat heart. Circ Res. 2009;104:639–649. doi: 10.1161/CIRCRESAHA.108.181909. [DOI] [PubMed] [Google Scholar]
- 75.Tian Q, Kaestner L, Lipp P. Noise-free visualization of microscopic calcium signaling by pixel-wise fitting. Circ Res. 2012;111:17–27. doi: 10.1161/CIRCRESAHA.112.266403. [DOI] [PubMed] [Google Scholar]
- 76.Gehi AK, Stein RH, Metz LD, Gomes JA. Microvolt t-wave alternans for the risk stratification of ventricular tachyarrhythmic events: A meta-analysis. J Am Coll Cardiol. 2005;46:75–82. doi: 10.1016/j.jacc.2005.03.059. [DOI] [PubMed] [Google Scholar]
- 77.Tsutsui H, Ide T, Hayashidani S, Suematsu N, Utsumi H, Nakamura R, Egashira K, Takeshita A. Greater susceptibility of failing cardiac myocytes to oxygen free radical-mediated injury. Cardiovasc Res. 2001;49:103–109. doi: 10.1016/s0008-6363(00)00197-8. [DOI] [PubMed] [Google Scholar]
- 78.Kinugawa S, Tsutsui H, Hayashidani S, Ide T, Suematsu N, Satoh S, Utsumi H, Takeshita A. Treatment with dimethylthiourea prevents left ventricular remodeling and failure after experimental myocardial infarction in mice: Role of oxidative stress. Circ Res. 2000;87:392–398. doi: 10.1161/01.res.87.5.392. [DOI] [PubMed] [Google Scholar]
- 79.Ide T, Tsutsui H, Kinugawa S, Suematsu N, Hayashidani S, Ichikawa K, Utsumi H, Machida Y, Egashira K, Takeshita A. Direct evidence for increased hydroxyl radicals originating from superoxide in the failing myocardium. Circ Res. 2000;86:152–157. doi: 10.1161/01.res.86.2.152. [DOI] [PubMed] [Google Scholar]
- 80.Mallat Z, Philip I, Lebret M, Chatel D, Maclouf J, Tedgui A. Elevated levels of 8-iso-prostaglandin F2α in pericardial fluid of patients with heart failure: A potential role for in vivo oxidant stress in ventricular dilatation and progression to heart failure. Circulation. 1998;97:1536–1539. doi: 10.1161/01.cir.97.16.1536. [DOI] [PubMed] [Google Scholar]
- 81.Maltsev VA, Sabbah HN, Higgins RSD, Silverman N, Lesch M, Undrovinas AI. Novel, ultraslow inactivating sodium current in human ventricular cardiomyocytes. Circulation. 1998;98:2545–2552. doi: 10.1161/01.cir.98.23.2545. [DOI] [PubMed] [Google Scholar]
- 82.Huang B, El-Sherif T, Gidh-Jain M, Qin D, El-Sherif N. Alterations of sodium channel kinetics and gene expression in the postinfarction remodeled myocardium. J Cardiovasc Electrophysiol. 2001;12:218–225. doi: 10.1046/j.1540-8167.2001.00218.x. [DOI] [PubMed] [Google Scholar]
- 83.Maltsev VA, Undrovinas AI. A multi-modal composition of the late Na+ current in human ventricular cardiomyocytes. Cardiovasc Res. 2006;69:116–127. doi: 10.1016/j.cardiores.2005.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moreno JD, Clancy CE. Pathophysiology of the cardiac late Na current and its potential as a drug target. J Mol Cell Cardiol. 2012;52:608–619. doi: 10.1016/j.yjmcc.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Saint DA, Ju YK, Gage PW. A persistent sodium current in rat ventricular myocytes. J Physiol. 1992;453:219–231. doi: 10.1113/jphysiol.1992.sp019225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pieske B, Maier LS, Piacentino V, III, Weisser J, Hasenfuss G, Houser S. Rate dependence of [Na+]i and contractility in nonfailing and failing human myocardium. Circulation. 2002;106:447–453. doi: 10.1161/01.cir.0000023042.50192.f4. [DOI] [PubMed] [Google Scholar]
- 87.Despa S, Islam MA, Weber CR, Pogwizd SM, Bers DM. Intracellular Na+ concentration is elevated in heart failure but Na/K pump function is unchanged. Circulation. 2002;105:2543–2548. doi: 10.1161/01.cir.0000016701.85760.97. [DOI] [PubMed] [Google Scholar]
- 88.Sossalla S, Wagner S, Rasenack EC, Ruff H, Weber SL, Schöndube FA, Tirilomis T, Tenderich G, Hasenfuss G, Belardinelli L, Maier LS. Ranolazine improves diastolic dysfunction in isolated myocardium from failing human hearts - role of late sodium current and intracellular ion accumulation. J Mol Cell Cardiol. 2008;45:32–43. doi: 10.1016/j.yjmcc.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 89.Sossalla S, Maurer U, Schotola H, Hartmann N, Didie M, Zimmermann WH, Jacobshagen C, Wagner S, Maier LS. Diastolic dysfunction and arrhythmias caused by overexpression of camkiiδc can be reversed by inhibition of late Na+ current. Basic Res Cardiol. 2010;106:263–272. doi: 10.1007/s00395-010-0136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ward CA, Giles WR. Ionic mechanism of the effects of hydrogen peroxide in rat ventricular myocytes. J Physiol. 1997;500 ( Pt 3):631–642. doi: 10.1113/jphysiol.1997.sp022048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dumaine R, Wang Q, Keating MT, Hartmann HA, Schwartz PJ, Brown AM, Kirsch GE. Multiple mechanisms of Na+ channel--linked long-QT syndrome. Circ Res. 1996;78:916–924. doi: 10.1161/01.res.78.5.916. [DOI] [PubMed] [Google Scholar]
- 92.Viswanathan PC, Balser JR. Inherited sodium channelopathies: A continuum of channel dysfunction. Trends in cardiovascular medicine. 2004;14:28–35. doi: 10.1016/j.tcm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 93.Antzelevitch C, Belardinelli L. The role of sodium channel current in modulating transmural dispersion of repolarization and arrhythmogenesis. J Cardiovasc Electrophysiol. 2006;17 (Suppl 1):S79–S85. doi: 10.1111/j.1540-8167.2006.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bonatti R, Silva AF, Batatinha JA, Sobrado LF, Machado AD, Varone BB, Nearing BD, Belardinelli L, Verrier RL. Selective late sodium current blockade with GS-458967 markedly reduces ischemia-induced atrial and ventricular repolarization alternans and ecg heterogeneity. Heart Rhythm. 2014;11:1827–1835. doi: 10.1016/j.hrthm.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 95.Wagner S, Seidler T, Picht E, Maier LS, Kazanski V, Teucher N, Schillinger W, Pieske B, Isenberg G, Hasenfuss G, Kögler H. Na+-Ca2+ exchanger overexpression predisposes to reactive oxygen species-induced injury. Cardiovasc Res. 2003;60:404–412. doi: 10.1016/j.cardiores.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 96.Fredj S, Lindegger N, Sampson KJ, Carmeliet P, Kass RS. Altered na+ channels promote pause-induced spontaneous diastolic activity in long QT syndrome type 3 myocytes. Circ Res. 2006;99:1225–1232. doi: 10.1161/01.RES.0000251305.25604.b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nagy N, Kormos A, Kohajda Z, Szebeni A, Szepesi J, Pollesello P, Levijoki J, Acsai K, Virag L, Nanasi PP, Papp JG, Varro A, Toth A. Selective Na+/Ca2+ exchanger inhibition prevents Ca2+ overload-induced triggered arrhythmias. Br J Pharmacol. 2014;171:5665–5681. doi: 10.1111/bph.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ahern GP, Hsu SF, Klyachko VA, Jackson MB. Induction of persistent sodium current by exogenous and endogenous nitric oxide. J Biol Chem. 2000;275:28810–28815. doi: 10.1074/jbc.M003090200. [DOI] [PubMed] [Google Scholar]
- 99.Maltsev VA, Reznikov V, Undrovinas NA, Sabbah HN, Undrovinas A. Modulation of late sodium current by Ca2+, calmodulin, and CaMKII in normal and failing dog cardiomyocytes: Similarities and differences. Am J Physiol Heart Circ Physiol. 2008;294:H1597–1608. doi: 10.1152/ajpheart.00484.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aiba T, Hesketh GG, Liu T, Carlisle R, Villa-Abrille MC, O’Rourke B, Akar FG, Tomaselli GF. Na+ channel regulation by Ca2+/calmodulin and Ca2+/calmodulin-dependent protein kinase II in guinea-pig ventricular myocytes. Cardiovasc Res. 2010;85:454–463. doi: 10.1093/cvr/cvp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yao L, Fan P, Jiang Z, Viatchenko-Karpinski S, Wu Y, Kornyeyev D, Hirakawa R, Budas GR, Rajamani S, Shryock JC, Belardinelli L. Nav1.5-dependent persistent Na+ influx activates CaMKII in rat ventricular myocytes and N1325S mice. Am J Physiol Cell Physiol. 2011;301:C577–586. doi: 10.1152/ajpcell.00125.2011. [DOI] [PubMed] [Google Scholar]
- 102.Ashpole NM, Herren AW, Ginsburg KS, Brogan JD, Johnson DE, Cummins TR, Bers DM, Hudmon A. Ca2+/calmodulin-dependent protein kinase II (CaMKII) regulates cardiac sodium channel Nav1.5 gating by multiple phosphorylation sites. J Biol Chem. 2012;287:19856–19869. doi: 10.1074/jbc.M111.322537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hund TJ, Koval OM, Li J, Wright PJ, Qian L, Snyder JS, Gudmundsson H, Kline CF, Davidson NP, Cardona N, Rasband MN, Anderson ME, Mohler PJ. A βiv-spectrin/CaMKII signaling complex is essential for membrane excitability in mice. J Clin Invest. 2010;120:3508–3519. doi: 10.1172/JCI43621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Undrovinas AI, Belardinelli L, Undrovinas NA, Sabbah HN. Ranolazine improves abnormal repolarization and contraction in left ventricular myocytes of dogs with heart failure by inhibiting late sodium current. J Cardiovasc Electrophysiol. 2006;17 (Suppl 1):S169–S177. doi: 10.1111/j.1540-8167.2006.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Antzelevitch C, Belardinelli L, Zygmunt AC, Burashnikov A, Di Diego JM, Fish JM, Cordeiro JM, Thomas G. Electrophysiological effects of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation. 2004;110:904–910. doi: 10.1161/01.CIR.0000139333.83620.5D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sossalla S, Maurer U, Schotola H, Hartmann N, Didie M, Zimmermann WH, Jacobshagen C, Wagner S, Maier LS. Diastolic dysfunction and arrhythmias caused by overexpression of CaMKIIδc can be reversed by inhibition of late Na+ current. Basic Res Cardiol. 2011;106:263–272. doi: 10.1007/s00395-010-0136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hwang H, Arcidi JM, Jr, Hale SL, Simkhovich BZ, Belardinelli L, Dhalla AK, Shryock JC, Kloner RA. Ranolazine as a cardioplegia additive improves recovery of diastolic function in isolated rat hearts. Circulation. 2009;120:S16–21. doi: 10.1161/CIRCULATIONAHA.108.844167. [DOI] [PubMed] [Google Scholar]
- 108.Song Y, Shryock JC, Wu L, Belardinelli L. Antagonism by ranolazine of the pro-arrhythmic effects of increasing late ina in guinea pig ventricular myocytes. J Cardiovasc Pharmacol. 2004;44:192–199. doi: 10.1097/00005344-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 109.Wu L, Shryock JC, Song Y, Li Y, Antzelevitch C, Belardinelli L. Antiarrhythmic effects of ranolazine in a guinea pig in vitro model of long-qt syndrome. J Pharmacol Exp Ther. 2004;310:599–605. doi: 10.1124/jpet.104.066100. [DOI] [PubMed] [Google Scholar]
- 110.Dhalla AK, Wang WQ, Dow J, Shryock JC, Belardinelli L, Bhandari A, Kloner RA. Ranolazine, an antianginal agent, markedly reduces ventricular arrhythmias induced by ischemia and iscehmia-reperfusion. Am J Physiol Heart Circ Physiol. 2009 doi: 10.1152/ajpheart.00173.2009. [DOI] [PubMed] [Google Scholar]
- 111.Morita N, Lee JH, Xie Y, Sovari A, Qu Z, Weiss JN, Karagueuzian HS. Suppression of re-entrant and multifocal ventricular fibrillation by the late sodium current blocker ranolazine. J Am Coll Cardiol. 2011;57:366–375. doi: 10.1016/j.jacc.2010.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rastogi S, Sharov VG, Mishra S, Gupta RC, Blackburn B, Belardinelli L, Stanley WC, Sabbah HN. Ranolazine combined with enalapril or metoprolol prevents progressive lv dysfunction and remodeling in dogs with moderate heart failure. Am J Physiol Heart Circ Physiol. 2008;295:H2149–2155. doi: 10.1152/ajpheart.00728.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, Murphy SA, Budaj A, Varshavsky S, Wolff AA, Skene A, McCabe CH, Braunwald E. Effects of ranolazine on recurrent cardiovascular events in patients with non-ST-elevation acute coronary syndromes: The MERLIN-TIMI 36 randomized trial. JAMA. 2007;297:1775–1783. doi: 10.1001/jama.297.16.1775. [DOI] [PubMed] [Google Scholar]
- 114.Karwatowska-Prokopczuk E, Wang W, Cheng ML, Zeng D, Schwartz PJ, Belardinelli L. The risk of sudden cardiac death in patients with non-st elevation acute coronary syndrome and prolonged QTc interval: Effect of ranolazine. Europace. 2013;15:429–436. doi: 10.1093/europace/eus400. [DOI] [PubMed] [Google Scholar]
- 115.Scirica BM, Morrow DA, Hod H, Murphy SA, Belardinelli L, Hedgepeth CM, Molhoek P, Verheugt FW, Gersh BJ, McCabe CH, Braunwald E. Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non ST-segment elevation acute coronary syndrome: Results from the metabolic efficiency with ranolazine for less ischemia in non ST-elevation acute coronary syndrome thrombolysis in myocardial infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation. 2007;116:1647–1652. doi: 10.1161/CIRCULATIONAHA.107.724880. [DOI] [PubMed] [Google Scholar]
- 116.Bunch TJ, Mahapatra S, Murdock D, Molden J, Weiss JP, May HT, Bair TL, Mader KM, Crandall BG, Day JD, Osborn JS, Muhlestein JB, Lappe DL, Anderson JL. Ranolazine reduces ventricular tachycardia burden and ICD shocks in patients with drug-refractory ICD shocks. Pacing Clin Electrophysiol. 2011;34:1600–1606. doi: 10.1111/j.1540-8159.2011.03208.x. [DOI] [PubMed] [Google Scholar]
- 117.Moss AJ, Zareba W, Schwarz KQ, Rosero S, McNitt S, Robinson JL. Ranolazine shortens repolarization in patients with sustained inward sodium current due to type-3 long-QT syndrome. J Cardiovasc Electrophysiol. 2008;19:1289–1293. doi: 10.1111/j.1540-8167.2008.01246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.De Ferrari GM, Maier LS, Mont L, Schwartz PJ, Simonis G, Leschke M, Gronda E, Boriani G, Darius H, Guillamon Toran L, Savelieva I, Dusi V, Marchionni N, Quintana Rendon M, Schumacher K, Tonini G, Melani L, Giannelli S, Alberto Maggi C, John Camm A. Ranolazine in the treatment of atrial fibrillation: Results of the dose-ranging raffaello (Ranolazine in atrial fibrillation following an electrical cardioversion) study. Heart Rhythm. 2015:S1547–5271. doi: 10.1016/j.hrthm.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 119.Maier LS, Zhang T, Chen L, DeSantiago J, Brown JH, Bers DM. Transgenic camkiiδc overexpression uniquely alters cardiac myocyte Ca2+ handling: Reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res. 2003;92:904–911. doi: 10.1161/01.RES.0000069685.20258.F1. [DOI] [PubMed] [Google Scholar]
- 120.Qi X, Yeh YH, Chartier D, Xiao L, Tsuji Y, Brundel BJ, Kodama I, Nattel S. The calcium/calmodulin/kinase system and arrhythmogenic afterdepolarizations in bradycardia-related acquired long-QT syndrome. Circ Arrhythm Electrophysiol. 2009;2:295–304. doi: 10.1161/CIRCEP.108.815654. [DOI] [PubMed] [Google Scholar]
- 121.Tsuji Y, Hojo M, Voigt N, El-Armouche A, Inden Y, Murohara T, Dobrev D, Nattel S, Kodama I, Kamiya K. Ca2+-related signaling and protein phosphorylation abnormalities play central roles in a new experimental model of electrical storm. Circulation. 2011;123:2192–2203. doi: 10.1161/CIRCULATIONAHA.110.016683. [DOI] [PubMed] [Google Scholar]
- 122.Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, Price EE, Jr, Thiel W, Guatimosim S, Song LS, Madu EC, Shah AN, Vishnivetskaya TA, Atkinson JB, Gurevich VV, Salama G, Lederer WJ, Colbran RJ, Anderson ME. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005;11:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 123.Ling H, Zhang T, Pereira L, Means CK, Cheng H, Gu Y, Dalton ND, Peterson KL, Chen J, Bers D, Brown JH. Requirement for ca2+/calmodulin-dependent kinase II in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice. J Clin Invest. 2009;119:1230–1240. doi: 10.1172/JCI38022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Khoo MS, Li J, Singh MV, Yang Y, Kannankeril P, Wu Y, Grueter CE, Guan X, Oddis CV, Zhang R, Mendes L, Ni G, Madu EC, Yang J, Bass M, Gomez RJ, Wadzinski BE, Olson EN, Colbran RJ, Anderson ME. Death, cardiac dysfunction, and arrhythmias are increased by calmodulin kinase II in calcineurin cardiomyopathy. Circulation. 2006;114:1352–1359. doi: 10.1161/CIRCULATIONAHA.106.644583. [DOI] [PubMed] [Google Scholar]
- 125.Xie LH, Chen F, Karagueuzian HS, Weiss JN. Oxidative-stress-induced afterdepolarizations and calmodulin kinase II signaling. Circ Res. 2009;104:79–86. doi: 10.1161/CIRCRESAHA.108.183475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wagner S, Dantz C, Flebbe H, Azizian A, Sag CM, Engels S, Mollencamp J, Dybkova N, Islam T, Shah AM, Maier LS. NADPH oxidase 2 mediates angiotensin II-dependent cellular arrhythmias via PKA and CaMKII. J Mol Cell Cardiol. 2014;75:206–215. doi: 10.1016/j.yjmcc.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 127.Zhao Z, Fefelova N, Shanmugam M, Bishara P, Babu GJ, Xie LH. Angiotensin II induces afterdepolarizations via reactive oxygen species and calmodulin kinase II signaling. J Mol Cell Cardiol. 2011;50:128–136. doi: 10.1016/j.yjmcc.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Luo M, Guan X, Luczak ED, Lang D, Kutschke W, Gao Z, Yang J, Glynn P, Sossalla S, Swaminathan PD, Weiss RM, Yang B, Rokita AG, Maier LS, Efimov IR, Hund TJ, Anderson ME. Diabetes increases mortality after myocardial infarction by oxidizing camkii. J Clin Invest. 2013;123:1262–1274. doi: 10.1172/JCI65268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Erickson JR, Pereira L, Wang L, Han G, Ferguson A, Dao K, Copeland RJ, Despa F, Hart GW, Ripplinger CM, Bers DM. Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature. 2013;502:372–376. doi: 10.1038/nature12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Coultrap SJ, Bayer KU. Nitric oxide induces Ca2+-independent activity of the Ca2+/calmodulin-dependent protein kinase II (CaMKII) J Biol Chem. 2014;289:19458–19465. doi: 10.1074/jbc.M114.558254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gutierrez DA, Fernandez-Tenorio M, Ogrodnik J, Niggli E. NO-dependent CaMKII activation during β-adrenergic stimulation of cardiac muscle. Cardiovasc Res. 2013;100:392–401. doi: 10.1093/cvr/cvt201. [DOI] [PubMed] [Google Scholar]
- 132.Jian Z, Han H, Zhang T, Puglisi J, Izu LT, Shaw JA, Onofiok E, Erickson JR, Chen YJ, Horvath B, Shimkunas R, Xiao W, Li Y, Pan T, Chan J, Banyasz T, Tardiff JC, Chiamvimonvat N, Bers DM, Lam KS, Chen-Izu Y. Mechanochemotransduction during cardiomyocyte contraction is mediated by localized nitric oxide signaling. Sci Signal. 2014;7:ra27. doi: 10.1126/scisignal.2005046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dybkova N, Wagner S, Backs J, Hund TJ, Mohler PJ, Sowa T, Nikolaev VO, Maier LS. Tubulin polymerization disrupts cardiac β-adrenergic regulation of late INa. Cardiovasc Res. 2014;103:168–177. doi: 10.1093/cvr/cvu120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mangmool S, Shukla AK, Rockman HA. Beta-arrestin-dependent activation of Ca2+/calmodulin kinase II after β1-adrenergic receptor stimulation. J Cell Biol. 2010;189:573–587. doi: 10.1083/jcb.200911047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pereira L, Cheng H, Lao DH, Na L, van Oort RJ, Brown JH, Wehrens XHT, Chen J, Bers DM. Epac2 mediates cardiac β1-adrenergic-dependent sarcoplasmic reticulum Ca2+ leak and arrhythmia. Circulation. 2013;127:913–922. doi: 10.1161/CIRCULATIONAHA.12.148619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Erickson JR, Patel R, Ferguson A, Bossuyt J, Bers DM. Fluorescence resonance energy transfer-based sensor camui provides new insight into mechanisms of calcium/calmodulin-dependent protein kinase ii activation in intact cardiomyocytes. Circ Res. 2011;109:729–738. doi: 10.1161/CIRCRESAHA.111.247148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Anderson ME, Braun AP, Schulman H, Premack BA. Multifunctional Ca2+/calmodulin-dependent protein kinase mediates Ca2+-induced enhancement of the L-type Ca2+ current in rabbit ventricular myocytes. Circ Res. 1994;75:854–861. doi: 10.1161/01.res.75.5.854. [DOI] [PubMed] [Google Scholar]
- 138.Xiao RP, Cheng H, Lederer WJ, Suzuki T, Lakatta EG. Dual regulation of Ca2+/calmodulin-dependent kinase II activity by membrane voltage and by calcium influx. Proc Natl Acad Sci U S A. 1994;91:9659–9663. doi: 10.1073/pnas.91.20.9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yuan W, Bers DM. Ca-dependent facilitation of cardiac Ca current is due to Ca-calmodulin-dependent protein kinase. Am J Physiol. 1994;267:H982–993. doi: 10.1152/ajpheart.1994.267.3.H982. [DOI] [PubMed] [Google Scholar]
- 140.Anderson ME, Braun AP, Wu Y, Lu T, Wu Y, Schulman H, Sung RJ. KN-93, an inhibitor of multifunctional Ca2+/calmodulin-dependent protein kinase, decreases early afterdepolarizations in rabbit heart. J Pharmacol Exp Ther. 1998;287:996–1006. [PubMed] [Google Scholar]
- 141.Wu Y, MacMillan LB, McNeill RB, Colbran RJ, Anderson ME. CaM kinase augments cardiac L-type Ca2+ current: A cellular mechanism for long QT arrhythmias. Am J Physiol. 1999;276:H2168–2178. doi: 10.1152/ajpheart.1999.276.6.H2168. [DOI] [PubMed] [Google Scholar]
- 142.Koval OM, Guan X, Wu Y, Joiner ML, Gao Z, Chen B, Grumbach IM, Luczak ED, Colbran RJ, Song LS, Hund TJ, Mohler PJ, Anderson ME. Cav1.2 β-subunit coordinates CaMKII-triggered cardiomyocyte death and afterdepolarizations. Proc Natl Acad Sci U S A. 2010;107:4996–5000. doi: 10.1073/pnas.0913760107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bezzina C, Veldkamp MW, van Den Berg MP, Postma AV, Rook MB, Viersma JW, van Langen IM, Tan-Sindhunata G, Bink-Boelkens MT, van Der Hout AH, Mannens MM, Wilde AA. A single Na+ channel mutation causing both long-QT and brugada syndromes. Circ Res. 1999;85:1206–1213. doi: 10.1161/01.res.85.12.1206. [DOI] [PubMed] [Google Scholar]
- 144.Veldkamp MW, Viswanathan PC, Bezzina C, Baartscheer A, Wilde AAM, Balser JR. Two distinct congenital arrhythmias evoked by a multidysfunctional Na+ channel. Circ Res. 2000;86:91e–97. doi: 10.1161/01.res.86.9.e91. [DOI] [PubMed] [Google Scholar]
- 145.Wehrens XHT, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res. 2004;94:e61–e70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- 146.Simmerman HK, Collins JH, Theibert JL, Wegener AD, Jones LR. Sequence analysis of phospholamban. Identification of phosphorylation sites and two major structural domains. J Biol Chem. 1986;261:13333–13341. [PubMed] [Google Scholar]
- 147.Bers DM, Eisner DA, Valdivia HH. Sarcoplasmic reticulum Ca2+ and heart failure: Roles of diastolic leak and Ca2+ transport. Circ Res. 2003;93:487–490. doi: 10.1161/01.RES.0000091871.54907.6B. [DOI] [PubMed] [Google Scholar]
- 148.Lindner M, Erdmann E, Beuckelmann DJ. Calcium content of the sarcoplasmic reticulum in isolated ventricular myocytes from patients with terminal heart failure. J Mol Cell Cardiol. 1998;30:743–749. doi: 10.1006/jmcc.1997.0626. [DOI] [PubMed] [Google Scholar]
- 149.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 150.Curran J, Brown KH, Santiago DJ, Pogwizd S, Bers DM, Shannon TR. Spontaneous ca waves in ventricular myocytes from failing hearts depend on Ca2+-calmodulin-dependent protein kinase II. J Mol Cell Cardiol. 2010;49:25–32. doi: 10.1016/j.yjmcc.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang T, Guo T, Mishra S, Dalton ND, Kranias EG, Peterson KL, Bers DM, Brown JH. Phospholamban ablation rescues sarcoplasmic reticulum Ca2+ handling but exacerbates cardiac dysfunction in CaMKIIδc transgenic mice. Circ Res. 2010;106:354–362. doi: 10.1161/CIRCRESAHA.109.207423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Respress JL, van Oort RJ, Li N, Rolim N, Dixit SS, deAlmeida A, Voigt N, Lawrence WS, Skapura DG, Skardal K, Wisloff U, Wieland T, Ai X, Pogwizd SM, Dobrev D, Wehrens XH. Role of RyR2 phosphorylation at S2814 during heart failure progression. Circ Res. 2012;110:1474–1483. doi: 10.1161/CIRCRESAHA.112.268094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.van Oort RJ, McCauley MD, Dixit SS, Pereira L, Yang Y, Respress JL, Wang Q, De Almeida AC, Skapura DG, Anderson ME, Bers DM, Wehrens XHT. Ryanodine receptor phosphorylation by calcium/calmodulin-dependent protein kinase II promotes life-threatening ventricular arrhythmias in mice with heart failure. Circulation. 2010;122:2669–2679. doi: 10.1161/CIRCULATIONAHA.110.982298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fischer TH, Eiringhaus J, Dybkova N, Forster A, Herting J, Kleinwachter A, Ljubojevic S, Schmitto JD, Streckfuss-Bomeke K, Renner A, Gummert J, Hasenfuss G, Maier LS, Sossalla S. Ca2+/calmodulin-dependent protein kinase II equally induces sarcoplasmic reticulum Ca2+ leak in human ischaemic and dilated cardiomyopathy. Eur J Heart Fail. 2014;16:1292–1300. doi: 10.1002/ejhf.163. [DOI] [PubMed] [Google Scholar]
- 155.Oestreich EA, Malik S, Goonasekera SA, Blaxall BC, Kelley GG, Dirksen RT, Smrcka AV. Epac and phospholipase Cε regulate Ca2+ release in the heart by activation of protein kinase Cε and calcium-calmodulin kinase II. J Biol Chem. 2009;284:1514–1522. doi: 10.1074/jbc.M806994200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Curran J, Hinton MJ, Rios E, Bers DM, Shannon TR. β-adrenergic enhancement of sarcoplasmic reticulum Ca leak in cardiac myocytes is mediated by calcium/calmodulin-dependent protein kinase. Circ Res. 2007;100:391–398. doi: 10.1161/01.RES.0000258172.74570.e6. [DOI] [PubMed] [Google Scholar]
- 157.Pereira L, Metrich M, Fernandez-Velasco M, Lucas A, Leroy J, Perrier R, Morel E, Fischmeister R, Richard S, Benitah JP, Lezoualc’h F, Gomez AM. The cAMP binding protein Epac modulates Ca2+ sparks by a Ca2+/calmodulin kinase signalling pathway in rat cardiac myocytes. J Physiol. 2007;583:685–694. doi: 10.1113/jphysiol.2007.133066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Neef S, Dybkova N, Sossalla S, Ort KR, Fluschnik N, Neumann K, Seipelt R, Schöndube FA, Hasenfuss G, Maier LS. CaMKII-dependent diastolic SR Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ Res. 2010;106:1134–1144. doi: 10.1161/CIRCRESAHA.109.203836. [DOI] [PubMed] [Google Scholar]
- 159.Purohit A, Rokita AG, Guan X, Chen B, Koval OM, Voigt N, Neef S, Sowa T, Gao Z, Luczak ED, Stefansdottir H, Behunin AC, Li N, El-Accaoui RN, Yang B, Swaminathan PD, Weiss RM, Wehrens XH, Song LS, Dobrev D, Maier LS, Anderson ME. Oxidized Ca2+/calmodulin-dependent protein kinase II triggers atrial fibrillation. Circulation. 2013;128:1748–1757. doi: 10.1161/CIRCULATIONAHA.113.003313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, Sun Q, Wieland T, Ravens U, Nattel S, Wehrens XH, Dobrev D. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation. 2012;125:2059–2070. doi: 10.1161/CIRCULATIONAHA.111.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li N, Wang T, Wang W, Cutler MJ, Wang Q, Voigt N, Rosenbaum DS, Dobrev D, Wehrens XH. Inhibition of CaMKII phosphorylation of RyR2 prevents induction of atrial fibrillation in FKBP12.6 knockout mice. Circ Res. 2012;110:465–470. doi: 10.1161/CIRCRESAHA.111.253229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 163.Prosser BL, Ward CW, Lederer WJ. X-ros signaling: Rapid mechano-chemo transduction in heart. Science. 2011;333:1440–1445. doi: 10.1126/science.1202768. [DOI] [PubMed] [Google Scholar]
- 164.Bovo E, Lipsius SL, Zima AV. Reactive oxygen species contribute to the development of arrhythmogenic Ca2+ waves during β-adrenergic receptor stimulation in rabbit cardiomyocytes. J Physiol. 2012;590:3291–3304. doi: 10.1113/jphysiol.2012.230748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation. 1995;91:1512–1519. doi: 10.1161/01.cir.91.5.1512. [DOI] [PubMed] [Google Scholar]
- 166.Hayashi M, Denjoy I, Extramiana F, Maltret A, Buisson NR, Lupoglazoff JM, Klug D, Hayashi M, Takatsuki S, Villain E, Kamblock J, Messali A, Guicheney P, Lunardi J, Leenhardt A. Incidence and risk factors of arrhythmic events in catecholaminergic polymorphic ventricular tachycardia. Circulation. 2009;119:2426–2434. doi: 10.1161/CIRCULATIONAHA.108.829267. [DOI] [PubMed] [Google Scholar]
- 167.Sy RW, Gollob MH, Klein GJ, Yee R, Skanes AC, Gula LJ, Leong-Sit P, Gow RM, Green MS, Birnie DH, Krahn AD. Arrhythmia characterization and long-term outcomes in catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2011;8:864–871. doi: 10.1016/j.hrthm.2011.01.048. [DOI] [PubMed] [Google Scholar]
- 168.Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, Sorrentino V, Danieli GA. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- 169.Dalal D, Nasir K, Bomma C, Prakasa K, Tandri H, Piccini J, Roguin A, Tichnell C, James C, Russell SD, Judge DP, Abraham T, Spevak PJ, Bluemke DA, Calkins H. Arrhythmogenic right ventricular dysplasia: A united states experience. Circulation. 2005;112:3823–3832. doi: 10.1161/CIRCULATIONAHA.105.542266. [DOI] [PubMed] [Google Scholar]
- 170.Lahat H, Pras E, Olender T, Avidan N, Ben-Asher E, Man O, Levy-Nissenbaum E, Khoury A, Lorber A, Goldman B, Lancet D, Eldar M. A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in bedouin families from israel. Am J Hum Genet. 2001;69:1378–1384. doi: 10.1086/324565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Roux-Buisson N, Cacheux M, Fourest-Lieuvin A, Fauconnier J, Brocard J, Denjoy I, Durand P, Guicheney P, Kyndt F, Leenhardt A, Le Marec H, Lucet V, Mabo P, Probst V, Monnier N, Ray PF, Santoni E, Tremeaux P, Lacampagne A, Faure J, Lunardi J, Marty I. Absence of triadin, a protein of the calcium release complex, is responsible for cardiac arrhythmia with sudden death in human. Hum Mol Genet. 2012;21:2759–2767. doi: 10.1093/hmg/dds104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sedej S, Heinzel FR, Walther S, Dybkova N, Wakula P, Groborz J, Gronau P, Maier LS, Vos MA, Lai FA, Napolitano C, Priori SG, Kockskamper J, Pieske B. Na+-dependent SR Ca2+ overload induces arrhythmogenic events in mouse cardiomyocytes with a human CPVT mutation. Cardiovasc Res. 2010;87:50–59. doi: 10.1093/cvr/cvq007. [DOI] [PubMed] [Google Scholar]
- 173.Liu N, Ruan Y, Denegri M, Bachetti T, Li Y, Colombi B, Napolitano C, Coetzee WA, Priori SG. Calmodulin kinase II inhibition prevents arrhythmias in RyR2(R4496C+/−) mice with catecholaminergic polymorphic ventricular tachycardia. J Mol Cell Cardiol. 2011;50:214–222. doi: 10.1016/j.yjmcc.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 174.Dybkova N, Sedej S, Napolitano C, Neef S, Rokita AG, Hunlich M, Brown JH, Kockskamper J, Priori SG, Pieske B, Maier LS. Overexpression of CaMKIIδc in RyR2 R4496C+/− knock-in mice leads to altered intracellular Ca2+ handling and increased mortality. J Am Coll Cardiol. 2011;57:469–479. doi: 10.1016/j.jacc.2010.08.639. [DOI] [PubMed] [Google Scholar]
- 175.Venetucci L, Denegri M, Napolitano C, Priori SG. Inherited calcium channelopathies in the pathophysiology of arrhythmias. Nature reviews Cardiology. 2012;9:561–575. doi: 10.1038/nrcardio.2012.93. [DOI] [PubMed] [Google Scholar]
- 176.van der Werf C, Wilde AA. Catecholaminergic polymorphic ventricular tachycardia: From bench to bedside. Heart. 2013;99:497–504. doi: 10.1136/heartjnl-2012-302033. [DOI] [PubMed] [Google Scholar]
- 177.Leenhardt A, Denjoy I, Guicheney P. Catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol. 2012;5:1044–1052. doi: 10.1161/CIRCEP.111.962027. [DOI] [PubMed] [Google Scholar]
- 178.Duygu B, Poels EM, da Costa Martins PA. Genetics and epigenetics of arrhythmia and heart failure. Frontiers in genetics. 2013;4:219. doi: 10.3389/fgene.2013.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bers DM. Ca2+-calmodulin-dependent protein kinase II regulation of cardiac excitation-transcription coupling. Heart Rhythm. 2011;8:1101–1104. doi: 10.1016/j.hrthm.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Backs J, Olson EN. Control of cardiac growth by histone acetylation/deacetylation. Circ Res. 2006;98:15–24. doi: 10.1161/01.RES.0000197782.21444.8f. [DOI] [PubMed] [Google Scholar]
- 181.Wu X, Zhang T, Bossuyt J, Li X, McKinsey TA, Dedman JR, Olson EN, Chen J, Brown JH, Bers DM. Local insp3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling. J Clin Invest. 2006;116:675–682. doi: 10.1172/JCI27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Awad S, Al-Haffar KM, Marashly Q, Quijada P, Kunhi M, Al-Yacoub N, Wade FS, Mohammed SF, Al-Dayel F, Sutherland G, Assiri A, Sussman M, Bers D, Al-Habeeb W, Poizat C. Control of histone H3 phosphorylation by CaMKIIΔ in response to haemodynamic cardiac stress. J Pathol. 2015;235:606–618. doi: 10.1002/path.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Awad S, Kunhi M, Little GH, Bai Y, An W, Bers D, Kedes L, Poizat C. Nuclear camkii enhances histone H3 phosphorylation and remodels chromatin during cardiac hypertrophy. Nucleic Acids Res. 2013;41:7656–7672. doi: 10.1093/nar/gkt500. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 184.Bers DM. Calcium fluxes involved in control of cardiac myocyte contraction. Circ Res. 2000;87:275–281. doi: 10.1161/01.res.87.4.275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.